Abstract
Medicinal plants largely serve as a source of bioactive compounds in traditional medicines to cure various diseases. The present study was aimed at chemical composition, antioxidant, antimicrobial, cytotoxic and antihemolytic potential of five different extracts of G. hispida and H. crispum (Boraginaceae). G. hispida methanolic extract displayed highest number (eleven) of polyphenolic compounds by using high performance liquid chromatography (HPLC). Functional groups were identified by Fourier-transformed infrared spectroscopy (FTIR) and elements (Si, Fe, Ba, Mg, Ti, Ca, Mg and Cr) were observed by using laser-induced breakdown spectroscopy (LIBS) which were also highly expressed in G. hispida as compared to H. crispum. Antioxidant activity was determined via six assays and antibacterial activity was observed in decreasing order of methanol > ethanol > chloroform > ethyl acetate > n-Hexane in both species. Cytotoxic potential was investigated against brine shrimps and then liver (HepG2) and skin (HT144) cancer cell lines which was detected highest in the G. hispida ethanolic extract (50.76 % and 72.95 %). However, H. crispum chloroform extract revealed highest (31.869 μg/mL) antihemolytic activity and its methanolic extract indicated highest (13.5 %) alpha-amylase inhibitory potential. Altogether, results suggested that both species could be used effectively in food and drug industries owing to the presence of vital bioactive compounds and elements. In future, we recommend to isolate active compounds and to perform in vivo biological assays to further validate their potential biological applications.
Keywords: Boraginaceae, HPLC, Functional groups, LIBS, Anticancer, Human erythrocytes
1. Introduction
Different research and floristic studies have indicated the existence of ∼ 500,000 plants species on earth and about 120,000 species are used in medicines to treat different diseases (Kallassy, 2019). Plants are the richest source of medicine in both developed and developing countries (Iqbal et al., 2018a, Iqbal et al., 2018b). Natural products derived from these plants serve as valuable starting points for drug discovery (Rodrigues et al., 2016). Plant-derived compounds exhibit remarkable biological potential as compared to synthetic chemical products (Espinosa-Leal et al., 2018). Up till now, scientific community is trying to isolate natural products from medicinal plants for treating different human diseases (Panthi et al., 2020, Zahra et al., 2021a). Plants and their active compounds are also rich source of natural antioxidants. The phytochemical constituents present inside the plants like flavonoids, polyphenols, tannins, carotenoids and terpenes exhibit antioxidant activities by quenching free radicals produced in the body (Zahra et al., 2021b, Ali et al., 2021a, Ali et al., 2021b).
Similarly, plant-derived antimicrobials compounds are also one of the most promising sources considered biosafe and biocompatible when compared with synthetic compounds (Casciaro et al., 2019). Use of crude extract of medicinal plants is also gaining attention to treat infectious diseases caused by microorganism (Phan et al., 2019). Plants generally produce many bioactive compounds which act as antimicrobial agents against different pathogenic organisms (Toiu et al., 2019). Due to the antimicrobial property of medicinal plants, these can be utilized as pharmacological agents and could be favorable approach for treating infectious diseases (Mickymaray, 2019).
Natural healing agents are largely used to overcome unwanted side effects of synthetic drugs worldwide. Pakistan comprises of ∼ 6000 species of higher plants, of which about 700 species are commonly used for medicinal purposes (Shinwari, 2010). The current study is focusing on the medicinal flora of district Dera Ghazi Khan (DG Khan) which lies in the sub-tropical continental plain of Pakistan, which has sandy soil and harsh climatic conditions. People living in the DG Khan mainly rely on medicinal plants to treat different diseases (Imran et al., 2013). Species belonging to family Boraginaceae exhibit great economic and medicinal values (Dresler et al., 2017). Heliotropium crispum aqueous paste is used against infectious and malarial agents while Gastrocotyle hispida is used as a refreshing drink like tea and leaves decoction is used as diuretic and in the treatment of rheumatism (Shahat et al., 2019, Khan et al., 2016).
Keeping in view the ethno-pharmacological importance and phytochemical values of G. hispida and H. crispum, present study was aimed at exploring the biochemical and elemental composition and biological potential of two species collected specifically from Dera Ghazi Khan, Pakistan. It is located at 70.38° longitude, 30.03° latitude, 699 m altitude and is very hot in summers (42 °C) with very little rainfall. Dominant species of this region mainly belongs to family Fabaceae, Solanaceae, Asteraceae, Poaceae and Brassicaceae (Gulshan et al., 2012). Hence, plant extracts were prepared using various polarity-based solvents followed by the compound identification which was done by performing HPLC, functional groups were ascertained via FTIR spectroscopy and elemental analysis was carried out using LIBS technique. Furthermore, biological potential was determined by conducting antioxidant, antimicrobial, cytotoxicity, antihemolytic and α-amylase inhibitory assays.
2. Materials and methods
2.1. Plant collection and extracts preparation
G. hispida (Acc. No.130864) and H. crispum (Acc. No. 130865) were collected from DG Khan, Pakistan during March 2019. Plants were washed, air-dried, powdered and extracted with ethanol, methanol, ethyl acetate, chloroform and n-Hexane (30 g/300 mL each) for five days. Subsequently, extracts were filtered and dried at reduce pressure using rotary evaporator and were stored at 4 °C for future use (Harborne, 1973).
2.2. Qualitative phytochemical analysis
Initially some phytochemical tests were carried out using standard protocols to determine the presence and/or absence of terpenoids and steroids (Libermann’s test), flavonoids (Alkaline reagent test), saponins (Foam test), phenols (Ferric chloride test), glycosides (Salkowski test), alkaloids (Mayer’s reagent), quinones (2 mL extract + 2 mL sulphuric acid – red colour) and coumarins (2 mL extract + 3 mL NaOH – yellow colour) (Raman, 2006, Harborne, 1973).
