Abstract
Drug-resistant pathogens form the main threat to global health during the current century. Annually, a lot of patients die in hospitals due to infection with one or more drug-resistant bacteria especially Staphylococcus aureus (MRSA). In the absence of new effective antimicrobial drugs, the number of deaths said to be increased. Searching for new antibiotics in our backyard form a part of scientist strategies to solve such serious health problem. Insects consider one of such interesting sources of the new era of antimicrobial drugs. Cockroaches as an example can live and adapt in a polluted area for a long time, so through this work field cockroach, Blattella vaga was collected from two semi-wild areas around Riyadh, Saudi Arabia for isolation of gut bacteria searching for new antimicrobial agents. Three species of bacteria were identified from field cockroach gut: Bacillus licheniformis, Bacillus subtilis, and Kocuria rosea. The three species were isolated, purified, and tested for their antimicrobial activity against four drug-resistant pathogens (three bacteria: Salmonella enterica (ATCC25566), Staphylococcus aureus (MRSA) (Clinical strain), and Streptococcus mutans (RCMB 017(1) ATCC ® 25175™) and one fungus: Candida albicans (RCMB005003(1) ATCC® 10231™)). The results show no antimicrobial activity of Bacillus subtilis and very good activity Bacillus licheniformis and Kocuria rosea. Bacillus licheniformis gives very effective activity against Candida albicans while Kocuria rosea is effective against MRSA and Streptococcus mutans. None of the gut isolated bacteria show any activity against Salmonella enterica. Such results revealed that the metabolites of these bacteria could be used as substitutes to the already used antibiotics to overcome the problem of multidrug-resistant human pathogens.
Keywords: Drug-resistant pathogens, Cockroaches, Animicrobial activity, Insects, MRSA
1. Introduction
Searching for new antimicrobial drugs forms the main challenge of microbiologists and pharmacologists during this century (Coates et al., 2002, Thomson et al., 2004, Al-Waili et al., 2012). With the presence of a huge number of drug resistance pathogens, the normal sources of such drugs become drained (Mandal et al., 2014, Farha and Brown, 2019, Noori et al., 2012, Noori et al., 2013 May 1). The rapid world spread of multi- and pan-resistant bacteria form a global medical and development threat that facing public health today (Odonkor and Addo, 2011, Ansari et al., 2013 Jul 1, Ansari et al., 2017, Meo et al., 2017). The same case can be applied to pathogenic fungi (Arendrup, 2014, Omar et al., 2014 Jun 1). The pressure of the absence of effective antimicrobial agents for many common diseases motivates scientists to search for alternative sources for new antibiotics (Clardy et al., 2006, Ansari et al., 2016 Jan 2, Ansari et al., 2016 Feb 18, Khan et al., 2018, Khan et al., 2019, Mohammed et al., 2019). Seaweeds, algae, and deep-sea soil form a part of such new sources for future drugs (Montaser and Luesch, 2011, Singh et al., 2015), but recently insects and their microbiomes were found to be rich sources of antibiotics that can be used in human medication (Chevrette et al., 2019).
Insects as the dominant group of animals that live on earth today have very complicated relations with all other living forms (Nasser, 2015, Adly et al., 2019). One of such interesting relations is their rapport with microorganisms including bacteria. The insect gut is considered a very specialized environment for a bacterial colonization that potentially provides many beneficial services to their hosts (Lee et al. 2012). Many insect species depend on gut bacteria for many basic functions such as protecting their insect hosts against pathogens, parasitoids, and other parasites by synthesizing specific peptides or modifying the way by which their immune systems work (Engel and Moran, 2013, Khan et al., 2017). Insect gut bacteria have been documented to exhibit antagonistic activity against several human pathogens (Voirol et al., 2018, Al-Ghamdi et al., 2020, Khan et al., 2020, Anjum et al., 2021), but many insect groups rarely screen for such interesting relation before and one of such group is Cockroaches (Ankrah et al., 2017). Cockroaches are one of the oldest insects that survive on earth millions of years ago, suggesting their ability to adapt and resist environmental threats including pathogenic agents (Kamal et al., 2020).
