Abstract
Liver transplantation (LT) remains the gold standard treatment for end stage liver disease in the pediatric population. For liver based metabolic disorders (LBMDs), the decision for LT is predicated on a different set of paradigms. With improved outcomes post-transplantation, LT is no longer merely life saving, but has the potential to also significantly improve quality of life. This review summarizes the clinical presentation, medical treatment and indications for LT for some of the common LBMDs. We also provide a practical update on the dilemmas and controversies surrounding the indications for transplantation, surgical considerations and prognosis and long terms outcomes for pediatric LT in LBMDs. Important progress has been made in understanding these diseases in recent years and with that we outline some of the new therapies that have emerged.
Keywords: Pediatric metabolic liver disease, Liver transplantation, Liver based metabolic disorders, Inherited, Cell therapy, Gene therapy
Core Tip: The decision for liver transplantation (LT) in liver based metabolic disorders (LBMDs) is not straightforward. As outcomes from pediatric LT continue to improve, transplantation is no longer merely life saving, but also potentially significantly improves the child’s quality of life. We herein discuss the clinical presentation, medical and surgical treatment for some of the common LBMDs. We provide a practical update on the indications, dilemmas and controversies for LT and the long-term outcomes for children with LBMDs.
INTRODUCTION
Liver transplantation (LT) remains the standard of care for children with end-stage liver disease. With advances in the perioperative transplant management, the outcomes after pediatric LT continue to improve-with better survival rates[1] and quality of life measures[2].
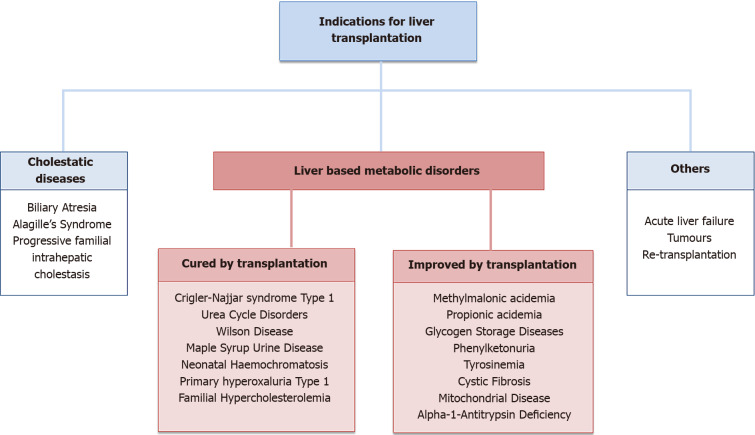
Indications for pediatric LT can be broadly divided in to three main groups (Figure 1). (1) Cholestatic diseases, such as biliary atresia and other conditions leading to biliary cirrhosis are the most common indications for LT in the pediatric population[3]; (2) Inherited metabolic liver diseases constitutes a wider group of diseases, in which inborn errors of liver metabolism lead to severe intra- or extra-hepatic manifestations. Within this group of conditions, LT results in a cure in some, whilst others have an improved quality of life after transplantation, without necessarily being cured from their primary illness; and (3) The third group is more varied, with indications of acute liver failure, tumors and re-transplantations.
Figure 1.
Indications for liver transplantation.
Some of the more common liver based metabolic disorders (LBMDs) are exemplified below.
LBMDS CURED BY LT
Crigler-Najjar syndrome type 1
Crigler-Najjar syndrome (CNS) type 1 is secondary to a total deficiency of the uridine diphosphoglucuronate glucuronosyltransferase activity[4]. This results in a severe indirect hyperbilirubinaemia from birth, with an otherwise normal liver biochemistry. It is an extremely rare familial disease affecting one per 600000-1000000 live births worldwide. It has an autosomal recessive inheritance pattern and is caused by biallelic mutations of the UGT1A1 gene[5].
Natural history and medical treatment: The build up of unconjugated bilirubin, which deposits in the brain, eventually leads to kernicterus, which is irreversible in most cases. Exchange transfusion in the neonatal period and plasmapheresis in older children, may be indicated for acute episodes of severe hyperbilirubinaemia.
Intensive phototherapy is the mainstay of treatment for CNS type 1, particularly in the newborn period. It is less effective in older children and adults due to skin thickness, pigmentation and lower body surface are to body mass[6].
Other treatments include bilirubin-binding agents such as orlistat–a lipase inhibitor which works better in tandem with calcium phosphate. Both of these agents help in the excretion of bilirubin through the gut[7,8]. Other pharmacological agents with limited evidence for efficacy include enzyme-inducing agents (phenobarbital), choleretics (ursodiol) and heme-oxygenase inhibitors (tin-protoporphyrin and zinc-protoporphyrin).
LT: At present, the only definitive treatment for CNS type 1 is LT. The two main types of LT include orthotopic LT (OLT) and auxiliary partial OLT (APOLT). The host liver is replaced with a whole or partial liver graft in OLT, whilst in APOLT only part of the native liver is removed and replaced with the graft. APOLT has the theoretical advantage for future novel therapies directed at native hepatocytes, such as gene replacement and genome editing[4].
The transplant provides the child with a normal liver with normal UGT1A1 enzymatic activity, thereby completely normalizing bilirubin levels and providing the child with a normal quality of life. LT is advisable before neurological damage occurs[9]. As the outcomes of transplantation in infants are now similar to children, transplantation is indicated in the first few years of life to prevent prolonged impairment to the child and family.
Future research implications: In recent years, allogenic hepatocyte transplantation has become an attractive alternative to LT[10]. Normal hepatocytes are transplanted via the portal vein or peritoneal space. Encouraging results have been observed with a reduction in bilirubin levels and reduced need for phototherapy[11]. Issues still exist around the longevity of the transplanted cells–which decreases after a few months, limited supply and cell quality. Mesenchymal stem cell therapy has shown some promise in animal models and may provide a new alternative treatment in the future[12].
Ex vivo and in vivo gene therapy is another new avenue for treatment of CNS type 1. Different approaches including infusing autologous liver or induced pluripotent stem cells into the liver and in vivo gene replacement using a vector delivery system have been proposed, but there remain little safety and efficacy data[4].
Urea cycle disorders
Urea cycle disorders (UCDs) are a group of disorders secondary to defects of urea synthesis and related metabolic pathways. UCDs result from a deficiency in either one of the six enzymes [n-acetylglutamine synthetase (NAGS), carbamoylphosphate synthetase I (CPS1), ornithine transcarbamylase (OTC), argininosuccinate synthase, argininosuccinate lyase (ASL), and arginase 1] or two mitochondrial transporters of the urea cycle pathway or metabolites of the amino acids related to the urea cycle[13]. The liver is central to these metabolic pathways, and plays a key role in removing waste from protein catabolism. The defect in the pathway leads to life threatening hyperammonaemia[14]. It is the most common IEM based in the liver with an incidence of 1 in 30000–46000 Live births. All UCDs are inherited in an autosomal recessive manner apart from OTC deficiency, which is inherited in an X-linked manner.
Natural history and medical treatment: Clinical findings are secondary to hyperammonaemia including seizures, coma, cerebral edema and death, with long-term neurodevelopmental implications in survivors. The severity of symptoms can be variable, with some presenting with fatal hyperammonaemia in infancy to asymptomatic adults. In the neonatal period, symptoms occur within hours to days after birth. Initially, neonates with UCD may present with non-specific features such as poor feeding, vomiting, lethargy and tachypnea, but quickly progress to coma and death secondary to hyperammonaemia. NAGS, CPS1 and OTC deficiencies, have the poorest outcomes with neonatal onset of hyperammonaemia and death within the first year of life[15]. Some children may have a delayed presentation with less severe features such as mild gastrointestinal or neurological symptoms. The long-term outcome is dependent on the number of episodes of hyperammonaemia (due to non-adherence, infections and lack of compliance to diet).
The medical management of UCDs requires multidisciplinary input and is complex. The treatment strategy for acute hyperammonaemia is three-fold[16]: (1) Reduce blood ammonia levels through hemodialysis or hemofiltration; (2) Reversal of the catabolic state through caloric and arginine supplementation; and (3) Elimination of excess nitrogen pharmacologically (e.g. benzoate and phenylbutyrate)
In the long term, a diet restrictive of protein, alongside supplementation with essential amino acids is key. Medications to increase waste nitrogen excretion are also important[17]. Despite aggressive and prompt medical treatment, not all episodes of acute hyperammonaemia can be avoided, and the risk of neurological damage remains.
