Abstract
Background
People with unstable angina and non‐ST elevation myocardial infarction (UA/NSTEMI) are managed with a combination of medical therapy, invasive angiography and revascularisation. Specifically, two approaches have evolved: either a 'routine invasive' strategy whereby all patients undergo coronary angiography shortly after admission and, if indicated, coronary revascularisation; or a 'selective invasive' (also referred to as 'conservative') strategy in which medical therapy alone is used initially, with a selection of patients for angiography based upon evidence of persistent myocardial ischaemia. Uncertainty exists as to which strategy provides the best outcomes for these patients. This Cochrane review is an update of a Cochrane review originally published in 2006, to provide a robust comparison of these two strategies in the early management of patients with UA/NSTEMI.
Objectives
To determine the benefits and harms associated with the following. 1. A routine invasive versus a conservative or 'selective invasive' strategy for the management of UA/NSTEMI in the stent era. 2. A routine invasive strategy with and without glycoprotein IIb/IIIa receptor antagonists versus a conservative strategy for the management of UA/NSTEMI in the stent era.
Search methods
We searched the following databases and additional resources up to 25 August 2015: the Cochrane Central Register of Controlled Trials (CENTRAL) on the Cochrane Library, MEDLINE and EMBASE, with no language restrictions.
Selection criteria
We included prospective randomised controlled trials (RCTs) that compared invasive with conservative or 'selective invasive' strategies in participants with acute UA/NSTEMI.
Data collection and analysis
Two review authors screened the records and extracted data in duplicate. Using intention‐to‐treat analysis with random‐effects models, we calculated summary estimates of the risk ratio (RR) with 95% confidence intervals (CIs) for the primary endpoints of all‐cause death, fatal and non‐fatal myocardial infarction (MI), combined all‐cause death or non‐fatal MI, refractory angina and re‐hospitalisation. We performed further analysis of included studies based on whether glycoprotein IIb/IIIa receptor antagonists were used routinely. We assessed the heterogeneity of included trials using Pearson χ² (Chi² test) and variance (I² statistic) analysis. Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, we assessed the quality of the evidence and the GRADE profiler (GRADEPRO) was used to import data from Review Manager 5.3 (Review Manager) to create Summary of findings (SoF) tables.
Main results
Eight RCTs with a total of 8915 participants (4545 invasive strategies, 4370 conservative strategies) were eligible for inclusion. We included three new studies and 1099 additional participants in this review update. In the all‐study analysis, evidence did not show appreciable risk reductions in all‐cause mortality (RR 0.87, 95% CI 0.64 to 1.18; eight studies, 8915 participants; low quality evidence) and death or non‐fatal MI (RR 0.93, 95% CI 0.71 to 1.2; seven studies, 7715 participants; low quality evidence) with invasive strategies compared to conservative (selective invasive) strategies at six to 12 months follow‐up. There was appreciable risk reduction in MI (RR 0.79, 95% CI 0.63 to 1.00; eight studies, 8915 participants; moderate quality evidence), refractory angina (RR 0.64, 95% CI 0.52 to 0.79; five studies, 8287 participants; moderate quality evidence) and re‐hospitalisation (RR 0.77, 95% CI 0.63 to 0.94; six studies, 6921 participants; moderate quality evidence) with routine invasive strategies compared to conservative (selective invasive) strategies also at six to 12 months follow‐up.
Evidence also showed increased risks in bleeding (RR 1.73, 95% CI 1.30 to 2.31; six studies, 7584 participants; moderate quality evidence) and procedure‐related MI (RR 1.87, 95% CI 1.47 to 2.37; five studies, 6380 participants; moderate quality evidence) with routine invasive strategies compared to conservative (selective invasive) strategies.
The low quality evidence were as a result of serious risk of bias and imprecision in the estimate of effect while moderate quality evidence was only due to serious risk of bias.
Authors' conclusions
In the all‐study analysis, the evidence failed to show appreciable benefit with routine invasive strategies for unstable angina and non‐ST elevation MI compared to conservative strategies in all‐cause mortality and death or non‐fatal MI at six to 12 months. There was evidence of risk reduction in MI, refractory angina and re‐hospitalisation with routine invasive strategies compared to conservative (selective invasive) strategies at six to 12 months follow‐up. However, routine invasive strategies were associated with a relatively high risk (almost double the risk) of procedure‐related MI, and increased risk of bleeding complications. This systematic analysis of published RCTs supports the conclusion that, in patients with UA/NSTEMI, a selectively invasive (conservative) strategy based on clinical risk for recurrent events is the preferred management strategy.
Keywords: Female; Humans; Male; Angina, Unstable; Angina, Unstable/mortality; Angina, Unstable/surgery; Angina, Unstable/therapy; Angioplasty, Balloon, Coronary; Angioplasty, Balloon, Coronary/adverse effects; Cause of Death; Coronary Angiography; Coronary Artery Disease; Coronary Artery Disease/therapy; Myocardial Infarction; Myocardial Infarction/mortality; Myocardial Infarction/surgery; Myocardial Infarction/therapy; Platelet Glycoprotein GPIIb‐IIIa Complex; Platelet Glycoprotein GPIIb‐IIIa Complex/antagonists & inhibitors; Randomized Controlled Trials as Topic; Sex Factors; Stents
Plain language summary
Routine invasive versus conservative strategies for unstable angina and non‐ST elevation myocardial infarction in the stent era
Background
People with prolonged or recurrent chest pain may have a condition called unstable angina (UA) or suffer a certain type of heart attack called non‐ST elevation myocardial infarction (NSTEMI). People with either of these two conditions may be managed by either one of two treatment strategies: the routine invasive strategy, or the conservative or 'selective invasive strategy'. With the first approach, patients have a catheter (a long, patent tube) inserted into the arteries that bring blood to the heart muscle itself, called the coronary arteries. The main objective behind inserting this catheter (in other words, to perform a procedure called coronary angiography) is to look for thickening and hardening of the vessel. If a significant narrowing or a complicated plaque is found, then the artery may be dilated by inserting a balloon catheter that can be inflated wherever the vessel is particularly narrow, so as to open the vessel and improve blood flow. The vessel is held open by inserting a metallic stent. In some cases, the region of vessel narrowing is not amenable to this approach and surgery to bypass it is required. With the other, conservative or 'selective invasive' strategy, patients are initially treated with drugs, and only those who continue to suffer further chest pain or who demonstrate evidence of ongoing coronary artery narrowing via other non‐invasive tests, such as stress testing or imaging, undergo coronary angiography and revascularisation if indicated. In this Cochrane review, researchers examined the available evidence to determine which strategy is better.
Study characteristics
We included randomised controlled trials that compared routine invasive strategies to conservative strategies in patients with UA and NSTEMI in the stent era. We searched the available literature up to 25 August 2015.
Key results
We included eight studies with 8915 participants: five trials were in the review version published in 2010, and three were new trials. Of the included participants with UA and NSTEMI, there were 4545 in the invasive strategy arm and 4370 in the conservative strategy arm. Evidence failed to show appreciable risk reduction in all‐cause mortality and death or non‐fatal myocardial infarction (MI) with routine invasive management strategy compared to conservative strategies. There was appreciable risk reduction in MI, refractory angina and re‐hospitalisation with routine invasive strategies compared to conservative strategies, but this was associated with an increased risk of procedure‐related MI and bleeding complications.
Quality of evidence for primary outcomes
The quality of the evidence in this review update ranged from low quality to moderate quality. Low quality evidence was as a result of serious risk of bias and uncertainty surrounding the effect, while moderate quality evidence was only due to serious risk of bias.
Conclusions
The debate continues as to which strategy is better. The invasive strategy reduces the incidence of further chest pain or re‐hospitalisation. Also, long‐term follow‐up from three studies suggests that it lowers the risk of a heart attack over the next three to five years. However, the invasive strategy also is associated with double the risk of heart attack during or soon after initial treatment, as well as an increased risk of bleeding. In summary, the published scientific research suggests that the invasive strategy may have particular benefit in those patients who are at high risk for recurrent events, and that patients at low risk for a recurrent event may even suffer harm from such an approach.
Summary of findings
Summary of findings for the main comparison. Routine invasive strategies versus selective invasive strategies (conservative) for unstable angina and non‐ST elevation myocardial (UA/NSTEMI) infarction in the stent era.
| Routine invasive strategies versus selective invasive strategies (conservative) for UA/NSTEMI infarction in the stent era | |||||
| Participant or population: participants with UA/NSTEMI in the stent era Settings: hospital setting Intervention: routine invasive strategies Comparison: selective invasive strategies (conservative) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Selective invasive strategies (conservative) | Routine invasive strategies | ||||
| Death Follow‐up: 6 to 12 months1 | Study population | RR 0.87 (0.64 to 1.18) | 8915 (8 studies) | ⊕⊕⊝⊝ low2,3 | |
| 42 per 1000 | 36 per 1000 (27 to 49) | ||||
| Moderate risk population | |||||
| 39 per 1000 | 34 per 1000 (25 to 46) | ||||
| Myocardial infarction Follow‐up: 6 to 12 months1 | Study population | RR 0.79 (0.63 to 1) | 8915 (8 studies) | ⊕⊕⊕⊝ moderate2 | |
| 78 per 1000 | 62 per 1000 (49 to 78) | ||||
| Moderate risk population | |||||
| 89 per 1000 | 70 per 1000 (56 to 89) | ||||
| Death or non‐fatal Myocardial infarction Follow‐up: 6 to 12 months1 | Study population | RR 0.93 (0.71 to 1.2) | 7715 (7 studies) | ⊕⊕⊝⊝ low2,3 | |
| 113 per 1000 | 105 per 1000 (80 to 135) | ||||
| Moderate risk population | |||||
| 109 per 1000 | 101 per 1000 (77 to 131) | ||||
| Refractory angina Follow‐up: 6 to 12 months1 | Study population | RR 0.64 (0.52 to 0.79) | 8287 (5 studies) | ⊕⊕⊕⊝ moderate2 | |
| 325 per 1000 | 208 per 1000 (169 to 257) | ||||
| Moderate risk population | |||||
| 129 per 1000 | 83 per 1000 (67 to 102) | ||||
| Rehospitalisation Follow‐up: 6 to 12 months1 | Study population | RR 0.77 (0.63 to 0.94) | 6921 (6 studies) | ⊕⊕⊕⊝ moderate2 | |
| 286 per 1000 | 220 per 1000 (180 to 269) | ||||
| Moderate risk population | |||||
| 122 per 1000 | 94 per 1000 (77 to 115) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; UA: unstable angina; NSTEMI: non ST segment myocardial infarction. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1 Intermediate end points. 2 Downgraded by one due to possible risk of bias due to lack of blinding. 3 Downgraded by one due to imprecision with effect size overlapping the line of no effect and appreciable benefit or harm.
Summary of findings 2. Routine invasive strategies versus selective invasive strategies (conservative) for unstable angina and non‐ST elevation myocardial infarction (UA/NSTEMI) in the stent era.
| Routine invasive strategies versus selective invasive strategies (conservative) for UA/NSTEMI in the stent era | |||||
| Participant or population: participants with UA/NSTEMI in the stent era Settings: hospital setting Intervention: routine invasive strategies Comparison: selective invasive strategies (conservative) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Selective invasive strategies (conservative) | Routine invasive strategies | ||||
| Complications of angiography or revascularization Bleeding | Study population | RR 1.73 (1.3 to 2.31) | 7584 (6 studies) | ⊕⊕⊕⊝ moderate1 | |
| 42 per 1000 | 72 per 1000 (54 to 96) | ||||
| Moderate risk population | |||||
| 27 per 1000 | 47 per 1000 (35 to 62) | ||||
| Complications of angiography or revascularization Procedure‐related myocardial infarction | Study population | RR 1.87 (1.47 to 2.37) | 6380 (5 studies) | ⊕⊕⊕⊝ moderate1 | |
| 30 per 1000 | 57 per 1000 (45 to 72) | ||||
| Moderate risk population | |||||
| 29 per 1000 | 54 per 1000 (43 to 69) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; UA: unstable angina; NSTEMI: non ST segment myocardial infarction. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1 Downgraded by one due to possible risk of bias due to lack of blinding.
Background
Diagnosis of acute coronary syndromes
Acute coronary syndrome (ACS) encompasses three disorders of related aetiology: ST‐elevation myocardial infarction (STEMI); non‐STEMI (NSTEMI); and unstable angina (UA). Management of STEMI differs from that for UA and NSTEMI, which can be considered a single clinical entity (UA/NSTEMI). The pathogenesis of UA/NSTEMI involves five non‐exclusive causative factors: non‐occlusive thrombus on pre‐existing plaque; dynamic obstruction; progressive mechanical obstruction; inflammation; and secondary UA associated with increased cardiac workload (Braunwald 1998). Of these factors, thrombus formation on pre‐existing plaque, which is an acute plaque change, is the most common. Indeed, most patients with ACS have an acute change in coronary atherosclerotic plaques, with STEMI usually associated with complete occlusion of the involved vessel(s) (DeWood 1980) and UA/NSTEMI usually associated with subtotal occlusion (DeWood 1986; TIMI‐IIIA 1993). The distinction between UA and NSTEMI depends on the presence of myocardial infarction (MI), as determined by markers of myocardial damage such as troponin I (TnI), troponin T (TnT) and creatine kinase‐myocardial band (CK‐MB).
Compared to STEMI, NSTEMI has a lower 30‐day mortality rate, but more recurrent ischaemia and a similar one‐year mortality rate (Armstrong 1998). UA/NSTEMI is much more common than STEMI; in the USA, for example, 1.4 million patients per year are admitted to hospital with ACS, approximately 70% of them with UA/NSTEMI (Rosamond 2008). Whereas emergency percutaneous coronary revascularisation is now a commonly‐used therapy for treating STEMI (Antman 2004; Cucherat 2003), the role of angiography and possible subsequent revascularisation is less clear in UA/NSTEMI patients. Treatment of UA/NSTEMI initially involves medical therapy followed by one of two variations on the management strategy, which results in differing rates of angiography and revascularisation. In this Cochrane review update we review the medical therapies for UA/NSTEMI briefly before we focus on the management strategies of patients with UA/NSTEMI.
Initial medical management of UA/NSTEMI
Medical treatments, as outlined in the American College of Cardiology Foundation (ACCF) and American Heart Association (AHA) (Anderson 2007; Jneid 2012) and European Society of Cardiology (ESC) guidelines (Hamm 2011), fall into the two major categories: anti‐ischaemic and anti‐platelet/anti‐coagulation. Anti‐ischaemic therapies include bed rest, nitroglycerin, beta blockers (or certain non‐dihydropyridine calcium antagonist if beta blockers are contraindicated) and angiotensin‐converting enzyme (ACE) inhibitors. Anti‐platelet or anti‐coagulation therapies include aspirin, P2Y12 receptor inhibitor treatment (clopidogrel, prasugrel and ticagrelor), heparin and glycoprotein IIb/IIIa receptor antagonists. Published randomised clinical trials support the use of most of these specific therapies. Among the anti‐ischaemic treatments, beta blockers have proven efficacy in patients with evolving MI (Hjalmarson 1982; Yusuf 1988), as well as in patients with UA/NSTEMI (Gottlieb 1986; Muller 1984; Théroux 1985). Non‐dihydropyridine calcium channel antagonists have proven efficacy in ACS (Boden 1991; Gibson 1986; Pepine 1998; Tijssen 1987), and are particularly useful in patients with contraindications to beta‐blockers. Both the early and late administration of ACE inhibitors can be beneficial for MI (EUROPA 2003; HOPE 2000; Rodrigues 2003). Of the anti‐platelet or anti‐coagulation treatments, aspirin exhibits a consistent benefit for UA/NSTEMI as demonstrated in several clinical trials (Cairns 1985; Lewis 1983; RISC 1990; Theroux 1988). Similarly, clopidogrel is a beneficial adjunct to aspirin (CURE 2001). Subsequently, prasugrel, TRITON‐TIMI 38, and ticagrelor, PLATO, have been identified as alternatives to clopidogrel. Heparin, in its various forms, or fondaparinux are also beneficial in UA/NSTEMI (Gurfinkel 1995; Mehta 2008; Neri Serneri 1990; RISC 1990; Theroux 1993). The glycoprotein IIb/IIIa receptor antagonists have proven efficacy in the medical treatment of UA/NSTEMI (Boersma 2002; PRISM‐PLUS Trial; PURSUIT 1998; Roffi 2002; Topol 1999). However, this class of drugs appears to have differential effects depending on the patients' risk level and bleeding propensity, and high‐risk patients obtain the greatest benefit; consequently, their use should be highly‐selective (ACUITY; EARLY ACS 2009). The glycoprotein IIb/IIIa receptor antagonists warrant special mention regarding their use in invasive procedures. We expand upon this concept later in this review update.
Management following initial medical treatment: what is the role of early coronary angiography and revascularization?
Two different treatment strategies may be adopted after the initial medical treatment of UA/NSTEMI.
A routine invasive strategy of coronary angi ography and, if indicated, revascularisation in most or all patients who have no contraindication to such an approach.
A 'selective invasive' or 'ischaemia guided' (conservative) strategy, in which patients undergo coronary angiography and revascularisation only if there is evidence of recurrent ischaemia. Examples are recurrent infarction, angina at rest, dynamic ST changes on electrocardiograph (ECG), and definitive inducible ischaemia on provocative testing.
Proponents of the routine invasive strategy argue that the early determination of coronary anatomy can be used to tailor revascularisation therapy, avoid lengthy hospital stays and prevent further events. Those with significant coronary disease evident on angiography can be treated expeditiously according to their angiographic findings, which may include revascularisation via percutaneous coronary intervention (PCI) comprised of coronary angioplasty and coronary stenting, or coronary artery bypass grafting (CABG). Proponents of the conservative or 'selective invasive' strategy argue that medical therapy can stabilise patients. Stress testing can identify patients at risk for future events and who would therefore benefit most from an invasive intervention. This strategy may also limit the costs and complications of invasive procedures. The evidence for the relative advantages and disadvantages of these two approaches is the subject of this Cochrane review.
Interpretation of the evidence from trials: changes in contemporary clinical practice
In routine clinical practice, the outcomes of invasive coronary procedures vary depending upon a number of factors, including clinical expertise (Singh 2000), volume of procedures performed (Magid 2000), and methods and protocols used, especially regarding pharmacological and procedural co‐interventions. Of particular importance in contemporary practice is the use of coronary artery stents (Al Suwaidi 2004), which improves outcomes and reduces complications when used with invasive procedures. Stenting is associated with fewer major adverse cardiovascular events and a reduced need for emergency cardiac surgery (Al Suwaidi 2004). Specifically, the reduction in target vessel revascularisation associated with stenting is of particular relevance to trials with longer durations of follow‐up.
Upstream glycoprotein IIb/IIIa receptor antagonist use is controversial. Though these drugs initially seemed beneficial (EPIC 1994; EPILOG 1997; EPISTENT 1998; Karvouni 2003; Simoons 1997), their routine application has been associated with an increased risk of non‐life‐threatening bleeding (ACUITY; EARLY ACS 2009), which has resulted in a paradigm shift from routine to highly‐selective use. In the ACUITY study, patients with UA/NSTEMI treated with an early invasive strategy, bivalirudin without routine glycoprotein IIb/IIIa receptor antagonists, demonstrated significantly reduced rates of major bleeding with non‐inferior outcomes in ischaemia endpoints compared to standard heparin or bivalirudin with mandatory glycoprotein IIb/IIIa receptor antagonists (Stone 2006; Stone 2007). However, the substitution of bivalirudin for heparin with glycoprotein IIb/IIIa receptor antagonists probably should not be undertaken unless patients have been pretreated with a thienopyridine prior to angiography (Stone 2006).
Why it is important to do this review
UA/NSTEMI is a common hospital presentation and carries a significant risk of mortality and recurrent ischaemic events. Older landmark meta‐analyses (Bavry 2006; Mehta 2005) include pre‐stent era trials and do not consider more recently published studies, and thus limit their application to contemporary management of this serious disease. This Cochrane review evaluates the relative merits of the two above‐noted strategies in the modern era with relevance to patients, physicians and healthcare systems.
Objectives
To determine the benefits and harms associated with the following.
A routine invasive versus a conservative or 'selective invasive' strategy for the management of UA/NSTEMI in the stent era.
A routine invasive strategy with and without glycoprotein IIb/IIIa receptor antagonists versus a conservative strategy for the management of UA/NSTEMI in the stent era.
Methods
Criteria for considering studies for this review
Types of studies
We only considered studies undertaken in the stent era for inclusion. If we had included non‐stent studies, the analysis would underestimate the benefits of a routine invasive strategy on endpoints such as recurrent angina and rehospitalisation (e.g. due to chest pain). We included randomised controlled trials (RCTs) that compared invasive and selectively invasive strategies in participants with unstable angina and non‐ST elevation myocardial infarction (UA/NSTEMI), and measured at least one of this review's outcomes. The revascularisation approaches in the included studies were percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), as required. We investigated the effect of glycoprotein IIb/IIIa receptor antagonist use on outcomes further by undertaking two separate analyses on trials according to routine versus selective use of glycoprotein IIb/IIIa receptor antagonists during PCI. Thus, the analyses we undertook were as follows.
All studies that deployed stents routinely in revascularisation procedures using PCI, regardless of glycoprotein IIb/IIIa receptor antagonist use.
Stents and glycoprotein IIb/IIIa receptor antagonists deployed routinely in revascularisation procedures using PCI.
Stents deployed routinely in revascularisation procedures using PCI with selective glycoprotein IIb/IIIa receptor antagonists use.
It is important to note that, in recent years, a number of studies have focused on the optimal timing of an invasive strategy. While these studies fulfil many of the criteria for inclusion, often they did not randomise patients to a medically‐managed conservative or 'selective invasive' strategy. Consequently, we have generally excluded these studies from the current analysis.
Types of participants
Men and women, at least 18 years of age, who had an episode of angina with an accelerating pattern of pain at rest. The index episode of pain must have occurred within 72 hours of randomisation. Furthermore, the patients must have exhibited at least one of the following.
New ST depression.
Transient (< 20 minute) ST elevation.
Ischaemic T‐wave inversion or T‐wave inversion in at least two contiguous leads.
Elevated levels of cardiac markers; e.g. troponin's or creatine kinase‐myocardial band (CK‐MB).
Coronary artery disease (CAD), as determined by a history of catheterisation, revascularisation, or acute coronary syndromes (ACS).
The included studies generally excluded patients if they had any of the following.
Persistent ST elevation (i.e. > 20 minutes).
Secondary causes of acute myocardial ischaemia (e.g. anaemia, thyrotoxicosis, acute pulmonary infection, fever, tachyarrhythmias, uncontrolled hypertension).
Secondary causes of cardiac biomarker elevation or altered kinetics (e.g. renal insufficiency, acute non‐cardiac disease etc.).
Serious systemic disease or major co‐morbidities that would preclude an invasive approach.
Severe congestive heart failure or cardiogenic shock.
Arrhythmias that required immediate catheterisation.
Refractory symptoms.
Intolerance of anticoagulation and anti‐platelet therapy.
Coronary revascularization procedure within the previous 30 days.
Types of interventions
All patients with UA/NSTEMI were initially treated with some or all of the medical therapies we discussed in the 'Background' section; we have summarised these in Table 3. Following initial medical therapy, patients were randomised to either routine or selective invasive treatment. The two treatment strategies differed with regard to the use of angiography and subsequent revascularisation rates.
1. Detailed characteristics of included studies, rates of angiography and revascularisation.
| Study characteristic | TACTICS‐TIMI 18 | ICTUS | RITA‐3 | FRISC‐II | VINO | Italian Elderly ACS | LIPSIA‐NSTEMI | OASIS 5 |
| Year of publication | 2001 | 2005 | 2002 | 1999 | 2001 | 2012 | 2012 | 2012 |
| Total number of participants | 2220 | 1200 | 1810 | 2457 | 131 | 313 | 602 | 184 |
| Stent use in invasive arm % | 83 | 88 | 88 | 61 | 47 | 50 | 73 | 52 |
| Men % | 66 | 74 | 62 | 70 | 80 | 50 | 67 | 0 |
| Mean age | 62 | 62 | 63 | 65 | 66 | 82 | 70 | 68 |
| Trial duration | 6 months | 4 years | 5 years | 5 years | 6 months | 1 year | 6 months | 2 years |
| Diabetes mellitus % | 28 | 14 | 13 | 13 | 25 | 36 | 38 | 25 |
| Myocardial infarction (MI) on trial enrolment % | 54 | 100 | 75 | 58 | 100 | 100 | 100 | 67 |
| Previous MI % | 29 | 23 | 28 | 23 | 26 | 31 | 20 | 22 |
| ST depression % | 39 | 48 | 37 | 46 | 47 | NA | 62 | 47 |
| Mortality in conservatively managed participants at end of follow‐up % (note different trial durations) | 3.5 | 7.7 | 14 | 10.1 | 13.4 | 13.8 | 6.5 | 2.2 |
| Mortality in conservatively managed participants expressed as an average mortality per year of follow‐up %/year | 7.0 | 1.9 | 2.8 | 2.0 | 26.8 | 13.8 | 13 | 1.1 |
| MI rate in conservatively managed participants at end of follow‐up % (note different trial durations) | 6.9 | 12.3 (as per trial definition) | 6.2 | 17.7 | 14.9 | 10.7 | 11.5 | 13.3 |
| Glycoprotein 2b/3a receptor antagonist use in invasive arm % | 94 | 94 | 9 | 10 | 0 | 17 | 99 | 12 |
| Revascularization at end of follow‐up invasive/conservative % | 61/44 | 81/58 | 61/38 | 80/52 | 78/39 | 58/31 | 84/70 | 64/49 |
| Difference in revascularization rates at end of follow‐up between the 2 strategies % | 17 | 23 | 23 | 28 | 39 | 27 | 14 | 15 |
| Percentage of revascularization procedures in the invasive group being CABG % | 22 | 24 | 42 | 41 | 35 | 7 | 10 | 21 |
| Medical co‐interventions (% of participants enrolled) | Aspirin: 98; unfractionated heparin: 99; beta‐blocker: 82; statin: 52; clopidogrel: 0 (this was a criterion for exclusion) | Aspirin: 100 as per protocol, enoxaparin: 100 as per protocol, statin: 92, clopidogrel: 55 | Aspirin: 92; enoxaparin: 84; unfractionated heparin: 11; beta‐blocker: 72; calcium channel antagonist: 35; ACE inhibitor: 18; statin: 45 | Aspirin: 93; dalteparin 50; unfractionated heparin: 50; beta‐blocker: 79; calcium channel antagonist: 20; statin: 56 | Aspirin: 100 as per protocol, heparin: 100 as per protocol; beta‐blocker: 76; calcium channel antagonist: 9; ACE inhibitor: 47; statin: 43 | During index admission: aspirin: 96; ticlopidine: 3.2; clopidogrel: 90; unfractionated heparin: 24; enoxaparin: 50; bivalirudin: 2.5; fondaparinux: 7; at discharge: aspirin: 91; ticlopidine: 2; clopidogrel: 76; beta‐blockers: 60; ACEi: 80; statins: 80 | Beta‐blockers: 99; ACEi/ARB: 99; Asprin: 100; clopidogrel/prasugrel: 99; statins: 98; tirofiban: 99 | Aspirin: 99; clopidogrel: or ticlopedin: 81; dual antiplatelet therapy: 80; UFH: 10; enoxaparin: 50; fondaparinux: 50; ACE inhibitor or ARB: 76; beta‐blocker: 94; lipid‐lowering drug: 87 |
The two management strategies compared were as follows.
Routine invasive strategy: routine angiography with or without revascularisation in all patients. This was performed in all eligible patients unless they had contraindications to angiography.
Conservative or 'selective invasive' strategy: angiography with or without revascularisation only in eligible patients with evidence of cardiac ischaemia; e.g. recurrent ischaemia, dynamic electrocardiograph (ECG) changes or a positive stress test.
Revascularisation modalities included PCI or CABG, depending on the angiographic findings. CABG is indicated in lieu of PCI when any one of the following criteria is met.
Three vessel disease and an ejection fraction (EF) of less than 0.50.
Two vessel disease with proximal left anterior descending involvement and EF of less than 0.50 or ischaemia.
Left main CAD.
Types of outcome measures
Primary outcomes
Death: all causes.
Myocardial infarction (MI) (this endpoint only included non‐fatal MI in the review protocol, but the review includes fatal and non‐fatal MI).
Death (all causes) or non‐fatal MI.
Refractory angina.
Secondary outcomes
Rehospitalisation for ACS.
Complications of angiography or revascularisation (e.g. bleeding, procedure‐related MI, stroke).
Differentiating peri‐PCI cardiac biomarker leaks from the outcome measure 'non‐fatal myocardial infarction' warrants further comment. A universal definition of MI, including peri‐procedural MI, has only recently been adopted and defines peri‐procedural MI as a biomarker increase to three times the upper reference limit (Thygesen 2007). Unfortunately, as summarised in Table 4, the included studies inconsistently defined peri‐procedural MI and this limited the interpretation of this outcome data across the included trials. The TACTICS‐TIMI 18, OASIS 5 and Italian Elderly ACS definitions most closely match the current universal definition. Furthermore, not all included studies involved the routine measurement of cardiac biomarkers following PCI. We have discussed this point further under the heading 'Outcomes'.
