Abstract
In this study, concentrations of disinfection byproducts (DBPs) and COVID-19 related pharmaceuticals in wastewater effluents and surface water were measured two weeks, three months and eight months after the lockdown in Wuhan. Little temporal variation in DBP concentrations suggested intensified disinfection during the COVID-19 pandemic had limited impacts on the occurrence of DBPs in the aquatic environment. In contrast, the pandemic led to a significant increase in concentrations of lopinavir and ritonavir in wastewater effluents and surface water. The high detection frequency of these pharmaceuticals in surface water after the lockdown highlighted their mobility and persistence in the aquatic environment. The initial ecological risk assessment indicated moderate risks associated with these pharmaceuticals in surface water. As the global situation is still rapidly evolving with a continuous surge in the number of confirmed COVID-19 cases, our results suggest a pressing need for monitoring COVID-19 related pharmaceuticals as well as a systematic evaluation of their ecotoxicities in the aquatic environment.
Keywords: COVID-19 pandemic, DBPs, Pharmaceuticals, Wastewater, Surface water, Ecological risk
Graphical abstract

1. Introduction
The outbreak of the coronavirus disease-2019 (COVID-19) caused by the Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) has resulted in unprecedented damage to human health and the global economy (Sarkodie and Owusu, 2021; Tisdell, 2020). As of August 12th 2021, WHO reports that there have been 204,644,849 confirmed cases of COVID-19 and the global situation is still rapidly evolving with a continuous surge in the number of confirmed cases (WHO, 2021). While most scientific studies focus on virus transmission (Chan et al., 2020; Q. Li et al., 2020; Tian et al., 2020) and deactivation (Nardell and Nathavitharana, 2020; Wang et al., 2020; Zhang et al., 2020), the emerging impacts of the COVID-19 pandemic on the aquatic environment due to the excessive use of disinfectants and COVID-19 related pharmaceuticals are of increasing concern and merit further investigation (Bandala et al., 2021; Chu et al., 2021).
Intensified disinfection procedures are performed during wastewater treatment and sanitation of healthcare facilities, streets and buildings during the COVID-19 pandemic in China, mainly using chlorine-based disinfectants (Chu et al., 2021; Li et al., 2021). For example, large quantities of chlorine-containing solutions were sprayed in streets and public places to prevent the spread of SARS-CoV-2 virus during Wuhan's lockdown. Elevated dose of chlorine was also applied to disinfect municipal wastewater (J. Li et al., 2020). Excessive use of chlorine-based disinfectants could possibly lead to the release of chlorine residual into surface water. The concentrations of chlorine residual were reported to increase up to 0.4 mg/L in some lakes in China during February and March 2020 (Yin et al., 2020). Chlorine residual entering natural water bodies can react with natural organic matter (NOM), forming a suite of disinfection byproducts (DBPs) including regulated trihalomethanes (THMs) and haloacetic acids (HAAs) (Wang et al., 2017) as well as unregulated but more toxic species such as haloacetaldehydes (HALs), haloacetonitriles (HANs), and halonitromethanes (HNMs) (Furst et al., 2019). The presence of chlorine residual and DBPs in surface water could adversely impact aquatic organisms (Fisher et al., 1999; Richardson et al., 2007). Two recent studies reported the presence of DBPs in surface water during the COVID-19 pandemic (Li et al., 2021; Wang et al., 2021). However, with limited information existing on the DBP concentrations in surface water before the COVID-19, it is difficult to evaluate the impacts of elevated disinfectant use on DBP occurrence based on these data.
In addition to DBPs, the release of COVID-19 related pharmaceuticals into surface water is a pressing concern. Since the outbreak of the COVID-19 pandemic, several therapeutic drugs have been used to treat the ever-growing number of COVID-19 patients (C. Liu et al., 2020; Wu et al., 2020). A large portion of these drugs are excreted from human body and subsequently discharged unchanged into wastewater (Liu and Wong, 2013; Nannou et al., 2020). As conventional wastewater treatment plants are not specifically designed for removing these compounds (Rodriguez-Narvaez et al., 2017; Xu et al., 2007), an enormous amount of drug residues are expected to be released into receiving surface water as the number of COVID-19 infected and hospitalized cases peaks. The significant presence of pharmaceuticals in surface water likely poses high risks to aquatic ecosystems due to their biological activity and persistence in the environment (Godoy and Kummrow, 2017; Huang et al., 2021; Nannou et al., 2020). Chen et al. investigated the post-pandemic occurrence of a wide range of pharmaceuticals and personal care products (PPCPs) in surface water two months after Wuhan's lockdown and reported that ribavirin and azithromycin had higher detection frequencies and concentrations than historically reported (Chen et al., 2021). However, these values can hardly represent the level of pharmaceutical concentrations during the lockdown, as the persistence of many PPCPs in the aquatic environment is generally on the order of several weeks (Huang et al., 2021; Kuroda et al., 2021). Up to now, limited information is available on the occurrence of COVID-19 related pharmaceuticals in surface water, which is a significant knowledge gap requiring further exploration.
