Abstract
Macrobrachium lamarrei (H. Milne-Edwards, 1837) is a well-known freshwater prawn species of Bangladesh. The aim of the research is to explore various reproductive aspects (size at sexual maturity, reproductive period and fecundity) of M. lamarrei in the Ganges River, Bangladesh through October 2012 to September 2013. We also study the environmental parameters and their impact on reproduction of M. lamarrei. A total 391 (ovigerous = 141, non-ovigerous = 250) female specimens were collected using Drag net. The TL50 (the TL at which 50% of individuals become mature) was calculated by a logistic equation as 5.20 cm. Based on the availability of ovigerous females the spawning season was February-November with the peak June-July. Further, 50% and 90% ovigerous females were observed when Fulton’s condition factor (KF) was 0.85 and 1.03, respectively. The total fecundity (FT) was ranged from 65 to 370 where TL was 4.20–6.40 cm and BW was 0.84–2.50 g. Fecundity was found to be highly correlated with TL (r2 ≥ 0.96, rs = 0.96, p < 0.0001) and BW (r2 ≥ 0.88, rs = 0.93, p < 0.0001). Temperature (rp = 0.82, p = 0.009), dissolved oxygen (DO) (rp = −0.83, p = 0.0007), pH (rp = 0.80, p = 0.0014) and total alkalinity (rp = −0.87, p = 0.0002), were highly correlated with ovigerous females. The average temperature on peak spawning season was 32 °C. Also, the spawning period connected with the peak rainfall and showed a notable relation between rainfall and ovigerous females. In addition, exploration of long data series pointed that yearly average air temperature is rising by 0.029 °C yr−1, whereas yearly average rainfall is falling by 2.96 mm yr−1. Therefore, the result will be helpful for the sustainable management and conservation of M. lamarrei through fixed permissible mesh size and establishment of a ban period in the Ganges River, Bangladesh and adjoining ecosystems.
Keywords: Climate change, Environmental parameters, Macrobrachium lamarrei, Size at sexual maturity, Spawning season
1. Introduction
Macrobrachium is one of the most mosaic freshwater genera under the family Palaemonidae. Among the crustacean fishery Palaemonidae is considered as a highly significant commercial resource worldwide (Mantelatto and Barbosa, 2005, De Grave and Fransen, 2011, Hossain et al., 2012a, Ara et al., 2014, Molina et al., 2020). Macrobrachium lamarrei is a member of this family that has a lucrative significance due to its rapid growth, succinct life span and is an extremely important part of the catch nature in Bangladesh (Sharma and Subba, 2005). Amongst several freshwater prawns existing in Bangladesh, M. lamarrei is renowned one, usually called as “Kuncho river prawn” which is an important source of foreign exchange (Ara et al., 2014). It is also known as Kuncho chingri in India (Sharma and Subba, 2005). This kuncho river prawn is distributed in Bangladesh, India, Pakistan, and Nepal (Sharma and Subba, 2005, Ara et al., 2014). It is usually found in beels, rivers and ponds (Kibria, 1983, Ara et al., 2014). This prawn is omnivorous and feeds on all kinds of sustenance, living or dead such as micro-plankton, algae, fish fleshes, decomposing plants and animals (Sharma and Subba, 2005). M. lamarrei is considered a least concern species in both Bangladesh (IUCN Bangladesh, 2015) and worldwide (IUCN, 2020). Kuncho chingri comprises huge economic importance as it has better taste, high price, and growing demand in the global market (Hussain and Manohar, 2016). Prawns are a key source of protein after fish and play a significant role in the aquatic ecosystems by reprocessing dead organic substance (Raghunathan and Valarmathi, 2005). But the production of the prawn is deteriorating owing to use of pesticides in crop fields and habitat damage. So, it is important to increase the production potentiality of this species. Therefore, an exhaustive study of reproductive biology of this prawn is needed (Sarkar et al. 2012a).
Mainly fisheries management depends on precise assessment of biological parameters (Tracey et al., 2007). Appropriate management systems for protection of the aquatic organisms are mostly relying on the information of reproductive criterion (Hardie et al., 2007). Reproductive criterions are an essential apparatus to have idea about systems of biological conservation of an aquatic organism (Hussain and Manohar, 2016).
In the mating process of Macrobrachium sp., the male deposits sperm on the underside of the female thorax between the walking legs. The female extrudes the eggs that pass through the sperm. The female brings the fertilized eggs with her before they are hatched; the period may vary but is usually less than three weeks (Wynne, 2000). In the natural environment, Macrobrachium sp. may spawn 3 to 4 times per year (Ling, 1969) or more than 4 times (Rao, 1991).
The size of maturity in fishes may vary across population from environmental condition and can help to determine the smallest catchable size of fishes as well as find out the reason for variations of maturity size (Templeman, 1987, Lucifora et al., 1999, Hasan et al., 2021, Mawa et al., 2021). Estimation of spawning/breeding season in fish is essential mainly for the conservation of mature individuals from heavy fishing pressure or other reasons (Templeman, 1987).
Fecundity is defined as the number of oocytes released from female pleopods in a single spawning season (Ramirez-Llodra, 2002). To well-understanding of the population dynamics, the information of fecundity is needed (Lagler, 1956). It is very essential for assessment of reproductive potential and providing information about the number of recruits produced in a period and the reproductive capability of the species (Hussain and Manohar, 2016, Sabbir et al., 2021). In the management strategies of prawn hatcheries, it is used to evaluate the reproductive capacity of brood prawns (Sharma and Subba, 2005).
Worldwide climate alteration responsible for the changing of aquatic ecosystem facilities those are essential for human welfare (Lorenzo and Galassi, 2017). Climate change puts extra stress on freshwater ecosystems (water quality, flow regime, and food web) interactions that have previously been seriously stressed by human activities (Doll et al., 2009, Sarkar et al., 2019). The dispersal limits of the maximum benthic organisms are distinguished by various environmental factors stand-in their whole life cycles (Bertini et al., 2010). Environmental issues (temperature and rainfall) and hydrological parameters (e.g. Do, pH, and alkalinity) have continuous effects on growth and reproduction of aquatic organisms ((Lappalainen et al., 2008, Shoji et al., 2011, Britton et al., 2013). In addition, the growth rate of aquatic organisms is reduced with the decrease of water temperature (Blanck and Lamouroux, 2007, Lappalainen et al., 2008, Carmona-Catot et al., 2011). The temperature is considered as the key to control every phase of reproduction (Pankhurst and Porter, 2003). Besides, temperature is the basic abiotic factor regulating the movements of larvae of fish and crustacean (Jakobsen et al., 2009).
Knowledge about the reproductive feature of M. lamarrei is scarce in the scientific papers. There are some works that have been done on various aspects for Macrobrachium species in water bodies worldwide (Table 1). Hence, it is the first study in which investigates the effect of environmental variation and hydrological parameters on the reproduction of M. lamarrei in Ganges River or anywhere else in the world. Consequently, the goal of this study is to investigate different reproductive features of M. lamarrei and the impact of environmental variation on their reproductive activities in the Ganges River, (NW) Bangladesh.
Table 1.
