Graphical abstract
Keywords: Low sodium, Maillard peptide, Seasoning salt, Salty taste, Sodium content, Sensory evaluation
Highlights
-
•
The structural and physicochemical properties of a low sodium MRPs were investigated..
-
•
Different MRPs low sodium seasoning salts had better flavor characteristics.
-
•
CMS had the largest bulk density and the highest sensory score.
-
•
SMS had good hygroscopicity and thermal stability during storage.
-
•
TMS had the highest solubility, which is significant for its use as seasoning salt.
Abstract
In recent years, Maillard peptides have attracted considerable attention of food researchers due to their distinct flavor properties in food processing. We investigated the structure and flavor properties of the newly developed low-sodium seasoning salt with sesame seed hydrolysate Maillard products (SSH-MRPs), cysteine Maillard products (Cys-MRPs), methionine Maillard products (Met-MRPs), and thiamine Maillard products (Thi-MRPs). Compared to the control group, the Cys-MRPs salt (CMS) had the smallest angle of repose, the highest bulk density, and the highest sensory score. The seasoning salt with SSH-MRPs (SMS) had appreciable hygroscopicity and thermal stability. The seasoning salt with Thi-MRPs (TMS) had the highest solubility. These MRPs seasoning salts showed better flavor characteristics and physicochemical properties, suggesting that MRPs can replace part of NaCl to develop new low sodium seasoning salts and promote their application in food flavoring systems.
1. Introduction
With the upsurge in economic development and improvement in the standard of living, consumers have become more aware of the relationship between diet and health (Pateiro, Munekata, Cittadini, Dominguez, & Lorenzo, 2021). According to China's per capita salt intake (more than two times the World Health Organization's recommended level), it is considered among the countries with the highest salt intake (He et al., 2017). It is known that the intake of salt with high sodium content not only has a serious impact on blood pressure, but can also lead to different degrees of damage to the heart, liver, kidney, and other organs (Taladrid, Laguna, Bartolome, & Moreno-Arribas, 2020). Therefore, it is essential to comprehensively reduce the consumption of high-salt products and promote the consumption of healthier foods with minimal salt content (Grillo, Salvi, Coruzzi, Salvi, & Parati, 2019). Many strategies have been reckoned to reduce the salt content in processed foods (Yang et al., 2018), and some studies even emphasized the complete replacement of NaCl with other chloride salts (Wu et al., 2016, Hurst et al., 2021, Ayed et al., 2021). However, these changes led to a significant negative impact on the product properties (Zhang et al., 2020). Therefore, researchers shifted their focus on the formulation of salt substitutes, flavor enhancers (Ahmad et al., 2017), and naturally salty tasting products (Taladrid, Laguna, Bartolome, & Moreno-Arribas, 2020). Currently, research studies on the development of low sodium meat products are also increasing, mainly using salt substitutes, such as potassium, magnesium, calcium, salty peptides, etc. (Pateiro et al., 2021, Zhao et al., 2021, Pinton et al., 2020). Liu et al. (2019) reported that KCl, l-lysine and l-histidine could partially replace sodium in dry-cured beef, which still retained the salty taste without any significant effect on the color and other characteristics of beef. In addition, there was no significant effect of NaCl, KCl, and glycine on the popularity of roasted peanuts with the decrease in sodium content (Pujols, Ardoin, Chaiya, Tuuri, & Prinyawiwatkul, 2019). Amorim et al. (2018) reported the antihypertensive properties of whey peptide extract in a new coating of cashew nuts with reduced salt content.
Since potassium and magnesium salts can lead to metal bitterness and do not have the salty taste comparable to sodium chloride, their promotion in the market is not very successful (Vidal et al., 2020). On the other hand, peptide salt (a new type of low-sodium compound salt) combines with NaCl by using peptides, which can have a strong and salty taste with the function of supplementing amino acids. Maillard flavor peptides produced by the Maillard reaction not only have the properties of fresh, salty taste and low bitterness, but they are also endowed with antibacterial, anti-allergic, anti-bacterial and anti-inflammatory properties (Fu, Zhang, Soladoye, & Aluko, 2020). Therefore, Maillard flavor peptides salt is a natural, nutritious, and low-sodium compound salt with the unique flavor of MRPs (Maillard reaction products). The Maillard reaction of peptides and reducing sugars can improve the flavor characteristics of MRPs. MRPs with different model systems and conditions have different flavors. MRPs from flaxseed meal added with cysteine showed better flavor and meaty taste (Wei, Thakur, Liu, Zhang, & Wei, 2018), and the MRPs from peony seed meal hydrolysate under monosaccharide had better caramel taste (Shang et al., 2020). In addition, the saltiness and flavor of sesame seed meal MRPs were relatively consistent under different sulfur compounds, and there was no significant difference (Shen et al., 2021). Previous studies have reported that MRPs are used as flavor enhancers in foods (Chen et al., 2019), but few studies have been conducted to replace the high-sodium salt. Mixing MRPs with salt can reduce the sodium content, change the physical properties of salt, and result in the unique flavor, saltiness, and other taste characteristics of MRPs.
So far, there are limited studies on low sodium salt, and most of them focus on potassium salt and magnesium instead of sodium salt. In our recent study, four types of MRPs (SSH-MRPs, Cys-MRPs, Met-MRPs, and Thi-MRPs) were prepared by adding various sulfur-containing substances to sesame seed meal (Shen et al., 2021). In continuation, the previously reported MRPs were used in combination with NaCl, KCl, sugar, and monosodium glutamate to develop the low salt MRPs model system. Compared with MRPs, the low sodium seasoning salt added with MRPs have good color and flavor characteristics. Furthermore, the color, solubility, sodium content, bulk density, angle of repose, hygroscopicity, thermal stability, particle size, and microstructure of the different MRPs-salt model systems were determined. The effects of the different MRPs on the structure and physical properties of the salt were investigated. In addition, the taste characteristics of low sodium salt prepared with different MRPs were analyzed by sensory evaluation.