2.3. Total phenolic and flavonoid contents
For TPC, gallic acid was added in Folin-ciocalteu reagent (90 µL) and NaCO3 solution (90 µL) to prepare the calibration curve. Then, 20 µL of each extract was added in the same reagent and the absorbance was recorded. Data obtained was expressed as mg GAE/g sample (Chlopicka et al., 2012). Similarly, Aluminum Chloride Colorimetric Method was used to measure Flavonoid contents and the results were recorded as mg QE/g sample (Chang et al., 2002).
2.4. High performance liquid chromatography (HPLC) analysis
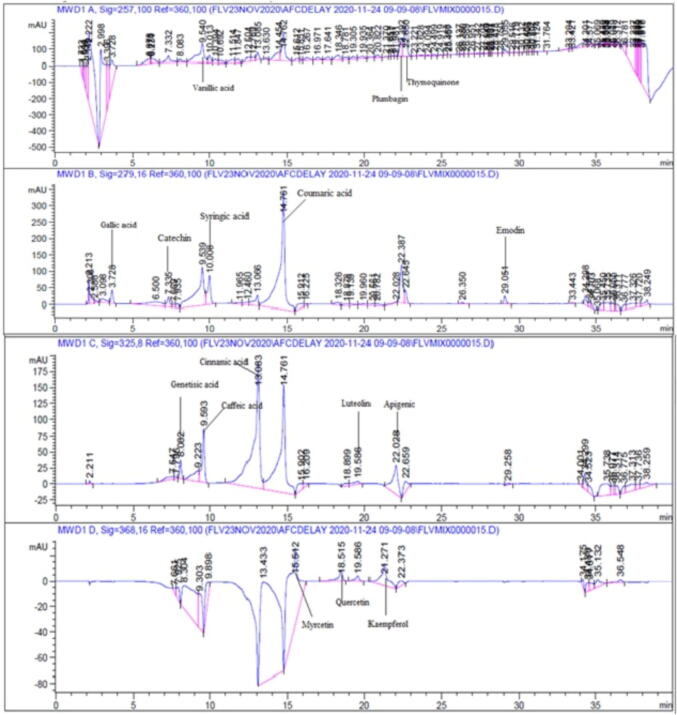
Polyphenol detection was performed by HPLC analysis of G. hispida and H. crispum extracts using previously optimized protocol of Qasim et al. (2017). A Zorbex-C8 analytical column (4.6 × 250 nm) and a diode array detector were fitted with the HPLC system (DAD, Agilent technologies, Germany). Two mobile phases including acetonitrile: methanol: water: acetic acid (5:10:85:1) and acetonitrile: methanol: acetic acid (40:60:1) were used with a flow rate of 1 mL/min. Samples were filtered using membrane filter (0.45 µm) and then 20 µg/mL sample was injected via an injection port into the column. Vanillic acid (257 nm), Thymoquinone (2 5 7), Plumbagin (2 5 7) Gallic acid (2 7 9), Syringic (2 7 9), Coumaric acid (2 7 9), Catechin (279 nm), Emodin (2 7 9), Caffeic acid (325 nm), Gentisic (325 nm), Cinnamic acid (3 2 5), Luteolin (3 2 5), Apigenic (3 2 5) Quercetin (368 nm), Myricetin (368 nm) and Kaempferol (368 nm) were used as standards and detected at respective wavelengths. Compound identification was done by comparing the UV absorption spectra and retention time of samples with the standards.
2.5. Fourier-Transform infrared (FTIR) spectroscopy
FTIR spectrometer Perkin Elmer- Spectrum 65 (FTIR model) was used for the identification of the characteristic functional groups in plants. This technique provides information from the obtained absorption spectrum. Plant material was powdered and subjected to a pressure of about 5 × 106 Pa in an evacuated dye. Spectras were recorded at 4000–400 cm−1 frequency and peak values were recorded (Meenambal et al., 2012).
2.6. Laser-induced breakdown spectroscopy (LIBS)
LIBS analysis was done using standard protocol as suggested by Zafar et al. (2016). Q-switched Nd: YAG laser (Brilliant-B Quantel, France) was used at 532 nm wavelength and repetition rate of 10 Hz was carried out for ablation. Laser energy was measured using energy meter and the sample was kept on the rotating stage in order to obtain the homogenous plasma. Four spectrometers (wavelength 250–870 nm) were used to detect radiations and the emission spectra was assessed with the help of laser that was synchronized with the spectrometer.
2.7. Determination of antioxidant assays
2.7.1. Reducing power assay
Protocol of Oyaizu (1986) was followed to observe the reducing potential of selected plant extracts. Each extract (200 µL) was added in the phosphate buffer (500 µL) and potassium ferricyanide (500 µL) and then incubated (20 min) at 50 °C. About 500 µL of TCA was added in each sample using micropipettes followed by centrifugation for 10 min. Almost 100 µL of 0.1 % FeCl3 was added in the supernatant and absorbance was recorded at 630 nm.
2.7.2. Cupric ions reducing antioxidant capacity
About 0.01 M CuCl2 solution (10 µL), 7.5 mM ethanol neocuproine solution (10 µL) and ammonium acetate buffer solution (1.0 M) of 10 µL were added in gallic acid and plant extracts (20 μL). Volume was adjusted upto 1 mL using dH2O and absorbance was recorded at 515 nm (Apak et al., 2004).
2.7.3. Phosphomolybdate assay
Reaction mixture was prepared by dissolving 28 mM sodium phosphate (1.68 g), 4 mM ammonium molybdate (0.25 g) and sulphuric acid (1.63 mL) in dH2O (50 mL). Each extract (50 µL) was dissolved in the reaction mixture and then incubated at 95 °C. Finally, absorbance was taken at 630 nm (Prieto et al., 1999).
