Abstract
Mammalian ovaries contain a large number of immature follicles. Follicular culture can contribute to the production of fertile oocytes from latent immature follicles, providing a useful tool for exploring the developmental competencies and related factors that oocytes acquire during growth. However, the potential of oocytes produced by follicular culture is limited. Herein, the optimal follicular culture conditions for the addition of polyvinylpyrrolidone to the medium and oxygen concentration were investigated. Polyvinylpyrrolidone with a high molecular weight (≥ 360,000) and a 7% oxygen concentration were found to increase the blastocyst formation rate by more than 20% compared with conventional culture conditions. Although the developmental ability of oocytes produced by follicular culture remained inferior to that of in vivo-derived oocytes, these findings may pave the way for enhanced production of fertile oocytes in vitro and for studying the process of full developmental potency acquisition by oocytes.
Keywords: Culture, Developmental competence, Oocyte, Oocyte growth
The first successful in vitro production of mammalian fertile oocytes collected from ovarian follicles was reported in mice by Cross and Brinster in 1970 [1]. Since then, in vitro maturation (IVM), in vitro fertilization (IVF), and culture of fertilized eggs have been used to understand the mechanisms of meiotic maturation and early development, as well as for assisted reproductive technology in various animals. Several studies have reported that oocytes with full developmental potency can be produced from mouse fetal primordial germ cells, non-growing oocytes of newborn mice, and growing oocytes of juvenile mice via ovarian and/or follicle culture (OC/FC) [2,3,4,5,6]. However, the developmental ability of the resultant oocytes is poor; therefore, the protocol needs to be revised. Previous reports have demonstrated that the FC medium supplemented with the high-molecular-weight (MW) compound polyvinylpyrrolidone (PVP; MW = 360,000 [360K]) at a concentration of 2% and 4% efficiently produced blastocysts after FC, IVM, and IVF in mice [7], cows [8], and pigs [9], respectively, although no study has evaluated the optimal MW of PVP for murine FC. Furthermore, a 5% oxygen concentration was reported as the optimal condition, compared with 10%, 15%, and 20% oxygen, for producing mouse oocytes with high developmental ability by FC [10]. In contrast, bovine follicle growth was restricted by a 5% oxygen culture condition, whereas FC under 5% oxygen for the first 4 days, followed by FC under 20% oxygen for an additional 10 days improved oocyte growth and developmental ability to the blastocyst stage compared with oocytes produced by FC for 14 days under a constant 20% oxygen condition [11]. However, it remains unclear whether bovine FC conditions are optimal for murine FCs. In addition, the fact that two gas conditions must be prepared for FC may not be widely applicable in all laboratories. Therefore, to identify a simple and efficient FC system that can serve as a general tool for studying oogenesis, in this study, we explored the optimal conditions for FC by evaluating the effects of PVP MW and oxygen concentration on oocyte competency.