Available works on Macrobrachium species from worldwide water bodies.
| Aspect | Water bodies/country | Species | References |
|---|---|---|---|
| Growth | India | Macrobrachium malcolmsonii | Kanaujia et al. (1997) |
| Bhopal | Macrobrachium lamarrei | Hussain et al. (2017) | |
| Condition factor and length-weight relationship | Indus River | Macrobrachium malcolmsonii | Soomro et al. (2012) |
| Bangladesh | Macrobrachium lamarrei | Ara et al. (2014) | |
| Fish Biology | Godavari and Hooghle River | Macrobrachium malcolmsonii | Rajyalashmi (1980) |
| Biratnagar, Nepal | Macrobrachium lamarrei | Sharma and Subba (2005) | |
| Bangladesh | Macrobrachium lamarrei, Macrobrachium malcolmsonii, Macrobrachium dolichodactylus and Macrobrachium dayanus | Saifullah et al. (2005) | |
| Life history | Ganga River | Macrobrachium malcolmsonii | Hossain et al. (2012a) |
| Reproductive biology | Sao Sebastiao, Brazil | Macrobrachium olfersi | Mossolin and Bueno (2002) |
| Ganga River | Macrobrachium gangeticum and Macrobrachium malcolmsonii | Prasad and Kanaujia (2006) | |
| Egypt | Macrobrachium rosenbergii | Habashy (2010) | |
| West Bengal, India | Macrobrachium dayanum | Sarkar et al. (2012b) | |
| India | Macrobrachium malcolmsonii | Mukheerjee et al. (2015) | |
| Upper Lake at Bhopal | Macrobrachium lamarrei | Hussain and Manohar (2016) | |
| Upper Lake at Bhopal | Macrobrachium lamarrei | Hussain and Manohar (2017) | |
| Grande River | Macrobrachium amazonicum | Paschoal et al. (2019) | |
| Fecundity | Bangalore, South India | Macrobrachium lamarrei | Shakuntala (1977) |
| Bangladesh | Macrobrachium lamarrei | Paul (2001) | |
| Santa Catarina Island, Brazil | Macrobrachium potiuna and M. olfersi | Nazari et al. (2003) | |
| Bangladesh | Macrobrachium lamarrei | Bhuiyan et al. (2008) | |
| Bangladesh | Macrobrachium sp. (M. rosenbergii, M. malcolmsonii and M. lamarrei) | Rashid et al. (2013) | |
| Population Dynamics | Tropical reservoir, India | Macrobrachium malcolmsonii | Khan et al. (2009) |
| First record | Gujarat, India | Macrobrachium lamarrei | Purohit and Vachhrajani (2018) |
2. Materials and method
2.1. Sampling
Our research was conducted in the Ganges River (Latitude 24° 22′ N; Longitude 88° 35′ E), NW Bangladesh (Fig. 1). Monthly samples of M. lamarrei were collected from the fisher’s catch landed at different points of the Ganges River (Godagari, Jahajghat, Charghat), Rajshahi, NW Bangladesh during the sampling period (October 2012 to September 2013). The prawns were caught during daytime 9:30 to 11:30 am using Drag net (2 mm) from different fishing points between mentioned areas.
Fig. 1.
The study site in the Ganges River, northwestern Bangladesh. The area from where Macrobrachium lamarrei was collected is indicated by rectangle.
2.2. Prawn measurement
A total 391 (ovigerous = 141, non-ovigerous = 250) specimens were collected from the study site and then fixed in 10% formalin. The fixed specimens were then taken out one by one, later to be weighed, measured and sexes were identified by the presence and absence of appendix masculina on 2nd pleopod (Tiwari, 1955, Cai and Naiyaneter, 2004). Individual total length (TL) was noted up to 0.01 cm using a digital slide caliper and weight was measured (BW, nearest 0.01 g) using an electric balance.
2.3. Size at first sexual maturity
Ovigerous females were pointed out by the bearing of embryos (external oocytes) attached on its pleopods. TL50 denoted the length wherein 50% of the individual specimens were matured. In order to analysis of TL50, a logistic curve was applied by plotting the percentage of ovigerous female (POF) against TL class as POF = 100/[1 + exp{-f (TLm -TL50)}] (King, 2007).
2.4. Spawning season and condition
The percentage occurrence of monthly ovigerous females was observed for estimation of spawning and peak spawning season of M. lamarrei. And KF was used to assess the relationship between POF and KF by logistic model (King, 2007). And KF was calculated by: KF = 100×(W/L3) (Fulton, 1904).
2.5. Fecundity
Firstly, forceps was used to eradicate the ovum mass from the female’s brood sack and then count the number of oocytes. The fecundity was estimated by actual counting method of (Lagler et al., 1967). Relationship between (i) TL vs. FT and (ii) BW vs. FT. were estimated by the formula: (i) ln FT = m + n ln TL; FT = m × TLn and (ii) ln FT = m + n ln BW; FT = m × BWn. Where, FT means total fecundity, m and n is constant parameters in the linear regression analysis. The coefficient of determination (r2) of each of the relationships was also estimated.
2.6. Climato-hydrological parameters
Hydrological data were collected in every month from sampling station by HACH (HQ40d) digital multi-meter parameter to assess dissolved oxygen (mg/l), temperature (°C), pH, total dissolved solids (TDS) mg/l and total alkalinity (mg/l). Climatological data (rainfall and air temperature) were obtained from the meteorological station of Dhaka, Bangladesh.
2.7. Statistical analyses
Statistical analyses were prepared by Past 4.03, Excel program and Graphpad Prism 6.5 with 5% significant level. Normality of the data was confirmed by Shapiro-Wilk normality test, and violin-box plot. Besides, Spearman rank test was performed to assess the relationship between POF and KF. Pearson correlation and Spearman rank test used to evaluate the impact of the environmental and hydrological factors on POF for detecting the vital influence of environment on ovigerous female and spawning.
3. Results
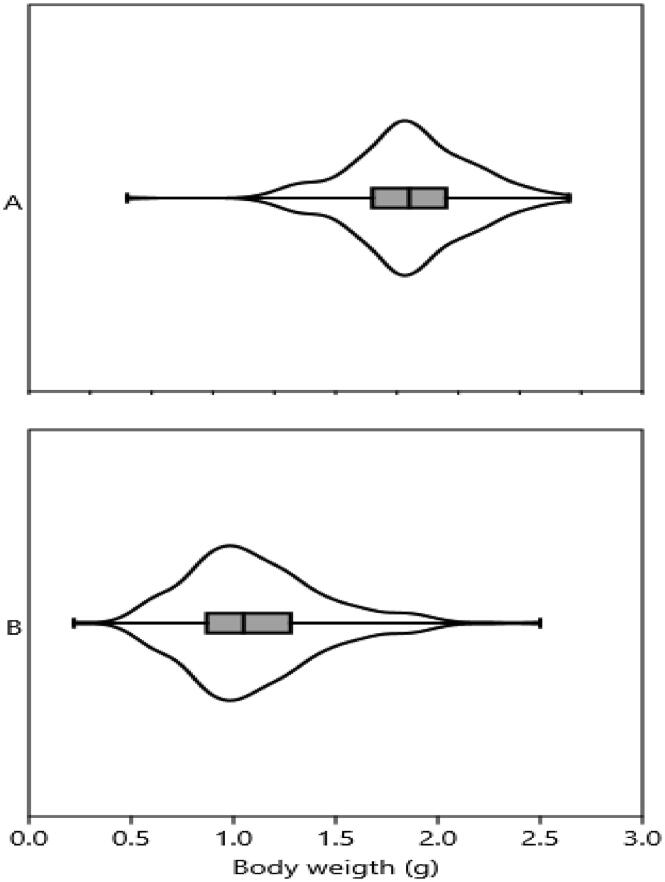
A total 391 (ovigerous = 141, non-ovigerous = 250) female specimens of M. lamarrei were analyzed, total length was ranged from 2.80 to 6.40 cm, body weight was 0.22–2.50 g and egg weight was 0.05–0.30 g (Table 2). Data (TL, BW) did not pass the normality (p < 0.0001, Fig. 2).
Table 2.
Descriptive statistics on the total length (cm), body weight (g) and egg weight (g) measurements of Macrobrachium lamarrei in the Ganges River.
| Characters | Mean ± SD | 95 %CI | Min | Max | n |
|---|---|---|---|---|---|
| Total length TL (cm) | 5.11 ± 0.49 | 5.06–5.16 | 2.80 | 6.40 | 391 |
| Body weight BW (g) | 1.10 ± 0.33 | 1.06–1.12 | 0.22 | 2.50 | |
| Egg weight EW (g) | 0.11 ± 0.05 | 0.09–0.12 | 0.05 | 0.30 | 52 |
n, sample size; Min, minimum; Max, maximum, SD, standard deviation; CI, confidence intervals.
Fig. 2.
Checking the normality of data (A) total length and (B) body weight of Macrobrachium lamarrei in the Ganges River (Data comes from a normal distribution, the box will be symmetrical with the mean and median in the center. If the data meets the assumption of normality, there should also be few outliers).
