BACKGROUND:
We describe the implementation of enhanced recovery after surgery (ERAS) programs designed to minimize postoperative nausea and vomiting (PONV) and pain and reduce opioid use in patients undergoing selected procedures at an ambulatory cancer surgery center. Key components of the ERAS included preoperative patient education regarding the postoperative course, liberal preoperative hydration, standardized PONV prophylaxis, appropriate intraoperative fluid management, and multimodal analgesia at all stages.
METHODS:
We retrospectively reviewed data on patients who underwent mastectomy with or without immediate reconstruction, minimally invasive hysterectomy, thyroidectomy, or minimally invasive prostatectomy from the opening of our institution on January 2016 to December 2018. Data collected included use of total intravenous anesthesia (TIVA), rate of PONV rescue, time to first oral opioid, and total intraoperative and postoperative opioid consumption. Compliance with ERAS elements was determined for each service. Quality outcomes included time to first ambulation, postoperative length of stay (LOS), rate of reoperation, rate of transfer to acute care hospital, 30-day readmission, and urgent care visits ≤30 days.
RESULTS:
We analyzed 6781 ambulatory surgery cases (2965 mastectomies, 1099 hysterectomies, 680 thyroidectomies, and 1976 prostatectomies). PONV rescue decreased most appreciably for mastectomy (28% decrease; 95% confidence interval [CI], –36 to –22). TIVA use increased for both mastectomies (28%; 95% CI, 20-40) and hysterectomies (58%; 95% CI, 46-76). Total intraoperative opioid administration decreased over time across all procedures. Time to first oral opioid decreased for all surgeries; decreases ranged from 0.96 hours (95% CI, 2.1-1.4) for thyroidectomies to 3.3 hours (95% CI, 4.5 to –1.7) for hysterectomies. Total postoperative opioid consumption did not change by a clinically meaningful degree for any surgery. Compliance with ERAS measures was generally high but varied among surgeries.
CONCLUSIONS:
This quality improvement study demonstrates the feasibility of implementing ERAS at an ambulatory surgery center. However, the study did not include either a concurrent or preintervention control so that further studies are needed to assess whether there is an association between implementation of ERAS components and improvements in outcomes. Nevertheless, we provide benchmarking data on postoperative outcomes during the first 3 years of ERAS implementation. Our findings reflect progressive improvement achieved through continuous feedback and education of staff.
See Article, p 1387
KEY POINTS.
Question: What are the rates of postoperative nausea and vomiting (PONV) and opioid use in patients undergoing ambulatory cancer surgeries with enhanced recovery after surgery (ERAS) programs?
Findings: We report low rates of PONV rescue and time to first oral opioids in our practice which incorporates ERAS.
Meaning: ERAS programs are applicable in the ambulatory setting and outcomes reflect an iterative process of continuous improvement.
Enhanced recovery programs (ERPs), or enhanced recovery after surgery (ERAS) programs, are multidisciplinary care pathways that standardize and optimize perioperative care to improve postoperative outcomes. ERAS, originally developed for inpatient surgeries, include multiple elements such as preoperative optimization of comorbid conditions, standardized multimodal analgesic and anesthetic regimens, and early postoperative resumption of diet and mobilization.1 These measures are intended to reduce length of stay (LOS), speed functional recovery, and minimize postoperative nausea and vomiting (PONV) and pain. Though the latter goals are equally desirable and beneficial for ambulatory surgery, few ERAS implementations have been reported in the outpatient or short-stay settings. Benchmarking data on the processes and outcomes of ambulatory ERAS will facilitate quality improvement as ambulatory surgery facilities expand their scope of services.
At the Josie Robertson Surgery Center (JRSC), a freestanding ambulatory surgical facility of Memorial Sloan Kettering Cancer Center (MSK) designed to perform complex cancer surgeries in the ambulatory setting,2 we have implemented ERAS for several ambulatory extended recovery (AXR) procedures. AXR refers to select complex ambulatory procedures after which the patient is permitted a single overnight hospital stay. These include mastectomy with and without immediate tissue expander reconstruction, minimally invasive (laparoscopic and robot-assisted) hysterectomy, thyroidectomy, and minimally invasive prostatectomy. These ERAS programs were developed by surgery-specific workgroups, led by anesthesiologists and including surgeons, nurses, advanced practice providers, pharmacists, nutritionists, and physical therapists. As few ambulatory ERAS had yet been reported in the literature, these pathways were based on broad guidelines from the ERAS Society (erassociety.org), American Society of Enhanced Recovery (aserhq.org), and American Society of Regional Anesthesia and Pain Medicine (asra.com), and the literature regarding other efforts to improve recovery following these and similar procedures3–13 in both the inpatient and short-stay settings. After careful consideration of each procedure in this ambulatory setting, each ERAS was created and approved by workgroup consensus. Furthermore, as various outcome data and practice patterns were observed, these protocols were periodically modified based on the new literature and the consensus of the clinicians involved.
Here, we present our ERAS and provide benchmarking data on postoperative outcomes evolving over the first 3 years of their implementation. Our findings reflect progressive improvement achieved through training, education, and adaptation of staff to changing clinical practices.
METHODS
All patients for the designated surgeries were placed on the enhanced recovery track (Table 1). For each ERAS, an electronic order set was created to initiate standardized pre- and postoperative practices. For intraoperative care, an educational guide was posted in each operating room and regularly updated. Before implementation and each revision, staff were educated about ERAS elements. Staff were initially educated at departmental meetings with specific ERAS pathway information. Hard copies of pathways were immediately available in the operating rooms for reference, as well as in an electronic version. Any updates or revisions were communicated to staff by e-mail and subsequently updated simultaneously in all versions.
Table 1.
