Abstract
Hyperglycemia as a common metabolic disorder in diabetes led to oxidative stress, inflammation and other complications. Natural and manufactured antioxidants alleviates the side effects of diabetes. The purpose of current study is to investigate the effect of pyrroloquinoline quinine (PQQ) as an antioxidant on the content of glucose-induced oxidative stress generation in the cells of the human hepatocellular liver carcinoma (HepG2) by inhibiting advanced glycation end products (AGEs) formation. The HepG2 cells were exposed to high dose (50 mM) of glucose (HG) only and with PQQ (HG + PQQ). Treatment with high dose increased AGEs formation, expression of receptor for advanced glycation endproducts (RAGE), reactive oxygen species ROS production, and oxidative stress markers in treated HepG2 cells. Interestingly, PQQ significantly reduced AGEs formation and (RAGE) expression, ROS formation, and inflammation induced by glucose. In conclusion, PQQ has a potentiail role as an antioxidant to reduce the oxidative damage during hyperglycemia by AGEs inhibition.
Keywords: Pyrroloquinoline quinone, Glucose, Oxidative stress, Cancer
1. Introduction
Diabetes is a chronic disease that impacts several individuals around the globe (Gupta et al., 2019, Singh et al., 2020, Syed et al., 2020, Syed et al., 2021). Throughout this disorder, insulin resistance, secretion, or both severely impair glucose metabolism leading to hyperglycemia (Samadder et al., 2011). Hyperglycemia result in oxidative stress, inflammations and other disorders in body organs (Kapoor and Kakkar, 2012). Hyperglycemia also leds to generation of free radicals that exert an essential role in the complexities of diabetes and cause cellular dysfunction. A natural body system defines oxidative stress and keep the balance between production and removing free radical Oxidative stress includes aging and a variety of illnesses such as cardiovascular conditions, cancer, and complications of diabetes (Atalay and Laaksonen, 2002). Abnormal cellular metabolism in a person with diabetes has been documented to generate free oxygen radicals and develop antioxidant capacity (Antunes and Cadenas, 2000, Nishikawa et al., 2000).
Advanced glycation end products (AGEs), non-enzymatic binding products of free sugar reduction, and reactive carbonyls to proteins are produced within the body during homeostasis (Njoroge et al., 1987, Reddy et al., 1995, Syed et al., 2020). AGEs formation levels have been related to redox balances. Excessive accumulation of AGEs occurs in pathological conditions such as hyperglycemia (Hu et al., 2020, Syed et al., 2020, Yamagishi and Matsui, 2011). AGEs' role in cancer initiation and progression is attracting ever more attention. AGE treatment of various cancer cell lines promotes cell proliferation, migration, and invasion (Jiao et al., 2011, Sparvero et al., 2009, van Heijst et al., 2005). Oxidative stress is a discrepancy between the reactive oxygen species (ROS) and the antioxidant protection system. ROS acts as signal molecules at some rates to promote cell proliferation, apoptosis, and gene expression (Chan, 2001, Finkel, 1998). High level of ROS result oxidative damage, which inhance the progression of diabetes and cancer diseases (Moloney and Cotter, 2018, Newsholme et al., 2016, Syed et al., 2016a, Syed et al., 2016b). High levels of AGE lead to increased ROS generation (Rochette et al., 2014, Volpe et al., 2018). The liver is the main organ of metabolism and management of glucose. In addition, hyperglycemia causes liver dysfunction thus, reducing the production of (ROS) and inhance the normal function of liver prevent liver disorders. According to previous reports, oxidant stress is the biochemical trigger for hepatic dysfunction in diabetic rats, (Cusi, 2009). Morever, mitochondria is one of the cell organels that response to high level of ROS and high glucose-induced oxidative stress (Sun et al., 2012, Xu et al., 2012). However, controlling hyperglycemia by insulin or other kind of medications causes various complications, like fatty liver (Kandhare et al., 2012, Zhang and Liu, 2011). The side effects of hyperglycemia can be reduced by using antioxidants (Kapoor and Kakkar, 2012, Vincent et al., 2004).