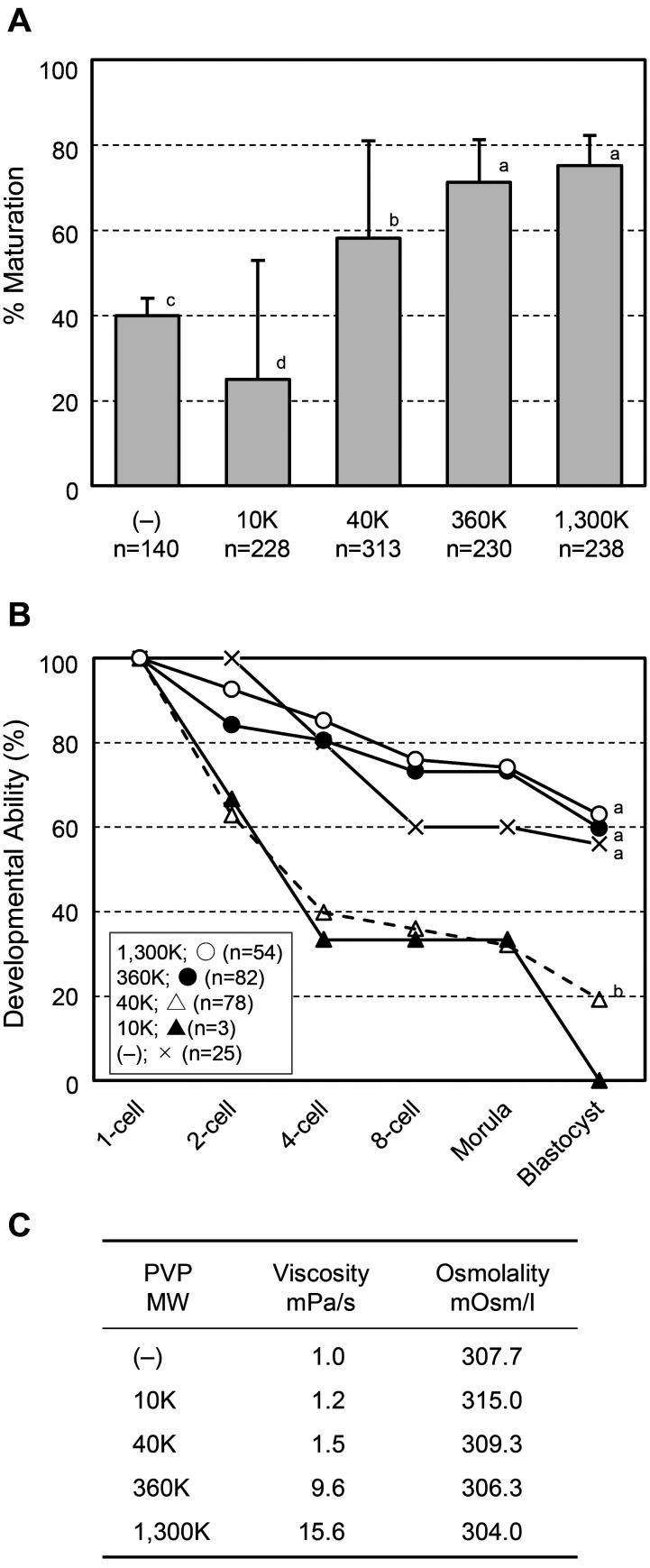
First, we compared the developmental ability of oocytes produced by FC using media containing PVP with an MW of 10K, 40K, 360K, or 1,300K at a concentration of 2%. A PVP-free medium was used as a negative control. Secondary follicles isolated from 10-day-old mice were treated with 0.1% collagenase for 20 min and then subjected to FC in an atmosphere of 5% CO2 and 95% air. On day 12 of FC, cumulus-oocyte complexes (COCs) were collected from the resultant follicles. The COCs obtained from each experimental group were subjected to IVM for 17 h. In the PVP 10K group, only 25.0% (57/228) of the oocytes matured into metaphase during the second meiosis (MII) stage, which was significantly lower than the number of oocytes obtained in the other groups (Fig. 1A, P < 0.01). Oocytes produced in the medium containing PVP 40K matured to the MII stage at a higher frequency (58.1%, 182/313) than those produced in the PVP-free medium (Fig. 1A; 40.0%, 56/140, P < 0.01). Notably, PVP with MW ≥ 360K yielded the largest number of MII oocytes from secondary follicles among all experimental groups (Fig. 1A; 360K, 71.3%, 164/230; 1,300K, 75.2%, 179/238, P < 0.01). Following IVF, the developmental ability of the oocytes produced by FC was also assessed. Approximately 60% of the normally fertilized oocytes developed into the blastocyst stage in the PVP 1,300K (63.0%, 34/54), PVP 360K (59.8%, 49/82), and PVP-free (56.0%, 14/25) groups (Fig. 1B). In contrast, the ability to develop into blastocysts was significantly reduced in the PVP 40K (15/78, 19.2%, P < 0.01) group compared with that in the PVP 1,300K, PVP 360K, and PVP-free groups (Fig. 1B). In this series of experiments, no blastocyst was observed in the PVP 10K group owing to the small number of samples (0/3, Fig. 1B). These findings indicate that PVP with an MW lower than 40K has detrimental effects on COC development and subsequent oocyte competency, although the reason remains unclear. Next, the viscosity and osmolality of the media were measured. The viscosity of the medium supplemented with PVP increased depending on the MW of the compound, especially when PVP 360K and PVP 1,300K were added (Fig. 1C). In contrast, the addition of PVP had little effect on the osmotic pressure of the medium (Fig. 1C). Thus, a highly viscous medium containing PVP 360K or PVP 1,300K was determined to be the most optimal condition for FC in this experiment.
