Abstract
Purpose:
The objective of this work was to evaluate phantom dosimetry of a novel kilovoltage (kV) x-ray source, which employs a stationary tungsten anode and a linearly swept scanning electron-beam. The source utilizes converging x-ray collimation along with orthogonal mechanical rotation to distribute surface flux over large area. In this study, this was investigated as a potential solution to fast-falloff limitations expected with kV radiotherapy. This was done with the aim of future clinical development of a lower-cost radiotherapy alternative to megavoltage (MV) linac systems.
Methods:
Radiochromic film was employed for dosimetry on the kV x-ray source of the Linear-Converging Radiotherapy System (LCRS). The source utilizes charge particle optics to magnetically deflect and focus an electron beam along a stationary, reflection tungsten target in an ultra-high-vacuum stainless-steel chamber. Resulting x-rays were collimated into converging beamlets that span a large planar angle and converge at the system isocenter. In this study, radiochromic film dosimetry was done at 140 and 145 kVp for a designated planning treatment volume (PTV) of 4-cm diameter. An acrylic phantom was employed for dose distribution measurements of stationary and rotational delivery. Film dosimetry was evaluated in planes parallel to the source x-ray window at various depths, as well as in the plane of gantry rotation.
Results:
At 140 and 145 kVp and using a collimated 4-cm square field at depth, lesion-to-skin dose ratio was shown to improve with additional beams from different relative source positions, where the different beams are focused at the same isocenter and do not overlap at the phantom surface. It was only possible to achieve a 1:1 Dmax-to-surface ratio with four delivery beams, but the ratio improved to 4:1 with 12 beams, focused at the same isocenter depth of 7.8 cm in an acrylic phantom. For the tests conducted, the following Dmax-to-surface ratios were obtained: 0.4:1 lesion-to-skin ratio for stationary delivery from one entry beam, 0.71:1 lesion-to-skin ratio was obtained for two beams, 1.07:1 ratio for four beams, and 4:1 for 12 beams. Dose-depth profiles were evaluated for stationary and rotational dosimetry. Additionally, rotational dosimetry was measured for a case more analogous to a clinical scenario, where the isocenter was located at an off-center simulated lesion.
Conclusions:
The results demonstrate potential dose-depth improvements with kV arc therapy by distributing the surface flux with a wide converging beam along with perpendicular mechanical source rotation of the LCRS. The system delivered tolerable dose to a large surface area when a threshold of multiple, separated beams was reached. The radiochromic film data supports the feasibility of the construct of the LCRS kV radiotherapy system design.
Keywords: Kilovoltage x-rays, radiotherapy, dosimetry, arc therapy
1. Introduction
Kilovoltage (kV) radiotherapy offers many practical benefits when compared to megavoltage (MV) radiotherapy, including reduced room shielding as well as lower acquisition and installation costs1. kV energy units would be better suited for mobile transport in a trailer thereby providing greater access to healthcare in regions with limited resources. The overall kV system complexity is lower than MV radiotherapy systems, which utilize complex linear accelerator designs2. Additionally, kV radiotherapy can be performed with real-time, beam’s-eye-view x-ray imaging using the same treating source and without further development of new flat-panel x-ray detectors needed for the higher energy MV beams3. For these reasons, kV radiotherapy is promising for lower middle-income countries (LMIC), which have a shortage of radiotherapy systems4. The linear converging radiotherapy source (LCRS) system described in this report takes full advantage of these benefits. It utilizes charged-particle optics, i.e., a magnetically controlled, scanning electron beam in an ultra-high vacuum chamber, to rapidly switch between tungsten targets for therapy or imaging. Imaging may be generated via illumination of an opposing flat panel detector5. Furthermore, the ability to rapidly change between imaging and therapy tungsten targets, coupled with the programmable deflection and focusing of the scanning electron-beam system, enables precise tumor-tracking during treatment6,7. The LCRS system is capable of both cone-beam CT (CBCT) imaging and real-time digital 3D tomosynthesis, utilizing technology developed for another project, the 130 kVp TumoTrak™ x-ray source3.
Historically, optimization of radiotherapy dosimetry has been driven by 3D and 4D image-based planning and intensity modulation with beam angulation, e.g., volumetric modulated arc therapy (VMAT)8,9. However, disadvantages of MV beams include requirements for shielding bunkers with 2-m of concrete housing walls1. This added infrastructure cost has largely excluded its use in most settings globally. Complexity reduction is key to expanding clinical implementation of radiotherapy. This is feasible with a kV therapy unit where estimated cost reductions of over 50% compared to conventional MV devices are possible.