LT: LT offers a practical cure for UCDs as the metabolic defect is predominantly or exclusively within the liver. A long waiting list duration is associated with long-term risk of cognitive delay[18]. As such LT should be considered in children with UCD to prevent progressive neurologic injury and improve cognitive outcomes. Post-transplantation, patients are allowed a normal diet without taking nitrogen scavengers[19]. LT should be offered early to patients with severe UCDs, poorly controlled with medical interventions to prevent long term neurological damage. Living related transplantation offers the advantage of optimal timing after confirmation of the donor phenotype[20].
Future research implications: Allogenic hepatocyte transplantation has been shown to have a sustained partial correction of the metabolic defect in OTC and ASL deficiency patients[21,22]. Another promising treatment for UCD is gene therapy and has seen many years of preclinical evaluation, but concerns still remain around the safety of the application[23].
Maple syrup urine disease
Maple syrup urine disease (MSUD) is an autosomal recessive disease, secondary to mutations in six gene loci where branched-chain alpha-ketoacid dehydrogenase complex is encoded. This results in the inability of the body to fully breakdown the essential amino acids valine, leucine and isoleucine. It has an estimated incidence of 1 in 185000 live births[24].
Natural history and medical treatment: There are five distinct clinical phenotypes of MSUD, without clear correlation of genotype-phenotype. Classic MSUD manifests in the neonatal period with delayed development, feeding difficulties, failure to thrive, opisthotonus, “bicycling” movements and maple syrup odor[25]. Metabolites accumulate and are excreted in the urine, sweat and ear cerumen, leading to the sweet odor of maple syrup. If left untreated, irreversible neurological damage and metabolic crisis occurs.
The most common medical treatment for patients with MSUD is dietary restriction of the affected amino acids, with supplementation[26]. Despite aggressive treatment, many patients will still experience episodes of metabolic decompensation during acute illness or stress, with risk of developing cerebral edema. Acute metabolic decompensation management includes effectively treating the underlying stressor, restricting protein intake, ample caloric support, supplementation with cofactors, elimination of toxic metabolites and correcting metabolic abnormalities[27].
LT: In patients with recurrent metabolic crises and high risk of cerebral edema, despite optimal medical treatment, LT should be considered[28]. LT is curative and significantly improves quality of life in children with MSUD. Patients can immediately cease protein-restricted diet and are safe from catabolic crisis[28]. Preexisting neuro-disability does not get reversed but LT offers neurological function stability and risk of cerebral edema is greatly reduced[29].
Domino transplantation where the explanted liver is used for another recipient without the underlying disease, has been used successfully in MSUD[30-32]. The new liver provides the metabolic protection in the MSUD patient, whilst the domino recipient has a normal systemic metabolism of branched amino acids and can counter the effects of an MSUD liver. This helps with organ allocation and diminishes the impact of the original transplant in the overall pool of organs[33].
Future research implications: Sodium phenylbutyrate (NaPBA) is commonly used for treatment in patients with UCD. In a cohort of 533 patients with UCD, Burrage et al[34] showed a reduction in branched chain amino acids and suggested follow up studies to investigate it’s utility in MSUD[34]. Studies are currently ongoing to assess its efficacy in MSUD patients.
Animal studies have shown encouraging therapeutic results using hepatocyte transplantation with partial metabolic correction of MSUD in a murine model[35]. Whilst promising, this intervention still warrants further clinical investigation.
Wilson disease
Wilson disease (WD) is secondary to mutations of the gene ATP7B on chromosome 13, which codes for the transmembrane ATP7B transporter, involved in the transport of copper, incorporation of copper to the protein caeruloplasmin and excretion of excessive copper into bile. Excess copper in the liver leads to liver destruction, diffusion in to blood and eventually deposition in the other organs[36]. It is an autosomal recessive condition with a prevalence of 1 in 30000 people. An age-phenotypic presentation has been observed with hepatic presentations seen in the younger age groups (< 10 years: 83%, 10-18 years: 52%, > 18 years: 24%), whilst a neuropsychiatric presentation was more common in the older age groups (> 18 years: 74%, 10-18 years: 48%, < 10 years: 17%). The median age of presentation is 13.2 years (range 3–74 years), but children are rarely symptomatic before the age of 5 years[37].
Natural history and medical treatment: The clinical features in the pediatric population depend mainly on the predominant organ involved (liver and brain). The deposition of copper in various site of the body leads to the plethora of clinical presentations.
The majority of children present with liver disease, ranging from an asymptomatic rise in transaminases, acute hepatitis, acute liver failure, acute on chronic liver failure, chronic hepatitis, cirrhosis, fatty liver disease or malignancy[38]. It is important to remember that the finding of another cause of liver dysfunction such as acute viral hepatitis or non-alcoholic steatohepatitis, does not necessarily rule out Wilson’s disease[39].
Up to 25% of children and adolescents present with acute or decompensated liver failure[40]. The presentation is similar to that of acute hepatitis, but the condition leads to rapid deterioration, with a high mortality. Symptoms include severe jaundice, Coombs- negative hemolytic anemia, deranged coagulation, ascites, encephalopathy and renal failure. Children present with very high serum bilirubin, rise in liver enzymes, low serum alkaline phosphatase and defective synthetic functions.
By the time children present with neurological symptoms, most already have liver disease, although may not be overtly symptomatic. Subtle signs may start from a young age such as deterioration of school performance or handwriting and dysarthria. Neurological signs tend to be wide-ranging and variable. Behavioral and psychological changes are very common in WD and make up for roughly one-third of presenting symptoms.
Medical therapy is mainly focused around the copper chelation. Main drugs currently in use include D penicillamine, trientine, zinc and ammonium tetrathiomolybdate. Treatment should be commenced as soon as the child is diagnosed, as untreated WD can be fatal. In patients with acute liver failure or advanced liver disease, LT is the only effective therapy.
LT: The liver disease is cured by LT and extra-hepatic symptoms generally improve after LT, particularly neurological signs. LT is the only option for patients with acute liver failure with encephalopathy secondary to WD. In children with liver dysfunction without encephalopathy, but are unresponsive to medical treatment, the indications are less clear. The Wilson Index is helpful in identifying children with decompensated liver failure, with a 93% sensitivity and 98% specificity[41].
Future research implications: Animal models have shown that restoration of 30%-50% of metabolic function may protect the rest of liver cells. This raises the possibility of gene therapy and hepatocyte transplantation as a potential therapeutic option in children with WD[42]. For patients with acute liver failure secondary to WD, hepatocyte transplantation may be used as transient support until chelation treatment shows its effect or as a definitive cure through repopulation of the liver by healthy donor cells as seen in animal models of WD[43].
LBMDS IMPROVED BY LT
Methylmalonic acidemia and propionic acidemia
Methylmalonic acidemia (MMA) and propionic acidemia (PA) are the commonest forms of organic acidemias resulting from defective catabolism of the amino acids[44]. MMA is an autosomal recessive disorder secondary to the complete or partial deficiency of methylmalonyl-CoA mutase. MMA is also caused by several inborn errors of cobalamin or B12 metabolism. It is rare with an incidence of 1 in 80000 live births[45]. PA is also an autosomal recessive disorder due to a defect in the enzyme propionil-CoA carboxylase[46].
Natural history and medical treatment: The presentation can be divided in to three categories: (1) Neonatal presentation with signs of sleepiness, encephalopathy, coma, hypotonia and hepatomegaly; (2) Infantile form with recurrent metabolic crisis and neurological changes; and (3) Chronic presentation with developmental delay, failure to thrive and recurrent infections[44]. Neurological signs include epilepsy, developmental delay and dystonia resulting from lesions in the basal ganglia. Methylmalonate is also nephrotoxic and can lead to progressive renal disease and end-stage renal failure by adolescence. Investigations in these patients show ketoacidosis, hyperammonaemia and hyperglycinemia. Urine organic acids will reveal propionyl-CoA derivative or methylmalonate.
Each metabolic crisis requires correction and maintenance therapy is dependent on dietary restriction of protein (low protein and high caloric diet with continuous overnight feeding), supplementation with amino acids, carnitine, metronidazole (reduce production of prioprionate in the gut) and cobalamine[47]. Intensive clinical management with aggressive treatment with dialysis and haemofiltration is often needed to minimize neurological sequelae. Despite early detection and maximal medical therapy, many children develop significant neurological, renal and cardiac complications.
LT: LT provides the deficient enzyme in MMA, but the overall biochemical defect is not entirely corrected as the enzyme is expressed in most cells in the body. Neurological and renal function may deteriorate further after LT in some MMA patients[48]. Kidney transplantation has had long-term success in reducing MMA levels and avoiding metabolic crisis in moderate forms of the disease[49]. In more severe forms, combined liver and kidney transplantation may reduce frequency of metabolic crises, severity of illness and probably decreases risk of further neurological deterioration, but does not abolish it entirely[45]. With that in mind, the indications for liver and/or kidney transplantation are still unclear in MMA.
