Abstract
This retrospective study aimed to explore the effect of enteral nutrition (EN) on immune and inflammatory factors after liver cancer surgery (LCS).
It was retrospectively conducted on enrolled LCS patients between January 2017 and May 2020. The medical records of 528 patient case records were collected and reviewed. After selection, a total of 80 eligible patient case records were finally included. All those patients received routine diet, and they were allocated to a treatment group (n = 40) and a control group (n = 40). In addition, patients in the treatment group also received EN. The primary outcomes were immune factors (CD4+, CD8+, CD4+/CD8+) and inflammatory factors (interleukin-1, interleukin-6, and tumor necrosis factor-α). The secondary outcomes were postoperative hospital stay (day), time to first bowel sounds (hour), time to first flatus (day), time to first defecation (day), and complications.
There were not significant differences in CD4+/CD8+ (P = .34), postoperative hospital stay (P = .39), and time to first bowel sounds (P = .17) between 2 groups. However, there were significant differences in CD4+ (P < .01), CD8+ (P < .01), interleukin-1 (P < .01), interleukin-6 (P < .01), tumor necrosis factor-α (P < .01), time to first flatus (P < .01), and time to first defecation (P < .01) between 2 groups. As for complications, there were not significant differences between 2 groups (P > .05).
The results of this study found that EN may benefit for patients after LCS during the recovery period. Future high quality prospective studies are needed to warrant the present conclusion.
Keywords: enteral nutrition, immune factor, inflammatory factor, liver cancer
1. Introduction
Primary liver cancer (PLC) is one of the most common malignancy.[1–4] It is also the most common of cancer-related mortality around the world.[5–7] It was reported that there were 841,080 new PLC cases globally each year.[8] In China, there were 392,869 new cases, which accounted for about 46.7% of all new cases annually worldwide.[9] It is mostly composed of hepatocellular carcinoma (HCC), and about 75% to 85% of all PLC cases are HCC.[8–11] The 5-year overall survival rate of HCC ranged between 11.2% and 14.0% from 2012 to 2015 in China.[9]
Previous studies have reported that malnutrition is one of the most healthy disorders in cancer patients.[12–14] Studies also suggest that there is firmly association between malnutrition and clinical cancer, such as PLC.[12–14] The other studies have reported that optimization of nutritional status may benefit liver function enhancement, and affect the success of liver cancer surgery (LCS).[15–18] Other studies have reported that enteral nutrition (EN) may impact the immune and inflammatory factors after LCS.[19–21] However, there is still insufficient evidence regarding this topic. Thus, the present study investigated the effect of EN for patients after LCS.
2. Methods
2.1. Ethic approval
This study was approved by the Ethics Committee of Longgang District Maternal and Child Health Hospital. The written informed consent was waived due to the completed patient records.
2.2. Study design
A retrospective cohort study was carried out on patients after LCS in Longgang District Maternal and Child Health Hospital. We inspected the medical records of 80 patients who were admitted from January 2017 to May 2020. We assigned patients into 2 different groups based on the different managements they received. All 80 patients in both treatment group (n = 40) and control group (n = 40) received routine diet. In addition, patients in the treatment group also received EN. The clinical retrospective data of characteristics, demographics, clinical endpoints, laboratory tests, treatments, and controls were summarized and analyzed.
2.3. Patients
All patient case records fulfilled the following eligibility criteria. The inclusion criteria were as follows: age between 18 and 80 years old; confirmed diagnoses of PLC by histopathological examination[3,5]; and completed liver resection for PLC without surgical contraindication. Exclusion criteria were: a history of other malignant tumors; mental disorder; neoadjuvant chemotherapy or radiotherapy before surgery; severe diabetes and renal dysfunction; poor glycemic control; surgical contraindications; and incomplete information of patient records.
2.4. Treatment schedule
All patients in both groups received routine diet. Before surgery, all patients received low-fat diet. After surgery, they were given dietary guidance, liquid food, and semi-liquid diet.
In addition to the routine diet, patients in the treatment group also received EN suspension (TP-MCT, 1 kcal/mL, 500 mL each bottle). The amount of oral administration was 500 mL daily for 3 consecutive days before the surgery.
