Abstract
Background:
Anthropometric measures, including obesity, are important risk factors for breast and endometrial cancers in postmenopausal women. It is unknown whether these risk factors are associated with androgen metabolism, another risk factor for these cancers.
Methods:
Using baseline data from 1,765 postmenopausal women in the Women’s Health Initiative Observational Study, we conducted a cross-sectional analysis examining associations between anthropometric measures (current body mass index [BMI], waist-to-hip ratio [WHR], height, and recalled BMI at age 18) and serum androgen metabolites. Twelve androgens/androgen metabolites were quantified using liquid chromatography-tandem mass spectrometry. Geometric means of androgen/androgen metabolite concentrations were estimated using linear regression, adjusting for potential confounders and stratified by hormone therapy (HT) use.
Results:
Regardless of HT use, higher current BMI (≥30 vs. <25 kg/m2) was associated with higher serum concentrations of DHEAS, 5α-reduced glucuronide metabolites (ADT-G, 3α-diol-3G, 3α-diol-17G), and DHEAS:DHEA ratio (all p-trend≤0.02). BMI was also positively associated with unconjugated estrone:androstenedione and unconjugated estradiol:testosterone ratios among never/former HT users (all p-trend<0.001) but not in current users (p-int<0.001). WHR was positively associated with adrenal androgens and 5α-reduced glucuronide metabolites in obese women only (BMI≥30 kg/m2; all p-trend≤0.01). BMI at age 18 was inversely associated with adrenal androgens (DHEA, DHEAS, androstenedione, testosterone) and 5α-reduced glucuronide metabolites in never/former HT users (all p-trend<0.06). Height was not associated with androgen metabolites.
Conclusions:
Current BMI is associated with androgen metabolism among postmenopausal women.
Impact:
This study contributes to our understanding of the link between obesity and cancer risk in postmenopausal women.
Keywords: BMI, WHR, height, adiposity, androgen, estrogen, AM, sex hormones, postmenopausal
INTRODUCTION
Obesity is an important risk factor for female cancers including breast (1), ovarian (2), and endometrial cancers (3) in postmenopausal women. One of potential mechanisms that may explain the obesity-cancer relationships in women is sex steroid hormone synthesis and metabolism. Adipose tissues can produce estrogens by converting androgens via aromatase activity (4). Consistently, studies have reported elevated circulating levels of estrogens in postmenopausal obese (vs. normal weight) women (5-10). In our previous analysis of estrogen metabolites in the Women’s Health Initiative Observational Study (WHI-OS), we further observed associations between current body mass index (BMI) and metabolism of estrogens (methylation of catechol estrogen metabolites) (10). Some studies also suggest that obesity-induced hyperinsulinemia may stimulate androgen production (11-13). However, little is known about the associations of obesity with androgen metabolism beyond aromatization to estrogens. It is also unclear whether other anthropometric measures including waist-to-hip ratio (WHR), BMI at age 18 years, and height are associated with androgen metabolism, independent of current BMI, among postmenopausal women. High WHR indicates abdominal obesity and is associated with elevated risk of endometrial and postmenopausal breast cancers (3,14,15). BMI at age 18 and height indicate early nutritional status and adolescent exposure to proliferative hormones. Understanding associations of anthropometric measures with serum androgen metabolism will improve our understanding of the potential mechanisms through which these risk factors influence cancer risk in postmenopausal women.
Androgens, as well as estrogens, are proliferative hormones that play important roles in breast (16-18), ovarian (19,20), and endometrial (21-29) carcinogenesis. Because androgens can be converted to estrogens, circulating levels of androgens may also reflect a reservoir of precursor substrates for estrogens. Some studies have shown that circulating adrenal androgens (androstenedione, testosterone, dehydroepiandrosterone [DHEA], dehydroepiandrosterone sulfate [DHEAS]) were associated with increased breast (16,30,31), ovarian (21-27) and endometrial cancer risks (19). Testosterone and androstenedione can also be metabolized to 5α-reduced metabolites and 5β-reduced metabolites. Although adrenal androgens were more commonly evaluated in previous studies, circulating levels of total 5α-reduced glucuronide metabolites (androsterone-glucuronide [ADT-G], 5α-androstane-3α,17β diol-3-glucuronide [3α-diol-3G], 3α-diol-17-glucuronide [3α-diol-17G]) are believed to better reflect tissue-level androgenic activity (32,33) as these metabolites cannot be converted back to adrenal androgens or aromatized to estrogens. Further, when metabolites are sulfated (e.g., dihydrotestosterone sulfate [DHTS]), the metabolites become less bioactive and may act as a reservoir for more potent androgenic forms (e.g., DHT). In the WHI-OS, we previously measured 12 individual androgens/androgen metabolites in serum and found associations between circulating 5α-reduced glucuronide metabolites (ADT-G, 3α-diol-3G, 3α-diol-17G) and an increased non-serous ovarian cancer risk (28). Similar positive associations were also observed with endometrial cancer risk but were not statistically significant (19). To elucidate the effects of anthropometric risk factors on androgen metabolism patterns, we conducted a cross-sectional analysis examining the associations of several anthropometric measures (current BMI, current WHR, BMI at age 18, and height) with 12 serum androgens/androgen metabolites among postmenopausal women in the WHI-OS at baseline. Because the concentrations of androgens/androgen metabolites and the associations with anthropometric measures may vary by hormone therapy (HT) use, we examined associations separately by HT use.
METHODS
Study population
This analysis included participants from a nested case-control study of ovarian and endometrial cancers in the WHI-OS (34,35). The WHI-OS is an ongoing cohort study of 93,676 postmenopausal women who were recruited at ages 50-79 years from 40 clinical centers in the USA from 1993 to 1998 (36,37). At study enrollment, trained medical staff conducted anthropometric measurements (height, weight, waist and hip circumferences) and collected blood samples. Baseline self-administered questionnaires were used to collect information on sociodemographic characteristics, medical history, reproductive factors, and lifestyle factors.
Details of the nested case-control study are described elsewhere (34,35). In brief, cases were women with ovarian or endometrial cancer diagnosed between baseline and 2012. At the date of diagnosis (index date) for each case, controls were selected among cancer-free women matched to the case based on age at baseline (5-year categories), year of blood draw (1993-1996, 1997-1998), self-identified race/ethnicity (White, Black, Hispanic, other/unknown), hysterectomy at baseline or during follow-up prior to the index date (for ovarian controls only), and HT use (never, ≤1 year since last HT use, >1 year since last HT use, current). Both cases and controls had no history of cancer (except non-melanoma skin cancer) at baseline, bilateral oophorectomy, or hysterectomy (for endometrial controls only), and had ≥1.1 mL serum sample available.
Of the 1,824 participating women, we excluded women who had missing information on any anthropometric measures (n=49) and had missing values for at least one androgens/androgen metabolite (n=10). After exclusion, 1,765 women (489 cases and 432 controls among never/former HT users, 442 cases and 402 controls among current HT users) were included in the analysis. Because all serum samples were collected at baseline prior to any cancer diagnosis, we included both cases and controls in this cross-sectional analysis. We also accounted for case-control selection criteria using inverse probability sampling weights.
Anthropometric assessment
Height, weight, waist circumference, and hip circumference were measured at baseline. Height was measured on a stadiometer to the nearest 0.1 cm, and weight was measured on a balance-beam scale to the nearest 0.1 kg. Waist circumference was measured at the narrowest part of the torso and hip circumference was measured at the site of maximum extension of the buttocks over non-binding undergarments to the nearest 0.5 cm using a measuring tape. All anthropometric measurements were conducted by clinic staff following standardized protocols. We calculated BMI by dividing weight (kg) by height squared (m2). BMI at age 18 was calculated using recalled weight and height. WHR was calculated as baseline waist circumference (cm) divided by hip circumference (cm). Based on the World Health Organization obesity classification, current BMI was categorized into three groups: <25.0 (normal), 25.0-29.9 (overweight), ≥30.0 kg/m2 (obesity). Height was categorized into 5-cm intervals: <160, 160-164, ≥165 cm. WHR and BMI at age 18 were categorized into tertiles.
Laboratory assays
All serum samples were collected at baseline. Details of the assay methods are described elsewhere (28). Briefly, serum concentrations of 12 individual androgens/androgen metabolites (four adrenal androgens: DHEA, DHEAS, androstenedione, testosterone; seven 5α-reduced metabolites: 5α-androstanedione, DHT, DHTS, ADT, ADT-G, 3α-diol-3G, 3α-diol-17G; one 5β-reduced metabolite: etiocholanolone-glucuronide [Etio-G]) were quantified by stable isotope dilution high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) (Thermo Fisher, San Jose, CA; Shimadzu Scientific Instruments, Columbia, MD) (38). Only Etio-G was included from 5β-reduced metabolites because concentrations of other 5β-reduced metabolites were very low in serum, did not have internal standards, or bind very weakly to the androgen receptor. Because studies suggested that combined circulating levels of ADT-G and androstanediol-glucuronide metabolites (3α-diol-3G, 3α-diol-17G) may indicate androgenic activity in tissue (32,33), we summed the concentrations of ADT-G, 3α-diol-3G, and 3α-diol-17G as a marker of tissue-level androgenic activity. Combined and unconjugated concentrations of estradiol and estrone were previously quantified using an independent LC-MS/MS assay (20,39). We refer to estrone and estradiol as “parent estrogens” because they serve as precursors to downstream estrogen metabolites. Unconjugated estrone and estradiol are the most potent forms of parent estrogens. Using the measures on androgens/androgen metabolites and estrogens, we calculated five different ratios: DHEAS:DHEA, DHTS:DHT, DHT:testosterone, unconjugated estrone:androstenedione, and unconjugated estradiol:testosterone ratios. We included two estrogen-to-androgen ratios (unconjugated estrone:androstenedione, unconjugated estradiol:testosterone) to estimate the extent of aromatase activity (conversion of androstenedione and testosterone into estrone and estradiol, respectively). Laboratory coefficients of variation (CV) of the assay were <11% and intraclass correlation coefficients (ICC) ranged from 0.77 to 0.997 (28).