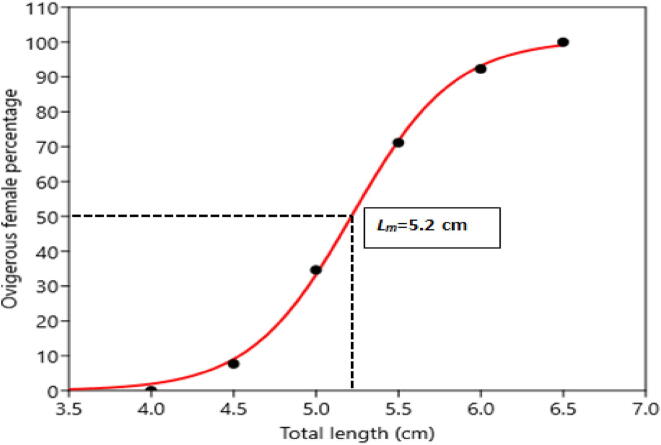
3.1. Size at first sexual maturity
According to the logistic model, the size at sexual maturity of M. lamarrei (Lm) was 5.20 cm (Fig. 3).
Fig. 3.
Adjusted percentage of mature females of Macrobrachium lamarrei versus total length showing the logistic curve fitted to the data.
3.2. Spawning season and condition
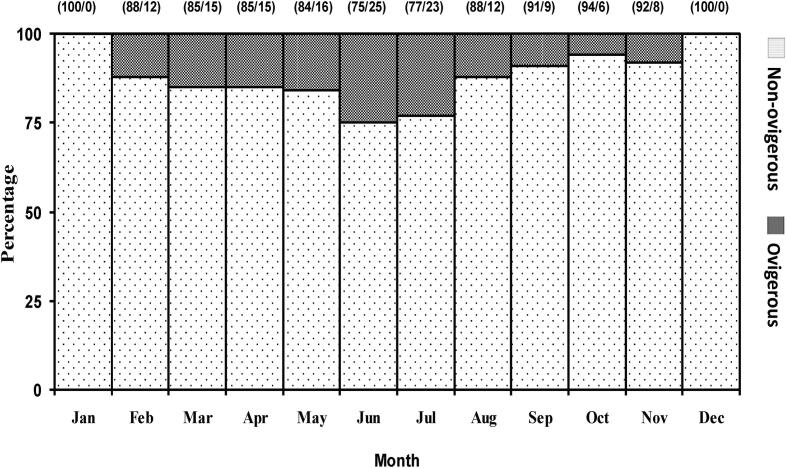
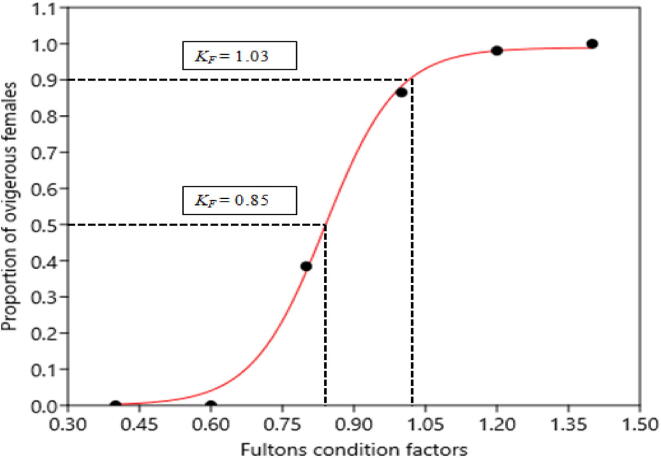
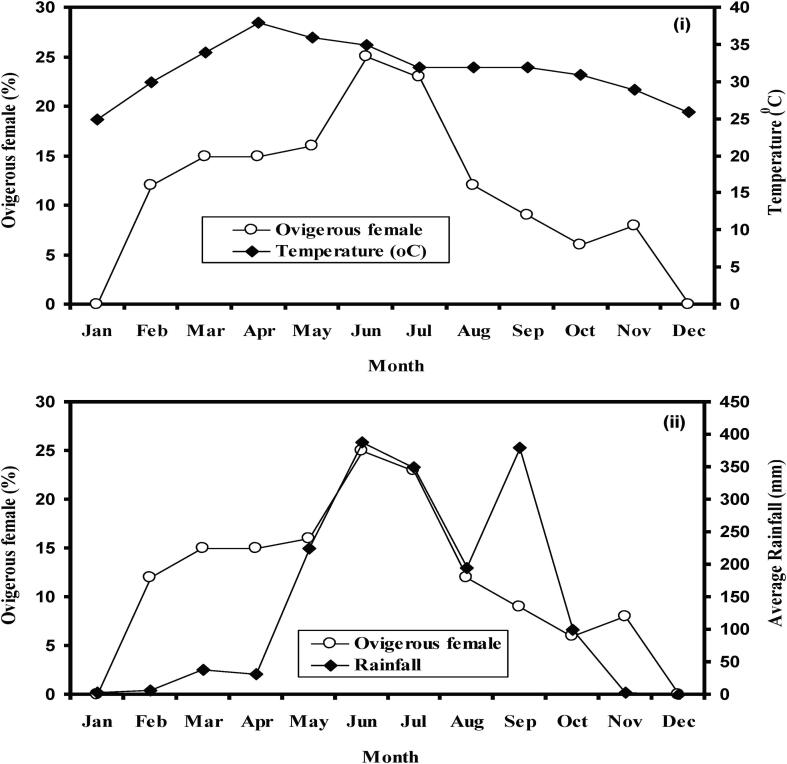
Monthly changes in POF were recorded (Fig. 4). Ovigerous females were found from February-November with the highest percentage in June-July. No ovigerous female was observed during December 2012 to January 2013. So, the spawning season of M. lamarrei was February-November and the peak was in June-July. According to the logistic model 50% ovigerous females were found when KF was 0.85 and 90% ovigerous females were found when KF was 1.03 (Fig. 5). In addition, spawning season of Macrobrachium sp. from world-wide water bodies and fishes in Ganges River was given in Table 3.
Fig. 4.
Monthly changes in percentage occurrence of ovigerous and non-ovigerous females of Macrobrachium lamarrei in the Ganges River. The number of females examined in each month is given in parentheses.
Fig. 5.
Adjusted percentage of mature females of Macrobrachium lamarrei versus Fulton’s condition factor showing the logistic curve fitted to the data.
Table 3.
Spawning season of Macrobrachium species from worldwide water bodies and fishes from the Ganges River.
