Abstract
Background:
The nucleus basalis of Meynert (NBM), representing the major source of cerebral cholinergic innervations, is vulnerable to neurodegeneration in Alzheimer’s and Parkinson’s disease.
Objective:
To determine associations between NBM properties and cognitive outcomes in patients with multiple sclerosis (PwMS).
Methods:
84 PwMS and 19 controls underwent 3T MRI, the Paced Auditory Serial Addition Test (PASAT) and subtests of the Brief International Cognitive Assessment for MS (BICAMS). NBM volume, fractional anisotropy, mean diffusivity (MD), axial diffusivity and radial diffusivity (D⊥) were calculated. Analyses assessed relationships between cognition and NBM measures. Linear regressions evaluated the prognostic value of baseline measures in predicting cognitive change over 3 years of follow-up (n=67).
Results:
Cognitive tests correlated with NBM diffusivity in PwMS (range r=−0.29 to r=−0.40, p<0.05). After accounting for NBM volume, NBM MD and D⊥ explained additional variance (adjusted R2 range: 0.08 to 0.20, p<0.05). Correlations between NBM imaging metrics and cognitive tests remained significant when including imaging parameters of other cognitive key brain regions in the models. After controlling for age, education, and baseline cognitive test score, NBM measures predicted change in cognition over follow-up in 5 of 10 and 2 of 10 assessments in the relapsing-remitting sample (n=43) (adjusted R2 range from 0.23 to 0.38, p<0.05) and secondary progressive sample (adjusted R2 of 0.280 and 0.183), respectively.
Conclusions:
NBM damage is linked to cognitive impairment in PwMS.
Keywords: multiple sclerosis, nucleus basalis of Meynert, cognition, diffusion tensor imaging, atrophy
Introduction
Magnetic resonance imaging (MRI) has helped unravel the underlying pathology leading to cognitive dysfunction in patients with multiple sclerosis (MS). Yet, many unknowns remain. For example, the nucleus basalis of Meynert (NBM), a group of basal forebrain neurons that represents the major source of cholinergic innervations for the cerebral cortex and subcortical, structures, including the hippocampal complex and amygdala,[1–5] has received little attention in the MS field. The NBM plays a role in control of attention and arousal, cognitive processing speed and memory.[2–4, 6–8]
A link between a cholinergic imbalance and neuroinflammation is described in other neurodegenerative diseases, such as Parkinson’s disease (PD) and Alzheimer’s disease (AD),[9] with the NBM being particularly vulnerable. For example, compared to healthy controls (HCs), PD patients have more NBM volume loss over time, associated with cognitive impairment.[6, 10, 11] Positron emission tomography studies show increased cholinergic neuronal degeneration in the basal forebrain of PD patients with cognitive impairment.[4, 12] Diffusion tensor imaging (DTI) reveals increased NBM diffusivity in PD.[6, 11, 12] Similar findings were also seen in AD and mild cognitive impairment.[1, 7, 8, 13, 14]
As the NBM and associated cognitive status has yet to be studied in the MS field, we aimed to characterize the association between NBM tissue properties and impaired cognition in MS for one of the first times. We hypothesized that compared to HCs, patients with MS (PwMS) would present with increased NBM injury, as reflect by lower NBM volume and increased tissue diffusivity, which would be associated with worse cognition.
Methods
Study population:
This is a retrospective analysis of a prospective study investigating personality changes in MS. Participants were enrolled between August 2009 and July 2010. 84 PwMS, including 54 relapsing-remitting (RR) MS patients and 30 secondary progressive (SP) MS patients as well as 19 age- and sex-matched healthy controls (HC) were included. Inclusion criteria for MS patients were a) being diagnosed with RRMS or SPMS according to the 2010 McDonald Criteria,[15] b) age between 20 and 65 years, c) having no other medical conditions associated with alterations in brain pathology, d) 3T MRI acquired with a standardized protocol, and e) completion of cognitive testing. Healthy control subjects were recruited by local advertisements and from friends or family members of the patients. HCs reported no relevant medical history that might be associated with clinical disability or imaging features. The study was approved by the local Institutional Review Board and written informed consent was obtained from all participants.
MRI acquisition and analysis:
Subjects underwent an MR scan on a 3T GE Signa Excite scanner (GE Healthcare Systems, Milwaukee, WI). A 2D T2-weighted fluid-attenuated inversion recovery image (FLAIR) sequence with 1x1x3 mm3 voxels, a 3D T1-weighted sequence with 1 mm3 isotropic voxels as well as a 2D diffusion-weighted imaging sequence with 2.5x2.5x3 mm3 (upsampled on the scanner to 1.25x1.25x3 mm3) voxels with 15 diffusion-weighted directions at b = 800 s/mm2 and one b = 0 s/mm2 image were acquired.
T2 lesion volume was calculated using a semi-automated contour/thresholding technique with the T2-weighted FLAIR. [16] T1 hypointensities on the 3D T1-weighted image were filled prior to subsequent processing. Normalized whole brain (WB), gray matter (GM), white matter (WM) as well as cortical volumes were obtained using SIENAX,[17] while thalamic and hippocampal volumes were calculated using FIRST.[18]
Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (D∥) and radial diffusivity (D⊥) were calculated after correction for subject motion and eddy current distortion. The b=0 image was nonlinearly registered to a T2-weighted MNI atlas using Advanced Normalization Tools. The inverse warp brought a probabilistic map of the NBM into native diffusion-weighted imaging space. Proper alignment was ensured for each case by visually inspecting the warped NBM map. The resulting probability map was used to derive weighted DTI-derived measures (Figure 1). The normalized NBM volume was calculated using SIENAX-derived tissue partial volume estimate images with the probabilistic NBM atlas.
Figure 1:
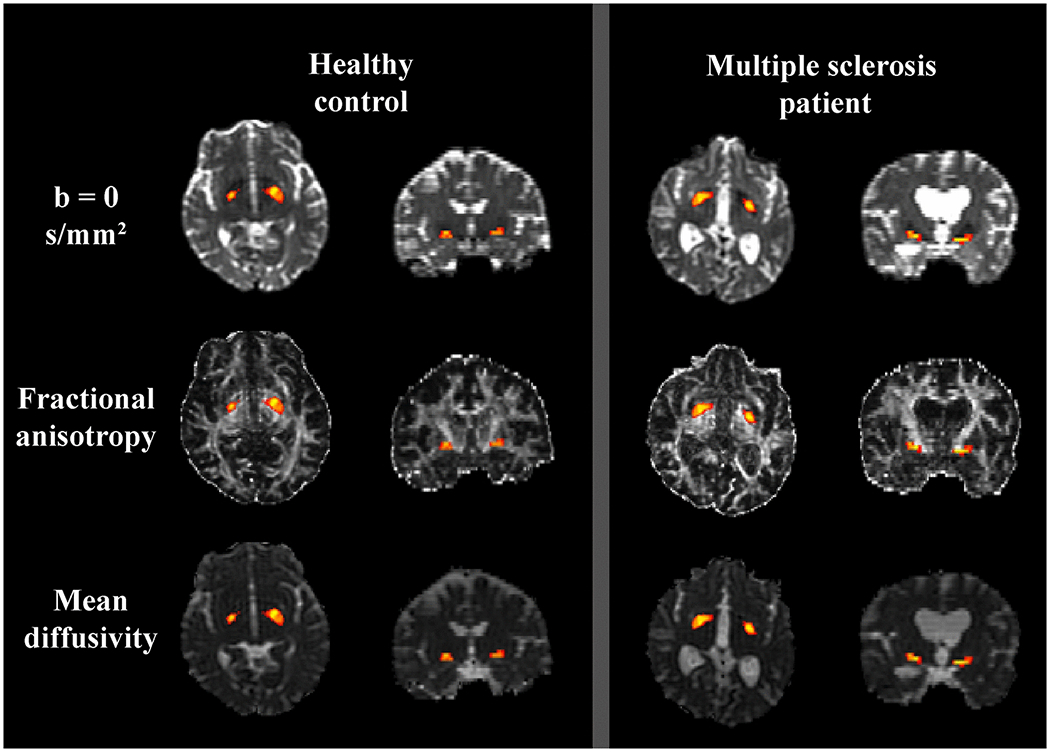
Axial and sagittal planes from a 48-year-old female healthy control is shown in the left column. On the right, images from a 46-year-old female multiple sclerosis patient with a relapsing-remitting disease course are shown. The warped probabilistic nucleus basalis of Meynert (NBM) atlas is overlaid on top of the corresponding diffusion images. Yellow indicates increased probability of corresponding to the NBM.