Statistical analyses
Because the concentrations of androgens and estrogens differ between never/former vs. current HT users (Supplementary Table 1), we stratified all analyses by HT use (n=921 never/former vs. n=844 current users) and examined the variation in associations by HT use. For all analyses, study participants were weighted by inverse probability sampling weights to represent the entire cohort as described by Li and Gail (40). Sampling weights accounted for the case-control sampling fractions. Sampling weight were 1 for all cases, and for controls depended on their strata defined by matching factors. After log transformation of data to improve normality, we fit inverse probability weighted multivariable linear regression to estimate geometric means (GMs) and 95% confidence intervals (CIs) of individual androgens/androgen metabolite concentrations (pmol/L) according to exposure categories (current BMI, WHR, BMI at age 18, height), adjusting for potential confounders. In multivariable models, we included age at blood draw, calendar year at blood draw, race/ethnicity, smoking status, alcohol drinking, parity, family history of breast and ovarian cancers, time since menopause, moderate- to vigorous-intensity physical activity, and HT use. Additional adjustment for history of oral contraceptive use did not change results and thus was not included in the final models. For WHR, BMI at age 18, and height, we additionally adjusted for current BMI to examine the associations independent of current BMI. For current BMI, we compared the models with and without additional adjustment for WHR. We tested for trend using Wald tests for continuous exposure variables. The percent change in GMs from the lowest to highest exposure categories was estimated by taking the ratio of GM difference between the two categories over the GM of the lowest category, multiplied by 100. We tested for the difference using Wald tests. To examine whether the associations of WHR vary by BMI, we also stratified the analyses by current BMI (≥30 kg/m2 [obese] vs. <30 kg/m2 [nonobese]). We tested for an interaction between current BMI and WHR using Wald test for product terms. Because adrenal androgens (DHEA, DHEAS, androstenedione, testosterone) can serve as precursors for parent estrogens (estrone and estradiol) during aromatization, we compared the mean proportions of adrenal androgens out of summed concentrations of adrenal androgens and parent estrogens across BMI categories, with adjustment for the summed concentration of adrenal androgens and parent estrogens. This approach estimates the association with replacement of androgens for estrogens, while holding the summed concentration constant. We tested for any difference across BMI categories using a global F test. In sensitivity analysis, we conducted analyses separately in cases and controls to investigate the variation in associations by the case-control status.
All statistical tests were two-sided with a 5% type I error rate. Q values reflecting the false discovery rate (FDR) were calculated to account for multiple comparisons (18 tests per exposure). Analyses were conducted using SURVEY procedures in SAS version 9.4 software (SAS Institute, Cary, NC, USA).
RESULTS
Study population characteristics
The mean age at baseline was 64.5 years in never/former HT users and 61.3 years in current HT users. Ninety percent of never/former HT users and 94% of current HT users were White. Compared with never/former HT users, current HT users had lower concentrations of adrenal androgens (DHEA, DHEAS, androstenedione, testosterone) and 5α-reduced glucuronide metabolites (ADT-G, 3α-diol-3G, 3α-diol-17G), and higher levels of DHEAS:DHEA, DHT:testosterone, unconjugated estrone:androstenedione, and unconjugated estradiol:testosterone ratios (Supplementary Table 1). Current HT users (vs. never/former users) also had lower mean BMI (26.5 v s. 27.0 kg/m2) and WHR (0.79 vs. 0.81) at baseline. Among never/former HT users, women with higher current BMI (≥30.0 vs. <25.0 kg/m2) were less likely to be parous, current smokers, and physically active (Table 1). Among current HT users, women with higher current BMI were more likely to be parous, non-drinkers, and have family history of breast or ovarian cancer, and less likely to be physically active.
Table 1.
Characteristics of study population according to current BMI, stratified by current use of hormone therapy: the Women’s Health Initiative Observational Study
| Characteristics | Never/former hormone therapy users (N=921, weighted N=29,839) |
Current hormone therapy users (N=844, weighted N=24,010) |
|||||
|---|---|---|---|---|---|---|---|
| Current BMI (kg/m2) | Current BMI (kg/m2) | ||||||
| <25.0 | 25.0-29.9 | ≥30.0 | <25.0 | 25.0-29.9 | ≥30.0 | ||
| N (weighted % a) | N (weighted % a) | ||||||
| Hormone therapy use | Never | 209 (56.6) | 181 (56.1) | 230 (59.6) | NA | NA | NA |
| Former | 125 (43.4) | 101 (43.9) | 75 (40.4) | NA | NA | NA | |
| Current | NA | NA | NA | 426 (100.0) | 251 (100.0) | 167 (100.0) | |
| Age at baseline blood draw | <55 years | 38 (11.7) | 18 (5.0) | 22 (7.1) | 44 (15.0) | 28 (17.3) | 26 (19.5) |
| 55-59 years | 49 (13.0) | 56 (18.6) | 69 (24.8) | 96 (28.2) | 56 (21.6) | 36 (27.7) | |
| 60-64 years | 76 (21.9) | 58 (16.6) | 84 (23.3) | 100 (19.8) | 60 (24.6) | 48 (29.0) | |
| 65-69 years | 65 (19.3) | 69 (27.2) | 63 (26.2) | 98 (20.2) | 44 (18.6) | 28 (14.8) | |
| 70-74 years | 68 (22.0) | 50 (22.2) | 46 (10.8) | 67 (11.6) | 41 (13.8) | 21 (6.5) | |
| 75-79 years | 38 (12.1) | 31 (10.5) | 21 (7.8) | 21 (5.2) | 22 (4.0) | 8 (2.4) | |
| White | 307 (93.7) | 246 (86.5) | 262 (88.5) | 397 (90.4) | 242 (96.3) | 158 (96.3) | |
| Calendar year at blood draw | 1993 - 1996 | 201 (59.9) | 173 (61.4) | 191 (62.6) | 282 (65.9) | 144 (54.1) | 96 (63.0) |
| 1997 - 1998 | 133 (40.1) | 109 (38.6) | 114 (37.4) | 144 (34.1) | 107 (45.9) | 71 (37.0) | |
| Smoking status | Never | 175 (49.4) | 150 (55.6) | 150 (48.3) | 213 (48.5) | 116 (40.1) | 79 (47.9) |
| Former | 126 (39.2) | 117 (38.5) | 141 (46.1) | 195 (45.7) | 127 (55.8) | 80 (44.7) | |
| Current | 33 (11.4) | 15 (5.9) | 14 (5.6) | 18 (5.8) | 8 (4.0) | 8 (7.4) | |
| Alcohol drinking | Non-drinker | 81 (23.9) | 78 (33.8) | 103 (29.9) | 79 (18.8) | 55 (23.2) | 46 (30.5) |
| <1 drink/week | 93 (32.2) | 95 (24.9) | 105 (34.3) | 122 (29.5) | 84 (31.9) | 72 (43.5) | |
| 1-6 drink/week | 98 (24.1) | 67 (24.1) | 60 (24.4) | 138 (31.0) | 75 (33.0) | 37 (17.7) | |
| ≥7 drink/week | 62 (19.8) | 42 (17.3) | 37 (11.4) | 87 (20.7) | 37 (11.9) | 12 (8.2) | |
| Parous | 280 (82.3) | 249 (91.7) | 244 (78.8) | 368 (83.4) | 225 (91.8) | 143 (90.7) | |
| Family history of breast or ovarian cancer | 73 (19.2) | 56 (21.8) | 55 (20.7) | 82 (17.6) | 47 (16.6) | 35 (24.2) | |
| Time since menopause | <10 years | 104 (31.5) | 92 (29.6) | 95 (29.1) | 164 (44.7) | 96 (42.5) | 78 (46.1) |
| 10-20 years | 124 (33.9) | 102 (40.2) | 120 (38.5) | 168 (33.6) | 91 (35.1) | 57 (34.8) | |
| 20+ years | 92 (31.0) | 80 (26.0) | 68 (26.2) | 94 (21.7) | 64 (22.5) | 32 (19.1) | |
| Missing | 14 (3.6) | 8 (4.2) | 22 (6.2) | 0 (0) | 0 (0) | 0 (0) | |
| Moderate-to-vigorous intensity physical activity | 0 MET-hr/wk | 35 (11.8) | 54 (16.7) | 102 (36.1) | 43 (14.9) | 46 (16.4) | 50 (29.3) |
| 0.1 – 9.9 MET-hr/wk | 101 (29.2) | 91 (28.9) | 98 (33.5) | 122 (26.3) | 86 (41.1) | 63 (42.5) | |
| ≥10.0 MET-hr/wk | 198 (59.1) | 137 (54.4) | 105 (30.4) | 261 (58.8) | 119 (42.5) | 54 (28.3) | |
Percentages reflect weighted counts and refer to the study cohort.
Abbreviations: BMI=body mass index; MET=metabolic equivalent of task; NA=not applicable
Current body mass index
Among both never/former and current HT users, higher current BMI (≥30.0 vs. <25.0 kg/m2) was associated with higher concentrations of DHEAS, 5α-reduced glucuronide metabolites (ADT-G, 3α-diol-3G, 3α-diol-17G), and DHEAS:DHEA ratio (all p-trend≤0.02; Table 2). Higher current BMI was also positively associated with the estrogen-to-androgen ratios among never/former HT users (GM=50.0 vs. 36.9 unconjugated estrone:androstenedione; 38.5 vs. 17.0 unconjugated estradiol:testosterone; all p-trend<0.001) but not among current HT users (GM=172 vs. 201, p-trend=0.13; GM=97.7 vs. 91.9, p-trend=0.57; respectively). For the estrogen-to-androgen ratios, the interactions by HT use were statistically significant (all p-int<0.001). The associations did not change after additional adjustment for current WHR (Supplementary Table 2).
Table 2.