2.5. Outcome measurements
The primary outcomes consisted of immune factors (CD4+, CD8+, CD4+/CD8+), inflammatory factors (interleukin-1 [IL-1], interleukin-6 [IL-6], and tumor necrosis factor-α [TNF-α]). The secondary outcomes included postoperative hospital stay (day), time to first bowel sounds (hour), time to first flatus (day), time to first defecation (day), and complications. We measured all available laboratory findings 7 days after surgery, as well as other outcome indicators postsurgery.
2.6. Statistical analysis
Data were analyzed by SAS package v.9.3 (SAS Institute Inc., Cary, NC). All data were summarized by descriptive statistics of continuous values (mean and standard deviation) and categorical values (number and percentages). We analyzed all continuous data using t test or Wilcoxon test, and categorical data using Pearson chi-square test or Fisher exact test. We defined a 2-side P-value < .05 as having statistical significance.
3. Results
3.1. Patient case records selection process
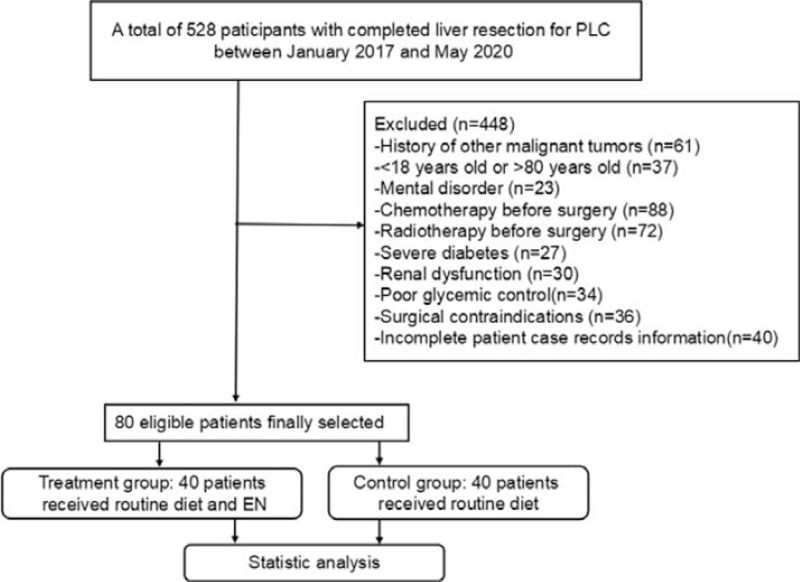
This retrospective study enrolled 528 patient case records with completed liver resection for PLC between January 2017 and May 2020 (Fig. 1). Of those, 448 patient case records were excluded because of the history of other malignant tumors (n = 61), <18 years old or >80 years old (n = 37), mental disorder (n = 23), chemotherapy before surgery (n = 88), radiotherapy before surgery (n = 72), severe diabetes (n = 27), renal dysfunction (n = 30), poor glycemic control (n = 34), surgical contraindications (n = 36), and incomplete patient case records information (n = 40). Finally, a total of 80 eligible patient case records were selected and were analyzed (Fig. 1).
Figure 1.
Flow chart of patient case records selection.
3.2. Demographics and clinical characteristics
A total of 80 cases were enrolled in this retrospective study. The characteristics and demographics of all patients are summarized in Table 1. There were not significant differences in all those values between 2 groups (P > .05, Table 1).
Table 1.
Patients’ characteristic and demographic data at baseline.
| Characteristics | Treatment group (n = 40) | Control group (n = 40) | P |
| Mean age, yr | 56.4 (10.2) | 57.1 (9.6) | .75 |
| Gender | |||
| Male | 23 (57.5) | 25 (62.5) | .65 |
| Female | 17 (42.5) | 15 (37.5) | – |
| Race (ethnicity) | |||
| Han | 40 (100.0) | 40 (100.0) | – |
| Occupation status | |||
| Employed | 14 (35.0) | 13 (32.5) | .81 |
| Unemployed | 6 (15.0) | 3 (7.5) | .30 |
| Retired | 20 (50.0) | 24 (60.0) | .37 |
| Body mass index, kg/m2 | 24.3 (2.0) | 24.6 (1.8) | .48 |
| Tumor size, cm | 3.9 (1.7) | 3.7 (1.6) | .59 |
| NRS2002 score | 3.6 (1.8) | 3.9 (1.9) | .47 |
| Child-Pugh grade | |||
| A | 38 (95.0) | 36 (90.0) | .40 |
| B | 2 (5.0) | 4 (10.0) | – |
| Drinking history | |||
| Yes | 21 (52.5) | 24 (60.0) | .50 |
| No | 19 (47.5) | 16 (40.0) | – |
| ALT, U/L | 28.9 (9.3) | 28.4 (9.9) | .82 |
| AST, U/L | 28.2 (7.7) | 28.0 (7.8) | .91 |
| TB, mmol/L | 14.0 (4.2) | 13.6 (5.1) | .70 |
| DB, mmol/L | 4.0 (1.8) | 3.8 (2.1) | .65 |
| INR | 1.07 (0.04) | 1.05 (0.06) | .08 |
| Type of hepatectomy (segments) | |||
| ≥4 | 6 (15.0) | 5 (12.5) | .75 |
| 2–3 | 15 (37.5) | 13 (32.5) | .64 |
| 1 | 19 (47.5) | 22 (55.0) | .50 |
Data are present as mean ± standard deviation or number (%).
