Abstract
Clearance of apoptotic cells, or ‘efferocytosis’, is essential for diverse processes including embryonic development, tissue turnover, organ regeneration, and immune cell development. The human body is estimated to remove approximately 1% of its body mass via apoptotic cell clearance daily. This poses several intriguing cell metabolism problems. For instance, phagocytes such as macrophages, must induce or suppress metabolic pathways to find, engulf, and digest apoptotic cells. Then, phagocytes must manage the potentially burdensome biomass of the engulfed apoptotic cell. Finally, phagocytes reside in complex tissue architectures that vary in nutrient availability, the types of dying cells or debris that require clearance, and the neighboring cells they interact with. Here, we review advances in our understanding of these three key areas of phagocyte metabolism. We end by proposing a model of efferocytosis that integrates recent findings and establishes a new paradigm for testing how efferocytosis prevents chronic inflammatory disease and autoimmunity.
Introduction and background
Apoptosis, and the phagocytic removal of apoptotic cells (ACs; termed ‘efferocytosis’), is an evolutionarily conserved process that occurs in all tissues throughout the lifespan of an organism and is essential for organismal development, homeostatic tissue turnover and regeneration, and immune system development and function (Boada-Romero et al., 2020; Doran et al., 2020; Morioka et al., 2019). Remarkably, the total cellular mass turnover in an adult (defined as a 20–30-year-old male, weighing 70kg measuring 170cm tall) is ~80 grams/day (~ 3.3×1011 cells/day) or ~230 million cells/minute, with an estimated two-fold increase in turnover in women and children (Sender and Milo, 2021). Although apoptosis probably dominates during development and homeostasis, different modes of cell death are also relevant, including necroptosis, pyroptosis, and ferroptosis (Boada-Romero et al., 2020; Rothlin et al., 2020), each likely inducing distinct metabolic pathways in phagocytes.
Efferocytosis is performed by three different categories of phagocytes: professional, non-professional, and specialized (Penberthy et al., 2018). Professional phagocytes, such as macrophages, are the main phagocyte responsible for efferocytosis (Blériot et al., 2020; Guilliams et al., 2020; Penberthy and Ravichandran, 2016). Non-professional phagocytes (e.g., mammary gland epithelial cells) are not inherently phagocytes, but are called into action during specific contexts such as post-lactation involution (Boada-Romero et al., 2020; Doran et al., 2020; Morioka et al., 2019; Serizier and McCall, 2017). Specialized phagocytes, such as Sertoli cells in the testes, typically feature both tissue-specific non-phagocytic and phagocytic functions (Galloway et al., 2019; Kwon and Freeman, 2020; Penberthy et al., 2018). Sertoli cells, for instance, constantly clear apoptotic germ cells (Penberthy et al., 2018). The majority of research studying metabolic regulation during efferocytosis focuses on macrophages. However, whenever possible, we will highlight the common and unique metabolic features of phagocytosis by non-professional and specialized phagocytes.
Tissue homeostasis depends on an orchestrated balance between removal of aged cells and replenishment with young cells, typically derived from metabolically active stem cells (Pellettieri and Alvarado, 2007). Efferocytosis is essential to this balance, as removal of ACs both opens up space and makes nutrients available for stem cells to thrive in often limited niches (Okabe and Medzhitov, 2016). Although the details of phagocyte metabolism are only beginning to be elucidated, it is apparent that each tissue constitutes a unique metabolic microenvironment (Bernier et al., 2020b; Viola et al., 2019; Zago et al., 2021). Macrophages, for instance, appear to repurpose ingested material to meet intrinsic metabolic demand (O’Neill et al., 2016; Tabas and Bornfeldt, 2020) and as a means to generate immunometabolites and regulate cytokine production (Makowski et al., 2020; O’Neill and Artyomov, 2019). Macrophages are also metabolically flexible, capable of operating in limited oxygen and nutrient environments and handling clearance of complex debris (Bernier et al., 2020b; Viola et al., 2019; Zago et al., 2021). In this review, we discuss efforts to understand the metabolic regulation of efferocytosis. We first focus on recent studies that elucidate unique metabolic requirements for the distinct steps of efferocytosis. We then highlight findings suggesting that phagocytes initiate unique programs in response to the ‘cargo’ they consume. Lastly, we briefly discuss work suggesting that phagocyte metabolism is informed by factors in the tissue microenvironment. Taking into consideration these findings, we end by proposing a new efferocytosis model that includes a hypothesis to explain one way in which efferocytosis prevents chronic inflammatory disease and autoimmunity.
Metabolic pathways initiated during early stages of efferocytosis
Removal of ACs by efferocytosis is a multistep process that, when disrupted, can result in inflammation, autoimmunity, developmental abnormalities, and neurodegenerative disease (Abdolmaleki et al., 2018; Arandjelovic and Ravichandran, 2015; Doran et al., 2020; Marquez-Ropero et al., 2020). Efferocytosis is thought to proceed via three key steps (Figure 1). First, cells undergo apoptosis and release numerous factors, termed ‘find-me’ signals, that recruit phagocytes to the site of death (Medina and Ravichandran, 2016). Once a phagocyte ‘arrives’, they must distinguish between dead and live cells. As part of the apoptosis program, cells expose phosphatidylserine (PS), which serves as an ‘eat-me’ signal for phagocytes (Segawa and Nagata, 2015). Phagocytes recognize PS via one of several receptors, triggering activation of the Rho family GTPase Rac1 and inducing AC internalization. It is clear that several distinct events, including changes in metabolism, are induced during the period from internalization of the AC and its final degradation in the phagolysosome (Elliott and Ravichandran, 2016). These latter stages, AC internalization and digestion, are discussed in greater detail in the next section. Here, we discuss the intercellular and intracellular metabolic pathways involved during early stages of efferocytosis.
Figure 1. Metabolic regulation during each stage of efferocytosis.
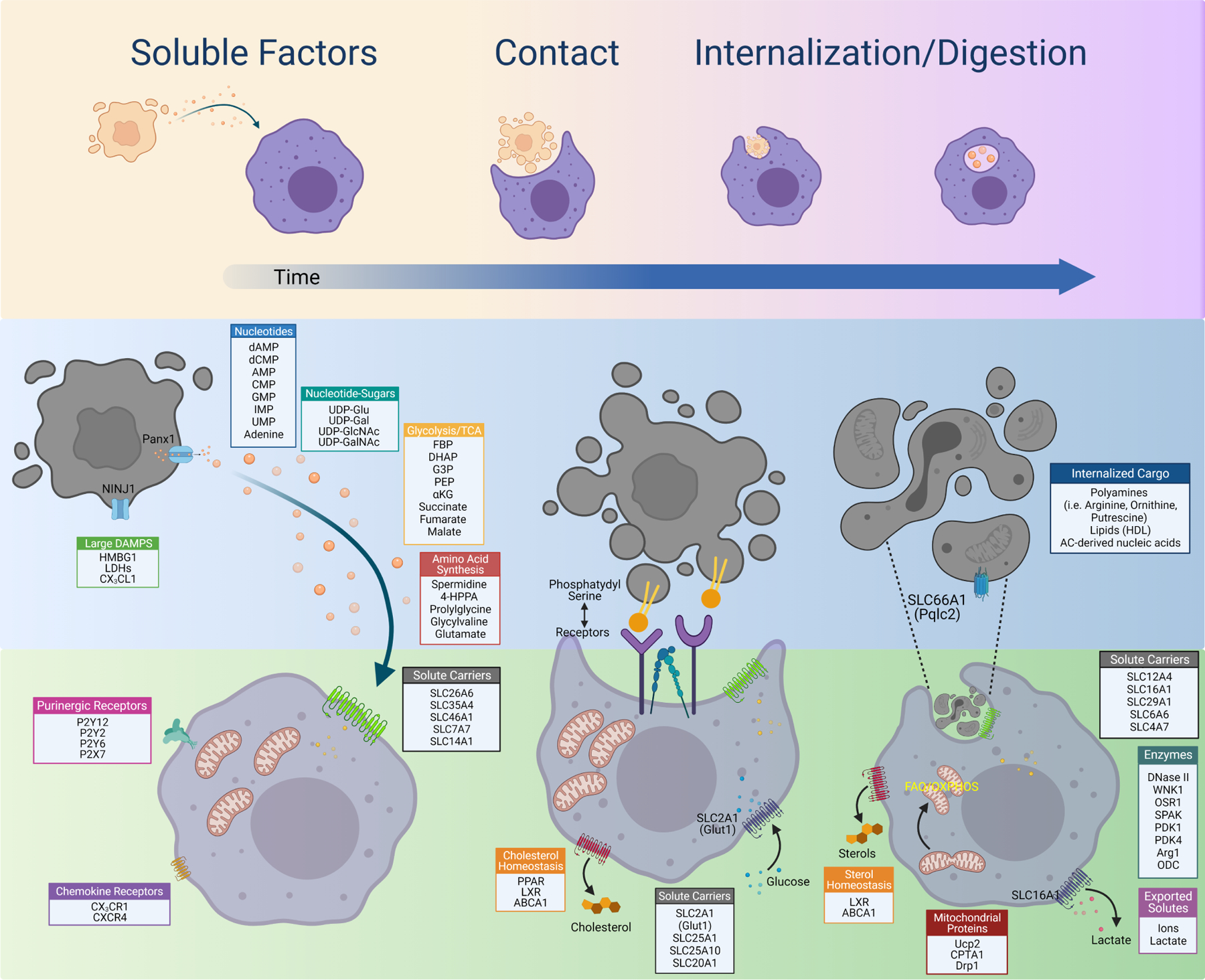
Shown are some of the pathways and proteins regulated during each stage of efferocytosis. Initially, soluble factors such as the find-me signal ATP or the polyamine spermidine are released from apoptotic cells and signal to neighboring phagocytes through as-yet unidentified mechanisms. Phosphatidylserine (PS)-mediated engagement with a PS receptor induces a change in metabolic programs, including upregulation of SLC2A1 and glucose uptake for aerobic glycolysis. PS engagement triggers internalization and digestion of the apoptotic cell, at which point phagocytes appear to switch to different metabolic programs that allow the phagocyte to manage engulfment of the first corpse and proceed to engulfment of subsequent corpses.
Metabolites released from apoptotic cells.
Apoptosis is a tightly controlled process, including regulation of transcript degradation and enzymatic activity (Liu et al., 2018; Tang et al., 2019). An essential component of this regulation involves caspases, cysteine-aspartic proteases, which carry out the core apoptosis program. As highlighted above, a key feature of the apoptosis program is the release of ‘find-me’ signals that attract phagocytes to help facilitate their removal (Figure 1). A series of elegant experiments revealed that one such ‘find-me’ signal is the nucleotide adenosine triphosphate (ATP), which is released via the caspase 3/7-activated channel Pannexin 1 (PANX1) (Chekeni et al., 2010; Elliott et al., 2009) which, together with the ATP-degradation products ADP, AMP, and adenosine, serve as potent anti-inflammatory metabolites (Antonioli et al., 2013; Szondy et al., 2017; Yamaguchi et al., 2014). Unlike channels involved in inflammatory forms of cell death that produce large pores, such as gasdermins (Rothlin et al., 2020), PANX1 forms a relatively small pore that excludes molecules larger than 1 kDa (Narahari et al., 2021).
Based on these findings, recent work explored if ACs release additional molecules in the <1 kDa range (Medina et al., 2020). The authors observed that ACs release dozens of metabolites, including the polyamine pathway end-product spermidine previously shown to act as an anti-inflammatory molecule (Madeo et al., 2018). PANX1-dependent metabolites triggered well-defined programs in both non-professional phagocytes in vitro and professional phagocytes in vivo as well as reduced joint swelling in a mouse model of arthritis and delayed allo-rejection of lung transplants (Medina et al., 2020). ACs also release sphingosine 1-phosphate (S1P) (Luo et al., 2016). S1P, in turn, induces erythropoietin signaling in macrophages which enhances phagocytic capacity. Macrophages lacking the erythropoietin receptor (EPOR) were less capable of clearing ACs in vitro, and mice bearing myeloid cell-specific deletion of EPOR exhibited lupus-like disease in aged mice (Luo et al., 2016). Collectively, these findings are consistent with previous studies showing that administration of exogenously-produced ACs is an effective therapeutic option in various animal models of transplantation tolerance (Dangi et al., 2018) and autoimmunity and chronic inflammatory disease (Saas et al., 2017; Veras et al., 2015).
The majority (~80%) of metabolites released from ACs appear to be released via PANX1-independent routes (Medina et al., 2020). Given the dramatic physiological changes experienced by cells undergoing apoptosis (Atkin-Smith and Poon, 2017), we speculate that both current-sensitive channels and solute-regulated transporters (e.g., solute carrier (SLC) proteins) are involved in the regulated release of metabolites by ACs. This work raises several intriguing questions. For instance, in tissues with cell types exhibiting unique metabolic situations (such as red blood cells) or tissues with unique metabolic environment conditions (such as the central nervous system), what are the dying cell secretomes and how do they affect the functions of their neighbors? Are there disease sequelae related to alterations in the AC metabolic secretome? In the harsh environments of metastatic cancers where cell death is abundant (Bianchini et al., 2016), do dying cancer cells secrete unique factors that support its own growth? Finally, how does metabolite release from inflammatory forms of cell death differ from the AC metabolite secretome, and how is it regulated? For instance, recent work suggests that both necroptotic cells (Gerlach et al., 2020; Hosseini et al., 2021) and ferroptotic cells (Katikaneni et al., 2020; Riegman et al., 2020) release potent lipid mediators that assist in damaged cell clearance and wound healing. How dying cells regulate metabolite release across different forms of cell death will be an interesting area of investigation moving forward.
