Abstract
Background:
Excessive neutrophil inflammation is the hallmark of cystic fibrosis (CF) airway disease. Novel technologies for characterizing neutrophil dysfunction may provide insight into the nature of these abnormalities, revealing a greater mechanistic understanding and new avenues for CF therapies that target these mechanisms.
Methods:
Blood was collected from individuals with CF in the outpatient clinic, CF individuals hospitalized for a pulmonary exacerbation, and non-CF controls. Using microfluidic assays and advanced imaging technologies, we characterized 1) spontaneous neutrophil migration using microfluidic motility mazes, 2) neutrophil migration to and phagocytosis of Staphylococcal aureus particles in a microfluidic arena, 3) neutrophil swarming on Candida albicans clusters, and 4) Pseudomonas aeruginosa-induced neutrophil transepithelial migration using micro-optical coherence technology (µOCT).
Results:
Participants included 44 individuals: 16 Outpatient CF, 13 Hospitalized CF, and 15 Non-CF individuals. While no differences were seen with spontaneous migration, CF neutrophils migrated towards S. aureus particles more quickly than non-CF neutrophils (p<0.05). CF neutrophils, especially Hospitalized CF neutrophils, generated significantly larger aggregates around S. aureus particles over time. Hospitalized CF neutrophils were more likely to have dysfunctional swarming (p<0.01) and less efficient clearing of C. albicans (p<0.0001). When comparing trans-epithelial migration towards Pseudomonas aeruginosa epithelial infection, Outpatient CF neutrophils displayed an increase in the magnitude of transmigration and adherence to the epithelium (P<0.05).
Conclusions:
Advanced technologies for characterizing CF neutrophil function reveal significantly altered migratory responses, cell-to-cell clustering, and microbe containment. Future investigations will probe mechanistic basis for abnormal responses in CF to identify potential avenues for novel anti-inflammatory therapeutics.
Keywords: cystic fibrosis, neutrophil, inflammation, micro-fluidics, micro-optical coherence tomography
Introduction:
Cystic fibrosis (CF), a genetic disease caused by a mutated cystic fibrosis transmembrane conductance regulator (CFTR) gene that results in a dysfunctional or absent epithelial chloride channel, is characterized by maladaptive neutrophilic inflammation. Clinically, individuals with CF have chronic airway infections, with thick neutrophil-laden mucus, which leads to a progressive decline in lung function, ultimately resulting in early death from respiratory failure. As neutrophils are key drivers of the hyper-inflammatory state associated with CF, there remains considerable interest in understanding how CF neutrophils function in comparison to non-CF neutrophils. It is well established that CF airways have increased IL-8 and neutrophil elastase, reflecting the increased neutrophil presence. Leukotriene B4 (LTB4) is also increased in the airways of adults with CF (1). Interestingly, children with CF with negative bronchoalveolar lavage (BAL) cultures have LTB4 levels comparable to healthy controls, but children with CF with BAL cultures growing pathogenic bacteria have substantially more LTB4 than non-CF pediatric individuals growing the same pathogens (2), suggesting an excessive, dysregulated LTB4 response in CF individuals as a result of infection. CF neutrophils have also been reported to have increased migration to IL-8 (3), increased activation of the inflammasome with increased IL-1β production (4, 5), and dysfunctional phagolysosomes resulting in the ineffective killing of bacteria (6, 7). Prior research studying neutrophil transepithelial migration in CF has focused on the role of CF epithelium or the consequences of transepithelial migration on neutrophil phenotype (8), rather than the intrinsic properties of neutrophils from individuals with CF (3).
Characterizing neutrophil function has dramatically improved with the emergence of new technology. Microfluidic devices combined with time-lapse microscopy enable single-neutrophil resolution analysis using small amounts of whole blood. Such microfluidic tools have been applied to study the ability of neutrophils to squeeze through tight spaces (9–11), retrotax from tissues to circulation (12, 13), cooperate during swarming behavior (14, 15), phagocytose and kill pathogens (16), and spontaneously migrate in the absence of chemoattractants (17). Micro-optical coherence tomography (µOCT) is a novel label-free cross-sectional imaging technology with sub-cellular resolution that enables the visualization of cell migration through mucosal barriers in vitro and in vivo (18–21). Probing the functional aspects of neutrophils using novel technologies has dramatically advanced our understanding of immune responses in sepsis (17, 22), trauma and burn injuries (23, 24), diabetes (25), chronic granulomatous disease (15), organ transplant (9, 26, 27) and Alzheimer’s disease (28), and can play an important role in furthering our understanding of neutrophil dysfunction in CF.
Here, we aim to illuminate specific aspects of CF neutrophil dysfunction that may explain the accelerated influx of neutrophils with an aberrant function within the CF airways. We used advanced technologies to analyze several aspects of neutrophil activities, including spontaneous migration, migration towards infectious particles and whole pathogens, swarming and phagocytosis, and trans-epithelial migration. Further, we sought to ascertain differential neutrophil responses from individuals with CF seen in the outpatient clinic, individuals with CF experiencing a pulmonary exacerbation, and individuals without CF. Understanding these differences will provide valuable insights into disease pathogenesis and opportunities for anti-inflammatory therapies in CF.
Materials and Methods
Human subjects
Individuals with CF who were seen in the outpatient clinic or who were hospitalized for a pulmonary exacerbation, plus healthy individuals without CF, were recruited to participate in this IRB-approved protocol (MGH IRB # 2011P000620). After informed consent was obtained, blood was collected for processing. Medical information was extracted from the electronic medical record.
Spontaneous Neutrophil Motility Assay
Spontaneous neutrophil behavior from the blood was studied using previously validated microfluidic motility mazes (17). Microfluidic devices provided eight fields of view, each containing one motility maze. Devices were primed with Iscove’s Modified Dulbecco’s Medium (IMDM) containing 20% fetal bovine serum (FBS). Whole blood from subjects was diluted 1:1 in IMDM with 20% FBS, then 1 μL of diluted blood was pipetted into the device’s center. Using time-lapse brightfield microscopy (CytoSMART LUX2 system, Eindhoven, The Netherlands), neutrophil positions were recorded every 10 seconds, for 4 hours at 37 °C. Neutrophil motility parameters were analyzed by manually tracking the cells using ImageJ/Fiji (NIH) analysis software. The percentage of maze coverage was obtained by recording the total amount of channels and edges that were visited by neutrophils in 4 hours using a multi-point tool. The total percent of the maze covered was calculated using: (3.84*number of channels) + (0.58*number of edges), where 3.84 and 0.58 are the percentage of one channel and one edge, respectively. The overall percent maze coverage for a sample was then obtained by averaging the values obtained in all 8 mazes. The NSM score was calculated as previously defined: NSM = N*(R + A + O + AD)/1000 where N is the total number of neutrophils moving spontaneously (in all eight mazes), and R, A, O, AD are numerical values quantifying the retrotaxis, arrest, oscillations phenotypes and average distance migrated by the spontaneously migrating neutrophils, respectively(17).
Neutrophil Recruitment and Phagocytosis Assay
We probed direct interactions between S. aureus particles and neutrophils using previously validated microfluidic arenas (16). The device includes multiple chambers arrayed inside a larger channel and connected by narrow migration channels. Central reservoirs were primed with S. aureus particles labeled with Alexa Fluor 488 and fMLP (100 nM) in IMDM/ 20% FBS while the outer chamber was filled with IMDM/ 20%FBS only. A suspension of neutrophils was then prepared from a small volume of whole blood via enhanced red blood cell (RBC) sedimentation following mixing with heparinized media and HetaSep (2:2:1 ratio, StemCell, Vancouver, Canada) and then gently injected into the outer chamber of the device. Using fluorescent time-lapse microscopy, we monitored neutrophil recruitment and phagocytosis in response to labeled particles every 5 minutes over 5 hours. Neutrophil recruitment was tracked and analyzed using ImageJ/Fiji (NIH) analysis software with TrackMate automated tracking for fluorescent time-lapse images. Phagocytosis parameters were also measured using ImageJ/Fiji analysis software using automated thresholding to measure areas of phagocytosed at interval time points.
