Abstract
Cell-based approaches to tissue repair suffer from rapid cell death upon implantation, limiting the window for therapeutic intervention. Despite robust lineage-specific differentiation potential in vitro, the function of transplanted mesenchymal stromal cells (MSCs) in vivo is largely attributed to their potent secretome comprised of a variety of growth factors (GFs). Furthermore, GF secretion is markedly increased when MSCs are formed into spheroids. Native GFs are sequestered within the extracellular matrix (ECM) via sulfated glycosaminoglycans, increasing the potency of GF signaling compared to their unbound form. To address the critical need to prolong the efficacy of transplanted cells, we modified alginate hydrogels with sulfate groups to sequester endogenous heparin-binding GFs secreted by MSC spheroids. We assessed the influence of crosslinking method and alginate modification on mechanical properties, degradation rate, and degree of sulfate modification. Sulfated alginate hydrogels sequestered a mixture of MSC-secreted endogenous biomolecules, thereby prolonging the therapeutic effect of MSC spheroids for tissue regeneration. GFs were sequestered for longer durations within sulfated hydrogels and retained their bioactivity to regulate endothelial cell tubulogenesis and myoblast infiltration. This platform has the potential to prolong the therapeutic benefit of the MSC secretome and serve as a valuable tool for investigating GF sequestration.
Keywords: mesenchymal stromal cells, secretome, heparin-binding, spheroids, sulfate
Graphical Abstract
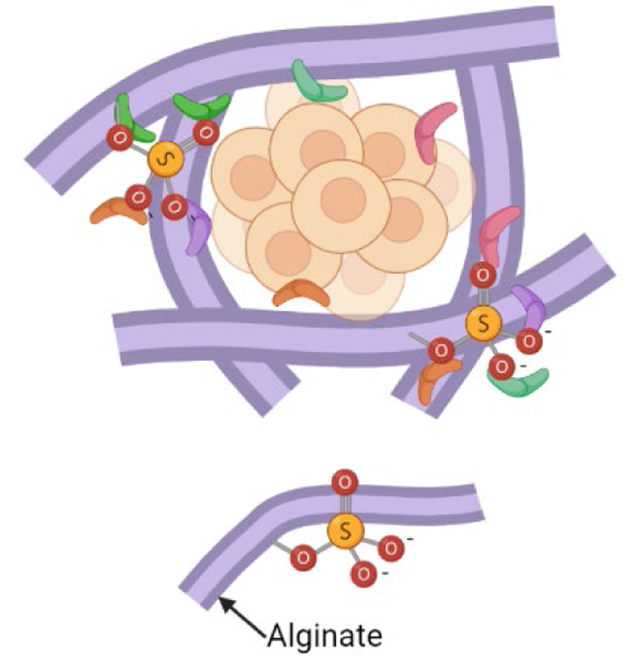
Spheroids of mesenchymal stromal cells (MSCs) secrete high levels of relevant growth factors (GFs) for tissue regeneration. Sulfated alginate can sequester these GFs through ionic interactions, allowing increased therapeutic potency to invading cells. This paper investigates how MSC spheroid-secreted GFs are retained in sulfated alginate and their ability to influence cell function in vitro.
1. Introduction
Cells are the building blocks of new and replacement tissues that offer a therapeutic benefit often unmatched by cell-free approaches. Consequently, a multitude of tissue engineering strategies have employed the delivery of cells to wound sites for improved regeneration.[1] Mesenchymal stromal cells (MSCs) are commonly used for this application due to their ease of harvest from adult patients, trilineage potential, and their therapeutic secretome that recruits host cells to the tissue site, promotes angiogenesis, and modulates the local inflammatory microenvironment.[2] The MSC secretome is composed of a complex mixture of tissue-reparative cytokines and endogenous growth factors (GFs) including VEGFA, IGF-1, and IL-8. Despite their robust differentiation potential in vitro, it is widely assumed that MSCs primarily contribute to tissue regeneration and repair via this potent secretome.[3]
Cell-based tissue engineering approaches are limited by rapid cell death upon implantation into a harsh microenvironment, thereby reducing the duration and overall potential of this strategy.[4] In contrast to monodisperse cells, the aggregation of MSCs into spheroids results in prolonged cell viability and enhanced secretion of endogenous GFs.[5, 6] The behavior of MSC spheroids can be further influenced by the biophysical properties of carriers used to transplant cells. For example, hydrogel stiffness and adhesivity can be tuned to upregulate GF production by MSC spheroids.[6, 7] However, the therapeutic effects of MSC spheroids are limited by their survival and rapid diffusion of endogenous GFs from many cell carriers and delivery vehicles.
To address the challenge of short-term cellular contributions to cell-based therapies, there is a critical need for the development of advanced biomaterials that prolong or retain the bioactivity of endogenous GFs or other bioactive moieties.[8, 9] Beyond tuning the morphological and mechanical properties of biomaterials, one strategy is designed to mimic a key role of the native extracellular matrix (ECM): retention and presentation of endogenous GFs secreted by neighboring cells. The presentation and release of GFs from biomaterials can be regulated by controlling pore density and gel composition, covalently tethering GFs to materials, and sequestering GFs via charged polymers.[10–12] Heparan sulfate (HS), a negatively charged glycosaminoglycan that reversibly binds positively charged GFs, is a key GF-binding constituent of the native ECM. HS function has been mimicked using naturally occurring sulfated polymers (e.g., heparin) or modifying polymers such as polyethylene glycol (PEG) and alginate with functional sulfate groups.[13, 14] Sulfated alginate has been used as a GF delivery vehicle for applications in hind limb ischemia and myocardial infarction, demonstrating a 3-fold greater retention of entrapped GFs compared to unmodified alginate.[15] However, this platform was utilized to deliver a single GF for a therapeutic application, which severely limits the potential of endogenous GFs to be retained in the platform. MSC spheroids are under investigation for the utility of their secretome,[16] a complex mixture of endogenous GFs, and cells are commonly transplanted using engineered hydrogels.[17, 18] Thus, it is imperative to develop and characterize the ability of biomaterial platforms to sequester the endogenous secretome produced by entrapped MSC spheroids.
We hypothesized that alginate hydrogels could be modified to support survival of entrapped MSC spheroids and sequester components of the endogenous secretome as a means to prolong their therapeutic potential. We sought to independently control initial moduli, degradation rate, and degree of sulfation of these modified hydrogels to tune the production and retention of endogenous GFs. We then investigated the capacity of sulfated alginate hydrogels to capture the bioactive MSC spheroid secretome and enhance cell infiltration to demonstrate the utility of this platform.
2. Materials and Methods
2.1. Modification of alginate with functional groups
PRONOVA UP VLVG alginate (<75,000 g/mol; NovaMatrix Sandvika, Norway) was modified with sulfate groups on the polymer backbone as described.[19] Briefly, dried VLVG was dissolved in formamide at 2.5% (w/v). Chlorosulfonic acid (HSO3Cl; Sigma Chemical, St. Louis, MO) at 0–2% (v/v) was added to the solution and incubated at 60°C for 2.5 hrs while stirring. Alginate was then precipitated out of solution in cold acetone and dissolved in ultrapure water overnight. Sodium hydroxide was added dropwise to the alginate solution until each sample reached a neutral pH (~7). The solutions were pipetted into 3500 Da molecular weight cut off (MWCO) dialysis tubing (Spectrum Laboratories, New Brunswick, NJ). The tubes were maintained in 2 L of 100 mM sodium chloride solution for 12 hrs and then ultrapure water the next 2.5 days, with water changes every 6–12 hrs. The alginate was then recovered from the dialysis tubing, filtered through a 0.22 μm pore filter, and lyophilized for up to 7 days until dry.
PRONOVA UP MVG (>200,000 g/mol; NovaMatrix Sandvika) was oxidized as previously described.[20] Alginate was dissolved overnight in ultrapure water at a 1% (w/v) solution and reacted with 1 mM of sodium periodate for 1% oxidation or 5 mM for 5% oxidation. The reaction was quenched after 17 hrs in darkness with stirring using an equimolar amount of ethylene glycol. The alginate was then dialyzed in ultrapure water, filtered, and lyophilized.
PRONOVA UP VLVG and oxidized PRONOVA UP MVG were modified with Arg-Gly-Asp (RGD) through standard carbodiimide chemistry.[21] Alginate was first dissolved in a 1% (w/v) solution in MES buffer (0.1 M MES, 0.3 M NaCl pH 6.5) overnight. The next day, N-(3-Dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC) and N-Hydroxysulfosuccinimide sodium salt (Sulfo-NHS) were added to the reaction at a ratio of 2:1 per gram of alginate. The peptide G4RGDSP (Commonwealth Biotechnologies, Richmond, VA) was then added to the reaction to achieve a degree of substitution (DS) of 2. The resulting RGD-alginate was put in a dialysis tube (6–8 kDa MWCO, Spectrum Laboratories) in a water bath for three days. The solution was sterile filtered and lyophilized for 4 days.
2.2. Detection of sulfate groups on the alginate polymer
Sulfate modification of alginate was confirmed via 1H NMR as described.[21] Briefly, lyophilized alginate samples were dissolved in D2O at 3.33% (w/v) concentration and recorded using an 800 MHz Bruker Avance III NMR spectrometer. Alginate sulfate modification was further verified through 1,9-Dimethyl-Methylene Blue zinc chloride double salt (DMMB) assay.[22] The DMMB solution pH was reduced to 1.5 to eliminate false readings from the negatively charged alginate polymer. Sulfated alginate was compared via DMMB assay with a sample of decellularized ECM derived from human MSCs and commercially available heparin sodium salt (Fisher Scientific, Hampton, NH). ECM was decellularized as previously described, including treatment with a detergent solution and deoxyribonuclease.[23]
2.3. Sulfated hydrogel synthesis
All alginate was dissolved at a concentration of 25 mg/mL in PBS. Alginate hydrogels were produced by combining sulfated alginate with non-sulfated alginate in a ratio of 1:7 (sulfated:non-sulfated). The sulfated alginate consisted of VLVG alginate reacted with 0, 1 or 2% chlorosulfonic acid. The non-sulfated alginate was composed of 1 part RGD-modified VLVG alginate and 1 part oxidized (either 1% or 5%) RGD-modified MVG, as described in Table 1. A visual representation of the gel composition can be seen in Figure 2A. After mixing via gentle tube rotation for 10 min, the alginate mixture was pipetted into 8 mm diameter circular silicone molds sandwiched between two dialysis membranes (6–8 kDa MWCO, Spectrum Laboratories). A solution of either 6 mM BaCl2 and 200 mM CaCl2 (high elastic modulus) or 3 mM BaCl2 and 100 mM CaCl2 (low elastic modulus) was pipetted onto the top dialysis membrane for 5 min. The gels were then flipped, and the ionic solution was pipetted onto the other dialysis membrane for an additional 5 min. Both dialysis membranes were removed, and the gels were placed in a bath of the same ionic solution for an additional 10 min. Gels were then removed from the mold and used immediately.
