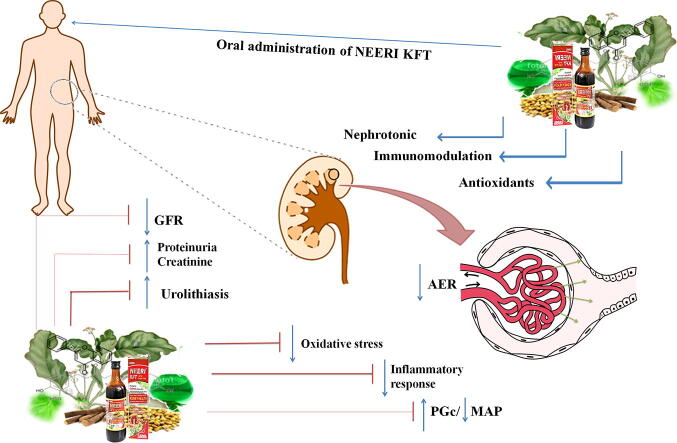
Graphical abstract
Keywords: CKD, NEERI-KFT, Herbal Medicine, Nephroprotection, Oxidative stress, Inflammation
Abstract
Chronic Kidney Disease (CKD) is a major health problem characterized by kidney dysfunction with progressive segmental glomerulosclerosis to end-stage renal disease (ESRD). Due to lack of scientific data and comprehensive reports, the current systematic review provides an inclusive understanding and prospective associated with phytopharmacology of NEERI-KFT in CKD. The data was collected from more than five databases such as Science Direct, Google Scholar, Elsevier, PubMed, Springer, ACS publication etc using keywords like CKD/Kidney disease, epidemiology/prevalence, modern therapies for CKD management, NEERI-KFT and its role in kidney disease. The study was performed based on scientific reports screened by experts according to inclusion and exclusion criteria. The pre-clinical and clinical findings suggested that NEERI-KFT has promising effects as nephroprotective and considered safe and well effective in primary care of kidney against disease. Phytopharmacological evaluation of NEERI-KFT suggest that it exhibit substantial potential against oxidative and inflammatory stress induced apoptosis by exerting antioxidants, nephroprotective and immunomodulatory effects. Hence, it can be enlighten that NEERI-KFT have potential herbs which exerts significant antioxidants, nephroprotective and immunomodulatory effects in the patients associated with renal dysfunction or CKD thus improving altered renal architecture and renal physiology. Clinically, it is concluded that NEERI-KFT works kidney malfunction and cease ESRD progression or even reduce the number of dialysis.
1. Introduction
The term CKD is used to describe kidney dysfunction with progressive segmental glomerulosclerosis (Fogo, 2015). The burden of kidney disease with high magnitude and impacts is defined better in developed and developing countries. Many settings emphasize to curb the development of acute kidney injury (AKI) or chronic kidney disease (CKD) and access the subspecialty care against kidney failure with renal replacement therapy (RRT). As per the report of the center for disease control and prevention (CDCP) 2021, 15% of adults and 14% of women are estimated to have CKD (more than 1 in 7) and most appeared in peoples aged 65 years (older 38%, 45–64-12%, 18–44-6% (Centers for Disease Control and Prevention, 2019). In India, CKD prevalence is estimated with 0.79% to 1.4% rate with 181 per million population incidence rate till 2005 in central Indiana (Rajapurkar and Dabhi, 2010) while India CKD registry claims more than 31% of peoples having CKD with diabetes among 52,273 adults (Tatapudi et al., 2019). A report published in 2015 “Start India Project” stated more than 40% of peoples having CKD among Type 2 diabetes mellitus (T2DM) patients and one CKD patient among five hypertensive subjects (Hussain et al., 2019).
Besides, progressive and continuous exposure of common varieties of drugs and/or environmental toxins results in ESRD or CKD (Todi and Majumdar, 2019). CKD is associated with a high prevalence risk factors in the patients such as hypertension,(Pugh et al., 2019) chronic obstructive pulmonary disease (COPD) (Gai et al., 2019), diabetes mellitus (Hahr and Molitch, 2015) depression (Shirazian, 2019). The recent data revealed the prevalence of CKD is not only stealing human health but also a cause of high morbidity and mortality due to these factors (Snider et al., 2019).
The modern system of medicine deals with many therapies like angiotensin-converting enzyme inhibitors (ACEis) or angiotensin-receptor blockers (ARBs), mineralocorticoid-receptor antagonists, sodium/glucose cotransporter 2 (SGLT2), statins, beta-blockers, H2-blockers, cardiovascular drugs, anti-fibrotic and anti-inflammatory and vitamins like Vitamin-B, C, D, etc are used for the management of kidney function through multimechanistic approaches by normalizes glomerular hyperfiltration, inhibiting renin-angiotensin system, reduce blood pressure, albuminuria, low-density lipoprotein cholesterol (LDLc) and C-reactive protein (CRP), systemic vascular resistance, creatinine level, improves antioxidant defense system, prevention of podocyte hypertrophy and inflammatory loss (Breyer and Susztak, 2016), (Yuan et al., 2016). Despite the significant progress of these therapies, the results for the treatment of kidney patients are still far from perfect. Due to the lack of availability and several adverse events associated with the use of synthetic drugs, such as hyperkalemia, gynecomastia, neurocognitive, fatigue, nausea, constipation erectile dysfunction, etc, prompts the researcher for the development of newer drugs from natural sources which can protect distorted function even improve overall architect of the kidney (Peltzer and Pengpid, 2019).
The trends in the selection of medicine have been shifted from synthetic to herbal medicine. In the last few decades, there has been an exponential growth in the field of herbal medicine and the medicines are gaining popularity in both developed and developing countries, because of their natural origin, easy availability and lesser side effects. The traditional system of medicine is known for millennia and highly esteemed throughout the globe as a therapeutic regimen for the prevention of diseases (Parveen et al., 2020).
NEERI-KFT is one of the famous traditional Indian Ayurvedic poly herbal sugar-free formulation own license by Aimil Pharmaceuticals India Ltd for its manufacturing. The formulation is comprised with different twenty herbs like Amaranthus spinosus, Boerhaavia diffusa, Butea monosperma, Crataeva nurvala, Carica papaya, Moringa oleifera, Nelumbo nucifera, Tinospora cordifolia, Solenum nigrum, Tribulus terrestris, Veteveria zizanioides, etc (Table 2). The formulation is targeted to reverse distorted renal architect and physiology in the patients associated with acute or chronic kidney functional insufficiency. The pre-clinical investigations reveal the phytopharmacological significance of “NEERI-KFT” possesses complexity of phytoconstituents with radicalized diversity in therapeutic values. Anti-oxidant, immunomodulator and nephroprotective activity are the reported primary function of NEERI-KFT intended to prevent the progressive deterioration of kidney function, restore the physiological strength of nephron with overall normalcy in the altered biochemical of kidney such as serum proteins, serum creatinine, serum urea while phytochemical evaluation revealed phenols, flavonoids, alkaloids, and glycosides are the active principle constituents which overcome the onsets of kidney disease (Gupta et al, 2018).
Table 2.
Ethnopharmacological and current evidences against NEERI-KFT ingredients for kidney/urinary, urolithiatic disorder via antioxidant and anti-inflammatory action.
| Sr. No. | Ingredients | Traditional claim |
Modern claim |
||||
|---|---|---|---|---|---|---|---|
| API | UPI | Dose (O.A/BW) | Exposer | Mechanism of action | References | ||
| 1. | Boerhavia diffusa L. (Root) | Mutrala (Diuretic), Vatakantaka/sothahara (Anti-inflammation) API, Part-I, Vol-I, V & IX, 140, 211 & 103 |
– | 800 mg/kg. | Gentamicin (80 mg /Kg BW, I.P) | Restore antioxidant defensive potential, renal function capacity and speeds up recovery from glomerulonephritis, tubular cell toxicity, and altered intraglomerular hemodynamics with a reduction in inflammatory and oxidative responses. | (Padmini, 2013) |
| 2. | Cichorium intybus L. (Stem) | – | Mudir-e-Baul (Diuretic) UPI, Part-I, Vol-VI, 96 |
3.4 mg/kg | Gentamicin (80 mg/Kg BW, I.M) | Reconditions the function of glomerular, peritubular, blood vessel congestion with the presence of inflammatory cells causing severe necrosis | (Emamiyan et al., 2018) |
| 3. | Solanum nigrum L. (Leaf) | Prameha (Urinary disorder/ increased frequency and turbidity of urine) API, Part-I, Vol-II, 68 |
Muhallil-e-auram (Anti-inflammatory) UPI, Part-I, Vol-VI, 93 |
200 mg/kg | Amphotericin B (10 mg/Kg BW, I.P) | Improve biochemical, antioxidants enzymatic system and restore the functions of glomerular and reduced acute interstitial nephritis due to oxidative stress | (Geo and Baskaran, 2011) |
| 4. | Tinospora cordifolia L. (Stem) | Prameha (Urinary disorder/ increased frequency and turbidity of urine) API, Part-I, Vol-I, 41 |
Mudirr-e-Baul (Diuretic) UPI, Part-I, Vol-I, 31 |
200 mg/kg | Aflatoxin B1 (2 Μg/30 G BW, O.A) | Decrease lipid peroxidation and protein carbonyl content, normalizing the levels of cellular antioxidants, improve architect of glomeruli, and intraglomerular hemodynamics altered due to oxidative and inflammatory stress. | (Gupta and Sharma, 2011) |
| 5. | Nelumbo nucifera G. (Flower) | Mutra virajaniya (Urinary depigmenter) API, Part-I, Vol-II, 70 |
– | 100 mg/kg | Gentamicin (100 mg/Kg BW, I.P) | Enhances speedy restoration of renal cellular functions via antioxidative action in gentamicin mediated nephrotoxic rat. | (Dubey et al., 2014) |
| 6. | Butea monosperma Lam. (Flower) | Mutrakrcchra (Dysuria) and Prameha (Urinary disorder/ increased frequency and turbidity of urine) API, Part-I, Vol-V, 162 |
Mudirr-e-Baul (Diuretic) UPI, Part-I, Vol-II, 83 |
400 mg/kg | Gentamicin (100 mg/Kg/BW ,I.P) | Preserve podocyte and tubulointerstitial inflammation and microcysts via antioxidative action, normalize biochemical and histopathology altered due to oxidative stress. | (Sonkar et al., 2014) |
| 7. | Tribulus terrestris L. (Fruit) | Mutrakrcchra (Dysuria)API, Part-I, Vol-1, 40 | Mudirr-e-Baul (Diuretic), Mufattit-e-Hasat (anti-lithotriptic) UPI, Part-I, Vol-I, 53 |