Overview of Ambulatory Surgery ERAS
| Phase | Category | Intervention | Mastectomy | MIS hysterectomy | Thyroidectomy | MIS prostatectomy |
|---|---|---|---|---|---|---|
| Preoperative | Optimization of comorbidities | Varies depending on patient’s condition | X | X | X | X |
| Patient education | Discussion regarding postoperative course (sore throat, nausea, and pain), ambulation | X | X | X | X | |
| Hydration | Clear liquids up to 2 h before scheduled arrival | X | X | X | X | |
| PONV prophylaxis | Aprepitant 40 mg orally, for patients with Apfel score of 4 | X | X | X | ||
| Multimodal analgesia | Gabapentin 300 mg orally, immediately before surgery | X | X | X | ||
| Paravertebral, serratus anterior, and PEC1 block | X | |||||
| Intraoperative | Fluid management | 1–3 mL/kg-IBW/h maintenance | X | X | X | Fluid restriction until bladder closure |
| Anesthesia | Total intravenous anesthesia | O | O | O | O | |
| Multimodal analgesia | Acetaminophen 1 g IV at start | X | X | X | X | |
| Ketorolac 15–30 mg IV | X | X | X | |||
| Local anesthesia infiltration | X | X | X | X | ||
| PONV prophylaxis | Dexamethasone 4 mg IV at start Ondansetron 4 mg IV at end | X | X | X + dexamethasone 8 mg IV | X | |
| Intubation recovery | 4% lidocaine 1–2 mL via endotracheal tube at start of closure | X | ||||
| Postoperative | Multimodal analgesia | Acetaminophen 1 g orally, every 8 h to maximum 3 g in 24 h | X | X | X | X |
| Gabapentin 300 mg orally, at night | X | X | X | |||
| Diclofenac 75 mg orally, at night | X | X | X | |||
| Postextubation | Benzocaine lozenges | X | ||||
| Ambulation | Patients encouraged to walk as soon as they felt able | X | X | X | X | |
| Diet | Patients encouraged to resume full diet as soon as they felt able | X | X | X | X |
O indicates optional measures and X indicates standard measures (encouraged but applied at clinicians’ discretion).
Abbreviations: ERAS, enhanced recovery after surgery; IBW, ideal body weight; IV, intravenous; MIS, minimally invasive surgery; PEC1, pectoralis 1; PONV, postoperative nausea.
ERAS Common Elements
All patients are evaluated in advance at presurgical testing clinics, where perioperative ERAS orders are placed and the need for comorbidity optimization is noted. Patients with an American Society of Anesthesiologists (ASA) physical status IV are redirected to have surgery at the main MSK hospital.
Quality perioperative education continues to be beneficial for patients with improved satisfaction, knowledge level, well-being, and reduced anxiety level.14 Patient education started in the surgeon’s office, with dedicated nursing staff instructing the ERAS patients on their pathway based on our guidelines. Education about milestones and clinical pathway is again reiterated by the preoperative nursing staff to the ambulatory patient and documented in the medical chart.
All patients were allowed up to 12 ounces of water 2 hours before arrival at the ambulatory center. NPO status was guided by both ASA guidelines and hospital recommendations. Carbohydrate loading or immunonutrition drinks were not part of the ambulatory ERAS. PONV risk is assessed using the Apfel score15; for those with a score of 4, preoperative aprepitant16,17 is ordered per the standardized MSK antiemetic guideline (Supplemental Digital Content, Figure 1, http://links.lww.com/AA/D318). Preoperative oral gabapentin 300 mg is ordered for patients <65 years of age undergoing mastectomy, hysterectomy, or prostatectomy to reduce postoperative pain and opioid consumption.18,19 Although higher doses have been reported,19 we selected 300 mg because the side effects of dizziness and sedation may be exacerbated in the ambulatory setting. To maintain hydration, patients are encouraged to drink clear liquids up to 2 hours before their scheduled arrival.
All patients receive a general anesthetic, either volatile anesthesia (sevoflurane or desflurane) or total intravenous anesthesia (TIVA) per the clinician’s discretion. TIVA was encouraged as part of all 4 ERAS for patients at increased risk of PONV.20–22 PONV reduction with TIVA is not novel in ambulatory surgery.23 A recent meta-analysis of 229 randomized controlled trials (RCTs) on the efficacy of propofol versus inhalational agents in both ambulatory and in-patient surgical procedures showed perioperative TIVA providing a better patient experience.24 However, Schaefer et al25 did not show a difference in overall risk of PONV when single-drug antiemetic prophylaxis was added to inhalational anesthesia compared to TIVA. To minimize intraoperative opioids, patients are given acetaminophen 1 g intravenous (IV) and ketorolac 15–30 mg intravenous (IV), if there are no surgical contraindications. All patients receive dexamethasone (4 mg IV) and ondansetron (4 mg IV) intraoperatively as PONV prophylaxis. Neuromuscular blocking agents and reversal were used per clinician discretion. Postanesthesia care aims to minimize use of opioids, facilitate early ambulation, and commence oral intake before discharge. Standardized postoperative orders include nonopioid pain medications (ketorolac, diclofenac, acetaminophen, and an evening dose of gabapentin) and antiemetics. Intravenous opioid medications are converted to oral as soon as patients tolerate oral intake. Early diet resumption was incorporated in all surgical services postoperatively. Mobility and ambulation were encouraged by the postanesthesia care unit (PACU) recovery nursing staff with specific instructions for patients to complete “laps” around the floor. Patients remain in the same physical room on arrival from operating room to discharge home. Postoperative LOS was defined as the time from patient entry into the PACU to discharge home or transfer from the facility.
Patients are encouraged to ambulate within a few hours postoperatively and discharged when their postanesthesia score, reflecting consciousness, oxygen saturation, hemodynamic stability, pain, and nausea/vomiting, reaches 8 of 10 on 2 consecutive assessments (Supplemental Digital Content, Figure 2, http://links.lww.com/AA/D318).
Surgery-Specific Considerations
Some ERAS include additional considerations for specific procedures. Mastectomy patients having immediate reconstruction are offered preoperative paravertebral nerve blocks, with or without fascial plane blocks, to reduce postoperative pain,26 and lorazepam postoperatively for chest wall tightness distinct from surgical pain. For thyroidectomies, smaller endotracheal tubes are used to minimize the risk of laryngeal edema, recurrent laryngeal nerve dysfunction, and throat discomfort: size 6.0 tubes for patients <180 cm (5′ 11″) and size 7.0 for taller patients. Also, to reduce PONV, thyroidectomy patients receive dexamethasone 8 mg IV intraoperatively instead of the usual 4 mg dose.27,28 Lidocaine 4% is sprayed down the endotracheal tube before surgical closure to reduce the risk of coughing and bleeding on emergence.29 Finally, benzocaine lozenges are provided in the PACU. We revised the thyroidectomy and hysterectomy ERAS in 2018 to advise TIVA use with propofol and dexmedetomidine in patients with Apfel scores of 3 or 4. For minimally invasive prostatectomies, fluid administration is restricted from surgical incision until bladder closure to facilitate visualization and minimize airway and facial edema from the steep Trendelenburg positioning.30 After that point, fluid deficits are replenished with IV fluids up to 2 L. Routine use of orogastric tubes was minimized unless necessary, as in steep Trendelenburg cases in robotic hysterectomies and prostatectomies.
