Abstract
Objective Leiomyoma with bizarre nuclei (LBN) is a variant of uterine leiomyoma, which has replaced the previous category of “atypical leiomyoma” and must be distinguished from smooth muscle tumors of uncertain malignant potential (STUMP). However, previously published series of “atypical leiomyoma” might have included both LBN and STUMP, due to the lack of strict diagnostic criteria. Based on such hypothesis, we aimed to define the risk of recurrence in LBN.
Study Design A systematic review and meta-analysis was performed by searching 4 electronic databases for all studies assessing the outcome of patients with “atypical leiomyoma” or LBN. The pooled absolute risk of recurrence was calculated. The included studies were subdivided into two subgroups based on the criteria used: “LBN + STUMP” or “LBN-only”.
Results Twelve studies with 433 patients were included. The pooled risk of recurrence was 5.5% overall. The funnel plot showed two cluster of studies which superimposed to the two subgroups. In the LBN + STUMP cluster/subgroup, the pooled risk of recurrence was 7.7%. In the LBN-only cluster/subgroup, the pooled risk of recurrence was 1.9%. Statistical heterogeneity was null in all analyses.
Conclusion Our results show a risk of recurrence of 1.9% for LBN; higher recurrence rates in older studies are likely due to the inclusion of STUMPs.
Key words: prognosis, diagnosis, sarcoma, myoma, leiomyoma, myomata
Zusammenfassung
Zielsetzung Leiomyom mit bizarrem Zellkern (LBN) ist eine Variante von uterinen Leiomyomen, welche die frühere Kategorie „atypisches Leiomyom“ ersetzt; sie sollte von glattmuskulären Tumoren mit unsicherem malignen Potenzial (STUMP) unterschieden werden. Weil aber strenge diagnostischen Kritierien bei früheren Studien fehlten, kann es sein, dass früher veröffentlichte Serien von atypischen Leiomyomen sowohl LBN als auch STUMP umfassen. Basierend auf dieser Hypothese war das Ziel unserer Studie, die Gefahr eines Rezidivs bei LBN zu definieren.
Studiendesign Eine systematische Überprüfung und eine Metaanalyse wurden durchgeführt. Hierzu wurden 4 elektronische Datenbanken nach Studien durchsucht, die das Outcome von Patientinnen mit atypischen Leiomyomen bzw. LBN evaluierten. Das gepoolte absolute Risiko eines Rezidivs wurde berechnet. Die eingeschlossenen Studien wurden in 2 Untergruppen unterteilt: eine LBN + STUMP-Gruppe und eine Nur-LBN-Gruppe.
Ergebnisse Zwölf Studien mit insgesamt 433 Patientinnen wurden gefunden und evaluiert. Das gepoolte Risiko eines Rezidivs betrug insgesamt 5,5%. Der Funnel Plot zeigte 2 Cluster von Studien, die sich mit den 2 Untergruppen überlagerten. In der LBN + STUMP-Cluster/Untergruppe betrug das gepoolte Risiko für ein Rezidiv 7,7%. In der Nur-LBN-Cluster/Untergruppe betrug das gepoolte Risiko eines Rezidivs dagegen 1,9%. Die statistische Heterogenität betrug bei allen Analysen null.
Schlussfolgerung Unsere Ergebnisse zeigen, dass die Gefahr eines Rezidivs bei LBN 1,9% beträgt; die höheren Rezidivraten in älteren Studien beruhen wahrscheinlich auf dem Einschluss von STUMP.