500 mg/kg | Cisplatin (5.5 mg/Kg, BW,I.P) | Improve renal epithelial damage, inflammation, and glomerular morphology as well as reduce biochemical biomarkers urea and creatinine levels to normal and reduced onslaughts of oxidative stress. | (Raoofi et al., 2015) |
| 8. | Albizzia lebbeck L. (Stem) | – | – | 400 mg/kg | Streptozotocin 60 mg/Kg,BW,I.P) | Improve antioxidants defense system and acute interstitial nephritis altered by streptozotocin-induced nephrotoxicity. | (Ahmed et al., 2014) |
| 9. | Pterocarpus santalinus L. (Stem) | – | Bol-ud-dam(Heamaturia) Tanqueehul Mufradat (Tanqeehul mufradat, Pg-169) |
250 mg/kg | Alcohol (20% O.A) | Protection from degeneration, necrotic changes of tissue with distortion of normal architecture, and tissue congestion due to oxidative stress with the speedy recovery of antioxidant enzyme activities and restoration of Na+/K+-ATPase activity to near-normal levels. | (Bulle et al., 2016) |
| 10. | Curcuma longa L. (Rhizome) | Prameha (Urinary disorder/ increased frequency and turbidity of urine) API, Part-I, Vol-I, 61 |
Muhallil-e-Auram (Anti-inflammatory) Tanqueehul Mufradat (Tanqeehul mufradat, Pg-108) |
200 mg /kg | Gentamicin (80 mg/Kg/BW, I.P) | Curcumin treatment exerted anti-apoptosis and anti-oxidative effects by up-regulating Nrf2/HO-1 and Sirt1 expression | (Jogdand et al., 2017) |
| 11. | Moringa oleifera Lam. (Leaf) | Mutrasarkara (Normalization of glucose in urine), API, Part-I, Vol-IV, 111 | – | 200 mg/kg | Acetaminophenon (400 mg/Kg/BW, I.P) | Improve disorganized glomerulus, tubular dilation with moderate tubular casting and inflammation as well as restore the function of the antioxidant system. | (Karthivashan et al., 2016) |
| 12. | Vetiveria zizanioides (Stem) | Mutrakrcchra (Dysuria) API, Part-I, Vol-III, 221 |
– | NA NA |
NA | NA | NA |
| 13. | Hemidesmus indicus L. (Stem) | Raktavikara (Blood detoxification) API, Part-I, Vol-I, 107 |
– | 500 mg/kg | Cisplatin (12 mg/Kg /BW I.P) | Restore the function of renal tubule against glomerulonephritis, antioxidant defense system, and altered intraglomerular hemodynamics in cisplatin-mediated nephrotoxic rats | (Sandeep and Krishnan Nair, 2010) |
| 14. | Coriandrum sativum L. (Fruit) | Mutrala (Diuretic) API, Part-I, Vol-I, 31 | Muhallil-e-waram (Anti-inflammatory) UPI, Part I, Vol-I, 57 |
400 mg/kg | Gentamicin 100 mg/Kg/BW,I.P) | Preserve the absorption function distorted due to damaged glomerular and tubular part of the kidney | (Lakhera et al., 2015) |
| 15. | Crataeva nurvala Buch. (Stem) | Raktavikara (Disorders of blood) API, Part-I, Vol-VI, 87 |
– | 700 mg/kg | Cisplatin (5 mg/Kg,BW, IP) | Reduce intracellular accumulation and covalent bonding of its highly reactive metabolite promoted by lipid peroxidation, | (T.T. et al., 2011) |
| 16. | Amaranthus spinosus L. (Seed) | – | – | 450 mg/kg | Ccl4 (3 ml /Kg ,BW, SC) | Reduce glomerular and tubular degenerations with evident in the endothelial lining of glomerular tuft and the epithelial lining of renal tubules. Bowman’s capsule was observed along with swollen collecting dust, distinct intertubular connective tissue and thickening of collecting tubules are an indicator of constant irritation | (Kengar et al., 2017) |
| 17. | Rheum emodi Wall. (Rhizome) | – | Mudirr-e-Baul(Diuretric) UPI, Part-I, Vol-II, 92 |
350 mg/kg | Gentamicin (100 mg/Kg,BW, I.P) | Possess antioxidants defense against oxidative stress and increase the affinity of proximal tubule with low-molecular tubular proteinuria, calciurea, and in some cases glucosuria. | (Alam et al., 2005) |
| 18. | Cucumis utilissimus/melo Roxb., (Seeds) | Mutrakrcchra (Dysuria) API, Part-I, Vol-II, 39 |
Mudirr-e-Baul (Diuretic) UPI, Part-I, Vol-IV, 64 |
500 mg/kg | Gentamicin (100 mg/Kg/BW, I.P) | C. melo extract reversed plasmacytic infiltration the action of toxicant with improved thinning, and edema in renal parenchyma as well as tubular atrophy, and glomeruli | (Saleem et al., 2019) |
| 19. | Carica papaya L. (fruit pulp) | Mutraroga (Urinery diseases), API, Part-I, Vol-VI, 89 | – | 500 mg/kg | Paracetamol (1 g/Kg, BW, O.A) | Improve bowman's capsule with moderate leukocyte infiltration; normalized glomerular filtrate, reabsorption from the proximal tubules, reflected the structure of the epithelial lining, brush border in some tubules implies improvement in the functioning of the nephrons as well as reduction of the biochemical markers in the blood. | (Naggayi et al., 2015) |
| 20 | Piper cubeba L. (Fruit) | – | – | 810 mg/kg | Gentamycin (80 mg/Kg BW, I.M) | Improve interstitial edema, a mild degree of glomerular congestion, and few congested blood vessels. Mild tubular damage was observed. The tissue was sparsely infiltrated by chronic inflammatory cells | (Ahmad et al., 2012) |
In this systematic review, a comprehensive prospectus based on polyherbal sugar-free syrupy formulation “NEERI-KFT” has been described concerning its and herbal ingredients role in management of kidney disease through multimechanistic approaches as per pre-clinical, clinical scientific evidences.
2. Methods
The studies were conducted according to the preferred reported items/checklist from the systematic reviews or research articles statements published in national and international journals.
2.1. Search strategy
The information was gathered through a prospective comprehensive literature search using more than five electronic databases such as Science Direct, Google Scholar, Elsevier, PubMed, Springer, ACS publication from published database between 2000 and 2020 for the selection of recent and impactful information for the study. The combination of keywords used in electronic databases was as follows: Epidemiology and or prevalence of CKD globally, CKD/Kidney disease, nephrotoxicity, nephroprotection, the modern system of medicine & therapies for CKD treatment, the traditional system of medicine & medicinal plants for CKD treatment, NEERI-KFT, phytochemistry, antioxidant activity, nephroprotective activity, anti-inflammatory activity and oxidative and inflammatory stress associated with pathophysiology of CKD, etc. The reference list of all included papers was checked for other potentially relevant citations. The selection of study was restricted to articles in English because of the language barrier, time efficiency, and high cost for the translation. To achieve a comprehensive search of relevant studies, the publication history of the journal was searched as systematic reviews with the hyphenated keyword “Role of medicinal plants in management of CKD; A Systematic review” and the relevant data was extracted to access in the selection process and summarize as potential information on NEERI-KFT and its herbal ingredients.
2.2. Criteria’s for original studies
2.2.1. Inclusion criteria
The review or research articles published in national and international journals concerning with prevalence of CKD, NEERI-KFT and its ingredients, molecular approaches for oxidative and inflammatory stress with comprehensive discussion of the study were added in the original study.
2.2.1.1. Exclusion criteria
Studies such as duplicate publication, abstract and research published before 2000, unauthentic ethno-botanical/ethno-medicinal reports lacking study areas/localities, informant’s involvement, data of untargeted plant and disease and from non-open access journal articles or partially accessed articles were removed to avoid misappropriate comprehensives.
2.2.1.2. Study selection
All the authors independently evaluated the studies through their inclusion criteria by screening the title and abstract of each record and retrieved their full text if necessary. The selection of the study includes only good indexed journal's publication (research and review articles) related to CKD for the trending input. The screening of search outputs was performed based on the title and the abstracts of identified journal articles/theses. Besides, out of thousands reports, only suitable reports were downloaded and critically inspected to elaborate the discussion concerning preclinical and clinical evaluation of NEERI-KFT and its and mechanistic approach against oxidative and inflammatory stress induced CKD.
3. Review findings
3.1. Modern medicine for CKD management
Angiotensin-converting enzyme inhibitors (ACEs) or angiotensin-receptor blockers (ARBs) provides an effective aid in the treatment of diabetic nephropathy, ameliorate albuminuria and normalize eGFR (Breyer and Susztak, 2016) while mineralocorticoid-receptor antagonists reduce proteinuria, high blood pressure, cardiovascular events and eGFR mortality (Ward et al., 2015). Sodium/glucose co-transporter 2 (SGLT2) inhibitors reduce plasma glucose, blood pressure, body weight (BW), proteinuria and significant reduction in eGFR (Dekkers and Gansevoort, 2020). Statins therapy is accounted to reduce cardiovascular events in pre-dialysis CKD patients, low-density lipoprotein cholesterol (LDLc) and C-reactive protein (CRP) which irreversibly decrease the risk of renal failure (Zhao et al., 2019). Beta-blockers and H2-blockers/H2 receptor antagonists seem to be more effective in the reduction of creatinine levels and ameliorate eGFR (Pachon et al., 1989). Moreover, vitamins like Vitamin-B, C, D repair tubular cell toxicity ameliorates fluoride nephrotoxicity, aids renal excretion of other toxins, and improves the antioxidant defense system. Vitamins also play a major role in the prevention of podocyte hypertrophy and regulate inflammatory cytokines responsible for podocyte loss (Ward et al., 2015), (Malihi et al., 2019). Despite, the progressive utilization of modern medicine got limitations because of its lower production rate and the occurrence of serious harms during the treatment of kidney disease. Modern medicine for the management of CKD and their adverse events enlisted in Table 1.
Table 1.