Data Collection
This retrospective study was approved by the MSK institutional review board (IRB) with waiver of written informed consent. Data were collected for all ERAS cases performed between the opening of JRSC (January 4, 2016) to December 31, 2018. When patients underwent >1 procedure at JRSC during the study period, the first case was retained, and subsequent cases were excluded.
Patient demographics extracted from the electronic medical record (EMR) included age, gender, ASA physical status, and body mass index (BMI). Medication administration was obtained from the electronic anesthesia record and the medication administration record.
Clinical outcomes analyzed included percentage of patients receiving any opioid in PACU, total intraoperative and postoperative opioid consumption measured in oral morphine milligram equivalents (MME), time from PACU arrival to first oral opioid, and percentage of patients requiring PONV rescue, defined as the administration of any antiemetic drug in the PACU.
Quality outcomes included time to first ambulation and LOS. Ambulation was measured using our real-time location system (RTLS; Midmark Corp., Traverse City, MI). All JRSC patients wear RTLS badges, which are detected by sensors placed throughout the facility, approximately 3 m apart. First ambulation is recorded as the time a patient’s badge is first detected outside of their PACU room. Postoperative LOS was defined as the time from patient entry into the PACU to discharge home or transfer from the facility.
Additional quality outcomes gathered from the EMR included transfer to the main hospital for escalation of care, reoperation, and hospital readmissions and urgent care center (UCC) visits to MSK within 30 days. Reoperation was defined as a return to an operating room either at JRSC or the MSK main hospital on postoperative day 0 or 1. ERAS protocol compliance was determined using the EMR.
Statistical Analysis
To estimate outcomes over time, we used a general additive model (GAM). The GAM allows for nonlinearity of relationships between the mean of the response variable and sum of smooth functions of the explanatory variable, in this case date of surgery.31 We estimated the association between date of surgery and proportion of patients using TIVA and requiring a PONV rescue medication using GAMs separately for each surgery type. Time to first oral opioid and total postoperative opioid, by date of surgery, were also estimated using GAMs with a logit link separately for each surgery type.
Compliance was defined as whether the required task was performed and the compliance rates for various analgesic and antiemetic medications were summarized by surgery type. Patients who had medically recognized contraindications to these various medications were excluded from the compliance rate calculation. Real-time interactive compliance dashboards were created to track compliance over time and to drive clinical practice and not for data analysis purposes (Supplemental Digital Content, Figure 3, http://links.lww.com/AA/D318). All calculations were performed using R version 3.5.1 with GAM implementation using the “mgcv” package (R Foundation for Statistical Computing, Vienna, Austria). This article adheres to the applicable Standards for Quality Improvement Reporting Excellence (SQUIRE) reporting guidelines.
RESULTS
From January 4, 2016 to December 31, 2018, 6781 ERAS-directed procedures were performed at JRSC. Only the first case for each patient was retained, for a total of 6720 cases. Patient and surgical characteristics are reported in Table 2 and are consistent with a healthy outpatient population. Changes in direct outcomes for each ERAS over the study period are shown in Figures 1–4 (grouped by ERAS-directed procedure), compliance rates in Supplemental Digital Content, Table 1, http://links.lww.com/AA/D318; Supplemental Digital Content, Figure 3, http://links.lww.com/AA/D318; and Supplemental Digital Content, quality indicators in Table 2, http://links.lww.com/AA/D318.
Table 2.
Patient and Surgical Characteristics by Surgery Type
| Characteristic | Mastectomy, n = 2965 | Minimally invasive hysterectomy, n = 1099 | Thyroidectomy, n = 680 | Minimally invasive prostatectomy, n = 1976 |
|---|---|---|---|---|
| Age (y) | 50 (43–61) | 57 (48–65) | 46 (35–57) | 62 (57–67) |
| Female | 2912 (98%) | 1099 (100%) | 505 (74%) | 0 (0%) |
| ASA physical status | ||||
| I–II | 1745 (59%) | 649 (59%) | 469 (69%) | 1222 (62%) |
| III | 1218 (41%) | 450 (41%) | 211 (31%) | 749 (38%) |
| IV | 2 (<0.1%) | 0 (0%) | 0 (0%) | 5 (0.3%) |
| BMI | 25 (22–29) | 28 (24–34) | 27 (23–32) | 28 (26–31) |
| Apfel score ≥3 | 2723 (92%) | 1011 (92%) | 479 (70%) | 77 (3.9%) |
| TIVA used | 948 (32%) | 207 (19%) | 58 (8.5%) | 26 (1.3%) |
Data are presented as median (IQR) or n (%).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; IQR, interquartile range; TIVA, total intravenous anesthesia.
