Abstract
In vitro maturation (IVM) is an important reproductive technology used to produce embryos in vitro. However, the developmental potential of oocytes sourced for IVM is markedly lower than those matured in vivo. Previously, NAD+-elevating treatments have improved oocyte quality and embryo development in cattle and mice, suggesting that NAD+ is important during oocyte maturation. The aim of this study was to examine the effects of nicotinic acid (NA), nicotinamide (NAM) and nicotinamide mononucleotide (NMN) on oocyte maturation and subsequent embryo development. Porcine oocytes from small antral follicles were matured for 44 h in a defined maturation medium supplemented with NA, NAM and resveratrol or NMN. Mature oocytes were artificially activated and presumptive zygotes cultured for 7 days. Additionally, oocytes were matured without treatment then cultured for 7 days with NMN. Supplementing the IVM medium with NA improved maturation and blastocyst formation while NAM supplementation improved cleavage rates compared with untreated controls. Supplementing the IVM or embryo culture media with NMN had no effect on maturation or embryo development. The results show that supplementing the maturation medium with NA and NAM improved maturation and developmental potential of porcine oocytes.
Keywords: Embryo development, In vitro maturation, Niacin, Oocyte quality, Pig
In vitro maturation (IVM) of oocytes has become a routine procedure for the in vitro production of embryos in livestock species [1] and is gaining increasing attention as a fertility treatment in humans [2]. However, the quality of embryos derived from IVM oocytes is relatively low in comparison to their in vivo-derived counterparts, with only a limited proportion of IVM oocytes attaining full developmental potential [3]. While recent improvements to IVM and oocyte culture systems have dramatically improved in vitro embryo production rates, they are still considered inadequate compared with the follicular environment of in vivo-matured oocytes [2, 4].
Oocyte quality and quantity is the rate limiting factor in the production of embryos in vitro [5]. Unlike most cells that undergo constant regeneration from precursor stem cells, the number of oocytes contained within the ovaries is finitely set during prenatal development [6, 7] and determines the reproductive lifespan of the female. Within the ovaries of cycling sows and pubertal gilts, gonadotropin-responsive antral follicles are recruited to undergo development during the follicular phase, with the follicles either undergoing atresia or being selected to continue development until eventual ovulation [8]. Many of the small-sized follicles fail to escape atresia. In pre-pubertal gilts, approximately half of the antral follicles apparent on the surface of the ovaries are less than 3 mm in diameter [9]. There is a lack of coordination between nuclear and cytoplasmic maturation during IVM of porcine oocytes harvested from small antral follicles (< 3 mm in diameter) [10], leading to reduced developmental potential compared with oocytes from larger antral follicles [11, 12]. As a result, porcine in vitro embryo production programs commonly utilise oocytes harvested from medium to large antral follicles (3–6 mm in diameter). The effective use of oocytes from the pool of small antral follicles carries great potential to increase the productivity of embryo production programs and enhance the reproductive capacity of genetically valuable females in all species. Therefore, it is imperative to gain a greater understanding of the mechanisms involved in oocyte maturation.
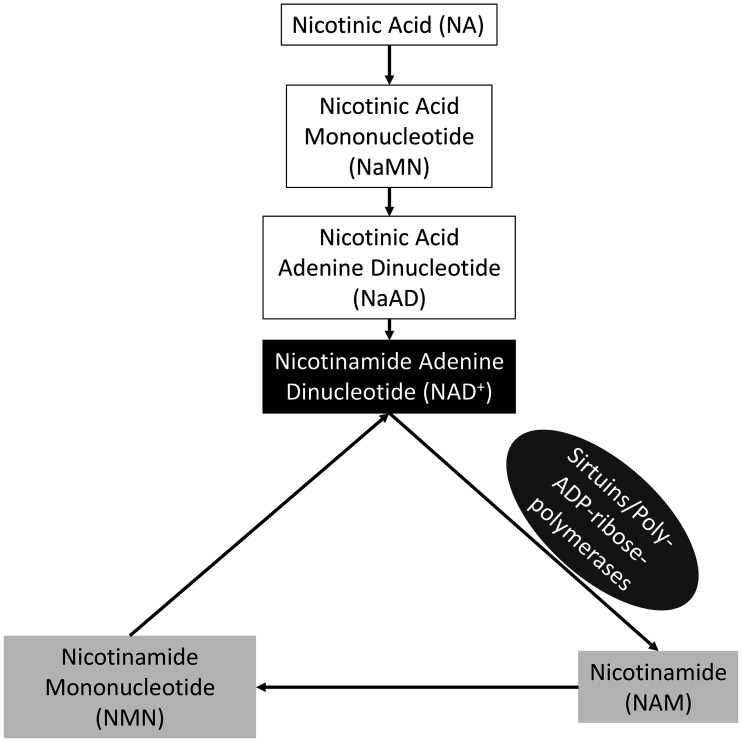
Recent studies have shown that nicotinamide adenine dinucleotide production is essential for the acquisition of oocyte developmental competence. Nicotinamide adenine dinucleotide (NAD+) is an essential cofactor implicated in many cellular processes and can be synthesized through one of three pathways in mammalian cells; catalytic conversion of the amino acid tryptophan (Trp) via the de novo synthesis pathway, recycling of NAD+ metabolites via the salvage pathway, and metabolism of niacin via the Preiss-Handler pathway [13,14,15] (Fig. 1). In humans and mice, NAD+ deficiency brought about by aberrant expression of de novo synthesis pathway enzymes leads to birth defects [16]. In addition, supplementation of NAD+ precursors, including nicotinamide mononucleotide (NMN) and nicotinic acid (NA), both in vitro and in vivo improved mouse oocyte quality and embryo development [5, 17, 18]. Furthermore, Sirtuins (SIRTs) and poly-ADP-ribose polymerases (PARPs) consume NAD+, and are known to play essential roles during oocyte maturation [19, 20]. The aim of this study was to determine the contribution of the different NAD+ synthesis pathways to the nuclear and cytoplasmic maturation of porcine oocytes from small antral follicles. Here we demonstrate that the addition of specific concentrations of NAD+ precursors to the maturation media of porcine oocytes improved aspects of their developmental potential.