Modern medicine for the management of CKD and their adverse events.
| Class | Drug | Mode of action | Associated adverse events | References |
|---|---|---|---|---|
| Angiotensin-converting enzyme inhibitors (ACEis) or angiotensin-receptor blockers (ARBs) | Captopril Benazepril |
Normalize glomerular hyperfiltration, inhibition of the renin-angiotensin system | Hyperkalemia and hypotension | (Breyer and Susztak, 2016), (Ward et al., 2015), (Xiang et al., 2019) |
| Mineralocorticoid-receptor antagonists | Spironolactone, eplerenone, Finerenone | Reduce blood pressure, eGFR, and albuminuria | Hyperkalemia, Gynecomastia | |
| Sodium/glucose cotransporter 2 (SGLT2) | Empagliflozin canagliflozin | Albuminuria reduction, decrease in eGFR, Hemodynamic | Hyperkalemia | |
| Statins | Atorvastatin, simvastatin | Reductions in low-density lipoprotein cholesterol (LDLc) and C-reactive protein (CRP) | Neurocognitive | (Zhao et al., 2019), (Herrera-Gómez et al., 2019) |
| Beta-blockers | Carvedilol and nebivolol, metoprolol | Reduction in systemic vascular resistance | Fatigue, nausea, and constipation erectile dysfunction | (Sinha and Agarwal, 2019), (Kover, 2012) |
| H2-blockers | Cimetidine, omeprazole | H2 receptor antagonists, reduction in creatinine levels and estimated glomerular filtration rate | Delirium, drowsiness, and somnolence | (Pino and Azer, 2019), (Cheema, 2019) |
| Cardiovascular drugs | Digoxin, Digitoxin | Reduction in systemic vascular resistance | Hyperkalemia, automaticity, and inotropy | (Yamagami et al., 2019) |
| Anti-fibrotic and anti-inflammatory | Pentoxifylline, Pirfenidone | Stimulation of methylxanthine phosphodiesterase inhibitor, RAS inhibition | Anorexia, dyspepsia, gastroesophageal reflux syndrome and constipation | (Ward et al., 2015), (Donate-Correa et al., 2019) |
| Vitamins | Vitamin-B, C, D | Repairs tubular cell toxicity ameliorates fluoride nephrotoxicity, aids renal excretion of other toxins and improves antioxidant defense system, Prevention of podocyte hypertrophy and loss, regulation of NF-κB | Hypercalcemia, hypercalciuria, elevated uric acid and oxalate level | (Ward et al., 2015), (Malihi et al., 2019), (Hon, 2014) |
3.2. Need of herbal medicine for CKD management
Traditional medicines are dealing with several phytoconstituents which play a crucial role in the treatment of disease through their catalytic and synergistic potential (Nasri and Rafieian-Kopaei, 2013). The increasing utilization of herbal medicine is attributed as alternative treatments in developed and developing countries because of their natural origin, least or no side effects, affordability and accessibility (D. Q. Chen et al., 2018). However, increasing incidence and high prevalence of herbal medicine as complementary and alternative medicine (CAM) among the peoples is on the peak for the treatment of kidney and associated ailments. CAM may provide a new therapeutic option for the patients with ESRD and may mitigate symptoms and improve health-related quality of life. In the such conditions where dialysis or kidney transplant remains the only option for the patient with ESRD or complete loss of kidney function, CAM may provide an effective treatment by ameliorating biomarkers, progressive segmental glomerulosclerosis and eGFR (Castelino et al., 2019).
3.3. NEERI-KFT for CKD management
NEERI-KFT is a traditional Indian sugar-free Ayurvedic polyherbal formulation which is well known for incredible dimensionality in therapeutic values against kidney malfunction. Aimil Pharmaceuticals India Ltd owns the license to fabricate and facilitate the NEERI-KFT out of the globe (Tiwari et al., 2016). The integrated clinical expertise was profiled on NEERI-KFT and developed the availability of the best clinical evidence which suggests that NEERI-KFT significantly reduces serum creatinine, blood urea, and serum uric acid as compared to placebo group and described its well-tolerated effect with no adverse hematological or biochemical abnormalities occurred by any subjects during clinical trial. The phytochemical screening revealed phenol, tannins, amino acid, glycoside, etc are the main principal components of NEERI-KFT while stability assessment reveals no changes even after six months of observation concerning physical, chemical, and microbial disability (Gupta et al, 2018). In a pre-clinical investigation of “NEERI KFT” demonstrated significant amelioration of biochemical and histology of damaged kidney via reducing oxidative and inflammatory stress. Further, the author claims antioxidant, nephroprotective, and immunomodulatory effect are the primary function of NEERI-KFT which produce multiple curative and preventive effect in kidney dysfunction by correcting damaged renal architect and strengthen kidney functions to acumen kidney’s associated ailments. However, emerging evidence on each herbs of NEERI-KFT suggests its regenerative and reparative capacity for kidneys against deleterious effect through their nephroprotective and anti-oxidant/inflammatory activity as shown in Table 2.
3.3.1. Herbal ingredients and reported therapeutic efficacy of NEERI-KFT for kidney disease
The emerging evidence on NEERI-KFT and its herbal ingredients deal with the varieties of phytochemical constituents includes phenolic, flavonoids, tannins, saponins, alkaloids, and glycoside. As per the record of NEERI-KFT, it is composed of verities of herbal medicine along with panchtarinmool as a classical formulation. Due to a lack of phytopharmacological comprehensive reports on NEERI-KFT, an attempt has been made to include phytopharmacological studies that have been conducted on herbal ingredients of NEERI-KFT at a single platform. The herbal ingredients of the NEERI-KFT and their ethnopharmacological claim as per traditional scripters (Ayurvedic pharmacopeia of India (API), and Unani pharmacopeia of India (UPI) and current evidenced have summarized in Table 2.
3.3.2. Reported chemical constituents of NEERI-KFT herbal ingredients for management of CKD
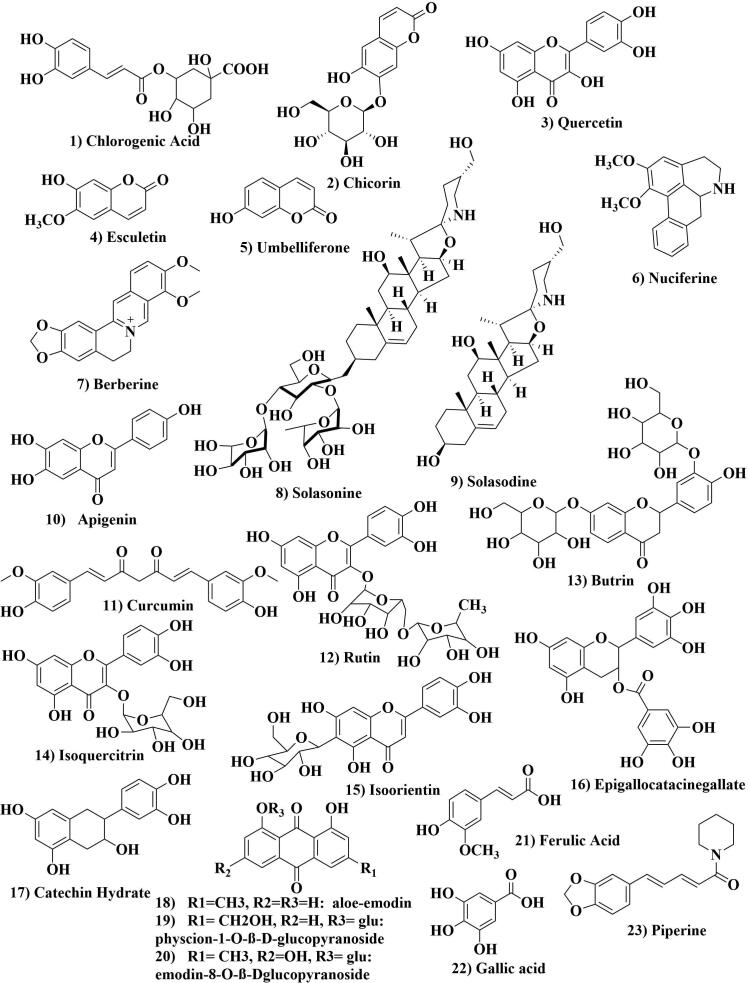
Phytopharmacological evaluation based on single constituents of NEERI-KFT or its herbs still is a lack in the establishment with complete phytochemical analysis. Although there is no published report deals with phytochemistry/analysis to identify chemical constituents of NEERI-KFT. An extensive search of the database was made on the phytochemistry of herbal ingredients reported to concern nephroprotective activity. Except few herbal ingredients, no evidences were found on individual phytoconstituents or even isolated and then subjected to their nephroprotective activity from medicinal plants. A comprehensive report was drafted based on cross-referencing of phytoconstituents pertaining NEERI-KFT/herbal ingredients for easy accessibility of phytopharmacological data for nephroprotective activity. The evidence revealed many nephroprotective phytoconstituents belong to diverse as phenol, flavonoids, alkaloids, glycosides, etc. which protect the kidney from distortion by oxidative and inflammatory stress. Further, to evaluate the majority of phytoconstituents responsible for nephroprotective action, target-based phytopharmacological analysis is needed which would be included as scientific proof concerning NEERI-KFT phytopharmacology. The reported chemical constituents of herbal ingredients of NEERI-KFT and their chemical structure are described in Fig. 1 and Table 3.
Fig. 1.
Chemical structures of reported phytoconstituents of NEERI-KFT herbs for management of kidney disease via antioxidant, nephroprotective and anti-inflammatory action.
Table 3.
Reported phytoconstituents of NEERI-KFT herbal ingredients for possible management of kidney disease via nephroprotective, antioxidant, immunomodulatory or anti-inflammatory action.