Cockroaches belong to the order Blattodea of the class Insecta with about 4000 known species worldwide (Cochran, 2009). Several associated bacterial families have been reported from cockroaches, particularly members of the family Enterobacteriaceae, but species of Staphylococcaceae and Mycobacteriaceae have also been found. Streptomyces, Bacillus, Enterococcus, and Pseudomonas were the most reported genera from the cockroach gut (Guzman and Vilcinskas, 2020, Anjum et al., 2018, Al-Ghamdi et al., 2018). Such diversity of gut microbiota may be playing an important role in cockroaches' health and fitness (Cruden and Markovetz, 1984, Tinker and Ottesen, 2016). The field cockroach, Blattella vaga Hebard (Blattodea: Ectobiidae), is one of the species for which we have insufficient knowledge of their gut microbiota (Lee, 2000). It was described in 1935 by Morgan Hebard from specimens collected in Arizona and California (Hebard 1935). This species resembles the other established Blattella spp. in North America: the Asian cockroach, B. asahinai Mizukubo, and the German cockroach, B. germanica (L.) (Atkinson et al. 1991). Adults of these species are similar in length, with a pair of longitudinal stripes on their pronotum. Both B. asahinai and B. vaga live outdoors in wild and semi-wild habitats, fly to lights, and can become peridomestic pests (Helfer, 1987, Atkinson et al., 1991). The clear natural habitat of Blattella vaga encourages the search for new bacterial symbionts that could form a source of antimicrobial agents. So, this work aims to isolate and identify gut bacteria from the wild field cockroach Blattella vaga from Saudi Arabia and to test their antagonistic activity against some drug-resistant microorganisms to handle the problems of antimicrobial resistance among human pathogens.
2. Materials and methods
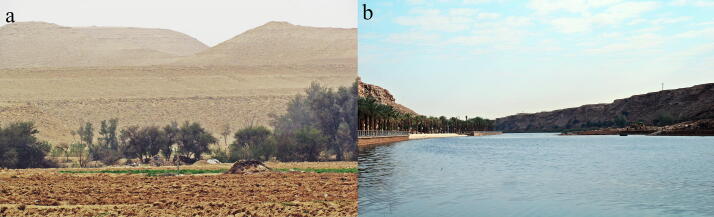
Adult field cockroaches Blattella vaga Hebard, 1935, were collected from two different environmental sites around Riyadh province, Saudi Arabia (Wadi Namar Dam 24.571488°N, 46.679726°E, and Wadi Hanifa 24.777°N, 46.530555°E), during Spring and summer of 2020 (Fig. 1). The adult cockroaches were collected live using hand picking methods by examining humus around trees and shrubs visually. A total of 74 individuals were collected, while only some of them were used to perform the experiments. The identification of insects was performed using the standard taxonomic key (Choate et al., 2008).
Fig. 1.
a. North wadi Hanifa Habitat; b. Wadi Namar Dam Habitat.
Cockroaches were dissected and their guts were removed using a sterile blade and forceps. The gut was completely crushed in a mortar with 1 mL sterile distilled water. Around 200 μL of the sample was inoculated into the nutrient agar medium then incubated at 37 °C for 24 hrs. Colonies with different Morphology were selected and further sub-cultured on an appropriate medium. All the isolated samples were stored at 4 °C for 20–25 min, and the isolated colonies were subjected to perform phenotypic analysis, which includes analysis of color, consistency, surface texture, appearance, and opaqueness. All bacterial isolates were stained with Gram’s dye for the identification of whether Gram-positive or Gram-negative bacteria (Buommino et al., 2021). The isolated bacteria were identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry (Vitek MALDI-TOF MS), the technique that depends on the biochemical activities and protein profile of each isolate (Bates et al., 2016).
Part of a single colony was transferred to an individual spot on the 48-well Vitek Mass spectrometry disposable slides MS-DS. Each spot was covered with 1 µL ready-to-use Vitek MS alpha-cyano-4-hydroxycinnamic acid (HCCA) matrix. The target plate was then read, the spectra were acquired using the MALDI-TOF Vitek MS (bioMérieux) and analyzed on Vitek Mass spectrometry in vitro diagnostic MS-IVD system. The protein profiles of each specimen with an m/z of 3000 to 15,000 were produced, and the profiles were further matched with the Vitek MS reference CE-IVD certified database (>20,000 spectra). Matching results with confidence percentages of 94% to 98% confidence were considered for genus level, results of >98% confidence were considered for species level, but < 94% confidence was unacceptable for identification.
Multidrug-resistant (MDR) bacteria and fungi were used and obtained from the medical city, King Khalid University Hospital, College of Medicine, King Saud University, Riyadh Saudi Arabia. Gram-positive bacteria utilized were methicillin-resistant Staphylococcus aureus (MRSA) (clinical isolate), and Streptococcus mutans Clark (RCMB 017(1) ATCC ® 25175™), and the Gram-negative bacteria utilized was Salmonella enterica (ATCC®. 25566™). The human pathogenic fungi utilized was Candida albicans (RCMB005003(1) ATCC® 10231™).
