ABSTRACT
The most common genetic and molecular process leading to sporadic colorectal cancer is chromosomal instability. By contrast, mismatch repair deficiency, which results in high levels of microsatellite instability or lack of mismatch repair (MMR) protein expression on immunohistochemistry (IHC), is the predominant cancer pathway in patients with Lynch syndrome (LS). Importantly, patients with LS may still develop sporadic tumors through chromosomal instability. Testing tumors with IHC staining helps expand the spectrum of LS-related tumors. In this series, we describe 4 cancers in patients with LS that are not typical of the syndrome. Lack of MMR protein expression on IHC staining confirmed that 2 cancers are related to LS, expanding the spectrum of LS-related tumors, and the presence of MMR protein expression on IHC in the other 2 cases confirmed that they were sporadic and not related to mismatch repair deficiency and, thus, not related to LS.
INTRODUCTION
Lynch syndrome (LS), the most common hereditary cancer syndrome, is caused by germline pathogenic variants (PVs) in 1 of several DNA mismatch repair (MMR) genes—MLH1, MSH2, MSH6, and PMS2. Patients with LS have a markedly increased lifetime risk of colorectal and endometrial cancer and a lesser risk of known extracolonic LS-associated cancers, including gastric, small bowel, pancreatic, and urothelial carcinomas.1 On a molecular level, tumors associated with LS show high levels of microsatellite instability (MSI) and loss of expression of the affected MMR proteins by immunohistochemistry (IHC).2 With the widespread interrogation of tumors for evidence of mismatch repair deficiency (MMRd), application of MSI polymerase chain reaction, and MMR IHC, which are both prognostic and guide therapy, tumors not previously included in the LS tumor spectrum are being discovered. In this case series, we describe 4 cancers (2 tumors are secondary to a deficiency in MMR and 2 are sporadic, without MMRd). The 2 with MMRd have either been rarely or never reported in patients with LS and highlight the utility of IHC staining in identifying LS-related tumors.
CASE REPORT 1
Patient 1: Esophageal adenocarcinoma
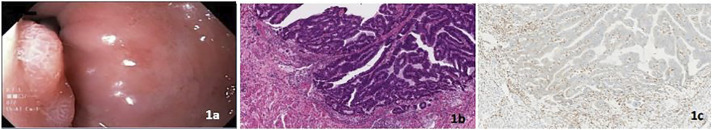
A 56-year-old White woman with LS due to a MSH2 PV had a personal history of melanoma, basal cell carcinoma, sebaceous adenocarcinoma, and Barrett's esophagus (BE). She was under esophagogastroduodenoscopy (EGD) surveillance for LS. Her family history was significant for colon and breast cancer. An EGD 16 months after her previous EGD revealed a 12-mm nodule above the gastroesophageal junction. Endoscopic resection revealed stage 1 esophageal adenocarcinoma, ie, MSI-H with loss of MSH2 protein expression by IHC (Figure 1).
Figure 1.
(A) Endoscopic image of distal esophageal nodule, (B) invasive esophageal adenocarcinoma (hematoxylin and eosin stain, 100× magnification), and (C) absence of nuclear MSH2 protein expression in the invasive esophageal adenocarcinoma with retained expression in background stromal cells (MSH2 stain, 100× magnification).
CASE REPORT 2
Patient 2: Gastric neuroendocrine tumor
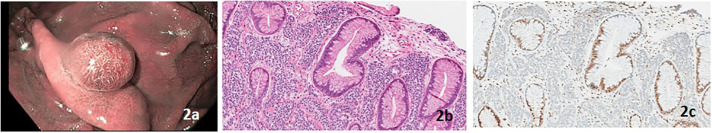
A 67-year-old White man with LS due to a MSH2 PV had a personal history of papillary urothelial carcinoma and gastric fundic gland polyposis. His family history was significant for colon and breast cancer. An EGD 1 year after his previous EGD revealed an 8-mm polyp in a background of fundic gland polyposis (Figure 2). The polyp was resected and confirmed to be a type 3 gastric neuroendocrine tumor, ie, MSI-H with loss of MSH2 protein expression on IHC (Figure 2).