Table 1.
Composition of the different hydrogel groups.
| Hydrogel component (percent volume in gel) | ||||
|---|---|---|---|---|
| Hydrogel Group | MVG (43.75%) | VLVG (43.75%) | Sulfated VLVG (12.5%) | Crosslinker |
| High moduli, slow degrading | 1% oxidized RGD modified | RGD modified | Sulfate modified | 6 mM BaCl2 200 mM CaCl2 |
| Low moduli, slow degrading | 1% oxidized RGD modified | RGD modified | Sulfate modified | 3 mM BaCl2 100 mM CaCl2 |
| High moduli, fast degrading | 5% oxidized RGD modified | RGD modified | Sulfate modified | 6 mM BaCl2 200 mM CaCl2 |
| Low moduli, fast degrading | 5% oxidized RGD modified | RGD modified | Sulfate modified | 3 mM BaCl2 100 mM CaCl2 |
2.4. Mechanical testing and degradation
We tested the shear storage moduli of 8 mm diameter gels using a Discovery HR2 Hybrid Rheometer (TA Instruments, New Castle, DE) with a stainless steel, cross hatched, 8 mm plate geometry. We performed an oscillatory strain sweep ranging from 0.004% to 4% strain on each gel to obtain the linear viscoelastic region (LVR) before failure.[21] At least 5 data points were collected for the LVR and averaged to obtain gel shear storage modulus. Gels were measured after 1, 14, or 21 days in serum-free αMEM at standard sterile culture conditions (37°C, 21% O2, 5% CO2). After testing, gels were frozen and lyophilized for 24 hrs or until dry. The dry mass of the gels was determined using a Mettler Toledo XPR2 Microbalance (Mettler-Toledo, Columbus, OH).
2.5. Cell culture and MSC spheroid formation
Human MSCs (RoosterBio, Frederick, MD) were cultured in αMEM (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS) (Biotechne, Minneapolis, MN) and 1% penicillin-streptomycin (pen/strep) (Gemini Bio Products, West Sacramento, CA) in standard conditions. MSCs were used at passage 3–5 for all experiments. TdTomato-expressing human hTERT/cdk4 immortalized myoblasts (Institute of Myology, Paris, France) were cultured in 1:4 ratio of Media 199 (Thermo Fisher, Chicago, IL) and DMEM (Invitrogen) supplemented with 20% FBS, 1% pen/strep, 25 μg/mL fetuin, 5 ng/mL of hEGF, 0.5 ng/mL of bFGF, 5 μg/mL of insulin, and 0.2 μg/mL dexamethasone (all from Millipore Sigma, Burlington, MA) under the same conditions.[24] Myoblasts were cultured in flasks until 80% confluence or lower to discourage cell fusion and myotube formation. Human dermal microvascular endothelial cells (HDMECs, Lot #437592; Lonza, Basel, Switzerland) were cultured in complete endothelial cell growth media (EGM2-MV). HDMECs were cultured for 6 days under standard conditions or until 75–80% confluent before use, and cells were used between passage 4–5 for all experiments.
MSC spheroids were produced using the forced aggregation technique as we described.[21, 25] Once cultured to confluence, MSCs were trypsinized and centrifuged down into 2,000 μm pores made out of 1.5% agarose to form 40,000-cell spheroids. After 48 hrs in static conditions to enable spheroid formation, MSC spheroids were pipetted into the alginate solutions at 800,000 cells per gel and crosslinked into hydrogels as described above.
2.6. Retention of proteins in modified alginate gels
Hydrogels were loaded with 1 μg HGF (PeproTech, Rocky Hill, NJ) as a model protein to verify that sulfate groups bind heparin-binding GFs. As a control experiment, another set of hydrogels was loaded with 1 μg IGF-1 (PeproTech), a non-heparin binding GF, to verify the specificity of GF retention. All gels were cultured in serum-free media collected at various time points for GF quantification. HGF and IGF detection was quantified using Human Quantikine ELISA Kits specific for each GF (R&D Systems, Minneapolis, MN) and assessed on a Synergy HTX Multi-Mode Reader (BioTek, Winooski, VT) at 450 nm. To quantify hydrogel-retained cytokines, MSC spheroids were cultured in sulfated or non-sulfated hydrogels for 3 days, then collected in 1 mg/mL alginate lyase (Millipore Sigma) in a 1.4 M NaCl solution. MSC spheroid-secreted GFs were quantified and identified using the IsoLight System (IsoPlexis, Branford, CT) with the Human Innate Immune cytokine panel.
2.7. Endothelial cell tubulogenesis assay
MSC spheroids were entrapped in alginate sulfated with either 0, 1, or 2% HSO3Cl and cultured for 21 days. The media was refreshed every 2–3 days and subsequently stored at −20°C for later use. Before use, the conditioned media was thawed on ice, vortexed briefly, then centrifuged at 700xg for 8 min to pellet any cell debris or ECM. Immediately before use, the media was warmed to 37°C, and 200 μL of supernatant was used for experimentation.
Matrigel (Corning, Corning, NY) was pipetted into a 48-well plate and incubated at 37°C for 1 hr to ensure gelation. HDMECs were trypsinized and resuspended at a concentration of 30,000 cells per well in either complete EGM2 as the positive control, GF-deficient media as the negative control, or conditioned media for the experimental groups. Tubule formation proceeded for 6 hrs. The media was then removed, a 2 mM calcein AM solution was added to each well, and plates were incubated for an additional 30 min. Each well was imaged in 3 different locations; network length and branch number were quantified using the ImageJ Angiogenesis Analyzer plugin. Network length was quantified as the sum of all segments in the network, and branches were defined as segments linked to one junction, a bifurcation point, and one extremity, a segment with a free end.[26]
2.8. Myoblast infiltration into hydrogels
MSC spheroids were loaded into sulfated alginate hydrogels at 800,000 cells per gel (20 spheroids per gel; 40,000 cells per spheroid). The gels were cultured in complete αMEM for 4 days to allow for accumulation of the secretome within the hydrogel. To test the potential of GFs retained after cell death, gels were frozen at −80°C for 2 weeks, while live spheroid hydrogels were used immediately after the 4-day culture. Hydrogels were then placed in non-tissue culture treated 24-well plates and seeded with 1×106 tdTomato myoblasts in a 50:50 αMEM:myoblast media mixture. Hydrogels were moved to new plates after 24 hrs and media was refreshed every 2–3 days to prevent GFs secreted from non-adherent myoblasts to confound results. After 8 days, myoblast infiltration was characterized via confocal imaging. Z stacks of 100–300 μm depth were analyzed using Imaris software to quantify myoblast infiltration depth and sphericity.
2.9. Macrophage migration into hydrogels and subsequent polarization
Conditioned media was collected from 1×106 rat MSCs formed into 40k spheroids cultured in 100 μL of media for 3 days. Alginate was then dissolved into the collected media and formed into hydrogels. IC-21 macrophages (ATCC, Manassas, VA) were seeded in non-tissue culture-treated wells containing the hydrogels at 500,000 cells per well immediately following gel formation. Gels were maintained in RPMI 1640 (ATCC) supplemented with 10% FBS. After 24 hrs, gels were moved to a new plate with fresh media, maintained in culture for another 24 hrs, and then collected.
Cells were recovered from alginate via digestion at 37°C in a 200 mM sodium citrate and 100 mM EDTA solution in PBS supplemented with 3% FBS, pH 6.8, at a 10:1 v:v ratio with alginate. Gels were digested for 45 min until alginate appeared dissolved. Solutions were filtered through a 100 μm filter to create a single cell suspension, and macrophage polarization was characterized using flow cytometry (Attune NxT, Life Tech). Following Fcγ receptor blocking (1:40, TruStain FcX, BioLegend, San Diego, CA), cells were stained with antibodies against CD86 (1:160, eBioscience, Hatfield, United Kingdom, #47-0862-82) and CD206 (1:40, eBioscience #48-2061-82). Cellular viability was evaluated with fixable Zombie Aqua (1:250, Life Tech, Carlsbad, CA). Cells were then fixed with 2% PFA, permeabilized with 0.1% Triton-X, and stained for intracellular markers, iNOS (1:500, eBioscience #12-5920-82) and Arginase-1 (1:500, eBioscience #53-3697-82), overnight at 4°C with gentle agitation.
Macrophages with an M1 phenotype were characterized by CD86+iNOS+ populations and M2 phenotypes by CD86-CD206+ARG1+ populations. The frequency of each type of macrophage was quantified as a function of sulfation. Polarization controls (data not shown) consisted of IC-21s seeded on TC wells in monolayer treated with basal media (M0), 200 ng/mL LPS (M1), and 20 ng/mL IL-4 (M2) for 24 hrs. Cells were lifted with cold 2.5 mM EDTA solution in PBS and cell scraping. Cells were filtered and stained as described.
2.10. Statistical analysis
Data are presented as means ± standard deviation. All experiments represent at least three independent experiments unless otherwise noted. Statistical analysis was performed using a one-way or two-way analysis of variance with Bonferroni correction for multiple comparisons in Prism 9 software (GraphPad, San Diego, CA); p-values less than 0.05 were considered statistically significant. Significance is denoted by alphabetical letterings; groups with no significance are linked by the same letters, while groups with significance do not share the same letters.
3. Results
3.1. Addition of sulfate groups to alginate increases retention of growth factors in hydrogels
Alginate was reacted with chlorosulfonic acid (HSO3Cl), resulting in the substitution of hydroxyl groups with sulfate groups (Fig. 1A). The success of this reaction was evaluated using 1H NMR analysis to verify peak shifts. Protons on the 1 and 5 positions on the G block of alginate (G1, G5) and the proton in the 1 position on the M block (M1) were identified and labeled. We observed increased substitution of sulfate groups on the alginate backbone with increasing concentration of HSO3Cl. All peaks exhibited a shift upfield with increasing sulfate modification, indicative of shielding or increase in electron density (Fig. 1B). This verifies that increasing the concentration of HSO3Cl in the reaction results in alginate possessing higher negative charges due to the addition of sulfate groups. Subsequently, three concentrations were explored (0%, 1%, and 2% HSO3Cl) and identified as non-sulfated, low-sulfated, and high-sulfated alginate, respectively.
Figure 1. Quantification of the amount and functionality of sulfate-modified alginate.