2.7.4. DPPH radical scavenging assay
Initially, 3.9 mg of DPPH (3.9 mg) was mixed in methanol (100 mL) followed by the incubation (30 min) in dark. Then, DPPH solution (180 µL) was mixed with the sample solution (20 µL) and ascorbic acid was used as a standard. The absorbance was measured at 517 nm using UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan). Percentage inhibition was calculated using formula provided below and then IC50 values were determined (Tepe et al., 2005).
% Scavenging activity = Abscontrol × Abssample/Abscontrol × 100
2.7.5. ABTS radical scavenging assay
ABTS stock solution (7 mM) and potassium persulphate (2.45 mM) were added together and after 12–16 h, solution was diluted (i.e 0.70 ± 0.02 absorbance). Then ABTS+ solution (160 µL) was mixed with different plant concentrations (10 µL) and absorbance was determined at 734 nm (Loziene et al., 2007). Percentage inhibition and IC50 values were expressed using same formula which was used for DPPH assay.
2.7.6. NBT (or superoxide radical scavenging) assay
In this assay, Na2CO3 (1 mL), 24 mM NBT (0.4 mL), plant extract (1 mL), EDTA solution (0.2 mL) and hydroxylamine hydrochloride (0.4 mL) were added in eppendorf tubes and placed at 25 °C for incubation (15 min). Then, the absorbance (540 nm) was measured and % inhibition and IC50 value were calculated (Munir and Sarfraz, 2014).
2.8. Antimicrobial assay
Antibacterial potential was evaluated against Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 90271), Bacillus subtilis (ATCC19659), Escherichia coli (ATCC 33456) and Klebsiella pneumonia (ATCC 1705) by using agar-disc diffusion assay (Jo et al., 2004). Bacterial strains were cultured in nutrient broth medium, incubated (37 °C) for 18 h (hr) and then standardized by regulating OD to 0.5 (1 × 108 CFU/mL). Plant extracts of different concentration (3.7, 11.11, 33.33, and 100 μg/mL) was used for this method. Zones<8 mm were not considered significant and MIC values were calculated. Similarly, Fusarium solani (FCBP 0291), Aspergillus niger (FCBP 0918), Aspergillus flavus (FCBP0064), Candida albicans (FCBP 478) and Mucor racemosus (FCBP 0300) were treated with varying doses (3.7, 11.11, 33.33 and 100 μg/mL) of plant extracts to assess antifungal activity. Strains were sub-cultured for 24 hr in SDA media at 25 °C. Fungal plates were placed in incubator for 24 hr after treatment with plant extracts and then inhibition zones were measured. Clotrimazole was used as a standard drug and MIC values were measured.
2.9. Brine shrimp cytotoxicity assay
Cytotoxicity potential was investigated at 62, 125, 250, 500 and 1000 μg/mL using standard protocol. Each extract was added in a separate vial and the final volume was brought to 5 mL using saline solution. Afterwards, ten (10) shrimps were taken in each vial (after 24 hr) and placed in incubator at 32 °C. Number of survivors were counted after 24 hr and then, LC50 values and % mortality was determined (Mehwish et al., 2019).
2.10. Anti-cancer assay
HepG2 (Liver cancer), HT144 (Skin cancer) cell lines were grown in DMEM supplemented with 10 percent FBS along with 1 percent antimycotic (ABAM) and maintained in a tissue culture cabinet under 37 °C conditions with 95 percent air and 5 percent CO2 (Manassas, VA, United States). MTT assay was carried out to detect the inhibitory effect of G. hispida and H. crispum extract on HepG2 (Liver cancer) and HT144 (Skin cancer) cell line. The reactant cell lines were held for 24 hr at a density of (5 × 104) cells/well in 96-well plates, followed by the addition of plant extracts. Media were subsequently extracted and 5 mg/mL of MTT reagents were loaded along with sterile PBS, followed by 4 h incubation. In addition, the MTT solution was extracted and DMSO was used to dissolve precipitate formazan. Finally, absorbance was measured at 570 nm.
2.11. Antihemolytic assay
Six milliliters of human blood were taken in EDTA vials and centrifuged (5 min) at 1000 × g. The obtained pellet was washed (3x) with 0.2 M PBS (pH 7.4) and then 0.5 mL of each extract (100–1000 μg/mL in PBS) was added to 1 mL of erythrocyte suspension followed by the incubation (20 min). Then H2O2 solution (0.5 mL) was added in the samples and then centrifuged for 10 min. Absorbance of supernatant was measured at 540 nm using H2O2 as a negative control and quercetin as a standard with PBS. Study approval (#BEC-FBS-QAU2019-143) was taken from the Bioethical Committee of Quaid-i-Azam University, Islamabad (Yang et al., 2005).
2.12. Alpha-amylase iinhibition assay
Initially, a reaction mixture of 15 μL phosphate buffer, 25 μL alpha-amylase enzyme (0.14 U/mL), 10 μL extract sample (4 mg/mL DMSO) and 40 μL starch solution were incubated (50 °C) for 30 min. Subsequently, 20 μL of 1 M HCl and 90 μL of the iodine reagent (5 mM phosphate buffer potassium iodide) were applied to each well. Acarbose was used as a positive control and absorbance was noted at 540 nm (Khalil et al., 2018). The inhibition of alpha-amylase was measured using following formula:
% α-amylase inhibition = Abscontrol – Abssample/ Abscontrol × 100
2.13. Statistical analyses
All experiments were performed thrice and mean ± standard error was calculated. ANOVA was performed using statistix version 8.1 and mean values were compared by Tukey LSD (Steel et al., 1996). IC50 values were calculated using Graphpad prism while LC50 values were determined via probit analysis program (Finney, 1952). Moreover, origin pro 7 was used for the interpretation of spectras obtained from LIBS.