Neuropsychological Testing:
All subjects underwent cognitive assessment with the Paced Auditory Serial Addition Test (PASAT, 3s version), the Symbol Digit Modalities Test (SDMT), measuring cognitive processing speed, the Brief Visuospatial Memory Test Revised (BVMT-R), testing visual learning and memory, and the California Verbal Learning Test second edition (CVLT-2), measuring verbal learning and memory.[19–21] For BVMT-R and CVLT-2 four sub-scores were evaluated (total learning, delayed recall, discrimination index and retention percentage). Patients were classified as cognitively impaired when z-scored test results were below the pre-defined clinical cut-off score of −1.5 in at least one of the cognitive assessments. Cognitive testing was available in a subset of patients (n=67) after a mean of 3.1 ± 0.2 years of follow-up.
Statistical Analysis:
Statistical analyses were performed using SPSS version 24.0. Differences in the demographic variables between the groups were analyzed using Fisher’s Exact test, Students’ t-test and Mann-Whitney U-test, as appropriate. MRI metrics and neuropsychological testing were compared between groups using analysis of covariance (ANCOVA). Analyses comparing PwMS and HCs were corrected for age and sex for imaging outcomes, whereas neuropsychological measures were additionally corrected for education.
Partial correlations between DTI metrics of the NBM and NP outcomes were calculated correcting for age and education and adjusted for false discovery rate using the Benjamini-Hochberg procedure.
Linear regression analysis was performed with cognitive score as the dependent variable, age and education forced into block 1, NBM volume forced into block 2 and the NBM DTI metrics into block 3. For the last block, forward selection was used. The model was then reanalyzed by changing the variable forced into the second block with one of the following: WB volume, cortical volume, thalamic volume, hippocampal volume, T2 lesion volume or MD of the normal-appearing whole brain (NAWB) tissue.
Finally, additional linear regression analyses were performed in the subset of patients for which follow-up data was available to assess whether baseline imaging measures could predict change in cognitive state. Age, education, and baseline test score were forced into the first block while imaging outcomes (i.e., NBM volume and DTI metrics, WB volume, NAWB MD, cortical volume, thalamic volume, and hippocampal volume) were forward selected in the second block. Change in cognitive score over three years was the dependent variable. As cognitive changes appear to evolve differently between phenotypes,[22] we assessed RRMS and SPMS groups separately.
P values < 0.05 using two-tailed tests were considered as statistically significant.
Data availability statement:
The data from this study are available on a reasonable request to the corresponding author.
Results
Demographic and clinical characteristics of patients with MS vs. HCs:
The MS patient group had a mean age of 46.6 ± 8.8 years, a mean disease duration of 15.5 ± 9.3 years and a median Expanded Disability Status Scale (EDSS) score of 3.5 (IQR: 2.5 – 6.0). 58.3% (n=49/84) of MS patients had objective cognitive impairment, as measured by the z-scored cognitive assessments. RRMS and SPMS patients differed significantly in age, disease duration and EDSS score, as expected but were matched with respect to education level. The two patient groups are represented separately in subsequent statistical analyses. The 19 HCs were group-matched for age and sex (Table 1).
Table 1:
Demographic characteristics of patients with MS and healthy controls.
| Total MS (n=84) | HC (n=19) | p value | RRMS (n=54, 64.3%) | SPMS (n=30, 35.7%) | p value | |
|---|---|---|---|---|---|---|
| Female, n (%) | 56 (66.7) | 11 (57.9) | 0.595a | 40 (74.1) | 16 (53.3) | 0.090a |
| Age in years, mean (SD), IQR | 46.6 (8.8), 41 – 53 | 42.8 (11.4), 33 – 51 | 0.107b | 43.4 (8.4), 36 – 50 | 52.4 (6.2), 49 – 57 | <0.001 b |
| Education in years, mean (SD), IQR | 14.4 (2.3), 12.25 – 16 | 15.6 (2.4), 13 – 18 | 0.043 c | 14.4 (2.3), 12 – 16 | 14.5 (2.3), 13 – 16 | 0.834c |
| Disease duration in years, mean (SD), IQR | 15.5 (9.3), 8.25 – 22 | N/A | N/A | 12.9 (8.1), 6 – 17 | 20.3 (9.6), 11 – 29 | <0.001 b |
| EDSS, median (IQR) | 3.5 (2.5 – 6.0) | N/A | N/A | 2.5 (1.5 – 4.0) | 6.0 (5.0 – 6.5) | <0.001 c |
|
Treatment status, n (%)Interferon beta-1a Glatiramer acetate Natalizumab Other DMT* No DMT Unknown |
29 (34.5) 15 (17.9) 18 (21.4) 4 (4.8) 17 (20.3) 1 (1.2) |
N/A | N/A | 18 (33.3) 8 (14.8) 14 (25.9) 2 (3.7) 12 (22.2) 0 (0) |
11 (36.7) 7 (23.3) 4 (13.3) 2 (6.7) 5 (16.7) 1 (3.3) |
0.570a |
Legend: MS: multiple sclerosis; RR: relapsing-remitting; SP: secondary progressive; HC: healthy controls; EDSS: Expanded Disability Status Scale; DMT: disease modifying treatment; IQR: interquartile range (25th – 75th percentile); SD: standard deviation.
p values represent total MS and HC group comparisons as well as RRMS and SPMS comparisons and were derived using Fisher’s Exact test
, Student’s t-test
and Mann-Whitney U-test
. Significant p values < 0.05 are represented in bold.
Other DMT include intravenous immunoglobulin, mycophenolate mofetil and mitoxantrone.
Neuropsychological outcomes in patients with MS vs. HCs:
Cognitive assessments revealed significantly lower scores in PwMS compared to HCs after adjusting for age, sex and education: PASAT (p=0.045), SDMT (p<0.001), CVLT-2 total learning (p<0.001), CVLT-2 delayed recall (p<0.001), CVLT-2 discrimination index (p=0.001), CVLT-2 retention (p=0.011), BVMT-R total learning (p<0.001) and BVMT-R delayed recall (p<0.001). Full details, as well as comparisons between RRMS and SPMS patients, are shown in Table 2.