Metabolic responses induced by contact with apoptotic cells.
We have long known that phagocyte receptor interaction with an AC is sufficient to induce anti-inflammatory cytokine production (discussed here (Henson et al., 2001)). Additionally, PS receptor engagement elicits distinct signaling and transcriptional programs within the phagocyte (Barth et al., 2017), It therefore seems likely that this early triggering event also induces distinct metabolic alterations (Figure 1). The earliest evidence of such changes came from the study of the lipid homeostasis regulator families liver x receptor (LXR) and peroxisomes proliferator-activated receptor (PPAR) in macrophages performing efferocytosis. Both LXRs and PPARs are nuclear receptor transcription factors that dimerize with retinoid X receptors (RXRs) in response to ligands (e.g., oxysterols by LXRs, unsaturated fatty acids by PPARs) to regulate lipid metabolism gene transcription (Grygiel-Górniak, 2014; Wang and Tontonoz, 2018). The importance of LXRs and PPARs for efferocytosis is conserved across all three types of phagocytes (Ershov and Bazan, 2000; Regueira et al., 2014) reviewed in detail elsewhere (Han and Ravichandran, 2011), however it is worth highlighting one proposed AC contact-dependent mechanism involving ABCA1. Across two studies, Ravichandran and colleagues found that PS receptor binding to ACs induced increased transcription and translation of ABCA1 in both professional and non-professional phagocytes (Fond et al., 2015; Kiss et al., 2006). Importantly, uncoupling PS contact signals and AC internalization using cytochalasin D, using PS liposomes that lack cholesterol content, or using carboxylate-modified polystyrene beads as a biomass-less AC surrogate, all induced phagocyte cholesterol efflux to apolipoprotein A-I. Contrarily, the cholesterol derived from ACs was distributed throughout phagocytes. Collectively, these studies suggest that phagocytes prepare for engulfment by first effluxing a portion of its own cholesterol, in a sense making room for the future burden of the engulfed AC (Fond et al., 2015; Kiss et al., 2006).
It is worth highlighting from the above studies is that J774A.1 macrophages, a commonly used cell line typically cultured in high glucose (~25mM) DMEM and 10% fetal bovine serum, exhibit LXR-dependent ABCA1 upregulation in response to ACs, whereas isolated primary peritoneal macrophages cultured in a chemically-defined serum-free media (X-VIVO10), upregulated ABCA1 in a LXR-independent manner. On one hand, this highlights important technical differences that should be considered when studying phagocyte metabolism. On the other hand, these findings may also have interesting implications for phagocytes in different tissues (Guilliams et al., 2020; Viaud et al., 2018). A recent in vivo study revealed alveolar macrophage (AM) removal of apoptotic neutrophils depends on AC contact-dependent induction of the enzyme cholesterol 25-hydroxylase (CH25H), which is required for production of the side-chain oxysterol 25-hydroxycholesterol (25HC) (Madenspacher et al., 2020). Intriguingly, the contact-induced action of 25HC was dependent on LXR. Given a key homeostatic function of AMs is the removal of excess pulmonary surfactant, a complex lipoprotein that challenges AM cholesterol metabolism (Sallese et al., 2017), one possible explanation for the observed differences is that pre-exposure to certain lipids informs how PS signaling drives cholesterol efflux. One final point worth noting is that it remains unclear if LXR activation is solely induced by contact with ACs. For instance, a previous study demonstrated that LXR signaling induced MerTK expression, which in turn promoted continual efferocytosis (A-Gonzalez et al., 2009). However, unlike the previous studies, sterols within ACs were necessary to induce LXR activation and transcriptional upregulation of Mertk and Abca1. Collectively, these findings support the notion that phagocytes assume complex metabolic states throughout the efferocytosis process and pose intriguing questions about how these metabolic pathways contribute to the important pro-resolution response triggered by efferocytosis.
Cellular metabolism is regulated both by substrate availability and the ability to import the requisite substrate. One way in which cells regulate substrate import is through the differential expression or post-translational regulation of solute carrier (SLC) transporters (César-Razquin et al., 2015; Zhang et al., 2018). As part of work profiling transcriptional changes during efferocytosis, Morioka et al. found that both professional and non-professional phagocytes differentially regulate numerous SLCs, many of which clustered into distinct functional programs including carbohydrate metabolism (Morioka et al., 2018). These SLC programs seemed to reflect broader transcriptional changes, including increased expression of glycolysis genes and decreased expression of oxidative phosphorylation (OXPHOS) genes. Furthermore, SLCs were differentially regulated during each step of efferocytosis (discussed further below), suggesting that cellular metabolism is tightly controlled during each step of efferocytosis.
Does contact with an AC actually induce a specific metabolic response in phagocytes? Indeed, Morioka et al. found that AC contact induced rapid upregulation of the primary glucose transporter SLC2A1 (and other glycolytic metabolism genes), which corresponded to significantly increased glucose uptake and catabolism via aerobic glycolysis (Morioka et al., 2018). Removing glucose or preventing glucose transport during efferocytosis significantly reduced phagocytic capacity whereas increased glucose or SLC2A1 availability boosted phagocytic capacity in both professional and non-professional phagocytes. Furthermore, genetic disruption of SLC2A1 decreased efferocytosis in in vivo mouse and zebrafish models, and worsened plaque size in a mouse model of atherosclerosis. Mechanistically, the authors found that aerobic glycolysis fuels ATP requirements for actin polymerization at the emerging phagocytic cup (Morioka et al., 2018). These findings are consistent with recent work demonstrating that retinal pigmented epithelial (RPE) cells, a specialized phagocyte responsible for clearing photoreceptor outer segments (OS) in the retina, upregulate glucose transport in response to contact with OS via the PS receptor MerTK (Wang et al., 2019). In the case of RPE, however, some of the glucose is passed on to photoreceptors to support OS synthesis, suggesting that specialized phagocytes might co-opt PS signaling to contribute to tissue metabolic homeostasis in unique ways. Increased glycolysis in macrophages performing efferocytosis was observed in subsequent studies, although it seems likely to be restricted to this initial stage of efferocytosis (discussed further below) (Bae et al., 2011; Zhang et al., 2019).
The above results are surprising given past work suggesting that the macrophage pro-inflammatory response preferentially relies on enhanced glycolysis (O’Neill et al., 2016). Although controversial (Wang et al., 2018), pro-resolving macrophage identity may also depend on glycolysis (Covarrubias et al., 2016; Huang et al., 2016). Such discrepancies may relate to differences in in vitro culture conditions or tissue environment. For instance, AMs stimulated with the toll-like receptor (TLR) agonist lipopolysaccharide (LPS) in vivo do not require glycolysis to initiate a pro-inflammatory response (Woods et al., 2020). One final consideration is that culturing cells in high glucose (e.g., ~25 mM) for an extended period results in downregulation of SLC2A1 and reduced reliance on aerobic glycolysis for efferocytosis (Park et al., 2011; Pavlou et al., 2018). Interestingly, a recent study observed that macrophages upregulate both glycolysis and OXPHOS during efferocytosis, relying on the latter for production of pro-resolving cytokines (Zhang et al., 2019). These findings support the notion that each stage of efferocytosis relies on different metabolic pathways (or different combinations of metabolic pathways). Moving forward, it will be interesting to map out the specifics of pathways induced by AC-secreted metabolites and contact-initiated metabolic events, especially the more challenging question of determining how these two processes interact within complex tissue environments in vivo.
Metabolic pathways induced by internalization and digestion of apoptotic cells
Interaction between PS and PS receptor triggers a series of tightly orchestrated steps that includes maturation of the nascent phagosome, fusion of the phagosome to lysosomes (phagolysosome), pH-dependent degradation of macromolecules, efflux of solutes from phagolysosomes, and resorption of the phagosomal membrane (Levin et al., 2016; Niedergang and Grinstein, 2018). In this section, we discuss work investigating the metabolic rewiring induced during internalization/digestion of an AC (Figure 1). Additionally, we will highlight recent findings that identify metabolic pathways required for phagocytes to engulf successive ACs, a phenomenon known as ‘continual’ efferocytosis.
As discussed above, PS-mediated contact induces rapid upregulation of SLC2A1, glucose uptake, and ATP generation via aerobic glycolysis (Morioka et al., 2018). A major byproduct of aerobic glycolysis is lactic acid (lactate), which is a robust anti-inflammatory and wound-healing metabolite (Colegio et al., 2014; Hoque et al., 2014; Iraporda et al., 2015; Iraporda et al., 2016; Porporato et al., 2012; Trabold et al., 2003). Cells rely on the monocarboxylate transporter (MCT, or SLC16) family of transporters to excrete or consume lactate or on hydroxycarboxylic acid receptor 1 (HCAR1, or GPR81) for ligand-induced signaling (Sun et al., 2017). Interestingly, internalization/digestion of ACs, but not contact, induced significant upregulation of SLC16A1 (Morioka et al., 2018). Furthermore, reduction or inhibition of SLC16A1 significantly reduced efferocytosis capacity in professional and non-professional phagocytes. These findings are confusing, given that SLC2A1 and aerobic glycolysis is driven by contact, not AC internalization. One possibility is that phagocytes express sufficient SLC16A1 (or other SLC16s) to cope with the initial glycolysis-induced accumulation of lactate production, and that SLC16A1 upregulation corresponds to its additional role in maintaining intracellular pH (Sun et al., 2017). This hypothesis is consistent with the observation that phagocytes upregulate an intracellular pH regulation program, including SLC4A7 that was independently shown to regulate intracellular pH during bacterial phagocytosis (Sedlyarov et al., 2018). Both this hypothesis, and if lactate is obtained from ACs, however remain unknown.
Canonically, macrophages are thought to depend on mitochondrial respiration for pro-resolving function (O’Neill et al., 2016). Given the importance of efferocytosis in inflammation resolution, several studies have focused on mitochondrial metabolism. For instance, Park et al. found that the mitochondrial inner membrane protein uncoupling protein 2 (UCP2, or SLC25A8) is upregulated upon internalization of an AC (Park et al., 2011). UCP2 upregulation was dependent on the presence of biomass, as PS liposomes or carboxylate beads failed to induce increased UCP2 protein. Importantly, loss of UCP2 diminished phagocyte ability to perform ‘continual’ efferocytosis in professional, non-professional, and specialized phagocytes, including by thymic macrophages and Sertoli cells in Slc25a8−/− mice in vivo. Similar results were observed in a mouse model of atherosclerosis, as transplantation of Slc25a8−/− bone marrow into Ldlr−/− mice fed an atherogenic diet exhibited significantly larger lesion size and more necrotic cells (Blanc et al., 2003). Mechanistically, Park et al. propose that UCP2 is required to dissipate the mitochondrial proton gradient during efferocytosis, thus ‘uncoupling’ cellular respiration and mitochondrial ATP synthesis and lowering the mitochondrial membrane potential (Park et al., 2011). However, subsequent work has suggested that UCP2 does not act as an uncoupling protein, but instead acts as a metabolic switch, promoting fatty acid oxidation (FAO) over glycolysis (reviewed in (Diano and Horvath, 2012)). This alternative role for UCP2 might be a mechanistic link to explain the efferocytosis stage-specific timing of the equally-important aerobic glycolysis and FAO/OXPHOS (Zhang et al., 2019).
What is the specific role of FAO and OXPHOS during efferocytosis and how does internalization/digestion of ACs contribute to it? Recently, Thorp and colleagues explored this question in efferocytotic macrophages (Zhang et al., 2019). They observed that sugar metabolism (including glycolysis) and fatty acid metabolism are upregulated during efferocytosis. Intriguingly, the production of some anti-inflammatory/pro-resolving mediators, especially IL-10, was dependent on FAO in vitro. The authors found that carnitine palmitoyltransferase (CPT) enzymes were required to catabolize fatty acids into acetyl-CoA, which in turn fueled the TCA cycle and IL-10 production. Through a series of elegant mechanistic experiments, Thorp and colleagues found that mitochondrial electron transport chain (ETC) activity was necessary for IL-10 production. Specifically, AC internalization/digestion-induced IL-10 production and OXPHOS, but not efferocytosis itself, required mitochondrial Rieske iron-sulfur protein (RISP) (Zhang et al., 2019). Absence of RISP resulted in a decreased ratio of oxidized to reduced nicotinamide adenine dinucleotide (NAD+ and NADH, respectively) which is required (together with NAD+ pool size) for optimal mitochondrial function (Stein and Imai, 2012). Efferocytosis-induced NAD+ production, in turn, drove activity of lysine deacetylase sirtuins which were required for accessibility and PBX1-binding to the Il10 promoter (Zhang et al., 2019). Importantly, myeloid-specific deletion of RISP resulted in significantly decreased survival in a myocardial infarction model.