Schlüsselwörter: Prognose, Diagnose, Sarkom, Myom, Leiomyom, Myome
Introduction
Smooth-muscle tumors are the most common gynecologic neoplasms 1 , 2 , 3 . They are categorized as leiomyoma, leiomyosarcoma or smooth muscle tumors of uncertain malignant potential (STUMP) 4 , 5 , 6 . The malignant potential of gynecologic smooth muscle tumors is currently assessed based on histomorphological features, out of which the most important are cytologic atypia, mitotic index and coagulative tumor cell necrosis (CTCN) 7 . According to the Stanford criteria, the presence of at least two among significant (i.e. at least moderate) cytologic atypia, mitotic index ≥ 10/10 HPF and CTCN is sufficient to qualify the tumor as leiomyosarcoma 4 , 7 . If the mitotic index is ≥ 10/10 HPF in the absence of the other two criteria, the tumor is categorized as a benign leiomyoma variant (i.e. mitotically active leiomyoma) 5 . If there is CTCN in the absence of the other two criteria, the tumor is labeled as STUMP 5 . Instead, the classification may be more difficult for tumors showing significant atypia but no mitotic index ≥ 10/10 HPF or CTCN. According to the current WHO classifications of gynecological neoplasms, these tumors would fall into two different categories: “STUMP” and “leiomyoma with bizarre nuclei” (LBN). LBN has replaced the former category of “atypical leiomyoma” and had also been termed as “symplastic” or pleomorphic“ leiomyoma, due to the frequent occurrence of large cells with bizarrely shaped, multiple or multilobulated nuclei and intranuclear cytoplasmic pseudoinclusions 5 , 8 . However, previous studies assessing so-called ”atypical leiomyoma“ did not adopt the diagnostic criteria of LBN. As a result, many published series might have lumped LBN and STUMP together under the definition of ”atypical leiomyoma”. This might be the cause for the high heterogeneity in the recurrence rates reported in the literature 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 . The consequence is that the risk of recurrence of LBN remains poorly defined. The aim of this systematic review and meta-analysis was to improve the definition of the risk of recurrence in LBN.
Materials and Methods
Study protocol
This systematic review were designed a priori based on previous studies 32 , 33 . Each stage (electronic search, study selection, data extraction, risk of bias assessment and data analysis) was performed independently by three authors. Disagreements, if any, were resolved by consensus. This review was reported following the PRISMA statement 34 .
Search strategy and study selection
Web of Sciences, PubMed, Scopus, and Google Scholar were searched from 1994 (year of publication of the Stanford criteria 7 ) to April 2020 for all studies assessing case series of “atypical leiomyoma” and LBN. The following combination of text words was used: (atypical OR pleomorphic OR symplastic OR bizarre) AND leiomyoma. Relevant references were also retrieved from eligible studies.
Inclusion criteria were: significant atypia and a mitotic index < 10/10 HPF.
Exclusion criteria were: sample size < 5; outcome data not reported; reviews.
Data extraction
Original data were not modified. The main data extracted were the number of included patients, the criteria for defining atypia and the number of cases which recurred. Further data extracted were country, selection criteria, period of enrollment, pathology review, type of intervention, follow-up information, characteristics of recurrent cases. The studies were subdivided into two subgroup based on the selection criteria:
the first subgroup included studies that selected leiomyomas based on the presence of any significant atypia under the definition of “atypical leiomyoma”; based on the assumption that these studies included both LBN and STUMP, we will refer to this subgroup as “LBN + STUMP”
the second subgroup included studies that selected leiomyomas based on the presence of bizarre nuclei (large cells with bizarrely shaped, multiple or multilobulated nuclei and intranuclear cytoplasmic pseudoinclusions); we will refer to this subgroup as “LBN-only”.
Risk of bias within studies assessment
According to the QUADAS-2 35 , four domains were assessed in each study:
Patient selection (i.e. if selection criteria and period of recruitment were reported);
Index test (i.e. histological slides were reviewed);
Reference standard (i.e. if outcome data were clearly reported);
Flow (i.e. if the flow of patients, including the number of eligible and excluded patients, with reasons, was reported).
For each domain, the judgement was categorized as “low”, “unclear” or “high risk of bias”, as previously described 32 , 33 .
Data analysis
The risk of recurrence was calculated as the number of recurrent cases divided by the total number of patients, as previously described 36 . The risk of recurrence, for each study and as pooled estimate, was reported graphically on forest plots with 95% confidence interval (CI). Both the Mantel-Haenszel fixed effect model and the DerSimonian-Laird random effect model were used to pool data. Statistical heterogeneity among studies was quantified according to the inconsistency index (I 2 ), as previously described 32 , 33 , 36 . The risk of bias across studies (publication bias) was assessed by using a funnel plot. Comprehensive Meta-Analysis (Biostat,14 North Dean Street, Englewood, NJ 07631, USA) was used for the analysis.