Wuhan experienced a 76-day city lockdown from January 23rd, 2020 to April 8th, 2020 in great efforts to curb the spread of SARS-CoV-2 virus along with unprecedented mass use of disinfectants and pharmaceuticals. While the collection of water samples was not allowed during the lockdown period, this study was aimed to investigate the occurrence and temporal variation of disinfection byproducts and pharmaceuticals in domestic wastewater and surface water after the lockdown in Wuhan. To this end, three sampling events were conducted: (1) two weeks after the lockdown when there were a few hospitalized COVID-19 patients and ongoing intensified disinfection during wastewater treatment and sanitation of healthcare facilities, streets and public areas; (2) three months after the lockdown when there were no COVID-19 patients but ongoing intensified disinfection for healthcare facilities and wastewater treatment plants; (3) eight months after the lockdown which could represent conditions before the outbreak of COVID-19 pandemic as no infected cases were reported over the past six months and disinfection practices returned to the normal level (Hubei Province, 2021). The outcome of this study fills the knowledge gap about the occurrence of COVID-19 related pharmaceuticals in surface water and provides information to evaluate the impacts of the COVID-19 pandemic on the aquatic environment regarding DBPs and pharmaceuticals. Based on the measured concentrations, the ecological risk of COVID-19 related pharmaceuticals in surface water is assessed.
2. Materials and methods
2.1. Sample collection
Three sampling events were conducted on April 24th (two weeks after the lockdown), July 9th (three months after the lockdown) and December 10th (eight months after the lockdown) in Wuhan. Duplicate grab samples were taken from effluents and 1 km downstream of two large municipal wastewater treatment plants and 13 sampling sites located in five rivers and four lakes in the morning and in the afternoon to capture the daily water quality variation (Fig. S1). The flow rates and water levels of the rivers and lakes during the three sampling events are provided in Table S1. The two wastewater treatment plants are operating at capacities of approximately 0.5 and 0.6 million m3/d, respectively. One wastewater treatment plant applies chlorination for disinfection while the other uses ultraviolet (UV) irradiation and chlorination. Wuhan features a subtropical monsoon climate with 15.8 °C–17.5 °C mean annual temperature, with rainy season occurring in June and July. To minimize the effect of rainfall, all three sampling events were conducted when no or little precipitation (<4 mm/day) was observed in the previous seven days (Fig. S2). Still, it is worth noting that samples collected during the second sampling event was likely to be more diluted than the others. General water quality parameters are detailed in Table S2. Wastewater influent samples were not collected in this study due to the possible presence of SARS-CoV-2 without proper disinfection or dilution. Water samples were collected into amber glass bottles without headspace and transported on ice to the laboratory within 24 h. Certain sample bottles contained ascorbic acid to quench chlorine residuals and 1 N sulfuric acid was added to acidify samples for DBP analysis (Zeng et al., 2016). Samples were then filtered through 0.45 μm glass fiber filters in the laboratory and stored at 4 °C for subsequent analysis.
2.2. Analytical methods
Duplicate samples, together with laboratory and field blanks, were extracted for DBP and pharmaceutical analysis. A total of 21 halogenated DBPs in six classes including the four regulated trihalomethanes (THM4), nine haloacetic acids (HAAs), four haloacetonitriles (HANs), one haloacetaldehydes (HAL: trichloroacetaldehyde (TCAL)), two haloketones (HKs) and one halonitromethane (HNM: trichloronitromethane (chloropicrin; TCNM)) were extracted by modified US EPA Methods 551.1 (US EPA, 1995) and 552.3 (US EPA, 2003) and analyzed by gas chromatography–mass spectrometry (GC–MS) with 0.2 μg/L method reporting limits as described in previous studies (Chuang and Mitch, 2017; Szczuka et al., 2019; Zhang et al., 2019). Three pharmaceuticals recommended in the treatment plan for the novel coronavirus disease (COVID-19) of China including lopinavir, ritonavir and chloroquine (X. Liu et al., 2020) were selected in this study due to their massive use in China and around the globe. Lopinavir, ritonavir and chloroquine were extracted by solid phase extraction (SPE) and analyzed by high performance liquid chromatography−triple quadrupole mass spectrometry (HPLC-MS/MS; Nexera XR LC-20AD HPLC system coupled to an ABSciex QTrap 5500 MS) in the multiple reaction monitoring mode (MRM) with 1 ng/L reporting limits. Additional analytical details are provided in Text S1.
