Abstract
Objective
The aim of this study was to assess the accuracy of the OptiBP mobile application based on an optical signal recorded by placing the patient’s fingertip on a smartphone’s camera to estimate blood pressure (BP). Measurements were carried out in a general population according to existing standards of the Association for the Advancement of Medical Instrumentation (AAMI), the European Society of Hypertension (ESH) and the International Organization for Standardization (ISO).
Methods
Participants were recruited during a scheduled appointment at the hypertension clinic of Lausanne University Hospital in Switzerland. Age, gender and BP distribution were collected to fulfill AAMI/ESH/ISO universal standards. Both auscultatory BP references and OptiBP were measured and compared using the opposite arm simultaneous method as described in the 81060-2:2018 ISO norm.
Results
A total of 353 paired recordings from 91 subjects were analyzed. For validation criterion 1, the mean ± SD between OptiBP and reference BP recordings was respectively 0.5 ± 7.7 mmHg and 0.4 ± 4.6 mmHg for SBP and DBP. For validation criterion 2, the SD of the averaged BP differences between OptiBP and reference BP per subject was 6.3 mmHg and 3.5 mmHg for SBP and DBP. OptiBP acceptance rate was 85%.
Conclusion
The smartphone embedded OptiBP cuffless mobile application fulfills the validation requirements of AAMI/ESH/ISO universal standards in a general population for the measurement of SBP and DBP.
Keywords: application, blood pressure, cuffless, international standards, optical signal, smartphone, validation
Introduction
Hypertension is the most important modifiable risk factor for cardiovascular diseases [1]. Its prevalence has been increasing and is expected to reach 1.5 billion people worldwide by 2025 [2]. Undiagnosed and uncontrolled hypertension are two of the most significant contributing factors to morbidity and mortality [3]. Auscultatory and automated oscillometric sphygmomanometers are the current reference techniques to measure blood pressure (BP) and diagnose hypertension whether they are performed at the doctors’ office or at home. However new technologies have recently emerged including cuffless approaches using smartphone-based medical applications. In the context of chronic disease diagnosis, treatment and follow-up, these smartphone-based solutions, defining mobile health, have been shown to be useful and effective [4–6]. Although many applications estimating BP can be easily downloaded, none of them has been validated following a strict reference international protocol nor approved as a medical device by the Food and Drug Administration (FDA) [7,8]. Validation and access to smartphone-based solutions to measure BP would allow widespread diagnosis of hypertension and consequently could improve the management of hypertensive patients in various settings. This is particularly true in low-income countries, where BP devices are scarce, but smartphones widely available [9]. It is with this in mind that the OptiBP mobile app (Biospectal SA, Lausanne, CH) has been developed to provide a BP estimation using a dedicated pulse wave analysis (PWA) algorithm applied to photoplethysmography signals derived from images acquired with a smartphone’s camera [10] (Fig. 1). The algorithm is based on the analysis of the morphology of the recorded photoplethysmography waveforms to estimate BP. This technique has been applied to photoplethysmography signal acquired with an oximeter finger clip and tested against an invasive reference (arterial line) in the context of general anesthesia induction [11]. In a recent study, the accuracy of the algorithm applied to photoplethysmography signals derived from images of the smartphone’s camera was assessed in a small patient cohort against auscultatory references during a stability protocol [12]. In the present study, we evaluate the OptiBP mobile app as a whole (app-based preprocessing, processing and postprocessing as well as PWA algorithm) on an extended sample of the general population that was used in our previous study [12].
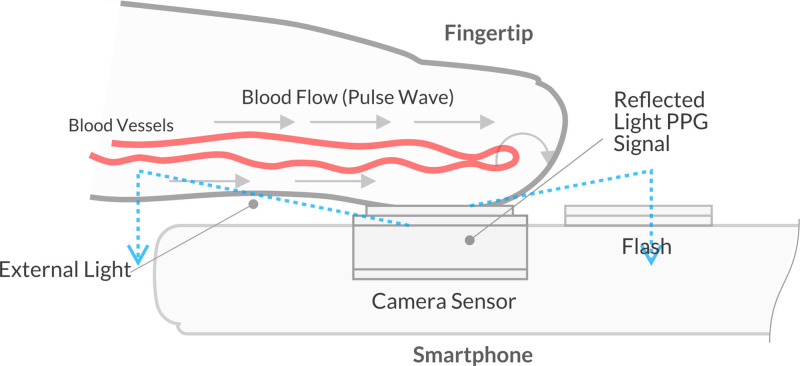
Fig. 1.