| Sr. no | Plants | Therapeutically active constituents | Associated pharmacological activity and or mechanism of action | References |
|---|---|---|---|---|
| 1. | Boerhavia diffusa L. | Quercetin | Reduces inflammatory and oxidative stress induced by MAPK signaling and ROS and improve anti-oxidative defense mechanism. | (Sánchez-González et al., 2017), (Sharma and Sahai, 2017) |
| 2. | Cichorium intybus L. | Chlorogenic Acid, Chicorin, Esculetin, Umbelliferone | Improve histological alterations in exposed kidney tissues arisen by oxidative and inflammatory stress. | (Metwally et al., n.d.), (Q, 2016), (Zaman et al., 2017), (Das et al., 2016), (Kisiel and Zielińska, 2001), (Satmbekova et al., 2018) |
| 3. | Solanum nigrum L. | Solasonine Solasodine | Normalize Na + and K + ion concentration, inhibit nephrolithiasis and improve dirutic effect. | (Pharma et al., 2010), (Gu et al., 2018), (Xiang et al., 2018) |
| 4. | Tinospora cordifolia L. | Berberine | Reduces cisplatin induced ROS inflammation by regulation expression of nuclear factor-kappa B (NF-kB), tumor necrosis factor-a (TNF-a), cyclooxygenase-2 (COX-2) and nitric oxide synthase (iNOS), along with p53. | (Domitrović et al., 2013), (Upadhyay et al., 2010) |
| 5. | Nelumbo nucifera G. | Nuciferine | Improves potassium oxonate-induced hyperuricemia through preserving action of renal urate transport-related proteins and suppresses renal inflammation by regulation of differentiation factor 88/NF-kappaB (TLR4/MyD88/NF-κB) signaling. | (Wang et al., 2015), (Paudel and Panth, 2015) |
| 6. | Butea monosperma Lam. | Butrin | Mitigates increased levels of lipid peroxidation and ameliorate glutathione, SOD, and catalase in different toxicant model. | (Nair et al., 2013), (G.Rajeswari, 2013) |
| 7. | Tribulus terrestris L. | Rutin | Improve cisplatin-induced interstitial congestion, focal mononuclear cell inflammatory, cell infiltrate, and acute tubular injury and apoptotic cells. It attenuates cisplatin-induced alteration in gene expression and structural and functional changes in the kidney. | (Alhoshani et al., 2017), (Yadava and Tiwari, 2005) |
| 8. | Albizzia lebbeck L. | Quercetin Apigenin |
Ameliorates biomarkers and antioxidant enzyme and reduce the levels of TNF-α, IL-1β and TGFβ in the kidney. Additionally, inhibits the activations of CYP2E1, phospho-NF-κB, p65 and phospho-P38 MAPK in toxicant-induced renal injury | (He et al., 2016), (Sánchez-González et al., 2017), (Verma et al., 2013) |
| 9. | Pterocarpus santalinus L. | Epigallocatacinegallate | Improved renal function with efficient reduction in lipid peroxidation levels and ameliorates kidney biomarkers and antioxidants enzymes. | (Profile, 2017), (Katiyar et al., 2016) |
| 10. | Curcuma longa L. | Curcumin | Ameliorate kidney biomarkers and antioxidants enzymes while reduce malondialdehyde (MDA). It suppresses verities of inflammatory stimulus includes iNOS, BAX, TNF-α and Caspase3 gene expressions turn to apoptosis. | (Abd El-Kader and Taha, 2020) |
| 11. | Moringa oleifera Lam. | Isoquercitrin | Ameliorates urinary excretion of electrolytes, or plasmatic levels of Na+ and K+ ions. | (Gasparotto Junior et al., 2011), (Q, 2016), (Vergara-Jimenez et al., 2017) |
| 12. | Vetiveria zizanioides | Isoorientin | Mitigates SIRT1- and SIRT6-mediated Nrf2 activation and promotion in oxidative stress, inflammation and apoptosis induced by nephrotoxicity. | (Fan et al., 2020), (Prajna et al., 2013) |
| 13. | Hemidesmus indicus L. | Steroid, Saponin, Resine Tannine | Showed nephroprotective effect against gentamicin induced nephrotoxicity | (Q, 2016) |
| 14. | Coriandrum sativum L. | Caffeic Acid Quercetin Chlorogenic Acid |
Exerts significant protective effect by amelioration of kidney biomarkers, oxidative and inflammatory stress thus improving distorted architect of the kidney. | (Kinra et al., 2019), (Sánchez-González et al., 2017), (Metwally et al., n.d.) |
| 15. | Crataeva nurvala Buch. | Catechin Hydrate | Renal structural and functional abnormalities accompanied by toxicants with renal oxidative stress efficiently reduced by Catechins. Catechin improves renal-reduced antioxidative defense enzymes. Hence it showed considerably nephroprotective action against toxicant-induced nephrotoxicity by preventing distracted renal structural, functional abnormalities and oxidative stress. | (Sardana et al., 2015), (Khattar and Wal, 2012) |
| 16. | Amaranthus spinosus L. | Rutin Caffeic Acid Quercetin Isoquercetrin |
Protect kidney function against varieties of inflammatory cytokines and free radicals oxidant species. | (Kinra et al., 2019), (Sánchez-González et al., 2017), (Gasparotto Junior et al., 2011), (Q, 2016), (Tanmoy et al., 2014) |
| 17. | Rheum emodi Wall. | Aloe-emodin, physcion-1-O-ß-D-glucopyranoside, emodin-8-O-ß-D-glucopyranoside | Emodins having protective action on cell viability and restored the cisplatin-induced glutathione depletion and total antioxidant capacity in a dose-dependent manner | (Waly et al., 2013), (ZHENG et al., 2013), (Zargar et al., 2011) |
| 18. | Cucumis utilissimus L./ Cucumis melo | Catechin Quercetin |
Improve antioxidative enzyme level and prevents progression of glomerulonephritis due to oxidative stress induced by free radicals oxygen species. | (Sardana et al., 2015), (Sánchez-González et al., 2017), (Metwally et al., n.d.), (Q, 2016), (Ullah et al., 2015) |
| 19. | Carica papaya L. | Ferulic Acid Gallic acid |
Reduces the level of BUN, creatinine, MDA (Malondialdehyde), MPO (Myeloperoxidase), TOS (Total Oxidative Status), PtNT (Protein Nitrotyrosine), prevent oxidative stress, increasing antioxidative status and restore histopathological injuries of renal tissue. | (Bami et al., 2017), (Rolim et al., 2018), (Singh and Ali, 2011) |
| 20. | Piper cubeba L. | Piperine | It improves toxicant-induced alteration in kidney histopathology (kidney damage, necrosis of tubules) along with the significant reduction of BUN, creatinine, and MDA by amelioration in the SOD and GPx in the kidney. | (Sudjarwo et al., 2017), (Nahak and Sahu, 2011) |
3.3.3. Elaboration of the study of Neeri KFT against oxidative stress-induced CKD (pre-clinical and clinical investigations)
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are normally generated in living organism cells because of normal cellular metabolism or maintenance of cellular homeostasis. However, the excess production of ROS/RNS is harmful or constructing adverse oxidative modifications into cell/components results in mitochondrial dysfunction, oxidative autophagy, DNA damage (Cenini et al., 2019). ROS/RSN and clumps tyrosine-containing protein products (Advanced Oxidation Protein Products—AOPPs) produce counteract effect over the function of kidney results in podocyte injury, progressive focal segmental glomerulosclerosis (FSGS), and sustained tubulointerstitial fibrosis (Daenen et al., 2019), (Duni et al., 2019).
Free radicals such as superoxide radical (•O2), hydroxyl radical (•OH), nitric oxide (•NO), peroxynitrite (•ONOO) degrades the enzymes such as NADH dehydrogenase, cytochrome c oxidase and ATP synthase and results in the shutdown of mitochondrial energy production (Panday et al., 2015). Mitochondria enzyme catalysis system and anti-oxidant enzyme defense system together accounted for in redox balance, failure of these systems may lead sustainability in oxidative stress and causes acute to chronic kidney disease (Duni et al., 2019), (Granata et al., 2015). The well-understood mechanism of mitochondrial ROS generation includes subsequent disruption of iron-sulfur (Fe-S) centers, releasing iron, and promoting processes that impair mitochondrial function along with distortion/collapse of mitochondrial membrane channels lead further ROS generation (Gordan et al., 2018). Increased ROS induces accumulation of uncoupling protein-2 (UCP2) interferes with ATP synthase and reduces ATP synthesis in renal tubular epithelial cells. The consequent reduction in ATP, autophagy induced apoptosis or necrosis occur followed by the formation of the apoptosome, activation of cytochrome C, caspase activation, and consequently apoptosis (Duni et al., 2019), (Granata et al., 2015), (Peng et al., 2019).
Autophagy or macroautophagy formation of the phagophore occurs by the wrapping of cellular organelles during nutrient starvation. In starvation, the canonical autophagic stimulus inhibits the mammalian target of rapamycin (mTOR) to allows the activation of ULK1 and ULK2, which together with ATG proteins form the complex that localizes on the phagophore to induce the autophagosome nucleation (Mathiassen et al., 2017). The formation of double-membrane structure induced via Beclin 1 forming distinct phosphatidylinositol 3-kinase complexes and the elongation of the phagophore, principally driven by ATG9, determines the formation of a double-membrane vesicle creating the autophagosome, which then fuses with the lysosome (Lin et al., 2019). The introduction of target protein inside the autophagosome is generally mediated by binding with the light chain 3-II (LC3-II) which localizes at autophagosome surface through phosphatidylethanolamine (PE) post-translational modification. The process of autophagy is selective in principle as many adaptor proteins allow LC3-II to recognize specific targets. Besides, p62/sequestrosome 1 (SQSTRM1) has been characterized as the selective mediator of ubiquitinated protein degradation via autophagy (Ciccarone et al., 2019).
Further, oxidative stress-induced cellular deprivation and autophagy responsible to lose the ability of blood vessel dilation and reversely cause glomerular hypertension or hyper-filtration which accompanied with podocyte and endothelial injury along with resistance in mesangial cell proliferation. The ability to perform autoregulation by nephrons, which can be lost due to induced oxidative stress and may cause chronic glomerulonephritis (Lin et al., 2019). Emerging evidence based on pre-clinical and clinical investigations on NEERI-KFT and its ingredients suggests its immense role in inhibiting autoxidation by free radicals through improvement in the antioxidative system and prevent progressive deleterious effects on kidney/renocytes (Gupta et al, 2018, Kumar et al., 2015). A study conducted by Sharma et al., 2020 reported that NEERI-KFT significantly (p ≤ 0.05) ameliorates the kidney function markers and oxidative stress markers elevated against the methotrexate-induced nephrotoxicity in the rat (Sharma et al., 2020)
3.3.4. Implications of NEERI-KFT against inflammatory stress-induced CKD
In inflammation, germline-encoded pattern recognition receptors (PRRs) are the host sensors that recognize damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) followed by activation of toll-like receptors (TLRs), intracellular nod-like receptors (NLRs), retinoic acid-inducible gene (RIGs) receptors, etc which play a major role to provoke an inflammatory response (Rayego-Mateos et al., 2019), (Kaneko et al., 2019). Inflammatory cytokines like tumor necrosis factor-α (TNF-α) interleukin-1β (IL-1β), IL-6 and their coordinated interaction with adopter protein like myeloid differentiation factor-88 (MyD88) activate most intracellular signaling pathways which includes nuclear factor kappa-B (NF-κB), Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway and mitogen-activated protein kinase (MAPK) (Lee et al., 2019), (Zhang et al., 2018)
During cellular stress, the NF-κB transcription factor coordinates with several associated transcription proteins including p50, p52, p65, RelA/B etc which activation depends on the nature of stimuli like pathogen-derived effectors, pro-inflammatory cytokines, and enzymes (Kumar et al., 2015). IκB kinases (IKK) which present in the cytoplasm are an integral part of the transduction cascade of NF-κB that induced cellular response to inflammation. IκB kinase (IKK) is derived from two kinase subunits including IKKα and IKKβ, with a regulatory subunit, IKKγ. NF-κB pathway activation is regulated by IκB phosphorylation and activation of IκB kinase (IKK) uses a similar mechanism of signal transduction as used by PRRs (Song et al., 2019). IκB phosphorylation represents its reversal effect after degradation through proteasome and results in the subsequent deliverance of NF-κB to translocate into the nuclear region and stimulate gene transcription. Pro-inflammatory cytokine production and inflammatory cell recruitment are regulated by this pathway which plays a critical role in the inflammatory response (Zhang and Sun, 2015).