| Water bodies | Species | Spawning season | Peak spawning season | Reference |
|---|---|---|---|---|
| Bangladesh | Macrobrachium lamarrei | May-August | – | Paul (2001) |
| Sao Sebastiao, Brazil | Macrobrachium olfersi | Continuous | Warmer and rainy months | Mossolin and Bueno (2002) |
| Biratnagar, Nepal | Macrobrachium lamarrei | March-November | – | Sharma and Subba (2005) |
| Upper Lake, Bhopal | Macrobrachium lamarrei | Continuous | June and November | Hussain and Manohar (2016) |
| Ganga River | Macrobrachium malcolmsonii | Continuous | August and September | Prasad and Kanaujia (2006) |
| Bangladesh | Macrobrachium lamarrei | April-June | – | Bhuiyan et al. (2008) |
| West Bengal | Macrobrachium lamarrei | Continuous | – | Sarkar et al. (2012b) |
| Upper Lake, Bhopal | Macrobrachium lamarrei | Continuous | May- June and November – December | Hussain and Manohar (2017) |
| Grande River | Macrobrachium amazonicum | Continuous | – | Paschoal et al. (2019) |
| Ganga River | Macrobrachium lamarrei | February-November | June-July | Present study |
| Habitat/River | Species | Spawning season | Peak spawning season | Reference |
| Ganges | Channa punctatus | June - October | – | Prasad et al. (2011) |
| Ganges | Rita rita | May -September | July and September | Alam et al. (2016) |
| Ganges/Padma | Mystus vittatus | March -September | June | Jasmine and Molina (2016) |
| Ganges | Ompok bimaculatus | June-August | July | Praveen et al. (2017) |
| Ganges/Padma | Eutropiichthys vacha | April- August | June-July | Khatun et al. (2019) |
3.3. Fecundity indices
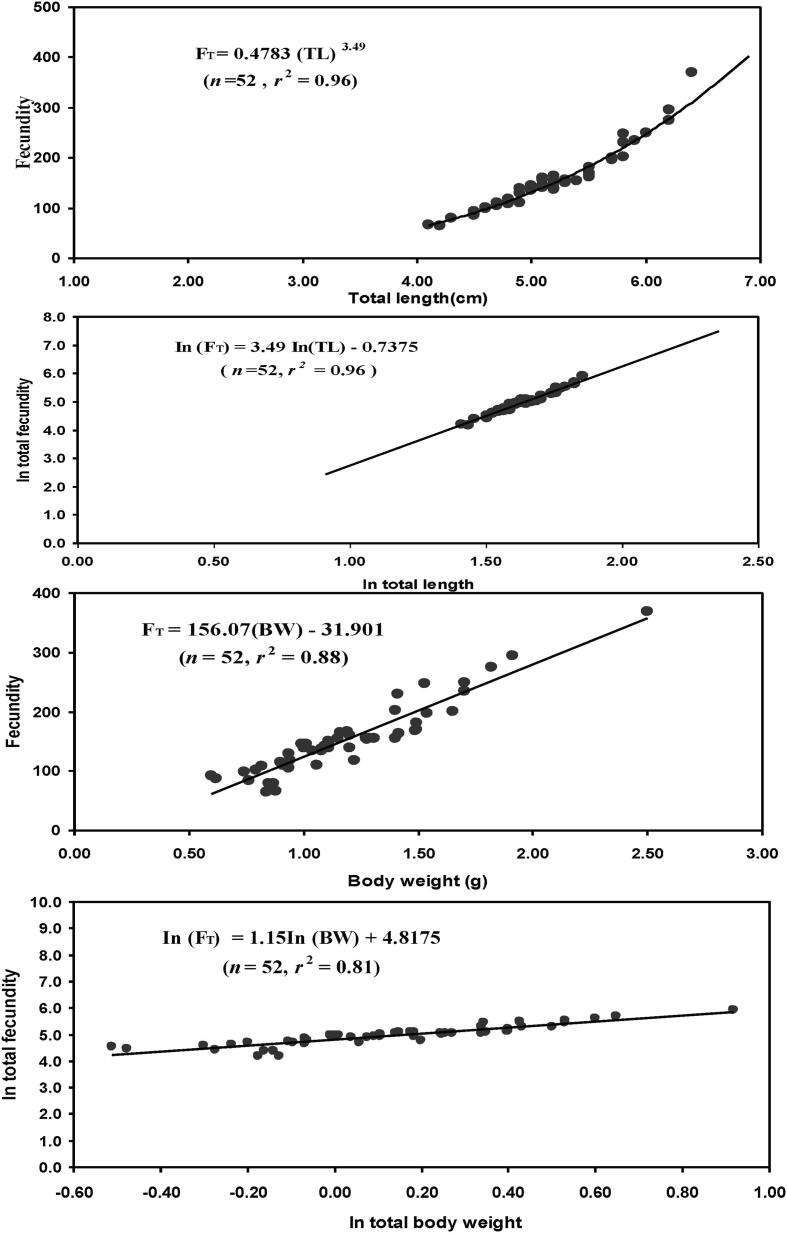
For the calculation of fecundity (FT), a total of 52 females (4.20–6.40 cm TL) were used. FT ranged from 65 to 370 (mean ± SD, 154 ± 59). Significant correlation observed between total fecundity vs. total length (r2 ≥ 0.96, rs = 0.96, p < 0.0001) and total fecundity vs. body weight (r2 ≥ 0.88, rs = 0.93, p < 0.0001).The relative fecundity ranged from 76 to 163 (mean 128 ± 19). Also, significant linear relationships were found for natural log (ln) transfer of FT - TL, FT - BW. Above relationships were shown in (Fig. 6).
Fig. 6.
The relationships between (i) total length vs. fecundity (ii) body weight vs. fecundity (iii) ln total length vs. ln fecundity (iv) ln body weight vs. ln fecundity of Macrobrachium lamarrei in the Ganges River.
3.4. Climato-hydrological parameters
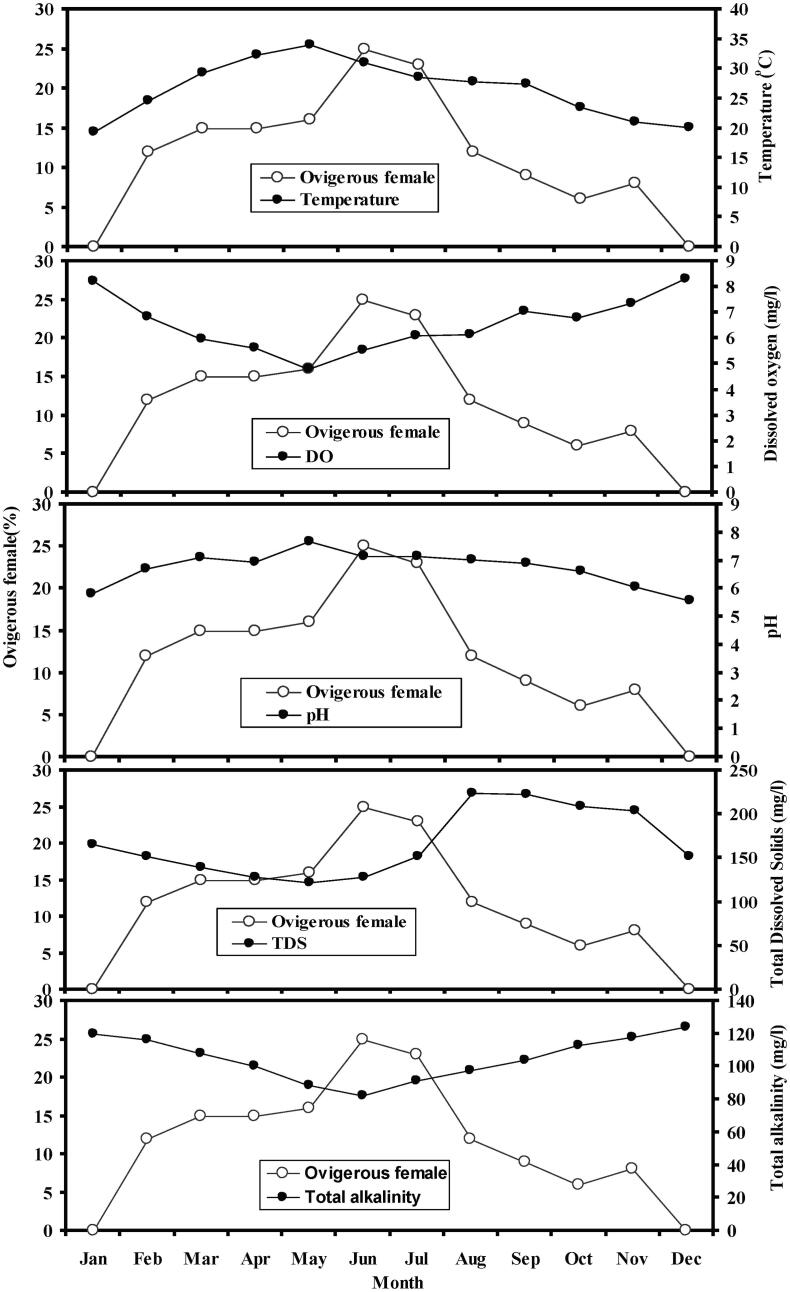
In this study, according to Pearson correlation test, temperature (rp = 0.828, p = 0.009), dissolved oxygen (DO) (rp = −0.83, p = 0.0007), pH (rp = 0.80, p = 0.0014) and total alkalinity (rp = −0.87, p = 0.0002), were highly correlated with ovigerous females except TDS (rp = −0.43, p = 0.1606) (Table 4, Fig. 7). In April–July, we observed the highest water temperature (average 32 °C) when an ovigerous female was in elevation. The lowest temperature was in January and this time no ovigerous females were absent.
Table 4.
Relationship between hydrological parameters with Ovigerous female of Macrobrachium lamarrei (H. Milne-Edwards, 1837) in the Ganges River.
| Relationship | rs value | 95% CL of rs | p values | Significance |
|---|---|---|---|---|
| GSI vs. Temperature | 0.82 | 0.48 to 0.95 | 0.0009 | * |
| GSI vs. DO | −0.83 | −0.95 to −0.50 | 0.0007 | * |
| GSI vs. PH | 0.80 | −0.44 to 0.94 | 0.0014 | * |
| GSI vs. TDS | −0.43 | −0.80 to 0.18 | 0.1606 | ns |
| GSI vs. Total alkalinity | −0.87 | −0.96 to −0.60 | 0.0002 | * |
GSI, Gonadosomatic index; DO, Dissolved Oxygen; TDS, Total Dissolved Solids; rs, Spearman rank correlation values; CL, confidence limit; p, shows the level of significance; ns, not significant; * significant (p ≤ 0.05).