Pyrroloquinoline quinone (PQQ) beneficial effects in reducing hyperglycemia have not been investigated as causing oxidative stress in an in vitro. In vivo, PQQ has been demonstrated to shield living cells from oxidative damage, and in vitro, it was shown to protect biomolecules from artificially induced reactive oxygen species (Misra et al., 2012). A study reported that PQQ treatment with PQQ reduced the ROS, oxidative stress levels, ameliorated mtDNA damage, and increased the mitochondrial membrane potential (MMP) (Masudul et al., 2021). It serves as a nutrient and vitamin to enable living cells development and defend themselves while they are under stress (Misra et al., 2012). The protective activity of PQQ due to its antioxidant effect (Decker, 1995, Misra et al., 2012, Nunome et al., 2008, Tao et al., 2007).
Therefore, this research work investigated the preventive effects of (PQQ) as an antioxidant on the content of glucose-induced (ROS) production in the (HepG2) cell line by inhibiting AGEs formation.
2. Material and methods
2.1. Cell culture
The used (PQQ) was obtained from Sigma Aldrich Chemical Co., St., USA. The hepatocytes, HepG2 cells were purchased from the American Type Culture Collection (Manassas, VA, USA) and were upheld in DMEM (Sigma), having 10% FBS (Gibco), kept at 37 °C and 5% CO2. It was incubated for 24 h, and when there was semi-confluent cells, it was exposed to PQQ (100 nM) (Yamada et al., 2020) and to the 50 mMD-Glucose (HG). Subsequently, the cells proceeded for various assays after 24 h incubation.
2.2. Estimation of advanced glycation end products AGE
The AGEs level was estimated in HepG2 cells using the ELISA kit. In brief, the HepG2 cells were cultured in 6-wells culture plates and treated with HG (50 mM) with and without PQQ (100 nM).
2.3. Estimation of reactive oxygen species (ROS)
The (ROS) was estimated in cells by using DCFDA, and the image was captured under fluorescent microscope and by fluorometry (Syed et al., 2016a, Syed et al., 2016b). The formation of a fluorescent product was measured at an excitation wavelength of 488 nm and an emission wavelength of 530 nm using fluorescent microscope EVOS FL auto (Life technology, USA).
2.4. Estimation of oxidative stress
Stress markers like malonaldehyde (MDA), and reduced glutathione (GSH), were estimated as per the described protocol (Dai et al., 2019, Prabhakar et al., 2012). SOD was measured by using a SOD kit (item no. 706002, Cayman chemical) according to manufactory protocol.
2.5. Estimation of tumor necrosis factor-α (TNF-α) and cytokines
The TNF-α, interleukin (IL-1β) and interleukin 6 (IL-6) were determined using ELISA kit according to the manufacturer’s instructions.
2.6. Real-time PCR
The RNA from cells were isolated using the TRIzol (Sigma Aldrich) method as described previously (Syed et al., 2016a, 2016b). The primers were obtained from Eurofins Scientific, Luxembourg.
2.7. Data analysis
The obtained results in the present research was written as Mean ± SEM. The data were analyzed by one-way ANOVA followed by a Tukey's tests using GraphPad Prism 8.0 software. p < 0.05 value was set as statistically significant.
3. Result
3.1. The levels of AGEs and RAGE
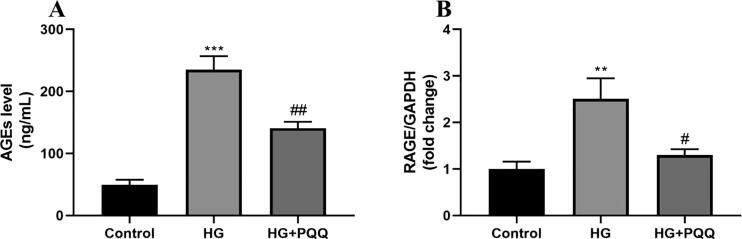
The level of AGEs was estimated in cell lysate by ELISA (Fig. 1A). The high glucose group (HG) exposed cells demonstrated significantly enhanced levels in the HG group as compared to the control group. At the same time, the PQQ treated group showed significantly decreased level as compared to the HG group. The mRNA (Fig. 1B) expression of RAGE measured by qPCR which further supported the above AGEs level.
Fig. 1.
Effect of PQQ on (A) AGEs level and (B) mRNA RAGE expression. Data are shown as Mean ± S.E.M, *Control vs HG; #HG vs HG + PQQ. **P < 0.01, ***P < 0.001; #P < 0.05, ##P < 0.01.