Furthermore, MAPKs belong to the family of serine/threonine protein kinases, stimulates cellular response against a variety of stimulus includes mitogens osmotic stress, heat shock and inflammatory cytokines (such as TNF-α, IL-1, and IL-6) which play role in the regulation of cell proliferation, differentiation, cell survival and apoptosis (Lee et al., 2019). Among this process, ERKs are mainly triggered by mitogens and many differentiation signals, while JNK and p38 are triggered by inflammatory stimuli and stress (Zhang et al., 2018). In this pathway, serine/threonine protein kinases deal with a variety of MAPKs include extracellular-signal-regulated kinase ERK1/2, MAP Kinase, p38 MAP Kinase, and c-Jun N-terminal kinases (JNK) (Lee et al., 2019). MAPKs are the complex of main three proteins includes MAPK, MAPK1, and MAPK2. MAPK2 is responsible to phosphorylate and activate MAPK1 and with the same, it turns to phosphorylate and activates MAPKs. Phosphorylation of MAPK1 and MAPK2 trigger ERK1/2, MAPK4 and MAPK7 trigger JNK, and MAPK3 and MAPK6 trigger p38. The whole process occurs in the cytoplasm and nucleus. The protein p38 transcription factors generally remain inactive in the non-phosphorylated state of MAPKs, becoming rapidly activated through MAPKs phosphorylation which initiates the inflammatory response and leads to apoptosis (Kurtzeborn et al., 2019), (L. Chen et al., 2018).
In the JAK-STAT pathway, the signal transduction mechanism responsible for gene expression which is controlled by varieties of extracellular and intracellular factors like leptin and growth hormone, growth factors, interferons, and various cytokines (O’Shea et al., 2015). The JAKs associated proteins are an integral part of tyrosine kinases and derive four members (JAK1, 2, 3, and TYK2). The cytoplasmic domain of the cytokine receptor coordinated to JAKs is activated through phosphorylation of tyrosine residues and brings conformational change to create binding sites for STATs. The cytoplasmic STATs recruited by the phosphorylated site of phosphotyrosine residues are translocated to the nucleus after the pre-dimerization process. The complex stimulates transcription of the targeted inflammatory genes are responsible for inflammation or programmed cell death/necrosis (Moshapa et al., 2019), (Matsui and Meldrum, 2012), (Alunno et al., 2019).
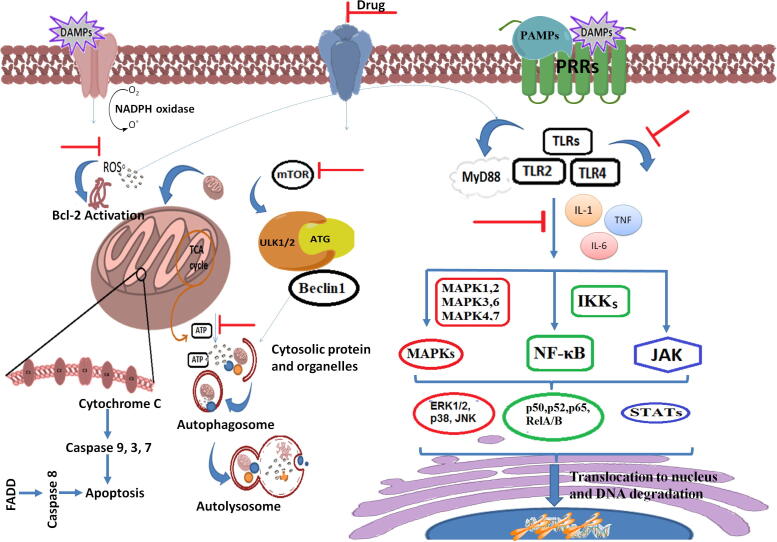
The dysregulation of NF-κB, MAPK, or JAK-STAT resulting in cytokine secretion is associated with inflammatory, autoimmune, and metabolic diseases. A variety of transcription factors regulates multiple genes induced inflammation, includes TNF-α IL-1, IL-6, interferons, transforming growth factor (TGF), colony-stimulating factor (CSF), and chemokines (Kim et al., 2020), (Steven et al., 2019). The previous findings based on phytopharmacological evaluation of NEERI-KFT showed strong evidences against PAMPs/DAMPs induced inflammation. A molecular mechanistic representation emphasized the oxidative and inflammatory distortion of renocyte and how NEERI-KFT tackles to prevent the progressive damage of kidney is shown in Fig. 2.
Fig. 2.
A probable mechanistic therapeutic approach of NEERI-KFT against oxidative and inflammatory stress induced by PAMPs and DAMPs in management of kidney.
4. Discussion
In particular, the ongoing prevalence of CKD laid a most diseased condition causing high morbidity and mortality. Besides, the development of new therapy remained a challenge for the treatment of CKD (Hill et al., 2016). If seen as knowing, a novel therapy should be considered with aimed to minimize progression of glomerulonephritis with improved outcomes in the architect of kidney and economics (Matthew and Katalin, 2016). However, many more attempts have been made from the last decade to prevent progressive economic fatality for the treatment of kidney disease (Peltzer and Pengpid, 2019). Researchers are working to explore the protective attributes using modern scientific approaches targeted for the development of economic and effective therapy which unravel the problem of CKD (Casanova et al., 2017). Although many targeted therapies in modern medicine employed to cure focal segmental glomerulosclerosis/nephritis. However, these medications are associated with serious adverse effects and resistance or even relapse of disease after medication discontinuation are commonly seen (Xiang et al., 2019). Due to the several limitations associated with the use of existing synthetic drugs, the search for newer drugs for CKD from natural sources can be the approach. Herbal medicine shows a multidimensional mechanistic approach due to complex matrix of phytoconstituents which cease progression of CKD by ameliorating eGFR, biochemical, cardiovascular disruption, biological antioxidants and inflammation (Kumela Goro et al., 2019), (Peltzer and Pengpid, 2019). NEERI-KFT is a polyherbal sugar-free syrup formulation provide an effective care for management of CKD via exerting therapeutic value as antioxidants, nephroprotection, and immunomodulation (Tiwari et al., 2016).
Moreover, oxidative stress is frequently observed as a mortality and diagnostic factor to target the pathophysiological stage of CKD. Sustainability in the progressive production of free radicals is commonly thought to be liable for the renal cellular or tissue injury results in CKD (Panday et al., 2015). Oxidative stress is associated with a variety of stimuli markers includes advanced glycation end products (AGEs) malondialdehyde (MDA), oxidized low-density lipoprotein, and 8-hydroxyde-oxyguanosine. These markers are generally thought to be responsible for the elevation of free radicals in circulating blood and/or tissue in CKD patients (Rapa et al., 2020).
NEERI-KFT works as a potent suppressor of oxidative stress, oxidative stress-induced autophagy or inflammation results in apoptosis/necrosis. In acute and chronic renal disease, the immune system/inflammation both plays a crucial role in regulation of progressive distortion of kidney function. Inflammatory cytokines such as TNF-α, IL-1, IL-6 are induced in kidney inflammation and cause down regulation of kidney function by multiplying the activation of fas-associated death domain (FADD) and TNFR-associated death domain (TRADD) (Zhang et al., 2018). The signaling pathway leads to exacerbation of the inflammatory state thought to be responsible for various other associated complications such as heart failure, coronary artery calcification, anemia, and so on. With this consideration, NEERI-KFT or its ingredients exert anti-inflammatory action by regulation of inflammatory cytokines results in strengthen the function of kidney concerning amelioration of biochemical imbalance and histology of the distorted kidney. Further, it can be suggested that NEERI-KFT can be an alternative and complimentary therapy at ESRD or complete loss of kidney function where frequent dialysis of renal transplant remains only a single option for continued survival CKD patent (Gupta et al, 2018).
5. Future prospective
Traditional and modern systems of medicine deal with the many therapies for the management of CKD. For the inhibition of subsequent progression of CKD, a drug should be much effective as nephroprotective without having liabilities to produce any serious harm. The obvious palliative choice for patients suffering from kidney disease is the only way to cure the progressive prevalence of CKD. Apart from this, a multidisciplinary approach is required to reduce morbidity and mortality among patients with CKD. CKD is recognized as a major health problem affecting peoples at a large scale in both developed and developing countries. Drug accessibility, availability, acceptability, and economy can be the major challenge for the economic sorrow of peoples for the treatment of kidney disease. Although still, large numbers of the peoples rely on herbal medicine for the treatment of illness due to their accessibility, availability, acceptability, and economically sound. Plant-derived metabolites or diet, vitamins, minerals can exhibit beneficial effects to manifest numerous complications associated with inflammation and oxidative stress. Although, quality, safety and efficacy based assessment of herbal medicine can be a reason to still not emerging with herbal medicine or natural products. The development of herbal medicine with scientific validation and known therapeutics and regulatory aspects can provide and formalizes us to the natural path which not only mitigates illnesses burden but also make the medicinal system up to affordable economic.
6. Conclusion
In this systematic review, the emerging evidence demonstrates sustainability and severity of CKD is strongly hyphenated with exacerbation of the signaling pathway of free radicals induced oxidative stress and cytokines induced inflammatory stress. NEERI-KFT (a polyherbal formulation) is responsible to superimpose the action of upstream varieties of oxidants and inflammation through its antioxidant, anti-inflammatory and nephroprotective action. However, herbal medicine-based phytopharmacological evaluation also demonstrated that NEERI-KFT exhibits immense protection from segmental glomerulosclerosis (FSGS) and tubulointerstitial fibrosis or nephritis. Hence, the study proves that NEERI-KFT strengthens the kidney against acute or chronic glomerulonephritis due to upstream oxidants and inflammatory cytokines. Besides, it can be effective to reduce the number of dialyzes or even transplantation of an ESRD patient.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Gaurav Gautam, Email: gautamgaurav878@gmail.com.
Ikshit Sharma, Email: ikshit.sharma@aimilpharmaceuticals.com.
Anil Kumar Sharma, Email: ak.sharma@aimilpharmaceuticals.com.
Sayeed Ahmad, Email: sahmad_jh@yahoo.co.in.