Table 2:
Magnetic resonance imaging in NBM and neuropsychological metrics of patients with MS and healthy controls.
| MS (n=84) | HC (n=19) | p value | RRMS (n=54) | SPMS (n=30) | p value | |
|---|---|---|---|---|---|---|
|
| ||||||
| T2 LV in mL median (IQR) | 9.75 (3.82 – 25.86) | 0.21 (0.14 – 1.71) | <0.001 | 9.59 (3.20 – 23.47) | 10.93 (5.44 – 26.24) | 0.806 |
|
| ||||||
| Normalized NBM volume in mL | 0.47 (0.05) | 0.48 (0.06) | 0.379 | 0.47 (0.05) | 0.47 (0.05) | 0.904 |
|
| ||||||
| Normalized WB volume in mL | 1454.12 (90.03) | 1509.55 (71.03) | 0.034 | 1474.46 (87.36) | 1417.52 (84.23) | 0.363 |
|
| ||||||
| Normalized GM volume in mL | 735.92 (57.15) | 754.96 (42.54) | 0.361 | 749.79 (54.20) | 710.95 (54.56) | 0.290 |
|
| ||||||
| Normalized WM volume in mL | 718.20 (49.76) | 754.59 (34.49) | 0.008 | 724.67 (48.89) | 706.57 (50.02) | 0.678 |
|
| ||||||
| Normalized cortical volume in mL | 597.14 (46.87) | 616.39 (38.27) | 0.212 | 609.02 (44.64) | 575.76 (43.72) | 0.263 |
|
| ||||||
| Normalized thalamus volume in mL | 18.23 (2.58) | 20.42 (1.43) | 0.001 | 18.70 (2.49) | 17.38 (2.56) | 0.448 |
|
| ||||||
| Normalized hippocampus volume in mL | 9.01 (1.28) | 9.43 (1.13) | 0.109 | 9.16 (1.16) | 8.75 (1.45) | 0.563 |
|
| ||||||
| NBM FA in unitless measures | 0.25 (0.03) | 0.26 (0.03) | 0.331 | 0.25 (0.03) | 0.25 (0.04) | 0.715 |
|
| ||||||
| NBM MD in mm2/s * 1000 | 0.63 (0.05) | 0.60 (0.04) | 0.008 | 0.63 (0.05) | 0.64 (0.06) | 0.543 |
|
| ||||||
| NBM D∥ in mm2/s * 1000 | 0.92 (0.07) | 0.89 (0.05) | 0.062 | 0.91 (0.06) | 0.92 (0.08) | 0.706 |
|
| ||||||
| NBM D⊥ in mm2/s * 1000 | 0.49 (0.06) | 0.45 (0.04) | 0.005 | 0.49 (0.05) | 0.50 (0.07) | 0.507 |
|
| ||||||
| SDMT | 48.15 (15.49) | 64.84 (9.87) | <0.001 | 49.35 (15.08) | 46.00 (16.25) | 0.755 |
|
| ||||||
| CVLT-2 | ||||||
| total learning | 50.76 (12.74) | 66.21 (6.34) | <0.001 | 51.85 (11.92) | 48.80 (14.10) | 0.738 |
| delayed recall | 10.81 (3.47) | 14.53 (1.39) | <0.001 | 11.17 (3.32) | 10.17 (3.69) | 0.729 |
| discrimination index | 3.06 (0.87) | 3.83 (0.21) | 0.001 | 3.06 (0.86) | 3.04 (0.90) | 0.644 |
| retention | 0.87 (0.14) | 0.97 (0.04) | 0.011 | 0.88 (0.15) | 0.86 (0.14) | 0.716 |
|
| ||||||
| BVMT-R | ||||||
| total learning | 19.37 (7.30) | 27.00 (4.94) | <0.001 | 19.56 (7.79) | 19.03 (6.46) | 0.843 |
| delayed recall | 7.49 (2.76) | 10.11 (1.56) | <0.001 | 7.52 (2.86) | 7.43 (2.61) | 0.516 |
| discrimination index | 5.50 (1.00) | 5.95 (0.23) | 0.071 | 5.39 (1.12) | 5.70 (0.70) | 0.324 |
| retention | 0.91 (0.13) | 0.94 (0.08) | 0.306 | 0.92 (0.12) | 0.88 (0.15) | 0.981 |
|
| ||||||
| PASAT | 40.57 (14.77) | 48.26 (8.84) | 0.045 | 39.85 (15.24) | 41.87 (14.05) | 0.942 |
All cells show the mean value and the respective standard deviation in brackets, unless otherwise specified.
Legend: NBM: nucleus basalis of Meynert; MS: multiple sclerosis; RR: relapsing-remitting; SP: secondary progressive; HC: healthy controls; LV: lesion volume; WB: whole brain; GM: gray matter; WM: white matter; FA: fractional anisotropy; MD: mean diffusivity; D∥: axial diffusivity; D⊥: radial diffusivity; SDMT: Symbol Digit Modalities Test; CVLT-2: California Verbal Learning Test second edition; BVMT-R: Brief Visuospatial Memory Test Revised; PASAT: Paced Auditory Serial Addition Test; IQR: interquartile range (25th – 75th percentile).
p values represent MS and HC group comparisons as well as RRMS and SPMS comparisons and were derived using analysis of covariance (ANCOVA), adjusted for age and sex for imaging measures and age, sex, and education for neuropsychological measures.
Significant p values < 0.05 are represented in bold.
Differences in MRI metrics between patients with MS and HCs:
PwMS showed a significantly lower WM volume (p=0.008), WB volume (p=0.034) and normalized thalamic volume (p=0.001) compared to HCs. As expected, PwMS had significantly higher T2 lesion volume (p<0.001). NBM DTI revealed significantly increased MD (p=0.008) and D⊥ (p=0.005) in the patient group. Additional details are provided in Table 2.
Correlation between NBM metrics and clinical associations in patients with MS:
When considering the PwMS group as a whole, after controlling for age and education and adjusting for false discovery rate, SDMT score of PwMS was significantly associated with NBM MD (r=−0.38, p<0.001) and D⊥ (r=−0.40, p<0.001). BVMT-R total learning scores were significantly associated with NBM MD (r=−0.33, p=0.031) and D⊥ (r=−0.37, p=0.030), and PASAT scores of PwMS were significantly correlated with NBM MD (r=−0.37, p=0.008) and NBM D⊥ (r=−0.39, p<0.001). Correlations between cognitive assessments and NBM metrics are separately shown for RRMS and SPMS patients in Table 3. Associations between NBM DTI and SDMT, CVLT-2 total learning, BVMT-R total learning as well as PASAT are shown in supplement Figure e-1, Figure e-2, Figure e-3 and Figure e-4. For descriptive purposes, correlations between NBM measures and other imaging-derived measures are shown in supplement Table e-1. Finally, disease duration was not associated with NBM metrics when considering the entire PwMS cohort, but a significant association was found in normalized NBM volume when considering only the SPMS cohort (r=−0.41, p= 0.026, Table e-2).