2. Materials and methods
2.1. Preparation of MRPs low sodium seasoning salt model system
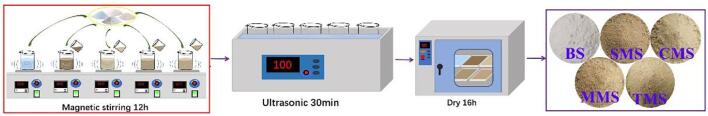
Sesame seed meal hydrolysate and its MRPs were prepared in our recent study using cysteine (Cys-MRPs), methionine (Met-MRPs), and thiamine (Thi-MRPs). In addition, a sesame seed meal hydrolysate solution without any sulphur containing substance (SSH-MRPs) was prepared at the same concentration (Shen et al., 2021). The previously prepared MRPs solutions (15 mL) were individually mixed with 24 g NaCl, 12 g KCl, 2 g monosodium glutamate, and 0.8 g sugar (quadruplicate were prepared with the same weight of all ingredients) in a beaker filled with 400 mL hot water (Pujols, Ardoin, Chaiya, Tuuri, & Prinyawiwatkul, 2019). The obtained solution was rotated in a magnetic stirrer at 800 r/min for 12 h. After 30 min of sonication, it was transferred to the culture dish and dried in a constant temperature oven at 50 ℃ for 16 h. The samples were ground into a fine powder and then placed into the oven until the salt powder was completely dried. The obtained samples were stored in a polyethylene bag at − 20 ℃. The five seasoning salts were named BS (control group: its composition is 24 g NaCl, 12 g KCl, 2 g monosodium glutamate, and 0.8 g sugar.), SMS (with SSH-MRPs), CMS (with Cys-MRPs), MMS (with Met-MRPs), and TMS (with Thi-MRPs). The sesame seed meal was purchased from Laowangjia Sesame Oil Co., Ltd. (Hefei, China), Alcalase (200,000 U/g) and Flavourzyme (20, 000 U/g) were purchased from Novozymes (Beijing, China). l-cysteine, d-xylose, l-methionine, and thiamine were purchased from Macklin Biochemical Co., Ltd.(Shanghai, China). Other solvents and chemicals were of analytical grade or chromatographic grade. Fig. 1 depicts the systematic preparation procedure of five different types of low sodium seasoning salts.
Fig. 1.
Production process of low sodium seasoning salt from different Maillard reaction products.
2.2. Determination of color
At first, 5 g of MRPs seasoning salt powder was added to the culture dish, and the color was determined using the colorimeter NR-200 (Shenzhen 3NH Technology Co., Ltd., China) (Wei et al., 2019, Ni et al., 2021). Here, the L* value indicates the brightness of the color, a* value indicates the degree of red and green color (+red, −green), b* value indicates the degree of yellow and blue color (+yellow, −blue) and ΔE represents the total color difference. Each group of samples was tested three times, and the average value was taken using the following formula.
Where L* 0, a* 0, and b* 0 are color parameters of the control group.
2.3. Sodium and potassium content
The method for determining sodium and potassium content was adapted from a previous study (Hurst et al., 2021). Where 0.5 g-5 g (to the nearest 0.001 g) samples were weighed into digestion utensils made of glass or polytetrafluoroethylene. Samples containing ethanol or carbon dioxide were first heated at low temperature on an electric hot plate to remove ethanol or carbon dioxide, then 10 mL of a mixed acid (perchloric acid and nitric acid) was added and placed under a lid for 1 h or overnight. The samples were placed on an adjustable temperature-controlled electric hot plate for digestion. After the samples turned brown and black, mixed acid was added after cooling, until white smoke was emitted, and the colorless and transparent or slightly yellow digestion liquid was obtained. The absorbance value of potassium or sodium was measured by injecting the blank solution and then measuring solution into the atomic absorption spectrometer (ICE3400, Thermo Fisher, USA). The concentration of potassium or sodium in the solution under study was determined according to the standard curve. The contents of K and Na in the sample were obtained by substituting them into the calculation formula.
where: X: the content of tested element in sample; ρ: the determination of mass concentration of elements in solution; ρ0: the determination of mass concentration of elements in blank solution; V: the volume of sample solution; the dilution ratio of sample solution; the mass of sample.
2.4. Bulk density and angle of repose
Bulk density refers to the mass of powder or particles per unit volume. As the bulk density increases, it decreases the adhesive power and improves the flowability of the powder. Following the previously reported method (Hurst et al., 2021), 5 g of spice salt powder of different MRP was weighed accurately and the mass was noted as M. The powder was poured into a 10-ml graduated cylinder at a time, and the graduated cylinder was shaken from a height of 2.5 cm on the experimental platform every 2 s until there was almost no change in the powder volume in the cylinder. At this time, the powder volume was recorded as V and the bulk density was calculated according to the following formula:
The angle of repose is often used to indicate the fluidity of particles. The smaller the angle of repose, the better the quality of the particles. The angle of repose was measured using the fixed funnel method. Three funnels were connected in series and fixed on an iron frame. The height between the bottom edge of the lowest funnel opening and the coordinate paper was 3 cm. Particles were carefully poured into the top funnel along the funnel wall until the tip of the cone formed on the coordinate paper touched the funnel mouth. The diameter (2R) at the bottom of the cone and the height (H) of the cone were measured with the coordinate paper and measured in parallel three times to obtain the average value (He et al., 2020). The angle of repose α was calculated using the following formula:
2.5. Solubility and hygroscopicity analysis
To assess the solubility of low-sodium seasoning salt, 6 g of accurately weighed salt powder was placed in a 50 mL centrifuge tube and 10 mL of distilled water was added followed by shaking for 90 min and centrifugation at 8000 rpm for 10 min. The resulting supernatant was transferred to the culture dish and the residue was dried to constant weight and weighed exactly at 50 ℃ (Huang et al., 2019).
where: m1: the weight of the sample (g); m2: the weight of residue(g).
Hygroscopicity refers to the ability or degree of a material to absorb water under certain conditions of temperature and humidity. A certain number of particles was dried in a 50 ℃ oven to obtain the constant weight. The glass desiccator saturated with sodium chloride solution a 25 ℃ was incubated for 24 h, and the RH (relative humidity) in the desiccator was maintained at 75%. The 2 mm thick dry particles were placed in the NaCl supersaturated solution after weighing accurately (the lid of the weighing bottle was open), and stored in the 25 ℃ incubator. The particles were weighed regularly for 12 h, 24 h, 36 h, 48 h, 60 h, 72 h, 84 h, 96 h, 108 h, and 120 h. Each sample was measured three times in parallel to obtain the average value. The hygroscopic fraction was used as the ordinate and time as abscissa (Aslan and Ertaş, 2021).
where: Mi: the weight of salt after water absorption every 12 h (g); M0: the initial weight of salt (g).