2. Definitions of myocardial infarction in the included studies.
| Study name | Definition for non‐procedural myocardial infarction (MI) | Definition of procedural MI | More than one definition of MI? | Definition of MI used in this review |
| RITA‐3 | Clinical symptoms, ECG changes and CK‐MB or Toponin > 2 x upper limit of normal > 24 hours postrandomisation | Clinical symptoms, ECG changes and CK‐MB or Toponin > 2 x upper limit of normal > 24 hours postrandomisation | Yes | As per trial definition |
| ICTUS | CK‐MB > upper limit of normal or a 50% decline from a peak value followed by subsequent rise to a value greater than the upper limit of normal. An increased troponin above the upper limit of normal was also used beyond one year of follow‐up | CK‐MB > upper limit of normal or a 50% decline from a peak value followed by subsequent rise to a value greater than the upper limit of normal. New Q waves on the electrocardiogram were used to define MI associated with coronary artery bypass grafting | Yes | In various publications, the trial authors report the MI end point as the following.
We utilized spontaneous MI for our MI endpoint, death/spontaneous MI for our death or MI composite and procedural MI is reported as a safety endpoint. Since the prognostic value of peri‐procedural infarctions is still debated, 'spontaneous' MI is our preferred endpoint since this allows for consistency with the other trials |
| TACTICS‐TIMI 18 | CK‐MB > upper limit of normal or > 50% over previous | CK‐MB > 3 times upper limit of normal or > 50% over previous | No | As per trial definition |
| Italian Elderly ACS | Cardiac ischaemic symptoms at rest within 42 hours before randomisation, together with Ischemic ECG changes (transient or persistent ST‐segment elevation or depression > 0.5mm but < 1 mm in the case of ST‐elevation, or persistent and definite T‐wave inversion > 1 mm in at least 2 contiguous leads) and/or any elevation of CK‐MB or cTn (> upper limit of normal) | Recurrent infarction within first 72 hours: Ischemic ECG changes (new Q‐waves > 0.04 s in 2 or more contiguous leads which is not an ambiguous change from baseline) CK‐MB > upper limit of normal and increased > 50% over previous value Following PCI: CK‐MB elevation > 3 times upper limit of normal and increased by at least 50% over the previous value. In 72 hours following CABG: both biomarker and ECG criteria if the CK‐MB is > 5 times upper limit of normal but < 10 times upper limit of normal; if the cardiac markers are > 10 times upper limit of normal, ECH criteria are not required. |
No | As per trial definition |
| LIPSIA‐NSTEMI | Ischaemic symptoms that were increasing or occurred at rest, with the last episode < 24 hours before randomisation plus elevated troponin T level ≥ 0.1 ng/mL | In‐hospital re‐MI was defined by the occurrence of any of the following: new Q waves in ≥ 2 contiguous leads plus ischaemic symptoms > 20 mins; or new ST‐segment elevation in ≥ 2 contiguous leads plus ischaemic symptoms > 20 mins; or elevation of CK‐MB > 5 upper limit of normal in those with CK‐MB > 5 times upper limit of normal at randomisation an increase > 50% was required for re‐MI definition | No | As per trial definition |
| VINO | Recurrent ischaemic chest pain lasting > 20 minutes, new ECG changes and CK‐MB > 1.5 times the upper limit of normal after 72 hours postrandomisation | Recurrent ischaemic chest pain lasting > 20 minutes, new ECG changes and CK‐MB > 1.5 times the upper limit of normal after 72 hours postrandomisation | No | As per trial definition |
| FRISC‐II | Two or three of the following criteria: chest pain, ECG changes or elevated markers of myocardial damage. Marker definitions: CK‐MB mass > upper limit of normal or CK, CK‐B, CK‐MB activity > 2 times upper limit of normal in 1 sample of CK‐MB activity > upper limit of normal in 2 samples | Either 2 or 3 of the following criteria: chest pain, ECG changes or elevated markers of myocardial damage. Marker definitions: CK‐MB mass > 1.5 times upper limit of normal or CK, CK‐B, CK‐MB activity > 3 times upper limit of normal in 1 sample of CK‐MB activity > 2 times upper limit of normal in 2 samples | No | As per trial definition |
| OASIS 5 | Typical rise and fall of biochemical marker of myocardial necrosis (including troponin, CK‐MB, CK) to greater than 2 x ULN (if markers were already elevated, > 50% of the lowest recovery biomarker level from the index infarction) and at least one of the following.
|
Typical rise and fall of biochemical marker of myocardial necrosis (including troponin, CK‐MB, CK) to greater than 3 x ULN if within 48 hours of PCI or 5 x ULN if within 48 hours of CABG and at least one of the following.
|
No | As per trial definition |
MI = myocardial infarction
Search methods for identification of studies
The previous version of this review (Hoenig 2010) included: the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 1 of 12, 2008) on the Cochrane Library, MEDLINE (1996 to February 2008) and EMBASE (1996 to February 2008). The review applied a restriction of 1996 onwards because of low rates of stent use prior to that year (see Appendix 1 for search strategies).
This review update utilised similar search strategies for CENTRAL (Issue 7 of 12, 2015) on the Cochrane Library, MEDLINE (OVID, 1946 to August week 3, 2015) and EMBASE (OVID, 1980 to 2015 week 33), restricted from 2008 through to the date of the searches, 25 August 2015 (see Appendix 2 for search strategies). We searched the reference lists of all retrieved articles and contacted experts in the field to identify additional potentially‐eligible studies. We did not apply any language restrictions.
Data collection and analysis
Selection of studies
Two review authors of a previous version of this review (Hoenig 2010)(MRH, JAD) identified and independently selected studies published between 1996 and 2008 for inclusion. Two authors of this review update (JPF, DLW) identified studies published between 2008 and 2015. We considered a study to be eligible for inclusion if it prospectively enrolled eligible participants with an acute presentation of UA/NSTEMI and randomised their management to either a routine invasive strategy or conservative/'selective invasive' strategy in patients with an acute presentation of UA/NSTEMI. We have mentioned specific exclusion criteria in the 'Types of studies' section.
Data extraction and management
Two of the original review authors (MRH, JAD) extracted data from the 1996 to 2008 searches independently onto data extraction sheets. They resolved any disagreements first by consensus and then by consultation with CNA and IAS. We updated the literature searches for the period 2008 to 2015. Two review authors (JPF, DLW) independently extracted relevant data using double data entry.
'Risk of bias' assessment
Two review authors independently assessed the risk of bias in all included studies. Please refer to the 'Characteristics of included studies' tables for the quality assessment guidelines for the included studies.
Statistical considerations
We analysed data on an ITT basis. Where appropriate, we combined data from all trials using the meta‐analysis software in Review Manager (RevMan) (RevMan 2014). All outcome measures of this Cochrane review were dichotomous. We combined data using a random‐effects model to determine a summary estimate of the risk ratio (RR) with a 95% confidence interval (CI). We assessed heterogeneity using the Pearson χ² (Chi²) test (P < 0.10) for all endpoints and the I² statistic for selected endpoints (Higgins 2003). We displayed the I² statistic on the forest plots for all analyses. Furthermore, we performed sensitivity analysis for various prespecified variables that may present sources of inter‐study heterogeneity. Since this meta‐analysis contained a small number of included studies and we previously identified many potential sources of heterogeneity (Hoenig 2010), we did not undertake meta‐regression. As such, we felt that individual patient data meta‐analysis would be more appropriate (Thompson 2005). This also would avoid aggregation bias. Given the discrepant definitions of MI between the included studies (Table 4), we used mortality at end of follow‐up when we assessed publication bias or heterogeneity via sensitivity analysis. As stated under the 'Types of studies' section, we analysed all included studies further by assigning them to one of two analyses, depending on the routine use of glycoprotein IIb/IIIa receptor antagonists. We compared the invasive strategy versus the conservative strategy within each analysis.
Summary of findings
The GRADE approach was employed to interpret findings and the GRADE profiler (GRADEPRO) allowed us to import data from Review Manager 5.3 (Review Manager) to create 'Summary of findings' tables.
Results
Description of studies
The original literature search performed for this review (1996 to 2008) yielded 2221 hits (Hoenig 2010). Of these, we selected 31 papers that reported on 14 studies for closer attention. We excluded one study because it was based on a registry and hence contained observational data (MITI 2000). We excluded another study because it was a post‐hoc analysis of a trial that compared hirudin to heparin in acute coronary syndrome (ACS) patients (GUSTO2b 2003). We excluded four trials because they were undertaken in the pre‐stent era or did not encourage the routine use of stents in the invasive strategy (MATE 1998; TIMI‐3b; VANQWISH 1998; Zhao 2005). Moreover, some studies included patients with ST elevation myocardial infarction (STEMI) but did not report outcomes separately for unstable angina (UA)/non‐ST elevation myocardial infarction (NSTEMI) (Eisenberg 2005; MATE 1998). As stated earlier, studies from the pre‐stent era underestimate the value of the invasive strategy and are irrelevant to current practice. Also, we excluded two studies because of inappropriate participant selection or trial design (ISAR‐COOL; TRUCS 2000). More details on the excluded studies can be found in the 'Characteristics of excluded studies' table. We deemed five studies appropriate for inclusion and we have described these in the 'Characteristics of included studies' table. We analysed these five studies together in Analysis 1. Two studies used a glycoprotein IIb/IIIa antagonist routinely in the routine invasive arm (ICTUS; TACTICS‐TIMI 18), and we analysed these two studies together via the prespecified Analysis 2 (see the 'Types of studies' section). The three remaining studies satisfied this Cochrane review's stent requirement but did not routinely use glycoprotein IIb/IIIa antagonists in participants randomised to the routine invasive strategy. We analysed these together as Analysis 3 (FRISC‐II; RITA‐3; VINO).
The literature search update (2008 to 2015) yielded 1929 hits, of which 27 were potentially relevant articles. Of these, we added five articles, which related to three studies (Italian Elderly ACS; LIPSIA‐NSTEMI; OASIS 5), to the previous analysis. The Italian Elderly ACS and OASIS 5 studies were also relevant to Analysis 2, and the LIPSIA‐NSTEMI trial was relevant to Analysis 3. We excluded a number of important randomised controlled trials (RCTs) from the update due to the absence of randomisation to a management arm consistent with the 'selective invasive' strategy as defined in this Cochrane review. These studies generally focused on the optimal timing of an invasive strategy (e.g. ABOARD; ELISA; ISAR‐COOL; OPTIMA; TIMACS; Zhang 2010), or optimal use of antithrombotic medication (e.g. ACUITY; EARLYACS; PLATO; TRITON‐TIMI 38). A published conference abstract, Dimitrov 2013, eluded to a potentially relevant study. However, as the full text publication was unavailable at the time of the literature search, we classified this study as 'ongoing' and excluded it from the analyses (see the 'Characteristics of ongoing studies' section).
Design
All included studies were RCTs. Due to the procedural nature of the intervention, we presumed that the participants and treating clinicians were not blinded to the intervention. However, a blinded committee could assess outcomes. The 'Characteristics of included studies' table describes the trial design features and includes information on intention‐to treat (ITT) analysis and losses to follow‐up.
Populations
The included studies were heterogeneous in their participant selection criteria. The inclusion criteria were comprised of different combinations of the following core criteria: chest pain, electrocardiograph (ECG) changes, increased level(s) of cardiac marker(s) or a documented history of coronary artery disease (CAD). We have outlined the specific criteria for each included study in the 'Characteristics of included studies' table. Clearly, since the included studies used different criteria, the trials randomised participants at different levels of risk. Elevated troponin levels (Antman 1996; Galvani 1997; Lindahl 1996) or ECG changes (Cannon 1997) forebode a worse prognosis for unstable angina and non‐ST elevation myocardial infarction (UA/NSTEMI). As such, trials that recruited these participants could be expected to exhibit higher event rates. For example, the VINO study, which had the highest mortality rate (26.8% per year of follow‐up) and the Italian Elderly ACS study, which had the second highest mortality rate (13.8% per year follow‐up), randomised participants who had chest pain, ECG changes and elevated cardiac markers; whereas in TACTICS‐TIMI 18, 27% of the trial participants had accelerating or prolonged chest pain with a history of CAD as the sole entry criteria. Additionally, the Italian Elderly ACS only included participants who were 65 years of age or older, with the attendant increased morbidity and mortality that comes with aging. In contrast, the entry criteria for the RITA‐3 study were explicitly aimed at intermediate‐risk participants and the OASIS 5 substudy exclusively recruited female participants.
Interventions
Within the routine invasive strategy, all participants were randomised to receive angiography, regardless of symptom status. In contrast, with the conservative/'selective invasive' strategy, angiography only was performed in participants with clinical or investigational evidence of ischaemia. It is important to note that angiography is a component of both strategies, and that angiography in the conservative arm did not represent a 'cross‐over', as long as it was preceded by myocardial ischaemia or evidence of CAD.
Time to interventions
The times to angiography after randomisation in the routine invasive arms were: mean 6.2 hours in VINO, median 22 hours in TACTICS‐TIMI 18, median 23 hours in ICTUS, mean 24 hours in the Italian Elderly ACS, median two days in RITA‐3, median 51 hours in OASIS 5 and mean four days in FRISC‐II. The invasive strategy in the LIPSIA‐NSTEMI trial included both an immediate invasive strategy and an early invasive strategy with respective mean randomisation to sheath insertion times of 1.1 and 18.3 hours.
The FRISC‐II trial authors cited observational data to justify delayed angiography and postulated that a period of "plaque passivation" prior to angiography would be beneficial. However, the ISAR‐COOL trial subsequently compared an 'early invasive' (angiography within six hours of randomisation) to 'delayed invasive' (angiography in three to five days) strategy in UA/NSTEMI patients and found that early angiography produced superior outcomes. Since that time, a number of RCTs have evaluated the optimal timing of an invasive approach (ABOARD; ELISA; LIPSIA‐NSTEMI; OPTIMA; TIMACS; Zhang 2010). However, at present, there is insufficient evidence to indicate the optimal time for invasive management in general populations and such a discussion is beyond the scope of this Cochrane review.
Criteria for ischaemia
There were important differences between the included trials in the criteria for ischaemia that mandated angiography within the selectively invasive arm. In particular, the FRISC‐II criteria were widely criticised for being more stringent than those of the other studies, thereby exaggerating any benefit conferred by the invasive strategy. Furthermore, FRISC‐II did not utilise nuclear imaging or pharmacologic stress testing in its selectively invasive strategy arm. Indeed, application of the FRISC‐II criteria to the VANQWISH 1998 study, which recruited similar participants, suggests that significant CAD was under‐detected in the selectively invasive arm of the FRISC‐II study (Goyal 2002). Similarly, the LIPSIA‐NSTEMI trial applied strict criteria for intervention in the conservative arm, including evidence of refractory ischaemia by clinical, ECG and stress testing, and an ejection fraction (EF) via echocardiography of less than 45%. Consequently, 70% of those randomised to the selectively invasive arm underwent an invasive strategy (versus 84% amongst those randomised to receive an invasive strategy). Conversely, the Italian Elderly ACS did not require an objective ischaemia measure and relied on symptoms alone. This resulted in lower rates of invasive management in the conservative arm (31% versus 58% amongst those randomised to an invasive strategy).
Outcomes
Commonly reported outcomes included death, myocardial infarction (MI) and recurrent angina. Death was reported as all‐cause mortality. The definition of MI varied between the included studies, but included a combination of chest pain, ECG changes and elevated cardiac biomarkers. Not all studies reported peri‐percutaneous coronary intervention (peri‐PCI) cardiac biomarker leaks without other criteria as an endpoint, but were included as a safety outcome where data were available. We have summarised the variable definitions of MI in Table 4, which show that some studies required clinical or ECG changes, or both, for MI endpoints, whereas others only required an increased cardiac marker. Importantly, the ICTUS and LIPSIA‐NSTEMI trial protocols mandated the routine measurement of creatine kinase‐myocardial band (CK‐MB) after PCI, and peak levels constituted the endpoint of MI for both and the primary outcome for the LIPSIA‐NSTEMI trial. Such an assessment has the benefit of allowing quantification of overall myocardial necrosis, including that associated with the intervention itself. However, the significance of peri‐PCI biomarker leaks is a subject of considerable debate (Bhatt 2005; Cutlip 2005).
The other included trials did not specify the routine measurement of CK‐MB after PCI per protocol. Fortunately the ICTUS trial authors reported 'spontaneous' and 'peri‐procedural' MI as separate endpoints (de Winter 2005; Hirsch 2007; Windhausen 2007b). Extraction of data from ICTUS, which combined spontaneous and procedural MI into a single MI endpoint, caused significant heterogeneity in a previous version of this meta‐analysis (Hoenig 2010). Hence, to maximize consistency between trials, we analysed 'spontaneous' MI from the ICTUS trial with our MI endpoint and reported peri‐procedural MI as a safety endpoint. This minimised heterogeneity during meta‐analysis and also is justifiable since the significance of peri‐procedural biomarker leaks is still a subject of contention. Fortunately, endpoints such as death are indisputable. Follow‐up was six months in TACTICS‐TIMI 18, VINO and LIPSIA‐NSTEMI; one year in the Italian Elderly ACS; two years in the OASIS 5 substudy; three years for MI but four years for mortality in ICTUS; five years in FRISC‐II; and five years in RITA‐3. We have summarised the characteristics of the included studies in the 'Characteristics of included studies' table and in Table 3.
Risk of bias in included studies
We summarised the risk of bias of the included studies in the 'Characteristics of included studies' table, Figure 1 and Figure 2.
1.
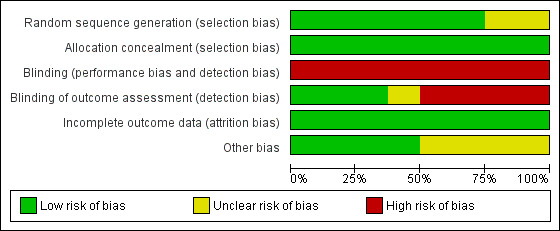
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
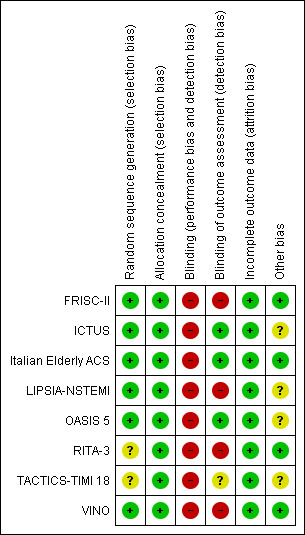
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged six of the included studies to be of low risk of bias as they generated the random sequence adequately (FRISC‐II; ICTUS; Italian Elderly ACS; LIPSIA‐NSTEMI; OASIS 5; VINO). Two trials are of unclear risk of bias for random sequence generation (RITA‐3; TACTICS‐TIMI 18) as they did not provide details on the method of randomisation.
All trials were judged to be of low risk of allocation concealment.
Blinding
All included studies were judged to be of high risk of performance bias. The blinding of outcome assessors was done in three trials (ICTUS; Italian Elderly ACS; OASIS 5) and they were therefore judged to be of low risk detection bias. One trial (TACTICS‐TIMI 18) was of unclear risk of bias in this domain and four trials (FRISC‐II; LIPSIA‐NSTEMI; RITA‐3; VINO) were judged to be of high risk of bias as outcome assessors were not blinded.
Incomplete outcome data
All trials were judged to be of low risk of attrition bias.
Other potential sources of bias
We judged four of the studies (FRISC‐II; Italian Elderly ACS; RITA‐3; VINO) to be at low risk of other biases and the other four studies (ICTUS; LIPSIA‐NSTEMI; OASIS 5; TACTICS‐TIMI 18) at unclear risk of bias.
Effects of interventions
The baseline participant characteristics were equivalent between the two randomised groups across all included studies. We analysed TACTICS‐TIMI 18, ICTUS and LIPSIA‐NSTEMI together in Analysis 2, since they involved the routine use of both glycoprotein IIb/IIIa receptor antagonists and stents. Analysis 3 included studies that only used stenting routinely, and included RITA‐3, FRISC‐II, the Italian Elderly ACS, the OASIS 5 substudy and VINO. Since the trials reported outcomes after different durations of follow‐up, we categorised the endpoints for meta‐analysis as being index, early, intermediate or late. 'Index' endpoints indicate follow‐up over the course of the initial hospitalisation. 'Early' endpoints indicate a follow‐up of up to four months. 'Intermediate' endpoints indicate a follow‐up from six to 12 months. 'Late' endpoints indicate a follow‐up greater than or equal to two years. In studies that supplied endpoints at various time points within a given category, we used the latest follow‐up outcomes. For example, if a trial provided outcomes at six and 12 months of follow‐up, we used the 12‐month data for intermediate analysis. In the 'Summary of findings' table, we reported an all‐study analysis that involved all included studies for each outcome. This all‐study analysis was seen in studies that reported six to 12 months follow‐up period (i.e. intermediate endpoints).
Analysis 1: studies that deployed stents routinely in revascularisation procedures using PCI, regardless of glycoprotein IIb/IIIa receptor antagonist use
This analysis included all eight studies undertaken in the stent era, regardless of glycoprotein IIb/IIIa receptor antagonist use (FRISC‐II; ICTUS; Italian Elderly ACS; LIPSIA‐NSTEMI; OASIS 5 substudy; TACTICS‐TIMI 18; RITA‐3; VINO). The commonly reported outcomes for this analysis are presented in Summary of findings' table 1 (Table 1) and complications of angiography or revascularization in Summary of findings' table 2 (Table 2).
Death: all causes (index, early, intermediate, late)
Risk of index death significantly increased with a routine invasive versus conservative or selective invasive strategy (risk ratio (RR) 1.54, 95% confidence interval (CI) 1.02 to 2.34; six trials, 8094 participants; Analysis 1.1). Early (RR 1.18, 95% CI 0.70 to 2.00; four trials, 4345 participants; Analysis 1.2), intermediate (RR 0.87, 95% CI 0.64 to 1.18; eight trials, 8915 participants; Analysis 1.3) and late (RR 0.90, 95% CI 0.76 to 1.08; three trials, 5467 participants; Analysis 1.4) death were not significantly affected by an invasive strategy. There was no evidence of any heterogeneity in inter‐study effect sizes across all time points for this endpoint.
1.1. Analysis.
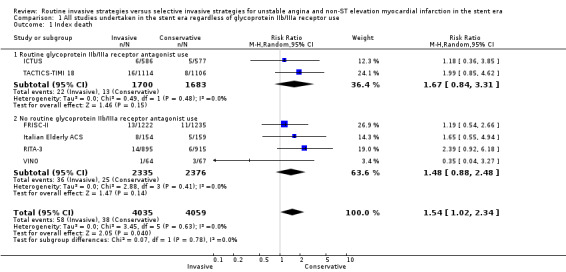
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 1 Index death.
1.2. Analysis.
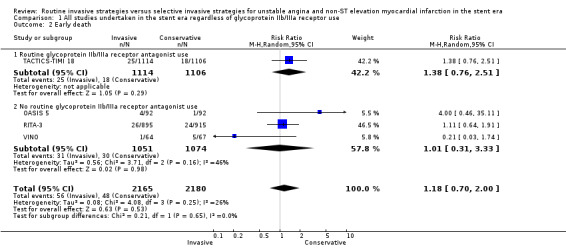
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 2 Early death.
1.3. Analysis.
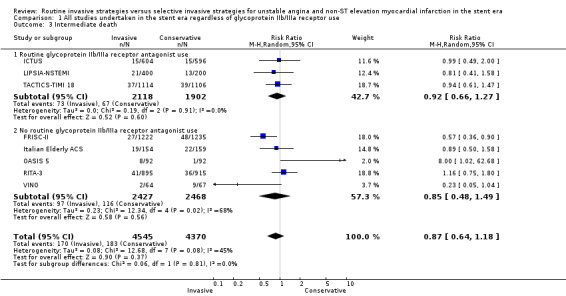
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 3 Intermediate death.
1.4. Analysis.
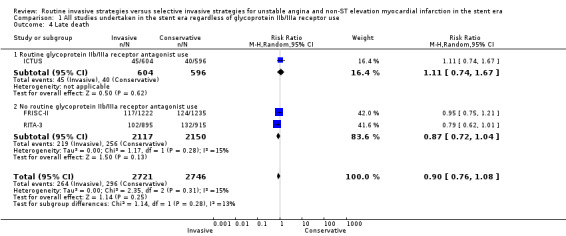
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 4 Late death.
The death rates standardised to years of study duration, shown in Table 3, were 1.1% to 2.8% per year for the OASIS 5 substudy, RITA‐3, FRISC‐II and ICTUS; whereas TACTICS‐TIMI 18 had a rate of 7%; the Italian Elderly ACS study and LIPSIA‐NSTEMI trial 13% to 14%; and VINO had a rate of 27%. For the most part, the levels of risk were concordant with the inclusion criteria employed by each study, as described in the 'Characteristics of included studies' table, with the exception of ICTUS. As already discussed, mortality increases as troponin concentrations increase in patients with ACS (Antman 1996). The ICTUS trial exclusively enrolled participants with a troponin T (TnT) greater than 0.03 ng/mL and, as such, would be expected to observe a higher mortality rate. Indeed, in TACTICS‐TIMI 18, the six‐month mortality rate for participants with a TnT greater than 0.01 ng/mL was 4% (Morrow 2001). Since the ICTUS trial recruited participants with a TnT greater than 0.03 ng/mL and had a longer duration of 12 months, the standardised mortality would be expected to be greater than 4%. Indeed, in FRISC‐II, participants with a TnT value greater than 0.03 ng/mL had a 12‐month mortality rate of 4.2% (Diderholm 2002). Hence, the ICTUS participants appear to have experienced a lower than expected event rate, based upon the event rates reported for other included trials. Differences between trials in baseline medical therapy do not appear to explain why mortality in the ICTUS trial was less than the other trials.High rates of background medical therapy seen in both ICTUS and TACTICS‐TIMI 18. This observation highlights the importance of global risk stratification over the selection of a single high‐risk characteristic when predicting the risk of future events.
MI (index, early, intermediate, late)
The incidence of MIs during the index hospitalisation was not significantly affected by an invasive strategy (RR 1.08, 95% CI 0.65 to 1.80; seven trials, 8694 participants; Analysis 1.5), though significant heterogeneity was identified at this time point (P = 0.003, I² statistic = 70%). Possible reasons for this heterogeneity include the use of glycoprotein IIb/IIIa receptor antagonists in TACTICS‐TIMI 18 and the unique definition of MI the VINO trial authors used, which excluded any events within the first 72 hours of randomisation (Table 4). Both early (RR 0.68, 95% CI 0.43 to 1.08; four trials, 4345 participants; Analysis 1.6) and intermediate (RR 0.79, 95% CI 0.63 to 1.00; eight trials, 8915 participants; Analysis 1.7) MI endpoints revealed a non‐significant trend towards reduction with an invasive strategy. Interestingly, the previous version of this review, Hoenig 2010. identified statistically significant reductions at the early and intermediate endpoints associated with an invasive versus conservative strategy, so that the current analysis represents a reduced effect. Late MI rates were drawn from three studies with these data — FRISC‐II (five years), RITA‐3 (five years) and ICTUS (three years) — and as in the previous meta‐analysis, they remained significantly decreased in those treated invasively (RR 0.78, 95% CI 0.67 to 0.92; three trials, 5467 participants; Analysis 1.8).
1.5. Analysis.
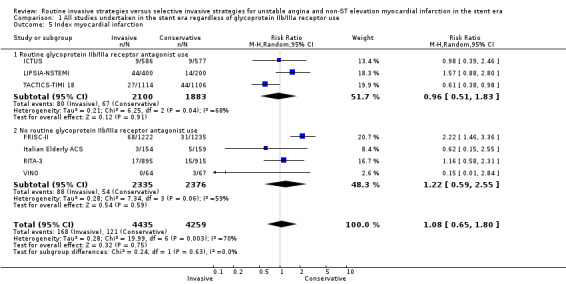
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 5 Index myocardial infarction.
1.6. Analysis.
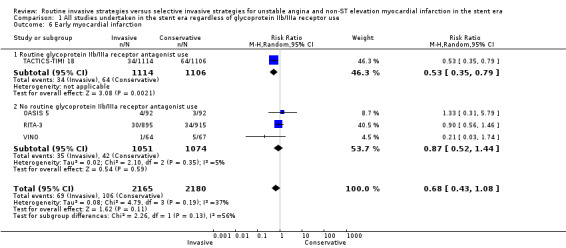
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 6 Early myocardial infarction.
