Abstract
BACKGROUND:
Postmenopausal pregnenolone and/or progesterone levels in relation to endometrial and ovarian cancer risks have been infrequently evaluated. To address this, we utilized a sensitive and reliable assay to quantify pre-diagnostic levels of seven markers related to endogenous hormone metabolism.
METHODS:
Hormones were quantified in baseline serum collected from postmenopausal women in a case-cohort study nested within the Breast and Bone Follow-up to the Fracture Intervention Trial (B~FIT). Women using exogenous hormones at baseline (1992–1993) were excluded. Incident endometrial (n=65) and ovarian (n=67) cancers were diagnosed during 12 follow-up years and compared with a subcohort of 345 women (no hysterectomy) and 413 women (no oophorectomy), respectively. Cox models with robust variance were used to estimate cancer risk.
RESULTS:
Circulating progesterone levels were not associated with endometrial [tertile(T)3 vs. T1 Hazard Ratio [HR] (95% confidence interval [CI]): 1.87 (0.85–4.11), p-trend 0.17] or ovarian cancer risk [1.16 (0.58–2.33), 0.73]. Increasing levels of the progesterone-to-estradiol ratio were inversely associated with endometrial cancer risk [T3 vs T1: 0.29 (0.09–0.95), 0.03]. Increasing levels of 17-hydroxypregnenolone were inversely associated with endometrial cancer risk [0.40 (0.18–0.91), 0.03] and positively associated with ovarian cancer risk [3.11 (1.39–6.93), 0.01].
CONCLUSIONS:
Using sensitive and reliable assays, this study provides novel data that endogenous progesterone levels are not strongly associated with incident endometrial or ovarian cancer risks. 17-hydroxypregnenolone was positively associated with ovarian cancer and inversely associated with endometrial cancer.
IMPACT:
While our results require replication in large studies, they provide further support of the hormonal etiology of endometrial and ovarian cancers.
Background
Our understanding of the potential role of sex hormones in the etiology of endometrial and ovarian cancers comes largely from epidemiologic research on exogenous hormone use. In terms of endometrial cancer, estrogen only menopausal hormone therapy is contraindicated among women that have not had a hysterectomy due to estrogen’s proliferative effect on the endometrium and subsequent increased endometrial cancer incidence [1]. In women with an intact uterus, a regimen of estrogen plus continuous progestin (at least 14 days per month of progestin) hormone therapy decreases endometrial cancer risk [2, 3], supporting the well-established role of progesterone ameliorating the proliferative effect of estradiol on the endometrium during the menstrual cycle [3, 4]. Ovarian cancer incidence is increased with the use of either formulation of menopausal hormone therapy [5–7].
Supporting the effects of exogenous hormones on endometrial and ovarian cancer risk, epidemiologic data provide compelling evidence of increased endometrial cancer and non-serous ovarian cancer risk with higher levels of postmenopausal endogenous circulating estrogens [8, 9]. While the role of progesterone or the progesterone-to-estradiol ratio in postmenopausal women remains unexplored. Sex steroid hormone metabolism begins with the conversion of cholesterol to pregnenolone and progesterone. Pregnenolone, produced by the adrenal glands, is a precursor to many steroid hormones. In addition to being metabolized to progesterone, pregnenolone is the starting substrate for the production of dehydroepiandrosterone (DHEA), androstenedione, testosterone, estrogens and other hormones. Given these metabolic relationships, the roles of pregnenolone, progesterone, and related metabolites in cancer risk warrant evaluation. Further, the progestogens (both endogenous progesterone and exogenous progestins in oral contraceptives) mitigate the proliferative effect of estradiol on the endometrium during the menstrual cycle [3, 4]. Risk factor associations, namely reduced ovarian and endometrial cancer risks with pregnancy and oral contraceptive use, have also contributed to the hypothesis that progesterone may be inversely associated with risk of endometrial and ovarian cancers [10, 11]. In vitro data from ovarian and endometrial cancer cells lines indicate that progesterone induces apoptosis [12]. However, the influence of progesterone and progesterone-related metabolites on postmenopausal cancer risk has been infrequently studied, primarily due to low levels in circulation, large sample volume required for assays, and low sensitivity of the assays. Pregnenolone has also been infrequently evaluated as a biomarker for cancer risk.
Given the understudied relationships between pregnenolone and progesterone and gynecologic cancer risk, we measured progesterone, its precursor pregnenolone, and five of their metabolites, using a sensitive and reliable liquid chromatography-tandem mass spectrometry (LC/MS-MS) assay, in a case-cohort study of postmenopausal women not using exogenous hormones at blood draw within the Breast and Bone Follow-Up to the Fracture Intervention Trial (B~FIT).
Materials and Methods
We conducted a case-cohort study within B∼FIT, a longitudinal cohort of participants screened for the Fracture Intervention Trial (FIT), which has previously been described [13, 14]. The case-cohort design was selected to assess the relationship between estrogen metabolism and multiple cancer endpoints [14–16]. Estradiol was associated with increased endometrial cancer risk, while estrogen metabolism was not associated with ovarian cancer in this population [14]. Residual sample volume was accessed to evaluate pregnenolone and progesterone metabolism and multiple cancer endpoints. Associations of pregnenolone, progesterone, and related metabolites with breast and colorectal cancers in this postmenopausal study population have been previously published [13, 17].
In brief, FIT was a randomized, placebo-controlled trial designed to test the efficacy of alendronate in reducing fracture rates in women with low bone mineral density [18]. In 1992–1993, 22,695 postmenopausal women (ages 55–80) were screened for participation in FIT at 11 clinical centers in the US [19]. The screening exam included a bone mineral density scan, baseline serum sample collection, clinical examination including measured anthropometrics, and an extensive health history questionnaire that ascertained information on demographic, lifestyle, hormonal, and reproductive factors. Serum samples were stored at −20 °C for three years and then transferred to −70 °C for long-term storage. Trial results from FIT were reported in 1996 and 1998, and a subset of participants who had used alendronate for at least three years were invited to participate in the FIT Long-Term Extension Trial (FLEX) [19–21].
