Abstract
Background:
Subsequent thyroid cancer (STC) is one of the most common malignancies in childhood cancer survivors. We aimed to evaluate the polygenic contributions to STC risk and potential utility in improving risk prediction.
Methods:
A polygenic risk score (PRS) was calculated from 12 independent single-nucleotide polymorphisms associated with thyroid cancer risk in the general population. Associations between PRS and STC risk were evaluated among survivors from St. Jude Lifetime Cohort (SJLIFE) and were replicated in survivors from Childhood Cancer Survivor Study (CCSS). A risk prediction model integrating the PRS and clinical factors, initially developed in SJLIFE, and its performance were validated in CCSS.
Results:
Among 2,370 SJLIFE survivors with a median follow-up of 28.8 (interquartile range [IQR]=21.9–36.1) years, 65 (2.7%) developed STC. Among them, the standardized PRS was associated with an increased rate of STC (relative rate [RR]=1.57, 95% CI=1.24–1.98, p<0.001). Similar associations were replicated in 6,416 CCSS survivors among whom 121 (1.9%) developed STC during median follow-up of 28.9 (IQR=22.6–34.6) years (RR=1.52, 95% CI=1.25–1.83, p<0.001). A risk prediction model integrating the PRS with clinical factors showed better performance than the model considering only clinical factors in SJLIFE (p=0.004, AUC=83.2% vs. 82.1%, at age 40), which was further validated in CCSS (p=0.010, AUC=72.9% vs. 70.6%).
Conclusions:
Integration of the PRS with clinical factors provided a statistically significant improvement in risk prediction of STC, although the magnitude of improvement was modest.
Impact:
PRS improves risk stratification and prediction of STC, suggesting its potential utility for optimizing screening strategies in survivorship care.
Keywords: Polygenic risk score, Subsequent thyroid cancer, Childhood cancer, Survivorship
INTRODUCTION
Following the successful treatment of childhood cancer, survivors often experience subsequent malignancies requiring further therapy and clinical care. Of these, the most common endocrine malignancy observed in survivors of childhood cancer are subsequent thyroid cancers (STC),(1) which accounts for approximately 10% of all subsequent malignancies.(2) The predominant form of STC is differentiated thyroid cancer, which includes both papillary and follicular carcinoma.(3) Among childhood cancer survivors, occurrence of STC has been reported largely attributable to radiotherapy (RT) for childhood cancer that exposes the thyroid gland.(4) Importantly, STC occurrence also demonstrates a dose-related increase in risk that declines after 30Gy.(5) Therefore, periodic surveillance for thyroid cancer among childhood cancer survivors treated with neck-RT is highly recommended.(6) However, debate remains over the necessity and benefit of routine STC screening with ultrasonography versus routine palpation, considering STC’s favorable prognosis as well as potential harms associated with discovery of benign thyroid nodules. In recently published consensus recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group and the PanCareSurFup Consortium, the expert panel found no evidence to support superiority of one screening modality over the other, and therefore recommended shared decision making between the health care provider and survivor to make this determination.(7) As such, both clinical practitioners and survivors of childhood cancer may benefit from methods to enhance precision in risk stratification and individual risk prediction via the integration of genetic susceptibility of STC with clinical risk factors such as childhood cancer treatment exposures and doses.
Polygenic contributions to the risk of de novo thyroid cancer in the general population have been studied by genome-wide association studies (GWAS), resulting in the identification of 12 independent common risk alleles within populations of European ancestry (without known exposure to radiotherapy or chemotherapy).(8–13) Notably, the estimated effect sizes (i.e., per-allele odds ratios (ORs)) range between 1.20 and 1.81, which are relatively larger than most cancer GWAS findings. For example, the effect size for 172 common breast cancer risk loci ranges between 1.03 and 1.31.(14) This difference points to an allelic architecture of genetic susceptibility for thyroid cancer, involving a smaller number of risk loci, some of which may have higher estimated effect sizes as compared to other more common adult carcinomas (e.g., breast,(15) colon,(16) and prostate(17)).
We hypothesized that the polygenic risk score (PRS) based on all the common risk alleles, which were identified within the general population presumably without exposure to radiotherapy or chemotherapeutic agents for de novo thyroid cancer risk, could be informative for assessing the risk of STC among survivors of childhood cancer. In this study, we established a risk prediction model by integrating a PRS with commonly used clinical risk factors identified among survivors in the St. Jude Lifetime Cohort (SJLIFE).(18) We further validated our integrated prediction model within an independent cohort of survivors from Childhood Cancer Survivors Study (CCSS).(19)
MATERIALS AND METHODS
Study population and data collection
The SJLIFE study is a retrospective cohort study initiated in 2007 with prospective clinical follow-up and ongoing enrollment of 5-year survivors of all childhood cancer who were treated at St. Jude Children’s Research Hospital (SJCRH) since its establishment in 1962.(18) Among 3,006 SJLIFE survivors with whole-genome sequencing (WGS) data as previously described(14), a total 2,370 survivors were available for further statistical analyses based on the exclusion criteria (Supplementary Methods, Supplementary Figure S1A). The demographic and clinical characteristics including treatment information were abstracted from the self-reported questionnaires and patients’ medical records, respectively. Subsequent malignancies including STC were clinically ascertained. The current report of SJLIFE is based on follow-up through 2018. All SJLIFE study participants provided written informed consent. The SJLIFE study protocol was approved by the Institutional Review Board (IRB) at SJCRH.
For replication/validation of findings from SJLIFE, data from survivors in the CCSS cohort, a multicenter retrospective cohort study with prospective follow-up, was used.(19,20) A total of 6,416 (4,188 original + 2,228 expansion) CCSS cohort were available for analyses after the exclusions (Supplementary Methods, Supplementary Figure S1B). The demographic and clinical characteristics were obtained in CCSS from self- or proxy-reported questionnaires, death certificate, and medical records. Treatment data included all treatments received within the first five years following childhood cancer diagnosis. Subsequent malignancies including STC were identified by self-reported questionnaires and subsequently confirmed by pathology reports. The CCSS survivors were followed up through 2019. The CCSS study participants provided the informed consent and the study protocol was approved by the IRB at each participating center.
SNP extraction and PRS calculation
A total of 32 single-nucleotide polymorphism (SNP) associations with thyroid cancer risk in the general population were downloaded from NHGRI-EBI GWAS catalog on December 8th, 2019(21) (Supplementary Table S1), many of which are highly correlated. We subsequently excluded studies using individuals from non-European ancestry. If multiple studies have reported association findings for the same SNP or SNPs with strong pairwise linkage disequilibrium (LD, r2>0.8), estimates from the study with the largest sample size were used. A total of 12 SNPs (rs11693806, rs2466076, rs1588635, rs368187, rs116909374, rs12129938, rs6793295, rs73227498, rs7902587, rs2289261, and rs56062135) remained after curation. The PRS was calculated as a weighted sum of the number of risk alleles carried by an individual, in which their weights were taken as the natural logarithm of the estimated ORs of the corresponding loci, and then standardized as a z-score with a mean of 0 and a standard deviation (SD) of 1. To compare cumulative incidence curves for groups with different genetic risks, the PRS was categorized into tertiles (cutoffs: 2.73 and 3.33).