References
- Abd El-Kader M., Taha R.I. Comparative nephroprotective effects of curcumin and etoricoxib against cisplatin-induced acute kidney injury in rats. Acta Histochem. 2020 doi: 10.1016/j.acthis.2020.151534. [DOI] [PubMed] [Google Scholar]
- Ahmad, Q.Z., Jahan, N., Ahmad, G., Tajuddin, 2012. Nephroprotective effect of Kabab chini (Piper cubeba) in gentamycin-induced nephrotoxicity. Saudi J. Kidney Dis. Transpl. https://doi.org/10.4103/1319-2442.98159 [DOI] [PubMed]
- Ahmed D., Kumar V., Verma A., Gupta P.S., Kumar H., Dhingra V., Mishra V., Sharma M. Antidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of Albizzia Lebbeck Benth. stem bark (ALEx) on streptozotocin induced diabetic rats. BMC Complement. Altern. Med. 2014 doi: 10.1186/1472-6882-14-243. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Alam M.M.A., Javed K., Jafri M.A. Effect of Rheum emodi (Revand Hindi) on renal functions in rats. J. Ethnopharmacol. 2005 doi: 10.1016/j.jep.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Alhoshani A.R., Hafez M.M., Husain S., Al-Sheikh A.M., Alotaibi M.R., Al Rejaie S.S., Alshammari M.A., Almutairi M.M., Al-Shabanah O.A. Protective effect of rutin supplementation against cisplatin-induced Nephrotoxicity in rats. BMC Nephrol. 2017 doi: 10.1186/s12882-017-0601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alunno A., Padjen I., Fanouriakis A., Boumpas D.T. Pathogenic and Therapeutic Relevance of JAK/STAT Signaling in Systemic Lupus Erythematosus: Integration of Distinct Inflammatory Pathways and the Prospect of Their Inhibition with an Oral Agent. Cells. 2019;8:898. doi: 10.3390/cells8080898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bami, E., Ozakpınar, O.B., Ozdemir-Kumral, Z.N., Köroglu, K., Ercan, F., Cirakli, Z., Sekerler, T., Izzettin, F.V., Sancar, M., Okuyan, B., 2017. Protective effect of ferulic acid on cisplatin induced nephrotoxicity in rats. Environ. Toxicol. Pharmacol. https://doi.org/10.1016/j.etap.2017.06.026 [DOI] [PubMed]
- Breyer M.D., Susztak K. Developing Treatments for Chronic Kidney Disease in the 21st Century. Semin. Nephrol. 2016;36:436–447. doi: 10.1016/j.semnephrol.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulle S., Reddy V.D., Hebbani A.V., Padmavathi P., Challa C., Puvvada P.K., Repalle E., Nayakanti D., Aluganti Narasimhulu C., Nallanchakravarthula V. Nephro-protective action of P. santalinus against alcohol-induced biochemical alterations and oxidative damage in rats. Biomed. Pharmacother. 2016 doi: 10.1016/j.biopha.2016.09.103. [DOI] [PubMed] [Google Scholar]
- Casanova A.G., Vicente-Vicente L., Hernández-Sánchez M.T., Pescador M., Prieto M., Martínez-Salgado C., Morales A.I., López-Hernández F.J. Key role of oxidative stress in animal models of aminoglycoside nephrotoxicity revealed by a systematic analysis of the antioxidant-to-nephroprotective correlation. Toxicology. 2017;385:10–17. doi: 10.1016/j.tox.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Castelino L.R., Nayak-Rao S., Shenoy M.P. Prevalence of use of complementary and alternative medicine in chronic kidney disease: A cross-sectional single-center study from South India. Saudi J. Kidney Dis. Transpl. 2019;30:185–193. doi: 10.4103/1319-2442.252909. [DOI] [PubMed] [Google Scholar]
- Cenini G., Lloret A., Cascella R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Chronic Kidney Disease in the United States, 2019. Cdc. 2019;1:1–6. [Google Scholar]
- Cheema E. Investigating the association of proton pump inhibitors with chronic kidney disease and its impact on clinical practice and future research: A review. J. Pharm. Policy Pract. 2019;12:1–9. doi: 10.1186/s40545-019-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D.Q., Hu H.H., Wang Y.N., Feng Y.L., Cao G., Zhao Y.Y. Natural products for the prevention and treatment of kidney disease. Phytomedicine. 2018;50:50–60. doi: 10.1016/j.phymed.2018.09.182. [DOI] [PubMed] [Google Scholar]
- Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9:7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone F., Castelli S., Ciriolo M.R. Oxidative stress-driven autophagy across onset and therapeutic outcome in hepatocellular carcinoma. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/6050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daenen K., Andries A., Mekahli D., Van Schepdael A., Jouret F., Bammens B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019;34:975–991. doi: 10.1007/s00467-018-4005-4. [DOI] [PubMed] [Google Scholar]
- Das, S., Vasudeva, N., Sharma, S., 2016. Cichorium intybus : A concise report on its ethnomedicinal, botanical, and phyto pharmacological aspects . Drug Dev. Ther. https://doi.org/10.4103/2394-6555.180157
- Dekkers, C.C.J., Gansevoort, R.T., 2020. Sodium-glucose cotransporter 2 inhibitors: extending the indication to non-diabetic kidney disease? Nephrol. Dial. Transplant. https://doi.org/10.1093/ndt/gfz264 [DOI] [PMC free article] [PubMed]
- Domitrović R., Cvijanović O., Pernjak-Pugel E., Škoda M., Mikelić L., Crnčević-Orlić Ž. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 2013 doi: 10.1016/j.fct.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Donate-Correa J., Tagua V.G., Ferri C., Martín-Núñez E., Hernández-Carballo C., Ureña-Torres P., Ruiz-Ortega M., Ortiz A., Mora-Fernández C., Navarro-González J.F. Pentoxifylline for Renal Protection in Diabetic Kidney Disease. A Model of Old Drugs for New Horizons. J Clin. Med. 2019;8:287. doi: 10.3390/jcm8030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey, T., Srivastav, A.K., Nagar, H., Mishra, B.K., 2014. Nephroprotective activity of Nelumbo nucifera Gaertn . roots , leaves and flowers on * Correspondence for Author : E mail : INTRODUCTION :
- Duni A., Liakopoulos V., Roumeliotis S., Peschos D., Dounousi E. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: Untangling ariadne’s thread. Int. J. Mol. Sci. 2019;20:1–23. doi: 10.3390/ijms20153711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamiyan M.Z., Vaezi G., Tehranipour M., Shahrohkabadi K., Shiravi A. Preventive effects of the aqueous extract of Cichorium intybus L. flower on ethylene glycol-induced renal calculi in rats. Avicenna J. phytomedicine. 2018;8:170–178. doi: 10.22038/ajp.2017.21952.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, X., Wei, W., Huang, J., Liu, X., Ci, X., 2020. Isoorientin Attenuates Cisplatin-Induced Nephrotoxicity Through the Inhibition of Oxidative Stress and Apoptosis via Activating the SIRT1/SIRT6/Nrf-2 Pathway. Front. Pharmacol. https://doi.org/10.3389/fphar.2020.00264 [DOI] [PMC free article] [PubMed]
- Fogo A.B. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat. Rev. Nephrol. 2015;11:76–87. doi: 10.1038/nrneph.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajeswari G. ISOLATION AND NEPHROPROTECTIVE ACTIVITY OF BUTRIN FROM ALCOHOLIC FLOWER EXTRACT OF BUTEA MONOSPERMA. Int. Res. J. Pharm. Appl. 2013;3:187–191. [Google Scholar]
- Gai Z., Wang T., Visentin M., Kullak-Ublick G.A., Fu X., Wang Z. Lipid accumulation and chronic kidney disease. Nutrients. 2019;11:11–12. doi: 10.3390/nu11040722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparotto Junior A., Gasparotto F.M., Boffo M.A., Lourenço E.L.B., Stefanello M.É.A., Salvador M.J., Da Silva-Santos J.E., Marques M.C.A., Kassuya C.A.L. Diuretic and potassium-sparing effect of isoquercitrin - An active flavonoid of Tropaeolum majus L. J. Ethnopharmacol. 2011 doi: 10.1016/j.jep.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Geo, A., Baskaran, X., 2011. Nephroprotective activity of aqueous extract of Solanum nigrum in Amphotericin B induced Wister rats. Int. J. Appl. Bioresearch.
- Gordan R., Wongjaikam S., Gwathmey J.K., Chattipakorn N., Chattipakorn S.C., Xie L.H. Involvement of cytosolic and mitochondrial iron in iron overload cardiomyopathy: an update. Heart Fail. Rev. 2018;23:801–816. doi: 10.1007/s10741-018-9700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata S., Dalla Gassa A., Tomei P., Lupo A., Zaza G. Mitochondria: a new therapeutic target in chronic kidney disease. Nutr. Metab. 2015;12:1–51. doi: 10.1186/s12986-015-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X.Y., Shen X.F., Wang L., Wu Z.W., Li F., Chen B., Zhang G.L., Wang M.K. Bioactive steroidal alkaloids from the fruits of Solanum nigrum. Phytochemistry. 2018 doi: 10.1016/j.phytochem.2017.12.020. [DOI] [PubMed] [Google Scholar]
- Gupta et al, 2018. Clinical assessment of “Neeri KFT” in chronic kidney disease patients. Int. J. Green Pharm. 12, 122–129.