The idea behind transplantation is to provide the deficient enzyme through the graft and correct renal failure if present. It is important to remember that transplantation does not cure MMA but may reduce the frequency of crisis and improve the child’s quality of life. Pre-transplant assessment should include a thorough neurological assessment as transplantation does potentially have the risk of further neurological deterioration. Dietary restriction of protein should be continued as a precaution against future metabolic decompensation and late complications after transplantation[45].
In PA, LT also only partially corrects the metabolic defect[50]. However, the improvement seen appears to be more significant in PA compared to MMA–diet can usually be normalized, no further metabolic crises and neurocognitive function remains as it is pre-transplantation[51]. LT should be indicated in patients with recurrent metabolic crises despite optimal medical therapy, with a view of preventing further neurological deterioration and cardiac complications[50].
Future therapies: The role of new and novel treatments such as genetic modification, hepatocyte transplantation and chronic medical therapies remains uncertain[52].
Glycogen storage diseases
Glycogen storage diseases (GSD) constitutes a group of mainly autosomal recessive metabolic disorders, caused by the accumulation of either an abnormal amount or type of glycogen. It has an incidence of 1 in 20000 to 40000 live births. Various enzymes of glycogen metabolism are potentially involved, with 12 types of GSD recognized – seven of which have an enzymatic defect in the liver. Types I, III, IV, VI and IX are associated with severe liver disease[53-55].
Natural history and medical treatment: Typically, it presents with fasting hypoglycemia, hepatomegaly and growth retardation. In the GSD type I, hepatocellular adenomas with risk of transformation to hepatocellular carcinoma has been found[55,56], particularly in those with pre-existing adenomatous nodules. In GSD type III, some patients may progress to liver cirrhosis, whilst some develop hepatocellular carcinoma[54]. GSD IV patients have a variable phenotype and some develop liver cirrhosis and hepatocellular carcinoma early on. Extrahepatic manifestations such as renal dysfunction in GSD type 1, myopathy in GSD type III and IV may also be present. It is important to distinguish between subtypes for optimal management. Diagnosis is through enzyme assays in the liver other tissues and mutation analysis. Presence of PAS-positive glycogen staining in liver biopsy samples is useful in confirming the diagnosis.
Treatment for liver GSD includes dietary changes and medical treatment when symptoms are not corrected by diet. In GSD type I, continuous overnight enteral drip-feeding is used to avoid fasting hypoglycemia and regular oral cornstarch intake is used for prolonged glucose release and have significantly improved metabolic control[57]. Other pharmacotherapy may be needed such as allopurinol for hyperuricaemia, angiotensin converting enzyme inhibitors for proteinuria and granulocyte-colony stimulating factor for neutropenia in GSD type Ib[58]. In patients with GSD types III, VI and IX, a high protein diet alongside uncooked cornstarch is standard therapy. Whilst metabolic control is generally successful with medical therapy, long-term complications still occur[15]. Adherence is also a common issue in children and adolescents and may not be tolerated in many, results in a higher rate of complications.
LT: In patients with very poor metabolic control despite optimal medical therapy, those with multiple recurrent adenomas with increasing size, progressive liver cirrhosis and/ or hepatic failure, LT should be considered. In children with GSD type IV, LT is generally the best option for treatment, particularly in those that develop liver cirrhosis[59]. Children with GSD are also living longer and despite medical treatment, many develop long-term complications. With the outcomes of LT improving, including better biochemical and clinical parameters, LT offers the potential to be both preventative and curative for patients with GSD.
Indications for LT in GSD can be summarized as: (1) Correction of LBMD when medical therapy is unsuccessful or impairs quality of life; (2) Cirrhosis and complications; and (3) Liver tumors such as adenoma and hepatocellular carcinoma.
LT corrects the enzymatic defect, but the extrahepatic manifestations often complicate post-transplantation management[59].
Future therapies: There has been limited experience with hepatocyte transplantation, but initial reports are positive[60,61]. Gene therapy has been developed in animal models, but there remains insufficient data for clinical trials[62].
Phenylketonuria
Phenylketonuria (PKU) is a rare autosomal recessive condition secondary to mutations in the phenylalanine hydroxylase gene (PAH). This results in a deficiency of PAH, an enzyme in the liver that converts phenylalanine (Phe) to tyrosine. The incidence is roughly 1 in 10000 and does vary by ethnic group, being higher in Caucasians.
Natural history and medical treatment: The lack of this enzyme results in abnormally high levels of phenylalanine in the brain, causing intellectual problems, developmental delay and psychiatric issues. Universal newborn screening in most developed nations has led to early detection and significantly reduced the number of children with intellectual disability secondary to PKU. Despite ongoing and early treatment of patients with PKU, majority of patients will still have a lower intellectual ability compared to family members and suffer from mental health issues[63,64].
Medical therapy consists of restriction of phenylalanine intake and supplementation with phenylalanine-free amino acid mixtures to ensure adequate protein intake[65]. The diet needed is extremely restrictive and include mainly fruits, vegetables and low protein modified foods such as bread, rice and pasta[66]. Dietary treatment, when maintained in childhood and well into adulthood has been shown to result in markedly improved outcomes at a cognitive and psychiatric level for patients. However, adherence to this strict regime is not ideal, particularly in adolescents and in adulthood.
Dietary modification has evolved with the introduction of glycomacropeptides (GMP), which are proteins contained in “whey”. These contain very little phenylalanine, which makes them suitable for replacing amino acid substitutes. Compliance has been shown to be improved with GMP compared to traditional amino acid foods[67]. The medication sapropterin, a form of tetrahydrobiopterin cofactor of phenylalanine hydroxylase has a success rate of up to 55% in PKU patients[68]. Patients with milder form of disease are more likely to respond to this drug. Another recent pharmaceutical drug known as peglyated phenylalanine ammonia lyase or pegvaliase, an enzyme substitute therapy has been assessed in Phase 2 and Phase 3 clinical trials[69]. Over 24 mo, patients showed a 69% decrease in Phe levels from baseline but almost all patients had mild to severe adverse events[70].
LT: Whole liver LT is not thought to be acceptable in majority of patients and physicians due to the availability of non-surgical treatment options.
Future therapies: Gene therapy has been shown to be successful in mouse models but no studies have reported trials in patients yet[71]. A variation of gene therapy is gene-editing techniques (Crispr/Cas9 or TALENS) to repair common mutations or insert active gene into “safe” areas of the gene. The development of an expressive synthetic RNA for the PAH gene is in development, but not with human subjects[72].
Cell-based therapies including hepatocyte and stem cell transplantation have been considered viable alternatives[73]. One patient has received hepatocyte transplantation with temporary improvement in Phe levels[74].
DILEMMAS AND CONTROVERSIES
The decision for transplantation in LBMDs remains a complex one. Whilst the distinctions between each group of LBMDs is relatively arbitrary and may overlap, the indication for LT is one that must be carefully considered.
In a disease process such as biliary atresia, the risk-benefit decision for LT may be relatively simple. In a child with failed portoenterostomy, with progressive liver disease and poor survival beyond 36 mo of life, LT offers long-term survival of over 80% in biliary atresia patients[75]. Therefore the risk/benefit decision is based on quantitative improved survival outcomes.
Indications for LT for LBMDs however, are based on a different set of paradigms. Some LBMDs result in progressive liver disease, leading to cirrhosis and liver failure, therefore making LT a life-saving procedure, whilst some LBMDs do not cause liver injury, but the toxic intermediary metabolites have significant extra-hepatic effects.
LT remains the mainstay of treatment for LBMDs causing life-threatening illness such as the neonatal form of the UCD OTC deficiency, primary hyperoxaluria and CNS type 1[6,76,77]. The enzymatic defects in these conditions are well documented and present with severe clinical phenotypes manifesting in life-threatening complications. LT offers a replacement for the hepatic enzymes, therefore providing a life-saving metabolic cure.
With improvement in the outcomes and reduced risks associated with LT, LT has become an attractive treatment strategy for a significant number of other LBMDs with a considerably more complicated phenotype and risk/benefit profile. The utility of LT as a life improving vs life saving treatment modality raises a number of important questions. This paradigm shift of improving quality of life as opposed to saving lives has dramatically changed the plethora of diseases for which LT may be considered appropriate therapy. The blurring of lines between standard medical therapy and more aggressive surgical intervention, increasingly poses complex decisions for the transplant community[78].