Geometric means (pmol/L) and 95% confidence intervals (CI) of serum androgens/androgen metabolites by current BMI in postmenopausal women, stratified by hormone therapy use: the Women’s Health Initiative Observational Study
| Never/former hormone therapy users | Current hormone therapy users | p-intd | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current BMI | p -trenda |
%Δb | p-diffc | Current BMI | p- trenda |
%Δb | p-diffc | ||||||
| <25.0 kg/m2 | 25.0-29.9 kg/m2 | ≥30.0 kg/m2 | <25.0 kg/m2 | 25.0-29.9 kg/m2 | ≥30.0 kg/m2 | ||||||||
| Median (kg/m2) | 22.5 | 27.1 | 33.7 | 22.4 | 26.9 | 33.0 | |||||||
| N | 334 | 282 | 305 | 426 | 251 | 167 | |||||||
| Weighted N e | 13209 | 8962 | 7669 | 10976 | 7553 | 5481 | |||||||
| Geometric means (95% CI) | Geometric means (95% CI) | ||||||||||||
| Adrenal androgens | |||||||||||||
| DHEA | 4.58 (3.98, 5.26) | 4.74 (4.16, 5.40) | 4.83 (4.20, 5.55) | 0.34 | 5.5 | 0.51 | 4.05 (3.56, 4.59) | 3.99 (3.39, 4.71) | 4.37 (3.73, 5.11) | 0.36 | 7.9 | 0.35 | 0.98 |
| DHEAS | 952 (811, 1118) | 1060 (905, 1241) | 1170 (982, 1394) | 0.02f | 22.9 | 0.03 | 911 (794, 1044) | 941 (777, 1139) | 1171 (987, 1389) | 0.01f | 28.5 | 0.01f | 0.82 |
| Androstenedione | 1.37 (1.24, 1.52) | 1.39 (1.26, 1.54) | 1.46 (1.30, 1.64) | 0.31 | 6.6 | 0.29 | 1.25 (1.14, 1.36) | 1.18 (1.05, 1.32) | 1.29 (1.16, 1.44) | 0.70 | 3.2 | 0.57 | 0.81 |
| Testosterone | 0.56 (0.50, 0.64) | 0.60 (0.53, 0.68) | 0.57 (0.49, 0.65) | 0.31 | 1.8 | 0.93 | 0.49 (0.44, 0.55) | 0.50 (0.44, 0.57) | 0.54 (0.48, 0.61) | 0.24 | 10.2 | 0.21 | 0.66 |
| 5α-reduced metabolites | |||||||||||||
| 5α-androstanedione | 1.31 (1.19, 1.45) | 1.26 (1.13, 1.40) | 1.31 (1.16, 1.48) | 0.65 | 0 | 0.98 | 1.29 (1.18, 1.42) | 1.27 (1.14, 1.42) | 1.56 (1.37, 1.77) | 0.03 | 20.9 | 0.01f | 0.29 |
| DHT | 0.19 (0.17, 0.21) | 0.18 (0.16, 0.20) | 0.19 (0.17, 0.21) | 0.49 | 0 | 0.83 | 0.21 (0.19, 0.23) | 0.21 (0.18, 0.24) | 0.21 (0.18, 0.23) | 0.67 | 0 | 0.81 | 0.18 |
| DHTS | 0.92 (0.82, 1.04) | 0.98 (0.86, 1.11) | 1.08 (0.93, 1.26) | 0.05 | 17.4 | 0.04 | 0.99 (0.85, 1.15) | 0.95 (0.82, 1.11) | 1.00 (0.85, 1.18) | 0.52 | 1.0 | 0.88 | 0.50 |
| ADT | 0.52 (0.49, 0.56) | 0.54 (0.49, 0.58) | 0.55 (0.50, 0.60) | 0.26 | 5.8 | 0.30 | 0.49 (0.46, 0.53) | 0.52 (0.48, 0.56) | 0.50 (0.46, 0.54) | 0.69 | 2.0 | 0.80 | 0.50 |
| ADT-G | 16.6 (14.3, 19.2) | 20.4 (17.5, 23.8) | 24.4 (20.3, 29.3) | <0.001g | 47.0 | <0.001g | 13.9 (11.8, 16.5) | 16.2 (13.1, 20.0) | 20.4 (16.9, 24.7) | 0.001g | 46.8 | <0.001g | 0.94 |
| 3α-diol-3G | 1.20 (1.02, 1.41) | 1.37 (1.16, 1.61) | 1.74 (1.42, 2.14) | <0.001g | 45.0 | <0.001g | 1.08 (0.94, 1.24) | 1.32 (1.11, 1.56) | 1.69 (1.42, 2.00) | <0.001g | 56.5 | <0.001g | 0.41 |
| 3α-diol-17G | 0.95 (0.83, 1.08) | 1.23 (1.08, 1.40) | 1.46 (1.25, 1.71) | <0.001g | 53.7 | <0.001g | 0.72 (0.63, 0.83) | 0.85 (0.71, 1.02) | 1.02 (0.86, 1.22) | <0.001g | 41.7 | <0.001g | 0.70 |
| Marker of tissue-level androgenic activity | |||||||||||||
| Sum of ADT-G, 3α-diol-3G, 3α-diol-17G | 19.2 (16.7, 22.1) | 23.5 (20.3, 27.2) | 28.1 (23.5, 33.5) | <0.001g | 46.4 | <0.001g | 16.1 (13.8, 18.9) | 18.8 (15.3, 22.9) | 23.4 (19.5, 28.1) | 0.001g | 45.3 | <0.001g | 0.97 |
| 5β-reduced metabolites | |||||||||||||
| Etio-G | 32.1 (27.1, 38.1) | 33.5 (28.4, 39.6) | 36.0 (29.5, 44.0) | 0.27 | 12.2 | 0.28 | 31.8 (27.7, 36.7) | 34.8 (29.4, 41.2) | 38.7 (32.5, 46.1) | 0.10 | 21.7 | 0.07 | 0.59 |
| Ratios | |||||||||||||
| DHEAS: DHEA | 208 (188, 230) | 224 (200, 250) | 242 (216, 272) | 0.02f | 16.3 | 0.02 | 225 (203, 249) | 236 (207, 268) | 268 (241, 299) | 0.01f | 19.1 | 0.004f | 0.70 |
| DHTS: DHT | 4.86 (4.26, 5.55) | 5.45 (4.65, 6.39) | 5.78 (4.88, 6.85) | 0.03 | 18.9 | 0.06 | 4.71 (4.02, 5.52) | 4.59 (3.91, 5.38) | 4.85 (4.02, 5.86) | 0.71 | 3.0 | 0.77 | 0.15 |
| DHT: Testosterone | 0.34 (0.30, 0.38) | 0.30 (0.26, 0.34) | 0.33 (0.28, 0.39) | 0.15 | −2.9 | 0.80 | 0.43 (0.38, 0.48) | 0.41 (0.36, 0.48) | 0.38 (0.33, 0.45) | 0.42 | −11.6 | 0.20 | 0.51 |
| Unconjugated estrone: Androstenedione | 36.9 (32.3, 42.1) | 44.1 (38.6, 50.5) | 50.0 (42.4, 58.9) | <0.001g | 35.5 | <0.001g | 201 (171, 237) | 185 (147, 233) | 172 (137, 216) | 0.13 | −14.4 | 0.24 | <0.001g |
| Unconjugated estradiol: Testosterone | 17.0 (13.7, 20.9) | 23.0 (19.0, 27.7) | 38.5 (31.3, 47.2) | <0.001g | 126.5 | <0.001g | 91.9 (74.6, 113) | 91.9 (73.1, 116) | 97.7 (77.1, 124) | 0.57 | 6.3 | 0.67 | <0.001g |
Adjusted for age at blood draw (<55, 55–59, 60–64, 65–69, 70–74, 75–79 years), calendar year at blood draw (1993–1996, 1997–1998), race (White, non-White), smoking status (never, former, current), moderate- to vigorous-intensity physical activity (0, 0.1–9.9, ≥10 metabolic equivalents of task-h/week), alcohol drinking (non-drinker, <1 drink/week, 1-6 drink/week, ≥7 drink/week), parous (yes, no), family history of breast and ovarian cancer (yes, no), and time since menopause (<10 years, 10–19 years, ≥20 years, missing). Additionally adjusted for hormone therapy use (never, former) among women never/former hormone therapy use.
p-trend was estimated using the Wald test for continuous BMI (kg/m2)
%Δ indicates the percentage change in GMs of androgens/androgen metabolite levels, comparing women with current BMI ≥30 vs. <25 kg/m2
p-diff was estimated using the Wald test and indicates a p-value for comparing androgens/androgen metabolite levels of women with current BMI ≥30 vs. <25 kg/m2
p-int was estimated using the Wald test for an interaction term between current BMI and hormone therapy use.
Weighted n reflects weighted counts and refers to the study cohort
False discovery rate (FDR) q value <0.05 and ≥0.01
False discovery rate (FDR) q value <0.01
Among never/former HT users, the proportion of adrenal androgens out of summed concentrations of adrenal androgens and parent estrogens was also lower in obese women (current BMI ≥30.0 vs. <25.0 kg/m2: 66.7% vs. 72.2%, respectively; p=0.01; Figure 1), while holding the summed concentrations constant. No association with proportion of adrenal androgens (vs. parent estrogens) was found among current HT users (current BMI ≥30.0 vs. <25.0 kg/m2: 30.3% vs. 25.5%, p=0.08; Supplementary Figure 1).
Figure 1. Proportions of adrenal androgens (DHEA, DHEAS, androstenedione, testosterone) and parent estrogens (estradiol and estrone) out of summed concentrations of adrenal androgens and parent estrogens according to current body mass index (BMI) categories among postmenopausal women not using hormone therapy.
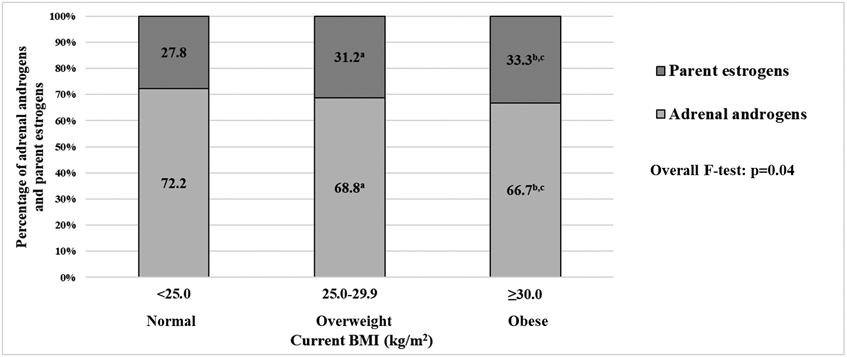
Adjusted for age at blood draw (<55, 55–59, 60–64, 65–69, 70–74, 75–79 years), calendar year at blood draw (1993–1996, 1997–1998), race (White, non-White), smoking status (never, former, current), moderate- to vigorous-intensity physical activity (0, 0.1–9.9, ≥10 metabolic equivalents of task-h/week), alcohol drinking (non-drinker, <1 drink/week, 1-6 drink/week, ≥7 drink/week), parous (yes, no), family history of breast and ovarian cancer (yes, no), time since menopause (<10 years, 10–19 years, ≥20 years, missing), and hormone therapy use (never, former).
a P-value for comparing proportions of adrenal androgens between women with current BMI 25.0-29.9 vs. <25.0 kg/m2 was 0.10.
b P-value for comparing proportions of adrenal androgens between women with current BMI ≥30.0 vs. <25.0 kg/m2 was 0.01.
c P-value for comparing proportions of adrenal androgens between women with current BMI ≥30.0 vs. 25.0-29.9 kg/m2 was 0.31.
Current waist-to-hip ratio
In models without adjustment for current BMI, current WHR (highest vs. lowest tertiles) was positively associated with serum 5α-reduced glucuronide metabolites (ADT-G, 3α-diol-3G, 3α-diol-17G) and, among never/former HT users, unconjugated estradiol:testosterone ratio (all p-trend≤0.01; Supplementary Table 3). After additional adjustment for current BMI, the associations were substantially attenuated (Table 3). The overall patterns of the associations were similar between never/former and current HT users.
Table 3.