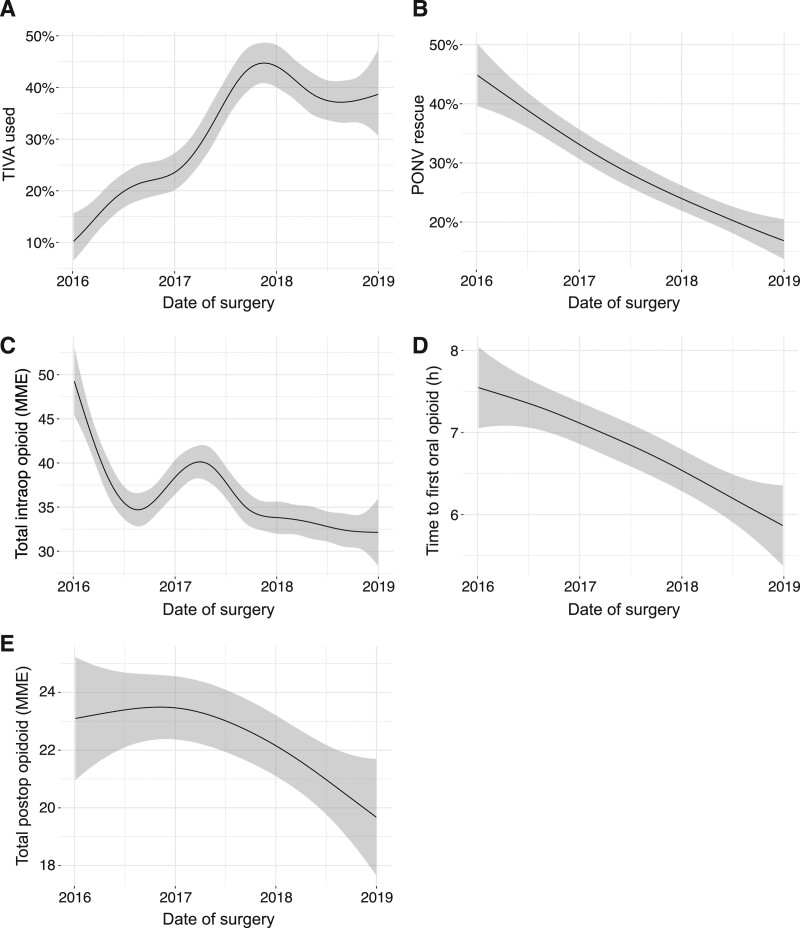
Among mastectomies, use of TIVA increased by 28% (95% CI, 20-40) (Figure 1A) and rates of PONV rescue decreased by 28% (95% CI, 22-36) (Figure 1B), consistent with this change in practice. Intraoperative opioid use also decreased by 17 MME (95% CI, 12-23) (Figure 1C). Time to convert to oral opioids decreased by 1.7 hours (95% CI, 0.42-2.4) (Figure 1D), while total postoperative opioid consumption did not change meaningfully (Figure 1E).
Figure 1.
Clinical outcomes of mastectomies from January 1, 2016 to December 31, 2018 visualized using a generalized additive model. A, TIVA use; (B) PONV rescue; (C) total intraoperative opioid administered (MME); (D) time to first oral opioid; and (E) total postoperative opioid administered (MME). Shaded area represents 95% confidence interval. MME indicates morphine milligram equivalents; PONV, postoperative nausea and vomiting; TIVA, total intravenous anesthesia.
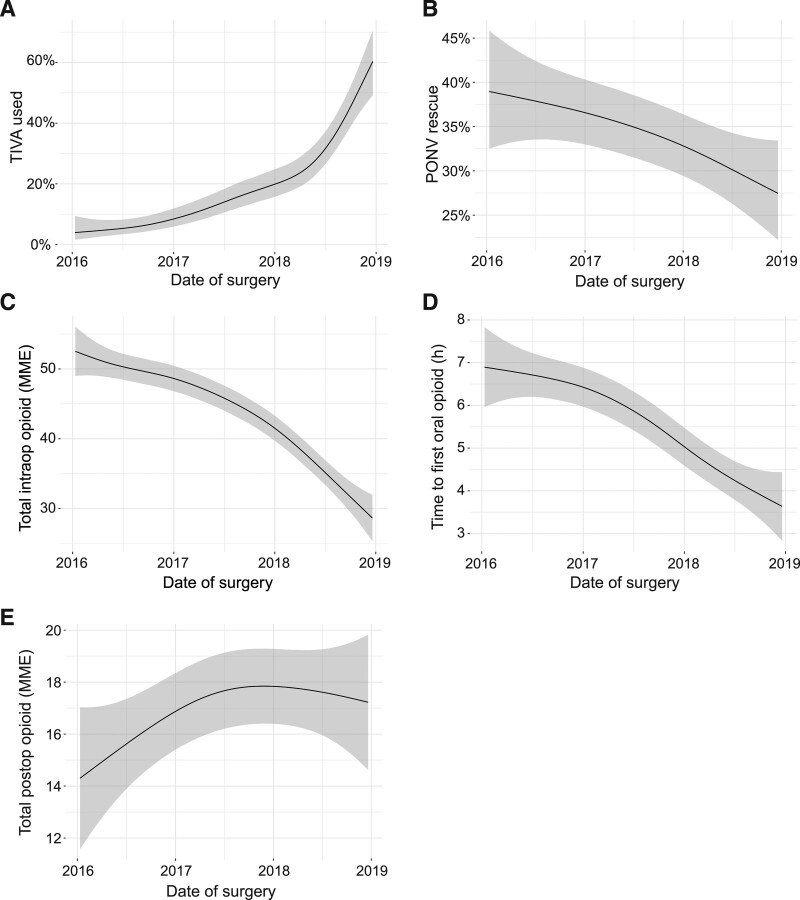
Similarly, among minimally invasive hysterectomies, TIVA use also increased, in this case by 56% (95% CI, 46-76) (Figure 2A), with concomitant decrease in PONV rescue by 11% (95% CI, 0.01-24) (Figure 2B), and intraoperative opioid use decreased by 24 MME (95% CI, 20-36). Time to convert to oral opioids decreased by 3.3 hours (95% CI, 1.7-4.5) (Figure 2D). Total postoperative opioid consumption increased slightly, by 2.9 MME, but the upper bound of the CI does not exclude the possibility that the increase was clinically meaningful (95% CI, 0.23-6.4) (Figure 2E).
Figure 2.
Clinical outcomes of minimally invasive hysterectomies from January 1, 2016 to December 31, 2018 visualized using a generalized additive model. A, TIVA use; (B) PONV rescue; (C) total intraoperative opioid administered (MME); (D) time to first oral opioid; and (E) total postoperative opioid administered (MME). Shaded area represents 95% confidence interval. MME indicates morphine milligram equivalents; PONV, postoperative nausea and vomiting; TIVA, total intravenous anesthesia.
