Abstract
Aims:
Patients who present with fragility fractures are consistently under-evaluated and under-treated for underlying osteoporosis. This point-of-care represents a lost opportunity to prevent future fractures. This 2-arm study evaluated the success by an orthopaedic department in osteoporosis evaluation and initiating treatment.
Methods:
Patients over the age of 50 years with a fragility fracture of the hip were candidates for inclusion. Exclusion criteria included end-of-life care and moderate or severe dementia. Patients were prospectively randomized into 1 of 2 groups. The Letter group received a letter at the time of discharge encouraging their primary care physician to start medication for osteoporosis (Letter group). The intervention group had 4 interventions including printed information, a DEXA scan, a specific treatment recommendation, and monthly phone calls for 4 months (Intervention group). The primary outcome measure was whether the patient was on recommended treatment at 4 months from the fracture.
Results:
There were 200 patients in the study, 100 in the Letter, and 100 in the Intervention group. Sixteen patients were removed from the study since they either died (9) within 4 months of their fracture, were transferred for end-of-life care (7), and 4 dropped out. This left 180 patients for analysis. The Letter group had only 6 patients (6.2%) on recommended treatment compared with the Intervention group with 64 patients (77.1%). This was statistically significant (P < 0.0001).
Conclusion:
Osteoporosis is a worldwide epidemic. Internationally, only about 20% of patients after a hip fracture are treated for their underlying weak bone. The most effective systems use a fracture liaison service (FLS) model. We present a 4-part intervention program that uses an FLS coordinator within the orthopaedic department. We encourage orthopaedic programs to adopt this or other models with the goal of taking the first step toward responsibility for bone health.
An FLS program within an orthopaedic department can successfully initiate treatment for underlying osteoporosis.
Keywords: FLS, fracture liason service, fragility hip fracture, osteoporosis
1. Introduction
Patients who present with fragility fractures are consistently under-evaluated and under-treated for underlying osteoporosis.[1] This point of care represents a lost opportunity to prevent future fractures.[2] The medical field treats the fracture as if the fall is the problem, but bone quality is the underlying issue. Studies have consistently shown that after a fragility fracture, the risk of a secondary fracture is significantly increased.[3] There is strong evidence that recommended pharmacologic treatment reduces the risk of these secondary fractures.[2,4] Despite this widespread knowledge, the recommendation of the International Osteoporosis Foundation and World Health Organization to begin medication is not being followed.[5] Orthopaedics treats the patients for their fractures and primary care physicians focus on general well-being, but no one is taking care of the bone health. Strategies to convince primary care to assume accountability have not succeeded. On the other hand, strategies where a fracture care coordinator takes responsibility have shown success.[6] This prospective 2-arm study evaluated the success of effort by an academic orthopaedic department in osteoporosis evaluation and treatment. When a patient has a fragility fracture of the hip, we know what medication should be started.[4] The challenge is to create a system where patients actually get started on medication for their underlying weak bone. We believe that when orthopaedics takes partial responsibility, that we can get the patient started on the road toward stronger bone. The interventions described in this manuscript provide a realistic framework for a motivated orthopaedist to change the culture for holistic treatment of fragility fractures. We test the effectiveness of an orthopaedic-driven intervention program in getting patients started on osteoporosis treatment.
2. Materials and methods
The trial was registered at ClinicalTrials.gov before Helsinki approval (201497CTIL). All patients who were hospitalized for a low-energy hip fracture at our Level I trauma center were candidates for the study. After meeting inclusion criteria, patients were randomized into 1 of 2 levels of evaluation and treatment. All patients in the study were consented prior to randomization.
The Letter group was given at the time of discharge a summary with a recommendation for evaluation and treatment by their family physician. The letter included a recommendation that the family doctor evaluate the patient for osteoporosis and that the patient should be started on appropriate medication. This was termed the Letter group. Recommended treatment was defined as one of the 3 treatment options approved by the ministry of health after a hip fracture (Appendix B).
The other group had 4 interventions (Intervention group).
-
1.
The patient was given an informational handout explaining osteoporosis and the importance of treatment (Appendix A).