Geometric means (pmol/L) and 95% confidence intervals (CI) of serum androgens/androgen metabolites by current waist-to-hip ratio in postmenopausal women, stratified by hormone therapy use: the Women’s Health Initiative Observational Study
| Never/former hormone therapy users | Current hormone therapy users | p-intd | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Waist-to-hip ratio | p- trenda |
%Δb | p-diffc | Waist-to-hip ratio | p- trenda |
%Δb | p-diffc | ||||||
| T1 | T2 | T3 | T1 | T2 | T3 | ||||||||
| Median (range) | 0.74 (0.49-0.77) | 0.81 (0.78-0.83) | 0.89 (0.84-1.37) | 0.73 (0.38-0.74) | 0.78 (0.75-0.80) | 0.86 (0.81-1.22) | |||||||
| N | 306 | 308 | 307 | 281 | 282 | 281 | |||||||
| Weighted N e | 10270 | 9779 | 9790 | 7813 | 7361 | 8836 | |||||||
| Geometric means (95% CI) | Geometric means (95% CI) | ||||||||||||
| Adrenal androgens | |||||||||||||
| DHEA | 4.97 (4.25, 5.81) | 4.40 (3.83, 5.05) | 4.79 (4.23, 5.42) | 0.83 | −3.6 | 0.67 | 4.20 (3.56, 4.94) | 4.01 (3.46, 4.64) | 4.13 (3.59, 4.76) | 0.76 | −1.7 | 0.86 | 0.66 |
| DHEAS | 1070 (893, 1282) | 1044 (874, 1247) | 1058 (910, 1231) | 0.66 | −1.1 | 0.90 | 881 (723, 1075) | 1005 (864, 1169) | 991 (854, 1151) | 0.45 | 12.5 | 0.27 | 0.34 |
| Androstenedione | 1.48 (1.32, 1.66) | 1.25 (1.14, 1.38) | 1.48 (1.33, 1.65) | 0.75 | 0.0 | 0.99 | 1.26 (1.11, 1.43) | 1.24 (1.13, 1.36) | 1.22 (1.10, 1.36) | 0.79 | −3.2 | 0.66 | 0.88 |
| Testosterone | 0.68 (0.58, 0.78) | 0.50 (0.44, 0.57) | 0.57 (0.50, 0.66) | 0.31 | −16.2 | 0.05 | 0.52 (0.45, 0.60) | 0.51 (0.45, 0.57) | 0.49 (0.44, 0.55) | 0.69 | −5.8 | 0.52 | 0.50 |
| 5α-reduced metabolites | |||||||||||||
| 5α-androstanedione | 1.44 (1.31, 1.59) | 1.34 (1.20, 1.49) | 1.19 (1.07, 1.33) | 0.11 | −17.4 | 0.002f | 1.37 (1.20, 1.57) | 1.35 (1.21, 1.50) | 1.32 (1.20, 1.46) | 0.77 | −3.6 | 0.68 | 0.22 |
| DHT | 0.21 (0.19, 0.23) | 0.18 (0.16, 0.19) | 0.18 (0.17, 0.19) | 0.05 | −14.3 | 0.01 | 0.21 (0.18, 0.24) | 0.21 (0.19, 0.24) | 0.20 (0.18, 0.23) | 0.35 | −4.8 | 0.62 | 0.29 |
| DHTS | 0.98 (0.85, 1.12) | 1.00 (0.87, 1.15) | 1.00 (0.88, 1.14) | 0.84 | 2.0 | 0.77 | 0.95 (0.80, 1.13) | 0.98 (0.84, 1.16) | 0.99 (0.85, 1.15) | 0.95 | 4.2 | 0.72 | 0.82 |
| ADT | 0.55 (0.51, 0.60) | 0.52 (0.48, 0.57) | 0.53 (0.49, 0.58) | 0.97 | −3.6 | 0.45 | 0.50 (0.46, 0.54) | 0.51 (0.47, 0.55) | 0.49 (0.46, 0.53) | 0.56 | −2.0 | 0.86 | 0.78 |
| ADT-G | 19.6 (16.5, 23.3) | 19.0 (16.1, 22.4) | 21.6 (18.4, 25.2) | 0.16 | 10.2 | 0.35 | 14.0 (11.4, 17.2) | 15.4 (12.9, 18.4) | 17.1 (14.2, 20.5) | 0.18 | 22.1 | 0.11 | 0.48 |
| 3α-diol-3G | 1.33 (1.10, 1.60) | 1.33 (1.13, 1.57) | 1.57 (1.29, 1.89) | 0.14 | 18.0 | 0.13 | 1.07 (0.90, 1.27) | 1.26 (1.08, 1.48) | 1.35 (1.18, 1.56) | 0.05 | 26.2 | 0.02 | 0.33 |
| 3α-diol-17G | 1.12 (0.97, 1.31) | 1.15 (1.00, 1.32) | 1.27 (1.10, 1.47) | 0.11 | 13.4 | 0.17 | 0.70 (0.59, 0.83) | 0.82 (0.70, 0.95) | 0.88 (0.76, 1.02) | 0.003f | 25.7 | 0.01 | 0.17 |
| Marker of tissue-level androgenic activity | |||||||||||||
| Sum of ADT-G, 3α-diol-3G, 3α-diol-17G | 22.5 (19.1, 26.6) | 21.8 (18.6, 25.6) | 24.9 (21.5, 29.0) | 0.15 | 10.7 | 0.30 | 16.2 (13.4, 19.6) | 17.9 (15.1, 21.1) | 19.7 (16.6, 23.4) | 0.12 | 21.6 | 0.08 | 0.43 |
| 5β-reduced metabolites | |||||||||||||
| Etio-G | 34.4 (28.4, 41.7) | 32.1 (26.7, 38.5) | 34.8 (29.3, 41.4) | 0.75 | 1.2 | 0.91 | 32.4 (27.2, 38.7) | 34.8 (29.6, 40.9) | 34.3 (29.9, 39.4) | 0.89 | 5.9 | 0.58 | 0.68 |
| Ratios | |||||||||||||
| DHEAS: DHEA | 215 (192, 242) | 237 (212, 265) | 221 (200, 244) | 0.34 | 2.8 | 0.70 | 210 (183, 241) | 251 (225, 280) | 240 (217, 265) | 0.16 | 14.3 | 0.07 | 0.36 |
| DHTS: DHT | 4.69 (4.03, 5.46) | 5.72 (4.90, 6.68) | 5.57 (4.81, 6.46) | 0.29 | 18.8 | 0.06 | 4.56 (3.76, 5.54) | 4.60 (3.89, 5.44) | 4.87 (4.15, 5.72) | 0.49 | 6.8 | 0.54 | 0.63 |
| DHT: Testosterone | 0.31 (0.27, 0.35) | 0.35 (0.30, 0.40) | 0.31 (0.27, 0.36) | 0.72 | 0.0 | 0.87 | 0.40 (0.35, 0.47) | 0.42 (0.37, 0.48) | 0.41 (0.36, 0.47) | 0.76 | 2.5 | 0.80 | 0.90 |
| Unconjugated estrone: Androstenedione | 45.0 (39.2, 51.7) | 43.6 (38.6, 49.2) | 42.4 (36.7, 49.0) | 0.39 | −5.8 | 0.43 | 194 (156, 241) | 206 (171, 248) | 177 (146, 215) | 0.57 | −8.8 | 0.48 | 0.07 |
| Unconjugated estradiol: Testosterone | 23.3 (19.0, 28.7) | 25.3 (21.1, 30.4) | 26.1 (21.4, 31.8) | 0.25 | 12.0 | 0.31 | 82.6 (65.1, 105) | 97.1 (77.4, 122) | 95.6 (78.2, 117) | 0.20 | 15.7 | 0.27 | 0.08 |
Adjusted for age at blood draw (<55, 55–59, 60–64, 65–69, 70–74, 75–79 years), calendar year at blood draw (1993–1996, 1997–1998), race (White, non-White), smoking status (never, former, current), moderate- to vigorous-intensity physical activity (0, 0.1–9.9, ≥10 metabolic equivalents of task-h/week), alcohol drinking (non-drinker, <1 drink/week, 1-6 drink/week, ≥7 drink/week), parous (yes, no), family history of breast and ovarian cancer (yes, no), time since menopause (<10 years, 10–19 years, ≥20 years, missing), and current BMI (kg/m2, continuous). Additionally adjusted for hormone therapy use (never, former) among women never/former hormone therapy use.
p-trend was estimated using the Wald test for continuous waist-to-hip ratio
%Δ indicates the percentage change in GMs of androgens/androgen metabolite levels, comparing women at the highest vs. lowest tertiles of waist-to-hip ratio.
p-diff was estimated using the Wald test and indicates a p-value for comparing androgens/androgen metabolite levels of women at the highest vs. lowest tertiles of waist-to-hip ratio.
p-int was estimated using the Wald test for an interaction term between waist-to-hip ratio and hormone therapy use.
Weighted n reflects weighted counts and refers to the study cohort.
False discovery rate (FDR) q value <0.05 and ≥0.01
When stratified by current BMI (≥30 vs. <30 kg/m2) among never/former HT users, current WHR (highest vs. lowest tertiles) was positively associated with DHEA (GM=4.87 vs. 3.65 pmol/L), DHEAS (1242 vs. 745 pmol/L), androstenedione (1.62 vs. 1.45 pmol/L), DHTS (1.32 vs. 0.86 pmol/L), ADT-G (28.8 vs. 15.6 pmol/L), 3α-diol-3G (2.57 vs. 1.09 pmol/L), 3α-diol-17G (1.51 vs. 1.21 pmol/L), and DHTS:DHT ratio (7.22 vs. 3.98) in women with higher BMI but not in women with lower BMI, in models additionally adjusted for current BMI as a continuous variable within each stratum (Table 4). However, the interaction by current BMI was not statistically significant for these metabolites. Among current HT users, no association for current WHR was observed in the analysis stratified by current BMI (Supplementary Table 4).
Table 4.