3. Results
3.1. Phytochemical analysis
3.1.1. Qualitative tests
Results revealed that secondary metabolites are present in most of the methanolic and ethanolic extracts as compared to the chloroform, ethyl acetate and n-Hexane extracts which revealed the presence of some compounds. Among these, flavonoids, glycosides, phenols, hormones, terpenoids, saponins and tannins were observed in most of the extracts, while alkaloids, quinone and coumarins were present in only few extracts (Table 1).
Table 1.
Qualitative phytochemical analysis of methanol, ethanol, ethyl acetate, chloroform and n-Hexane extracts of G. hispida and H. crispum.
|
Phytochemicals |
H. crispum |
G. hispida |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCM | HCE | HCEA | HCCHL | HCHEX | GHM | GHE | GHEA | GHCHL | GHHEX | |
| Alkaloids | + | + | + | – | + | + | + | + | – | + |
| Flavonoid | + | + | + | + | + | + | + | + | + | + |
| Glycosides | + | + | + | + | + | + | + | + | + | + |
| Phenols | + | + | + | + | + | + | + | + | + | + |
| Steroids | + | + | + | + | + | + | + | + | + | + |
| Terpenoids | + | + | + | + | + | + | + | + | + | + |
| Saponins | + | + | + | + | + | + | + | + | + | + |
| Tannins | + | + | + | + | + | + | + | + | + | + |
| Quinone | + | + | – | – | – | + | + | – | – | – |
| Coumarin | + | + | – | + | + | + | + | – | + | + |
‘+’ and ‘-’ indicates the presence and absence of secondary metabolites in selected plant extracts. (HCM: H. crispum methanolic extract; HCE: H. crispum ethanolic extract; HCEA: H. crispum ethyl acetate extract; HCCHL: H. crispum chloroform extract; HCHEX: H. crispum n-Hexane extract; GHM: G. hispida methanolic extract; GHE: G. hispida ethanolic extract; GHEA: G. hispida ethyl acetate extract; GHCHL: G. hispida chloroform extract; GHHEX: G. hispida n-Hexane extract).
3.1.2. Total phenolic and flavonoid contents
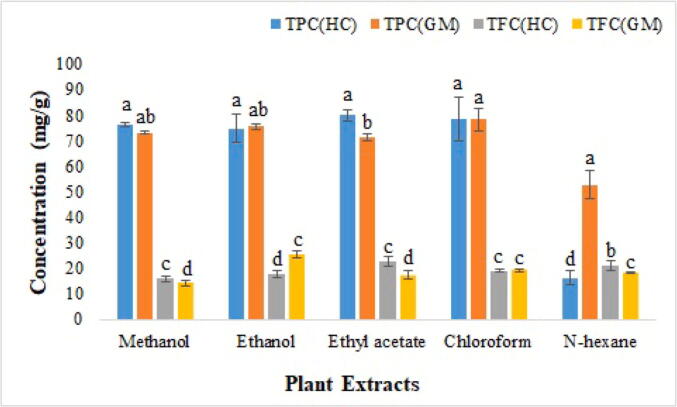
In case of H. crispum, phenolic contents were found highest in the ethyl acetate extract (79.889 ± 1.97 mg GAE/g) and lowest in n-Hexane extract (16.286 ± 2.69 mg GAE/g) while among G. hispida highest TPC was detected in the chloroform extract (78.272 ± 4.41 mg GAE/g) while lowest in n-Hexane extract (52.708 ± 5.53 mg GAE/g) respectively. Similarly, H. crispum also showed highest TFC in ethyl acetate extract (22.685 ± 2.05 mg QE/g) and lowest in methanolic extract (15.934 ± 1.13 mg QE/g). However, G. hispida showed highest TFC in ethanolic extract (25.338 ± 1.44 mg QE/g) and lowest in methanolic extract (14.183 ± 1.04 mg QE/g) as shown in Fig. 1.
Fig. 1.
Total phenolic (mg GAE/g) and flavonoid (mg QE/g) contents observed in different extracts of H. crispum and G. hispida. Data represents the mean of three replicates, error bars indicates standard deviation and each letter (a-d) indicates significance at P < 0.05 by using ANOVA.
3.1.3. Hplc analysis
HPLC analysis of methanolic, ethanolic and ethyl acetate extracts of H. crispum and G. hispida was performed and their chromatographs were compared with the standards. Presence or absence of sixteen (16) compounds in G. hispida and H. crispum extracts is presented in table 2. Highest number of compounds were expressed in G. hispida methanolic extract namely Plumbagin, Thymoquinone, Gallic acid, Vanillic acid, Coumaric acid, Syringic acid, Gentisic, Caffeic acid, Luteolin, Myrcetin, Quercetin followed by H. crispum ethanolic extract (Plumbagin, Thymoquinone, Vanillic acid, Gallic acid, Syringic acid, Cinnamic acid, Coumaric acid and Caffeic acid). Chromatogram of standards and G. hispida methanolic extract derived at different wavelengths is presented in Fig. 2a and 2b.
Fig. 2.
RP-HPLC chromatograms indicating the existence of polyphenolic compounds at different wavelengths (a) Chromatograms of standards (b) Chromatograms of G. hispida methanolic extract.