Previously, Monte Carlo (MC) radiotherapy planning yielded encouraging models of dose-distribution profiles using arc delivery based on a kV x-ray system. That study was modeled for an 80–120 kV inverse geometry fluoroscopy system11. It was validated by matching MC modeling to calibrated Gafchromic™ film exposure measurement in a solid-water phantom. This agreed (within 5%) for an actual dose rate delivering 1 Gy/15 min at 6-cm depth in the phantom. More recently, the same team expanded its MC modeling to a 200–225 kV system and showed that the dose delivery time and quality could approach that of standard MV VMAT radiotherapy for a simulated lung cancer and a breast cancer patient12. A study published in 2017 provides simulated results of kV arc therapy in full rotation configurations about cylindrical phantoms at 200 kV, with dose rate and lesion-to-skin ratios presented therein13. In 2019, the first prototype ring gantry with the 200 kVp LCRS design was installed in a shielded laboratory space5. In this study, the authors expand upon the initial developments of kV radiotherapy and their preliminary investigations by presenting calibrated radiochromic film dosimetry of the LCRS beam in simple phantom geometries.
2. Materials and Methods
2.A. LCRS description
The LCRS (Fig. 1) uses a magnetically focused and deflected electron beam swept along a linear path of reflection tungsten target within an ultra-high vacuum, stainless steel chamber. It produces x-rays up to 200 kVp and 200 mA beam current. The x-rays are collimated to create a linear array of converging beamlets that span a large angle in the long axis of the patient table (red triangular polygon in Fig. 1a). The beamlets converge to a designated size and shape at the system isocenter. This geometry distributes the x-ray flux over a large area of skin when combined with mechanical gantry rotation in the orthogonal plane.
Figure 1:
(a) Model of the shroud-covered LCRS mounted on a ring gantry showing an instance during kilovoltage arc therapy on an anthropomorphic figure. (b) Photograph of the prototype LCRS system. A fixture is mounted to the collimator for holding slab phantoms at adjustable distances from the source. (c) Schematic drawing of the inner configuration of the LCRS x-ray source.
For treatment, the angle and speed of the gantry rotation, in a plane perpendicular to the plane of the converging beamlets, is controlled during x-ray production according to a customized treatment plan. The planar convergence of the therapy beamlets, coupled with perpendicular gantry rotation, delivers an inverted pyramid of dose. The apex of the pyramidal dose distribution is at the system isocenter, which is positioned at a treatment site, Fig. 1(a). This arrangement thus enables favorable kV dose distributions at depth by using multiple non-coplanar orientations of the therapeutic beams, which reduce skin and superficial doses.
The novel x-ray source employs charged-particle optics to make it possible for the LCRS to rapidly distribute x-rays over a wide area in a clinically practical timeframe. After a thermionic cathode emits electrons, a focused beam of electrons is scanned across dwell locations along a stationary anode by use of a custom compact deflection coil system14. The design more readily dissipates the heat generated by the high power employed than is possible with a conventional rotating-anode x-ray tube. LCRS is designed to maintain a 40-kW continuous operation heat load by means of heatsink and water cooling.
For this study, the incident electron-beam was programmed to dwell at 41 discrete locations along the kV bremsstrahlung producing tungsten target. The dwell time was programmed for 1 ms at each dwell location. With respect to Fig. 1(c), the tungsten targets, the dwell locations, and collimator are oriented perpendicular to the plane of the page. There was a 0.13 ms travel-time covering 1-cm spacing between each location. The magnet coils were calibrated to achieve accurate positioning relative to apertures in the lead therapy-collimator. The collimator apertures correspond to each of the electron-beam dwell locations along the tungsten target.
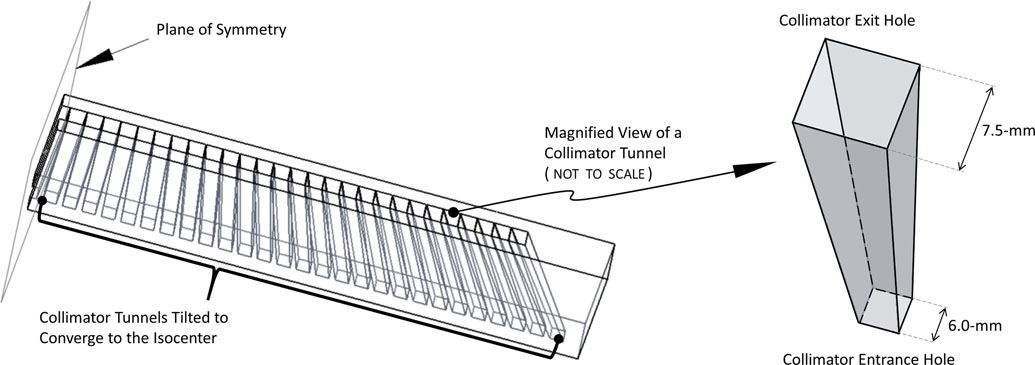
The dimensions of the individual tunnels of the collimator diverge from small entrance apertures to larger exit holes. In addition, each tunnel is tilted, along its length, to converge to the collimator’s focal plane at the system’s isocenter. Thus, this type of collimator geometry and resulting beamlets has been labeled as “diverging-converging”. A schematic of the collimator design is presented in Fig. 2. Here, the term beamlet refers to the discrete flux resulting from x-rays passing through an individual collimator hole per the above, after generation in the tungsten target. As shown in Fig. 3, the collimated x-ray beamlets were made to converge to a focal plane at a designated distance from the collimator exit. An irradiated film showing the beamlets exiting the collimator in the plane of collimation convergence is shown in the bottom-right of Fig. 3 for better visualization. The 41 dwell locations were evenly spaced by 1-cm spacing linearly along the tungsten target, utilizing 41-cm of the 53-cm total designed tungsten target length. The resultant collimated x-ray beam covered a range of 42.1°, providing one, albeit limited, direction of arc therapy. When coupled with perpendicular gantry rotation, there are effectively two axes of arc therapy: the axis corresponding to the collimated convergence of beamlets, and the axis corresponding to gantry rotation. This delivery distribution, enabled by both electromagnetic and mechanical control, provides a large area of spreading of superficial dose. For the collimator designed and used in this study, each individual beamlet was collimated to diverge (as described previously), such that the x-ray beam shape and size is approximately a 4-cm square in the plane parallel to the x-ray window at the system isocenter. Each of the collimator holes formed beamlets that diverged out from 0.75-cm square at the collimator exit while converging to the same 4-cm square distribution at the system isocenter. The delivered x-ray beamlet shapes and collimation can be adjusted using alternative collimator designs. In the results presented throughout, only a 4-cm square focus (measured at the isocenter) collimator was used.