Geometric means (pmol/L) and 95% confidence intervals (CI) of serum androgens/androgen metabolites by current waist-to-hip ratio in postmenopausal women, stratified by current BMI (<30 vs. ≥30 kg/m2) among never/former hormone therapy users
| Current BMI <30 kg/m2 | Current BMI ≥30 kg/m2 | p-intd | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Waist-to-hip ratio | p- trenda |
%Δb | p- diffc |
Waist-to-hip ratio | p- trenda |
%Δb | p-diffc | ||||||
| T1 | T2 | T3 | T1 | T2 | T3 | ||||||||
| Median (range) | 0.74 (0.53-0.78) | 0.81 (0.78-0.84) | 0.88 (0.84-1.37) | 0.75 (0.49-0.78) | 0.81 (0.78-0.84) | 0.90 (0.84-1.17) | |||||||
| N | 270 | 209 | 137 | 36 | 99 | 170 | |||||||
| Weighted N e | 9325 | 7672 | 5174 | 945 | 2107 | 4616 | |||||||
| Geometric means (95% CI) | Geometric means (95% CI) | ||||||||||||
| Adrenal androgens | |||||||||||||
| DHEA | 5.05 (4.28, 5.97) | 4.30 (3.64, 5.08) | 4.39 (3.75, 5.15) | 0.10 | −13.1 | 0.17 | 3.65 (2.73, 4.87) | 3.97 (3.07, 5.14) | 4.87 (3.80, 6.24) | 0.003f | 33.4 | 0.06 | 0.97 |
| DHEAS | 1047 (866, 1267) | 982 (802, 1202) | 885 (730, 1073) | 0.26 | −15.5 | 0.16 | 745 (543, 1024) | 1093 (802, 1489) | 1242 (925, 1669) | 0.003f | 66.7 | 0.003f | 0.53 |
| Androstenedione | 1.46 (1.30, 1.63) | 1.23 (1.10, 1.37) | 1.38 (1.22, 1.57) | 0.32 | −5.5 | 0.51 | 1.45 (1.16, 1.80) | 1.20 (1.01, 1.43) | 1.62 (1.33, 1.96) | 0.01f | 11.7 | 0.30 | 0.43 |
| Testosterone | 0.68 (0.58, 0.79) | 0.49 (0.42, 0.56) | 0.56 (0.48, 0.66) | 0.16 | −17.6 | 0.07 | 0.68 (0.55, 0.85) | 0.53 (0.43, 0.65) | 0.63 (0.52, 0.75) | 0.36 | −7.4 | 0.42 | 0.20 |
| 5α- reduced metabolites | |||||||||||||
| 5α-androstanedione | 1.44 (1.30, 1.60) | 1.35 (1.19, 1.53) | 1.16 (1.03, 1.30) | 0.05 | −19.4 | 0.001f | 1.48 (1.19, 1.83) | 1.30 (1.08, 1.58) | 1.32 (1.08, 1.61) | 0.79 | −10.8 | 0.30 | 0.98 |
| DHT | 0.21 (0.19, 0.24) | 0.18 (0.16, 0.20) | 0.17 (0.16, 0.19) | 0.01 | −19.0 | 0.01 | 0.22 (0.18, 0.25) | 0.16 (0.14, 0.19) | 0.18 (0.15, 0.22) | 0.90 | −18.2 | 0.10 | 0.10 |
| DHTS | 0.93 (0.81, 1.07) | 0.99 (0.86, 1.15) | 0.90 (0.77, 1.05) | 0.27 | −3.2 | 0.75 | 0.86 (0.57, 1.29) | 1.07 (0.76, 1.51) | 1.32 (0.94, 1.84) | 0.03 | 53.5 | 0.02 | 0.36 |
| ADT | 0.56 (0.51, 0.61) | 0.52 (0.47, 0.57) | 0.50 (0.45, 0.55) | 0.51 | −10.7 | 0.04 | 0.47 (0.39, 0.56) | 0.55 (0.47, 0.64) | 0.58 (0.51, 0.65) | 0.16 | 23.4 | 0.03 | 0.91 |
| ADT-G | 18.0 (14.9, 21.7) | 16.2 (13.7, 19.1) | 17.0 (14.2, 20.4) | 0.91 | −5.6 | 0.65 | 15.6 (10.5, 23.1) | 24.7 (16.6, 36.5) | 28.8 (19.6, 42.4) | 0.01 f | 84.6 | 0.003f | 0.64 |
| 3α-diol-3G | 1.12 (0.94, 1.35) | 1.10 (0.93, 1.29) | 1.20 (1.00, 1.45) | 0.63 | 7.1 | 0.56 | 1.09 (0.73, 1.63) | 2.05 (1.46, 2.89) | 2.57 (1.80, 3.67) | 0.01 f | 136 | <0.001g | 0.30 |
| 3α-diol-17G | 1.02 (0.86, 1.19) | 0.99 (0.84, 1.17) | 1.11 (0.93, 1.32) | 0.39 | 8.8 | 0.42 | 1.21 (0.88, 1.66) | 1.48 (1.06, 2.07) | 1.51 (1.08, 2.12) | 0.28 | 24.8 | 0.22 | 0.89 |
| Marker of androgenic activity | |||||||||||||
| Sum of ADT-G, 3α-diol-3G, 3α-diol-17G | 20.5 (17.1, 24.5) | 18.5 (15.8, 21.7) | 19.8 (16.6, 23.5) | 0.94 | −3.4 | 0.76 | 18.3 (12.5, 26.8) | 29.1 (20.1, 42.1) | 34.0 (23.8, 48.6) | 0.01 f | 85.8 | 0.002f | 0.58 |
| 5β- reduced metabolites | |||||||||||||
| Etio-G | 32.8 (26.8, 40.1) | 29.8 (24.5, 36.1) | 29.0 (23.5, 35.8) | 0.56 | −11.6 | 0.36 | 25.2 (16.7, 38.2) | 34.8 (24.3, 49.9) | 43.2 (30.6, 61.0) | 0.09 | 71.4 | 0.02 | 0.60 |
| Ratios | |||||||||||||
| DHEAS: DHEA | 207 (183, 234) | 228 (200, 261) | 201 (175, 231) | 0.63 | −2.9 | 0.72 | 204 (162, 258) | 275 (230, 330) | 255 (220, 296) | 0.40 | 25.0 | 0.07 | 0.37 |
| DHTS: DHT | 4.32 (3.66, 5.10) | 5.45 (4.58, 6.47) | 5.14 (4.28, 6.16) | 0.52 | 19.0 | 0.13 | 3.98 (2.66, 5.95) | 6.69 (4.58, 9.76) | 7.22 (5.04, 10.3) | 0.04 | 81.4 | <0.001g | 0.90 |
| DHT: Testosterone | 0.32 (0.27, 0.37) | 0.37 (0.32, 0.44) | 0.31 (0.26, 0.37) | 0.68 | −3.1 | 0.84 | 0.32 (0.25, 0.40) | 0.30 (0.24, 0.39) | 0.29 (0.22, 0.38) | 0.50 | −9.4 | 0.49 | 0.02 |
| Unconjugated estrone: Androstenedione | 39.5 (34.3, 45.5) | 40.4 (35.4, 46.2) | 41.2 (35.2, 48.1) | 0.61 | 4.3 | 0.63 | 61.9 (43.4, 88.2) | 60.2 (46.0, 78.7) | 51.7 (36.1, 74.1) | 0.19 | −16.5 | 0.31 | 0.51 |
| Unconjugated estradiol: Testosterone | 16.7 (13.3, 21.1) | 20.2 (16.1, 25.4) | 20.5 (15.8, 26.5) | 0.11 | 22.8 | 0.12 | 47.5 (33.4, 67.5) | 40.2 (30.2, 53.5) | 45.5 (32.6, 63.7) | 0.003 f | 33.4 | 0.06 | 0.44 |
Adjusted for age at blood draw (<55, 55–59, 60–64, 65–69, 70–74, 75–79 years), calendar year at blood draw (1993–1996, 1997–1998), race (White, non-White), smoking status (never, former, current), moderate- to vigorous-intensity physical activity (0, 0.1–9.9, ≥10 metabolic equivalents of task-h/week), alcohol drinking (non-drinker, <1 drink/week, 1-6 drink/week, ≥7 drink/week), parous (yes, no), family history of breast and ovarian cancer (yes, no), time since menopause (<10 years, 10–19 years, ≥20 years, missing), hormone therapy use (never, former), and current BMI (kg/m2, continuous).
p-trend was estimated using the Wald test for continuous waist-to-hip ratio
%Δ indicates the percentage change in GMs of androgens/androgen metabolite levels, comparing women at the highest vs. lowest tertiles of waist-to-hip ratio.
p-diff was estimated using the Wald test and indicates a p-value for comparing androgens/androgen metabolite levels of women at the highest vs. lowest tertiles of waist-to-hip ratio.
p-int was estimated using the Wald test for an interaction term between waist-to-hip ratio and current BMI
Weighted n reflects weighted counts and refers to the study cohort.
False discovery rate (FDR) q value <0.05 and ≥0.01
False discovery rate (FDR) q value <0.01
Body mass index at age 18 years
In models without adjustment for current BMI, BMI at age 18 was inversely associated with adrenal androgens (DHEA, DHEAS, androstenedione, testosterone) and positively associated with estrogen-to-androgen ratios (unconjugated estrone:androstenedione, unconjugated estradio:testosterone) among never/former HT users (all p-trend≤0.05; (Supplementary Table 5). In current HT users, similar patterns of associations were observed but the associations were not statistically significant. After additional adjustment for current BMI, BMI at age 18 (highest vs. lowest tertiles) was inversely associated with adrenal androgens and 5α-reduced glucuronide metabolites among both never/former and current HT users (Table 5). Among current HT users, BMI at age 18 was also positively associated with DHT:testosterone ratio (GM=0.45 vs. 0.38; p-trend=0.01). The positive associations between BMI at age 18 and estrogen-to-androgen ratios disappeared after adjustment for current BMI.
Table 5.