- Gupta, R., Sharma, V., 2011. Ameliorative effects of Tinospora Cordifolia root extract on histopathological and biochemical changes induced by Aflatoxin-B 1 in mice kidney. Toxicol. Int. https://doi.org/10.4103/0971-6580.84259 [DOI] [PMC free article] [PubMed]
- Hahr, A.J., Molitch, M.E., 2015. Management of diabetes mellitus in patients with chronic kidney disease. Clin. Diabetes Endocrinol. https://doi.org/10.1186/s40842-015-0001-9 [DOI] [PMC free article] [PubMed]
- He, X., Li, C., Wei, Z., Wang, J., Kou, J., Liu, W., Shi, M., Yang, Z., Fu, Y., 2016. Protective role of apigenin in cisplatin-induced renal injury. Eur. J. Pharmacol. https://doi.org/10.1016/j.ejphar.2016.07.003 [DOI] [PubMed]
- Herrera-Gómez F., Chimeno M.M., Martín-García D., Lizaraso-Soto F., Maurtua-Briseño-Meiggs Á., Grande-Villoria J., Bustamante-Munguira J., Alamartine E., Vilardell M., Ochoa-Sangrador C., Álvarez F.J. Cholesterol-Lowering Treatment in Chronic Kidney Disease: Multistage Pairwise and Network Meta-Analyses. Sci. Rep. 2019;9:1–20. doi: 10.1038/s41598-019-45431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill N.R., Fatoba S.T., Oke J.L., Hirst J.A., O’Callaghan C.A., Lasserson D.S., Hobbs F.D.R. Global prevalence of chronic kidney disease - A systematic review and meta-analysis. PLoS One. 2016 doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon S.L. Vitamin C (Ascorbic Acid) Encycl. Toxicol. Third Ed. 2014;962–963 doi: 10.1016/B978-0-12-386454-3.01250-1. [DOI] [Google Scholar]
- Hussain S., Habib A., Najmi A.K. Limited knowledge of chronic kidney disease among type 2 diabetes mellitus patients in india. Int. J. Environ. Res. Public Health. 2019;16 doi: 10.3390/ijerph16081443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogdand S.D., Shinde R., Sinha V., Chandrakar N. Int. J. Basic Clin; Pharmacol: 2017. Nephroprotective effect of turmeric on oxidative stress, renal histopathology and toxicity induced by gentamicin. https://doi.org/10.18203/2319-2003.ijbcp20172028. [Google Scholar]
- Kaneko N., Kurata M., Yamamoto T., Morikawa S., Masumoto J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019;39:1–38. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthivashan G., Kura A.U., Arulselvan P., Isa N.M., Fakurazi S. The modulatory effect of Moringa oleifera leaf extract on endogenous antioxidant systems and inflammatory markers in an acetaminophen-induced nephrotoxic mice model. PeerJ. 2016 doi: 10.7717/peerj.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar D., Singh V., Ali M. Phytochemical and pharmacological profile of Pterocarpus marsupium: a review. Pharma Innov. J. 2016 [Google Scholar]
- Kengar S., Thorat D., Jadhav J. Nephroprotective effect of Amaranthus spinosus root extract in carbon tetrachloride induced histological toxicity in male albino rat. Int. J. Drug Dev. 2017;9:5–7. [Google Scholar]
- Khattar V., Wal A. Int. J. Pharm. Pharm; Sci: 2012. Utilities of crataeva nurvala. [Google Scholar]
- Kim D.Y., Lim S.G., Suk K., Lee W.H. Mitochondrial dysfunction regulates the JAK-STAT pathway via LKB1-mediated AMPK activation ER-stress-independent manner. Biochem. Cell Biol. 2020 doi: 10.1139/bcb-2019-0088. [DOI] [PubMed] [Google Scholar]
- Kinra M., Arora D., Mudgal J., Pai K.S.R., Mallikarjuna Rao C., Nampoothiri M. Effect of Caffeic Acid on Ischemia-Reperfusion-Induced Acute Renal Failure in Rats. Pharmacology. 2019;103:315–319. doi: 10.1159/000497474. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Zielińska K. Guaianolides from Cichorium intybus and structure revision of Cichorium sesquiterpene lactones. Phytochemistry. 2001 doi: 10.1016/S0031-9422(01)00072-3. [DOI] [PubMed] [Google Scholar]
- Kover A.J. Beta blockers. 5-Minute Anesth. Consult. 2012:2–5. [Google Scholar]
- Kumar D., Singla S.K., Puri V., Puri S. The restrained expression of NF-kB in renal tissue ameliorates folic acid induced acute kidney injury in mice. PLoS One. 2015 doi: 10.1371/journal.pone.0115947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumela Goro K., Desalegn Wolide A., Kerga Dibaba F., Gashe Fufa F., Wakjira Garedow A., Edilu Tufa B., Mulisa Bobasa E. Patient Awareness, Prevalence, and Risk Factors of Chronic Kidney Disease among Diabetes Mellitus and Hypertensive Patients at Jimma University Medical Center. Ethiopia. Biomed Res. Int. 2019;2019:11–12. doi: 10.1155/2019/2383508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzeborn K., Kwon H.N., Kuure S. MAPK/ERK signaling in regulation of renal differentiation. Int. J. Mol. Sci. 2019;20:1–30. doi: 10.3390/ijms20071779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhera, A., Ganeshpurkar, A., Bansal, D., Dubey, N., 2015. Chemopreventive role of Coriandrum sativum against gentamicin-induced renal histopathological damage in rats. Interdiscip. Toxicol. https://doi.org/10.1515/intox-2015-0015 [DOI] [PMC free article] [PubMed]
- Lee J., An J.N., Hwang J.H., Lee H., Lee J.P., Kim S.G. P38 MAPK activity is associated with the histological degree of interstitial fibrosis in IgA nephropathy patients. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T.-A., Wu V.C.-C., Wang C.-Y. Autophagy in Chronic Kidney Diseases. Cells. 2019;8:61. doi: 10.3390/cells8010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malihi Z., Wu Z., Lawes C.M.M., Scragg R. Adverse events from large dose vitamin D supplementation taken for one year or longer. J. Steroid Biochem. Mol. Biol. 2019;188:29–37. doi: 10.1016/j.jsbmb.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Mathiassen S.G., De Zio D., Cecconi F. Autophagy and the cell cycle: A complex landscape. Front. Oncol. 2017;7:1–31. doi: 10.3389/fonc.2017.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui F., Meldrum K.K. The role of the Janus kinase family/signal transducer and activator of transcription signaling pathway in fibrotic renal disease. J. Surg. Res. 2012 doi: 10.1016/j.jss.2012.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew B.D., Katalin S. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug Discov. 2016;15:568–588. doi: 10.1002/cncr.27633.Percutaneous. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwally, D.M., Habotta, O.A., Amin, H.K., Ahmed, E., Moneim, A., El-khadragy, M., Arabia, S., Arabia, S., Medicine, F., n.d. Nephroprotective effects of chlorogenic acid against sodium arsenite-induced oxidative stress , inflammation , and apoptosis. https://doi.org/10.1002/jsfa.10565 [DOI] [PubMed]
- Moshapa F.T., Riches-Suman K., Palmer T.M. Therapeutic Targeting of the Proinflammatory IL-6-JAK/STAT Signalling Pathways Responsible for Vascular Restenosis in Type 2 Diabetes Mellitus. Cardiol. Res. Pract. 2019;2019:1–2. doi: 10.1155/2019/9846312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naggayi, M., Mukiibi, N., Iliya, E., 2015. The protective effects of aqueous extract of carica papaya seeds in paracetamol induced nephrotoxicity in male wistar rats. Afr. Health Sci. https://doi.org/10.4314/ahs.v15i2.37 [DOI] [PMC free article] [PubMed]
- Nahak, G., Sahu, R.K., 2011. Phytochemical evaluation and antioxidant activity of Piper cubeba and Piper nigrum. J. Appl. Pharm. Sci.
- Nair, A.S., Vidhya, K.M., Saranya, T.R., Sreelakshmy, K.R., Nair, S.C., 2013. International Research Journal of Pharmaceutical and Applied Sciences (IRJPAS) 3, 192–196.
- Nasri H., Rafieian-Kopaei M. Tubular kidney protection by antioxidants. Iran. J. Public Health. 2013;42:1194–1196. [PMC free article] [PubMed] [Google Scholar]
- O’Shea J.J., Schwartz D.M., Villarino A.V., Gadina M., McInnes I.B., Laurence A. The JAK-STAT Pathway: Impact on Human Disease and Therapeutic Intervention. Annu. Rev. Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachon J., Lorber M.I., Bia M.J. Effects of H2-receptor antagonists on renal function in cyclosporine-treated renal transplant patients. Transplantation. 1989 doi: 10.1097/00007890-198902000-00011. [DOI] [PubMed] [Google Scholar]
- Padmini, P., 2013. An Experimental Study of Biochemical and Histopathological Study on Gentamycin Induced Renal Failure in Albino Rat And The Effectiveness Of Punarnava (BOERHAEVIA Diffusa) On Reversal Of Renal Damage. IOSR J. Dent. Med. Sci. https://doi.org/10.9790/0853-0961721
- Panday A., Sahoo M.K., Osorio D., Batra S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen R., Khan N., Zahiruddin S., Ibrahim M., Anjum V., Parveen B., Khan M.A. TLC-Bioautographic Evaluation for High-Throughput Screening and Identification of Free Radical Scavenging and Antidiabetic Compounds from Traditional Unani Medicinal Plant: Citrullus colocynthis Schrad. J. AOAC Int. 2020 doi: 10.5740/jaoacint.19-0287. [DOI] [PubMed] [Google Scholar]
- Paudel, K.R., Panth, N., 2015. Phytochemical profile and biological activity of Nelumbo nucifera. Evidence-based Complement. Altern. Med. https://doi.org/10.1155/2015/789124 [DOI] [PMC free article] [PubMed]
- Peltzer K., Pengpid S. The use of herbal medicines among chronic disease patients in Thailand: A cross-sectional survey. J. Multidiscip. Healthc. 2019;12:573–582. doi: 10.2147/JMDH.S212953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W., Huang J., Zheng Y., Ding Y., Li S., Zhang J., Lyu J., Zeng Q. UCP2 silencing aggravates mitochondrial dysfunction in astrocytes under septic conditions. Mol. Med. Rep. 2019;20:4459–4466. doi: 10.3892/mmr.2019.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharma, D., Patel, V.B., Rathod, I.S., Patel, J.M., Brahmbhatt, M.R., 2010. Scholars Research Library Anti-urolithiatic and natriuretic activity of steroidal constituents of Solanum xanthocarpum, pdfs.semanticscholar.org.
- Pino, M.A., Azer, S.A., 2019. Cimetidine 1–6. [PubMed]
- Prajna J., Richa J., Dipjyoti C. HPLC Quantification of Phenolic Acids from Vetiveria zizanioides (L.) Nash and Its Antioxidant and Antimicrobial Activity. J. Pharm. 2013 doi: 10.1155/2013/270472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profile, S.E.E., 2017. Attenuation of Cypermethrin Induced Nephrotoxicity by (-) Epigallocatechin Gallate (EGCG) in Male Wistar Rats Islamiah College Some of the authors of this publication are also working on these related projects : Cigarette smoke induced lung toxicity V.
- Pugh D., Gallacher P.J., Dhaun N. Management of Hypertension in Chronic Kidney Disease. Drugs. 2019;79:365–379. doi: 10.1007/s40265-019-1064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Q, Z.A., 2016. Herbs as Nephroprotective Agent : An Over View with Reference to Unani System of Medicine 1, 1–3.