Fingertip on the smartphone’s camera [12]. OptiBP app utilizes image data generated from volumetric blood flow changes via light passing through the fingertip, reflecting off of blood flowing through the vessels, and then passing to the phone camera’s image sensor.
Due to the current lack of any international standards for the validation of cuffless BP measuring devices, this study is based on the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) universal standards [13,14] with modifications imposed by cuffless measurements. The objective of the study was to determine the accuracy of the OptiBP smartphone app using standards adapted to cuffless devices. OptiBP mobile app is not yet approved by the FDA.
Methods
Study approval
The study has been approved by the local ethic committee (Commission cantonale d’éthique de la recherche sur l’être humain, 1012 Lausanne, Switzerland, CER-VD no. 2018-01656) and registered under number NCT03875248 at www.clinicaltrials.gov on 14 March 2019. We obtained written informed consent of all enrolled subjects. The protocol was conducted in accordance with the Declaration of Helsinki.
Participants
One hundred participants older than 18 years from the outpatient hypertension clinic of Lausanne University Hospital in Switzerland and from the University of Lausanne were recruited. The following subject’s features were collected: gender, age, height, weight, known hypertension and its stage as well as midarm circumference. The subjects’ characteristics are shown in Table 1, and the requirements for gender distribution were fulfilled. Special populations as described in AAMI/ESH/ISO universal standards (ISO 81060-2:2018) were excluded as well as subjects with the following cardiovascular conditions: myocardial infarction of less than 1 week, pulmonary embolism, arrhythmia and decompensated heart failure.
Table 1.
Participants’ characteristics
| Participants’ characteristics (n = 91 subjects) | Mean | SD | Range |
|---|---|---|---|
| Age (years) | 52.9 | 15.9 | 21–81 |
| Height (cm) | 171.6 | 10.2 | 154–202 |
| Weight (kg) | 75.7 | 18.4 | 45–152 |
| BMI (kg/m2) | 25.4 | 4.8 | 17.2–44.6 |
| Gender (M/F) | 42/49 | ||
| Reference recordings (n = 353) | |||
| SBP (mmHg) | 122.1 | 19.8 | 81–171 |
| DBP (mmHg) | 78.3 | 12.7 | 56–109 |
Test device
The OptiBP app was installed on a Samsung Galaxy S7 (Samsung GEC, 26, Sangil-ro 6-gil, Gagdong-gu, Seoul, Korea) to estimate BP by applying the fingertip on its camera. The methodology of the optical signal acquisition and its initial processing has been published recently [12].
Reference blood pressure
A dual-head (Y-tube) stethoscope was used by two independent and blinded experienced observers. A validated sphygmomanometer (A&D UM-101, A&D Company, Ltd., Toshima Ku, Tokyo, Japan) [15,16], which had been calibrated before the study initiation was used for simultaneous reference auscultatory SBP and DBP measurements. The size of the reference cuff was adapted to the circumference of the subject’s midarm. Two different cuffs with inflatable bladder dimensions 14 × 25 cm and 16 × 32 cm respectively were used so that the length corresponds to 75–100% of the subject’s midarm circumference and the width’s 37–50%.
Validation team
The team was conducted by a supervisor and two trained observers who were experienced in measurement research and were standardized for their agreement in BP measurement before the study initiation [13,14]. During the study, each observer took the measurements without knowing the extent or the nature of their potential disagreement as described in AAMI/ESH/ISO universal standards.