Figure 2:
The LCRS diverging-converging x-ray therapy collimator design, shown as a transparent schematic on the left. A magnified view of an individual diverging collimator tunnel is shown on the right.
Figure 3:
Irradiated Gafchromic™ EBT3 films in the collimation converging plane obtained in air with the LCRS, overlaid onto a simple schematic to assist in visualizing the convergence of 41 beamlets to the system isocenter. The section a-a shown in top-middle was the designed and measured collimated beam shape and size, in a parallel plane to the source window at the isocenter. The 41 beamlet exposures at the exit of the collimator in the collimation converging plane are shown in the photograph in the top-right and scanned film image in bottom-right.
Figure 4 shows an illustration of LCRS arc therapy. The kV x-ray beamlets shown distributed along the straight-line source are collimated to aim at a focal plane while diverging to conform to the borders of the planning treatment volume (PTV) shown in dark-grey at the system isocenter. The lighter-color bands (yellow) represent the beamlets’ intersection with the surface of the cylindrical phantom. In contrast to the centered single beamlet representing conventional planar delivery of arced radiotherapy (indicated in purple), the LCRS flux distribution is widely spread, out of the plane of the gantry, over the superficial tissue (yellow). The purple band of surface entry area is less than the much larger surface area encompassed by and between the two yellow surface bands. This is a key characteristic of LCRS which enables kV radiotherapy to be applied to deep-seated lesions.
Figure 4:
Illustration of LCRS applied in rotational arc therapy showing the double-spreading distribution of the skin dose. The PTV is dark-grey and the spread region from LCRS treatment is shown in yellow along the cylindrical phantom, whereas the purple center beam represents a similar rotational dose distribution with strictly co-planar beamlets. The skin dose is spread over the Z-direction (head-to-foot) by the converging x-ray beamlet design of LCRS collimator, and when combined with mechanical gantry rotation, the system can deliver an enhanced volume of dose distribution.
The LCRS source was still undergoing both high-voltage conditioning and vacuum conditioning during the data collection, which reduced the stability of operation at full system power of 200 kV, 200 mA. Therefore, the source was maintained at lower energy and beam current to improve stability of dose delivery and ensure a complete data set for the initial studies.
2.B. Procedures
GAFChromic™ film has been shown to be an effective tool for dosimetry in the kV energy range15,16,17, and it was selected for this study to measure and demonstrate planar dose distributions of LCRS. The film was handled per the methodology of the AAPM TG-55 report18.
GAFChromic™ EBT3 dosimetry film (batch number 10151801) was exposed by the linear-converging radiotherapy system (LCRS). The LCRS x-ray beam was filtered through 1 mm of stainless steel and 2 mm of water. The LCRS was operated under constant-wave (CW) electron source operation at up to 145 kVp. The scanning electron beam system was programmed with a deflection waveform of 41 dwell spots, on a stationary reflection tungsten target, of 4-mm diameter, 1 ms dwell time each, and an average beam current of 20 mA. The average run duration was 20 minutes. The total mAs for each experiment was calculated by numerical integration of the beam current at each time increment.