Neutrophil Swarming Assay
We examined neutrophil swarming responses using in vitro microscale arrays, previously validated to allow the interrogation of swarming responses to live microbial clusters (15). Briefly, poly-l-lysine/ZETAG spots of 100 µm diameter are printed in arrays on glass slides. We place 16-well attachments to these slides (Grace Bio-labs), then add live C. albicans constitutively expressing far-red protein (29) to the wells. C. albicans is incubated for 5 minutes to allow adherence to the wells. Then, excess yeast is washed off with PBS. Slides are screened for complete patterning of yeast on the arrays and minimal non-specific binding before use. Primary human neutrophils from non-CF, CF-outpatient, and CF-hospitalized individuals are isolated from whole peripheral blood by magnetic separation according to the manufacturer’s protocol (Stemcell). Neutrophils are stained with Hoechst prior to use. Five hundred thousand neutrophils are added to each well as appropriate, and then swarming responses are followed via fluorescent time-lapse microscopy for at least 12 hours. Fungal growth and swarm area are quantified at the specified time points (16 hours for fungal growth) by manually tracing the area of fungal growth, including any hyphae, or the area covered by neutrophils in ImageJ/Fiji (NIH). Fungal growth is manually outlined using the brightfield and far-red fluorescent channels to accurately ascertain coverage by fungi at each cluster site. Swarm area is manually outlined using the DAPI fluorescent channel.
µOCT imaging of the neutrophil transepithelial migration assay
Neutrophil migration across an infected airway was studied using an inverted air-liquid interface (ALI) in vitro co-culture model and protocol as previously reported (19, 20). Paired experiments (n=7) were performed sequentially with CF-outpatient and non-CF neutrophils, with variation in the order of analysis from experiment to experiment. Sub-cellular resolution, 3D time-lapse µOCT (18, 19) videos of the neutrophil migration were recorded by scanning the µOCT imaging beam over a 1×1 mm lateral width every 10 minutes for 2 hours. Using binarized µOCT images, volumes representing neutrophils in the apical compartment were quantified over time to study the kinetics of neutrophil transepithelial migration. The volume of neutrophil migration was converted to numerical counts based on the pre-determined mean diameter of neutrophils, (Supplemental Fig. 1a; 13.5 ± 2.2 µm, mean ± S.D.), which was similar between the CF-outpatient and non-CF groups. The mean neutrophil column area, representing a cluster of neutrophils migrating across the epithelium in unison, was determined from en face images at approximately 10 µm below the monolayers. Adherent neutrophil columns in binarized en face images were segmented with Watershed algorithm. Image processing and calculations were performed using ImageJ (30) and Matlab (Mathworks Inc).
Myeloperoxidase Activity
Cell-associated myeloperoxidase (MPO) activity / neutrophil was quantified using a colorimetric assay employing the peroxidase substrate 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS). First, the number of neutrophils in the assay was determined by counting a sample on a hemocytometer. Then, neutrophils are lysed with 0.5% triton X-100 and the lysate is transfered to a 96-well plate. After H2O2 and ABTS substrate solutions are added to the plate, the peroxidase activity is determined on a microplate reader from the optical density determined at wavelength of 405 nm (31). Supplemental Fig. 1b displays a representative standard curve for CF and non-CF individuals.
Statistical methods
Results were analyzed using one-way ANOVA with Tukey’s multiple comparison test, Kruskal-Wallis with Dunn’s post-test, and paired t-tests in Prism (GraphPad Software Inc.). Categorical comparisons were completed using Fischer’s exact test.
Additional methodologic detail can be found in Supplemental Materials.
Results
Twenty-nine individuals with CF and 15 individuals without CF (Non-CF) were enrolled in the study. Individuals with CF were defined as having two CF-causing mutations, positive sweat chloride results, and a clinical diagnosis of CF. Non-CF controls were defined as individuals over 18 years of age without any medical history of autoimmune disease or immunodeficiency, anti-inflammatory medication use, or symptoms of illness. The average age was 27.4 years and 35.6 years for the Non-CF and CF individuals, respectively. One-third of Non-CF individuals were male, whereas the CF participants were more equally gender-matched (Table 1).
Table 1:
Individuals enrolled in three groups Non-CF, CF-outpatients, and CF-hospitalized.
| Clinical demographics (N=44) | Non-CF (n=15) | CF-outpatient (n=16) | CF-hospitalized (n=13) |
|---|---|---|---|
| Age, years, mean (SD) | 27.4 (6.2) | 39.1 (13.8) | 31.4 (10.9) |
| Male, % (frequency) | 33 (5) | 50 (8) | 54 (7) |
| Most recent FEV1, % predicted, mean (SD) | 51.5 (18.1) | 39.7 (17.3) | |
| Most recent FVC, % predicted, mean (SD) | 66.4 (15.6) | 57.8 (19.9) | |
| Most recent BMI (kg/m2), mean (SD) | 25.5 (5.1) | 19.8 (2.8) | |
| Co-morbidities, % (frequency) | |||
| Pancreatic insufficiency | 93.8 (15) | 100 (13) | |
| CF-related diabetes | 37.5 (6) | 38.5 (5) | |
| Liver disease | 12.5 (2) | 7.7 (1) | |
| Implanted central venous access | 12.5 (2) | 46.2 (6) | |
| Allergic bronchopulmonary aspergillosis | 12.5 (2) | 0 (0) | |
| Autoimmunity | 12.5 (2) | 0 (0) | |
| History of cancer | 18.8 (3) | 7.7 (1) | |
| Genotype, % (frequency) | |||
| F508del/F508del | 25 (4) | 30.8 (4) | |
| F508del/Minimal Function | 62.5 (10) | 38.5 (5) | |
| Gating | 0 (0) | 23.1 (3) | |
| Null/Null | 6.3 (1) | 7.7 (1) | |
| Minimal Function (no F508del) | 6.3 (1) | 0 (0) | |
Individuals with CF were comprised of two groups: individuals seen in the outpatient clinic with baseline symptomatology (CF-outpatients) and individuals hospitalized during a pulmonary exacerbation (CF-hospitalized). There was no significant difference in age or lung function between the CF groups. However, CF-hospitalized trended younger (31.4 vs. 39.1 years, CF-hospitalized vs. outpatient, respectively), with lower lung function (FEV1 39.7% vs. 51.5% predicted). CF-hospitalized had lower body mass index (BMI) (19.8 vs 25.5 kg/m2, Students t-test, P=0.001), reflecting more advanced disease. Additionally, more CF-hospitalized individuals had catheters implanted for central venous access (46.2% vs. 12.5%, Fischer’s exact test, P<0.001). Overall, CF co-morbidities and genotypes were reflective of the CF population, with the most common co-morbidities being pancreatic insufficiency and CF-related diabetes (Table 1).
The genotype for all CF individuals was documented. At the time of this study, CFTR modulators, which are new therapies that improve CFTR production and/or function, were available for individuals with two F508del mutations (lumacaftor/ivacaftor and tezacaftor/ivacaftor) and individuals with one gating mutation (ivacaftor). None of the CF-outpatients were taking CFTR modulators, compared to five of the CF-hospitalized group.
To understand differences that may contribute to the neutrophilic, hyper-inflamed state of the CF lung, we studied neutrophil functionality in a series of assays reflecting key aspects of neutrophil antimicrobial defense in the CF lung. Neutrophil spontaneous migration, migration towards and engulfment of microbial particles, swarming around and killing of a live pathogen, and migration across an infected airway were all examined with CF and non-CF neutrophils.
CF neutrophils do not have increased spontaneous migration.
First, we sought to assess whether CF neutrophils display differences in motility. We employed a recently designed microfluidic assay that monitors spontaneous neutrophil migration in one microliter of diluted blood for 4 hours (Figure 1A). To assess whether CF neutrophils were more likely to be active during a pulmonary exacerbation, we included neutrophils from both CF-outpatient and CF-hospitalized groups. We measured no significant differences in neutrophil velocity between Non-CF neutrophils and neutrophils from either CF-outpatient or CF-hospitalized individuals (Figure 1B). We also quantified maze coverage, as a measure of the ability of neutrophils to patrol large areas and found no difference between the three groups of donors (Figure 1C). Neutrophil spontaneous migration (NSM) scores were calculated for each patient group based on several parameters, such as the number of neutrophils, distance traveled, and phenotypic migration parameters (Supplementary methods). We measured no differences in motility between neutrophils from the three groups of donors (Figure 1D). The relationship between NSM score and maze coverage was highly linear (R2=0.81).