Procedure
The protocol was based on the AAMI/ESH/ISO universal standard (ISO 81060-2:2018) with minor adjustments due to our cuffless approach [13,14]. The opposite arm simultaneous method was chosen to conduct the study (Fig. 2). Subject preparation consisted of five min relaxation in an isolated and quiet room with a comfortable temperature. All subjects had their backs, elbows and forearms supported. Legs were uncrossed, feet flat on the floor and bladder was empty. The appropriate cuff size was selected and the smartphone was positioned at the level of the left ventricle of the heart.
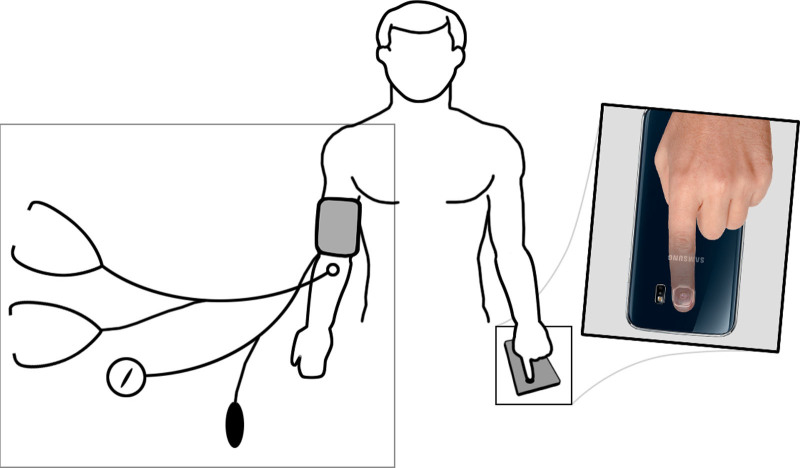
Fig. 2.
Setting for opposite arm simultaneous method according to ISO 81060-2:2018
The SBP and DBP reference measurements were obtained three times on the right and left arm and averaged to obtain systolic and diastolic lateral differences. Subjects with SBP difference >15 mmHg or DBP difference >10 mmHg between arms were excluded.
For each measurement, reference BP values were measured and optical signals were acquired simultaneously using the reference method on one arm and the smartphone on the fingertip of the opposite arm. The starting arm side was alternated between subjects according to the recruitment number: odd-numbered subjects started with the reference device on the right arm and even-numbered subjects on the left arm. After the first four valid measurements, sides of measurement were changed. Then, the first 49 participants had three additional measurements on the other arm side and four for the last 51 participants (amended protocol approved by the local ethic committee and Swissmedic). The ISO specification requires the two observers to have a maximal disagreement in their measure of SBP and DBP of 4 mmHg and successive reference recordings on the same arm to have variations of max 12 mmHg for SBP and 8 mmHg for DBP. In case of failure of the above, the measurements were discarded and repeated. The protocol included a maximum of nine sequential BP measurements for each participant taken every 2 min including repeated measurements. The amended protocol is summarized in Table 2.
Table 2.