1.7. Analysis.
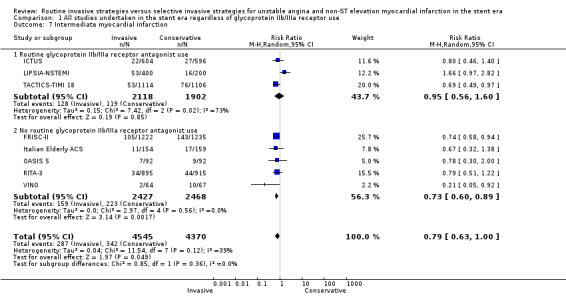
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 7 Intermediate myocardial infarction.
1.8. Analysis.
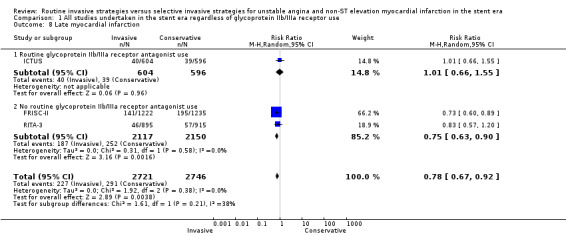
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 8 Late myocardial infarction.
Death (all causes) or non‐fatal MI (index, early, intermediate, late)
Index death or non‐fatal MI, as a composite outcome, was not decreased in those treated via a routine invasive approach (RR 1.14, 95% CI 0.59 to 2.21; four trials, 6618 participants; Analysis 1.9); however, we noted significant heterogeneity (P = 0.001, I² statistic = 81%) and possible reasons include those already discussed for components of the composite outcome. Early death or non‐fatal MI, based on 30‐day TACTICS‐TIMI 18 data and VINO data, was significantly decreased with an invasive strategy (RR 0.64, 95% CI 0.45 to 0.92; two trials, 2351 participants; Analysis 1.10). We observed a trend towards a decreased incidence of intermediate death or non‐fatal MI, again as a composite outcome, with the routine invasive strategy and included data from all included studies except for ICTUS (RR 0.93, 95% CI 0.71 to 1.20; seven trials, 7715 participants; Analysis 1.11). Again, as was the case for the intermediate MI component of this composite outcome, we noted heterogeneity (P = 0.007, I² statistic = 66%). As with intermediate MI alone (see the previous paragraph), this represents a loss of significance relative to the previous version of this review (Hoenig 2010), due to the influence of the OASIS 5 substudy and LIPSIA‐NSTEMI studies. Late death or non‐fatal MI was not significantly decreased (RR 0.89, 95% CI 0.73 to 1.08; three trials, 5467 participants; Analysis 1.13). The late follow‐up for this composite endpoint was perhaps less important, given the independent benefit observed for the MI endpoint at late follow‐up and the 'dilution' of this effect observed with incorporation of mortality into a composite outcome.
1.9. Analysis.
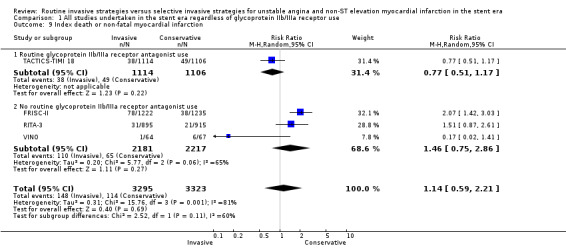
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 9 Index death or non‐fatal myocardial infarction.
1.10. Analysis.
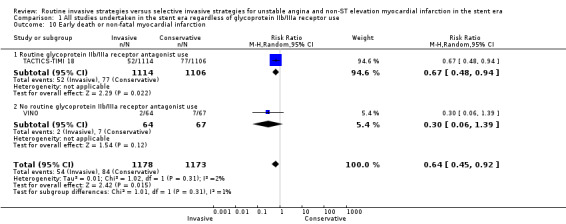
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 10 Early death or non‐fatal myocardial infarction.
1.11. Analysis.
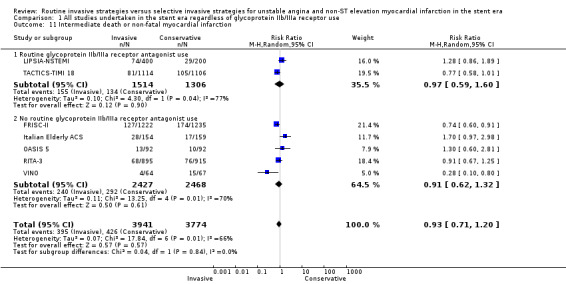
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 11 Intermediate death or non‐fatal myocardial infarction.
1.13. Analysis.
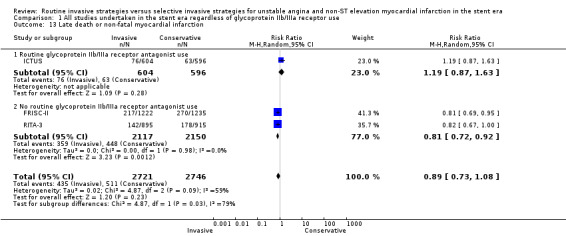
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 13 Late death or non‐fatal myocardial infarction.
Four included studies reported gender‐specific data for males and five studies reported it for females, and subanalysis of intermediate death or non‐fatal MI demonstrated a statistically‐significant benefit of a routine invasive strategy only in males (male: RR 0.73, 95% CI 0.62 to 0.87; four trials, 4454 participants; female: RR 0.87, 95% CI 0.65 to 1.16; five trials, 2521 participants; Analysis 1.12). Late (five‐year) follow‐up from the FRISC‐II trial also showed that the invasive strategy only significantly benefited males (RR 0.70, 95% CI 0.59 to 0.86). These subgroup analyses should be interpreted with caution, and we have explored them further in the Discussion.
1.12. Analysis.
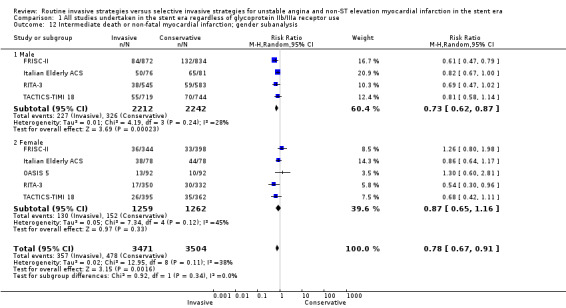
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 12 Intermediate death or non‐fatal myocardial infarction; gender subanalysis.
Refractory angina (early, intermediate)
An invasive strategy decreased early refractory angina, based upon four‐month data from RITA‐3 (RR 0.47, 95% CI 0.32 to 0.68). Intermediate refractory angina was significantly decreased using a routine invasive strategy (RR 0.64, 95% CI 0.52 to 0.79; five trials, 8287 participants; Analysis 1.14), although we found significant heterogeneity at this time point (P < 0.0003, I² statistic = 81%), which was driven by the ICTUS results. The null effect for this endpoint in ICTUS was surprising, given that this study recruited only troponin‐positive participants. Indeed, retrospective analysis of troponin‐positive participants from the TACTICS‐TIMI 18 trial revealed that 94% of troponin‐positive participants had significant angiographic CAD, 79% of whom were revascularised (PCI or coronary artery bypass grafting (CABG)) during the index hospitalisation (Dokainish 2005). Hence, the trial participants in ICTUS would be expected to exhibit high rates of angiographic CAD and to experience considerable symptomatic improvement with an invasive strategy. One possible explanation for this difference in outcomes is that 20% of the participants enrolled in ICTUS underwent PCI or CABG prior to randomisation, which potentially resulted in artificially‐improved outcomes in the conservative arm as these participants likely had the most to gain from a routine invasive strategy.
1.14. Analysis.
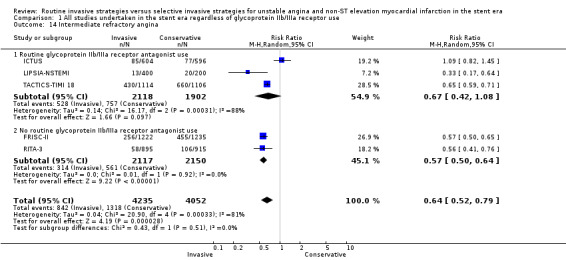
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 14 Intermediate refractory angina.
Rehospitalisation for ACS (early, intermediate, late)
The invasive strategy was associated with a significantly decreased rate of rehospitalisation at the intermediate time point (RR of 0.77, 95% CI 0.63 to 0.94; six trials, 6921 participants; Analysis 1.15), albeit with significant heterogeneity (P = 0.05, I² statistic = 54%). ICTUS provided late follow‐up on rehospitalisation at three years, at which point no significant benefit persisted (RR 0.79, 95% CI 0.56 to 1.12). This attenuation of earlier significance was unsurprising, when we considered the narrowing in the difference in revascularisation rates between the two strategies in ICTUS, from a 36% difference at initial hospitalisation between revascularisation in the routine invasive versus conservative or selective invasive strategies, to only 23% at the termination of follow‐up.
1.15. Analysis.
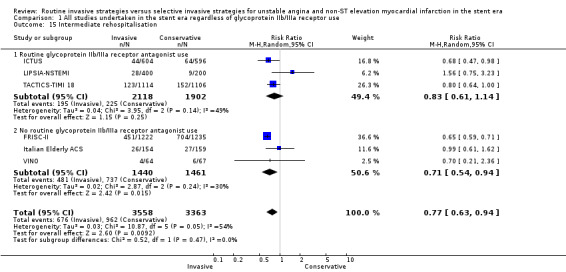
Comparison 1 All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use, Outcome 15 Intermediate rehospitalisation.
Analysis 2: routine use of both stents and glycoprotein IIb/IIIa receptor antagonists in revascularisation procedures using PCI
This analysis examined routine use of both stents and glycoprotein IIb/IIIa receptor antagonists, and included three trials (ICTUS; LIPSIA‐NSTEMI; TACTICS‐TIMI 18).
Death: all causes (index, early, intermediate, late)
There was no difference between the treatment strategies at any of the time points assessed. Data from TACTICS‐TIMI 18 and ICTUS at hospitalisation (for index death) and from TACTICS‐TIMI 18 at 30 days (for early death) exhibited a trend toward increased index death (RR 1.67, 95% CI 0.84 to 3.31; two trials, 3383 participants; Analysis 1.1) and early death (RR 1.38, 95% CI 0.76 to 2.51; one trial, 2220 participants; Analysis 1.2) in the invasive arm, but this did not reach statistical significance. Intermediate death was no different between the treatment strategies when we combined six‐month data from the LIPSIA‐NSTEMI trial and TACTICS‐TIMI 18 and 12‐month data from ICTUS (RR 0.92, 95% CI 0.66 to 1.27; three trials, 4020 participants; Analysis 1.3). In TACTICS‐TIMI 18, a routine invasive strategy did not reduce the risk of death, even in higher‐risk participants with troponin I (TnI) levels greater than 0.1 ng/mL. Late follow‐up from ICTUS (four years) revealed no benefit of a routine invasive strategy on the death endpoint at late follow‐up (RR 1.11, 95% CI 0.74 to 1.67; one trial, 1200 participants; Analysis 1.4).
MI (index, early, intermediate, late)
Based on the LIPSIA‐NSTEMI, TACTICS‐TIMI 18 and ICTUS data, the routine invasive strategy exhibited no significant difference in MI rate during the index hospitalisation (RR 0.96, 95% CI 0.51 to 1.83; three trials, 3983 participants; Analysis 1.5). Hence, there did not appear to be an early hazard to an invasive strategy when glycoprotein IIb/IIIa receptor antagonists were used upstream of PCI. Early MI was reduced by an invasive strategy, based on TACTICS‐TIMI 18 data at 30 days (RR 0.53, 95% CI 0.35 to 0.79; one trial, 2220 participants; Analysis 1.6). Intermediate MI was unaffected by an invasive strategy using data for spontaneous MI from LIPSIA‐NSTEMI, ICTUS and TACTICS‐TIMI 18 (RR 0.95, 95% CI 0.56 to 1.60; three trials, 4020 participants; Analysis 1.7). As already discussed, the TACTICS‐TIMI 18 trial authors did not routinely measure CK‐MB post‐PCI (Table 4). Late follow‐up from ICTUS (three years) demonstrated no benefit of an early invasive strategy on the rate of spontaneous MI (RR 1.01, 95% CI 0.66 to 1.55; one trial, 1200 participants; Analysis 1.8).
Death (all causes) or non‐fatal MI (index, early, intermediate, late)
Data for this composite endpoint at index and early (30‐day) time points were only available from TACTICS‐TIMI 18. There was no difference between the treatment strategies at index admission (RR 0.77, 95% CI 0.51 to 1.17; one trial, 2220 participants; Analysis 1.9). However, the invasive strategy was associated with significant early benefit (RR 0.67, 95% CI 0.48 to 0.94; one trial, 2220 participants; Analysis 1.10). Baseline troponin levels were available from 1826 of 2220 trial participants, and these data formed the basis for the prespecified subgroup analysis based on TnT levels greater than (troponin positive) or less than (troponin negative) 0.01 ng/mL. Upon subgroup analysis, the early (30 day) benefit of a routine invasive strategy only achieved statistical significance in troponin‐positive participants (RR 0.50, 95% CI 0.32 to 0.79). Troponin‐negative participants received no significant benefit at 30‐days follow‐up (RR 0.95, 95% CI 0.44 to 2.06), although this CI overlapped with those of troponin‐positive participants. Although the TACTICS‐TIMI 18 trial authors prespecified this subgroup analysis based on troponin, it should nevertheless be interpreted with caution.
Contrary to the early results, at intermediate (six‐month) follow‐up, adoption of a routine invasive strategy yielded no benefit (RR 0.97, 95% CI 0.59 to 1.60; two trials, 2820 participants; Analysis 1.11). The results of this subgroup analysis changed when the TACTICS‐TIMI 18 authors used a different cardiac biomarker. With subgroup analysis based on a TnI cut‐off of 0.1 ng/mL, troponin‐positive participants showed early (30 day) and intermediate (six month) benefits of an invasive strategy, with a RR of 0.47 (95% CI 0.30 to 0.73) and a RR of 0.67 (95% CI 0.47 to 0.96) respectively. The TACTICS‐TIMI 18 authors prespecified such subgroup analysis based on troponin, but should nevertheless be interpreted with caution. The ICTUS trial suggested no benefit of a routine invasive strategy at late follow‐up regardless of baseline risk (RR 1.19, 95% CI 0.87 to 1.63; one trial, 1200 participants; Analysis 1.13); we have explored this further in the Discussion.
Analysis 3: routine stent use in revascularisation procedures using PCI with selective glycoprotein IIb/IIIa receptor antagonist use
This analysis included five trials (FRISC‐II; Italian Elderly ACS; the OASIS 5 substudy; RITA‐3, VINO).
Death: all causes (index, early, intermediate, late)
There was a non‐significant trend towards increased death rate at index hospitalisation (RR 1.48, 95% CI 0.88 to 2.48; four trials, 4711 participants; Analysis 1.1) and no effect on early death (RR 1.01, 95% CI 0.31 to 3.33; three trials, 2125 participants; Analysis 1.2) in the invasive strategy group. Intermediate death at six to 12 months was not significantly improved by an invasive strategy (RR 0.85, 95% CI 0.48 to 1.49; five trials, 4895 participants; Analysis 1.3). However, we noted significant heterogeneity was noted (P=0.02, I² statistic = 68%). This may have been driven by the stringent criteria set by the FRISC‐II group to define failure of conservative therapy; and by the large benefit of an invasive strategy observed in the small VINO study, which randomised patients with the highest death rates of all five studies (Table 3). The FRISC‐II trial authors undertook subgroup analysis based on the presence of TnT greater than or less than 0.03 ng/mL and the presence of ST depression on the admission ECG. Mortality assessed at one year was not affected by an invasive strategy in this retrospective analysis, even in the group of participants with both TnT greater than 0.03 ng/mL and ST depression, although the numbers of participants may have been too small to detect any difference. Only FRISC‐II and RITA‐3 provided follow‐up data for late death at five years, and was not significantly improved by an invasive strategy (RR 0.87, 95% CI 0.72 to 1.04; two trials, 4267 participants; Analysis 1.4).
MI (index, early, intermediate, late)
There were no differences in index MI rates between the two strategies (RR 1.22, 95% CI 0.59 to 2.55; four trials, 4711 participants; Analysis 1.5), although we found significant heterogeneity (P = 0.06, I² statistic = 59%). The FRISC‐II data show a significant hazard for this endpoint in the routine invasive group (RR 2.22, 95% CI 1.46 to 3.36). Importantly, the four studies in this analysis did not undertake routine cardiac biomarkers measurements post‐PCI, as the ICTUS and LIPSIA‐NSTEMI did, and used clinical symptoms as a diagnostic criterion (Table 4). Significant heterogeneity may be due to the distinct VINO definition of MI, which excluded events within 72 hours of randomisation when calculating this endpoint. Early MI, based on 30‐day VINO and OASIS 5 data, and four‐month RITA‐3 data, was not significantly altered by a routine invasive strategy (RR 0.87, 95% CI 0.52 to 1.44; three trials, 2125 participants; Analysis 1.6). Intermediate (six‐month data from VINO and 12‐month data from the Italian Elderly ACS, FRISC‐II, OASIS 5 and RITA‐3 studies) and late MI (five‐year FRISC‐II and RITA‐3 data) significantly decreased with a routine invasive strategy (RR 0.73, 95% CI 0.60 to 0.89; five trials, 4895 participants; Analysis 1.7; and RR 0.75, 95% CI 0.63 to 0.90; two trials, 4267 participants; Analysis 1.8, respectively).
Death (all causes) or non‐fatal MI (index, early, intermediate, late)
Death or non‐fatal MI at index hospitalisation did not differ between strategies (RR 1.46, 95% CI 0.75 to 2.86; three trials, 4398 participants; Analysis 1.9). Notably, this contrasts with the FRISC‐II data, from which a significant hazard of the routine invasive strategy was identified for this endpoint (RR 2.07, 95% CI 1.42 to 3.03). There was no significant benefit with a routine invasive strategy with respect to early death or non‐fatal MI based on 30‐day VINO data (RR 0.30, 95% CI 0.06 to 1.39; one trial, 131 participants; Analysis 1.10). Similarly, there was no difference between strategies at the intermediate time point (RR 0.91, 95% CI 0.62 to 1.32; five trials, 4895 participants; Analysis 1.11) with analysis of data from FRISC‐II, RITA‐3, OASIS 5 and Italian Elderly ACS, in addition to VINO. However, drawing on five‐year results from FRISC‐II and RITA‐3, we noted a significant benefit for this composite outcome with a routine invasive strategy (RR 0.81, 95% CI 0.72 to 0.92; two trials, 4267 participants; Analysis 1.13).
The FRISC‐II data revealed that the intermediate (six to 12‐month) benefit of a routine invasive strategy was only significant in participants with ST depression at entry, who exhibited a RR of 0.66 (95% CI 0.50 to 0.88). There was no benefit from a routine invasive strategy in participants without ST depression, although such retrospective subgroup analysis should be interpreted with caution. Furthermore, FRISC‐II troponin subgroup analysis identified a RR of 0.71 (95% CI 0.53 to 0.93) at 12 months in troponin‐positive participants (TnT greater than 0.1 ng/mL), whereas participants with a TnT of less than 0.1 ng/mL only trended towards benefit with a RR of 0.77 (95% CI 0.53 to 1.11). Again, the CIs of these subgroup analyses overlap and the results should be regarded with caution. In a separate report, the FRISC‐II trial authors undertook subgroup analysis based on the presence of TnT greater than versus less than 0.03 ng/mL and the presence of ST depression on admission ECG. The intermediate (one‐year) death or non‐fatal MI endpoint was only significantly decreased in the group of participants with both TnT greater than 0.03 ng/mL and ST depression greater than 0.1 mV (RR 0.60, 95% CI 0.43 to 0.82). Likewise, the FRISC‐II trial authors stratified participants by FRISC score when they reported late (five‐year) outcomes for this endpoint. We have explored these findings in the Discussion.
Safety endpoints
Procedure‐related MI
Analysis of data from FRISC‐II, RITA‐3, ICTUS,Italian Elderly ACS and LIPSIA‐NSTEMI showed that the invasive strategy was associated with an increased risk of procedure‐related MI (RR 1.87, 95% CI 1.47 to 2.37; five trials, 6380 participants; Analysis 2.1). We did not identify any heterogeneity, despite the different diagnostic criteria used for MI: routine measurement of CK‐MB post‐PCI in ICTUS and LIPSIA‐NSTEMI; and the FRISC‐II, RITA‐3 and Italian Elderly ACS studies also included either clinical or ECG criteria, or both, to define MI (Table 4). As already discussed, the significance of a peri‐procedural cardiac biomarker leak is the subject of considerable debate, but can be modified by background medications, including use of glycoprotein IIb/IIIa receptor antagonists (Cutlip 2005). Notably, the increased rate of procedure‐related MI seen in participants subjected to a routine invasive strategy did not translate into any increased long‐term mortality.
2.1. Analysis.
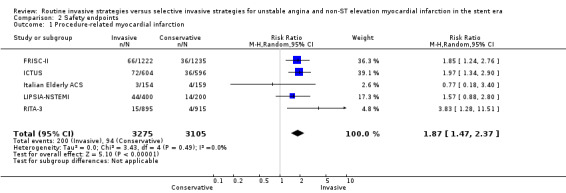
Comparison 2 Safety endpoints, Outcome 1 Procedure‐related myocardial infarction.
Bleeding
The invasive strategy was associated with an increased risk of bleeding (RR 1.73, 95% CI 1.30 to 2.31; six trials, 7584 participants; Analysis 2.2), although we noted considerable variability in bleeding definitions between the included studies that reported this endpoint (FRISC‐II; ICTUS,Italian Elderly ACS; LIPSIA‐NSTEMI; OASIS 5; RITA‐3). Numerous studies of people with UA/NSTEMI have identified major bleeding as a harbinger of a poor ultimate outcome. The ICTUS trial authors reported major bleeding, which was defined as: fatal bleeding, intracranial bleeding, need for transfusion, a decrease in haemoglobin by 4.8 g/dL, or bleeding causing haemodynamic compromise. Major bleeding occurred in 3.1% and 1.7% (P = not significant) of participants randomised to a routine invasive versus conservative strategy, respectively, during the initial hospitalisation. On four‐year follow‐up, mortality was 18.6% in the 29 participants with major bleeding during initial hospitalisation, versus just 7.5% in the 1171 participants without an in‐hospital major bleed (RR 2.68, 95% CI 1.08 to 6.61).
2.2. Analysis.
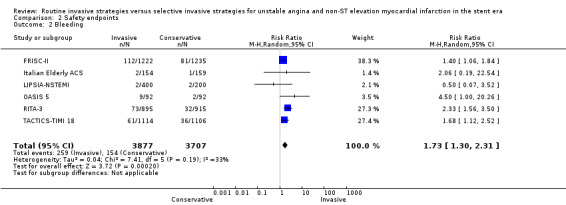
Comparison 2 Safety endpoints, Outcome 2 Bleeding.
Stroke
Data from five trials revealed no statistically‐significant hazard for stroke with a routine invasive strategy (RR 0.84, 95% CI 0.38 to 1.86) (ICTUS; Italian Elderly ACS; LIPSIA‐NSTEMI; OASIS 5; TACTICS‐TIMI 18).
Contrast reactions
Typically, 1% of participants assigned to an invasive strategy experienced a contrast allergy. The rate in the conservative strategy depended on the proportion that underwent subsequent angiography, and this depended upon the population risk level. Contrast‐induced renal failure was not reported; however, this outcome can be modified by the patient's baseline renal function, hydration status and administration of sodium bicarbonate.
Sensitivity analysis
We chose a random‐effects model to analyse the results, as it provides a more conservative estimate of effect size in the presence of a small number of studies and variable risk levels among randomised participants. Table 3 highlights important differences between the included studies, which guided our choice of sensitivity analysis based on the exclusion of certain studies. We did not subject recurrent angina and rehospitalisation endpoints to sensitivity analysis, because RR estimates were the most consistent and robust findings of this meta‐analysis and, in general, were not associated with significant heterogeneity.
Time to angiography
As previously discussed, time to angiography in the invasive arm could influence outcomes, and optimal timing remains unclear (Navarese 2013). Indeed, the ISAR‐COOL study found that, in participants with UA/NSTEMI, a 'delayed invasive' strategy with angiography three to five days postrandomisation approximately doubled the risk of death or non‐fatal MI over that observed in participants with an 'early invasive' strategy in whom angiography was performed within six hours of randomisation. The excess events in the late invasive arm occurred prior to angiography; this was observed despite background anti‐thrombotic therapy which included aspirin, clopidogrel, tirofiban and heparin. Notably, this study randomised a high risk population with roughly two thirds of participants positive for troponin and ST depression on ECG (ISAR‐COOL). We have presented the times to angiography in the 'Characteristics of included studies' table and can be categorised into an 'immediate invasive', 'early invasive' or 'delayed invasive' strategy. The ICTUS, TACTICS‐TIMI 18, VINO and Italian Elderly ACS studies generally employed angiography within 24 hours of randomisation; whereas in the FRISC‐II, OASIS 5 and RITA‐3 studies, angiography was typically delayed for at least two days.
The increasing interest in timing of invasive strategies has resulted in more considered comparisons of timing to determine optimal management. The most recently performed study of the included clinical trials, the LIPSIA‐NSTEMI trial, randomised participants to immediate, early and selective invasive approaches with median times from randomisation to angiography of 1.1, 18.6 and 67.2 hours respectively.
Mortality rates In the conservative arm
We have presented mortality rates in Table 3 as the mortality rate in the conservative arm divided by the number of years of follow‐up. The OASIS 5 substudy, ICTUS, FRISC‐II and RITA‐3 had mortality rates of 1.1% to 2.8% per year of follow‐up, while TACTICS‐TIMI 18 had a rate of 7%, the Italian Elderly ACS and LIPSIA‐NSTEMI trials had rates between 13% and 14%, and VINO had a rate of 27%. Hence, we analysed the data for OASIS 5, ICTUS, FRISC‐II and RITA‐3 separately, and the data for TACTICS‐TIMI 18, the Italian Elderly ACS, LIPSIA‐NSTEMI and VINO. When we analysed the high‐mortality and low‐mortality rate studies separately, the previously‐reported findings were significantly different, which is likely due to the inclusion of the more recent Italian Elderly ACS and LIPSIA‐NSTEMI studies. These studies, as we will discuss below, included participant cohorts of high‐risk for interventional complications.
Percentage of trial participants with a positive troponin
Findings on subgroup analysis suggest that a positive troponin may identify high‐risk patients likely to experience a particular benefit with a routine invasive strategy. While the VINO, ICTUS and LIPSIA‐NSTEMI trials only recruited participants with positive cardiac biomarkers, the percentage of biomarker‐positive participants in the Italian Elderly ACS, OASIS 5 substudy, FRISC‐II, RITA‐3 and TACTICS‐TIMI 18 studies ranged between 50% and 80% (Table 3). We analysed the studies that only randomised biomarker‐positive participants separately (ICTUS; LIPSIA‐NSTEMI; VINO) and exhibited a null effect for mortality at all time points. However, this finding should not undermine the potential hazards of a routine invasive strategy and the importance of risk stratification to select high‐risk patients who may experience meaningful benefits that outweigh any potential harm.
CABG as a mode of revascularization in the invasive arm
We have described the rates of CABG as a mode of revascularisation in the invasive arms in Table 3. The OASIS 5 substudy, ICTUS and TACTICS‐TIMI 18 identified rates of approximately 20%, while RITA‐3, FRISC‐II and VINO had rates of approximately 40%. Consistent with international UA/NSTEMI management guidelines (Hamm 2011; Jneid 2012) and subsequent increasing use of PCI, we observed the lowest CABG rates in the two studies with the most recent recruitment of participants, Italian Elderly ACS and LIPSIA‐NSTEMI, with rates of 7% and 10% respectively. Performing a sensitivity analysis on the basis of high or low rates of CABG in the invasive arm used the same data already utilised in Analyses 2 and 3; hence, the findings were identical to those already described.
Difference in revascularisation rates between the treatment arms
We have presented the absolute percentage differences in revascularisation rate between the routine invasive and conservative arms of each trial in Table 3. The Italian Elderly ACS, FRISC‐II and VINO exhibited higher absolute differences in revascularisation rate (27% to 39%) relative to the other trials (14% to 23%). When we pooled the former trials, we noted a non‐significant trend towards benefit with a routine invasive strategy at all time points except the index hospitalisation. Conversely, as the difference between rates of revascularisation narrows — as is seen for instance in the LIPSIA‐NSTEMI study, which had the highest invasive rate reported to date amongst participants randomised to a conservative management strategy (70%) — any benefit derived from a routine invasive strategy may diminish.
Discussion
Summary of findings
Eight randomised controlled trials (RCTs) with a total of 8915 participants (4545 invasive strategies, 4370 conservative strategies) were eligible for inclusion.