Figure 1: CF neutrophils do not exhibit increased spontaneous migration in whole blood.
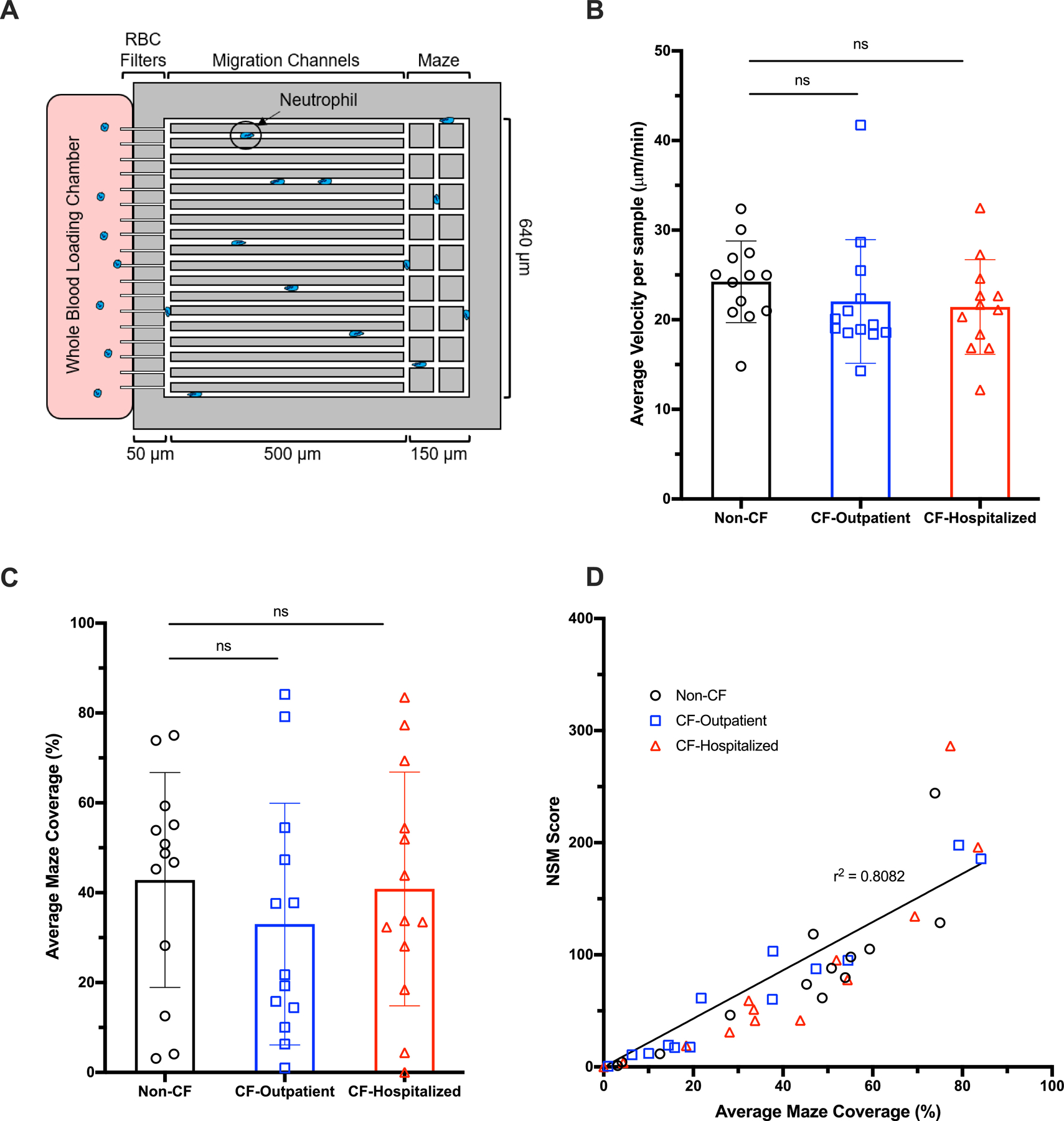
A drop of diluted blood was loaded into a microfluidic device composed of 8 migratory mazes filled with media. Spontaneous neutrophil migration parameters were studied in the absence of chemoattractants. (A) Schematic representation of the device showing spontaneous neutrophil migration from the whole blood loading chamber to the migratory maze composed of red blood cell (RBC) filters, migration channels, and a maze. (B) The average velocity of neutrophils in non-CF (N=13), CF-Outpatient (N=13), and CF-Hospitalized (N=12) were comparable (non-parametric Kruskal-Wallis with Dunn’s test). (C) Percentages of maze coverage in non-CF, CF-Outpatient, and CF-Hospitalized were similar (parametric ANOVA with Tukey’s test). (D) The relationship between NSM score and maze coverage was highly linear (R2=0.81), with no differences between neutrophils from the three different groups. NSM= neutrophil spontaneous migration
CF neutrophils display aberrant phagocytosis of microbe particles.
We then tested the ability of neutrophils to chemotax towards fMLP sources and to phagocytose fluorescently labeled Staphylococcus aureus particles inside microfluidic arenas (Figure 2A, Supplemental Video 1, Supplemental Video 2). We quantified the number of neutrophils entering the arenas then measured the size of particle aggregates inside the neutrophils. Particle aggregates < 100 µm2 primarily represent events involving a single neutrophil (Figure 2B, left two columns). Particle aggregates > 100 µm2 are usually associated with the swarming of multiple neutrophils (Figure 2B, right two columns). Larger particle aggregates form when multiple neutrophils carrying particles converge to one spot. When assessing neutrophil migration towards S. aureus particles, CF neutrophils are recruited more quickly into the arena than non-CF neutrophils (Figure 2C). The percent incidence of various sized phagocytosed particle aggregates was determined for each patient neutrophil group following the 5-hour incubation (Figure 2D). We found that non-CF neutrophils display the greatest percentage of particle aggregates less than 15 µm2, as compared to CF groups (Figure 2E), whereas CF neutrophils show a higher percentage of particle aggregates greater than 100 µm2 (Figure 2F). These results suggest that the phagocytosis load is more heterogeneous among CF compared to non-CF neutrophils. We also observed significantly larger phagocytosed particle aggregates formed inside both CF-outpatient and CF-hospitalized neutrophils within the first 30 minutes. The average aggregate size continued to increase throughout the 5-hour incubation in CF-hospitalized neutrophils (Figure 2G). Additionally, when large aggregates were formed, their size was significantly larger in CF-hospitalized neutrophils than both non-CF and CF-outpatient (Figure 2H).