Figure 2.
(A) Narrow band image of proximal gastric polyp, (B) gastric well-differentiated neuroendocrine tumor (hematoxylin and eosin stain, 100× magnification), and (C) absence of nuclear MSH2 protein expression in the well-differentiated neuroendocrine tumor with retained expression in the overlying benign gastric mucosa (MSH2 stain, 100× magnification).
CASE REPORT 3
Patient 3: Small bowel neuroendocrine tumor
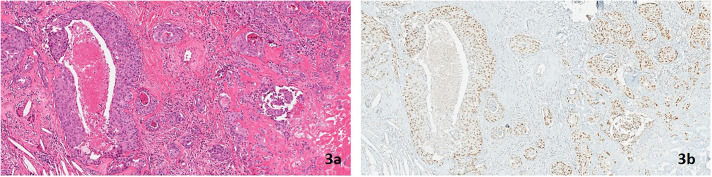
A 62-year-old White woman with LS due to a MSH2 PV had a personal history of endometrial cancer and 2 discrete metachronous colon cancers, for which she underwent partial bowel resections. Her family history was significant for colon and endometrial cancer. Surveillance colonoscopy revealed a small nodule on the ileal aspect of the ileocolic anastomosis. Resection of the nodule revealed a stage 1 well-differentiated neuroendocrine tumor (Figure 3) IHC revealed retention of MSH2 protein expression (Figure 3).
Figure 3.
(A) Microscopic focus of well-differentiated neuroendocrine tumor at an ileal anastomosis (hematoxylin and eosin stain, 200× magnification) and (B) retained MSH2 nuclear protein expression in the well-differentiated neuroendocrine tumor (MSH2 stain, 200× magnification).
CASE REPORT 4
Patient 4: Salivary duct carcinoma ex pleomorphic adenoma
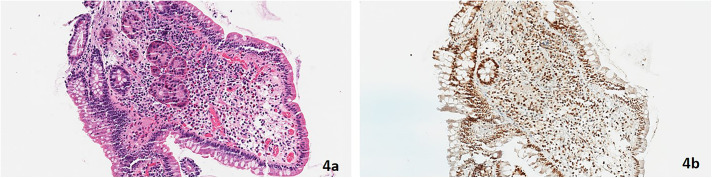
A 43-year-old White man with LS due to a MSH2 PV had no personal history of cancer. His family history was significant for colon cancer in his father. A right parotid gland mass was resected and revealed a stage 4a salivary duct carcinoma ex pleomorphic adenoma with multilevel ipsilateral cervical metastases (Figure 4). IHC revealed retention of MSH2 protein expression (Figure 4). This was the first of several cancer diagnoses, including bladder urothelial, prostate, keratoacanthomas (×2), sebaceous adenocarcinoma, and numerous cutaneous basal and squamous cell carcinomas. Muir-Torre syndrome is the moniker given to patients with LS and sebaceous gland neoplasms (Figures 3 and 4).
Figure 4.
(A) Salivary duct carcinoma ex pleomorphic adenoma (hematoxylin and eosin stain, 100× magnification) and (B) retained MSH2 nuclear protein expression in the salivary duct carcinoma ex pleomorphic adenoma (MSH2 stain, 100× magnification).
DISCUSSION
This case series adds to the literature by expanding the LS-associated tumor spectrum and highlights the utility of lack of MMR protein expression on IHC staining to identify tumors with evidence of MMRd, which may alert the clinician to the possibility of LS. Alternatively, MMR protein expression on IHC staining is not suggestive of genesis through MMRd and most likely represents sporadic cancers, which also occur in patients with LS. Only 2 cases of esophageal cancer in patients with LS have been previously described. Both tumors occurred in patients with a MSH2 PV and lacked protein expression of MSH2 on IHC. The adenocarcinomas in both cases appeared as a polypoid lesion in the proximal esophagus and were presumed to arise from gastric heterotopia.3,4 Our case of esophageal adenocarcinoma originated from Barrett's esophagus in the distal esophagus. No previous studies have described Barrett's esophagus–related esophageal cancer in LS.