2.6. Thermal stability analysis
According to previous study (Yu et al., 2018), the thermal stability of low-sodium seasoning salt was investigated by measuring the degree of browning of seasoning salt. The five kinds of salts were prepared in 0.1 g/mL salt solution and heated at 100 ℃ for 0–30 min, respectively from 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, and 30 min, after which an appropriate amount of each solution was taken out every 2 min and transferred to ice water for rapid cooling. Then, each solution was centrifuged at 8000 rpm/min for 15 min, and the absorbance values of the supernatant at 420 nm and 294 nm (diluted 30 times) were measured by microplate reader and UV-2100 spectrophotometer, respectively.
2.7. Particle size distribution
The particle size distribution of MRPs seasoning salt was determined using a laser particle size analyzer (MS 2000; Malvern Instruments, Ltd.; Worcestershire, UK) (Ren et al., 2021). The particle size distribution of low sodium seasoning salt was evaluated according to the volume weight average diameter D (4, 3) (Franco et al., 2016).
2.8. Scanning electron microscopy (SEM)
A certain amount of freeze-dried MRP seasoning salt powder was fixed on the conductive adhesive and placed in a vacuum gilding apparatus for gilding (10 Ma, 30 s). The morphology of the MRPs spice salt powder after vacuum freeze-drying was observed using a high-resolution field emission SEM (Regulus 8230, Hitachi, Japan) at magnification range of 1000 × and 2000 × (Boulemkahel et al., 2021).
2.9. Sensory evaluation
For sensory study, some modifications were made to the previous method (Wei et al., 2020). We conducted sensory analysis on five low sodium seasoning salts. For this, an appropriate amount of model salt samples was taken in a cup followed by pouring an appropriate amount of hot water (about 80℃) to prepare a 1% solution (w/v). The resulting samples were tasted after the samples were completely dissolved. The sensory evaluation team was selected from Laboratory of Sensory Science, Hefei University of Technology (Hefei, China) by confirming the criteria for descriptive analysis. The expert team was composed of 14 experts experienced in flavor evaluation (six males and eight females, aged 23–40 years). Before the formal sensory evaluation, the team members received a relevant training: firstly, they received a three-day training (twice a day, each training lasted 1 h) to distinguish and describe the taste characteristics of food. This allowed the team members to identify the different flavors. Secondly, the team members were asked to describe the eight taste characteristics of the analytical model salt (flavor, sweety, salty, bitterness, mouthfulness, astringency, umami, and overall preference). The team members rated the samples from 0 to 10.
2.10. Statistical evaluation
Duncan's test was conducted using SPSS Statistics 20.0 (SPSS, Inc., Chicago, IL, USA). Each experiment was repeated three times, and significance was measured at ± 5% (P ≤ 0.05). Results were expressed as mean ± standard deviation and ANOVA was used for overall data analysis.
3. Result and discussion
3.1. Color analysis
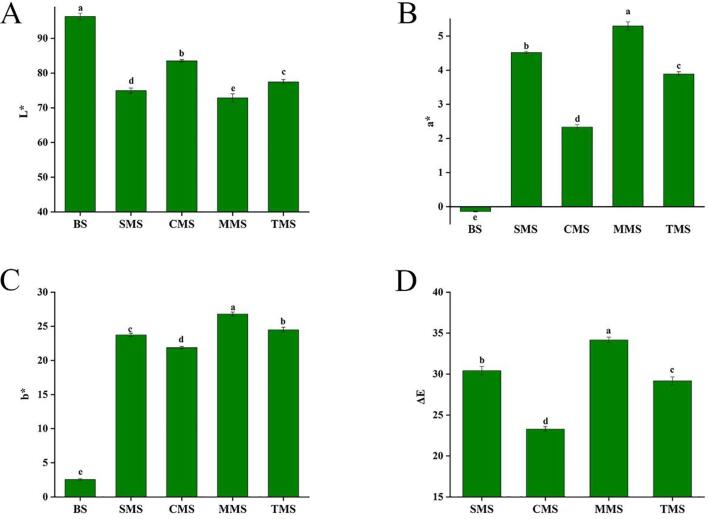
Color is an important sensory attribute of any food that can accurately reflect the apparent color of seasoning salt and affect the acceptance of consumers (Vargas-Ramella et al., 2021). There are significant differences in the composition of different MRPs and salts (P < 0.05). As shown in Fig. 2, among the four MRPs, CMS had the largest L* value indicating its brightness, while MMS had the smallest L* value, so the surface brightness of MMS was low. The L* value of SMS was close to that of MMS, and significantly different from that of the other seasoning salts (P < 0.05). The a* value and b* value of CMS was the lowest, indicating that the seasoning salts added with Cys-MRPs had better color characteristics (green and blue). In addition, the salt without MRPs was used as the ΔE control. The color difference values of the samples in the four MRPs seasoning salt were all greater than 3.0, indicating that the color difference between the seasoning salt powder and the control group was significant (P < 0.05), and these results were consistent with the previous results of the appearance color map (Shen et al., 2021). In addition, compared with the Maillard reaction product derived from sesame meal protein hydrolysate, L*, a*, b* and ΔE increased in varying degrees, indicating that the low sodium seasoning salt added with MRPs has better brightness and color characteristics. The order of overall color difference was MMS > SMS > TMS > CMS, indicating that the color of CMS was lighter, while the color of MMS was the most different from that of the control group, which might be due to the color of MRPs. In our previous report, cysteine was able to produce many intermediate compounds that inhibited the progress of the Maillard reaction and thus produced fewer melanoids, therefore, the color of Cys-MRPs seasoning salt was lighter (Wei et al., 2019).
Fig. 2.
Color comparison of low sodium seasoning salts from different Maillard reaction products. The values followed by different letters were significantly different (P < 0.05).