2.3. Ecological risk assessment
In this study, the risk quotients (RQs) of COVID-19 related pharmaceuticals were calculated to estimate their ecological risks (Godoy et al., 2018; Li et al., 2021). RQ is derived from the ratio between the measured environmental concentration (MEC) of individual pharmaceutical and its predicted no-effect concentration (PNEC) based on Eq. (1). The concentrations of pharmaceuticals below the method reporting limits (<1 ng/L) were treated as zero to avoid incorrect conclusion and bias. As experiment-based ecotoxicity data was only available for chloroquine (Zurita et al., 2005), chronic ecotoxicity data of lopinavir and ritonavir were obtained from ECOSAR V2.0, a computer program used for structure-activity relationship prediction of aquatic toxicity (Table S5) (US EPA, 2020). The lowest ecotoxicity value of each compound towards three taxonomic groups (daphnia, algae and fish) was chosen for a conservative estimate and divided by the assessment factor (AF) of 1000 to calculate the PNEC according to previous studies (Escher et al., 2011; Kuroda et al., 2021).
| (1) |
3. Results and discussion
3.1. DBPs
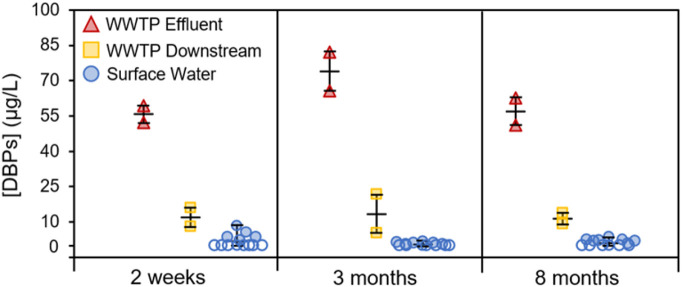
Fig. 1 and Table S6 illustrate the total concentrations of the 21 measured DBPs in wastewater effluents and surface water after the lockdown in Wuhan. Although intensified disinfection was performed during wastewater treatment till three months after the lockdown as indicated by the elevated chlorine residual in wastewater effluents (Table S2), DBP concentrations were generally similar two weeks, three months and eight months after the lockdown with the average concentrations of 55.9 μg/L, 74.0 μg/L and 56.9 μg/L, respectively. DBP concentrations measured two weeks after the lockdown were within the range of data reported in a recent study that collected samples around the same time (Li et al., 2021). Higher DBP concentrations measured three months after the lockdown were attributed to elevated DOC values occurred in the wastewater effluents (Table S2). The relative proportions of different DBP classes in wastewater effluents were fairly consistent across three sampling events (Fig. S3). THMs (43%–57%) and HAAs (22%–38%) dominated DBP speciation, followed by haloacetaldehydes (7.5%–11.0%), haloacetonitriles (2.4%–7.8%), and haloketones (2.4%–5.1%). This trend was consistent with previous studies investigating DBP occurrence in wastewater effluents and drinking waters (Chuang et al., 2019; Krasner et al., 2009; Richardson et al., 1999). DBP concentrations decreased rapidly over short distance (1 km) downstream of wastewater discharge points with the concentrations ranging from 5.9 μg/L to 21.7 μg/L. Higher loss was observed for THMs (83%–94%) than HAAs (58%–79%) since THMs exhibit higher volatilization and adsorption rates in the aquatic environment compared with HAAs (Jin et al., 2012). DBP concentrations occurred in surface water were much lower than those in wastewater effluents. The average concentrations of DBPs in surface water were 1.9 μg/L, 0.7 μg/L and 1.2 μg/L two weeks, three months and eight months after the lockdown, respectively, with detection frequencies varying from 38% to 54%. DBP concentrations observed three months after the lockdown were likely to be underestimated due to the dilution by rainwater. THMs and HAAs were the two dominant species accounting for >90% of the total DBP concentrations. DBPs, especially volatile species such as THMs, HANs, and HNMs, decay fast in the aquatic environment due to the coexistence of many mechanisms (e.g., volatilization, hydrolysis, adsorption, photolysis, and biodegradation) (Jin et al., 2012) and the occurrence of DBPs in natural water bodies is generally assumed to be insignificant. The low concentrations of DBPs detected in this study were consistent with recent studies reporting DBPs in surface water during the COVID-19 pandemic (Li et al., 2021; Wang et al., 2021). While disinfection practices returned to the normal level eight months after the lockdown, the similar level of DBP concentrations observed two weeks and three months after the lockdown suggests that the intensified disinfection did not result in a significant increase in DBP concentrations in surface water, likely due to a combined effect of dilution and fast decay rates of DBPs in natural watersheds.
Fig. 1.
Total DBP concentrations measured in effluents and 1 km downstream of 2 municipal wastewater treatment plants (WWTPs) and 13 surface water bodies two weeks, three months and eight months after the lockdown in Wuhan. Symbols represent the average concentrations of duplicate samples measured in each sampling site. Hollow symbols represent non-detectable samples. Horizontal bars represent the maximum, average, and minimum values.