3.2. FTIR analysis
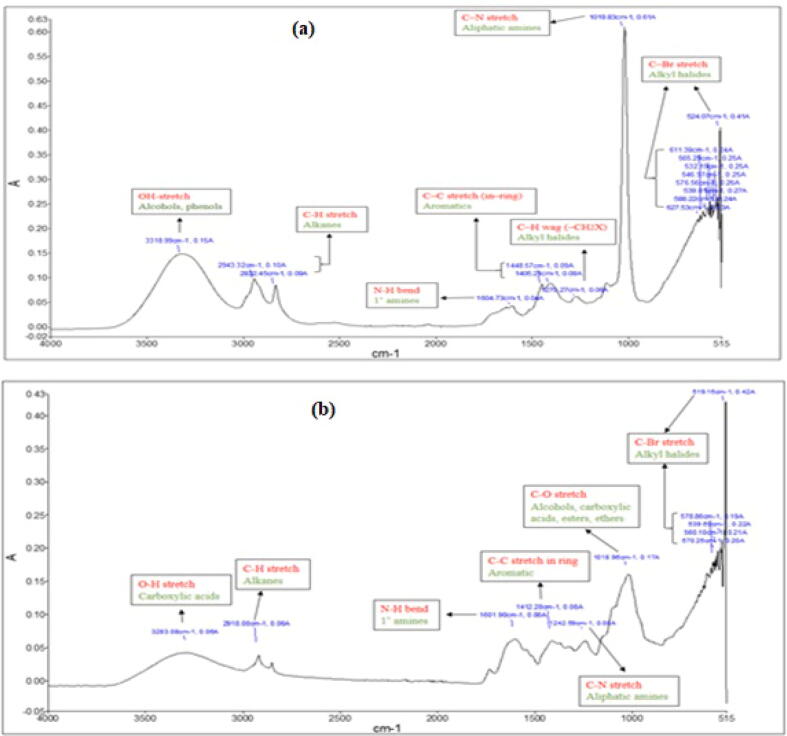
FTIR spectroscopy was performed to determine the bioactive functional groups present in plant extracts. In both species, total seven prominent peaks were observed indicating the presence of seven different kinds of bonds i.e. OH-stretch, C-H stretch, C-C stretch, N-H bend, C-H wag (-CH2X), C-O stretch, C-N stretch and C-Br stretch (Fig. 3a and b).
Fig. 3.
FTIR spectra of two species of family Boraginaceae showing the presence of functional groups at different peaks (a) FTIR spectrum of G. hispida(b) FTIR spectrum of H. crispum.
3.3. LIBS analysis
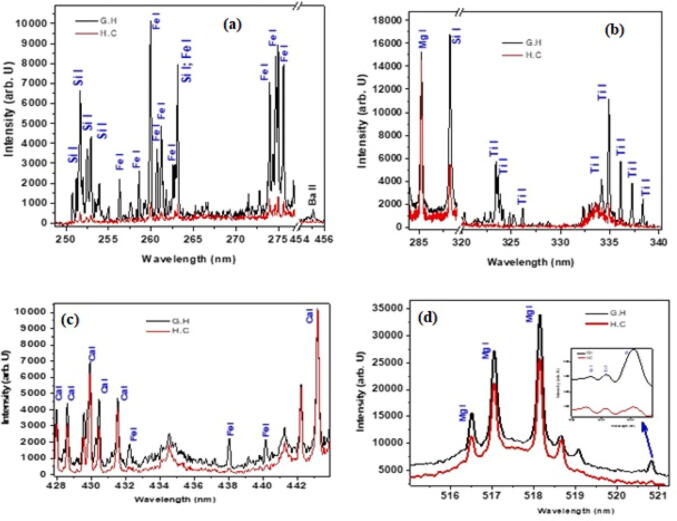
In present study, the elemental analysis of selected plant samples was observed using LIBS spectra and the spectral lines were identified using NIST database. Emission spectrum revealed that Si, Fe and Ba were present in both plants, but their concentration was much higher in G. hispida as compared to H. crispum. Emission spectrum presented in Fig. 4 clearly shows the signature of Mg, Si and Ti. It seems that the concentration of Mg was almost comparable in both plants, but the concentration of Si and Ti was higher in G. hispida as compared to H. crispum. A very small concentration of heavy metal chromium (Cr) was also detected in G. hispida. Moreover, Ca and Mg were also detected in both plants with comparable concentration (Fig. 4).
Fig. 4.
The emission spectrum of H. crispum and G. hispida covering the wavelength region from (a) 250–280 nm (b) 285–340 nm (c) 428–442 nm (d) 516–521 nm.
3.4. Biological assays
3.4.1. Antioxidant assays
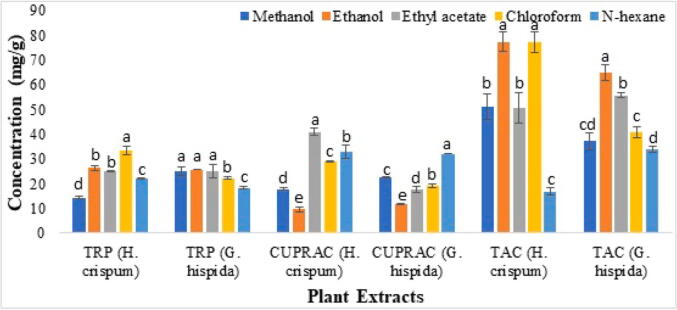
Highest reducing potential was observed in H. crispum chloroform extracts (33.379 ± 1.66 mg/g) and lowest in the methanolic extract of H. crispum (14.243 ± 0.50 mg/g) while in G. hispida highest reducing potential was observed in methanolic extract and lowest in n-Hexane extract. In case of CUPRAC assay, highest activity was recorded in the ethyl acetate extract of H. crispum (40.943 ± 1.50 mg/g) and lowest in the H. crispum ethanolic extract (09.620 ± 0.91 mg/g) while in G. hispida highest activity was observed in n-Hexane and lowest in ethanolic extracts. However, TAC was found highest in H. crispum ethanolic extract (78.128 ± 6.79 mg/g) and lowest in H. crispum n-Hexane extract (16.877 ± 1.64 mg/g) respectively. Similarly, TAC was revealed highest in ethanolic extract of G. hispida and lowest in n- hexane extract. Additionally, free radicals (DPPH, ABTS and SOR) scavenging activities of the methanolic and chloroform extracts of H. crispum and G. hispida was determined and the results were compared with ascorbic acid which is used as a standard. Among both plants, highest DPPH scavenging activity was observed in methanolic extract of G. hispida (12.636 ± 2.07 μg/mL) and lowest in the chloroform extract of G. hispida (34.480 ± 3.62 μg/mL). Similarly, highest NBT activity was determined in the methanolic extract of H. crispum (38.556 ± 5.34 μg/mL) and lowest in the chloroform extract of G. hispida (119.23 ± 5.80 μg/mL). Moreover, highest ABTS scavenging activity was found in the methanolic extract of H. crispum (7.980 ± 2.94 μg/mL) and lowest in the methanolic extract of G. hispida (29.083 ± 0.37 μg/mL) (Table 3).