3.2. The effects of PQQ on ROS and oxidative stress markers
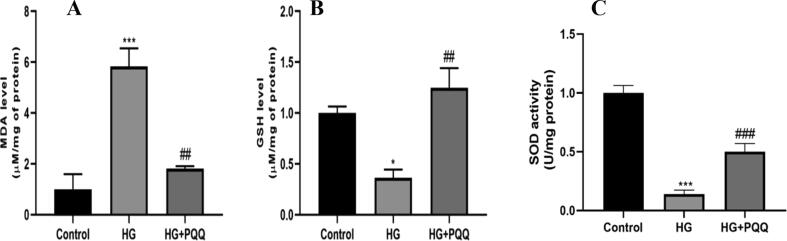
The ROS activity was substantially increased in the HG exposed cells. On treatment with PQQ, the ROS level was substantially decreased as compared to the diseased (HG exposed) group (Fig. 2A, B). The level of oxidative stress markers like MDA and GSH were measured to be abnormal in the HG group as compared to the control group. Additionally, the cells treated with PQQ had shown significant protection against oxidative stress by showing the level comparable to the control group (Fig. 3A, B). The level of SOD activity was significantly reduced in HG exposed cells as compared to the control group. On treatment with PQQ, it showed a significant increse as compared to the HG group. (Fig. 3C).
Fig. 2.
Effect of PQQ on ROS production was determined using DCFDA by (A) microscopic imaging (magnification 40x), and (B) fluorometry. Data are shown as Mean ± S.E.M, *Control vs HG; #HG vs HG + PQQ. **P < 0.01; ##P < 0.01.
Fig. 3.
Effect of PQQ on oxidative stress marker (A) MDA level, (B) GSH level and SOD activity (C). Data are shown as Mean ± S.E.M, *Control vs HG; #HG vs HG + PQQ. *P < 0.05, ***P < 0.001; ##P < 0.01; ###P < 0.01.
3.3. The effects of PQQ on the inflammatory cytokine markers
The level of inflammatory markers, including TNFα, IL6, and IL1β revealed significantly enhanced level in HG exposed cells as compared to the control group. On treatment with PQQ, it showed a significant decrease as compared to the HG group. The expression of the cytokinesat the mRNA level measured by qPCR supports the cytokine level determined by ELISA kit (Fig. 4).
Fig. 4.
Effect of PQQ on inflammatory markers (A) TNF-α, (B) IL6, (C) IL-1β, (D) mRNA TNF-α expression, (E) mRNA IL6 expression, and (F) mRNA IL-1β expression. Data are shown as Mean ± S.E.M, *Control vs HG; #HG vs HG + PQQ. **P < 0.01, ***P < 0.001; #P < 0.05; ###P < 0.001.
4. Discussion
It has been documented that PQQ exerts protecting effects on oxidative stress-induced cell damage in the brain, liver and heart by reducing oxidative stress and ROS (He et al., 2003, Nunome et al., 2008, Pandey et al., 2014, Tao et al., 2007). In the present research, we concentrated on PPQ's inhibitory effect on the cell model of HG-induced cell damage by inhibiting AGEs formation. In this research, we have used hepatocyte, HepG2 cells and high glucose concentration as an in vitro model of the toxicity of glucose in liver cell lines. However, when the HepG2 cells were incubated with a HG (50 mM), we observed a substantial reduction in cell viability. Several studies have utilized a high glucose concentration (50 mM) as an in vitro model to investigate toxicity caused by hyperglycemia that simulated the in vivo state of diabetic ketoacidosis in acute or untreated diabetes (Chandrasekaran et al., 2010, Greene et al., 1992, Jiang et al., 2015). The development of induced hyperglycemia-ROS in different cell types plays a vital role in the pathogenesis of diabetic complications (Giugliano et al., 1996, Ha and Lee, 2000, Volpe et al., 2018). LPO and protein oxidation can result in damage to the membrane, impairment of the production of ATP, and other essential cell functions. As a result, the the total damage leads to the beginning of death signals in the cell via apoptosis or necrosis resulting in damage of tissues (Shi et al., 2005, Tandon et al., 2004). In vivo, PQQ has been demonstrated to shield living cells from oxidative damage, and in vitro, it was shown to protect biomolecules from artificially induced reactive oxygen species (Misra et al., 2012). It serves as a nutrient and vitamin to enable living cells development and defend themselves while they are under stress (Misra et al., 2012). The protective activity of PQQ due to its antioxidant effect (Decker, 1995, Misra et al., 2012, Nunome et al., 2008, Tao et al., 2007). PQQ showed antioxidant activity as depicted by ROS activity (Fig. 2), MDA, GSH level ans SOD activity (Fig. 3). We initially examined an oxidative stress indicators after exposure to hyperglycemic conditions of the HepG2 