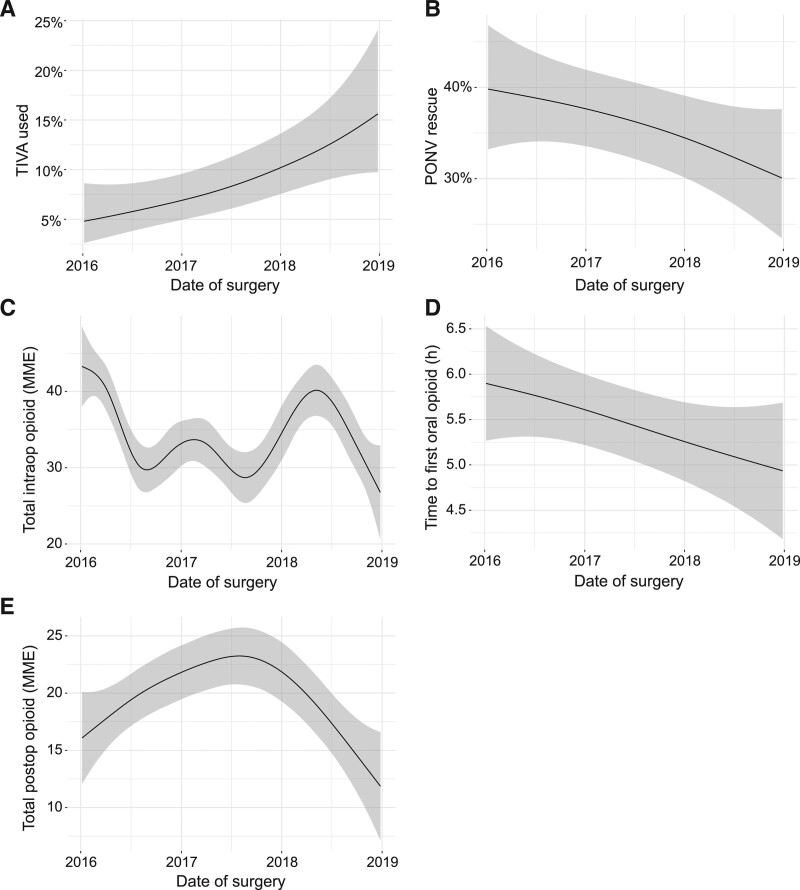
Analogous patterns were observed for thyroidectomies: TIVA use increased by 11% (95% CI, 2.3-49) (Figure 3A), and PONV rescue decreased by 9.7% (95% CI, 0.00-31) (Figure 3B). Intraoperative opioid use decreased by 16 MME (95% CI, 6.2-24), with variability over time, possibly reflecting medication shortages (eg, remifentanil shortage in 2017) and change in ERAS protocol in mid-2018 (Figure 3C). Time to convert to oral opioids decreased by 0.96 hours (95% CI, 1.4-2.1) (Figure 3D) and total postoperative opioid consumption did not change (Figure 3E).
Figure 3.
Clinical outcomes of thyroidectomies from January 1, 2016, to December 31, 2018 visualized using a generalized additive model. A, TIVA use; (B) PONV rescue; (C) total intraoperative opioid administered (MME); (D) time to first oral opioid; and (E) total postoperative opioid administered (MME). Shaded area represents 95% confidence interval. MME indicates morphine milligram equivalents; PONV, postoperative nausea and vomiting; TIVA, total intravenous anesthesia.
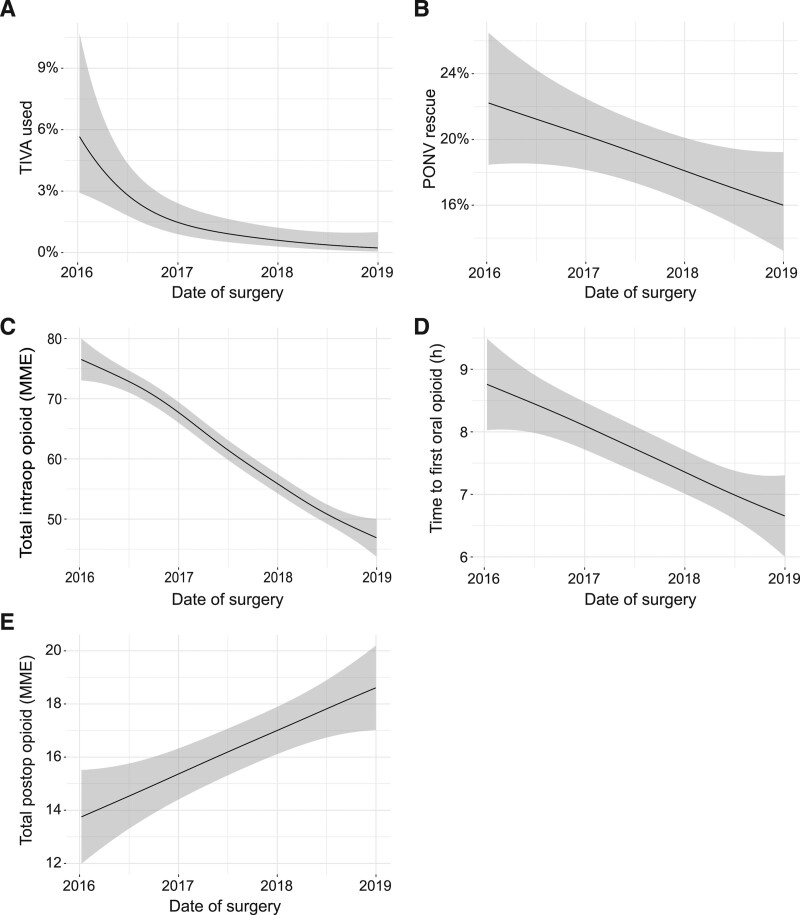
As PONV risk among patients undergoing prostatectomy is lower, TIVA use was not encouraged in this ERAS and did not increase (Figure 4A); PONV rescue rates did not meaningfully decrease (Figure 4B). Intraoperative opioid use decreased by 30 MME (95% CI, 20-34) (Figure 4C). Time to convert to oral opioids decreased by 2.1 hours (95% CI, 1.0-3.1) (Figure 4D). Total postoperative opioid consumption increased by 4.9 MME (95% CI, 3.2-7.4) (Figure 4E).
Figure 4.