Table 3.
IC50 values of radical scavenging activities observed in the methanolic and chloroform extracts of G. hispida and H. crispum in comparison with standard.
| Plant Extracts | DPPH scavenging activity | NBT scavenging activity | ABTS scavenging activity |
|---|---|---|---|
| HCM | 20.256 ± 0.35bc | 38.556 ± 5.34bc | 7.980 ± 2.94c |
| HCCHL | 26.720 ± 3.07b | 99.323 ± 9.33a | 12.520 ± 0.18b |
| GHM | 12.636 ± 2.07d | 49.610 ± 2.15b | 29.083 ± 0.37a |
| GHCHL | 34.480 ± 3.62a | 119.23 ± 5.80a | 13.443 ± 0.11b |
| Ascorbic acid | 16.913 ± 2.57 cd | 32.250 ± 4.67c | 2.804 ± 0.29d |
The data is expressed as mean ± SD (n = 3) and the alphabetical letters (a-d) indicates significant difference (P < 0.05) from each other (DPPH = 2,2-diphenyl-1picrylhydrazyl; NBT = Nitro Blue Tetrazolium; ABTS = 2, 2′-azino-bis −3- ethylbenzthiazoline-6-sulphonic acid; HCM: H. crispum methanolic extract; HCCHL: H. crispum chloroform extract; GHM: G. hispida methanolic extract; GHHCL: G. hispida chloroform extract). Altogether, H. crispum and G. hispida extracts made in polar solvents showed more antioxidant potential than in non-polar extracts (Fig. 5).
Fig. 5.
Total reducing power (TRP), cupric ions reducing antioxidant capacity (CUPRAC) and total antioxidant capacity (TAC) of the selected plant extracts. The data is the mean of three replicates (SD ± 3) and each letter (a-e) indicates significance at P < 0.05 [Total reducing power and total antioxidant capacity expressed as ascorbic acid equivalent (mg AE/g extract); Cupric ions reducing assay expressed as gallic acid equivalent (mg GA/g extract)].
3.4.2. Antimicrobial activity
Antibacterial activities of the plant extracts were evaluated against five bacterial strains including Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, Escherichia coli and Klebsiella pneumonia. H. crispum revealed highest activity in its ethyl acetate (10.66 ± 0.57 mm), chloroform (10.33 ± 0.72 mm) and n-Hexane (15.33 ± 0.57 mm) extracts against E. coli. While G. hispida extracts showed highest activity in the methanolic (11.3 ± 0.57 mm) and chloroform (19.0 ± 1.01 mm) extracts against B. subtilis. Overall, among all extracts n-Hexane extracts showed minimum activity against most of the bacterial strains. Furthermore, results revealed that selected plant extracts did not possess significant antifungal potential and thus, considered as the most resistant strains (Table 4).
Table 4.
Antibacterial and antifungal activity of five different extracts of G. hispida and H. crispum.
| Microbial strains |
G. hispida |
H. crispum |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methanol | Ethanol | Ethyl acetate | Chloroform | n-Hexane | Methanol | Ethanol | Ethyl acetate | Chloroform | n-Hexane | Control | ||
| Bacterial strains | ||||||||||||
| S. aureus | ZOI | 7.6 ± 1.5 | 7.6 ± 2.0 | 8.0 ± 1.0 | 8.0 ± 1.0 | NI | 10.6 ± 0.1 | 9.1 ± 0.4 | NI | 8.6 ± 0.2 | NI | 32.0 ± 2.8 |
| MIC | 625 | 625 | 625 | 312.5 | – | 150 | 312.5 | – | 312.5 | – | – | |
| P. aeruginosa | ZOI | 8.6 ± 1.1 | 8.6 ± 0.5 | NI | 7.6 ± 0.5 | NI | NI | 7.0 ± 1.0 | 7.3 ± 1.1 | 9.3 ± 0.5 | NI | 30.0 ± 1.5 |
| MIC | 312.5 | 312.5 | – | 625 | – | – | 312 | 625 | 150 | – | – | |
| B. subtilis | ZOI | 11.3 ± 0.5 | NI | NI | 19.0 ± 1.0 | NI | 8.3 ± 0.5 | NI | NI | NI | NI | 31.0 ± 2.0 |
| MIC | 150 | – | – | 75 | – | 312.5 | – | – | – | – | – | |
| E. coli | ZOI | 8.1 ± 0.5 | NI | 7.1 ± 1.1 | NI | NI | 9.3 ± 0.5 | NI | 10.6 ± 0.5 | 10.3 ± 0.7 | 15.33 ± 0.57 | 29.0 ± 1.5 |
| MIC | 312.5 | – | 625 | – | – | 312.5 | – | 150 | 150 | 75 | – | |
| K. pneumonia | ZOI | NI | 7.4 ± 1.0 | NI | NI | 7.0 ± 1.1 | NI | NI | NI | 7.3 ± 0.5 | NI | 28.0 ± 1.8 |
| MIC | – | 625 | – | – | 625 | – | – | – | 625 | – | – | |
| Fungal strain | ||||||||||||
| F. solani | ZOI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 32.0 ± 1.8 |
| MIC | – | – | – | – | – | – | – | – | – | – | – | |
| A. niger | ZOI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 33.0 ± 1.8 |
| MIC | – | – | – | – | – | – | – | – | – | – | – | |
| A. fumigatus | ZOI | NI | NI | NI | NI | NI | NI | NI | NI | 8.0 + 1.2 | NI | 30.0 ± 2.6 |
| MIC | – | – | – | – | – | – | – | – | 312.5 | – | – | |
| Mucor racemosus | ZOI | NI | NI | NI | NI | NI | NI | NI | NI | NI | NI | 28.0 ± 2.0 |
| MIC | – | – | – | – | – | – | – | – | – | – | – | |
ZOI: Zone of inhibition (mm ± SD); MIC: Minimum inhibitory concentration (µg/mL); NI: No inhibition; Positive control: Oxytetracycline (Antibacterial standard) and Chloramphenicol (Antifungal standard).