Results
Study selection and characteristics
Twelve studies with 433 patients were included 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 . The full process of study selection is reported in Supplementary Fig. S1 . Six studies were included in the “LBN + STUMP” subgroup 7 , 9 , 10 , 12 , 13 , 14 , and 6 studies were included in the “LBN-only” subgroup 8 , 11 , 15 , 16 , 17 , 18 . Characteristics of the included studies are shown in Table 1 .
Table 1 Characteristics of the included studies.
| Study | Country | Period of enrollment | Sample size | Definition | Selection criteria |
|---|---|---|---|---|---|
| Bell 1994 | USA | 1970 – 1991 | 49 | “Atypical leiomyoma” | < 10 mitoses/10 HPF + significant atypia (any type) |
| Downes 1997 | USA | 1971 – 1995 | 24 | “Bizarre leiomyoma” | < 10 mitoses/10 HPF + bizarre nuclei |
| Sung 2009 | Korea | 1994 – 2007 | 15 | “Atypical leiomyoma” | < 10 mitoses/10 HPF + significant atypia (any type) |
| Ly 2013 | USA | 1992 – 2003 | 51 | “Atypical leiomyoma” | < 10 mitoses/10 HPF + significant atypia (any type) |
| Croce 2014 | USA | 2000 – 2011 | 59 | Leiomyoma with bizarre nuclei | < 10 mitoses/10 HPF + bizarre nuclei |
| Liang 2015 | China | 1997 – 2009 | 25 | “Atypical leiomyoma” | < 10 mitoses/10 HPF + significant atypia (any type) |
| Kalogiannidis 2016 | Greece | 2002 – 2012 | 5 | “Atypical leiomyoma” | < 10 mitoses/10 HPF + significant atypia (any type) |
| Ubago 2016 | USA, China | 1993 – 2015 | 43 | “Atypical leiomyoma” | < 10 mitoses/10 HPF + significant atypia (any type) |
| Chow 2017 | Hong Kong | unclear | 18 | Leiomyoma with bizarre nuclei | < 10 mitoses/10 HPF + bizarre nuclei |
| Bennett 2017 | USA | 1990 – 2014 | 26 | Leiomyoma with bizarre nuclei | < 10 mitoses/10 HPF + bizarre nuclei |
| Kefeli 2018 | Turkey | 2002 – 2016 | 26 | Leiomyoma with bizarre nuclei | < 10 mitoses/10 HPF + bizarre nuclei |
| Gregova 2019 | Czech Republic | 1998 – 2017 | 92 | Leiomyoma with bizarre nuclei | < 10 mitoses/10 HPF + bizarre nuclei |
Risk of bias assessment
For the “Patient selection” domain, one study was considered at unclear risk of bias, because the period of enrollment was not reported 18 , while the other studies were considered at low risk of bias. For the “Index test” and “reference standard” domains, all studies were considered at low risk of bias. For the “Flow” domain, one study was considered at unclear risk of bias, since the number of eligible patients excluded in the selection process was not reported 10 . The other studies were considered at low risk of bias (Supplementary Fig. S2 ).
Risk of recurrence
Overall, the recurrence rate was 5.5% (95% CI 3.5 – 8.5%) ( Fig. 1 ). The funnel plot revealed two different clusters of studies: the studies of the first clusters showed a risk of recurrence above the pooled result 7 , 9 , 10 , 12 , 13 , 14 , while the studies of the second cluster showed a risk of recurrence below the pooled result 8 , 11 , 15 , 16 , 17 , 18 ( Fig. 2 ). The first cluster of studies overlapped with the “LBN + STUMP” subgroup. In this cluster/subgroup, the pooled risk of recurrence was 7.7% (95% CI 4.6 – 12.5%) ( Fig. 3 ); all recurrences in this group occurred locally, after hysterectomy (37.5%) or after myomectomy (62.5%). The second cluster of studies overlapped with the “LBN-only” subgroup. In this cluster/subgroup, the pooled risk of recurrence was 1.9% (95% CI 0.8 – 4.7%) ( Fig. 4 ); all recurrences occurred locally and after myomectomy. Statistical heterogeneity was null (I 2 = 0%) in all analyses.
