Abstract
Background
Fear of dental pain is a major barrier to treatment for children who need dental care. The use of preoperative analgesics has the potential to reduce postoperative discomfort and intraoperative pain. We reviewed the available evidence to determine whether further research is warranted and to inform the development of prescribing guidelines. This is an update of a Cochrane review published in 2012.
Objectives
To assess the effects of preoperative analgesics for intraoperative or postoperative pain relief (or both) in children and adolescents undergoing dental treatment without general anaesthesia or sedation.
Search methods
We searched the following electronic databases: Cochrane Oral Health's Trials Register (to 5 January 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2015, Issue 12), MEDLINE via OVID (1946 to 5 January 2016), EMBASE via OVID (1980 to 5 January 2016), LILACS via BIREME (1982 to 5 January 2016) and the ISI Web of Science (1945 to 5 January 2016). We searched ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform for ongoing trials to 5 January 2016. There were no restrictions regarding language or date of publication in the searches of the electronic databases. We handsearched several specialist journals dating from 2000 to 2011.
We checked the reference lists of all eligible trials for additional studies. We contacted specialists in the field for any unpublished data.
Selection criteria
Randomised controlled clinical trials of analgesics given before dental treatment versus placebo or no analgesics in children and adolescents up to 17 years of age. We excluded children and adolescents having dental treatment under sedation (including nitrous oxide/oxygen) or general anaesthesia.
Data collection and analysis
Two review authors assessed titles and abstracts of the articles obtained from the searches for eligibility, undertook data extraction and assessed the risk of bias in the included studies. We assessed the quality of the evidence using GRADE criteria.
Main results
We included five trials in the review, with 190 participants in total. We did not identify any new studies for inclusion from the updated search in January 2016.
Three trials were related to dental treatment, i.e. restorative and extraction treatments; two trials related to orthodontic treatment. We did not judge any of the included trials to be at low risk of bias.
Three of the included trials compared paracetamol with placebo, only two of which provided data for analysis (presence or absence of parent‐reported postoperative pain behaviour). Meta‐analysis of the two trials gave arisk ratio (RR) for postoperative pain of 0.81 (95% confidence interval (CI) 0.53 to 1.22; two trials, 100 participants; P = 0.31), which showed no evidence of a benefit in taking paracetamol preoperatively (52% reporting pain in the placebo group versus 42% in the paracetamol group). One of these trials was at unclear risk of bias, and the other was at high risk. The quality of the evidence is low. One study did not have any adverse events; the other two trials did not mention adverse events.
Four of the included trials compared ibuprofen with placebo. Three of these trials provided useable data. One trial reported no statistical difference in postoperative pain experienced by the ibuprofen group and the control group for children undergoing dental treatment. We pooled the data from the other two trials, which included participants who were having orthodontic separator replacement without a general anaesthetic, to determine the effect of preoperative ibuprofen on the severity of postoperative pain. There was a statistically significant mean difference in severity of postoperative pain of ‐13.44 (95% CI ‐23.01 to ‐3.88; two trials, 85 participants; P = 0.006) on a visual analogue scale (0 to 100), which indicated a probable benefit for preoperative ibuprofen before this orthodontic procedure. However, both trials were at high risk of bias. The quality of the evidence is low. Only one of the trials reported adverse events (one participant from the ibuprofen group and one from the placebo group reporting a lip or cheek biting injury).
Authors' conclusions
From the available evidence, we cannot determine whether or not preoperative analgesics are of benefit in paediatric dentistry for procedures under local anaesthetic. There is probably a benefit in using preoperative analgesics prior to orthodontic separator placement. The quality of the evidence is low. Further randomised clinical trials should be completed with appropriate sample sizes and well defined outcome measures.
Keywords: Adolescent; Child; Humans; Acetaminophen; Acetaminophen/therapeutic use; Analgesics, Non‐Narcotic; Analgesics, Non‐Narcotic/therapeutic use; Dental Care; Dental Care/adverse effects; Dental Care for Children; Dental Care for Children/adverse effects; Ibuprofen; Ibuprofen/therapeutic use; Orthodontics, Corrective; Orthodontics, Corrective/adverse effects; Pain; Pain/prevention & control; Preoperative Care; Preoperative Care/methods; Randomized Controlled Trials as Topic; Tooth Extraction; Tooth Extraction/adverse effects
Plain language summary
Painkillers, such as paracetamol and ibuprofen, before dental treatment in children and adolescents for reducing pain after treatment
Review question
Does giving children painkillers such as paracetamol and ibuprofen before dental treatment help reduce pain after the treatment?
Background
Dental pain is common after dental procedures and can lead to increased fear of dental treatment, avoidance of dental treatment and other associated problems. Reduction of pain is important, particularly in children and adolescents. One way of managing this might be to give painkillers before treatment so that the painkillers can start to work right away.
Review authors working with Cochrane Oral Health conducted this updated review to look at evidence for using painkillers in children, aged up to 17 years, undergoing treatment without sedation or general anaesthetic, but who may have had a local anaesthetic. The treatments included extracting teeth, restoring teeth and fitting braces.
Study characteristics
We searched several electronic databases to 5 January 2016, as well as doing some searching by hand. We included five studies in the review, which had 190 participants in total. We did not find any new studies between the previous Cochrane review in 2012 and our updated search in January 2016.
Three included studies related to dental treatment (fillings and tooth extractions) and two related to orthodontic treatment (braces). Three of the five included studies compared paracetamol to a placebo (sugar tablet) and four of them compared ibuprofen to a placebo.
Key results
From the available evidence, we could not determine whether or not painkillers before treatment are of benefit for children and adolescents having dental procedures under local anaesthetic. There is probably a benefit in giving painkillers before braces are fitted. Only one study reported an adverse event (one participant in each group had a lip or cheek biting injury). More research is needed.
Quality of the evidence
None of the included studies were at low risk of bias. The quality of the evidence is low.
Summary of findings
Summary of findings for the main comparison. Paracetamol (acetaminophen) versus placebo for additional pain relief in children and adolescents having any dental treatment under local anaesthetic.
| Paracetamol (acetaminophen) versus placebo for additional pain relief in children and adolescents having dental treatment under local anaesthetic | ||||||
| Patient or population: children and adolescents having dental treatment Settings: hospital Intervention: paracetamol versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Preoperative paracetamol | |||||
| Postoperative pain (dichotomous measure: parent report of presence of pain‐related behaviours) Follow‐up: mean 6.5 hours | 520 per 1000 | 421 per 1000 (276 to 634) | RR 0.81 (0.53 to 1.22) | 100 (2 studies1) | ⊕⊕⊝⊝ low2, 3 | See footnotes |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; GRADE: Grading of Recommendations Assessment, Development and Evaluation. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Baygin 2011 (unclear risk of bias) also compared paracetamol with placebo for primary tooth extraction and found a statistically significant reduction in pain that favoured paracetamol. 2 Two studies: one at high risk of bias and one at unclear risk of bias. We downgraded by one level for risk of bias. 3We downgraded by one level for imprecision.
Summary of findings 2. Ibuprofen versus placebo for additional pain relief in children and adolescents having orthodontic separator placement (no local anaesthetic).
| Ibuprofen versus placebo for additional pain relief in children and adolescents having orthodontic separator placement (no local anaesthetic) | |||||
| Patient or population: children and adolescents having dental treatment Settings: hospital Intervention: ibuprofen versus placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Preoperative ibuprofen | ||||
| Postoperative pain (continuous measure: self report using visual analogue scale) Follow‐up: 2 hours | The mean severity of pain in the control groups ranged from 19 to 25 mm | The mean severity of postoperative pain in the intervention groups was 13.44 mm lower (23.01 to 3.88 lower) | 85 (2 studies1) | ⊕⊕⊝⊝ low2, 3 | See footnotes |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; GRADE: Grading of Recommendations Assessment, Development and Evaluation. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1Two further studies compared ibuprofen and placebo. Baygin 2011 (unclear risk of bias) reported a statistically significant reduction in pain at two hours following primary tooth extraction, but Primosch 1995 (high risk of bias) found no difference between the groups of children following tooth extraction (data unavailable). 2Two small studies were at high risk of bias. We downgraded by one level for risk of bias. 3We downgraded by one level for imprecision.