cells. We observed elevated development of ROS along with oxidative stress markers after exposure to 50 mM glucose compared to control group, as shown in the results (Fig. 2, Fig. 3). Interestingly, this increase in ROS is due to the increased level of AGEs formation and RAGE expression, which demonstrated the role of oxidative stress in cell damage caused by hyperglycemia. These data support earlier reports on the role of glucose-mediated oxidative stress in complications with diabetes (Chandrasekaran et al., 2010, Haidari et al., 2013, Kang et al., 2011, Nelson et al., 2012). It has been documented that the risk of development of complications in diabetic patients with increase in LPO and plasma GSH enzymes has a direct relationship (Anwer et al., 2012, Rabbani et al., 2010, Safhi et al., 2019). Moreover, PQQ can increases the activity of antioxidant enzymes, inhibits the production of ROS and MDA, reduces the expression of inflammatory genes. Furthermore, these data also suggest that PQQ controls mitochondrial activity through directly affecting the NADH dehydrogenase (Lixia et al., 2021). The most effective way of minimizing complications of diabetes is strict glycemic control, but this control can not be accomplished in most cases (Molitch et al., 1993, Vincent et al., 2004). Instead of additional therapy, it is important to use antioxidants to inhibit the pathological pathways that lead to complications caused by hyperglycemia (Chugh et al., 2001, Krishan and Chakkarwar, 2011). As indicated in the data, following exposure to a increased concentration of glucose (50 mM), PQQ at a concentration of 100 nM significantly decrease AGEs level and RAGE expression in the HepG2 cell line. This effect was likely due to the inhibition of ROS formation caused by glucose. In HepG2 cells, we measured the rate of ROS formation which showed a significant increase in the high glucose treated group compared to the control group. Curiously, due to antioxidant properties, PQQ prevented the development of glucose-induced ROS. In HepG2 cells PQQ strongly inhibited MDA, which was induced by hyperglycemia. We performed the GSH assay as the significant antioxidant cell that might scavenge H2O2 and other ROS (Shaki and Pourahmad, 2013). Our data showed that GSH oxidation induced by 50 mM glucose was significantly reversed with PQQ. In another study, red wine treatment substantially prevented the oxidative stress caused by streptozocin in the rat brains (Montilla et al., 2005). The exposure of hepatocytes (rat primary cells) to a increased concentration of glucose (40 mM) resulted in reduced cell viability, increased ROS formation, and reduction of hepatocyte antioxidant material. In primary rat hepatocytes, however, treatment with PQQ has reversed high glucose-induced oxidative stress and cell death (Kapoor and Kakkar, 2012). The liver is well known to be very susceptible to oxidative damage under hyperglycemic conditions, which may lead to damage to the liver cells of diabetic patients in a chronic state (Pourkhalili et al., 2011). Antioxidant treatment may also be used as a preventive technique to attenuate the side effects of hyperglycemia.
5. Conclusion
Current results showed that PQQ ameliorates the production of ROS, reduced oxidative stress and inflammation due to hyperglycaemia. Thus this study illustrated the potential role of PQQ as an effecvtive candidate for attenuation of oxidative damage to hyperglycemia by AGEs inhibition.
6. Availability of data
Data will be available on request to corresponding or firstauthor.
7. Authors' contributions
Saud Alarifi and Gadah Albasher were performed cell culture and treatments. Mohammed AL- Zharani, Norah M. Alhoshani and Norah Saad AL-Johani were evaluated the Oxidative Damage. Abdullah A. Alkahtane and Nada H. Aljarba were determined the inflammatory markers. Md Saquib Hasnain was involved in data interpretation and writing initial draft of manuscript. Saad Alkahtani were involved in the conception and design of the study, data interpretation, and critically revised the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no (RG-1441-018).