Fig. 1.
Meiotic and developmental competence of oocytes produced by follicular culture (FC) with medium containing polyvinylpyrrolidone (PVP) compounds of various molecular weights (MW). (A) Percentage of oocytes that reached the second meiosis stage after FC and in vitro maturation (IVM). Error bars indicate the standard deviation. Statistical significance was analyzed by Tukey’s test. Different letters represent a significant difference (P < 0.01). (B) Percentage of normally fertilized oocytes that developed into each stage after FC, IVM, and in vitro fertilization. Statistical significance was analyzed by Tukey’s test. Different letters represent a significant difference (P < 0.01). (C) Viscosity and osmolality of the medium containing various PVP compounds.
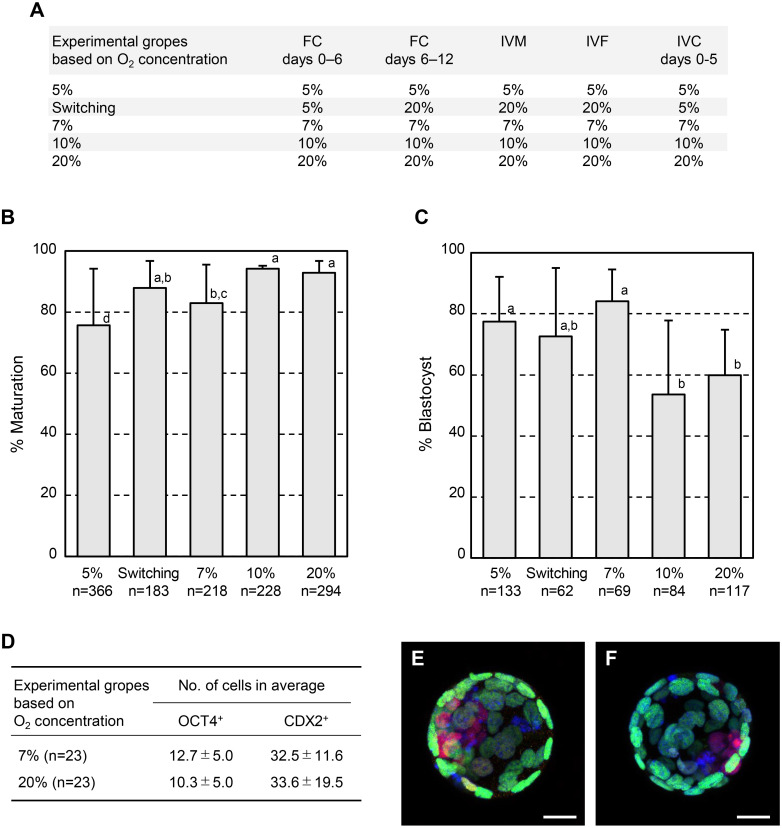
In the next series of experiments, we investigated the optimal O2 concentration for mouse FC. The preliminary study after the first series of experiments showed that prolonged collagenase treatment effectively recovered COCs and produced MII-stage oocytes. Insufficient collagenase treatment resulted in theca cell layers remaining in some follicles, leading to the inability to aspirate COCs with glass capillaries. Therefore, secondary follicles isolated from 10-day-old mice were treated with 0.1% collagenase for 28 min. These were then cultured in a medium containing 2% PVP 360K under 5%, 7%, 10%, and 20% O2 for 12 days. Additionally, some follicles were cultured under 5% O2 for 6 days and subsequently cultured under 20% O2 for another 6 days (Fig. 2A, see Methods section), which were referred to as the switching group. At the end of the FC, the obtained COCs were subjected to IVM. Although more than 70% of the oocytes produced under a 5% O2 concentration matured into the MII stage (75.7%, 277/366), this outcome was significantly worse than that of the 10% O2 (94.3%, 215/228, P < 0.01), 20% O2 (92.9%, 273/294, P < 0.01), 7% O2 (83.0%, 181/218, P < 0.05), and switching (88.0%, 161/183, P < 0.01, Fig. 2B) groups. The maturation rate in the switching group was comparable to that in the 10% and 20% O2 groups. Oocytes produced under the 7% O2 condition also matured into the MII stage as well as the switching group, although the rate was significantly lower than that in the 10% and 20% O2 groups (P < 0.01, Fig. 2B). In contrast to meiotic competency, oocytes that were able to reach the MII stage at a low O2 