Background
Description of the condition
Pain is a multidimensional sensory experience that is unpleasant (Pozos‐Guillen 2007), and has strong cognitive and emotional components. It may vary in intensity (mild, moderate or severe), quality (sharp, burning or dull), duration (transient, intermittent or persistent) and referral (superficial or deep, localised or diffuse) (Pozos‐Guillen 2007).
Many people associate dental care with pain. An experience of poorly managed pain related to dental treatment can cause people to avoid seeking further treatment, and make them more difficult to treat (Carr 1999). The management of pain is of particular importance in paediatric dentistry where patient perceptions of dental treatment are being established.
Description of the intervention
Pain control is routinely achieved through the use of local anaesthetic (LA) solutions injected into the soft tissues. However, Ashkenazi 2007 showed that 38% of treated children still reported postoperative dental pain, with the highest incidence being after root canal therapy, stainless steel crowns and extractions.
The use of preoperative analgesics to manage postoperative pain in adults is well established in medicine (Toms 2009). Preoperative oral analgesics are also commonly used in oral surgery for adults to supplement the analgesic effect of LA, e.g. following removal of impacted third molars (Weil 2007). Pain is usually of short duration and reaches its maximum intensity in the early postoperative period (Seymour 1985). It is during this time period that analgesics are frequently prescribed.
Use of preoperative oral analgesics for children undergoing dental treatment under general anaesthesia (GA) is also routine, but their value is unclear. Use of preoperative oral analgesics for children having dental treatment without GA is not routine; no guidelines or recommendations exist and there has been no formal review of the evidence to date.
Why it is important to do this review
Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important ones to maintain on the Cochrane Library (Worthington 2015). The paediatric dentistry expert panel identified this review as a priority title (Cochrane Oral Health priority review portfolio).
Use of preoperative analgesics in children undergoing dental treatment either with or without LA has the potential to reduce postoperative discomfort. It might also reduce intraoperative pain. Reviewing the available evidence will determine whether further research on this topic is warranted and help inform the development of prescribing guidelines if appropriate.
Objectives
To assess the effects of preoperative analgesics for intraoperative or postoperative pain relief (or both) in children and adolescents undergoing dental treatment without general anaesthesia or sedation.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials (RCTs) (including cluster‐RCTs and cross‐over trials where the order was randomised). We excluded quasi‐RCTs.
Types of participants
We included children and adolescents aged up to 17 years having dental treatment including orthodontic treatment, fillings, removal of the nerve from a tooth and extraction of a tooth.
We excluded children and adolescents having dental treatment under sedation (including nitrous oxide/oxygen) or general anaesthesia (GA).
Types of interventions
Intervention group
Analgesics given before dental treatment.
Control group
Placebo or no analgesics.
Both intervention and control groups may include local anaesthetics (LAs).
Types of outcome measures
Primary outcomes
Postoperative pain measures (either expressed as intensity of pain or presence or absence of pain)
Secondary outcomes
Intraoperative pain
Preoperative and postoperative anxiety measures
Patient satisfaction
Parental satisfaction
Cost
Completion of treatment (yes/no)
Adverse events
Search methods for identification of studies
Electronic searches
To identify studies for inclusion in this review, we developed detailed search strategies for each database searched. We based these on the search strategy we developed for MEDLINE (OVID) but revised it appropriately for each database. The search strategy used a combination of controlled vocabulary and free‐text terms and was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying RCTs in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We have provided details of the MEDLINE search in Appendix 1. We linked the EMBASE search to Cochrane Oral Health's filter for identifying RCTs, and we linked the LILACS search to the Brazilian Cochrane Centre filter. We searched the following databases.
Cochrane Oral Health's Trials Register (to 5 January 2016) (see Appendix 2)
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2015, Issue 12) (see Appendix 3)
MEDLINE via OVID (1946 to 5 January 2016) (see Appendix 1)
EMBASE via OVID (1980 to 5 January 2016) (see Appendix 4)
LILACS via BIREME Virtual Health Library (1982 to 5 January 2016) (see Appendix 5)
ISI Web of Science (1945 to 5 January 2016) (see Appendix 6)
We did not impose any restrictions on either language or date of publication in the electronic searches.
We searched the following trials registries for ongoing trials (see Appendix 7 for details of the search strategy).
ClinicalTrials.gov (to 5 January 2016)
The World Health Organization International Clincial Trials Registry Platform (WHO ICTRP) (who.int/ictrp/) (to 5 January 2016)
Searching other resources
We handsearched the following journals for the period 2000 to April 2011.
International Journal of Paediatric Dentistry
Dental Update
Pediatric Dentistry
Journal of Dentistry for Children
American Academy of Pediatric Dentistry
Journal of American Dental Association
British Dental Journal
The Angle Orthodontist
American Journal of Orthodontics and Dentofacial Orthopedics
We checked the reference lists of all eligible trials for additional studies.
We contacted specialists in the field for unpublished data.
Data collection and analysis
Selection of studies
Two review authors assessed titles and abstracts for inclusion in the review. We selected the papers suitable for inclusion in this review using our inclusion criteria. We extracted information relevant to the objectives and outcome measures into a specially designed data extraction form. We resolved any disagreements by discussion. We did not mask the journal or authors' names before paper screening or data extraction. We listed the full‐text articles that were excluded with the reason for exclusion in ‘Characteristics of excluded studies’ tables. We summarised the flow of studies using a PRISMA diagram.
Data extraction and management
We included all studies that met the inclusion criteria regardless of the study quality. We collected descriptive data where available in addition to that already outlined. We used these data to provide contextual information for the main outcomes to aid interpretation of the results.
Year study started (if unavailable, year it was published)
Country where the study was conducted
Previous treatment of participants
Assessment of risk of bias in included studies
We assessed the risk of bias in the included trials using the methodology set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed included trials on the following criteria.
Generation of random sequence
Concealed allocation of treatment
Blinding of participants/caregivers
Incomplete outcome data
Selective reporting
Other bias
We tabulated a description of the 'Risk of bias' items for each included trial, along with a judgement of either low, high or unclear risk of bias. We have provided the criteria for 'Risk of bias' judgements regarding allocation concealment below, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Low risk of bias: adequate concealment of the allocation (e.g. sequentially numbered, sealed, opaque envelopes or centralised or pharmacy‐controlled randomisation)
Uncertain risk of bias: uncertainty about whether the allocation was adequately concealed (e.g. where the trial authors did not describe the method of concealment or did not describe it in sufficient detail to allow a definite judgement)
High risk of bias: inadequate allocation concealment (e.g. open random number lists or quasi‐randomisation such as alternate days, date of birth or case record number)
We performed a summary 'Risk of bias' assessment for the primary outcome (across domains) (Higgins 2011). Within a study, we gave a summary assessment of low risk of bias when there was a low risk of bias for all key domains, unclear risk of bias when there was an unclear risk of bias for one or more key domains, and high risk of bias when there is a high risk of bias for one or more key domains.
Measures of treatment effect
For binary outcomes (e.g. successful completion of treatment), we presented the estimates of effect of the preoperative analgesia as risk ratios (RRs) with their associated 95% confidence intervals (CIs). For continuous outcomes, we used mean differences and their 95% CIs.
Unit of analysis issues
The unit of analysis was the unit of allocation, i.e. the child.
Dealing with missing data
We planned to manage missing data as per the recommendations in the Cochrane Handbook for Systematic Revews of Interventions (Higgins 2011).
Whenever possible, we planned to contact the original study investigators to request missing data.
We made explicit the assumptions of any methods the trial authors used to cope with missing data: for example, that the data are assumed missing at random, or that missing values were assumed to have a particular value such as a poor outcome.
We planned to perform sensitivity analyses to assess how sensitive results were to reasonable changes in the assumptions that were made.
We aimed to address the potential impact of missing data on the review findings in the 'Discussion' section.
Assessment of heterogeneity
We performed Cochran's test for heterogeneity and calculated the I² statistic (which describes the percentage total variation across studies that is due to heterogeneity rather than chance) for each meta‐analysis, in addition to the pooled effect estimate and its associated 95% CI.