3.4.3. Cytotoxic activity
In case of H. crispum, highest brine shrimp cytotoxicity was recorded in its methanolic (LC50 −8.4980 ppm), ethanolic (LC50 − 8.3640 ppm) and chloroform (LC50 −10.817 ppm) extract while among different extracts of G. hispida highest activity was revealed in n-Hexane (LC50 − 42.380 ppm) and methanolic (LC50 −75.883 ppm) extracts. However, chloroform extracts showed lowest activity in both species and vincristine sulfate (control) displayed 0.839 ppm LC50 value (Table 5).
Table 5.
Cytotoxicity of brine shrimps observed at different concentrations of selected plant extracts and their LC50 values.
| Plants | Solvents used for extracts | Percentage mortality at different doses |
LC50 (ppm) | 95 % Confidence Interval | ||||
|---|---|---|---|---|---|---|---|---|
| 62 | 125 | 250 | 500 | 1000 | ||||
| H. crispum | Methanol | 86.66 | 93.33 | 100.0 | 100.0 | 100.0 | 8.4980 | 2.7070 – 26.683 |
| Ethanol | 73.33 | 86.66 | 86.66 | 100.0 | 100.0 | 8.3640 | 2.2820 – 30.654 | |
| Ethyl acetate | 43.33 | 50.00 | 80.00 | 96.66 | 100.0 | 93.162 | 59.329 – 146.28 | |
| Chloroform | 46.66 | 63.33 | 80.00 | 96.66 | 100.0 | 78.107 | 47.551 – 128.29 | |
| N. hexane | 80.00 | 86.66 | 90.00 | 96.66 | 100.0 | 10.817 | 3.5780 – 32.703 | |
| G. hispida | Methanol | 40.00 | 70.00 | 80.00 | 90.00 | 100.0 | 75.883 | 42.196 – 136.46 |
| Ethanol | 23.33 | 50.00 | 76.66 | 86.66 | 100.0 | 129.86 | 82.146 – 205.30 | |
| Ethyl acetate | 23.33 | 33.33 | 63.33 | 70.00 | 96.66 | 178.16 | 112.97 – 280.97 | |
| Chloroform | 30.00 | 50.00 | 76.66 | 83.33 | 100.0 | 119.71 | 70.435 – 203.47 | |
| N. hexane | 63.33 | 83.33 | 100.0 | 100.0 | 100.0 | 42.380 | 23.657 – 75.923 | |
LC50: Lethal concentratin which causes death of fifty percent (one half) of group of tested animals.
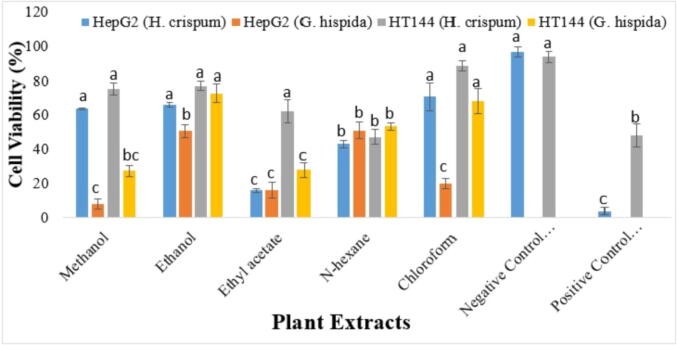
Results of anticancer assay revealed that G. hispida ethanolic extract exhibited highest cell viability against HepG2 (50.76 %) and HT144 (72.95 %) cell lines. Similarly, ethyl acetate extract of H. crispum also showed significant viability (16.14 % cell viability) against HepG2 cell lines. However, other H. crispum extracts were determined as ineffective against cancer cell lines while G. hispida extracts showed moderate anticancer activity (Fig. 6).
Fig. 6.
Percentage viabilities of HepG2 and HT144 cells in five different extracts of H. crispum and G. hispida relative to untreated control (mean ± SD). Each letter (a-c) indicates significance at P < 0.05 using ANOVA.
3.4.4. Antihemolytic activity
Results revealed highest antihemolytic activity in the H. crispum chloroform extract (31.869 μg/mL) followed by H. crispum ethanolic extract (18.634 μg/mL) and G. hispida methanolic extract (14.991 μg/mL) respectively. However, extracts of both species prepared in ethyl acetate indicated lowest antihemolytic potential (8.227 μg/mL and 9.560 μg/mL) as shown in Table 6.
Table 6.