Fig. 1.
NAD+ biosynthetic pathways. Two of the three pathways through which NAD+ can be synthesised in cells include the Preiss-Handler pathway and more commonly through the salvage of other metabolites. Nicotinic acid is consumed in feed and is the initial metabolite for the Preiss-Handler pathway. NAD+ is consumed by Sirtuins and Poly-ADP-ribose-polymerases, the first reaction in the salvage pathway. The metabolites in white boxes represent metabolites in the Preiss-Handler pathway while the metabolites in the light grey boxes represent those from the salvage pathway. NAD+ in the black box represents the end point for both NAD+ biosynthetic pathways.
Materials and Methods
Chemicals and media preparation
All chemicals and reagents were purchased from Sigma-Aldrich (Australia) unless otherwise specified. The medium used to wash the cumulus oocyte complexes (COCs) was HEPES-buffered 199 (H199) medium (Gibco, Grand Island, NY, USA) supplemented with 1 mg ml–1 PVA, 1 mM GlutaMAX (Gibco), 75 µg ml–1 penicillin G and 50 µg ml–1 streptomycin sulphate. The IVM medium used was porcine oocyte medium (POM) [21], supplemented with 10 ng ml–1 epidermal growth factor (EGF), 10 IU ml–1 equine chorionic gonadotrophin (eCG; Pregnecol; Bioniche Animal Health, Armidale, Australia) and 10 IU ml–1 human chorionic gonadotrophin (hCG; Chorulon; Intervet Australia, Bendigo East, Australia) as described by our group previously [22]. The POM used for the first 22 h of IVM also contained 1 mM dibutyryl cyclic adenosine-monophosphate (db-cAMP). Tyrode’s albumin lactate pyruvate-polyvinyl alcohol (TALP-PVA) medium [23] was used for the parthenogenetic activation of mature oocytes. Porcine zygote medium containing 3 mg ml–1 BSA (PZM-3) [24] was used for the in vitro culture of embryos. The metabolites NA (N4126), nicotinamide (NAM; 72340), NMN and Resveratrol (Res; R5010) were supplemented in the maturation media and used as NAD+ pathway and Sirtuin modulators.
Ovary collection and in-vitro maturation
Ovaries were collected from prepubertal gilts at a local abattoir and transported in 38°C saline solution supplemented with antibiotic-antimycotic (Gibco). Ovaries were transferred to fresh 38°C saline solution supplemented with antibiotic-antimycotic upon arrival at the laboratory. The contents of small antral follicles (1–3 mm diameter) were aspirated into warm vacutainer tubes via a 21 G needle using a vacuum pump at a flow rate of 1 L min–1. Cumulus-oocyte complexes (COCs) were recovered from the collected material using a stereomicroscope and oocytes with an evenly distributed cytoplasm and a minimum of 3 layers of cumulus cells were selected for in vitro maturation (IVM). The COCs were washed twice in H199 media, followed by a final wash in POM supplemented with db-cAMP and incubated in a four well NUNC dish (Nunc A/S, Roskilde, Denmark) with POM media supplemented with db-cAMP for 22 h in their respective treatments (40–50 COCs/500 µl well). Incubation was carried out at 38.5°C in a humidified atmosphere of 6% CO2 in air. Following 22 h of maturation, the oocytes were transferred to fresh POM media in a separate four well NUNC dish in their respective treatments without db-cAMP for a further 22 h. Following maturation, oocytes were stripped of cumulus cells by brief exposure to 1 mg ml–1 hyaluronidase and gentle aspiration with a narrow bore glass pipette.
Parthenogenetic activation and embryo culture
Following cumulus cell removal, mature oocytes, indicated by the presence of a polar body, were held in 100 µl droplets of TALP-PVA media for 30 min, then activated with the addition of 5 µM ionomycin for 5 min. Oocytes were then washed three times and incubated for 3 h in PZM-3 supplemented with 2 mM 6-dimethylaminopurine and 7.5 µg ml–1 cytocholasin B. Presumptive zygotes were washed three times with PZM-3 and then cultured in 50 µl droplets of PZM-3 under mixed gas conditions (6% CO2, 5% O2, 89% N2) for 7 days. Embryo culture droplets were supplemented with 10% fetal calf serum on day 4 following activation.
Fluorescent staining of embryos and total cell counts
On day 7, all blastocysts were fixed in absolute ethanol supplemented with 10 µg ml–1 Hoechst 33342. All fixed embryos were mounted on microscope slides and viewed under a fluorescence microscope (Olympus BX61; Olympus Corporation, Tokyo, Japan; 405–450 nm). Total cell counts were determined for each embryo. Embryos that formed an obvious blastocoele and had at least 12 cells were classified as blastocysts.
Experimental design
Oocytes were exposed to treatments for the entire duration of maturation, and each experiment was replicated at least three times on separate days. Stock solutions of NMN (10 mM), NA (20 mM), NAM (50 mM) and Res (2 µM) were generated by dissolving the metabolite in water (NMN, NA and NAM) or ethanol (Res) and 20 µl aliquots were stored at –20°C until use. For each treatment group, the rates of maturation, cleavage, blastocyst formation and blastocyst hatching were calculated as the proportions of oocytes with a discernible polar body from total oocytes, oocytes that cleaved from total matured oocytes, embryos with an obvious blastocoele from total cleaved embryos, and blastocysts that breached the zona pellucida from total blastocysts, respectively.
Experiment 1: Effect of nicotinic acid dose on oocyte maturation
a) Cumulus-oocyte complexes were randomly allocated to the control (n = 120), 5 µM NA (n = 120), 10 µM NA (n = 120) or 20 µM NA (n = 120) treatment groups. Oocytes were matured in their respective treatments for the entire duration of IVM before artificial activation and embryo culture. Resulting blastocysts were fixed and stained, and total cell numbers were determined.
b) Cumulus-oocyte complexes were randomly allocated to the control (n = 145), 20 µM NA (n = 149), 50 µM NA (n = 144), 100 µM NA (n = 135) or 200 µM NA (n = 139) treatment groups. Oocytes were matured in their respective treatments for the entire duration of IVM before artificial activation and embryo culture. Resulting blastocysts were fixed and stained, and total cell numbers were determined.