Geometric means (pmol/L) and 95% confidence intervals (CI) of serum androgens/androgen metabolites by BMI at age 18, stratified by hormone therapy use: the Women’s Health Initiative Observational Study
| Never/former hormone therapy users | Current hormone therapy users | p-intd | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI at age 18 | p- trenda |
%Δb | p- diffc |
BMI at age 18 | p- trenda |
%Δb | p- diffc |
||||||
| T1 | T2 | T3 | T1 | T2 | T3 | ||||||||
| Median (range) | 18.4 (8.7-19.4) | 20.2 (19.5-21.3) | 22.8 (21.4-51.5) | 18.3 (11.5-19.3) | 20.3 (19.4-20.9) | 22.1 (21.0-22.1) | |||||||
| N | 307 | 306 | 308 | 277 | 283 | 284 | |||||||
| Weighted N e | 10583 | 10136 | 9121 | 8248 | 8030 | 7731 | |||||||
| Geometric means (95% CI) | Geometric means (95% CI) | ||||||||||||
| Adrenal androgens | |||||||||||||
| DHEA | 5.30 (4.57, 6.14) | 4.47 (3.91, 5.12) | 4.52 (3.93, 5.19) | 0.005f | −14.7 | 0.06 | 4.44 (3.85, 5.13) | 3.94 (3.37, 4.61) | 3.87 (3.33, 4.51) | 0.21 | −12.8 | 0.12 | 0.68 |
| DHEAS | 1197 (1001, 1431) | 1030 (868, 1221) | 979 (837, 1144) | 0.001f | −18.2 | 0.03 | 1130 (972, 1313) | 908 (748, 1101) | 863 (720, 1034) | 0.02 | −23.6 | 0.01f | 0.83 |
| Androstenedione | 1.50 (1.36, 1.67) | 1.39 (1.24, 1.56) | 1.36 (1.22, 1.50) | 0.002f | −9.3 | 0.08 | 1.27 (1.15, 1.42) | 1.25 (1.12, 1.40) | 1.17 (1.04, 1.33) | 0.08 | −7.9 | 0.30 | 0.90 |
| Testosterone | 0.60 (0.53, 0.69) | 0.57 (0.49, 0.65) | 0.57 (0.49, 0.66) | 0.02f | −5.0 | 0.46 | 0.55 (0.49, 0.62) | 0.49 (0.43, 0.55) | 0.47 (0.41, 0.54) | 0.04 | −14.5 | 0.06 | 0.86 |
| 5α-reduced metabolites | |||||||||||||
| 5α-androstanedione | 1.27 (1.14, 1.42) | 1.28 (1.16, 1.43) | 1.35 (1.21, 1.50) | 0.39 | 6.3 | 0.39 | 1.42 (1.28, 1.58) | 1.31 (1.17, 1.46) | 1.29 (1.14, 1.45) | 0.76 | −9.2 | 0.19 | 0.72 |
| DHT | 0.18 (0.16, 0.20) | 0.18 (0.17, 0.20) | 0.19 (0.18, 0.21) | 0.89 | 5.6 | 0.26 | 0.21 (0.19, 0.23) | 0.20 (0.18, 0.23) | 0.21 (0.19, 0.24) | 0.61 | 0.0 | 0.78 | 0.49 |
| DHTS | 1.03 (0.90, 1.19) | 0.93 (0.80, 1.07) | 1.03 (0.91, 1.17) | 0.55 | 0.0 | 0.96 | 1.03 (0.88, 1.20) | 0.96 (0.81, 1.13) | 0.94 (0.81, 1.11) | 0.34 | −8.7 | 0.35 | 0.50 |
| ADT | 0.54 (0.50, 0.58) | 0.57 (0.52, 0.61) | 0.51 (0.47, 0.55) | 0.18 | −5.6 | 0.25 | 0.51 (0.47, 0.55) | 0.50 (0.46, 0.54) | 0.50 (0.46, 0.54) | 0.29 | −2.0 | 0.61 | 0.69 |
| ADT-G | 23.0 (19.4, 27.2) | 19.7 (16.6, 23.4) | 18.6 (16.0, 21.6) | 0.02f | −19.1 | 0.03 | 19.5 (16.3, 23.3) | 14.6 (12.0, 17.9) | 13.1 (10.7, 16.1) | 0.002f | −32.8 | 0.001f | 0.12 |
| 3α-diol-3G | 1.55 (1.31, 1.84) | 1.38 (1.12, 1.69) | 1.38 (1.17, 1.62) | 0.02f | −11.0 | 0.18 | 1.45 (1.25, 1.68) | 1.14 (0.96, 1.35) | 1.17 (0.99, 1.37) | 0.09 | −19.3 | 0.02 | 0.99 |
| 3α-diol-17G | 1.30 (1.12, 1.51) | 1.23 (1.06, 1.43) | 1.07 (0.94, 1.22) | 0.06 | −17.7 | 0.02 | 0.95 (0.81, 1.10) | 0.77 (0.66, 0.91) | 0.72 (0.61, 0.84) | 0.001f | −24.2 | 0.003f | 0.13 |
| Marker of tissue-level androgenic activity | |||||||||||||
| Sum of ADT-G, 3α-diol-3G, 3α-diol-17G | 26.3 (22.4, 30.9) | 22.9 (19.4, 27.0) | 21.4 (18.5, 24.7) | 0.01f | −18.6 | 0.02 | 22.2 (18.7, 26.3) | 16.9 (14.0, 20.4) | 15.5 (12.9, 18.7) | 0.002f | −30.2 | 0.002f | 0.15 |
| 5β-reduced metabolites | |||||||||||||
| Etio-G | 35.7 (29.4, 43.2) | 32.8 (27.1, 39.9) | 33.5 (28.5, 39.3) | 0.01f | −6.2 | 0.49 | 37.9 (32.7, 44.0) | 31.7 (26.6, 37.9) | 32.2 (26.9, 38.5) | 0.24 | −15.0 | 0.13 | 0.83 |
| Ratios | |||||||||||||
| DHEAS: DHEA | 226 (204, 250) | 230 (206, 257) | 217 (194, 242) | 0.12 | −4.0 | 0.51 | 254 (228, 283) | 231 (204, 261) | 223 (197, 252) | 0.09 | −12.2 | 0.07 | 0.41 |
| DHTS: DHT | 5.75 (4.92, 6.72) | 5.07 (4.31, 5.97) | 5.31 (4.56, 6.19) | 0.71 | −7.7 | 0.41 | 4.93 (4.16, 5.84) | 4.69 (4.00, 5.50) | 4.46 (3.76, 5.28) | 0.24 | −9.5 | 0.29 | 0.31 |
| DHT: Testosterone | 0.30 (0.26, 0.34) | 0.32 (0.28, 0.38) | 0.34 (0.29, 0.39) | 0.07 | 13.3 | 0.07 | 0.38 (0.33, 0.43) | 0.42 (0.37, 0.48) | 0.45 (0.39, 0.52) | 0.01f | 18.4 | 0.04 | 0.49 |
| Unconjugated estrone: Androstenedione | 42.5 (36.4, 49.6) | 41.9 (36.7, 47.7) | 46.1 (40.0, 53.0) | 0.29 | 8.5 | 0.33 | 172 (140, 211) | 186 (153, 226) | 221 (180, 272) | 0.03 | 28.5 | 0.06 | 0.88 |
| Unconjugated estradiol: Testosterone | 25.1 (20.6, 30.7) | 26.5 (21.7, 32.5) | 23.6 (19.2, 29.0) | 0.99 | −6.0 | 0.58 | 81.2 (64.4, 102) | 90.5 (72.5, 113) | 114 (89.5, 145) | 0.15 | 40.4 | 0.02 | 0.42 |
Adjusted for age at blood draw (<55, 55–59, 60–64, 65–69, 70–74, 75–79 years), calendar year at blood draw (1993–1996, 1997–1998), race (White, non-White), smoking status (never, former, current), alcohol drinking (non-drinker, <1 drink/week, 1-6 drink/week, ≥7 drink/week), parous (yes, no), family history of breast and ovarian cancer (yes, no), time since menopause (<10 years, 10–19 years, ≥20 years, missing), moderate- to vigorous-intensity physical activity (0, 0.1–9.9, ≥10 metabolic equivalents of task-h/week), and current BMI (kg/m2, continuous). Additionally adjusted for hormone therapy use (never, former) among women not using hormone therapy use.
p-trend was estimated using the Wald test for continuous BMI at age 18 (kg/m2)
%Δ indicates the percentage change in GMs of androgens/androgen metabolite levels, comparing women at the highest vs. lowest tertiles of BMI at age 18.
p-diff was estimated using the Wald test and indicates a p-value for comparing androgens/androgen metabolite levels of women at the highest vs. lowest tertiles of BMI at age 18.
p-int was estimated using the Wald test for an interaction term between BMI at age 18 and hormone therapy use.
Weighted n reflects weighted counts and refers to the study cohort.
False discovery rate (FDR) q value <0.05 and ≥0.01
Adult height
Among both never/former and current HT users, height was not associated with any of the androgens/androgen metabolites (Supplementary Table 6).
When adjusting for multiple comparisons, most of the associations remained significant at 5% FDR (Table 2-5). Similar results were observed in cases and controls (Supplementary Table 7-8).
DISCUSSION
In this study, we conducted a comprehensive analysis of the relationships between anthropometric measures and 12 serum androgens/androgen metabolites in postmenopausal women. In this analysis, we found that higher current BMI was associated with higher circulating levels of sulfated adrenal androgens (DHEAS) and 5α-reduced glucuronide metabolites (ADT-G, 3α-diol-3G, 3α-diol-17G), suggesting increased androgen production (and/or increased retention) and tissue-level androgenic activity. Among never/former HT users, current BMI was also positively associated with the estrogen-to-androgen ratios and the relative proportions of parent estrogens (vs. adrenal androgens), suggesting an elevated conversion of androgens to estrogens in obese women. Current WHR was not independently associated with androgens/androgen metabolites among women with low current BMI (<30 kg/m2) but was positively associated with circulating adrenal androgens and 5α-reduced glucuronide metabolites in women with high current BMI (≥30 kg/m2). With adjustment for current BMI, BMI at age 18 was inversely associated with adrenal androgens and 5α-reduced glucuronide metabolites. Height was not associated with any of the androgens/androgen metabolites.
Our findings of positive associations between current BMI and the relative proportion of parent estrogens (vs. adrenal androgens) are in line with biologic and epidemiologic evidence on the relationship between postmenopausal adiposity and estrogens (4,10,41,42). In postmenopausal women, their ovaries stop producing estradiol and other potent estrogens, leading to reduced circulating levels. In postmenopausal obese women, adipose tissues produce estrogens via aromatization of androgens (4,42), leading to elevated circulating levels of estradiol (from conversion of testosterone) and estrone (from conversion of androstenedione) as shown in a previous pooled analysis (41). Our previous analysis in the WHI-OS also identified strong positive associations between current BMI and serum parent estrogens among never/former HT users (10). To further investigate the association of postmenopausal adiposity on aromatase activity, in the present study we compared the serum concentrations of parent estrogens vs. adrenal androgens, while holding the summed concentration constant. Because androgens are converted to estrogens during aromatization, the proportion of estrogens should increase while the proportion of androgens decrease in the setting of elevated aromatase activity. In the present study, postmenopausal obese (vs. nonobese) women had a higher proportion of serum parent estrogens (vs. adrenal androgens), suggesting an increased replacement of androgens for estrogens. Our data further support the increased aromatization associated with postmenopausal adiposity. Higher concentrations of estrogens compared with those of androgens may stimulate cellular proliferation at a greater extent, leading to increased cancer risks (19,28). Among current HT users, there was no difference in the proportions of parent estrogens according to current BMI, possibly due to the negative feedback that suppresses the aromatization of androgens in the presence of excess estrogens (from exogenous source). Among both never/former and current HT users, we also observed that circulating levels of adrenal androgens are elevated in obese women and most adrenal androgens were present in sulfated forms (DHEAS). In postmenopausal obese women, androgen production (or retention) may also be stimulated, and the excess androgens get stored as sulfated forms to be used later. Consistently, animal studies have demonstrated an increased aromatase activity (43) and hyperandrogenism (44) in obese mice. Studies also suggest that hyperinsulinemia may contribute to elevated androgen production in obese women (44-47). Together, our findings suggest that postmenopausal adiposity may be associated with estrogen production by stimulating androgen production/retention (i.e., increased total concentrations of precursor substrates for estrogens) and promoting aromatase activity (i.e., faster conversion of androgens to estrogens), particularly in women not using HT.