This study raises several important questions. First, do the FAO/OXPHOS-dependent findings in the previous study rely on initial macrophage activation? The authors used thioglycolate-elicited peritoneal macrophages, which have been shown to consume significantly more oxygen than freshly isolated (non-elicited) peritoneal macrophages basally and during phagocytosis of dead S. aureus (Cohen et al., 1981; Pavlou et al., 2017). Taken together with their analysis of cardiac macrophages isolated from mice experiencing myocardial infarction, these findings pose an intriguing hypothesis that FAO (and ETC)-dependent IL-10 production is important during resolution of inflammation but not during homeostatic efferocytosis. These findings also imply that phagocytes utilize fatty acids acquired from engulfed ACs to drive resolution of inflammation, though future experiments will require stable isotope tracing to formally demonstrate this. Second, what is the relevance of oxygen availability to efferocytosis in settings where oxygen is limited? During myocardial infarction, Thorp and colleagues observed that the vast majority of cardiac macrophages are found in regions of higher oxygenation. However, oxygen availability is much lower in other contexts where efferocytosis is prevalent, such as the brain or liver at homeostasis (Carreau et al., 2011; Norris et al., 2019; Place et al., 2017; Stuart et al., 2018) or in disease settings such as the tumor microenvironment or atherosclerotic plaque (Carmona-Fontaine et al., 2017; Daulatzai, 2017; Marsch et al., 2013). The beating heart, however, exhibits a range of ~80–120 mmHg O2 (~10–15%) (Broten and Feigl, 1992; Dole and Nuno, 1986) which, assuming unrestrained access for cardiac macrophages, would result in an intracellular oxygen level of ~50–80 mmHg O2 (~7–10%; (Stuart et al., 2018)). This is a strikingly high potential level of intracellular oxygen availability compared to the consensus measurements of oxygen across healthy cell types in vivo (~9.9–19 mmHg O2, or 1.3–2.5%) (Carreau et al., 2011; Stuart et al., 2018). Cellular oxygen availability is an important consideration as it was recently shown to dramatically affect cellular lipid metabolism (Jain et al., 2020). Finally, the finding that AC-induced IL-10 production depends on FAO/OXPHOS through a PBX1-dependent mechanism is in line with previous results suggesting that metabolites secreted from ACs induce PBX1 expression and subsequent IL-10 production by macrophages (Chung et al., 2007; Medina et al., 2020; Yamaguchi et al., 2014). Contrarily, Thorp and colleagues found that production of TGFβ, previously shown to be elicited by AC contact (Huynh et al., 2002; Lucas et al., 2006), does not depend on the RISP pathway. Although the dynamics of these processes are unclear, these findings collectively suggest remarkable metabolic flexibility in phagocytes during each step of efferocytosis, including the possibility that different metabolic adaptations induce production of different pro-resolving cytokines.
In addition to metabolites released by ACs, engulfed AC content also contributes to phagocyte metabolism (Figure 1). For instance, Tabas and colleagues observed that efferocytotic macrophages exhibit increases in arginine and ornithine (Yurdagul et al., 2020), consistent with previous work suggesting that pro-resolving macrophages have increased conversion of arginine to ornithine via the enzyme arginase 1 (Arg1) (Puleston et al., 2019; Singer and Chandel, 2019; Viola et al., 2019). Interestingly, inhibition or deletion of Arg1 failed to affect initial efferocytosis capacity in naïve macrophages or macrophages treated with the Arg1-inducer IL-4, but instead reduced ability to perform continual efferocytosis (Yurdagul et al., 2020). The hydrolysis of arginine via Arg1 into ornithine serves as the initiating step for a series of metabolic pathways, including polyamine synthesis. Yurdagul, et al., observed that IL-4-treated macrophages increased production of putrescine during efferocytosis. This increased production was dependent on both Arg1 and the enzyme ornithine decarboxylase (ODC1), which catalyzes the decarboxylation of ornithine to putrescine. Fascinatingly, arginine (and ornithine) derived from internalized ACs was recycled via the lysosome cationic amino acid exporter PQLC2 (SLC66A1) which was necessary for continual efferocytosis in vitro. Importantly, mice with myeloid-specific deletion of Arg1 exhibited both diminished efferocytosis in the dexamethasone-induced thymic clearance model in vivo and resulted in significantly worse disease in a mouse model of atherosclerosis regression (Yurdagul et al., 2020).
Why internalization/digestion of the first and subsequent apoptotic corpses use different pathways remains unknown but might relate to the differences in cellular metabolism discussed above. The above findings relied on induction of Arg1 using IL-4, further evidence that pro-resolving macrophages may rely on different metabolic pathways to resolve inflammation and to perform homeostatic efferocytosis. It is worth highlighting that these findings are consistent with recent work from Rothlin and colleagues, who found that macrophages require both IL-4/13 and AC contact/internalization for resolution of inflammation and tissue repair (Bosurgi et al., 2017). Thus, it will be important to continue to tease out the metabolic programs used by phagocytes during homeostatic efferocytosis.
Importance of ion transport for ‘healthy’ efferocytosis.
Although much of the literature focuses on macromolecule recycling and degradation in phagocytes following engulfment, it is often overlooked that the bulk of the content in the phagolysosome is solutes and water (Levin et al., 2016; Niedergang and Grinstein, 2018). Macropinocytosis (the ‘drinking’ of extracellular fluid) is required for cellular metabolism and homeostasis, including in phagocytic cells (Freeman et al., 2020; Palm et al., 2015). Through this mechanism alone, a phagocyte can internalize 25% of their cell volume per hour (King and Smythe, 2020; Steinman et al., 1976). Despite continuous endocytic and efferocytotic processes that results in internalization of fluids and ions from both inside and outside of the AC, a phagocyte’s cell size/volume remains remarkably stable (Davidson and Wood, 2020; Perry et al., 2019). Understanding how phagocytes handle internalized water and extracellular (or AC-derived) ions during efferocytosis has important implications for phagocyte metabolism. Below, we briefly summarize two recent studies that highlight the importance of ion flux regulation during internalization/digestion of ACs.
Mitochondrial function is regulated at multiple levels, including morphologically (reviewed in (Wai and Langer, 2016)). Under excess nutrient availability, mitochondria fragment (‘fission’) mediated by several mitochondrial proteins, including dynamin-related protein 1 (DRP1). Mitochondrial fission is also observed in cells experiencing impaired OXPHOS or in response to mitochondrial damage. Contrarily, mitochondria undergo ‘fusion’ when metabolic stress is induced by nutrient withdrawal. Mitochondrial fusion triggers functional complementation which improves OXPHOS efficiency and prevents mitophagy. Mitochondrial fusion is primarily mediated by the mitofusin proteins MFN1 and MFN2. Interestingly, a recent study found that DRP1-mediated mitochondrial fission is required for continual efferocytosis by macrophages (Wang et al., 2017). Mechanistically, the authors found that fragmented mitochondria localize to the AC-containing phagolysosome, but surprisingly, facilitate release of Ca2+ from ER stores into the cytoplasm. This Ca2+ release was necessary for appropriate turnover of vesicles and replenishment of plasma membrane for phagosome formation around subsequent engulfed ACs. Perturbation of mitochondrial fission resulted in altered phagolysosomal processing of internalized ACs, including defective sealing of the initial phagosome and delayed lysosomal acidification and AC degradation (Wang et al., 2017). Myeloid cell-specific deletion of DRP1 resulted in diminished clearance of ACs in the dexamethasone-induced efferocytosis model and more severe disease in a mouse model of atherosclerosis. Taken together, these results suggest that mitochondrial dynamics-mediated calcium flux is necessary for efficient degradation of internalized ACs. It seems likely that mitochondrial dynamics during efferocytosis also affects phagocyte metabolism, as the authors observed that knockdown of the mitochondrial fusion mediator MFN1 resulted in hyper-fragmented mitochondria (suggesting increased aerobic glycolysis (Buck et al., 2016)) and enhanced efferocytosis.
Building on the notion that each step of efferocytosis induces unique cellular responses (Figure 1) (Morioka et al., 2018), Ravichandran and colleagues sought to determine if unique transcriptional programs are induced in response to AC internalization (Perry et al., 2019). Indeed, AC internalization induced differential regulation of hundreds of unique genes. However, analysis of programs induced or suppressed revealed only one program, chloride transport/cell volume regulation, that was specifically induced in response to AC internalization. This program included the electroneutral chloride transporter NKCC1 (SLC12A2) which transports extracellular chloride into the cytosol (Delpire and Gagnon, 2018) as well as kinases OSR1 and SPAK. NKCC1 is controlled via a signaling cascade which begins with detection of low intracellular chloride levels by the chloride-sensing kinase WNK1. WNK1, in turn, phosphorylates and activates OSR1/SPAK. OSR1/SPAK subsequently phosphorylates NKCC1, opening the transporter. Importantly, genetic or small molecule perturbation of NKCC1, WNK1, or OSR1/SPAK significantly enhanced efferocytosis in professional and non-professional phagocytes in vitro and by macrophages, via small molecule perturbation of WNK1 or NKCC1, in vivo (Perry et al., 2019). Collectively these findings suggest that the WNK1-OSR1/SPAK-NKCC1 pathway acts as a physiological ‘brake’ on continual efferocytosis.
Is there any consequence to removing this physiological ‘brake’ on efferocytosis? NKCC1-deficient efferocytotic phagocytes exhibited a striking loss of the canonical efferocytosis-induced anti-inflammatory program. Furthermore, NKCC1-deficient phagocytes exhibited markedly increased induction of pro-inflammatory and oxidative stress programs in vitro, a phenomenon not seen in TIM4-overexpressing phagocytes that also exhibit boosted efferocytosis (Perry et al., 2019). Aberrant oxidative stress and production of pro-inflammatory cytokines was also observed using small molecules targeting NKCC1 or WNK1 in two in vivo models of efferocytosis. Collectively, these data suggests that the WNK1-OSR1/SPAK-NKCC1 pathway protects phagocytes from excessive AC uptake and enforces the anti-inflammatory response induced during efferocytosis. It remains unclear, however, if this pathway is relevant for maintenance of homeostasis or resolution of inflammation. Furthermore, it will be interesting to see if additional pathways function during AC internalization/digestion. One alluring example is SLC37A2, a putative glucose-6-phosphate exchanger, which was recently identified as required for phagolysosomal shrinkage in efferocytotic phagocytes (Villani et al., 2019). Thus, we hypothesize that ion and water transport systems are important contributors to phagocyte metabolic homeostasis by both regulating intracellular organelle dynamics during efferocytosis and the safe metabolic breakdown of internalized corpses.
Efferocytosis within a specific tissue environment
Phagocytes, and in turn efferocytosis, are heavily influenced by various factors in their respective tissue environment (Figure 2). Most (if not all) tissues exhibit turnover of cells that are native to that particular tissue, and these cells often have unique metabolic features (Morioka et al., 2019). Furthermore, some tissues exhibit remarkably high cellular turnover, and cellular turnover in other tissues is regulated during development or according to a circadian cycle (Boada-Romero et al., 2020; Doran et al., 2020). For example, the spleen serves as an interface between the blood and lymph, where tissue-resident splenic red pulp macrophages (RPMs) are estimated to remove approximately 109 red blood cells (RBCs) daily. This poses a unique challenge for RPMs because each spent RBC contains excess heme that, if left uncleared, would jeopardize homeostasis. Interestingly, RPMs are programmed early during development via a process that may include phagocytosis of heme (Haldar et al., 2014; Le Blanc et al., 2012). This program includes specialized machinery such as heme oxygenase 1 (Hmox1) which catalyzes the recycling of iron from heme (Klei et al., 2017; Nairz et al., 2017). Similarly, bone marrow-resident macrophages are responsible for removal of large quantities of nuclei that are expelled from enucleated RBCs during erythropoiesis (de Back et al., 2014; Klei et al., 2017) especially during the developmental period of definitive erythropoiesis (Yoshida et al., 2005). Most tissues feature multiple professional phagocyte types (Figure 2). Generally speaking, these phagocytes located in various tissues perform efferocytosis of unique targets and reside in distinct locations within a tissue, therefore we anticipate that the metabolic pathways important for efferocytosis will be informed by the nature of the tissue and what they clear.
Figure 2. The tissue environment of phagocytes under high burden.