3.2. Pharmaceuticals
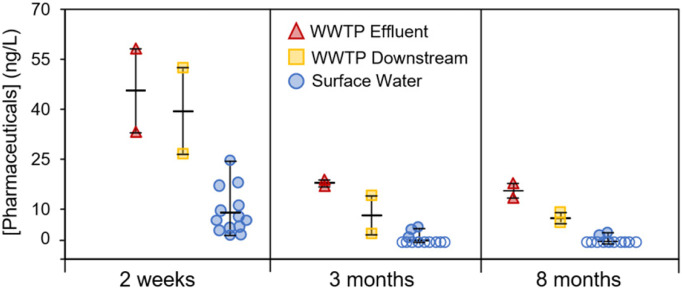
The concentrations of three pharmaceuticals recommended in the China's COVID-19 treatment plan including lopinavir, ritonavir and chloroquine (X. Liu et al., 2020) were determined in this study. While chloroquine was below its method reporting limit (<1 ng/L), concentrations of lopinavir and ritonavir in wastewater effluents and surface water two weeks, three months and eight months after the lockdown are depicted in Fig. 2 and Table 1 . The concentrations measured eight months after the lockdown served as a benchmark to evaluate the impacts of the COVID-19 pandemic on the environmental occurrence of pharmaceuticals in this study since there were no infected cases over the past six months and the persistence of these compounds in aquatic environment is on the order of several weeks without continuous inputs (Huang et al., 2021; Kuroda et al., 2021). Elevated pharmaceutical concentrations were detected two weeks after the lockdown (45.8 ng/L) in wastewater effluents when there were a few remaining hospitalized COVID-19 patients compared to those observed three months (18.0 ng/L) and eight months (15.6 ng/L) after the lockdown. Lopinavir was the dominant specie contributing >80% to the total concentrations due to the higher doses administered in COVID-19 treatment (800 mg/person/day for lopinavir vs 200 mg/person/day for ritonavir) (Fig. S4) (WHO, 2020). In contrast to DBPs, moderate decreases in pharmaceutical concentrations were observed downstream of wastewater discharge points. The concentrations and detection frequencies of pharmaceuticals in surface water two weeks after the lockdown were also much higher than those detected three months and eight months after the lockdown. Pharmaceuticals were detected in all surface water resources two weeks after the lockdown, with concentrations ranging from 2.1 ng/L to 24.5 ng/L (average 8.9 ng/L). The detection of these compounds in surface water indicated their ability to transport over long distances from the point of emission (Huang et al., 2021). These measured concentrations were generally higher than predicted pharmaceutical concentrations in surface water during the COVID-19 reported in one recent study (lopinavir: 7.1 ng/L; ritonavir: 2.6 ng/L) (Kuroda et al., 2021). In contrast, pharmaceutical concentrations ranged from ND (not detectable, <1 ng/L) to 4.4 ng/L and ND to 2.8 ng/L three months and eight months after the lockdown with detection frequencies of ~20%. These results suggest that mass use of lopinavir and ritonavir during the pandemic could lead to an increase in their concentrations in wastewater effluents and even surface water. It is important to note that much higher pharmaceutical concentrations were expected during the lockdown when the number of COVID-19 infected and hospitalized cases peaked.
Fig. 2.
Total pharmaceutical concentrations measured in effluents and 1 km downstream of 2 municipal wastewater treatment plants (WWTPs) and 13 surface water bodies two weeks, three months and eight months after the lockdown in Wuhan. Symbols represent the average concentrations of duplicate samples measured in each sampling site. Hollow symbols represent non-detectable samples. Horizontal bars represent the maximum, average, and minimum values.
Table 1.
Pharmaceutical concentrations measured in effluents and 1 km downstream of municipal wastewater treatment plants and surface water in Wuhan two weeks, three months and eight months after the lockdown.
| Pharmaceutical conc. (ng/L) | Wastewater eff. |
Downstream |
Surface water |
||||
|---|---|---|---|---|---|---|---|
| Average | Range | Average | Range | Average | Range | DF%a | |
| Two weeks after the lockdown | |||||||
| Lopinavir | 41.0 | 29.2–52.7 | 31.5 | 12.9–50 | 4.7 | ND–14.5 | 77% |
| Ritonavir | 4.8 | 4.0–5.5 | 5.8 | 2.3–9.4 | 4.2 | 2.0–10.0 | 100% |
| Chloroquine | NDb | ND | ND | ND | ND | ND | ND |
| Three months after the lockdown | |||||||
| Lopinavir | 15.5 | 14.3–16.6 | 8.2 | 2.4–14.0 | 0.5 | ND–4.0 | 15% |
| Ritonavir | 2.5 | 2.4–2.6 | ND | ND | 0.3 | ND–2.0 | 15% |
| Chloroquine | ND | ND | ND | ND | ND | ND | ND |
| Eight months after the lockdown | |||||||
| Lopinavir | 13.0 | 10.7–15.2 | 5.7 | 2.5–8.9 | 0.3 | 0–2.8 | 15% |
| Ritonavir | 2.6 | 2.5–2.8 | 1.8 | 0–3.5 | 0.1 | 0–1.2 | 8% |
| Chloroquine | ND | ND | ND | ND | ND | ND | ND |
All the samples were analyzed in duplicate.