Table 3:
Partial correlation matrix of magnetic resonance imaging in NBM and neuropsychological testing in patients with MS.
| NBM FA | NBM MD | NBM D∥ | NBM D⊥ | Normalized NBM volume | |
|---|---|---|---|---|---|
|
| |||||
| SDMT | |||||
| RRMS | 0.16 (0.411) | −0.41 (0.033) | −0.31 (0.156) | −0.44 (0.033) | 0.17 (0.392) |
| SPMS | 0.13 (0.623) | −0.34 (0.222) | −0.21 (0.442) | −0.37 (0.181) | 0.36 (0.181) |
|
| |||||
| CVLT-2 | |||||
| total learning | |||||
| RRMS | 0.04 (0.843) | −0.33 (0.121) | −0.29 (0.174) | −0.33 (0.121) | 0.17 (0.391) |
| SPMS | 0.13 (0.626) | −0.23 (0.403) | −0.14 (0.598) | −0.25 (0.392) | 0.28 (0.342) |
| delayed recall | |||||
| RRMS | 0.06 (0.730) | −0.27 (0.181) | −0.21 (0.342) | −0.29 (0.171) | 0.18 (0.392) |
| SPMS | 0.15 (0.572) | −0.36 (0.181) | −0.24 (0.392) | −0.38 (0.181) | 0.25 (0.392) |
| discrimination index | |||||
| RRMS | 0.13 (0.528) | −0.41 (0.033) | −0.34 (0.117) | −0.42 (0.033) | 0.18 (0.392) |
| SPMS | −0.02 (0.921) | −0.36 (0.181) | −0.25 (0.392) | −0.38 (0.181) | 0.10 (0.695) |
| retention | |||||
| RRMS | 0.06 (0.752) | −0.09 (0.626) | −0.03 (0.898) | −0.12 (0.533) | 0.29 (0.173) |
| SPMS | 0.26 (0.381) | −0.46 (0.117) | −0.29 (0.342) | −0.50 (0.086) | 0.02 (0.921) |
|
| |||||
| BVMT-R | |||||
| total learning | |||||
| RRMS | 0.09 (0.633) | −0.31 (0.144) | −0.21 (0.338) | −0.35 (0.117) | 0.13 (0.520) |
| SPMS | 0.35 (0.203) | −0.41 (0.158) | −0.23 (0.392) | −0.46 (0.117) | 0.36 (0.181) |
| delayed recall | |||||
| RRMS | 0.17 (0.392) | −0.32 (0.144) | −0.20 (0.362) | −0.36 (0.100) | 0.11 (0.572) |
| SPMS | 0.15 (0.577) | −0.34 (0.222) | −0.24 (0.392) | −0.35 (0.215) | 0.22 (0.411) |
| discrimination index | |||||
| RRMS | 0.12 (0.564) | −0.11 (0.577) | −0.06 (0.755) | −0.13 (0.520) | 0.11 (0.577) |
| SPMS | 0.16 (0.562) | −0.37 (0.181) | −0.23 (0.392) | −0.40 (0.170) | −0.05 (0.864) |
| retention | |||||
| RRMS | 0.10 (0.583) | −0.17 (0.392) | −0.15 (0.438) | −0.17 (0.392) | −0.07 (0.724) |
| SPMS | 0.27 (0.363) | −0.10 (0.711) | −0.19 (0.478) | −0.02 (0.935) | 0.05 (0.864) |
|
| |||||
| PASAT | |||||
| RRMS | 0.20 (0.362) | −0.44 (0.033) | −0.30 (0.156) | −0.49 (0.005) | 0.06 (0.758) |
| SPMS | 0.03 (0.900) | −0.29 (0.338) | −0.22 (0.411) | −0.29 (0.338) | 0.30 (0.338) |
All cells show the correlation coefficient r and the respective p value in brackets.
Legend: NBM: nucleus basalis of Meynert; MS: multiple sclerosis; RR: relapsing-remitting; SP: secondary progressive; FA: fractional anisotropy; MD: mean diffusivity; D∥: axial diffusivity; D⊥: radial diffusivity; SDMT: Symbol Digit Modalities Test; CVLT-2: California Verbal Learning Test second edition; BVMT-R: Brief Visuospatial Memory Test Revised; PASAT: Paced Auditory Serial Addition Test.
All neuropsychological measures were controlled for age and education and adjusted for false discovery rate using the Benjamini-Hochberg procedure.
Significant correlations with corrected p values < 0.05 are shown in bold while original p values < 0.05 that did not survive corrections for multiple comparisons are shown in italics.
Linear regressions in patients with MS:
For SDMT and BVMT-R total learning, NBM D⊥ significantly explained additional variance after controlling for age, education, and NBM volume for both RRMS and SPMS groups. For CVLT-2 total learning and PASAT, NBM D⊥ and MD explained additional variance, respectively, after controlling for the aforementioned variables in the RRMS cohort but no NBM DTI metrics were retained as significant for the SPMS cohort. Table 4 shows the results of linear regression analyses separately for RRMS and SPMS patients for all of the cognitive assessments.
Table 4:
Linear regression analysis of neuropsychological measures in patients with MS and the influence of NBM metrics.
| Variables | R2 | Adjusted R2 | ΔR2 | p value | |
|---|---|---|---|---|---|
|
| |||||
| SDMT | RRMS: | ||||
| Model 1: Age, education | 0.03 | −0.01 | 0.03 | 0.507 | |
| Model 2: Normalized NBM volume | 0.05 | 0.00 | 0.03 | 0.239 | |
| Model 3: NBM D⊥ | 0.23 | 0.17 | 0.18 | 0.001 | |
| SPMS: | |||||
| Model 1: Age, education | 0.08 | 0.01 | 0.08 | 0.316 | |
| Model 2: Normalized NBM volume | 0.20 | 0.11 | 0.12 | 0.058 | |
| Model 3: NBM D⊥ | 0.33 | 0.22 | 0.13 | 0.038 | |
|
| |||||
| CVLT-2 total learning | RRMS: | ||||
| Model 1: Age, education | 0.06 | 0.02 | 0.06 | 0.217 | |
| Model 2: Normalized NBM volume | 0.09 | 0.03 | 0.03 | 0.234 | |
| Model 3: NBM MD | 0.19 | 0.12 | 0.10 | 0.017 | |
| SPMS: | |||||
| Model 1: Age, education | 0.13 | 0.07 | 0.13 | 0.151 | |
| Model 2: Normalized NBM volume | 0.20 | 0.11 | 0.07 | 0.147 | |
|
| |||||
| BVMT-R total learning | RRMS: | ||||
| Model 1: Age, education | 0.02 | −0.02 | 0.02 | 0.682 | |
| Model 2: Normalized NBM volume | 0.03 | −0.03 | 0.02 | 0.363 | |
| Model 3: NBM D⊥ | 0.15 | 0.08 | 0.12 | 0.013 | |
| SPMS: | |||||
| Model 1: Age, education | 0.02 | −0.05 | 0.02 | 0.756 | |
| Model 2: Normalized NBM volume | 0.15 | 0.05 | 0.13 | 0.058 | |
| Model 3: NBM D⊥ | 0.36 | 0.26 | 0.21 | 0.008 | |
|
| |||||
| Delayed recall CVLT-2 | RRMS: | ||||
| Model 1: Age, education | 0.07 | 0.04 | 0.07 | 0.141 | |
| Model 2: Normalized NBM volume | 0.10 | 0.05 | 0.03 | 0.211 | |
| Model 3: NBM D⊥ | 0.18 | 0.11 | 0.07 | 0.043 | |
| SPMS: | |||||
| Model 1: Age, education | 0.18 | 0.11 | 0.18 | 0.075 | |