In the all‐study analysis, evidence did not show appreciable risk reductions in all‐cause mortality (RR 0.87, 95% CI 0.64 to 1.18; eight studies, 8915 participants; low quality evidence) and death or non‐fatal myocardial infarction (MI) (RR 0.93, 95% CI 0.71 to 1.2; seven studies, 7715 participants; low quality evidence) with routine invasive strategies compared to conservative (selective invasive) strategies at six to 12 months follow‐up. There was appreciable risk reduction in MI (RR 0.79, 95% CI 0.63 to 1; eight studies, 8915 participants; moderate quality evidence), refractory angina (RR 0.64, 95% CI 0.52 to 0.79; five studies, 8287 participants; moderate quality evidence) and rehospitalisation (RR 0.77, 95% CI 0.63 to 0.94; six studies, 6921 participants; moderate quality evidence) with routine invasive strategies compared to conservative (selective invasive) strategies also at six to 12 months follow‐up.
There were increased risks in bleeding (RR 1.73, 95% CI 1.3 to 2.31; six studies, 7584 participants; moderate quality evidence) and procedure‐related MI (RR 1.87, 95% CI 1.47 to 2.37; five studies, 6380 participants; moderate quality evidence) with routine invasive strategies compared to conservative (selective invasive) strategies. Low quality evidence was as a result of serious risk of bias and imprecision in the estimate of effect, while moderate quality evidence was only due to serious risk of bias.
The risk of index death (during the initial hospitalisation for unstable angina and non‐ST elevation myocardial infarction (UA/NSTEMI)) was high when an invasive strategy was adopted from the outset, with a RR of 1.54 (95% CI 1.02 to 2.34). However, early death (less than four months), intermediate death (six to 12 months) and late death (four to five years) were not influenced by management strategy. Though index MI was not significantly improved with an invasive strategy, we identified significant heterogeneity within this analysis, possibly driven by the different levels of risk, different rates of background medical therapies and different criteria for ischaemia in the studies included in the analysis. Early MI data from trials that routinely used glycoprotein IIb/IIIa receptor antagonists revealed a significant benefit of a routine invasive strategy (RR 0.53, 95% CI 0.35 to 0.79), though in the all‐study combined analysis, this failed to achieve statistical significance (RR 0.68, 95% CI 0.43 to 1.08).
In comparison to previous versions of this Cochrane review (Hoenig 2006; Hoenig 2010), the inclusion of OASIS 5, Italian Elderly ACS and LIPSIA‐NSTEMI studies resulted in the loss of significant benefit in the all‐study analysis of a routine invasive approach at the intermediate MI endpoint (RR 0.79, 95% CI 0.63 to 1.00). However, we observed a significant difference between strategies at the endpoint with the exclusion of the studies employing routine glycoprotein IIb/IIIa (RR 0.73, 95% CI 0.60 to 0.89). With a routine invasive strategy, the significant reduction observed in late MI remained unchanged (RR 0.78, 95% CI 0.67 to 0.92), driven by studies that did not employ routine glycoprotein IIb/IIIa strategy (RR 0.75, 95% CI 0.63 to 0.90).
Regarding the composite endpoint of death or non‐fatal MI, although a routine invasive approach was not beneficial at the time of index hospitalisation, we observed a significant benefit at the early time point (RR 0.64, 95% CI 0.45 to 0.92). The all‐study analysis of the this endpoint at the intermediate time point lost significance (RR 0.93, 95% CI 0.71 to 1.20). However, an unchanged benefit remained significant amongst males (RR 0.73, 95% CI 0.62 to 0.87). Late death or non‐fatal MI was unaffected by management strategy (RR 0.89, 95% CI 0.73 to 1.08). The studies that reported the death or MI endpoint suggest that any benefits of a routine invasive strategy were significant only in trial participants with high‐risk characteristics, primarily positive troponin or dynamic ischaemic electrocardiograph (ECG) changes on admission or secondary diabetes mellitus, renal insufficiency, reduced EF to less than 40%, early postinfarction angina, recent percutaneous coronary intervention (PCI), prior coronary artery bypass grafting (CABG), or intermediate to high Global Registry of Acute Coronary Events (GRACE) risk score (Hamm 2011). These markers of risk may have identified populations with higher event rates and, hence, enhanced the power to detect differences between the two strategies. The CIs between subgroups overlapped, and these findings from post‐hoc analyses should be interpreted with appropriate caution.
We observed a statistically‐significant benefit for both refractory angina and rehospitalisation (RR 0.64, 95% CI 0.52 to 0.79; and RR 0.77, 95% CI 0.63 to 0.94, respectively) at the intermediate time point, both driven by the benefit of an invasive strategy in studies that did not use routine glycoprotein IIb/IIIa inhibitors. However, the narrowing difference in revascularisation rates between the two strategies over time reduces the hospitalisation benefit longer term.
Regarding safety endpoints, the invasive strategy was associated with a 1.9‐fold increase in the RR of the variably defined procedurally‐related MI endpoint, as well as a 1.8‐fold increase in the RR of bleeding. This bleeding was mainly due to wound site bleeding, but was difficult to grade due to inter‐trial differences in the definition of bleeds and reporting of data. No increase risk of stroke was noted (RR 0.84, 95% CI 0.38 to 1.86).
Discussion of findings on subgroup analysis
Cardiac troponin status of participants
The baseline cardiac biomarker status of the patient serves as an important tool for risk stratification, though the ideal marker and definitions remain unclear and have evolved over time with advances in technology ( creatine kinase‐myocardial band (CK‐MB) versus troponin versus high‐sensitivity troponin). The TACTICS‐TIMI 18 trial had the prespecified intention of testing the 'troponin hypothesis': that is, to test whether benefit from an invasive strategy was limited to troponin‐positive participants. Data for the death or non‐fatal MI endpoint from TACTICS‐TIMI 18 and FRISC‐II suggest that only high‐risk participants with a positive troponin benefited from a routine invasive strategy with respect to this endpoint. However, the CI for this subgroup analysis showed overlap with that of troponin‐negative participants. The Italian Elderly ACS reinforced the importance of baseline troponin status on treatment effect, and identified a significant reduction in the primary endpoint (composite of death, MI, disabling stroke and repeat hospital stay for cardiovascular causes or severe bleeding within one year) amongst participants with an elevated troponin on admission (hazard ratio (HR) 0.43, 95% CI 0.23 to 0.80) but not in those with normal troponin (HR 1.67, 95% CI 0.75 to 3.70). Data from VINO, which only included participants with clinical symptoms, ECG changes and positive cardiac biomarkers, revealed a significant 72% risk ratio reduction in this endpoint at six months. However, the ICTUS trial, which also exclusively enrolled troponin‐positive participants, had an unexpectedly low baseline mortality rate relative to the other included studies (Table 3). This may be partly due to optimal medical therapy in the ICTUS trial versus other included trials wherein, in both trial arms, early use of clopidogrel and intensive lipid‐lowering therapy was recommended to treating clinicians. Alternatively, this may be a statistical outlier given the large CIs for mortality in these studies. Disparate event rates in participants with positive troponin highlights the importance of global risk stratification as opposed to using cardiac biomarkers as a single risk index. Indeed, in a retrospective analysis of the FRISC‐II data (Diderholm 2002), death or non‐fatal MI experienced a significant 40% risk ratio reduction only in participants with both troponin T (TnT) greater than 0.03 ng/mL and ST depression on admission ECG. Hence, although ICTUS participants all had a TnT of greater than 0.03 ng/mL, this sole criterion did not necessarily identify a risk level that might benefit from invasive treatment. The risk associated with troponin elevation has been shown to be a continuous variable and therefore classification as a dichotomous variable may dilute its predictive power.
Though some have argued that the prognostic value of troponin is greater than that of CK‐MB (Montalescot 2009; Saenger 2008; Thompson 1979), troponin may be overly sensitive for the detection of re‐infarction and only elevated CK‐MB has been correlated with evidence of myocardial necrosis (Lim 2011). Retrospective analysis performed by the TACTICS‐TIMI 18 trial authors highlights the limitations of purely using a positive troponin to predict event rates. Analysis of the invasive arm revealed that 6% of the participants with a positive troponin test did not have significant angiographic coronary artery disease (CAD), defined as greater than 50% stenosis of any coronary artery (Dokainish 2005). At six months, these participants had a 3.1% rate of death or re‐infarction, compared to 0% among those with a negative troponin and no angiographic CAD. As would be expected, troponin‐positive participants with angiographic CAD had a high rate of death or re‐infarction (8.6%) at six months. Interestingly, participants with angiographic CAD who had a negative troponin had a 5.8% rate of death or re‐infarction at six months, which is clearly higher than that for troponin‐positive participants without angiographic CAD. Hence, troponin alone cannot be used to risk stratify patients. Moreover, this analysis highlights the limitations of angiography in the assessment of plaque burden. In general in unstable angina studies, positive troponin status has been shown to correlate with complex coronary lesions on angiography and reduced coronary flow (Benamer 1999; Heeschen 1999a; Hochman 1999), but should not be used alone to identify those at high‐risk. However, absolute values of troponin exhibit a linear relationship with subsequent risk of coronary events. Troponin positivity has also been shown to predict benefit from glycoprotein IIb/IIIa receptor antagonists (Hamm 1999; Heeschen 1999b), an early invasive strategy in the elderly (Italian Elderly ACS), and remains a critical element of risk stratification.
ST depression on admission
As previously mentioned, ECG changes on admission forebode a worse prognosis in UA/NSTEMI patients. Indeed, data from the TIMI III Registry shows that patients with ST depression on admission ECG have a 2.5‐fold increased risk of death or MI within one year (Cannon 1997). In the ICTUS and TACTICS‐TIMI 18 trials, ST depression was an independent predictor of failure of medical therapy with the conservative strategy (Sabatine 2006; Windhausen 2007b). As discussed above, on post‐hoc analysis of FRISC‐II data, the benefit of a routine invasive strategy on the endpoint of death or non‐fatal MI only achieved statistical significance in participants with ST depression on admission ECG. In FRISC‐II and the TIMI III Registry, the prevalence rates for triple‐vessel and left main artery disease were approximately 50% and 66%, respectively, in participants who had ST depression on admission ECG. Similarly, the TACTICS‐TIMI 18 study identified an odds ratio for three‐vessel disease of 1.79 in participants with ST deviation of 0.05 to 0.09 mV, and an odds ratio of 1.91 in those with a ST deviation greater than 0.10 mV versus those with a ST deviation less than 0.05 mV. Hence, the ECG can be used as a tool to identify patients who are likely to benefit from revascularisation. Analysis of the FRISC‐II data demonstrated that ST depression was still a predictor of benefit from an invasive strategy, even after baseline differences were accounted for (Holmvang 2003). Furthermore, this analysis also suggested that the benefits of a routine invasive strategy were further amplified with increasing amplitude of ST depression in an increasing number of ECG leads.
Data from TACTICS‐TIMI 18 confirms the utility of ST segment changes in identifying a higher‐risk population that may benefit from an invasive strategy. Unfortunately, we could not obtain data for the composite endpoint of death or non‐fatal MI, but the study includes data for the endpoint of death or non‐fatal MI or rehospitalisation for ACS. Using this endpoint, the RR was 0.62 (95% CI 0.53 to 0.74) in participants with baseline ST changes, while no effect was observed in those without such changes. The ICTUS data show a trend towards decreased rates of (spontaneous) MI at one year in those randomised to a routine invasive strategy, with a risk ratio of 0.74 (95% CI 0.40 to 1.38). However, the events were few and CIs were wide. In light of the potential implications of ST‐depression on treatment effect, we have provided the percentages of trial participants with ST depression on index ECG in Table 3, with the highest rates of 62% reported in the LIPSIA‐NSTEMI study. In general the studies eligible for analysis did not provide data for subgroup analysis of ST depression and troponin status. While subgroup analyses may identify populations with increased risk, and hence provide increased power to detect statistical significance, such post‐hoc analyses should be interpreted with caution.
Gender
Disparate outcomes of a routine invasive strategy based on gender has been a source of controversy. The all‐study (Analysis 1) gender subanalysis for intermediate death or non‐fatal MI revealed a benefit of routine invasive strategy confined to males. However, the number of women in the studies was lower than the number of men, and the decreased power to detect any advantage of routine invasive strategy is highlighted by the comparatively wide CIs. TACTICS‐TIMI 18 identified no significant interaction between gender and outcomes based on treatment strategy. Conversely, the FRISC‐II and RITA‐3 trials found significant benefit of a routine invasive strategy for death or MI amongst men, but not women. Supporting this, the OASIS 5 substudy, which randomly assigned 184 women to a routine or selective invasive strategy, identified significantly more deaths after one year (HR 9.01, 95% CI 1.11 to 72.90) and higher rates of major bleeding at 30 days (HR 11.45, 95% CI 1.43 to 91.96) with a routine invasive strategy.
Confounding comparison and interpretation of these results, women had less severe CAD across the studies analysed, and were less likely to have an elevated troponin level than men (Clayton 2004; Glaser 2002; Lagerqvist 2001). Moreover, in FRISC‐II and RITA‐3, women in the conservative arm had a better prognosis than men in the conservative arm. A retrospective analysis of TACTICS‐TIMI 18 data suggests that, after adjusting for differences in baseline characteristics, the benefits of an early invasive strategy in women were the same as those seen in men (Glaser 2002). In contrast, similar analyses undertaken by FRISC‐II and RITA‐3 trial authors failed to demonstrate any benefit of an invasive strategy in women, even after they adjusted for baseline characteristics. The RITA‐3 analysis suggested that women had better outcomes than men when managed conservatively and did not benefit from an invasive strategy, even when those with high‐risk features were analysed separately (Clayton 2004). Women in TACTICS‐TIMI 18 and RITA‐3 were less likely than men to undergo CABG, even when trials adjusted for the presence of three‐vessel or left anterior descending artery disease (Clayton 2004; Glaser 2002). Notably in FRISC‐II, where the rates of CABG were similar in both men and women, the one‐year mortality rate in participants undergoing CABG was 9.9% in women versus just 1.2% in men (Lagerqvist 2001). Higher operative CABG mortality has been observed in women enrolled in observational studies and this discrepancy could not be accounted for by age, co‐morbidities or smaller body surface area (Blankstein 2005). These retrospective analyses should be interpreted with appropriate caution. They highlight the importance of further research to determine the optimal treatment strategy in women, and the importance of risk stratification, especially in women who are less likely to have angiographic CAD when compared to their male counterparts, and requisite caution extrapolating results from men to women.
Other subgroups
We have discussed other subgroups of interest that we did not prespecify in our protocol, Hoenig 2004, as a narrative review in this section.
Elderly
The elderly (aged over 65 years) comprise the majority of hospital admissions for UA/NSTEMI. Given the higher risk of recurrent events in this group compared to counterparts who are younger, increased absolute risk may translate into a greater absolute risk reduction with improved understanding of the relative benefits of invasive versus conservative management (Alexander 2007). Despite this, there is a deficit of knowledge regarding the management of elderly patients, since the included studies in this Cochrane review generally excluded participants over 75 years of age.
The Italian Elderly ACS was the only RCT to specifically compare treatment strategies in elderly participants with non‐ST‐elevation ACS. Here, we did not identify any statistical difference between early aggressive and initially‐conservative groups for the primary endpoint of composite death, MI, disabling stroke and repeat hospital stay for cardiovascular or bleeding causes (HR 0.80, 95% CI 0.53 to 1.19) or for mortality (HR 0.87; 95% CI 0.49 to 1.56), MI (HR 0.67, 95% CI 0.33 to 1.36) or repeat hospitalisation (HR 0.81, 95% CI 0.45 to 1.46) when examined alone. However, stratification of participants dependent on baseline troponin revealed a significant reduction in the primary endpoint amongst troponin‐positive (HR 0.43, 95% CI 0.23 to 0.80) versus troponin‐negative participants (HR 1.67, 95% CI 0.75 to 3.70; P = 0.03). Implicit in this is the lost advantage of an early invasive strategy in troponin‐negative elderly, who can be safely managed conservatively. These findings are supported by the collaborative analysis of individual data from the FRISC II‐ICTUS‐RITA 3 (FIR) trials (Damman 2012), where a 29% reduction in cardiovascular death or MI was reported with a routine invasive strategy in participant greater than or equal to 75 years old, with sustained benefit still demonstrated at long‐term follow‐up.
A retrospective analysis of the TACTICS‐TIMI 18 trial showed that those over 65 years of age were more likely to have high‐risk features, such as elevated troponin levels, ST‐deviation, diabetes and congestive heart failure (Bach 2004). Indeed, 90% of those over 65 years old had intermediate to high‐risk TIMI scores (score greater than or equal to three), versus just 63% of those under 65 years of age. Overall, the routine invasive strategy reduced early and intermediate death or MI when compared to conservative management amongst those over 65 years of age, with risk ratios of 0.58 (95% CI 0.37 to 0.92) and 0.64 (95% CI 0.45 to 0.93), respectively. The invasive strategy did not significantly benefit those under 65 years old, which suggests that benefit increases with age, though the CIs were wide and overlapped. However, major bleeding was higher with the invasive strategy in those over 65 (RR 1.74, 95% CI 1.12 to 2.70), while no such hazard was observed in those under 65 years of age.
Reassuringly, in both the Italian Elderly ACS and the retrospective analysis of the TACTICS‐TIMI 18 trial, stroke was not increased with an invasive strategy in the elderly, and in fact demonstrated a trend towards decreased events with the routine invasive strategy adopted in TACTICS‐TIMI 18. The results of this type of analysis are unsurprising, given that the elderly are at increased risk of events. Therefore, retrospective analysis should have greater power to identify benefits of an intervention with absolute event rates.
The FRISC‐II trial authors also published risk ratio estimates for participants based on age; and while the risk estimate was only significant in those over 65 years old, the risk estimate for those under 65 years of age was similar and the CIs overlapped (Lagerqvist 2006). However, the results from TACTICS‐TIMI 18 and FRISC‐II differ from older excluded studies such as TIMI‐3b, which showed a significant hazard of intervention in younger trial participants. This point again reinforces the reasoning behind only including studies that were undertaken in the stent era, since older studies are irrelevant to contemporary practice.
The 2012 American College of Cardiology Foundation/American Heart Associate (ACCF/AHA) focused update, Jneid 2012, and the 2011 European Society of Cardiology (ESC) guidelines, Hamm 2011, on the management of UA/NSTEMI endorse an early invasive strategy (Level B evidence) for elderly patients, despite increased early procedural risks. Moreover, since elderly patients recruited into clinical trials generally have fewer cardiovascular risk factors, fewer co‐morbidities, and better haemodynamics and renal function than community‐dwelling elderly, event rates and benefits from a routine invasive strategy might be even greater in the 'real world'. Registry data support the use of the routine invasive strategy in the elderly, and there is no stroke hazard as a consequence of routine intervention reported in contemporary registries (Bauer 2007).
However, in the real world, acute coronary care for the elderly is provided within the context of the health and co‐morbid status of the patient. These factors also need to be considered for therapeutic decision‐making. Despite the lack of any statistically‐significant benefit with an invasive strategy in younger age groups, this is not to say that younger patients with high‐risk features would not benefit from a routine invasive approach. Age is included in the TIMI risk score, which integrates several prognostic variables readily available from the clinical history and first‐line investigations (Antman 2000). Similarly, retrospective analyses from the included studies have suggested that diabetes, peripheral arterial disease and a history of previous coronary artery bypass grafting are co‐morbid conditions associated with an increased risk of events and, hence, the potential for enhanced benefit from an early invasive strategy, as well as a more favourable risk‐benefit ratio (Januzzi 2005; Kugelmass 2006; Norhammar 2004). However, as with age, there is co‐variation with other indicators of high‐risk. Consequently, while retrospective analyses that focus on a single indicator of higher risk are interesting, a universal and easily‐applied method of risk stratification that can be utilised by the practicing physician would be of greater interest.
Diabetics
The relative benefit of adopting a routine invasive strategy has been a contentious issue amongst people with diabetes, who have both an increased risk of recurrent cardiovascular events and an increased risk of intervention due to co‐morbid conditions. A collaborative meta‐analysis of RCTs, which incorporated 9904 participants, compared conservative verse invasive treatment strategies between diabetic and non‐diabetic patients (O'Donoghue 2012). Although an invasive strategy yielded similar reductions in diabetic and non‐diabetic participants in overall cardiovascular events, the reduction in recurrent non‐fatal MI was greater in diabetic participants. The data presented by O'Donoghue 2012 support the 2012 ACCF/AHA focused update (Jneid 2012; Level B evidence) and the 2011 ESC guidelines (Hamm 2011; Level A evidence), which recommend use of an invasive strategy for people with diabetes who present with UA/NSTEMI.
The importance of global risk stratification
As the above discussion highlights, and as subgroup analyses have illustrated, risk stratification is an integral component of managing patients with UA/NSTEMI. The goal of risk stratification is to identify patients with a high likelihood of complicated CAD who are at increased risk of recurrent coronary events or premature death, and to offer such patients the benefits of revascularisation. However, the clinical distinction between UA and NSTEMI does not adequately stratify high‐risk patients (Zaacks 1999). Consequently, the current 2012 ACCF/AHA focused update, Jneid 2012, and the 2011 ESC guidelines, Hamm 2011, recommend using several parameters for risk stratification; as occurs with the application of risk scoring tools, for example the TIMI risk score (Antman 2000). To underscore this point, in a post‐hoc analysis of the FRISC‐II data, participants with troponin T of greater than 0.03 ng/mL and ST depression experienced a statistically‐significant benefit with a routine invasive strategy, whereas participants with only one of these variables did not (Diderholm 2002). Only TACTICS‐TIMI 18 undertook subgroup analyses based on TIMI risk scores, and stratified the participants into three categories based on their TIMI risk score: low‐, intermediate‐ or high‐risk. In this study, only intermediate‐ and high‐risk participants benefited from the invasive strategy, regarding the primary composite endpoint of death or non‐fatal MI or rehospitalisation for ACS. Unfortunately, data for the composite endpoint of death or non‐fatal MI were unavailable and therefore we could not incorporate them into this Cochrane review.
The TIMI score was extracted from the unfractionated heparin arm of the TIMI 11B trial (TIMI 11B 1999). It was validated in the enoxaparin arm of TIMI 11B and in both arms of the ESSENCE 1997 trial. The risk score was shown to be a valid predictor of the composite endpoint encompassing all‐cause mortality, MI and urgent revascularisation within 14 days of randomisation. Importantly, the TIMI score also predicted each of the components of this composite endpoint (Antman 2000). The TIMI risk score was subsequently validated in the TIMI III Registryof unselected UA/NSTEMI patients and was shown to predict the endpoint of death, MI or recurrent ischaemia and the components of the composite outcome at both six weeks and one year (Scirica 2002). Further, the TIMI risk score was validated for the death, MI or recurrent ischaemia composite endpoint for up to six months in the PRISM‐PLUS Trial; and it was shown to predict benefit from tirofiban, even in participants with a negative CK‐MB (Morrow 2002). Hence, this versatile risk score is able to identify patients with high event rates who may also benefit from an invasive strategy. Intuitively, one would expect that patients with higher TIMI scores, and therefore a higher risk for mortality and recurrent events, have more extensive CAD on angiography. This has been confirmed in a retrospective analysis of patients with UA/NSTEMI (Garcia 2004). The PRISM‐PLUS Trial authors also confirmed these findings by a retrospective analysis, and found the TIMI score to correlate with impaired epicardial artery blood flow and the presence of visible thrombus on angiography (Mega 2005). Although there are other published risk scores for UA/NSTEMI (de Araújo Gonçalves 2005), the TIMI risk score is perhaps the most widely used. In addition, the low event rates in ICTUS, which exclusively enrolled troponin‐positive participants, highlight the importance of considering multiple variables in risk stratification. Indeed, on five‐year follow‐up by the RITA‐3 trial authors, nine factors other than treatment group emerged as multi‐variate predictors of death or non‐fatal MI (Fox 2005). When the logistic coefficients for the risk factors were added and the study population divided into quartiles based upon risk score, participants in the highest quartile of risk score experienced substantially greater benefit from an invasive strategy. Similarly, the FRISC‐II trial authors developed a FRISC score, ranging from zero to seven, with one point alloted for each of seven factors: age of over 70 years, male sex, diabetes, previous MI, ST depression, increased troponin, and increased interleukin‐6 or C‐reactive protein (Lagerqvist 2005). Having a medium to high‐risk (score of three to seven) predicted benefit from an early invasive strategy, with risk ratios of 0.64 (95% CI 0.51 to 0.80) at two years and 0.75 (95% CI 0.64 to 0.89) at five years for the composite endpoint of death or non‐fatal MI (FRISC‐II). Low‐risk patients (score zero to two) did not benefit and had a trend towards harm for the composite endpoint of death or non‐fatal MI, with a RR of 1.62 (95% CI 0.71 to 3.69) at two years and 1.26 (95% CI 0.66 to 2.40) at five years (FRISC‐II). In contrast, the ICTUS trial authors confirmed the prognostic utility of the FRISC score, but were unable to predict benefit from an early invasive strategy in this trial; even participants with the highest FRISC scores (five to seven) derived no benefit from an early invasive approach (RR 1.30, 95% CI 0.69 to 2.47), in terms of the late death or MI endpoint.
Current 'real world' event rates in patients with UA/NSTEMI compared to rates observed in the included trials
The largest multinational registry, the GRACE registry, which collects data from 30 countries, has reported mortality rates in patients hospitalised with various forms of acute coronary syndrome (ACS). Entry criteria for this registry include a history of chest pain and one of the following: ischaemic ECG changes, increased cardiac biomarkers or a documented history of CAD. The in‐hospital mortality rates for patients recruited between 1999 and 2002 were 5.9% for patients with NSTEMI and 2.7% for patients with unstable angina. Also, the six‐month post‐discharge mortality rates were 6.2% and 3.6% for NSTEMI and unstable angina, respectively (Goldberg 2004). Furthermore, rehospitalisation rates six months post‐discharge were roughly 20%. Another report from the GRACE registry, which included patients recruited between 1999 and 2003, reported the six‐month post‐discharge mortality rates as 11.6% for NSTEMI and 6.8% for unstable angina (Van de Werf 2005). Clearly, the mortality rates from this real‐world registry are higher than those observed in the studies included in our meta‐analysis, as shown in Table 3. However, these patients did not receive optimal medical management in that only approximately 50% of NSTEMI patients received ACE inhibitors, heparin or statins (Goldberg 2004). While over 90% of patients received aspirin and over 80% received beta blockers, it is unlikely that many would have received clopidogrel as the patients studied were entered into the registry prior to publication of the CURE trial (CURE 2001); that is, before use of clopidogrel for UA/NSTEMI became accepted as standard therapy. Similarly, participants enrolled in the UA/NSTEMI trials received higher rates of medical therapy than participants enrolled in the CRUSADE registry (Kandzari 2005). However, the discrepancy in mortality rates between the participants in the included studies of this Cochrane review and registry‐reported mortality rates is arguably too high to be explained by advances in the medical management of UA/NSTEMI alone. Another explanation may be that selection and recruitment protocols may bias trials towards enrolling participants with a risk lower than that seen in unselected participants entered into registries. While analysis of available data suggests that high‐risk patients may benefit from an invasive strategy, this absolute benefit is likely to narrow as early medical therapies and risk stratification procedures for UA/NSTEMI improve, combined with the appropriate use of deferred coronary angiography and revascularisation. Novel medical therapies, such as prasugrel (TRITON‐TIMI 38) and ticagrelor (PLATO) instead of clopidogrel, continue to decrease absolute event rates in patients with UA/NSTEMI. Consequently, future trials of invasive versus conservative management for UA/NSETMI will be required as novel medical therapies are adopted. It is likely that only progressively higher‐risk patients will continue to benefit from routine invasive intervention in the future. A report from the GRACE registry has shown that increasing use of evidence‐based therapies has translated into reduced event rates over time (Fox 2007b). However, the lack of benefit observed for several endpoints in this review may be due to lower‐risk patients having been selected for trial enrolment.