Fig. 1.
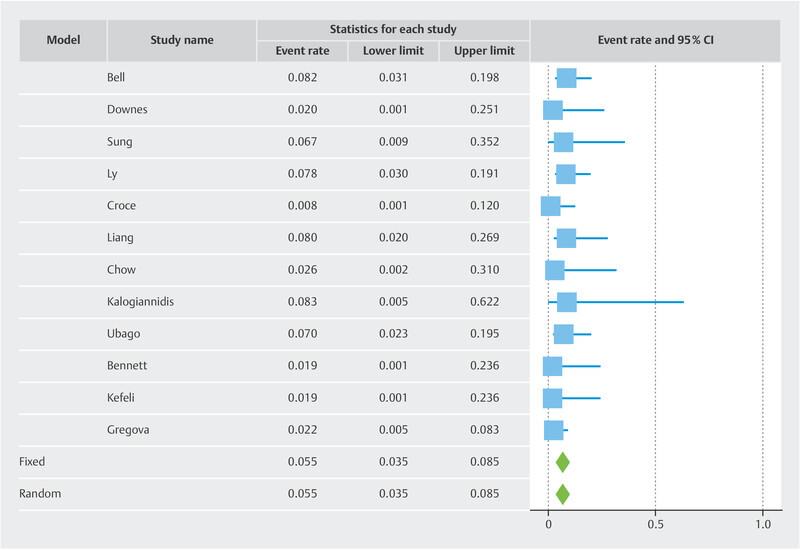
Forest plot reporting the rate or recurrence of published series of so-called “atypical leiomyoma” and leiomyoma with bizarre nuclei, lumped together.
Fig. 2.
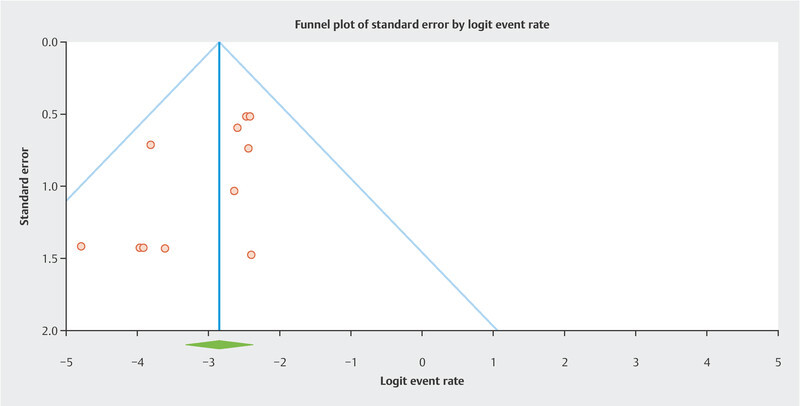
Funnel plot for the assessment of the risk of bias across studies. Two different clusters of studies are evident.
Fig. 3.
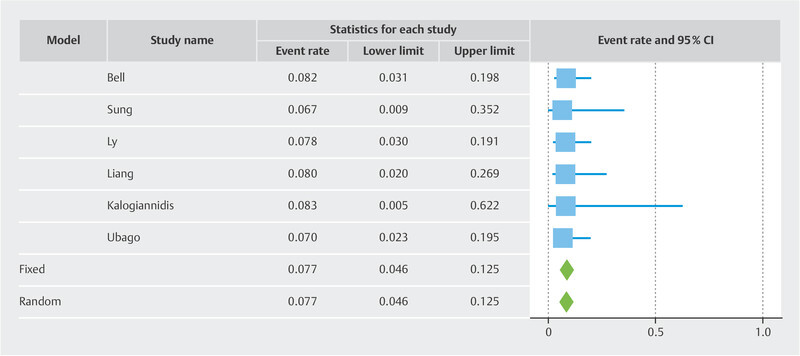
Forest plot reporting the rate or recurrence in published series of so-called “atypical leiomyoma”.
Fig. 4.