DF: Detection Frequency.
ND: Not Detectable.
3.3. Ecological risk assessment
As lopinavir and ritonavir were widely detected in surface water two weeks after the lockdown, their risk quotients (RQs) were calculated as the ratios between the measured concentrations and predicted no-effect concentrations (PNECs) to assess their ecological risks (Fig. 3 and Table S7) (Godoy et al., 2018; Li et al., 2021). The risk is classified into four levels based on RQ values: RQ < 0.1, insignificant risk (no adverse effect expected); 0.1 < RQ < 1, low risk; 10 > RQ > 1, moderate risk (probable adverse effect); RQ > 10, high risk (adverse effect) (Aydin et al., 2019; Godoy et al., 2018). Previous studies have reported EC50 values of chloroquine towards various aquatic species (Race et al., 2020; Rendal et al., 2011; Zurita et al., 2005). However, experiment-based ecotoxicity data was not available for lopinavir and ritonavir and thus was estimated by ECOSAR in this study (Table S5). Ritonavir showed higher chronic toxicity to aquatic organisms as indicated by its lower PNEC (2.9 ng/L) compared with lopinavir (4.5 ng/L). The PNECs were similar with those values reported in previous studies (Kuroda et al., 2021). The RQ values of lopinavir and ritonavir in surface water ranged from 0 to 3.2 (average 1.0) and from 0.7 to 3.4 (average 1.5) two weeks after the lockdown, respectively. Moderate risks were observed in around 50% of surface water resources two weeks after the lockdown. It is important to note that elevated RQ values were expected during the lockdown periods when infected and hospitalized cases peaked, causing much higher pharmaceutical concentrations in surface water. The RQ values of lopinavir and ritonavir dropped below 1 three months and eight months after the lockdown. While the results highlight the ecological risks associated with these pharmaceuticals in surface water, there were some limitations in the initial ecological risk assessment conducted in this study. The RQ values were calculated using estimated values not measured toxicity data and the ecological risk of each pharmaceutical was assessed independently. Furthermore, the metabolites of these pharmaceuticals were not considered. Although chloroquine was not detected, the environmental risks of its metabolites are still worthy of further research.
Fig. 3.
Risk quotient (RQ) values of lopinavir and ritonavir in 13 surface water bodies two weeks, three months and eight months after the lockdown in Wuhan. Symbols represent the average concentrations of duplicate samples measured in each sampling site. Hollow symbols represent samples with RQ of 0. Horizontal bars represent the maximum, average, and minimum values.
4. Conclusions
The emerging impacts of the COVID-19 pandemic on the aquatic environment remain unelucidated at present and require further investigation. In this study, temporal variation in concentrations of DBPs and COVID-19 related pharmaceuticals occurring in wastewater effluents and surface water was determined two weeks, three months and eight months after the lockdown in Wuhan. Due to limited information available on the occurrence of DBPs and pharmaceuticals before the pandemic, concentrations measured eight months after the lockdown were used to represent pre-pandemic levels. The similar level of DBP concentrations observed two weeks, three months and eight months after the lockdown suggests that intensified disinfection had limited impacts on the occurrence of DBPs in the aquatic environment. However, much higher concentrations of lopinavir and ritonavir were observed in both wastewater effluents and surface water two weeks after the lockdown when there were still COVID-19 patients. The detection of these pharmaceuticals in all 13 surface water resources raises concerns on their potential ecotoxicological effects to aquatic lives. The initial ecological risk assessment conducted in this study indicates moderate risks associated with these pharmaceuticals present in surface water. The presence of pharmaceuticals in the surface water is alarming since pharmaceutical concentrations are expected to increase significantly during the lockdown periods when the number of infected cases is dozens of times higher, exacerbating the ecological risks. As many other pharmaceutical drugs have been used to counteract the effects of COVID-19 and consumption of pharmaceuticals varies significantly depending on the phases of the pandemic, more comprehensive studies are needed to monitor these compounds and evaluate their ecotoxicities with measured toxicity data to fully understand the potential risks of these pharmaceuticals on the aquatic environment.
CRediT authorship contribution statement
Zhong Zhang: Conceptualization, Methodology, Validation, Investigation, Writing – original draft, Writing – review & editing. Yang Zhou: Conceptualization, Methodology, Investigation, Writing – review & editing. Lanfang Han: Investigation, Resources. Xiaoyu Guo: Investigation, Visualization. Zihao Wu: Methodology. Jingyun Fang: Resources, Methodology. Banglei Hou: Resources, Methodology. Yanpeng Cai: Conceptualization, Investigation. Jin Jiang: Conceptualization, Resources. Zhifeng Yang: Conceptualization, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by funding from the National Natural Science Foundation of China (52000040, 21906030), the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2019ZT08L213), and Postdoctoral Science Foundation of China (2020T130123). We would like to thank Wuhan Ecological Environment Monitoring Center for help with collecting samples.