Statistical analysis
The cumulative incidence of STC by age was estimated for SJLIFE and CCSS survivors in each tertile of the PRS. Death was considered as a competing risk event. Gray’s method (22) was used to evaluate statistical significance of the differences in cumulative incidence curves across three tertiles. We employed the Fine and Gray proportional subdistribution hazards model (23) to construct a clinical base model encompassing demographic and treatment variables in the SJLIFE study (Supplementary Methods). The final clinical model included the following covariates: attained age; age at primary diagnosis; sex; and the derived 8-category treatment groups (Supplementary Table S2). Then, the standardized PRS was added as a continuous independent variable to the final clinical model to formulate the full integrated model. The adjusted subdistribution hazard ratio was reported as relative rate (RR). The RR of STC by one SD increase in the PRS was estimated by the maximum likelihood method and its inference including 95% confidence intervals (CIs) and p-values were calculated using the standard large-sample inference methods. The analyses were also stratified by the neck-RT exposure status. In order to evaluate model-predicted lifetime risk, we estimated the cumulative incidence of STC at age of 20, 30, 40 and 50 years for each risk profile (n=192 profiles) comprised of the combinations of sex (2 categories), age-at-diagnosis (4), treatment combinations (8), and PRS tertiles (3). A replication analysis of the integrated model was conducted with the CCSS data using the integrated model from SJLIFE including the same definitions of variables and adjusting for the same set of covariates: the purpose of this replication analysis was to examine the consistency between SJLIFE and CCSS regarding the associations of the PRS with the STC rate.
To evaluate risk prediction performance of the SJLIFE models, we considered the final clinical model and the integrated model (the final clinical model plus the PRS) of SJLIFE and validated in CCSS. We estimated time-dependent receiver operating characteristic (ROC) curves(24) and compared predictive power by time-specific area under the ROC curves (AUCs) and its weighted average, Harrell’s Concordance (C) statistic(25), between the two models at ages of 40 and 50 years. Data analysis and visualization were performed using SAS 9.4 (SAS Institute Inc, Cary, NC, USA) and R 3.5.1(26). All statistical tests were two-sided and p-value<0.05 was set as the threshold for statistical significance.
RESULTS
Characteristics of study populations
Among 2,370 SJLIFE survivors (median time from diagnosis: 28.8 years, interquartile range [IQR]: 21.9–36.1 years), 65 (2.7%) were subsequently diagnosed with thyroid cancer (Table 1). The median age at primary cancer diagnosis and follow-up was 7.1 years (IQR: 3.1–13.1 years) and 36.6 years (IQR: 30.3–44.1 years), respectively. A total of 1,265 (53.4%) were male. Childhood cancer diagnoses comprised leukemia (36.6%), central nervous system (CNS) tumors (10.5%), lymphoma (20.0%), sarcoma (12.9%), and non-CNS embryonal tumors (16.5%). For radiotherapy, 20.1% survivors received neck-RT. For chemotherapy potentially impacting STC risk, 58.3%, and 35.4% survivors were exposed to anthracyclines, and epipodophyllotoxins, respectively.
Table 1.
Demographics and treatment characteristics in SJLIFE and CCSS
| Characteristics | SJLIFE | CCSS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Total (N=2,370, 100%) | Survivors with STC (N=65, 2.7%) | Survivors without STC (N=2,305, 97.3%) | Total (N=6,416, 100%) | Survivors with STC (N=121, 1.9%) | Survivors without STC (N=6,295, 98.1%) | |||||||
|
| ||||||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
| Age at diagnosis, years | ||||||||||||
| 0–4 | 922 | (38.9%) | 19 | (29.2%) | 903 | (39.2%) | 2,391 | (37.3%) | 30 | (24.8%) | 2,361 | (37.5%) |
| 5–9 | 538 | (22.7%) | 5 | (7.7%) | 533 | (23.1%) | 1,423 | (22.2%) | 21 | (17.4%) | 1,402 | (22.3%) |
| 10–14 | 532 | (22.4%) | 25 | (38.5%) | 507 | (22.0%) | 1,440 | (22.4%) | 47 | (38.8%) | 1,393 | (22.1%) |
| ≥15 | 378 | (15.9%) | 16 | (24.6%) | 362 | (15.7%) | 1,162 | (18.1%) | 23 | (19.0%) | 1,139 | (18.1%) |
| Sex | ||||||||||||
| Men | 1,265 | (53.4%) | 27 | (41.5%) | 1,238 | (53.7%) | 3,058 | (47.7%) | 41 | (33.9%) | 3,017 | (47.9%) |
| Women | 1,105 | (46.6%) | 38 | (58.5%) | 1,067 | (46.3%) | 3,358 | (52.3%) | 80 | (66.1%) | 3,278 | (52.1%) |
| Diagnosis | ||||||||||||
| Leukemia | 868 | (36.6%) | 19 | (29.2%) | 849 | (36.8%) | 1,713 | (26.7%) | 25 | (20.7%) | 1,688 | (26.8%) |
| Acute lymphoblastic leukemia | 802 | (33.8%) | 16 | (24.6%) | 786 | (34.1%) | 1,536 | (23.9%) | 21 | (17.4%) | 1,515 | (24.1%) |
| Acute myeloid leukemia | 63 | (2.7%) | 3 | (4.6%) | 60 | (2.6%) | 144 | (2.2%) | 1 | (0.8%) | 143 | (2.3%) |
| Other leukemia | 3 | (0.1%) | - | (0.0%) | 3 | (0.1%) | 33 | (0.5%) | 3 | (2.5%) | 30 | (0.5%) |
| CNS tumors | 249 | (10.5%) | 5 | (7.7%) | 244 | (10.6%) | 1,160 | (18.1%) | 19 | (15.7%) | 1,141 | (18.1%) |