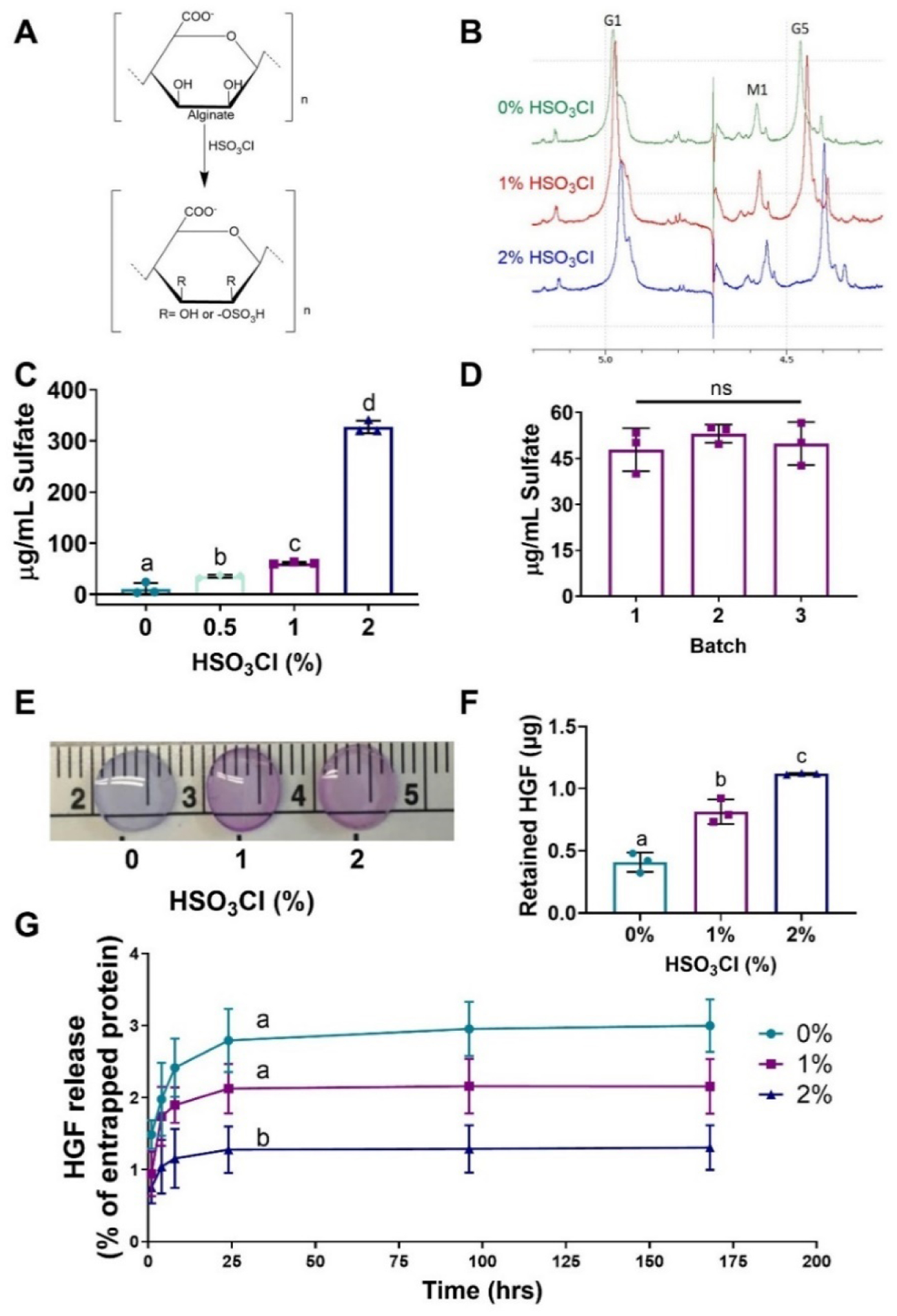
(A) The reaction of alginate with chlorosulfonic acid yields sulfated alginate. (B) Sulfate modification of alginate was verified by 1H NMR. (C, D) Sulfate modification was quantified at different chlorosulfonic acid concentrations and between different batches via DMMB assay. (E) DMMB staining of crosslinked hydrogels exhibited uniform staining throughout the gel. (F) Recombinant HGF was entrapped in sulfated alginate hydrogels and the amount retained after 7 days was quantified. (G) Quantification of HGF release confirms that sulfate groups are functional and retain this heparin binding GF. Data are mean ± SD (n=3–4). Groups with statistically significant differences do not share the same letters; ns denotes no significance among all groups.
Sulfate modification was further confirmed using a DMMB assay, a protocol used to determine the GAG content in physiological samples. In agreement with 1H NMR analysis, increases in the HSO3Cl correlated with greater sulfate modification of the alginate. Differences among the hydrogels were apparent with as low as 0.5% HSO3Cl, indicating the efficiency of this reaction (Fig. 1C). To further validate this sulfate modification method, we evaluated batch-to-batch variation of modification with 1% HSO3Cl using the DMMB assay. Sulfation was similar among three batches, confirming the reproducibility of this modification method (Fig. 1D). Crosslinked hydrogels were stained with DMMB to qualitatively observe the spatial distribution of sulfate in a 3D hydrogel. DMMB exhibits a blue color until it is bound to sulfate and turns pink. Gels exhibited relatively homogenous staining, with sulfate-bound pink staining most apparent in the 2% HSO3Cl gels, 0% HSO3Cl gels exhibiting minimal blue staining, and 1% HSO3Cl exhibiting a combination of both blue and pink staining (Fig. 1E). The sulfate concentration detected on the alginate was also compared to heparin and decellularized ECM via DMMB assay (Supplemental Fig. 1). All sulfated alginate groups exhibited lower sulfate modification than heparin but higher sulfate concentrations than decellularized ECM secreted by human MSCs. Collectively, these data demonstrate the efficiency, reproducibility, and tunability of this method to modify alginate with sulfate groups.
Upon verification of sulfate modification of the alginate, we sought to demonstrate that the sulfate groups were functional and could sequester GFs. We loaded all hydrogels with known quantities of HGF and monitored its release over 7 days. Compared to the non-sulfated control, HGF was retained within sulfated alginate longer, exhibiting slower release curves over 7 days compared to non-sulfated (p<0.0001) and 1% (p=0.046) hydrogels (Fig. 1F,G). We confirmed the functionality of sulfated alginate to retain GFs by studying the encapsulation and release of a non-heparin binding GF, IGF-1. These curves revealed no differences in release rate, indicating sulfation had no effect on the retention of non-heparin binding GFs (Supplemental Fig. 2). These data demonstrate that sulfate-modified alginate can effectively retain heparin binding growth factors, while non-heparin binding growth factors readily diffuse from the gel into the surrounding environment.
3.2. Sulfate modification, degradation and initial moduli can be decoupled
Hydrogels were prepared from a mixture of sulfated VLVG, non-sulfated VLVG, and oxidized non-sulfated MVG alginates (Fig. 2A). 1% oxidized MVG gels were dual-crosslinked using either 6 mM BaCl2 and 200 mM CaCl2 to achieve a high initial modulus or 3 mM BaCl2 and 100 mM CaCl2 for a low initial modulus (Table 1). Since sulfate groups can inhibit the formation of ionic crosslinks, BaCl2 was used to strengthen hydrogel formation, as it is able to crosslink both guluronic (G) and mannuronic (M) acid blocks in alginate, while CaCl2 primarily crosslinks only G blocks.[27] A higher concentration of CaCl2 was supplemented with BaCl2 to further increase crosslinking since higher concentrations of BaCl2 can be cytotoxic.[28] On day 1, we observed significant differences in initial elastic moduli between the high moduli (14.5 ± 3.2 kPa) and low moduli groups (7.5 ± 2.4 kPa; p<0.0001) regardless of the level of sulfation (Fig. 2B). Next, we assessed hydrogel degradation by quantifying changes in moduli at day 14 (Fig. 2C,D) and measuring dry mass over time (Supplemental Fig. 3). Both groups exhibited significant reductions in moduli compared to initial elastic modulus by day 14, with the low moduli group decreasing to 1.8 ± 0.9 kPa and the high moduli decreasing to 3.3 ± 0.9 kPa (p<0.0001 for both groups). We did not detect differences in modulus as a function of sulfate modification at either time point. These data confirm that the degree of sulfate modification on the backbone of alginate can be controlled without sacrificing the ability to tune initial moduli.
Figure 2. Hydrogel formulation and modulation of mechanical and degradation properties.
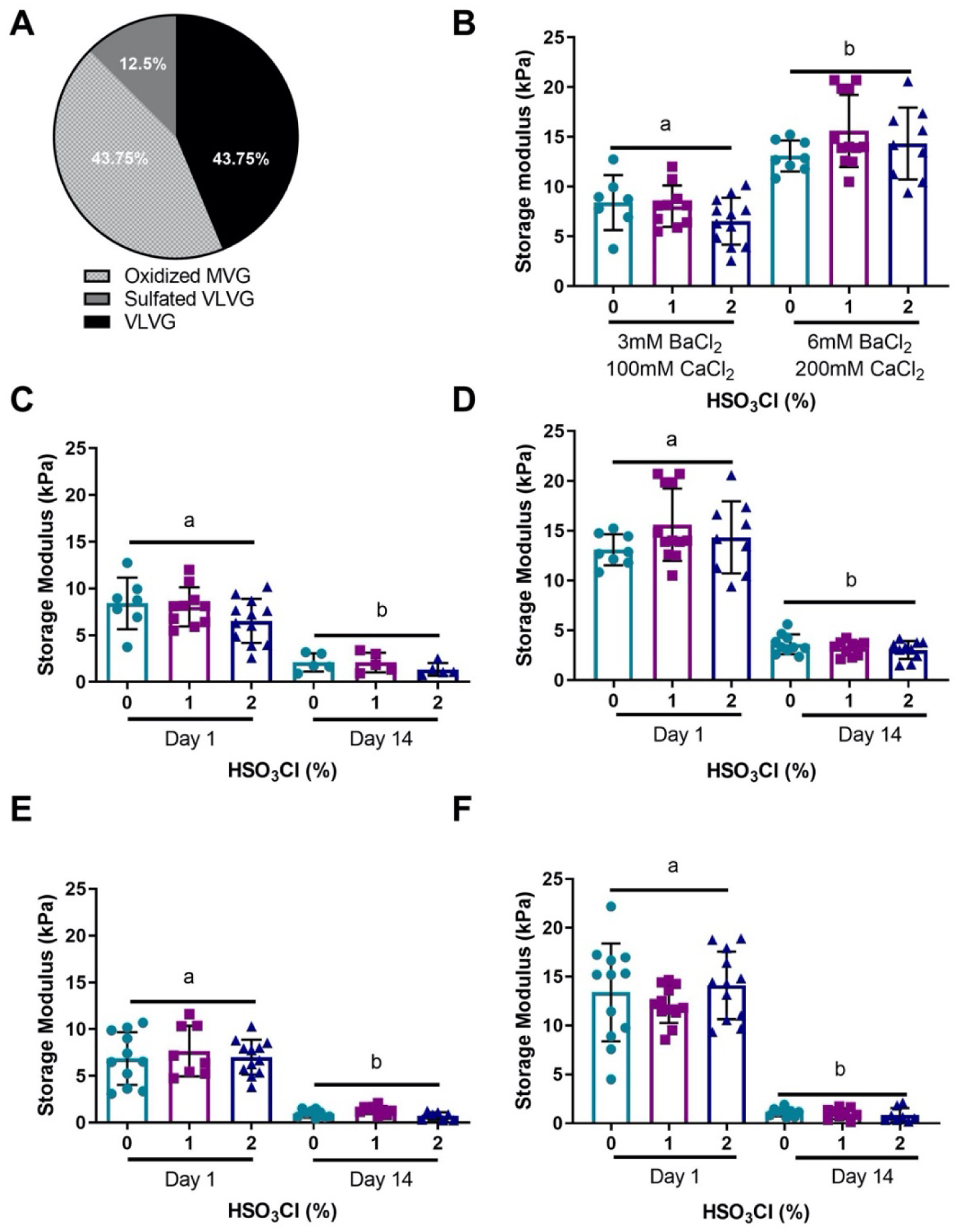
(a) Composition of sulfated alginate hydrogels. (b) Initial moduli of sulfated alginate can be regulated by varying the concentration of ionic crosslinkers. A 3 mM BaCl2 and 100 mM CaCl2 concentration produced a hydrogel with low initial modulus, and 6 mM BaCl2 and 200 mM CaCl2 produced a high initial modulus. The moduli for 1% oxidized (c) low initial moduli, and (d) high initial moduli decreased to similar levels by day 14. Increasing the oxidation percentage of the MVG alginate from 1% to 5% caused the day 14 moduli to decrease further for (e) low and (f) high initial moduli groups without affecting initial moduli. Data are mean ± SD (n=7–12). Groups with statistically significant differences do not share the same letters.