B∼FIT (N = 15,595) includes female volunteers originally screened for FIT at 10 of the 11 FIT centers. Women who refused or withdrew from the FIT trial were excluded (n = 7100). Vital status and cause of death was determined using the National Death Index Plus (NDI+). From 2001 to 2004, surviving women were contacted by mail and/or telephone and invited to complete a follow-up questionnaire (64% of eligible women completed the BFIT questionnaire), which asked about cancer diagnoses, other health outcomes and reproductive surgeries that occurred since they were screened for FIT, family history of cancer, detailed hormone use, and preventive screening procedures. Women who reported an incident cancer were asked to give permission for medical record review of those events. In addition, all B∼FIT women from clinical sites located in states with cancer registries (Florida, Maryland, North Carolina, Oregon, and Tennessee) or in Surveillance Epidemiology and End Results (SEER) registry areas (Northern California, Washington, and Iowa) were linked to the registry to identify and confirm cancer diagnoses (73% of subjects resided in areas with registry linkage, of which 29% were SEER registry areas). Approximately 93% of the endometrial and 70% of the ovarian cancer cases reported among B∼FIT participants were confirmed by medical record or registry linkage. All women provided written informed consent. B∼FIT was approved by the Institutional Review Board (IRB) of each participating site and the University of California San Francisco Coordinating Center, as well as the National Cancer Institute.
Eligibility Criteria and Subcohort Selection
Details on the case-cohort design (including study flowchart) and findings from analyses of multiple cancer endpoints have previously been published [13, 15, 16]. Prior to selecting the subcohort from the overall B∼FIT cohort (N = 15,595), the following exclusion criteria were applied to determine the eligible population: 1) no available baseline unthawed serum sample (n = 872); 2) missing age at screening (n = 13); 3) ineligible for breast cancer analysis due to history of bilateral mastectomy (n = 623); 4) reported use of postmenopausal hormones (oral, injection, or patch) within four months of their FIT interview/blood draw (n = 45); and 5) a previous history of any cancer other than non-melanoma skin cancer before FIT baseline (n = 258). As a result, 13,784 participants were eligible for selection for the case-cohort study. This included women randomized in the FIT trial, as well as women screened for but not included in this trial. The subcohort (N = 515) was randomly selected from among these eligible women (N = 13,784), within 10-year age and geographical clinic strata, irrespective of case status. In determining the analytic population for the analysis of endometrial and ovarian cancer risk [14], additional exclusion criteria were applied to the subcohort. Women who reported a history of hysterectomy at baseline or unknown hysterectomy status (n = 153) or a bilateral oophorectomy or unknown oophorectomy status at baseline (n = 75) were excluded from the subcohort for endometrial and ovarian cancer analyses, respectively. Additionally, women were excluded from the analytic population based on the following: missing dates for calculation of follow-up time (endometrial: n = 1 case, 3 non-cases; ovarian: 13 non-cases), issues with sample vials (endometrial: 13 non-cases; ovarian: 11 non-cases), insufficient volume for additional assays (endometrial: 1 case, 1 non-case; ovarian: 3 non-cases).
The final study population for the endometrial cancer analysis included 410 postmenopausal women, including 65 incident endometrial cases, of whom, 4 were diagnosed among women sampled as part of the subcohort. The resulting subcohort consisted of 341 women who did not develop endometrial cancer during follow-up and 4 women that did. The final study population for the ovarian cancer analysis included 480 postmenopausal women, with 67 incident ovarian cancer cases and 413 subcohort members; no ovarian cancer cases were diagnosed during follow-up in the subcohort.
Laboratory Assays
Progesterone, and related metabolite levels are significantly lower among postmenopausal women as compared to premenopausal women, posing a challenge for radioimmunoassay. Furthermore, available commercial kits measure only progesterone or 17-hydroxyprogesterone, and do not measure the other major metabolites, which may also play a role in the carcinogenic process [22, 23]. The liquid-chromatography-tandem mass spectrometry (LC-MS/MS) technique developed by the Cancer Research Technology Program, Leidos Biomedical Research, Inc., Frederick National Laboratory for Cancer Research quantifies seven endogenous hormones, specifically progesterone, its precursor pregnenolone, and their major metabolites providing a more comprehensive assessment of endogenous progesterone exposure with documented reproducibility (CVs <3% and ICCs>98%) and sensitivity (lower limit of detection 0.1 ng/dL) [13, 24].
Stable isotope dilution high performance LC-MS/MS was used to quantify the following hormones: pregnenolone, progesterone, and their major metabolites in serum: 17-hydroxypregnenolone, 17-hydroxyprogesterone, 3α-dihydroprogesterone (3αHP), 5α-dihydroprogesterone (5αP), and 20α-dihydroprogesterone (20αHP). Details of the assay method have been published [13, 24]. Total estradiol was previously quantified using an independent LC-MS/MS assay [15].
For all hormones the lower limit of quantitation of the assay was 0.5 ng/dL and the lower limit of detection was 0.1 ng/dL. No samples in the current study had undetectable levels for any of the hormones measured. Laboratory coefficients of variation (CVs) of blinded duplicate samples within and across batches were <3.5% for all hormones measured. Intraclass correlation coefficients (ICCs) were >0.97.
Statistical Analysis
Pregnenolone, progesterone, and their major metabolites were analyzed individually. The relative concentration of progesterone-to-estradiol was also evaluated given progesterone prevents estradiol induced proliferation in the uterus. Spearman rank correlations of the hormones measured were evaluated among women in the subcohort.
We evaluated possible deviations from linearity of the hormone-cancer association using a five-knot spline with knots at the 10th, 25th, 50th, 75th, and 90th percentiles for each hormone based on the distribution in the subcohort; otherwise, hormones were modeled in tertiles based on the distribution in the respective subcohorts. Cox proportional hazards regression with robust variance adjustment to account for the case-cohort design was used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the relationship between each hormone measure (or the progesterone-to-estradiol ratio) and cancer risk. Age was used as the time metric for all analyses. For the subcohort, women entered the analysis at baseline age and contributed person-time until first diagnosis of endometrial cancer or ovarian cancer (event) or censoring (age at death or end of follow-up). Cancer cases not included in the subcohort entered the analysis six months prior to their age at diagnosis, contributing information only to their risk set. Exposure curves from survivor function plots were parallel suggesting no deviation from proportional hazards.