3.2. Sodium and potassium content analysis
Compared with the control group, the sodium content was significantly reduced after using KCl, monosodium glutamate, sugar and MRPs (Table 1). The actual sodium content of the control group (248.69 mg/g) was slightly higher than the theoretical sodium content (243.27 mg/g), which may be due to the hydrolysis of sodium glutamate into monosodium glutamate. The sodium contents of SMS, CMS, MMS and TMS were 167.87, 187.31, 204.76, and 220.83 mg/g, respectively, which were significantly lower than the control group. This may be due to the combination of sodium ions with different MRPs in different degrees and precipitation after centrifugation. Similarly, the potassium content in the spice salt system model decreased at different degrees compared to the theoretical value because there was no other substance to provide potassium except KCl, and the trend was consistent with the sodium content. The addition of MRPs, KCl, monosodium glutamate and sugar could not only reduce the sodium content in the salt but it also increased the potassium content of the seasoning salt. Potassium regulates the appropriate osmotic pressure and acidbase balance of body fluid in cells and participates in the metabolism of sugar and protein in cells (Vargas-Ramella et al., 2021, Zhang et al., 2020).
Table 1.
Physical properties of different types of low sodium seasoning salt.
| BS | SMS | CMS | MMS | TMS | |
|---|---|---|---|---|---|
| Theoretical Na value (mg/g) | 243.27a | 235.18b | 235.18b | 235.18b | 235.18b |
| Actual Na value (mg/g) | 248.69 ± 9.48a | 167.87 ± 6.44e | 187.31 ± 7.39d | 203.76 ± 7.44c | 220.83 ± 8.58b |
| Theoretical K value (mg/g) | 161.93a | 157.11b | 157.11b | 157.11b | 157.11b |
| Actual K value (mg/g) | 155.57 ± 5.95a | 101.98 ± 3.84d | 113.52 ± 4.11c | 125.18 ± 4.42b | 128.05 ± 5.17b |
| Bulk density (g/ml) | 0.93 ± 0.02c | 1.02 ± 0.04b | 1.12 ± 0.01a | 1.03 ± 0.02b | 0.91 ± 0.01d |
| Angle of repose (°) | 35.44 ± 0.40c | 36.51 ± 0.44b | 32.36 ± 0.77e | 38.25 ± 0.39a | 34.44 ± 0.41d |
| Solubility(g/100 g) | 51.48 ± 0.47a | 40.60 ± 0.27e | 42.59 ± 0.59d | 46.74 ± 0.43c | 48.33 ± 0.67b |
Note: Means within different letters were significantly (p < 0 0.05) different on the same line. BS, blank-salt; SMS, SSH-MRPs-salt; CMS, Cys-MRPs-salt; MMS, Met-MRPs-salt; TMS, Thi-MRPs-salt.
3.3. Bulk density and angle of repose
The bulk density of the powder is the mass per unit volume of the micro powder, which is one of the indices that reflects the filling ability of the powder (Hurst, Ayed, Derbenev, Hewson, & Fisk, 2021). It can be seen from Table 1 that the bulk densities of the five salts ranged from 0.9 g/ml to 1.2 g/ml, in the order CMS > MMS > SMS > BS > TMS. The bulk densities of the SMS and MMS samples were close to each other, and there was a significant difference between them and the other three salts (P < 0.05). The difference in bulk density between the different seasoning salts may be caused by the different addition of MRPs. As mentioned earlier, the seasoning salts with MRP addition have more pores, which may be another reason for the higher bulk density of the seasoning salts in the MRPs model. To some extent, the bulk density reflects the compactness of the salt particles. A higher bulk density can hold more samples for the same volume, which is important for packaging, transportation, material handling, and processing of salt powder in the food industry (Adeloye, Osho, & Idris, 2020).
Angle of repose is the acute angle between the inclined plane of natural accumulation of powder and the lower horizontal plane in static equilibrium condition. The magnitude of the angle of repose directly reflects the flow ability of the powder. The smaller the angle of repose, the better the flow ability is reported (He et al., 2020). As shown in Table 1, there was a significant difference in the angle of repose between different seasoning salts (P < 0.05). The angle of repose of CMS was the smallest, 32.36° and that of MMS was the largest, 38.25°. The angle of repose of the above five kinds of salt was<40°, which indicates that better fluidity related to the raw materials of MRPs constitutes low sodium seasoning salt. In a previous report, the angle of repose of Mexican pine nut powder was smaller than that of sesame seed powder and melon seed powder (Pensamiento-Niño et al., 2019).
3.4. Solubility and hygroscopicity analysis
Solubility refers to the solubility of samples in an aqueous solution, which is an important parameter of salt powder quality. Solubility is related to powder processing, chemical composition, particle size, capillarity, porosity, and external conditions. It is one of the important indices to evaluate the characteristics of instant powder (Li, Masatcioglu, & Koksel, 2019). The solubility of the five different seasoning salts was shown in Table 1. The solubility of TMS was significantly different from that of other system models (P < 0.05), except for the control group, and its solubility value was the highest, reaching 48.33 g/100 g. While SMS had the lowest solubility value of 40.6 g/100 g. The possible reason might be that sulfur-containing MRPs were added to the other three salts, which contain highly soluble compounds. The solubility of TMS particles was good which might be related to the particle size of the powder. There is no fixed standard for solubility, but since seasoning salt is used in food seasoning, therefore, higher solubility brings a better seasoning effect (Alissa, Hung, Hou, Lim, & Ciou, 2020).