Protocol using the opposite arm simultaneous method based on ISO 81060-2:2018
| Protocol (opposite arm simultaneous method) |
|---|
| Lateral difference determination (LD): 3 reference measurements on each arm |
| Measurements #0: reference (R0) and OptiBP (T0) measurements |
| Screening and calibration for the first arm (if signal quality is satisfactory) |
| Measurement #1: reference (R1) and OptiBP (T1) measurements |
| Measurement #2: reference (R2) and OptiBP (T2) measurements |
| Measurement #3: reference (R3) and OptiBP (T3) measurements |
| Interchange arm sides |
| Measurement #4: reference (R4) and OptiBP (T4) measurements |
| Calibration for the second arm (if signal quality is satisfactory) |
| Measurement #5: reference (R5) and OptiBP (T5) measurements |
| Measurement #6: reference (R6) and OptiBP (T6) measurements |
| Measurement #7: reference (R7) and OptiBP (T7) measurements |
| Extra measurement #8: reference (R8) and OptiBP (T8) measurements |
Optical signal analysis
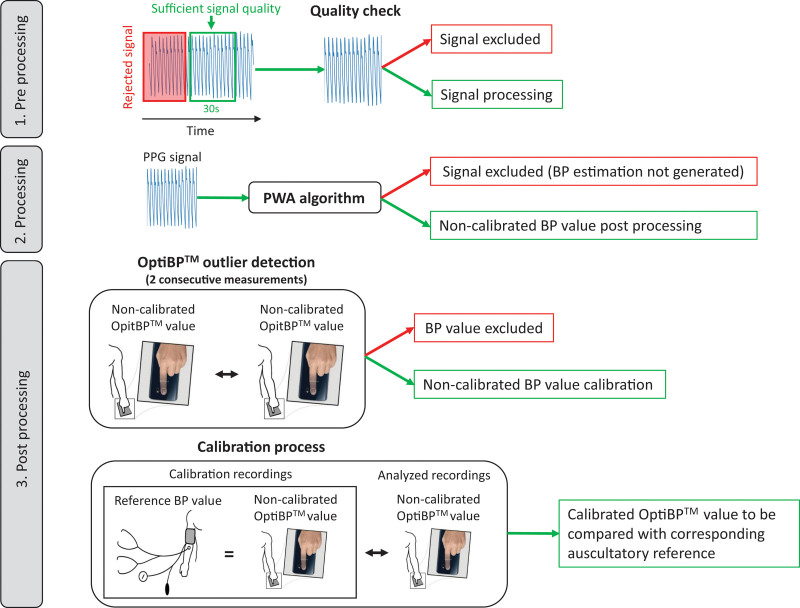
The data was acquired and preprocessed using the OptiBP app, then postprocessed and blindly analyzed offline to extract values of SBP and DBP as described in Fig. 3. An in-app preprocessing step, applied to each 1-min sequence of images acquired, identified the starting time of a 30-s window based on an initial quality criteria defined by the similarity between consecutive pulse waves during 1 s. The selected 30 s of recording were then further processed by the OptiBP app to assess the overall signal quality. Low-quality measurements were automatically excluded at this point, with the remaining measurements being processed by the PWA algorithm to extract uncalibrated estimates of BP. Despite the in-app raw photoplethysmography data quality check, it may happen that the PWA algorithm was unable to find reliable physiologic features required to provide a BP estimate. These measurements were therefore also excluded prior to the statistical analysis. Then, consecutive noncalibrated measurements on the same arm were compared to detect outliers. Two consecutive measurements in a 2-minutes interval with a difference of respectively >20 and >10 mmHg on SBP and DBP were classified as outliers by OptiBP and also excluded for the analysis. Finally, raw BP estimations for each arm were calibrated using the first available signal on the same arm to estimate BP values and test their accuracy against the reference measurement. This last step was performed following the exact same protocol as that used in the preliminary study [12].
Fig. 3.
OptiBP analysis.