| Astrocytoma or glioma | 123 | (5.2%) | - | (0.0%) | 123 | (5.3%) | 720 | (11.2%) | 5 | (4.1%) | 715 | (11.4%) |
| Medulloblastoma or PNET | 62 | (2.6%) | 4 | (6.2%) | 58 | (2.5%) | 271 | (4.2%) | 8 | (6.6%) | 263 | (4.2%) |
| Ependymoma | 25 | (1.1%) | 1 | (1.5%) | 24 | (1.0%) | ||||||
| Other CNS tumors | 39 | (1.6%) | - | (0.0%) | 39 | (1.7%) | 169 | (2.6%) | 6 | (5.0%) | 163 | (2.6%) |
| Lymphoma | 474 | (20.0%) | 32 | (49.2%) | 442 | (19.2%) | 1,373 | (21.4%) | 43 | (35.5%) | 1,330 | (21.1%) |
| Hodgkin lymphoma | 289 | (12.2%) | 30 | (46.2%) | 259 | (11.2%) | 849 | (13.2%) | 38 | (31.4%) | 811 | (12.9%) |
| Non-Hodgkin lymphoma | 185 | (7.8%) | 2 | (3.1%) | 183 | (7.9%) | 524 | (8.2%) | 5 | (4.1%) | 519 | (8.2%) |
| Sarcoma | 306 | (12.9%) | 3 | (4.6%) | 303 | (13.1%) | 1,028 | (16.0%) | 20 | (16.5%) | 1,008 | (16.0%) |
| Ewing sarcoma | 84 | (3.5%) | 2 | (3.1%) | 82 | (3.6%) | 194 | (3.0%) | 5 | (4.1%) | 189 | (3.0%) |
| Osteosarcoma | 82 | (3.5%) | - | (0.0%) | 82 | (3.6%) | 314 | (4.9%) | 10 | (8.3%) | 304 | (4.8%) |
| Rhabdomyosarcoma | 76 | (3.2%) | 1 | (1.5%) | 75 | (3.3%) | ||||||
| Non-rhabdomyosarcoma | 64 | (2.7%) | - | (0.0%) | 64 | (2.8%) | ||||||
| Soft tissue sarcoma | 520 | (8.1%) | 5 | (4.1%) | 515 | (8.2%) | ||||||
| Non-CNS Embryonal | 401 | (16.5%) | 5 | (7.7%) | 396 | (16.8%) | 1108 | (17.3%) | 12 | (9.9%) | 1096 | (17.4%) |
| Wilms tumor | 152 | (6.4%) | 1 | (1.5%) | 151 | (6.6%) | 621 | (9.7%) | 5 | (4.1%) | 616 | (9.8%) |
| Neuroblastoma | 119 | (5.0%) | 1 | (1.5%) | 118 | (5.1%) | 487 | (7.6%) | 7 | (5.8%) | 480 | (7.6%) |
| Germ cell tumor | 45 | (1.9%) | 1 | (1.5%) | 44 | (1.9%) | ||||||
| Retinoblastoma | 66 | (2.8%) | 2 | (3.1%) | 64 | (2.8%) | ||||||
| Hepatoblastoma | 16 | (0.7%) | - | (0.0%) | 16 | (0.7%) | ||||||
| Others | ||||||||||||
| Melanoma | 15 | (0.6%) | - | (0.0%) | 15 | (0.7%) | ||||||
| Carcinomas | 26 | (1.1%) | - | (0.0%) | 26 | (1.1%) | ||||||
| Other | 34 | (1.4%) | 1 | (1.5%) | 33 | (1.4%) | 34 | (0.5%) | 2 | (1.7%) | 32 | (0.5%) |
| Radiation therapy | ||||||||||||
| Neck-RT dose, Gy | ||||||||||||
| None | 1,894 | (79.9%) | 18 | (27.7%) | 1,876 | (81.4%) | 5,062 | (78.9%) | 48 | (39.7%) | 5,014 | (79.7%) |
| >0-<20 | 59 | (2.5%) | 6 | (9.2%) | 53 | (2.3%) | 175 | (2.7%) | 9 | (7.4%) | 166 | (2.6%) |
| ≥20-<30 | 247 | (10.4%) | 31 | (47.7%) | 216 | (9.4%) | 397 | (6.2%) | 28 | (23.1%) | 369 | (5.9%) |
| ≥30 | 170 | (7.2%) | 10 | (15.4%) | 160 | (6.9%) | 802 | (12.5%) | 36 | (29.8%) | 766 | (12.2%) |
| Chemotherapy | ||||||||||||
| Anthracycline dose, tertiles | ||||||||||||
| None | 988 | (41.7%) | 30 | (46.2%) | 958 | (41.6%) | 3,784 | (59.0%) | 72 | (59.5%) | 3,712 | (59.0%) |
| 1st tertile | 464 | (19.6%) | 2 | (3.1%) | 462 | (20.0%) | 361 | (5.6%) | 5 | (4.1%) | 356 | (5.7%) |
| 2nd tertile | 456 | (19.2%) | 19 | (29.2%) | 437 | (19.0%) | 937 | (14.6%) | 13 | (10.7%) | 924 | (14.7%) |
| 3rd tertile | 462 | (19.5%) | 14 | (21.5%) | 448 | (19.4%) | 1,334 | (20.8%) | 31 | (25.6%) | 1,303 | (20.7%) |
| Epipodophyllotoxin dose, tertiles | ||||||||||||
| None | 1,532 | (64.6%) | 43 | (66.2%) | 1,489 | (64.6%) | 5,693 | (88.7%) | 112 | (92.6%) | 5,581 | (88.7%) |
| 1st tertile | 271 | (11.4%) | 10 | (15.4%) | 261 | (11.3%) | 269 | (4.2%) | 2 | (1.7%) | 267 | (4.2%) |
| 2nd tertile | 282 | (11.9%) | 6 | (9.2%) | 276 | (12.0%) | 342 | (5.3%) | 6 | (5.0%) | 336 | (5.3%) |
| 3rd tertile | 285 | (12.0%) | 6 | (9.2%) | 279 | (12.1%) | 76 | (1.2%) | 1 | (0.8%) | 75 | (1.2%) |
| Treatment group | ||||||||||||
| Epipodophyllotoxin & Anthracycline 2–3 tertiles without neck-RT | 247 | (10.2%) | 7 | (10.8%) | 240 | (10.2%) | 392 | (6.1%) | 5 | (4.1%) | 387 | (6.1%) |
| Neck-RT >0-<20 Gy without Epipodophyllotoxin | 39 | (1.6%) | 3 | (4.6%) | 36 | (1.5%) | 132 | (2.1%) | 7 | (5.8%) | 125 | (2.0%) |
| Neck-RT ≥20-<30 Gy without Epipodophyllotoxin | 294 | (12.1%) | 22 | (33.8%) | 172 | (7.3%) | 360 | (5.6%) | 27 | (22.3%) | 333 | (5.3%) |
| Neck-RT ≥30 Gy without Epipodophyllotoxin | 135 | (5.6%) | 8 | (12.3%) | 127 | (5.4%) | 741 | (11.5%) | 36 | (29.8%) | 705 | (11.2%) |
| Neck-RT >0-<20 Gy with Epipodophyllotoxin | 20 | (0.8%) | 3 | (4.6%) | 17 | (0.7%) | 43 | (0.7%) | 2 | (1.7%) | 41 | (0.7%) |
| Neck-RT ≥20-<30 Gy with Epipodophyllotoxin | 53 | (2.2%) | 9 | (13.8%) | 44 | (1.9%) | 37 | (0.6%) | 1 | (0.8%) | 36 | (0.6%) |
| Neck-RT ≥30 Gy with Epipodophyllotoxin | 35 | (1.4%) | 2 | (3.1%) | 33 | (1.4%) | 41 | (0.6%) | - | (0.0%) | 41 | (0.7%) |
| None of the above | 1,647 | (67.9%) | 11 | (16.9%) | 1,636 | (69.3%) | 4,670 | (72.8%) | 43 | (35.5%) | 4,627 | (73.5%) |
| PRS | ||||||||||||
| 1st tertile | 791 | (33.4%) | 9 | (13.8%) | 782 | (33.9%) | 2,201 | (34.3%) | 22 | (18.2%) | 2,179 | (34.6%) |
| 2nd tertile | 789 | (33.3%) | 23 | (35.4%) | 766 | (33.2%) | 2,132 | (33.2%) | 40 | (33.1%) | 2,092 | (33.2%) |
| 3rd tertile | 790 | (33.3%) | 33 | (50.8%) | 757 | (32.8%) | 2,083 | (32.5%) | 59 | (48.8%) | 2,024 | (32.2%) |
|
| ||||||||||||
| Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | Median | (IQR) | |
|
| ||||||||||||