Data synthesis
We attempted formal data synthesis in the form of meta‐analysis for trials with similar outcome measures that we judged to have sufficiently similar experimental procedures and participants. We combined RRs (for binary data) and mean differences (for continuous data) using fixed‐effect models (we would have used random‐effects models had there been more than three pooled trials). The use of a systemically delivered intervention means that there cannot be split‐mouth trials. It is likely that most or all of the trials will be of a parallel group design; however, it is theoretically possible that some data may be of a related nature if the same participants receive multiple courses of treatment under different treatment arms. In the event that trials had included paired data, we would have combined these with the data from the parallel group trials using the method of Elbourne 2002. We would have used the approaches described by Follmann 1992 to estimate the standard errors (SEs) for those studies where the trial(s) did not explicitly report the SE, but it was appropriate to attempt to derive or estimate the SE.
Subgroup analysis and investigation of heterogeneity
We proposed to conduct the following subgroup analyses if data were available.
Age
Use of local anaesthesia
Dental procedure
Type of analgesic
Sensitivity analysis
We planned to use sensitivity analyses and meta‐analysis regression to explore, quantify and control for sources of heterogeneity between included studies where possible. Such sources of heterogeneity might have included, but were not limited to, participant characteristics and the nature of the interventions.
Summarising findings and assessing the quality of the evidence
We presented data using 'Summary of findings' tables, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where available, we used the following outcomes.
Differences in intraoperative and postoperative pain measures between test and control groups
Differences in preoperative and postoperative anxiety measures between test and control groups
We used illustrative means.
We presented key results for each comparison and outcome in 'Summary of findings' tables. We presented the quality of the evidence for each comparison and outcome, which we assessed using Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria, as either high, moderate, low or very low quality.
Results
Description of studies
Results of the search
In the original review, we identified 1344 records at the first stage and one further study after we checked the references from potentially included studies. In our January 2016 update, we identified another 347 records after de‐duplication. From the total of 1691 records, we rejected 1613 after we screened the titles and a further 61 after we checked the abstracts. We excluded 12 studies after we extracted information relevant to the objectives from the full‐text articles into a specially designed 'data extraction form'. We therefore included five studies in the review. There were no significant disagreements between review authors during the process. For the flow of studies, see Figure 1.
1.
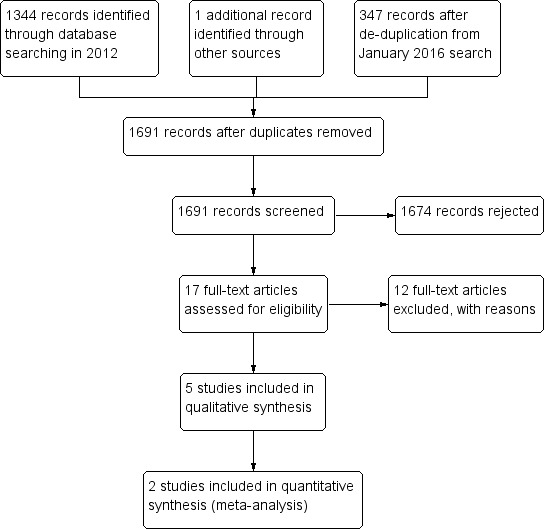
Study flow diagram
Included studies
Details of the five included studies are in the 'Characteristics of included studies' table.
Design
All five included studies were parallel group randomised controlled trials (RCTs). Three of the five included studies looked at preoperative analgesics and their effect on postoperative pain following restorative and extraction treatments under local anaesthesia (LA) (Baygin 2011; Primosch 1993; Primosch 1995). The remaining two studies looked at preoperative analgesics before orthodontic separator placement without LA (Bernhardt 2001; Law 2000).
Sample sizes
The number of children analysed in the five studies ranged from 41 to 63 (190 in total). Only one study, Baygin 2011, reported sample size calculations. None of the included trials used an intention‐to‐treat analysis.
Setting
Four studies were carried out in the USA and one in Turkey (Baygin 2011).
Participants
Studies included children aged from two to 16 years of age, and each study had a different age range of participants.
Interventions
The five included studies compared the following preoperative interventions with a placebo (including lactose tablet).
Paracetamol versus placebo (Baygin 2011; Primosch 1993; Primosch 1995)
Ibuprofen versus placebo (Baygin 2011; Bernhardt 2001; Law 2000; Primosch 1995)
Outcomes
None of the included trials assessed anxiety or behaviour at baseline. All studies used some measure of postoperative pain as the main outcome. Outcome variables were either ordinal (e.g. severity of pain) or categorical in nature (e.g. presence or absence of pain). Methods used for statistical analysis included both non‐parametric and parametric tests.
In two studies, parents recorded the prevalence of their child's postoperative pain‐related behaviour and analgesic use at six hours (Primosch 1993), and at seven hours (Primosch 1995). In one further study, Baygin 2011, parents recorded pain and the need for postoperative analgesics at five‐, six‐ and 24‐hour intervals.
Three studies on older children used self‐reporting scales, as described below.
Pain scale (0 to 4) for four hours after the procedure (Baygin 2011)
Pain incidence and severity (on chewing, biting, fitting back teeth together, fitting front teeth together) recorded on a 10 cm visual analogue scale at the following time intervals postoperatively: 2 hours, 6 hours, bedtime, the day after, 2, 3 and 7 days (Bernhardt 2001).
Pain incidence and severity (on chewing, biting, when fitting back teeth together, when fitting front teeth together) recorded on a visual analogue scale at the following time intervals postoperatively: 2 hours, 6 hours, 24 hours, 2, 3 and 7 days (Law 2000).
Only two studies reported pain severity (Bernhardt 2001; Law 2000).
Excluded studies
We have provided the reasons for the exclusion of 12 studies in the 'Characteristics of excluded studies' table. Nine of the 12 studies involved participants in nine of the 12 included studies were over 17 years of age, one dealt with postoperative analgesia, one was not a RCT and one study did not have a placebo arm.
Risk of bias in included studies
Allocation
Sequence generation
In one study, Baygin 2011, the participants selected a number and an independent person had previously numbered and anonymised the drug containers. We rated this study as at low risk of bias. The remaining included studies did not report the method of sequence generation and we assessed them as at unclear risk of bias.
Concealment of allocation
In Baygin 2011, it appeared that the trial authors took steps to conceal the allocation sequence. However, it was unclear how the trial authors maintained allocation concealment and blinding whilst ensuring participants took the correct dose at the correct time. We contacted the study authors but received no response. The remaining studies did not describe allocation concealment. Thus we assessed all included studies as being at unclear risk of bias.
Blinding
Two studies blinded both the participant and operator to the therapy (Law 2000; Primosch 1995); Baygin 2011 blinded the participants and investigators; Bernhardt 2001 blinded participants and assessors; and Primosch 1993 blinded only the participants to the interventions.
It was unclear how Baygin 2011 and Primosch 1993 performed blinding (see previous comments for Baygin 2011). It was unclear whether Bernhardt 2001 blinded the operators. Law 2000 and Primosch 1995 described the method of blinding well.
We assessed one study as at unclear risk of bias (Primosch 1993), and the remaining studies at low risk of bias.
Incomplete outcome data
In Bernhardt 2001, the trial authors excluded 22 participants from the trial analysis, although they completed the outcome questionnaire, because they took rescue medication. The trial authors reported that these participants were evenly distributed between the groups. It is difficult to estimate the effect inclusion of these participants might have had on the results. Forty‐eight participants from Law 2000 were lost between when they were consented and when the trial authors recorded data. It is unclear which groups they were lost from and therefore what impact this had on the results. Thus we assessed the two studies as being at high risk of bias (Bernhardt 2001; Law 2000). The remaining three studies included all participants in their analyses and we assessed them as at low risk of bias.
Selective reporting
One study stated that the parents of participants recorded the presence or absence of postoperative pain‐related behaviours, but the trial authors did not report these data in the paper. Therefore we assessed it as being at high risk of bias (Primosch 1995). All other included studies reported the important outcomes and we judged them as at low risk of bias.