Footnotes
Peer review under responsibility of King Saud University.
References
- Antunes F., Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000;475:121–126. doi: 10.1016/s0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- Anwer T., Sharma M., Pillai K.K., Khan G. Protective effect of Withania somnifera against oxidative stress and pancreatic beta-cell damage in type 2 diabetic rats. Acta Pol Pharm. 2012;69:1095–1101. [PubMed] [Google Scholar]
- Atalay M., Laaksonen D.E. Diabetes, oxidative stress and physical exercise. J. Sports Sci. Med. 2002;1:1. [PMC free article] [PubMed] [Google Scholar]
- Chan P.H. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K., Swaminathan K., Chatterjee S., Dey A. Apoptosis in HepG2 cells exposed to high glucose. Toxicol. Vitr. 2010;24:387–396. doi: 10.1016/j.tiv.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Chugh S.N., Dhawan R., Kishore K., Sharma A., Chugh K. Glibenclamide vs gliclazide in reducing oxidative stress in patients of noninsulin dependent diabetes mellitus–a double blind randomized study. J. Assoc. Physicians India. 2001;49:803–807. [PubMed] [Google Scholar]
- Cusi K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2009;16:141–149. doi: 10.1097/MED.0b013e3283293015. [DOI] [PubMed] [Google Scholar]
- Dai Y., Zhang J., Xiang J., Li Y., Wu D., Xu J. Calcitriol inhibits ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in hyperosmotic stress stimulated human corneal epithelial cells. Redox Biol. 2019;21:101093. doi: 10.1016/j.redox.2018.101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker E.A. The role of phenolics, conjugated linoleic acid, carnosine, and pyrroloquinoline quinone as nonessential dietary antioxidants. Nutr. Rev. 1995;53:49–58. doi: 10.1111/j.1753-4887.1995.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Finkel T. Oxygen radicals and signaling. Curr. Opin. Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- Giugliano D., Ceriello A., Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- Greene D.A., Sima A.A.F., Stevens M.J., Feldman E.L., Lattimer S.A. Complications: neuropathy, pathogenetic considerations. Diabetes Care. 1992;15:1902–1925. doi: 10.2337/diacare.15.12.1902. [DOI] [PubMed] [Google Scholar]
- Gupta A.P., Syed A.A., Garg R., Goand U.K., Singh P., Riyazuddin M., Valicherla G.R., Husain A., Gayen J.R. Pancreastatin inhibitor PSTi8 attenuates hyperinsulinemia induced obesity and inflammation mediated insulin resistance via MAPK/NOX3-JNK pathway. Eur. J. Pharmacol. 2019;864 doi: 10.1016/j.ejphar.2019.172723. [DOI] [PubMed] [Google Scholar]
- Ha H., Lee H.B. Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int. 2000;58:S19–S25. doi: 10.1046/j.1523-1755.2000.07704.x. [DOI] [PubMed] [Google Scholar]
- Haidari F., Omidian K., Rafiei H., Zarei M., Shahi M.M. Green tea (Camellia sinensis) supplementation to diabetic rats improves serum and hepatic oxidative stress markers. Iran. J. Pharm. Res. IJPR. 2013;12:109. [PMC free article] [PubMed] [Google Scholar]
- He K., Nukada H., Urakami T., Murphy M.P. Antioxidant and pro-oxidant properties of pyrroloquinoline quinone (PQQ): implications for its function in biological systems. Biochem. Pharmacol. 2003;65:67–74. doi: 10.1016/s0006-2952(02)01453-3. [DOI] [PubMed] [Google Scholar]
- Hu R., Wang Ming-qing, Ni S., Wang Ming, Liu L., You H., Wu X., Wang Y., Lu L., Wei L. Salidroside ameliorates endothelial inflammation and oxidative stress by regulating the AMPK/NF-κB/NLRP3 signaling pathway in AGEs-induced HUVECs. Eur. J. Pharmacol. 2020;867 doi: 10.1016/j.ejphar.2019.172797. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Yuan Y., Zhou J., Wu Y., Zhou Q., Gui S., Wang Y. Apoptotic events induced by high glucose in human hepatoma HepG2 cells involve endoplasmic reticulum stress and MAPK’s activation. Mol. Cell. Biochem. 2015;399:113–122. doi: 10.1007/s11010-014-2238-5. [DOI] [PubMed] [Google Scholar]