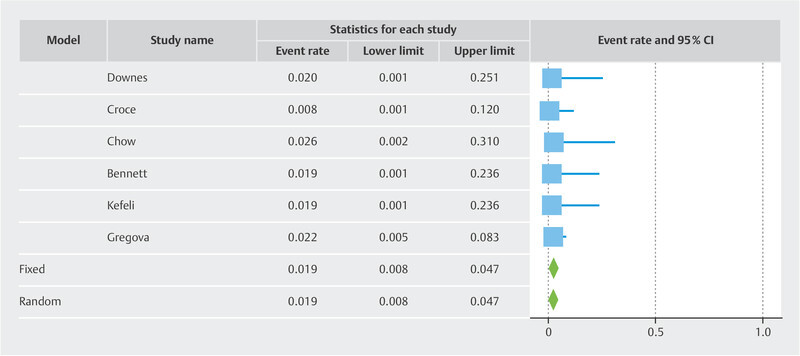
Forest plot reporting the rate or recurrence in published series of leiomyoma with bizarre nuclei.
Comment
Main findings and interpretation
This study showed that, pooling all the published studies on “atypical leiomyoma” or “LBN”, the overall risk of recurrence in was 5.5%. However, the risk of recurrence was 7.7% in studies that included generically “atypical leiomyoma” (which likely included both LBN and STUMP), while it was 1.9% in the studies that only included LBN.
The classification of leiomyomas exhibiting significant atypia in the absence of increased mitotic index or CTCN have been subject of debate. These tumors have variably been regarded as a stage in the development of leiomyosarcoma, as benign lesions with degenerative changes, as STUMPs 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 . In the study by Bell et al., they have been termed as “atypical leiomyoma with low malignant potential” 7 . In the subsequent studies, these tumors have been either listed in STUMP series 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 or treated separately in case series 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 . The current WHO classifications include the category of “LBN” among the benign variant of leiomyomas 5 . In many studies, LBN was considered as a synonym of atypical leiomyoma 8 , 11 , 15 , 16 , 17 . However, the published studies on “atypical leiomyoma” did not use strict criteria for LBN, apparently including all smooth muscle tumors with significant atypia but with neither CTCN nor a mitotic index ≥ 10/10 HPF 7 , 9 , 10 , 12 , 13 , 14 . We assumed that these studies actually included both LBN and STUMPs under the definition of “atypical leiomyoma”. As a result, the risk of recurrence of LBN is currently not well defined.
By pooling all the published studies on “atypical leiomyoma” or “LBN”, this meta-analysis found an overall risk of recurrence of 5.5%. This result seems to contrast with the benign nature of LBN, although not so much to warrant a STUMP diagnosis. Indeed, the STUMP category is characterized by a higher overall risk of recurrence 6 . Furthermore, STUMPs may have metastatic spread and may recur as leiomyosarcoma 6 . In our series, only 3 cases recurred outside the uterus, and none distant from the primary site. Although the heterogeneity among studies was null, the funnel plot showed two different clusters of studies with different risk of recurrence. The first cluster was composed of studies that included so-called “atypical leiomyoma”, independently of the type of cytologic atypia 7 , 9 , 10 , 12 , 13 , 14 ; this subgroup showed a risk of recurrence of 7.7%; all recurrences outside the uterus (i.e., after hysterectomy) occurred in this subgroup. The second cluster was composed of studies that only selected cases exhibiting bizarre nuclei (i.e., with large cells with bizarrely shaped, multiple or multilobulated nuclei and intranuclear cytoplasmic pseudoinclusions) 8 , 11 , 15 , 16 , 17 , 18 ; in this subgroup, the risk of recurrence was considerably lower (1.9%), with all recurrences being local and after myomectomy. Remarkably, none of the included studies, regardless of the subgroup, reported any recurrence outside the pelvis.
These results support that the biological behavior of LBN is consistent with a benign lesion, with no concerns of distant spread and an overall low risk of local recurrence. Our findings also support that the higher recurrence rates observed in older studies are due to the inclusion of STUMP. In fact, smooth muscle tumors with significant atypia in the absence of increased mitotic index or CTCN represent the majority of cases labeled as STUMP in the literature; these STUMPs shave shown a considerable risk of recurrence (about 20%) 37 . The recurrence rate of 7.7% observed in the series of so-called “atypical leiomyoma” appears therefore consistent with an admixture of LBN and STUMP.