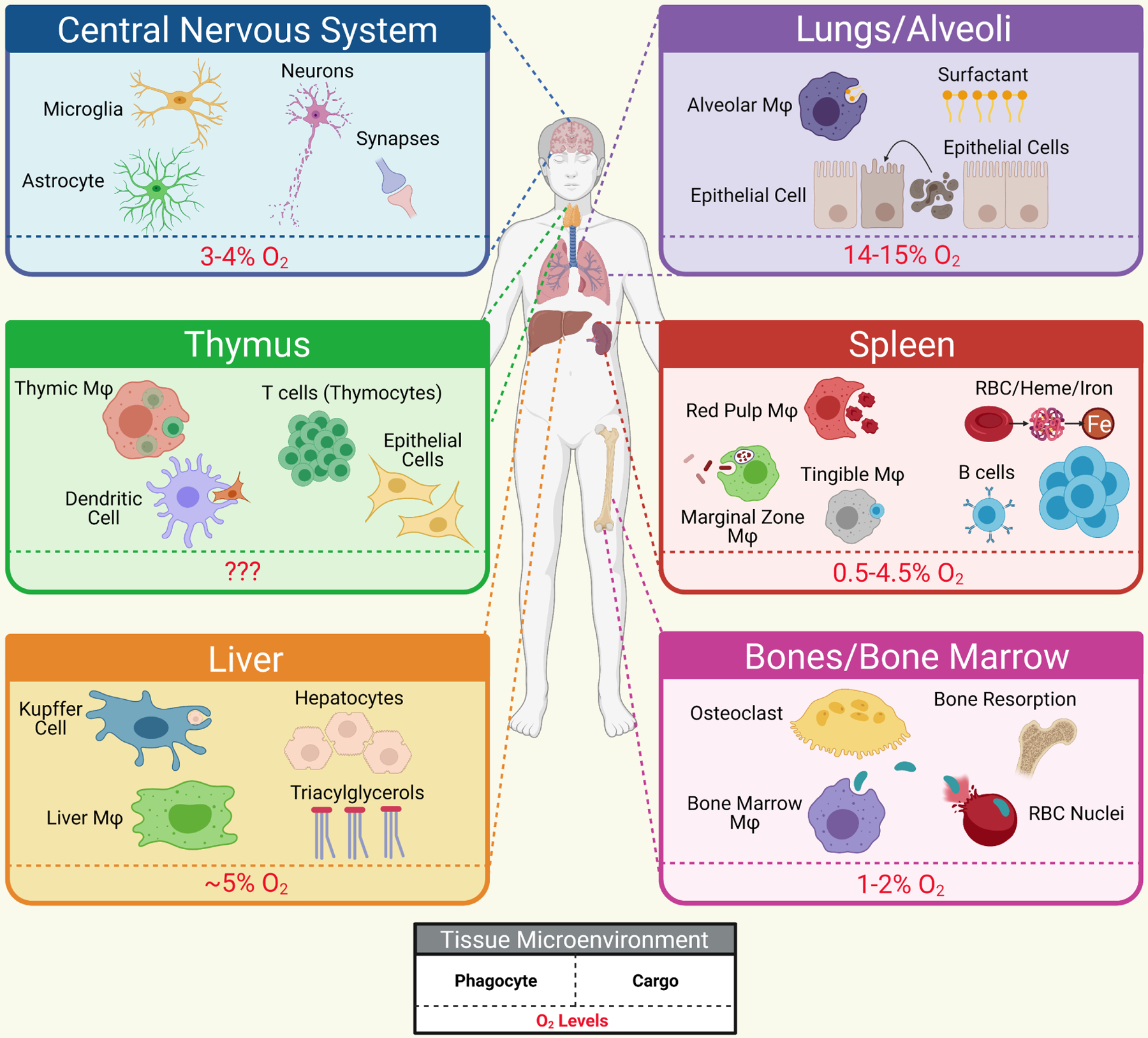
Some tissues experience continued high burden phagocytosis, including removal of unique tissue material (such as surfactant in the alveoli of lungs) on top of removal of apoptotic cells. Shown are a selection of representative tissues, some of the cell and material types they are responsible for clearing, and the oxygen levels that these phagocytes must cope with, all of which likely impact their metabolic function. Mφ – macrophage.
Phagocyte metabolism during efferocytosis may also be informed by the ‘waves’ of apoptosis that tissues experience. For instance, the developing central nervous system (CNS) undergoes an approximate two-fold reduction in neurons (Galloway et al., 2019; Hutchins and Barger, 1998). Furthermore, CNS region-specific waves of apoptosis continue throughout adulthood, which require clearance by both professional (microglia) and non-professional (astrocytes) phagocytes (Ayata et al., 2018; Damisah et al., 2020; Diaz-Aparicio et al., 2020; Lu et al., 2011; Sierra et al., 2010). The CNS is an excellent example of a tissue that features both limited nutrient availability and low oxygen, especially deeper into the CNS parenchyma (Carreau et al., 2011). This is, in part, informed by the high metabolic demand of neurons (Jha and Morrison, 2018). The CNS is also lipid- and glutamine-rich, which may regulate microglia efferocytotic activity (Bernier et al., 2020b; Chausse et al., 2020; Ghosh et al., 2018). Likely due to these factors, microglia are remarkably metabolically flexible, both capable of using various substrates to support metabolic demand during efferocytosis and safely handle the potentially toxic metabolic products present in dying neurons and oligodendrocytes (Berghoff et al., 2021; Bernier et al., 2020a; Zhang et al., 2020). Interestingly, phagocytes in the spleen and bone marrow also experience physiologic hypoxia. Phagocytes under physiologic hypoxia that engulf apoptotic neutrophils or senescent erythrocytes synthesize a unique pro-resolving lipid mediator, resolvin E4, derived via the eicosapentaenoic acid pathway (Norris et al., 2019). The authors suggest that physiologic hypoxia drives enhanced synthesis of resolvin E4 via lipolysis of recycled erythrocyte membrane phospholipids. Thus, together with what a phagocyte ‘eats’, their metabolism is also likely regulated by the nature of the environment itself, including nutrient and oxygen availability (Figure 2). We hypothesize that, together with the aforementioned factors, additional factors such as extracellular ion levels (Vodnala et al., 2019) and acidity (Corbet et al., 2016) will affect phagocyte metabolism during efferocytosis.
Metabolites and metabolic regulators released from efferocytotic phagocytes.
Do efferocytotic phagocytes release factors other than cytokines that affect neighboring cells? As discussed above, we have known for some time that efferocytotic phagocytes release pro-resolving lipid mediators, hormones, and other trophic factors (Diaz-Aparicio et al., 2020; Fadok et al., 1998; Norris et al., 2019; Werfel and Cook, 2018), and the identity of these secreted lipid mediators likely inform the ‘neighborhood’ of the difference between pro-resolving efferocytosis and inflammatory modes of cell death such as necroptosis or ferroptosis (Gerlach et al., 2020; Hosseini et al., 2021; Katikaneni et al., 2020; Riegman et al., 2020).
Beyond lipid mediators and cytokines, much less is known about the efferocytosis (or phagocytosis) secretome. In the lung, both macrophages and airway epithelial cells (non-professional phagocytes) contribute to clearance of ACs and smaller particulate. Intriguingly, efferocytotic macrophages (or macrophages treated with IL-4) release the insulin-like growth factor 1 (IGF-1) which signals via its cognate IGF-1 receptor in epithelial cells, decreasing epithelial cell efferocytotic capacity (Han et al., 2016). Surprisingly, IGF-1 signaling instead increased uptake of small vesicular bodies by non-professional phagocytes. Together with IGF-1, efferocytotic (or IL-4 stimulated) macrophages also released microvesicles that were engulfed by non-professional phagocytes, which was necessary to dampen and resolve lung inflammation in a mouse model of allergen exposure (Han et al., 2016). This study raises several questions. Given the role of IGF-1 in stimulating cellular metabolism (Clemmons, 2012), what is the effect of IGF-1 signaling on the metabolic program of non-professional phagocytes? Also, what, if anything, is contained in the microvesicles released by efferocytotic macrophages? Several potential candidates have been identified, including genomic DNA (Sisirak et al., 2016; Torralba et al., 2018), mitochondrial DNA (Torralba et al., 2018), and unique lipid species (Hough et al., 2018). Finally, it has been long known that in tissues, non-professional phagocytes appear to preferentially engulf smaller apoptotic bodies and particulate (Kerr et al., 1972). Despite the observation from Kerr and colleagues over 50 years ago, we still do not understand this phenomenon, including if there are metabolic differences between efferocytotic professional and non-professional phagocytes or between the phagocytosis of small bodies versus whole ACs.
Beyond IGF-1, efferocytotic professional and non-professional phagocytes release the aerobic glycolysis byproduct lactate via SLC16A1 which contributes to the establishment of pro-resolving macrophage identity in vitro (Morioka et al., 2018). This may also explain the abundance of pro-resolving tumor-associated macrophages present in highly glycolytic tumors (Carmona-Fontaine et al., 2017; Colegio et al., 2014). Ultimately though, very little is known about the nature of the efferocytosis metabolite secretome, let alone its purpose in vivo during homeostasis or resolution and prevention of disease.
The Failure to Appropriately Digest (FAD) model of autoimmunity and inflammatory disease
A prevailing view is that efferocytosis proceeds relatively ‘silently’ (Failure to Appropriately Clear, FAC; Figure 3A) (Morioka et al., 2019). In this view, homeostatic efferocytosis is performed passively in the background of the normal function of a tissue, with the release of cytokines and other factors serving to aid in keeping the ‘neighborhood’ calm. In the case of an immune or sterile insult, efferocytosis serves to dampen the immune response and prevent subsequent damage. In this model, chronic inflammatory disease and autoimmunity (collectively termed ‘inflammatory disease’) arise because of deficient uptake of ACs. Uncleared ACs subsequently release inflammatory mediators, such as damage-associated molecular patterns (DAMPs) (Doran et al., 2020; Morioka et al., 2019). Studies in animals suggest that global loss of certain PS receptors or PS bridging molecules manifest in diseases of defective clearance, including retinitis pigmentosa or lupus-like disease (Fourgeaud et al., 2016; Penberthy and Ravichandran, 2016; Rothlin et al., 2020). Furthermore, certain human diseases such as Systemic Lupus Erythematosus (SLE, a model efferocytosis disease), present with uncleared ACs (Arandjelovic and Ravichandran, 2015). It is likely that reduced ability to clear ACs is a key pathogenic driver of certain inflammatory disease. For instance, atherosclerosis, a leading cause of morbidity and death in the world (Virani et al., 2021), presents with necrotic core formation in the progressing arterial plaque, ultimately impairing resolution of coronary artery disease (Kavurma et al., 2017; Kojima et al., 2017; Tabas and Bornfeldt, 2020). The emergence of necrotic cells in atherosclerosis progression has been causally linked to a failure to appropriately clear ACs across numerous animal studies (reviewed elsewhere (Bäck et al., 2019; Kasikara et al., 2018)). However, many inflammatory diseases do not present with accumulated ACs, suggesting phagocytes remain capable of efferocytosis. Previous reports suggest that only a fraction of patients suffering from SLE exhibit increased AC numbers (Baumann et al., 2002; Gaipl et al., 2005; Ren et al., 2003). Furthermore, this work found that only a subgroup of SLE patients have macrophages that present with decreased, albeit modestly, efferocytotic capacity. The relative dearth of diseases directly caused by loss-of-function mutations in efferocytosis-associated proteins is understandable when considering the remarkable redundancy in PS receptors (Arandjelovic and Ravichandran, 2015; Penberthy and Ravichandran, 2016). Furthermore, germline deletion of DNase II, the lysosomal nuclease responsible for phagocyte digestion of AC-derived DNA, leads to embryonic lethality because of failed definitive erythropoiesis (Kawane et al., 2001), suggesting that mammals evolved numerous redundancies to prevent the developmentally catastrophic result of defective AC uptake.
Figure 3. Quiet efferocytosis versus failure to appropriately digest models of inflammatory disease.