- Jiao L., Taylor P.R., Weinstein S.J., Graubard B.I., Virtamo J., Albanes D., Stolzenberg-Solomon R.Z. Advanced glycation end products, soluble receptor for advanced glycation end products, and risk of colorectal cancer. Cancer Epidemiol. Prev. Biomarkers. 2011;20:1430–1438. doi: 10.1158/1055-9965.EPI-11-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandhare A.D., Raygude K.S., Ghosh P., Ghule A.E., Bodhankar S.L. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia. 2012;83:650–659. doi: 10.1016/j.fitote.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Kang, K.A., Kim, J.S., Zhang, R., Piao, M.J., Maeng, Y.H., Kang, M.Y., Lee, I.K., Kim, B.J., Hyun, J.W., 2011. KIOM-4 protects against oxidative stress-induced mitochondrial damage in pancreatic β-cells via its antioxidant effects. Evidence-based Complement. Altern. Med. eCAM 2011. [DOI] [PMC free article] [PubMed]
- Kapoor R., Kakkar P. Protective role of morin, a flavonoid, against high glucose induced oxidative stress mediated apoptosis in primary rat hepatocytes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishan P., Chakkarwar V.A. Diabetic nephropathy: aggressive involvement of oxidative stress. J. Pharm Educ. Res. 2011;2 [Google Scholar]
- Misra H.S., Rajpurohit Y.S., Khairnar N.P. Pyrroloquinoline-quinone and its versatile roles in biological processes. J. Biosci. 2012;37:313–325. doi: 10.1007/s12038-012-9195-5. [DOI] [PubMed] [Google Scholar]
- Molitch M.E., Steffes M.W., Cleary P.A., Nathan D.M. Baseline analysis of renal function in the Diabetes Control and Complications Trial. Kidney Int. 1993;43:668–674. doi: 10.1038/ki.1993.96. [DOI] [PubMed] [Google Scholar]
- Moloney, J.N., Cotter, T.G., 2018. ROS signalling in the biology of cancer. In: Seminars in Cell & Developmental Biology. Elsevier, pp. 50–64. [DOI] [PubMed]
- Montilla P., Barcos M., Munoz M.C., Bujalance I., Muñoz-Castañeda J.R., Tunez I. Red wine prevents brain oxidative stress and nephropathy in streptozotocin-induced diabetic rats. J. Biochem. Mol. Biol. 2005;38:539. doi: 10.5483/bmbrep.2005.38.5.539. [DOI] [PubMed] [Google Scholar]
- Nelson L.E., Valentine R.J., Cacicedo J.M., Gauthier M.-S., Ido Y., Ruderman N.B. A novel inverse relationship between metformin-triggered AMPK-SIRT1 signaling and p53 protein abundance in high glucose-exposed HepG2 cells. Am. J. Physiol. Physiol. 2012;303:C4–C13. doi: 10.1152/ajpcell.00296.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme P., Cruzat V.F., Keane K.N., Carlessi R., de Bittencourt Jr P.I.H. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016;473:4527–4550. doi: 10.1042/BCJ20160503C. [DOI] [PubMed] [Google Scholar]
- Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., Yorek M.A., Beebe D., Oates P.J., Hammes H.-P. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Njoroge F.G., Sayre L.M., Monnier V.M. Detection of D-glucose-derived pyrrole compounds during Maillard reaction under physiological conditions. Carbohydr. Res. 1987;167:211–220. doi: 10.1016/0008-6215(87)80280-x. [DOI] [PubMed] [Google Scholar]
- Nunome K., Miyazaki S., Nakano M., Iguchi-Ariga S., Ariga H. Pyrroloquinoline quinone prevents oxidative stress-induced neuronal death probably through changes in oxidative status of DJ-1. Biol. Pharm. Bull. 2008;31:1321–1326. doi: 10.1248/bpb.31.1321. [DOI] [PubMed] [Google Scholar]