-
2.
The patient had a Dual-energy X-ray absorptiometry (DEXA) scan performed, usually before their discharge from the hospital. The patient was given the result to bring to their family doctor after discharge.
-
3.
A specific treatment recommendation was given by the patient to their family physician (Appendix B). This was based on an algorithm determined by the study endocrinologist (N.S.).
-
4.
Monthly phone calls were made by the research assistant encouraging the patient and/or their family to get started on treatment.
The primary outcome is the fraction of patients who get started on recommended pharmacologic treatment within 4 months after their fragility fracture.
2.1. Inclusion/exclusion criteria
All patients over the age of 50 admitted with a fragility-related hip fracture were considered for inclusion. A fragility fracture is defined here as a fracture resulting from a low-energy fall typically occurring while standing or walking. Hip fractures included those in the subcapital, femoral neck, intertrochanteric or subtrochanteric region. Fractures of the trochanter alone, those involving the shaft or peri-prosthetic region, were not included. Exclusion criteria also included patients with a fracture sustained in a non-low energy fall, those with metastatic cancer or known metabolic bone disease or patients in end-of-life care. Patients unable to undergo consent because of dementia were excluded, but if their dementia was mild and consent could be obtained, they were included.
2.2. Randomization and data management
All patients who met the inclusion criteria were first consented then randomized into one of the 2 pathways described above. Randomization was done using a table provided by our statistician created by a randomization program. No steps were used to conceal the sequence but the patients were randomized without any deviations from the series. The research assistant used the sequence to assign participants into 1 of the 2 interventions.
Data was entered into the database created using the Research Electronic Data Capture (RedCAP) from Vanderbilt University.
2.3. DEXA study
During the hospitalization, a DEXA was performed for those patients in the Intervention Group who had not had a DEXA scan for the prior 24 months or whose DEXA scan results were not available. We use the Hologic Discovery system at our institution. The standard protocol is to scan the opposite or nonfractured hip, the lumbar spine and the distal forearm. If the opposite hip has been fractured in the past, then only the spine and distal forearm were scanned. Bone mineral density used the T-score and was adjusted for the site of the scan.
2.4. Laboratory evaluation and medication treatment algorithm
Laboratory evaluation as recommended by our endocrinologist was performed on admission and before starting therapy (Table 1). The medications for osteoporosis listed below were given only after calcium was within the normal range.
Table 1.
Recommended laboratory screening tests
| Laboratory test | Abbreviation |
|---|---|
| Calcium, albumin (repeat every 2 weeks until normal level) | Ca, Alb |
| Creatinine (creatinine clearance needs to be calculated. Renal failure defined as clearance<30 mL per minute) | Cr |
| Electrolytes (sodium, potassium bicarbonate, chloride) | Lytes |
| Complete blood count | CBC |
| Thyroid stimulating hormone | TSH |
| Parathyroid hormone (if calcium elevated) | PTH |
| Vitamin D level recommended but not done for this study | Vit D |
All patients in both groups with the exception of those with defined renal failure were given a loading dose of Vitamin D consisting of 50,000 IU given orally then continued Vitamin D at 1000 IU per day during and after their hospitalization. If the patient had defined renal failure then no loading dose was given but Vitamin D in the form of Alpha D3 0.25 micrograms orally was given instead and continued.
There are many medications available for osteoporosis treatment. For the purpose of this study, zolendronate was the preferred treatment.[1] Patients who fractured their hip while on medication such as a bisphosphonate (if treatment started 12 months prior or more) were considered as having failed treatment. For these patients, teriparatide (Forteo by Lilly) was the primary recommendation. Teriparatide is given 20 μg subcutaneously once a day (for 2 years). For those patients who are unwilling or unable to have daily subcutaneous injections, denosumab (Prolia by Amgen) was offered as an alternative.
Patients with defined renal failure (except for those with end-stage renal failure) were started on denosumab with a subcutaneous injection of 60 mg every 6 months. Any patient who did not fit the algorithm criteria was evaluated by the endocrinologist participating in the study (N.S.) and recommendation for treatment was individualized.