These findings support that two types of atypia exist in uterine smooth muscle tumors: the atypia of LBN, characterized by extremely bizarre features (striking pleomorphism, multinucleation, pseudoinclusions) but devoid of malignant potential, and the atypia of STUMP, which may be less striking but reflects a more aggressive behavior. In this regard, even the atypia of leiomyosarcoma may be less striking than that of LBN 5 . However, differentiating between LBN and STUMP may not be obvious. Other features, not included in the Stanford criteria, may justify a STUMP diagnosis when present; these include atypical mitoses, infiltrative margins and vascular intrusion 29 . In addition, the 2020 WHO classification established an objective criterion for such differential diagnosis, indicating a mitotic index of 0 – 4/10 HPF for LBN and 5 – 9/10 HPF for STUMP 5 . However, the studies included in this meta-analysis were published before 2020 and did not adopt such thresholds, despite showing a clear difference in the recurrence rate. Further studies are warranted in this field.
Strengths and limitations
To the best of our knowledge, this is the first meta-analysis assessing the risk of recurrence in LBN. We also separated the studies based on the inclusion criteria, highlighting that studies assessing so-called “atypical leiomyoma” likely included both LBN and STUMP. The overall sample size (N = 433) may be considered adequate, given the rarity of these tumors. The main limitation to our results is the impossibility to review the morphological features of the individual cases, precluding to assess the different types of atypia and other relevant morphological features. However, we hope that our result may encourage further research in this field.
Conclusion
LBN shows a biological behavior consistent with a benign lesions, with a risk of local recurrence < 2% and no evident risk of recurrence outside uterus. The higher recurrence rates reported in previous series of so-called “atypical leiomyomas” are likely due to the inclusion of both LBN and STUMP. Discriminating between LBN and STUMP is therefore highly relevant in terms of prognosis and patient management. Further studies are encouraged to improve the prognostic stratification and differential diagnosis of uterine smooth muscle tumors.
Financial Support
No financial support was received for this study.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Supporting Information
References
- 1.Mazzei P, Piccolo A, Nugnes L. Metabolic profile of intact tissue from uterine leiomyomas using high-resolution magic-angle-spinning 1 H NMR spectroscopy . NMR Biomed. 2010;23:1137–1145. doi: 10.1002/nbm.1540. [DOI] [PubMed] [Google Scholar]
- 2.De Falco M, Staibano S, Mascolo M. Leiomyoma pseudocapsule after pre-surgical treatment with gonadotropin-releasing hormone agonists: relationship between clinical features and immunohistochemical changes. Eur J Obstet Gynecol Reprod Biol. 2009;144:44–47. doi: 10.1016/j.ejogrb.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 3.De Falco M, Staibano S, DʼArmiento F P. Preoperative treatment of uterine leiomyomas: clinical findings and expression of transforming growth factor-beta3 and connective tissue growth factor. J Soc Gynecol Investig. 2006;13:297–303. doi: 10.1016/j.jsgi.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Denschlag D, Ackermann S, Battista M J. Sarcoma of the Uterus. Guideline of the DGGG and OEGGG (S2k Level, AWMF Register Number 015/074, February 2019) Geburtshilfe Frauenheilkd. 2019;79:1043–1060. doi: 10.1055/a-0882-4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Classification of Tumours Editorial Board Female Genital Tumours Lyon (France)International Agency for Research on Cancer; 2020(WHO classification of tumours series, 5th ed., vol. 4) [Google Scholar]