Results
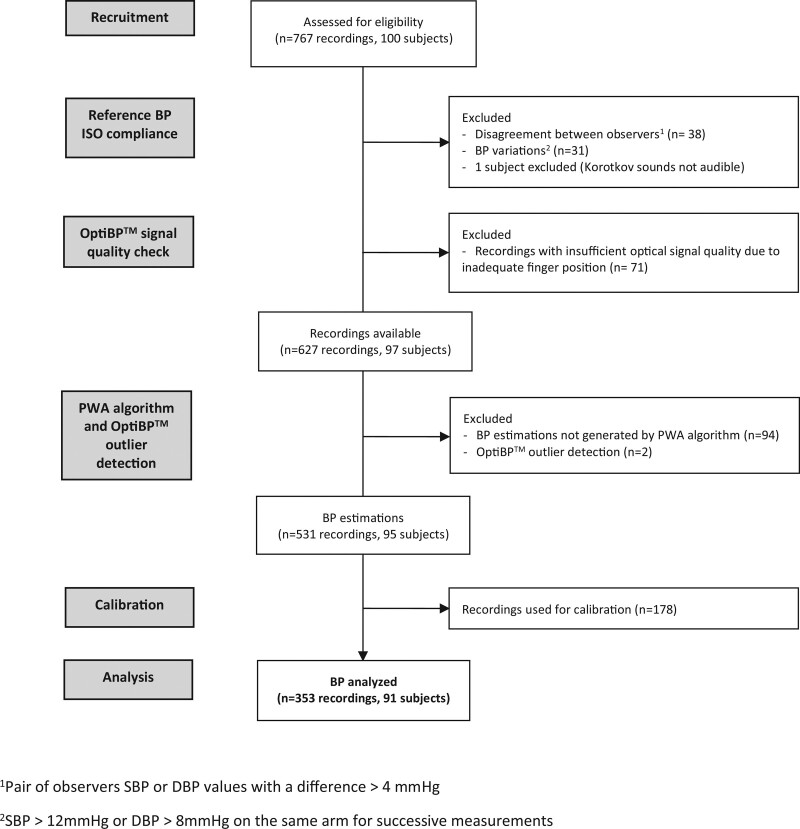
In total, 100 subjects were recruited and 91 were analyzed, for a total of 353 readings. One participant was excluded because of the impossibility to measure BP auscultatory (Korotkov sounds not audible).
Concerning the reference measurements, the mean BP difference between the simultaneous observers’ measurements was respectively 0.6 ± 3.1 and 0.3 ± 2.2 mmHg for SBP and DBP. Forty-four out of 767 BP recordings with inter-observer disagreement >4 mmHg were excluded including six recordings with both SBP and DBP disagreements. Thirty-one reference BP measurements were also excluded due to BP variations. Among the initial 767 recordings, 698 were analyzed by OptiBP. The distribution of the reference BP measurements is presented in Table 3.
Table 3.
Distribution of the reference blood pressure measurements
| Reference BP distribution (n = 627 recordings) | # of recordings | % of recordings | AAMI/ESH/ISO requirement (%) | ||
|---|---|---|---|---|---|
| Systolic | SBP ≥160 mmHg | 31 | 4.9 | ≥5 | FAIL |
| SBP ≥140 mmHg | 151 | 24.1 | ≥20 | PASS | |
| SBP ≤100 mmHg | 77 | 12.3 | ≥5 | PASS | |
| Diastolic | DBP ≥100 mmHg | 39 | 6.2 | ≥5 | PASS |
| DBP ≥85 mmHg | 206 | 32.9 | ≥20 | PASS | |
| DBP ≤60 mmHg | 46 | 7.3 | ≥5 | PASS | |
Concerning the recordings acquired with the OptiBP app, 71 of the 698 recordings were rejected during its preprocessing quality evaluation. For the remaining 627 recordings, two measurements were excluded by the outlier rejection procedure and 94 recordings could not be analyzed because the PWA algorithm was unable to identify reliable physiologic features to provide a BP estimation. OptiBP acceptance rate was 85% for the 627 recordings. Finally, 178 recordings were used for calibration and 353 analyzed to test OptiBP accuracy against reference measurements. Reasons for data exclusions are summarized in Fig. 4.
Fig. 4.
Consort flow diagram.