Furthermore, LBMDs are relatively rare, and a detailed understanding in to the natural progression/history is still lacking. There is also a diverse genotype and phenotype correlation for many of these rare disorders. The risk/benefit consideration is made even more complicated for a given individual as the inherent risks of a condition are not always well-defined.
SURGICAL CONSIDERATIONS
As more children receive transplants for LBMDs, organ allocation is an important consideration. In the United States, the Pediatric end-stage liver disease score and Model for End Stage Liver Disease score are used to prioritize candidates for LT. These scoring systems are centered mainly on worsening biochemical parameters which progress with advancing liver failure. In many LBMDs, there is typically no evidence of progressive liver disease and as such predicting risk of which candidate is most likely to benefit for LT can be challenging. As we expand the indication for LT for metabolic conditions, the issue of organ allocation must also be addressed.
The issue of scarcity of donor organs has led to optimization of the available grafts through various surgical techniques such as reduction of an adult donor graft in children, particularly through split liver grafts[79], auxiliary transplantation and the use of heterozygous donors.
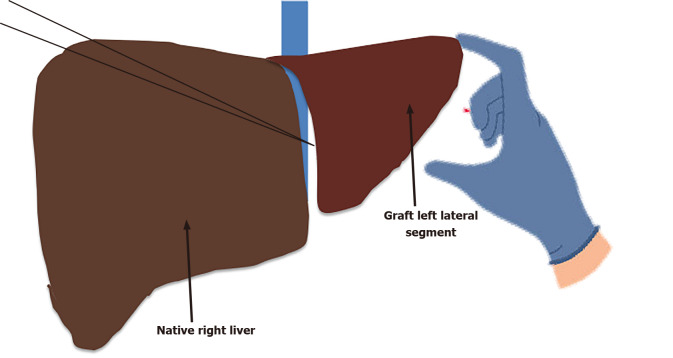
Auxiliary transplantation[80]–where the whole or partial left lobe of a living or deceased donor is transplanted in an orthotopic site whilst preserving the right lobe of the recipient[81] is increasingly being used (Figure 2). Whilst technically challenging, advantages are two fold; (1) It allows the native liver to continue functioning normally, aside from the enzymatic defect, serving as a safety net should the graft fail; and (2) It may serve as a bridge to gene therapy, a new and novel developing area of metabolic medicine. Despite the initial discouraging results with higher mortality and morbidity, more recent studies[82,83] from experienced centers have shown comparable outcomes to whole LT and successful weaning of immunosuppression with native liver regeneration[84]. Study by Sze et al[85], showed that of the 96 paediatric LT patients with LBMDs, 14 (13%) children had auxiliary transplantation. Of these, 11 children had noncirrhotic LBMDs (CNS type 1, OTC, familial hypercholesterolism, proprionic acidemia). Long term patient and graft survival was not statistically different to standard orthotopic LT at 1 and 10-years post-transplantation[85]. Cautious selection of patients for auxiliary transplantation is vital as LBMDs that lead to cirrhosis or produce abnormal enzymes or proteins such as primary hyperoxaluria should not be treated with auxiliary transplantation as the underlying abnormality results in disease progression[86].
Figure 2.
Auxiliary liver transplantation for select liver based metabolic disorders.
Living related living transplant using relatives as donors has emerged as a solution to the scarcity of donor organs. In Japan, where there are no deceased donors, living related donor LT for metabolic disorders is a key option[87]. As described above, most metabolic disorders have an autosomal recessive inheritance pattern. Parents, who are obligate carriers of the recipient’s disorders, become potentially heterozygous donors. Kasahara et al[20], conducted an extensive review from a Japanese multicenter registry of living related LT[20]. Among the patients transplanted for metabolic conditions, 95% of donors were parents who were carriers of the recipients’ disorders. Indications for transplantation were WD in 30%, UCD in 29%, MMA in 10% and GSD 7.7%[88]. The outcome reported after using heterozygous donors was excellent with better long-term survival rate, especially in WD and UCD. Other studies have also demonstrated the safety of heterozygous donors for LT in LBMDs with excellent metabolic correction[89,90].
As previously discussed, LT for organic acidurias is not curative, but may improve quality of life. Combined liver and kidney transplantation can be considered in patients with MMA and PA with frequent metabolic decompensation episodes in spite of rigorous medical therapy, based on highly individualized criteria[47]. The experience with combined liver and kidney transplantation in this cohort of patients remains limited. In MMA patients specifically, it has become an effective treatment modality with favorable graft survival and short-term outcomes, and good survival rates[45,91,92]. Combined liver and kidney transplantation does not cure the disease, but leads to partial correction of the metabolic derangement and improvement in clinical features. Medical therapy is generally continued, although less stringent than pre-transplantation, in order to lower the risk of renal and neurological worsening[47]. Choice of immunosuppressive therapy that is renal-sparing is encouraged and neurological side effects from medication need to be carefully monitored[93].
OUTCOMES AND PROGNOSIS
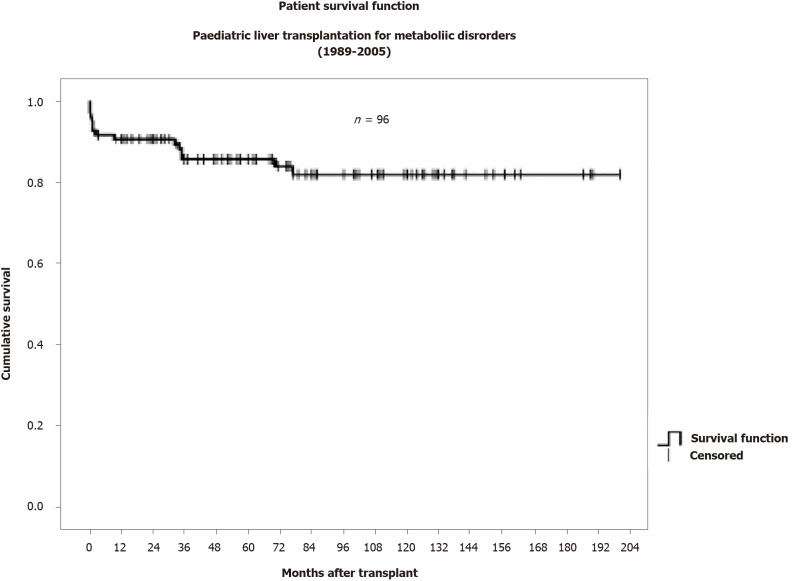
With LBMDs constituting roughly 15%-25% of LT in the pediatric population, it is important to consider the outcomes of these children. Single and multicenter studies have suggested that their outcomes are comparable if not better than those transplanted for decompensated cirrhosis or other forms of chronic liver disease with excellent survival rate of > 82% at 10 years[85,94] (Figure 3) (Graph data from King’s College Hospital, 2009).
Figure 3.
Patient survival for pediatric liver transplantation for metabolic disorders (data from King’s College Hospital, London).
Some studies, however, have shown that chronic rejection is a common problem in LT for LBMDs, often leading to re-transplantation[85,95]. Re-transplantation is associated with higher morbidity and mortality. Immunosuppression regimes are important in maintaining long-term allograft health, but may also contribute to potentially serious complications over time.
Optimization of immunosuppression can be challenging and is not standardized[85,96]. In children receiving LT for LBMDs, the optimal use of immunosuppressive agents is to achieve a balance between minimizing risks of allograft rejection and secondary toxicity[97]. Renal impairment specifically is frequently seen in these children. Thus, choosing an immunosuppressive agent with minimal nephrotoxic potential is important. The use of basiliximab, a chimeric anti-IL2 receptor antibody, has been shown to be an effective renal-sparing agent with delayed entry and lower early target trough levels of calcineurin inhibitors (CNI) in children with renal impairment[98]. Mycophenolate mofetil (MMF) is also a CNI sparing agent, useful in children with CNI toxicity. Induction with monoclonal antibodies such as Basilixumab as an induction, followed by the use of MMF may be a helpful renal-sparing strategy in children with renal dysfunction.
The overall prognosis for children receiving transplantation for LBMDs must account for both allograft and extrahepatic complications. Meaningful survival in all pediatric LT recipients should be a state of complete physical, mental and social wellbeing[99]. Long-term management of children transplanted for LBMDs must include aspects such as growth and nutrition, neurological outcomes and psychosocial well-being.