concentration showed higher potency to develop into the blastocyst stage. The ability of the resultant oocytes to develop into blastocysts was higher in the 5% (77.4%, 103/133) and 7% O2 (84.1%, 58/69) groups than in the 10% (53.6%, 45/84, P < 0.01) and 20% O2 (59.8%, 70/117, P < 0.01, Fig. 2C) groups. No significant difference in the developmental ability of embryos produced under switching conditions (72.6%, 45/62) was observed compared to the other groups (Fig. 2C). Combining the maturation and development rates, 7% O2 was the most efficient setting for producing blastocysts from secondary follicles (69.8%), followed by the switching group (63.9%). Under 7% O2, poor meiotic competency was counteracted by a higher potency to develop into the blastocyst stage. Finally, to compare the quality of blastocysts obtained from the 7% and 20% O2 conditions, the number of cells that consisted of blastocysts was analyzed. No significant differences in the mean numbers of OCT4+ inner cell mass cells (7%, 12.7 ± 5.0; 20%, 10.3 ± 5.0), CDX2+ trophectodermal cells (7%, 32.5 ± 11.6; 20%, 33.6 ± 19.5), and OCT4+CDX2+ cells (7%, 0.9 ± 1.2; 20%, 0.4 ± 0.7) were observed between the 7% and 20% O2 groups on day 4.5 (Fig. 2D–2F). Thus, the quality of embryos that were able to develop into the blastocyst stage was almost the same.
Fig. 2.
Meiotic and developmental competence of oocytes produced by follicular culture (FC) under various oxygen concentrations. (A) Conditions of FC, in vitro maturation (IVM), in vitro fertilization (IVF), and in vitro culture (IVC) of embryos tested in this study. (B) Percentage of oocytes that reached the second meiosis stage after FC and IVM. Error bars indicate the standard deviation. Statistical significance was analyzed by Tukey’s test. Different letters represent a significant difference (a vs. bc, a vs. d, ab vs. d, P < 0.01; bc vs. d, P < 0.05). (C) Percentage of normally fertilized oocytes that developed into each stage after FC, IVM, and IVF. Error bars indicate the standard deviation. Statistical significance was analyzed by Tukey’s test. Different letters represent a significant difference (P < 0.01). (D) Numbers of OCT4+ and CDX2+ cells in the resultant blastocysts. (E, F) Immunostaining images of blastocysts produced under (E) 7% and (F) 20% O2 conditions using anti-OCT4 (green) and anti-CDX2 (magenta) antibodies. Nuclei (blue) were counterstained with DAPI. Bars indicate 20 µm.