In order to decouple the interplay between sulfate modification and degradation, we incorporated our sulfated alginate into a faster degrading hydrogel composed of 5% oxidized MVG (Table 1). The initial moduli for these hydrogels were 13.2 ± 3.7 kPa for the high moduli group and 7.1 ± 2.4 kPa for the low moduli group, similar to moduli for the slower degrading 1% oxidized gels. As expected, the moduli of the 5% oxidized high group decreased more by 14 days to 1 ± 0.5 kPa (Fig. 2E,F). These changes were further confirmed by measuring the dry mass degradation of the high initial moduli group (Supplemental Fig. 3A,B). The dry mass decreased significantly for all gels from day 1 to 14 in the low moduli group except those modified with 1% HSO3Cl (Supplemental Figure 3B). To verify that degradation did not significantly influence the availability of sulfate groups, we performed a DMMB assay on gels after 14 days to determine if degrees of sulfate modification could be detected. All formulations exhibited clear differences in sulfate modification between 0% and 2% HSO3Cl groups, with all except the high moduli 1% oxidation group exhibiting statistical differences between all groups (Supplemental Fig. 4). We also measured swelling ratio and mesh size of sulfated hydrogels. Sulfation did not appreciably influence these parameters, confirming that hydrogel physical properties are independent of modification with sulfate groups (Supplemental Fig. 5A,B). To investigate how hydrogel properties change over longer periods of time, we measured the moduli and mesh size at day 21. As predicted, the moduli decreased, and mesh size increased after 21 days, however there were no differences between sulfated and non-sulfated hydrogels. These data verify that the degree of sulfate modification, degradation rate, and initial modulus can be independently tuned.
3.3. MSC spheroids entrapped in sulfated alginate are viable and secreted GFs are retained
All subsequent experiments were carried out with 1% oxidized hydrogels crosslinked with 6 mM BaCl2 and 200 mM CaCl2, as this composition exhibited the most durable hydrogels at later time points. To verify that our dual ionic crosslinking method did not adversely affect cell viability, we entrapped human MSC spheroids within our hydrogels and evaluated viability after 8 and 21 days (Fig. 3A). At both time points, we observed high viability of the MSC spheroids and no observable differences between sulfated and non-sulfated groups when stained with LIVE/DEAD stain, indicating that neither the exposed sulfate groups nor ionic crosslinking method were detrimental toward cell viability (Fig. 3B–C). We detected comparable metabolic activity via alamarBlue between groups at day 21 (Fig. 3D).
Figure 3. Spheroid-produced GFs are retained within sulfated alginate hydrogels.
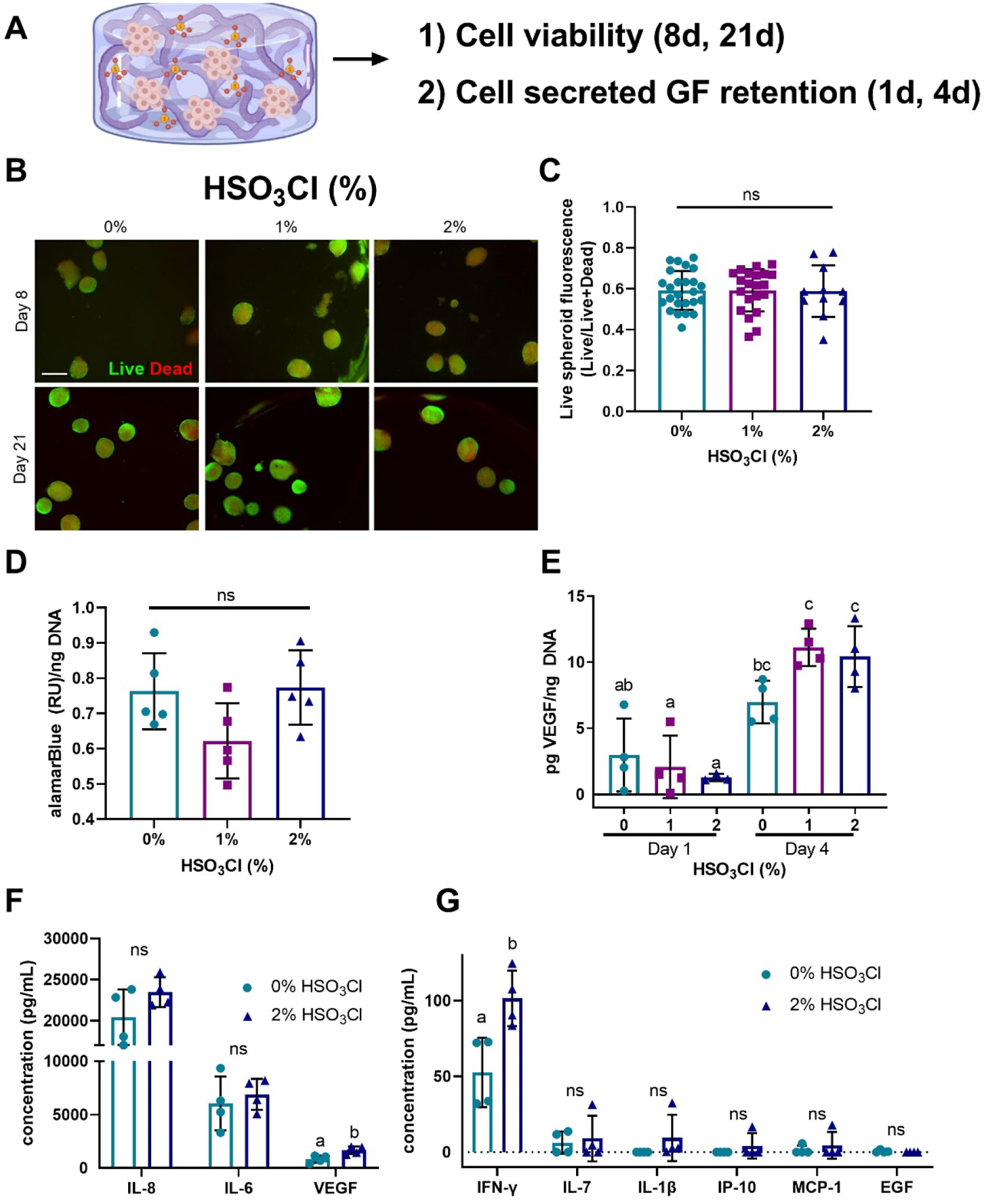
(a) MSC spheroids were entrapped in sulfated alginate hydrogels. (b) No differences were observed in cell viability by LIVE/DEAD stain of MSC spheroids entrapped in sulfated and non-sulfated alginate on day 8 (scale bar = 500μm). Live cells are green, dead cells are red. (c) Ratio of live cell fluorescence to total cell fluorescence revealed no differences. (d) Metabolic activity is comparable for MSC spheroids entrapped in sulfated and non-sulfated alginate on day 8. (e) MSC-secreted VEGF is retained in sulfated alginate hydrogels on days 1 and 4. (f-g) Retained MSC spheroid cytokines in sulfated and non-sulfated hydrogels. Data are mean ± SD (n=4–5). Groups with statistically significant differences do not share the same letters; ns denotes no significance among all groups.
We then sought to determine if sulfated alginate could retain cell-secreted GFs. After 24 hours, the retained MSC-secreted VEGF in all gels was equivalent. However, the amount of VEGF retained in the 1% and 2% gels increased significantly from day 1 to day 4 (p<0.0001 and p=0.0002), whereas the 0% group exhibited no change in VEGF retention (Fig. 3E). These data confirm the functionality of sulfated hydrogels to sequester both recombinant GFs as well as endogenous, cell-secreted VEGF at higher levels than non-sulfated hydrogels. We examined the diversity of GFs and cytokines within sulfated gels to assess the efficiency of MSC secretome retention. A variety of factors were retained at higher levels in sulfated alginate compared to unmodified gels. The greatest cytokine signals were derived from IL-8, IL-6, and VEGF, with sulfated gels exhibiting significantly higher levels of VEGF compared to their non-sulfated counterparts. Cytokines detected at lower concentrations exhibited similar trends, with heparin binding factors such as interferon gamma (IFN-γ) present in significantly increased concentrations in sulfated gels (Fig. 3F–G). However, non-heparin binding GFs such as epidermal growth factor (EGF) exhibited no increase in retention. These data indicate that sulfated alginate hydrogels can retain an array of cytokines secreted by MSC spheroids.