| Age at diagnosis, years | 7.1 | (3.1–13.1) | 12.0 | (4.2–14.9) | 6.9 | (3.1–13.1) | 7.5 | (3.3–13.6) | 11.1 | (5.0–14.3) | 7.4 | (3.2–13.6) |
| Age at follow-up, years | 36.6 | (30.3–44.1) | 42.2 | (37.7–48.6) | 36.5 | (30.1–43.9) | 36.5 | (30.2–44.2) | 42.6 | (36.7–49.3) | 36.4 | (30.1–44.1) |
| Length of follow-up, years | 28.8 | (21.9–36.1) | 31.6 | (26.7–37.9) | 28.8 | (21.9–36.1) | 28.9 | (22.6–34.6) | 32.5 | (25.7–38.4) | 28.8 | (22.5–34.5) |
Abbreviations: SJLIFE (St. Jude Lifetime cohort study), CCSS (Childhood Cancer Survivor Study), STC (subsequent thyroid cancer), CNS (central nervous system), PNET (primitive neuroectodermal tumor), RT (radiotherapy), PRS (polygenic risk score), IQR (interquartile range)
Among 6,416 CCSS survivors (median time from diagnosis: 28.9 years, IQR: 22.6–34.6), 121 (1.9%) survivors were subsequently diagnosed with thyroid cancer. The median age at primary cancer diagnosis was 7.5 years (IQR: 3.3–13.6 years) and the median follow-up was 36.5 years (IQR: 30.2–44.2 years). A total of 3,058 (47.7%) were male. Childhood cancer diagnoses comprised leukemia (26.7%), central nervous system (CNS) tumors (18.1%), lymphoma (21.4%), sarcoma (16.0%), and non-CNS embryonal tumors (17.3%). Regarding the treatment variables required for the replication analysis in the CCSS, 21.1%, 41.0% and 11.3% survivors were exposed to neck-RT, anthracyclines and epipodophyllotoxins, respectively.
We further tested the difference of several characteristics including age at diagnosis, sex, primary diagnosis, treatment exposures and treatment group between survivors included in the analysis versus those excluded (Supplementary Table S3). We found that primary diagnosis was significantly different (P<0.001) in SJLIFE, whereas age at diagnosis, treatment exposures, and treatment group were significantly different (P<0.001) in CCSS.
Cumulative incidence of STC by neck-RT and PRS tertiles
The cumulative incidence curves of STC showed statistically significant differences by PRS tertiles among SJLIFE survivors (p=0.002, Figure 1A). Survivors with PRS in the third tertile had the highest cumulative incidence compared to survivors >25 years old with a PRS in the second or the first tertile. Among SJLIFE survivors (Supplementary Table S4), the cumulative incidence for those with PRS in the first, second and third tertiles were 0.4% (95% CI=0.0–0.9), 1.2% (95% CI=0.3–2.0), and 2.1% (95% CI=1.0–3.3) by age 30 years, 1.6% (95% CI=0.4–2.8), 2.8% (95% CI=1.3–4.2), 4.8% (95% CI=2.9–6.6), by age 40 years, and 2.7% (95% CI=0.8–4.5), 6.7% (95% CI=3.4–10.0), 8.1% (95% CI=5.0–11.2) by age 50 years, respectively. Among 65 SJLIFE survivors who developed STC, 9 had a PRS in the first tertile, 23 in the second tertile and 33 in the third tertile. When stratified by neck-RT, the cumulative incidence of STC showed statistically significant differences by neck-RT exposure among SJLIFE survivors (p<0.001, Supplementary Figure S2A, Supplementary Table S5). Furthermore, the cumulative incidence of STC differed across PRS tertiles in survivors exposed to neck-RT (p=0.013, Figure 1B) but not in survivors without neck-RT exposure (p=0.29, Figure 1C). The cumulative incidence was as high as 22.0% by age 50 years for SJLIFE survivors who were previously exposed to neck-RT and had PRS in the third tertile (Supplementary Table S4).
Figure 1.
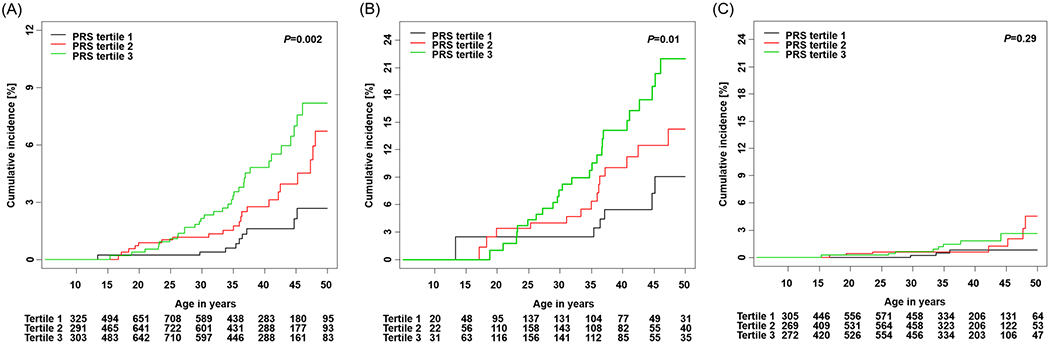
Cumulative incidence of STC by PRS tertiles in SJLIFE. (A) Overall survivors (B) Survivors with neck-RT (C) Survivors without neck-RT
A similar pattern of cumulative incidence of STC by PRS tertiles was observed among CCSS survivors (p<0.001, Figure 2A). Among the CCSS survivors (Supplementary Table S4), the cumulative incidences of STC for survivors with PRS in the first, second and third tertiles were 0.3% (95% CI=0.0–0.5), 1.0% (95% CI=0.5–1.5), 1.4% (95% CI=0.8–1.9) by age 30 years, 1.4% (95% CI=0.8–2.0), 2.5% (95% CI=1.6–3.3), 3.5% (95% CI=2.5–4.5) by age 40 years, and 1.6% (95% CI=0.9–2.4), 3.5% (95% CI=2.2–4.9), 5.2% (95% CI=3.6–6.8) by age 50 years, respectively. Among 121 CCSS survivors who developed STC, 22 had a PRS in the first tertile, 40 in the second tertile and 59 in the third tertile. When stratified by neck-RT, the cumulative incidence of STC showed statistically significant differences by neck-RT exposure among CCSS survivors (p<0.001, Supplementary Figure S2B, Supplementary Table S5). Furthermore, statistically significant differences of cumulative incidence across the PRS tertiles were observed among survivors previously exposed to neck-RT (p=0.040, Figure 2B) and survivors not exposed to neck-RT (p<0.001, Figure 2C). CCSS survivors exposed to neck-RT showed a distinct pattern where survivors with a PRS in the second tertile had the highest incidence before age 35 years but survivors with a PRS in the third tertile became the highest incidence group after age 35 years. The cumulative incidence was as high as 10.0% by age 50 years for CCSS survivors who were previously exposed to neck-RT and had a PRS in the third tertile (Supplementary Table S4).