Models were adjusted for a priori selected confounding factors based on knowledge of the literature and study design considerations as follows: clinic (ten sites), trial participation status (screenee-only, FIT, or FIT+FLEX), body mass index (BMI) (kg/m2, continuous), parity (nulliparous, 1 birth, ≥2 births), oral contraceptive use (never, ever, missing), years postmenopausal at blood draw (<10, 10–19, ≥20 years), and duration of prior menopausal hormone therapy use (never/<1 year, 1–4 years, 5–9 years, ≥10 years, defined as estrogen only use for endometrial cancer analyses and estrogen and/or progestin use for ovarian cancer analyses).
In exploratory analyses we evaluated endometrial cancer risk with the cross-classification of estradiol and progesterone tertiles with the middle (second tertile) for both hormones serving as the reference group to facilitate the estimation of risk with high progesterone and low estradiol and vice versa. We conducted the following sensitivity analyses: 1) censoring cancer cases that were not confirmed by medical record and/or cancer registry linkage (endometrial: n=8; ovarian: n=20 excluded), and 2) restricting the analytic study population to women who were screened but did not participate in FIT or FIT/FLEX (endometrial: n=58 cases, n=252 subcohort; ovarian: n=57, n=308 subcohort).
All p-values were based on two-sided tests and a nominal p-value<0.05 was considered statistically significant. Analyses were conducted in SAS version 9.4 (Cary, North Carolina).
Results
Endometrial Cancer
Endometrial cancer cases and women in the subcohort were mostly non-Hispanic White (>95%) and predominantly consisted of women screened but not selected for participation in the FIT trial (>73%, Table 1). As expected, endometrial cancer cases included a higher proportion of women with obese BMI (38.5% >30 kg/m2) than women in the subcohort (22.9%). Women eligible for the endometrial cancer analysis were on average 67 years of age at blood draw. The time between blood draw and endometrial cancer diagnosis was 6.3 years (range 6 months-11.7 years). Unadjusted geometric mean hormone levels were generally similar comparing endometrial cancer cases to the subcohort, except for 17-hydroxypregnenolone, 20αHP, and the ratio of progesterone-to-estradiol (Table 2).
Table 1.
Demographic characteristics of women with endometrial or ovarian cancer and women in the respective subcohort from a case-cohort study within B~FIT.
| Endometrial Cases (n=65) | Subcohort (n=345) | Ovarian Cases (n=67) | Subcohort (n=413) | |
|---|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) | n (%) |
| Age | ||||
| <65 | 17 (26.2) | 135 (39.1) | 21 (31.3) | 165 (40.0) |
| ≥65 | 48 (73.9) | 210 (60.9) | 46 (68.7) | 248 (60.1) |
| Race/ethnicity | ||||
| Non-Hispanic White | 64 (98.5) | 329 (95.4) | 65 (97.0) | 394 (95.4) |
| Other | 1 (1.5) | 16 (4.6) | 2 (3.0) | 19 (4.6) |
| Group | ||||
| Screened | 58 (89.2) | 252 (73.0) | 57 (85.1) | 308 (74.6) |
| FIT | 6 (9.2) | 73 (21.2) | 10 (14.9) | 83 (20.1) |
| FIT + FLEX | 1 (1.5) | 20 (5.8) | 0 (0.0) | 22 (5.3) |
| BMI (kg/m2) | ||||
| <25.0 | 19 (29.2) | 157 (45.5) | 25 (37.3) | 176 (42.6) |
| 25.0–29.9 | 19 (29.2) | 108 (31.3) | 24 (35.8) | 132 (32.0) |
| >30.0 | 25 (38.5) | 79 (22.9) | 18 (26.9) | 102 (24.7) |
| Missing | 2 (3.1) | 1 (0.3) | 0 (0.0) | 3 (0.7) |
| Parity | ||||
| Nulliparous | 13 (20.0) | 33 (9.6) | 8 (11.9) | 40 (9.7) |
| 1 birth | 9 (13.9) | 41 (11.9) | 7 (10.5) | 45 (10.9) |
| ≥2 births | 43 (66.2) | 271 (78.6) | 52 (77.6) | 328 (79.4) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Oral contraceptive use | ||||
| Never | 38 (58.5) | 160 (46.4) | 25 (37.3) | 195 (47.2) |
| Ever | 9 (13.9) | 51 (14.8) | 7 (10.5) | 59 (14.3) |
| Missing | 18 (27.7) | 134 (38.8) | 35 (52.2) | 159 (38.5) |
| Years postmenopausal at blood draw | ||||
| <10 | 6 (9.2) | 44 (12.8) | 7 (10.5) | 44 (10.7) |
| 10–19 | 35 (53.9) | 150 (43.5) | 18 (26.9) | 163 (39.5) |
| 20+ | 22 (33.9) | 149 (43.2) | 41 (61.2) | 203 (49.2) |
| Missing | 2 (3.1) | 2 (0.6) | 1 (1.5) | 3 (0.7) |
| Duration of prior estrogen only (endometrial analysis) or estrogen and/or progestin (ovarian analysis) menopausal hormone therapy use | ||||
| Never or <1 year | 48 (73.9) | 279 (80.9) | 51 (76.1) | 326 (78.9) |
| 1–4 years | 7 (10.8) | 44 (12.8) | 7 (10.5) | 53 (12.8) |
| 5–9 years | 4 (6.2) | 12 (3.5) | 4 (6.0) | 20 (4.8) |
| ≥10 years | 6 (9.2) | 10 (2.9) | 4 (6.0) | 14 (3.4) |
| Missing | 0 (0.0) | 0 (0.0) | 1 (1.5) | 0 (0.0) |
Table 2:
Geometric mean (GM) serum concentrations and 95% Confidence Intervals (CI) of pregnenolone, progesterone, and related metabolites (pmol/l) among postmenopausal endometrial and ovarian cancer cases and subcohort members, a case-cohort study within B~FIT.