Clinical outcomes of minimally invasive prostatectomies from January 1, 2016 to December 31, 2018 visualized using a generalized additive model. A, TIVA use; (B) PONV rescue; (C) total intraoperative opioid administered (MME); (D) time to first oral opioid; and (E) total postoperative opioid administered (MME). Shaded area represents 95% confidence interval. MME indicates morphine milligram equivalents; PONV, postoperative nausea and vomiting; TIVA, total intravenous anesthesia.
Across all procedures, we noted that a considerable proportion of patients (ranging from 16% to 25%) received no opioid at all in the PACU (data not shown).
Compliance with ERAS measures was generally high and improved over time (Supplemental Digital Content, Table 1, http://links.lww.com/AA/D318; Supplemental Digital Content, Figure 3, http://links.lww.com/AA/D318). Compliance for mastectomy elements was 93% or greater except for aprepitant (76%) and ketorolac (46%). For hysterectomy, compliance was 82% or greater except for aprepitant at 74%. For thyroidectomies, compliance was 85% or greater except for aprepitant (75%) and lidocaine (46%). Compliance was 93% or greater for prostatectomies except for ketorolac (83%).
Time to first ambulation was similar across surgeries (approximately 5–6 hours) (Supplemental Digital Content, Table 2, http://links.lww.com/AA/D318). PACU LOS was similar for most surgeries, averaging approximately 20 hours, except hysterectomy, which was shorter, at 17.6 hours (interquartile range [IQR] 7.8–20.8). Reoperation rates were <1%, with the exception of mastectomies (3.2%). Hospital transfer rates were <2% for all surgeries. Of the 30-day MSK UCC visits (made by 6.8% of all patients), 58% occurred >7 days after surgery.
DISCUSSION
We demonstrated the feasibility of implementing ERAS for complex ambulatory cancer surgeries and provide the evolving outcomes of 6720 surgical cases over 36 months. In addition, we report compliance, an important component of an effective ERAS. Our results establish benchmarks for specific outcomes following 4 advanced ambulatory surgery procedures using ERAS and can be used by other facilities as they expand their scope of services. Furthermore, we demonstrate continued improvement resulting from increasing protocol compliance as well as data-driven incremental changes to these programs. Importantly, accelerating recovery after surgery allows patients to receive their next chemotherapy treatment sooner, potentially further improving long-term outcomes.32
We report clinically meaningful decreases in total intraoperative opioid administered and in time to transition to oral analgesics across all 4 procedures. Patients switch from intravenous to oral pain medications when their level of pain can be adequately managed with oral medications, making this transition an important component of ambulatory surgery pain management33 and a surrogate for improved analgesia. We also note that a considerable proportion of patients (ranging from 16% to 25% among the 4 procedures) received no opioid at all in the PACU. This suggests a successful trend toward opioid minimization in ambulatory patients undergoing the prescribed pathways.
In this study, we report median time to first ambulation (approximately 5–6 hours), which has not been previously examined in the ambulatory surgical literature. The success of ambulatory surgery depends on patients’ functional recovery, and early mobilization is believed to reduce pulmonary complications and thrombotic events,30 especially in the cancer patient population. Our metric for this outcome was automated via RTLS, making it less subjective than observations; this system can also quantify the distance ambulated until discharge. Future study may examine the relationships between mobilization, time to discharge, and postoperative recovery trajectory and complications, perhaps using novel technology to follow patients after discharge.34
Rates of inpatient readmissions (2.6%) and urgent care visits (6.8%) within 30 days are comparable to the 1 prior report from Steiner et al35 on readmissions (2.7%) and emergency room visits (5.9%) after ambulatory surgery, despite the higher complexity of procedures at our center and their exclusion of cancer surgeries. Note that our rates are only for patients returning to MSK facilities and do not capture patients going to local emergency rooms or hospitals, and thus underestimate the overall rate of patients’ postdischarge acute care.
Compliance exceeded 90% for the majority of ERAS elements across all programs (Table 3) and improved over time for nearly all ERAS components for which compliance was initially <90% (Supplemental Digital Content, Figure 3, http://links.lww.com/AA/D318). This high compliance reflects deliberate efforts including preimplementation and periodic education of staff, real-time compliance dashboards (Supplemental Digital Content, Figure 3, http://links.lww.com/AA/D318), maintaining updated summary materials in all operating rooms, and, importantly, regular, open discussion of outcome data and mitigation strategies with clinical staff.11
Many factors contributed to ERAS noncompliance. First, not every patient agreed to a peripheral nerve block or to preoperative medications. Second, in the early phase of ERAS implementation, there was not always clear understanding among the staff as to which cases were covered by an ERAS. Interventions that were new to clinicians, such as preoperative administration of aprepitant and gabapentin, were not consistently ordered. In addition, ketorolac was not unanimously supported by surgeons, as some felt that its benefits did not outweigh the risk of postoperative bleeding. Finally, intermittent drug shortages affected compliance values. Taken together, these factors made it impossible to reach 100% compliance for all ERAS measures.
LIMITATIONS
All patients undergoing AXR procedures at JRSC were managed using ERAS, so we were unable to compare ERAS outcomes to those of other protocols. Before JRSC’s opening, these procedures were performed by many of the same surgeons but were conducted at the main hospital, which treats both inpatients and outpatients, without standardized clinical pathways or dedicated ambulatory surgery perioperative nursing staff; these and other confounding variables prevent meaningful comparisons. Therefore, we chose to report changes in outcomes among JRSC patients over the 3 years following the implementation of the program. We report opioid consumption rather than pain scores because the latter are inherently subjective and are affected by activity level, stress, anxiety among other factors at time of pain assessment. Similarly, we quantified objective rates of PONV using antiemetic rescue rather than nursing assessments of patient nausea and vomiting in the postoperative period because these are also subjective. We recognize that our ERAS procedures are not standard for many ambulatory surgery facilities and therefore our results are limited, yet those practices contemplating expanding their scope of services may find our processes adaptable and results encouraging.
We highlight the multifactorial, interrelated, and temporal factors potentially contributing to clinical outcomes. However, as our study did not include a concurrent control arm, we cannot determine whether similar trends would have resulted without these interventional factors. Ideally, we would have designed a randomized trial to assess the effect of different interventions on the outcomes, however, we made many small changes over time to our ERAS protocol. We recognize this as a limitation and have since started up various clinically integrated randomized trials to assess specific interventions. In contrast to a research protocol, a continuous quality improvement process may not allow determination of which of several overlapping changes are responsible for improvement in outcomes, but our experience reveals steady progress toward improving those outcomes. We do not claim that any specific interventions were responsible for improvements seen in Figures 1–4 but that outcomes improved concurrently with our continually evolving ERAS program. Future studies to ascertain causal relationships between individual ERAS interventions and the outcomes should be conducted.