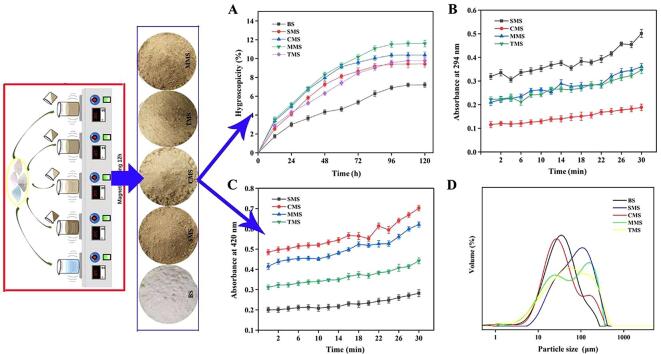
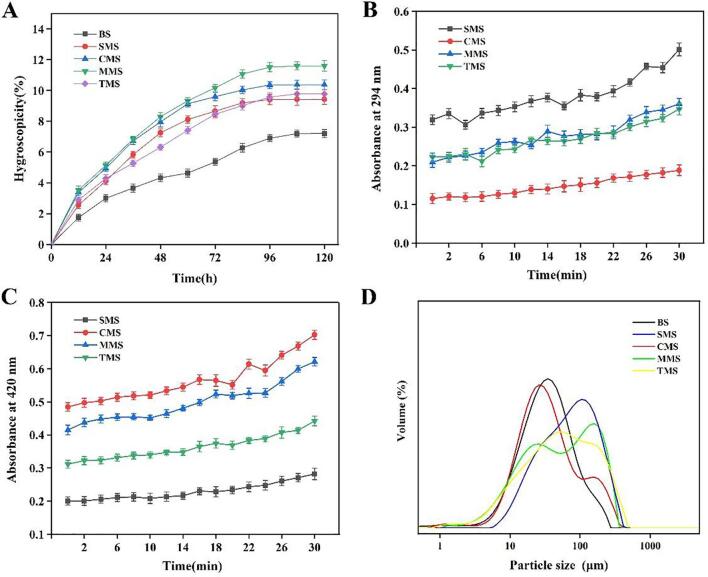
The stronger the hygroscopicity of the powder, the easier it is to cause agglomeration of the material, resulting in physical and chemical changes such as decreased fluidity and deliquescence of the material, reducing the biological stability of the material, and making the product difficult to be accepted by consumers (Laokuldilok, Thakeow, Kopermsub, & Utama-ang, 2016). Under the conditions of 25 ℃ and 75% RH, the hygroscopic percentage curves of five kinds of seasoning salts are shown in Fig. 3A. The hygroscopic percentage of seasoning salts prepared by different methods increased with time. In the first 60 h, the trend of the hygroscopic curves for seasoning salt added with Cys-MRPs and Met-MRPs was roughly the same, and after that, the hygroscopic percentage of MMS changed greatly. At 96 h, the hygroscopic percentage of SMS did not change at first, and the hygroscopic percentage of the three MRPs seasoning salts did not change at 108 h. The hygroscopic percentage of all system models did not change after 120 h. The hygroscopicity of seasoning salt added with MRPs was higher than that of the control group (P < 0.05), and the addition of Met-MRPs had the greatest effect on the hygroscopicity of seasoning salt. The hygroscopicity of the powder was related to a variety of substances in the powder, and the type and quantity of compounds contained in different MRPs were very different, which may be the reason for the difference in hygroscopicity (U‐Chupaj et al., 2021). The above results showed that compared with the control group, the seasoning salt added with MRPs had poor hygroscopicity, but the maximum hygroscopicity of the seasoning salt of all model systems was less than 15%, so it can be assumed that the hygroscopicity of low sodium seasoning salt can meet the needs of production and processing.
Fig. 3.
A, Hygroscopicity comparison of low sodium seasoning salts from different Maillard reaction products. B, Comparison of thermal stability of low sodium seasoning salts from different Maillard reaction products (A294). C, Comparison of thermal stability of low sodium seasoning salts from different Maillard reaction products (A420). D, Comparison of particle size distribution of low sodium seasoning salts from different Maillard reaction products.
3.5. Thermal stability analysis
The low sodium seasoning salt was added to MRPs, so the browning degree of the compound salt under heating can reflect the thermal stability of the low sodium seasoning salt (Shang et al., 2020). The change of browning degree of seasoning salt with heating time can be seen in Fig. 3B and Fig. 3C. The absorbance at 294 nm and 420 nm represented the content of the intermediate compound and final product, respectively. In the initial stage of heating, A294 and A420 of SMS, CMS, and TMS showed minute change, which indicated that short time heating had little effect on seasoning salt. With the extension of heating time, the absorbance of seasoning salt solution at 420 nm and 294 nm showed an upward trend, especially during the last 10 min of the reaction, and the browning degree changed to a great extent. A294 of SMS, MMS and TMS changed significantly, while the A294 of CMS changed the least, indicating that more intermediates were produced in the three seasoning salts (Shen et al., 2021) With the extension of heating time, the browning degree of seasoning salt gradually increased, and Maillard products gradually accumulated. Compared with other seasoning salts added with sulfur-containing amino acid MRPs, the seasoning salt added with single hydrolysate MRPs had the lowest rate change of A294 and A420, indicating that SMS had better thermal stability in the heating process. The change rate of A294 and A420 in MMS was the highest, followed by CMS. This may be due to the high content of free amino acids in Met-MRPs and Cys-MRPs. A large number of amino acids exacerbated the process of the Maillard reaction, which was reported in our previous study (Shang et al., 2020).
3.6. Particle size distribution
The addition of MRPs changed the particle size distribution of low sodium seasoning salt. It can be seen from Fig. 3D that the distribution of different low sodium seasoning salt was different. The control group showed a single peak distribution. The particle size distribution of seasoning salts with MRPs was in the range of 0.1–240 μm. SMS showed an almost single peak distribution, with the main peak of about 100–300 µm, and its volume average particle size was the largest among all low sodium seasoning salt model systems, reaching 88.89 µm. The particle size distribution of CMS, MMS, and TMS with sulfur-containing MRPs had two peaks, the first peak was between 10 and 100 μm, and the second peak was between 100 and 300 μm. The two peaks of MMS were obvious, indicating the heterogeneity of particle groups and the coexistence of fine and coarse particles. Compared with the control group, the particle size of low sodium seasoning salt increased significantly after adding MRPs, and the average particle size of SMS was more than twice that of the control group. These differences may be due to the type of raw materials and grinding, which may have a significant impact on a variety of physical and chemical properties (Huang et al., 2020, Huang et al., 2019).