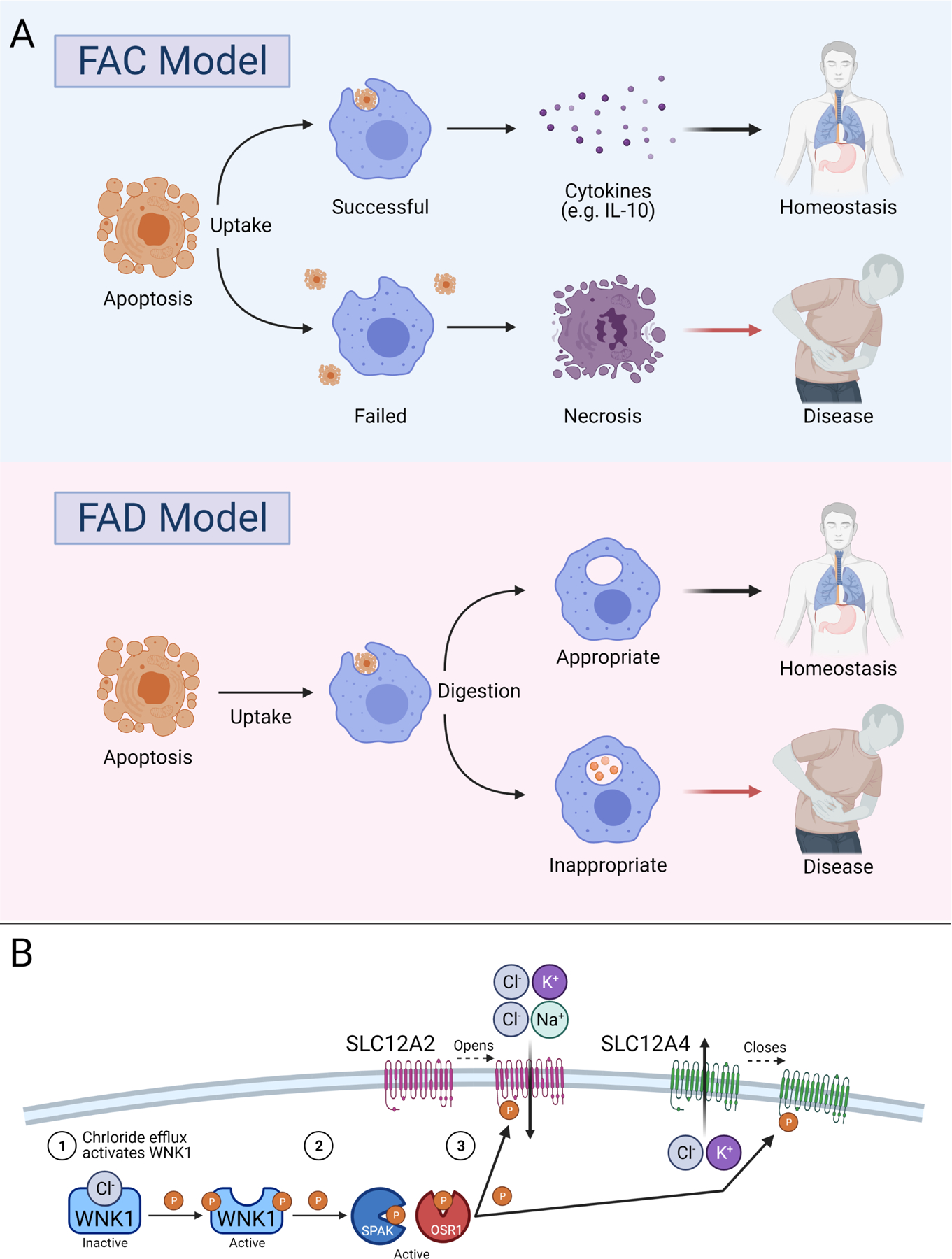
(A) Depiction of failure to appropriately clear (FAC) vs. failure to appropriately digest (FAD) models. In QE, cells undergo apoptosis and are either detected and engulfed (Successful) or not (Failed). Successful clearance induces production of anti-inflammatory cytokines (e.g., IL-10), maintaining homeostasis. Failed clearance results in necrosis of apoptotic cells, production of inflammatory molecules (e.g., HMGB1), and induction of inflammatory disease. In FAD, apoptotic cells are engulfed by phagocytes. Digestion of apoptotic cell material in the phagolysosome (Appropriate) induces programs in phagocytes to maintain homeostasis. Failed or incomplete digestion of apoptotic cell material (Inappropriate), on the other hand, induces inflammatory programs in phagocytes, contributing to inflammatory disease.
(B) Rapid response circuits (RRC) consist of sensors, such as the kinase WNK1, and effectors, such as the SLC12 transporters, that are required for ‘healthy’ digestion of apoptotic cells (FAD model). In RRCs, internalization of apoptotic cells (1) induces a change in cellular state. For instance, apoptotic cell internalization induces chloride efflux. The phagocyte senses this change (2), such as WNK1 directly sensing a decrease in cytosolic chloride. Sensor proteins then induce a switch in the circuit (3). WNK1 activates and initiates a signaling cascade resulting in SLC12A2 opening and SLC12A4 closing, allowing for influx of extracellular chloride and blocking efflux of intracellular chloride. If chloride levels are deemed safe, then the circuit closes. Otherwise, the circuit remains open until homeostasis is achieved.
Intriguingly, there is emerging evidence suggesting that inflammatory disease may also arise due to events that occur during the internalization and digestion of ACs (Hsu et al., 2012; Martinez et al., 2011; Martinez et al., 2016; Perry et al., 2019). In the proposed model (Failure to Appropriately Digest, FAD; Figure 3A), we first define efferocytosis as a perilous process, characterized by uptake of an abundance of dangerous metabolic biomass, including oxidized lipids, nucleotides/nucleosides, proteins and amino acids, and ions, all of which can induce inflammatory responses in phagocytes (Liston and Masters, 2017). We propose that phagocytes, in order to maintain internal homeostasis, must actively detect and assess the nature of the engulfed content and employ compensatory intracellular responses to adjust to these specific changes in biomass (termed rapid response circuits (RRCs) Figure 3B). Inappropriate digestion, then, occurs when these mechanisms either falter or are overwhelmed, resulting in activation of the phagocyte, aberrant secretion of inflammatory cytokines, and subsequent breakdown of immune homeostasis. Below, we briefly discuss results from independent studies that support the FAD model. We include discussion of the FAD model in the context of inflammatory disease and beyond. We end by laying out an example of a RRC and use this as a model for defining putative RRCs.
A hallmark of SLE is the presence of the LE cell (Hargraves et al., 1948). LE cells appear under histological analysis as a phagocyte containing a pyknotic AC nucleus, which has since been identified as a phagocyte containing an undigested AC (Ruiz-Argüelles and Alarcón-Segovia, 2001; Schmidt-Acevedo et al., 2000). Despite this knowledge, it was only recently shown that genetic perturbation of a phagolysosomal component resulted in lupus-like disease in mice (Martinez et al., 2016). Specifically, Green and colleagues found that germline deletion of rubicon (RUBCN) or NADPH oxidase-2 (NOX2), both required for phagosome-lysosome fusion (Martinez et al., 2011; Martinez et al., 2015), resulted in lupus-like symptoms in aged mice (Martinez et al., 2016). Importantly, RUBCN-deficient phagocytes exhibited an inability to digest ACs, resulting in aberrant production of IIL-1β and IL-6 and failed production of IL-10. This work serves as a proof-of-principle that appropriate digestion of ACs is necessary to prevent inflammatory disease.
As noted, ACs contain numerous molecules that pose a metabolic burden on phagocytes, including the potentially dangerous nucleotides. In the phagolysosome, nucleotides are broken down into nucleosides via DNase and RNase enzymes (Fujiwara et al., 2016). Once generated, nucleosides require ‘salvage’ via one of the lysosome-associated equilibrative nucleoside transporters (ENTs, or SLC29s) (Baldwin et al., 2004). Intriguingly, patients with various mutations in SLC29A3 (ENT3) develop one of a host of inflammatory histiocytoses, including H syndrome, pigmentary hypertrichosis and non-autoimmune insulin-dependent diabetes, Faisalabad histiocytosis, sinus histiocytosis with massive lymphadenopathy, and Rosai Dorfman Disease (Avitan-Hersh et al., 2011; Elbarbary et al., 2013; Morgan et al., 2010a; Morgan et al., 2010b; Rafiq et al., 2017). Mice globally lacking Slc29a3 present similar symptoms to patients bearing mutant SLC29A3, Interestingly, SLC29A3-deficient mice exhibit undigested AC material in the phagolysosome, accumulation of enlarged LAMP1+ lysosomes, and increased lysosomal pH (Hsu et al., 2012). Consistent with this work, SLC29s are regulated in response to AC internalization, albeit with different SLC29 expression depending on the type and tissue location of phagocyte ((Morioka et al., 2018; Perry et al., 2019) and ImmGen (Aguilar et al., 2020)). One final point worth noting is that the above histiocytosis diseases are classified as lysosomal storage diseases (LSDs). Thus, these studies both provide support for the FAD model and suggest that LSDs may also be efferocytosis diseases that present with a failure to appropriate digest engulfed ACs.
Together with nucleoside salvage, phagocytes also cope with the ingested proteins and amino acids of the engulfed AC. Although the nutrient-sensing function of mTORC1 at the lysosome is well-studied (Efeyan et al., 2012), far less is known about how cells, especially phagocytes, recycle excess amino acids. One potentially relevant family of transporters is the cationic amino acid transporter (SLC7) family (Fotiadis et al., 2013). SLC7A7, the y+LAT1 subunit of the system y+L amino acid transporter, has been identified as essential for tissue macrophage survival in zebrafish in vivo (Demy et al., 2020; Rossi et al., 2015). Surprisingly, however, macrophages from patients with a mutation in SLC7A7 or macrophages in Slc7a7-deficient zebrafish develop and engulf ACs normally (Barilli et al., 2012; Demy et al., 2020). Instead, efferocytosis induces expression of Slc7a7 in macrophages which is thought to assist efferocytotic macrophages cope with high metabolic demand and prevent cell death (Demy et al., 2020). These findings are compelling because patients with mutations in SLC7A7 (and mice lacking SLC7A7) develop lysinuric protein intolerance (LPI), an autosomal-recessive disorder characterized by hepato-splenomegaly, osteoporosis, alveolar proteinosis, and hemophagocytic lymphohistiocytosis (Bodoy et al., 2019; Borsani et al., 1999; Ogier de Baulny et al., 2012; Stroup et al., 2020). Recent studies of LPI patients suggest that the observed inflammatory sequalae may be caused by macrophage activation, including hyperproduction of nitric oxide and inflammatory cytokines (Mauhin et al., 2017; Ogier de Baulny et al., 2012). As with many SLCs, SLC7A7 is abundantly expressed in specific TRM populations, including AMs, Kupffer cells, RPMs, and osteoclasts (from ImmGen (Aguilar et al., 2020)), consistent with the primary organs affected in LPI. Finally, it is worth noting that inflammatory macrophages downregulate Arg1, the enzyme necessary for catabolism of AC-derived arginine (discussed above; (Yurdagul et al., 2020)). We speculate that, within diseases that present with failure to appropriately clear ACs such as atherosclerosis, there may also be a role for appropriate metabolism of AC content, such as arginine.
Phagocyte rapid response circuits.
Together with the internalized AC, phagocytes also consume ions and water from the extracellular milieu. If the FAD model is correct, then phagocytes must have mechanisms in place to rapidly sense and response to changes in solutes and ions (and presumably water) that occur during efferocytosis. In the case of the WNK1-OSR1/SPAK-NKCC1 pathway, WNK1 responds to intracellular chloride change within minutes, inducing regulation of a signaling cascade that results in the opening of NKCC1 (Perry et al., 2019). Collectively, WNK1 forms a RRC with OSR1/SPAK and NKCC1/KCC1 (Figure 3B). Functionally, internalization/digestion of an AC induces a change in cellular state, such as decreased intracellular chloride. The change in cellular state is detected by a sensor protein (WNK1 detects decrease in cytosolic chloride) inducing a switch in the circuit (KCC1 closes, NKCC1 opens, stopping chloride efflux and starting chloride influx). If levels remain unsafe the circuit remains open (WNK1 remains active). Once safe levels are reached and homeostasis is achieved, the circuit closes (chloride binds and deactivates WNK1). Perturbation of this circuit results in ‘unhealthy’ overeating of ACs, which induces a hyper-inflammatory response by phagocytes. Thus, the WNK1-OSR1/SPAK-KCC1/NKCC1 pathway is a model RRC and provides additional support for the FAD model.
It remains unclear if and how the other SLCs discussed above fit into RRCs. Are each of these SLCs regulated via phosphorylation (or other post-translational modification, PTM) by sensor proteins similar to how WNK1 regulates KCC1/NKCC1? SLC activity can be regulated by PTM in many ways, including plasma membrane localization (César-Razquin et al., 2015; Zhang et al., 2018). On the other hand, certain SLCs appear to function via concentration gradient or as sensors themselves (Rebsamen et al., 2015; Wang et al., 2015; Wyant et al., 2017). In such cases, one could imagine a RRC that involves an enzymatic sensor that converts the elevated substrate into a product that is either usable by the phagocyte or more easily removable. The bulk of studies related to metabolite transport have focused on SLCs, likely because SLCs constitute the second largest family of proteins, essentially involved in transport of most ions and metabolites, with new transport specificities being defined regularly (Kory et al., 2020; Kory et al., 2018; Luongo et al., 2020). However, are non-SLC transporters or channels involved in RRCs? It is possible, for instance, that Ca2+ flux via MCU forms a RRC with one of many Ca2+ sensors. Lastly, tissue-specific phagocytes, especially TRMs, exhibit distinct transporter expression patterns that can vary with age. It will be important to map the tissue-specific RRCs in order to better understand the manifestation of tissue restricted inflammatory disease.
Conclusions
In this Review, we have summarized recent findings related to metabolic regulation during efferocytosis. Efferocytosis consists of a series of orchestrated steps, beginning with the death of a cell and ending with the internalization and degradation of that dead cell by a phagocyte, with each step exhibiting different metabolic features. We also briefly discuss the idea that phagocytes do not operate in isolation, but rather exist in a complex tissue environment that requires removal of cell types with unique metabolic loads. We especially highlight recent research seeking to understand the metabolic regulation of AC internalization and digestion, as phagocytes face the immense challenge of meeting their own metabolic demands while simultaneously managing the metabolic burden of an engulfed cell. In lieu of this point, we propose a new efferocytosis model to explain how autoimmunity and inflammatory disease might arise. This model, the failure to appropriately digest (FAD) model, suggests that efferocytosis is inherently dangerous, and that autoimmunity and inflammatory disease can arise when phagocytes are unable to handle the biomass of the engulfed AC. As part of this model, we propose that phagocytes rely on specific programs, termed rapid response circuits (RRCs), to sense the change in a given solute (such as a metabolite or ion) and respond by adjusting the flux or production of that solute. We hypothesize that the success or failure of phagocyte RRCs underlies the maintenance of tissue, and ultimately organismal, homeostasis.