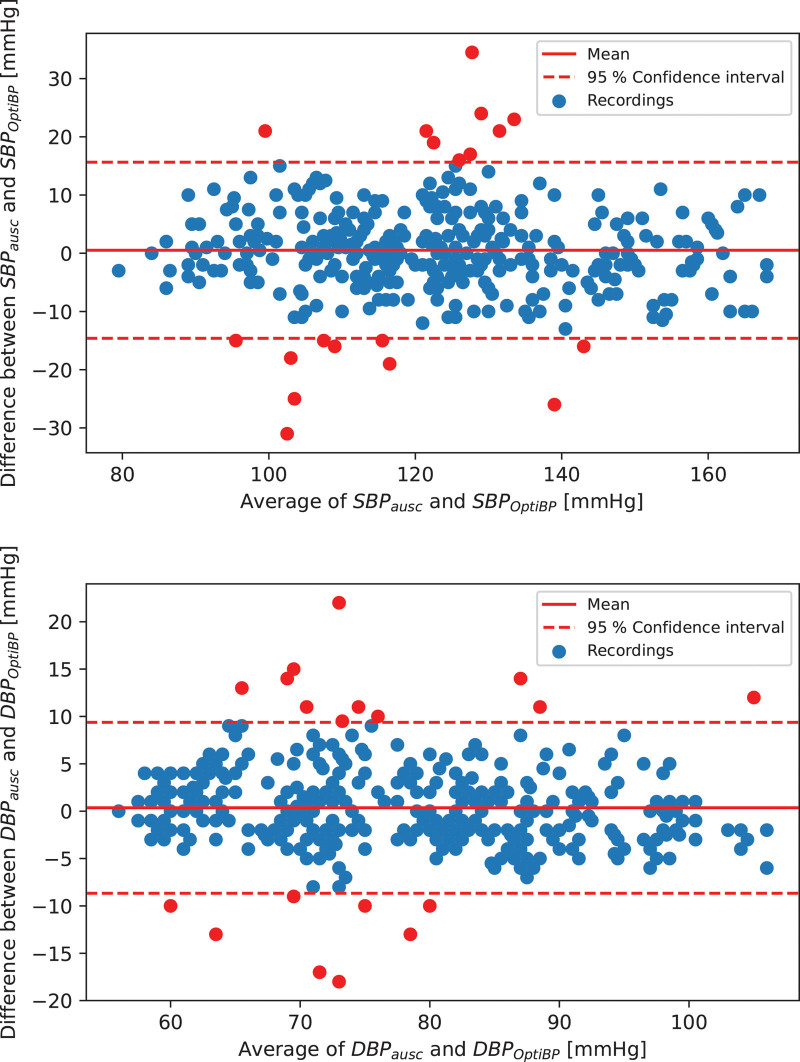
The results for validation criteria 1 and 2 of the 353 analyzed recordings are summarized in Table 4. Criteria 1 validation results show a cohort-wise error of 0.5 ± 7.7 mmHg for the SBP and 0.4 ± 4.6 mmHg for the DBP. The results for criteria 2 show an SD of the average error per subject of 6.3 mmHg for the SBP and 3.5 mmHg for the DBP. Standardized Bland–Altman scatterplots of the OptiBP-reference BP differences against their average value are shown in Fig. 5.
Table 4.
Validation study results
| OptiBP results | AAMI/ESH/ISO requirement | |||
|---|---|---|---|---|
| SBP | DBP | |||
| Criterion 1 (353 recordings) | ||||
| Mean BP difference (mmHg) | 0.5 | 0.4 | ≤ 5 | PASS |
| SD BP difference (mmHg) | 7.7 | 4.6 | ≤ 8 | PASS |
| Criterion 2 (91 subjects) | ||||
| SD of average BP difference (mmHg, SBP/DBP) | 6.3 | 3.5 | ≤ 6.92/6.95 | PASS |
Fig. 5.
Standardized Bland–Altman scatterplots of the OptiBP-Reference BP differences against their average.
Discussion
The practical aspects of using a smartphone as an interface to estimate BP show numerous advantages. Smartphones are widely available and used with the potential to allow quick and efficient implementation of a new cuffless approach based on a mobile app. As of now, an initial calibration is necessary and could be done at the doctors’ office or at a healthcare facility, depending on the local setting. This may further participate in the patients’ understanding of his hypertensive disease while strengthening the relationship with his health practitioner. Lastly, by simply applying a fingertip on a smartphone’s camera during 1 min in the patient’s own setting, the OptiBP can estimate BP at various times of the day whereas minimizing complexity. Its tolerance is excellent and no adverse events have been reported during the existing studies. As with any other BP device, training to assure correct measurements of BP is of importance to obtain adequate readings. In our setting, finger positioning is essential.