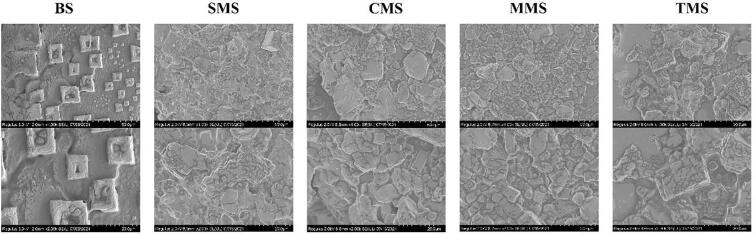
3.7. Scanning electron microscopy (SEM)
The SEM images of the five different seasoning salts at 1000 and 2000 magnification were shown in Fig. 4, which was consistent with the results of particle size. The addition of different MRPs had a great influence on the texture and structure of the seasoning salt. The powder particles of the control group had different sizes and showed an angular polygonal structure. At the same time, some depressions, cracks, and many holes of different depths are observed on the surface. Therefore, the structure was relatively loose and the size was 30–50 μm. Compared with the control group, the particles of the MRPs seasoning salt model were bigger, more incomplete, more irregular in shape, and more small holes formed, which may be caused by the high pressure in the grinding process (He et al., 2020). In addition, the four MRPs seasoning salt particles were closely connected with each other. The small particles were closely packed together to form a cluster, and the particles of SMS showed a better particle uniformity, close to the control group. This may be due to the stronger adsorption on the surface of low sodium seasoning salt powder added with MRPS, and the increased interaction force, which led to the aggregation of molecules and the dense distribution phenomenon (Hurst, Ayed, Derbenev, Hewson, & Fisk, 2021).
Fig. 4.
Scanning electron microscopy images of model salts: (A) BS, (B) SMS, (C) CMS, (D) MMS, (E) TMS. The magnification of each low sodium seasoning salt is × 1000 and × 2000.
3.8. Sensory evaluation
The sensory evaluation of five different seasoning salts by 4 trained panelists (six males and eight females, aged 23–48 years) were selected from the Laboratory of Sensory Science, Hefei University of Technology (Hefei, China) by confirming the criteria for descriptive analysis. In terms of the important sensory characteristics, the saltiness of all MRPs seasoning salts had a different increasing trend compared with the control group. CMS had the strongest saltiness score, which may be due to the salty peptide produced after the Maillard reaction (Selamassakul, Laohakunjit, Kerdchoechuen, Yang, & Maier, 2020). For umami, the four kinds of seasoning salts containing MRPs were significantly different from the control group, and the seasoning salt containing Cys-MRPs had the strongest umami, which may be due to the higher presence of umami amino acids in Cys-MRPs, and it still retained its original umami after compounding with NaCl and KCl (Shang et al., 2020). In addition, compared with the control group, CMS, MMS, TMS three MRPs seasoning salt model had a better contribution in flavor, because there were more sulfur-containing amino acids and sulfur-containing substances in Cys-MRPs, Met-MRPs, and Thi-MRPs, which contributed to the caramel and meat-like flavors (Shen et al., 2021). Due to the addition of white granulated sugar in the low sodium salt model system, the expert group reported different degrees of sweetness in the five seasoning salts. In terms of bitterness and astringency, there was not much difference between the five kinds of salt (P greater than 0.05), and these two types of sensations were all low (Fig. 5). CMS and TMS had the highest mouthfulness score, and there was little difference between them. In terms of overall preference, the order from small to large was TMS < SMS < BS < MMS < CMS. CMS was selected as the salt with the highest preference by the expert group because of its relatively high saltiness, umami, flavor, and relatively low bitterness and astringency.
Fig. 5.
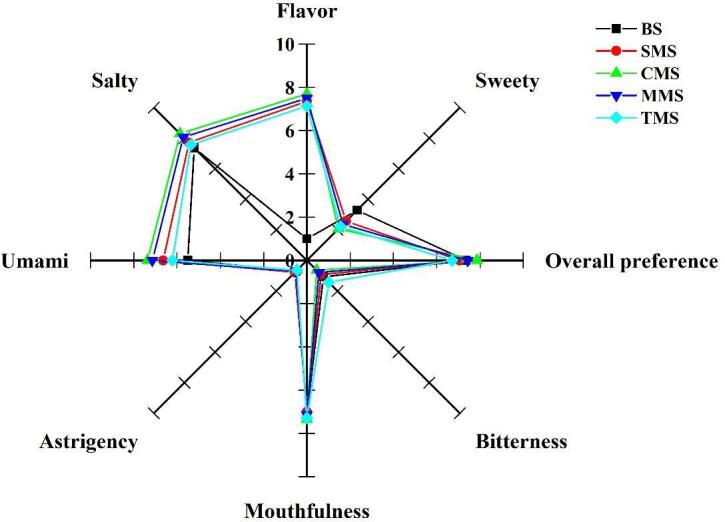
Sensory evaluation of low sodium seasoning salts from different Maillard reaction products.
4. Conclusion
To conclude, we explored a new type of low sodium seasoning salt in combination with different MRPs with NaCl, KCl, monosodium glutamate and sugar, and compared their structural properties. In different combinations, CMS showed the smallest angle of repose, the largest bulk density, and the highest sensory score in MRPs seasoning salt model system. SMS had appreciable hygroscopicity and thermal stability which can offer a unique advantage in transportation and storage. In addition, TMS had the highest solubility, which is of great significance as a condiment. Our results revealed that, compared with the control group, different MRPs low sodium seasoning salts exhibited better flavor characteristics and physicochemical properties, and the overall properties of CMS were improved, indicating that these MRPs can replace part of NaCl to develop new low sodium seasoning salts. Further research is warranted to explore these MRPs as salt substitutes and food flavor agents in food processing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of Ningxia Province (2021AAC02019), the Youth talent cultivation project of North Minzu University (2021KYQD27), the Major Projects of Science and Technology in Anhui Province (201903a06020021, 18030701158, 18030701144, 18030701161, 1804h07020147, 201904a06020008), the National Natural Science Foundation of China (31850410476).
Contributor Information
Long-Teng Hu, Email: 2019171251@mail.hfut.edu.cn.
Yi Shen, Email: 2019111359@mail.hfut.edu.cn.
Kiran Thakur, Email: kumarikiran@hfut.edu.cn.
Li Jiang, Email: lijiang@hfut.edu.cn.
Jian-Guo Zhang, Email: zhangjianguo@hfut.edu.cn.