The purified colonies of isolated gut bacteria were cultured on nutrient broth medium, which was prepared by adding 15 gm of a mixture of beef Extract-Peptone-Sodium Chloride with 1 L of distilled water, which was mixed to dissolve completely and sterilized by the autoclave at 121 °C for 15 min; incubation was then done at 37 °C for 24 h in a shaker incubator before testing their antagonistic activity (Bertino-Grimaldi et al., 2013). The agar well diffusion method was used to test the antagonistic activity of the gut-isolated bacteria against the selected human pathogens (using nutrient agar media for testing bacteria and malt extract agar media for testing fungus) (Bhat & Nalawade, 2016). Then the plates were incubated for 24 h at 37 °C for testing pathogenic bacteria and 28 °C for testing pathogenic fungus. Negative control of using free media was used to ensure the accuracy of results. The zones of inhibition of pathogenic bacteria and fungus were measured by a transparent ruler. Three replicates for each test were done for every evaluated pathogenic species.
3. Results
Three species of gram-positive bacteria were identified using Vitek (MALDI-TOF MS) from the gut of adult field cockroaches Blattella vaga for the first time: Bacillus licheniformis, Bacillus subtilis of Family Bacillaceae, and Kocuria rosea of Family Micrococcaceae. The results show somewhat difference in bacterial symbionts between the two sites of collections as the cockroach collected from Wadi Hanifa has only two species Bacillus licheniformis and Bacillus subtilis while that of Wadi Namar Dam have the three species (Table.1). The culture indicates the domination of Bacillus subtilis on the bacterial gut symbionts of Blattella vaga.
Table 1.
Summarized list of isolated symbiont bacteria of Blattella vaga through the two collecting sites and the frequency of their colony occurrence during culture.
| Environmental sites | Identification of gut bacteria | Frequency of occurrence of colony |
|---|---|---|
| Wadi Hanifa | Bacillus licheniformis | 33%* |
| Bacillus subtilis | 43%** | |
| Wadi Namar Dam | Bacillus licheniformis | 33%* |
| Bacillus subtilis | 43%** | |
| Kocuria rosea | 24% | |
| Total no. of bacterial isolates = 100 | 100% | |
* Bacillus licheniformis, isolated from two different environmental sites so take the same percentage of occurrence
** Bacillus subtilis, isolated from two different environmental sites so take the same percentage of occurrence
The evaluation of the antimicrobial activity of the three bacterial symbionts against the selected drug-resistant pathogens indicated no effect of Bacillus subtilis against any tested microbes. On the other hand, the Bacillus licheniformis show great effect against Candida albicans (Pathogenic fungus) while no antimicrobial activity against pathogenic bacteria. In contrast, Kocuria rosea shows a good effect against two pathogenic bacteria MRSA and Streptococcus mutans, and no effect against pathogenic fungi. No of all tested bacteria give any antimicrobial activity against Salmonella enterica (Table 2) (Figs. 2& 3).
Table 2.
Antimicrobial activity of isolated gut bacteria against tested pathogenic agents.
| Isolated gut bacteria | Pathogenic agents | |||
|---|---|---|---|---|
| Growth Inhibition Zone (mm) | ||||
| Candida albicans | MRSA | Salmonella enterica | Streptococcus mutans | |
| Bacillus licheniformis | 29 ± 0.5 | 0 | 0 | 0 |
| Bacillus subtilis | 0 | 0 | 0 | 0 |
| Kocuria rosea | 0 | 12 ± 0.4 | 0 | 11 ± 0.9 |
Fig. 2.
The inhibitory effect of gut isolated symbiont (Green color) against selective drug-resistant pathogens (Red colors); the inhibition zone indicated with a red spot against clear negative controls.
Fig. 3.
The graph shows the inhibitory effect of gut bacterial symbionts against the selected drug-resistant pathogens the columns represent the diameter of the inhibition zone.
4. Discussion
Our public health systems today are facing several serious problems and drug-resistant pathogenic agents form one of headache and sophisticated issue of such problems that far from completely resolved (Buhner, 2012, Dheda et al., 2017, Hosni et al., 2020). Finding new alternatives to the present antimicrobial drugs become the very important and urgent target of researchers to achieve a sustainable level of infection control through the global health systems (Boucher, H. W., Ambrose, P. G., Chambers, H. F., Ebright, R. H., Jezek, A., Murray, B. E., & Infectious Diseases Society of America, 2017, Coates et al., 2002). Several natural and artificial sources were tested to accomplish such poupous (Rajanbabu and Chen, 2011, Sargiya et al., 2017). Insects and their associated microflora form a very good store to the new era of antimicrobial and antipathogenic medications (Amer et al., 2021, Al-Ghamdi et al., 2021, Masry et al., 2021).