2.B.1. Film calibration
The film was calibrated with respect to a PTW 30013 farmer probe (PTW North America Corporation, Brooklyn, NY) alongside EBT3 film in a parallel plane to the x-ray source window located at the system isocenter at 140 kVp tube energy. Protocols for calibration recommended by GAFchromic™ were followed throughout19. The probe was positioned at isocenter at a depth of 1.27 cm in an acrylic slab phantom. Following exposure, the film was scanned on an HP™ Officejet™ Pro 8710 flatbed scanner at multiple measured doses. The film was scanned at 600 dpi resolution, and 5×5 spatial mean smoothing was employed. Calibration curves of the form were fit to the red and green channel data sets, where X(D, n) was the scanner response on channel n for a measured dose D in mGy, and a, b, c were the fitting parameters. The film was calibrated in the range of 0.1 to 8 Gy. EBT3 film has an optimal dose range of 0.2 to 10 Gy20. Further details about the film calibration are available as supplementary information.
2.B.2. Experimental setup
Stationary irradiation
A fixturing mechanism was designed to mount directly to the therapy collimator and hold a stack of up to twelve 15.6-cm x 15.6-cm x 1.27-cm acrylic slabs at an adjustable distance from the source (Fig. 5). The EBT3 films were placed in between the acrylic slabs, which had a measured density of ρ=1.24 ± 0.01 g/cm3 and estimated mass energy-absorption coefficient of μen/ρ=2.30e-2 to 2.53e-2 cm2/g.21 EBT3 film has a thickness of 278-μm20, therefore the total stacked height for one slab and one film is approximately 1.30-cm. Film dosimetry for a static gantry angle of the LCRS was done with the stack of acrylic and film placed in a parallel plane to the source window (X-Z plane, coronal plane relative to the patient table top) as shown in Fig. 5(b).
Figure 5:
Setup for film dosimetry in a parallel plane to the source window (coronal plane relative to the table top): (a) Illustration showing the setup for film exposures of the converging beamlets measured in a parallel plane to the x-ray source window (not to scale). (b) Photograph of the experimental setup with the system coordinates for reference.
The collimation focal plane was located at a fixed 40-cm from the collimator exit in the Y-direction (floor-to ceiling) as shown in Fig. 5(a), and it was 50-cm from the stationary tungsten target surface, corresponding to designed source-to-axis distance (SAD) of the LCRS of 50 cm. It was positioned at a depth of 7.8-cm into the phantom. Dose distributions in planes parallel to the x-ray source window were collected for a single delivery angle at depths of film in the acrylic phantom of 2.6-cm, 5.2-cm, and 7.8-cm. The gantry position was maintained in a static orientation at the 6 o’clock position with the primary direction of x-rays being upward (floor-to-ceiling) for obtaining the dose distributions during static delivery tests. These tests were done to provide insight into the converging beam distribution at depths proximal to, and at the system isocenter.
Rotational irradiation
In addition to the distributions obtained with films oriented in the plane parallel to the x-ray source window (X-Z plane, coronal relative to the table top) from a single rotational angle as described above, rotational dosimetry was performed with the films oriented in the plane of gantry rotation (X-Y plane, axial plane relative to the table top) to allow for a controlled rotational testbed via phantom rotation. The stack of acrylic with film was rotated to simulate gantry rotation. This was done to ensure a fixed collimated beam distribution in space relative to the source throughout rotation. In the first rotational tests, a square-cross-section phantom was employed for 4 orthogonal x-ray delivery angles with isocenter at 7.8-cm deep in the phantom.
A better representation of the potential effectiveness for kV arc therapy with LCRS was obtained with a cylindrical shaped phantom via additional dose delivery angles. Utilizing 15.6-cm by 15.6-cm square acrylic slabs, six circular discs of diameter 15.6-cm and thickness of 1.27-cm were milled to shape and placed as the center six slabs within the phantom, as shown in Fig. 6(a). A piece of radiochromic film was cut into a 15.6-cm circle and placed between the centermost two acrylic disc slabs. The phantom with film was placed relative to the source as shown in Fig. 6(b), with the isocenter at 7.8-cm depth into the phantom. Dose was delivered from 12 angles at 145 kVp tube energy using 9,000 – 12,000 mAs at each angle. The quantity of mAs from each delivery angle varied during the experiment due to differences in beam current and time of exposure. The delivery angles were positioned every 30° around a full 360° rotation of the cylindrical phantom about the Z-axis. The angular markings were made from a printed pattern, but were rotated by hand, and thus may have introduced some minor rotational positioning inaccuracy.
Figure 6:
The modified stack of acrylic used as a phantom for rotational film dosimetry, with the center 6 slabs cut to circular cross-sections of 15.6-cm diameter (a), and as installed on the positioning fixture attached to LCRS (b). The EBT3 Gafchromic film was cut to a 15.6-cm circle shape and placed at the center of the stack, which was located at the system isocenter when installed onto the fixture for the concentric rotational dosimetry.