Fig. 7.
Relationship between ovigerous females and environmental parameters of Macrobrachium lamarrei in the Ganges River, northwestern Bangladesh.
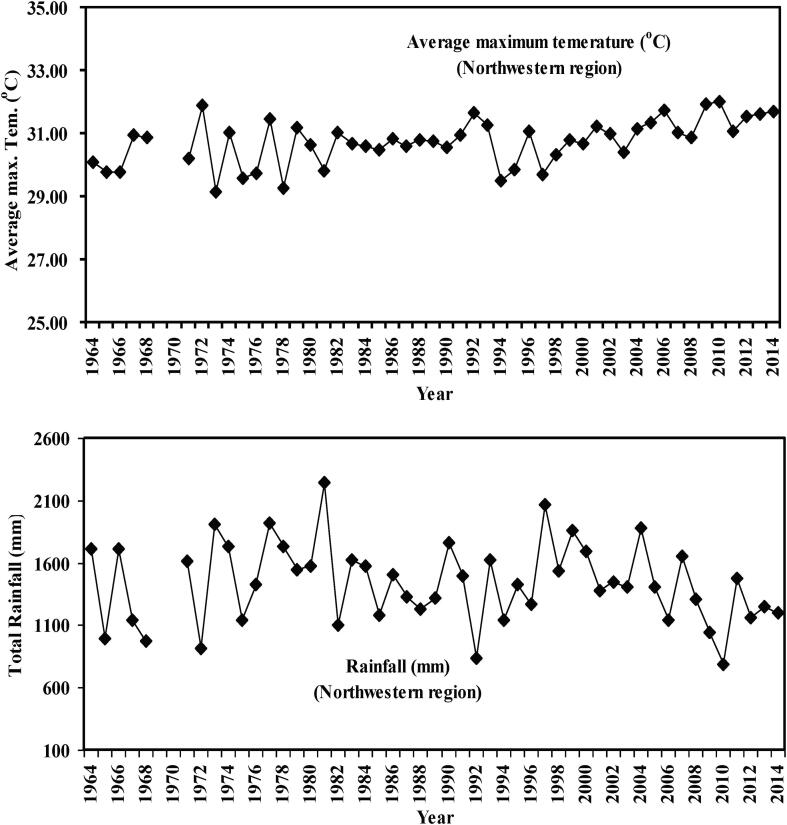
A long data series (1964 to 2014) of air temperature and of rainfall were arranged to observe its fluctuation over time. The yearly average air temperature was rising up to 0.029 °C yr−1 (r2 = 0.350) while rainfall indicated dropping trend by 2.96 mm yr−1 (r2 = 0.018) (Fig. 8). However, air temperature (rs = 0.8248, p = 0.0015) and rainfall (rs = 0.6925, p = 0.0152) was recorded significantly related with POF (Fig. 9).
Fig. 8.
Annual average maximum temperature (oC) and rainfall (mm) in the northwestern region, northwestern Bangladesh during 1964 to 2014.
Fig. 9.
Relationships between (i) temperature vs. ovigerous females and (ii) rainfall vs. ovigerous female of Macrobrachium lamarrei in the Ganges River.
4. Discussion
Information on reproductive features is vital to assess the life cycle and stocks of fish as well as aquatic organisms (Hossain et al., 2017). Our research presents the most comprehensive investigation on reproductive biology of M. lamarrei. The Lm is an essential tool for fisheries management and planning (Lucifora et al., 1999, Hossain et al., 2012b, Hossain et al., 2013, Hossain et al., 2016). In the present study, the Lm was 5.02 cm (TL) for M. lamarrei. Whereas, Hussain and Manohar (2017) reported the Lm was 4.70 cm (TL) from the upper lake Bhopal, India which is lower than our results. However, Lm may vary due to population thickness, water temperature, perceived length and food availability (Hossain et al., 2012c).
Reproduction cycle and time of spawning was estimated by several models including direct observation of spawning, brooding of eggs over time, appearance of ovary and maturation stages over time (King, 2007), relative weight of gonad (TL vs. gonadosomatic index, modified gonadosomatic index and dobrial index) over time (Hossain et al., 2017, Ahamed et al., 2018, Khatun et al., 2019, Sabbir et al., 2021) and histological studies (Chelemal et al., 2009, Hussain and Manohar, 2017, Lucano-Ramírez et al., 2019). The spawning seasons of M. lamarrei was estimated by the percentage of mature females present in the catch or by changes in gonadal indices (Garcia, 1985, Erisman et al., 2012). However, in the current study we observed that the ovigerous females of M. lamarrei were found throughout the year except the month of December and January which means the spawning season was February-November with the peak of June and July. Sharma and Subba (2005) reported that females of M. lamarrei were not having mature eggs in the month of December, January and February from Biratnagar, Nepal which is close agreement with our present study. Hussain and Manohar, 2016, Hussain and Manohar, 2017 and Sarkar et al. (2012b) reported that M. lamarrei was a continuous breeder from Bhopal and West Bengal in India respectively, which is also almost similar to our findings. Whereas, Bhuiyan et al. (2008) recorded spawning season was April-June from Rajshahi, Bangladesh and Paul (2001) reported May-August from Natore, Bangladesh which is dissimilar to our present study. Hussain and Manohar, 2016, Hussain and Manohar, 2017 reported two peak seasons (May–June and November–December) in a year for M. lamarrei from Bhopal, India. However, this disparity may be occurring due to environmental factors, population densities, and food availability (Khatun et al., 2019, Sabbir et al., 2021).
Condition factor discloses several biological interfaces of aquatic organisms like maturity status and level of fitness (Hossen et al., 2019, Sabbir et al., 2020). In this study, KF was found significantly related to POF and our observed KF range was 0.85–1.03 which is suitable for the spawning of M. lamarrei.
Fecundity is used for predicting how many of broods are requisite to producing preferred quantities of offspring (Sharma and Subba, 2005). In addition, it helps to estimate the smallest number of broods needed to sustain the recruitment and offspring survival rates. In the present study, the FT of M. lamarrei varied from 65 to 370 (154 ± 59). Saifullah et al. (2005) documented the total fecundity (FT) ranged from 141 to 328 in the North East and North West regions, Bangladesh. Likewise, Shafi and Quddus, 1982, Sharma and Subba, 2005, Hussain and Manohar, 2016 recorded fecundity was ranged from 2250 to 16300, 82–308, and 69–143 in Biratnagar, Nepal, Bhopal, India and Bangladesh respectively. However, the numbers of eggs for many crustaceans’ species are highly variable (Khmeleva and Golobev, 1986). This variation occurs due to some factor i.e. age, length, weight and environmental condition (Sharma and Subba, 2005).
The success of crustaceans can be ascribed to the point that most of them bear their ovum till hatching and this is why offspring survival rate is high. The sizes of the mother control the number of ovum/egg carried by a female. In numerous crustaceans, the number of eggs has been observed to be linear to the size of the female (Vogt, 2012, Subramoniam, 2013). In addition, in the current study we observed that the total fecundity (FT) has significant relationship with TL and BW which was supported by Saifullah et al. (2005). Whereas, Sharma and Subba (2005) observed insignificant relationship between total length and total fecundity which was dissimilar with our findings and significant relationship between body weight and total fecundity which was support our present findings.