- Pandey S., Singh A., Kumar P., Chaudhari A., Nareshkumar G. Probiotic Escherichia coli CFR 16 producing pyrroloquinoline quinone (PQQ) ameliorates 1, 2-dimethylhydrazine-induced oxidative damage in colon and liver of rats. Appl. Biochem. Biotechnol. 2014;173:775–786. doi: 10.1007/s12010-014-0897-z. [DOI] [PubMed] [Google Scholar]
- Pourkhalili N., Hosseini A., Nili-Ahmadabadi A., Hassani S., Pakzad M., Baeeri M., Mohammadirad A., Abdollahi M. Biochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic rats. World J. Diabetes. 2011;2:204. doi: 10.4239/wjd.v2.i11.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar P.V., Reddy U.A., Singh S.P., Balasubramanyam A., Rahman M.F., Indu Kumari S., Agawane S.B., Murty U.S.N., Grover P., Mahboob M. Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats. J. Appl. Toxicol. 2012;32:436–445. doi: 10.1002/jat.1775. [DOI] [PubMed] [Google Scholar]
- Rabbani S.I., Devi K., Khanam S. Pioglitazone, a Ppar-γ ligand inhibited the nicotinamidestreptozotocin induced sperm abnormalities in type-2 diabetic wistar rats. Pak. J. Pharm. Sci. 2010:23. [PubMed] [Google Scholar]
- Reddy S., Bichler J., Wells-Knecht K.J., Thorpe S.R., Baynes J.W. N. epsilon.-(Carboxymethyl) lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry. 1995;34:10872–10878. doi: 10.1021/bi00034a021. [DOI] [PubMed] [Google Scholar]
- Rochette L., Zeller M., Cottin Y., Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta (BBA)-General Subj. 2014;1840:2709–2729. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Safhi M.M., Qumayri H.M., Masmali A.U.M., Siddiqui R., Alam M.F., Khan G., Anwer T. Thymoquinone and fluoxetine alleviate depression via attenuating oxidative damage and inflammatory markers in type-2 diabetic rats. Arch. Physiol. Biochem. 2019;125:150–155. doi: 10.1080/13813455.2018.1443141. [DOI] [PubMed] [Google Scholar]
- Samadder A., Chakraborty D., De A., Bhattacharyya S.S., Bhadra K., Khuda-Bukhsh A.R. Possible signaling cascades involved in attenuation of alloxan-induced oxidative stress and hyperglycemia in mice by ethanolic extract of Syzygium jambolanum: drug-DNA interaction with calf thymus DNA as target. Eur. J. Pharm. Sci. 2011;44:207–217. doi: 10.1016/j.ejps.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Shaki F., Pourahmad J. Mitochondrial toxicity of depleted uranium: protection by beta-glucan. Iran. J. Pharm. Res. IJPR. 2013;12:131. [PMC free article] [PubMed] [Google Scholar]
- Shi H., Wang X., Luo Y., Su Y. Electron paramagnetic resonance evidence of hydroxyl radical generation and oxidative damage induced by tetrabromobisphenol A in Carassius auratus. Aquat. Toxicol. 2005;74:365–371. doi: 10.1016/j.aquatox.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Singh P., Reza M.I., Syed A.A., Garg R., Husain A., Katekar R., Goand U.K., Riyazuddin M., Gupta A.P., Gayen J.R. PSTi8 with metformin ameliorates perimenopause induced steatohepatitis associated ER stress by regulating SIRT-1/SREBP-1c axis. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparvero L.J., Asafu-Adjei D., Kang R., Tang D., Amin N., Im J., Rutledge R., Lin B., Amoscato A.A., Zeh H.J. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.-Q., Zhao J., Zhang T., Qu L., Wang X., Xue B., Li X.-J., Mu Y.-M., Lu J.-M. Protective effects of Salvianolic acid B on Schwann cells apoptosis induced by high glucose. Neurochem. Res. 2012;37:996–1010. doi: 10.1007/s11064-011-0695-8. [DOI] [PubMed] [Google Scholar]