Finally, eccentric rotational dosimetry was tested utilizing the same 15.6-cm diameter circular-cross-section phantom. Although not accurate to human anatomy, this configuration better demonstrates the potential for a typical treatment of breast and chest lesions than the previous examples. Here, the fixture was adjusted to position the collimated convergence of x-ray beamlets at a depth of 4.1-cm into the acrylic phantom. The fixture and phantom were allowed to rotate together about that axis, i.e., at the system isocenter and a depth of 4.1-cm into the cylindrical phantom. Two example eccentric delivery angles are shown in Fig. 7. Film dosimetry was collected with a circular-cut film of 15.6-cm diameter placed between the center two slabs of the phantom. The film was exposed at evenly spaced angles in 40° increments, as measured by digital level to ± 0.1°. After the first three angles of film exposure over an entry-angle range totaling 80°, the film was scanned and placed back at the same position in the phantom for two additional beams from non-overlapping angles of delivery, thereby measuring the dose over a 160° total range.
Figure 7:
Photograph of the experimental setup for eccentric rotational dosimetry at an angle relative to the x-ray source of +40° (a) and −80° (b).
3. Results
3.A. Static gantry, single angle dose distributions
The results for stationary irradiation with LCRS are shown in Fig. 8. The film exposure at the isocenter is shown in planes parallel to the source, along with the film exposures from two more superficial depths proximal to the collimator focal plane. Linear dose profiles were obtained along the vertical line paths through each scanned film that were positioned at the indicated depths.
Figure 8:
LCRS dose distribution in planes parallel to the source window (coronal relative to the table top) at depths in acrylic of 2.6-cm, 5.2-cm, and 7.8-cm. The graph shows the linear distribution across the exposed film from top to bottom (“head-to-foot”). The system isocenter, corresponding to the collimator focal plane, was positioned at a depth of 7.8-cm (the rightmost film). This data was collected at 140 kVp and 24,600 mAs.
3.B. Rotational dosimetry with square cross-section phantom
The results from rotational dosimetry experiments with square cross-section acrylic phantom for one, two, and four delivery beams focused at the isocenter but non-overlapping at the phantom surface, are shown in Fig. 9, for a film placed in the plane of rotation.
Figure 9:
Rotational film dosimetry of LCRS with a square acrylic phantom. (a) Photograph of the stack of square acrylic slabs with film centered at the isocenter in the plane of gantry rotation (axial). This entire stack of acrylic slabs and film was rotated about the same axis of rotation as the gantry to simulate gantry rotation. Both the source and gantry were maintained fixed. (b) Graph of the linear distribution of dose along the paths shown in the films from bottom to top of the images. The black curve representing dose delivered from one angle is equivalent to a depth-dose profile for the stationary LCRS source. (c) Film dosimetry centered at the isocenter in the plane of gantry rotation for one angle of delivery (left), two orthogonal angles of delivery (middle), and four angles of delivery (right). Source was operated at 140 kVp with 51,700 mAs combined total dose delivered from all 4 angles.
From Fig. 9(b), it can be readily observed that the ratio of dose at lesion-to-skin, or isocenter-to-surface, improved as expected with additional relative beam delivery angles from the x-ray source, where the different beams are focused at the same isocenter and not overlapping at the phantom surface. The lesion-to-skin dose ratio was 0.40:1 for one delivery beam, 0.71:1 for two beams, and 1.07:1 for four beams.
3.C. Concentric rotational dosimetry with circular cross-section phantom
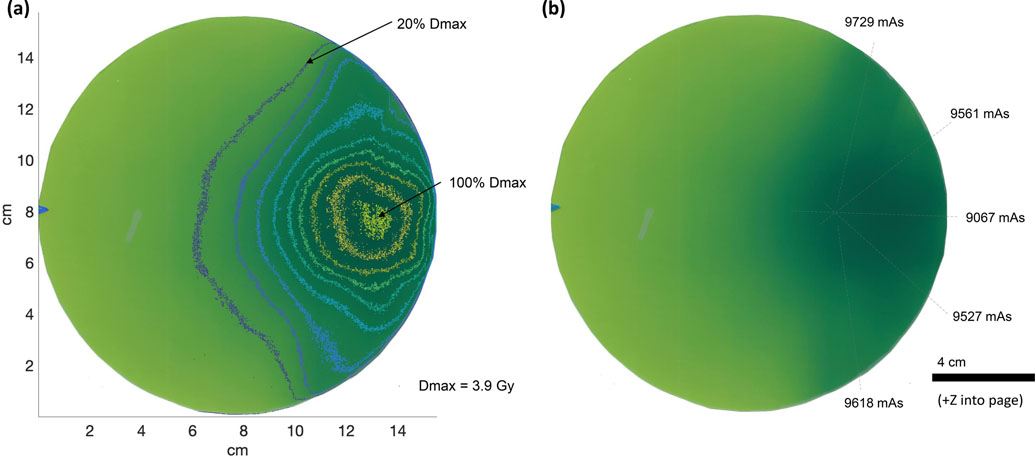
Rotational dosimetry was performed for circular-cross section phantom with delivery from 12 equally spaced angles about the full 360° rotation. Figure 10(a) shows the dose distribution obtained after delivery from all 12 angles was completed, with isodose lines (IDLs) to indicate levels of the maximum measured dose, Dmax = 7.0 Gy. Linear dose distributions along the centerline and nearby lines are shown in Fig. 10(b), where the corresponding vertical line paths are shown overlaid on the film in Fig. 10(c). The mAs for each delivery angle are also indicated in Fig. 10(c). The film was placed in the plane of rotation.