3.4. MSC spheroid-secreted GFs are effectively retained in sulfated hydrogels
We indirectly assessed the bioactivity of sequestered factors within sulfated alginate gels by collecting conditioned media from sulfated alginate hydrogels with entrapped MSC spheroids at 4 and 21 days. We stimulated HDMECs seeded on Matrigel with this media, and tubule formation was quantified after 6 hours (Fig. 4A). We hypothesized that sulfated alginate hydrogels would sequester more GFs, resulting in fewer GFs in the conditioned media and thus, inferior tubule formation. Tubule formation was most visibly affected when stimulated by 21-day conditioned media. Tubule formation was greatest using conditioned media from unmodified alginate, followed by 1% sulfated gels and virtually no tubule formation was observed using media from 2% sulfated hydrogels (Fig. 4B). Day 4 conditioned media elicited no statistical differences in tubule length among conditioned media from the different gels, though HDMECs stimulated by media from 2% sulfated gels trended lower. The day 21 conditioned media showed clearer results, with statistically greater tubule length for the unmodified gels compared to 2% sulfated alginate (p<0.001) (Fig. 4C). Tubule branch number exhibited similar trends to the tubule length data, although no significant differences were observed among alginates (Fig. 4D). To further verify growth factors from the FBS in the media were not contributing to these results, we analyzed a control group exposed to media incubated with acellular hydrogels and found this spurred limited tubule formation (data not shown). These data indicate that GFs are retained for longer durations in more sulfated alginate gels, while non- or lower-sulfated hydrogels elute factors that enhance HDMEC tubule formation. As previously stated, mesh size and moduli were comparable for all groups at day 21, supporting the fact that the observed differences are not due to variations in hydrogel degradation, but rather sequestration of the GFs by sulfate groups.
Figure 4. GFs eluting from sulfated alginate are functional and stimulate tubule formation.
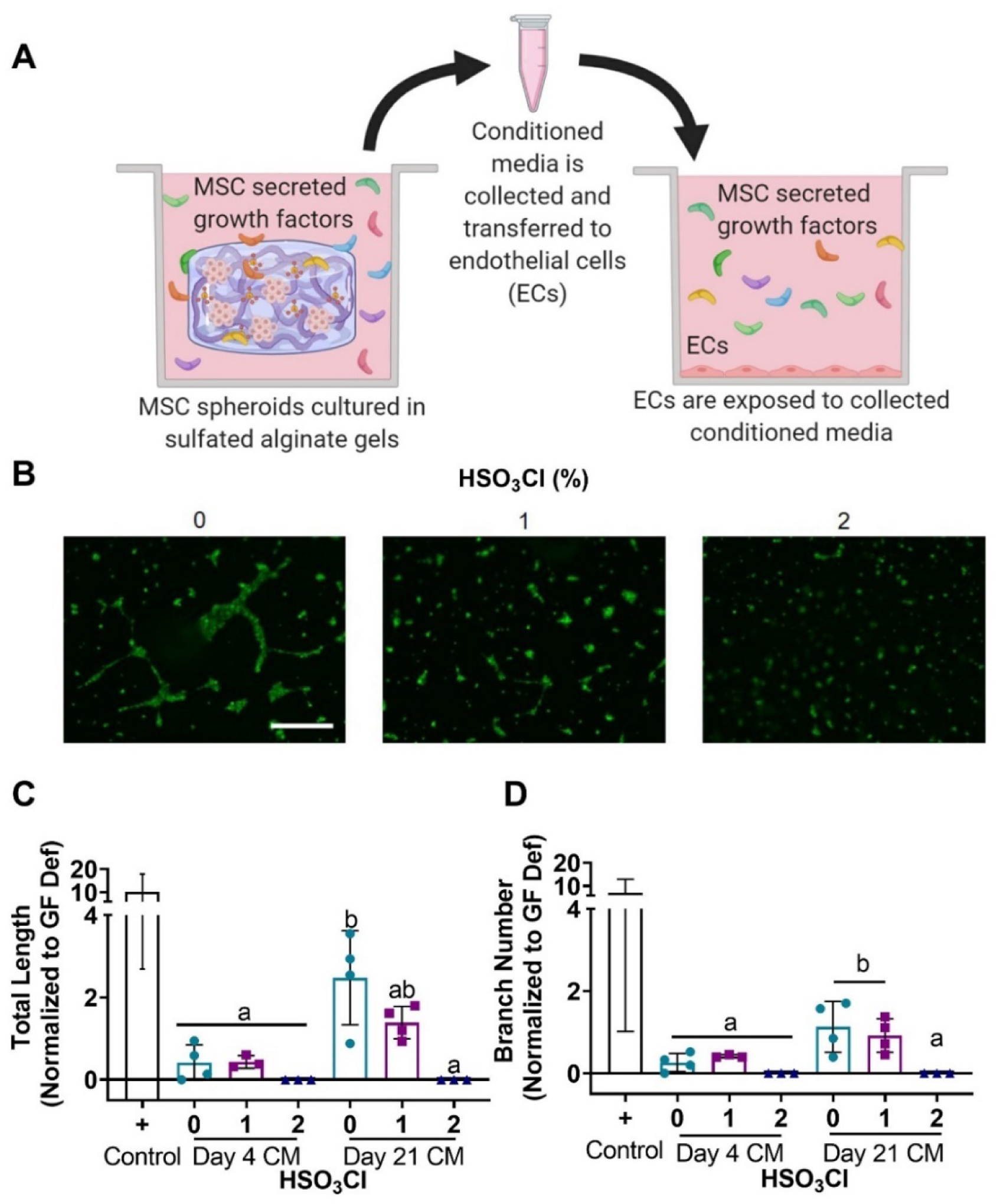
(a) Conditioned media was collected from sulfated hydrogels loaded with MSC spheroids and pipetted on HDMECs to establish the retention of function GFs indirectly by determining tubule formation. (b) Calcein AM stain of HDMECs exposed to day 21 conditioned media (scale bar = 500 μm). Quantification of (c) total tubule length and (d) branch number from HDMEC images confirm that more GFs are eluted from non- and low-sulfated alginate hydrogels. Positive control is tubule formation in complete endothelial cell growth media. All values were normalized to the negative control that was performed in growth factor deficient media. Data are mean ± SD (n=3–4). Groups with statistically significant differences do not share the same letters; ns denotes no significance among all groups.
3.5. MSC spheroids entrapped in sulfated alginate stimulate myoblast infiltration
Having established differences in the bioactivity of GFs eluted from sulfated hydrogels, we investigated the bioactivity of GFs retained within the hydrogel. We cultured MSC spheroids for 4 days to enable accumulation of the MSC spheroid secretome within the sulfated hydrogels. We then assessed the invasion of tdTomato-expressing human myoblasts into the gels over 8 days via confocal microscopy (Fig. 5A). Confocal z-stack images confirmed greater numbers of myoblasts within 2% sulfated hydrogels that also exhibited a more elongated morphology. In contrast, myoblasts in unmodified and 1% hydrogels were sparse, rounded, and often remained at the periphery of the hydrogel (Fig. 5B). Image quantification of myoblast invasion confirmed visual observations, with significantly higher fluorescence, corresponding to higher myoblast infiltration, detected in 2% sulfated hydrogels compared to 0% (p=0.001) and 1% (p=0.001) groups (Fig. 5C). We observed significant increases in the depth of myoblast invasion as a function of sulfate modification (Fig. 5D). Myoblasts that invaded the 2% sulfated alginate gels exhibited significantly lower sphericity compared to lower sulfated and non-sulfated groups (p<0.0001 for both), in agreement with representative images of increased spreading and elongation in 2% gels (Fig. 5E).
Figure 5. MSC spheroids entrapped in sulfate-modified alginate hydrogels enhance myoblast infiltration.
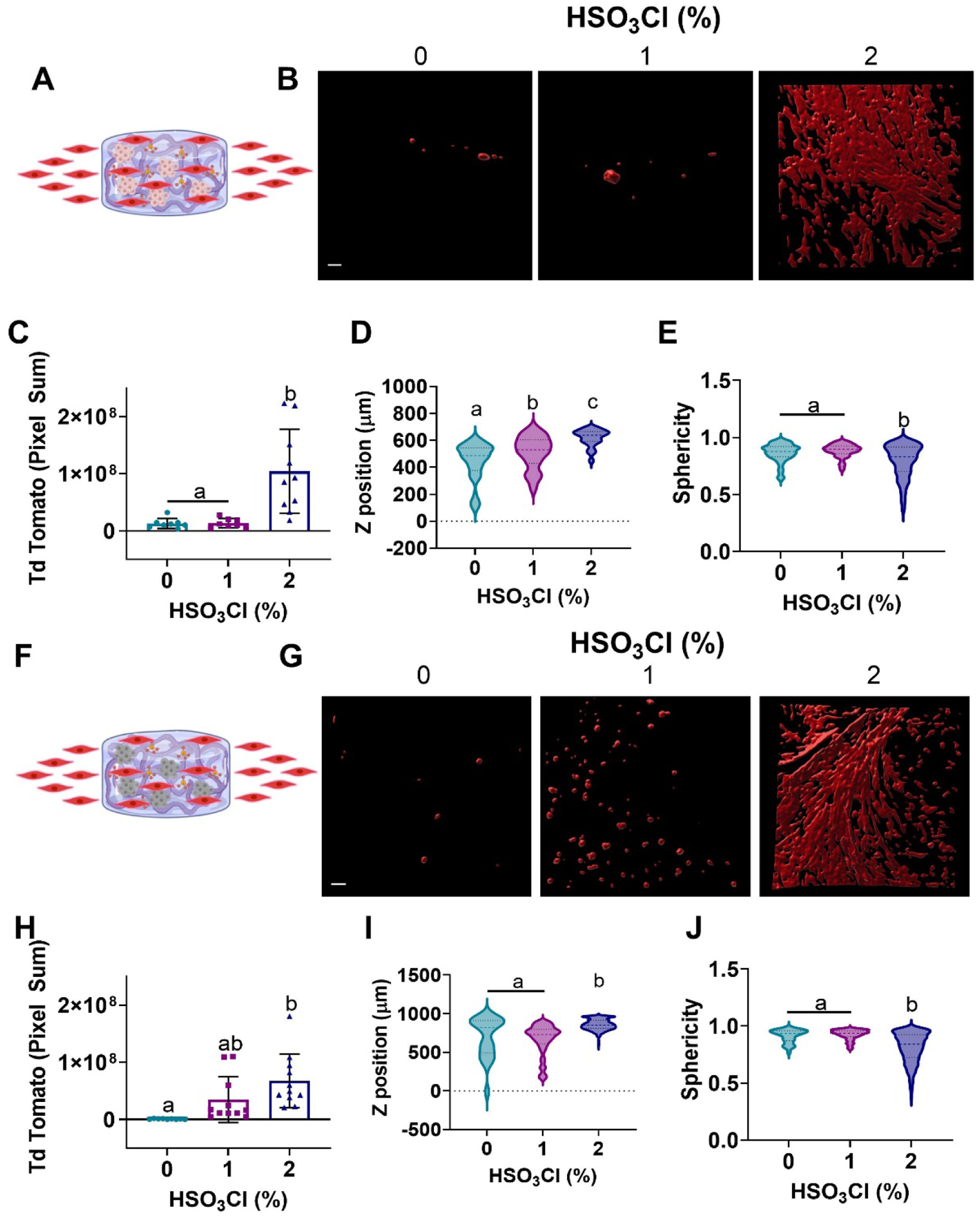
(a) MSC spheroids were entrapped and cultured in alginate hydrogels for 4 days, then tdTomato-expressing myoblasts were introduced to the media and cultured for an additional 8 days. (b) Representative z stack 3D reconstruction images of tdTomato myoblasts within MSC spheroid gels on day 8 (scale bar = 100 μm). (c) Pixel sum of tdTomato fluorescence, (d) depth of penetration and (e) sphericity of the infiltrating myoblasts were quantified from the z stacks, confirming greater myoblast invasion and elongation in 2% sulfated gels. (f-j) The experiment was repeated with spheroids that were killed after 4 days of culture to confirm that GF retention induced myoblast infiltration. Data are mean ± SD (n=9–1145). Groups with statistically significant differences do not share the same letters; ns denotes no significance among all groups.