Figure 2: CF neutrophils display differences in microbe-like particle phagocytosis:
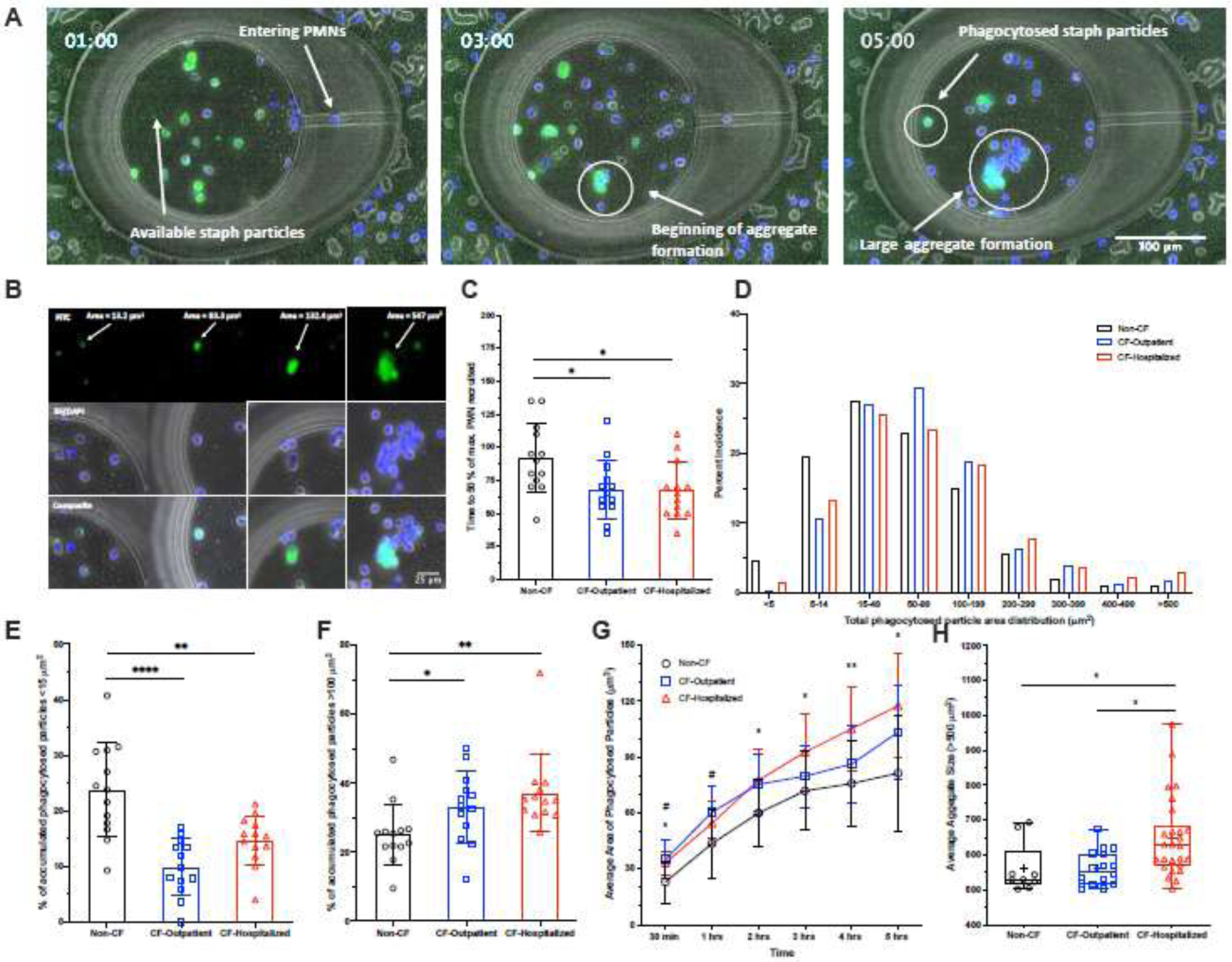
S. aureus bio-particles with fMLP were loaded into microfluidic chambers. Buffy coat-containing neutrophils stained with Hoechst were loaded around these chambers to observe host-pathogen interactions, specifically neutrophil recruitment to and phagocytosis of these particles. (A) A panel of images taken at 1, 3 and 5 hours showing the distribution of neutrophils (Hoechst, blue) and phagocytosis behaviors in response to S. aureus particles (FITC, green), which are a faint green, becoming brighter with increased aggregation. (B) A panel (left to right) showing small areas of phagocytosed particles formed by individual neutrophils to larger aggregates of S. aureus particles formed by multiple neutrophils. Phagocytosed S. aureus particles are shown in the FITC channel, while neutrophils are shown in the BF/DAPI channel. (C) Non-CF neutrophils take longer on average to reach 50% of the maximum number of neutrophils recruited at 5 hours compared to CF-outpatient and CF-hospitalized individuals. (D) The distribution of phagocytosed particles and aggregate sizes formed after 5 hours from Non-CF and CF individuals. Comparisons were made between each group for the percent of accumulated, phagocytoses S. aureus particles that were (E) <15µm or (F) >100µm in size at 5 hours. (G) Average phagocytosed particle sizes measured over time. (H) When large aggregates (>500µm) were formed, average size of aggregate was compared between groups. An ordinary one-way ANOVA with Tukey’s multiple comparison test was used to test for significance. *P<0.05
CF neutrophils display altered swarming phenotypes and deficient microbial restriction.
Neutrophil swarming assays, comprised of microscale arrays of living microbes as swarming targets, facilitated comparative qualitative and quantitative measurement of neutrophil swarming and capacity to restrict pathogen survival/growth (15). DAPI-stained neutrophils were added to microtiter plates containing Candida albicans expressing far-red fluorescent protein; swarming patterns and fungal coverage was quantified over 12 hours (Figure 3A). Although C. albicans is not typically considered pathogenic in CF, it is a useful microbe for testing neutrophil function and swarming behaviors (15). No differences in swarming patterns were seen when comparing non-CF neutrophil responses with combined CF patient groups (Figure 3B). However, we detected significant heterogeneity of neutrophil swarm size in the CF-hospitalized group, which appeared to be bimodal. The size of the neutrophil swarms was either smaller or larger in CF-hospitalized as compared to either non-CF or CF-outpatient neutrophils (Figure 3C, Supplemental Figure 2, Supplemental Video 3). Of note, these differences did not correlate with whether the CF-hospitalized individual was taking a CFTR modulator. Further, there were no significant differences between groups in the neutrophils ability to generate LTB4 (Figure 3D), a key factor driving neutrophil swarming(32). Regardless of neutrophil swarm size, CF-hospitalized neutrophils appeared to be defective in their ability to restrict pathogens. CF-hospitalized neutrophil swarms allowed significantly more growth of C. albicans than the non-CF and CF-outpatient swarms (Figure 3E-F). Together, these results demonstrate that neutrophil swarming function is significantly altered in the CF-hospitalized individuals.
Figure 3. CF-hospitalized neutrophils display dysfunctional swarming dynamics and function:
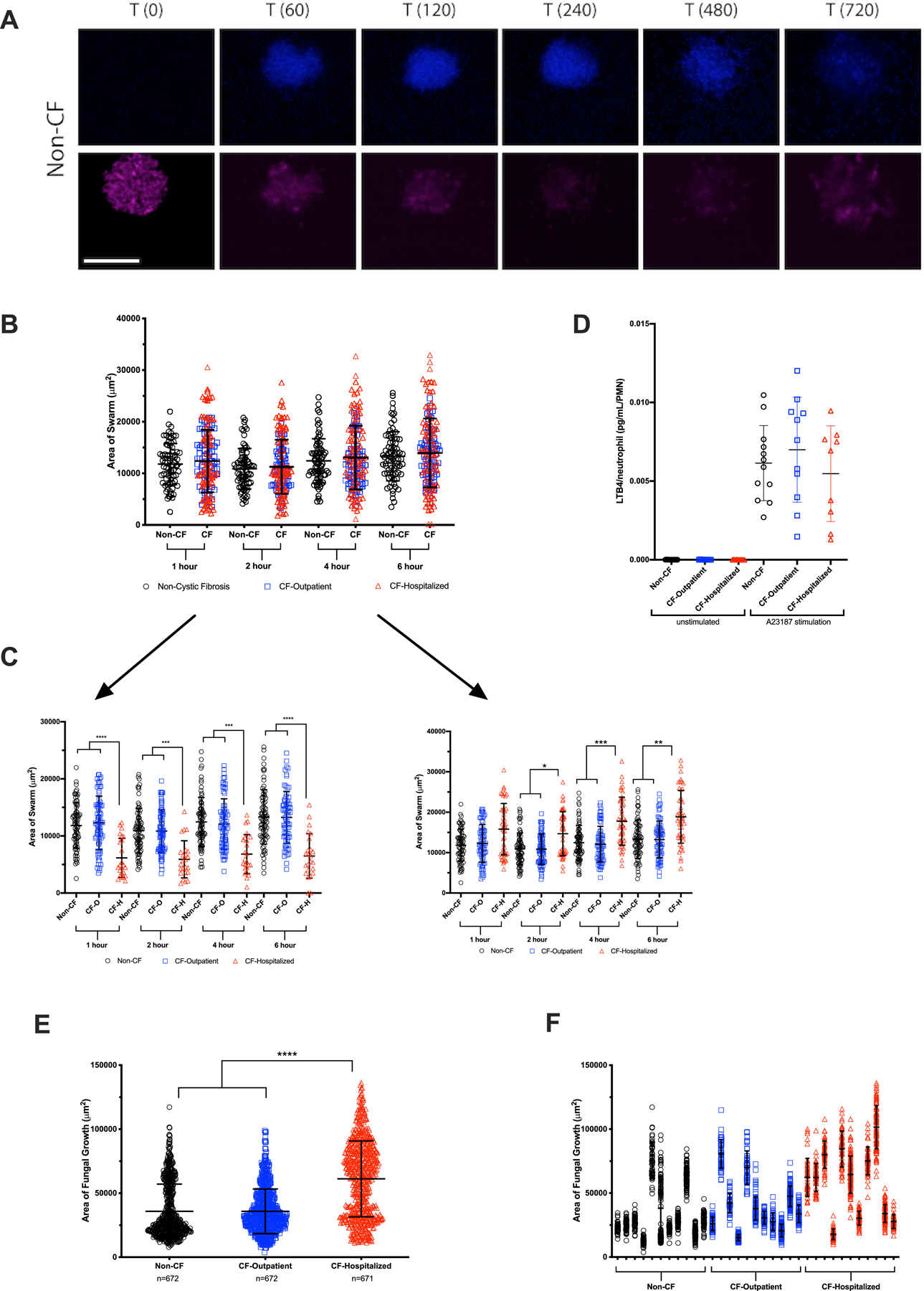
Live C. albicans were patterned in 100 um diameter clusters on poly-l-lysine/Zetag arrays. Purified human neutrophils were stained with Hoechst, then added to the arrays to observe host-pathogen interactions, particularly swarming responses. (A) A panel of images showing a typical swarming response of neutrophils (Hoechst, blue) to a cluster of C. albicans (pink) is shown. (B) The area covered by individual neutrophil swarms was measured at specific timepoints for non-CF and CF-individuals. N=77 non-CF and 143 CF swarms. (C) The CF population was split into CF-outpatient and CF-hospitalized populations. CF-hospitalized displayed two aberrant swarming phenotypes, one with significantly smaller swarms than non-CF or