ALT = alanine aminotransferase, AST = aspartate aminotransferase, AKP = alkaline phosphatase, DB = direct bilirubin, INR = international normalized ratio, TB = total bilirubin.
3.3. Laboratory findings
Laboratory results for all admitted patients with PLC after surgery are presented in Tables 2 and 3. The patients in the treatment group showed better enhancement of immune factors (CD4+, P < .01; CD8+, P < .01; Table 2) and inflammatory factors (IL-1, P < .01; IL-6, P < .01; and TNF-α, P < .01; Table 3), than those of patients in the control group. However, we did not identify significant difference in CD4+/CD8+ between 2 groups (P = .34, Table 2).
Table 2.
Comparison of immune factors after surgery between 2 groups.
| Immune factors | Treatment group (n = 40) | Control group (n = 40) | P |
| CD4+ | 31.9 (2.1) | 28.3 (1.8) | <.01 |
| CD8+ | 30.5 (1.6) | 27.7 (1.9) | <.01 |
| CD4+/CD8+ | 1.05 (0.15) | 1.02 (0.13) | .34 |
Data are present as mean ± standard deviation.
Table 3.
Comparison of inflammatory factors after surgery between 2 groups.
| Inflammatory factors | Treatment group (n = 40) | Control group (n = 40) | P |
| IL-1, pg/mL | 50.3 (9.5) | 61.6 (10.4) | <.01 |
| IL-6, pg/mL | 97.7 (13.6) | 155.4 (30.7) | <.01 |
| TNF-α, pg/mL | 53.2 (8.8) | 65.0 (10.3) | <.01 |
Data are present as mean ± standard deviation.
IL-1 = interleukin-1, IL-6 = interleukin-6, TNF-α = tumor necrosis factor-α.
3.4. Clinical findings
Although there were not significant differences of postoperative hospital stay (day) (P = .39, Table 4), and time to first bowel sounds (hour) (P = .17, Table 4) between 2 groups, patients in the treatment group still achieved shorter time to first flatus (day) (P < .01, Table 4) and time to first defecation (day) (P < .01, Table 4), than those of patients in the control group.
Table 4.
Comparison of secondary outcomes after surgery between 2 groups.
| Secondary outcomes | Treatment group (n = 40) | Control group (n = 40) | P |
| Postoperative hospital stay, d | 16.9 (3.0) | 17.5 (3.3) | .39 |
| Time to first bowel sounds, h | 10.3 (4.5) | 11.8 (5.2) | .17 |
| Time to first flatus, d | 1.4 (0.4) | 2.2 (0.6) | <.01 |
| Time to first defecation, d | 3.2 (0.8) | 2.1 (0.5) | <.01 |
Data are present as mean ± standard deviation.
3.5. Complications
All recorded complications are presented in Table 5. No significant differences of all complications were found between 2 groups (P > .05; Table 5).
Table 5.
Comparison of complications between 2 groups.
| Complications | Treatment group (n = 40) | Control group (n = 40) | P |
| Lung infection | 1 (2.5) | 0 (0) | .50 |
| Ascites | 0 (0) | 1 (2.5) | .50 |
| Postoperative hemorrhage | 2 (5.0) | 0 (0) | .29 |
| Hepatic failure | 0 (0) | 1 (2.5) | .50 |
Data are present as number (%).