To further verify that the effect of infiltration was due to GFs sequestered by the sulfated alginate and not the MSC spheroids themselves, we repeated the experiment by killing the MSC spheroids via flash freezing after 4 days in culture. Hydrogels were thawed, and myoblasts were introduced in the same manner as the previous experiment (Fig. 5F). After 8 days, we observed similar infiltration in gels containing dead MSC spheroids, with myoblast infiltration and elongation corresponding to the degree of sulfate modification (Fig. 5G). Quantification of these images indicated increased myoblast infiltration in higher sulfated hydrogels, with trends mirroring those studies using viable MSC spheroids and significant increases in the 2% sulfated hydrogels compared to non-sulfated alginate (p=0.001) (Fig. 5H). Z-position and sphericity were in agreement with data using live cells, evidenced by significant increases in infiltration depth and myoblast elongation within 2% sulfated hydrogels (p<0.0001 for all) (Fig. 5I–J). We performed a control experiment using acellular sulfated hydrogels to investigate if myoblast infiltration was influenced by the sulfate groups themselves in the absence of MSC spheroids (Supplemental Fig. 6). These data indicate that sulfation alone is sufficient to induce myoblast migration, even in the absence of cells. Indeed, penetration depth of the myoblasts increased in acellular hydrogels, suggesting an increase in migration of cells. However, pixel sum for the 2% sulfated hydrogels decreased in acellular conditions. Thus, acellular sulfated hydrogels enable enhanced migration but lower overall engraftment of myoblasts. These data support the hypothesis that sulfated alginate sequesters endogenous, cell-secreted GFs, and these bound GFs retain their bioactivity.
3.6. Macrophage migration and differentiation are not influenced by alginate sulfation
Given the first-response nature of immune cells to sites of injury and the capability of immune cells to secrete pro-inflammatory cytokines, we evaluated the response of macrophages in the presence of sulfated alginate hydrogels containing MSC spheroid-conditioned media. Live macrophages and their polarization were quantified using flow cytometry (Fig. 6A). We observed a nonsignificant trend in the number of macrophages recovered from alginate gels with increasing degrees of sulfation (Fig. 6B). We characterized the polarization of macrophages seeded on sulfated alginate gels and found no statistical differences in the number of M1 or M2 macrophages as a function of sulfation (Fig. 6C,D). Furthermore, the frequency of macrophages with a well-defined M1 phenotype after interaction with our materials is significantly lower than the M1-polarized control group (data not shown for control group). This may indicate either a limited pro-inflammatory response by macrophages to sulfated alginate groups with retained growth factors or may demonstrate the anti-inflammatory action of the growth factors retained by the material. Distinguishing between these mechanisms would require further investigation, but ultimately these data demonstrate that the immunomodulatory signaling provided by retained growth factors is not abrogated by the local pro-inflammatory response of infiltrating macrophages.
Figure 6. MSC spheroid conditioned media entrapped in sulfate-modified alginate hydrogels exhibited no significant increases in macrophage invasion.
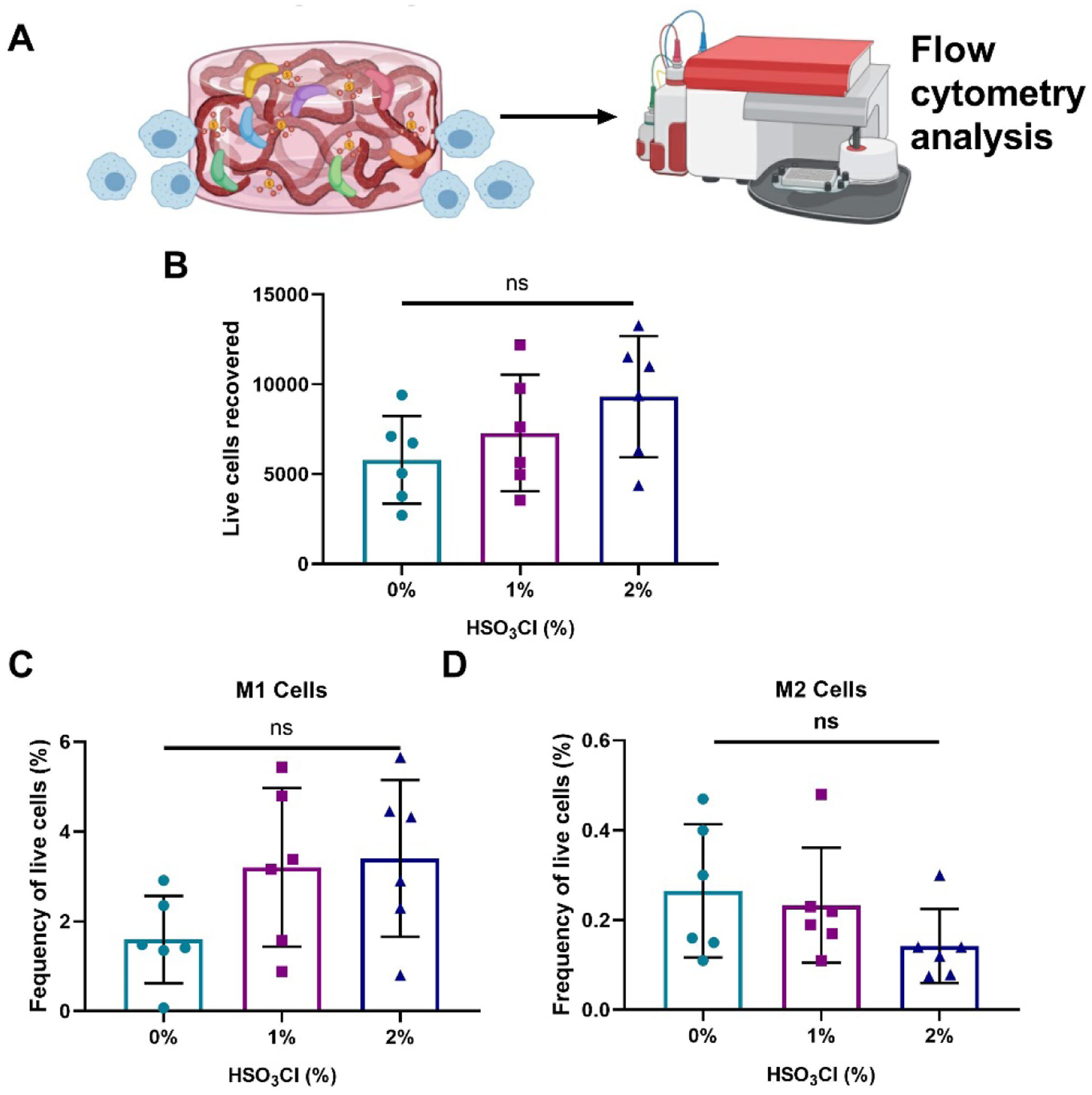
(a) MSC spheroid conditioned media was entrapped in sulfated and non-sulfated hydrogels, and IC-21 macrophages were then seeded on the hydrogels. (b) Total macrophages recovered from the hydrogel. (c) M1 polarized macrophages (CD86+, iNOS+) and (d) M2 polarized macrophages (CD86-, CD206+, ARG1+) were quantified via flow cytometry. Data are mean ± SD (n=6), ns denotes no significance among all groups.
4. Discussion
Poor cell viability upon transplantation remains a critical challenge for the success of cell-based approaches to tissue repair and regeneration. MSCs are widely acknowledged to support tissue repair upon implantation through their potent secretome, yet the utility of MSCs persists only while cells remain viable. We developed a biomaterial platform that supports the viability of entrapped MSC spheroids while sequestering a variety of endogenous secreted GFs for prolonged presentation. To our knowledge, this is the first study to explore utilizing GF sequestering moieties to retain and prolong the therapeutic benefit of the MSC spheroid secretome. We designed a unique crosslinking method that facilitates the tunable regulation of initial elastic moduli, degradation, and degree of sulfate modification within alginate hydrogels. Our results demonstrate that increasing the sulfate modification of alginate improves the retention of MSC spheroid-secreted GFs. Importantly, sulfated hydrogels enhanced myoblast infiltration even when MSC spheroids were no longer viable. These findings highlight how our platform could aid in solving challenges related to rapid cell death upon transplantation in cellular therapies.
The addition of GF sequestering moieties to biomaterials has been reported by several groups.[29, 30] Heparin is a well-characterized anticoagulant that has been thoroughly investigated in the biomedical field as a GF delivery agent. Foundational studies of heparin immobilization were initially motivated by increasing blood compatibility on the surface of biomaterials. These methods leveraged both the localized, sustained release of heparin[31] and direct covalent binding to the material surface[32], though the latter was more advantageous for long-term results. These studies ultimately led to the successful design of adhesion methods for bioactive heparin that have been implemented commercially in polytetrafluoroethylene (PTFE) vascular grafts.[33]
Heparin has been used for GF delivery due to its ability to bind bioactive factors strongly and reversibly. Heparin has been incorporated into various hydrogel polymers, including polyethylene glycol (PEG)[34], fibrin[35], poly(lactic-co-glycolic) acid (PLGA)[36] and alginate[37] for effective delivery of therapeutic GFs for tissue engineering applications. Notably, heparin was co-polymerized with PEG to form hydrogels that delivered basic fibroblast growth factor (bFGF) to MSCs for up to five weeks, resulting in increased cellular proliferation, adhesion, and osteogenic differentiation.[38] Although heparin incorporation is an effective method for GF sequestration, alternative approaches have been evaluated to avoid the complexity, cost, and antithrombotic effects of using the full heparin molecule. For example, the modification of alginate macromer with sulfate groups derived from sulfonic acid treatment capitalizes on the polymer’s high degree of material tunability.[13, 19] Like heparin, sulfated alginate can be used for controlled GF delivery. The formulation has been successfully used to sequester and release BMP-2[39] and FGF-2[40], resulting in increased cell differentiation and response compared to GFs released from unmodified alginate.