Zhao-Jun Wei, Email: zjwei@hfut.edu.cn.
References
- Adeloye J.B., Osho H., Idris L.O. Defatted coconut flour improved the bioactive components, dietary fibre, antioxidant and sensory properties of nixtamalized maize flour. Journal of Agriculture and Food Research. 2020;2:100042. doi: 10.1016/j.jafr.2020.100042. [DOI] [Google Scholar]
- Ahmad S.R., Sharma B.D., Irshad A., Kumar R.R., Malav O.P., Talukder S. Improving the functional characteristics of low salt restructured buffalo meat fillets with the use of maize flour as an extender. SKUAST Journal of Research. 2017;2017(19):187–191. [Google Scholar]
- Alissa K., Hung Y.-C., Hou C.Y., Lim G.C.W., Ciou J.-Y. Developing New Health Material: The Utilization of Spray Drying Technology on Avocado (Persea Americana Mill.) Seed Powder. Foods. 2020;9(2):139. doi: 10.3390/foods9020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim M., Pereira J.O., Silva L.B., Ormenese R.C.S.C., Pacheco M.T.B., Pintado M. Use of whey peptide fraction in coated cashew nut as functional ingredient and salt replacer. LWT-Food Science and Technology. 2018;92:204–211. [Google Scholar]
- Aslan M., Ertaş N. Foam drying of aquafaba: Optimization with mixture design. Journal of Food Processing and Preservation. 2021;45(3) doi: 10.1111/jfpp.v45.310.1111/jfpp.15185. [DOI] [Google Scholar]
- Ayed C., Lim M., Nawaz K., Macnaughtan W., Sturrock C.J., Hill S.E. The role of sodium chloride in the sensory and physico-chemical properties of sweet biscuits. Food Chemistry-X. 2021;9:100115. doi: 10.1016/j.fochx.2021.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulemkahel S., Betoret E., Benatallah L., Rosell C.M. Effect of low pressures homogenization on the physico-chemical and functional properties of rice flour. Food Hydrocolloids. 2021;112:106373. doi: 10.1016/j.foodhyd.2020.106373. [DOI] [Google Scholar]
- Chen H., Cui H., Zhang M., Hayat K., Yu J., Xia S. Improving the Flavor and Oxidation Resistance of Processed Sunflower Seeds with Maillard Peptides. Food and Bioprocess Technology. 2019;12(5):809–819. [Google Scholar]
- Franco T.S., Perussello C.A., Ellendersen L.N., Masson M.L. Effects of foam mat drying on physicochemical and microstructural properties of yacon juice powder. LWT-Food Science and Technology. 2016;66:503–513. [Google Scholar]
- Fu Y.u., Zhang Y., Soladoye O.P., Aluko R.E. Maillard reaction products derived from food protein-derived peptides: Insights into flavor and bioactivity. Critical Reviews in Food Science and Nutrition. 2020;60(20):3429–3442. doi: 10.1080/10408398.2019.1691500. [DOI] [PubMed] [Google Scholar]
- Grillo A., Salvi L., Coruzzi P., Salvi P., Parati G. Sodium intake and hypertension. Nutrients. 2019;11:1970. doi: 10.3390/nu11091970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Zheng J., Liu F., Woo M.W., Xiong H., Zhao Q. Fabrication and characterization of oat flour processed by different methods. Journal of Cereal Science. 2020;96:103123. doi: 10.1016/j.jcs.2020.103123. [DOI] [Google Scholar]
- He Y., Yang W.H., Liu S.J., Gan L.L., Zhang F., Mu C.H. Interactions between angiotensin-converting enzyme-2 polymorphisms and high salt intake increase the risk of hypertension in the Chinese Wa population. International Journal of Clinical and Experimental Pathology. 2017;10:11159–11168. [PMC free article] [PubMed] [Google Scholar]
- Huang S., Martinez M.M., Bohrer B.M. The Compositional and Functional Attributes of Commercial Flours from Tropical Fruits (Breadfruit and Banana) Foods. 2019;8(11):586. doi: 10.3390/foods8110586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Liang K.-H., Liu Q.i., Qiu J.u., Wang J., Zhu H. Superfine grinding affects physicochemical, thermal and structural properties of moringa oleifera leaf powders. Industrial Crops and Products. 2020;151:112472. doi: 10.1016/j.indcrop.2020.112472. [DOI] [Google Scholar]
- Hurst K.E., Ayed C., Derbenev I.N., Hewson L., Fisk I.D. Physicochemical design rules for the formulation of novel salt particles with optimised saltiness. Food Chemistry. 2021;360:129990. doi: 10.1016/j.foodchem.2021.129990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laokuldilok N., Thakeow P., Kopermsub P., Utama-ang N. Optimisation of microencapsulation of turmeric extract for masking flavour. Food Chemistry. 2016;194:695–704. doi: 10.1016/j.foodchem.2015.07.150. [DOI] [PubMed] [Google Scholar]
- Li X., Masatcioglu M.T., Koksel F. Physical and functional properties of wheat flour extrudates produced by nitrogen injection assisted extrusion cooking. Journal of Cereal Science. 2019;89:102811. doi: 10.1016/j.jcs.2019.102811. [DOI] [PubMed] [Google Scholar]
- Liu S., Zhang Y., Zhou G., Bao Y., Ren X., Zhu Y. Protein degradation, color and textural properties of low sodium dry cured beef. International Journal of Food Properties. 2019;22(1):487–498. [Google Scholar]
- Ni Z.J., Liu X., Xia B., Hu L.T., Thakur K., Wei Z.J. Effects of sugars on the flavor and antioxidant properties of the Maillard reaction products of camellia seed meals. Food Chemistry: X. 2021;11 doi: 10.1016/j.fochx.2021.100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateiro M., Munekata P.ES., Cittadini A., Domínguez R., Lorenzo J.M. Metallic-based salt substitutes to reduce sodium content in meat products. Current Opinion in Food Science. 2021;38:21–31. [Google Scholar]