Editor: Jay Gan
Footnotes
Sampling locations; Flow rates and water levels of the sampling sites; Daily temperature and precipitation during the sampling events; Basic water quality; additional materials and methods; disinfection byproduct and pharmaceutical concentrations; ecotoxicity data and risk quotients of pharmaceuticals. Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.151409.
Appendix A. Supplementary data
Supplementary material
References
- Aydin S., Aydin M.E., Ulvi A., Kilic H. Antibiotics in hospital effluents: occurrence, contribution to urban wastewater, removal in a wastewater treatment plant, and environmental risk assessment. Environ. Sci. Pollut. Res. 2019;26(1):544–558. doi: 10.1007/s11356-018-3563-0. [DOI] [PubMed] [Google Scholar]
- Bandala E.R., Kruger B.R., Cesarino I., Leao A.L., Wijesiri B., Goonetilleke A. Impacts of COVID-19 pandemic on the wastewater pathway into surface water: a review. Sci. Total Environ. 2021;774 doi: 10.1016/j.scitotenv.2021.145586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J.F.W., Yuan S.F., Kok K.H., To K.K.W., Chu H., Yang J., Xing F.F., Liu J.L., Yip C.C.Y., Poon R.W.S., Tsoi H.W., Lo S.K.F., Chan K.H., Poon V.K.M., Chan W.M., Ip J.D., Cai J.P., Cheng V.C.C., Chen H.L., Hui C.K.M., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.P., Lei L., Liu S.T., Han J., Li R.W., Men J., Li L., Wei L., Sheng Y.Q., Yang L.H., Zhou B.S., Zhu L.Z. Occurrence and risk assessment of pharmaceuticals and personal care products (PPCPs) against COVID-19 in lakes and WWTP-river-estuary system in Wuhan, China. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu W.H., Fang C., Deng Y., Xu Z.X. Intensified disinfection amid COVID-19 pandemic poses potential risks to water quality and safety. Environ. Sci. Technol. 2021;55(7):4084–4086. doi: 10.1021/acs.est.0c04394. [DOI] [PubMed] [Google Scholar]
- Chuang Y.H., Mitch W.A. Effect of ozonation and biological activated carbon treatment of wastewater effluents on formation of N-nitrosamines and halogenated disinfection byproducts. Environ. Sci. Technol. 2017;51(4):2329–2338. doi: 10.1021/acs.est.6b04693. [DOI] [PubMed] [Google Scholar]
- Chuang Y.H., Szczuka A., Mitch W.A. Comparison of toxicity-weighted disinfection byproduct concentrations in potable reuse waters and conventional drinking waters as a new approach to assessing the quality of advanced treatment train waters. Environ. Sci. Technol. 2019;53(7):3729–3738. doi: 10.1021/acs.est.8b06711. [DOI] [PubMed] [Google Scholar]
- Escher B.I., Baumgartner R., Koller M., Treyer K., Lienert J., McArdell C.S. Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res. 2011;45(1):75–92. doi: 10.1016/j.watres.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Fisher D.J., Burton D.T., Yonkos L.T., Turley S.D., Ziegler G.P. The relative acute toxicity of continuous and intermittent exposures of chlorine and bromine to aquatic organisms in the presence and absence of ammonia. Water Res. 1999;33(3):760–768. [Google Scholar]
- Furst K.E., Coyte R.M., Wood M., Vengosh A., Mitch W.A. Disinfection byproducts in Rajasthan, India: are trihalomethanes a sufficient indicator of disinfection byproduct exposure in low-income countries? Environ. Sci. Technol. 2019;53(20):12007–12017. doi: 10.1021/acs.est.9b03484. [DOI] [PubMed] [Google Scholar]
- Godoy A.A., Kummrow F. What do we know about the ecotoxicology of pharmaceutical and personal care product mixtures? A critical review. Crit. Rev. Env. Sci. Tec. 2017;47(16):1453–1496. [Google Scholar]
- Godoy A.A., Domingues I., Arsénia Nogueira A.J., Kummrow F. Ecotoxicological effects, water quality standards and risk assessment for the anti-diabetic metformin. Environ. Pollut. 2018;243:534–542. doi: 10.1016/j.envpol.2018.09.031. [DOI] [PubMed] [Google Scholar]
- Huang C., Jin B., Han M., Yu Y., Zhang G., Arp H.P.H. The distribution of persistent, mobile and toxic (PMT) pharmaceuticals and personal care products monitored across chinese water resources. J. Hazard. Mater. Lett. 2021;2 [Google Scholar]
- Hubei Province Health Commission of Hubei Province. 2021. http://wjw.hubei.gov.cn/ (accessed 19th June, 2021)
- Jin W.B., Zhou J., Chen B.Y., Zhu X.S., Cui C.W. Modeling volatilization and adsorption of disinfection byproducts in natural watersheds. J. Environ. Monitor. 2012;14(11):2990–2999. doi: 10.1039/c2em30617d. [DOI] [PubMed] [Google Scholar]