Through the present study, the gut symbiont of wild cockroach Blattella vaga was isolated and identified for the first time to test their antimicrobial activity. There were clear differences in such bacterial symbionts between the studied cockroaches in the two different collection sites. Recently, a study that evaluates the antagonistic activity of gut bacteria of American cockroach Periplaneta americana L. shows similar results, as the habitat of American cockroach individuals affects greatly the associated bacterial species of cockroach gut (Amer et al., 2021). Also, the same results appear during the study of symbionts of other insects like the associated bacteria of house fly (Rady et al., 2007).
Here, Kocuria rosea occurs only on individuals collected from Wadi Namar Dam. The Kocuria rosea naturally occurs on human skin and the surface of some species of spiny plants; it is also known as an opportunistic pathogenic agent that causes urinary tract infections in people with a very weak immune system (Halpern et al., 2011, Kandi et al., 2016). The presence of a huge lake on the Wadi Namar Dam could explain the presence of such bacteria on the gut of Blattella vaga individuals on this site and the absence of its individuals of Wadi Hanifa (Fig. 1). The contamination of Wadi Namar Dam water with sewage could indicate the transfer of this species of bacteria to Blattella vaga individuals that live in the surrounding habitat. On the other hand, Bacillus licheniformis and Bacillus subtilis are common soil bacteria species (Kambourova et al., 2001). Bacillus licheniformis is also a common inhabitor of bird plumage especially on ground-dwelling birds (like sparrows) and aquatic species (like ducks) (Whitaker et al., 2005) while Bacillus subtilis is known from the gut of several animal species including insects such as honey bees (Sudhagar et al., 2017). So, the occurrence of these species on the gut of Blattella vaga through the two collection sites consider a normal thing, especially that these species collected from other cockroach gut before (Amer et al., 2021).
The relative cleanness of the wild habitat of Blattella vaga effects on antimicrobial activity of its bacterial symbionts. As environmental heterogeneity of insect habitats helps in increasing the antagonistic effect of its bacterial symbionts toward pathogens (Iqbal et al., 2014). The data that also supported through the studying of associated bacteria of the American cockroach gut (Amer et al., 2021). The isolated Bacillus subtilis show no antipathogenic activity to all microbes tested, although this species of bacteria was known before the antibiotic era as an immunostimulatory aid for the treatment of gastrointestinal and urinary tract diseases (Ciprandi et al., 1986). Bacillus licheniformis display great antimicrobial activity against Candida albicans the pathogenic fungi which causing Candidiasis. Candidiasis is the disease caused by the Candida yeast that infects the mouth, skin, or vagina (Pappas et al., 2004). Kocuria rosea the third isolated bacteria of gut Blattella vaga from Wadi Namar Dam show good anti-microbial activity against Staphylococcus aureus (MRSA) -the bacteria that cause several skin infections causing abscesses (boils), furuncles, and cellulitis- and Streptococcus mutans – the common mouth bacteria with significant contributor to tooth decay- (Wang and Kuramitsu, 2005, Holland et al., 2014).
Several previous studies have discussed cockroaches as sources for several alternative drugs (Whitten and Ratcliffe, 1999, Basseri et al., 2019, Kim et al., 2020). Also, some works illustrate the antimicrobial activity of some species of gut-associated bacteria of cockroaches against multidrug-resistant pathogenic human microbes (Akbar et al., 2018, Amer et al., 2021), but this is the first time to point out the antipathogenic activity of gut symbionts of Blattella vaga. The result looks very promising especially for developing antifungal agents from Bacillus licheniformis against Candida albicans. For achieving this aim, further chemical analysis of the nature of such antimicrobial activity should be done and more clinical evaluation must occur before the production of the final pharmaceutical product based on the present results. Also, several future studies are needed to screening the antimicrobial activities of gut-associated bacteria of several insect groups.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program. The author also would like to thank Dr. Mohamed Nasser, Department of Entomology, Faculty of Science, Ain Shams University for his help in insect identification.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adly E., Nasser M., Soliman D., Gustafsson D.R., Shehata M. New records of chewing lice (Phthiraptera: Amblycera, Ischnocera) from Egyptian pigeons and doves (Columbiformes), with description of one new species. Acta Trop. 2019;190:22–27. doi: 10.1016/j.actatropica.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Akbar N., Siddiqui R., Iqbal M., Sagathevan K., Khan N.A. Gut bacteria of cockroaches are a potential source of antibacterial compound (s) Lett. Appl. Microbiol. 2018;66(5):416–426. doi: 10.1111/lam.12867. [DOI] [PubMed] [Google Scholar]