Experiment 2: Effect of nicotinamide mononucleotide dose on oocyte maturation and embryo culture
a) Cumulus-oocyte complexes were randomly allocated to the control (n = 190), 0.1 µM NMN (n = 193), 1 µM NMN (n = 196), 10 µM NMN (n = 193) or 100 µM NMN (n = 195) treatment groups. Oocytes were matured in their respective treatments for the entire duration of IVM before artificial activation and embryo culture. Resulting blastocysts were fixed and stained, and total cell numbers were determined.
b) Cumulus-oocyte complexes were matured in IVM medium without NMN supplementation. Rather, the embryo culture medium was supplemented with different doses of NMN. Activated oocytes were randomly allocated to the control (n = 103), 0.1 µM NMN (n = 103), 1 µM NMN (n = 104), 10 µM NMN (n = 104) or 100 µM NMN (n = 102) treatment groups. Parthenotes were cultured in their respective treatments for 7 days, fixed and stained, and total cell numbers of blastocysts were determined.
Experiment 3: Effects of nicotinamide and resveratrol on oocyte maturation
Maturation medium was supplemented with NAM and Res individually, or in combination, using similar doses to those described previously [24,25,26,27]. Cumulus-oocyte complexes were randomly allocated to either the control (n = 126), NAM (5 µM; n = 120), Res (2 nM; n = 127) or NAM+Res (5 µM and 2 nM respectively; n = 129) treatment groups. Oocytes were matured in their respective treatments in IVM medium for the entire duration of IVM before artificial activation and embryo culture. Resulting blastocysts were fixed and stained, and total cell numbers were determined.
Statistical analysis
The software package R (version 3.6.1; 2019; Vienna, Austria) was used to detect differences in the rates of oocyte maturation, cleavage, blastocyst formation and blastocyst hatching, and blastocyst cell numbers. The proportional data was subjected to a logit transformation using the car package (version 3.0-3) and then a residual maximum likelihood regression using the lmerTest package (version 3.1-0) with treatment group as factor and replicate as random effect. When significant differences were detected, Tukey’s test was used to determine the differences between treatment groups using the emmeans (version 1.3.5.1) package. The blastocyst cell number data were subjected to a two-sample unequal variances T-test to identify differences between treatment groups. Values are presented as the mean ± SEM and a P-value ≤ 0.05 was considered significant.
Results
Effect of nicotinic acid on oocyte quality and embryo development
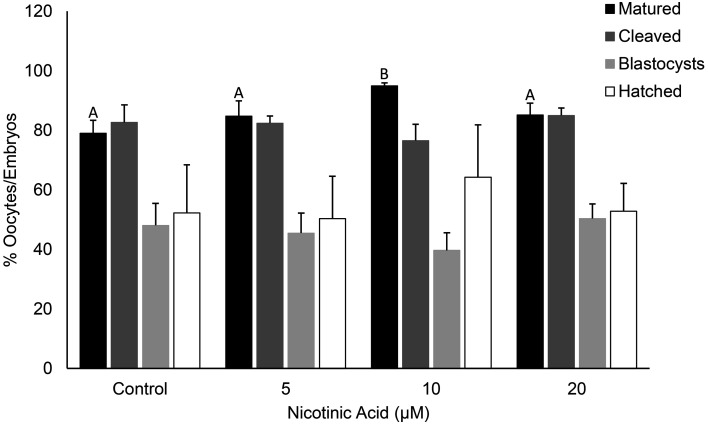
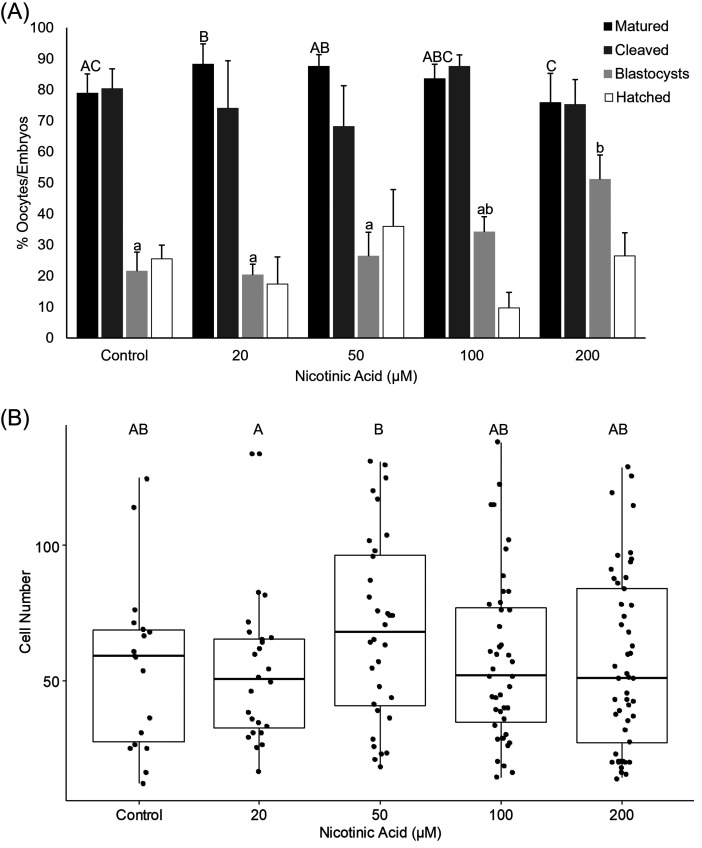
The addition of 10 µM of NA in the low dose experiment (0, 5, 10 and 20 µM) resulted in a greater proportion of oocytes reaching the MII stage of maturation compared with the control, 5 and 20 µM treatments (P < 0.05; Supplementary Table 1; Fig. 2). The rates of cleavage, blastocyst formation, blastocyst hatching and total blastocyst cell number did not differ among the groups (Supplementary Table 1; Fig. 2). In the high dose experiment (20, 50, 100 and 200 µM), the addition of 20 µM NA resulted in a greater proportion of oocytes that reached MII compared with the control and 200 µM groups (P < 0.05 and 0.01 respectively; Supplementary Table 2; Fig. 3(A)). The 200 µM treatment also resulted in a lower maturation rate compared with the 20 µM and 50 µM treatments (P < 0.05) but was not different to the control and 100 µM treatments (P > 0.05; Supplementary Table 2; Fig. 3(A)). However, of the oocytes that reached the MII stage in the 200 µM group, a significantly greater blastocyst formation rate was observed compared with the control (P < 0.01), 20 µM (P < 0.01) and 50 µM (P < 0.05) groups (Supplementary Table 2; Fig. 3(A)). The 50 µM group had blastocysts with greater cell numbers compared with the 20 µM group (P < 0.05; Supplementary Table 2; Fig. 3(B)).