The general paucity of enrolment of participants with cardiogenic shock or an advanced Killip class in the included studies may mean that the results of this systematic review are not applicable to this high‐risk subset. Advanced Killip class has been identified as an independent predictor of mortality in patients with NSTEMI (Khot 2003), while Killip class and congestive heart failure (development of or history of) were shown to predict death and the composite of death or MI in the GRACE registry (Fox 2006). Indeed, the current ACCF/AHA guidelines for UA/NSTEMI recommend using signs of heart failure as markers of increased risk (Jneid 2012). However, most of the included studies did not report Killip class, EF or brain natriuretic peptides among their baseline characteristics; and the event rates in the included studies indicate that participants with cardiogenic shock were excluded. Two exceptions to this are the FRISC‐II trial, in which 13% of participants were reported to have an EF of less than 45% at baseline, and the VINO trial, in which 53% of the sample were reported to be Killip class of II or III at baseline. This high percentage of participants with pulmonary oedema in VINO may explain why this trial had the highest standardised mortality rates of the included studies (Table 3); and, while being a small trial, identified a robust benefit for routine invasive strategy. Observational data have revealed that Killip class II and III patients enjoyed a significant mortality benefit (at 30 days and six months) from an invasive strategy, while Killip class I patients did not benefit (Rott 2001). The SHOCK 1999 trial (302 participants), which recruited STEMI patients with cardiogenic shock, uncovered a significant mortality benefit for a routine invasive versus conservative strategy at six months, with mortality rates of 50.3% and 63.1% (P = 0.027), respectively (SHOCK 1999). These are consistent with observations from the GRACE registry (Dauerman 2002). Similarly, elevated N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) has been shown to predict a poor prognosis in patients with UA/NSTEMI, independently of age, Killip class or left ventricular EF (Jernberg 2004). In a retrospective subgroup analyses from FRISC‐II (2017 participants), NT‐proBNP measured at median of 39 hours after symptom presentation correlated (correlation coefficient, r) with TnT (r = 0.53, P < 0.001), interleukin‐6 (r = 0.29, P < 0.001) and the severity of coronary disease on angiography (Jernberg 2003). A relationship between higher brain natriuretic peptide (BNP) and more severe angiographic coronary disease was also evident in a small retrospective analysis from the TACTICS‐TIMI 18 trial, which also demonstrated higher BNP to be associated with higher TIMI frame counts, consistent with reduced myocardial perfusion (Sadanandan 2004). In FRISC‐II, NT‐proBNP predicted two‐year mortality independently of TnT, interleukin‐6 and left ventricular EF, but failed to predict the incidence of MI. Importantly, this retrospective subgroup analysis from the FRISC‐II trial authors suggested that the early invasive strategy only improved two‐year mortality in participants within the highest tertile for NT‐proBNP (greater than 906 ng/L for men, greater than 1345 ng/L for women) and with an interleukin‐6 concentration greater than 5 ng/mL (absolute risk reduction of 7.3%; RR 0.46, 95% CI 0.21 to 1.00). Such retrospective analyses are hypothesis‐generating and by no means definitive. A similar analysis from TACTICS‐TIMI 18 (1676 participants) measured BNP instead of NT‐proBNP, and dichotomised participants at a BNP of greater than 80 ng/L. The analysis found that participants with an elevated BNP exhibited greater seven‐day and six‐month mortality (2.5% versus 0.7%, P < 0.01; and 8.4% versus 1.8%, P < 0.01 respectively). However, BNP was not shown to predict any benefit from invasive management (Morrow 2003). This may be due to the relatively short follow‐up performed in the TACTICS‐TIMI 18 study, which was only six months (Table 3). The ICTUS trial authors also examined the prognostic influence of NT‐proBNP measured a median of 13 hours after presentation in a 1141‐participant subgroup extracted from the main trial (Windhausen 2007a). In the highest quartile (greater than 1170 ng/L for men, greater than 2150 ng/L for women), one‐year mortality was 7.3%, compared to just 1.1% among participants in the lower three quartiles. However, as with the retrospective analyses from the FRISC‐II and TACTICS‐TIMI 18 trials, NT‐proBNP failed to predict MI and, in contrast to FRISC‐II, elevated NT‐proBNP did not predict any benefit from an early invasive strategy in the ICTUS cohort (Windhausen 2007a). Hence, the role of natriuretic peptides and the assessment of patients for clinical features of congestive heart failure in UA/NSTEMI need to be further elucidated. In the interim, patients with features of congestive heart failure need to be considered at high‐risk for death and managed aggressively. The GRACE investigators identified predictors of a poor prognosis that were derived from and validated in cohorts enrolled in GRACE from 1999 to 2002 and 2002 to 2003, respectively (Eagle 2004). The investigators identified nine variables — older age, history of MI, history of heart failure, increased heart rate, lower systolic blood pressure, elevated serum creatinine, elevated cardiac biomarkers, ST depression and not undergoing PCI — as independent predictors of increased six‐month mortality across the ACS spectrum. Of particular note is that the GRACE risk score incorporates renal function, which is an important, practical risk prognosticator in UA/NSTEMI that was not considered when the TIMI risk score was derived (Antman 2000). In a retrospective subgroup analysis of the FRISC‐II trial, creatinine clearance was estimated from serum creatinine using the Cockcroft‐Gault formula (Johnston 2006). In conservatively‐managed patients, the rates of death or MI for creatinine clearances of less than 69 mL/min, 69 to 90 mL/min and greater than 90 mL/min were 22.4%, 14.6% and 11.6%, respectively. The corresponding event rates in the invasive group were 14.6% (P < 0.01 versus conservative treatment), 9.9% (P = 0.048) and 11.2% (P = not significant), respectively. Indeed, there was a significant interaction between treatment strategy and outcomes in patients with a creatinine clearance of less than 90 mL/min. These data are indeed sobering, since patients with renal dysfunction are often denied aggressive therapy in the real world, possibly because of clinician concerns about bleeding risk and a poor prognosis regardless of therapy. These data are particularly relevant to clinicians practicing in countries where an estimate of glomerular filtration rate is mandatory on adult electrolyte panels, as is standard in the USA and Australia. Hence, risk stratification is an integral part of the management of patients with UA/NSTEMI and needs to be considered carefully in future prospective RCTs on the topic. Moreover, the roles of estimated glomerular filtration rate and NT‐proBNP as risk prognosticators over and above established markers such as the TIMI risk score need to be further evaluated.
Current 'real world' management of patients with UA/NSTEMI, emphasising the relationship between patient risk and subsequent management
Despite the extensive literature that exists on risk stratification, real‐world data from the GRACE registry has shown that high‐risk patients are no more likely to receive enoxaparin or glycoprotein IIb/IIIa receptor antagonists or to undergo catheterisation and PCI than low‐risk patients (Oliveira 2007). In a different analysis from the GRACE registry that only included participants recruited with direct access to a catheterisation laboratory, there was an inverse relationship between the level of patient risk (measured as a GRACE risk score) and the frequency of angiography and PCI (Fox 2007a; Ranasinghe 2011). Indeed, the rates of cardiac catheterisation in low‐, medium‐ and high‐risk patients with UA/NSTEMI were 72%, 68% and 51%, respectively, while the rates of PCI were 40%, 35% and 25%, respectively (Fox 2007a). In addition, thienopyridines and glycoprotein IIb/IIIa receptor antagonists were more commonly used in low‐risk patients than medium‐ or high‐risk patients with similar findings in a Canadian registry (Yan 2007). Likewise, diabetics with UA/NSTEMI, despite their higher risk, are not treated more aggressively than non‐diabetics (Franklin 2004). The reasons for the discrepancy between patient risk and treatment have been unclear, but recent data from a Canadian registry suggest that the most common reason for under‐utilization of an invasive strategy in high‐risk patients is the treating physician's underestimation of patient risk (Lee 2008). In this regard, a focused initiative to educate physicians on risk stratification could enhance quality of care in patients with UA/NSTEMI. It is also important to recognise that access and distance to cardiac catheterisation services are established predictors of treatment strategy.
Quality of life endpoints
Although not an initial outcome of this systematic review, this section provides a narrative discussion of health‐related quality of life (HRQOL) outcomes. Four studies (Eisenberg 2005; RITA‐3; FRISC‐II; TACTICS‐TIMI 18) specifically compared HRQOL and functional status following invasive versus non‐invasive management for NSTEMI. One trial (Eisenberg 2005) selected change in QOL as a primary endpoint, and the other three (RITA‐3; FRISC‐II; TACTICS‐TIMI 18) had HRQOL measures as secondary endpoints. In the primary endpoint trial, which included only 88 participants (Eisenberg 2005), there was no difference between the two groups at 12 months in terms of the level of peak exercise reached on an endurance exercise treadmill (7.8 versus 6.7 metabolic equivalents). Functional status was improved in the invasive group (Duke Activity Status Index scores 4.3 versus −3.5, P = 0.04), as was angina‐specific quality of life, assessed using the Seattle Angina Questionnaire measure of anginal stability (21.6 versus −5.3, P = 0.020), anginal frequency (22.9 versus 2.3, P = 0.02) and treatment satisfaction (11.2 versus −10.3, P = 0.02).
In the RITA‐3 trial, Kim 2005 assessed HRQOL was assessed with the Short Form‐36 (SF‐36), Seattle Angina Questionnaire (SAQ), EuroQOL Visual Analogue Scale (EQ‐VAS) and EuroQOL 5‐Dimensional Classification (EQ‐5D) scale at baseline, four months and one year follow‐up. Mean changes from baseline EQ‐VAS scores were better for the invasive versus non‐invasive strategy at four months (treatment difference of 3.0, P < 0.001) and one year (2.3, P < 0.01). The EQ‐5D utility scores were also higher in the invasive group at four months (treatment difference 0.036, P < 0.01) but not at one year (0.016, P = 0.20). For the SF‐36, the invasive strategy group scored significantly better at four months for physical function, physical role function, emotional role function, social function, vitality and general health. The SAQ scores for exertional capacity, anginal stability and frequency, treatment satisfaction and disease perception were significantly better for the invasive strategy group at both four months and one year, though attenuated at the last follow‐up. The study authors concluded that improvements in HRQOL associated with the invasive strategy were most likely due to improved in anginal symptoms.
In theFRISC‐II trial, Janzon 2004 measured HRQOL was measured using the generic Medical Outcomes Study SF‐36 and the disease‐specific Angina Pectoris Quality of Life Questionnaire (APQLQ) at baseline and three, six and 12 months follow‐up. The invasively‐treated group reported a significantly better quality of life in all eight scales and both component scores (physical and mental) of the SF‐36 at three and six months of follow‐up (P < 0.01) relative to the non‐invasively treated group. These differences remained at 12 months follow‐up, with significance in seven of the scales and in the physical component score. The invasive group scored significantly higher on all five subscales of the APQLQ scores at three months (P < 0.01), and on four subscales at six months (P < 0.05), but only on one subscale at one year.
Regarding the TACTICS‐TIMI 18 trial, Weintraub 1999 planned to assess health status using some measure of utility in order to perform cost‐effectiveness evaluations of invasive versus non‐invasive strategy, but subsequent publications failed to disclose HRQOL data (Mahoney 2002). From the available evidence, it would appear that improvements in HRQOL as a result of an invasive strategy are modest and last on average no more than 12 months, with anginal relief likely the key determinant of improved HRQOL.
Findings from studies in the pre‐stent era and other reviews on this topic
We excluded two large trials that were undertaken during the pre‐stent era (TIMI‐3b; VANQWISH 1998). The early invasive arm of TIMI‐3b involved cardiac catheterisation an average of 36 hours after randomisation and coronary revascularisation by coronary angioplasty or CABG. The early invasive strategy had no effect on the hard clinical endpoints of death, MI, stroke or the composite of death or MI. As is consistent with more recent clinical trials, the early invasive strategy reduced recurrent hospitalisation at both six weeks and one year, with RRs of 0.54 (95% CI 0.40 to 0.74) and 0.79 (95% CI 0.68 to 0.93), respectively (TIMI‐3b). In TIMI‐3b, a routine invasive strategy did not reduce the need for anti‐angina medications at one year. In contrast, the VANQWISH 1998 study demonstrated increased risk associated with the early invasive strategy, which involved cardiac catheterisation an average of 48 hours after randomisation. In fact, the early invasive strategy was associated with an increased risk ratio of mortality prior to hospital discharge, and at one month and one year (RR 3.47 (95% CI 1.41 to 8.52); RR 2.53, 95% CI 1.19 to 5.42; and RR 1.60, 95% CI 1.08 to 2.37, respectively) (VANQWISH 1998). Similarly, increased risk was associated with the early invasive strategy for the composite endpoint of death or non‐fatal MI. The hazard of an early invasive strategy on these endpoints ceased to be significant by the end of the study (average 23 months). Forty‐four percent of participants in the invasive arm of this trial underwent a revascularisation procedure, of which 47% involved CABG. The mortality associated with CABG in the invasive arm was 11.6%, compared to 3.4% in the conservative arm. Braunwald 2003 has cited this discrepancy as an explanation for the increased mortality in the early invasive arm of the VANQWISH 1998 trial. Unsurprisingly, rates of background medical therapy were low by contemporary standards.
Two older meta‐analyses on this topic that included the aforementioned pre‐stent era trials, plus trials that we excluded for reasons other than low stent use, reached different conclusions than the ones presented here (Choudhry 2005; Mehta 2005). These reviews did not include the most recent trials, including the OASIS 5 substudy, LIPSIA‐NSTEMI, Italian Elderly ACS and ICTUS studies. A subsequent meta‐analysis included the early trials and the one‐year results from ICTUS (Bavry 2006). The review by Mehta 2005 associated an invasive strategy with an increased risk of mortality during the period from randomisation to hospital discharge (RR 1.61, 95% CI 1.14 to 2.27). When Mehta 2005 analysed the outcomes from hospital discharge to end of follow‐up, the early invasive strategy was associated with reductions in death and non‐fatal MI (RR 0.78, 95% CI 0.64 to 0.94; and RR 0.56, 95% CI 0.47 to 0.68, respectively). When they analysed trial data from randomisation to the end of follow‐up, the invasive strategy exhibited no effect on mortality, but induced a reduction in non‐fatal MI (RR 0.77, 95% CI 0.67 to 0.89). This Cochrane review analysed the endpoints at certain time points, since we felt that combining outcomes collected from studies of short duration (six months) with those of longer duration (five years) would not provide a meaningful point estimate (see the 'Characteristics of included studies' table). All reviews consistently found a significant reduction in recurrent angina and rehospitalisation with an invasive strategy (Bavry 2006; Choudhry 2005; Mehta 2005). More recently, a meta‐regression analysis that included the earlier studies but excluded VANQWISH 1998 revealed the benefit of an invasive strategy — with respect to the endpoint of death or the composite of death or MI — to be related to the comparator odds ratio for events in the conservative group (Tarantini 2007). This implies that the benefit of an invasive strategy relates to the level of baseline risk in the comparator group. One meta‐analysis has been published since the publication of late follow‐up data from the ICTUS trial. This report, which included the older studies, identified no benefit of an invasive strategy on the endpoints of death, MI, or the composite of death or MI (Qayyum 2008). The findings from our analysis differ, because we excluded older studies and utilised the reported 'spontaneous' MI endpoint for our analysis, in light of the controversy surrounding the routine peri‐procedural biomarker assessment undertaken by the ICTUS trial authors.
Relevant international guidelines for management of UA/NSTEMI
Both the current AHA (Anderson 2007; Jneid 2012) and ESC (Hamm 2011) guidelines make class 1 recommendations for an invasive strategy in patients who are symptomatic or are considered (Level A evidence). The AHA guidelines also endorse the option of treating stabilised but high‐risk (for example troponin‐positive) patients conservatively, however with only Class IIb recommendation.
Limitations
We have limited the included studies in this Cochrane review to those from the post‐stent era. However, a number of changes in practice have occurred over this time which limit the applicability of findings from this meta‐analysis to contemporary practice. Of greatest significance, during the post‐stent era, is that the routine use of glycoprotein IIb/IIIa inhibitors gained acceptance (Boersma 2002). Subsequently, however this practice has been discredited due to the association with increased bleeding (EARLY ACS 2009). As such, we have presented analyses of studies in which glycoprotein IIb/IIIa use was routine (Analyses 1 and 2) as of historical interest, with Analysis 3 being the most relevant to contemporary practice. Additionally, across this time period there has been controversy regarding access site efficacy. In particular, the prospective randomised RIFLE‐STEACS study reported significant benefit for a transradial verse transfemoral approach for major adverse cardiac and cerebrovascular events (MACCE), non‐CABG‐related bleeding and overall net adverse clinical event (NACE) rate among STEMI patients. Subsequently, the transradial access approach has emerged as the preferred strategy for PCI and has been adopted as routine practice for invasive strategies in ACSs. Despite this, a transfemoral strategy was predominant amongst the included studies in this meta‐analysis, which potentially resulted in increased adverse events in the routine invasive strategy compared with contemporary practice. Additionally, improved outcomes have also resulted from refinement in stent design with second‐generation drug‐eluting stents forming the cornerstone of modern PCI practice (Sarno 2012). Both the predominance of a transfemoral approach and use of earlier generation stents amongst the included studies in this meta‐analysis potentially and differentially diminish outcomes in the routine invasive arm compared with contemporary practice, thus obscuring any comparison with selective invasive strategies.
These limitations of the current body of evidence form important considerations for future trials designed to compare a routine verse selective invasive strategy in UA/NSTEMI. We recommend that such studies employ a transradial access route, an antithrombotic regime consisting of aspirin and ticagrelor across all study participants and second generation drug‐eluting stents to most accurately reflect current practice and difference between routine and selective invasive strategies. Finally, where possible, analyses should allow for the discrete assessment of outcome measures for NSTEMI and UA as the heterogeneity of combined UA/NSTEMI may mask important intervention risk‐benefit differences between the two entities.
Quality of the evidence
The GRADE approach was employed to interpret findings and the GRADE profiler (GRADEPRO) allowed us to import data from Review Manager 5.3 (Review Manager) to create 'Summary of findings' tables. The quality of evidence for routine invasive strategies versus selective invasive strategies (conservative) for unstable angina and non‐ST elevation myocardial (UA/NSTEMI) infarction in the stent era (Table 1 and Table 2) ranged from moderate to low across the different outcomes. This was mainly due to risk of bias and imprecise results.
Authors' conclusions
Implications for practice.
The most important new finding of this Cochrane review update is the identification of a significantly increased risk of index death with a routine invasive approach. Conversely, consistent with previous versions of this review (Hoenig 2006; Hoenig 2010), adopting a routine invasive strategy for the management of UA/NSTEMI patients results in a significant reduction in risk, from six to 12 months of follow‐up, for the two endpoints of refractory angina and rehospitalisation. While the invasive strategy is associated with an almost two‐fold increase in the risk of peri‐procedural myocardial infarction (MI), the data also suggest a significant risk reduction in the rate of MI assessed at three to five years. The importance of peri‐procedural infarction continues to be a subject of dispute, with recent suggestions that this endpoint lacks prognostic significance. Hence, the early risks associated with a routine invasive strategy must be weighed against potential long‐term benefits in clinical endpoints. However, longer term follow‐up of more contemporary trials may find this benefit to be attenuated with more optimal use of medical therapies, the deployment of more rigorous risk stratification protocols in the days immediately following onset of the acute event, and a more conservative/selective invasive approach with high invasive percentages. The benefits of a routine invasive strategy may be more meaningful in higher‐risk patients, among whom the number needed to treat for an additional beneficial outcome (NNTB) should be less.
The increased risk of index death noted with the inclusion of the Italian Elderly ACS and the LIPSIA‐NSTEMI trials in this review update must be interpreted in the context of the disproportionate representation of populations with known adverse predictors. Increased mortality risk has been associated with both elderly populations (Italian Elderly ACS) and routine glycoprotein IIb/IIIa inhibitor use (LIPSIA‐NSTEMI), both of which contribute to excess event rates among people treated using a routinely invasive strategy. A more considered approach in the elderly population and a selective use of glycoprotein IIb/IIIa inhibitors is now recommended in most international guidelines (Hamm 2011; Jneid 2012). The finding that inclusion of two such populations resulted in an overall increase in index mortality rate associated with an invasive strategy strengthens the current recommendations. Due to the attendant bleeding risk and subsequent increased morbidity/mortality, glycoprotein IIb/IIIa inhibitors should only be used selectively in people undergoing invasive treatment. Similarly, in elderly populations, a selective invasive approach is appropriate, as invasive interventions carry increased risk, with decisions based upon markers of risk — in particular, troponin status.
In the all‐study analysis, evidence incorporating intermediate endpoints with all included studies (or most of the studies) failed to show appreciable benefit with invasive strategies for unstable angina and non‐ST elevation MI compared to conservative strategies in all‐cause mortality and death or non‐fatal MI at six to 12 months. Evidence showed risk reduction in MI, refractory angina and rehospitalisation with routine invasive strategies compared to conservative (selective invasive) strategies at six to 12 months follow‐up. However, routine invasive strategies were seen to be associated with a relatively high risk (almost double the risk) of procedure‐related MI, and increased risk of bleeding complications. This systematic analysis of published RCTs supports the conclusion that, in people with unstable angina and non‐ST elevation MI (UA/NSTEMI), a selectively invasive (conservative) strategy based on clinical risk for recurrent events is the preferred management strategy.
Implications for research.
This Cochrane review highlights the need for further research on treatment strategies for UA/NSTEMI. To date, published trials have enrolled heterogeneous populations of patients with variable levels of risk and event rates, subjected to a variety of co‐interventions, and used outcome measures subject to variable definition and timing. Risk stratification of the participants in each trial based upon a validated risk system (e.g. the TIMI risk score) would allow for more meaningful meta‐analyses of available data, and provide a risk score or an absolute event rate above which an invasive strategy is expected to significantly improve outcomes. Clearly as medical therapies for UA/NSTEMI improve, progressively less absolute benefit is likely to be gained via aggressive interventions. Hence, the level of baseline risk at which an invasive intervention becomes warranted is likely to be a moving target. Another major limitation to the analyses undertaken in this review is the under‐powering of trials in terms of assessing the effects of an invasive strategy on all‐cause mortality, due to the short length of follow‐up. Inadequate numbers also hinder the interpretation of subgroup analyses. This could be addressed in future clinical trials by ensuring that sufficient events accrue by way of larger sample sizes, the enrolment of higher‐risk participants, and longer durations of follow‐up. Finally, further research is required to better define the benefits and hazards of an invasive strategy in females.
Given the support for a routine invasive strategy in the management of non‐ST‐elevation ACS, it has become difficult to justify the inclusion of a selectively invasive (conservative) arm when designing RCTs, and this is reflected in the paucity of recent research comparing a selective versus routine invasive approach. However, our findings suggest that including a selectively invasive arm is required. Using a routine invasive strategy amongst the elderly requires particular investigation. In light of the results of the ACUITY and EARLY ACS 2009 studies, studies responsible for discrediting the routine use of glycoprotein IIb/IIIa inhibitors as part of an invasive strategy, it is also prudent to re‐evaluate conservative and invasive management strategies in light of the significant potential confounding effect observed in a number of influential studies. Indeed, these studies warrant repeating, albeit employing a highly‐selective glycoprotein IIb/IIIa inhibitor strategy.
What's new
| Date | Event | Description |
|---|---|---|
| 26 August 2015 | New search has been performed | Three new studies met the inclusion criteria of this Cochrane review. |
| 26 August 2015 | New citation required and conclusions have changed | We re‐ran the search strategies for the last published review (with minor amendments). We included new articles related to three studies, Italian Elderly ACS, LIPSIA‐NSTEMI and OASIS 5, in the meta‐analysis. This resulted in a change to the review conclusions. |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 27 February 2009 | New citation required but conclusions have not changed | Change of review authors. |
| 27 February 2009 | New search has been performed | We updated the literature search to February 2008, and identified 22 additional potentially relevant references. We excluded five references that reported on two studies. The remaining 14 references were additional reports of already included studies. We added long‐term follow‐up data from the ICTUS trial. |
| 27 October 2008 | Amended | We converted to a new review format. |
| 5 March 2006 | New citation required and conclusions have changed | We made substantive amendments to the review. |
Acknowledgements
We thank Dr Michel R Hoenig (MRH) and Dr Jenny A Doust (JAD) for their contributions to previous versions of this Cochrane review. JAD contributed to the design of the review, extracted data, assessed the quality of included studies and assisted in data interpretation (1996 to 2008). In addition to the contributions of JAD, MRH was the primary author of previous versions of this Cochrane review (Hoenig 2004; Hoenig 2006; Hoenig 2010). We thank Professor Shah Ebrahim of the Cochrane Heart Group for his helpful comments and assistance in editing the protocol of this review (Hoenig 2004).
Appendices
Appendix 1. Search strategies 2008
CENTRAL on the Cochrane Library
#1 MeSH descriptor Angina, Unstable explode all trees #2 unstable next angina in All Text #3 coronary next syndrome* in All Text #4 MeSH descriptor Myocardial Infarction explode all trees #5 myocardial next infarct* in All Text #6 heart next infarct* in All Text #7 nstemi in All Text #8 unstable next coronary in All Text #9 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8) #10 (ischaemi* in All Text near/6 guid* in All Text) #11 (ischemi* in All Text near/6 guid* in All Text) #12 (early in All Text near/6 invasive in All Text) #13 (invasive in All Text near/6 conservative in All Text) #14 (angiography in All Text near/6 invasive in All Text) #15 (angiography in All Text near/6 conservative in All Text) #16 (ischemi* in All Text near/6 strateg* in All Text) #17 (ischaemi* in All Text near/6 strateg* in All Text) #18 (conservative in All Text near/6 strateg* in All Text) #19 (conservative in All Text near/6 therap* in All Text) #20 (conservative in All Text near/6 treatment* in All Text) #21 (conservative in All Text near/6 management in All Text) #22 (interventional in All Text near/6 strateg* in All Text) #23 (interventional in All Text near/6 therap* in All Text) #24 (interventional in All Text near/6 treatment* in All Text) #25 (interventional in All Text near/6 management in All Text) #26 (invasive in All Text near/6 strateg* in All Text) #27 (invasive in All Text near/6 therap* in All Text) #28 (invasive in All Text near/6 treatment* in All Text) #29 (invasive in All Text near/6 management in All Text) #30 (triage in All Text near/6 angiograph* in All Text) #31 (#10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19) #32 (#20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30) #33 (#31 or #32) #34 (#9 and #33)
MEDLINE (on Ovid)
1 Myocardial Infarction/ 2 exp Angina, Unstable/ 3 Acute Coronary Syndrome/ 4 unstable angina$.tw. 5 coronary syndrome$.tw. 6 myocardial infarction$.tw. 7 or/1‐6 8 (intervention$ adj2 (strateg$ or therapy or therapies or treatment$)).tw. 9 (conservative adj2 (strateg$ or therapy or therapies or treatment$)).tw. 10 (invasive adj2 (strateg$ or therapy or therapies or treatment$)).tw. 11 8 or 9 or 10 12 7 and 11 ( 13 (isch?emia adj2 guide$).tw. 14 ((invasive or conservative) adj2 management).tw. 15 11 or 13 or 14 16 7 and 15 17 randomized controlled trial.pt. 18 controlled clinical trial.pt. 19 Randomized controlled trials/ 20 random allocation/ 21 double blind method/ 22 single‐blind method/ 23 or/17‐22 24 exp animal/ not humans/ 25 23 not 24 26 clinical trial.pt. 27 exp Clinical Trials as Topic/ 28 (clin$ adj25 trial$).ti,ab. 29 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. 30 placebos/ 31 placebo$.ti,ab. 32 random$.ti,ab. 33 research design/ 34 or/26‐33 35 34 not 24 36 35 not 25 37 comparative study.pt. 38 exp evaluation studies/ 39 follow up studies/ 40 prospective studies/ 41 (control$ or prospectiv$ or volunteer$).ti,ab. 42 or/37‐41 43 42 not 24 44 43 not (25 or 36) 45 25 or 36 or 44 46 45 and 16
EMBASE (on Ovid)
1 exp heart infarction/ 2 exp unstable angina pectoris/ 3 Acute Coronary Syndrome/ 4 unstable angina$.tw. 5 coronary syndrome$.tw. 6 myocardial infarct$.tw. 7 heart infarct$.tw. 8 nstemi.tw. 9 unstable coronary.tw. 10 or/1‐8 11 (isch?emi$ adj3 guid$).tw. 12 (early adj3 invasive$).tw. 13 (early adj3 conservative$).tw. 14 (isch?emi$ adj3 strateg$).tw. 15 (conservative adj3 (strateg$ or therapy or therapies or treatment$ or management)).tw. 16 (interventional adj3 (strateg$ or therapy or therapies or treatment$ or management)).tw. 17 (invasive adj3 (strateg$ or therap$ or treatment$ or management)).tw. 18 (triage adj3 angiograph$).tw. 19 or/11‐18 20 10 and 19 21 controlled study/ 22 clinical trial/ 23 major clinical study/ 24 random$.tw. 25 randomized controlled trial/ 26 trial$.tw. 27 compar$.tw. 28 control$.tw. 29 follow‐up.tw. 30 blind$.tw. 31 double blind procedure/ 32 placebo$.tw. 33 clinical article/ 34 placebo/ 35 doubl$.tw. 36 or/21‐35 37 20 and 36 38 limit 37 to yr="1996 ‐ 2008"
MEDLINE (Ovid) search for 2006 review version
#1 explode 'Myocardial‐Infarction' / #2 explode 'Angina‐Unstable' / #3 unstable angina$ #4 coronary syndrome$ #5 myocardial infarct$ #6 myocardial infarction heart infarct$ #7 nstemi #8 unstable coronary #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 ischaemi$ adj3 guid$ #11 ischemi$ adj3 guid$ #12 early adj3 invasive #13 invasive adj3 conservative #14 ischemi$ adj3 strateg$ #15 ischaemi$ adj3 strateg$ #16 conservative adj3 strateg$ #17 conservative adj3 therap$ #18 conservative adj3 treatment$ #19 conservative adj3 management #20 interventional adj3 strateg$ #21 interventional adj3 therap$ #22 interventional adj3 treatment$ #23 interventional adj3 management #24 invasive adj3 strateg$ #25 invasive adj3 therap$ #26 invasive adj3 treatment$ #27 invasive adj3 management #28 triage adj3 angiograph$ #29 #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 #30 #9 and #29
We used a randomised controlled trial (RCT) filter as described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
Appendix 2. Search strategies 2015
We applied the RCT filter for MEDLINE is the Cochrane sensitivity‐maximising RCT filter, and for EMBASE, terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
CENTRAL on the Cochrane Library
#1 MeSH descriptor: [Myocardial Infarction] explode all trees
#2 MeSH descriptor: [Acute Coronary Syndrome] this term only
#3 MeSH descriptor: [Angina, Unstable] explode all trees
#4 unstable next angina*
#5 coronary next syndrome*
#6 myocardial next infarct*
#7 heart next infarct*
#8 nstemi
#9 unstable next coronary
#10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9
#11 (interven* near/6 (strateg* or therap* or treatment* or management))
#12 (conservative near/6 (strateg* or therap* or treatment* or management))
#13 (invasive near/6 (strateg* or therap* or treatment* or management))
#14 ((ischaemi* or ischemi*) near/6 (guid* or strateg*))
#15 early near/6 invasive
#16 invasive near/6 conservative
#17 angiography near/6 (invasive or conservative)
#18 triage near/6 angiograph*
#19 #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18
#20 #10 and #19
Publication years 2008‐2015
MEDLINE (on Ovid)
1. Myocardial Infarction/
2. exp Angina, Unstable/
3. Acute Coronary Syndrome/
4. unstable angina$.tw.