The impact of environmental change on aquatic organisms will differ with habitat, latitude, water features, and in riverine schemes, current regimes (Pankhurst and Munday, 2011). Globally, in the current years it has been reported that freshwater aquatic species could change their distribution in response to climate change (Sarkar et al., 2019). The reproductive strategy of M. lamarrei was affected by the environmental conditions. In our study, the peak values of ovigerous females (%) followed from June-July and at the same time water temperature is also high which confirms that water temperature has an effect on prawn reproduction. During our study, the lowest temperature was found in January, and the highest was in April-June and there was substantial connection between temperature and ovigerous females. The spawning period was started when temperature is above than 25 °C. In this study, the highest ovigerous female (%) was observed when the average temperature was 32 °C. In case of Macrobrachium rosenbergii suitable temperature for spawning was 25–29.5 °C under a laboratory condition in Egypt recorded by Habashy (2010). We found DO, pH and total alkalinity ranges around 5.52–6.10 mg/l, 7.13 and 82.14–90.90 mg/l, respectively from peak spawning time of M. lamarrei. So, these ranges are supposed to be optimum for reproduction of M. lamarrei. There was no work on these types for M. lamarrei but Habashy (2010) research on these aspect for M. rosenbergii and found suitable ranges DO and pH was 6.00–6.80 mg/l and 7.5–8.4 , respectively from Egypt (under a laboratory condition). The analysis of climate data (1964–2014) indicated temperature is increasing and rainfall is reducing day by day in the northwestern district of Bangladesh which alarmingly forecast the shifting of the spawning season of this species.
Spawning events are normally related to climatic conditions (Wilding et al., 2000). In the current study, we observed a highly significant relationship between rainfall vs. ovigerous females (%). The uppermost rainfall was noted in the month of June (389 mm) and this is the peak spawning season of M. lamarrei. The lowest rainfall was observed in November–January when the ovigerous female (%) was also lowest. As it is the first work on this aspect, so comparison is not possible.
5. Conservation strategies
The Lm was 5.20 cm of M. lamarrei in the Ganges River that’s mean 50% prawn spawn at this length so, smaller than this sizes are strongly prohibited to catch and bigger than this sizes are recommended for exploitation. Catching of this species should be stop during their peak spawning season. The suitable range of KF (0.85–1.03) should be maintained in hatchery for artificial breeding of this species. In addition, 80% of female may spawn at 32◦C temperature so, this temperature could be maintained for artificial breeding. Year by year temperature is rising and rainfall is decreasing that`s why in future spawning may be shifted. So, short term management policies should be established for the management and conservation of the wild stock of M. lamarrei.
6. Conclusion
Our research will be helpful to set the mesh size of nets based on Lm and establishment of ban period during peak spawning season is essential for the existence/conservation of M. lamarrei year after year in the Ganges River and worldwide water bodies. As well, a long series data of climate change showed the ups and down of temperature and rainfall which have significant impact on the reproduction of M. lamarrei. Therefore, it can be imagined that the spawning season might be shifted in the future which should be taken into consideration for successful management of M. lamarrei in the Ganges River as well as worldwide water bodies.
Declaration of Competing Interest
The author declare that there is no conflict of interest.
Acknowledgements
We would like to thank Bangladesh fisheries research institute for their cordial support.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2021.06.077.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ahamed F., Saha N., Ahmed Z.F., Hossain M.Y., Ohtomi J. Reproductive biology of Apocryptes bato (Gobiidae) in the Payra River, southern Bangladesh. J. Appl. Ichthyol. 2018;34:1169–1175. [Google Scholar]
- Alam A., Joshi K.D., Das S.C. Feeding and reproductive behaviour of the river catfish Rita rita (Hamilton, 1822) in the river Ganga, India. Indian J. Anim. Sci. 2016;86(6):736–740. [Google Scholar]
- Ara M.G., Nobi M.N., Ahmed Z.F., Fatema M.K. Length-weight relationship and growth pattern inference of a small indigenous freshwater prawn, Macrobrachium lamarrei (H. Milne-Edwards, 1837) in Bangladesh. Res. Agri. Live. Fish. 2014;1:137–145. [Google Scholar]
- Bertini G., Fransozo A., Negreiros-Fransozo M.L. Brachyuran soft-bottom assemblage from marine shallow waters in the southeastern Brazilian littoral. Mar Biodiv. 2010;4:277–291. [Google Scholar]
- Bhuiyan S.S., Joadder M.A.R., Bhuiyan A.S. Occurrence of fishes and non-fin fishes of the River Padma near Rajshahi, Bangladesh. Univ. J. Zool. Rajshahi Univ. 2008;27:99–100. [Google Scholar]
- Blanck A., Lamouroux N. Large–scale in traspecific variation in life–history traits of European fresh water fish. J. Biogeogr. 2007;34:862–875. [Google Scholar]
- Britton J.R., Davies G.D., Pegg J. Spatial variation in the somatic growth rates of European barbel Barbus barbus: a UK perspective. Ecol. Freshw. Fish. 2013;22:21–29. [Google Scholar]
- Cai Y., Naiyaneter P. The freshwater prawns of the genus Macrobrachium Bate, 1868, of Thailand (Crustacea: Decapoda: Palaemonidae. J. Nat. Hist. 2004;38:581–649. [Google Scholar]
- Carmona-Catot G., Benito J., García-Berthou E. Comparing latitudinal and upstream downstream gradients: life history traits of invasive mosquito fish. Divers. Distrib. 2011;17:214–224. [Google Scholar]
- Chelemal M., Jamili S., Sharifpour I. Reproductive biology and histological studies in Abu Mullet, Liza abu in the water of the Khozestan province. J. Fish. Aquat. Sci. 2009;4:1–11. [Google Scholar]
- De Grave S., Fransen C.H.J.M. Carideorum Catalogus: The recent species of the dendrobranchiate, stenopodidean, procarididean and caridean shrimps (Crustacea: Decapoda) Zool. Med. Leiden. 2011;85:195–589. [Google Scholar]
- Doll P., Fiedler K., Zhang J. Global-scale analysis of river¨ flow alterations due to water withdrawals and reservoirs. Hydrol. Earth. Syst. Sci. 2009;13:2413–2432. [Google Scholar]
- Erisman, B., Aburto-Oropeza, O., Gonzalez-Abraham, C., Mascareñas –Osorio, I., Moreno-Báez, M., Hastings-Spatiotemporal, P.A., 2012. Dynamics of a fish spawning aggregation and its fishery in the Gulf of California. Sci. Rep. 2, 284. [DOI] [PMC free article] [PubMed]
- Fulton, T.W., 1904. The rate of growth of fishes, 22nd annual report, Part III. Edinburg: Fisheries board of Scotland.
- Garcia, S., 1985. Reproduction, stock assessment models and population parameters in exploited penaeid shrimp populations. In: Rothlisberg, P. C., Hill, B. J., Staples, D. J. (Eds.). Second Australian Natl. prawn Seminar, NPS2, Cleveland, Australia, 139–158.
- Habashy M.M. On the breeding behavior and reproduction of the freshwater prawn, Macrobrachium rosenbergii (Decapoda-Crustacea) under laboratory conditions. African J. Biol. Sci. 2010;6(2):63–73. [Google Scholar]
- Hardie S.A., White R.W., Barmuta L.A. Reproductive biology of the threatened golden Galaxias galaxias Auratus Johnston and the influence of Lake Hydrology. J. Fish. Biol. 2007;71:1820–1840. [Google Scholar]
- Hasan M.R., Hossain M.Y., Mawa Z., Tanjin S., Rahman M.A., Sarkar U.K., Ohtomi J. Evaluating the size at sexual maturity for 20 fish species (Actinopterygii) in wetland (Gajner Beel) ecosystem, north-western Bangladesh through multi-model approach: A key for sound management. Acta. Ichthyol. Piscat. 2021;51:29–36. [Google Scholar]
- Hossain M.Y., Ohtomi J., Jaman A., Saleha J., Robert L.V.J. Life history traits of the monsoon river prawn Macrobrachium malcolmsonii (Milne-Edwards, 1844) (Palaemonidae) in the Ganges (Padma) River, northwestern Bangladesh. J. Freshw. Ecol. 2012;27:131–142. [Google Scholar]
- Hossain, M.Y., Jewe,l M.A.S., Nahar, L., Rahman, M.M., Naif, A., Ohtomi, J., 2012b. Gonadosomatic index-based size at first sexual maturity ofthe catfish Eutropiichthys vacha (Hamilton, 1822) in the Ganges River (NW Bangladesh). J. Appl. Ichthyol. 28, 601–605.