Fig. 2.
The effect of NA at low doses on the rates of oocyte maturation, cleavage, blastocyst formation and blastocyst hatching. Bars labelled with different letters indicate statistical differences (P < 0.05).
Fig. 3.
The effect of NA at high doses on (A) the rates of oocyte maturation, cleavage, blastocyst formation and blastocyst hatching, and (B) total blastocyst cell number. Bars and plots labelled with different letters indicate statistical differences (P < 0.05).
Effect of nicotinamide mononucleotide on oocyte quality and embryo development
NMN supplementation of IVM medium did not significantly affect the rates of oocyte maturation, cleavage, blastocyst formation or blastocyst hatching at any concentration (Table 1). Furthermore, there was no difference in the total cell number of blastocysts among any of the treatment groups (Table 1).
Table 1. The effects of nicotinamide mononucleotide (NMN) supplementation during IVM on oocyte quality and embryo development.
| NMN (µM) |
Oocytes matured (%) |
Oocytes cleaved (%) |
Blastocyst formation (%) |
Hatching blastocysts (%) |
Total blastocyst cell number |
|---|---|---|---|---|---|
| Control | 72.2 ± 9.5 | 92.9 ± 3.2 | 23.0 ± 10.0 | 15.8 ± 11.8 | 78.0 ± 7.3 |
| 0.1 | 76.5 ± 7.0 | 89.9 ± 2.9 | 29.8 ± 9.0 | 20.1 ± 7.4 | 65.5 ± 6.1 |
| 1 | 77.2 ± 5.1 | 89.8 ± 2.4 | 23.3 ± 6.1 | 30.7 ± 2.4 | 71.6 ± 6.9 |
| 10 | 78.0 ± 3.6 | 91.5 ± 3.0 | 28.2 ± 5.4 | 15.8 ± 3.4 | 79.3 ± 7.0 |
| 100 | 68.5 ± 8.6 | 89.7 ± 5.3 | 22.1 ± 4.6 | 25.4 ± 4.6 | 75.1 ± 10.3 |
Data presented as mean ± SEM.
Supplementation of embryo culture medium with 0.1, 1, 10 and 100 µM NMN had no significant effect on any aspect of embryo development, including the rates of cleavage, blastocyst formation or blastocyst hatching (Table 2). Likewise, there was no difference in the total cell number of blastocysts among any of the treatment groups (Table 2).
Table 2. The effects of nicotinamide mononucleotide (NMN) supplementation during embryo culture on embryo development.
| NMN (µM) |
Oocytes cleaved (%) |
Blastocyst formation (%) |
Hatching blastocysts (%) |
Total blastocyst cell number |
|---|---|---|---|---|
| Control | 58.4 ± 10.4 | 19.4 ± 2.5 | 15.3 ± 9.7 | 62.2 ± 16.1 |
| 0.1 | 53.5 ± 17.9 | 17.7 ± 12.0 | 27.8 ± 14.7 | 62.7 ± 9.4 |
| 1 | 52.7 ± 18.8 | 23.5 ± 3.7 | 20.7 ± 11.6 | 59.2 ± 5.8 |
| 10 | 61.4 ± 9.8 | 20.0 ± 6.9 | 47.6 ± 29.0 | 64.1 ± 7.5 |
| 100 | 61.6 ± 15.2 | 36.5 ± 7.8 | 22.2 ± 2.8 | 74.4 ± 8.6 |
Data presented as mean ± SEM.
Effects of nicotinamide and resveratrol on oocyte quality and embryo development
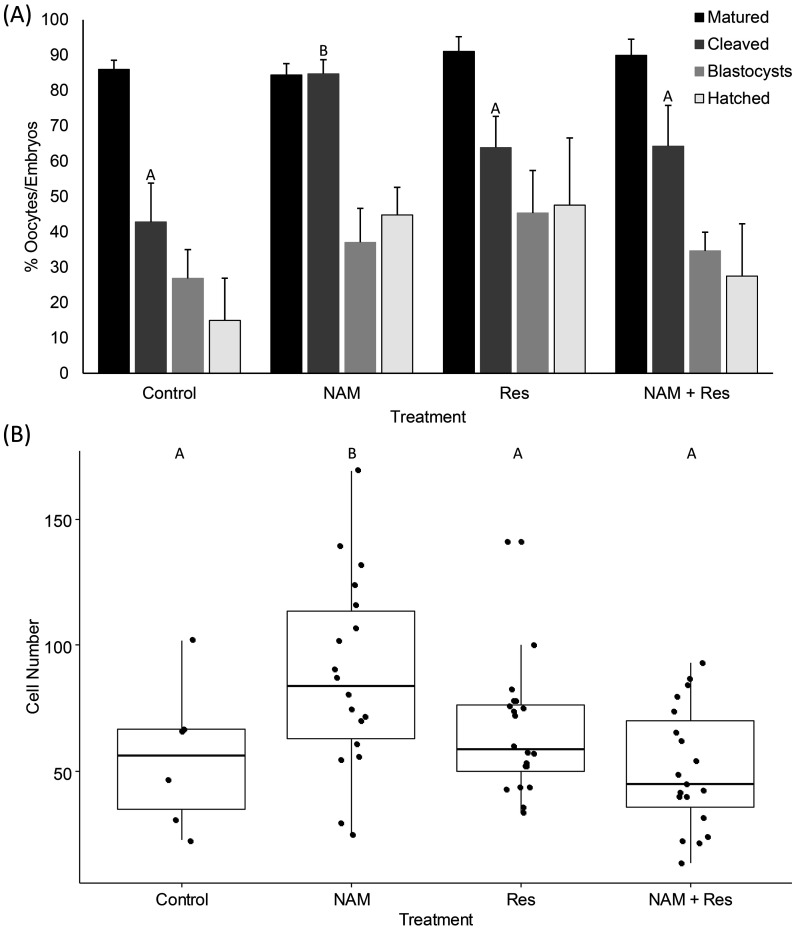
Supplementation of the maturation media with NAM, Res or NAM+Res had no effect on oocyte maturation, however, a greater proportion of oocytes underwent cleavage when they were matured with NAM alone compared to the control, Res and NAM+Res groups (P < 0.05; Supplementary Table 3; Fig. 4(A)). In addition, blastocysts derived from oocytes matured with NAM alone contained a greater number of cells compared with those of the control, Res and NAM+Res groups (P < 0.05; Supplementary Table 3; Fig. 4(B)).