5. coronary syndrome$.tw.
6. myocardial infarction$.tw.
7. heart infarct*.tw.
8. nstemi.tw.
9. (unstable adj2 coronary).tw.
10. or/1‐9
11. (interven$ adj2 (strateg$ or therap$ or treatment$ or management)).tw.
12. (conservative adj2 (strateg$ or therap$ or treatment$ or management)).tw.
13. (invasive adj2 (strateg$ or therap$ or treatment$ or management)).tw.
14. (early adj2 invasive).tw.
15. ((ischaemi* or ischemi*) adj4 (guid* or strateg*)).tw.
16. (invasive adj4 conservative).tw.
17. (angiography adj4 (invasive or conservative)).tw.
18. (triage adj4 angiograph*).tw.
19. or/11‐18
20. 10 and 19
21. randomized controlled trial.pt.
22. controlled clinical trial.pt.
23. randomized.ab.
24. placebo.ab.
25. drug therapy.fs.
26. randomly.ab.
27. trial.ab.
28. groups.ab.
29. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
30. exp animals/ not humans.sh.
31. 29 not 30
32. 20 and 31
33. limit 32 to yr="2008 ‐Current"
EMBASE (on Ovid)
1. exp heart infarction/
2. exp unstable angina pectoris/
3. acute coronary syndrome/
4. unstable angina$.tw.
5. coronary syndrome$.tw.
6. myocardial infarct$.tw.
7. heart infarct*.tw.
8. nstemi.tw.
9. (unstable adj2 coronary).tw.
10. or/1‐9
11. (conservative adj3 (strateg$ or therap$ or treatment$ or management)).tw.
12. (interven$ adj3 (strateg$ or therap$ or treatment$ or management)).tw.
13. (invasive adj3 (strateg$ or therap$ or treatment$ or management)).tw.
14. (early adj2 invasive).tw.
15. ((ischaemi* or ischemi*) adj4 (guid* or strateg*)).tw.
16. (invasive adj4 conservative).tw.
17. (triage adj4 angiograph*).tw.
18. (angiography adj4 (invasive or conservative)).tw.
19. or/11‐18
20. 10 and 19
21. random$.tw.
22. factorial$.tw.
23. crossover$.tw.
24. cross over$.tw.
25. cross‐over$.tw.
26. placebo$.tw.
27. (doubl$ adj blind$).tw.
28. (singl$ adj blind$).tw.
29. assign$.tw.
30. allocat$.tw.
31. volunteer$.tw.
32. crossover procedure/
33. double blind procedure/
34. randomized controlled trial/
35. single blind procedure/
36. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35
37. (animal/ or nonhuman/) not human/
38. 36 not 37
39. 20 and 38
40. limit 39 to yr="2008 ‐Current"
Data and analyses
Comparison 1. All studies undertaken in the stent era regardless of glycoprotein IIb/IIIa receptor use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Index death | 6 | 8094 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.02, 2.34] |
| 1.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 2 | 3383 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.84, 3.31] |
| 1.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 4 | 4711 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.88, 2.48] |
| 2 Early death | 4 | 4345 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.70, 2.00] |
| 2.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 1 | 2220 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.76, 2.51] |
| 2.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 3 | 2125 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.31, 3.33] |
| 3 Intermediate death | 8 | 8915 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.64, 1.18] |
| 3.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 3 | 4020 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.66, 1.27] |
| 3.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 5 | 4895 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.48, 1.49] |
| 4 Late death | 3 | 5467 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.76, 1.08] |
| 4.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 1 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.74, 1.67] |
| 4.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 2 | 4267 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.04] |
| 5 Index myocardial infarction | 7 | 8694 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.65, 1.80] |
| 5.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 3 | 3983 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.51, 1.83] |
| 5.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 4 | 4711 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.59, 2.55] |
| 6 Early myocardial infarction | 4 | 4345 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.08] |
| 6.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 1 | 2220 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.79] |
| 6.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 3 | 2125 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.52, 1.44] |
| 7 Intermediate myocardial infarction | 8 | 8915 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 1.00] |
| 7.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 3 | 4020 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.56, 1.60] |
| 7.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 5 | 4895 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.60, 0.89] |
| 8 Late myocardial infarction | 3 | 5467 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.67, 0.92] |
| 8.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 1 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.55] |
| 8.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 2 | 4267 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.63, 0.90] |
| 9 Index death or non‐fatal myocardial infarction | 4 | 6618 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.59, 2.21] |
| 9.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 1 | 2220 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.51, 1.17] |
| 9.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 3 | 4398 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.75, 2.86] |
| 10 Early death or non‐fatal myocardial infarction | 2 | 2351 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.45, 0.92] |
| 10.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 1 | 2220 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.48, 0.94] |
| 10.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.06, 1.39] |
| 11 Intermediate death or non‐fatal myocardial infarction | 7 | 7715 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.20] |
| 11.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 2 | 2820 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.59, 1.60] |
| 11.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 5 | 4895 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.62, 1.32] |
| 12 Intermediate death or non‐fatal myocardial infarction; gender subanalysis | 5 | 6975 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.67, 0.91] |
| 12.1 Male | 4 | 4454 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.62, 0.87] |
| 12.2 Female | 5 | 2521 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.65, 1.16] |
| 13 Late death or non‐fatal myocardial infarction | 3 | 5467 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.73, 1.08] |
| 13.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 1 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.87, 1.63] |
| 13.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 2 | 4267 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.72, 0.92] |
| 14 Intermediate refractory angina | 5 | 8287 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.52, 0.79] |
| 14.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 3 | 4020 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.42, 1.08] |
| 14.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 2 | 4267 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.50, 0.64] |
| 15 Intermediate rehospitalisation | 6 | 6921 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.63, 0.94] |
| 15.1 Routine glycoprotein IIb/IIIa receptor antagonist use | 3 | 4020 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.61, 1.14] |
| 15.2 No routine glycoprotein IIb/IIIa receptor antagonist use | 3 | 2901 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.54, 0.94] |
Comparison 2. Safety endpoints.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Procedure‐related myocardial infarction | 5 | 6380 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [1.47, 2.37] |
| 2 Bleeding | 6 | 7584 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.30, 2.31] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
FRISC‐II.
| Methods | Prospective, randomised, multicentre trial with parallel groups. Invasve and non‐invasive treatments compared by factorial design. | |
| Participants | 2457 participants with anginal pain within the last 48 hours and ST depression or elevated cardiac markers. Overall impression of participant risk level: intermediate‐high. | |
| Interventions | Conservative arm: aspirin, beta blocker, statin, ACEI, dalteparin or UFH.
Invasive arm: as above and routine angiography (average time to angiography: 4 days). 10% glycoprotein 2b/3a receptor antagonist use. Each strategy further randomised to placebo or dalteparin in a double‐blind fashion. |
|
| Outcomes | Death all causes (6, 12, 24 months, 5 years), MI (6, 12, 24 months, 5 years), refractory angina (6 months), death or non‐fatal MI (6, 12, 24 months, 5 years), rehospitalisation (6 weeks, 6, 12 months), procedural MI, bleeding, contrast allergy. | |
| Notes | Sponsored by Pharmacia and Upjohn (a subsidiary of Pfizer). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation algorithm was not disclosed. An independent organisation performed randomisation. There were no significant differences in baseline characteristics of the groups, which supports minimal selection bias. |
| Allocation concealment (selection bias) | Low risk | An independent organisation performed randomisation by telefax (Clinical Data Care, Lund, Sweden). |
| Blinding (performance bias and detection bias) All outcomes | High risk | Allocation to invasive and non‐invasive strategies was open (allocation to long‐term dalteparin treatment with placebo was double‐blinded). |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was equivalent between groups. The trial randomised 1222 participants to invasive, with 32 lost to 6‐month follow‐up (2.62%) compared with 1235 participants randomised to conservative management and 49 lost to 6‐month follow‐up (3.96%). The trial used intention‐to‐treat (ITT) analysis. |
| Other bias | Low risk | The sponsoring pharmaceutical company employed continuous source‐data verification of all case‐record forms by external monitors. An independent clinical‐event committee and a data and safety monitoring board adjudicated adverse events. |
ICTUS.
| Methods | Prospective, randomised, multicentre trial. | |
| Participants | 1200 participants with accelerating angina or angina at rest in the preceding 24 hours and an elevated cardiac troponin T > 0.3 µg/L and either ischaemic ECG changes or a documented history of coronary artery disease (CAD) (previous catheterization, history of myocardial infarction (MI) or positive exercise test). Overall impression on level of risk in participants: high risk; all participants had a positive troponin test on randomisation. | |
| Interventions | Conservative arm: aspirin, enoxaparin, statin, clopidogrel. Invasive arm: as above, abciximab and routine angiography (median time to angiography: 23 hours) postrandomisation. 94% glycoprotein 2b/3a receptor antagonist use. | |
| Outcomes | Death all causes (1, 3 and 4 years), MI (1 and 3 years), rehospitalisation (1 and 3 years), major bleeding during the index admission. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted‐block randomisation, with stratification according to site, with block size randomly chosen to be 4, 6 or 8. Baseline characteristics were comparable between groups. |
| Allocation concealment (selection bias) | Low risk | Eligibility was confirmed prior to contacting a central telephone system for allocation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Members of an independent clinical endpoints committee, who were blinded to treatment allocation of participants, adjudicated endpoints. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Six participants were lost to follow‐up. The trial used ITT analysis. |
| Other bias | Unclear risk | Clopidogrel was more common at discharge for early invasive (61%) versus selective invasive (49%) strategies. There was sponsorship from Eli Lilly, Sanofi‐Synthelabo, Aventis, Pfizer and Medtronic. Sponsors were reported to have had no involvement in the design of the study, data collection or analysis, or the writing of the manuscript. |
Italian Elderly ACS.
| Methods | Prospective, randomised, multicentre trial | |
| Participants | 313 participants with symptoms suggestive of acute myocardial ischaemia at rest within 48 hours before randomisation and ischaemic ECG changes (transient or persistent ST‐segment elevation or depression > 0.5 mm but < 1 mm in the case of ST‐elevation or persistent and definite T wave inversion > 1 mm in at least 2 contiguous leads) and/or elevate levels (> upper limit of normal) of creatine kinase‐myocardial band (CK‐MB) or cTn. Overall impression of level of risk in participants: high risk; all participants were elderly (≥ 75 years of age). |
|
| Interventions | Early aggressive strategy (coronary angiography and, when indicated, revascularization within 72 hours) or initially conservative strategy (angiography and revascularization only for recurrent ischaemia). | |
| Outcomes | All‐cause death (6 months, 1 year), MI (6 months, 1 year), rehospitalisation (6 month, 1 year), major bleeding (6 months, 1 year), days spent in hospital (6 months, 1 year), stroke (6 month, 1 year). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated list, stratified by the centre, and randomly balanced every 4, 6 or 8 participants for each centre. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation was immediately made available to the investigator upon registering the participant on the website http://elderly.altavianet.it. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were collected in a web‐based case report form. This was audited/supervised by study monitors who visit study centres. An independent event adjudication committee adjudicated all serious adverse events on the basis of the review of the original source documents. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants were lost to follow‐up and 2 participants withdrew. Attrition was equal between groups. All recruited participants were accounted for in analysis of each group on an ITT basis. |
| Other bias | Low risk | There was no industry sponsorship. |
LIPSIA‐NSTEMI.
| Methods | Prospective, randomised, multicentre trial comparing immediate versus early versus selective invasive strategies. | |
| Participants | 602 participants with NSTEMI (ischaemic symptoms that were increasing or occurred at rest, with the last episode < 24 hours before randomisation plus elevated troponin T level ≥ 0.1 ng/mL) were admitted across 6 tertiary care centres with 24 hour PCI facilities. Overall impression of level of risk in participants: high risk; all participants with elevated troponin (T level ≥ 0.1 ng/mL). |
|
| Interventions | Immediate invasive strategy: < 2 hours after randomisation; early invasive strategy: 10 to 48 hours after randomisation; selective invasive only if refractory ischaemia. | |
| Outcomes | Primary endpoint: peak creatine kinase (CK)‐myocardial band (MB) activity during index admission. Secondary clinical endpoints were the composite of death and non‐fatal infarction; death, non‐fatal infarction and refractory ischaemia; death, non‐fatal infarction, refractory ischaemia and rehospitalisation for unstable angina within 6 months. | |
| Notes | Though results were expressed in terms of the 3 groups of randomisation (immediate versus early versus selective invasive) for the purposes of this review, the immediate and early invasive strategies were grouped and considered "early invasive", whereas the criteria for the selective invasive was most consistent with a conservative strategy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation system utilising permuted block randomisation performed with stratification according to site. |
| Allocation concealment (selection bias) | Low risk | Centralised web‐based allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants were lost to follow‐up, 1 each from the immediate invasive and the selective invasive groups. The trial used ITT analysis. |
| Other bias | Unclear risk | The study was supported by free tirofiban medication from MSD SHARP & DOHME GmbH, and Iroko Pharmaceutical. |
OASIS 5.
| Methods | Randomised, multicentre, prospectively designed substudy of the OASIS 5 trial (a double‐blinded trial in which fondaparinux was compared with enoxaparin in participants with UA/NSTEMI). | |
| Participants | 184 female participants were recruited when the OASIS 5 main trial was stopped. These participants presented to hospital with symptoms of UA or MI without persistent ST elevation and at least 2 of: age ≥ 60 years, troponin T or I or CK‐MB above the upper limit of normal or ECG changes compatible with ischaemia (ST depression ≥ 1 mm in 2 contiguous leads or T wave inversion > 3 mm or any dynamic ST shift or transient ST elevation). Overall impression of level of risk in participants: intermediate risk. |
|
| Interventions | Conservative/selective invasive arm: with coronary angiography only if symptoms or signs of severe ischaemia. Invasive arm: routine coronary angiography within 4 days of admission and, if appropriate, revascularisation within 7 days of admission. |
|
| Outcomes | Primary endpoint was the composite of death, MI or stroke at 2 years. Secondary outcomes included the following.
|
|
| Notes | Recruitment ceased early and sample sizes curtailed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated permuted block randomisation, stratified according to study centre using predetermined site specific randomisation ratios of 1:1, 1:2 or 2:1 for early intervention:delayed intervention in block sizes of 2 and 4. There were no significant differences in baseline characteristics of groups. |
| Allocation concealment (selection bias) | Low risk | Allocations were concealed at the Canadian Cardiovascular Collaboration Project Office, Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Canada and accessed via 24‐hour computerized telephone service. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A central committee of clinicians blinded to the allocated management strategy adjudicated death classified by cause, MI, refractory ischaemia, stroke and major bleeding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nine participants were lost to long‐term follow‐up, equally distributed between routine invasive (5) and selective invasive (4) strategies. The trial used ITT analysis. |
| Other bias | Unclear risk | Curtailment in sample size as well as follow‐up time. Sponsored by Sanofi‐Aventis, Organon and GlaxoSmithKline. The sponsor reportedly did not have a role in the study design; the collection, analysis, or interpretation of the data; the preparation, review or approval of the manuscript. |
RITA‐3.
| Methods | Prospective, randomised mulitcentre trial with parallel groups. | |
| Participants | 1810 participants with chest pain within the last 72 hours, a documented history of CAD, and one of the following: ischaemic ECG changes or Q waves suggesting previous MI or proven CAD on angiogram. The trial excluded those with probable evolving MI or those with elevated cardiac biomarkerss (2x) before randomisation. Overall impression on level of risk in participants: intermediate | |
| Interventions | Conservative arm: aspirin, beta blocker, enoxaparin Invasive arm: as above and routine angiography (median time to angiography: 2 days). 25% glycoprotein 2b/3a receptor antagonist use | |
| Outcomes | Death all causes (4, 12, 24 months, 5 years), MI (4, 12, 24 months, 5 years), refractory angina (4,12 mo), death or non‐fatal MI (4, 12, 24 months, 5 years), procedural bleeding and MI | |
| Notes | Recruitment from November 1997 to October 2001. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The methodology of randomisation was not disclosed. The baseline characteristics between groups were comparable. |
| Allocation concealment (selection bias) | Low risk | Central telephone service. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for at 2 years, 99.8% at 3 years and 59% at 5 years follow‐up. The trial used ITT analysis. |
| Other bias | Low risk | We did not detect any other sources of bias. |
TACTICS‐TIMI 18.
| Methods | Prospective, randomised, multicentre trial with parallel groups. | |
| Participants | 2220 participants with angina (accelerating or prolonged) at rest in preceding 24 hours and at least 1 of the following: ischaemic ECG changes, elevated cardiac markers or documented CAD (previous catheterisation, revascularisation or MI) Overall impression on level of risk in participants: variable; subanalyses reported on TIMI risk score and troponin status | |
| Interventions | Conservative arm: aspirin, beta blocker, UFH, tirofiban, statin Invasive arm: as above and routine angiography (median time to angiography: 22 hours). 94% glycoprotein 2b/3a receptor antagonist use | |
| Outcomes | Death all causes (30 days, 6 months), refractory angina (6 months), death or MI (30 days, 6 months), rehospitalisation (30 days, 6 months) | |
| Notes | Recruitment between December 1997 and December 1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methodology of randomisation was not disclosed. Baseline characteristics between groups were comparable. |
| Allocation concealment (selection bias) | Low risk | Centralised system. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | A blinded committee adjudicated endpoints. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for by the end of the trial; the trial used ITT analysis. |
| Other bias | Unclear risk | Sponsored by Merck. |
VINO.
| Methods | Prospective, randomised, multicentre trial with parallel groups. | |
| Participants | 131 participants with ischaemic chest pain lasting more than 20 mins (within the preceding 24 hours) and ECG changes and elevated cardiac markers Overall impression on level of risk in participants: high; all participants were cardiac biomarker positive | |
| Interventions | Conservative arm: aspirin, beta blocker, UFH Invasive arm: as above and routine angiography (average time to angiography: 6.2 hours). 0% glycoprotein 2b/3a receptor antagonist use | |
| Outcomes | Death all causes (30 days, 6 months), MI (30 days, 6 months), death or non‐fatal MI (30 days, 6 months), rehospitalisation (30 days, 6 months) | |
| Notes | Recruitment commenced May 1998. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequentially numbered envelopes. |
| Allocation concealment (selection bias) | Low risk | Sealed envelope. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for by the end of the trial; the trial used ITT analysis. |
| Other bias | Low risk | We did not detect any other sources of bias. |
ACEI = angiotensin converting enzyme inhibitors; UFH = unfractionated heparin; MI = myocardial infarction; ITT = intention to treat; ECG = electrocardiogram; UA = unstable angina; STEMI = ST segment elevation myocardial infarction; CAD = coronary artery disease
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ABOARD | This trial randomised 352 participants with acute coronary syndrome (ACS) without ST‐segment elevation and a TIMIscore of > 3 to an immediate or delayed invasive strategy. |
| ACUITY | This trial randomised 13819 participants with acute coronary syndromes (ACS) undergoing an invasive strategy to 1 of 3 antithrombotic regimes: unfractionated heparin or enoxaparin plus a glycoprotein IIb/IIIa inhibitor, bivalirudin plus a glycoprotein IIb/IIIa inhibitor, or bivalirudin alone. |
| EARLYACS | This trial randomly assigned 9492 participants with UA/NSTEMI, all of whom were assigned to an invasive strategy, to receive either early routine administration of eptifibatide or to delayed provisional administration. |
| Eisenberg 2005 | This trial included participants with STEMI and, while index and late death are reported, outcomes for UA/NSTEMI are not reported separately. Also, this was a trial of 88 participants where the primary endpoints related to quality of life. |
| ELISA | This trial randomised 220 participants with ACS to early angiography without tirofiban pretreatment (early strategy) or to delayed angiography after 24 to 48 hours of pre‐treatment with tirofiban (late strategy). |
| GUSTO2b 2003 | This was a post‐hoc analysis from a trial designed to compare hirudin to heparin in UA/NSTEMI participants. |
| Hsin 2010 | Participants were randomised to early invasive and early conservative treatment arms. The early‐conservative treatment arm was managed medically for the first 48 hours before undergoing routine coronary angiogram at 48 hours after enrolment, consistent with a "delayed invasive" rather than true conservative management. Thus this study does not meet the review;s criteria for conservative management strategy. |
| ISAR‐COOL | This trial included UA/NSTEMI participants that were all due to have angiography. This trial compared 2 invasive strategies depending on whether angiography was undertaken at < 6 hours or at 3 to 5 days. Hence, this trial compared 2 different invasive strategies i.e. early or delayed invasive and is inappropriate for this review. |
| MATE 1998 | This trial was undertaken in the pre‐stent era and included participants with STEMI. |
| MITI 2000 | This was not a randomised controlled trial (RCT). The data are extracted from a registry. |
| OPTIMA | This trial randomised 251 participants with non‐ST‐elevation ACS who were eligible for PCI to either immediate or deferred (24 to 48 hours) PCI. |
| Teixeira 2009 | This was not a RCT but an observational comparative study. |
| TIMACS | This study randomised 3031 participants with all forms of ACS (not specifically UA/NSTEMI patients), to undergo early (< 36 hours) or delayed (> 36 hours) intervention. |
| TIMI‐3b | This trial was undertaken in the pre‐stent era. |
| TRUCS 2000 | This trial was deemed inappropriate to this review since the included participants were admitted with recurrent angina 48 hours after the index case of unstable angina. Hence, the participants in this trial had all been managed conservatively for at least 48 hours after their index chest pain, and had to suffer another bout of angina before randomisation was considered. The included studies in this review require that participants were randomised at index presentation. This study, by definition, only considered participants with Braunwald class IIIb or IIIc unstable angina, and is therefore dissimilar enough from the included studies to warrant exclusion. |
| VANQWISH 1998 | This trial was undertaken in the pre‐stent era and included participants treated with thrombolysis. |
| Yu 2011 | All participants underwent coronary angiogram prior to randomisation into PCI and conservative therapy. Thus, this study does not meet the review's criteria for conservative management. |
| Zhang 2010 | Eight hundred and fifteen non‐ST‐elevation ACS patients undergoing an invasive strategy were randomly assigned to undergo early (< 24hrs) or delayed (> 24hrs) intervention. |
| Zhao 2005 | This study doesn't meet this review's stent requirement. |
UA = unstable angina; NSTEMI = non ST segment myocardial infarction; STEMI = ST segment elevation myocardial infarction; ACS = acute coronary syndrome; PCI = percutaneous coronary intervention
Characteristics of ongoing studies [ordered by study ID]
Dimitrov 2013.
| Trial name or title | Timing of invasive strategy in acute coronary syndrome without ST segment elevation in groups of patients with different ischemic risk |
| Methods | Randomized controlled trial |
| Participants | 178 participants with UA/NSTEMI |
| Interventions | Early invasive (coronary angiography‐SCAG and percutaneous intervention‐PCI in the first 24 hours after admission); selective invasive (attempt for medical stabilization and proceeding to SCAG only in case of angina recurrence and/or evidence of inducible myocardial ischaemia |
| Outcomes | Choice of an early invasive strategy in participants with acute coronary syndrome without ST elevation in the presence of high risk features is associated with a reduced incidence of MACE compared to a selective invasive strategy. In the subgroups of participants without high risk characteristics the advantages of early versus selective are not as clear |
| Starting date | |
| Contact information | University Hospital St. Ekaterina, Sofia, Bulgaria |
| Notes | Full study not yet analysed/published. We extracted details from a conference abstract |
UA = unstable angina; NSTEMI = non ST segment myocardial infarction; SCAG = Selective coronary angiography; PCI = Percutaneous coronary intervention; MACE = Major adverse cardiac events
Differences between protocol and review
We modified the title of the review to reflect evolution in practice. "Routine invasive" replaces "early invasive" and "selective invasive" replaces "conservative". We used the GRADE methodology to assess the quality of evidence and included 'Summary of findings' tables, though we did not specify this in the published Cochrane protocol (Hoenig 2004).
Contributions of authors
JPF is the primary author of the review update, and extracted and analysed data (2008 to 2015). JN screened results and prepared the 'Summary of findings' tables and performed the GRADE assessments. CNA and IAS designed the protocol and provided advice regarding the final manuscript. DLW is an author of the review update, and extracted and analysed the data (2008 to 2015).
Sources of support
Internal sources
-
The Prince Charles Hospital Foundation (TPCHF), Australia.
Grant support
-
The University of Queensland, Australia.
Scholarship
External sources
-
The Cardiac Society of Australia and New Zealand (CSANZ), Australia.