- Pensamiento-Niño C.A., Hernández-Santos B., Herman-Lara E., Juárez-Barrientos J.M., Martínez-Sánchez C.E., Ramírez-Rivera E.J. Physical, mechanical, functional and chemical properties of Mexican pink pinion (Pinus pinea L.) Journal of Food Science and Technology-Mysore. 2019;56(2):763–774. doi: 10.1007/s13197-018-3536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton M.B., dos Santos B.A., Correa L.P., Leães Y.S.V., Cichoski A.J., Lorenzo J.M. Ultrasound and low-levels of NaCl replacers: A successful combination to produce low-phosphate and low-sodium meat emulsions. Meat Science. 2020;170:108244. doi: 10.1016/j.meatsci.2020.108244. [DOI] [PubMed] [Google Scholar]
- Pujols K.D., Ardoin R., Chaiya B., Tuuri G., Prinyawiwatkul W. Low-sodium roasted peanuts: Effects of salt mixtures (NaCl, KCl and glycine) on consumer perception and purchase intent. International Journal of Food Science & Technology. 2019;54:2754–2762. [Google Scholar]
- Ren Y., Setia R., Warkentin T.D., Ai Y. Functionality and starch digestibility of wrinkled and round pea flours of two different particle sizes. Food Chemistry. 2021;336:127711. doi: 10.1016/j.foodchem.2020.127711. [DOI] [PubMed] [Google Scholar]
- Selamassakul O., Laohakunjit N., Kerdchoechuen O., Yang L., Maier C.S. Bioactive peptides from brown rice protein hydrolyzed by bromelain: Relationship between biofunctional activities and flavor characteristics. Journal of Food Science. 2020;85(3):707–717. doi: 10.1111/1750-3841.15052. [DOI] [PubMed] [Google Scholar]
- Shang Y.-F., Cao H., Wei C.-K., Thakur K., Liao A.-M., Huang J.-H. Effect of sugar types on structural and flavor properties of peony seed derived Maillard reaction products. Journal of Food Processing and Preservation. 2020;44(3) doi: 10.1111/jfpp.v44.310.1111/jfpp.14341. [DOI] [Google Scholar]
- Shen Y.i., Hu L.-T., Xia B., Ni Z.-J., Elam E., Thakur K. Effects of different sulfur-containing substances on the structural and flavor properties of defatted sesame seed meal derived Maillard reaction products. Food chemistry. 2021;365:130463. doi: 10.1016/j.foodchem.2021.130463. [DOI] [PubMed] [Google Scholar]
- Taladrid D., Laguna L., Bartolomé B., Moreno-Arribas M.V. Plant-derived seasonings as sodium salt replacers in food. Trends in Food Science & Technology. 2020;99:194–202. [Google Scholar]
- U‐Chupaj J., Malila Y., Gozzi G., Vannini L., Dellarosa N., Laghi L. Influence of non-phosphate and low-sodium salt marination in combination with tumbling process on properties of chicken breast meat affected by white striping abnormality. Journal of Food Science. 2021;86(2):319–326. doi: 10.1111/1750-3841.15565. [DOI] [PubMed] [Google Scholar]
- Vargas-Ramella M., Lorenzo J.M., Domínguez R., Pateiro M., Munekata P.E.S., Campagnol P.C.B. Effect of NaCl Partial Replacement by Chloride Salts on Physicochemical Characteristics, Volatile Compounds and Sensorial Properties of Dry-Cured Deer Cecina. Foods. 2021;10(3):669. doi: 10.3390/foods10030669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal V.A.S., Paglarini C.S., Freitas M.Q., Coimbra L.O., Esmerino E.A., Pollonio M.A.R. Q Methodology: An interesting strategy for concept profile and sensory description of low sodium salted meat. Meat Science. 2020;161:108000. doi: 10.1016/j.meatsci.2019.108000. [DOI] [PubMed] [Google Scholar]
- Wei C.-K., Ni Z.-J., Thakur K., Liao A.-M., Huang J.-H., Wei Z.-J. Color and flavor of flaxseed protein hydrolysatesMaillard reaction products: Effect of cysteine, initial pH, and thermal treatment. International Journal of Food Properties. 2019;22(1):84–99. [Google Scholar]
- Wei C.K., Ni Z.J., Thakur K., Liao A.M., Huang J.H., Wei Z.J. Aromatic effects of immobilized enzymatic oxidation of chicken fat on flaxseed (Linum usitatissimum L.) derived Maillard reaction products. Food Chemistry. 2020;306 doi: 10.1016/j.foodchem.2019.125560. [DOI] [PubMed] [Google Scholar]
- Wei C.-K., Thakur K., Liu D.-H., Zhang J.-G., Wei Z.-J. Enzymatic hydrolysis of flaxseed (Linum usitatissimum L.) protein and sensory characterization of Maillard reaction products. Food Chemistry. 2018;263:186–193. doi: 10.1016/j.foodchem.2018.04.120. [DOI] [PubMed] [Google Scholar]
- Wu H., Yan W., Zhuang H., Huang M., Zhao J., Zhang J. Oxidative stability and antioxidant enzyme activities of dry-cured bacons as affected by the partial substitution of NaCl with KCl. Food Chemistry. 2016;201:237–242. doi: 10.1016/j.foodchem.2016.01.025. [DOI] [PubMed] [Google Scholar]
- Yang G.H., Zhou X., Ji W.J., Liu J.X., Sun J., Shi R., Jiang T.M., Li Y.M. Effects of a low salt diet on isolated systolic hypertension: A community-based population study. Medicine. 2018;97 doi: 10.1097/MD.0000000000010342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., He S., Tang M., Zhang Z., Zhu Y., Sun H. Antioxidant activity and sensory characteristics of Maillard reaction products derived from different peptide fractions of soybean meal hydrolysate. Food Chemistry. 2018;243:249–257. doi: 10.1016/j.foodchem.2017.09.139. [DOI] [PubMed] [Google Scholar]
- Zhang D., Li H.J., Emara A.M., Wang Z.F., Chen X.S., He Z.F. Study on the mechanism of KCl replacement of NaCl on the water retention of salted pork. Food Chemistry. 2020;332 doi: 10.1016/j.foodchem.2020.127414. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Wang S., Li D., Zhou Y. Effect of xanthan gum on the quality of low sodium salted beef and property of myofibril proteins. Food Science and Human Wellness. 2021;10(1):112–118. [Google Scholar]