The in vitro environment is assumed to induce mechanical stresses that differ from those in the in vivo environment. Some studies have explored the optimal culture conditions for premature ovarian follicles. Polyacrylamide gel and polysaccharides such as xanthan and locust gum are reportedly available as substrates for porcine FC [12, 13]. An intriguing point is that polymeric compounds commonly assist follicular development. Three-dimensional culture systems using alginate or extracellular matrix-derived soft hydrogels have also been shown to be effective for follicle development [14, 15], which may be owing to their capacity to relieve mechanical stresses, such as shear stress, tensile stress, impact, and vibration during FC. As no difference was observed in the developmental competency of oocytes produced by media containing PVP 360K and PVP 1,300K, we hypothesized that PVP 360K may reach a maximal viscosity potential to relieve mechanical stress (Fig. 1). In addition, the viscosity of the medium may enhance the support of granulosa cells to acquire the meiotic and developmental competence of oocytes during FC by not dispersing the factors secreted from the follicles. In contrast, the atmospheric O2 concentration (20% O2), which is widely adopted for culture conditions, can also be a physiological stress because O2 concentrations in female reproductive organs and follicular fluid are within the range of 1.4%–10.5% [16,17,18,19]. Cell proliferation results in O2 consumption. The data presented are in line with a previous report showing that bovine oocytes cultured under 5% O2 conditions were smaller than those cultured under 20% O2 conditions [11]. The meiotic competency of the oocytes produced under a low O2 concentration was lower than that of oocytes produced under atmospheric O2 concentration (Fig. 2), presumably because of a delay in the proliferation of granulosa cells and oocyte growth. During ATP production in mitochondria, reactive oxygen species (ROS) are generated by incomplete oxygen reduction [20]. Therefore, FC under a low O2 concentration mimics the in vivo environment but might represent a trade-off between granulosa cell growth and ROS accumulation in vitro. Under switching conditions, FC is believed to incorporate the advantages of both cell growth and low ROS accumulation. However, in this study, FC under the 7% O2 condition was shown to elicit oocyte competency similar to that observed under the switching condition (Fig. 2). This finding may be useful for simple culture conditions that do not require two different incubators.
In conclusion, addition of PVP 360–1,300K to the medium and culture under a constant 7% O2 condition is a better, simpler, and more effective strategy for producing oocytes from mouse secondary follicles than the conventional procedures.
Methods
Animals
B6D2F1 (C57BL/6N × DBA/2) mice (CLEA Japan Inc., Tokyo, Japan) were used for the FC and IVF experiments. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Tokyo University of Agriculture (approval numbers: 2020048 and 2019063).
Growth, maturation, and development of oocytes
All culture experiments for oocyte and embryo production were conducted as previously described [21]. For FC, secondary follicles from 10-day-old mice were isolated and then treated with 0.1% (w/v) collagenase type I (295 U/mg, #LS004196, Worthington Biochemicals, Lakewood, NJ, USA) in L15 medium (#L5520, Sigma-Aldrich, St. Louis, MO, USA) for 20 min (assessment of PVP MW) or 28 min (assessment of O2 concentration) at 37°C. The secondary follicles were cultured on a Transwell-COL insert membrane (#3492, Corning, Corning, NY, USA) with alpha-minimum essential medium (MEM) (#32571-036, Gibco, Waltham, MA, USA) supplemented with 2% (w/v) PVP (Sigma-Aldrich), 5% (v/v) fetal bovine serum (FBS; #F7524, Sigma-Aldrich), and 0.1 IU/ml Follistim (#2413405A1023, recombinant follicle stimulating hormone; MSD, Kenilworth, NJ, USA) for 12 days at 37°C. In the first experiment, the optimal MW of PVP for the FC medium was examined using 10K (#PVP10), 40K (#PVP40), 360K (#PVP360), and 1,300K (#437190) compounds. The FC was performed under 5% CO2 and 95% air (20% O2) at 37°C. In the second experiment, the optimal O2 concentrations were determined to be 5%, 7%, 10%, and 20%. A lower O2 concentration than that in the atmosphere was modulated by the N2 supply. In every experimental group, conditions of 5% CO2 and 37°C were maintained. The switching of O2 was performed as previously described, with minor modifications (Fig. 2) [11]. Briefly, FC from days 0 to 6 was conducted under 5% O2 conditions. Thereafter, upon FC until day 12, IVM and IVF were switched from 5% to 20% O2. The fertilized eggs were cultured again under 5% O2.