Figure 2.
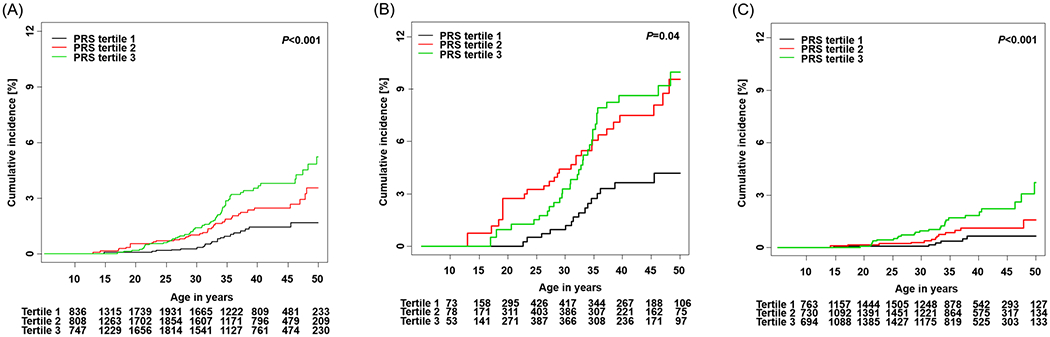
Cumulative incidence of STC by PRS tertiles in CCSS. (A) Overall survivors (B) Survivors with neck-RT (C) Survivors without neck-RT
Abbreviations: STC (subsequent thyroid cancer), SJLIFE (St. Jude Lifetime Cohort Study), RT (radiation therapy), CCSS (Childhood Cancer Survival Study)
In addition, we calculated the model-predicted lifetime risk (cumulative incidence) of STC at age of 20, 30, 40 and 50 years for each risk profile (n=192 profiles). (Supplementary Table S6).
Association of the PRS with STC risk
The base clinical model with the treatment groups and other clinical characteristics was built with the SJLIFE data (Supplementary Table S2). The base clinical model was validated in CCSS (Supplementary Table S7). We assessed whether the PRS based on the 12 SNPs was associated with the risk of developing STC among childhood cancer survivors (Table 2). In SJLIFE, the PRS was statistically significantly associated with an increased rate of STC among all survivors (RR=1.57, 95% CI=1.24–1.98, p<0.001) and among survivors with prior neck-RT exposure (RR=1.68, 95% CI=1.29–2.18, p<0.001). However, no significant association was observed among survivors with no prior neck-RT exposure. The associations between the PRS and STC rates were replicated in CCSS overall (RR=1.52, 95% CI=1.25–1.83, p<0.001), survivors with neck-RT (RR=1.42, 95% CI=1.09–1.85, p=0.009) and survivors without prior neck-RT (RR=1.66, 95% CI=1.26–2.20, p<0.001) (Table 3).
Table 2.
Multivariable model fits for STC rates in association with the standardized PRS and clinical risk factors in SJLIFEa
| Characteristics | Overall survivors | Survivors with neck-RT | Survivors without neck-RT | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| RR | (95% CI) | P | RR | (95% CI) | P | RR | (95% CI) | P | |
| Age at diagnosis, years | |||||||||
| 0–4 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| 5–9 | 0.28 | (0.10–0.78) | 0.015 | 0.26 | (0.07–1.03) | 0.06 | 0.33 | (0.07–1.50) | 0.15 |
| 10–14 | 1.20 | (0.64–2.23) | 0.58 | 1.46 | (0.64–3.34) | 0.37 | 0.67 | (0.21–2.09) | 0.49 |
| ≥15 | 0.61 | (0.31–1.20) | 0.15 | 0.74 | (0.32–1.71) | 0.49 | 0.47 | (0.12–1.82) | 0.28 |
| Sex | |||||||||
| Men | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| Women | 1.50 | (0.89–2.53) | 0.13 | 1.17 | (0.63–2.17) | 0.62 | 2.97 | (1.05–8.39) | 0.039 |
| Treatment group | |||||||||
| Anthracycline dose in the 2–3 tertiles with Epipodophyllotoxin without neck-RT | 4.81 | (1.83–12.65) | 0.001 | - | 5.23 | (1.82–15.03) | 0.002 | ||
| Neck-RT >0-<20 Gy without Epipodophyllotoxin | 7.25 | (2.08–25.32) | 0.002 | 1.00 | (Ref.) | - | |||
| Neck-RT ≥20-<30 Gy without Epipodophyllotoxin | 14.55 | (7.03–30.09) | <0.001 | 1.95 | (0.60–6.36) | 0.27 | - | ||
| Neck-RT ≥30 Gy without Epipodophyllotoxin | 5.61 | (2.14–14.74) | <0.001 | 0.73 | (0.19–2.76) | 0.65 | - | ||
| Neck-RT >0-<20 Gy with Epipodophyllotoxin | 35.94 | (10.20–126.56) | <0.001 | 4.52 | (0.94–21.76) | 0.06 | - | ||
| Neck-RT ≥20-<30 Gy with Epipodophyllotoxin | 28.50 | (11.19–72.60) | <0.001 | 3.56 | (0.96–13.19) | 0.06 | - | ||
| Neck-RT ≥30 Gy with Epipodophyllotoxin | 13.32 | (2.96–60.01) | <0.001 | 1.72 | (0.29–10.13) | 0.55 | - | ||
| None of the above | 1.00 | (Ref.) | (Ref.) | ||||||
| Standardized PRSb, continuous (per one standard deviation) | 1.57 | (1.24–1.98) | <0.001 | 1.68 | (1.29–2.18) | <0.001 | 1.36 | (0.85–2.15) | 0.24 |
Abbreviations: PRS (polygenic risk score), STC (subsequent thyroid cancer), RT (radiotherapy), RR (relative rate), and SJLIFE (St. Jude Lifetime Cohort Study).
Adjusted for attained age modeled by restricted cubic splines.
Standardized PRS was calculated as a weighted sum of the number of risk alleles carried by survivors and standardized by a mean of 0 and a SD of 1.
Table 3.