GF delivery alone is often insufficient for tissue regeneration due to unintended side effects when using GFs at supraphysiological concentrations, prohibitive cost of these GFs, and poor replication of endogenous signaling that occurs with a multitude of GFs presented to neighboring cells.[41] MSCs produce a complex secretome composed of multiple GFs at lower concentrations than routinely used for therapeutic applications.[42] Although the incorporation of sulfate groups onto polymers such as alginate has been reported, previous studies have focused on delivering a single recombinant GF, while neglecting to capitalize on the complex and physiologically tuned MSC secretome.[19, 39] The innovation of our work is in sequestering a diverse array of factors secreted by MSC spheroids as opposed to pre-loading individual recombinant GFs for delivery.
To investigate this aspect further, we characterized MSC spheroid-secreted factors retained in sulfated and non-sulfated hydrogels. We found increased retention of an array of heparin binding growth factors and cytokines in sulfated hydrogels, verifying that this platform is capable of retaining several therapeutic biomacromolecules. These included high levels of heparin binding cytokines IL-8, IL-6, and VEGF, which are in agreement with other reports describing the composition of the MSC spheroid secretome.[43, 44] We also detected increased retention of INF-γ in sulfated hydrogels, corresponding with previous work indicating that INF-γ has high heparin affinity.[45] This is especially promising considering INF-γ sequestered by heparin and collagen has enhanced ability to increase MSC cytokine secretion and integrin binding.[46] When evaluating the capacity for matricellular proteins to be sequestered, we did not detect the retention of collagen within sulfated alginate gels (data not shown). Identification of potent cytokines within this platform indicates MSC spheroid loaded sulfated alginate has high therapeutic potential, which we further evaluated through a series of in vitro studies.
Upon accumulation of endogenous factors of the MSC secretome, we observed increased adherence, spreading, and invasion of myoblasts into sulfated alginate compared to unmodified hydrogels. It is important to note that the sulfate-modified alginate is likely retaining MSC spheroid-secreted GFs as well as myokines that may further contribute to myoblast infiltration. Indeed, we observed myoblast infiltration from the acellular sulfated hydrogel control groups, indicating myokine and serum GF retention is likely contributing to our results. These results are in agreement with previous observations of the bioactivity of sequestered GF within hydrogels.[38] A possible limitation to this platform is that the sulfated alginate will retain proinflammatory cytokines produced by the injured tissue when implanted in a wound site. To investigate this possibility and any immunological repercussions, we assessed macrophage invasion and polarization on sulfated and non-sulfated hydrogels loaded with MSC spheroid conditioned media. We found no significant increase in adherence of macrophages to sulfated hydrogels, nor did our data indicate that sulfated hydrogels induced a greater M1 or M2 phenotype. The high secretion of chemokines such as IL-8 and IP-10 by MSC spheroids and their subsequent retention by more sulfated materials may be involved in the homing of immune cells to 2% sulfated alginate hydrogels. However, this hypothesis warrants further investigation and is beyond the scope of this work. Overall, these data confirm that sulfated groups do not enhance pro-inflammatory responses from macrophages and therefore are likely not adhering excessive pro-inflammatory cytokines. Future studies are required to evaluate the efficacy of sulfated alginate in vivo and determine if preloading hydrogels with MSC secretome is necessary so endogenous GFs produced by the tissue do not dominate the hydrogel.
When formed into spheroids, MSCs exhibit enhanced viability, differentiation, and increased secretion of immunomodulatory and reparative GFs compared to monodisperse MSCs.[44, 47–49] The MSC secretome can also be influenced by the biophysical properties of the surrounding ECM. Fibrin hydrogels were designed to increase the concentration of VEGF or PGE2 within the MSC spheroid secretome [16], and degree of alginate RGD modification influences MSC spheroid migration, GF secretion, and differentiation.[21] These findings highlight the importance of the mechanical and chemical tunability of sulfated alginate, as these properties can be leveraged to manipulate secretome and cell function for different applications that may be further amplified through GF sequestration.
We used a dual ionic crosslinking technique to independently modulate the physical and chemical properties of sulfated alginate hydrogels. Few studies have directly measured and established this tunability using an ionically crosslinked sulfated alginate hydrogel, likely because the negatively charged sulfate groups can interfere with ionic crosslinking, influencing hydrogel degradation and moduli. By crosslinking with both CaCl2 and BaCl2, we produced a divalent cation combination strong enough to overcome these issues. CaCl2 and BaCl2 have been successfully utilized for bio-printing applications of alginate[50], although not with this reported simultaneous crosslinking technique. One possible limitation of using BaCl2 as a crosslinker is that it can injure myofibers, and injection of the chemical has been well established as a muscle injury model.[51] Our crosslinker concentration is well below that of the concentration used for muscle injury, yet we have not tested our hydrogel in vivo to verify the BaCl2 will not induce adverse effects. Using this method, we demonstrated that sulfate modification can be modulated independently of initial elastic moduli and degradation. Although not explicitly explored in this paper, modification with different adhesion sites and densities could further be pursued as another independent factor, as adhesion peptide degree of substitution does not influence alginate mechanical properties.[21, 52]
These studies establish an exciting platform of study, facilitating the independent regulation of mechanical properties, adhesive moiety type and concentration, and sulfate modification within alginate hydrogels using simple and well-established chemical modifications. This platform can be used to further interrogate the effect of higher sulfate modification levels, as previous studies indicate sulfate concentration on the alginate backbone increases when using up to 3.5% HSO3Cl.[53] Sulfated alginate also exhibits promising translational potential, as alginate is FDA approved for wound healing and as a food additive. The sulfated alginate described herein requires significantly lower concentrations of sulfate than that contained in heparin (Supplemental Fig. 1) while still inducing significant biological differences. This is advantageous, as heparin levels that exceed the effective threshold for VEGF delivery correlated with reductions in network formation by endothelial cells.[54] These data emphasize the importance of striking a balance between sequestration and delivery of GF.
5. Conclusion
We established and characterized a sulfated alginate hydrogel platform with independent tunability of sulfate modification, initial elastic moduli, and degradation rate. We determined that sulfated alginate could bind both recombinant and cell-secreted GFs. Sulfated alginate sequestered a multitude of bioactive GFs from the secretome of entrapped MSC spheroids. These studies indicate that sulfate groups aid in retaining and increasing the therapeutic effect of the MSC spheroid secretome within the hydrogel, potentially prolonging the therapeutic effect of cells transplanted for their secretome.
Supplementary Material
6. Acknowledgements
Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number R01 DE025475 and R01 DE025899 to JKL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in the decision to publish, or preparation of the manuscript. Human hTERT/cdk4 immortalized myoblasts were gifted from AB at the Institute of Myology. MGG was supported by a National Science Foundation Graduate Research Fellowship (2016228876).
Footnotes
7. Disclosure Statement
The authors have nothing to disclose.
Contributor Information
Marissa Gionet-Gonzales, Department of Biomedical Engineering, University of California, Davis, Davis, CA 95616.
Alena Casella, Department of Biomedical Engineering, University of California, Davis, Davis, CA 95616.
Daphne Diloretto, Department of Biomedical Engineering, University of California, Davis, Davis, CA 95616.
Clara Ginnell, Department of Biomedical Engineering, University of California, Davis, Davis, CA 95616.
Katherine H. Griffin, School of Veterinary Medicine, University of California, Davis, CA, 95616, USA Department of Orthopaedic Surgery, UC Davis Health, Sacramento, CA 95817.
Anne Bigot, Universite de Paris, Institut de Myologie, Paris, France 75013.
J. Kent Leach, Department of Biomedical Engineering, University of California, Davis, Davis, CA 95616; Department of Orthopaedic Surgery, UC Davis Health, Sacramento, CA 95817.