Fig. 4.
The effect of NAM and Res, alone and in combination, on (A) the rates of oocyte maturation, cleavage, blastocyst formation and blastocyst hatching, and (B) total blastocyst cell number. Bars and plots labelled with different letters indicate statistical differences (P < 0.05).
Discussion
Using porcine oocytes from small antral follicles as a model of poor oocyte quality, the results of this study provide insights into the pathways through which NAD+ precursors may be utilised to support oocyte maturation and embryo development. Oocytes treated with NA and NAM exhibited enhanced meiotic progression and developmental competency. The findings of the present study add to the growing body of evidence that NAD+ production is critical for optimal oocyte maturation and embryo development, and current embryo in vitro production systems may be improved by supplementing the media with NAD+ precursors.
Oocytes from small antral follicles treated with NA exhibited improved meiotic maturation rates and developmental potential. At lower concentrations (10 µM) meiotic maturation was enhanced, while at higher concentrations (200 µM) development to the blastocyst stage was superior. Consistent with this, previous studies have also shown an improvement in oocyte quality when mouse follicles cultured in vitro were supplemented with NA [29]. Bovine oocytes treated with 400 µM NA also exhibited significant improvements in meiotic progression and developmental competence following vitrification [30], and blastocyst formation was significantly improved when porcine oocytes isolated from large antral follicles were supplemented with 600 µM niacin during IVM [31]. There was no effect on aged, in vitro matured murine oocytes at low doses of NA [18], however, a significant increase in intrinsic oocyte NAD+ levels in the oocytes from aged mice compared with control mice was observed [18]. Nicotinic acid is acquired through the diet [32] and the production of NAD+ from NA is much more efficient than that of tryptophan [33]. As such, supplementing the maturation media with NA in this study potentially provided the oocyte with a much greater capacity to produce NAD+ than was possible from tryptophan alone. Together the findings suggest that the effective concentration of NA for improving oocyte quality differs between species and oocytes from follicles of different diameter [18, 29, 30].
Embryos derived from oocytes treated with 5 µM NAM displayed an increased rate of embryo cleavage and had greater blastocyst cell numbers compared with the control group. Previous studies have examined the effects of much greater concentrations of NAM on oocyte maturation, and detrimental impacts were reported [26, 28]. Murine oocytes treated with 5 mM NAM had delayed meiotic progression, although the timing of polar body extrusion remained unaffected [26]. Exposure of porcine oocytes to 5 mM NAM reduced the incidence of polar body extrusion and increased the rate of abnormal meiotic spindle assembly [28]. In addition, porcine embryos treated with 5 mM of NAM had a decreased blastocyst formation rate and total blastocyst cell numbers compared with control embryos [34]. While high doses of NAM exert negative effects on oocytes and embryos by inhibiting the Sirtuin family of NAD+-dependent deacetylases, at the low dose of 5 µM examined here, NAM improved the developmental potential of porcine oocytes [28, 35], suggesting that oocyte processes are promoted by NAM supplementation below a certain threshold concentration. Precisely what that threshold concentration is requires further investigation.
The effects of NAM were diminished in the presence of resveratrol. Resveratrol is a phenol with well-known antioxidant properties and is a potent activator of SIRT1 [36]. In porcine oocytes grown in vitro, supplementation of IVM medium with resveratrol promoted the production of ATP and improved blastocyst formation compared with the control [37]. Interestingly, the effect of resveratrol on oocyte ATP production was diminished in the presence of the SIRT1 inhibitor EX527 [37]. As NAM and resveratrol are both mediators of Sirtuins and PARPs, through target proteins such as PGC1α and FOXO [38, 39], the interaction observed in the present study is likely due to their effects being exerted on shared pathways.
Supplementation of the maturation or embryo culture media with NMN did not significantly improve any aspect of oocyte maturation or embryo development. NMN is a secondary NAD+ metabolite in the salvage pathway, and is converted from NAM by nicotinamide phosphoribosyl transferase (NamPRT) [14, 15]. Beneficial effects of NMN treatment on oocyte quality have previously been reported. Supplementing the drinking water of aged mice with NMN resulted in an improvement in oocyte quality through increased NAD+ levels and restoration of spindle assembly [5, 17]. Furthermore, in vitro fertilization rates and blastocyst formation were enhanced, mitochondrial function was restored, DNA damage was reduced and apoptosis was inhibited in oocytes from aged mice administered NMN via intraperitoneal injection compared with aged control mice [17]. Additionally, supplementing the embryo culture medium improved the developmental potential of aged mouse oocytes [5]. Pig oocytes matured in vitro depend more on glucose as an energy substrate while mice utilise pyruvate more readily [40, 41], potentially contributing to the variation in effects on oocyte quality. NAD+ is consumed by ADP-ribose polymerases, which play an important role in carbohydrate metabolism [42], however, the function of mitochondrial ADP-ribose polymerases in carbohydrate metabolism remains under scrutiny. The high concentration of lipids in porcine oocytes compared with mouse oocytes may also reflect the differences in NMN supplementation. Fatty acid oxidation is vital for oocyte maturation in the pig but not in the mouse [43]. The production of NAD+ from NMN via the salvage pathway may promote oocyte quality through pyruvate metabolism in mouse oocytes, but may have no impact on glucose metabolism or fatty acid oxidation in porcine oocytes from small follicles.