- Al-Ghamdi A., Khan K.A., Ansari M.J., Almasaudi S.B., Al-Kahtani S. Effect of gut bacterial isolates from Apis mellifera jemenitica on Paenibacillus larvae infected bee larvae. Saudi journal of biological sciences. 2018;25(2):383–387. doi: 10.1016/j.sjbs.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghamdi A., Al-Abbadi A.A., Khan K.A., Ghramh H.A., Ahmed A.M., Ansari M.J. In vitro antagonistic potential of gut bacteria isolated from indigenous honey bee race of Saudi Arabia against Paenibacillus larvae. J. Apic. Res. 2020;59(5):825–833. [Google Scholar]
- Al-Ghamdi A.A., Al-Ghamdi M.S., Ahmed A.M., Mohamed A.S.A., Shaker G.H., Ansari M.J., Dorrah M.A., Khan K.A., Ayaad T.H. Immune investigation of the honeybee Apis mellifera jemenitica broods: a step toward production of a bee-derived antibiotic against the American foulbrood. Saudi Journal of Biological Sciences. 2021;28(3):1528–1538. doi: 10.1016/j.sjbs.2020.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Waili N, Salom K, Al-Ghamdi A, Ansari MJ. Antibiotic, pesticide, and microbial contaminants of honey: human health hazards. Antibiotic, Pesticide, and Microbial Contaminants of Honey: Human Health Hazards“, The Scientific World Journal, vol. 2012, Article ID 930849, 9 pages, 2012. https://doi.org/10.1100/2012/930849 [DOI] [PMC free article] [PubMed]
- Amer A., Hamdy B., Mahmoud D., Elanany M., Rady M., Alahmadi T., AlAshaal S. Antagonistic Activity of Bacteria Isolated from the Periplaneta americana L. Gut against Some Multidrug-Resistant Human Pathogens. Antibiotics. 2021;10(3):294. doi: 10.3390/antibiotics10030294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankrah N.Y., Luan J., Douglas A.E. Cooperative metabolism in a three-partner insect-bacterial symbiosis revealed by metabolic modeling. J. Bacteriol. 2017;199(15) doi: 10.1128/JB.00872-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum S.I., Shah A.H., Aurongzeb M., Kori J., Azim M.K., Ansari M.J., Bin L. Characterization of gut bacterial flora of Apis mellifera from north-west Pakistan. Saudi journal of biological sciences. 2018;25(2):388–392. doi: 10.1016/j.sjbs.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum S.I., Aldakheel F., Shah A.H., Khan S., Ullah A., Hussain R., Khan H., Ansari M.J., Mahmoud A.H., Mohammed O.B. Honey bee gut an unexpected niche of human pathogen. Journal of King Saud University-Science. 2021;33(1) [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Usmani S., Al-Waili N.S., Sharma D., Nuru A., Al-Attal Y. Effect of jujube honey on Candida albicans growth and biofilm formation. Arch. Med. Res. 2013 Jul 1;44(5):352–360. doi: 10.1016/j.arcmed.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Usmani S., Al-Waili N., Nuru A., Sharma D., Khan K.A., Kaur M., Omer M. In vitro evaluation of the effects of some plant essential oils on Paenibacillus larvae, the causative agent of American foulbrood. Biotechnol. Biotechnol. Equip. 2016 Jan 2;30(1):49–55. [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Al-Waili N., Adgaba N., Khan K.A., Amro A. Antimicrobial Activity of Dracaena cinnabari resin from Soqotra Island on multi drug resistant human pathogens. Afr. J. Tradit. Complement. Altern. Med. 2016 Feb 18;13(1):123–127. [Google Scholar]
- Ansari M.J., Al-Ghamdi A., Usmani S., Khan K.A., Alqarni A.S., Kaur M., Al-Waili N. In vitro evaluation of the effects of some plant essential oils on Ascosphaera apis, the causative agent of Chalkbrood disease. Saudi journal of biological sciences. 2017;24(5):1001–1006. doi: 10.1016/j.sjbs.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendrup M.C. Update on antifungal resistance in A spergillus and C andida. Clin. Microbiol. Infect. 2014;20:42–48. doi: 10.1111/1469-0691.12513. [DOI] [PubMed] [Google Scholar]
- Atkinson T.H., Wadleigh R.W., Koehler P.G., Patterson R.S. Pyrethroid Resistance and synergism in a field strain of the German cockroach (Dictyoptera: Blaitellidae) J. Econ. Entomol. 1991;84(4):1247–1250. doi: 10.1093/jee/84.4.1247. [DOI] [PubMed] [Google Scholar]