In the present analysis, we examined current BMI in relation to androgen metabolism beyond aromatization to estrogens. Among both never/former and current HT users, we observed positive associations between current BMI and serum 5α-reduced glucuronide metabolites, the androgen metabolism profiles that have previously been associated with higher endometrial (19) and non-serous ovarian cancer risks (28). However, current BMI was not associated with serum Etio-G, one of major 5β-reduced metabolites. These findings suggest that postmenopausal obesity may alter 5α-reductase activity but not 5β-reductase activity. Studies have suggested that 5α-reduced glucuronide metabolites may better reflect the androgenic activity in tissue (32,33). The 5α-reduced metabolites are also believed to have higher biologic activity than 5β-reduced metabolites and thus increased levels of 5α-reduced metabolites may indicate a more carcinogenic androgen metabolism profile. While the BMI-cancer relationships in postmenopausal women have been explained primarily by estrogenic, inflammatory, and metabolic pathways, the fourth mechanism, namely androgenic pathway, may also influence cancer risk. Postmenopausal obesity may have both estrogen-dependent (estrogen production) and estrogen-independent (androgen production and tissue-level androgenic activity) effects. Androgen signaling may contribute to cancer risk by stimulating cellular proliferation and inhibiting apoptosis (48-50). Circulating levels of androgens have been associated with increased breast (16,30,31), ovarian (21-28) and endometrial cancer risks (19) in postmenopausal women. While it is yet unclear whether the associations vary by tumor subtypes, some studies suggested that the associations may be restricted to non-serous (vs. serous) ovarian cancer (28) and stronger for hormone receptor-positive (vs. negative) breast cancer risks (31,51). Further studies are needed to formally investigate the mediating effects of androgen metabolism in the obesity-cancer relationship in postmenopausal women.
In addition to current BMI, we also observed associations between current WHR and androgen metabolism. In women with high current BMI, WHR may be a better proxy of abdominal and visceral adiposity, an independent risk factor for endometrial and postmenopausal breast cancers (3,14,15). Among these women with high current BMI, WHR was positively associated with circulating adrenal androgens and 5α-reduced metabolites. The associations are unlikely to be driven by residual effects of BMI because we additionally adjusted for current BMI as a continuous variable in the models. Based on our data, abdominal fat distribution may be independently associated with androgen metabolism by stimulating androgen production and promoting tissue-level activity of androgens. However, given that we did not observe any association between WHR and estrogen-to-androgen ratios, it is likely that abdominal adiposity may not independently contribute to the increased aromatization in obese women. Consistently, previous studies have shown that women with abdominal body fat have higher androgen production rates and an increased amount of free-testosterone, whereas women with lower-body fat have an increased amount of estrone from peripheral aromatization (9).
Lastly, we also found associations between BMI at age 18 and serum androgen metabolism, independent of current BMI. After adjustment for current BMI, BMI at age 18 was inversely associated with adrenal androgens and 5α-reduced glucuronide metabolites, whereas current BMI was positively associated with these metabolites. Our data suggest that obesity during early adulthood may be differentially associated with androgen metabolism. We also observed that the positive association with the estrogen-to-androgen ratios disappeared after additional adjustment for current BMI, suggesting that associations between obesity and aromatization are likely to be specific to postmenopausal obesity and not obesity in early adulthood. As obesity in early and later adulthood also show differential associations with breast cancer risk in multiple studies (52,53), further studies are needed to investigate the effects of timing of obesity on hormone metabolism.
We acknowledge several limitations of this study. We used a single serum sample collected at baseline from each participant. If serum concentrations of androgens/androgen metabolites widely fluctuate within individuals, our data may not reflect participants’ usual circulating levels of androgens/androgen metabolites. However, in a study of 12 postmenopausal women, we observed that most androgens/androgen metabolite concentrations were highly stable over a two-year period. We also calculated BMI at age 18 based on recalled data but related measurement error is unlikely to be related to serum androgen/androgen metabolite levels. Given a cross-sectional design of the study, it is also difficult to clarify the temporal relationship of our results. Some studies suggest the opposite direction of causality (excess androgens increase body fat accumulation) is also possible (54). Therefore, further studies are needed to examine the changes in androgen/androgen metabolites with weight gain using a longitudinal study design. We also did not have information on polycystic ovary syndrome or other conditions (e.g., congenital adrenal hyperplasia) that may be related to abnormal concentrations of androgens/androgen metabolites and thus we were not able to adjust in the analysis. Further, because the WHI-OS participants were highly selected volunteers, our study findings may not be generalizable to overall postmenopausal women. Our study participants were highly motivated women who were mostly White. Because the relationship between BMI and body composition varies by race (e.g., greater body fat in Asian vs. White women for a given BMI) (55), the association between BMI and androgens/androgen metabolites may also vary by race. Given the increased obesity prevalence among the US women since the start of the WHI, it is also possible that the circulating levels of estrogens and androgens may differ in future studies that use more recently collected data. Lastly, due to the number of tests, some of the observed associations may be due to chance (e.g., false positives). However, when we accounted for multiple comparison using FDR, most associations remained significant at a 5% FDR level.
This study has several strengths. By using measured data on BMI and WHR at baseline, we reduced measurement error in exposures. Use of a high-performance LC-MS/MS assay also allowed highly sensitive evaluation of serum androgens/androgen metabolites. We also examined both absolute (e.g., DHT) and relative (e.g., DHTS:DHT ratio) concentrations of androgens/androgen metabolites. While the associations with absolute concentrations may provide insights into the biologic mechanisms, the associations with relative concentrations may help us understand the pathway metabolism that can inform cancer risk. Finally, we increased the validity of our results by using a large sample and careful adjustment for potential confounders.
In summary, we observed positive associations between current BMI and circulating levels of adrenal androgens, 5α-reduced glucuronide metabolites, and proportions of parent estrogens (vs. adrenal androgens) among postmenopausal women. Our data suggest that postmenopausal obesity may be associated with increased androgen production (or retention), tissue-level androgenic activity, and aromatization of androgens. Similar androgen metabolism profiles have previously been associated with higher ovarian and endometrial cancer risks (19,28) and thus prospective studies are needed to confirm the mediating role of androgen metabolism in the obesity-cancer relationship.
Supplementary Material
ACKNOWLEDGEMENT:
The WHI program is supported by contracts from the National Heart, Lung and Blood Institute, NIH (Program Office: Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller). The authors thank the WHI Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle WA) and the WHI investigators (Garnet Anderson, Ross Prentice, Andrea LaCroix, Charles Kooperberg, JoAnn E. Manson, Barbara V. Howard, Marcia L. Stefanick, Rebecca Jackson, Cynthia A. Thomson, Jean Wactawski-Wende, Marian Limacher, Jennifer Robinson, Lewis Kuller, Sally Shumaker, Robert Brunner) for their dedication, and the study participants for making the program possible. A full list of WHI investigators can be found at https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
FUNDING:
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, 75N92021D00005. This study was also supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics of the National Cancer Institute. H.O. was supported by the National Research Foundation of Korea (NRF) grant (2019R1G1A1004227, 2019S1A3A2099973).
Abbreviations:
- ADT-G
androsterone-glucuronide
- BMI
body mass index
- CV
coefficients of variation
- DHEA
dehydroepiandrosterone
- DHEAS
dehydroepiandrosterone sulfate
- DHTS
dihydrotestosterone sulfate
- Etio-G
etiocholanolone-glucuronide
- FDR
false discovery rate
- HT
hormone therapy
- ICC
intraclass correlation coefficients
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- WHI-OS
Women’s Health Initiative Observational Study
- WHR
waist-to-hip ratio
- 3α-diol-3G
5α-androstane-3α,17β diol-3-glucuronide
- 3α-diol-17G
3α-diol-17-glucuronide
Footnotes
Ethical Approval and Consent to participate
The Office of Human Subjects Research at the National Institutes of Health approved this analysis. As required by the WHI protocol, informed consent was obtained from all study subjects. Informed consent documents and procedures were approved by the Institutional Review Board (IRB) of the Fred Hutchinson Cancer Research Center, and by the IRBs of each of the participating clinical centers.
Conflict of interest statement: Authors declare no conflict of interest.