4. Discussion
PLC is a very common malignant disorder,[1–4] which often causes leading mortality and morbidity.[5–7] Patients with this condition are often reported to experience malnutrition, especially for patients with LCS. EN has reported to benefit patients with LCS in improving liver function, and impacting the success of LCS.[15–18] Previous studies have reported that EN has a stronger immune improvement effect and can reduce the concurrent infection rate.[22,23] In addition, EN also exerts a regulatory effect on immune function, which mainly based on the cellular immunity.[22,23] The other study has reported that EN can enhance patients’ nutritional statuses and regulate their inflammation during the perioperative period.[24] There is limited study on immune and inflammatory factors after LCS. Thus, this retrospective study investigated the effect of EN on immune and inflammatory factors after LCS.
Previous study has found that increase in levels of CD4+, CD8+ can help promote the recovery of postoperative immune function and improve the status of immunosuppression, which helps to prevent postoperative infection.[25] The other studies have reported that decrease in levels of IL-1, IL-6, and TNF-α can lower inflammatory reaction, and reduce postoperative infection.[26–28] The results of this retrospective study showed that EN can not significantly improve CD4+/CD8+, postoperative hospital stay (day), and time to first bowel sounds (hour) for patients with LCS. However, EN can significantly enhance immune factors (CD4+, CD8+), inflammatory factors (IL-1, IL-6, and TNF-α); and shorten time to first flatus (day) and time to first defecation (day) for patients with LCS. Laboratory findings showed that immune factors (CD4+, CD8+) were increased, and inflammatory factors (IL-1, IL-6, and TNF-α) were lowered in the treatment group, which indicates EN may benefit patients after LCS during the recovery period. With respect to the complications, there were not significant differences in all complications between 2 groups. It means that EN may have safe profile for the management of patients after LCS.
Several limitations may exist in this retrospective study. At first, this study only inspected the short-term effect of EN on patients with LCS. So, further studies should focus on its longer term effect. Secondly, this retrospective study could not utilize randomization and blinding, which may increase the selection bias. Finally, it was retrospectively designed based on a relatively small sample size, thus, it may affect the findings of this study.
5. Conclusion
This study found that EN may benefit patients with LCS. Further prospective studies are still encouraged to warrant the present findings.
Author contributions
Conceptualization: Yao Xu, Feng-xiang Wei.
Data curation: Yao Xu, Feng-xiang Wei.
Formal analysis: Yao Xu.
Methodology: Feng-xiang Wei.
Project administration: Feng-xiang Wei.
Resources: Yao Xu.
Software: Yao Xu.
Supervision: Feng-xiang Wei.
Validation: Yao Xu, Feng-xiang Wei.
Visualization: Yao Xu, Feng-xiang Wei.
Writing – original draft: Yao Xu, Feng-xiang Wei.
Writing – review & editing: Yao Xu, Feng-xiang Wei.
Footnotes
Abbreviations: EN = enteral nutrition, HCC = hepatocellular carcinoma, IL-1 = interleukin-1, IL-6 = interleukin-6, LCS = liver cancer surgery, PLC = primary liver cancer, TNF-α = tumor necrosis factor-α.
How to cite this article: Xu Y, Wei Fx. A retrospective study of enteral nutrition on immune and inflammatory factors after liver cancer surgery. Medicine. 2021;100:44(e27718).