2.5. Determination of compliance
Patients or their families were contacted each month until medication started for up to 4 months from the hip fracture. Often these phone calls served as important reminders and encouragement to begin treatment. Treatment was verified using our hospital online connection to the health management database. Specifically, in Israel, there is national health care with 4 competing management plans (kupot). Our hospital provides access to laboratory and medication information via this online link so compliance can be verified.
2.6. Statistical analysis—power of study
The study was initially powered assuming 20% recommended treatment at 4 months in the Letter group. We assumed that we could improve recommended treatment to 50% using the interventions described above. This required a minimum of 100 patients in each group. One hundred eighteen patients in each group or 236 patients in total were required to achieve 94% power, assuming that 10% of patients would be lost to follow-up at 4 months and 5% mortality. As the study progressed, we had a lower mortality than predicted and no patients were lost to follow-up at 4 months so we stopped the study at 200 patients. Baseline characteristics were compared with Chi-square and Fishers exact tests for categorical data, depending on the distribution of the data. BMI comparison was done with a T-test. Logistic regression was used to analyze differences in recommended treatment rates.
2.7. Completion of study
At the completion of the study, patients in the Letter group that did not have either DEXA evaluation and/or proper medication treatment were encouraged to do so. Specifically, a letter was sent to the participant outlining the recommendations for continued care and they were asked to bring it to their primary care physician to complete their evaluation and treatment.
3. Results
The enrollment period was from February 21, 2017 to September 15, 2018 when 200 patients were reached. The age range was 51 to 95 years with an average age of 79.2 (± 9.2) years. Seventy-two percent were female.
During the enrollment period, there were 618 low-energy hip fractures of which 305 were eligible for enrollment (see flow diagram in Fig. 1). Most of the patients not eligible were excluded due to moderate or severe dementia, an exclusion criterion for this study. Sixty-six percent of those eligible agreed to participate in the study.
Figure 1.
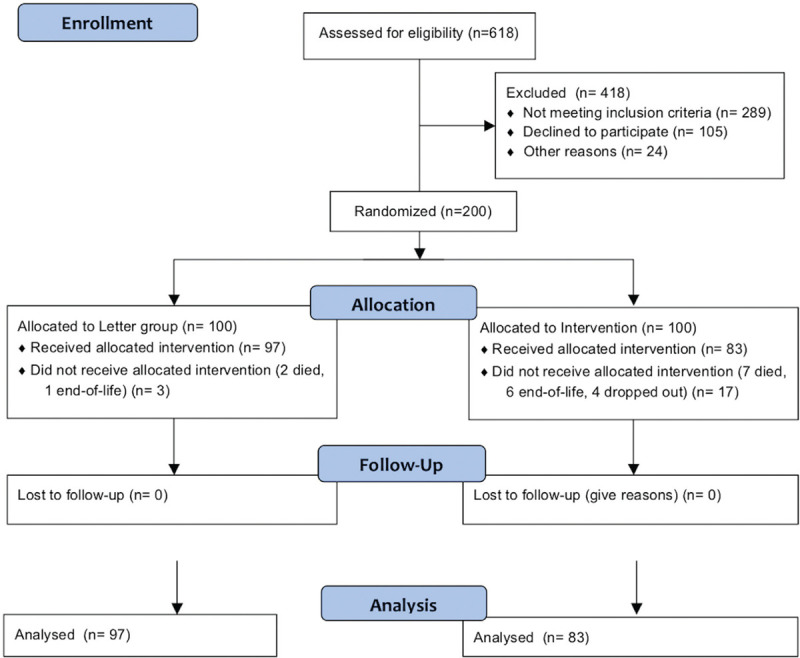
Flow diagram.
There were 100 patients enrolled into the Letter group. Three of these patients were removed from the study, 2 died, and another was diagnosed as end-of-life before the 4-month outcome measure. There were 100 patients enrolled into the intervention group. Thirteen were removed from the study (7 died and 6 diagnosed as end-of-life before the 4-month period) and 4 dropped out of the study. The analysis is based on the remaining 180 patients. Baseline characteristics were not different between groups (Table 2).