| Endometrial Cancer | |||||
| Cases (n=65) | Subcohort (n=345) | P value | |||
| Hormone measures (pmol/l) | GM | 95% CI | GM | 95% CI | |
| Pregnenolone | 417.18 | (373.04–466.54) | 463.83 | (441.86–486.89) | 0.09 |
| 17 OH-Pregnenolone | 2263.17 | (2,059.05–2,487.53) | 2594.89 | (2,490.60–2,703.55) | 0.01 |
| Progesterone | 140.40 | (129.93–151.71) | 138.75 | (134.16–143.49) | 0.78 |
| 17 OH-Progesterone | 391.90 | (350.30–438.44) | 402.75 | (383.60–422.84) | 0.66 |
| 5αP | 202.91 | (179.28–229.65) | 220.04 | (208.53–232.19) | 0.24 |
| 3αHP | 57.47 | (50.03–66.02) | 62.19 | (58.56–66.04) | 0.31 |
| 20αHP | 162.37 | (150.11–175.64) | 147.08 | (142.15–152.18) | 0.02 |
| Estradiol | 75.20 | (65.50–84.89) | 47.96 | (43.75–52.17) | <0.0001 |
| Progesterone:estradiol ratio* | 2.38 | (2.00–2.82) | 3.57 | (3.32–3.85) | <0.0001 |
| Ovarian Cancer | |||||
| Cases (n=67) | Subcohort (n=413) | P value | |||
| GM | 95% CI | GM | 95% CI | ||
| Pregnenolone | 457.04 | (409.30–510.34) | 462.78 | (442.78–483.67) | 0.84 |
| 17 OH-Pregnenolone | 2917.50 | (2,655.90–3,204.86) | 2612.65 | (2,516.20–2,712.80) | 0.03 |
| Progesterone | 146.33 | (136.58–156.77) | 139.84 | (136.04–143.76) | 0.23 |
| 17 OH-Progesterone | 407.23 | (364.40–455.11) | 403.12 | (385.57–421.47) | 0.87 |
| 5αP | 232.76 | (207.99–260.48) | 218.51 | (208.88–228.58) | 0.31 |
| 3αHP | 68.88 | (60.04–79.02) | 64.10 | (60.67–67.72) | 0.34 |
| 20αHP | 151.17 | (140.35–162.83) | 148.51 | (144.16–152.99) | 0.66 |
| Estradiol | 55.06 | (42.09–68.04) | 50.03 | (44.81–55.24) | 0.48 |
| Progesterone:estradiol ratio* | 3.48 | (2.93–4.13) | 3.55 | (3.31–3.80) | 0.83 |
Ratio calculated as pmol/L progesterone divided by pmol/L estradiol.
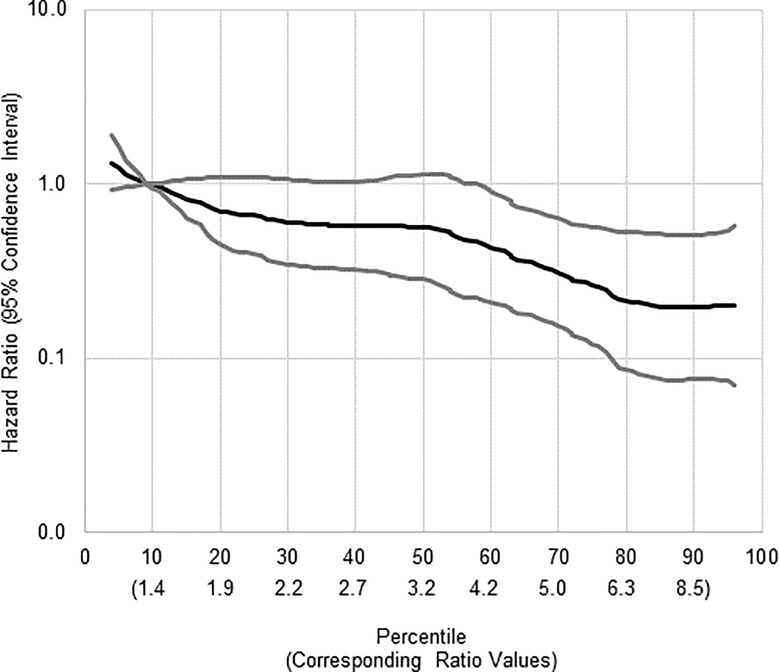
Adjusted analyses evaluating associations of pregnenolone, progesterone, and related metabolites with endometrial cancer risk were mostly null (Table 3). However, increasing levels of 17-hydroxypregnenolone were inversely associated with risk of endometrial cancer (tertile (T)3 vs T1 HR (95% CI): 0.40 (0.18–0.91; p-trend=0.03). The 17-hydroxypregnenolone-endometrial cancer association was robust to excluding cases with blood draws within 2 and 5 years of diagnosis (excluding n=8 cases with blood draws 6 months to <2 years of diagnosis: T3 vs T1: 0.30 (0.12–0.76), and excluding n=22 cases with blood draws 6 months to <5 years prior to diagnosis: T3 vs T1: 0.27 (0.07–0.95)). The observed difference in geometric mean hormone levels by case-subcohort status for 20αHP was not apparent in multivariable adjusted Cox proportional hazards regression models. Increasing levels of progesterone relative to estradiol, as modeled by the progesterone-to-estradiol ratio, were also inversely associated with risk (T3 vs T1: 0.29 (0.09–0.95); p-trend 0.03); this association was linear in a 5-knot spline (Figure 1). Given that the ratio association is largely driven by the strong effect of estradiol on endometrial cancer risk (Spearman correlation for estradiol with the ratio of progesterone-to-estradiol: rho=−0.92, Supplemental Table 1) we further modeled the cross-classification of estradiol and progesterone tertiles with the second or middle tertiles as the reference group (Supplemental Table 2). Endometrial cancer risk was substantially reduced for the highest tertile progesterone and lowest tertile estradiol (HR (95% CI) for T3 progesterone/T1 estradiol vs T2 progesterone/T2 estradiol): 0.21 (0.05–0.93), p-interaction 0.97). Other associations were not statistically significant, but the pattern of risk estimates was consistent with high progesterone potentially offsetting the elevated risks associated with high levels of estradiol. In particular, endometrial cancer risk estimates were elevated for the highest tertile of estradiol and the first or second tertile progesterone (HR (95% CI) T1 progesterone/T3 estradiol vs. T2 progesterone/T2 estradiol: 1.41 (0.49–4.03) and T2 progesterone/T3 estradiol vs. T2 progesterone/T2 estradiol: 1.66 (0.57–4.86) while the risk estimate for the highest tertile of estradiol and the highest tertile progesterone was 0.80 (95% CI: 0.24–2.67, vs. T2 progesterone/T2 estradiol).