For IVM, collected COCs were cultured in alpha-MEM (#11900-024, Gibco) supplemented with 5% (v/v) FBS, 0.1 IU/ml Follistim, 1.2 IU/ml human chorionic gonadotropin (2413402X3084, ASKA Pharmaceutical, Tokyo, Japan), and 4 ng/mL epidermal growth factor (#53003-018, Gibco) for 17 h. Meiotic competence was evaluated by the first polar body extrusion.
Sperm samples were collected from the epididymides of the adult males. The IVF was performed in TYH medium (#DR01031, LSI Medience, Tokyo, Japan). Normally fertilized oocytes with two pronuclei were cultured in KSOM+AA medium (#MR-121-D, Millipore, Burlington, MA, USA) for 5 days.
Immunofluorescent staining
Blastocysts at 4.5 days after IVF were fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature. Blastocysts were permeabilized in blocking solution containing 10% (v/v) FBS (Sigma-Aldrich), 3% (w/v) bovine serum albumin (#A9647, Sigma-Aldrich), and 0.2% (v/v) Triton X-100 (Nacalai Tesque, Kyoto, Japan) in PBS overnight at 4°C. Blastocysts were incubated with the primary antibody (rabbit polyclonal anti-OCT4 antibody, 1:100 dilution, #2840, Cell Signaling Technology, Danvers, MA, USA; mouse monoclonal anti-CDX2 antibody, 1:100 dilution, #MU392A-UC, BioGenex, Fremont, CA, USA) overnight at 4°C. After washing, the blastocysts were incubated with the secondary antibody (Alexa Fluor 594-conjugated donkey anti-rabbit IgG antibody, 1:500 dilution, #A-21207, Thermo Fisher Scientific, Waltham, MA, USA; Alexa Fluor 488-conjugated goat anti-mouse IgG antibody, 1:500 dilution, #A-11001, Thermo Fisher Scientific) for 1 h at room temperature. After washing, the blastocysts were mounted on glass slides using DAPI-containing mounting medium (Vector Laboratories, Burlingame, CA, USA) and coverslips. Fluorescent Z-stack images were acquired at 2-µm thickness using a Nikon A1 confocal microscope (Nikon, Tokyo, Japan).
Measurement of viscosity and osmolality
The viscosity and osmolality were measured at 20°C. Viscosity was measured using a Ubbelohde viscometer according to the manufacturer’s protocol (Shibata Scientific Technology, Soka, Japan) (Supplementary Material). The osmolality of each medium was measured using an osmometer (Vogel, Bamberg, Germany) according to the manufacturer’s protocol. Viscosity and osmolality were measured at least three times for each medium.
Statistical analysis
Data from three or more independent culture experiments were analyzed statistically. Data are presented as the mean ± standard deviation (SD) for each group. Tukey’s test was used for multiple comparisons among experimental groups. The Student’s t-test was used for comparisons of cell numbers between the two experimental groups. Differences were considered statistically significant at P < 0.05.
Conflict of interests
The authors declare that there are no conflicts of interests.
Supplementary
Acknowledgments
We thank Dr. Yuji Hirao (Institute of Livestock and Grassland Science, NARO, Japan), Prof. Seiichi Taguchi, and Dr. Ayaka Hiroe (Department of Chemistry for Life Science and Agriculture, Tokyo University of Agriculture, Japan) for their helpful comments. We are also grateful to the members of the Animal Life Science Research Center at the Tokyo University of Agriculture for their contributions to animal care. This work was supported in part by KAKENHI (18H05547 to YO).