Table 2.
Baseline characteristics of Letter versus Intervention groups
| Letter Group N = 97 | Intervention Group N = 83 | ||
|---|---|---|---|
|
|
|||
| Baseline characteristic | N (%)/mean (Std) | ||
| Age | |||
| <70 | 13 (13.4) | 18 (21.7) | 0.136 |
| 70–79 | 26 (26.8) | 28 (33.7) | |
| 80–89 | 47 (48.5) | 33 (39.8) | |
| > = 90 | 11 (11.3) | 4 (4.8) | |
| Gender | |||
| Male | 29 (29.9) | 21 (25.3) | 0.493 |
| Female | 98 (70.1) | 62 (74.7) | |
| Type of fracture | |||
| Femoral neck | 14 (14.4) | 9 (10.8) | 0.236 |
| Intertrochanteric | 56 (57.7) | 58 (69.9) | |
| Subcapital | 24 (24.7) | 16 (19.3) | |
| Subtrochanteric | 3 (3.1) | 0 | |
| BMI | 25.5 (4.83) | 26.1 (5.2) | 0.385 |
In the Letter group, there were 6 of 97 patients (6.2%) on recommended medication at 4 months postinjury. In the intervention group, there were 64 of 83 patients (77.1%) on recommended medication at 4 months postinjury. The difference between Letter and intervention was significant (P < 0.0001) with an OR of 51.1 (CI 19.3–134.9). We did not find any differences in treatment rates between age groups (P = 0.089) or gender (P = 0.131). Multivariate analysis controlling for age and gender showed similar results (OR = 52.6 CI 19.3–143.5; P < 0.0001). In the intervention group, there were 19 patients who did not get treatment. There were 4 whose doctor recommended against, 1 whose family refused, 5 for medical reasons, 1 who was willing but not able, 5 who were unwilling, and 3 for unknown reasons.
Overall, there were 14 patients on medication for osteoporosis when they were admitted with hip fractures (7.8%). There were 5 in the Letter group (5.1%) and 9 in the intervention group (10.8%). This was not significantly different (P = 0.16). In the 5 patients in the Letter group on medication at the time of their fracture, none were on medication at 4 months. In the 9 patients on medication in the intervention group, 8 were on medication at 4 months.
Although DEXA was part of the intervention protocol, only 78.3% of the patients in the intervention group had the DEXA performed. Reasons for not performing the DEXA included scheduling issues during or after their hospitalization, poor mobility, obesity, and MRSA status. When we compared treatment rate within the intervention group in those that received the DEXA (81.5% on recommended medication) versus those that did not have the DEXA (61.1% on recommended medication), the difference in treatment rate did not reach statistical significance (P = 0.075). This study was powered for differences between the intervention and letter groups and not within the intervention group and would have required 104 patients instead of 83 (in the intervention group) to reach 80% power.
The metabolic work-up for patients in this study is described in the Methods section. Overall 7.8% of the patients had a potentially treatable underlying cause identified (high PTH or low TSH levels). An additional 23% of the patients had lab results that altered medical management (for example elevated TSH levels treated with thyroid supplement).
4. Discussion
Our study interventions provided results that are at least as good as those we found published by other orthopaedic departments. Edwards et al[5] did a prospective randomized effectiveness study but their 2 arms were in different hospitals, which could introduce confounding variables in interpreting the results. Their intervention group included starting medication. In our national health care system the cost of medication is provided by the one of the 4 kupot and not by the hospital. This leaves primary care actually responsible for ordering the medication. Their study had 35% lost to follow-up and 67% success in those successfully contacted. Our study had none lost to follow-up and 77% success. Gardner et al[1] in a prospective, randomized study involving 80 patients had a 42% success rate in their intervention group. However, they defined success as the primary care physician either starting bisphosphonate therapy or just ordering a DEXA scan. If one looks at receiving medication as the criteria for success, their success rate was 29% for intervention. We defined success as those patients only started on medication.