Table 3.
Risk of endometrial and ovarian cancer across tertile of hormone concentration or ratio of hormone concentrations in a case-cohort study within B~FIT.
| Endometrial Cancer Cases (n=65) | Subcohort (n=345) | Ovarian Cancer Cases (n=67) | Subcohort (n=413) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnenolone | n | % | n | % | HR | (95% CI) | p-trend | n | % | n | % | HR | (95% CI) | p-trend |
| Tertile(T) 1 | 23 | 35.4 | 113 | 32.8 | 1.00 | ref | 0.18 | 23 | 34.3 | 137 | 33.2 | 1.00 | ref | 0.62 |
| T2 | 27 | 41.5 | 119 | 34.5 | 1.01 | (0.50–2.06) | 23 | 34.3 | 140 | 33.9 | 0.82 | (0.41–1.64) | ||
| T3 | 15 | 23.1 | 113 | 32.8 | 0.59 | (0.26–1.31) | 21 | 31.3 | 136 | 32.9 | 0.83 | (0.43–1.61) | ||
| 17 OH-Pregnenolone | ||||||||||||||
| T1 | 29 | 44.6 | 113 | 32.8 | 1.00 | ref | 0.03 | 12 | 17.9 | 136 | 32.9 | 1.00 | ref | 0.01 |
| T2 | 25 | 38.5 | 118 | 34.2 | 0.84 | (0.41–1.72) | 27 | 40.3 | 140 | 33.9 | 2.74 | (1.25–5.97) | ||
| T3 | 11 | 16.9 | 114 | 33.0 | 0.40 | (0.18–0.91) | 28 | 41.8 | 137 | 33.2 | 3.11 | (1.39–6.93) | ||
| Progesterone | ||||||||||||||
| T1 | 17 | 26.2 | 113 | 32.8 | 1.00 | ref | 0.17 | 20 | 29.9 | 137 | 33.2 | 1.00 | ref | 0.73 |
| T2 | 28 | 43.1 | 119 | 34.5 | 2.03 | (0.97–4.28) | 24 | 35.8 | 139 | 33.7 | 1.23 | (0.60–2.52) | ||
| T3 | 20 | 30.8 | 113 | 32.8 | 1.87 | (0.85–4.11) | 23 | 34.3 | 137 | 33.2 | 1.16 | (0.58–2.33) | ||
| 17 OH-Progesterone | ||||||||||||||
| T1 | 23 | 35.4 | 114 | 33.0 | 1.00 | ref | 0.86 | 25 | 37.3 | 136 | 32.9 | 1.00 | ref | 0.45 |
| T2 | 21 | 32.3 | 118 | 34.2 | 1.07 | (0.48–2.42) | 21 | 31.3 | 140 | 33.9 | 0.75 | (0.38–1.48) | ||
| T3 | 21 | 32.3 | 113 | 32.8 | 1.07 | (0.51–2.27) | 21 | 31.3 | 137 | 33.2 | 0.74 | (0.37–1.47) | ||
| 5αP | ||||||||||||||
| T1 | 30 | 46.2 | 113 | 32.8 | 1.00 | ref | 0.28 | 17 | 25.4 | 136 | 32.9 | 1.00 | ref | 0.28 |
| T2 | 19 | 29.2 | 119 | 34.5 | 0.69 | (0.35–1.40) | 27 | 40.3 | 140 | 33.9 | 1.87 | (0.91–3.86) | ||
| T3 | 16 | 24.6 | 113 | 32.8 | 0.64 | (0.29–1.39) | 23 | 34.3 | 137 | 33.2 | 1.59 | (0.77–3.30) | ||
| 3αHP | ||||||||||||||
| T1 | 22 | 33.8 | 113 | 32.8 | 1.00 | 0.99 | 17 | 25.4 | 137 | 33.2 | 1.00 | ref | 0.07 | |
| T2 | 27 | 41.5 | 118 | 34.2 | 1.67 | (0.73–3.83) | 19 | 28.4 | 140 | 33.9 | 1.02 | (0.47–2.21) | ||
| T3 | 16 | 24.6 | 114 | 33.0 | 1.05 | (0.45–2.44) | 31 | 46.3 | 136 | 32.9 | 1.79 | (0.91–3.54) | ||
| 20αHP | ||||||||||||||
| T1 | 15 | 23.1 | 113 | 32.8 | 1.00 | ref | 0.18 | 22 | 32.8 | 136 | 32.9 | 1.00 | ref | 0.92 |
| T2 | 22 | 33.8 | 119 | 34.5 | 1.61 | (0.73–3.52) | 20 | 29.9 | 141 | 34.1 | 0.92 | (0.46–1.83) | ||
| T3 | 28 | 43.1 | 113 | 32.8 | 1.73 | (0.83–3.63) | 25 | 37.3 | 136 | 32.9 | 1.01 | (0.53–1.95) | ||
| Estradiol | ||||||||||||||
| T1 | 7 | 10.8 | 113 | 32.8 | 1.00 | ref | 0.002 | 15 | 22.4 | 136 | 32.9 | 1.00 | ref | 0.28 |
| T2 | 21 | 32.3 | 119 | 34.5 | 3.24 | (1.27–8.29) | 26 | 38.8 | 141 | 34.1 | 1.57 | (0.72–3.41) | ||
| T3 | 37 | 56.9 | 113 | 32.8 | 4.63 | (1.80–11.91) | 26 | 38.8 | 136 | 32.9 | 1.53 | (0.72–3.29) | ||
| Progesterone:estradiol ratio | ||||||||||||||
| T1 | 35 | 53.8 | 113 | 32.8 | 1.00 | ref | 0.03 | 22 | 32.8 | 137 | 33.2 | 1.00 | ref | 0.64 |
| T2 | 23 | 35.4 | 119 | 34.5 | 0.79 | (0.37–1.67) | 28 | 41.8 | 139 | 33.7 | 1.38 | (0.71–2.67) | ||
| T3 | 7 | 10.8 | 113 | 32.8 | 0.29 | (0.09–0.95) | 17 | 25.4 | 137 | 33.2 | 0.92 | (0.42–2.01) | ||
Cox proportional hazards models used age as the timescale and adjusted for clinic (ten sites), trial participation status (screenee-only, FIT, or FIT+FLEX), body mass index (continuous), parity (nulliparous, 1 birth, ≥2 births), oral contraceptive use (never, ever, missing), years postmenopausal at blood draw (<10, 10–19, ≥20 years), and duration of prior menopausal hormone therapy use (never/<1 year, 1–4 years, 5–9 years, ≥10 years, defined as estrogen only use for endometrial cancer analyses and estrogen and/or progestin use for ovarian cancer analyses).