- Basseri H., Bakhtiyari R., Hashemi S.J., Baniardelani M., Shahraki H., Hosainpour L. Antibacterial/antifungal activity of extracted chitosan from American cockroach (Dictyoptera: Blattidae) and German cockroach (Blattodea: Blattellidae) J. Med. Entomol. 2019;56(5):1208–1214. doi: 10.1093/jme/tjz082. [DOI] [PubMed] [Google Scholar]
- Bates M.K., Phillips D.S., O’Bryan J. Thermo Fish. Sci; Anshorbgrt: 2016. Shaker agitation rate and orbit affect growth of cultured bacteria; p. 816. [Google Scholar]
- Bertino-Grimaldi D., Medeiros M.N., Vieira R.P., Cardoso A.M., Turque A.S., Silveira C.B., Martins O.B. Bacterial community composition shifts in the gut of Periplaneta americana fed on different lignocellulosic materials. Springerplus. 2013;2:609. doi: 10.1186/2193-1801-2-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat K.G., Nalawade T.M. Antimicrobial activity of endodontic medicaments and vehicles using agar well diffusion method on facultative and obligate anaerobes. Int. J. Clin. Pediatr. Dent. 2016;9:335–341. doi: 10.5005/jp-journals-10005-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher, H. W., Ambrose, P. G., Chambers, H. F., Ebright, R. H., Jezek, A., Murray, B. E., & Infectious Diseases Society of America White paper: developing antimicrobial drugs for resistant pathogens, narrow-spectrum indications, and unmet needs. J. Infect. Dis. 2017;216(2):228–236. doi: 10.1093/infdis/jix211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhner S.H. Storey Publishing; 2012. Herbal antibiotics: natural alternatives for treating drug-resistant bacteria. [Google Scholar]
- Buommino E., Vollaro A., Nocera F., Lembo F., DellaGreca M., De Martino L., Catania M. Synergistic effect of abietic acid with oxacillin against methicillin-resistant Staphylococcus pseudintermedius. Antibiotics. 2021;10:80. doi: 10.3390/antibiotics10010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrette M.G., Carlson C.M., Ortega H.E., Thomas C., Ananiev G.E., Barns K.J., Currie C.R. The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 2019;10(1):1–11. doi: 10.1038/s41467-019-08438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choate P., Burns S., Olsen L., Richman D., Perez O., Patnaude M., McFarland C., McManamy K., Pluke R.A. Dichotomous key for the identification of the cockroach fauna (Insecta: Blattaria) of Florida. Fla. Entomol. 2008;72:612–617. [Google Scholar]
- Ciprandi G., Scordamaglia A., Venuti D., Caria M., Canonica G.W. In vitro effects of Bacillus subtilis on the immune response. Chemioterapia: international journal of the Mediterranean Society of. Chemotherapy. 1986;5(6):404–407. [PubMed] [Google Scholar]
- Clardy J., Fischbach M.A., Walsh C.T. New antibiotics from bacterial natural products. Nat. Biotechnol. 2006;24(12):1541–1550. doi: 10.1038/nbt1266. [DOI] [PubMed] [Google Scholar]
- Coates A., Hu Y., Bax R., Page C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discovery. 2002;1(11):895–910. doi: 10.1038/nrd940. [DOI] [PubMed] [Google Scholar]
- Cochran D.G. Academic Press; 2009. Blattodea:(cockroaches). In Encyclopedia of insects; pp. 108–112. [Google Scholar]
- Cruden D.L., Markovetz A.J. Microbial aspects of the cockroach hindgut. Arch. Microbiol. 1984;138:131–139. doi: 10.1007/BF00413013. [DOI] [PubMed] [Google Scholar]
- Dheda K., Gumbo T., Maartens G., Dooley K.E., McNerney R., Murray M., Warren R.M. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. The lancet Respiratory medicine. 2017;5(4):291–360. doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- Engel P., Moran N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013;37:699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- Farha M.A., Brown E.D. Drug repurposing for antimicrobial discovery. Nat. Microbiol. 2019;4(4):565–577. doi: 10.1038/s41564-019-0357-1. [DOI] [PubMed] [Google Scholar]
- Guzman J., Vilcinskas A. Bacteria associated with cockroaches: Health risk or biotechnological opportunity? Appl. Microbiol. Biotechnol. 2020;104:10369–10387. doi: 10.1007/s00253-020-10973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M., Waissler A., Dror A., Lev-Yadun S. Biological warfare of the spiny plant: introducing pathogenic microorganisms into herbivore's tissues. Adv. Appl. Microbiol. 2011;74:97–116. doi: 10.1016/B978-0-12-387022-3.00008-2. [DOI] [PubMed] [Google Scholar]
- Hebard, M. (1935). Studies in the Orthoptera of Arizona. Part I. New genera, species and geographic races. Transactions of the American Entomological Society (1890-),61(2), 111-153.