Multivariable model fits for STC rates in association with the standardized PRS and clinical risk factors in CCSSa
| Characteristics | Overall survivors | Survivors with neck-RT | Survivors without neck-RT | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| RR | (95% CI) | P | RR | (95% CI) | P | RR | (95% CI) | P | |
| Age at diagnosis, years | |||||||||
| 0–4 | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| 5–9 | 0.70 | (0.40–1.23) | 0.21 | 0.83 | (0.40–1.91) | 0.65 | 0.46 | (0.18–1.17) | 0.10 |
| 10–14 | 0.92 | (0.56–1.50) | 0.74 | 0.90 | (0.46–1.86) | 0.77 | 0.79 | (0.35–1.77) | 0.57 |
| ≥15 | 0.40 | (0.21–0.75) | 0.004 | 0.25 | (0.10–0.61) | <0.001 | 0.82 | (0.36–1.89) | 0.65 |
| Sex | |||||||||
| Men | 1.00 | (Ref.) | 1.00 | (Ref.) | 1.00 | (Ref.) | |||
| Women | 1.69 | (1.15–2.47) | 0.007 | 1.51 | (0.93–2.45) | 0.10 | 2.17 | (1.17–4.05) | 0.015 |
| Treatment group | |||||||||
| Anthracycline 2–3 tertiles without neck-RT | 2.21 | (0.88–5.57) | 0.09 | - | 2.19 | (0.87–5.54) | 0.10 | ||
| Neck-RT >0-<20 Gy without Epipodophyllotoxin | 5.04 | (2.25–11.28) | <0.001 | 1.00 | (Ref.) | - | |||
| Neck-RT ≥20-<30 Gy without Epipodophyllotoxin | 8.07 | (4.84–13.47) | <0.001 | 1.66 | (0.72–3.83) | 0.23 | - | ||
| Neck-RT ≥30 Gy without Epipodophyllotoxin | 4.49 | (2.68–7.52) | <0.001 | 1.03 | (0.45–2.36) | 0.94 | - | ||
| Neck-RT >0-<20 Gy with Epipodophyllotoxin | 8.76 | (2.14–35.88) | 0.003 | 1.77 | (0.36–8.73) | 0.48 | - | ||
| Neck-RT ≥20-<30 Gy with Epipodophyllotoxin | 3.76 | (0.51–27.47) | 0.19 | 0.81 | (0.10–6.72) | 0.85 | - | ||
| Neck-RT ≥30 Gy with Epipodophyllotoxin | - | - | - | ||||||
| None of the above | 1.00 | (Ref.) | 1.00 | (Ref.) | |||||
| Standardized PRSb, continuous (per one standard deviation) | 1.52 | (1.25–1.83) | <0.001 | 1.42 | (1.09–1.85) | 0.009 | 1.66 | (1.26–2.20) | <0.001 |
Abbreviations: PRS (polygenic risk score), STC (subsequent thyroid cancer), RT (radiotherapy), RR (relative rate), and CCSS (Childhood Cancer Survivor Study)
Adjusted for attained age modeled by restricted cubic splines
Standardized PRS was calculated as a weighted sum of the number of risk alleles carried by survivors and standardized by a mean of 0 and a SD of 1
Evaluation of a risk prediction model of STC with the PRS included
We first compared two risk prediction models of STC: a base clinical model considering the treatment groups and other clinical characteristics and an integrated model additionally including the PRS (Table 4). In the SJLIFE survivors, the integrated model with the PRS performed better than the base clinical model at age of 40 years (C-statistic=84.2%, AUC=0.83 vs. C-statistic=82.8%, AUC=0.82, p=0.004) and at age of 50 years (C-statistic=83.4%, AUC=0.82 vs. C-statistic=82.1%, AUC=0.81, p=0.022). The CCSS replication data showed better performance of the integrated model with the PRS than the base clinical model at age of 40 years (C-statistic=73.0%, AUC=0.73 vs. C-statistic=70.7%, AUC=0.71, p=0.010) and age of 50 years (C-statistic=72.7%, AUC=0.72 vs. C-statistic=70.5%, AUC=0.69, p=0.006).
Table 4.
Comparison of risk prediction models for STC between the base clinical model and the integrated model including the PRS
| SJLIFE discovery | CCSS replicationc | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Clinical modela | Integrated modelb | P | Clinical modela | Integrated modelb | P | |
| Age at 40 years | 0.004 | 0.010 | ||||
| C-statistic | 82.8% | 84.2% | 70.7% | 73.0% | ||
| AUC | 0.82 | 0.83 | 0.71 | 0.73 | ||
| Age at 50 years | 0.022 | 0.006 | ||||
| C-statistic | 82.1% | 83.4% | 70.5% | 72.7% | ||
| AUC | 0.81 | 0.82 | 0.69 | 0.72 | ||
Abbreviations: STC (subsequent thyroid cancer), PRS (polygenic risk score), SJLIFE (St. Jude Lifetime Cohort Study), CCSS (Childhood Cancer Survivor Study), and AUC (area under curve)
The base clinical model included age at diagnosis, attained age modeled by restricted cubic splines, sex, and combined treatment group
The integrated model included PRS on top of the clinical model
The CCSS replication analysis used the exact same model as SJLIFE discovery including the regression coefficients and variable definitions
DISCUSSION
Implementation of precision care that focuses surveillance and interventions on populations at high risk of adverse outcomes represents a priority among multidisciplinary health care professionals monitoring childhood cancer survivors. Our study demonstrates that the PRS constructed from thyroid cancer associated genetic variants established in the general population can effectively identify survivors with high STC risk, although the risk prediction model integrating the PRS with cancer treatment risk factors only provided a marginal improvement over risk prediction of STC utilizing a clinical model that includes cancer therapy exposures. Nonetheless, these findings, which were validated in an independent cohort of childhood cancer survivors, provide a basis for future addition of newly discovered thyroid cancer risk loci that could eventually be useful for identifying individuals at the highest and lowest risk for STC and inform future discussions regarding risk-based surveillance strategies.
In the current study, it is evident that the PRS can stratify survivors with different STC risk levels as observed in the distinct cumulative incidence curves corresponding to PRS tertiles, in both SJLIFE and CCSS. The magnitude of differences in cumulative incidence is substantial. For instance, by age of 40 years, SJLIFE survivors with a PRS in the third tertile had a 6-fold increased STC rate compared to survivors with a PRS in the first tertile suggesting that survivors with a PRS in the third tertile may benefit more from a recommendation of imaging screening to facilitate early diagnosis. The rate of STC increased by 1.5-fold per standard deviation change of the PRS after adjusting for other clinical risk factors. The effect sizes for the PRS differed little between survivors with and without radiation exposure to neck. Moreover, we found the prediction model integrating the PRS with clinical risk factors had small but statistically significant improvement of accuracy in predicting STC risk over the model considering only clinical risk factors across the entire age range in SJLIFE. The improved performance was further validated using CCSS study.
Clinically, if we follow the surveillance guideline for carriers of pathogenic/likely pathogenic germline variants in PTEN gene(27–29), using a 35% lifetime risk as the threshold for recommendation of ultrasonography surveillance (USS) for STC may be reasonable. To illustrate the value of considering PRS, take male survivors at age of 50 years (Supplementary Table S6D) as an example: for those diagnosed between 0 and 4 years and treated with Neck RT <20 Gy plus epipodophyllotoxin, considering PRS would exclude approximately one-third of survivors who would have met criteria for USS without the additional consideration of the PRS; for those diagnosed between 0 and 4 years and treated with Neck RT between 20 and 30 Gy plus epipodophyllotoxin, considering PRS would exclude approximately two-thirds from USS; for those diagnosed between 10 and 14 years and treated with Neck RT <30 Gy plus epipodophyllotoxin, considering PRS would exclude approximately one-third from USS; for those diagnosed ≥ 15 years old and treated with Neck RT < 20 Gy plus epipodophyllotoxin, considering PRS would include an additional one-third for USS.