Scholarship
Declarations of interest
JPF has no known conflicts of interest. JN has no known conflicts of interest. IAS has no known conflicts of interest. CNA received travel grants from Boston Scientific, J&J, Medtronic, MSD and Eli Lilly. DLW has been an investigator on a number of clinical research trials in the area of ACS and PCI. All funds in this regard have been paid to the institution to cover research costs. He did some paid proctoring work in the field of TAVI, which might not be directly relevant to this work.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
FRISC‐II {published data only}
- Diderholm E, Andrén B, Frostfeldt G, Genberg M, Jernberg T, Lagerqvist B, et al. ST depression in ECG at entry indicates severe coronary lesions and large benefits of an early invasive treatment strategy in unstable coronary artery disease; the FRISC II ECG sub study. The Fast Revascularisation during InStability in Coronary artery disease. European Heart Journal 2002;23(1):41‐9. [DOI] [PubMed] [Google Scholar]
- Diderholm E, Andrén B, Frostfeldt G, Genberg M, Jernberg T, Lagerqvist B, et al. The prognostic and therapeutic implications of increased troponin T levels and ST depression in unstable coronary artery disease: the FRISC II invasive troponin T electrocardiogram substudy. American Heart Journal 2002;143(5):760‐7. [DOI] [PubMed] [Google Scholar]
- FRagmin and Fast Revascularisation during InStability in Coronary artery disease Investigators. Invasive compared with non‐invasive treatment in unstable coronary‐artery disease: FRISC II prospective randomised multicentre study. Lancet 1999;354(9180):708‐15. [PubMed] [Google Scholar]
- Holmvang L, Clemmensen P, Lindahl B, Lagerqvist B, Venge P, Wagner G, et al. Quantitative analysis of the admission electrocardiogram identifies patients with unstable coronary artery disease who benefit the most from early invasive treatment. Journal of the American College of Cardiology 2003;41(6):905‐15. [DOI] [PubMed] [Google Scholar]
- Lagerqvist B, Husted S, Kontny F, Näslund U, Ståhle E, Swahn E, et al. A long‐term perspective on the protective effects of an early invasive strategy in unstable coronary artery disease: two‐year follow‐up of the FRISC‐II invasive study. Journal of the American College of Cardiology 2002;40(11):1902‐14. [DOI] [PubMed] [Google Scholar]
- Lagerqvist B, Husted S, Kontny F, Ståhle E, Swahn E, Wallentin L. 5‐year outcomes in the FRISC‐II randomised trial of an invasive versus a non‐invasive strategy in non‐ST‐elevation acute coronary syndrome: a follow‐up study. Lancet 2006;368(9540):998‐1004. [DOI] [PubMed] [Google Scholar]
- Lagerqvist B, Säfström K, Ståhle E, Wallentin L, Swahn E, FRISC II Study Group Investigators. Is early invasive treatment of unstable coronary artery disease equally effective for both women and men? FRISC II Study Group Investigators. Journal of the American College of Cardiology 2001;38(1):41‐8. [DOI] [PubMed] [Google Scholar]
- Wallentin L, Lagerqvist B, Husted S, Kontny F, Ståhle E, Swahn E. Outcome at 1 year after an invasive compared with a non‐invasive strategy in unstable coronary‐artery disease: the FRISC II invasive randomised trial. FRISC II Investigators. Fast Revascularisation during Instability in Coronary artery disease. Lancet 2000;356(9223):9‐16. [DOI] [PubMed] [Google Scholar]
ICTUS {published data only}
- Hirsch A, Windhausen F, Tijssen JG, Verheugt FW, Cornel JH, Winter RJ. Long‐term outcome after an early invasive versus selective invasive treatment strategy in patients with non‐ST‐elevation acute coronary syndrome and elevated cardiac troponin T (the ICTUS trial): a follow‐up study. Lancet 2007;369(9564):827‐35. [DOI] [PubMed] [Google Scholar]
- Windhausen F, Hirsch A, Tijssen JG, Cornel JH, Verheugt FW, Klees MI, et al. ST‐segment deviation on the admission electrocardiogram, treatment strategy, and outcome in non‐ST‐elevation acute coronary syndromes. A substudy of the Invasive versus Conservative Treatment in Unstable coronary Syndromes (ICTUS) Trial. Journal of Electrocardiology 2007;40(5):408‐15. [DOI] [PubMed] [Google Scholar]
- Winter RJ, Windhausen F, Cornel JH, Dunselman PH, Janus CL, Bendermacher PE, et al. Early invasive versus selectively invasive management for acute coronary syndromes. New England Journal of Medicine 2005;353(11):1095‐104. [DOI] [PubMed] [Google Scholar]
Italian Elderly ACS {published data only}
- Savonitto S, Cavallini C, Petronio AS, Murena E, Antonicelli R, Sacco A, et al. Early aggressive versus initially conservative treatment in elderly patients with non‐ST‐segment elevation acute coronary syndrome: a randomized controlled trial. JACC: Cardiovascular Interventions 2012;5(9):906‐16. [DOI] [PubMed] [Google Scholar]
- Savonitto S, Servi S, Petronio AS, Bolognese L, Cavallini C, Greco C, et al. Early aggressive vs. initially conservative treatment in elderly patients with non‐ST‐elevation acute coronary syndrome: the Italian Elderly ACS study. Journal of Cardiovascular Medicine 2008;9(3):217‐26. [DOI] [PubMed] [Google Scholar]
LIPSIA‐NSTEMI {published data only}
- Thiele H, Rach J, Klein N, Pfeiffer D, Hartmann A, Hambrecht R, et al. Optimal timing of invasive angiography in stable non‐ST‐elevation myocardial infarction: the Leipzig Immediate versus early and late PercutaneouS coronary Intervention triAl in NSTEMI (LIPSIA‐NSTEMI Trial). European Heart Journal 2012;33(16):2035‐43. [DOI] [PubMed] [Google Scholar]
OASIS 5 {published data only}
- Swahn E, Alfredsson J, Afzal R, Budaj A, Chrolavicius S, Fox K, et al. Early invasive compared with a selective invasive strategy in women with non‐ST‐elevation acute coronary syndromes: a substudy of the OASIS 5 trial and a meta‐analysis of previous randomized trials. European Heart Journal 2012;33(1):51‐60. [DOI] [PubMed] [Google Scholar]
RITA‐3 {published data only}
- Clayton TC, Pocock SJ, Henderson RA, Poole‐Wilson PA, Shaw TR, Knight R, et al. Do men benefit more than women from an interventional strategy in patients with unstable angina or non‐ST‐elevation myocardial infarction? The impact of gender in the RITA 3 trial. European Heart Journal 2004;25(18):1641‐50. [DOI] [PubMed] [Google Scholar]
- Fox KA, Poole‐Wilson P, Clayton TC, Henderson RA, Shaw TR, Wheatley DJ, et al. 5‐year outcome of an interventional strategy in non‐ST‐elevation acute coronary syndrome: the British Heart Foundation RITA 3 randomised trial. Lancet 2005;366(9489):914‐20. [DOI] [PubMed] [Google Scholar]
- Fox KA, Poole‐Wilson PA, Henderson RA, Clayton TC, Chamberlain DA, Shaw TR, et al. Interventional versus conservative treatment for patients with unstable angina or non‐ST‐elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Randomized Intervention Trial of unstable Angina. Lancet 2002;360(9335):743‐51. [DOI] [PubMed] [Google Scholar]
TACTICS‐TIMI 18 {published data only}
- Cannon CP, Weintraub WS, Demopoulos LA, Vicari R, Frey MJ, Lakkis N, et al. Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. New England Journal of Medicine 2001;344(25):1879‐87. [DOI] [PubMed] [Google Scholar]
- Glaser R, Herrmann HC, Murphy SA, Demopoulos LA, DiBattiste PM, Cannon CP, et al. Benefit of an early invasive management strategy in women with acute coronary syndromes. JAMA 2002;288(24):3124‐9. [DOI] [PubMed] [Google Scholar]
- Morrow DA, Cannon CP, Rifai N, Frey MJ, Vicari R, Lakkis N, et al. Ability of minor elevations of troponins I and T to predict benefit from an early invasive strategy in patients with unstable angina and non‐ST elevation myocardial infarction: results from a randomized trial. JAMA 2001;286(19):2405‐12. [DOI] [PubMed] [Google Scholar]
- Sabatine MS, Morrow DA, McCabe CH, Antman EM, Gibson CM, Cannon CP. Combination of quantitative ST deviation and troponin elevation provides independent prognostic and therapeutic information in unstable angina and non‐ST‐elevation myocardial infarction. American Heart Journal 2006;151(1):25‐31. [DOI] [PubMed] [Google Scholar]
VINO {published data only}
- Spacek R, Widimský P, Straka Z, Jiresová E, Dvorák J, Polásek R, et al. Value of first day angiography/angioplasty in evolving Non‐ST segment elevation myocardial infarction: an open multicenter randomized trial. The VINO Study. European Heart Journal 2002;23(3):230‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ABOARD {published data only}
- Montalescot G, Cayla G, Collet JP, Elhadad S, Begui F, Breton H, et al. Immediate vs. Delayed intervention for acute coronary syndromes: a randomized clinical trial. JAMA 2009;302(9):947‐54. [DOI] [PubMed] [Google Scholar]
ACUITY {published data only}
- Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, et al. Bivalirudin for patients with acute coronary syndromes. New England Journal of Medicine 2006;355(21):2203‐16. [DOI] [PubMed] [Google Scholar]
EARLYACS {published data only}
- Giugliano RP, White JA, Bode C, Armstrong PW, Montalescot G, Lewis BS, et al. Early versus delayed, provisional eptifibatide in acute coronary syndrome. New England Journal of Medicine 2009;360(21):2176‐90. [DOI] [PubMed] [Google Scholar]
Eisenberg 2005 {published data only}
- Eisenberg MJ, Teng FF, Chaudhry MR, Ortiz J, Sobkowski W, Ebrahim I, et al. Impact of invasive management versus noninvasive management on functional status and quality of life following non‐Q‐wave myocardial infarction: a randomized clinical trial. American Heart Journal 2005;149(5):813‐9. [DOI] [PubMed] [Google Scholar]
ELISA {published data only}
- van't Hof AW, Vries ST, Dambrink JH, Miedema K, Suryapranta H, Hoorntje JC, et al. A comparison of two invasive strategies in patients with non‐ST elevation acute coronary syndromes: results of the Early or Late Intervention in unStable Angina (ELISA) pilot Study. European Heart Journal 2003;24(15):1401‐5. [DOI] [PubMed] [Google Scholar]
GUSTO2b 2003 {published data only}
- Cho L, Bhatt DL, Marso SP, Brennan D, Holmes DR Jr, Califf RM, et al. An invasive strategy is associated with decreased mortality in patients with unstable angina and non‐ST‐elevation myocardial infarction: GUSTO IIb trial. American Journal of Medicine 2003;114(2):106‐11. [DOI] [PubMed] [Google Scholar]
Hsin 2010 {published data only}
- Hsin HT, Li AH, Yeih DF, Lai CL, Chiu YW, Liao PC, et al. Two‐year follow‐up of Tirofiban‐based management of non‐ST‐elevation acute coronary syndrome ‐ a single center study. Acta Cardiologica Sinica 2010;26:19‐27. [Google Scholar]
ISAR‐COOL {published data only}
- Neumann FJ, Kastrati A, Pogatsa‐Murray G, Mehilli J, Bollwein H, Bestehorn HP, et al. Evaluation of prolonged antithrombotic pretreatment ("cooling‐off" strategy) before intervention in patients with unstable coronary syndromes: a randomized controlled trial. JAMA 2003;290(12):1593‐9. [DOI] [PubMed] [Google Scholar]
MATE 1998 {published data only}
- McCullough PA, O'Neill WW, Graham M, Stomel RJ, Rogers F, David S, et al. A prospective randomized trial of triage angiography in acute coronary syndromes ineligible for thrombolytic therapy. Results of the medicine versus angiography in thrombolytic exclusion (MATE) trial. Journal of the American College of Cardiology 1998;32(3):596‐605. [DOI] [PubMed] [Google Scholar]
MITI 2000 {published data only}
- Scull GS, Martin JS, Weaver WD, Every NR. Early angiography versus conservative treatment in patients with non‐ST elevation acute myocardial infarction: MITI Investigators. Myocardial Infarction Triage and Intervention. Journal of the American College of Cardiology 2000;35(4):895‐902. [DOI] [PubMed] [Google Scholar]
OPTIMA {published data only}
- Riezebos RK, Ronner E, Ter Bals E, Slagboom T, Smits PC, Berg JM, et al. Immediate verse deferred coronary angioplasty in non‐ST‐segment elevation acute coronary syndromes. Heart 2009;95(10):807‐12. [DOI] [PubMed] [Google Scholar]
Teixeira 2009 {published data only}
- Teixeira R, Lourenço C, Baptista R, Jorge E, António N, Monteiro S, et al. Invasive versus conservative strategy in non‐ST elevation acute coronary syndromes: data from a single Portuguese center. Revista Portuguesa de Cardiologia 2009;28(4):355‐73. [PubMed] [Google Scholar]
TIMACS {published data only}
- Mehta SR, Granger CB, Boden WE, Steg PG, Bassand JP, Faxon DP, et al. Early versus delayed invasive intervention in acute coronary syndromes. New England Journal of Medicine 2009;360(21):2165‐75. [DOI] [PubMed] [Google Scholar]
TIMI‐3b {published data only}
- Anderson HV, Cannon CP, Stone PH, Williams DO, McCabe CH, Knatterud GL, et al. One‐year results of the Thrombolysis in Myocardial Infarction (TIMI) IIIB clinical trial. A randomized comparison of tissue‐type plasminogen activator versus placebo and early invasive versus early conservative strategies in unstable angina and non‐Q wave myocardial infarction. Journal of the American College of Cardiology 1995;26(7):1643‐50. [DOI] [PubMed] [Google Scholar]
TRUCS 2000 {published data only}
- Michalis LK, Stroumbis CS, Pappas K, Sourla E, Niokou D, Goudevenos JA, et al. Treatment of refractory unstable angina in geographically isolated areas without cardiac surgery. Invasive versus conservative strategy (TRUCS study). European Heart Journal 2000;21(23):1954‐9. [DOI] [PubMed] [Google Scholar]
VANQWISH 1998 {published data only}
- Boden WE, O'Rourke RA, Crawford MH, Blaustein AS, Deedwania PC, Zoble RG, et al. Outcomes in patients with acute non‐Q‐wave myocardial infarction randomly assigned to an invasive as compared with a conservative management strategy. Veterans Affairs Non‐Q‐Wave Infarction Strategies in Hospital (VANQWISH) Trial Investigators. New England Journal of Medicine 1998;338(25):1785‐92. [DOI] [PubMed] [Google Scholar]
- Heggunje PS, Wade MJ, O'Rourke RA, Kleiger PC, Deedwania PC, Lavori PW, et al. Early invasive versus ischaemia‐guided strategies in the management of non‐Q wave myocardial infarction patients with and without prior myocardial infarction. Results of Veterans Affairs Non‐Q Wave Infarction Strategies in Hospital (VANQWISH) trial. European Heart Journal 2000;21(24):2014‐25. [DOI] [PubMed] [Google Scholar]
Yu 2011 {published data only}
- Yu DQ, Lin SG, Zhou YL, Li G, Tan N, Dong HJ, et al. Vulnerable plaque burden post pharmacological and interventional treatments in patients with acute coronary syndrome and borderline lesion: intravascular ultrasound follow up results. Chung‐Hua Hsin Hsueh Kuan Ping Tsa Chih [Chinese Journal of Cardiology] 2011;39(2):137‐41. [PubMed] [Google Scholar]
Zhang 2010 {published data only}
- Zhang J, Qiao SB, Zhu J, Chinese Cooperative Group of the Timing of Intervention in Acute Coronary Syndrome. Outcome of patients with non‐ST segment elevation acute coronary syndrome undergoing early or delayed intervention. Zhonghua Xin Xue Guan Bing Za Xhi 2010;38(10):865‐9. [PubMed] [Google Scholar]
Zhao 2005 {published data only}
- Jiang LQ, Zhao MZ, Hu DY. Predictive value of positive troponin I in clinical prognosis of non‐ST‐segment elevation acute coronary syndrome. Zhonghua Nei Ke Za Zhi 2005;44(5):350‐2. [PubMed] [Google Scholar]
- Zhao MZ, Hu DY, Jiang LQ, Wu Y, Zhu TG, Hao HJ, et al. The effect of early invasive strategy on early and late outcomes in high‐risk non‐ST‐segment elevation acute coronary syndromes. Zhonghua Nei Ke Za Zhi 2005;44(10):737‐40. [PubMed] [Google Scholar]
- Zhao MZ, Hu DY, Ma CS, Jiang LQ, Huo Y, Zhu TG, et al. The relationship between TIMI (thrombolysis in myocardial infarction) risk score and efficacy of conservative or interventional strategy in patients with non‐ST‐segment elevation acute coronary syndromes. Zhonghua Xin Xue Guan Bing Za Zhi 2006;34(11):1001‐4. [PubMed] [Google Scholar]
- Zhao MZ, Hu DY, Zhang FC, Wu Y, Yan MY, Wang SW, et al. The changes of electrocardiogram and impact of early invasive strategy in patients with acute coronary syndromes without ST‐segment elevation. Zhonghua Yi Xue Za Zhi 2005;85(13):879‐82. [PubMed] [Google Scholar]
References to ongoing studies
Dimitrov 2013 {published data only}
- Dimitrov N, Simova I, Mateev H, Radkova M, Pavlov P, Tasheva I. Timing of invasive strategy in acute coronary syndrome without ST segment elevation in groups of patients with different ischemic risk. American Journal of Cardiology 2013;111(Issue 7, Supplement):55B. [Google Scholar]
Additional references
Al Suwaidi 2004
- Al Suwaidi J, Holmes DR Jr, Salam AM, Lennon R, Berger PB. Impact of coronary artery stents on mortality and nonfatal myocardial infarction: meta‐analysis of randomized trials comparing a strategy of routine stenting with that of balloon angioplasty. American Heart Journal 2004;147(5):815‐22. [DOI] [PubMed] [Google Scholar]
Alexander 2007
- Alexander KP, Newby LK, Cannon CP, Armstrong PW, Gibler WB, Rich MW, et al. Acute coronary care in the elderly, part I: Non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation 2007;115(19):2549‐69. [DOI] [PubMed] [Google Scholar]
Anderson 2007
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2007;116(7):e148‐304. [DOI] [PubMed] [Google Scholar]
Antman 1996
- Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, et al. Cardiac‐specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. New England Journal of Medicine 1996;335(18):1342‐9. [DOI] [PubMed] [Google Scholar]
Antman 2000
- Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non‐ST elevation MI: A method for prognostication and therapeutic decision making. JAMA 2000;284(7):835‐42. [DOI] [PubMed] [Google Scholar]
Antman 2004
- Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation 2004;110(9):e82‐e292. [PubMed] [Google Scholar]
Armstrong 1998
- Armstrong PW, Fu Y, Chang W‐C, Topol EJ, Granger CB, Betriu A, et al. Acute coronary syndromes in the GUSTO‐IIb trial: prognostic insights and impact of recurrent ischemia. Circulation 1998;98(18):1860‐8. [DOI] [PubMed] [Google Scholar]
Bach 2004
- Bach RG, Cannon CP, Weintraub WS, DiBattiste PM, Demopoulos LA, Anderson HV, et al. The effect of routine, early invasive management on outcome for elderly patients with non‐ST‐segment elevation acute coronary syndromes. Annals of Internal Medicine 2004;141(3):186‐95. [DOI] [PubMed] [Google Scholar]
Bauer 2007
- Bauer T, Koeth O, Jünger C, Heer T, Wienbergen H, Gitt A, et al. Effect of an invasive strategy on in‐hospital outcome in elderly patients with non‐ST‐elevation myocardial infarction. European Heart Journal 2007;28(23):2873‐8. [DOI] [PubMed] [Google Scholar]
Bavry 2006
- Bavry AA, Kumbhani DJ, Rassi AN, Bhatt DL, Askari AT. Benefit of early invasive therapy in acute coronary syndromes: a meta‐analysis of contemporary randomized clinical trials. Journal of the American College of Cardiology 2006;48(7):1319‐25. [DOI] [PubMed] [Google Scholar]
Benamer 1999
- Benamer H, Steg PG, Benessiano J, Vicaut E, Gaultier CJ, Aubry P, et al. Elevated cardiac troponin I predicts a high‐risk angiographic anatomy of the culprit lesion in unstable angina. American Heart Journal 1999;137(5):815‐20. [DOI] [PubMed] [Google Scholar]
Bhatt 2005
- Bhatt DL, Topol EJ. Does creatinine kinase‐MB elevation after percutaneous coronary intervention predict outcomes in 2005? Periprocedural cardiac enzyme elevation predicts adverse outcomes. Circulation 2005;112(6):906‐15. [DOI] [PubMed] [Google Scholar]
Blankstein 2005
- Blankstein R, Ward RP, Arnsdorf M, Jones B, Lou YB, Pine M. Female gender is an independent predictor of operative mortality after coronary artery bypass graft surgery: contemporary analysis of 31 Midwestern hospitals. Circulation 2005;112 Suppl(9):I323‐7. [DOI] [PubMed] [Google Scholar]
Boden 1991
- Boden WE, Krone RJ, Kleiger RE, Oakes D, Greenberg H, Dwyer EJ Jnr, et al. Electrocardiographic subset analysis of diltiazem administration on long‐term outcome after acute myocardial infarction. The Multicenter Diltiazem Post‐Infarction Trial Research Group. American Journal of Cardiology 1991;67(5):335‐42. [DOI] [PubMed] [Google Scholar]
Boersma 2002
- Boersma E, Harrington RA, Moliterno DJ, White H, Theroux P, Werf F, et al. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta‐analysis of all major randomised clinical trials. Lancet 2002;359(9302):189‐98. [DOI] [PubMed] [Google Scholar]
Braunwald 1998
- Braunwald E. Unstable angina: an etiologic approach to management. Circulation 1998;98(21):2219‐22. [DOI] [PubMed] [Google Scholar]
Braunwald 2003
- Braunwald E. Application of current guidelines to the management of unstable angina and non‐ST‐elevation myocardial infarction. Circulation 2003;108 Suppl 1(16):III28‐37. [DOI] [PubMed] [Google Scholar]
Cairns 1985
- Cairns JA, Gent M, Singer J, Finnie KJ, Froggatt GM, Holder DA, et al. Aspirin, sulfinpyrazone, or both in unstable angina. Results of a Canadian multicenter trial. New England Journal of Medicine 1985;313(22):1369‐75. [DOI] [PubMed] [Google Scholar]
Cannon 1997
- Cannon CP, McCabe CH, Stone PH, Rogers WJ, Schactman M, Thompson BW, et al. The electrocardiogram predicts one‐year outcome of patients with unstable angina and non‐Q wave myocardial infarction: results of the TIMI III Registry ECG Ancillary Study. Thrombolysis in Myocardial Ischemia. Journal of the American College of Cardiology 1997;30(1):133‐40. [DOI] [PubMed] [Google Scholar]
Choudhry 2005
- Choudhry NK, Singh JM, Barolet A, Tomlinson GA, Detsky AS. How should patients with unstable angina and non‐ST‐segment elevation myocardial infarction be managed? A meta‐analysis of randomized trials. American Journal of Medicine 2005;118(5):465‐74. [DOI] [PubMed] [Google Scholar]
Clayton 2004
- Clayton TC, Pocock SJ, Henderson RA, Poole‐Wilson PA, Shaw TR, Knight R, et al. Do men benefit more than women from an interventional strategy in patients with unstable angina or non‐ST‐elevation myocardial infarction? The impact of gender in the RITA 3 trial. Eurpean Heart Journal 2004;25(18):1641‐50. [DOI] [PubMed] [Google Scholar]
Cucherat 2003
- Cucherat M, Bonnefoy E, Tremeau G. Primary angioplasty versus intravenous thrombolysis for acute myocardial infarction. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001560.pub2] [DOI] [PubMed] [Google Scholar]
CURE 2001
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐Segment Elevation. New England Journal of Medicine 2001;345(7):494‐502. [DOI] [PubMed] [Google Scholar]
Cutlip 2005
- Cutlip DE, Kuntz RE. Does creatinine kinase‐MB elevation after percutaneous coronary intervention predict outcomes in 2005? Cardiac enzyme elevation after successful percutaneous coronary intervention is not an independent predictor of adverse outcomes. Circulation 2005;112(6):916‐22. [PubMed] [Google Scholar]
Damman 2012
- Damman P, Clayton T, Wallentin L, Lagerqvist B, Fox KA, Hirsch A, et al. Effects of age on long‐term outcomes after a routine invasive or selective invasive strategy in patients presenting with non‐ST segment elevation acute coronary syndromes: a collaborative analysis of individual data from the FRISC II‐ICTUS‐RITA 3 (FIR) trials. Heart 2011;98(3):207‐13. [DOI] [PubMed] [Google Scholar]
Dauerman 2002
- Dauerman HL, Goldberg RJ, White K, Gore JM, Sadiq I, Gurfinkel E, et al. Revascularization, stenting, and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. American Journal of Cardiology 2002;90(8):838‐42. [DOI] [PubMed] [Google Scholar]
de Araújo Gonçalves 2005
- Araújo Gonçalves P, Ferreira J, Aguiar C, Seabra‐Gomes R. TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE‐ACS. European Heart Journal 2005;26(9):865‐72. [DOI] [PubMed] [Google Scholar]
de Winter 2005
- Winter RJ, Windhausen F, Cornel JH, Dunselman PH, Janus CL, Bendermacher PE, et al. Early invasive versus selectively invasive management for acute coronary syndromes. New England Journal of Medicine 2005;353(11):1095‐104. [DOI] [PubMed] [Google Scholar]
DeWood 1980
- DeWood MA, Spores J, Notske R, Mouser LT, Burroughs R, Golden MS, et al. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. New England Journal of Medicine 1980;303(16):897‐902. [DOI] [PubMed] [Google Scholar]
DeWood 1986
- DeWood MA, Stifter WF, Simpson CS, Spores J, Eugster GS, Judge TP, et al. Coronary arteriographic findings soon after non‐Q‐wave myocardial infarction. New England Journal of Medicine 1986;315(7):417‐23. [DOI] [PubMed] [Google Scholar]
Diderholm 2002
- Diderholm E, Andrén B, Frostfeldt G, Genberg M, Jernberg T, Lagerqvist B, et al. The prognostic and therapeutic implications of increased troponin T levels and ST depression in unstable coronary artery disease: the FRISC II invasive troponin T electrocardiogram substudy. American Heart Journal 2002;143(5):760‐7. [DOI] [PubMed] [Google Scholar]
Dokainish 2005
- Dokainish H, Pillai M, Murphy SA, DiBattiste PM, Schweiger MJ, Lotfi A, et al. Prognostic implications of elevated troponin in patients with suspected acute coronary syndrome but no critical epicardial coronary disease: a TACTICS‐TIMI‐18 substudy. Journal of the American College of Cardiology 2005;45(1):19‐24. [DOI] [PubMed] [Google Scholar]
Eagle 2004
- Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Werf F, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6‐month postdischarge death in an international registry. JAMA 2004;291(22):2727‐33. [DOI] [PubMed] [Google Scholar]
EARLY ACS 2009
- Giugliano RP, White JA, Bode C, Armstrong PW, Montalescot G, Lewis BS, et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. New England Journal of Medicine 2009;360(21):2176‐90. [DOI] [PubMed] [Google Scholar]
EPIC 1994
- Shadoff N, Valett N, Bates E, Galeana A, Knopf W, Shaftel J, et al. Use of a monoclonal antibody directed against the platelet glycoprotein IIb/IIIa receptor in high‐risk coronary angioplasty. The EPIC Investigation. New England Journal of Medicine 1994;330(14):956‐61. [DOI] [PubMed] [Google Scholar]
EPILOG 1997
- Topol EJ, Califf RM, Lincoff AM, Tcheng JE, Cabot CF, Weisman HF, et al. Platelet glycoprotein IIb/IIIa receptor blockade and low‐dose heparin during percutaneous coronary revascularization. The EPILOG Investigators. New England Journal of Medicine 1997;336(24):1689‐96. [DOI] [PubMed] [Google Scholar]
EPISTENT 1998
- Topol EJ, Lincoff AM, Califf RM, Tcheng JE, Cohen EA, Kleiman NS, et al. Randomised placebo‐controlled and balloon‐angioplasty‐controlled trial to assess safety of coronary stenting with use of platelet glycoprotein‐IIb/IIIa blockade. The EPISTENT Investigators. Lancet 1998;352(9122):87‐92. [DOI] [PubMed] [Google Scholar]
ESSENCE 1997
- Cohen M, Demers C, Gurfinkel EP, Turpie AG, Fromell GJ, Goodman S, et al. A comparison of low‐molecular‐weight heparin with unfractionated heparin for unstable coronary artery disease. Efficacy and Safety of Subcutaneous Enoxaparin in Non‐Q‐Wave Coronary Events Study Group. New England Journal of Medicine 1997;337(7):447‐52. [DOI] [PubMed] [Google Scholar]
EUROPA 2003
- Fox KM, EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double‐blind, placebo‐controlled, multicentre trial (the EUROPA study). Lancet 2003;362(9386):782‐8. [DOI] [PubMed] [Google Scholar]
Fox 2005
- Fox KA, Poole‐Wilson P, Clayton TC, Henderson RA, Shaw TR, Wheatley DJ, et al. 5‐year outcome of an interventional strategy in non‐ST‐elevation acute coronary syndrome: the British Heart Foundation RITA 3 randomised trial. Lancet 2005;366(9489):914‐20. [DOI] [PubMed] [Google Scholar]
Fox 2006
- Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Werf F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 2006;333(7587):1091‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2007a
- Fox KA, Anderson FA Jr, Dabbous OH, Steg PG, López‐Sendón J, Werf F, et al. Intervention in acute coronary syndromes: do patients undergo intervention on the basis of their risk characteristics? The Global Registry of Acute Coronary Events (GRACE). Heart 2007;93(2):177‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2007b
- Fox KA, Steg PG, Eagle KA, Goodman SG, Anderson FA Jr, Granger CB, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999‐2006. JAMA 2007;297(17):1892‐900. [DOI] [PubMed] [Google Scholar]
Franklin 2004
- Franklin K, Goldberg RJ, Spencer F, Klein W, Budaj A, Brieger D, et al. Implications of diabetes in patients with acute coronary syndromes. The Global Registry of Acute Coronary Events. Archives of Internal Medicine 2004;164(13):1457‐63. [DOI] [PubMed] [Google Scholar]