Figure 10:
LCRS rotational film dosimetry from 12 delivery angles rotated about a 15.6 cm diameter cylindrical phantom with film positioned at isocenter with center at the axis of rotation. Each rotation was 30° for 12 evenly spaced delivery angles. (a) Contour curves for IDLs as indicated. (b) Graph of the linear distributions from top to bottom across the film for line paths shown in (c). (c) The same scanned film shown with light-grey dashed lines and arrows to indicate the quantity of dose delivered from each of the 12 angles in mAs. Source operated at 145 kVp.
The 90% isodose line (IDL), was circular due to the overlap of beams from all delivery angles as they approach the isocenter. The diameter of the 90% IDL was measured to be 3.4±0.3 cm. At the lower 50% IDL, an irregular waviness was observed. The waviness was attributed primarily to the number of delivery angles and their effect on the overlapped regions. With higher number of delivery angles or constant rotation, the waviness at lower dose levels would be expected to smoothen. Additionally, the waviness dissipated at higher IDLs due to increased overlap of delivered dose. Apart from the waviness, the irregular nonuniformity of the lower IDLs was primarily attributed to the variation in beam current and time from each delivery angle.
Note that the 20% IDL was inconsistent as the dose at entrance (“skin” dose) varied from 20 – 28% around the circumference of the film. This was readily observable comparing the 20% and 30% contour curves of Fig. 10(a).
3.D. Eccentric rotational dosimetry with circular cross-section phantom
Figure 11 shows the dose distribution for delivery from three entrance angles over a total sweep of 80°, with isocenter at 4.1-cm depth in the phantom. The quantity of dose delivered from each angle in mAs ranged from 9000 – 9600 mAs, as indicated in Fig. 11(b). Dmax was 3.1 Gy for this data set.
Figure 11:
Eccentric rotational dosimetry with LCRS from three delivery angles over a total sweep of 80°. (a) Isodose lines from 20% to 100% IDL at 10% increments. (b) mAs delivered from each rotation angle. Source was operated at 145 kVp.
Figure 12 shows the dose distribution with two additional delivery angles at ±80° for a total of five delivery beams over a sweep of 160°. The dose delivered from each angle ranged from 9000 – 9800 mAs, as indicated in Fig. 12(b). Dmax was 3.9 Gy for this data set.
Figure 12:
Eccentric rotational dosimetry with LCRS from five delivery angles over a total sweep of 160°. (a) Isodose lines from 20% to 100% IDL at 10% increments. (b) mAs delivered from each rotation angle. Source was operated at 145 kVp.
The fluctuation of attenuation due to the depth of the convergence point varied with the delivery angles due to the eccentric geometry of the experiment. The centermost delivery angle had the shortest distance to travel through the phantom prior to hitting the system isocenter. The delivery angles from ±40° relative to center had to travel longer distances in the acrylic, and the delivery angles from ±80° had the longest distance to travel in the acrylic prior to reaching the system isocenter. The location of Dmax was observed at a shallower location than the axis of rotation and collimator focal plane at 4.1-cm depth, which was consistent with historical arcing techniques such as “past pointing.” In the latter case, arcing was performed with the isocenter beyond a lesion, to localize the most dose in a proximal pathologic lesion.
4. Discussion
Excessive skin dose has long been a barrier to acceptable kV radiotherapy of deep-seated cancer lesions. The LCRS was designed to enhance the lesion-to-skin x-ray dose ratio in spite of the faster kV fall-off. It delivers a set of beamlets, which are widely distributed over the skin while focused on a lesion in an inverted pyramid configuration. The work reported herein is the initial demonstration of a prototype device, which though underpowered, was nevertheless capable of delivering kV therapy in this clinically practical fashion. It was engineered to overcome the constraints of kV x-rays, including a lack of subsurface dose build-up effect, and composition sensitivity with greater attenuation in tissue by employing a converging array of beamlets. The fully conditioned device operating at greater energy is projected to compare favorably across a range of clinical scenarios. The lower energy dosimetry we have evaluated in the simplified geometry of the 15.6-cm diameter cylindrical, acrylic phantom used in this study, is encouraging for that next engineering step. In follow-on studies, this will also be addressed by comparison and validation with inverse optimized Monte Carlo simulated results such as those presented in previous papers by members of our team7, 11, 12, 13.
Although the main objective of this study was to evaluate the dose distributions within phantoms for LCRS, the dose rate can also be estimated from the film dosimetry data for LCRS at 140 and 145 kVp. Dose rate can be found by dividing the dose by the total time of delivery, where the delivery time can be projected from the beam quantity (mAs) divided by the operating beam current (mA). From Fig. 10, one can deduce the dose rate at isocenter for the 15.6-cm cylindrical acrylic phantom, which for a fully conditioned LCRS system operating at 200 mA would be 66 cGy/min at the depth of 7.8-cm.