CF-outpatient and the other with significantly larger swarms than non-CF or CF-outpatient. N=77 for non-CF and 71 for CF-outpatient swarms. N=24 for the “small” CF-hospitalized phenotype and N=48 for the CF-hospitalized “large” phenotype. (D) In parallel, neutrophils were isolated using the gelatin/RBC lysis method, then stimulated with DMSO or A23187. LTB4 was quantified by ELISA (E) The amount of C. albicans growth was quantified at 16 hours. CF-hospitalized neutrophils displayed a significant defect in restricting fungal growth compared to non-CF or CF-outpatient populations. (F) The fungal growth allowed by swarms from each donor is also displayed. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 by Kruskal-Wallis with Dunn’s post-test. Scale bar represents 100 µm.
CF neutrophils exhibit altered bacterial-induced transepithelial migration
High-resolution 3D time-lapse µOCT videos were used to study the differences between CF-outpatient and non-CF neutrophil migration across an airway epithelium pre-infected with Pseudomonas aeruginosa, strain PAO1 (Figure 4A). 3D µOCT images were acquired at 10-minute intervals over 2 hours after neutrophil migration was initiated by adding neutrophils to the basolateral side of infected airway epithelial cells (Figure 4B, Supplemental Video 4, Supplemental Video 5). The µOCT videos captured the dynamical process in which neutrophils entered and breached the epithelial barrier, migrating from the basolateral to the apical side. Neutrophil transepithelial migration occurred in clusters or columns of neutrophils, whereby columns would breach the epithelial barrier and remain aggregated on the apical side until individual neutrophils break free and descend to the bottom of the well.
Figure 4. Neutrophil transepithelial migration across a lung epithelial monolayer.
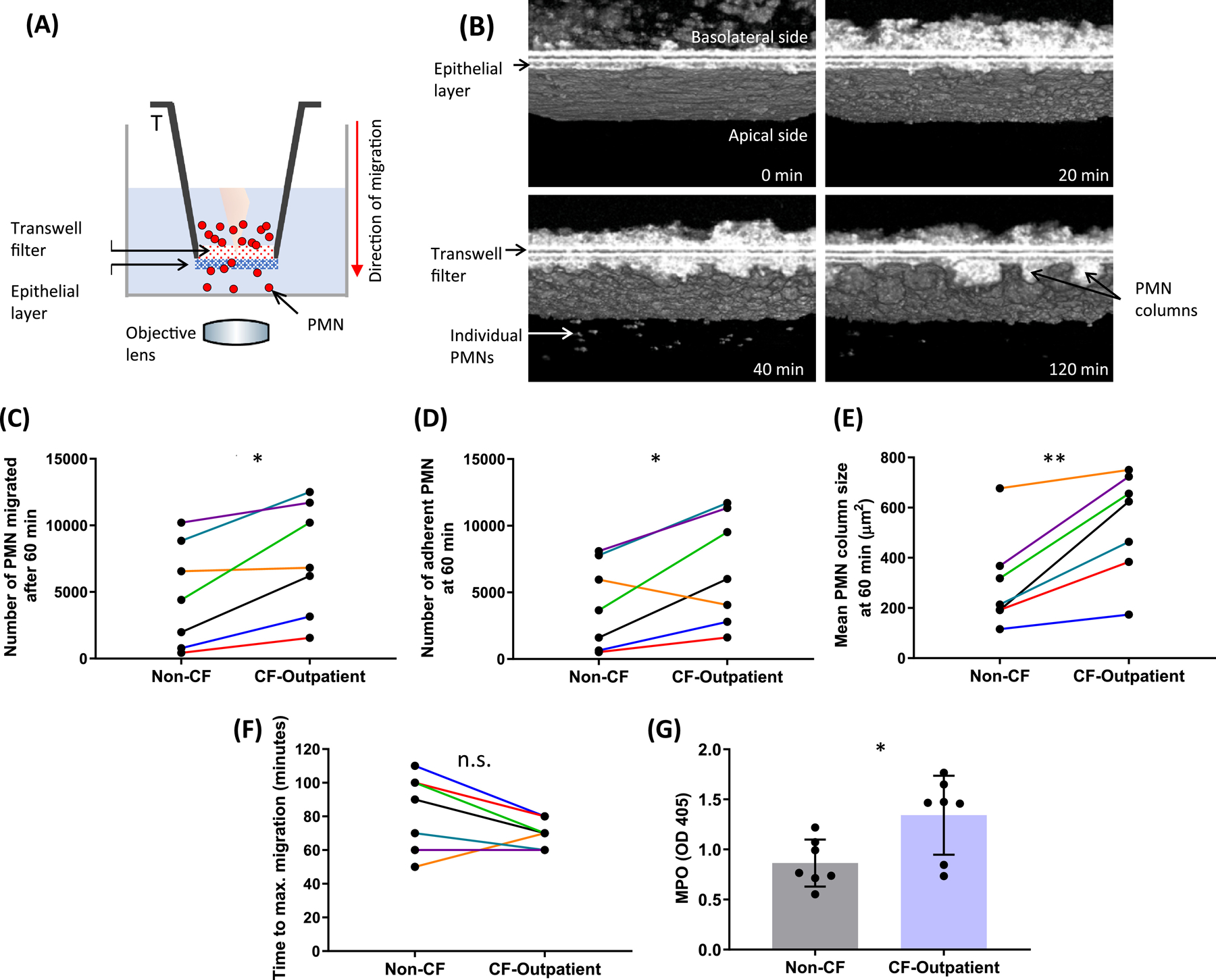
(A) Schematic illustrating µOCT imaging of the neutrophil transepithelial migration assay. (B) Aggregation and adherence of migrated neutrophils, as well as subsequent detachment of individual neutrophils are shown in representative 3D µOCT images captured at various time points after initiation of transepithelial migration. Using the 60-minute time point to compare paired experiments, we found that there was (C) a significantly greater number of CF-outpatient neutrophils that migrated and (D) adhered to the apical side of the lung-epithelial layer. (E) En face views taken about at 10 µm below the epithelial monolayer revealed that the mean area of adherent CF-outpatient neutrophil columns was significantly larger than that of non-CF neutrophils. (F) Maximal neutrophil migration occurred more rapidly in the CF-outpatient group, and G) unstimulated, unmigrated CF-outpatient neutrophils released greater MPO (as quantified by OD405) per 500,000 neutrophils than healthy controls. N = 7 non-CF, N = 7 CF-outpatient, paired-samples on the same day. * P < 0.05; ** P < 0.01. MPO = myeloperoxidase
There were multiple aspects of transepithelial migration that were found to be significantly different between CF-outpatient and non-CF neutrophils. We selected the 60-minute time point to compare paired experiments between CF and non-CF neutrophils. A significantly greater number of CF neutrophils migrated across the epithelial layer (Figure 4C; P<0.05) and remained adherent to the epithelium (Figure 4D; P<0.05) compared to non-CF neutrophils. In addition, CF neutrophils formed larger adherent neutrophil clusters, or “columns” compared with non-CF neutrophils (Figure 4E; Supplemental Figure 3; CF; P<0.01). The maximum number of migrated neutrophils varied between experiments, and the time to reach maximal influx of CF neutrophils trended toward more rapidly than with non-CF neutrophils (Figure 4F; CF 70.0 ± 8.2 minutes; non-CF 82.9 ± 22.9 minutes; P=0.11). Additionally, to assess enzymatic capability within neutrophils, MPO activity was quantified and compared between non-migrated, unstimulated neutrophils from CF and non-CF individuals. CF neutrophils exhibit more cell-associated MPO activity than non-CF neutrophils (Figure 4G; P<0.05), a finding that is consistent with previous reports (33). Representative standard curves display MPO generated across a pre-determined number of non-migrated, unstimulated neutrophils from a CF and non-CF individual (Supplemental Figure 4).