| Model 2: Normalized NBM volume | 0.23 | 0.14 | 0.05 | 0.192 | |
| Model 3: NBM D⊥ | 0.35 | 0.25 | 0.12 | 0.040 | |
|
| |||||
| Delayed recall BVMT-R | RRMS: | ||||
| Model 1: Age, education | 0.04 | 0.00 | 0.04 | 0.403 | |
| Model 2: Normalized NBM volume | 0.05 | −0.01 | 0.01 | 0.431 | |
| Model 3: NBM D⊥ | 0.17 | 0.10 | 0.12 | 0.010 | |
| SPMS: | |||||
| Model 1: Age, education | 0.04 | −0.04 | 0.04 | 0.618 | |
| Model 2: Normalized NBM volume | 0.08 | −0.03 | 0.05 | 0.267 | |
|
| |||||
| Discrimination index CVLT-2 | RRMS: | ||||
| Model 1: Age, education | 0.01 | −0.03 | 0.01 | 0.836 | |
| Model 2: Normalized NBM volume | 0.04 | −0.02 | 0.03 | 0.212 | |
| Model 3: NBM MD | 0.20 | 0.14 | 0.17 | 0.002 | |
| SPMS: | |||||
| Model 1: Age, education | 0.26 | 0.21 | 0.26 | 0.016 | |
| Model 2: Normalized NBM volume | 0.27 | 0.19 | 0.01 | 0.598 | |
|
| |||||
| Discrimination index BVMT-R | RRMS: | ||||
| Model 1: Age, education | 0.01 | −0.03 | 0.01 | 0.774 | |
| Model 2: Normalized NBM volume | 0.02 | −0.04 | 0.01 | 0.456 | |
| SPMS: | |||||
| Model 1: Age, education | 0.06 | −0.01 | 0.06 | 0.423 | |
| Model 2: Normalized NBM volume | 0.06 | −0.04 | 0.00 | 0.821 | |
| Model 3: NBM D⊥ | 0.22 | 0.09 | 0.15 | 0.038 | |
|
| |||||
| Retention CVLT-2 | RRMS: | ||||
| Model 1: Age, education | 0.03 | −0.01 | 0.03 | 0.480 | |
| Model 2: Normalized NBM volume | 0.11 | 0.06 | 0.08 | 0.038 | |
| SPMS: | |||||
| Model 1: Age, education | 0.05 | −0.02 | 0.05 | 0.519 | |
| Model 2: Normalized NBM volume | 0.05 | −0.06 | 0.00 | 0.911 | |
| Model 3: NBM D⊥ | 0.29 | 0.18 | 0.24 | 0.008 | |
|
| |||||
| Retention BVMT-R | RRMS: | ||||
| Model 1: Age, education | 0.13 | 0.10 | 0.13 | 0.027 | |
| Model 2: Normalized NBM volume | 0.14 | 0.08 | 0.00 | 0.637 | |
| SPMS: | |||||
| Model 1: Age, education | 0.03 | −0.04 | 0.03 | 0.670 | |
| Model 2: Normalized NBM volume | 0.03 | −0.08 | 0.00 | 0.815 | |
|
| |||||
| PASAT | RRMS: | ||||
| Model 1: Age, education | 0.03 | −0.01 | 0.03 | 0.472 | |
| Model 2: Normalized NBM volume | 0.03 | −003 | 0.00 | 0.697 | |
| Model 3: NBM D⊥ | 0.26 | 0.20 | 0.23 | <0.001 | |
| SPMS: | |||||
| Model 1: Age, education | 0.13 | 0.07 | 0.13 | 0.146 | |
| Model 2: Normalized NBM volume | 0.21 | 0.12 | 0.08 | 0.126 | |
Legend: MS: multiple sclerosis; RR: relapsing-remitting; SP: secondary progressive; NBM: nucleus basalis of Meynert; MD: mean diffusivity; D⊥: radial diffusivity; SDMT: Symbol Digit Modalities Test; CVLT-2: California Verbal Learning Test second edition; BVMT-R: Brief Visuospatial Memory Test Revised; PASAT: Paced Auditory Serial Addition Test; DTI: diffusion tensor imaging.
Model 1 forces age and education into the model.
Model 2 forces normalized NBM volume into the model while retaining demographics.
Model 3 adds NBM DTI metrics to the model with forward selection while retaining demographics and normalized NBM volume.
Significant p values < 0.05 are represented in bold.
Additional results controlling for other imaging measures rather than NBM volume (e.g., WB volume, NAWB MD, thalamic volume, hippocampal volume, cortical volume, T2 lesion volume) are shown in supplement Tables e-3 through e-8.
Predicting cognitive score change:
Of the 67 patients with cognitive scores available at follow-up, there were 43 RRMS and 24 SPMS patients. The 17 patients who were not evaluated at follow-up declined to participate or had moved out of the area. Results for each of the cognitive score changes are presented in Table 5. In the RRMS sample, NBM measures were retained for 5 of the 10 assessments (SDMT, PASAT, CVLT-2 delayed recall, CVLT-2 discrimination index, and CVLT-2 retention), while NAWB MD was retained for SDMT, CVLT-2 total learning and PASAT, and WB volume was retained for CVLT-2 delayed recall and BVMT-R discrimination index. The association between the normalized NBM volume at baseline and the change of the raw SDMT score from baseline over 3-year follow-up is shown in Figure 2. No imaging measures were retained as significant in the regression model for BVMT-R total learning, BVMT-R delayed recall, and BVMT-R retention. In the SPMS sample, NBM measures were retained for BVMT-R delayed recall and PASAT, while other measures explained additional variance for SDMT (cortical volume), BVMT-R discrimination index (T2 LV) and BVMT-R retention (T2 LV and hippocampal volume). No imaging measures were retained for the remaining five outcomes (all four CVLT-2 measures and BVMT-R total learning).
Table 5:
Predicting change of the cognitive score in patients with MS with NBM metrics and other imaging measures.
| Variables | R2 | Adjusted R2 | ΔR2 | p value | |
|---|---|---|---|---|---|
|
| |||||
| SDMT | RRMS: | ||||
| Model 1: Age, education, SDMT score at BL | 0.048 | −0.025 | 0.048 | 0.583 | |
| Model 2: + NBM volume | 0.307 | 0.234 | 0.259 | 0.001 | |
| Model 3: + NAWB MD | 0.390 | 0.308 | 0.083 | 0.031 | |
| SPMS: | |||||
| Model 1: Age, education, SDMT score at BL | 0.240 | 0.126 | 0.240 | 0.132 | |
| Model 2: + Cortical volume | 0.456 | 0.342 | 0.216 | 0.013 | |
|
| |||||
| CVLT-2 total learning | RRMS: | ||||
| Model 1: Age, education, CVLT-2 score at BL | 0.086 | 0.015 | 0.086 | 0.316 | |
| Model 2: + NAWB MD | 0.188 | 0.103 | 0.102 | 0.035 | |
| SPMS: | |||||