Efferocytosis is a complex, multi-step process that exerts a heavy metabolic burden on phagocytic cells. Here, Trzeciak et al., review studies that address the metabolic regulation of the unique steps of efferocytosis and integrate these findings into a new model suggesting how efferocytosis prevents autoimmunity and chronic inflammatory disease.
Acknowledgements
We thank members of the Perry laboratory for discussions related to this review. Conceptual discussions with members of the Ravichandran laboratory (University of Virginia) related to this work were essential. This work was supported by NIH NCI 5R00CA237728-03, a Parker Institute for Cancer Immunotherapy Career Development Award, a V Foundation Scholars Grant, and MSKCC Cancer Center Support Grant P30CA008748. Figures were created using BioRender.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests
The authors declare no competing financial interests.
References
- A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Díaz M, Gallardo G, et al. (2009). Apoptotic Cells Promote Their Own Clearance and Immune Tolerance through Activation of the Nuclear Receptor LXR. Immunity 31, 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdolmaleki F, Farahani N, Gheibi Hayat SM, Pirro M, Bianconi V, Barreto GE, and Sahebkar A (2018). The Role of Efferocytosis in Autoimmune Diseases. Front Immunol 9, 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar SV, Aguilar O, Allan R, Amir EAD, Angeli V, Artyomov MN, Asinovski N, Astarita J, Austen KF, Bajpai G, et al. (2020). ImmGen at 15. Nature Immunology 21, 700–703. [DOI] [PubMed] [Google Scholar]
- Antonioli L, Pacher P, Vizi ES, and Haskó G (2013). CD39 and CD73 in immunity and inflammation. Trends in molecular medicine 19, 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arandjelovic S, and Ravichandran KS (2015). Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 16, 907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin-Smith GK, and Poon IKH (2017). Disassembly of the Dying: Mechanisms and Functions. Trends in Cell Biology 27, 151–162. [DOI] [PubMed] [Google Scholar]
- Avitan-Hersh E, Mandel H, Indelman M, Bar-Joseph G, Zlotogorski A, and Bergman R (2011). A case of H syndrome showing immunophenotye similarities to Rosai-Dorfman disease. The American Journal of dermatopathology 33, 47–51. [DOI] [PubMed] [Google Scholar]
- Ayata P, Badimon A, Strasburger HJ, Duff MK, Montgomery SE, Loh Y-HE, Ebert A, Pimenova AA, Ramirez BR, Chan AT, et al. (2018). Epigenetic regulation of brain region-specific microglia clearance activity. Nature Neuroscience 21, 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäck M, Yurdagul A, Tabas I, Öörni K, and Kovanen PT (2019). Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nature Reviews Cardiology 16, 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H-B, Zmijewski JW, Deshane JS, Tadie J-M, Chaplin DD, Takashima S, and Abraham E (2011). AMP-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. 25, 4358–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, and Young JD (2004). The equilibrative nucleoside transporter family, SLC29. Pflugers Archiv : European journal of physiology 447, 735–743. [DOI] [PubMed] [Google Scholar]
- Barilli A, Rotoli BM, Visigalli R, Bussolati O, Gazzola GC, Gatti R, Dionisi-Vici C, Martinelli D, Goffredo BM, Font-Llitjós M, et al. (2012). Impaired phagocytosis in macrophages from patients affected by lysinuric protein intolerance. Molecular Genetics and Metabolism 105, 585–589. [DOI] [PubMed] [Google Scholar]
- Barth ND, Marwick JA, Vendrell M, Rossi AG, and Dransfield I (2017). The “Phagocytic Synapse” and Clearance of Apoptotic Cells. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, and Herrmann M (2002). Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. 46, 191–201. [DOI] [PubMed] [Google Scholar]
- Berghoff SA, Spieth L, Sun T, Hosang L, Schlaphoff L, Depp C, Düking T, Winchenbach J, Neuber J, Ewers D, et al. (2021). Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis. Nature Neuroscience 24, 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier L-P, York EM, Kamyabi A, Choi HB, Weilinger NL, and MacVicar BA (2020a). Microglial metabolic flexibility supports immune surveillance of the brain parenchyma. Nature Communications 11, 1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier L-P, York EM, and MacVicar BA (2020b). Immunometabolism in the Brain: How Metabolism Shapes Microglial Function. Trends in Neurosciences 43, 854–869. [DOI] [PubMed] [Google Scholar]
- Bianchini G, Balko JM, Mayer IA, Sanders ME, and Gianni L (2016). Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nature Reviews Clinical Oncology 13, 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc J, Alves-Guerra MC, Esposito B, Rousset S, Gourdy P, Ricquier D, Tedgui A, Miroux B, and Mallat Z (2003). Protective Role of Uncoupling Protein 2 in Atherosclerosis. 107, 388–390. [DOI] [PubMed] [Google Scholar]
- Blériot C, Chakarov S, and Ginhoux F (2020). Determinants of Resident Tissue Macrophage Identity and Function. Immunity 52, 957–970. [DOI] [PubMed] [Google Scholar]
- Boada-Romero E, Martinez J, Heckmann BL, and Green DR (2020). The clearance of dead cells by efferocytosis. Nature Reviews Molecular Cell Biology 21, 398–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoy S, Sotillo F, Espino-Guarch M, Sperandeo MP, Ormazabal A, Zorzano A, Sebastio G, Artuch R, and Palacín M (2019). Inducible Slc7a7 Knockout Mouse Model Recapitulates Lysinuric Protein Intolerance Disease. Int J Mol Sci 20, 5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsani G, Bassi MT, Sperandeo MP, De Grandi A, Buoninconti A, Riboni M, Manzoni M, Incerti B, Pepe A, Andria G, et al. (1999). SLC7A7, encoding a putative permease-related protein, is mutated in patients with lysinuric protein intolerance. Nat Genet 21, 297–301. [DOI] [PubMed] [Google Scholar]
- Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, et al. (2017). Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 356, 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broten TP, and Feigl EO (1992). Role of myocardial oxygen and carbon dioxide in coronary autoregulation. The American journal of physiology 262, H1231–1237. [DOI] [PubMed] [Google Scholar]
- Buck Michael D., O’Sullivan D, Klein Geltink, Ramon I, Curtis Jonathan D., Chang C-H, Sanin David E., Qiu J, Kretz O, Braas D, van der Windt Gerritje J.W., et al. (2016). Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell 166, 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Deforet M, Akkari L, Thompson CB, Joyce JA, and Xavier JB (2017). Metabolic origins of spatial organization in the tumor microenvironment. Proceedings of the National Academy of Sciences 114, 2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, and Kieda C (2011). Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 15, 1239–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- César-Razquin A, Snijder B, Frappier-Brinton T, Isserlin R, Gyimesi G, Bai X, Reithmeier Reinhart A., Hepworth D, Hediger Matthias A., Edwards Aled M., et al. (2015). A Call for Systematic Research on Solute Carriers. Cell 162, 478–487. [DOI] [PubMed] [Google Scholar]
- Chausse B, Kakimoto PA, and Kann O (2020). Microglia and lipids: how metabolism controls brain innate immunity. Seminars in Cell & Developmental Biology. [DOI] [PubMed] [Google Scholar]
- Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, et al. (2010). Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467, 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, and Ma X (2007). Interleukin-10 Expression in Macrophages during Phagocytosis of Apoptotic Cells Is Mediated by Homeodomain Proteins Pbx1 and Prep-1. Immunity 27, 952–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR (2012). Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am 41, 425–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Ryan JL, and Root RK (1981). The oxidative metabolism of thioglycollate-elicited mouse peritoneal macrophages: the relationship between oxygen, superoxide and hydrogen peroxide and the effect of monolayer formation. 127, 1007–1011. [PubMed] [Google Scholar]
- Colegio OR, Chu N-Q, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. (2014). Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbet C, Pinto A, Martherus R, Santiago de Jesus JP, Polet F, and Feron O (2016). Acidosis Drives the Reprogramming of Fatty Acid Metabolism in Cancer Cells through Changes in Mitochondrial and Histone Acetylation. Cell Metab 24, 311–323. [DOI] [PubMed] [Google Scholar]
- Covarrubias AJ, Aksoylar HI, Yu J, Snyder NW, Worth AJ, Iyer SS, Wang J, Ben-Sahra I, Byles V, Polynne-Stapornkul T, et al. (2016). Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. eLife 5, e11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damisah EC, Hill RA, Rai A, Chen F, Rothlin CV, Ghosh S, and Grutzendler J (2020). Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. 6, eaba3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangi A, Yu S, and Luo X (2018). Apoptotic cell-based therapies for promoting transplantation tolerance. Curr Opin Organ Transplant 23, 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daulatzai MA (2017). Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer’s disease. 95, 943–972. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, and Wood W (2020). Macrophages Use Distinct Actin Regulators to Switch Engulfment Strategies and Ensure Phagocytic Plasticity In Vivo. Cell Reports 31, 107692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Back DZ, Kostova EB, van Kraaij M, van den Berg TK, and van Bruggen R (2014). Of macrophages and red blood cells; a complex love story. Front Physiol 5, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpire E, and Gagnon KB (2018). Water Homeostasis and Cell Volume Maintenance and Regulation. Curr Top Membr 81, 3–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demy DL, Carrère M, Noche R, Tauzin M, Le Bris M, Baek C, Leshchiner I, Goessling W, and Herbomel P (2020). The cationic amino acid exporter Slc7a7 is induced and vital in zebrafish tissue macrophages with sustained efferocytic activity. Journal of cell science 133. [DOI] [PubMed] [Google Scholar]
- Diano S, and Horvath TL (2012). Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends in Molecular Medicine 18, 52–58. [DOI] [PubMed] [Google Scholar]
- Diaz-Aparicio I, Paris I, Sierra-Torre V, Plaza-Zabala A, Rodríguez-Iglesias N, Márquez-Ropero M, Beccari S, Huguet P, Abiega O, Alberdi E, et al. (2020). Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. The Journal of neuroscience : the official journal of the Society for Neuroscience 40, 1453–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dole WP, and Nuno DW (1986). Myocardial oxygen tension determines the degree and pressure range of coronary autoregulation. Circulation research 59, 202–215. [DOI] [PubMed] [Google Scholar]
- Doran AC, Yurdagul A, and Tabas I (2020). Efferocytosis in health and disease. Nature Reviews Immunology 20, 254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, and Sabatini DM (2012). Amino acids and mTORC1: from lysosomes to disease. Trends in molecular medicine 18, 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbarbary NS, Tjora E, Molnes J, Lie BA, Habib MA, Salem MA, and Njølstad PR (2013). An Egyptian family with H syndrome due to a novel mutation in SLC29A3 illustrating overlapping features with pigmented hypertrichotic dermatosis with insulin-dependent diabetes and Faisalabad histiocytosis. Pediatric diabetes 14, 466–472. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. (2009). Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott Michael R., and Ravichandran Kodi S. (2016). The Dynamics of Apoptotic Cell Clearance. Developmental Cell 38, 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershov AV, and Bazan NG (2000). Photoreceptor phagocytosis selectively activates PPARγ expression in retinal pigment epithelial cells. 60, 328–337. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, and Henson PM (1998). Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. The Journal of clinical investigation 101, 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond AM, Lee CS, Schulman IG, Kiss RS, and Ravichandran KS (2015). Apoptotic cells trigger a membrane-initiated pathway to increase ABCA1. The Journal of Clinical Investigation 125, 2748–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiadis D, Kanai Y, and Palacín M (2013). The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med 34, 139–158. [DOI] [PubMed] [Google Scholar]
- Fourgeaud L, Través PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, Callaway P, Zagórska A, Rothlin CV, Nimmerjahn A, et al. (2016). TAM receptors regulate multiple features of microglial physiology. Nature 532, 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman SA, Uderhardt S, Saric A, Collins RF, Buckley CM, Mylvaganam S, Boroumand P, Plumb J, Germain RN, Ren D, et al. (2020). Lipid-gated monovalent ion fluxes regulate endocytic traffic and support immune surveillance. 367, 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Wada K, and Kabuta T (2016). Lysosomal degradation of intracellular nucleic acids—multiple autophagic pathways. The Journal of Biochemistry 161, 145–154. [DOI] [PubMed] [Google Scholar]
- Gaipl US, Voll RE, Sheriff A, Franz S, Kalden JR, and Herrmann M (2005). Impaired clearance of dying cells in systemic lupus erythematosus. Autoimmunity Reviews 4, 189–194. [DOI] [PubMed] [Google Scholar]