Supplementation of the maturation media with NA, which is the precursor required for NAD+ production via the Preiss-Handler pathway, improved blastocyst formation, while activation of the NAD+ salvage pathway through NAM supplementation improved embryo cleavage, indicating that the small antral follicle oocytes were deficient in NAD+. In addition to the effects on cleavage and blastocyst formation, NA and NAM treatment during IVM increased the number of cells within blastocysts. Elevation of NAD+ leads to the activation of Sirtuins and PARPs that are involved in DNA damage signalling, cell cycle checkpoint monitoring and the induction of apoptosis [20, 44]. The increased number of cells in the blastocysts derived from NA and NAM treated oocytes may be due to enhanced repair of damaged DNA and thus improved cell survival or reduced apoptotic cell death. Differential staining of blastocysts would provide insight into the NAD+ biosynthetic pathways involved in determining the fate of blastocyst cells. Intriguingly, treating oocytes with NMN showed no effect on any aspect of oocyte quality. This suggests that the conversion of NMN to NAD+ is the main bottleneck and that NAM is the most limiting metabolite of the salvage pathway in porcine oocytes from small antral follicles.
Cumulus-oocyte complexes sourced from small antral follicles provide a useful model of poor oocyte quality in the pig [10, 11, 45]. Not all follicles retain the same potential to produce a viable oocyte [46]. Harvesting small antral follicles allows the selection of suboptimal oocytes resulting from inadequate maturation of the various follicular cells that lack the ability to support follicular growth and oocyte development. The coordination of cytoplasmic maturation appears vital in determining the developmental ability of an oocyte [47] and oocytes from small antral follicles lack the necessary cytoplasmic machinery to support embryo development [48]. As such, the production of NAD+ may play a vital role in promoting cytoplasmic maturation. The incidence of inadequate cytoplasmic maturation in prepubertal gilts is especially interesting in that oocytes harvested from sow ovaries display greater rates of complete cytoplasmic maturation and has been correlated with increasing follicle size [11]. Additionally, the capacity of the follicular cells to receive and respond to signals from the oocyte may be impaired in oocytes from small follicles [49] resulting in inadequate maturation of the cytoplasmic components. Comparing the developmental potential of oocytes from prepubertal gilts and sows and of small and large antral follicles treated with various NAD+ precursors would provide further insights into the role of NAD+ in the acquisition of developmental competence as follicular growth progresses, as well as the different pathways involved. The incidence of polyspermy is also a barrier to in vitro embryo production programs in the pig. More than 40% of inseminated oocytes show polyspermic penetration [50] and the percentage of oocytes fertilised by a single spermatozoon typically does not exceed 45% [51]. Also, IVM oocytes from prepubertal gilts are afflicted by higher rates of polyspermy than that of sows [52]. While implementing IVF provides information on the effects of IVM treatments on polyspermy, chemical activation was used in the present study to assess oocyte developmental potential. Chemical activation is commonly employed to evaluate oocyte quality in livestock species, and the treatments used here have previously been shown to effectively generate porcine diploid parthenotes [45, 53, 54]. Quantifying the change in the NAD+ content of porcine oocytes following treatment with the various NAD+ metabolites also requires further investigation. Intra-oocyte concentrations of NAD+ were markedly increased in murine oocytes treated with NA [18]. Therefore, determining the levels of NAD+ in porcine oocytes and cumulus cells would provide additional evidence that increasing NAD+ levels are responsible for the enhancement in oocyte quality and embryo development.
In conclusion, this study demonstrated that supplementing the maturation media with nicotinic acid and nicotinamide improved porcine oocyte maturation and developmental potential, and may be an effective strategy to increase NAD+ levels in oocytes of poor quality. Utilising oocytes from small antral follicles provided useful insights into the deficiencies that must be overcome to improve the acquisition of developmental competence. As such, NAD+ precursor supplementation has the potential to improve porcine IVM and embryo culture systems, enabling the production of more viable embryos from a restricted source of oocytes.
Conflict of interests
The authors declare no conflicts of interest.
Supplementary
Acknowledgments
The authors would like the acknowledge Dr. Lindsay Wu and Dr. Michael Bertoldo for providing one of the metabolites utilised in this study, and the Australian Research Council for supplying the funding to complete this research (LP160100824).