Other potential sources of bias
Gender was unevenly distributed between groups in Bernhardt 2001; there were 10 males and three females in group A, and four males and 10 females in group B. Therefore we assessed Bernhardt 2001 as at high risk of bias, and the remaining included studies as at low risk of bias.
Overall risk of bias
Overall, we assessed three studies as at high risk of bias (Bernhardt 2001; Law 2000; Primosch 1995). We judged the remaining two studies as at unclear risk of bias (Baygin 2011; Primosch 1993). See Figure 2 and Figure 3.
2.
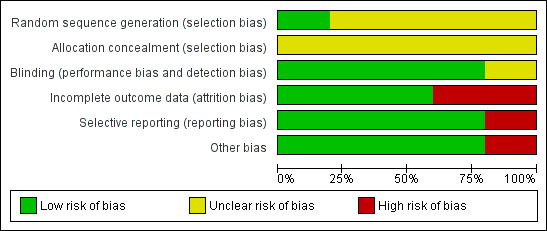
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
3.
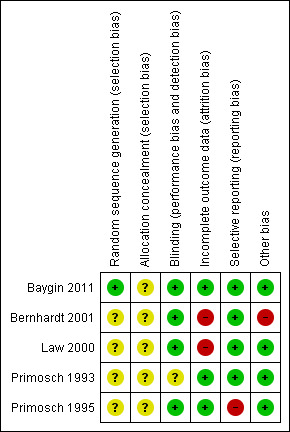
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Effects of interventions
Comparison 1.1: Paracetamol versus placebo
Postoperative pain
Three studies compared paracetamol with placebo (Baygin 2011; Primosch 1993; Primosch 1995). We were unable to use the data from Baygin 2011 as the trial presented data graphically with no standard deviations (SDs); however, the other studies provided dichotomous data on postoperative pain‐related behaviours. We assessed Baygin 2011 and Primosch 1993 as at unclear risk of bias and Primosch 1995 at high risk of bias.
In Baygin 2011, participants who had preoperative analgesics reportedly showed significantly lower pain scores (P < 0.05) than those who had the placebo at all time points (15 minutes, 1, 2, 3, 4, 5, 6 and 24 hours). Both Primosch 1993 and Primosch 1995 only presented the presence or absence of postoperative pain‐related behaviours, which the parents of participants recorded (dichotomous outcome) at six and seven hours respectively. These studies both included children having primary teeth extraction under LA, and Primosch 1993 also included children having restorations. The meta‐analysis of the two studies showed a non‐significant risk ratio (RR) of 0.81 (95% confidence interval (CI) 0.53 to 1.22), which showed no evidence of a benefit in taking paracetamol preoperatively (Analysis 1.1).
1.1. Analysis.
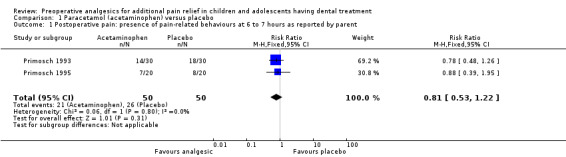
Comparison 1 Paracetamol (acetaminophen) versus placebo, Outcome 1 Postoperative pain: presence of pain‐related behaviours at 6 to 7 hours as reported by parent.
Adverse events
No adverse events were recorded in Primosch 1995. The other two studies did not mention adverse events.
Other secondary outcomes
The three studies did not measure intraoperative pain, preoperative and postoperative anxiety measures, patient satisfaction, parental satisfaction, cost or completion of treatment.
Comparison 2.1: Ibuprofen versus placebo
Postoperative pain
Four studies compared ibuprofen with placebo (Baygin 2011; Bernhardt 2001; Law 2000; Primosch 1995), and one of these studies gave ibuprofen postoperatively to both groups (Bernhardt 2001). Three of these studies provided useable data; Primosch 1995 provided dichotomous data, and Bernhardt 2001 and Law 2000 reported continuous data.
In Primosch 1995, there was no statistical difference in postoperative pain experienced by the ibuprofen group and the control group. The parents of the participants recorded the presence or absence of postoperative pain‐related behaviours but the trial authors did not report these data in the paper. We assessed this study as at high risk of bias.
Bernhardt 2001 reported that participants with preoperative ibuprofen felt significantly less pain two hours after treatment (P < 0.05), whereas Law 2000 reported that participants who had taken preoperative ibuprofen reported significantly less "pain to chewing" (P < 0.05) at two hours. Both studies included participants who were having orthodontic separator replacement without a general anaesthetic. We tried to obtain the raw data from Law 2000 and Bernhardt 2001 as the graph in the paper was difficult to read. Bernhardt 2001 provided data, which allowed us to meta‐analyse the two‐hour 'pain to chewing' data from that study with the two‐hour 'pain to chewing' data from Law 2000 to determine the effect of preoperative ibuprofen on the severity of postoperative pain. For Bernhardt 2001, we combined the intervention groups A and B. We found a statistically significant benefit for giving ibuprofen preoperatively with mean difference ‐13.44 (95% CI ‐23.01, to ‐3.88; 85 participants; P = 0.006) on a visual analogue scale (0 to 100), which indicated a benefit for preoperative ibuprofen before this orthodontic procedure (Analysis 2.1). We assessed both studies as at high risk of bias.
2.1. Analysis.
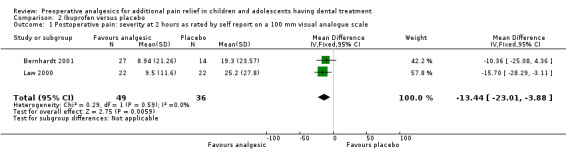
Comparison 2 Ibuprofen versus placebo, Outcome 1 Postoperative pain: severity at 2 hours as rated by self report on a 100 mm visual analogue scale.
Baygin 2011 reported that participants having preoperative analgesics showed significantly lower pain scores (P < 0.05) compared to the placebo at all time points (15 minutes, 1, 2, 3, 4, 5, 6 and 24 hours). We were unable to include data from this study report as the trial authors reported only the median graphically with no accompanying SDs. We assessed this study as at unclear risk of bias.
Adverse events
One participant from the ibuprofen and one from the placebo group reported a lip or cheek biting injury (Bernhardt 2001). No other adverse events were recorded for Bernhardt 2001 and Law 2000.
Other secondary outcomes
The four studies did not measure intraoperative pain, preoperative and postoperative anxiety measures, patient satisfaction, parental satisfaction, cost or completion of treatment.
Discussion
Summary of main results
This Cochrane review found that there were may be some benefits in using preoperative analgesics prior to orthodontic separator placement. It was difficult to reach firm conclusions as to the benefit of using preoperative analgesics before restorations or extractions under local anaesthetic (LA). In general we had difficulty in interpreting studies and comparing them due to differing outcome measures, interventions and treatment types. We were unable to reach any conclusions regarding the most effective analgesic.
Overall completeness and applicability of evidence
Sample
In all studies, the age range was applicable to paediatric dentistry. However, it could be argued that studies represented three different age groups. The children in the Bernhardt 2001 and Law 2000 studies were predominantly aged 12 years and over, those in Baygin 2011 ranged from 6 to 12 years, and those in the Primosch studies from 2 to 10 yearsPrimosch 1993; Primosch 1995). This would have influenced the recording and perception of pain, with the older age group intellectually much better equipped to self‐report on their sensations post‐treatment. In general it is beneficial to confine studies to limited age ranges or to include sufficient numbers of children from various age groups to permit analysis within age categories.
Pain assessment
Pain assessment remains difficult in young children because of their limited ability to understand assessment instructions and to articulate descriptions of their pain. The included studies used two approaches: parental report of presence or absence of pain and self‐reported severity or intensity of pain. Use of self‐reported intensity measures (e.g. visual analogue scale) does allow more information to be recorded and is reliable (Seymour 1985); however, it depends on sufficient intellectual development from the child to understand the question asked and use the scale appropriately (Shields 2003). Three included studies followed this approach (Baygin 2011; Bernhardt 2001; Law 2000). Bernhardt 2001 and Law 2000 used adolescent age groups, who might be expected to manage well with a self‐reported pain scale. Participants in Baygin 2011 ranged in age from 6 to 12 years, and it is possible that the younger children might have struggled with the concept of the pain scale. Both Primosch studies recorded parental‐reported presence or absence of pain (Primosch 1993; Primosch 1995), primarily because the trial included a very young age group (less than six years) (Gauvain‐Piquard 1987; Norden 1991; Swafford 1968). It is likely that this measure was less sensitive than self‐reported measures would have been in an older age group. It is also important to note that very young children might be confused between the feeling of numbness resulting from LA administration and a feeling of pain.