Discussion
Despite significant therapeutic advances with CFTR modulation, destructive airway inflammation remains a major concern for individuals with CF. Individuals on CFTR modulator therapy continue to develop pulmonary exacerbations (35), particularly those with advanced lung disease. Further, CFTR modulator therapy is not effective for all CF genotypes. Therefore, it is critical to continue to advance our understanding of airway inflammation in CF to provide insight into possible therapeutic avenues. In this study, we show that neutrophils from individuals with CF have increased bacterial-induced chemotaxis, altered neutrophil clustering behavior, and defective microbial containment and clearance.
Neutrophil migratory dysfunction in CF
Although CF neutrophils express abnormal surface markers that facilitate migration such as increased E-selectins (36) and P-selectins (37), with decreased shedding of L-selectins (38), and the CF airway milieu is plentiful with endogenous neutrophil chemotactic signals such as IL-8 (39), the migratory capacity of the CF neutrophil itself in this context is unclear. In our study, we found that in the absence of endothelial and epithelial interaction or chemotactic gradients, CF neutrophils did not exhibit any differences in migratory capacity. This contrasts with other hyper-inflammatory states with increased spontaneous neutrophil migration (22). However, the addition of an infectious signal stimulates increased neutrophil migration, as was seen with increased migration towards S. aureus particles and towards P. aeruginosa-infected epithelium. This could be because CF neutrophils are already primed by the presence of chronic infection to migrate quickly to infection sites.
Indeed, neutrophils pre-stimulated with LPS display increased migration toward a second chemotactic signal (40), likely mediated by the release of a secondary chemoattractant, such as LTB4, augmenting chemotactic signals for neutrophil migration (41, 42). In CF, priming of neutrophils by low level endotoxemia has proven to increase the release of IL-1β by the NOD-LRR-and pyrin domain-containing protein 3 (NLRP3) inflammasome in a CFTR-independent manner (5). IL-1β, a central pro-inflammatory cytokine, in turn, stimulates LTB4 release, augmenting chemotaxis.(43) Priming of CF neutrophils in the setting of chronic airway infection could increase CF chemotaxis towards infectious sites.
Dysfunctional neutrophil clustering in CF
Dense conglomerates of neutrophils and neutrophil byproducts are the hallmark of cellular inflammation seen in CF airways. However, neutrophil interactions with other neutrophils have not been well-defined in CF. Our study revealed that neutrophils from individuals with CF showed increased cluster formation around S. aureus particles, abnormal swarm patterns for CF-hospitalized neutrophils, and increased cluster/column formation following trans-epithelial migration. Accelerated neutrophil clustering marked by larger aggregates among CF neutrophils may reflect altered chemotaxis, changes in adhesion receptors, or changes in neutrophil-neutrophil communication. Neutrophil auto-activation increases ROS formation (44), feeding into the pro-inflammatory environment. Understanding the mechanisms driving the abnormal neutrophil clustering could provide insight into the hyperinflammatory environment seen in the CF airways.
Our studies also showed that CF neutrophils are more likely to remain adherent to the epithelial surface over long periods of time, forming large focal aggregates, as visualized by µOCT. This increased neutrophil-epithelial interaction in CF could imply altered neutrophilic expression of adhesion molecules, such as CD18(19), or rapid post-migratory neutrophil activation (i.e. NETosis(45)) near the epithelial surface, whereby the sticky, expelled DNA impedes migration of neutrophils. Additionally, when activated, neutrophils can cause bystander damage, expelling enzymatic materials either by exocytosis or NETosis(46). Clumping near the epithelial surface may theoretically result in damage to the epithelial surface, particularly when taking into account the increased rate of neutrophils migrating, the number of neutrophils adherent to the epithelium and the enzymatic activity within a neutrophil (MPO). This combination suggests a potential for increased epithelial damage associated with CF neutrophil recruitment, underscoring the possible implications of hyperinflammation in the CF airway.
Dysfunctional microbe containment and killing in CF
CF neutrophils are known to have defective bacterial killing, likely related to the inability of CFTR deficient lysosomes to acidify and kill pathogens (47). Our studies support abnormal microbial containment and clearance as well, shedding light on additional features of neutrophil dysfunction. CF neutrophils, particularly those from CF-hospitalized individuals, rapidly phagocytose a large number of S. aureus particles and appear to stimulate further neutrophil recruitment. Neutrophils from non-CF individuals are more likely to distribute the uptake of particles across multiple neutrophils and do not form large aggregates. Additionally, CF neutrophils from hospitalized individuals, with either increased or decreased swarming activity, had reduced ability to contain C. albicans growth. This result suggests that regardless of the neutrophil recruitment stimulus, the neutrophils themselves are less effective at clearing microbes. This finding in CF-hospitalized individuals overall suggests a deterioration of neutrophils’ microbial containment and killing in advanced disease and during a pulmonary exacerbation.
These observations highlight the need to better understand the mechanisms driving hyperinflammation in CF. Others have shown that low-level endotoxemia in CF, likely from chronic lung infection, alters immunometabolism within the neutrophil, resulting in increased IL-1β production via cleavage of pro-IL-1b by capsase-1 within the NLRP3 inflammasome.(5) This cleavage occurs in the LPS-rich CF lung and is significantly reduced in individuals with lung transplantation but unaffected by CFTR modulation, suggesting CF-independent mechanisms driving neutrophilic inflammation.(5) Research bridging mechanisms of airway inflammation in CF with highly advanced technology for characterizing neutrophil dysfunction are needed.
Our study had several limitations. First, these assays were developed and validated using pathogens or pathogenic strains not classically observed in the CF lung. However, the goal of this study was to assess neutrophil function in the context of CF. Future studies will be critical to determine whether more pathogenic strains or other pathogenic organisms elicit similar aberrations in CF neutrophils as detailed here. Additionally, neutrophils were isolated using a variety of methods, each with potential limitations. For assays using gelatin sedimentation and RBC lysis (transmigration, LTB4 production, MPO production), other immune cells purified together with the neutrophils might have impacted the observations. Additionally, future studies are needed to assess the contributions of CF ALI, rather than non-CF cell line-derived epithelium, towards neutrophil migration and activation.
The differences observed in this study do not distinguish between inherent abnormalities in CF neutrophils vs. acquired defects as CF study participants primarily included adults with more advanced disease. Although a subgroup of CF-hospitalized individuals was on CFTR modulators, this subgroup did not differentiate itself from those not on CFTR modulators. This lack of difference suggests the neutrophil defects seen are CFTR-independent; The effect of CFTR modulators on neutrophil function warrants further evaluation. Additionally, neutrophil abnormalities in the CF-hospitalized group may occur in other acute illness or pulmonary infections. Follow up studies, including non-CF bronchiectasis controls, could add clarity to our findings.