Figure 1.
Five-knot spline of the association between the progesterone-to-estradiol ratio and endometrial cancer risk.
Black line=hazard ratio, gray lines=95% confidence interval are plotted on the Y-axis. The X-axis reported as percentile of ratio of progesterone-to-estradiol (the corresponding ratio values for the percentile cutpoint are reported in italics below the percentile scale).
Ovarian Cancer
Ovarian cancer cases and subcohort members were mostly non-Hispanic White (>95%) and predominantly consisted of women screened but not selected for participation in the FIT trial (>74%, Table 1). Women eligible for the ovarian cancer analysis were on average 67.2 years of age at blood draw. The time between blood draw and ovarian cancer diagnosis was 6.4 years (range 6 months-12.2 years). Unadjusted geometric mean hormone levels were generally similar comparing ovarian cancer cases to women in the subcohort, with the exception of 17-hydroxypregnenolone, where we observed higher geometric mean levels in circulation for the cases (2917.5 pmol/L) compared with the subcohort (2612.7 pmol/L; p-value for comparison=0.03) (Table 2).
Neither pregnenolone nor progesterone was associated with risk of ovarian cancer. Analyses of progesterone metabolites also supported no association. 17-hydroxypregnenolone was positively associated with increased ovarian cancer risk (T3 vs T1: 3.11 (1.39–6.93); p-trend=0.01). The 17-hydroxypregnenolone-ovarian cancer association was robust to excluding cases with blood draws within 2 and 5 years of diagnosis (excluding n=4 cases with blood draws 6 months to <2 years of diagnosis: T3 vs T1: 2.90 (1.28–6.55), and excluding n=22 cases with blood draws 6 months to <5 years prior to diagnosis: T3 vs T1: 4.61 (1.57–13.55)). The association between the progesterone-to-estradiol ratio and ovarian cancer risk was also null. Risk estimates were similar in analyses limited to confirmed cases and screened women only.
Among the subcohort, the correlations of hormone levels were primarily low to weak (average spearman correlation 0.09), with only a few analytes demonstrating strong correlation (correlation coefficients 0.72 and 0.73 for progesterone with 17α-hydroxyprogesterone and 20α-dihydroprogesterone, respectively) (Supplemental Table 1). Hormone-cancer associations were similar in analyses limited to confirmed cases and screened women only.
Discussion
Results from this prospective study of pre-diagnostic levels of circulating pregnenolone, progesterone, and their metabolites in relation to subsequent endometrial and ovarian cancer risk provides preliminary evidence that progesterone likely mitigates at least part of the increased endometrial cancer risk observed with high levels of estradiol. In contrast, the progesterone or progesterone-to-estradiol association with ovarian cancer was not apparent. Interestingly, 17-hydroxypregnenolone, a precursor to DHEA, was associated with both endometrial and ovarian cancer risk, albeit in opposing directions.
It is established that progesterone prevents estrogen induced proliferation in the uterus during the luteal phase of the menstrual cycle [3, 4]. Hormone production by the ovaries dramatically decreases at menopause, the balance of progesterone and estrogen shifts where even small amounts of circulating estrogens may not be adequately counterbalanced by progesterone [25], resulting in thickening of the endometrium and, subsequently, a potential increase in endometrial cancer risk. In the uterus, progesterone and progestins decrease the number of proliferating cells, possible mechanisms include counteracting the endocrine effects of estrogen on DNA synthesis, inhibiting cellular growth, decreasing mitotic activity in the epithelial cells of the endometrium, promoting differentiation of the endometrium, and stimulating mechanical endometrial sloughing [26]. Progesterone also affects estrogen receptors and can favor the interconversion of estradiol to the much less biologically active estrogen, estrone [27].
The current findings provide the first prospective epidemiologic data that postmenopausal levels of progesterone relative to estradiol are inversely related to endometrial cancer risk. However, the strong inverse association for the ratio measure is primarily explained by high levels of estradiol conferring increased endometrial cancer risk. The evaluation of the cross-classification of progesterone and estradiol tertiles lends some support to the hypothesis that the highest progesterone levels may offset the increased endometrial cancer risk with high estradiol levels. Additional studies with a larger number of endometrial cancer cases are necessary to replicate this finding and to determine if an optimal level of progesterone exists that may offset the proliferative effects of estradiol on the postmenopausal endometrium. Systemic progestin therapy, and recently progestin containing intrauterine devices, have been used for conservative treatment of endometrial hyperplasia and low-grade endometrial cancers primarily among women wishing to maintain fertility [28]. The utility of this approach as first line treatment in postmenopausal women warrants further exploration and should include measurement of systemic endogenous and exogenous hormone levels to help inform optimal treatment regimens.