References
- 1.Lonergan P. In vitro maturation environment affects developmental outcome. In: Krisher, RL (eds.) Oocyte Physiology and Development in Domestic Animals. Wiley-Blackwell, U.S.A.: 2013. [Google Scholar]
- 2.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update 2008; 14: 159–177. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder AC, Eppig JJ. The developmental capacity of mouse oocytes that matured spontaneously in vitro is normal. Dev Biol 1984; 102: 493–497. [DOI] [PubMed] [Google Scholar]
- 4.Grupen CG. The evolution of porcine embryo in vitro production. Theriogenology 2014; 81: 24–37. [DOI] [PubMed] [Google Scholar]
- 5.Bertoldo MJ, Listijono DR, Ho WJ, Riepsamen AH, Goss DM, Richani D, Jin XL, Mahbub S, Campbell JM, Habibalahi A, Loh WN, Youngson NA, Maniam J, Wong ASA, Selesniemi K, Bustamante S, Li C, Zhao Y, Marinova MB, Kim LJ, Lau L, Wu RM, Mikolaizak AS, Araki T, Le Couteur DG, Turner N, Morris MJ, Walters KA, Goldys E, O’Neill C, Gilchrist RB, Sinclair DA, Homer HA, Wu LE. NAD+ repletion rescues female fertility during reproductive aging. Cell Reports 2020; 30: 1670–1681.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black JL, Erickson BH. Oogenesis and ovarian development in the prenatal pig. Anat Rec 1968; 161: 45–55. [DOI] [PubMed] [Google Scholar]
- 7.Kezele P, Nilsson E, Skinner MK. Cell-cell interactions in primordial follicle assembly and development. Front Biosci 2002; 7: d1990–d1996. [DOI] [PubMed] [Google Scholar]
- 8.Soede NM, Langendijk P, Kemp B. Reproductive cycles in pigs. Anim Reprod Sci 2011; 124: 251–258. [DOI] [PubMed] [Google Scholar]
- 9.Bagg MA, Vassena R, Papasso-Brambilla E, Grupen CG, Armstrong DT, Gandolfi F. Changes in ovarian, follicular, and oocyte morphology immediately after the onset of puberty are not accompanied by an increase in oocyte developmental competence in the pig. Theriogenology 2004; 62: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 10.Bagg MA, Nottle MB, Armstrong DT, Grupen CG. Effect of follicle size and dibutyryl cAMP on the cAMP content and gap junctional communication of porcine prepubertal cumulus-oocyte complexes during IVM. Reprod Fertil Dev 2009; 21: 796–804. [DOI] [PubMed] [Google Scholar]
- 11.Bagg MA, Nottle MB, Armstrong DT, Grupen CG. Relationship between follicle size and oocyte developmental competence in prepubertal and adult pigs. Reprod Fertil Dev 2007; 19: 797–803. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, You J, Hyun SH, Lee G, Lim J, Lee E. Developmental competence of morphologically poor oocytes in relation to follicular size and oocyte diameter in the pig. Mol Reprod Dev 2010; 77: 330–339. [DOI] [PubMed] [Google Scholar]
- 13.Belenky P, Bogan KL, Brenner C. NAD+ metabolism in health and disease. Trends Biochem Sci 2007; 32: 12–19. [DOI] [PubMed] [Google Scholar]
- 14.Bender DA, Olufunwa R. Utilization of tryptophan, nicotinamide and nicotinic acid as precursors for nicotinamide nucleotide synthesis in isolated rat liver cells. Br J Nutr 1988; 59: 279–287. [DOI] [PubMed] [Google Scholar]
- 15.Magni G, Orsomando G, Raffelli N, Ruggieri S. Enzymology of mammalian NAD metabolism in health and disease. Front Biosci 2008; 13: 6135–6154. [DOI] [PubMed] [Google Scholar]
- 16.Shi H, Enriquez A, Rapadas M, Martin EMMA, Wang R, Moreau J, Lim CK, Szot JO, Ip E, Hughes JN, Sugimoto K, Humphreys DT, McInerney-Leo AM, Leo PJ, Maghzal GJ, Halliday J, Smith J, Colley A, Mark PR, Collins F, Sillence DO, Winlaw DS, Ho JWK, Guillemin GJ, Brown MA, Kikuchi K, Thomas PQ, Stocker R, Giannoulatou E, Chapman G, Duncan EL, Sparrow DB, Dunwoodie SL. NAD deficiency, congenital malformations, and niacin supplementation. N Engl J Med 2017; 377: 544–552. [DOI] [PubMed] [Google Scholar]
- 17.Miao Y, Cui Z, Gao Q, Rui R, Xiong B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Reports 2020; 32: 107987. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Hu F, Zeng J, Han L, Qiu D, Wang H, Ge J, Ying X, Wang Q. NMNAT2-mediated NAD+ generation is essential for quality control of aged oocytes. Aging Cell 2019; 18: e12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DH, Lee HR, Kim MG, Lee JS, Jin SJ, Lee HT. The effect of poly(ADP-ribosyl)ation inhibition on the porcine cumulus-oocyte complex during in vitro maturation. Biochem Biophys Res Commun 2017; 483: 752–758. [DOI] [PubMed] [Google Scholar]
- 20.Tatone C, Di Emidio G, Barbonetti A, Carta G, Luciano AM, Falone S, Amicarelli F. Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum Reprod Update 2018; 24: 267–289. [DOI] [PubMed] [Google Scholar]
- 21.Yoshioka K, Suzuki C, Onishi A. Defined system for in vitro production of porcine embryos using a single basic medium. J Reprod Dev 2008; 54: 208–213. [DOI] [PubMed] [Google Scholar]
- 22.Lowe JL, Bathgate R, Grupen CG. Effect of carbohydrates on lipid metabolism during porcine oocyte IVM. Reprod Fertil Dev 2019; 31: 557–569. [DOI] [PubMed] [Google Scholar]
- 23.Grupen CG, Armstrong DT. Relationship between cumulus cell apoptosis, progesterone production and porcine oocyte developmental competence: temporal effects of follicular fluid during IVM. Reprod Fertil Dev 2010; 22: 1100–1109. [DOI] [PubMed] [Google Scholar]
- 24.Yoshioka K, Suzuki C, Tanaka A, Anas IM, Iwamura S. Birth of piglets derived from porcine zygotes cultured in a chemically defined medium. Biol Reprod 2002; 66: 112–119. [DOI] [PubMed] [Google Scholar]
- 25.Liu MJ, Sun AG, Zhao SG, Liu H, Ma SY, Li M, Huai YX, Zhao H, Liu HB. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil Steril 2018; 109: 900–907. [DOI] [PubMed] [Google Scholar]