| Model 1: Age, education, CVLT-2 score at BL | 0.174 | 0.056 | 0.174 | 0.249 | |
|
| |||||
| BVMT-R total learning | RRMS: | ||||
| Model 1: Age, education, BVMT-R score at BL | 0.123 | 0.055 | 0.123 | 0.160 | |
| SPMS: | |||||
| Model 1: Age, education, BVMT-R score at BL | 0.304 | 0.199 | 0.304 | 0.060 | |
|
| |||||
| Delayed recall CVLT-2 | RRMS: | ||||
| Model 1: Age, education, delayed CVLT-2 score at BL | 0.006 | −0.070 | 0.006 | 0.970 | |
| Model 2: + WB volume | 0.299 | 0.225 | 0.293 | <0.001 | |
| Model 3: + NBM volume | 0.387 | 0.305 | 0.089 | 0.026 | |
| SPMS: | |||||
| Model 1: Age, education, delayed CVLT-2 score at BL | 0.032 | −0.106 | 0.032 | 0.874 | |
|
| |||||
| Delayed recall BVMT-R | RRMS: | ||||
| Model 1: Age, education, delayed BVMT-R score at BL | 0.179 | 0.116 | 0.179 | 0.050 | |
| SPMS: | |||||
| Model 1: Age, education, delayed BVMT-R score at BL | 0.190 | 0.069 | 0.190 | 0.228 | |
| Model 2: + NBM FA | 0.406 | 0.280 | 0.215 | 0.017 | |
|
| |||||
| Discrimination index CVLT-2 | RRMS: | ||||
| Model 1: Age, education, CVLT-2 discrimination index at BL | 0.219 | 0.159 | 0.219 | 0.021 | |
| Model 2: + NBM volume | 0.308 | 0.235 | 0.089 | 0.033 | |
| SPMS: | |||||
| Model 1: Age, education, CVLT-2 discrimination index at BL | 0.185 | 0.069 | 0.185 | 0.221 | |
|
| |||||
| Discrimination index BVMT-R | RRMS: | ||||
| Model 1: Age, education, BVMT-R discrimination index at BL | 0.082 | 0.011 | 0.082 | 0.339 | |
| Model 2: + WB volume | 0.424 | 0.363 | 0.342 | <0.001 | |
| SPMS: | |||||
| Model 1: Age, education, BVMT-R discrimination index at BL | 0.295 | 0.190 | 0.295 | 0.067 | |
| Model 2: + T2 LV | 0.515 | 0.413 | 0.220 | 0.008 | |
|
| |||||
| Retention CVLT-2 | RRMS: | ||||
| Model 1: Age, education, CVLT-2 retention at BL | 0.037 | −0.037 | 0.037 | 0.681 | |
| Model 2: + Thalamic volume | 0.291 | 0.216 | 0.252 | 0.001 | |
| Model 3: + NBM volume | 0.392 | 0.310 | 0.102 | 0.017 | |
| Model 4: + NBM D∥ | 0.469 | 0.380 | 0.076 | 0.029 | |
| SPMS: | |||||
| Model 1: Age, education, CVLT-2 retention at BL | 0.123 | −0.002 | 0.123 | 0.420 | |
|
| |||||
| Retention BVMT-R | RRMS: | ||||
| Model 1: Age, education, BVMT-R retention at BL | 0.279 | 0.223 | 0.279 | 0.005 | |
| SPMS: | |||||
| Model 1: Age, education, BVMT-R retention at BL | 0.471 | 0.392 | 0.471 | 0.005 | |
| Model 2: + T2 LV | 0.581 | 0.492 | 0.110 | 0.038 | |
| Model 3: + Hippocampal volume | 0.679 | 0.590 | 0.098 | 0.030 | |
|
| |||||
| PASAT | RRMS: | ||||
| Model 1: Age, education, PASAT score at BL | 0.113 | 0.043 | 0.113 | 0.201 | |
| Model 2: + NAWB MD | 0.297 | 0.221 | 0.184 | 0.004 | |
| Model 3: + NBM volume | 0.371 | 0.284 | 0.074 | 0.047 | |
| SPMS: | |||||
| Model 1: Age, education, PASAT score at BL | 0.041 | −0.096 | 0.041 | 0.825 | |
| Model 2: + NBM FA | 0.319 | 0.183 | 0.278 | 0.010 | |
Legend: MS: multiple sclerosis; RR: relapsing-remitting; SP: secondary progressive; NBM: nucleus basalis of Meynert; NAWB: normal-appearing whole brain; WB: whole brain; MD: mean diffusivity; FA: fractional anisotropy; D∥: axial diffusivity; SDMT: Symbol Digit Modalities Test; CVLT-2: California Verbal Learning Test second edition; BVMT-R: Brief Visuospatial Memory Test Revised; PASAT: Paced Auditory Serial Addition Test; BL: baseline.
Model 1 forces age, education and the baseline score of the respective cognitive test into the model.
Models 2, 3 and 4 show which measures were retained as significant when NBM metrics, WB volume, NAWB MD, cortical volume, thalamic volume and hippocampal volume were added using forward selection while retaining demographics and baseline test score.
Significant p values < 0.05 are represented in bold.
Figure 2:
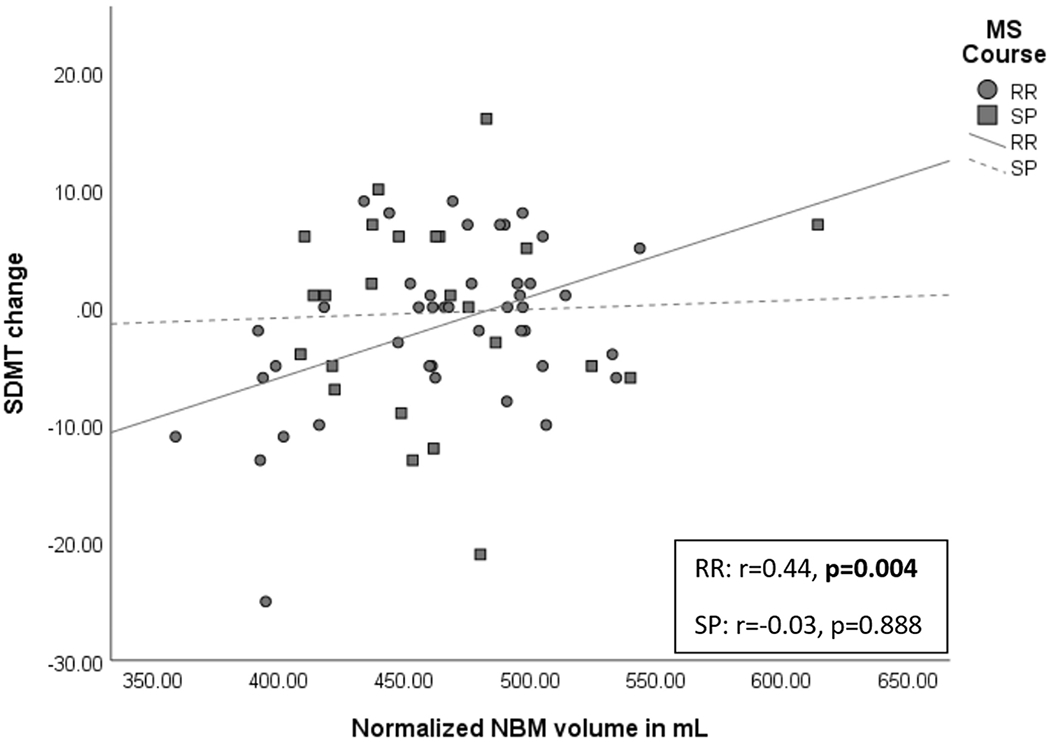
Scatter plot showing the association between the normalized NBM volume at baseline and the change of the raw SDMT score from baseline over 3-year follow-up in patients with RRMS and SPMS. Partial correlation r values and p values are shown after controlling for age and years of education.
In the HC sample, no NBM measures were associated with change of cognitive test scores (see Table e-9). Supplement Figure e-5 shows the nonsignificant association between SDMT change and NBM volume in HCs.
Discussion
In the present study, we investigated the association between damage to the NBM and cognitive state in PwMS.
We found significantly increased MD and D⊥ in the NBM of PwMS compared to HCs. As the NBM is associated with cognitive processes,[1, 2] a link between microstructural damage of the NBM and cognitive impairment seems reasonable. Increased NBM diffusivity was correlated with lower scores in all cognitive assessments.
Our findings are in line with previous investigations of the NBM microstructure in other neurodegenerative diseases, which revealed that increased MD and D⊥ levels of the NBM in patients with PD and an increased NBM MD level in patients with AD are associated with cognitive decline.[6, 13, 14] While direct interpretation of DTI measures is fraught with difficulty, especially in the gray matter, increased tissue damage is reflected by greater diffusivity.[23, 24] Nevertheless, our findings confirm that increased MD and D⊥ levels of the NBM are a structural correlate of cognitive impairment in neurodegenerative diseases and show that this finding includes PwMS.