- Helfer J.R. Dover Publications, Mineola, New York, USA; Cockroaches and their Allies: 1987. How to Know the Grasshoppers, Crickets. [Google Scholar]
- Holland T.L., Arnold C., Fowler V.G. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA. 2014;312(13):1330–1341. doi: 10.1001/jama.2014.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosni E.M., Nasser M.G., Al-Ashaal S.A. Modeling current and future global distribution of Chrysomya bezziana under changing climate. Sci Rep. 2020;10:4947. doi: 10.1038/s41598-020-61962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J., Siddiqui R., Khan N.A. Acanthamoeba and bacteria produce antimicrobials to target their counterpart. Parasites Vectors. 2014;7:56. doi: 10.1186/1756-3305-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambourova M., Tangney M., Priest F.G. Regulation of polyglutamic acid synthesis by glutamate in Bacillus licheniformis and Bacillus subtilis. Appl. Environ. Microbiol. 2001;67(2):1004–1007. doi: 10.1128/AEM.67.2.1004-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal M., Adly E., Alharbi S.A., Khaled A.S., Rady M.H., Ibrahim N.A. Exploring simplified methods for insect chitin extraction and application as a potential alternative bioethanol resource. Insects. 2020;11(11):788. doi: 10.3390/insects11110788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandi V., Palange P., Vaish R., Bhatti A.B., Kale V., Kandi M.R., Bhoomagiri M.R. Emerging bacterial infection: identification and clinical significance of Kocuria species. Cureus. 2016;8(8) doi: 10.7759/cureus.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K.A., Ansari M.J., Al-Ghamdi A., Nuru A., Harakeh S., Iqbal J. Investigation of gut microbial communities associated with indigenous honey bee (Apis mellifera jemenitica) from two different eco-regions of Saudi Arabia. Saudi journal of biological sciences. 2017;24(5):1061–1068. doi: 10.1016/j.sjbs.2017.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.U., Anjum S.I., Rahman K., Ansari M.J., Khan W.U., Kamal S., Khattak B., Muhammad A., Khan H.U. Honey: Single food stuff comprises many drugs. Saudi journal of biological sciences. 2018;25(2):320–325. doi: 10.1016/j.sjbs.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.U., Anjum S.I., Ansari M.J., Khan M.H.U., Kamal S., Rahman K., Shoaib M., Man S., Khan A.J., Khan S.U., Khan D. Antimicrobial potentials of medicinal plant’s extract and their derived silver nanoparticles: A focus on honey bee pathogen. Saudi journal of biological sciences. 2019;26(7):1815–1834. doi: 10.1016/j.sjbs.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K.A., Al-Ghamdi A.A., Ghramh H.A., Ansari M.J., Ali H., Alamri S.A., Al-Kahtani S.N., Adgaba N., Qasim M., Hafeez M. Structural diversity and functional variability of gut microbial communities associated with honey bees. Microb. Pathog. 2020;138 doi: 10.1016/j.micpath.2019.103793. [DOI] [PubMed] [Google Scholar]
- Kim, I. W., Lee, J. H., Seo, M., Lee, H. J., Baek, M., Kim, M. A., ... & Hwang, J. S. (2020). Anti-inflammatory activity of antimicrobial peptide periplanetasin-5 derived from the cockroach Periplaneta americana. [DOI] [PMC free article] [PubMed]
- Lee C.Y. Research Note Diversity of cockroach species and effect of sanitation on level of cockroach infestation in residential premises. Trop. Biomed. 2000;17:39–43. [Google Scholar]
- Lee S., Siddiqui R., Khan N.A. Animals living in polluted environments are potential source of antimicrobials against infec-tious agents. Pathog. Glob. Health. 2012;106:218–223. doi: 10.1179/2047773212Y.0000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S.M., Roy A., Ghosh A.K., Hazra T.K., Basak A., Franco O.L. Challenges and future prospects of antibiotic therapy: from peptides to phages utilization. Front. Pharmacol. 2014;5:105. doi: 10.3389/fphar.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masry S.H.D., Taha T.H., Botros W.A., Mahfouz H., Al-Kahtani S.N., Ansari M.J., Hafez E.E. Antimicrobial activity of camphor tree silver nano-particles against foulbrood diseases and finding out new strain of Serratia marcescens via DGGE-PCR, as a secondary infection on honeybee larvae. Saudi Journal of Biological Sciences. 2021;28(4):2067–2075. doi: 10.1016/j.sjbs.2021.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meo S.A., Al-Asiri S.A., Mahesar A.L., Ansari M.J. Role of honey in modern medicine. Saudi journal of biological sciences. 2017;24(5):975–978. doi: 10.1016/j.sjbs.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed S.E.A., Kabbashi A.S., Koko W.S., Ansari M.J., Adgaba N., Al-Ghamdi A. In vitro activity of some natural honeys against Entamoeba histolytica and Giardia lamblia trophozoites. Saudi journal of biological sciences. 2019;26(2):238–243. doi: 10.1016/j.sjbs.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaser R., Luesch H. Marine natural products: a new wave of drugs? Future Med. Chem. 2011;3(12):1475–1489. doi: 10.4155/fmc.11.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser M.G. Chewing lice: tiny insects in raging seas. Journal of Marine Science Research and Development. 2015;5 [Google Scholar]