Neuroendocrine tumors (NET) have been reported in various locations within the gastrointestinal tract in patients with LS, although their overall prevalence is low. To date, there are only 8 published reports of NET occurring in LS, including 2 in the pancreas,5,6 2 in the colon,7 1 in the rectum,7 and 3 in the small bowel.8 The abnormal MMR protein expression on IHC of the NETs in the colon, rectum, and in 1 pancreatic tumor was consistent with their germline PV.6–8 One patient with a pancreatic NET that lacked protein expression of MSH2 on IHC did not have germline PV testing but had a family history fulfilling the revised Bethesda Guidelines and a personal history of both colonic and endometrial carcinomas that lacked protein expression of MSH2 on IHC. To the best of our knowledge, the gastric NET presented here is the first reported case related to LS. Our case of an ileal NET did not develop through the MSI pathway based on the normal MSH2 protein expression on IHC.
The interrogation of tumors by MSI polymerase chain reaction and MMR protein expression by IHC is important for many reasons. When there is evidence of MSI-H or lack of MMR protein expression, it should raise the suspicion of LS and guide which patients should undergo additional genetic evaluation and consideration of germline testing for PV in the MMR genes. Their utility also extends to expanding the tumor spectrum in LS. Moreover, the identification of MSI-H tumors provides prognostic information and guides therapeutic choices in patients who harbor these cancers. Familiarity in these concepts is important for gastroenterologists, surgeons, oncologists, and pathologists.
DISCLOSURES
Author contributions: N. Farha, E. Savage, and CA Burke wrote the manuscript. N. Farha, E. Savage, J. Sleiman, and CA Burke revised the manuscript for intellectual content. CA Burke is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Natalie Farha, Email: nataliedfarha@gmail.com.
Erica Savage, Email: savagee2@ccf.org.
Joseph Sleiman, Email: sleimaj@ccf.org.
REFERENCES
- 1.National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Colorectal 2020. (https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf). Accessed October 20, 2021. [Google Scholar]
- 2.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: A consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Dis Colon Rectum. 2014;57(8):1025–48. [DOI] [PubMed] [Google Scholar]
- 3.Sweetser S, Chandan VS, Baron TH. Dysphagia in Lynch syndrome. Gastroenterology. 2013;145(5):945, 1167–8. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki H, Yamashita K, Nakase H. Pedunculated upper esophageal adenocarcinoma in Lynch syndrome. Clin Gastroenterol Hepatol. 2019;17(5):A20. [DOI] [PubMed] [Google Scholar]
- 5.Karamurzin Y, Zeng Z, Stadler ZK, et al. Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: A report of new cases and review of the literature. Hum Pathol. 2012;43(10):1677–87. [DOI] [PubMed] [Google Scholar]
- 6.Serracant Barrera A, Serra Pla S, Blázquez Maña CM, et al. Pancreatic non-functioning neuroendocrine tumor: A new entity genetically related to Lynch syndrome. J Gastrointest Oncol. 2017;8(5):E73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kidambi TD, Pedley C, Blanco A, Bergsland EK, Terdiman JP. Lower gastrointestinal neuroendocrine neoplasms associated with hereditary cancer syndromes: A case series. Fam Cancer. 2017;16(4):537–43. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Bigas MA, Vasen HF, Lynch HT, et al. Characteristics of small bowel carcinoma in hereditary nonpolyposis colorectal carcinoma. International Collaborative Group on HNPCC. Cancer. 1998;83(2):240–4. [DOI] [PubMed] [Google Scholar]