DEXA scans were included in this protocol though not required after a fragility fracture to initiate treatment. There were 2 purposes. First is to serve as a baseline so that effectiveness of treatment could later be evaluated. Second is because we suspected based on prior literature that compliance rates might be higher in the group who had the DEXA performed.[7] The DEXA was also helpful in identifying patients with an unexpectedly low bone density that were then referred for further evaluation with our endocrine department. Specifically, 5 patients had T-scores less than −3.5 and 1 patient age 58 had a T-score less than −3.1. When the DEXA was done while an inpatient, the hospital bore the cost since treatment is reimbursed via the DRG (diagnosis related group). When done as an outpatient, the kupot paid for the test.
Treatment for osteoporosis includes Vitamin D and calcium supplementation. However, this is not sufficient to prevent further loss of bone. Treatment for osteoporosis needs to include either an agent that reduces bone turnover such as bisphosphonates or an agent that actually increases bone density. Calcium needs to be corrected before such agents can be started and this protocol includes that requirement. Vitamin D level is ideally measured and corrected. The Horizon study started patients on zolendronic acid after hip fractures without requiring Vitamin D correction and showed improved refracture rates and reduced mortality.[2] We were concerned that the delay in requiring Vitamin D evaluation and correction before treatment might lead to a delay in starting treatment and lose the opportunity to get patients started on medication. For that reason and given the benign nature of Vitamin D, all patients in both Letter and intervention groups were just started on supplementation.
An important part of the protocol[8] was to make it easier for the primary care physician to initiate the prescription (Appendix B). In order to give them some flexibility, we gave 2 options for treatment. All 3 medications on the recommended list have been verified as appropriate options after hip fractures.[4] Zolendronic acid was the preferred option for a patient not already on medication. This is partially based on the proven effectiveness as shown in other studies[2,9,10] and also to reduce the concern for possible noncompliance for medications taken more frequently via the oral route (monthly or weekly). Teriparatide and denosumab although expensive options are both covered by the Israel National Healthcare system after fragility fractures of the hip. They would not normally be covered with fragility fractures of the spine or wrist.
Four months was chosen as the end point for starting medication. We believe that in these elderly patients, time is critical. We felt that if the patient did not start the medication by 4 months, he or she would be unlikely to start later. If they started medication at a much later date, for example 1 year, we would not be able to conclude that they had started because of our intervention.
There are 3 directions that can improve compliance with known guidelines.[1,5,11] The least effective appears to be for the orthopaedist to only recommend that the patient follow-up with their primary care physician (the Letter group). More success occurs when the orthopaedic department does part of the evaluation such as ordering the DEXA.[7] The maximum compliance occurs when management of bone health becomes the responsibility of the orthopaedic department.[1,5] The orthopaedic surgeon treating fragility fractures has a unique opportunity to help prevent additional fragility fractures. The literature shows that only when the orthopaedic surgeon gets involved in either evaluation or treatment or both does medication reliably get started.
Bunta et al[12] found that over a 10-year period, the number of secondary prevention programs in the USA increased from 14 sites to 177 sites showing that starting an intervention program is possible. Sietsema et al[13] evaluated the results of an orthopaedic based and nurse practitioner managed osteoporosis program in a large private orthopaedic practice in Michigan. They showed a reduction in secondary fractures and no increase in overall costs. This study confirms known effectiveness of FLS programs and shows that it can be successfully initiated in private practice programs and not just large health maintenance organizations.
The primary limitation of our study was the lack of inclusion of patients with moderate or severe dementia. We made this an exclusion criterion since a prospective study with monthly follow-up requires a patient who is able to provide consent and to be in monthly communication.
We do not feel that lack of reporting follow-up refracture rates or mortality in this study is a limitation. It is not the intent here to prove that treatment is effective. Our goal is to show that despite 20 years of literature that shows poor rates of initiating treatment, that it can be done effectively within an FLS framework and with only partial intervention by an interested orthopaedic trauma unit.