- Galloway DA, Phillips AEM, Owen DRJ, and Moore CS (2019). Phagocytosis in the Brain: Homeostasis and Disease. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach BD, Marinello M, Heinz J, Rymut N, Sansbury BE, Riley CO, Sadhu S, Hosseini Z, Kojima Y, Tang DD, et al. (2020). Resolvin D1 promotes the targeting and clearance of necroptotic cells. Cell Death & Differentiation 27, 525–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Castillo E, Frias ES, and Swanson RA (2018). Bioenergetic regulation of microglia. Glia 66, 1200–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygiel-Górniak B (2014). Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications--a review. Nutr J 13, 17–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilliams M, Thierry GR, Bonnardel J, and Bajenoff M (2020). Establishment and Maintenance of the Macrophage Niche. Immunity 52, 434–451. [DOI] [PubMed] [Google Scholar]
- Haldar M, Kohyama M, So Alex Y.-L., Kc W, Wu X, Briseño Carlos G., Satpathy Ansuman T., Kretzer Nicole M., Arase H, Rajasekaran Namakkal S., et al. (2014). Heme-Mediated SPI-C Induction Promotes Monocyte Differentiation into Iron-Recycling Macrophages. Cell 156, 1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han CZ, Juncadella IJ, Kinchen JM, Buckley MW, Klibanov AL, Dryden K, Onengut-Gumuscu S, Erdbrügger U, Turner SD, Shim YM, et al. (2016). Macrophages redirect phagocytosis by non-professional phagocytes and influence inflammation. Nature 539, 570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Claudia Z., and Ravichandran Kodi S. (2011). Metabolic Connections during Apoptotic Cell Engulfment. Cell 147, 1442–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargraves MM, Richmond H, and Morton R (1948). Presentation of two bone marrow elements; the tart cell and the L.E. cell. Proceedings of the staff meetings. Mayo Clinic 23, 25–28. [PubMed] [Google Scholar]
- Henson PM, Bratton DL, and Fadok VA (2001). Apoptotic cell removal. Current Biology 11, R795–R805. [DOI] [PubMed] [Google Scholar]
- Hoque R, Farooq A, Ghani A, Gorelick F, and Mehal WZ (2014). Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146, 1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini Z, Marinello M, Decker C, Sansbury BE, Sadhu S, Gerlach BD, Ramos RB, Adam AP, Spite M, and Fredman G (2021). Resolvin D1 Enhances Necroptotic Cell Clearance Through Promoting Macrophage Fatty Acid Oxidation and Oxidative Phosphorylation. 41, 1062–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough KP, Wilson LS, Trevor JL, Strenkowski JG, Maina N, Kim Y-I, Spell ML, Wang Y, Chanda D, Dager JR, et al. (2018). Unique Lipid Signatures of Extracellular Vesicles from the Airways of Asthmatics. Scientific Reports 8, 10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-L, Lin W, Seshasayee D, Chen Y-H, Ding X, Lin Z, Suto E, Huang Z, Lee WP, Park H, et al. (2012). Equilibrative Nucleoside Transporter 3 Deficiency Perturbs Lysosome Function and Macrophage Homeostasis. 335, 89–92. [DOI] [PubMed] [Google Scholar]
- Huang Stanley C.-C., Smith Amber M., Everts B, Colonna M, Pearce Erika L., Schilling Joel D., and Pearce Edward J. (2016). Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity 45, 817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins JB, and Barger SW (1998). Why neurons die: cell death in the nervous system. Anat Rec 253, 79–90. [DOI] [PubMed] [Google Scholar]
- Huynh M-LN, Fadok VA, and Henson PM (2002). Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. The Journal of clinical investigation 109, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraporda C, Errea A, Romanin DE, Cayet D, Pereyra E, Pignataro O, Sirard JC, Garrote GL, Abraham AG, and Rumbo M (2015). Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 220, 1161–1169. [DOI] [PubMed] [Google Scholar]
- Iraporda C, Romanin DE, Bengoa AA, Errea AJ, Cayet D, Foligné B, Sirard JC, Garrote GL, Abraham AG, and Rumbo M (2016). Local Treatment with Lactate Prevents Intestinal Inflammation in the TNBS-Induced Colitis Model. Front Immunol 7, 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain IH, Calvo SE, Markhard AL, Skinner OS, To T-L, Ast T, and Mootha VK (2020). Genetic Screen for Cell Fitness in High or Low Oxygen Highlights Mitochondrial and Lipid Metabolism. Cell 181, 716–727.e711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, and Morrison BM (2018). Glia-neuron energy metabolism in health and diseases: New insights into the role of nervous system metabolic transporters. Experimental Neurology 309, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasikara C, Doran AC, Cai B, and Tabas I (2018). The role of non-resolving inflammation in atherosclerosis. The Journal of Clinical Investigation 128, 2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katikaneni A, Jelcic M, Gerlach GF, Ma Y, Overholtzer M, and Niethammer P (2020). Lipid peroxidation regulates long-range wound detection through 5-lipoxygenase in zebrafish. Nature Cell Biology 22, 1049–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavurma MM, Rayner KJ, and Karunakaran D (2017). The walking dead: macrophage inflammation and death in atherosclerosis. Current opinion in lipidology 28, 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawane K, Fukuyama H, Kondoh G, Takeda J, Ohsawa Y, Uchiyama Y, and Nagata S (2001). Requirement of DNase II for Definitive Erythropoiesis in the Mouse Fetal Liver. 292, 1546–1549. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, and Currie AR (1972). Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. British journal of cancer 26, 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JS, and Smythe E (2020). Water loss regulates cell and vesicle volume. Science 367, 246–247. [DOI] [PubMed] [Google Scholar]
- Kiss RS, Elliott MR, Ma Z, Marcel YL, and Ravichandran KS (2006). Apoptotic Cells Induce a Phosphatidylserine-Dependent Homeostatic Response from Phagocytes. Current Biology 16, 2252–2258. [DOI] [PubMed] [Google Scholar]
- Klei TR, Meinderts SM, van den Berg TK, and van Bruggen R (2017). From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front Immunol 8, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Weissman IL, and Leeper NJ (2017). The Role of Efferocytosis in Atherosclerosis. 135, 476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kory N, uit de Bos J, van der Rijt S, Jankovic N, Güra M, Arp N, Pena IA, Prakash G, Chan SH, Kunchok T, et al. (2020). MCART1/SLC25A51 is required for mitochondrial NAD transport. 6, eabe5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kory N, Wyant GA, Prakash G, Uit de Bos J, Bottanelli F, Pacold ME, Chan SH, Lewis CA, Wang T, Keys HR, et al. (2018). SFXN1 is a mitochondrial serine transporter required for one-carbon metabolism. Science 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon W, and Freeman SA (2020). Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc S, Garrick MD, and Arredondo M (2012). Heme carrier protein 1 transports heme and is involved in heme-Fe metabolism. American journal of physiology. Cell physiology 302, C1780–1785. [DOI] [PubMed] [Google Scholar]
- Levin R, Grinstein S, and Canton J (2016). The life cycle of phagosomes: formation, maturation, and resolution. Immunological Reviews 273, 156–179. [DOI] [PubMed] [Google Scholar]
- Liston A, and Masters SL (2017). Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nature Reviews Immunology 17, 208–214. [DOI] [PubMed] [Google Scholar]
- Liu X, Fu R, Pan Y, Meza-Sosa KF, Zhang Z, and Lieberman J (2018). PNPT1 Release from Mitochondria during Apoptosis Triggers Decay of Poly(A) RNAs. Cell 174, 187–201.e112. [DOI] [PubMed] [Google Scholar]
- Lu Z, Elliott MR, Chen Y, Walsh JT, Klibanov AL, Ravichandran KS, and Kipnis J (2011). Phagocytic activity of neuronal progenitors regulates adult neurogenesis. Nature cell biology 13, 1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Stuart LM, Zhang A, Hodivala-Dilke K, Febbraio M, Silverstein R, Savill J, and Lacy-Hulbert A (2006). Requirements for Apoptotic Cell Contact in Regulation of Macrophage Responses. The Journal of Immunology 177, 4047–4054. [DOI] [PubMed] [Google Scholar]
- Luo B, Gan W, Liu Z, Shen Z, Wang J, Shi R, Liu Y, Liu Y, Jiang M, Zhang Z, et al. (2016). Erythropoeitin Signaling in Macrophages Promotes Dying Cell Clearance and Immune Tolerance. Immunity 44, 287–302. [DOI] [PubMed] [Google Scholar]
- Luongo TS, Eller JM, Lu MJ, Niere M, Raith F, Perry C, Bornstein MR, Oliphint P, Wang L, McReynolds MR, et al. (2020). SLC25A51 is a mammalian mitochondrial NAD(+) transporter. Nature 588, 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madenspacher JH, Morrell ED, Gowdy KM, McDonald JG, Thompson BM, Muse G, Martinez J, Thomas S, Mikacenic C, Nick JA, et al. (2020). Cholesterol 25-hydroxylase promotes efferocytosis and resolution of lung inflammation. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Eisenberg T, Pietrocola F, and Kroemer G (2018). Spermidine in health and disease. 359, eaan2788. [DOI] [PubMed] [Google Scholar]
- Makowski L, Chaib M, and Rathmell JC (2020). Immunometabolism: From basic mechanisms to translation. 295, 5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Ropero M, Benito E, Plaza-Zabala A, and Sierra A (2020). Microglial Corpse Clearance: Lessons From Macrophages. Front Immunol 11, 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch E, Sluimer JC, and Daemen MJ (2013). Hypoxia in atherosclerosis and inflammation. Current opinion in lipidology 24, 393–400. [DOI] [PubMed] [Google Scholar]
- Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, and Green DR (2011). Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proceedings of the National Academy of Sciences of the United States of America 108, 17396–17401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Cunha LD, Park S, Yang M, Lu Q, Orchard R, Li Q-Z, Yan M, Janke L, Guy C, et al. (2016). Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 533, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Martinez J, Malireddi RKS, Lu Q, Cunha LD, Pelletier S, Gingras S, Orchard R, Guan J-L, Tan H, Peng J, et al. (2015). Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nature Cell Biology 17, 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mauhin W, Habarou F, Gobin S, Servais A, Brassier A, Grisel C, Roda C, Pinto G, Moshous D, Ghalim F, et al. (2017). Update on Lysinuric Protein Intolerance, a Multi-faceted Disease Retrospective cohort analysis from birth to adulthood. Orphanet journal of rare diseases 12, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina CB, Mehrotra P, Arandjelovic S, Perry JSA, Guo Y, Morioka S, Barron B, Walk SF, Ghesquière B, Krupnick AS, et al. (2020). Metabolites released from apoptotic cells act as tissue messengers. Nature 580, 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina CB, and Ravichandran KS (2016). Do not let death do us part: ‘find-me’ signals in communication between dying cells and the phagocytes. Cell Death And Differentiation 23, 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NV, Morris MR, Cangul H, Gleeson D, Straatman-Iwanowska A, Davies N, Keenan S, Pasha S, Rahman F, Gentle D, et al. (2010a). Mutations in SLC29A3, encoding an equilibrative nucleoside transporter ENT3, cause a familial histiocytosis syndrome (Faisalabad histiocytosis) and familial Rosai-Dorfman disease. PLoS genetics 6, e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NV, Morris MR, Cangul H, Gleeson D, Straatman-Iwanowska A, Davies N, Keenan S, Pasha S, Rahman F, Gentle D, et al. (2010b). Mutations in SLC29A3, Encoding an Equilibrative Nucleoside Transporter ENT3, Cause a Familial Histiocytosis Syndrome (Faisalabad Histiocytosis) and Familial Rosai-Dorfman Disease. PLoS genetics 6, e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka S, Maueröder C, and Ravichandran KS (2019). Living on the Edge: Efferocytosis at the Interface of Homeostasis and Pathology. Immunity 50, 1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka S, Perry JSA, Raymond MH, Medina CB, Zhu Y, Zhao L, Serbulea V, Onengut-Gumuscu S, Leitinger N, Kucenas S, et al. (2018). Efferocytosis induces a novel SLC program to promote glucose uptake and lactate release. Nature 563, 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairz M, Theurl I, Swirski FK, and Weiss G (2017). “Pumping iron”-how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers Arch 469, 397–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahari AK, Kreutzberger AJ, Gaete PS, Chiu YH, Leonhardt SA, Medina CB, Jin X, Oleniacz PW, Kiessling V, Barrett PQ, et al. (2021). ATP and large signaling metabolites flux through caspase-activated Pannexin 1 channels. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedergang F, and Grinstein S (2018). How to build a phagosome: new concepts for an old process. Current Opinion in Cell Biology 50, 57–63. [DOI] [PubMed] [Google Scholar]
- Norris PC, Libreros S, and Serhan CN (2019). Resolution metabolomes activated by hypoxic environment. 5, eaax4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LAJ, Kishton RJ, and Rathmell J (2016). A guide to immunometabolism for immunologists. Nature Reviews Immunology 16, 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LAJ, and Artyomov MN (2019). Itaconate: the poster child of metabolic reprogramming in macrophage function. Nature Reviews Immunology 19, 273–281. [DOI] [PubMed] [Google Scholar]
- Ogier de Baulny H, Schiff M, and Dionisi-Vici C (2012). Lysinuric protein intolerance (LPI): a multi organ disease by far more complex than a classic urea cycle disorder. Mol Genet Metab 106, 12–17. [DOI] [PubMed] [Google Scholar]