- Rajapurkar, M., Dabhi, M., 2010. Burden of disease - prevalence and incidence of renal disease in India. Clin. Nephrol. https://doi.org/10.5414/cnp74s009 [DOI] [PubMed]
- Raoofi, A., Khazaei, M., Ghanbari, A., 2015. Protective effect of hydroalcoholic extract of tribulus terrestris on cisplatin induced renal tissue damage in male mice. Int. J. Prev. Med. https://doi.org/10.4103/2008-7802.151817 [DOI] [PMC free article] [PubMed]
- Rapa, S.F., Di Iorio, B.R., Campiglia, P., Heidland, A., Marzocco, S., 2020. Inflammation and oxidative stress in chronic kidney disease—potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21010263 [DOI] [PMC free article] [PubMed]
- Rayego-Mateos S., Goldschmeding R., Ruiz-Ortega M. Inflammatory and Fibrotic Mediators in Renal Diseases. Mediators Inflamm. 2019;2019:10–12. doi: 10.1155/2019/7025251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolim P.M., Fidelis G.P., Padilha C.E.A., Santos E.S., Rocha H.A.O., Macedo G.R. Phenolic profile and antioxidant activity from peels and seeds of melon (Cucumis melo L. var. reticulatus) and their antiproliferative effect in cancer cells. Brazilian J. Med. Biol. Res. 2018 doi: 10.1590/1414-431x20176069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M., Javed F., Asif M., Baig M.K., Arif M. HPLC analysis and in vivo renoprotective evaluation of hydroalcoholic extract of cucumis melo seeds in gentamicin-induced renal damage. Med. 2019 doi: 10.3390/medicina55040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-González P.D., López-Hernández F.J., Dueñas M., Prieto M., Sánchez-López E., Thomale J., Ruiz-Ortega M., López-Novoa J.M., Morales A.I. Differential effect of quercetin on cisplatin-induced toxicity in kidney and tumor tissues. Food Chem. Toxicol. 2017 doi: 10.1016/j.fct.2017.06.047. [DOI] [PubMed] [Google Scholar]
- Sandeep, D., Krishnan Nair, C.K., 2010. Amelioration of cisplatin-induced nephrotoxicity by extracts of Hemidesmus indicus and Acorus calamus. Pharm. Biol. https://doi.org/10.3109/13880200903116048 [DOI] [PubMed]
- Sardana, A., Kalra, S., Khanna, D., Balakumar, P., 2015. Nephroprotective effect of catechin on gentamicin-induced experimental nephrotoxicity. Clin. Exp. Nephrol. https://doi.org/10.1007/s10157-014-0980-3 [DOI] [PubMed]
- Satmbekova, D., Srivedavyasasri, R., Orazbekov, Y., Omarova, R., Datkhayev, U., Ross, S.A., 2018. Chemical and biological studies on Cichorium intybus L. Nat. Prod. Res. https://doi.org/10.1080/14786419.2017.1343319 [DOI] [PMC free article] [PubMed]
- Sharma, K., Sahai, M., 2017. Chemical constituents of Boerhavia diffusa leaves. J. Med. Plants Stud.
- Sharma, S., Baboota, S., Amin, S., Mir, S.R., 2020. Ameliorative effect of a standardized polyherbal combination in methotrexate-induced nephrotoxicity in the rat. Pharm. Biol. 10.1080/13880209.2020.1717549. [DOI] [PMC free article] [PubMed]
- Shirazian S. Depression in CKD: Understanding the Mechanisms of Disease. Kidney Int. Reports. 2019;4:189–190. doi: 10.1016/j.ekir.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, O., Ali, M., 2011. Phytochemical and antifungal profiles of the seeds of Carica papaya L. Indian J. Pharm. Sci. https://doi.org/10.4103/0250-474X.95648 [DOI] [PMC free article] [PubMed]
- Sinha A.D., Agarwal R. Clinical pharmacology of antihypertensive therapy for the treatment of hypertension in CKD. Clin. J. Am. Soc. Nephrol. 2019;14:757–764. doi: 10.2215/CJN.04330418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider J.T., Sullivan J., Van Eijndhoven E., Hansen M.K., Bellosillo N., Neslusan C., O’Brien E., Riley R., Seabury S., Kasiske B.L. Lifetime benefits of early detection and treatment of diabetic kidney disease. PLoS One. 2019;14:1–17. doi: 10.1371/journal.pone.0217487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song N., Thaiss F., Guo L. NFκB and kidney injury. Front. Immunol. 2019;10:1–25. doi: 10.3389/fimmu.2019.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkar, N., Ganeshpurkar, A., Yadav, P., Dubey, S., Bansal, D., Dubey, N., 2014. An experimetal evaluation of nephroprotective potential of Butea monosperma extract in albino rats. Indian J. Pharmacol. https://doi.org/10.4103/0253-7613.125190 [DOI] [PMC free article] [PubMed]
- Steven S., Frenis K., Oelze M., Kalinovic S., Kuntic M., Jimenez M.T.B., Vujacic-Mirski K., Helmstädter J., Kröller-Schön S., Münzel T., Daiber A. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019;2019:1–45. doi: 10.1155/2019/7092151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudjarwo, S.A., Eraiko, K., Sudjarwo, G.W., Koerniasari, 2017. Protective effects of piperine on lead acetate induced-nephrotoxicity in rats. Iran. J. Basic Med. Sci. https://doi.org/10.22038/IJBMS.2017.9487 [DOI] [PMC free article] [PubMed]
- T.T., S., V.H., B., P.P., A., U., J., R.J., O., 2011. Nephroprotective activity of ethanolic extract of stem barks of Crataeva nurvula Buch hum. Int. J. Pharm. Sci. Res.
- Tanmoy G., Arijit M., Tanushree S., Jagadish S., Kumar M.T. Pharmacological actions and phytoconstituents of Amaranthus spinosus Linn: A review. Int. J. Pharmacogn. Phytochem. Res. 2014 [Google Scholar]
- Tatapudi R.R., Rentala S., Gullipalli P., Komarraju A.L., Singh A.K., Tatapudi V.S., Goru K.B., Bhimarasetty D.M., Narni H. High Prevalence of CKD of Unknown Etiology in Uddanam. India. Kidney Int. Reports. 2019;4:380–389. doi: 10.1016/j.ekir.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M.K., Sharma S., Sharma I., Goyal P.K., Verma S.K., Barwal A. European Journal of Nephroprotective Role of Neeri-Kft (a Polyherbal Formulation) Against Gentamicin Induced Nephrotoxicity in Experimental Rat Model : a Pre-Clinical Study. 2016;3:410–417. [Google Scholar]
- Todi, S., Majumdar, A., 2019. Acute kidney injury, in: ICU Protocols: A Step-Wise Approach, Vol I. https://doi.org/10.1007/978-981-15-0898-1_46
- Ullah, N., Khan, S., Khan, A., Ahmad, W., Shah, Y., Ahmad, L., Ullah, I., 2015. A prospective pharmacological review of medicinal herbs, Cucumis melo and Berberis vulgaris, commonly used in the treatment of renal diseases in Pakistan. Acta Pol. Pharm. - Drug Res. [PubMed]
- Upadhyay, A., Kumar, K., Kumar, A., Mishra, H., 2010. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi) - validation of the Ayurvedic pharmacology through experimental and clinical studies. Int. J. Ayurveda Res. https://doi.org/10.4103/0974-7788.64405 [DOI] [PMC free article] [PubMed]
- Vergara-Jimenez M., Almatrafi M.M., Fernandez M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants. 2017 doi: 10.3390/antiox6040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S.C., Vashishth E., Singh R., Kumari A., Meena A.K., Pant P., Bhuyan G.C., Padhi M.M. A review on parts of Albizia lebbeck (L.) Benth. used as Ayurvedic drugs. Res. J. Pharm. Technol. 2013 [Google Scholar]
- Waly M.I., Ali B.H., Al-Lawati I., Nemmar A. Protective effects of emodin against cisplatin-induced oxidative stress in cultured human kidney (HEK 293) cells. J. Appl. Toxicol. 2013 doi: 10.1002/jat.1788. [DOI] [PubMed] [Google Scholar]
- Wang, M.X., Liu, Y.L., Yang, Y., Zhang, D.M., Kong, L.D., 2015. Nuciferine restores potassium oxonate-induced hyperuricemia and kidney inflammation in mice. Eur. J. Pharmacol. https://doi.org/10.1016/j.ejphar.2014.11.035 [DOI] [PubMed]
- Ward F., Holian J., Murray P.T. Drug therapies to delay the progression of chronic kidney disease. Clin. Med. J. R. Coll. Physicians London. 2015;15:550–557. doi: 10.7861/clinmedicine.15-6-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L., Wang Y., Yi X., He X. Anti-inflammatory steroidal glycosides from the berries of Solanum nigrum L. (European black nightshade) Phytochemistry. 2018 doi: 10.1016/j.phytochem.2018.01.019. [DOI] [PubMed] [Google Scholar]
- Xiang Y., Shi W., Li Z., Yang Y., Wang S.Y., Xiang R., Feng P., Wen L., Huang W. Efficacy and safety of spironolactone in the heart failure with mid-range ejection fraction and heart failure with preserved ejection fraction: A meta-analysis of randomized clinical trials. Medicine (Baltimore). 2019;98 doi: 10.1097/MD.0000000000014967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadava, R.N., Tiwari, L., 2005. A potential antiviral flavone glycoside from the seeds of Butea monosperma O. Kuntze. J. Asian Nat. Prod. Res. https://doi.org/10.1080/1028602042000204054 [DOI] [PubMed]
- Yamagami F., Tajiri K., Yumino D., Ieda M. Uremic Toxins and Atrial Fibrillation: Mechanisms and Therapeutic Implications. Toxins (Basel). 2019;11:1–12. doi: 10.3390/toxins11100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21 doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman R., Alam A., Choudhry S.S., Tariq M. Nephroprotective Agents Used in Unani Medicine-An Evidence Based Approach. J. Nephrol. Ther. 2017;07:1–12. doi: 10.4172/2161-0959.1000296. [DOI] [Google Scholar]
- Zargar B.A., Masoodi M.H., Ahmed B., Ganie S.A. Phytoconstituents and therapeutic uses of Rheum emodi wall. ex Meissn. Food Chem. 2011 doi: 10.1016/j.foodchem.2011.03.083. [DOI] [Google Scholar]
- Zhang C., Wang N., Xu Y., Tan H.Y., Li S., Feng Y. Molecular mechanisms involved in oxidative stress-associated liver injury induced by chinese herbal medicine: An experimental evidence-based literature review and network pharmacology study. Int. J. Mol. Sci. 2018;19:2018–2019. doi: 10.3390/ijms19092745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Sun S.C. NF-ΚB in inflammation and renal diseases. Cell Biosci. 2015;5:1–25. doi: 10.1186/s13578-015-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Du S., Shen S., Luo P., Ding S., Wang G., Wang L., Omboni S. Comparative efficacy and safety of lipid-lowering agents in patients with hypercholesterolemia: A frequentist network meta-analysis. Med. (United States) 2019;98:1–12. doi: 10.1097/MD.0000000000014400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG, Q., WU, H., GUO, J., NAN, H., CHEN, S., YANG, J., XU, X., 2013. Review of Rhubarbs: Chemistry and Pharmacology. Chinese Herb. Med. https://doi.org/10.7501/j.issn.1674-6384.2013.01.003