CONCLUSION
Pediatric LT has come leaps and bounds in the treatment of children with LBMDs. Where it has been previously viewed only as life-saving for some LBMDs, there is good reason to consider a shift in the utility of LT beyond metabolic rescue. It remains the gold standard for children with end stage liver disease. The success rate in most LBMDs is promising but the clinician plays a vital role in determining which patients are most suited for LT. The care pre- and post-transplantation is especially important. Pre-transplantation, identifying the most appropriate candidate for transplant will involve assessment of the severity of the primary disease, neurological status, and comorbidities which may affect transplant survival and ensuring that all alternative treatment modalities have be explored. It is important to remember that good metabolic control including ongoing dietary management and medical therapy supplements often results in better post-transplantation outcomes. A multidisciplinary network of professionals is key in the management of these children post LT, to ensure all aspects including growth and development, psychosocial well-being and nutrition are considered.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases.
Peer-review started: May 4, 2021
First decision: June 15, 2021
Article in press: August 20, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferrarese A, Yagi H S-Editor: Fan JR L-Editor: A P-Editor: Guo X
Contributor Information
Sunitha Vimalesvaran, Paediatric Liver GI and Nutrition Center, King's College Hospital, London SE5 9RS, United Kingdom.
Anil Dhawan, Paediatric Liver GI and Nutrition Center, King's College Hospital, London SE5 9RS, United Kingdom. anil.dhawan@kcl.ac.uk.
References
- 1.Venick RS, Farmer DG, Soto JR, Vargas J, Yersiz H, Kaldas FM, Agopian VG, Hiatt JR, McDiarmid SV, Busuttil RW. One Thousand Pediatric Liver Transplants During Thirty Years: Lessons Learned. J Am Coll Surg. 2018;226:355–366. doi: 10.1016/j.jamcollsurg.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Duffy JP, Kao K, Ko CY, Farmer DG, McDiarmid SV, Hong JC, Venick RS, Feist S, Goldstein L, Saab S, Hiatt JR, Busuttil RW. Long-term patient outcome and quality of life after liver transplantation: analysis of 20-year survivors. Ann Surg. 2010;252:652–661. doi: 10.1097/SLA.0b013e3181f5f23a. [DOI] [PubMed] [Google Scholar]
- 3.Arnon R, Annunziato RA, D'Amelio G, Chu J, Shneider BL. Liver Transplantation for Biliary Atresia: Is There a Difference in Outcome for Infants? J Pediatr Gastroenterol Nutr. 2016;62:220–225. doi: 10.1097/MPG.0000000000000986. [DOI] [PubMed] [Google Scholar]
- 4.Dhawan A, Lawlor MW, Mazariegos GV, McKiernan P, Squires JE, Strauss KA, Gupta D, James E, Prasad S. Disease burden of Crigler-Najjar syndrome: Systematic review and future perspectives. J Gastroenterol Hepatol. 2020;35:530–543. doi: 10.1111/jgh.14853. [DOI] [PubMed] [Google Scholar]
- 5.Ebrahimi A, Rahim F. Crigler-Najjar Syndrome: Current Perspectives and the Application of Clinical Genetics. Endocr Metab Immune Disord Drug Targets. 2018;18:201–211. doi: 10.2174/1871530318666171213153130. [DOI] [PubMed] [Google Scholar]
- 6.van der Veere CN, Sinaasappel M, McDonagh AF, Rosenthal P, Labrune P, Odièvre M, Fevery J, Otte JB, McClean P, Bürk G, Masakowski V, Sperl W, Mowat AP, Vergani GM, Heller K, Wilson JP, Shepherd R, Jansen PL. Current therapy for Crigler-Najjar syndrome type 1: report of a world registry. Hepatology. 1996;24:311–315. doi: 10.1002/hep.510240205. [DOI] [PubMed] [Google Scholar]
- 7.Van Der Veere CN, Schoemaker B, Bakker C, Van Der Meer R, Jansen PL, Elferink RP. Influence of dietary calcium phosphate on the disposition of bilirubin in rats with unconjugated hyperbilirubinemia. Hepatology. 1996;24:620–626. doi: 10.1002/hep.510240326. [DOI] [PubMed] [Google Scholar]
- 8.Nishioka T, Hafkamp AM, Havinga R, vn Lierop PP, Velvis H, Verkade HJ. Orlistat treatment increases fecal bilirubin excretion and decreases plasma bilirubin concentrations in hyperbilirubinemic Gunn rats. J Pediatr. 2003;143:327–334. doi: 10.1067/s0022-3476(03)00298-1. [DOI] [PubMed] [Google Scholar]
- 9.Schauer R, Stangl M, Lang T, Zimmermann A, Chouker A, Gerbes AL, Schildberg FW, Rau HG. Treatment of Crigler-Najjar type 1 disease: relevance of early liver transplantation. J Pediatr Surg. 2003;38:1227–1231. doi: 10.1016/s0022-3468(03)00273-2. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosino G, Varotto S, Strom SC, Guariso G, Franchin E, Miotto D, Caenazzo L, Basso S, Carraro P, Valente ML, D'Amico D, Zancan L, D'Antiga L. Isolated hepatocyte transplantation for Crigler-Najjar syndrome type 1. Cell Transplant. 2005;14:151–157. doi: 10.3727/000000005783983250. [DOI] [PubMed] [Google Scholar]
- 11.Jorns C, Nowak G, Nemeth A, Zemack H, Mörk LM, Johansson H, Gramignoli R, Watanabe M, Karadagi A, Alheim M, Hauzenberger D, van Dijk R, Bosma PJ, Ebbesen F, Szakos A, Fischler B, Strom S, Ellis E, Ericzon BG. De Novo Donor-Specific HLA Antibody Formation in Two Patients With Crigler-Najjar Syndrome Type I Following Human Hepatocyte Transplantation With Partial Hepatectomy Preconditioning. Am J Transplant. 2016;16:1021–1030. doi: 10.1111/ajt.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spitzhorn LS, Kordes C, Megges M, Sawitza I, Götze S, Reichert D, Schulze-Matz P, Graffmann N, Bohndorf M, Wruck W, Köhler JP, Herebian D, Mayatepek E, Oreffo ROC, Häussinger D, Adjaye J. Transplanted Human Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Support Liver Regeneration in Gunn Rats. Stem Cells Dev. 2018;27:1702–1714. doi: 10.1089/scd.2018.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seminara J, Tuchman M, Krivitzky L, Krischer J, Lee HS, Lemons C, Baumgartner M, Cederbaum S, Diaz GA, Feigenbaum A, Gallagher RC, Harding CO, Kerr DS, Lanpher B, Lee B, Lichter-Konecki U, McCandless SE, Merritt JL, Oster-Granite ML, Seashore MR, Stricker T, Summar M, Waisbren S, Yudkoff M, Batshaw ML. Establishing a consortium for the study of rare diseases: The Urea Cycle Disorders Consortium. Mol Genet Metab. 2010;100 Suppl 1:S97–105. doi: 10.1016/j.ymgme.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone WL, Basit H, Jaishankar GB. Urea Cycle Disorders. 2021 Aug 11. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. [PubMed] [Google Scholar]
- 15.Mazariegos G, Shneider B, Burton B, Fox IJ, Hadzic N, Kishnani P, Morton DH, McIntire S, Sokol RJ, Summar M, White D, Chavanon V, Vockley J. Liver transplantation for pediatric metabolic disease. Mol Genet Metab. 2014;111:418–427. doi: 10.1016/j.ymgme.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto S, Häberle J, Kido J, Mitsubuchi H, Endo F, Nakamura K. Urea cycle disorders-update. J Hum Genet. 2019;64:833–847. doi: 10.1038/s10038-019-0614-4. [DOI] [PubMed] [Google Scholar]
- 17.Häberle J, Burlina A, Chakrapani A, Dixon M, Karall D, Lindner M, Mandel H, Martinelli D, Pintos-Morell G, Santer R, Skouma A, Servais A, Tal G, Rubio V, Huemer M, Dionisi-Vici C. Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J Inherit Metab Dis. 2019;42:1192–1230. doi: 10.1002/jimd.12100. [DOI] [PubMed] [Google Scholar]
- 18.Ziogas IA, Wu WK, Matsuoka LK, Pai AK, Hafberg ET, Gillis LA, Morgan TM, Alexopoulos SP. Liver Transplantation in Children with Urea Cycle Disorders: The Importance of Minimizing Waiting Time. Liver Transpl . 2021 doi: 10.1002/lt.26186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim IK, Niemi AK, Krueger C, Bonham CA, Concepcion W, Cowan TM, Enns GM, Esquivel CO. Liver transplantation for urea cycle disorders in pediatric patients: a single-center experience. Pediatr Transplant. 2013;17:158–167. doi: 10.1111/petr.12041. [DOI] [PubMed] [Google Scholar]