We previously showed that the PRS based on established breast cancer risk loci identified from the general population (i.e., by comparing de novo breast cancer cases vs. non-cancer controls) was associated with risk of subsequent breast cancer among survivors of childhood cancer.(14) Our current study further generalizes this paradigm by demonstrating that the PRS based on the 12 SNPs previously discovered by thyroid cancer GWAS studies could inform STC risk in survivors of childhood cancer. Notably, the effect of each of the 12 SNPs was attenuated towards null when analyzing the STC among survivors of childhood cancer, possibly due to the strong effects of prior cancer treatment and different host genetics (Supplementary Table S8). A methodological study evaluating the generalizability of GWAS findings among childhood cancer survivors suggested that cancer treatments, including chemotherapy and RT, produce persistent changes on the methylome affecting methylation levels of CpG sites near disease/trait-associated genes and alter the expression of underlying genes or expressivity of risk alleles(30). However, despite the attenuated effect size observed in each SNP for STC risk in survivors of childhood cancer, joint contributions of all 12 SNPs in the PRS were still useful in risk stratification and prediction.
In general population case-control samples from UK Biobank, Liyanarachchi et al. reported that the top decile (91–100%) of the PRS, built by 10 SNPs identified from previous GWAS, conferred a 6.9-fold higher risk of thyroid cancer compared to the bottom decile (0–10%), and adding the 10-SNP PRS to the model significantly improved predictive ability with AUC of 0.69–0.75(31). There are few studies to evaluate the polygenic contributions for subsequent malignancies among survivors of childhood cancer(14,32) and one study for clinical prediction model, specifically for STC.(33) Our risk prediction model integrating the PRS, generated by the 12-GWAS SNPs, with the treatment exposure groups, improved the performance with AUC of 0.72–0.83, which was slightly better than the previously reported clinical model with AUC of 0.71–0.80.(33)
This study has several limitations. First, even though we included all survivors five or more years from the completion of primary diagnoses, they are still relatively young, with a median attained age of 36.6 years in SJLIFE and 36.5 years in CCSS. The young age of our cohorts is especially pertinent considering that thyroid cancer incidence increases with age in the general population and is most prevalent in the 65-to-74 age group.(34) Hence, longer follow-up is warranted to comprehensively evaluate the effect of the PRS on STC. Second, we did not have neck-RT dose for everyone, and we did not consider low dose scattering from nearby radiation fields. Third, since the study population was restricted to survivors of European ancestry, further validation in other race/ethnic groups is needed. We compared the difference of STC incidence between CEU survivors (i.e., survivors of European ancestry) (N=2,370 SJLIFE and 6,416 CCSS) and non-CEU survivors (i.e., survivors of other or mixed ancestries) excluded from the analysis (N=546 SJLIFE and 707). In SJLIFE, 65 of 2,370 CEU survivors (2.7%) and 4 of 546 non-CEU survivors (0.7%) developed STC and CEU survivors had 3.2-fold increased risk for STC than non-CEU survivors (RR=3.2, 95% CI=1.2–8.9, p=0.02) in multivariable analysis adjusted for age at diagnosis, attained age, and sex, and 2.7-fold increased risk when additionally adjusted for treatment group (RR=2.7, 95% CI=1.0–7.4, p=0.06). In CCSS, 121 of 6,416 CEU survivors (1.9%) and 8 of 707 non-CEU survivors (1.1%) developed STC but this was not statistically significant (p=0.34). Because non-CEU survivors represent a mixed population and a relatively small proportion of STC incidence, we had insufficient statistical power, to include non-CEU population in this analysis. Lastly, heterogeneity exists between SJLIFE, the discovery study, and CCSS, the replication study. It is notable that the incidence of STC is lower in CCSS than SJLIFE (especially among survivors with prior neck-RT exposure), potentially due to under reporting in CCSS, which relies on self-report and subsequent confirmation by pathology report. Nevertheless, our study is the largest (Ntotal =8,786) and the first to study the polygenic contributions to STC risk among childhood cancer survivors.
In summary, this study demonstrates that the PRS, generated from thyroid cancer risk loci identified in the general population, can further enhance STC risk stratification among childhood cancer survivors, beyond cancer-treatment factors. As more clinical indicators and new thyroid cancer-related loci are identified, we anticipate that findings from research like ours will enable more precise identification of survivors at highest and lowest risk for STC, and thus, begin to inform development of personalized surveillance strategies.
Supplementary Material
Acknowledgments:
This research was supported by funding from the American Lebanese Syrian Associated Charities to St. Jude Children’s Research Hospital and by grants (CA021765, CA195547, CA55727 and CA216354) from the National Institutes of Health to St. Jude Children’s Research Hospital. Genotyping for the CCSS original cohort was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
Abbreviation list
- AUCs
Area under the ROC curves
- CCSS
Childhood Cancer Survivor Study
- C
Concordance
- CIs
Confidence intervals
- GWAS
Genome-wide association studies
- IQR
Interquartile range
- LD
Linkage disequilibrium
- ORs
Odds ratios
- PRS
Polygenic risk score
- RT
Radiotherapy/radiation therapy
- ROC
Receiver operating characteristic
- RR
Relative rate
- SNP
Single-nucleotide polymorphism
- SD
Standard deviation
- SJCRH
St. Jude Children’s Research Hospital
- SJLIFE
St. Jude Lifetime Cohort
- STC
Subsequent thyroid cancer
- USS
Ultrasonography surveillance
- WGS
Whole-genome sequencing
Footnotes
Publisher's Disclaimer: Disclaimers: The funders of the study had no role in the design and conduct of the study; were not involved in collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of interest statement: We declare no competing interests.
Data sharing statement: The St. Jude Lifetime Cohort Study data are accessible through the St. Jude Cloud (https://stjude.cloud) through the link of cancer survivorship. The Childhood Cancer Survivor Study data are accessible throught the dbGaP (https://www.ncbi.nlm.nih.gov/gap/) with the accession number: phs001327.v2.p1.