CONCLUSIONS
Our study demonstrates the feasibility of implementing ERAS at an ambulatory surgery center. However, the study did not include either a concurrent or preintervention control, so that further studies are needed to assess whether there is an association between implementation of ERAS components and improvements in outcomes. Nevertheless, we demonstrate meaningful decreases in the time to transition to oral analgesic in this setting. The outcome data presented here, on a large cohort of more than 6720 complex ambulatory surgery cases with standardized ERAS care for 4 common surgical procedures, provide initial benchmarks for future ambulatory ERAS studies. In our experience, the 3 key elements for developing effective ERAS are (1) clearly defined pathway elements based on the best available information, (2) communication of compliance with these measures to providers, and (3) meaningful outcomes collected from clinical data. Future studies that directly compare individual ERAS interventions to standard of care in the same or different institutions are needed, as such studies can assess association and potentially even causation between ERAS implementation and outcomes of interest.
ACKNOWLEDGMENTS
The authors acknowledge Jessica Moore, MS, staff editor at MSK, for assistance in preparing the article.
DISCLOSURES
Name: Anoushka M. Afonso, MD.
Contribution: This author helped with the conceptual design, data acquisition and interpretation, and preparation and revision of the manuscript.
Name: Patrick J. McCormick, MD, MEng.
Contribution: This author helped with the conceptual design, data acquisition and interpretation, and preparation and revision of the manuscript.
Name: Melissa J. Assel, MS.
Contribution: This author helped with the conceptual design, data acquisition and interpretation, and preparation and revision of the manuscript.
Name: Elizabeth Rieth, MD.
Contribution: This author helped prepare and revise the manuscript.
Name: Kara Barnett, MD.
Contribution: This author helped prepare and revise the manuscript.
Name: Hanae K. Tokita, MD.
Contribution: This author helped prepare and revise the manuscript.
Name: Geema Masson, MD.
Contribution: This author helped prepare and revise the manuscript.
Name: Vincent Laudone, MD.
Contribution: This author helped prepare and revise the manuscript.
Name: Brett A. Simon, MD, PhD.
Contribution: This author helped prepare and revise the manuscript.
Name: Rebecca S. Twersky, MD, MPH.
Contribution: This author helped with conceptual design, data acquisition and interpretation, and preparation and revision of the manuscript.
This manuscript was handled by: Thomas R. Vetter, MD, MPH.
Supplementary Material
GLOSSARY
- 95% CI
- 95% confidence interval
- ASA
- American Society of Anesthesiologists
- AXR
- ambulatory extended recovery
- BMI
- body mass index
- EMR
- electronic medical record
- ERAS
- enhanced recovery after surgery
- ERP
- enhanced recovery program
- GAM
- general additive model
- IBW
- ideal body weight
- IQR
- interquartile range
- IRB
- institutional review board
- IV
- intravenous
- JRSC
- Josie Robertson Surgery Center
- LOS
- length of stay
- MME
- morphine milligram equivalents
- MSK
- Memorial Sloan Kettering Cancer Center
- NPO
- nil per os
- OR
- operating room
- PACU
- postanesthesia care unit
- PEC1
- pectoralis 1
- PONV
- postoperative nausea and vomiting
- RCTs
- randomized controlled trials
- RTLS
- real-time location system
- SQUIRE
- Standards for Quality Improvement Reporting Excellence
- TIVA
- total intravenous anesthesia
- UCC
- urgent care center
Funding: This research was supported in part by the National Cancer Institute, National Institutes of Health Cancer Center Support Grant P30 CA008748.
The authors declare no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website.
Reprints will not be available from the authors.
REFERENCES
- 1.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921–1928. [DOI] [PubMed] [Google Scholar]
- 2.Tokita H, Twersky R, Laudone V, et al. Complex cancer surgery in the outpatient setting: the Josie Robertson Surgery Center. Anesth Analg. 2020;131:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arsalani-Zadeh R, ElFadl D, Yassin N, MacFie J. Evidence-based review of enhancing postoperative recovery after breast surgery. Br J Surg. 2011;98:181–196. [DOI] [PubMed] [Google Scholar]
- 4.Chiu C, Aleshi P, Esserman LJ, et al. Improved analgesia and reduced post-operative nausea and vomiting after implementation of an enhanced recovery after surgery (ERAS) pathway for total mastectomy. BMC Anesthesiol. 2018;18:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temple-Oberle C, Shea-Budgell MA, Tan M, et al. ; ERAS Society. Consensus review of optimal perioperative care in breast reconstruction: enhanced recovery after surgery (ERAS) society recommendations. Plast Reconstr Surg. 2017;139:1056e–1071e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber WP, Barry M, Junqueira MJ, Lee SS, Mazzella AM, Sclafani LM. Initial experiences with a multidisciplinary approach to decreasing the length of hospital stay for patients undergoing unilateral mastectomy. Eur J Surg Oncol. 2011;37:944–949. [DOI] [PubMed] [Google Scholar]
- 7.Chapman JS, Roddy E, Ueda S, Brooks R, Chen LL, Chen LM. Enhanced recovery pathways for improving outcomes after minimally invasive gynecologic oncology surgery. Obstet Gynecol. 2016;128:138–144. [DOI] [PubMed] [Google Scholar]
- 8.Lee SJ, Calderon B, Gardner GJ, et al. The feasibility and safety of same-day discharge after robotic-assisted hysterectomy alone or with other procedures for benign and malignant indications. Gynecol Oncol. 2014;133:552–555. [DOI] [PubMed] [Google Scholar]