- Noori A.L., Al-Ghamdi A., Ansari M.J., Al-Attal Y., Salom K. Synergistic effects of honey and propolis toward drug multi-resistant Staphylococcus aureus, Escherichia coli and Candida albicans isolates in single and polymicrobial cultures. International journal of medical sciences. 2012;9(9):793. doi: 10.7150/ijms.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori A.L., Al Ghamdi A., Ansari M.J., Al-Attal Y., Al-Mubarak A., Salom K. Differences in composition of honey samples and their impact on the antimicrobial activities against drug multiresistant bacteria and pathogenic fungi. Arch. Med. Res. 2013 May 1;44(4):307–316. doi: 10.1016/j.arcmed.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Odonkor S.T., Addo K.K. Bacteria resistance to antibiotics: Recent trends and challenges. Int. J. Biol. Med. Res. 2011;2:1204–1210. [Google Scholar]
- Omar M.O., Moustafa A.M., Ansari M.J., Anwar A.M., Fahmy B.F., Al-Ghamdi A., Nuru A. Antagonistic effect of gut bacteria in the hybrid Carniolan honey bee, Apis Mellifera Carnica, against Ascosphaera apis, the causal organism of chalkbrood disease. Journal of Apicultural Science. 2014 Jun 1;58(1):17–27. [Google Scholar]
- Pappas P.G., Rex J.H., Sobel J.D., Filler S.G., Dismukes W.E., Walsh T.J., Edwards J.E. Guidelines for treatment of candidiasis. Clin. Infect. Dis. 2004;38(2):161–189. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- Rajanbabu V., Chen J.Y. Applications of antimicrobial peptides from fish and perspectives for the future. Peptides. 2011;32(2):415–420. doi: 10.1016/j.peptides.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Rady M., Fallata S., El deen, N, Environmental impact assessment of solid wastes on the housefly distribution and fly - borne diseases. Egy Soc Bioch and Mol biol. 2007;2(1):21–28. [Google Scholar]
- Sargiya H., Goyal P.K., Vyas B. Antimicrobial Drugs of Herbal Origin. International Journal of Pharmaceutical Erudition. 2017;7(1):1–10. [Google Scholar]
- Singh R.P., Kumari P., Reddy C.R.K. Antimicrobial compounds from seaweeds-associated bacteria and fungi. Appl. Microbiol. Biotechnol. 2015;99(4):1571–1586. doi: 10.1007/s00253-014-6334-y. [DOI] [PubMed] [Google Scholar]
- Sudhagar S., Reddy P.R., Nagalakshmi G. Influence of elevation in structuring the gut bacterial communities of Apis cerana Fab. Journal of Entomology and Zoology Studies. 2017;5(3):434–440. [Google Scholar]
- Tinker K.A., Ottesen E.A. The core gut microbiome of the american cockroach, Periplaneta americana, is stable and resilient to dietary shifts. Appl. Environ. Microbiol. 2016;82:6603–6610. doi: 10.1128/AEM.01837-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson C.J., Power E., Ruebsamen-Waigmann H., Labischinski H. Antibacterial research and development in the 21st Century–an industry perspective of the challenges. Curr. Opin. Microbiol. 2004;7(5):445–450. doi: 10.1016/j.mib.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Voirol L.R.P., Frago E., Kaltenpoth M., Hilker M., Fatouros N.E. Bacterial symbionts in lepidoptera: Their Diversity, transmission, and impact on the host. Front. Microbiol. 2018;9:556. doi: 10.3389/fmicb.2018.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B.Y., Kuramitsu H.K. Interactions between oral bacteria: inhibition of Streptococcus mutans bacteriocin production by Streptococcus gordonii. Appl. Environ. Microbiol. 2005;71(1):354–362. doi: 10.1128/AEM.71.1.354-362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J.M., Cristol D.A., Forsyth M.H. Prevalence and genetic diversity of Bacillus licheniformis in avian plumage. J. Field Ornithol. 2005;76(3):264–270. [Google Scholar]
- Whitten M.M., Ratcliffe N.A. In vitro superoxide activity in the haemolymph of the West Indian leaf cockroach, Blaberus discoidalis. J. Insect Physiol. 1999;45(7):667–675. doi: 10.1016/s0022-1910(99)00039-6. [DOI] [PubMed] [Google Scholar]