Successful FLS programs require orthopaedic involvement. The patients with low-energy fractures enter the health system through the orthopaedic department and provide the unique opportunity to intervene. It would be both logistically unrealistic and financially prohibitive for all fragility fractures to be evaluated and treated by an endocrine department. Successful FLS programs are designed with the guidance of endocrine but managed by allied health providers using an algorithm to guide recommendations.[6]
The 4 interventions described in this report can be used as a starting point for an orthopaedist interested in starting an FLS program in their department. Most orthopaedic surgeons are not interested in managing chronic care, including the treatment of osteoporosis. However, we suggest that an orthopaedic department is the ideal and possibly the only successful port of entry for a patient to receive this vital care they need for their weak bone.
The first-year mortality after a hip fracture is approximately 20%.[14] That is a worse prognosis than most cancers, and specifically comparable to thyroid and breast cancer.[15] For those that survive, over 80% of patients never get back to their preinjury level of function.[14] We owe it to our patients, our parents, our grandparents, and one day to ourselves, to take responsibility for bone health. We present an example of an effective FLS program that can be adapted or modified. We hope the orthopaedic community takes the opportunity to implement such a program.
Level of Evidence: Therapeutic Level I
Supplementary Material
Supplementary Material
References
- 1.Gardner MJ, Brophy RH, Demetrakopoulos D, et al. Interventions to improve osteoporosis treatment following hip fracture. J Bone Joint Surg. 2005;87:3–7. [DOI] [PubMed] [Google Scholar]
- 2.Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zolendronic Acid and clinical fractures and mortality after hip fracture. NEJM. 2007;357:1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klotzbuecher CM, Ross PD, Landsman PB, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Min Res. 2000;15:721–739. [DOI] [PubMed] [Google Scholar]
- 4.Crandall CJ, Newberry SJ, Diamant A, et al. Comparative effectiveness of pharmacologic treatments to prevent fractures. Ann Int Med. 2014;161:711–724. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BJ, Koval K, Bunta AD, et al. Addressing secondary prevention of osteoporosis in fracture care: follow-up to “own the bone”. J Bone Joint Surg Am. 2011;93:e87. [DOI] [PubMed] [Google Scholar]
- 6.Marsh D, Akesson K, Beaton DE, et al. IOF CSA Fracture Working Group. Coordinator-based systems for secondary fracture prevention in fragility fracture patients. Osteoporos Int. 2011;22:2051–2065. [DOI] [PubMed] [Google Scholar]
- 7.Rozental TD, Makhni EC, Day CS, et al. Improving evaluation and treatment for osteoporosis following distal radius fractures. A prospective randomized intervention. J Bone Joint Surg. 2008;90:953–961. [DOI] [PubMed] [Google Scholar]
- 8.Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2016;22 (supple 4):1–42. [DOI] [PubMed] [Google Scholar]
- 9.Bell KJL, Hayen A, Glasziou P, et al. Potential usefulness of BMD and bone turnover monitoring of Zoledronic Acid therapy among women with osteoporosis: Secondary analysis of randomized controlled trial data. J Bone Min Res. 2016;31:1767–1773. [DOI] [PubMed] [Google Scholar]
- 10.Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinal Metab. 2011;96:1727–1736. [DOI] [PubMed] [Google Scholar]
- 11.Dell RM, Greene D, Anderson D, et al. Osteoporosis disease management: what every orthopedic surgeon should know. J Bone Joint Surg. 2009;(suppl 6):79–86. [DOI] [PubMed] [Google Scholar]
- 12.Bunta AD, Edwards BJ, Macaulay WB, Jr, et al. Own the bone, a system-based intervention, improves osteoporosis care after fragility fractures. J Bone Joint Surg Am. 2016;98:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sietsema DL, Araujo AB, Wang L, et al. The effectiveness of a private orthopaedic practice-based osteoporosis management service to reduce the risk of subsequent fractures. J Bone Joint Surg Am. 2018;100:1819–1828. [DOI] [PubMed] [Google Scholar]
- 14.Cooper C. The crippling consequences of fractures and their impact on the quality of life. Am J Med. 1997;103:12S–19S. [DOI] [PubMed] [Google Scholar]
- 15.Lee YK, Lee YJ, Ha YC, et al. Five-year relative survival of patients with osteoporotic hip fracture. J Clin Endocrinol Metab. 2014;99:97–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