- 20.Kasahara M, Sakamoto S, Horikawa R, Koji U, Mizuta K, Shinkai M, Takahito Y, Taguchi T, Inomata Y, Uemoto S, Tatsuo K, Kato S. Living donor liver transplantation for pediatric patients with metabolic disorders: the Japanese multicenter registry. Pediatr Transplant. 2014;18:6–15. doi: 10.1111/petr.12196. [DOI] [PubMed] [Google Scholar]
- 21.Stéphenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317–1323. doi: 10.1053/j.gastro.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Stéphenne X, Najimi M, Smets F, Reding R, de Ville de Goyet J, Sokal EM. Cryopreserved liver cell transplantation controls ornithine transcarbamylase deficient patient while awaiting liver transplantation. Am J Transplant. 2005;5:2058–2061. doi: 10.1111/j.1600-6143.2005.00935.x. [DOI] [PubMed] [Google Scholar]
- 23.Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, Wilson JM, Batshaw ML. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Edelmann L, Wasserstein MP, Kornreich R, Sansaricq C, Snyderman SE, Diaz GA. Maple syrup urine disease: identification and carrier-frequency determination of a novel founder mutation in the Ashkenazi Jewish population. Am J Hum Genet. 2001;69:863–868. doi: 10.1086/323677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackburn PR, Gass JM, Vairo FPE, Farnham KM, Atwal HK, Macklin S, Klee EW, Atwal PS. Maple syrup urine disease: mechanisms and management. Appl Clin Genet. 2017;10:57–66. doi: 10.2147/TACG.S125962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morton DH, Strauss KA, Robinson DL, Puffenberger EG, Kelley RI. Diagnosis and treatment of maple syrup disease: a study of 36 patients. Pediatrics. 2002;109:999–1008. doi: 10.1542/peds.109.6.999. [DOI] [PubMed] [Google Scholar]
- 27.Hassan SA, Gupta V. Maple Syrup Urine Disease. 2021 Feb 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. [PubMed] [Google Scholar]
- 28.Strauss KA, Mazariegos GV, Sindhi R, Squires R, Finegold DN, Vockley G, Robinson DL, Hendrickson C, Virji M, Cropcho L, Puffenberger EG, McGhee W, Seward LM, Morton DH. Elective liver transplantation for the treatment of classical maple syrup urine disease. Am J Transplant. 2006;6:557–564. doi: 10.1111/j.1600-6143.2005.01209.x. [DOI] [PubMed] [Google Scholar]
- 29.Mazariegos GV, Morton DH, Sindhi R, Soltys K, Nayyar N, Bond G, Shellmer D, Shneider B, Vockley J, Strauss KA. Liver transplantation for classical maple syrup urine disease: long-term follow-up in 37 patients and comparative United Network for Organ Sharing experience. J Pediatr. 2012;160:116–21.e1. doi: 10.1016/j.jpeds.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanna A, Hart M, Nyhan WL, Hassanein T, Panyard-Davis J, Barshop BA. Domino liver transplantation in maple syrup urine disease. Liver Transpl. 2006;12:876–882. doi: 10.1002/lt.20744. [DOI] [PubMed] [Google Scholar]
- 31.Badell IR, Hanish SI, Hughes CB, Hewitt WR, Chung RT, Spivey JR, Knechtle SJ. Domino liver transplantation in maple syrup urine disease: a case report and review of the literature. Transplant Proc. 2013;45:806–809. doi: 10.1016/j.transproceed.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 32.Herden U, Li J, Fischer L, Brinkert F, Blohm M, Santer R, Nashan B, Grabhorn E. The first case of domino-split-liver transplantation in maple syrup urine disease. Pediatr Transplant. 2017;21 doi: 10.1111/petr.12993. [DOI] [PubMed] [Google Scholar]
- 33.Celik N, Squires JE, Soltys K, Vockley J, Shellmer DA, Chang W, Strauss K, McKiernan P, Ganoza A, Sindhi R, Bond G, Mazariegos G, Khanna A. Domino liver transplantation for select metabolic disorders: Expanding the living donor pool. JIMD Rep. 2019;48:83–89. doi: 10.1002/jmd2.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burrage LC, Jain M, Gandolfo L, Lee BH Members of the Urea Cycle Disorders Consortium, Nagamani SC. Sodium phenylbutyrate decreases plasma branched-chain amino acids in patients with urea cycle disorders. Mol Genet Metab. 2014;113:131–135. doi: 10.1016/j.ymgme.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skvorak KJ, Paul HS, Dorko K, Marongiu F, Ellis E, Chace D, Ferguson C, Gibson KM, Homanics GE, Strom SC. Hepatocyte transplantation improves phenotype and extends survival in a murine model of intermediate maple syrup urine disease. Mol Ther. 2009;17:1266–1273. doi: 10.1038/mt.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernando M, van Mourik I, Wassmer E, Kelly D. Wilson disease in children and adolescents. Arch Dis Child. 2020;105:499–505. doi: 10.1136/archdischild-2018-315705. [DOI] [PubMed] [Google Scholar]
- 37.Lin LJ, Wang DX, Ding NN, Lin Y, Jin Y, Zheng CQ. Comprehensive analysis on clinical features of Wilson's disease: an experience over 28 years with 133 cases. Neurol Res. 2014;36:157–163. doi: 10.1179/1743132813Y.0000000262. [DOI] [PubMed] [Google Scholar]
- 38.Sternlieb I. Wilson's disease: indications for liver transplants. Hepatology. 1984;4:15S–17S. doi: 10.1002/hep.1840040706. [DOI] [PubMed] [Google Scholar]
- 39.Roberts EA, Yap J. Nonalcoholic Fatty Liver Disease (NAFLD): Approach in the Adolescent Patient. Curr Treat Options Gastroenterol. 2006;9:423–431. doi: 10.1007/BF02738532. [DOI] [PubMed] [Google Scholar]
- 40.Pandit A, Bavdekar A, Bhave S. Wilson's disease. Indian J Pediatr. 2002;69:785–791. doi: 10.1007/BF02723693. [DOI] [PubMed] [Google Scholar]
- 41.Dhawan A, Taylor RM, Cheeseman P, De Silva P, Katsiyiannakis L, Mieli-Vergani G. Wilson's disease in children: 37-year experience and revised King's score for liver transplantation. Liver Transpl. 2005;11:441–448. doi: 10.1002/lt.20352. [DOI] [PubMed] [Google Scholar]
- 42.Irani AN, Malhi H, Slehria S, Gorla GR, Volenberg I, Schilsky ML, Gupta S. Correction of liver disease following transplantation of normal rat hepatocytes into Long-Evans Cinnamon rats modeling Wilson's disease. Mol Ther. 2001;3:302–309. doi: 10.1006/mthe.2001.0271. [DOI] [PubMed] [Google Scholar]
- 43.Filippi C, Dhawan A. Current status of human hepatocyte transplantation and its potential for Wilson's disease. Ann N Y Acad Sci. 2014;1315:50–55. doi: 10.1111/nyas.12386. [DOI] [PubMed] [Google Scholar]
- 44.Deodato F, Boenzi S, Santorelli FM, Dionisi-Vici C. Methylmalonic and propionic aciduria. Am J Med Genet C Semin Med Genet. 2006;142C:104–112. doi: 10.1002/ajmg.c.30090. [DOI] [PubMed] [Google Scholar]
- 45.Kasahara M, Horikawa R, Tagawa M, Uemoto S, Yokoyama S, Shibata Y, Kawano T, Kuroda T, Honna T, Tanaka K, Saeki M. Current role of liver transplantation for methylmalonic acidemia: a review of the literature. Pediatr Transplant. 2006;10:943–947. doi: 10.1111/j.1399-3046.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 46.Sass JO, Hofmann M, Skladal D, Mayatepek E, Schwahn B, Sperl W. Propionic acidemia revisited: a workshop report. Clin Pediatr (Phila) 2004;43:837–843. doi: 10.1177/000992280404300908. [DOI] [PubMed] [Google Scholar]
- 47.Baumgartner MR, Hörster F, Dionisi-Vici C, Haliloglu G, Karall D, Chapman KA, Huemer M, Hochuli M, Assoun M, Ballhausen D, Burlina A, Fowler B, Grünert SC, Grünewald S, Honzik T, Merinero B, Pérez-Cerdá C, Scholl-Bürgi S, Skovby F, Wijburg F, MacDonald A, Martinelli D, Sass JO, Valayannopoulos V, Chakrapani A. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet J Rare Dis. 2014;9:130. doi: 10.1186/s13023-014-0130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leonard JV, Walter JH, McKiernan PJ. The management of organic acidaemias: the role of transplantation. J Inherit Metab Dis. 2001;24:309–311. doi: 10.1023/a:1010395724012. [DOI] [PubMed] [Google Scholar]