- Okabe Y, and Medzhitov R (2016). Tissue biology perspective on macrophages. Nature Immunology 17, 9–17. [DOI] [PubMed] [Google Scholar]
- Palm W, Park Y, Wright K, Pavlova NN, Tuveson DA, and Thompson CB (2015). The Utilization of Extracellular Proteins as Nutrients Is Suppressed by mTORC1. Cell 162, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Han CZ, Elliott MR, Kinchen JM, Trampont PC, Das S, Collins S, Lysiak JJ, Hoehn KL, and Ravichandran KS (2011). Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature 477, 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlou S, Lindsay J, Ingram R, Xu H, and Chen M (2018). Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunology 19, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlou S, Wang L, Xu H, and Chen M (2017). Higher phagocytic activity of thioglycollate-elicited peritoneal macrophages is related to metabolic status of the cells. Journal of Inflammation 14, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellettieri J, and Alvarado AS (2007). Cell Turnover and Adult Tissue Homeostasis: From Humans to Planarians. 41, 83–105. [DOI] [PubMed] [Google Scholar]
- Penberthy KK, Lysiak JJ, and Ravichandran KS (2018). Rethinking Phagocytes: Clues from the Retina and Testes. Trends Cell Biol 28, 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy KK, and Ravichandran KS (2016). Apoptotic cell recognition receptors and scavenger receptors. Immunological Reviews 269, 44–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JSA, Morioka S, Medina CB, Iker Etchegaray J, Barron B, Raymond MH, Lucas CD, Onengut-Gumuscu S, Delpire E, and Ravichandran KS (2019). Interpreting an apoptotic corpse as anti-inflammatory involves a chloride sensing pathway. Nature Cell Biology 21, 1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place TL, Domann FE, and Case AJ (2017). Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radical Biology and Medicine 113, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porporato PE, Payen VL, De Saedeleer CJ, Préat V, Thissen JP, Feron O, and Sonveaux P (2012). Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 15, 581–592. [DOI] [PubMed] [Google Scholar]
- Puleston DJ, Buck MD, Klein Geltink RI, Kyle RL, Caputa G, O’Sullivan D, Cameron AM, Castoldi A, Musa Y, Kabat AM, et al. (2019). Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metabolism 30, 352–363.e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiq NK, Hussain K, and Brogan PA (2017). Tocilizumab for the Treatment of SLC29A3 Mutation Positive PHID Syndrome. Pediatrics 140. [DOI] [PubMed] [Google Scholar]
- Rebsamen M, Pochini L, Stasyk T, de Araújo MEG, Galluccio M, Kandasamy RK, Snijder B, Fauster A, Rudashevskaya EL, Bruckner M, et al. (2015). SLC38A9 is a component of the lysosomal amino acid sensing machinery that controls mTORC1. Nature 519, 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regueira M, Riera MF, Galardo MN, Pellizzari EH, Cigorraga SB, and Meroni SB (2014). Activation of PPAR α and PPAR β/δ regulates Sertoli cell metabolism. Molecular and cellular endocrinology 382, 271–281. [DOI] [PubMed] [Google Scholar]
- Ren Y, Tang J, Mok MY, Chan AWK, Wu A, and Lau CS (2003). Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. 48, 2888–2897. [DOI] [PubMed] [Google Scholar]
- Riegman M, Sagie L, Galed C, Levin T, Steinberg N, Dixon SJ, Wiesner U, Bradbury MS, Niethammer P, Zaritsky A, et al. (2020). Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nature Cell Biology 22, 1042–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F, Casano Alessandra M., Henke K, Richter K, and Peri F (2015). The SLC7A7 Transporter Identifies Microglial Precursors prior to Entry into the Brain. Cell Reports 11, 1008–1017. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Hille TD, and Ghosh S (2020). Determining the effector response to cell death. Nat Rev Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Argüelles A, and Alarcón-Segovia D (2001). Novel facts about an old marker: the LE cell. Scandinavian journal of clinical and laboratory investigation. Supplementum 235, 31–37. [DOI] [PubMed] [Google Scholar]
- Saas P, Bonnefoy F, Toussirot E, and Perruche S (2017). Harnessing Apoptotic Cell Clearance to Treat Autoimmune Arthritis. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallese A, Suzuki T, McCarthy C, Bridges J, Filuta A, Arumugam P, Shima K, Ma Y, Wessendarp M, Black D, et al. (2017). Targeting cholesterol homeostasis in lung diseases. Scientific Reports 7, 10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Acevedo S, Pérez-Romano B, and Ruiz-Argüelles A (2000). ‘LE cells’ result from phagocytosis of apoptotic bodies induced by antinuclear antibodies. Journal of autoimmunity 15, 15–20. [DOI] [PubMed] [Google Scholar]
- Sedlyarov V, Eichner R, Girardi E, Essletzbichler P, Goldmann U, Nunes-Hasler P, Srndic I, Moskovskich A, Heinz LX, Kartnig F, et al. (2018). The Bicarbonate Transporter SLC4A7 Plays a Key Role in Macrophage Phagosome Acidification. Cell Host Microbe 23, 766–774.e765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa K, and Nagata S (2015). An Apoptotic ‘Eat Me’ Signal: Phosphatidylserine Exposure. Trends in Cell Biology 25, 639–650. [DOI] [PubMed] [Google Scholar]
- Sender R, and Milo R (2021). The distribution of cellular turnover in the human body. Nat Med 27, 45–48. [DOI] [PubMed] [Google Scholar]
- Serizier SB, and McCall K (2017). Scrambled Eggs: Apoptotic Cell Clearance by Non-Professional Phagocytes in the Drosophila Ovary. Front Immunol 8, 1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJP, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, and Maletic-Savatic M (2010). Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell 7, 483–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BD, and Chandel NS (2019). Immunometabolism of pro-repair cells. The Journal of clinical investigation 129, 2597–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisirak V, Sally B, D’Agati V, Martinez-Ortiz W, Özçakar ZB, David J, Rashidfarrokhi A, Yeste A, Panea C, Chida Asiya S., et al. (2016). Digestion of Chromatin in Apoptotic Cell Microparticles Prevents Autoimmunity. Cell 166, 88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein LR, and Imai S. i. (2012). The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol Metab 23, 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Brodie SE, and Cohn ZA (1976). Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol 68, 665–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup BM, Marom R, Li X, Hsu CW, Chang CY, Truong LD, Dawson B, Grafe I, Chen Y, Jiang MM, et al. (2020). A global Slc7a7 knockout mouse model demonstrates characteristic phenotypes of human lysinuric protein intolerance. Hum Mol Genet 29, 2171–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart JA, Fonseca J, Moradi F, Cunningham C, Seliman B, Worsfold CR, Dolan S, Abando J, and Maddalena LA (2018). How Supraphysiological Oxygen Levels in Standard Cell Culture Affect Oxygen-Consuming Reactions. Journal of Oxidative Medicine and Cellular Longevity 2018, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Li H, Chen J, and Qian Q (2017). Lactic Acid: No Longer an Inert and End-Product of Glycolysis. 32, 453–463. [DOI] [PubMed] [Google Scholar]
- Szondy Z, Sarang Z, Kiss B, Garabuczi É, and Köröskényi K (2017). Anti-inflammatory Mechanisms Triggered by Apoptotic Cells during Their Clearance. Frontiers in immunology 8, 909–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, and Bornfeldt KE (2020). Intracellular and Intercellular Aspects of Macrophage Immunometabolism in Atherosclerosis. 126, 1209–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Berghe TV, Vandenabeele P, and Kroemer G (2019). The molecular machinery of regulated cell death. Cell Res 29, 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torralba D, Baixauli F, Villarroya-Beltri C, Fernández-Delgado I, Latorre-Pellicer A, Acín-Pérez R, Martín-Cófreces NB, Jaso-Tamame ÁL, Iborra S, Jorge I, et al. (2018). Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nature Communications 9, 2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabold O, Wagner S, Wicke C, Scheuenstuhl H, Hussain MZ, Rosen N, Seremetiev A, Becker HD, and Hunt TK (2003). Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 11, 504–509. [DOI] [PubMed] [Google Scholar]
- Veras FP, Peres RS, Saraiva ALL, Pinto LG, Louzada-Junior P, Cunha TM, Paschoal JAR, Cunha FQ, and Alves-Filho JC (2015). Fructose 1,6-bisphosphate, a high-energy intermediate of glycolysis, attenuates experimental arthritis by activating anti-inflammatory adenosinergic pathway. Scientific Reports 5, 15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud M, Ivanov S, Vujic N, Duta-Mare M, Aira L-E, Barouillet T, Garcia E, Orange F, Dugail I, Hainault I, et al. (2018). Lysosomal Cholesterol Hydrolysis Couples Efferocytosis to Anti-Inflammatory Oxysterol Production. 122, 1369–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani A, Benjaminsen J, Moritz C, Henke K, Hartmann J, Norlin N, Richter K, Schieber NL, Franke T, Schwab Y, et al. (2019). Clearance by Microglia Depends on Packaging of Phagosomes into a Unique Cellular Compartment. Developmental Cell 49, 77–88.e77. [DOI] [PubMed] [Google Scholar]
- Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, and Castegna A (2019). The Metabolic Signature of Macrophage Responses. Frontiers in immunology 10, 1462–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. (2021). Heart Disease and Stroke Statistics—2021 Update. 143, e254–e743. [DOI] [PubMed] [Google Scholar]
- Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha N-H, Lee P-H, Shin M, Patel SJ, Yu Z, et al. (2019). T cell stemness and dysfunction in tumors are triggered by a common mechanism. 363, eaau0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai T, and Langer T (2016). Mitochondrial Dynamics and Metabolic Regulation. Trends in Endocrinology & Metabolism 27, 105–117. [DOI] [PubMed] [Google Scholar]
- Wang B, and Tontonoz P (2018). Liver X receptors in lipid signalling and membrane homeostasis. Nature reviews. Endocrinology 14, 452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Zhang S, Vuckovic I, Jeon R, Lerman A, Folmes CD, Dzeja PP, and Herrmann J (2018). Glycolytic Stimulation Is Not a Requirement for M2 Macrophage Differentiation. Cell Metabolism 28, 463–475.e464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tsun Z-Y, Wolfson RL, Shen K, Wyant GA, Plovanich ME, Yuan ED, Jones TD, Chantranupong L, Comb W, et al. (2015). Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. 347, 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kini A, Wang Y, Liu T, Chen Y, Vukmanic E, Emery D, Liu Y, Lu X, Jin L, et al. (2019). Metabolic Deregulation of the Blood-Outer Retinal Barrier in Retinitis Pigmentosa. Cell Reports 28, 1323–1334.e1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Subramanian M, Yurdagul A, Barbosa-Lorenzi VC, Cai B, de Juan-Sanz J, Ryan TA, Nomura M, Maxfield FR, and Tabas I (2017). Mitochondrial Fission Promotes the Continued Clearance of Apoptotic Cells by Macrophages. Cell 171, 331–345.e322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werfel TA, and Cook R.S.J.S.i.I. (2018). Efferocytosis in the tumor microenvironment. 40, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods PS, Kimmig LM, Meliton AY, Sun KA, Tian Y, O’Leary EM, Gökalp GA, Hamanaka RB, and Mutlu GM (2020). Tissue-Resident Alveolar Macrophages Do Not Rely on Glycolysis for LPS-induced Inflammation. 62, 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyant GA, Abu-Remaileh M, Wolfson RL, Chen WW, Freinkman E, Danai LV, Vander Heiden MG, and Sabatini DM (2017). mTORC1 Activator SLC38A9 Is Required to Efflux Essential Amino Acids from Lysosomes and Use Protein as a Nutrient. Cell 171, 642–654.e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Maruyama T, Urade Y, and Nagata S (2014). Immunosuppression via adenosine receptor activation by adenosine monophosphate released from apoptotic cells. eLife 3, e02172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Kawane K, Koike M, Mori Y, Uchiyama Y, and Nagata S (2005). Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature 437, 754–758. [DOI] [PubMed] [Google Scholar]
- Yurdagul A, Subramanian M, Wang X, Crown SB, Ilkayeva OR, Darville L, Kolluru GK, Rymond CC, Gerlach BD, Zheng Z, et al. (2020). Macrophage Metabolism of Apoptotic Cell-Derived Arginine Promotes Continual Efferocytosis and Resolution of Injury. Cell Metabolism 31, 518–533.e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zago G, Saavedra PHV, Keshari KR, and Perry JSA (2021). Immunometabolism of Tissue-Resident Macrophages – An Appraisal of the Current Knowledge and Cutting-Edge Methods and Technologies. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Velmeshev D, Hashimoto K, Huang Y-H, Hofmann JW, Shi X, Chen J, Leidal AM, Dishart JG, Cahill MK, et al. (2020). Neurotoxic microglia promote TDP-43 proteinopathy in progranulin deficiency. Nature 588, 459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Weinberg S, DeBerge M, Gainullina A, Schipma M, Kinchen JM, Ben-Sahra I, Gius DR, Yvan-Charvet L, Chandel NS, et al. (2019). Efferocytosis Fuels Requirements of Fatty Acid Oxidation and the Electron Transport Chain to Polarize Macrophages for Tissue Repair. Cell Metabolism 29, 443–456.e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang Y, Sun K, Meng Z, and Chen L (2018). The SLC transporter in nutrient and metabolic sensing, regulation, and drug development. Journal of Molecular Cell Biology 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