The author reports no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Professional Committee for Prevention, Control of Hepatobiliary, Pancreatic Diseases of Chinese Preventive Medicine Association, Committee of Hepatology of Chinese Research Hospital Association, Society of Hepatology of Chinese Medical Association, et al.Guideline for stratified screening and surveillance of primary liver cancer (2020 edition). Chin J Oncol 2021;43:60–77. [DOI] [PubMed] [Google Scholar]
- [2].Qiu G, Jin Z, Chen X, Huang J. Interpretation of guidelines for the diagnosis and treatment of primary liver cancer (2019 edition) in China. Glob Health Med 2020;2:306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Liu Z, Suo C, Mao X, et al. Global incidence trends in primary liver cancer by age at diagnosis, sex, region, and etiology, 1990-2017. Cancer 2020;126:2267–78. [DOI] [PubMed] [Google Scholar]
- [4].Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep 2019;6:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Department of Medical Administration, National Health, Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition). Chin J Hepatol 2020;28:112–28. [Google Scholar]
- [6].Sharma R. Descriptive epidemiology of incidence and mortality of primary liver cancer in 185 countries: evidence from GLOBOCAN 2018. Jpn J Clin Oncol 2020;50:1370–9. [DOI] [PubMed] [Google Scholar]
- [7].Chen PY, Fang AP, Wang XY, et al. Adherence to the Chinese or American dietary guidelines is associated with a lower risk of primary liver cancer in china: a case-control study. Nutrients 2018;10:1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [9].Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health 2018;6:e555–67. [DOI] [PubMed] [Google Scholar]
- [10].Feng M, Pan Y, Kong R, Shu S. Therapy of primary liver cancer. Innovation (NY) 2020;1:100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 Edition). Liver Cancer 2018;7:235–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bo Y, Yao M, Zhang L, Bekalo W, Lu W, Lu Q. Preoperative Nutritional Risk Index to predict postoperative survival time in primary liver cancer patients. Asia Pac J Clin Nutr 2015;24:591–7. [DOI] [PubMed] [Google Scholar]
- [13].Hsuan CA. Hospice care nursing experience related to a patient with terminal stage liver cancer. J Nurs Sci 2009;56:98–104. [PubMed] [Google Scholar]
- [14].Wang JB, Abnet CC, Chen W, et al. Association between serum 25(OH) vitamin D, incident liver cancer and chronic liver disease mortality in the Linxian Nutrition Intervention Trials: a nested case-control study. Br J Cancer 2013;109:1997–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Merli M, Nicolini G, Angeloni S, Riggio O. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition 2002;18:978–86. [DOI] [PubMed] [Google Scholar]
- [16].Read JA, Choy ST, Beale PJ, Clarke SJ. Evaluation of nutritional and inflammatory status of advanced colorectal cancer patients and its correlation with survival. Nutr Cancer 2006;55:78–85. [DOI] [PubMed] [Google Scholar]
- [17].Huhmann MB, August DA. Perioperative nutrition support in cancer patients. Nutr Clin Pract 2012;27:586–92. [DOI] [PubMed] [Google Scholar]
- [18].Hammond JS, Guha IN, Beckingham IJ, Lobo DN. Prediction, prevention and management of postresection liver failure. Br J Surg 2011;98:1188–200. [DOI] [PubMed] [Google Scholar]
- [19].Zhang Y, Chen WJ. The effect of moxibustion combined with comprehensive nutritional intervention on the nutritional status and cellular immune function of patients with advanced liver cancer. Chin Modern Doctor 2020;58:88–91. [Google Scholar]
- [20].Fujian Medical University, He LS. The Effect of Intestinal Immune Microecological Nutrition Support on the Perioperative Nutritional Status and Immune Function of Patients with Liver Cancer. 2017;(Dissertation). [Google Scholar]
- [21].Li CL, Zeng YQ, Zhang P, et al. The abbreviated version of PG-SGA (PG-SGA SF) in the nutritional assessment of patients with liver cancer. Parent Enter Nutr 2019;26:266–70. [Google Scholar]
- [22].Braga M, Gianotti L, Nespoli L, Radaelli G, Di Carlo V. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg 2002;137:174–80. [DOI] [PubMed] [Google Scholar]
- [23].Yao D, Zheng L, Wang J, Guo M, Yin J, Li Y. Perioperative alanyl-glutamine-supplemented parenteral nutrition in chronic radiation enteritis patients with surgical intestinal obstruction: a prospective, randomized, controlled study. Nutr Clin Pract 2016;31:250–6. [DOI] [PubMed] [Google Scholar]
- [24].Di Caro S, Fragkos KC, Keetarut K, et al. Enteral nutrition in adult Crohn's disease: toward a paradigm shift. Nutrients 2019;11:2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Y. Change of nutritional status in treatment of primary liver cancer after TACE. Parenter Enter Nutr 2015;22:282–4. [Google Scholar]
- [26].Lin J, Liu P. Early enteral nutrition influence inflammatory factor and intestine permeability in severe acute pancreatitis patients. Jiangxi Med J 2011;46:308–10. [Google Scholar]
- [27].Zhang LH, Wang J, Sun GJ, et al. Effects of early enteral nutrition in patients with severe acute inflammatory cytokine levels and immune function. J Hainan Med Univ 2015;21:758–61. [Google Scholar]
- [28].Li D, Tu YJ, Chen Z, et al. Effects of early enteral nutrition on inflammatory factors and lymphocytes in patients with rectal cancer. Med J Natl Defend Forces Southwest China 2016;26:1428–30. [Google Scholar]