In the last decade, numerous studies have evaluated cuffless-based smartphone algorithm to estimate BP as stated recently by Lee et al. [8]. Among them, only two fulfilled the accuracy requirements of the ISO norm 81060–2:2013. In 2016, Gaurav et al. [17] developed a solution using photoplethysmography signals acquired via a smartphone to track continuously BP values. Although they reported accurate results in a large population, no validation study following a standardized international protocol has been conducted. More recently, Luo et al. [18] demonstrated that transdermal optical imaging was able to extract BP values by detecting facial blood flow changes using a smartphone’s camera in a controlled setting, using an external light source to ensure sufficient and uniform lightning. This novel technology passed the validation criteria in a cohort of normotensive participants. However, the inclusion of hypertensive and hypotensive patients is necessary to assess the clinical applicability of the approach. Comparison against a recognized gold standard is also required.
As of now, research in the field of cuffless BP measurements focused on the accuracy of BP estimations trying to follow strict international validation protocols edited for the traditional cuff-based oscillometric monitor. Although innovative solutions have shown encouraging results, the current validation processes are no longer adequate. The use of the smartphone as a potential medical device will trigger a paradigm shift in the standards as the main purpose for developing such widely available technologies has changed. A balance between accuracy and its ranges, signal stability and portability across brands and their various components will have to be considered to properly democratize smartphone-based BP measurements while putting patients at risk or falsely reassuring them.
Limitations
The main limitations to our study are related to the lack of existing standards to validate a noncontinuous cuffless BP monitor. Thus, minor adjustments had to be made to the ISO 81060-2:2018 protocol to be compatible with our mobile app. Furthermore, for the data processing, in cases of exclusion of one or several reference measurements of one subject (as described in the procedure section), the remaining valid recordings of the same subject were included for the data analysis contrary of ISO recommendations.
A second limitation concerns the studied population. The population does not precisely meet the criteria of BP values distribution requested by the standards with a few missing reference measurements with SBP ≥ 160 mmHg (5% requested versus 4.9% measured). However, Fig. 5 demonstrates that OptiBP error is not influenced by BP values and confirms its performance in low and high BP. Equally, our results apply to a general population as described in ISO standards. OptiBP needs further validation in greater and various subtypes of populations to allow democratization with medical precision. Recruitment of patients with specific medical conditions such as peripheral arterial disease, connective tissue or skin diseases is also necessary to further identify their potential impact on the signal quality analysis. Studies are underway in various settings to assess those specific points.
Lastly, our results do not provide information about the stability of the calibration over time as this was not the primary endpoint of this current study. This will be the subject of the next study, in which several recording sessions are carried out over various time periods.
Conclusion
This study demonstrates the accuracy of the OptiBP smartphone app to estimate the BP of 91 participants representative of a general population using a standardized protocol. Both validation criteria 1 and 2 of the AAMI/ESH/ISO universal standards (ISO 81060-2:2018) were fulfilled with a same-day calibration. This validation opens opportunities to improve hypertension awareness and could positively impact the diagnosis and follow-up of hypertensive patients worldwide.
Acknowledgements
Innosuisse - Swiss Innovation Agency, Project no. 32688.1 IP-ICT
Conficts of interest
M.P., G.B., A.L. and M.L. are with CSEM, the owners of the optical blood pressure monitoring technology and assignee of the related application (WO2016138965A1), of which M.P. and M.L. are inventors. U.C. and J-F.K. are working for Biospectal SA. P.S. is an advisor to Biospectal. Innosuisse - Swiss Innovation Agency, Project no. 32688.1 IP-ICT had no role in study design, data collection nor analysis, in the writing of the report nor in the decision to submit the article for publication. For the remaining authors, there are no conflicts of interest.
Footnotes
Dr. Gregoire Wuerzner and Prof. Patrick Schoettker contributed equally to the writing of this article and share last authorship.