The above found dose rate can be compared, albeit roughly, to the simulated Monte Carlo findings previously published for a 16.2-cm diameter water phantom13. In that study, the dose rate at 200 kV, 200 mA was found to be just over 150 cGy/min for a 4-cm lesion at the center of a 16.2-cm cylindrical water phantom13. Given the estimate dose rate from Fig. 10 as described above, found as 66 cGy/min for 15.6-cm acrylic phantom at 145 kV, the dose rate is lower than the simulated values. This is primarily due to the lower beam energy, where applying the square-law provides an extrapolated dose rate of 125 cGy/min, which is still low in comparison. If further corrections were applied to account for the difference in material attenuation of the acrylic used in this study compared to the water phantom of the simulated work, the results would approach better agreement. Of a similar note, the dose rate found at 145 kV of 66 cGy/min at depth of 7.8-cm is on the low end for treatment, and LCRS would not be beneficially practical for use for deeper seated lesions until it is fully conditioned to operate at 200 kV.
The lesion-to-skin ratio found in this study for full rotation about a 15.6-cm cylindrical phantom shows strong agreement with the previous simulated studies13. From the 2017 work, the lesion-to-skin ratio was found to be 4.1:1 for a 4-cm lesion at the center of a 16.2-cm cylindrical water phantom, at a beam energy of 200 kV 13. In the given study, the authors have found the lesion-to-skin ratio to be 4:1 for a 4-cm lesion at the center of a 15.6-cm cylindrical acrylic phantom, at 145 kV. The depth-dose distributions are only expected to improve slightly with increasing beam energy, whereas the dose rate itself will increase greatly, therefore the agreement to simulations are better for lesion-to-skin than for dose rate comparisons.
At the full-power designed tube energy of 200 kVp, one can expect increased dose rates and subtle improvement to the depth-dose distributions due to attenuation effects. At full tube beam current of 200 mA, the run durations would be 10x shorter than those used in this study to achieve the same dose. The authors have validated the system operation at 200 kVp, 200 mA settings for 2 minutes continuously. Future dosimetry with LCRS will be performed at the full power source operation. This is necessary for the system to be comparable in treatment quantity and quality to standard MV radiotherapy.
In addition, ongoing development of optimized collimator designs for different size lesions will enhance system capabilities and better conform to irregularly shaped treatment volumes. A 2-cm-square focus (as measured at isocenter) collimator is currently undergoing initial testing. Future work trade-off studies via Monte Carlo modeling will provide another tool for dosimetry optimization and collimator design. Additionally, work is being done to incorporate real-time imaging during radiotherapy with tumor tracking utilizing the onboard kV capabilities of LCRS.
5. Conclusions
The results from a static angle provide a representation of the dose distributions at depths superficial to and at the isocenter from the stationary LCRS source. To the authors’ knowledge, this is the first calibrated film dosimetry measured for linear, converging kV x-rays from a stationary-anode. However, it is apparent that static delivery is not suitable for treatment, with isocenter-to-surface ratio of only 0.4:1 for the stationary LCRS with 4-cm focus collimator at an isocenter depth of 7.8-cm. The needed improvement in dosimetry derives from additional perpendicular gantry rotation as demonstrated in this work. Rotational dosimetry results that were presented show this effect, where the lesion-to-skin ratio improved as expected with additional delivery angles, achieving a 1:1 ratio with four delivery angles, and reaching 4:1 with 12 delivery angles, all at the same isocenter depth in an acrylic phantom of 7.8-cm. The results demonstrated tolerable dose to a large surface area when a threshold of multiple separated beams was reached. The data warrants continued study and confirmed the feasibility of the design and construction of a kV radiotherapy system capable of required beamlet distributions and power.