The measurement of pain will be influenced by the baseline anxiety of the child (Versloot 2008), yet none of the included studies recorded this. Ideally this should always be recorded to either allow sampling of a high or low anxiety group, or to allow comparison of the effects of preoperative analgesics on postoperative pain in high‐ and low‐anxiety participants.
Dental treatment
The major difference in this Cochrane review was between the orthodontic studies with no LA and the restorative/extraction studies with LA. Arguably these types of studies should be examined separately.
Analgesics used
The included studies compared paracetamol and ibuprofen with each other or a placebo. These are commonly used over‐the‐counter medications for children and are appropriate for this use. Dosages were as recommended for the relevant age groups, but the time of administration varied. Primosch 1993 and Primosch 1995 gave the analgesics 15 to 20 minutes before the procedure, whilst Baygin 2011 gave ibuprofen 30 minutes before and paracetamol 60 minutes before. Bernhardt 2001 and Law 2000 gave ibuprofen one hour before. This could be a factor when considering efficacy, i.e. the earlier the analgesic is given, the less likely it is to be effective postoperatively.
Quality of the evidence
In common with many other Cochrane reviews, the quality of studies was found to be disappointing with poor reporting often the main problem. We rated the quality of the body of evidence regarding postoperative pain as low.
As we have mentioned above, randomisation and allocation were unclear and this has the potential to introduce bias into the study. Blinding was also unclear in one study (Primosch 1993). Ideally the operator, assessor and participant are blind to the intervention. Only one study reported adverse events (Bernhardt 2001). The other studies stated there were none or it is assumed there were none; however, it was not explicitly stated which, if any, adverse outcomes were measured. Moreover, there was no reporting of participant or parent satisfaction in any included study.
Only Baygin 2011 mentioned sample size calculations. Obviously without a sample size calculation it is difficult to comment on the size of these studies. However, there is a risk that they were underpowered.
In Bernhardt 2001, there was a very uneven distribution of male and female participants between groups. Although many studies have found that there are no differences in discomfort between genders after dental treatment (Jones 1984; Jones 1992; Kvam 1989; Ngan 1989), some studies have noted differences between genders (Denning 2000; Scheurer 1996). This is a potential source of bias.
Potential biases in the review process
We excluded several studies because the age range included adults. We did consider whether or not to contact the study authors for data relating to the children. However, in all cases, the children in these studies were only just within the age range (e.g. 15 years old). The intent of this Cochrane review was to investigate the effect of preoperative analgesics on children. We decided that inclusion of these data from adolescents in studies primarily designed to record outcomes in adults was not appropriate.
For the ibuprofen versus placebo comparison, we combined data from Bernhardt 2001 and Law 2000 even though they evaluated a slightly different comparison. In Law 2000, participants were given a placebo immediately after the procedure whereas in Bernhardt 2001 they were not. So the Bernhardt 2001 comparison in this review was preoperative ibuprofen with no treatment postoperatively versus preoperative placebo with no treatment postoperatively. The Law 2000 comparison for this review was preoperative ibuprofen with placebo postoperatively versus preoperative placebo with placebo postoperatively.
Agreements and disagreements with other studies or reviews
There are no other published reviews on this topic.
Authors' conclusions
Implications for practice.
From the available evidence, we cannot determine whether or not preoperative analgesics are of benefit in paediatric dentistry for procedures under local anaesthetic. There does seem to be some benefit in use of preoperative analgesics prior to orthodontic separator placement. The quality of the evidence is low.
Implications for research.
Based on the literature review and the results of this review, we suggest the following research.
Further randomised controlled clinical trials (RCTs) are needed to assess the efficacy of preoperative analgesia in children and adolescents having routine dental treatments. Follow‐up of participants may be necessary to determine if the effect of preoperative analgesia has reduced postoperative pain and anxiety, and thus modified the child's perception towards having dental treatments.
Trialists should report sample size calculations.
Trialists should record baseline anxiety.
Trialists should select well‐defined age ranges with appropriate outcome measures.
What's new
| Date | Event | Description |
|---|---|---|
| 21 June 2016 | New citation required and conclusions have changed | The updated search did not yield any new studies for inclusion; we found two new studies for exclusion. One meta‐analysis used new data unavailable to us at the time of the previous version of the review. We reassessed the quality of the evidence as 'low' rather than 'moderate'. |
| 5 January 2016 | New search has been performed | We updated the literature search. |
Acknowledgements
We thank the following peer reviewers for their comments on the previous version of this Cochrane review (Ashley 2012): Barbara Chadwick, Peter Day, Lasse A Skoglund and Jaap SJ Veerkamp. We also acknowledge Amal Behbehani for her contribution to the previous version of this review (Ashley 2012). We thank Anne Littlewood, Anne‐Marie Glenny and Helen Worthington of Cochrane Oral Health for their input to this version of the review, and Deirdre Walsh for copy editing. We thank Melissa Bernhardt for clarifying results from her study.
Appendices
Appendix 1. MEDLINE search strategy
exp DENTISTRY/
(dental$ or dentist$ or "oral surg$" or orthodont$ or pulpotom$ or pulpect$ or endodont$ or "pulp cap$").mp.
((dental or tooth or teeth) and (fill$ or restor$ or extract$ or remov$ or "cavity prep$" or caries or carious or decay$)).mp.
("root canal therapy" or "tooth replant$").mp.
or/1‐4
exp ANALGESICS/
analgesi$.mp.
exp ANTI‐INFLAMMATORY AGENTS, NON‐STEROIDAL/
("nonsteroidal anti‐inflammatory agent$" or "anti inflammatory agent$" or "nonsteroidal antiinflammatory agent$" or "non steroidal antiinflammatory agent$" or "Nonsteroidal analgesic$" or "anti‐inflammator$" or "aspirin‐like agent$" or NSAID$).mp.
IBUPROFEN/
(ibuprofen or brufen).mp.
exp Acetaminophen/ (acetaminophen or paracetamol).mp. or/6‐13 exp CHILD/ INFANT/ ADOLESCENT/ (child$ or infant$ or adolescen$).ab,sh,ti. (pediatric$ or paediatric$).ab,sh,ti. Dental Care for Children/ or/15‐20 5 and 14 and 21
We linked the above subject search to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized controlled clinical trials (RCTs) in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. Cochrane Oral Health Trials Register search strategy
From 5 January 2016, we searched the Cochrane Oral Health Trials Register using the Cochrane Register of Studies and the search strategy below.
1 ((analgesi* or "nonsteroidal anti‐inflammatory agent*" or "anti inflammatory agent*" or "nonsteroidal antiinflammatory agent*" or "non steroidal antiinflammatory agent*" or "nonsteroidal analgesic*" or anti‐inflammator* or "aspirin‐like agent*" or NSAID* or ibuprofen or brufen or acetaminophen or paracetamol):ti,ab) AND (INREGISTER) 2 ((child* or infant* or adolescent* or pediatric* or paediatric*):ti,ab) AND (INREGISTER) 3 (#1 and #2) AND (INREGISTER)
We performed previous searches of the Cochrane Oral Health Group Trials Register using the Procite software and the search strategy below.