Galvani 1997
- Galvani M, Ottani F, Ferrini D, Ladenson JH, Destro A, Baccos D, et al. Prognostic influence of elevated values of cardiac troponin I in patients with unstable angina. Circulation 1997;95(8):2053‐9. [DOI] [PubMed] [Google Scholar]
Garcia 2004
- Garcia S, Canoniero M, Peter A, Marchena E, Ferreira A. Correlation of TIMI risk score with angiographic severity and extent of coronary artery disease in patients with non‐ST‐elevation acute coronary syndromes. American Journal of Cardiology 2004;93(7):813‐6. [DOI] [PubMed] [Google Scholar]
Gibson 1986
- Gibson RS, Boden WE, Theroux P, Strauss HD, Pratt CM, Gheorghiade M, et al. Diltiazem and reinfarction in patients with non‐Q‐wave myocardial infarction. Results of a double‐blind, randomized, multicenter trial. New England Journal of Medicine 1986;315(7):423‐9. [DOI] [PubMed] [Google Scholar]
Glaser 2002
- Glaser R, Herrmann HC, Murphy SA, Demopoulos LA, DiBattiste PM, Cannon CP, et al. Benefit of an early invasive management strategy in women with acute coronary syndromes. JAMA 2002;288(24):3124‐9. [DOI] [PubMed] [Google Scholar]
Goldberg 2004
- Goldberg RJ, Currie K, White K, Brieger D, Steg PG, Goodman SG, et al. Six‐month outcomes in a multinational registry of patients hospitalized with an acute coronary syndrome (the Global Registry of Acute Coronary Events [GRACE]). American Journal of Cardiology 2004;93(3):288‐93. [DOI] [PubMed] [Google Scholar]
Gottlieb 1986
- Gottlieb SO, Weisfeldt ML, Ouyang P, Achuff SC, Baughman KL, Traill TA, et al. Effect of the addition of propranolol to therapy with nifedipine for unstable angina pectoris: a randomized, double‐blind, placebo‐controlled trial. Circulation 1986;73(2):331‐7. [DOI] [PubMed] [Google Scholar]
Goyal 2002
- Goyal A, Samaha FF, Boden WE, Wade MJ, Kimmel SE. Stress test criteria used in the conservative arm of the FRISC‐II trial underdetects surgical coronary artery disease when applied to patients in the VANQWISH trial. Journal of the American College of Cardiology 2002;39(10):1601‐7. [DOI] [PubMed] [Google Scholar]
Gurfinkel 1995
- Gurfinkel EP, Manos EJ, Mejaíl RI, Cerdá MA, Duronto EA, García CN, et al. Low molecular weight heparin versus regular heparin or aspirin in the treatment of unstable angina and silent ischemia. Journal of the American College of Cardiology 1995;26(2):313‐8. [DOI] [PubMed] [Google Scholar]
Hamm 1999
- Hamm CW, Heeschen C, Goldmann B, Vahanian A, Adgey J, Miguel CM, et al. Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. c7E3 Fab Antiplatelet Therapy in Unstable Refractory Angina (CAPTURE) Study Investigators. New England Journal of Medicine 1999;340(21):1623‐9. [DOI] [PubMed] [Google Scholar]
Hamm 2011
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndrome in patients presenting without persistent ST‐segment elevation. European Heart Journal 2011;32(23):2999‐3054. [DOI] [PubMed] [Google Scholar]
Heeschen 1999a
- Heeschen C, Brand MJ, Hamm CW, Simoons ML. Angiographic findings in patients with refractory unstable angina according to troponin T status. Circulation 1999;100(14):1509‐14. [DOI] [PubMed] [Google Scholar]
Heeschen 1999b
- Heeschen C, Hamm CW, Goldmann B, Deu A, Langenbrink L, White HD. Troponin concentrations for stratification of patients with acute coronary syndromes in relation to therapeutic efficacy of tirofiban. PRISM Study Investigators. Platelet Receptor Inhibition in Ischemic Syndrome Management. Lancet 1999;354(9192):1757‐62. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirsch 2007
- Hirsch A, Windhausen F, Tijssen JG, Verheugt FW, Cornel JH, Winter RJ. Long‐term outcome after an early invasive versus selective invasive treatment strategy in patients with non‐ST‐elevation acute coronary syndrome and elevated cardiac troponin T (the ICTUS trial): a follow‐up study. Lancet 2007;369(9564):827‐35. [DOI] [PubMed] [Google Scholar]
Hjalmarson 1982
- Hjalmarson A, Elmfeldt D, Herlitz J, Holmberg S, Málek I, Rydén L, et al. Effect on mortality of metoprolol in acute myocardial infarction. A randomized double‐blind trial [Effekt von metoprolol auf die mortalitat beim akuten myokardinfarkt]. Münchener Medizinische Wochenschrift 1982;124(1):32‐45. [PubMed] [Google Scholar]
Hochman 1999
- Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Werf F, et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes IIb Investigators. New England Journal of Medicine 1999;341(4):226‐32. [DOI] [PubMed] [Google Scholar]
Holmvang 2003
- Holmvang L, Clemmensen P, Lindahl B, Lagerqvist B, Venge P, Wagner G, et al. Quantitative analysis of the admission electrocardiogram identifies patients with unstable coronary artery disease who benefit the most from early invasive treatment. Journal of the American College of Cardiology 2003;41(6):905‐15. [DOI] [PubMed] [Google Scholar]
HOPE 2000
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin‐converting‐enzyme inhibitor, ramipril, on cardiovascular events in high‐risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. New England Journal of Medicine 2000;342(3):145‐53. [DOI] [PubMed] [Google Scholar]
Januzzi 2005
- Januzzi JL Jr, Buros J, Cannon CP, Tactics TIMI 18 Investigators. Peripheral arterial disease, acute coronary syndromes, and early invasive management: the TACTICS TIMI 18 trial. Clinical Cardiology 2005;28(5):238‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Janzon 2004
- Janzon M, Levin LA, Swahn E. Invasive treatment in unstable coronary artery disease promotes health‐related quality of life: results from the FRISC II trial. American Heart Journal 2004;148(1):114‐21. [DOI] [PubMed] [Google Scholar]
Jernberg 2003
- Jernberg T, Lindahl B, Siegbahn A, Andren B, Frostfeldt G, Lagerqvist B, et al. N‐terminal pro‐brain natriuretic peptide in relation to inflammation, myocardial necrosis, and the effect of an invasive strategy in unstable coronary artery disease. Journal of the American College of Cardiology 2003;42(11):1909‐16. [DOI] [PubMed] [Google Scholar]
Jernberg 2004
- Jernberg T, James S, Lindahl B, Johnston N, Stridsberg M, Venge P, et al. Natriuretic peptides in unstable coronary artery disease. European Heart Journal 2004;25(17):1486‐93. [DOI] [PubMed] [Google Scholar]
Jneid 2012
- Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE Jr, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non‐ST‐elevation myocardial infarction (updating the 2007 guidelines and replacing the 2011 focused update). Circulation 2012;126(7):875‐910. [DOI] [PubMed] [Google Scholar]
Johnston 2006
- Johnston N, Jernberg T, Lagerqvist B, Wallentin L. Early invasive treatment benefits patients with renal dysfunction in unstable coronary artery disease. American Heart Journal 2006;152(6):1052‐8. [DOI] [PubMed] [Google Scholar]
Kandzari 2005
- Kandzari DE, Roe MT, Chen AY, Lytle BL, Pollack CV Jr, Harrington RA, et al. Influence of clinical trial enrollment on the quality of care and outcomes for patients with non‐ST‐segment elevation acute coronary syndromes. American Heart Journal 2005;149(3):474‐81. [DOI] [PubMed] [Google Scholar]
Karvouni 2003
- Karvouni E, Katritsis DG, Ioannidis JP. Intravenous glycoprotein IIb/IIIa receptor antagonists reduce mortality after percutaneous coronary interventions. Journal of the American College of Cardiology 2003;41(1):26‐32. [DOI] [PubMed] [Google Scholar]
Khot 2003
- Khot UN, Jia G, Moliterno DJ, Lincoff AM, Khot MB, Harrington RA, et al. Prognostic importance of physical examination for heart failure in non‐ST‐elevation acute coronary syndromes: the enduring value of Killip classification. JAMA 2003;290(16):2174‐81. [DOI] [PubMed] [Google Scholar]
Kim 2005
- Kim J, Henderson RA, Pocock SJ, Clayton T, Sculpher MJ, Fox KA. Health‐related quality of life after interventional or conservative strategy in patients with unstable angina or non‐ST‐segment elevation myocardial infarction: one‐year results of the third Randomized Intervention Trial of unstable Angina (RITA‐3). Journal of the American College of Cardiology 2005;45(2):221‐8. [DOI] [PubMed] [Google Scholar]
Kugelmass 2006
- Kugelmass AD, Sadanandan S, Lakkis N, Dibattiste PM, Robertson DH, Demopoulos LA, et al. Early invasive strategy improves outcomes in patients with acute coronary syndrome with previous coronary artery bypass graft surgery: a report from TACTICS‐TIMI 18. Critical Pathways in Cardiology 2006;5(3):167‐72. [DOI] [PubMed] [Google Scholar]
Lagerqvist 2001
- Lagerqvist B, Säfström K, Ståhle E, Wallentin L, Swahn E. Is early invasive treatment of unstable coronary artery disease equally effective for both women and men? FRISC II Study Group Investigators. Journal of the American College of Cardiology 2001;38(1):41‐8. [DOI] [PubMed] [Google Scholar]
Lagerqvist 2005
- Lagerqvist B, Diderholm E, Lindahl B, Husted S, Kontny F, Ståhle E, et al. FRISC score for selection of patients for an early invasive treatment strategy in unstable coronary artery disease. Heart 2005;91(8):1047‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lagerqvist 2006
- Lagerqvist B, Husted S, Kontny F, Ståhle E, Swahn E, Wallentin L. 5‐year outcomes in the FRISC‐II randomised trial of an invasive versus a non‐invasive strategy in non‐ST‐elevation acute coronary syndrome: a follow‐up study. Lancet 2006;368(9540):998‐1004. [DOI] [PubMed] [Google Scholar]
Lee 2008
- Lee CH, Tan M, Yan AT, Yan RT, Fitchett D, Grima EA, et al. Use of cardiac catheterization for non‐ST‐segment elevation acute coronary syndromes according to initial risk: reasons why physicians choose not to refer their patients. Archives of Internal Medicine 2008;168(3):291‐6. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Lewis 1983
- Lewis HD Jr, Davis JW, Archibald DG, Steinke WE, Smitherman TC, Doherty JE 3rd, et al. Protective effects of aspirin against acute myocardial infarction and death in men with unstable angina. Results of a Veterans Administration Cooperative Study. New England Journal of Medicine 1983;309(7):396‐403. [DOI] [PubMed] [Google Scholar]
Lim 2011
- Lim CCS, Gaal WJ, Testa L, Cuculi F, Arnold JR, Karamitsos T, et al. With the 'universal definition', measurement of creatine kinase‐myocardial band rather than troponin allows more accurate diagnosis of periprocedural necrosis and infarction after coronary intervention. Journal of the American College of Cardiology 2011;57(6):653‐61. [DOI] [PubMed] [Google Scholar]
Lindahl 1996
- Lindahl B, Venge P, Wallentin L. Relation between troponin T and the risk of subsequent cardiac events in unstable coronary artery disease. The FRISC study group. Circulation 1996;93(9):1651‐7. [DOI] [PubMed] [Google Scholar]
Magid 2000
- Magid DJ, Calonge BN, Rumsfeld JS, Canto JG, Frederick PD, Every NR, et al. Relation between hospital primary angioplasty volume and mortality for patients with acute MI treated with primary angioplasty vs thrombolytic therapy. JAMA 2000;284(24):3131‐8. [DOI] [PubMed] [Google Scholar]
Mahoney 2002
- Mahoney EM, Jurkovitz CT, Chu H, Becker ER, Culler S, Kosinski AS, et al. Cost and cost‐effectiveness of an early invasive vs conservative strategy for the treatment of unstable angina and non‐ST‐segment elevation myocardial infarction. JAMA 2002;288(15):1851‐8. [DOI] [PubMed] [Google Scholar]
Mega 2005
- Mega JL, Morrow DA, Sabatine MS, Zhao XQ, Snapinn SM, DiBattiste PM, et al. Correlation between the TIMI risk score and high‐risk angiographic findings in non‐ST‐elevation acute coronary syndromes: observations from the Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM‐PLUS) trial. American Heart Journal 2005;149(5):846‐50. [DOI] [PubMed] [Google Scholar]
Mehta 2005
- Mehta SR, Cannon CP, Fox KA, Wallentin L, Boden WE, Spacek R, et al. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta‐analysis of randomized trials. JAMA 2005;293(23):2908‐17. [DOI] [PubMed] [Google Scholar]
Mehta 2008
- Mehta SR, Boden WE, Eikelboom JW, Flather M, Steg PG, Avezum A, et al. Antithrombotic therapy with fondaparinux in relation to interventional management strategy in patients with ST‐ and non‐ST‐segment elevation acute coronary syndromes: an individual patient‐level combined analysis of the Fifth and Sixth Organization to Assess Strategies in Ischemic Syndromes (OASIS 5 and 6) randomized trials. Circulation 2008;118(20):2038‐46. [DOI] [PubMed] [Google Scholar]
Montalescot 2009
- Montalescot G, Cayla G, Collet JP, Elhadad S, Beygui F, LeBreton H, et al. Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial. JAMA 2009;302(9):947‐54. [DOI] [PubMed] [Google Scholar]
Morrow 2001
- Morrow DA, Cannon CP, Rifai N, Frey MJ, Vicari R, Lakkis N, et al. Ability of minor elevations of troponins I and T to predict benefit from an early invasive strategy in patients with unstable angina and non‐ST elevation myocardial infarction: results from a randomized trial. JAMA 2001;286(19):2405‐12. [DOI] [PubMed] [Google Scholar]
Morrow 2002
- Morrow DA, Antman EM, Snapinn SM, McCabe CH, Theroux P, Braunwald E. An integrated clinical approach to predicting the benefit of tirofiban in non‐ST elevation acute coronary syndromes. Application of the TIMI Risk Score for UA/NSTEMI in PRISM‐PLUS. European Heart Journal 2002;23(3):223‐9. [DOI] [PubMed] [Google Scholar]
Morrow 2003
- Morrow DA, Lemos JA, Sabatine MS, Murphy SA, Demopoulos LA, DiBattiste PM, et al. Evaluation of B‐type natriuretic peptide for risk assessment in unstable angina/non‐ST‐elevation myocardial infarction: B‐type natriuretic peptide and prognosis in TACTICS‐TIMI 18. Journal of the American College of Cardiology 2003;41(8):1264‐72. [DOI] [PubMed] [Google Scholar]
Muller 1984
- Muller JE, Turi ZG, Pearle DL, Schneider JF, Serfas DH, Morrison J, et al. Nifedipine and conventional therapy for unstable angina pectoris: a randomized, double‐blind comparison. Circulation 1984;69(4):728‐39. [DOI] [PubMed] [Google Scholar]
Navarese 2013
- Navarese EP, Gurbel PA, Andreotti F, Tantry U, Jeong YH, Kozinski M, et al. Optimal timing of coronary invasive strategy in non‐ST‐segment elevation acute coronary syndrome: a systematic review and meta‐analysis. Annals of Internal Medicine 2013;158(4):261‐70. [DOI] [PubMed] [Google Scholar]
Neri Serneri 1990
- Neri Serneri GG, Gensini GF, Poggesi L, Trotta F, Modesti PA, Boddi M, et al. Effect of heparin, aspirin, or alteplase in reduction of myocardial ischaemia in refractory unstable angina. Lancet 1990;335(8690):615‐8. [DOI] [PubMed] [Google Scholar]
Norhammar 2004
- Norhammar A, Malmberg K, Diderholm E, Lagerqvist B, Lindahl B, Rydén L, et al. Diabetes mellitus: the major risk factor in unstable coronary artery disease even after consideration of the extent of coronary artery disease and benefits of revascularization. Journal of the American College of Cardiology 2004;43(4):585‐91. [DOI] [PubMed] [Google Scholar]
O'Donoghue 2012
- O'Donoghue ML, Vaidya A, Afsal R, Alfredsson J, Boden WE, Braunwald E, et al. An invasive or conservative strategy in patients with diabetes mellitus and non‐ST‐segment elevation acute coronary syndromes: a collaborative meta‐analysis of randomized trials. Journal of the American College of Cardiology 2012;60(2):106‐11. [DOI] [PubMed] [Google Scholar]
Oliveira 2007
- Oliveira GB, Avezum A, Anderson FA Jr, Budaj A, Dabbous OH, Goodman SG, et al. Use of proven therapies in non‐ST‐elevation acute coronary syndromes according to evidence‐based risk stratification. American Heart Journal 2007;153(4):493‐9. [DOI] [PubMed] [Google Scholar]
Pepine 1998
- Pepine CJ, Faich G, Makuch R. Verapamil use in patients with cardiovascular disease: an overview of randomized trials. Clinical Cardiology 1998;21(9):633‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
PLATO
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New England Journal of Medicine 2009;361(11):1045‐57. [DOI] [PubMed] [Google Scholar]
PRISM‐PLUS Trial
- Bazzino O, Barrero C, Garre L, Sosa A, Aylward P, Slany J, et al. Inhibition of the platelet glycoprotein IIb/IIIa receptor with tirofiban in unstable angina and non‐Q‐wave myocardial infarction. Platelet Receptor Inhibition in Ischemic Syndrome Management in Patients Limited by Unstable Signs and Symptoms (PRISM‐PLUS) Study Investigators. New England Journal of Medicine 1998;338(21):1488‐97. [DOI] [PubMed] [Google Scholar]
PURSUIT 1998
- Topol E, Califf R, Simoons M, Diaz R, Paolasso E, Klein W, et al. Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. New England Journal of Medicine 1998;339(7):436‐43. [Google Scholar]
Qayyum 2008
- Qayyum R, Khalid MR, Adomaityte J, Papadakos SP, Messineo FC. Systematic review: comparing routine and selective invasive strategies for the acute coronary syndrome. Annals of Internal Medicine 2008;148(3):186‐96. [DOI] [PubMed] [Google Scholar]
Ranasinghe 2011
- Ranasinghe I, Alprandi‐Costa B, Chow V, Elliott JM, Waites J, Counsell JT, et al. Risk stratification in the setting of Non‐ST elevation acute coronary syndrome 1999‐2007. American Journal of Cardiology 2011;108(5):617‐24. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RIFLE‐STEACS
- Romagnoli E, Biondi‐Zoccai G, Sciahbasi A, Politi L, Rigattieri S, Pendenza G, et al. Radial Versus Femoral Randomized Investigation in ST‐Segment Elevation Acute Coronary Syndrome: the RIFLE‐STEACS Study. Journal of the American College of Cardiology 2012;60(24):2481‐9. [DOI] [PubMed] [Google Scholar]
RISC 1990
- The RISC Group. Risk of myocardial infarction and death during treatment with low dose aspirin and intravenous heparin in men with unstable coronary artery disease. The RISC Group. Lancet 1990;336(8719):827‐30. [PubMed] [Google Scholar]
Rodrigues 2003
- Rodrigues EJ, Eisenberg MJ, Pilote L. Effects of early and late administration of angiotensin‐converting enzyme inhibitors on mortality after myocardial infarction. American Journal of Medicine 2003;115(6):473‐9. [DOI] [PubMed] [Google Scholar]
Roffi 2002
- Roffi M, Chew DP, Mukherjee D, Bhatt DL, White JA, Moliterno DJ, et al. Platelet glycoprotein IIb/IIIa inhibition in acute coronary syndromes. Gradient of benefit related to the revascularization strategy. European Heart Journal 2002;23(18):1441‐8. [DOI] [PubMed] [Google Scholar]
Rosamond 2008
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics‐‐2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117(4):e25‐146. [DOI] [PubMed] [Google Scholar]
Rott 2001
- Rott D, Behar S, Leor J, Hod H, Boyko V, Mandelzweig L, et al. Effect on survival of acute myocardial infarction in Killip classes II or III patients undergoing invasive coronary procedures. American Journal of Cardiology 2001;88(6):618‐23. [DOI] [PubMed] [Google Scholar]
Sabatine 2006
- Sabatine MS, Morrow DA, McCabe CH, Antman EM, Gibson CM, Cannon CP. Combination of quantitative ST deviation and troponin elevation provides independent prognostic and therapeutic information in unstable angina and non‐ST‐elevation myocardial infarction. American Heart Journal 2006;151(1):25‐31. [DOI] [PubMed] [Google Scholar]
Sadanandan 2004
- Sadanandan S, Cannon CP, Chekuri K, Murphy SA, Dibattiste PM, Morrow DA, et al. Association of elevated B‐type natriuretic peptide levels with angiographic findings among patients with unstable angina and non‐ST‐segment elevation myocardial infarction. Journal of the American College of Cardiology 2004;44(3):564‐8. [DOI] [PubMed] [Google Scholar]
Saenger 2008
- Saenger AK, Jaffe AS. Requiem for a heavyweight: the demise of creatine kinas‐MB. Circulation 2008;118(21):2200‐6. [DOI] [PubMed] [Google Scholar]
Sarno 2012
- Sarno G, Lagerqvist B, Frobert O, Nilsson J, Olivercrona G, Omerovic E, et al. Lower risk of stent thrombosis and restenosis with unrestricted used of 'new‐generation' drug‐eluting stents: a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). European Heart Journal 2012;33(5):606‐13. [DOI] [PubMed] [Google Scholar]
Scirica 2002
- Scirica BM, Cannon CP, Antman EM, Murphy SA, Morrow DA, Sabatine MS, et al. Validation of the thrombolysis in myocardial infarction (TIMI) risk score for unstable angina pectoris and non‐ST‐elevation myocardial infarction in the TIMI III registry. American Journal of Cardiology 2002;90(3):303‐5. [DOI] [PubMed] [Google Scholar]
SHOCK 1999
- Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. New England Journal of Medicine 1999;341(9):625‐34. [DOI] [PubMed] [Google Scholar]
Simoons 1997
- Simoons ML, Rutsch W, Vahanian A, Adgey J, Maseri A, Vassanelli C, et al. Randomised placebo‐controlled trial of abciximab before and during coronary intervention in refractory unstable angina: the CAPTURE Study. Lancet 1997;349(9063):1429‐35. [PubMed] [Google Scholar]
Singh 2000
- Singh M, Rihal CS, Berger PB, Bell MR, Grill DE, Garratt KN, et al. Improving outcome over time of percutaneous coronary interventions in unstable angina. Journal of the American College of Cardiology 2000;36(3):674‐8. [DOI] [PubMed] [Google Scholar]
Stone 2006
- Stone GW, McLaurin BT, Cox DA, Bertrand ME, Lincoff AM, Moses JW, et al. Bivalirudin for patients with acute coronary syndromes. New England Journal of Medicine 2006;355(21):2203‐16. [DOI] [PubMed] [Google Scholar]
Stone 2007
- Stone GW, White HD, Ohman EM, Bertrand ME, Lincoff AM, McLaurin BT, et al. Bivalirudin in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a subgroup analysis from the Acute Catheterization and Urgent Intervention Triage strategy (ACUITY) trial. Lancet 2007;369(9565):907‐19. [DOI] [PubMed] [Google Scholar]
Tarantini 2007
- Tarantini G, Razzolini R, Ramondo A, Napodano M, Manica A, Favaretto E, et al. Patient risk profile and benefit from an invasive approach in the initial management of non‐ST‐segment elevation acute coronary syndrome. Journal of Cardiovascular Medicine 2007;8(10):799‐802. [DOI] [PubMed] [Google Scholar]
Theroux 1988
- Theroux P, Ouimet H, McCans J, Latour JG, Joly P, Levy G, et al. Aspirin, heparin, or both to treat acute unstable angina. New England Journal of Medicine 1988;319(17):1105‐11. [DOI] [PubMed] [Google Scholar]
Theroux 1993
- Theroux P, Waters D, Qiu S, McCans J, Guise P, Juneau M. Aspirin versus heparin to prevent myocardial infarction during the acute phase of unstable angina. Circulation 1993;88(5 Pt 1):2045‐8. [DOI] [PubMed] [Google Scholar]
Thompson 1979
- Thompson PL, Fletcher EE, Katavatis V. Enzymatic indices of myocardial necrosis, influence on short‐ and long‐term prognosis after myocardial infarction. Circulation 1979;59:653‐661. [DOI] [PubMed] [Google Scholar]
Thompson 2005
- Thompson SG, Higgins JP. Treating individuals 4: can meta‐analysis help target interventions at individuals most likely to benefit?. Lancet 2005;365(9456):341‐6. [DOI] [PubMed] [Google Scholar]
Thygesen 2007
- Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Universal definition of myocardial infarction. Circulation 2007;116(22):2634‐53. [DOI] [PubMed] [Google Scholar]
Théroux 1985
- Théroux P, Taeymans Y, Morissette D, Bosch X, Pelletier GB, Waters DD. A randomized study comparing propranolol and diltiazem in the treatment of unstable angina. Journal of the American College of Cardiology 1985;5(3):717‐22. [DOI] [PubMed] [Google Scholar]
Tijssen 1987
- Tijssen JG, Lubsen J. Nifedipine and metoprolol in unstable angina: findings from the Holland Interuniversity Nifedipine/Metoprolol Trial (HINT). Journal of Cardiovascular Pharmacology 1987;10 Suppl 2:S15‐22; discussion S23‐4. [PubMed] [Google Scholar]
TIMI 11B 1999
- Antman EM, McCabe CH, Gurfinkel EP, Turpie AG, Bernink PJ, Salein D, et al. Enoxaparin prevents death and cardiac ischemic events in unstable angina/non‐Q‐wave myocardial infarction. Results of the thrombolysis in myocardial infarction (TIMI) 11B trial. Circulation 1999;100(15):1593‐601. [DOI] [PubMed] [Google Scholar]
TIMI III Registry
- Stone PH, Thompson B, Anderson HV, Kronenberg MW, Gibson RS, Rogers WJ, Diver DJ, Théroux P, Warnica JW, Nasmith JB, Kells C, Kleiman N, McCabe CH, Schactman M, Knatteruf GL, Braunwald E. Influence of race, sex, and age on management of unstable angina and non‐Q‐wave acute myocardial infarction: The TIMI III registry. Journal of the American Medical Association 1996;275(14):1104‐12. [PubMed] [Google Scholar]
TIMI‐IIIA 1993
- Braunwald E, Muller JE, McCabe CH, Knatterud GL, Thompson B, Prior MJ, et al. Early effects of tissue‐type plasminogen activator added to conventional therapy on the culprit coronary lesion in patients presenting with ischemic cardiac pain at rest. Results of the Thrombolysis in Myocardial Ischemia (TIMI IIIA) Trial. Circulation 1993;87(1):38‐52. [DOI] [PubMed] [Google Scholar]
Topol 1999
- Topol EJ, Byzova TV, Plow EF. Platelet GPIIb‐IIIa blockers. Lancet 1999;353(9148):227‐31. [DOI] [PubMed] [Google Scholar]
TRITON‐TIMI 38
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. The New England Journal of Medicine 2007;357(20):2001‐15. [DOI] [PubMed] [Google Scholar]
Van de Werf 2005
- Werf F, Gore JM, Avezum A, Gulba DC, Goodman SG, Budaj A, et al. Access to catheterisation facilities in patients admitted with acute coronary syndrome: multinational registry study. BMJ 2005;330(7489):441‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weintraub 1999
- Weintraub WS, Culler SD, Kosinski A, Becker ER, Mahoney E, Burnette J, et al. Economics, health‐related quality of life, and cost‐effectiveness methods for the TACTICS (Treat Angina With Aggrastat [tirofiban]] and Determine Cost of Therapy with Invasive or Conservative Strategy)‐TIMI 18 trial. Amercan Journal of Cardiology 1999;83(3):317‐22. [DOI] [PubMed] [Google Scholar]
Windhausen 2007a
- Windhausen F, Hirsch A, Sanders GT, Cornel JH, Fischer J, Straalen JP, et al. N‐terminal pro‐brain natriuretic peptide for additional risk stratification in patients with non‐ST‐elevation acute coronary syndrome and an elevated troponin T: an Invasive versus Conservative Treatment in Unstable coronary Syndromes (ICTUS) substudy. American Heart Journal 2007;153(4):485‐92. [DOI] [PubMed] [Google Scholar]
Windhausen 2007b
- Windhausen F, Hirsch A, Tijssen JG, Cornel JH, Verheugt FW, Klees MI, et al. ST‐segment deviation on the admission electrocardiogram, treatment strategy, and outcome in non‐ST‐elevation acute coronary syndromes A substudy of the Invasive versus Conservative Treatment in Unstable coronary Syndromes (ICTUS) Trial. Journal of Electrocardiology 2007;40(5):408‐15. [DOI] [PubMed] [Google Scholar]
Yan 2007
- Yan AT, Yan RT, Tan M, Fung A, Cohen EA, Fitchett DH, et al. Management patterns in relation to risk stratification among patients with non‐ST elevation acute coronary syndromes. Archives of Internal Medicine 2007;167(10):1009‐16. [DOI] [PubMed] [Google Scholar]
Yusuf 1988
- Yusuf S, Wittes J, Friedman L. Overview of results of randomized clinical trials in heart disease. II. Unstable angina, heart failure, primary prevention with aspirin, and risk factor modification. JAMA 1988;260(15):2259‐63. [PubMed] [Google Scholar]
Zaacks 1999
- Zaacks SM, Liebson PR, Calvin JE, Parrillo JE, Klein LW. Unstable angina and non‐Q wave myocardial infarction: does the clinical diagnosis have therapeutic implications?. Journal of the American College of Cardiology 1999;33(1):107‐18. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hoenig 2004
- Hoenig MR, Doust JA, Aroney CN, Scott IA. Early invasive versus ischemia‐guided strategies for unstable angina & non‐ST‐elevation myocardial infarction. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004815] [DOI] [PubMed] [Google Scholar]
Hoenig 2006
- Hoenig MR, Doust JA, Aroney CN, Scott IA. Early invasive versus conservative strategies for unstable angina & non‐ST‐elevation myocardial infarction in the stent era. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004815.pub2] [DOI] [PubMed] [Google Scholar]
Hoenig 2010
- Hoenig MR, Aroney CN, Scott IA. Early invasive versus conservative strategies for unstable angina and non‐ST elevation myocardial infarction in the stent era. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD004815.pub3] [DOI] [PubMed] [Google Scholar]