- 6.Gadducci A, Zannoni G F. Uterine smooth muscle tumors of unknown malignant potential: A challenging question. Gynecol Oncol. 2019;154:631–637. doi: 10.1016/j.ygyno.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Bell S W, Kempson R L, Hendrickson M R. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18:535–558. [PubMed] [Google Scholar]
- 8.Downes K A, Hart W R. Bizarre leiomyomas of the uterus: a comprehensive pathologic study of 24 cases with long-term follow-up. Am J Surg Pathol. 1997;21:1261–1270. doi: 10.1097/00000478-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Sung C O, Ahn G, Song S Y. Atypical leiomyomas of the uterus with long-term follow-up after myomectomy with immunohistochemical analysis for p 16INK4A, p 53, Ki-67, estrogen receptors, and progesterone receptors. Int J Gynecol Pathol. 2009;28:529–534. doi: 10.1097/PGP.0b013e3181a2b8d3. [DOI] [PubMed] [Google Scholar]
- 10.Ly A, Mills A M, McKenney J K. Atypical leiomyomas of the uterus: a clinicopathologic study of 51 cases. Am J Surg Pathol. 2013;37:643–649. doi: 10.1097/PAS.0b013e3182893f36. [DOI] [PubMed] [Google Scholar]
- 11.Croce S, Young R H, Oliva E. Uterine leiomyomas with bizarre nuclei: a clinicopathologic study of 59 cases. Am J Surg Pathol. 2014;38:1330–1339. doi: 10.1097/PAS.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 12.Liang Y, Zhang X, Chen X. Diagnostic value of progesterone receptor, p 16, p 53 and pHH3 expression in uterine atypical leiomyoma. Int J Clin Exp Pathol. 2015;8:7196–7202. [PMC free article] [PubMed] [Google Scholar]
- 13.Kalogiannidis I, Stavrakis T, Dagklis T. A clinicopathological study of atypical leiomyomas: Benign variant leiomyoma or smooth-muscle tumor of uncertain malignant potential. Oncol Lett. 2016;11:1425–1428. doi: 10.3892/ol.2015.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ubago J M, Zhang Q, Kim J J. Two Subtypes of Atypical Leiomyoma: Clinical, Histologic, and Molecular Analysis. Am J Surg Pathol. 2016;40:923–933. doi: 10.1097/PAS.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett J A, Weigelt B, Chiang S. Leiomyoma with bizarre nuclei: a morphological, immunohistochemical and molecular analysis of 31 cases. Mod Pathol. 2017;30:1476–1488. doi: 10.1038/modpathol.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kefeli M, Caliskan S, Kurtoglu E. Leiomyoma With Bizarre Nuclei: Clinical and Pathologic Features of 30 Patients. Int J Gynecol Pathol. 2018;37:379–387. doi: 10.1097/PGP.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 17.Gregová M, Hojný J, Němejcová K. Leiomyoma with Bizarre Nuclei: a Study of 108 Cases Focusing on Clinicopathological Features, Morphology, and Fumarate Hydratase Alterations. Pathol Oncol Res. 2019 doi: 10.1007/s12253-019-00739-5. [DOI] [PubMed] [Google Scholar]
- 18.Chow K L, Tse K Y, Cheung C L. The mitosis-specific marker phosphohistone-H3 (PHH3) is an independent prognosticator in uterine smooth muscle tumours: an outcome-based study. Histopathology. 2017;70:746–755. doi: 10.1111/his.13124. [DOI] [PubMed] [Google Scholar]
- 19.Atkins K A, Arronte N, Darus C J. The Use of p 16 in enhancing the histologic classification of uterine smooth muscle tumors. Am J Surg Pathol. 2008;32:98–102. doi: 10.1097/PAS.0b013e3181574d1e. [DOI] [PubMed] [Google Scholar]
- 20.Ip P P, Cheung A N, Clement P B. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): a clinicopathologic analysis of 16 cases. Am J Surg Pathol. 2009;33:992–1005. doi: 10.1097/PAS.0b013e3181a02d1c. [DOI] [PubMed] [Google Scholar]
- 21.Veras E, Malpica A, Deavers M T. Mitosis-specific marker phospho-histone H3 in the assessment of mitotic index in uterine smooth muscle tumors: a pilot study. Int J Gynecol Pathol. 2009;28:316–321. doi: 10.1097/PGP.0b013e318193df97. [DOI] [PubMed] [Google Scholar]