- 9.Nelson G, Altman AD, Nick A, et al. Guidelines for postoperative care in gynecologic/oncology surgery: enhanced recovery after surgery (ERAS®) Society recommendations–part II. Gynecol Oncol. 2016;140:323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: enhanced recovery after surgery (ERAS®) Society recommendations–part I. Gynecol Oncol. 2016;140:313–322. [DOI] [PubMed] [Google Scholar]
- 11.Stone R. Enhanced recovery after minimally invasive surgery (ERAmiS) for gynecology. Curr Obstet Gynecol Rep. 2018;7:39–50. [Google Scholar]
- 12.Dort JC, Farwell DG, Findlay M, et al. Optimal perioperative care in major head and neck cancer surgery with free flap reconstruction: a consensus review and recommendations from the enhanced recovery after surgery society. JAMA Otolaryngol Head Neck Surg. 2017;143:292–303. [DOI] [PubMed] [Google Scholar]
- 13.Musser JE, Assel MJ, Meeks JJ, et al. Ambulatory extended recovery: safely transitioning to overnight observation for minimally invasive prostatectomy. Urol Pract. 2015;2:121–125. [DOI] [PubMed] [Google Scholar]
- 14.Afonso AM, Tokita HK, McCormick PJ, Twersky RS. Enhanced recovery programs in outpatient surgery. Anesthesiol Clin. 2019;37:225–238. [DOI] [PubMed] [Google Scholar]
- 15.Apfel CC, Heidrich FM, Jukar-Rao S, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. 2012;109:742–753. [DOI] [PubMed] [Google Scholar]
- 16.Milnes V, Gonzalez A, Amos V. Aprepitant: a new modality for the prevention of postoperative nausea and vomiting: an evidence-based review. J Perianesth Nurs. 2015;30:406–417. [DOI] [PubMed] [Google Scholar]
- 17.de Morais LC, Sousa AM, Flora GF, Grigio TR, Guimarães GMN, Ashmawi HA. Aprepitant as a fourth antiemetic prophylactic strategy in high-risk patients: a double-blind, randomized trial. Acta Anaesthesiol Scand. 2018;62:483–492. [DOI] [PubMed] [Google Scholar]
- 18.Alayed N, Alghanaim N, Tan X, Tulandi T. Preemptive use of gabapentin in abdominal hysterectomy: a systematic review and meta-analysis. Obstet Gynecol. 2014;123:1221–1229. [DOI] [PubMed] [Google Scholar]
- 19.Rai AS, Khan JS, Dhaliwal J, et al. Preoperative pregabalin or gabapentin for acute and chronic postoperative pain among patients undergoing breast cancer surgery: a systematic review and meta-analysis of randomized controlled trials. J Plast Reconstr Aesthet Surg. 2017;70:1317–1328. [DOI] [PubMed] [Google Scholar]
- 20.Miller TE, Gan TJ. Total intravenous anesthesia and anesthetic outcomes. J Cardiothorac Vasc Anesth. 2015;29(suppl 1):S11–S15. [DOI] [PubMed] [Google Scholar]
- 21.Visser K, Hassink EA, Bonsel GJ, Moen J, Kalkman CJ. Randomized controlled trial of total intravenous anesthesia with propofol versus inhalation anesthesia with isoflurane-nitrous oxide: postoperative nausea with vomiting and economic analysis. Anesthesiology. 2001;95:616–626. [DOI] [PubMed] [Google Scholar]
- 22.Apfel CC, Korttila K, Abdalla M, et al. ; IMPACT Investigators. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar G, Stendall C, Mistry R, Gurusamy K, Walker D. A comparison of total intravenous anaesthesia using propofol with sevoflurane or desflurane in ambulatory surgery: systematic review and meta-analysis. Anaesthesia. 2014;69:1138–1150. [DOI] [PubMed] [Google Scholar]
- 24.Schraag S, Pradelli L, Alsaleh AJO, et al. Propofol vs. inhalational agents to maintain general anaesthesia in ambulatory and in-patient surgery: a systematic review and meta-analysis. BMC Anesthesiol. 2018;18:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaefer MS, Kranke P, Weibel S, Kreysing R, Kienbaum P. Total intravenous anaesthesia versus single-drug pharmacological antiemetic prophylaxis in adults: a systematic review and meta-analysis. Eur J Anaesthesiol. 2016;33:750–760. [DOI] [PubMed] [Google Scholar]
- 26.Abdallah FW, Morgan PJ, Cil T, et al. Ultrasound-guided multilevel paravertebral blocks and total intravenous anesthesia improve the quality of recovery after ambulatory breast tumor resection. Anesthesiology. 2014;120:703–713. [DOI] [PubMed] [Google Scholar]
- 27.Li B, Wang H. Dexamethasone reduces nausea and vomiting but not pain after thyroid surgery: a meta-analysis of randomized controlled trials. Med Sci Monit. 2014;20:2837–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee Y, Lin PC, Lai HY, Huang SJ, Lin YS, Cheng CR. Prevention of PONV with dexamethasone in female patients undergoing desflurane anesthesia for thyroidectomy. Acta Anaesthesiol Sin. 2001;39:151–156. [PubMed] [Google Scholar]
- 29.Jee D, Park SY. Lidocaine sprayed down the endotracheal tube attenuates the airway-circulatory reflexes by local anesthesia during emergence and extubation. Anesth Analg. 2003;96:293–297. [DOI] [PubMed] [Google Scholar]
- 30.Danic MJ, Chow M, Alexander G, Bhandari A, Menon M, Brown M. Anesthesia considerations for robotic-assisted laparoscopic prostatectomy: a review of 1,500 cases. J Robot Surg. 2007;1:119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hastie T, Tibshirani R. Generalized additive models. Statist Sci. 1986;1:297–310. [DOI] [PubMed] [Google Scholar]
- 32.Day AR, Middleton G, Smith RV, Jourdan IC, Rockall TA. Time to adjuvant chemotherapy following colorectal cancer resection is associated with an improved survival. Colorectal Dis. 2014;16:368–372. [DOI] [PubMed] [Google Scholar]
- 33.Cyriac JM, James E. Switch over from intravenous to oral therapy: a concise overview. J Pharmacol Pharmacother. 2014;5:83–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panda N, Solsky I, Huang EJ, et al. Using smartphones to capture novel recovery metrics after cancer surgery. JAMA Surg. 2019;155:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steiner CA, Maggard-Gibbons M, Raetzman SO, Barrett ML, Sacks GD, Owens PL. Return to acute care following ambulatory surgery. JAMA. 2015;314:1397–1399. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.