As it is well-known that cognitive impairment in PwMS involves widespread damage throughout the brain,[25] we sought to show that NBM can still inform on cognitive state in PwMS even after controlling for several key imaging measures. In this regard, we accounted for WB volume, reflecting overall atrophy as a result of disease progression, as well as NAWB MD, reflecting microstructural damage throughout the normal-appearing brain tissue. We showed that NBM MD and D⊥ explained incremental variance to the SDMT, BVMT-R total learning, CVLT-2 discrimination index as well as PASAT outcomes after accounting for the normalized WB volume and the MD of the NAWB, respectively. NBM MD was additionally retained for the CVLT-2 delayed recall after accounting for the normalized WB volume. Further, we showed that NBM MD and NBM D⊥ continue to explain additional variance in several cognitive tests, when controlling for hippocampal, thalamic or cortical GM volume. Our findings support the notion that the NBM represents an, as of now, scarcely studied region that explains cognitive status in PwMS.
NBM volume did not differ significantly between PwMS and HCs, suggesting that NBM atrophy is not an early feature of the disease, unlike that which has been shown in AD and PD.[6, 14] Another possibility is that our HC sample was atypical, and characterized by greater atrophy than would otherwise be expected. Nevertheless, lower NBM volume in the patient cohort tended to be associated with lower SDMT scores. We also showed that after controlling for NBM volume, NBM DTI metrics still added incremental variance in NP outcomes. We found that out of all of the investigated measures, including the non-NBM measures of WB, thalamic, hippocampal and cortical volumes in addition to T2 LV and NAWB MD, NBM damage was the most consistently predictive of change in cognitive status over time in the RRMS sample. This was reflected by an association between NBM metrics and a decrease in PASAT and SDMT as well as CVLT-2 and BVMT-R subtest scores. However, in the SPMS sample, baseline NBM measures were only predictive of change in cognition for BVMT-R delayed recall and PASAT, while changes in SDMT, BVMT-R discrimination index, and BVMT-R retention were associated with non-NBM imaging measures at baseline. Together with our cross-sectional results, these findings suggest that the NBM may play a key role in determining cognitive state in PwMS, at least in patients with a RRMS disease course. Our results point towards DTI and volumetric NBM assessments as potentially serving as imaging biomarkers of cognition in MS, particularly in the relapsing-remitting phase. The more limited number of associations between baseline NBM metrics and cognitive change in SPMS might possibly be explained by more extensive tissue damage throughout the brain in these patients; as the disease progresses, other factors, such as widespread network collapse, may have more of a role in determining subsequent cognitive decline.[26]
Previous studies have suggested that the cholinergic system, of which the NBM is an essential part, plays a key role in the regulation of neuro-immune interactions and immune responses.[27, 28] Recent data has shown significantly increased expression and activity of acetylcholine (ACh) hydrolyzing enzymes and significantly lower ACh levels in MS patients compared to HCs.[29, 30] The resulting dysregulated ACh homeostasis might be driven by NBM damage. The ACh imbalance is suggested to have a negative effect on the modulation of inflammatory processes and consequently disease outcome in MS.[31] This assumption has been confirmed in studies of experimental autoimmune encephalomyelitis where treatment with cholinesterase inhibitors reduced neuroinflammation and ameliorated disease symptoms.[27] The administration of cholinesterase inhibitors diminishes the ACh dysregulation possibly driven by NBM damage and therefore constitutes a promising avenue for novel pharmacological interventions aimed at improving cognition in MS patients.[32] In fact, it was previously shown in a single center trial that donepezil improved both learning and memory in PwMS with cognitive impairment.[33] These findings were not replicated in a multicenter randomized trial,[34] but secondary analyses suggested that there might be a benefit for the most severely impaired patients.
Our study is not without limitations. Longitudinal imaging studies will be needed to better understand the temporal association between NBM damage and cognitive decline in PwMS. Nevertheless, we showed a potential prognostic role of baseline NBM damage for predicting cognitive decline. Moreover, our diffusion protocol was relatively limited in terms of spatial and angular resolution. More advanced imaging protocols will allow for the calculation of quantitative measures that provide increased specificity to the underlying tissue properties compared to standard DTI metrics. Finally, 17 PwMS were lost to follow-up. However, they did not differ significantly in terms of baseline characteristics. Thus, it is unlikely that our results concerning the prediction of cognitive decline are biased. Finally, we found evidence of white matter, but not whole brain or global gray matter, atrophy in the MS sample. This may potentially represent a peculiarity of the current sample, either with respect to the healthy controls (being worse off than typical healthy controls) or the MS patients (being better off than the overall MS population). Nevertheless, we do not have reason to believe that this limits the generalizability of our NBM-specific findings in the MS sample.
We conclude that NBM assessment may represent a useful imaging marker to gain further insights into the structural correlates of cognitive status in patients with MS.
Supplementary Material
Financial Relationships/Potential Conflicts of Interest:
Franziska Hildesheim, Tom Fuchs, Dejan Jakimovski, and Niels Bergsland have nothing to disclose.
Ralph H. B. Benedict has received research support from Accorda, Novartis, Genzyme, Biogen Idec, and Mallinkrodt, and is on the speakers’ bureau for EMD Serono, and consults for Biogen Idec, Genentech, Roche, Sanofi/Genzyme, Takeda, NeuroCog Trials, and Novartis. Dr. Benedict also receives royalties for Psychological Assessment Resources.
Robert Zivadinov received personal compensation from EMD Serono, Sanofi, Bristol Myers Squibb, Keystone Heart and Novartis for speaking and consultant fees. He received financial support for research activities from Novartis, Protembis, Bristol Myers Squibb, Keystone Heart, Mapi Pharma, V-WAVE Medical and Boston Scientific.
Michael G. Dwyer has received consultant fees from Claret Medical and EMD Serono and research grant support from Novartis.
Bianca Weinstock-Guttman received honoraria as a speaker and/or as a consultant for Biogen Idec, EMD Serono, Genentech, Novartis, Mallinckrodt, Celgene, Abbvie. Dr Weinstock-Guttman received research funds from Biogen Idec, EMD Serono, Genentech, and Novartis.