- Krasner S.W., Westerhoff P., Chen B.Y., Rittmann B.E., Amy G. Occurrence of disinfection byproducts in United States wastewater treatment plant effluents. Environ. Sci. Technol. 2009;43(21):8320–8325. doi: 10.1021/es901611m. [DOI] [PubMed] [Google Scholar]
- Kuroda K., Li C., Dhangar K., Kumar M. Predicted occurrence, ecotoxicological risk and environmentally acquired resistance of antiviral drugs associated with COVID-19 in environmental waters. Sci. Total Environ. 2021;776 doi: 10.1016/j.scitotenv.2021.145740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang Y., Xiong H.S., Tan Z.J., Lv Z., Zheng K.K., Zou L.X., Luo G.B., Ye L., Zhang Z.H., Wang M. Investigation and optimization strategy on the operation of disinfection facilities in municipal WWTPs. China Water Wastew. 2020;36(8):7–19. [Google Scholar]
- Li Q., Guan X.H., Wu P., Wang X.Y., Zhou L., Tong Y.Q., Ren R.Q., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X.S., Xiang N.J., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W.X., Chen C.D., Jin L.M., Yang R., Wang Q., Zhou S.H., Wang R., Liu H., Luo Y.B., Liu Y., Shao G., Li H., Tao Z.F., Yang Y., Deng Z.Q., Liu B.X., Ma Z.T., Zhang Y.P., Shi G.Q., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z.J. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z.G., Song G.F., Bi Y.H., Gao W., He A., Lu Y., Wang Y.W., Jiang G.B. Occurrence and distribution of disinfection byproducts in domestic wastewater effluent, tap water, and surface water during the SARS-CoV-2 pandemic in China. Environ. Sci. Technol. 2021;55(7):4103–4114. doi: 10.1021/acs.est.0c06856. [DOI] [PubMed] [Google Scholar]
- Liu J.L., Wong M.H. Pharmaceuticals and personal care products (PPCPs): a review on environmental contamination in China. Environ. Int. 2013;59:208–224. doi: 10.1016/j.envint.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Liu C., Zhou Q.Q., Li Y.Z., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6(3):315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chen H., Shang Y., Zhu H., Chen G., Chen Y., Liu S., Zhou Y., Huang M., Hong Z., Xia J. Efficacy of chloroquine versus lopinavir/ritonavir in mild/general COVID-19 infection: a prospective, open-label, multicenter, randomized controlled clinical study. Trials. 2020;21(1):622. doi: 10.1186/s13063-020-04478-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannou C., Ofrydopoulou A., Evgenidou E., Heath D., Heath E., Lambropoulou D. Antiviral drugs in aquatic environment and wastewater treatment plants: a review on occurrence, fate, removal and ecotoxicity. Sci. Total Environ. 2020;699 doi: 10.1016/j.scitotenv.2019.134322. [DOI] [PubMed] [Google Scholar]
- Nardell E.A., Nathavitharana R.R. Airborne spread of SARS-CoV-2 and a potential role for air disinfection. J. Am. Med. Assoc. 2020;324(2):141–142. doi: 10.1001/jama.2020.7603. [DOI] [PubMed] [Google Scholar]
- Race M., Ferraro A., Galdiero E., Guida M., Nunez-Delgado A., Pirozzi F., Siciliano A., Fabbricino M. Current emerging SARS-CoV-2 pandemic: potential direct/indirect negative impacts of virus persistence and related therapeutic drugs on the aquatic compartments. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendal C., Kusk K.O., Trapp S. The effect of pH on the uptake and toxicity of the bivalent weak base chloroquine tested on Salix viminalis and daphnia magna. Environ. Toxicol. Chem. 2011;30(2):354–359. doi: 10.1002/etc.391. [DOI] [PubMed] [Google Scholar]
- Richardson S.D., Thruston A.D., Caughran T.V., Chen P.H., Collette T.W., Floyd T.L. Identification of new drinking water disinfection byproducts formed in the presence of bromide. Environ. Sci. Technol. 1999;33(19):3378–3383. [Google Scholar]
- Richardson S.D., Plewa M.J., Wagner E.D., Schoeny R., DeMarini D.M. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat. Res. Rev. Mutat. Res. 2007;636(1):178–242. doi: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Narvaez O.M., Peralta-Hernandez J.M., Goonetilleke A., Bandala E.R. Treatment technologies for emerging contaminants in water: a review. Chem. Eng. J. 2017;323:361–380. [Google Scholar]
- Sarkodie S.A., Owusu P.A. Global assessment of environment, health and economic impact of the novel coronavirus (COVID-19) Environ. Dev. Sustain. 2021;23(4):5005–5015. doi: 10.1007/s10668-020-00801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczuka A., Berglund-Brown J.P., Chen H.K., Quay A.N., Mitch W.A. Evaluation of a pilot anaerobic secondary effluent for potable reuse: impact of different disinfection schemes on organic fouling of RO membranes and DBP formation. Environ. Sci. Technol. 2019;53(6):3166–3176. doi: 10.1021/acs.est.8b05473. [DOI] [PubMed] [Google Scholar]