Data and materials availability:
Z.W. and Y.Y. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Turcotte LM, Whitton JA, Friedman DL, Hammond S, Armstrong GT, Leisenring W, et al. Risk of Subsequent Neoplasms During the Fifth and Sixth Decades of Life in the Childhood Cancer Survivor Study Cohort. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33(31):3568–75 doi 10.1200/jco.2015.60.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reulen RC, Frobisher C, Winter DL, Kelly J, Lancashire ER, Stiller CA, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. Jama 2011;305(22):2311–9 doi 10.1001/jama.2011.747. [DOI] [PubMed] [Google Scholar]
- 3.Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet 2016;388(10061):2783–95 doi 10.1016/S0140-6736(16)30172-6. [DOI] [PubMed] [Google Scholar]
- 4.Bhatti P, Veiga LH, Ronckers CM, Sigurdson AJ, Stovall M, Smith SA, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiation research 2010;174(6):741–52 doi 10.1667/rr2240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veiga LH, Holmberg E, Anderson H, Pottern L, Sadetzki S, Adams MJ, et al. Thyroid Cancer after Childhood Exposure to External Radiation: An Updated Pooled Analysis of 12 Studies . Radiation research 2016;185(5):473–84 doi 10.1667/rr14213.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Version 4.0. Monrovia, CA: Children’s Oncology Group; October 2013; Available on-link: www.survivorshipguidelines.org. [Google Scholar]
- 7.Clement SC, Kremer LCM, Verburg FA, Simmons JH, Goldfarb M, Peeters RP, et al. Balancing the benefits and harms of thyroid cancer surveillance in survivors of Childhood, adolescent and young adult cancer: Recommendations from the international Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Cancer treatment reviews 2018;63:28–39 doi 10.1016/j.ctrv.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Sigurdsson A, Bergthorsson JT, et al. Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nature genetics 2009;41(4):460–4 doi 10.1038/ng.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi M, Saenko VA, Rogounovitch TI, Kawaguchi T, Drozd VM, Takigawa-Imamura H, et al. The FOXE1 locus is a major genetic determinant for radiation-related thyroid carcinoma in Chernobyl. Human molecular genetics 2010;19(12):2516–23 doi 10.1093/hmg/ddq123. [DOI] [PubMed] [Google Scholar]
- 10.Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Masson G, He H, et al. Discovery of common variants associated with low TSH levels and thyroid cancer risk. Nature genetics 2012;44(3):319–22 doi 10.1038/ng.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohler A, Chen B, Gemignani F, Elisei R, Romei C, Figlioli G, et al. Genome-wide association study on differentiated thyroid cancer. The Journal of clinical endocrinology and metabolism 2013;98(10):E1674–81 doi 10.1210/jc.2013-1941. [DOI] [PubMed] [Google Scholar]
- 12.Mancikova V, Cruz R, Inglada-Perez L, Fernandez-Rozadilla C, Landa I, Cameselle-Teijeiro J, et al. Thyroid cancer GWAS identifies 10q26.12 and 6q14.1 as novel susceptibility loci and reveals genetic heterogeneity among populations. International journal of cancer 2015;137(8):1870–8 doi 10.1002/ijc.29557. [DOI] [PubMed] [Google Scholar]
- 13.Gudmundsson J, Thorleifsson G, Sigurdsson JK, Stefansdottir L, Jonasson JG, Gudjonsson SA, et al. A genome-wide association study yields five novel thyroid cancer risk loci. Nature communications 2017;8:14517 doi 10.1038/ncomms14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Liu Q, Wilson CL, Easton J, Mulder H, Chang TC, et al. Polygenic Determinants for Subsequent Breast Cancer Risk in Survivors of Childhood Cancer: The St Jude Lifetime Cohort Study (SJLIFE). Clinical cancer research : an official journal of the American Association for Cancer Research 2018;24(24):6230–5 doi 10.1158/1078-0432.Ccr-18-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michailidou K, Lindström S, Dennis J, Beesley J, Hui S, Kar S, et al. Association analysis identifies 65 new breast cancer risk loci. Nature 2017;551(7678):92–4 doi 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law PJ, Timofeeva M, Fernandez-Rozadilla C, Broderick P, Studd J, Fernandez-Tajes J, et al. Association analyses identify 31 new risk loci for colorectal cancer susceptibility. Nature communications 2019;10(1):2154 doi 10.1038/s41467-019-09775-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schumacher FR, Al Olama AA, Berndt SI, Benlloch S, Ahmed M, Saunders EJ, et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nature genetics 2018;50(7):928–36 doi 10.1038/s41588-018-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howell CR, Bjornard KL, Ness KK, Alberts N, Armstrong GT, Bhakta N, et al. Cohort profile: the St. Jude Lifetime Cohort Study (SJLIFE) for pediatric cancer survivors. Int J Epidemiol (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2009;27(14):2308–18 doi 10.1200/jco.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Wilson CL, Armstrong GT, Hudson MM, Zhang J, Nichols KE, et al. Association of Germline BRCA2 Mutations With the Risk of Pediatric or Adolescent Non-Hodgkin Lymphoma. JAMA Oncol 2019. doi 10.1001/jamaoncol.2019.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Research 2018;47(D1):D1005–D12 doi 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of statistics 1988:1141–54. [Google Scholar]
- 23.Austin PC, Fine JP. Practical recommendations for reporting F ine-G ray model analyses for competing risk data. Statistics in medicine 2017;36(27):4391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics 2005;61(1):92–105 doi 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15(4):361–87 doi . [DOI] [PubMed] [Google Scholar]
- 26.R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available on-line: https://www.R-project.org/. [Google Scholar]
- 27.Nagy R, Ganapathi S, Comeras I, Peterson C, Orloff M, Porter K, et al. Frequency of germline PTEN mutations in differentiated thyroid cancer. Thyroid 2011;21(5):505–10 doi 10.1089/thy.2010.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pilarski R PTEN Hamartoma Tumor Syndrome: A Clinical Overview. Cancers (Basel) 2019;11(6) doi 10.3390/cancers11060844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tischkowitz M, Colas C, Pouwels S, Hoogerbrugge N. Cancer Surveillance Guideline for individuals with PTEN hamartoma tumour syndrome. Eur J Hum Genet 2020;28(10):1387–93 doi 10.1038/s41431-020-0651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Im C, Qin N, Wang Z, Qiu W, Howell CR, Sapkota Y, et al. Generalizability of “GWAS hits” in clinical populations: Lessons from childhood cancer survivors. bioRxiv 2020:2020.02.02.930818 doi 10.1101/2020.02.02.930818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liyanarachchi S, Gudmundsson J, Ferkingstad E, He H, Jonasson JG, Tragante V, et al. Assessing thyroid cancer risk using polygenic risk scores. Proceedings of the National Academy of Sciences of the United States of America 2020;117(11):5997–6002 doi 10.1073/pnas.1919976117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Sun CL, Hageman L, Smith K, Singh P, Desai S, et al. Clinical and Genetic Risk Prediction of Subsequent CNS Tumors in Survivors of Childhood Cancer: A Report From the COG ALTE03N1 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35(32):3688–96 doi 10.1200/jco.2017.74.7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovalchik SA, Ronckers CM, Veiga LH, Sigurdson AJ, Inskip PD, de Vathaire F, et al. Absolute risk prediction of second primary thyroid cancer among 5-year survivors of childhood cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2013;31(1):119–27 doi 10.1200/jco.2012.41.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weeks KS, Kahl AR, Lynch CF, Charlton ME. Racial/ethnic differences in thyroid cancer incidence in the United States, 2007–2014. Cancer 2018;124(7):1483–91 doi 10.1002/cncr.31229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Z.W. and Y.Y. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.