8. References
- [1].Wang X, Rivera-Bolanos N, Jiang B, Ameer GA, Advanced functional biomaterials for stem cell delivery in regenerative engineering and medicine, Adv Funct Mater 29(23) (2019). [Google Scholar]
- [2].Caplan AI, Adult mesenchymal stem cells for tissue engineering versus regenerative medicine, J Cell Physiol 213(2) (2007) 341–7. [DOI] [PubMed] [Google Scholar]
- [3].Wechsler ME, Rao VV, Borelli AN, Anseth KS, Engineering the MSC secretome: a hydrogel focused approach, Adv Healthc Mater 10(7) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lam MT, Nauta A, Meyer NP, Wu JC, Longaker MT, Effective delivery of stem cells using an extracellular matrix patch results in increased cell survival and proliferation and reduced scarring in skin wound healing, Tissue Eng Part A 19(5–6) (2013) 738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Murphy KC, Fang SY, Leach JK, Human mesenchymal stem cell spheroids in fibrin hydrogels exhibit improved cell survival and potential for bone healing, Cell Tissue Res 357(1) (2014) 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ho SS, Hung BP, Heyrani N, Lee MA, Leach JK, Hypoxic preconditioning of mesenchymal stem cells with subsequent spheroid formation accelerates repair of segmental bone defects, Stem Cells 36(9) (2018) 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abdeen A, Weiss J, Lee J, Kilian K, Matrix composition and mechanics direct proangiogenic signaling from mesenchymal stem cells, Tissue Eng Part A 20(19–20) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lamichhane TN, Sokic S, Schardt JS, Raiker RS, Lin JW, Jay SM, Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine, Tissue Eng Part B Rev 21(1) (2015) 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gotterbarm T, Richter W, Jung M, Berardi Vilei S, Mainil-Varlet P, Yamashita T, Breusch SJ, An in vivo study of a growth-factor enhanced, cell free, two-layered collagen-tricalcium phosphate in deep osteochondral defects, Biomaterials 27(18) (2006) 3387–95. [DOI] [PubMed] [Google Scholar]
- [10].Silva AK, Richard C, Bessodes M, Scherman D, Merten OW, Growth factor delivery approaches in hydrogels, Biomacromolecules 10(1) (2009) 9–18. [DOI] [PubMed] [Google Scholar]
- [11].Brudno Y, Pezone MJ, Snyder TK, Uzun O, Moody CT, Aizenberg M, Mooney DJ, Replenishable drug depot to combat post-resection cancer recurrence, Biomaterials 178 (2018) 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bratt-Leal AM, Nguyen AH, Hammersmith KA, Singh A, McDevitt TC, A microparticle approach to morphogen delivery within pluripotent stem cell aggregates, Biomaterials 34(30) (2013) 7227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Freeman I, Kedem A, Cohen S, The effect of sulfation of alginate hydrogels on the specific binding and controlled release of heparin-binding proteins, Biomaterials 29(22) (2008) 3260–8. [DOI] [PubMed] [Google Scholar]
- [14].Benoit DS, Durney AR, Anseth KS, The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation, Biomaterials 28(1) (2007) 66–77. [DOI] [PubMed] [Google Scholar]
- [15].Ruvinov E, Leor J, Cohen S, The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction, Biomaterials 32(2) (2011). [DOI] [PubMed] [Google Scholar]
- [16].Murphy KC, Whitehead J, Zhou D, Ho SS, Leach JK, Engineering fibrin hydrogels to promote the wound healing potential of mesenchymal stem cell spheroids, Acta Biomater 64 (2017) 176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Whitehead J, Griffin KH, Gionet-Gonzales M, Vorwald CE, Cinque SE, Leach JK, Hydrogel mechanics are a key driver of bone formation by mesenchymal stromal cell spheroids, Biomaterials 269 (2021) 120607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Leach JK, Whitehead J, Materials-directed differentiation of mesenchymal stem cells for tissue engineering and regeneration, ACS Biomater Sci Eng 4(4) (2017) 1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Arlov Ø, Skjåk-Bræk G, Rokstad AM, Sulfated alginate microspheres associate with factor H and dampen the inflammatory cytokine response, Acta Biomater 42 (2016) 180–188. [DOI] [PubMed] [Google Scholar]
- [20].Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, Mooney DJ, Degradation of partially oxidized alginate and its potential application for tissue engineering, Biotechnol Prog 17(5) (2001) 945–50. [DOI] [PubMed] [Google Scholar]
- [21].Ho SS, Keown AT, Addison B, Leach JK, Cell migration and bone formation from mesenchymal stem cell spheroids in alginate hydrogels are regulated by adhesive ligand density, Biomacromolecules 18(12) (2017) 4331–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zheng C, Levenston ME, Fact versus artifact: avoiding erroneous estimates of sulfated glycosaminoglycan content using the dimethylmethylene blue colorimetric assay for tissue-engineered constructs, Eur Cell Mater 29 (2015) 224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Harvestine JN, Gonzalez-Fernandez T, Sebastian A, Hum NR, Genetos DC, Loots GG, Leach JK, Osteogenic preconditioning in perfusion bioreactors improves vascularization and bone formation by human bone marrow aspirates, Sci Adv 6(7) (2020) 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Thorley M, Duguez S, Mazza EMC, Valsoni S, Bigot A, Mamchaoui K, Harmon B, Voit T, Mouly V, Duddy W, Skeletal muscle characteristics are preserved in hTERT/cdk4 human myogenic cell lines, Skelet Muscle 6(1) (2016) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Whitehead J, Kothambawala A, Leach JK, Morphogen delivery by osteoconductive nanoparticles instructs stromal cell spheroid phenotype, Adv Biosyst 3(12) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vorwald CE, Murphy KC, Leach JK, Restoring vasculogenic potential of endothelial cells from diabetic patients through spheroid formation, Cell Mol Bioeng 11(4) (2018) 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mørch ÝA, Donati I, Strand BL, Skjåk-Bræk G, Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads, Biomacromolecules 7(5) (2006) 1471–1480. [DOI] [PubMed] [Google Scholar]
- [28].Dietz D, Elwell M, Davis W, Meirhenry E, Subchronic toxicity of barium chloride dihydrate administered to rats and mice in the drinking water, Fundam Appl Toxicol 19(4) (1992) 527–37. [DOI] [PubMed] [Google Scholar]
- [29].Impellitteri NA, Toepke MW, Lan Levengood SK, Murphy WL, Specific VEGF sequestering and release using peptide-functionalized hydrogel microspheres, Biomaterials 33(12) (2012) 3475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jha AK, Tharp KM, Ye J, Santiago-Ortiz JL, Jackson WM, Stahl A, Schaffer DV, Yeghiazarians Y, Healy KE, Enhanced survival and engraftment of transplanted stem cells using growth factor sequestering hydrogels, Biomaterials 47 (2015) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sung Ho K, Sung Wan K, Heparin release from hydrophobic polymers: (I) In vitro studies, Arch Pharm Res 9(4) (2020) 193–199. [Google Scholar]
- [32].Park K, Okano T, Nojiri C, Kim S, Heparin immobilization onto segmented polyurethane-urea surfaces--effect of hydrophilic spacers, J Biomed Mater Res 22(11) (1988) 977–92. [DOI] [PubMed] [Google Scholar]
- [33].Begovac P, Thomson R, Fisher J, Hughson A, Gällhagen A, Improvements in GORE-TEX vascular graft performance by Carmeda BioActive surface heparin immobilization, Eur J Vasc Endovasc Surg 25(5) (2003) 432–7. [DOI] [PubMed] [Google Scholar]
- [34].Tae G, Scatena M, Stayton P, Hoffman A, PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor, J Biomater Sci Polym Ed 17(1–2) (2006) 187–97. [DOI] [PubMed] [Google Scholar]
- [35].Jeon O, Ryu S, Chung J, Kim B, Control of basic fibroblast growth factor release from fibrin gel with heparin and concentrations of fibrinogen and thrombin, J Control Release 105(3) (2005) 249–59. [DOI] [PubMed] [Google Scholar]
- [36].Chung HJ, Kim HK, Yoon JJ, Park TG, Heparin immobilized porous PLGA microspheres for angiogenic growth factor delivery, Pharm Res 23(8) (2006) 1835–41. [DOI] [PubMed] [Google Scholar]
- [37].Tanihara M, Suzuki Y, Yamamoto E, Noguchi A, Mizushima Y, Sustained release of basic fibroblast growth factor and angiogenesis in a novel covalently crosslinked gel of heparin and alginate, J Biomed Mater Res 56(2) (2001) 216–221. [DOI] [PubMed] [Google Scholar]
- [38].Benoit DSW, Anseth KS, Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation, Acta Biomater 1(4) (2005) 461–70. [DOI] [PubMed] [Google Scholar]
- [39].Park J, Lee SJ, Lee H, Park SA, Lee JY, Three dimensional cell printing with sulfated alginate for improved bone morphogenetic protein-2 delivery and osteogenesis in bone tissue engineering, Carbohydr Polym 196 (2018) 217–224. [DOI] [PubMed] [Google Scholar]
- [40].Mhanna R, Becher J, Schnabelrauch M, Reis RL, Pashkuleva I, Sulfated alginate as a mimic of sulfated glycosaminoglycans: binding of growth factors and effect on stem cell behavior, Adv Biosyst 1(7) (2017). [DOI] [PubMed] [Google Scholar]
- [41].Ahangar P, Mills SJ, Cowin AJ, Mesenchymal Stem Cell Secretome as an Emerging Cell-Free Alternative for Improving Wound Repair, Int J Mol Sci 21(19) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kehl D, Generali M, Mallone A, Heller M, Uldry AC, Cheng P, Gantenbein B, Hoerstrup SP, Weber B, Proteomic analysis of human mesenchymal stromal cell secretomes: a systematic comparison of the angiogenic potential, NPJ Regen Med 4 (2019) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xie L, Mao M, Zhou L, Zhang L, Jiang B, Signal factors secreted by 2D and spheroid mesenchymal stem cells and by cocultures of mesenchymal stem cells derived microvesicles and retinal photoreceptor neurons, Stem Cells Int 2017 (2017) 2730472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Saiz AM Jr., Gionet-Gonzales MA, Lee MA, Leach JK, Conditioning of myoblast secretome using mesenchymal stem/stromal cell spheroids improves bone repair, Bone 125 (2019) 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lortat-Jacob H, Esterre P, Grimaud JA, Interferon-gamma, an anti-fibrogenic cytokine which binds to heparan sulfate, Pathol Res Pract 190(9–10) (1994) 920–2. [DOI] [PubMed] [Google Scholar]
- [46].Castilla-Casadiego DA, García JR, García AJ, Almodovar J, Heparin/Collagen coatings improve human mesenchymal stromal cell response to interferon gamma, ACS Biomater Sci Eng 5(6) (2019) 2793–2803. [DOI] [PubMed] [Google Scholar]
- [47].Murphy KC, Whitehead J, Falahee PC, Zhou D, Simon SI, Leach JK, Multifactorial experimental design to optimize the anti-inflammatory and proangiogenic potential of mesenchymal stem cell spheroids, Stem Cells 35(6) (2017) 1493–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Guo L, Zhou Y, Wang S, and Wua Y, Epigenetic changes of mesenchymal stem cells in three-dimensional (3D) spheroids, J Cell Mol Med 18(10) (2014) 2009–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ho SS, Murphy KC, Binder BYK, Vissers CB, Leach JK, Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels, Stem Cells Transl Med 5(6) (2016) 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tabriz A, Hermida M, Leslie N, Shu W, Three-dimensional bioprinting of complex cell laden alginate hydrogel structures, Biofabrication 7(4) (2015). [DOI] [PubMed] [Google Scholar]
- [51].Morton AB, Norton CE, Jacobsen NL, Fernando CA, Cornelison DDW, Segal SS, Barium chloride injures myofibers through calcium-induced proteolysis with fragmentation of motor nerves and microvessels, Skelet Muscle 9(1) (2019) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hung BP, Gonzalez-Fernandez T, Lin JB, Campbell T, Lee YB, Panitch A, Alsberg E, Leach JK, Multi-peptide presentation and hydrogel mechanics jointly enhance therapeutic duo-potential of entrapped stromal cells, Biomaterials 245 (2020) 119973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Arlov Ø, Aachmann FL, Sundan A, Espevik T, Skjåk-Bræk G, Heparin-Like properties of sulfated alginates with defined sequences and sulfation degrees, Biomacromolecules 15(7) (2014) 2744–2750. [DOI] [PubMed] [Google Scholar]
- [54].Limasale YDP, Atallah P, Werner C, Freudenberg U, Zimmermann R, Tuning the local availability of VEGF within glycosaminoglycan-based hydrogels to modulate vascular endothelial cell morphogenesis, Adv Funct Mater 30(44) (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