REFERENCES
- 1.Cheraghi Z, Poorolajal J, Hashem T, Esmailnasab N, Doosti Irani A. Effect of body mass index on breast cancer during premenopausal and postmenopausal periods: a meta-analysis. PLoS One 2012;7(12):e51446 doi 10.1371/journal.pone.0051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collaborative Group on Epidemiological Studies of Ovarian C. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med 2012;9(4):e1001200 doi 10.1371/journal.pmed.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aune D, Navarro Rosenblatt DA, Chan DS, Vingeliene S, Abar L, Vieira AR, et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Ann Oncol 2015;26(8):1635–48 doi 10.1093/annonc/mdv142. [DOI] [PubMed] [Google Scholar]
- 4.Folkerd EJ, James VH. Aromatization of steroids in peripheral tissues. J Steroid Biochem 1983;19(1B):687–90 doi 10.1016/0022-4731(83)90236-4. [DOI] [PubMed] [Google Scholar]
- 5.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, et al. Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. European journal of endocrinology / European Federation of Endocrine Societies 2004;150(2):161–71. [DOI] [PubMed] [Google Scholar]
- 6.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. Journal of the National Cancer Institute 1995;87(17):1297–302. [DOI] [PubMed] [Google Scholar]
- 7.Bruning PF, Bonfrer JM, Hart AA, van Noord PA, van der Hoeven H, Collette HJ, et al. Body measurements, estrogen availability and the risk of human breast cancer: a case-control study. International journal of cancer Journal international du cancer 1992;51(1):14–9. [DOI] [PubMed] [Google Scholar]
- 8.Newcomb PA, Klein R, Klein BE, Haffner S, Mares-Perlman J, Cruickshanks KJ, et al. Association of dietary and life-style factors with sex hormones in postmenopausal women. Epidemiology 1995;6(3):318–21. [DOI] [PubMed] [Google Scholar]
- 9.Kirschner MA, Samojlik E, Drejka M, Szmal E, Schneider G, Ertel N. Androgen-estrogen metabolism in women with upper body versus lower body obesity. J Clin Endocrinol Metab 1990;70(2):473–9 doi 10.1210/jcem-70-2-473. [DOI] [PubMed] [Google Scholar]
- 10.Oh H, Coburn SB, Matthews CE, Falk RT, LeBlanc ES, Wactawski-Wende J, et al. Anthropometric measures and serum estrogen metabolism in postmenopausal women: the Women's Health Initiative Observational Study. Breast Cancer Res 2017;19(1):28 doi 10.1186/s13058-017-0810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson GF, Magoffin DA, Dyer CA, Hofeditz C. The ovarian androgen producing cells: a review of structure/function relationships. Endocr Rev 1985;6(3):371–99 doi 10.1210/edrv-6-3-371. [DOI] [PubMed] [Google Scholar]
- 12.Poretsky L On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocr Rev 1991;12(1):3–13 doi 10.1210/edrv-12-1-3. [DOI] [PubMed] [Google Scholar]
- 13.Iuorno MJ, Nestler JE. The polycystic ovary syndrome: treatment with insulin sensitizing agents. Diabetes Obes Metab 1999;1(3):127–36 doi 10.1046/j.1463-1326.1999.00026.x. [DOI] [PubMed] [Google Scholar]
- 14.Connolly BS, Barnett C, Vogt KN, Li T, Stone J, Boyd NF. A meta-analysis of published literature on waist-to-hip ratio and risk of breast cancer. Nutr Cancer 2002;44(2):127–38 doi 10.1207/S15327914NC4402_02. [DOI] [PubMed] [Google Scholar]
- 15.Barberio AM, Alareeki A, Viner B, Pader J, Vena JE, Arora P, et al. Central body fatness is a stronger predictor of cancer risk than overall body size. Nat Commun 2019;10(1):383 doi 10.1038/s41467-018-08159-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Key T, Appleby P, Barnes I, Reeves G, Endogenous H, Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. Journal of the National Cancer Institute 2002;94(8):606–16 doi 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 17.Sampson JN, Falk RT, Schairer C, Moore SC, Fuhrman BJ, Dallal CM, et al. Association of Estrogen Metabolism with Breast Cancer Risk in Different Cohorts of Postmenopausal Women. Cancer Res 2017;77(4):918–25 doi 10.1158/0008-5472.CAN-16-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuhrman BJ, Schairer C, Gail MH, Boyd-Morin J, Xu X, Sue LY, et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. Journal of the National Cancer Institute 2012;104(4):326–39 doi 10.1093/jnci/djr531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michels KA, Brinton LA, Wentzensen N, Pan K, Chen C, Anderson GL, et al. Postmenopausal Androgen Metabolism and Endometrial Cancer Risk in the Women's Health Initiative Observational Study. JNCI Cancer Spectr 2019;3(3):pkz029 doi 10.1093/jncics/pkz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brinton LA, Trabert B, Anderson GL, Falk RT, Felix AS, Fuhrman BJ, et al. Serum Estrogens and Estrogen Metabolites and Endometrial Cancer Risk among Postmenopausal Women. Cancer Epidemiol Biomarkers Prev 2016;25(7):1081–9 doi 10.1158/1055-9965.EPI-16-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helzlsouer KJ, Alberg AJ, Gordon GB, Longcope C, Bush TL, Hoffman SC, et al. Serum gonadotropins and steroid hormones and the development of ovarian cancer. Jama 1995;274(24):1926–30. [PubMed] [Google Scholar]
- 22.Lukanova A, Lundin E, Akhmedkhanov A, Micheli A, Rinaldi S, Zeleniuch-Jacquotte A, et al. Circulating levels of sex steroid hormones and risk of ovarian cancer. International journal of cancer Journal international du cancer 2003;104(5):636–42 doi 10.1002/ijc.10990. [DOI] [PubMed] [Google Scholar]
- 23.Tworoger SS, Lee IM, Buring JE, Hankinson SE. Plasma androgen concentrations and risk of incident ovarian cancer. American journal of epidemiology 2008;167(2):211–8 doi 10.1093/aje/kwm278. [DOI] [PubMed] [Google Scholar]
- 24.Rinaldi S, Dossus L, Lukanova A, Peeters PH, Allen NE, Key T, et al. Endogenous androgens and risk of epithelial ovarian cancer: results from the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Epidemiol Biomarkers Prev 2007;16(1):23–9 doi 10.1158/1055-9965.EPI-06-0755. [DOI] [PubMed] [Google Scholar]
- 25.Ose J, Fortner RT, Rinaldi S, Schock H, Overvad K, Tjonneland A, et al. Endogenous androgens and risk of epithelial invasive ovarian cancer by tumor characteristics in the European Prospective Investigation into Cancer and Nutrition. International journal of cancer Journal international du cancer 2015;136(2):399–410 doi 10.1002/ijc.29000. [DOI] [PubMed] [Google Scholar]
- 26.Schock H, Surcel HM, Zeleniuch-Jacquotte A, Grankvist K, Lakso HA, Fortner RT, et al. Early pregnancy sex steroids and maternal risk of epithelial ovarian cancer. Endocr Relat Cancer 2014;21(6):831–44 doi 10.1530/ERC-14-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ose J, Poole EM, Schock H, Lehtinen M, Arslan AA, Zeleniuch-Jacquotte A, et al. Androgens are differentially associated with ovarian cancer subtypes in the Ovarian Cancer Cohort Consortium. Cancer Res 2017. doi 10.1158/0008-5472.CAN-16-3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trabert B, Michels KA, Anderson GL, Brinton LA, Falk RT, Geczik AM, et al. Circulating androgens and postmenopausal ovarian cancer risk in the Women's Health Initiative Observational Study. International journal of cancer Journal international du cancer 2019;145(8):2051–60 doi 10.1002/ijc.32157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trabert B, Brinton LA, Anderson GL, Pfeiffer RM, Falk RT, Strickler HD, et al. Circulating Estrogens and Postmenopausal Ovarian Cancer Risk in the Women's Health Initiative Observational Study. Cancer Epidemiol Biomarkers Prev 2016;25(4):648–56 doi 10.1158/1055-9965.EPI-15-1272-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baglietto L, Severi G, English DR, Krishnan K, Hopper JL, McLean C, et al. Circulating steroid hormone levels and risk of breast cancer for postmenopausal women. Cancer Epidemiol Biomarkers Prev 2010;19(2):492–502 doi 10.1158/1055-9965.EPI-09-0532. [DOI] [PubMed] [Google Scholar]
- 31.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. Journal of the National Cancer Institute 2004;96(24):1856–65 doi 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 32.Labrie F, Belanger A, Belanger P, Berube R, Martel C, Cusan L, et al. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. J Steroid Biochem Mol Biol 2006;99(4-5):182–8 doi 10.1016/j.jsbmb.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Labrie F Intracrinology in action: importance of extragonadal sex steroid biosynthesis and inactivation in peripheral tissues in both women and men. J Steroid Biochem Mol Biol 2015;145:131–2 doi 10.1016/j.jsbmb.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Brinton LA, Trabert B, Anderson GL, Falk RT, Felix AS, Fuhrman BJ, et al. Serum estrogens and estrogen metabolites and endometrial cancer risk among postmenopausal women in the Women’s Health Initiative Observational Study. . 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trabert B, Brinton LA, Anderson GL, Pfeiffer R, Falk RT, Strickler HD, et al. Circulating estrogens and postmenopausal ovarian cancer risk in the Women’s Health Initiative Observational Study. Cancer Epidemiol Biomarkers Prev 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women's Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Annals of epidemiology 2003;13(9 Suppl):S107–21. [DOI] [PubMed] [Google Scholar]
- 37.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Controlled clinical trials 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 38.Trabert B, Xu X, Falk RT, Guillemette C, Stanczyk FZ, McGlynn KA. Assay reproducibility of serum androgen measurements using liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol 2016;155(Pt A):56–62 doi 10.1016/j.jsbmb.2015.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X, Roman JM, Issaq HJ, Keefer LK, Veenstra TD, Ziegler RG. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal Chem 2007;79(20):7813–21 doi 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- 40.Li HL, Gail MH. Efficient Adaptively Weighted Analysis of Secondary Phenotypes in Case-Control Genome-Wide Association Studies. Hum Hered 2012;73(3):159–73 doi Doi 10.1159/000338943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. Journal of the National Cancer Institute 2003;95(16):1218–26 doi 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 42.Grodin JM, Siiteri PK, MacDonald PC. Source of estrogen production in postmenopausal women. J Clin Endocrinol Metab 1973;36(2):207–14 doi 10.1210/jcem-36-2-207. [DOI] [PubMed] [Google Scholar]
- 43.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4(3):329–46 doi 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Wu S, Divall S, Nwaopara A, Radovick S, Wondisford F, Ko C, et al. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes 2014;63(4):1270–82 doi 10.2337/db13-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blank SK, McCartney CR, Marshall JC. The origins and sequelae of abnormal neuroendocrine function in polycystic ovary syndrome. Hum Reprod Update 2006;12(4):351–61 doi 10.1093/humupd/dml017. [DOI] [PubMed] [Google Scholar]
- 46.Nestler JE. Insulin regulation of human ovarian androgens. Hum Reprod 1997;12 Suppl 1:53–62 doi 10.1093/humrep/12.suppl_1.53. [DOI] [PubMed] [Google Scholar]
- 47.Nestler JE, Jakubowicz DJ, de Vargas AF, Brik C, Quintero N, Medina F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J Clin Endocrinol Metab 1998;83(6):2001–5 doi 10.1210/jcem.83.6.4886. [DOI] [PubMed] [Google Scholar]
- 48.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. Journal of the National Cancer Institute 1998;90(23):1774–86 doi 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 49.Simitsidellis I, Gibson DA, Cousins FL, Esnal-Zufiaurre A, Saunders PT. A Role for Androgens in Epithelial Proliferation and Formation of Glands in the Mouse Uterus. Endocrinology 2016;157(5):2116–28 doi 10.1210/en.2015-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simitsidellis I, Saunders PTK, Gibson DA. Androgens and endometrium: New insights and new targets. Mol Cell Endocrinol 2018;465:48–60 doi 10.1016/j.mce.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 51.Sieri S, Krogh V, Bolelli G, Abagnato CA, Grioni S, Pala V, et al. Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: the ORDET cohort. Cancer Epidemiol Biomarkers Prev 2009;18(1):169–76 doi 10.1158/1055-9965.EPI-08-0808. [DOI] [PubMed] [Google Scholar]
- 52.Baer HJ, Tworoger SS, Hankinson SE, Willett WC. Body fatness at young ages and risk of breast cancer throughout life. American journal of epidemiology 2010;171(11):1183–94 doi 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hopper JL, Dite GS, MacInnis RJ, Liao Y, Zeinomar N, Knight JA, et al. Age-specific breast cancer risk by body mass index and familial risk: prospective family study cohort (ProF-SC). Breast Cancer Res 2018;20(1):132 doi 10.1186/s13058-018-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril 2006;85(5):1319–40 doi 10.1016/j.fertnstert.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 55.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord 1998;22(12):1164–71 doi 10.1038/sj.ijo.0800741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