In this novel prospective evaluation of pregnenolone, progesterone, their metabolites and ovarian cancer risk in postmenopausal women, we found that progesterone was not associated with ovarian cancer risk. However, additional research is needed to determine whether progesterone metabolism is an important factor in ovarian carcinogenesis. Both androgen and estrogen metabolism in postmenopausal women are associated with non-serous ovarian cancers [9, 29]. As such, any association with pregnenolone/progesterone-related metabolites may also be histotype specific. In the current study we were not able to evaluate associations by histotype. The hypothesized inverse association between progesterone and ovarian cancer risk is based predominantly on premenopausal exposures (i.e., pregnancy and oral contraceptive use) [10], which also show variation by histotype [30]. Additionally, premenopausal circulating hormones levels with ovarian cancer risk should also be evaluated.
The opposing associations we observed between 17-hydroxypregnenolone and endometrial and ovarian cancer risk are unexpected as many risk factors for these cancers overlap. 17-hydroxypregnenolone is a precursor to DHEA and downstream adrenal androgen metabolites [31]. Higher DHEA levels (relative to lower levels) were associated with increased endometrial cancer risk in postmenopausal women in a Women’s Health Initiative (WHI) Observational Study (OS) [32] and were unrelated to ovarian cancer risk [29] in the same study population. Neither of these studies, nor the case-cohort analyses presented here, included measures of pregnenolone, progesterone, and androgens in the same population. As such we are unable to confirm if higher 17-hydroxypregneolone levels are correlated with higher concentrations of downstream adrenal androgens. An endometrial cancer risk reduction with variation in the gene encoding 17alpha hydroxylase/17,20 lyase is suggested in a meta-analysis of seven studies [33]. 17alpha hydroxylase mediates the synthesis of 17-hydroxypregnenolone and 17-hydroxyprogesterone from their precursors (i.e., pregnenolone and progesterone, respectively). The reduction in risk suggested in the meta-analysis [33] supports the reduction in endometrial cancer risk that we observed with higher circulating 17-hydroxypregnenlone levels. In contrast, genetic variants encoding 17alpha hydroxylase have not been associated with ovarian cancer risk [34], and do not help to explain the substantially increased risk of ovarian cancer that we observed with the highest tertile levels of 17-hydroxypregnenolone relative to the lowest tertile. In addition to 17alpha hydroxylase, 3beta hydroxysteroid dehydrogenase (HSD) is also involved in catalyzing multiple biochemical steps in the hormone metabolism pathway relevant to the current study. 3beta HSD is involved in the conversion of pregnenolone to progesterone and 17-hydroxypregnenolone to 17-hydroxyprogesterone. There is limited evidence evaluating the association between the genes encoding 3beta HSD and either endometrial or ovarian cancer risk [35]. However, HSD3B1 and HSD3B2 were not differentially expressed comparing 47 pairs of endometrial cancer tissue with adjacent normal endometrium [36]. Additional research is needed to better understand whether genetic variation might contribute to the difference in circulating 17-hydroxypregnenolone levels observed in the current study as well as the opposing effects of 17-hydroxypregnenolone on ovarian and endometrial cancer risks. Future studies should also evaluate the role of pregnenolone, 17-hydroxypregnenolone, and the adrenal androgens (namely DHEA, androsterone, androstenedione, and testosterone) and endometrial and ovarian cancer risks further.
Strengths of this study include the use of pre-diagnostic serum to assess progesterone, its precursor and progesterone metabolites. Availability of previously measured estradiol enabled the evaluation of the progesterone-to-estradiol ratio. Other strengths included long follow-up and the use of an LC-MS/MS assay with high specificity and sensitivity that enabled measurement of progesterone among all postmenopausal women in our study population. Further, cases and subcohort members were randomly distributed across assay batches to ensure similar quality of exposure measurement. The current study is limited by a small number of cases and lack of information on histotype and grade of endometrial and ovarian cancers. Further, the small number of endometrial cancer cases limited our ability to precisely evaluate progesterone levels and endometrial cancer risk across levels of estradiol, or to evaluate other potential effect modifiers.
In conclusion, we were able to provide a novel evaluation of pre-diagnostic postmenopausal pregnenolone, progesterone, and related metabolite levels with endometrial and ovarian cancer risk using a sensitive and reliable LC-MS/MS assay. The ratio of progesterone-to-estradiol was associated with decreased endometrial cancer risk, but the association was largely driven by the strong estradiol-endometrial cancer association. However, the cross-classified analyses provided preliminary evidence that the elevated endometrial cancer risk with the highest tertile of estradiol may be reduced at high levels of progesterone. Progesterone was not associated with ovarian cancer risk. 17-hydroxypregnenolone was associated with decreased endometrial cancer risk and substantially elevated ovarian cancer risk in postmenopausal women. Additional investigations including large numbers of cancer cases are needed to further our understanding of progesterone metabolism in relation to endometrial and ovarian cancer, overall and by histotype.
Supplementary Material
Acknowledgements:
This work was supported in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (ZIA CP010126, Britton Trabert). B~FIT was funded by the National Cancer Institute (contract #N02-CP-01019), National Institutes of Health, Department of Health and Human Services. The original FIT study was funded by Merck Research Laboratories.