((analgesi* or "nonsteroidal anti‐inflammatory agent*" or "anti inflammatory agent*" or "nonsteroidal antiinflammatory agent*" or "non steroidal antiinflammatory agent*" or "nonsteroidal analgesic*" or anti‐inflammator* or "aspirin‐like agent*" or NSAID* or ibuprofen or brufen or acetaminophen or paracetamol) AND (child* or infant* or adolescent* or pediatric* or paediatric*))
Appendix 3. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Dentistry explode all trees #2 (dental* in All Text or dentist* in All Text or "oral surg*" in All Text or orthodont* in All Text or pulpotom* in All Text or pulpect* in All Text or endodont* in All Text or "pulp cap*" in All Text) #3 ((dental in All Text or tooth in All Text or teeth in All Text) and (fill* in All Text or restor* in All Text or extract* in All Text or remov* in All Text or "cavity prep*" in All Text or caries in All Text or carious in All Text or decay* in All Text)) #4 ("root canal therapy" in All Text or "tooth replant*" in All Text) #5 (#1 or #2 or #3 or #4) #6 MeSH descriptor Analgesics explode all trees #7 analgesi* in All Text #8 MeSH descriptor Anti‐inflammatory agents, non‐steroidal explode all trees #9 ("nonsteroidal anti‐inflammatory agent*" in All Text or "anti inflammatory agent*" in All Text or "nonsteroidal antiinflammatory agent*" in All Text or "non steroidal antiinflammatory agent*" in All Text or "nonsteroidal analgesic*" in All Text or "anti‐inflammator$" in All Text or "aspirin‐like agent*" in All Text or NSAID* in All Text) #10 MeSH descriptor Ibuprofen this term only #11 (ibuprofen in All Text or brufen in All Text) #12 MeSH descriptor Acetaminophen explode all trees #13 (acetaminophen in All Text or paracetamol in All Text) #14 (#6 or #7 or #8 or #9 or #10 or #11 or #12 or #13) #15 (child* in All Text or infant* in All Text or adolescent* in All Text) #16 (pediatric in All Text or paediatric in All Text) #17 MeSH descriptor Dental care for children this term only #18 (#15 or #16 or #17) #19 (#5 and #14 and #18)
Appendix 4. EMBASE via OVID search strategy
1. exp DENTISTRY/ 2. (dental$ or dentist$ or "oral surg$" or orthodont$ or pulpotom$ or pulpect$ or endodont$ or "pulp cap$").mp. 3. ((dental or tooth or teeth) and (fill$ or restor$ or extract$ or remov$ or "cavity prep$" or caries or carious or decay$)).mp. 4. ("root canal therapy" or "tooth replant$").mp. 5. or/1‐4 6. exp ANALGESIC AGENT/ 7. analgesi$.mp. 8. exp ANTI‐INFLAMMATORY AGENTS, NON‐STEROIDAL/ 9. ("nonsteroidal anti‐inflammatory agent$" or "anti inflammatory agent$" or "nonsteroidal antiinflammatory agent$" or "non steroidal antiinflammatory agent$" or "Nonsteroidal analgesic$" or "anti‐inflammator$" or "aspirin‐like agent$" or NSAID$).mp. 10. IBUPROFEN/ 11. (ibuprofen or brufen).mp. 12. PARACETAMOL/ 13. (paracetamol or acetaminophen).mp. 14. or/6‐13 15. exp CHILD/ 16. INFANT/ 17. ADOLESCENT/ 18. (child$ or infant$ or adolescen$).ab,sh,ti. 19. (pediatric$ or paediatric$).ab,sh,ti. 20. Dental Care for Children/ 21. or/15‐20 22. 21 and 14 and 5 We linked the above subject search to the Cochrane Oral Health Group filter for identifying RCTs in EMBASE via OVID.
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Appendix 5. LILACS via BIREME Virtual Health Library search strategy
(Mh Dentistry or Mh Odonologia or (dental$ or dentaria or dentist$ or "oral surg$" or orthodont$ or ortodonc$ or ortodont$ or pulpotom$ or pulpect$ or endodont$ or "root canal" or caries or carious)) [Words] and ((Mh Analgesics or Mh Analgesicos or analgesi$ or Mh Anti inflammatory agents or Mh Agentes Antiinflamatorios or Mh Antiinflamatorios or NSAID$ or anti‐inflammator$ or "anti inflammator$" or antiinflammator$ or Mh Ibuprofen or Mh Ibuprofeno or ibuprofen or brufen or Mh Paracetamol) and (child$ or nino$ or crianca$ or infant$ or lactante$ or lactente$ or adolescent$)) [Words]
We linked the above subject search to the Brazilian Cochrane Centre filter for LILACs via BIREME.
Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal)))and not (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL)))
Appendix 6. ISI Web of Knowledge search strategy
# 1 TS=(dental* or dentist* or "oral surg*" or orthodont* or pulpotom* or pulpect* or endodont* or "pulp cap*") # 2 TS=(dental or tooth or teeth) # 3 TS=(fill* or restor* or extract* or remov* or "cavity prep*" or caries or carious or decay*) # 4 #2 and #3 # 5 TS=("root canal" or "tooth replant*") # 6 #1 or #4 or #5 # 7 TS=(analgesi* or anti‐inflammator* or "anti inflammator*" or antiiinflammator* or NSAID* or "aspirin‐like agent*" or ibuprofen or brufen or paracetamol or acetaminophen) # 8 #6 and #7 # 9 TS=(child* or infant* or adolescent* or pediatric or paediatric) # 10 #8 and #9
Appendix 7. ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) search strategy
Advanced search:
Keywords: child and dental
Interventions: preoperative analgesic
Keywords: child and dental
Interventions: preoperative anti‐inflammatory
Data and analyses
Comparison 1. Paracetamol (acetaminophen) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain: presence of pain‐related behaviours at 6 to 7 hours as reported by parent | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.22] |
Comparison 2. Ibuprofen versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative pain: severity at 2 hours as rated by self report on a 100 mm visual analogue scale | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐13.44 [‐23.01, ‐3.88] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baygin 2011.
| Methods | RCT Parallel design |
|
| Participants | Location: Turkey Children needing primary molar tooth extraction, N = 45, age range 6 to 12 years Setting: paediatric dental clinic Group 1: 10 male, 5 female; mean age (years) 8.53 (standard deviation (SD) 1.60) Group 2: 8 male, 7 female; mean age (years) 9.33 (SD 1.4) Group 3: 7 male, 8 female; mean age (years) 9.33 (SD 2.2) |
|
| Interventions | Group 1: 100 mg/5 mL ibuprofen 30 minutes preoperatively by age (N = 15) Group 2: 250 mg/4 mL paracetamol 1 hour preoperatively by age (N = 5) Group 3: placebo 1 hour preoperatively (N = 15) All oral |
|
| Outcomes | 5 face scale (15 minutes, 1, 2, 3, 4, 5, 6 and 24 hours) | |
| Notes | It was difficult to determine the actual dosage of painkillers given. The trial authors stated in the discussion that ibuprofen varied from 4 to 10 mg/kg body weight and paracetamol from 7 to 15 mg/kg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants selected a number; an independent person had previously numbered and anonymised containers |
| Allocation concealment (selection bias) | Unclear risk | See above. The independent person then allocated drugs in lightproof anonymised containers that contained the premeasured volume. It is unclear how the participants were instructed regarding the time at which they took the solution and also the amount of the solution if they were blind to the drug they were taking. We sent an email to the trial authors for clarification |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blind" Participant and operator were blind to the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial authors evaluated all included participants |
| Selective reporting (reporting bias) | Low risk | The trial authors reported all expected outcomes |
| Other bias | Low risk | None apparent |
Bernhardt 2001.
| Methods | RCT Parallel design |
|
| Participants | Location: USA Orthodontic participants undergoing separator placement, N = 41 Setting: Department of Orthodontics Group A: 10 male, 3 female; mean age (years) 12.1 (SD 1.6) Group B: 4 male, 10 female; mean age (years) 13.5 (SD 1.9) Group C: 4 male, 8 female; mean age 12.8 (SD 1.5) |
|
| Interventions | Group A: 400 mg ibuprofen 1 hour preoperatively + 400 mg ibuprofen 6 hours after the initial dose (N = 13) Group B: 400 mg ibuprofen 1 hour preoperatively + placebo 6 hours after the initial dose (N = 14) Group C: placebo 1 hour preoperatively + 400 mg ibuprofen 6 hours after the initial dose (N = 14) All oral |
|
| Outcomes | Incidence and severity of pain at different time intervals postoperatively, using a visual analogue scale | |
| Notes | Authors concluded that further studies with additional postoperative doses needed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment method not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and assessors were blind to the therapy, but it was unclear whether the operator was blind to therapy |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Trial authors excluded 22/63 participants from the final analysis as these participants took rescue medication. Trial authors reported that these participants were evenly distributed between the groups |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | High risk | Gender unevenly distributed between groups |
Law 2000.