- 49.Lubrano R, Elli M, Rossi M, Travasso E, Raggi C, Barsotti P, Carducci C, Berloco P. Renal transplant in methylmalonic acidemia: could it be the best option? Pediatr Nephrol. 2007;22:1209–1214. doi: 10.1007/s00467-007-0460-z. [DOI] [PubMed] [Google Scholar]
- 50.Barshes NR, Vanatta JM, Patel AJ, Carter BA, O'Mahony CA, Karpen SJ, Goss JA. Evaluation and management of patients with propionic acidemia undergoing liver transplantation: a comprehensive review. Pediatr Transplant. 2006;10:773–781. doi: 10.1111/j.1399-3046.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- 51.Schlenzig JS, Poggi-Travert F, Laurent J, Rabier D, Jan D, Wendel U, Sewell AC, Revillon Y, Kamoun P, Saudubray JM. Liver transplantation in two cases of propionic acidaemia. J Inherit Metab Dis. 1995;18:448–461. doi: 10.1007/BF00710056. [DOI] [PubMed] [Google Scholar]
- 52.Chandler RJ, Chandrasekaran S, Carrillo-Carrasco N, Senac JS, Hofherr SE, Barry MA, Venditti CP. Adeno-associated virus serotype 8 gene transfer rescues a neonatal lethal murine model of propionic acidemia. Hum Gene Ther. 2011;22:477–481. doi: 10.1089/hum.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manzia TM, Angelico R, Toti L, Cillis A, Ciano P, Orlando G, Anselmo A, Angelico M, Tisone G. Glycogen storage disease type Ia and VI associated with hepatocellular carcinoma: two case reports. Transplant Proc. 2011;43:1181–1183. doi: 10.1016/j.transproceed.2011.01.129. [DOI] [PubMed] [Google Scholar]
- 54.Kishnani PS, Austin SL, Arn P, Bali DS, Boney A, Case LE, Chung WK, Desai DM, El-Gharbawy A, Haller R, Smit GP, Smith AD, Hobson-Webb LD, Wechsler SB, Weinstein DA, Watson MS ACMG. Glycogen storage disease type III diagnosis and management guidelines. Genet Med. 2010;12:446–463. doi: 10.1097/GIM.0b013e3181e655b6. [DOI] [PubMed] [Google Scholar]
- 55.Koeberl DD, Kishnani PS, Chen YT. Glycogen storage disease types I and II: treatment updates. J Inherit Metab Dis. 2007;30:159–164. doi: 10.1007/s10545-007-0519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuen WY, Quak SH, Aw MM, Karthik SV. Long-term outcome after liver transplantation in children with type 1 glycogen storage disease. Pediatr Transplant. 2021;25:e13872. doi: 10.1111/petr.13872. [DOI] [PubMed] [Google Scholar]
- 57.Parikh NS, Ahlawat R. Glycogen Storage Disease Type I. 2020 Aug 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan–. [PubMed] [Google Scholar]
- 58.Melis D, Fulceri R, Parenti G, Marcolongo P, Gatti R, Parini R, Riva E, Della Casa R, Zammarchi E, Andria G, Benedetti A. Genotype/phenotype correlation in glycogen storage disease type 1b: a multicentre study and review of the literature. Eur J Pediatr. 2005;164:501–508. doi: 10.1007/s00431-005-1657-4. [DOI] [PubMed] [Google Scholar]
- 59.Davis MK, Weinstein DA. Liver transplantation in children with glycogen storage disease: controversies and evaluation of the risk/benefit of this procedure. Pediatr Transplant. 2008;12:137–145. doi: 10.1111/j.1399-3046.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 60.Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, Giron G, Burlina AB. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- 61.Lee KW, Lee JH, Shin SW, Kim SJ, Joh JW, Lee DH, Kim JW, Park HY, Lee SY, Lee HH, Park JW, Kim SY, Yoon HH, Jung DH, Choe YH, Lee SK. Hepatocyte transplantation for glycogen storage disease type Ib. Cell Transplant. 2007;16:629–637. doi: 10.3727/000000007783465019. [DOI] [PubMed] [Google Scholar]
- 62.Salabarria SM, Nair J, Clement N, Smith BK, Raben N, Fuller DD, Byrne BJ, Corti M. Advancements in AAV-mediated Gene Therapy for Pompe Disease. J Neuromuscul Dis. 2020;7:15–31. doi: 10.3233/JND-190426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Waisbren SE, Azen C. Cognitive and behavioral development in maternal phenylketonuria offspring. Pediatrics. 2003;112:1544–1547. [PubMed] [Google Scholar]
- 64.Waisbren SE, Noel K, Fahrbach K, Cella C, Frame D, Dorenbaum A, Levy H. Phenylalanine blood levels and clinical outcomes in phenylketonuria: a systematic literature review and meta-analysis. Mol Genet Metab. 2007;92:63–70. doi: 10.1016/j.ymgme.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 65.MacDonald A, Rocha JC, van Rijn M, Feillet F. Nutrition in phenylketonuria. Mol Genet Metab. 2011;104 Suppl:S10–S18. doi: 10.1016/j.ymgme.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 66.Jameson E, Remmington T. Dietary interventions for phenylketonuria. Cochrane Database Syst Rev. 2020;7:CD001304. doi: 10.1002/14651858.CD001304.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ney DM, Stroup BM, Clayton MK, Murali SG, Rice GM, Rohr F, Levy HL. Glycomacropeptide for nutritional management of phenylketonuria: a randomized, controlled, crossover trial. Am J Clin Nutr. 2016;104:334–345. doi: 10.3945/ajcn.116.135293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qu J, Yang T, Wang E, Li M, Chen C, Ma L, Zhou Y, Cui Y. Efficacy and safety of sapropterin dihydrochloride in patients with phenylketonuria: A meta-analysis of randomized controlled trials. Br J Clin Pharmacol. 2019;85:893–899. doi: 10.1111/bcp.13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas J, Levy H, Amato S, Vockley J, Zori R, Dimmock D, Harding CO, Bilder DA, Weng HH, Olbertz J, Merilainen M, Jiang J, Larimore K, Gupta S, Gu Z, Northrup H PRISM investigators. Pegvaliase for the treatment of phenylketonuria: Results of a long-term phase 3 clinical trial program (PRISM) Mol Genet Metab. 2018;124:27–38. doi: 10.1016/j.ymgme.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Longo N, Zori R, Wasserstein MP, Vockley J, Burton BK, Decker C, Li M, Lau K, Jiang J, Larimore K, Thomas JA. Long-term safety and efficacy of pegvaliase for the treatment of phenylketonuria in adults: combined phase 2 outcomes through PAL-003 extension study. Orphanet J Rare Dis. 2018;13:108. doi: 10.1186/s13023-018-0858-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harding CO, Gillingham MB, Hamman K, Clark H, Goebel-Daghighi E, Bird A, Koeberl DD. Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria. Gene Ther. 2006;13:457–462. doi: 10.1038/sj.gt.3302678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lichter-Konecki U, Vockley J. Phenylketonuria: Current Treatments and Future Developments. Drugs. 2019;79:495–500. doi: 10.1007/s40265-019-01079-z. [DOI] [PubMed] [Google Scholar]
- 73.Harding C. Progress toward cell-directed therapy for phenylketonuria. Clin Genet. 2008;74:97–104. doi: 10.1111/j.1399-0004.2008.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stéphenne X, Debray FG, Smets F, Jazouli N, Sana G, Tondreau T, Menten R, Goffette P, Boemer F, Schoos R, Gersting SW, Najimi M, Muntau AC, Goyens P, Sokal EM. Hepatocyte transplantation using the domino concept in a child with tetrabiopterin nonresponsive phenylketonuria. Cell Transplant. 2012;21:2765–2770. doi: 10.3727/096368912X653255. [DOI] [PubMed] [Google Scholar]
- 75.Diem HV, Evrard V, Vinh HT, Sokal EM, Janssen M, Otte JB, Reding R. Pediatric liver transplantation for biliary atresia: results of primary grafts in 328 recipients. Transplantation. 2003;75:1692–1697. doi: 10.1097/01.TP.0000062570.83203.A3. [DOI] [PubMed] [Google Scholar]