- Hossain M.Y., Rahman M.M., Miranda R., Leunda P.M., Oscoz J., Jewel M.A.S., Ohtomi J. Size at first sexual maturity, fecundity, length–weight and length–length relationships of Puntius sophore (Cyprinidae) in Bangladeshi waters. J. Appl. Ichthyol. 2012;28:818–822. [Google Scholar]
- Hossain M.Y., Arefin M.S., Mohmud M.S., Hossain M.I., Jewel M.A.S., Rahman M.M., Ohtomi J. Length-weight relationships, condition factor, gonadosomatic index-based size at first sexual maturity, spawning season and fecundity of Aspidoparia morar (Cyprinidae) in the Jamuna River (Brahmaputra River distributary), northern Bangladesh. J. Appl. Ichthyol. 2013;29:1166–1169. [Google Scholar]
- Hossain M.Y., Naser S.M.A., Bahkali A.H., Yahya K., Hossen M.A., Elgorban A.M., Rahman M.M. Life history traits of the flying barb Esomus danricus (Hamilton, 1822) (Cyprinidae) in the Ganges River, Northwestern Bangladesh, Pakistan. J. Zool. 2016;48:399–408. [Google Scholar]
- Hossain M.Y., Hossen M.A., Islam M.S., Jasmine S., Nawer F., Rahman M.M. Reproductive biology of Pethia ticto (Cyprinidae) from the Gorai River (SW Bangladesh) J. Appl. Ichthyol. 2017;33:1007–1014. [Google Scholar]
- Hossen M.A., Paul A.K., Hossain M.Y., Ohtomi J., Sabbir W., Rahman O., Jasmin J., Khan M.N., Islam M.A., Rahman M.A., Khatun D., Kamruzzaman S.K. Estimation of biometric indices for Snakehead Channa punctata (Bloch, 1973) through multi-model inferences, Jordan. J. Biol. Sci. 2019;12:197–202. [Google Scholar]
- Hussain S., Manohar S. Reproductive aspects of freshwater prawn Macrobrachium lamarrei lamarrei H. M. Edwards 1837 in upper lake at Bhopal. Int. J. Fish. Aquat. Stu. 2016;4(6):208–211. [Google Scholar]
- Hussain, S., Manohar, S., 2017. Reproductive biology of Macrobrachium lamarrei (H. Milne-Edwards, 1837) from the upper Lake, Bhopal, India. J. Entomol. Zool. Stud. 5(2), 32–36.
- Hussain S., Ramteke N., Manohar S. Growth performance of Freshwater prawn Macrobrachium lamarrei lamarrei (HM Edward 1837) fed with commercial, supplementary plant and animal feeds. J. Entomol. Zool. Stud. 2017;5(2):28–31. [Google Scholar]
- IUCN Bangladesh, 2015. Red list of Bangladesh Volume 5: Freshwater Fishes. IUCN, International Union for Conservation of Nature, Bangladesh Country Office, Dhaka, Bangladesh, 360p.
- IUCN, 2020. IUCN red list of threatened species. Version 2020-1. IUCN red list of threatened species (Downloaded on 03 February 2020).
- Jakobsen, T., Fogarty, M.J., Mergrey, B.A., Moksness, E., 2009. Fish reproductive biology, John Wiley & Sons, Chichester, United Kingdom.
- Jasmine S., Molina M.A. Reproductive biology of Mystus vittatus (Bloch, 1794) in the Padma River, Bangladesh. Int. J. Fish. Aquat. Stud. 2016;4(5):666–669. [Google Scholar]
- Kanaujia D.R., Mohanty A.N., Tripathi S.D. Growth and production of Indian river prawn Macrobrachium malcolmsonii (H. Milne Edwards) under pond conditions. Aquaculture. 1997;154:79–85. [Google Scholar]
- Khan M.F., Panikkar P., Das A.K., Manna R.K., Singh D.N. Population dynamics of monsoon river prawn Macrobrachium malcolmsonii (Milne Edwards) in Wyra, a tropical reservoir in India. Asian. Fish. Sci. 2009;22:1201–1210. [Google Scholar]
- Khatun D., Hossain M.Y., Nawer F., Mostafa A.A., Al-Askar A.A. Reproduction of Eutropiichthys vacha (Schilbeidae) in the Ganges River (NW Bangladesh) with special reference to potential influence of climate variability. Environ. Sci. Pollut. Res. 2019;26:10800–10815. doi: 10.1007/s11356-019-04523-5. [DOI] [PubMed] [Google Scholar]
- Khmeleva N.N., Golobev A.D. La Production Chez Les Crustaces role dans les ecosystem et utilization. Infremer. 1986;198:p. [Google Scholar]
- Kibria G. Jahan Printing Press; Satkhira, Khulna: 1983. Bangladesher Chingri sampad O Chingri Chas (Shrimp fishery and shrimp culture) p. 126. [Google Scholar]
- King M. 2nd ed. Wiley-Blackwell Publishing; Oxford: 2007. Fisheries Biology, Assessment and Management. [Google Scholar]
- Lagler, K.F., 1956. Enumeration of fish eggs. In freshwater fishery biology. 2nd Edn. W.M. Brown company publishers. Dubuque, 106–110 pp.
- Lagler K.F., Bardach J.E., Miller R.R. John. Willey & Some, Inc.; New York, London, Sydney: 1967. Icthyology; pp. 271–274. [Google Scholar]
- Lappalainen J., Tarkan A.S., Harrod C. A meta-analysis of latitudinal variations in life-history traits of roach, Rutilus rutilus, over its geographical range: linear or non-linear relationships? Freshwater Biol. 2008;53:1491–1501. [Google Scholar]
- Ling, S.W., 1969. The general biology and development of Macrobrachium rosenbergii. FA0 Fisheries Report 3, 589–606.
- Lorenzo T.D., Galassi D.M.P. Effect of temperature rising on the stygobitic crustacean species Diacyclops belgicus: does global warming affect groundwater populations? Water. 2017;9:951. [Google Scholar]
- Lucano-Ramírez, G., Gómez-García, M.D.J., Ruiz-Ramírez, S., González-Sansón, G., Aguilar, Betancourt, C., Flores-Ortega, J.R., 2019. Reproductive characteristics of the sole Achirus mazatlanus (Pleuronectiformes: Achiridae) in the Barra de Navidad coastal lagoon, Jalisco, Mexico, Ciencias Marinas 45, 47–58.
- Lucifora L.O., Valero J.L., Garcia V.B. Length at maturity of the green-eye spurdog shark, Squalus mitsukuii (Elasmobranchii: Squalidae) from the SW Atlantic, with comparisons with other regions. Mar. Freshw. Res. 1999;50:629–632. [Google Scholar]
- Mantelatto F.L.M., Barbosa L.R. Population structure and relative growth of freshwater prawn Macrobrachium brasiliense (Decapoda, Palaemonidae) from São Paulo State, Brazil. Acta. Limnol. Bras. 2005;17:245–255. [Google Scholar]
- Mawa Z., Hossain M.Y., Hasan M.R., Tanjin S., Rahman M.A., Sarmin M.S., Habib K.A. First record on size at sexual maturity and optimum catchable length of 10 marine fishes from the Bay of Bengal (Bangladesh) through multi-models approach: a key for sound fisheries management. Environ. Sci. Pollut. Res. 2021:1–11. doi: 10.1007/s11356-021-13491-8. [DOI] [PubMed] [Google Scholar]
- Molina W.F., Costa G.W.W.F., Cunha I.M.C., Bertollo L.A.C., Ezaz T., Liehr T., Ciof M.B. Molecular cytogenetic analysis in freshwater prawns of the genus Macrobrachium (Crustacea: Decapoda: Palaemonidae) Int. J. Mol. Sci. 2020;21:2599. doi: 10.3390/ijms21072599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossolin E.C., Bueno S.L.S. Reproductive biology of Macrobrachium olfersi (Decapoda, palaemonidae) in Sao Sebastião, Brazil. J. Crust. Biol. 2002;22(2):367–376. [Google Scholar]
- Mukheerjee R., Kanaujia D.R., Pandey A.K. Breeding, larval biology, seed production and aquaculture of Indian river prawn, Macrobrachium malcolmsonii (H. Milne Edwards) Biochem. Cell. Arch. 2015;15:349–382. [Google Scholar]
- Nazari E.M., Simoes-Costa M.S., Muller Y.M.R., Ammar D., Dias M. Comparisons of fecundity, egg size, and egg mass volume of the freshwater prawns Macrobrachium potiuna and Macrobrachium olfersi (Decapoda, Palaemonidae) J. Crust. Boil. 2003;23(4):862–868. [Google Scholar]
- Pankhurst N.W., Munday P.L. Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 2011;62:1015–1026. [Google Scholar]
- Pankhurst N.W., Porter M.J.R. Cold and dark or warm and light: variations on the theme of environmental control of reproduction. Fish. Physiol. Biochem. 2003;28:385–389. [Google Scholar]
- Paschoal, L.R.P., de Oliveira, L.J.F., Andrioli, G.C., Zara, F.J., 2019. Reproductive biology of Macrobrachium amazonicum (Heller, 1862) populations with distinct phenotypes in Neotropical reservoirs during the ‘El Niño’ event. Mar. Freshwater. Res. https://doi.org/10.1071/MF18228.