| Methods | RCT Parallel design |
|
| Participants | Location: USA Orthodontic patients undergoing separator placement, N = 63 (including Group B) Setting: Department of Orthodontics Group A: 10 male, 12 female; mean age (years) 13.4 (SD 1.7) Group C: 9 male, 13 female; mean age (years) 13.1 (SD 1.8) |
|
| Interventions | Group A: 400 mg ibuprofen 1 hour preoperatively (and placebo immediately after the appointment) (N = 22) Group C: placebo 1 hour preoperatively (and placebo immediately after the appointment) (N = 22) All oral |
|
| Outcomes | Incidence and severity of pain at different time intervals postoperatively, using a visual analogue scale | |
| Notes | We did not report Group B as this group only received postoperative painkillers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigator, clinician and participants were blinded to the treatment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 111 participants consented, but 28 of these did not receive separators at next appointment, and 17 participants forgot to take pretreatment dose before appointment. Trial authors did not include 3 more participants for unspecified reasons. It was unclear from the paper which groups participants were lost from |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | None apparent |
Primosch 1993.
| Methods | RCT Parallel design |
|
| Participants | Location: USA Children undergoing variety of dental procedures, N = 60 Setting: paediatric dental clinic Mean age (years) 7.3 (range 4.6 to 10.5) |
|
| Interventions | Group 1: 80 mg paracetamol 20 minutes preoperatively (N = 30) Group 2: placebo 20 minutes preoperatively (N = 30) All oral |
|
| Outcomes | Prevalence of pain related behaviours postoperatively | |
| Notes | Groups further subdivided into restoration or extraction (N = 15 per subgroup) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Trial authors referred to allocation concealment but did not explain the method |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants were blinded to the therapy, but it is unclear whether assessors and operators were blinded as well |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from included participants were complete |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | None apparent |
Primosch 1995.
| Methods | RCT Parallel design |
|
| Participants | Location: USA Children requiring primary teeth extractions, N = 60 Setting: paediatric dental clinic Group 1: 13 male, 7 female; mean age (months) 80.3 (standard error (SE) 5.1); mean weight (kg) 24.5 (SE 2.3) Group 2: 12 male, 8 female; mean age (months) 78.9 (SE 4.8); mean weight (kg) 24.4 (SE 2.5) Group 3: 13 male, 7 female; mean age (months) 79.7 (SE 4.6); mean weight (kg) 23.6 (SE 1.9) |
|
| Interventions | Group 1: ibuprofen 15 minutes preoperatively; dose by age (N = 20) Group 2: paracetamol 15 minutes preoperatively; dose by age (N = 20) Group 3: placebo (N = 20) All oral |
|
| Outcomes | Prevalence of pain‐related behaviours postoperatively | |
| Notes | Trial authors concluded that clinical studies must continue to evaluate analgesic efficacy in children | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Containers of each solution...randomized" |
| Allocation concealment (selection bias) | Unclear risk | Trial authors did not describe the method of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and operators were blinded. Trial authors described this in sufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from included participants were complete |
| Selective reporting (reporting bias) | High risk | Parents of the participants recorded the presence or absence of postoperative pain‐related behaviours but the trial authors did not report these data in the paper |
| Other bias | Low risk | None apparent |
Abbreviations: SD: standard deviation; SE: standard error; N: number of participants.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arantes 2009 | Included participants over 17 years of age |
| Ashkenazi 2007 | Study ineligible (not designed as a randomised controlled trial) |
| Bird 2007 | Included participants over 17 years of age |
| Bradley 2007 | No placebo |
| Guan 2013 | Adult participants |
| Gupta 2014 | Participant age ranged from 15 to 22 years; we were unable to extract child data |
| McGaw 1987 | Postoperative analgesics only |
| Minor 2009 | Included participants over 17 years of age |
| Ngan 1994 | Included participants over 17 years of age |
| Patel 2011 | Participants were over 17 years of age |
| Polat 2005a | Included participants over 17 years of age |
| Polat 2005b | Included participants over 17 years of age |
Differences between protocol and review
In the 'Data synthesis' section of the protocol we stated: "Meta‐analyses will be restricted to studies at low (or lower) risk of bias" (Ashley 2010). We have removed this from the 'Data synthesis' section of the review and we considered all studies for potential inclusion in the meta‐analysis regardless of risk of bias.
We added adverse events as an outcome.
Contributions of authors
Paul F Ashley conceived the idea and appraised the risk of bias in the papers. Paul F Ashley, Susan Parekh, Amal Behbehani and Prabhleen Anand wrote the protocol (Ashley 2010). Amal Behbehani organised retrieval of papers, wrote to authors of papers for additional information, collected data for the review, screened the retrieved papers against the inclusion criteria and obtained and screened data on unpublished studies. Paul F Ashley and David R Moles provided additional data about papers. Paul F Ashley, Susan Parekh and Amal Behbehani screened the search results, extracted data from the papers and entered data into RevMan (RevMan 2014). David R Moles analysed the data. Paul F Ashley, Susan Parekh, Amal Behbehani and Prabhleen Anand wrote the review. Paul F Ashley and Laura CI MacDonald updated the review, including screening studies, editing background and discussion sections, obtaining data from trial authors, reanalysing data and reassessing evidence quality.
Sources of support
Internal sources
UCL, UK.
External sources
-
Cochrane Oral Health Group Global Alliance, Other.
Through our Global Alliance (http://oralhealth.cochrane.org/partnerships‐alliances), Cochrane Oral Health has received support from the following organisations: British Association for the Study of Community Dentistry, UK; British Association of Oral Surgeons, UK; British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; British Society of Periodontology, UK; Canadian Dental Hygienists Association, Canada; Mayo Clinic, USA; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland (NES); Royal College of Surgeons of Edinburgh, UK.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Oral Health Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
Declarations of interest
Paul F Ashley: none known Susan Parekh: none known David R Moles: none known Prabhleen Anand: none known Laura CI MacDonald: none known. Laura is a salaried member of staff with Cochrane Oral Health.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Baygin 2011 {published data only}
- Baygin O, Tuzuner T, Isik B, Kusgoz A, Tanriver M. Comparison of pre‐emptive ibuprofen, paracetamol, and placebo administration in reducing post‐operative pain in primary tooth extraction. International Journal of Paediatric Dentistry 2011;21(4):306‐13. [DOI] [PubMed] [Google Scholar]
Bernhardt 2001 {published data only}
- Bernhardt M. RE: FAO Melissa Bernhardt ‐ The effect of preemptive and/or postoperative ibuprofen therapy for orthodontic pain (personal communication). Email to: Cochrane Oral Health 8 June 2016.