Despite significant therapeutic advances with CFTR modulation, destructive airway inflammation remains a major concern for individuals with CF. Individuals on CFTR modulator therapy continue to develop pulmonary exacerbations(35), particularly those with advanced lung disease. Further, CFTR modulator therapy is not effective for all CF genotypes. Therefore, it is critical to continue to advance our understanding of airway inflammation in CF to provide insight into possible therapeutic avenues.
Conclusion
Individuals with CF display dysfunctional neutrophilic inflammatory responses, which could contribute to the progression of airway disease. Anti-inflammatories specifically targeting abnormal functional responses could play an important role, even for individuals who are eligible for CFTR modulator therapy, and especially during a pulmonary exacerbation.
Supplementary Material
Supplemental Video 1: Non-CF neutrophils (Hoechst, blue) migrated into the microfluidic arenas and phagocytose fluorescently labeled Staphylococcus aureus particles (green).
Supplemental Video 2: Neutrophils from a hospitalized CF individual (Hoechst, blue) migrated into the microfluidic arenas and phagocytose fluorescently labeled Staphylococcus aureus particles (green).
Supplemental Figure 1: Comparison of non-CF and CF neutrophil size, as measured by µOCT. Comparison by Student t-test, n.s = not significant.
Supplemental Figure 2: Swarming patterns (Hoechst, blue) and fungal growth (pink) are visualized in swarming assay over 12 hours. Swarming by the CF neutrophils are on left three panels, non-CF swarming is on the right sided panel. CF-hospitalized group showed two aberrant swarming phenotypes: one where neutrophil swarms were significantly smaller than those for non-CF and CF-outpatient neutrophils and one where they formed significantly larger swarms.
Supplemental Figure 3: µOCT visualization of neutrophils trans-epithelial migration, en-face view capturing neutrophil migration through the apical surface. CF neutrophils formed larger adherent neutrophil clusters or “columns” (B) as compared with non-CF neutrophils (A).
Supplemental Figure 4: Myeloperoxidase (MPO) per neutrophil (PMN). Representative standard curve for MPO generated from a pre-determined number of neutrophils from CF and non-CF individuals.
Supplemental Video 3: Neutrophil swarming patterns (Hoechst, blue, left) and fungal growth (pink, right) are captured in swarming assays over 12 hours. Non-CF neutrophils are in the top row. CF-hospitalized group showed two aberrant swarming phenotypes: reduced swarming (middle row) and increased swarming (bottom row).
Supplemental Video 4: Migration of non-CF neutrophils across airway epithelial cells infected with Pseudomonas aeruginosa. 3D µOCT images captures neutrophil migration at 10-minute intervals over 2 hours.
Supplemental Video 5: Migration of CF-Outpatient neutrophils across airway epithelial cells infected with Pseudomonas aeruginosa. 3D µOCT images captures neutrophil migration at 10-minute intervals over 2 hours.
Highlights:
Microfluidic assays and advanced imaging technologies provide novel insight toward neutrophil function in individuals with cystic fibrosis (CF).
Neutrophils from individuals with CF display dysfunctional migration, cell-to-cell clustering, and phagocytosis.
Differences were noted between individuals with CF who were well and those experiencing a pulmonary exacerbation.
Acknowledgments:
We would like to thank Drs. Allen Lapey, Shannon Fracchia, Isabel Neuringer, Lenny Sicilan and Chris Richards for their commitment to advancing care for individuals with CF and assistance in recruiting patients for this study. We also acknowledge funding from the National Heart Lung and Blood Institute (5K08HL143183 to LY), the Cystic Fibrosis Foundation (YONKER18Q0 to LY, TEARNE16XX0 to GT), the National Institute of General Medical Sciences (GM092804 to DI), the National Institute of Allergy and Infectious Diseases (R01AI095338 to BPH), and the National Institute of Child Health and Human Development (HD089939 to DI). Dr. Alex Hopke was supported by a fellowship from the Shriners Hospital for Children.
Abbreviations:
- LTB4
leukotriene B4
- MPO
myeloperoxidase
- µOCT
micro-optical coherence tomography
- NSM
Neutrophil spontaneous migration
- PMN
neutrophil
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: none
Conflict of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Konstan MW, Walenga RW, Hilliard KA, Hilliard JB. Leukotriene B4 markedly elevated in the epithelial lining fluid of patients with cystic fibrosis. Am Rev Respir Dis 1993;148(4 Pt 1):896–901. [DOI] [PubMed] [Google Scholar]
- 2.Ringholz FC, Buchanan PJ, Clarke DT, Millar RG, McDermott M, Linnane B, et al. Reduced 15-lipoxygenase 2 and lipoxin A4/leukotriene B4 ratio in children with cystic fibrosis. Eur Respir J 2014;44(2):394–404. [DOI] [PubMed] [Google Scholar]
- 3.Pizurki L, Morris MA, Chanson M, Solomon M, Pavirani A, Bouchardy I, et al. Cystic fibrosis transmembrane conductance regulator does not affect neutrophil migration across cystic fibrosis airway epithelial monolayers. Am J Pathol 2000;156(4):1407–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lara-Reyna S, Holbrook J, Jarosz-Griffiths HH, Peckham D, McDermott MF. Dysregulated signalling pathways in innate immune cells with cystic fibrosis mutations. Cell Mol Life Sci 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McElvaney OJ, Zaslona Z, Becker-Flegler K, Palsson-McDermott EM, Boland F, Gunaratnam C, et al. Specific Inhibition of the NLRP3 Inflammasome as an Antiinflammatory Strategy in Cystic Fibrosis. Am J Respir Crit Care Med 2019;200(11):1381–91. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Y, Song K, Painter RG, Aiken M, Reiser J, Stanton BA, et al. Cystic fibrosis transmembrane conductance regulator recruitment to phagosomes in neutrophils. J Innate Immun 2013;5(3):219–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robledo-Avila FH, Ruiz-Rosado JD, Brockman KL, Kopp BT, Amer AO, McCoy K, et al. Dysregulated Calcium Homeostasis in Cystic Fibrosis Neutrophils Leads to Deficient Antimicrobial Responses. J Immunol 2018;201(7):2016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forrest OA, Ingersoll SA, Preininger MK, Laval J, Limoli DH, Brown MR, et al. Frontline Science: Pathological conditioning of human neutrophils recruited to the airway milieu in cystic fibrosis. J Leukoc Biol 2018;104(4):665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boneschansker L, Jorgensen J, Ellett F, Briscoe DM, Irimia D. Convergent and Divergent Migratory Patterns of Human Neutrophils inside Microfluidic Mazes. Sci Rep 2018;8(1):1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Jodoin E, Jorgensen J, Lee J, Markmann JJ, Cataltepe S, et al. Progressive mechanical confinement of chemotactic neutrophils induces arrest, oscillations, and retrotaxis. J Leukoc Biol 2018;104(6):1253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Hossain M, Bogoslowski A, Kubes P, Irimia D. Chemotaxing neutrophils enter alternate branches at capillary bifurcations. Nat Commun 2020;11(1):2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamza B, Irimia D. Whole blood human neutrophil trafficking in a microfluidic model of infection and inflammation. Lab Chip 2015;15(12):2625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamza B, Wong E, Patel S, Cho H, Martel J, Irimia D. Retrotaxis of human neutrophils during mechanical confinement inside microfluidic channels. Integr Biol (Camb) 2014;6(2):175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reategui E, Jalali F, Khankhel AH, Wong E, Cho H, Lee J, et al. Microscale arrays for the profiling of start and stop signals coordinating human-neutrophil swarming. Nat Biomed Eng 2017;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopke A, Scherer A, Kreuzburg S, Abers MS, Zerbe CS, Dinauer MC, et al. Neutrophil swarming delays the growth of clusters of pathogenic fungi. Nat Commun 2020;11(1):2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellett F, Jalali F, Marand AL, Jorgensen J, Mutlu BR, Lee J, et al. Microfluidic arenas for war games between neutrophils and microbes. Lab Chip 2019;19(7):1205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellett F, Jorgensen J, Marand AL, Liu YM, Martinez MM, Sein V, et al. Diagnosis of sepsis from a drop of blood by measurement of spontaneous neutrophil motility in a microfluidic assay. Nat Biomed Eng 2018;2(4):207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Gardecki JA, Nadkarni SK, Toussaint JD, Yagi Y, Bouma BE, et al. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nat Med 2011;17(8):1010–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu KK, Kusek ME, Liu L, Som A, Yonker LM, Leung H, et al. Illuminating dynamic neutrophil trans-epithelial migration with micro-optical coherence tomography. Sci Rep 2017;8:45789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yonker LM, Mou H, Chu KK, Pazos MA, Leung H, Cui D, et al. Development of a Primary Human Co-Culture Model of Inflamed Airway Mucosa. Sci Rep 2017;7(1):8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung HM, Birket SE, Hyun C, Ford TN, Cui D, Solomon GM, et al. Intranasal micro-optical coherence tomography imaging for cystic fibrosis studies. Sci Transl Med 2019;11(504). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones CN, Moore M, Dimisko L, Alexander A, Ibrahim A, Hassell BA, et al. Spontaneous neutrophil migration patterns during sepsis after major burns. PLoS One 2014;9(12):e114509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otawara M, Roushan M, Wang X, Ellett F, Yu YM, Irimia D. Microfluidic Assay Measures Increased Neutrophil Extracellular Traps Circulating in Blood after Burn Injuries. Sci Rep 2018;8(1):16983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler KL, Ambravaneswaran V, Agrawal N, Bilodeau M, Toner M, Tompkins RG, et al. Burn injury reduces neutrophil directional migration speed in microfluidic devices. PLoS One 2010;5(7):e11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou HW, Petchakup C, Tay HM, Tam ZY, Dalan R, Chew DE, et al. Rapid and label-free microfluidic neutrophil purification and phenotyping in diabetes mellitus. Sci Rep 2016;6:29410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones CN, Dimisko L, Forrest K, Judice K, Poznansky MC, Markmann JF, et al. Human Neutrophils Are Primed by Chemoattractant Gradients for Blocking the Growth of Aspergillus fumigatus. J Infect Dis 2016;213(3):465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones CN, Ellett F, Robertson AL, Forrest KM, Judice K, Balkovec JM, et al. Bifunctional Small Molecules Enhance Neutrophil Activities Against Aspergillus fumigatus in vivo and in vitro. Front Immunol 2019;10:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J, Baik SH, Mook-Jung I, Irimia D, Cho H. Mimicry of Central-Peripheral Immunity in Alzheimer’s Disease and Discovery of Neurodegenerative Roles in Neutrophil. Front Immunol 2019;10:2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hopke A, Nicke N, Hidu EE, Degani G, Popolo L, Wheeler RT. Neutrophil Attack Triggers Extracellular Trap-Dependent Candida Cell Wall Remodeling and Altered Immune Recognition. PLoS Pathog 2016;12(5):e1005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusek ME, Pazos MA, Pirzai W, Hurley BP. In vitro coculture assay to assess pathogen induced neutrophil trans-epithelial migration. J Vis Exp 2014(83):e50823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013;498(7454):371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witko-Sarsat V, Allen RC, Paulais M, Nguyen AT, Bessou G, Lenoir G, et al. Disturbed myeloperoxidase-dependent activity of neutrophils in cystic fibrosis homozygotes and heterozygotes, and its correction by amiloride. J Immunol 1996;157(6):2728–35. [PubMed] [Google Scholar]
- 34.Strzepa A, Pritchard KA, Dittel BN. Myeloperoxidase: A new player in autoimmunity. Cell Immunol 2017;317:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volkova N, Moy K, Evans J, Campbell D, Tian S, Simard C, et al. Disease progression in patients with cystic fibrosis treated with ivacaftor: Data from national US and UK registries. J Cyst Fibros 2020;19(1):68–79. [DOI] [PubMed] [Google Scholar]
- 36.De Rose V, Oliva A, Messore B, Grosso B, Mollar C, Pozzi E. Circulating adhesion molecules in cystic fibrosis. Am J Respir Crit Care Med 1998;157(4 Pt 1):1234–9. [DOI] [PubMed] [Google Scholar]
- 37.Hubeau C, Lorenzato M, Couetil JP, Hubert D, Dusser D, Puchelle E, et al. Quantitative analysis of inflammatory cells infiltrating the cystic fibrosis airway mucosa. Clin Exp Immunol 2001;124(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Russell KJ, McRedmond J, Mukherji N, Costello C, Keatings V, Linnane S, et al. Neutrophil adhesion molecule surface expression and responsiveness in cystic fibrosis. Am J Respir Crit Care Med 1998;157(3 Pt 1):756–61. [DOI] [PubMed] [Google Scholar]
- 39.Noah TL, Black HR, Cheng PW, Wood RE, Leigh MW. Nasal and bronchoalveolar lavage fluid cytokines in early cystic fibrosis. J Infect Dis 1997;175(3):638–47. [DOI] [PubMed] [Google Scholar]
- 40.Boribong BP, Lenzi MJ, Li L, Jones CN. Super-Low Dose Lipopolysaccharide Dysregulates Neutrophil Migratory Decision-Making. Front Immunol 2019;10:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013;13(3):159–75. [DOI] [PubMed] [Google Scholar]
- 42.Majumdar R, Tavakoli Tameh A, Parent CA. Exosomes Mediate LTB4 Release during Neutrophil Chemotaxis. PLoS Biol 2016;14(1):e1002336. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Oliveira SH, Canetti C, Ribeiro RA, Cunha FQ. Neutrophil migration induced by IL-1beta depends upon LTB4 released by macrophages and upon TNF-alpha and IL-1beta released by mast cells. Inflammation 2008;31(1):36–46. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen GT, Green ER, Mecsas J. Neutrophils to the ROScue: Mechanisms of NADPH Oxidase Activation and Bacterial Resistance. Front Cell Infect Microbiol 2017;7:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado-Rizo V, Martinez-Guzman MA, Iniguez-Gutierrez L, Garcia-Orozco A, Alvarado-Navarro A, Fafutis-Morris M. Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview. Front Immunol 2017;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng Y, Herbert JA, Robinson E, Ren L, Smyth RL, Smith CM. Neutrophil-Airway Epithelial Interactions Result in Increased Epithelial Damage and Viral Clearance during Respiratory Syncytial Virus Infection. J Virol 2020;94(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol 2006;8(9):933–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1: Non-CF neutrophils (Hoechst, blue) migrated into the microfluidic arenas and phagocytose fluorescently labeled Staphylococcus aureus particles (green).
Supplemental Video 2: Neutrophils from a hospitalized CF individual (Hoechst, blue) migrated into the microfluidic arenas and phagocytose fluorescently labeled Staphylococcus aureus particles (green).
Supplemental Figure 1: Comparison of non-CF and CF neutrophil size, as measured by µOCT. Comparison by Student t-test, n.s = not significant.
Supplemental Figure 2: Swarming patterns (Hoechst, blue) and fungal growth (pink) are visualized in swarming assay over 12 hours. Swarming by the CF neutrophils are on left three panels, non-CF swarming is on the right sided panel. CF-hospitalized group showed two aberrant swarming phenotypes: one where neutrophil swarms were significantly smaller than those for non-CF and CF-outpatient neutrophils and one where they formed significantly larger swarms.
Supplemental Figure 3: µOCT visualization of neutrophils trans-epithelial migration, en-face view capturing neutrophil migration through the apical surface. CF neutrophils formed larger adherent neutrophil clusters or “columns” (B) as compared with non-CF neutrophils (A).
Supplemental Figure 4: Myeloperoxidase (MPO) per neutrophil (PMN). Representative standard curve for MPO generated from a pre-determined number of neutrophils from CF and non-CF individuals.
Supplemental Video 3: Neutrophil swarming patterns (Hoechst, blue, left) and fungal growth (pink, right) are captured in swarming assays over 12 hours. Non-CF neutrophils are in the top row. CF-hospitalized group showed two aberrant swarming phenotypes: reduced swarming (middle row) and increased swarming (bottom row).
Supplemental Video 4: Migration of non-CF neutrophils across airway epithelial cells infected with Pseudomonas aeruginosa. 3D µOCT images captures neutrophil migration at 10-minute intervals over 2 hours.
Supplemental Video 5: Migration of CF-Outpatient neutrophils across airway epithelial cells infected with Pseudomonas aeruginosa. 3D µOCT images captures neutrophil migration at 10-minute intervals over 2 hours.