- Paul S.K. Bengali. Pub. Monika Paul; Gurudaspur, Natore, Bangladesh: 2001. Shrimp and lobster: Biology; pp. 58–59. [Google Scholar]
- Prasad S., Kanaujia D.R. Availability and Breeding Behaviour of Ganga River Prawn Macrobrachium gangeticum (Bate) and Macrobrachium malcolmsonii (H.M. Edwards) Asian. Fish. Sci. 2006;19:377–388. [Google Scholar]
- Prasad L., Dwivedi A.K., Dubey V.K., Serajuddin M. Reproductive biology of freshwater murrel, Channa punctatus (Bloch, 1793) from river Varuna (A tributary of Ganga River) in India. J. Ecophysiol. Occup. Health. 2011;11(1/2):69. [Google Scholar]
- Praveen A., Sarkar U.K., Nagpure N.S., Mishra R.M., Kumar R., Awasthi A., Pandey B.K. Dynamics of reproductive ecology of the fish Ompok bimaculatus (Siluriformes: Siluridae) in six tropical rivers of the Ganges basin. India. 2017;9:73–85. [Google Scholar]
- Purohit, B., Vachhrajani, K.D., 2018. First record of freshwater shrimp, Macrobrachium lamarrei lamarrei (H. Milne Edwards, 1837) from Gujarat, India. J. Fish. 6 (3), 654–657.
- Raghunathan M.B., Valarmathi K. Check list of freshwater prawns (Crustacea, Decapoda, Natantia) in Tamil Nadu India. Hydrobiol. 2005;8(1):35–39. [Google Scholar]
- Rajyalashmi T. Comparative study of biology of fresh water prawn, Macrobrachium malcolmsonii Godavari and Hooghly River system. Indian Natn. Sci. Acad. 1980;45:72–89. [Google Scholar]
- Ramirez-Llodra E. Fecundity and life-history strategies in marine invertebrates. Adv. Mar. Biol. 2002;43:87–170. doi: 10.1016/s0065-2881(02)43004-0. [DOI] [PubMed] [Google Scholar]
- Rao K.J. Reproductive biology of the giant freshwater prawn Macrobrachium rosenbergii (de Man) from Lake Kolleru (Andhra Pradesh) Indian J. Anim. Sci. 1991;61:780–787. [Google Scholar]
- Rashid M.A., Shahjahan R.M., Begum R.A., Alam M.S., Ferdous Z., Kamruzzaman M. Fecundity and embryonic development in three Macrobrachium species. J. Entomol. 2013;1:72–83. [Google Scholar]
- Sabbir, W., Hossain, M.Y., Rahman, M.A., Hasan, M.R., Mawa, Z., Tanjin, S., Ohtomi, J., 2021. First report on reproductive features of the Hooghly croaker Panna heterolepis Trewavas, 1977 from the Bay of Bengal in relation to environmental factors. Environ. Sci. Pollut. R. 1, 1–8. [DOI] [PubMed]
- Sabbir W., Hossain M.Y., Rahman M.A., Hasan M.R., Mawa Z., Tanjin S., Hassan H.U., Ohtomi J. First report on condition factor of Panna heterolepis (Trewavas, 1977) in the Bay of Bengal (Southwestern Bangladesh) in relation to eco-climatic factors. Egypt J. Aquat. Biol. Fish. 2020;24(2):591–608. [Google Scholar]
- Saifullah A.S.M., Rahman M.S., Jabber S.M.A., Khan Y.S.A., Uddin N. Study on some aspects of biology of prawns from northeast and northwest regions of Bangladesh. Pak. J. Biol. Sci. 2005;8(3):425–428. [Google Scholar]
- Sarkar U.K., Naskar M., Srivastava P.K., Roy K., Sarkar S.D., Gupta S., Bose A.K., Nandy S.K., Verma V.K., Sudheesan D., Karnatak G. Climato-environmental influence on breeding phenology of native catfishes in river Ganga and modeling species response to climatic variability for their conservation. International Journal of Biometeorology. Int. J. Biometeorol. 2019;63:991–1004. doi: 10.1007/s00484-019-01703-3. [DOI] [PubMed] [Google Scholar]
- Sarkar U.K., Pathak A.K., Sinha R.K., Sivakumar K., Pandian A.K., Pandey A., Dubey V.K., Lakra W.S. Freshwater fish biodiversity in the River Ganga (India): Changing pattern, threats and conservation perspectives. Rev. Fish. Biol. Fisheries. 2012;22:251–272. [Google Scholar]
- Sarkar I., Basu A., Dutta S., Roy S. Male mating tactics and mating activity in freshwater prawn, Macrobrachium dayanum (Henderson, 1893) Paleomonidae: Caridae. Int. J. Aquat. Sci. 2012;3(2):56–70. [Google Scholar]
- Shafi M., Quddus M.M.A. Bangla academy; Dhaka: 1982. Bangladesher matshya sampad (Fishery resources of Bangladesh) p. 44. [Google Scholar]
- Shakuntala K. The Relation between Body Size and Number of Eggs in the Freshwater Prawn, Macrobrachium lamarrei (H. Milne Edwards) (Decapoda, Caridea) Crustaceana. 1977;33:17–22. [Google Scholar]
- Sharma A., Subba B.R. General biology of freshwater prawn, Macrobrachium lamarrei (Milne-Edwards, 1837) of Biratnagar, Nepal. Our Nature. 2005;3:31–41. [Google Scholar]
- Shoji J., Toshito S., Mizuno K., Kamimura Y., Hori H.K. Possible effects of global warming on fish recruitment: shifts in spawning season and latitudinal distribution can alter growth of fish early life stages through changes in day length. ICES. J. Mar. Sci. 2011;68:1165–1169. [Google Scholar]
- Soomro, A.N., Baloch, W.A., Chandio, T.J., Mohammd, A.W., Saddozai, S., 2012. Condition factor and length-weight relationship of monsoon river prawn Macrobrachium malcolmsonii malcolmsonii (H. Milne-Edwards, 1844) (Palaemonidae) in Lower Indus River. Pakistan J. Zool. 44(5), 1279–1283.
- Subramoniam T. Origin and occurrence of sexual and mating systems in Crustacea: A progression towards communal living and eusociality. J. Biosci. 2013;38:951–969. doi: 10.1007/s12038-013-9392-x. [DOI] [PubMed] [Google Scholar]
- Templeman W. Differences in sexual maturity and related characteristics between populations of thorny skate (Raja radiate) from the northwest Atlantic. J. NW. Atl. Fish. Sci. 1987;7:155–167. [Google Scholar]
- Tiwari K.K. Trend of evolution in the Hendersoni group of species of palaemon Fabricius. (Crustacea: Decapoda) Bull. Natl. Inst. Sci. India, New Delhi. 1955;7:189–197. [Google Scholar]
- Tracey S.R., Lyle J., Haddon M. Reproductive biology and per-recruit analyses of striped trumpeter (Latris lineata) from Tasmania, Australia: Implications for management. Fish. Res. 2007;84:358–368. [Google Scholar]
- Vogt G. Abbreviation of larval development and extension of brood care as key features of the evolution of freshwater Decapoda. Biol. Rev. Cambridge Philos. Soc. 2012 doi: 10.1111/j.1469-185X.2012.00241.x. [DOI] [PubMed] [Google Scholar]
- Wilding T., Young K., Pitkethley R. Environmental report 00/26. Environment Bay of plenty; Whakatane: 2000. Bay of plenty freshwater fish calendar. [Google Scholar]
- Wynne, F., 2000. Grow-out culture of freshwater prawns in Kentucky. Archived from the original on 21 August 2008. Retrieved 4 July 2005.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.