- Syed A.A., Lahiri S., Mohan D., Valicherla G.R., Gupta A.P., Kumar S., Maurya R., Bora H.K., Hanif K., Gayen J.R. Cardioprotective effect of ulmus wallichiana planchon in β-adrenergic agonist induced cardiac hypertrophy. Front. Pharmacol. 2016;7:510. doi: 10.3389/fphar.2016.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A.A., Lahiri S., Mohan D., Valicherla G.R., Gupta A.P., Riyazuddin M., Kumar S., Maurya R., Hanif K., Gayen J.R. Evaluation of anti-hypertensive activity of Ulmus wallichiana extract and fraction in SHR, DOCA-salt-and L-NAME-induced hypertensive rats. J. Ethnopharmacol. 2016;193:555–565. doi: 10.1016/j.jep.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Syed A.A., Reza M.I., Husain A., Singh P., Gayen J.R. Inhibition of NOX4 by Cissus quadrangularis extract protects from Type 2 diabetes induced-steatohepatitis. Phytomed. Plus. 2021:100021. doi: 10.1016/j.phyplu.2021.100021. [DOI] [Google Scholar]
- Syed A.A., Reza M.I., Shafiq M., Kumariya S., Singh P., Husain A., Hanif K., Gayen J.R. Naringin ameliorates type 2 diabetes mellitus-induced steatohepatitis by inhibiting RAGE/NF-κB mediated mitochondrial apoptosis. Life Sci. 2020;257:118118. doi: 10.1016/j.lfs.2020.118118. [DOI] [PubMed] [Google Scholar]
- Tandon R., Khanna R.D., Dorababu M., Goel R.K. Oxidative stress and antioxidants status in peptic ulcer and gastric carcinoma. Indian J. Physiol. Pharmacol. 2004;48:115–118. [PubMed] [Google Scholar]
- Tao R., Karliner J.S., Simonis U., Zheng J., Zhang J., Honbo N., Alano C.C. Pyrroloquinoline quinone preserves mitochondrial function and prevents oxidative injury in adult rat cardiac myocytes. Biochem. Biophys. Res. Commun. 2007;363:257–262. doi: 10.1016/j.bbrc.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heijst J.W.J., Niessen H.W.M., Hoekman K., Schalkwijk C.G. Advanced glycation end products in human cancer tissues: detection of Nε-(carboxymethyl) lysine and argpyrimidine. Ann. N. Y. Acad. Sci. 2005;1043:725–733. doi: 10.1196/annals.1333.084. [DOI] [PubMed] [Google Scholar]
- Vincent A.M., Russell J.W., Low P., Feldman E.L. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr. Rev. 2004;25:612–628. doi: 10.1210/er.2003-0019. [DOI] [PubMed] [Google Scholar]
- Volpe C.M.O., Villar-Delfino P.H., dos Anjos P.M.F., Nogueira-Machado J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9:1–9. doi: 10.1038/s41419-017-0135-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Nie L., Yin Y.-G., Tang J.-L., Zhou J.-Y., Li D.-D., Zhou S.-W. Resveratrol protects against hyperglycemia-induced oxidative damage to mitochondria by activating SIRT1 in rat mesangial cells. Toxicol. Appl. Pharmacol. 2012;259:395–401. doi: 10.1016/j.taap.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Nishii K., Kuwata K., Nakamichi M., Nakanishi K., Sugimoto A., Ikemoto K. Effects of pyrroloquinoline quinone and imidazole pyrroloquinoline on biological activities and neural functions. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S., Matsui T. Advanced glycation end products (AGEs), oxidative stress and diabetic retinopathy. Curr. Pharm. Biotechnol. 2011;12:362–368. doi: 10.2174/138920111794480534. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur. J. Pharmacol. 2011;670:325–332. doi: 10.1016/j.ejphar.2011.08.011. [DOI] [PubMed] [Google Scholar]
Further reading
- Liu Lixia, Zhang Yingyong, Liu Tao, Ke Chongrong, Huang Jianzhong, Yajuan Fu., Lin Zhang, Chen Fengjuan, Xiuqin Wu., Chen Qi. Pyrroloquinoline quinone protects against exercise-induced fatigue and oxidative damage via improving mitochondrial function in mice. FASEB J. 2021;35(4) doi: 10.1096/fj.202001977RR. [DOI] [PubMed] [Google Scholar]
- Hoque Masudul, Umehara Takashi, Kawai Tomoko, Shimada Masayuki. Adverse effect of superoxide-induced mitochondrial damage in granulosa cells on follicular development in mouse ovaries. Free Radical Biol. Med. 2021;163:344–355. doi: 10.1016/j.freeradbiomed.2020.12.434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available on request to corresponding or firstauthor.