Funding:
The research reported in this publication was funded by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- ACh
acetylcholine
- AD
Alzheimer’s disease
- ANCOVA
Analysis of Covariance
- BICAMS
Brief International Cognitive Assessment for MS
- BVMT-R
Brief Visuospatial Memory Test Revised
- CVLT-2
California Verbal Learning Test second edition
- DTI
diffusion tensor imaging
- D∥
axial diffusivity
- D⊥
radial diffusivity
- FA
fractional anisotropy
- FIRST
FMRIB’s Integrated Registration and Segmentation Tool
- FLAIR
fluid-attenuated inversion recovery
- GM
gray matter
- IQR
interquartile range
- LV
lesion volume
- MD
mean diffusivity
- MS
multiple sclerosis
- NAWB
normal-appearing whole brain
- NBM
nucleus basalis of Meynert
- NP
neuropsychological
- PASAT
Paced Auditory Serial Addition Test
- PD
Parkinson’s disease
- PwMS
patients with MS
- RRMS
relapsing-remitting multiple sclerosis
- SDMT
Symbol Digit Modalities Test
- SIENAX
Structural Image Evaluation, using Normalisation, of Atrophy – X-sectional
- SPMS
secondary progressive multiple sclerosis
- WM
white matter
- WB
whole brain
References
- 1.Nemy M, Cedres N, Grothe MJ, Muehlboeck JS, Lindberg O, Nedelska Z, et al. Cholinergic white matter pathways make a stronger contribution to attention and memory in normal aging than cerebrovascular health and nucleus basalis of Meynert. Neuroimage. 2020;211. [DOI] [PubMed] [Google Scholar]
- 2.Koulousakis P, Andrade P, Visser-Vandewalle V, Sesia T. The nucleus basalis of meynert and its role in deep brain stimulation for cognitive disorders: A historical perspective. J Alzheimer’s Dis. 2019;69(4):905–19. [DOI] [PubMed] [Google Scholar]
- 3.Mesulam M Cholinergic Circuitry of the Human Nucleus Basalis and Its Fate in Alzheimer̀s Disease. J Comp Neurol. 2013;521(18):4124–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohnen NI, Albin RL. The cholinergic system in Parkinson’s disease. Behav Brain Res. 2011;221(2):564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesulam M, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: Cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (Substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983;214:170–97. [DOI] [PubMed] [Google Scholar]
- 6.Schulz J, Pagano G, Fernández Bonfante JA, Wilson H, Politis M. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson’s disease. Brain. 2018;1501–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu SY, Jiang G, Zheng Z, Ma L, Wang B, Zeng Q, et al. A New Neural Pathway from the Ventral Striatum to the Nucleus Basalis of Meynert with Functional Implication to Learning and Memory. Mol Neurobiol. 2019;7222–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jethwa KD, Dhillon P, Meng D, Auer DP. Are linear measurements of the nucleus basalis of Meynert suitable as a diagnostic biomarker in mild cognitive impairment and Alzheimer disease? Am J Neuroradiol. 2019;1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tata AM, Velluto L, D’Angelo C, Reale M. Cholinergic System Dysfunction and Neurodegenerative Diseases: Cause or Effect? CNS & Neurological Disorders - Drug Targets. 2014;13(7). [DOI] [PubMed] [Google Scholar]
- 10.Gang M, Baba T, Hosokai Y, Nishio Y, Kikuchi A, Hirayama K, et al. Clinical and Cerebral Metabolic Changes in Parkinson’s Disease With Basal Forebrain Atrophy. Mov Disord. 2020;1–8. [DOI] [PubMed] [Google Scholar]
- 11.Hepp DH, Foncke EMJ, Berendse HW, Wassenaar TM, Olde Dubbelink KTE, Groenewegen HJ, et al. Damaged fiber tracts of the nucleus basalis of Meynert in Parkinson’s disease patients with visual hallucinations. Sci Rep. 2017;7(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohnen NI, Mueller MLTM, Kotagal V, Koeppe RA, Kilbourn MR, Gilman S, et al. Heterogeneity of cholinergic denervation in Parkinson’s disease without dementia. J Cereb Blood Flow Metab. 2012;32:1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brueggen K, Dyrba M, Barkhof F, Hausner L, Filippi M, Nestor PJ, et al. Basal forebrain and hippocampus as predictors of conversion to Alzheimer’s disease in patients with mild cognitive impairment-a multicenter DTI and volumetry study. J Alzheimer’s Dis. 2015;48(1):197–204. [DOI] [PubMed] [Google Scholar]
- 14.Teipel SJ, Meindl T, Grinberg L, Grothe M, Cantero JL, Reiser MF, et al. The Cholinergic System in Mild Cognitive Impairment and Alzheimeŕs Disease: An In Vivo MRI and DTI study. Hum Brain Mapp. 2011;32(9):1349–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zivadinov R, Heininen-Brown M, Schirda CV, Poloni GU, Bergsland N, Magnano CR, et al. Abnormal subcortical deep-gray matter susceptibility-weighted imaging filtered phase measurements in patients with multiple sclerosis. A case-control study. Neuroimage. 2012;59(1):331–9. [DOI] [PubMed] [Google Scholar]
- 17.Dwyer MG, Bergsland N, Zivadinov R. Improved longitudinal gray and white matter atrophy assessment via application of a 4-dimensional hidden Markov random field model. Neuroimage. 2014;90:207–17. [DOI] [PubMed] [Google Scholar]
- 18.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benedict RHB, Hulst HE, Bergsland N, Schoonheim MM, Dwyer MG, Weinstock-Guttman B, et al. Clinical significance of atrophy and white matter mean diffusivity within the thalamus of multiple sclerosis patients. Mult Scler J. 2013;19(11):1478–84. [DOI] [PubMed] [Google Scholar]
- 20.Benedict RHB, Amato MP, Boringa J, Brochet B, Foley F, Fredrikson S, et al. Brief International Cognitive Assessment for MS (BICAMS): international standards for validation. BMC Neurol. 2012;12(55):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benedict RHB, DeLuca J, Phillips G, LaRocca N, Hudson LD, Rudick R, et al. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult Scler J. 2017;1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brochet B, Ruet A. Cognitive Impairment in Multiple Sclerosis With Regards to Disease Duration and Clinical Phenotypes. Front. Neurol 2019;10:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lope-Piedrafita S Diffusion Tensor Imaging (DTI). In: Garcia-Martin ML, Lopez-Larrubia P, editors. Preclinical MRI: Methods and Protocols, Methods in Molecular Biology. 2018. p. 103–16. [DOI] [PubMed] [Google Scholar]
- 24.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–36. [DOI] [PubMed] [Google Scholar]
- 25.Eijlers AJC, Dekker I, Steenwijk MD, Meijer KA, Hulst HE, Pouwels PJW, et al. Cortical atrophy accelerates as cognitive decline worsens in multiple sclerosis. Neurology. 2019;93(14):E1348–59. [DOI] [PubMed] [Google Scholar]
- 26.Charalambous T, Tur C, Prados F, Kanber B, Chard DT, Ourselin S, et al. Structural network disruption markers explain disability in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2018;0:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nizri E, Hamra-Amitay Y, Sicsic C, Lavon I, Benner T. Anti-inflammatory properties of cholinergic up-regulation: A new role for acetylcholinesterase inhibitors. Neuropharmacology. 2006;50:540–547. [DOI] [PubMed] [Google Scholar]
- 28.Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23(1):41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reale M, Costantini E, Di Nicola M, D’Angelo C, Franchi S, D’Aurora M, et al. Butyrylcholinesterase and Acetylcholinesterase polymorphisms in Multiple Sclerosis patients: implication in peripheral inflammation. Scientific Reports. 2018;8:1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Bari M, Di Pinto G, Reale M, Mengod G, Tata AM. Cholinergic System and Neuroinflammation: Implication in Multiple Sclerosis. Cent Nerv Syst Agents Med Chem. 2017;17:1–7. [DOI] [PubMed] [Google Scholar]
- 31.Gatta V, Mengod G, Reale M, Tata AM. Possible Correlation between Cholinergic System Alterations and Neuro/Inflammation in Multiple Sclerosis. Biomedicines. 2020;8:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polachini CRN, Spanevello RM, Schetinger MRC, Morsch VM. Cholinergic and Purinergic systems: A key to multiple sclerosis? J Neurol Sci. 2018;392:8–21. [DOI] [PubMed] [Google Scholar]
- 33.Christodoulou C, Melville P, Scherl WF, Macallister WS, Elkins LE, Krupp LB. Effects of donepezil on memory and cognition in multiple sclerosis, J Neurol Sci. 2006;245:127–136. [DOI] [PubMed] [Google Scholar]
- 34.Krupp LB, Christodoulou C, Melville P, Scherl WF, Pai LY, Muenz LR, et al. Multicenter randomized clinical trial of donepezil for memory impairment in multiple sclerosis. Neurology. 2011;76(17):1500–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from this study are available on a reasonable request to the corresponding author.