- Bernhardt MK, Southard KA, Batterson KD, Logan HL, Baker KA, Jakobsen JR. The effect of pre‐emptive and/or postoperative ibuprofen therapy for orthodontic pain. American Journal of Orthodontics and Dentofacial Orthopedics 2001;120(1):20‐7. [DOI] [PubMed] [Google Scholar]
Law 2000 {published data only}
- Steen Law SL, Southard KA, Law AS, Logan HL, Jakobsen JR. An evaluation of preoperative ibuprofen for treatment of pain associated with orthodontic separator placement. American Journal of Orthodontics and Dentofacial Orthopedics 2000;118(6):629‐35. [DOI] [PubMed] [Google Scholar]
Primosch 1993 {published data only}
- Primosch R, Antony SJ, Courts FJ. The efficacy of preoperative analgesic administration for postoperative pain management of pediatric dental patients. Anesthesia and Pain Control in Dentistry 1993;2(2):102‐6. [PubMed] [Google Scholar]
Primosch 1995 {published data only}
- Primosch RE, Nichols DL, Courts FJ. Comparison of preoperative ibuprofen, acetaminophen, and placebo administration on parental report of postextraction pain in children. Pediatric Dentistry 1995;17(3):187‐91. [PubMed] [Google Scholar]
References to studies excluded from this review
Arantes 2009 {published data only}
- Arantes GM, Arantes VM, Ashmawi HA, Posso IP. Tenoxicam controls pain without altering orthodontic movement of maxillary canines. Orthodontics & Craniofacial Research 2009;12(1):14‐9. [DOI] [PubMed] [Google Scholar]
Ashkenazi 2007 {published data only}
- Ashkenazi M, Blumer S, Eli I. Post‐operative pain and use of analgesic agents in children following intrasulcular anaesthesia and various operative procedures. British Dental Journal 2007;202(5):E13. [DOI] [PubMed] [Google Scholar]
Bird 2007 {published data only}
- Bird SE, Williams K, Kula K. Preoperative acetaminophen vs ibuprofen for control of pain after orthodontic separator placement. American Journal of Orthodontics and Dentofacial Orthopedics 2007;132(4):504‐10. [DOI] [PubMed] [Google Scholar]
Bradley 2007 {published data only}
- Bradley RL, Ellis PE, Thomas P, Bellis H, Ireland AJ, Sandy JR. A randomized clinical trial comparing the efficacy of ibuprofen and paracetamol in the control of orthodontic pain. American Journal of Orthodontics and Dentofacial Orthopedics 2007;132(4):511‐7. [DOI] [PubMed] [Google Scholar]
Guan 2013 {published data only}
- Guan M, Wang EB, Cui NH, Liu Y, Ding B, Zhang W. Effect of flurbiprofen on preemptive analgesia in teeth extraction under intravenous sedation by midazolam. Zhonghua Kou Qiang Yi Xue Za Zhi [Chinese Journal of Stomatology] 2013;48(9):554‐5. [PubMed] [Google Scholar]
Gupta 2014 {published data only}
- Gupta M, Kandula A, Laxmikanth SM, Vyavahare SS, Reddy SB, Ramachandra CS. Controlling pain during orthodontic fixed appliance therapy with non‐steroidal anti‐inflammatory drugs (NSAID): a randomized, double‐blinded, placebo‐controlled study. Journal of Orofacial Orthopedics 2014;75(6):471‐6. [DOI] [PubMed] [Google Scholar]
McGaw 1987 {published data only}
- McGaw T, Raborn W, Grace M. Analgesics in pediatric dental surgery: relative efficacy of aluminum ibuprofen suspension and acetaminophen elixir. ASDC Journal of Dentistry for Children 1987;54(2):106‐9. [PubMed] [Google Scholar]
Minor 2009 {published data only}
- Minor V, Marris CK, McGorray SP, Yezierski R, Fillingim R, Logan H, et al. Effects of preoperative ibuprofen on pain after separator placement. American Journal of Orthodontics and Dentofacial Orthopedics 2009;136(4):510‐7. [DOI] [PubMed] [Google Scholar]
Ngan 1994 {published data only}
- Ngan P, Wilson S, Shanfeld J, Amini H. The effect of ibuprofen on the level of discomfort inpatients undergoing orthodontic treatment. American Journal of Orthodontics and Dentofacial Orthopedics 1994;106(1):88‐95. [DOI] [PubMed] [Google Scholar]
Patel 2011 {published data only}
- Patel S, McGorray SP, Yezierski R, Fillingim R, Logan H, Wheeler TT. Effects of analgesics on orthodontic pain. American Journal of Orthodontics and Dentofacial Orthopedics 2011;139(1):e53‐8. [DOI] [PubMed] [Google Scholar]
Polat 2005a {published data only}
- Polat O, Karaman AI. Pain control during fixed orthodontic appliance therapy. The Angle Orthodontist 2005;75(2):214‐9. [DOI] [PubMed] [Google Scholar]
Polat 2005b {published data only}
- Polat O, Karaman AI, Durmus E. Effects of preoperative ibuprofen and naproxen sodium on orthodontic pain. The Angle Orthodontist 2005;75(5):791‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Carr 1999
- Carr DB, Goudas LC. Acute pain. The Lancet 1999;353(9169):2051‐8. [DOI] [PubMed] [Google Scholar]
Denning 2000
- Denning J, Logan H. Gender differences in response to orthodontic separator placement. Journal of Dental Research 2000;79:326. [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Follmann 1992
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Gauvain‐Piquard 1987
- Gauvain‐Piquard A, Rodary C, Rezvani A, Lemerle J. Pain in children aged 2‐6 years: a new observation rating scale elaborated in a pediatric oncology unit ‐ preliminary report. Pain 1987;31(2):177‐88. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jones 1984
- Jones ML. An investigation into the initial discomfort caused by placement of an archwire. European Journal of Orthodontics 1984;6(1):48‐54. [DOI] [PubMed] [Google Scholar]
Jones 1992
- Jones M, Chan C. The pain and discomfort experienced during orthodontic treatment: a randomized controlled clinical trial of two initial aligning arch wires. American Journal of Orthodontics and Dentofacial Orthopedics 1992;102(4):373‐81. [DOI] [PubMed] [Google Scholar]
Kvam 1989
- Kvam E, Bondevik O, Gjerdet NR. Traumatic ulcers and pain in adults during orthodontic treatment. Community Dentistry and Oral Epidemiology 1989;17(3):154‐7. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org. [Google Scholar]
Ngan 1989
- Ngan P, Kess B, Wilson S. Perception of discomfort by patients undergoing orthodontic treatment. American Journal of Orthodontics and Dentofacial Orthopedics 1989;96(1):47‐53. [DOI] [PubMed] [Google Scholar]
Norden 1991
- Norden J. Concurrent validation of an objective pain scale for infants and children. Anesthesiology 1991;75:A934. [Google Scholar]
Pozos‐Guillen 2007
- Pozos‐Guillen A, Martinez‐Rider R, Aguirre‐Banuelos P, Perez‐Urizar J. Pre‐emptive analgesic effect of tramadol after mandibular third molar extraction: a pilot study. Journal of Oral and Maxillofacial Surgery 2007;65(7):1315‐20. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scheurer 1996
- Scheurer PA, Firestone AR, Bürgin WB. Perception of pain as a result of orthodontic treatment with fixed appliances. European Journal of Orthodontics 1996;18(4):349‐57. [DOI] [PubMed] [Google Scholar]
Seymour 1985
- Seymour RA, Meechan JG, Blair GS. An investigation into post‐operative pain after third molar surgery under local analgesia. The British Journal of Oral and Maxillofacial Surgery 1985;23(6):410‐8. [DOI] [PubMed] [Google Scholar]
Shields 2003
- Shields BJ, Palermo TM, Powers JD, Grewe SD, Smith GA. Predictors of a child's ability to use a visual analogue scale. Child: Care, Health and Development 2003;29(4):281‐90. [DOI] [PubMed] [Google Scholar]
Swafford 1968
- Swafford LI, Allen D. Pain relief in the pediatric patient. The Medical Clinics of North America 1968;52(1):131‐5. [Google Scholar]
Toms 2009
- Toms L, Derry S, Moore RA, McQuay HJ. Single dose oral paracetamol (acetaminophen) with codeine for postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001547.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Versloot 2008
- Versloot J, Veerkamp JS, Hoogstraten J. Pain behaviour and distress in children during two sequential dental visits: comparing a computerised anaesthesia delivery system and a traditional syringe. British Dental Journal 2008;205(1):E2. [DOI] [PubMed] [Google Scholar]
Weil 2007
- Weil K, Hooper L, Afzal Z, Esposito M, Worthington HV, Wijk A, et al. Paracetamol for pain relief after surgical removal of lower wisdom teeth. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004487.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision‐making. Evidence‐Based Dentistry 2015;16(3):69‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ashley 2010
- Ashley PF, Parekh S, Moles DR, Anand P, Behbehani A. Preoperative analgesics for additional pain relief in children and adolescents having dental treatment. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD008392] [DOI] [PubMed] [Google Scholar]
Ashley 2012
- Ashley PF, Parekh S, Moles DR, Anand P, Behbehani A. Preoperative analgesics for additional pain relief in children and adolescents having dental treatment. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD008392.pub2] [DOI] [PubMed] [Google Scholar]