- 26.Riepsamen A, Wu L, Lau L, Listijono D, Ledger W, Sinclair D, Homer H. Nicotinamide impairs entry into and exit from meiosis I in mouse oocytes. PLoS One 2015; 10: e0126194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, Kong X, Martins-Santos MES, Aleman G, Chaco E, Liu GE, Wu SY, Samols D, Hakimi P, Chiang CM, Hanson RW. Activation of SIRT1 by resveratrol represses transcription of the gene for the cytosolic form of phosphoenolpyruvate carboxykinase (GTP) by deacetylating hepatic nuclear factor 4α. J Biol Chem 2009; 284: 27042–27053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Ma R, Hu J, Ding X, Xu Y. Sirtuin inhibition adversely affects porcine oocyte meiosis. PLoS One 2015; 10: e0132941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Sun M, Yu L, Wang Y, Yao Y, Wang D. Niacin inhibits apoptosis and rescues premature ovarian failure. Cell Physiol Biochem 2018; 50: 2060–2070. [DOI] [PubMed] [Google Scholar]
- 30.Kafi M, Ashrafi M, Azari M, Jandarroodi B, Abouhamzeh B, Asl AR. Niacin improves maturation and cryo-tolerance of bovine in vitro matured oocytes: An experimental study. Int J Reprod Biomed 2019; 17: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Almubarak AM, Kim E, Yu IJ, Jeon Y. Supplementation with Niacin during in vitro maturation improves the quality of porcine embryos. Theriogenology 2021; 169: 36–46. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S, George S, Roy U, Ramachandran D, Kolthur-Seetharam U. NAD: a master regulator of transcription. Biochim Biophys Acta 2010; 1799: 681–693. [DOI] [PubMed] [Google Scholar]
- 33.Fukuwatari T, Ohta M, Kimtjra N, Sasaki R, Shibata K. Conversion ratio of tryptophan to niacin in Japanese women fed a purified diet conforming to the Japanese Dietary Reference Intakes. J Nutr Sci Vitaminol (Tokyo) 2004; 50: 385–391. [DOI] [PubMed] [Google Scholar]
- 34.Kwak SS, Cheong SA, Yoon JD, Jeon Y, Hyun SH. Expression patterns of sirtuin genes in porcine preimplantation embryos and effects of sirtuin inhibitors on in vitro embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology 2012; 78: 1597–1610. [DOI] [PubMed] [Google Scholar]
- 35.Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem 2002; 277: 45099–45107. [DOI] [PubMed] [Google Scholar]
- 36.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H, Taussig R, Brown AL, Kim MK, Beaven MA, Burgin AB, Manganiello V, Chung JH. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 2012; 148: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itami N, Shirasuna K, Kuwayama T, Iwata H. Resveratrol improves the quality of pig oocytes derived from early antral follicles through sirtuin 1 activation. Theriogenology 2015; 83: 1360–1367. [DOI] [PubMed] [Google Scholar]
- 38.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol 2016; 17: 679–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hwang ES, Song SB. Nicotinamide is an inhibitor of SIRT1 in vitro, but can be a stimulator in cells. Cell Mol Life Sci 2017; 74: 3347–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krisher RL, Brad AM, Herrick JR, Sparman ML, Swain JE. A comparative analysis of metabolism and viability in porcine oocytes during in vitro maturation. Anim Reprod Sci 2007; 98: 72–96. [DOI] [PubMed] [Google Scholar]
- 41.Wen J, Wang GL, Yuan HJ, Zhang J, Xie HL, Gong S, Han X, Tan JH. Effects of glucose metabolism pathways on nuclear and cytoplasmic maturation of pig oocytes. Sci Rep 2020; 10: 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopp AK, Grüter P, Hottiger MO. Regulation of glucose metabolism by NAD+ and ADP-ribosylation. Cells 2019; 8: 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paczkowski M, Silva E, Schoolcraft WB, Krisher RL. Comparative importance of fatty acid beta-oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biol Reprod 2013; 88: 111. [DOI] [PubMed] [Google Scholar]
- 44.Sousa FG, Matuo R, Soares DG, Escargueil AE, Henriques JA, Larsen AK, Saffi J. PARPs and the DNA damage response. Carcinogenesis 2012; 33: 1433–1440. [DOI] [PubMed] [Google Scholar]
- 45.Bertoldo M, Holyoake PK, Evans G, Grupen CG. Oocyte developmental competence is reduced in sows during the seasonal infertility period. Reprod Fertil Dev 2010; 22: 1222–1229. [DOI] [PubMed] [Google Scholar]
- 46.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod 1986; 1: 81–87. [DOI] [PubMed] [Google Scholar]
- 47.Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev 1996; 8: 485–489. [DOI] [PubMed] [Google Scholar]
- 48.Campbell KHS, Wilmut I. Totipotency and multipotentiality of cultured cells: applications and progress. Theriogenology 1997; 47: 63–72. [Google Scholar]
- 49.Procházka R, Sršeň V, Nagyová E, Miyano T, Flechon JE. Developmental regulation of effect of epidermal growth factor on porcine oocyte-cumulus cell complexes: nuclear maturation, expansion, and F-actin remodeling. Mol Reprod Dev 2000; 56: 63–73. [DOI] [PubMed] [Google Scholar]
- 50.Romar R, Cánovas S, Matás C, Gadea J, Coy P. Pig in vitro fertilization: Where are we and where do we go? Theriogenology 2019; 137: 113–121. [DOI] [PubMed] [Google Scholar]
- 51.Romar R, Funahashi H, Coy P. In vitro fertilization in pigs: New molecules and protocols to consider in the forthcoming years. Theriogenology 2016; 85: 125–134. [DOI] [PubMed] [Google Scholar]
- 52.Marchal R, Tomanek M, Terqui M, Mermillod P. Effects of cell cycle dependent kinases inhibitor on nuclear and cytoplasmic maturation of porcine oocytes. Mol Reprod Dev 2001; 60: 65–73. [DOI] [PubMed] [Google Scholar]
- 53.Grupen CG, Mau JC, McIlfatrick SM, Maddocks S, Nottle MB. Effect of 6-dimethylaminopurine on electrically activated in vitro matured porcine oocytes. Mol Reprod Dev 2002; 62: 387–396. [DOI] [PubMed] [Google Scholar]
- 54.Bagg MA, Nottle MB, Grupen CG, Armstrong DT. Effect of dibutyryl cAMP on the cAMP content, meiotic progression, and developmental potential of in vitro matured pre-pubertal and adult pig oocytes. Mol Reprod Dev 2006; 73: 1326–1332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.