- Tian Y., Rong L., Nian W.D., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020;51(9):843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdell C.A. Economic, social and political issues raised by the COVID-19 pandemic. Econ. Anal. Policy. 2020;68:17–28. doi: 10.1016/j.eap.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA . United States Environmental Protection Agency; Cincinnati, OH: 1995. Method 551.1: Determination of Chlorination Disinfection Byproducts, Chlorinated Solvents, and Halogenated Pesticides/Herbicides in Drinking Water by Liquid-Liquid Extraction and Gas Chromatography with Electron-Capture Detection, Revision 1.0. [Google Scholar]
- US EPA . United States Environmental Protection Agency; Cincinnati, OH: 2003. Method 552.3: Determination of Haloacetic Acids and Dalapon in Drinking Water by Liquid-Liquid Microextraction, Derivatization, and Gas Chromatography with Electron-Capture Detection, Revision 1.0. [Google Scholar]
- US EPA . United States Environmental Protection Agency; Washington, DC, USA: 2020. US EPA Ecological Structure-Activity Relationship Model (ECOSAR) Version 2.0. [Google Scholar]
- Wang X., Zhang H., Zhang Y., Shi Q., Wang J., Yu J., Yang M. New insights into trihalomethane and haloacetic acid formation potentials: correlation with the molecular composition of natural organic matter in source water. Environ. Sci. Technol. 2017;51(4):2015–2021. doi: 10.1021/acs.est.6b04817. [DOI] [PubMed] [Google Scholar]
- Wang J., Shen J., Ye D., Yan X., Zhang Y.J., Yang W.J., Li X.W., Wang J.Q., Zhang L.B., Pan L.J. Disinfection technology of hospital wastes and wastewater: suggestions for disinfection strategy during coronavirus disease 2019 (COVID-19) pandemic in China. Environ. Pollut. 2020;262 doi: 10.1016/j.envpol.2020.114665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.Y., Zhang X., Chen S.S., Meng F.B., Zhang D.Y., Liu Y., Li M., Liu X., Huang X., Qu J.H. Spatial variation of dissolved organic nitrogen in Wuhan surface waters: correlation with the occurrence of disinfection byproducts during the COVID-19 pandemic. Water Res. 2021;198 doi: 10.1016/j.watres.2021.117138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . WHO; 2020. Landscape analysis of therapeutics.https://www.who.int/blueprint/priority-diseases/key-action/Table_of_therapeutics_Appendix_17022020.pdf (accessed 19th June, 2021) [Google Scholar]
- WHO WHO Coronavirus (COVID-19) dashboard. 2021. https://covid19.who.int/
- Wu R., Wang L., Kuo H.-C.D., Shannar A., Peter R., Chou P.J., Li S., Hudlikar R., Liu X., Liu Z., Poiani G.J., Amorosa L., Brunetti L., Kong A.-N. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. Rep. 2020;6:56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W.H., Zhang G., Li X.D., Zou S.C., Li P., Hu Z.H., Li J. Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China. Water Res. 2007;41(19):4526–4534. doi: 10.1016/j.watres.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Yin W., Wang C., Zhang H., Lei P. Impact of the use of disinfectants on water environment in Wuhan during COVID-19 pandemic. Yangtze River. 2020;51(5):29–33. [Google Scholar]
- Zeng T., Plewa M.J., Mitch W.A. N-nitrosamines and halogenated disinfection byproducts in US full advanced treatment trains for potable reuse. Water Res. 2016;101:176–186. doi: 10.1016/j.watres.2016.03.062. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Chuang Y.H., Szczuka A., Ishida K.P., Roback S., Plumlee M.H., Mitch W.A. Pilot-scale evaluation of oxidant speciation, 1,4-dioxane degradation and disinfection byproduct formation during UV/hydrogen peroxide, UV/free chlorine and UV/chloramines advanced oxidation process treatment for potable reuse. Water Res. 2019;164 doi: 10.1016/j.watres.2019.114939. [DOI] [PubMed] [Google Scholar]
- Zhang D.Y., Ling H.B., Huang X., Li J., Li W.W., Yi C., Zhang T., Jiang Y.Z., He Y.N., Deng S.Q., Zhang X., Wang X.Z., Liu Y., Li G.H., Qu J.H. Potential spreading risks and disinfection challenges of medical wastewater by the presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of fangcang hospital. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurita J.L., Jos A., del Peso A., Salguero M., Lopez-Artiguez M., Repetto G. Ecotoxicological evaluation of the antimalarial drug chloroquine. Aquat. Toxicol. 2005;75(2):97–107. doi: 10.1016/j.aquatox.2005.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material