- 76.Morioka D, Kasahara M, Takada Y, Shirouzu Y, Taira K, Sakamoto S, Uryuhara K, Egawa H, Shimada H, Tanaka K. Current role of liver transplantation for the treatment of urea cycle disorders: a review of the worldwide English literature and 13 cases at Kyoto University. Liver Transpl. 2005;11:1332–1342. doi: 10.1002/lt.20587. [DOI] [PubMed] [Google Scholar]
- 77.Bergstralh EJ, Monico CG, Lieske JC, Herges RM, Langman CB, Hoppe B, Milliner DS IPHR Investigators. Transplantation outcomes in primary hyperoxaluria. Am J Transplant. 2010;10:2493–2501. doi: 10.1111/j.1600-6143.2010.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shneider BL, Vockley J, Mazariegos GV. Trading places: liver transplantation as a treatment, not a cure, for metabolic liver disease. Liver Transpl . 2011;17:628–630. doi: 10.1002/lt.22293. [DOI] [PubMed] [Google Scholar]
- 79.Cherukuru R, Reddy MS, Shanmugam NP, Rajalingam R, Kota V, Gunasekaran V, Narasimhan G, Kaliamoorthy I, Rela M. Feasibility and Safety of Split-Liver Transplantation in a Nascent Framework of Deceased Donation. Liver Transpl. 2019;25:450–458. doi: 10.1002/lt.25405. [DOI] [PubMed] [Google Scholar]
- 80.Rammohan A, Reddy MS, Narasimhan G, Rajalingam R, Kaliamoorthy I, Shanmugam N, Rela M. Auxiliary Partial Orthotopic Liver Transplantation for Selected Noncirrhotic Metabolic Liver Disease. Liver Transpl. 2019;25:111–118. doi: 10.1002/lt.25352. [DOI] [PubMed] [Google Scholar]
- 81.Terpstra OT, Schalm SW, Reuvers CB, Baumgartner D, Groenland TH, ten Kate FJ, Stibbe J, Terpstra JL, Weimar W, Willemse PJ. The role of auxiliary liver transplantation. Transplant Proc. 1987;19:4370–4372. [PubMed] [Google Scholar]
- 82.Faraj W, Dar F, Bartlett A, Melendez HV, Marangoni G, Mukherji D, Vergani GM, Dhawan A, Heaton N, Rela M. Auxiliary liver transplantation for acute liver failure in children. Ann Surg. 2010;251:351–356. doi: 10.1097/SLA.0b013e3181bdfef6. [DOI] [PubMed] [Google Scholar]
- 83.Weiner J, Griesemer A, Island E, Lobritto S, Martinez M, Selvaggi G, Lefkowitch J, Velasco M, Tryphonopoulos P, Emond J, Tzakis A, Kato T. Longterm outcomes of auxiliary partial orthotopic liver transplantation in preadolescent children with fulminant hepatic failure. Liver Transpl. 2016;22:485–494. doi: 10.1002/lt.24361. [DOI] [PubMed] [Google Scholar]
- 84.Kato T, Selvaggi G, Levi D, Hernandez E, Takahashi H, Velasco M, Moon J, Nishida S, Thompson J, Ruiz P, Sfakianakis G, Tzakis A. Routine use of auxiliary partial orthotopic liver transplantation for children with fulminant hepatic failure: Preliminary report. Transplant Proc. 2006;38:3607–3608. doi: 10.1016/j.transproceed.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 85.Sze YK, Dhawan A, Taylor RM, Bansal S, Mieli-Vergani G, Rela M, Heaton N. Pediatric liver transplantation for metabolic liver disease: experience at King's College Hospital. Transplantation. 2009;87:87–93. doi: 10.1097/TP.0b013e31818bc0c4. [DOI] [PubMed] [Google Scholar]
- 86.Ciria R, Davila D, Heaton N. Auxiliary liver transplantation in children. Curr Opin Organ Transplant. 2011;16:489–493. doi: 10.1097/MOT.0b013e32834a94cf. [DOI] [PubMed] [Google Scholar]
- 87.Oishi K, Arnon R, Wasserstein MP, Diaz GA. Liver transplantation for pediatric inherited metabolic disorders: Considerations for indications, complications, and perioperative management. Pediatr Transplant. 2016;20:756–769. doi: 10.1111/petr.12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morioka D, Kasahara M, Takada Y, Corrales JP, Yoshizawa A, Sakamoto S, Taira K, Yoshitoshi EY, Egawa H, Shimada H, Tanaka K. Living donor liver transplantation for pediatric patients with inheritable metabolic disorders. Am J Transplant. 2005;5:2754–2763. doi: 10.1111/j.1600-6143.2005.01084.x. [DOI] [PubMed] [Google Scholar]
- 89.Kim JS, Kim KM, Oh SH, Kim HJ, Cho JM, Yoo HW, Namgoong JM, Kim DY, Kim KH, Hwang S, Lee SG. Liver transplantation for metabolic liver disease: experience at a living donor dominant liver transplantation center. Pediatr Gastroenterol Hepatol Nutr. 2015;18:48–54. doi: 10.5223/pghn.2015.18.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sood V, Squires JE, Mazariegos GV, Vockley J, McKiernan PJ. Living Related Liver Transplantation for Metabolic Liver Diseases in Children. J Pediatr Gastroenterol Nutr. 2021;72:11–17. doi: 10.1097/MPG.0000000000002952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leal R, Costa J, Santos T, Galvão A, Santos L, Romãzinho C, Macário F, Alves R, Campos M, Furtado E, Mota A. Combined liver and kidney transplantation in two women with primary hyperoxaluria: Different roads led to different outcomes. Nefrologia. 2017;37:433–434. doi: 10.1016/j.nefro.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 92.Mc Guire PJ, Lim-Melia E, Diaz GA, Raymond K, Larkin A, Wasserstein MP, Sansaricq C. Combined liver-kidney transplant for the management of methylmalonic aciduria: a case report and review of the literature. Mol Genet Metab. 2008;93:22–29. doi: 10.1016/j.ymgme.2007.08.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molema F, Williams M, Langendonk J, Darwish-Murad S, van de Wetering J, Jacobs E, Onkenhout W, Brusse E, van der Eerden A, Wagenmakers M. Neurotoxicity including posterior reversible encephalopathy syndrome after initiation of calcineurin inhibitors in transplanted methylmalonic acidemia patients: Two case reports and review of the literature. JIMD Rep. 2020;51:89–104. doi: 10.1002/jmd2.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Arnon R, Kerkar N, Davis MK, Anand R, Yin W, González-Peralta RP SPLIT Research Group. Liver transplantation in children with metabolic diseases: the studies of pediatric liver transplantation experience. Pediatr Transplant. 2010;14:796–805. doi: 10.1111/j.1399-3046.2010.01339.x. [DOI] [PubMed] [Google Scholar]
- 95.Jain A, Mazariegos G, Kashyap R, Kosmach-Park B, Starzl TE, Fung J, Reyes J. Pediatric liver transplantation. A single center experience spanning 20 years. Transplantation. 2002;73:941–947. doi: 10.1097/00007890-200203270-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jain A, Mazariegos G, Kashyap R, Green M, Gronsky C, Starzl TE, Fung J, Reyes J. Comparative long-term evaluation of tacrolimus and cyclosporine in pediatric liver transplantation. Transplantation. 2000;70:617–625. doi: 10.1097/00007890-200008270-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miloh T, Barton A, Wheeler J, Pham Y, Hewitt W, Keegan T, Sanchez C, Bulut P, Goss J. Immunosuppression in pediatric liver transplant recipients: Unique aspects. Liver Transpl. 2017;23:244–256. doi: 10.1002/lt.24677. [DOI] [PubMed] [Google Scholar]
- 98.Mouzaki M, Yap J, Avinashi V, Babu A, Fu A, Deangelis M, Van Roestel K, Ghanekar A, Kamath B, Avitzur Y, Fecteau A, Jones N, Ling S, Grant D, Ng V. Basiliximab with delayed introduction of calcineurin inhibitors as a renal-sparing protocol following liver transplantation in children with renal impairment. Pediatr Transplant. 2013;17:751–756. doi: 10.1111/petr.12158. [DOI] [PubMed] [Google Scholar]
- 99.Williams R, Aithal G, Alexander GJ, Allison M, Armstrong I, Aspinall R, Baker A, Batterham R, Brown K, Burton R, Cramp ME, Day N, Dhawan A, Drummond C, Ferguson J, Foster G, Gilmore I, Greenberg J, Henn C, Jarvis H, Kelly D, Mathews M, McCloud A, MacGilchrist A, McKee M, Moriarty K, Morling J, Newsome P, Rice P, Roberts S, Rutter H, Samyn M, Severi K, Sheron N, Thorburn D, Verne J, Vohra J, Williams J, Yeoman A. Unacceptable failures: the final report of the Lancet Commission into liver disease in the UK. Lancet. 2020;395:226–239. doi: 10.1016/S0140-6736(19)32908-3. [DOI] [PubMed] [Google Scholar]