References
- 1.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Association. W.H. global status report on non-communicable diseases 2014. Vol. 2014. pp. 2. WHO Library Cataloguing; 1–281. [Google Scholar]
- 3.NCD Risk Factor Collaboration. Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet 2019; 394:639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel AA. Developing and evaluating mHealth solutions for chronic disease prevention in primary care. Circulation 2019; 139:392–394. [DOI] [PubMed] [Google Scholar]
- 5.Burke LE, Ma J, Azar KM, Bennett GG, Peterson ED, Zheng Y, et al.; American Heart Association Publications Committee of the Council on Epidemiology and Prevention, Behavior Change Committee of the Council on Cardiometabolic Health, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, Council on Quality of Care and Outcomes Research, and Stroke Council. Current science on consumer use of mobile health for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation 2015; 132:1157–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anglada-Martinez H, Riu-Viladoms G, Martin-Conde M, Rovira-Illamola M, Sotoca-Momblona JM, Codina-Jane C. Does mHealth increase adherence to medication? Results of a systematic review. Int J Clin Pract 2015; 69:9–32. [DOI] [PubMed] [Google Scholar]
- 7.Kumar N, Khunger M, Gupta A, Garg N. A content analysis of smartphone-based applications for hypertension management. J Am Soc Hypertens 2015; 9:130–136. [DOI] [PubMed] [Google Scholar]
- 8.Lee HY, Lee DJ, Seo J, Ihm SH, Kim KI, Cho EJ, et al.; Korean Society of Hypertension. Smartphone / smartwatch-based cuffless blood pressure measurement: a position paper from the Korean Society of Hypertension. Clin Hypertens 2021; 27:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Commission. E. Health care in your pocket: unlocking the potential of mHealth. 2014. https://ec.europa.eu/commission/presscorner/detail/en/IP_14_394. [Accessed February 9 2021].
- 10.Proença M, Caros SI, Lemay M, Verjus C. Method, apparatus and computer program for determining a blood pressure value 2016. [Google Scholar]
- 11.Ghamri Y, Proença M, Hofmann G, Renevey P, Bonnier G, Braun F, et al. Automated pulse oximeter waveform analysis to track changes in blood pressure during anesthesia induction: a proof-of-concept study. Anesth Analg 2020; 130:1222–1233. [DOI] [PubMed] [Google Scholar]
- 12.Schoettker P, Degott J, Hofmann G, Proença M, Bonnier G, Lemkaddem A, et al. Blood pressure measurements with the OptiBP smartphone app validated against reference auscultatory measurements. Sci Rep 2020; 10:17827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stergiou GS, Palatini P, Asmar R, Ioannidis JP, Kollias A, Lacy P, et al.; European Society of Hypertension Working Group on Blood Pressure Monitoring. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J Hypertens 2019; 37:459–466. [DOI] [PubMed] [Google Scholar]
- 14.ISO 81060-2:2018 international standard. Non-invasive sphygmomanometers - Part 2: Clinical investigation of intermittent automated measurement type. 2018.ISO (International Organization for Standardization). [Google Scholar]
- 15.Stergiou GS, Giovas PP, Gkinos CP, Tzamouranis DG. Validation of the A&D UM-101 professional hybrid device for office blood pressure measurement according to the International Protocol. Blood Press Monit 2008; 13:37–42. [DOI] [PubMed] [Google Scholar]
- 16.Pruijm MT, Wuerzner G, Glatz N, Alwan H, Ponte B, Ackermann D, et al. A new technique for simultaneous validation of two manual nonmercury auscultatory sphygmomanometers (A&D UM-101 and Accoson Greenlight 300) based on the International protocol. Blood Press Monit 2010; 15:322–325. [DOI] [PubMed] [Google Scholar]
- 17.Gaurav A, Maheedhar M, Tiwari VN, Narayanan R. Cuff-less PPG based continuous blood pressure monitoring: a smartphone based approach. Annu Int Conf IEEE Eng Med Biol Soc 2016; 2016:607–610. [DOI] [PubMed] [Google Scholar]
- 18.Luo H, Yang D, Barszczyk A, Vempala N, Wei J, Wu SJ, et al. Smartphone-based blood pressure measurement using transdermal optical imaging technology. Circ Cardiovasc Imaging 2019; 12:e008857. [DOI] [PubMed] [Google Scholar]