Footnotes
Conflict of interest statement: All authors declare no conflict of interest
REFERENCES
- 1.Cramer DW and Knapp RC, Review of epidemiologic studies of endometrial cancer and exogenous estrogen. Obstet Gynecol, 1979. 54(4): p. 521–6. [PubMed] [Google Scholar]
- 2.Trabert B, et al. , Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk? Int J Cancer, 2013. 132(2): p. 417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gompel A, Progesterone and endometrial cancer. Best Pract Res Clin Obstet Gynaecol, 2020. 69: p. 95–107. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead MI and Fraser D, The effects of estrogens and progestogens on the endometrium. Modern approach to treatment. Obstet Gynecol Clin North Am, 1987. 14(1): p. 299–320. [PubMed] [Google Scholar]
- 5.Trabert B, et al. , Ovarian cancer and menopausal hormone therapy in the NIH-AARP diet and health study. Br J Cancer, 2012. 107(7): p. 1181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsilidis KK, et al. , Menopausal hormone therapy and risk of ovarian cancer in the European prospective investigation into cancer and nutrition. Cancer Causes Control, 2011. 22(8): p. 1075–1084. [DOI] [PubMed] [Google Scholar]
- 7.Beral V, et al. , Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet, 2015. 385(9980): p. 1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinton LA, et al. , Serum Estrogens and Estrogen Metabolites and Endometrial Cancer Risk among Postmenopausal Women. Cancer Epidemiol Biomarkers Prev, 2016. 25(7): p. 1081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trabert B, et al. , Circulating Estrogens and Postmenopausal Ovarian Cancer Risk in the Women’s Health Initiative Observational Study. Cancer Epidemiol Biomarkers Prev, 2016. 25(4): p. 648–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risch HA, Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst, 1998. 90(23): p. 1774–86. [DOI] [PubMed] [Google Scholar]
- 11.Eliassen AH and Hankinson SE, Endogenous hormone levels and risk of breast, endometrial and ovarian cancers: prospective studies. Adv Exp Med Biol, 2008. 630: p. 148–65. [PubMed] [Google Scholar]
- 12.McGlorthan L, et al. , Progesterone induces apoptosis by activation of caspase-8 and calcitriol via activation of caspase-9 pathways in ovarian and endometrial cancer cells in vitro. Apoptosis, 2021. [DOI] [PubMed] [Google Scholar]
- 13.Trabert B, et al. , Association of Circulating Progesterone With Breast Cancer Risk Among Postmenopausal Women. JAMA Netw Open, 2020. 3(4): p. e203645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dallal CM, et al. , Estrogen Metabolism and Risk of Postmenopausal Endometrial and Ovarian Cancer: the B~FIT Cohort. Horm Cancer, 2016. 7(1): p. 49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dallal CM, et al. , Estrogen metabolism and breast cancer risk among postmenopausal women: a case-cohort study within B~FIT. Carcinogenesis, 2014. 35(2): p. 346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falk RT, et al. , Estrogen Metabolites Are Not Associated with Colorectal Cancer Risk in Postmenopausal Women. Cancer Epidemiol Biomarkers Prev, 2015. 24(9): p. 1419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michels KA, et al. , Endogenous progestogens and colorectal cancer risk among postmenopausal women. Cancer Epidemiol Biomarkers Prev, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Black DM, et al. , Design of the Fracture Intervention Trial. Osteoporos Int, 1993. 3 Suppl 3: p. S29–39. [DOI] [PubMed] [Google Scholar]
- 19.Black DM, et al. , Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet, 1996. 348(9041): p. 1535–41. [DOI] [PubMed] [Google Scholar]
- 20.Black DM, et al. , Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA, 2006. 296(24): p. 2927–38. [DOI] [PubMed] [Google Scholar]
- 21.Cummings SR, et al. , Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA, 1998. 280(24): p. 2077–82. [DOI] [PubMed] [Google Scholar]
- 22.Wiebe JP, Progesterone metabolites in breast cancer. Endocr Relat Cancer, 2006. 13(3): p. 717–38. [DOI] [PubMed] [Google Scholar]
- 23.Wiebe JP, et al. , The 4-pregnene and 5alpha-pregnane progesterone metabolites formed in nontumorous and tumorous breast tissue have opposite effects on breast cell proliferation and adhesion. Cancer Res, 2000. 60(4): p. 936–43. [PubMed] [Google Scholar]
- 24.Trabert B, et al. , Reproducibility of an assay to measure serum progesterone metabolites that may be related to breast cancer risk using liquid chromatography-tandem mass spectrometry. Horm Mol Biol Clin Investig, 2015. 23(3): p. 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catenaccio E, Mu W, and Lipton ML, Estrogen- and progesterone-mediated structural neuroplasticity in women: evidence from neuroimaging. Brain Struct Funct, 2016. 221(8): p. 3845–3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Key TJ and Pike MC, The dose-effect relationship between ‘unopposed’ oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer, 1988. 57(2): p. 205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui J, Shen Y, and Li R, Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med, 2013. 19(3): p. 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pal N, et al. , Treatment of Low-Risk Endometrial Cancer and Complex Atypical Hyperplasia With the Levonorgestrel-Releasing Intrauterine Device. Obstet Gynecol, 2018. 131(1): p. 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trabert B, et al. , Circulating androgens and postmenopausal ovarian cancer risk in the Women’s Health Initiative Observational Study. Int J Cancer, 2019. 145(8): p. 2051–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wentzensen N, et al. , Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. Journal of Clinical Oncology, 2016. 34(24): p. 2888–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soucy P and Luu-The V, Conversion of pregnenolone to DHEA by human 17alpha-hydroxylase/17, 20-lyase (P450c17). Evidence that DHEA is produced from the released intermediate, 17alpha-hydroxypregnenolone. Eur J Biochem, 2000. 267(11): p. 3243–7. [DOI] [PubMed] [Google Scholar]
- 32.Michels KA, et al. , Postmenopausal Androgen Metabolism and Endometrial Cancer Risk in the Women’s Health Initiative Observational Study. JNCI Cancer Spectr, 2019. 3(3): p. pkz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang X, et al. , CYP17A1 T-34C polymorphism is not associated with endometrial cancer risk. Tumour Biol, 2013. 34(5): p. 2583–7. [DOI] [PubMed] [Google Scholar]
- 34.Spurdle AB, et al. , CYP17 promotor polymorphism and ovarian cancer risk. Int J Cancer, 2000. 86(3): p. 436–9. [DOI] [PubMed] [Google Scholar]
- 35.Olson SH, Bandera EV, and Orlow I, Variants in estrogen biosynthesis genes, sex steroid hormone levels, and endometrial cancer: a HuGE review. Am J Epidemiol, 2007. 165(3): p. 235–45. [DOI] [PubMed] [Google Scholar]
- 36.Sinreih M, Hevir N, and Rizner TL, Altered expression of genes involved in progesterone biosynthesis, metabolism and action in endometrial cancer. Chem Biol Interact, 2013. 202(1–3): p. 210–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.