Supplementary Material
Acknowledgements
DB and MDW are the principal investigators of the project. TS is LCRS project manager and is responsible for the tube operation, experimental planning, and dosimetry. LP is the clinical medical lead expertise engineer. JK and MP are responsible for assembly, mechanical design, and testing. SS and JYH are responsible for design of the collimator and development of the electron beam deflection software. MB-C provided valuable modeling insight for design parameters and reviewed the manuscript. RR, VZ and HC are responsible for the design, simulation and analysis, and assembly of the electron beam optics. All authors have reviewed the manuscript and provided insight to the content of the study. It is gratefully acknowledged that portions of this work were supported in part by NIH/NCI/SBIR Phase II Award 2R44CA192498-04. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Footnotes
Declaration of Conflicting Interests
The authors, except MDW, MB-C, and RR, disclose that they are employees of Imatrex Inc. MDW is founder of Precision RT Inc., a subsidiary of Imatrex Inc, and owns shares in Precision RT Inc. Imatrex and Precision RT are the developers of the LCRS. MDW, MB-C, and RR are consultants of Imatrex Inc.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Martin M Shielding design methods for radiation oncology departments. Lecture presented at: American College of Medical Physics 25th Annual Meeting; May 4, 2008; Seattle, WA. [Google Scholar]
- 2.Ginzton E, Nunan C. History of microwave electron linear accelerator radiotherapy. Int J Radiat Oncol Biol Phys. 1985;11:205–216. 10.1016/0360-3016(85)90141-5. [DOI] [PubMed] [Google Scholar]
- 3.Partain L, Benedict S, Kim N, et al. Comparisons of 6 fps volume-rendered x-ray digital tomosynthesis TumoTrak™-guided to 2D MRI-guided radiotherapy of lung cancer. Proc SPIE. 2019;109483. 10.1117/12.2511993. [DOI] [Google Scholar]
- 4.Palta J, Azangwe G, White G. Low complexity (e.g. 60 Co teletherapy) is the appropriate level of radiotherapy for use in low-income countries. Med Phys. 2020;47:4671–4674. 10.1002/mp.14137. [DOI] [PubMed] [Google Scholar]
- 5.Stalbaum T, Boyd D, Weil MD, et al. 200 kV x-ray source for radiotherapy and imaging: preliminary results and discussion. Proc SPIE. 2020;11312. [Google Scholar]
- 6.Zankowski C, Adelsheim C, Pyyry J, Kuusela E. Radiotherapy treatment planning using artificial intelligence (ai) engines. Google Patents; 2019.
- 7.Breitkreutz D, Weil MD, Bazalova-Carter M. External beam radiation therapy with kilovoltage x-rays. Phys Med. 2020;79:103–112. [DOI] [PubMed] [Google Scholar]
- 8.Schell M, Maurer C, Seibert J, Yin F-F. Clinical Oncology: Radiation Physics as Applied to Clinical Radiation Oncology. 8th ed. Philadelphia, PA: Saunders; 2001. [Google Scholar]
- 9.Otto K Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35(1):310–7. 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 10.Prionas ND, McKenney SE, Stern RL, Boone JM. Kilovoltage rotational external beam radiotherapy on a breast computed tomography platform: A feasibility study. Int J Radiat Oncol Biol Phys. 2012;84(2):533–539. 10.1016/j.ijrobp.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bazalova M, Weil MD, Wilfley B, Graves E. Monte Carlo model of the scanning beam digital x-ray (SBDX) source. Phys Med Biol. 2012;57:7381–7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breitkreutz D, Renaud M-A, Seuntjens J, Weil MD, Zavgorodni S, Bazalova-Carter M. Inverse optimization of low-cost kilovoltage x-ray arc therapy plans. Med Phys. 2018;45:5161–5171. [DOI] [PubMed] [Google Scholar]
- 13.Breitkreutz D, Weil M, Zavgorodni S, Bazalova-Carter M. Monte Carlo simulations of a kilovoltage external beam radiotherapy system on phantoms and breast patients. Med Phys. 2017;44:6548–6559. [DOI] [PubMed] [Google Scholar]
- 14.Rand R, Ziskin V, inventors; Imatrex Inc, assignee. Compact deflecting magnet. U.S. Patent No. 10,332,718. June 25, 2019.
- 15.Fletcher CL, Mills JA. An assessment of GafChromic film for measuring 50 kV and 100 kV percentage depth dose curves. Phys Med Biol, 2008;53(11):N209. [DOI] [PubMed] [Google Scholar]
- 16.Steenbeke F, Gevaert T, Tournel K, et al. Quality assurance of a 50-kV radiotherapy unit using EBT3 GafChromic film: a feasibility study. Technol Cancer Res Treat. 2016;15(1):163–170. [DOI] [PubMed] [Google Scholar]
- 17.Giaddui T, Cui Y, Galvin J, Chen W, Yu Y, Xiao Y. Characteristics of Gafchromic XRQA2 films for kV image dose measurement. Med Phys. 2012;39(2):842–850. [DOI] [PubMed] [Google Scholar]
- 18.Niroomand-Rad A, Chiu-Tsao ST, Grams MP, et al. Report of AAPM Task Group 235 Radiochromic Film Dosimetry: An Update to TG-55. Med Phys. 2020;47(12). [DOI] [PubMed] [Google Scholar]
- 19.Lewis D, Micke A, Yu X, Chan MF. An efficient protocol for radiochromic film dosimetry with flatbed CCD scanners combining calibration and measurement in a single scan. Med Phys. 2012;39:6339–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GAFchromic™. GAFchromic dosimetry media, type EBT-3. GAFchromic website. Accessed November 10, 2020. http://www.gafchromic.com/documents/EBT3_Specifications.pdf [Google Scholar]
- 21.NIST. Mass energy-absorption coefficient: Polymethyl methacrylate. NIST Physical Measurement Laboratory website. Accessed February 14, 2021. https://physics.nist.gov/PhysRefData/XrayMassCoef/ComTab/pmma.html [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.