References
- 1.Cross PC, Brinster RL. In vitro development of mouse oocytes. Biol Reprod 1970; 3: 298–307. [DOI] [PubMed] [Google Scholar]
- 2.Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod 1996; 54: 197–207. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien MJ, Pendola JK, Eppig JJ. A revised protocol for in vitro development of mouse oocytes from primordial follicles dramatically improves their developmental competence. Biol Reprod 2003; 68: 1682–1686. [DOI] [PubMed] [Google Scholar]
- 4.Mochida N, Akatani-Hasegawa A, Saka K, Ogino M, Hosoda Y, Wada R, Sawai H, Shibahara H. Live births from isolated primary/early secondary follicles following a multistep culture without organ culture in mice. Reproduction 2013; 146: 37–47. [DOI] [PubMed] [Google Scholar]
- 5.Morohaku K, Hirao Y, Obata Y. Developmental competence of oocytes grown in vitro: Has it peaked already? J Reprod Dev 2016; 62: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morohaku K, Tanimoto R, Sasaki K, Kawahara-Miki R, Kono T, Hayashi K, Hirao Y, Obata Y. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc Natl Acad Sci USA 2016; 113: 9021–9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizumachi S, Aritomi T, Sasaki K, Matsubara K, Hirao Y. Macromolecular crowded conditions strengthen contacts between mouse oocytes and companion granulosa cells during in vitro growth. J Reprod Dev 2018; 64: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirao Y, Itoh T, Shimizu M, Iga K, Aoyagi K, Kobayashi M, Kacchi M, Hoshi H, Takenouchi N. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol Reprod 2004; 70: 83–91. [DOI] [PubMed] [Google Scholar]
- 9.Tasaki H, Iwata H, Sato D, Monji Y, Kuwayama T. Estradiol has a major role in antrum formation of porcine preantral follicles cultured in vitro. Theriogenology 2013; 79: 809–814. [DOI] [PubMed] [Google Scholar]
- 10.Eppig JJ, Wigglesworth K. Factors affecting the developmental competence of mouse oocytes grown in vitro: oxygen concentration. Mol Reprod Dev 1995; 42: 447–456. [DOI] [PubMed] [Google Scholar]
- 11.Hirao Y, Shimizu M, Iga K, Takenouchi N. Optimization of oxygen concentration for growing bovine oocytes in vitro: constant low and high oxygen concentrations compromise the yield of fully grown oocytes. J Reprod Dev 2012; 58: 204–211. [DOI] [PubMed] [Google Scholar]
- 12.Munakata Y, Kawahara-Miki R, Shirasuna K, Kuwayama T, Iwata H. Polyacrylamide gel as a culture substrate improves in vitro oocyte growth from porcine early antral follicles. Mol Reprod Dev 2017; 84: 44–54. [DOI] [PubMed] [Google Scholar]
- 13.Munakata Y, Sugimoto A, Shirasuna K, Kuwayama T, Iwata H. Xanthan gum and Locust bean gum gel supports in vitro development of porcine oocytes derived from early antral follicles. J Reprod Dev 2019; 65: 551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreeger PK, Deck JW, Woodruff TK, Shea LD. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 2006; 27: 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim EJ, Yang C, Lee J, Youm HW, Lee JR, Suh CS, Kim SH. The new biocompatible material for mouse ovarian follicle development in three-dimensional in vitro culture systems. Theriogenology 2020; 144: 33–40. [DOI] [PubMed] [Google Scholar]
- 16.Fischer B, Künzel W, Kleinstein J, Gips H. Oxygen tension in follicular fluid falls with follicle maturation. Eur J Obstet Gynecol Reprod Biol 1992; 43: 39–43. [DOI] [PubMed] [Google Scholar]
- 17.Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. J Reprod Fertil 1993; 99: 673–679. [DOI] [PubMed] [Google Scholar]
- 18.de Castro E Paula LA, Andrzejewski J, Julian D, Spicer LJ, Hansen PJ. Oxygen and steroid concentrations in preovulatory follicles of lactating dairy cows exposed to acute heat stress. Theriogenology 2008; 69: 805–813. [DOI] [PubMed] [Google Scholar]
- 19.Redding GP, Bronlund JE, Hart AL. Theoretical investigation into the dissolved oxygen levels in follicular fluid of the developing human follicle using mathematical modelling. Reprod Fertil Dev 2008; 20: 408–417. [DOI] [PubMed] [Google Scholar]
- 20.McKee T, McKee JR. Biochemistry: The Molecular Basis of Life. 6th International Edition, Oxford: Oxford University Press, 2015: pp. 770. [Google Scholar]
- 21.Morohaku K, Hirao Y, Obata Y. Development of fertile mouse oocytes from mitotic germ cells in vitro. Nat Protoc 2017; 12: 1817–1829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.