- 22.Dańska-Bidzińska A, Bakuła-Zalewska E, Nasierowska-Guttmejer A. [Smooth muscle tumor of uncertain malignant potential (STUMP)–clinico-pathomorphological analysis of the cases and literature review] Ginekol Pol. 2012;83:412–416. [PubMed] [Google Scholar]
- 23.DallʼAsta A, Gizzo S, Musarò A. Uterine smooth muscle tumors of uncertain malignant potential (STUMP): pathology, follow-up and recurrence. Int J Clin Exp Pathol. 2014;7:8136–8142. [PMC free article] [PubMed] [Google Scholar]
- 24.Croce S, Ribeiro A, Brulard C. Uterine smooth muscle tumor analysis by comparative genomic hybridization: a useful diagnostic tool in challenging lesions. Mod Pathol. 2015;28:1001–1010. doi: 10.1038/modpathol.2015.3. [DOI] [PubMed] [Google Scholar]
- 25.Slatter T L, Hsia H, Samaranayaka A. Loss of ATRX and DAXX expression identifies poor prognosis for smooth muscle tumours of uncertain malignant potential and early stage uterine leiomyosarcoma. J Pathol Clin Res. 2015;1:95–105. doi: 10.1002/cjp2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacanakgil B H, Deveci M, Karabuk E. Uterine Smooth Muscle Tumor of Uncertain Malignant Potential: Clinicopathologic-Sonographic Characteristics, Follow-Up and Recurrence. World J Oncol. 2017;8:76–80. doi: 10.14740/wjon1031w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esposito N N, Hunt J L, Bakker A. Analysis of allelic loss as an adjuvant tool in evaluation of malignancy in uterine smooth muscle tumors. Am J Surg Pathol. 2006;30:97–103. doi: 10.1097/01.pas.0000180424.75077.a3. [DOI] [PubMed] [Google Scholar]
- 28.Basaran D, Usubutun A, Salman M C. The Clinicopathological Study of 21 Cases With Uterine Smooth Muscle Tumors of Uncertain Malignant Potential: Centralized Review Can Purify the Diagnosis. Int J Gynecol Cancer. 2018;28:233–240. doi: 10.1097/IGC.0000000000001178. [DOI] [PubMed] [Google Scholar]
- 29.Gupta M, Laury A L, Nucci M R. Predictors of adverse outcome in uterine smooth muscle tumours of uncertain malignant potential (STUMP): a clinicopathological analysis of 22 cases with a proposal for the inclusion of additional histological parameters. Histopathology. 2018;73:284–298. doi: 10.1111/his.13515. [DOI] [PubMed] [Google Scholar]
- 30.Ha H I, Choi M C, Heo J H. A clinicopathologic review and obstetric outcome of uterine smooth muscle tumor of uncertain malignant potential (STUMP) in a single institution. Eur J Obstet Gynecol Reprod Biol. 2018;228:1–5. doi: 10.1016/j.ejogrb.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Karataşlı V, Çakır İ, Ayaz D. Clinicopathologic evaluation of uterine smooth muscle tumors of uncertain malignant potential (STUMP): A single center experience. J Gynecol Obstet Hum Reprod. 2019;48:637–642. doi: 10.1016/j.jogoh.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Travaglino A, Raffone A, Saccone G. Congruence Between 1994 WHO Classification of Endometrial Hyperplasia and Endometrial Intraepithelial Neoplasia System. Am J Clin Pathol. 2020;153:40–48. doi: 10.1093/ajcp/aqz132. [DOI] [PubMed] [Google Scholar]
- 33.Travaglino A, Raffone A, Saccone G. Nuclear expression of β-catenin in endometrial hyperplasia as marker of premalignancy. APMIS. 2019;127:699–709. doi: 10.1111/apm.12988. [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Shamseer L, Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whiting P F, Rutjes A W, Westwood M E. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 36.Travaglino A, Raffone A, Mascolo M. TCGA Molecular Subgroups in Endometrial Undifferentiated/Dedifferentiated Carcinoma. Pathol Oncol Res. 2020;26:1411–1416. doi: 10.1007/s12253-019-00784-0. [DOI] [PubMed] [Google Scholar]
- 37.Travaglino A, Raffone A, Gencarelli A. Stanford parameters stratify the risk of recurrence in gynecologic smooth muscle tumors of uncertain malignant potential. APMIS. 2021;129:283–290. doi: 10.1111/apm.13135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