Antihemolytic activity of different extracts of H. crispum and G. hispida observed against H2O2 induced hemolysis.
| Plants | Solvents used to prepare extracts | Absorbance at 560 nm concentration (μg/mL) |
% Inhibition of hemolysis at 1000 (μg/mL) | ||||
|---|---|---|---|---|---|---|---|
| 62.5 | 125 | 250 | 500 | 1000 | |||
| H. crispum | Methanol | 0.049 ± 0.004 | 0.052 ± 0.001 | 0.076 ± 0.015 | 0.099 ± 0.019 | 0.113 ± 0.024 | 11.056def |
| Ethanol | 0.051 ± 0.007 | 0.053 ± 0.004 | 0.063 ± 0.004 | 0.117 ± 0.011 | 0.191 ± 0.004 | 18.634c | |
| Chloroform | 0.050 ± 0.005 | 0.083 ± 0.019 | 0.150 ± 0.063 | 0.159 ± 0.014 | 0.326 ± 0.072 | 31.869b | |
| Ethyl acetate | 0.040 ± 0.037 | 0.049 ± 0.001 | 0.050 ± 0.001 | 0.068 ± 0.017 | 0.084 ± 0.026 | 8.227f | |
| n-Hexane | 0.064 ± 0.015 | 0.068 ± 0.020 | 0.071 ± 0.002 | 0.081 ± 0.026 | 0.107 ± 0.010 | 10.439ef | |
| G. hispida | Methanol | 0.047 ± 0.001 | 0.059 ± 0.001 | 0.077 ± 0.001 | 0.122 ± 0.010 | 0.153 ± 0.018 | 14.991 cd |
| Ethanol | 0.047 ± 0.005 | 0.050 ± 0.005 | 0.068 ± 0.005 | 0.080 ± 0.004 | 0.126 ± 0.003 | 12.292def | |
| Chloroform | 0.051 ± 0.003 | 0.056 ± 0.001 | 0.069 ± 0.009 | 0.077 ± 0.002 | 0.108 ± 0.004 | 10.601def | |
| Ethyl acetate | 0.049 ± 0.003 | 0.049 ± 0.001 | 0.060 ± 0.007 | 0.080 ± 0.021 | 0.098 ± 0.018 | 9.560ef | |
| n-Hexane | 0.059 ± 0.015 | 0.060 ± 0.007 | 0.085 ± 0.003 | 0.123 ± 0.003 | 0.134 ± 0.004 | 13.138de | |
| Quercetin | 0.570 ± 0.020 | 0.620 ± 0.040 | 0.790 ± 0.090 | 0.891 ± 0.010 | 1.012 ± 0.025 | 98.700a | |
Values in same column followed by different letter (a–f) are significantly different (P < 0.05) as detected by LSD - all pair-wise comparison test.
3.4.5. Alpha-amylase inhibition assay
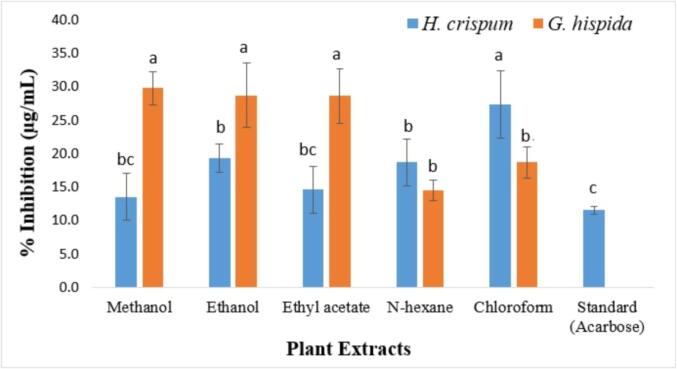
In this assay, acarbose was used as a standard which showed 11.513 % inhibition. Among all extracts, highest % inhibition was observed for the methanolic extract of H. crispum (13.5 %) followed by the n-Hexane extract of G. hispida (14.5 %) and ethyl acetate extract of G. hispida (14.5 %) respectively. However, all other extracts showed moderate alpha-amylase inhibitory potential (Fig. 7).
Fig. 7.
Alpha-amylase inhibitory potential of H. crispum and G. hispida extracts prepared in five different solvents. Data is expressed as mean of three replicates, error bars indicates standard deviation and each letter (a-c) indicates significance at P < 0.05 by using ANOVA.
4. Discussion
Plant natural products are gaining interest now-a-days for their biological activities due to the presence of phenolics, alkaloids and other compounds (Iqbal et al., 2018c, Abbasi et al., 2019, Abbasi et al., 2020). Ethno-medicinal and biological application of different species belonging to genus Heliotropium and Gastrocotyle (family Boraginaceae) have been reported extensively (Aïssaoui et al., 2019, Aslam et al., 2017, Bazzaz and Haririzadeh, 2003) but the two species namely H. crispum and G. hispida have not been explored so far. As a result, recent experiments have been planned to investigate the biological ability of the two species.
Various compounds occurring naturally in the plant kingdom are part of our daily diet and are used in pharmaceutical and cosmetic industries as well (Iqbal et al., 2017, Batool et al., 2019, Batool et al., 2020). Among these, flavonoids and polyphenols exhibit significant values and are detected in Boraginaceae family (Iqbal et al., 2005). Secondary metabolites derived from plants are also involved in regulating the mechanism of enzyme action for detoxification (Báidez et al., 2007, Abbasi et al., 2021). Flavonoids provides defense to the living systems by stabilizing the free radicals and act as anti-inflammatory agents (Iqbal et al., 2019, Karmakar and Halder, 2019, Iqbal et al., 2020). Results revealed that both species showed the presence of significant phenolic contents (greater than50 mg GAE/g) in all extracts except n-Hexane extracts which exhibited < 50 mg GAE/g phenolic contents. However, flavonoid contents were detected in the range of 25.338 ± 1.44 mg QE/g (G. hispida ethanolic extract) to 14.183 ± 1.04 mg QE/g (G. hispida methanolic extract) respectively (Table 1).
Different solvents are used to separate compounds and their extraction efficiency is determined by the solvent type and the extraction process (Goli et al., 2005). In present study, HPLC analysis revealed the presence of highest number of compounds in the G. hispida methanolic extract (eleven compounds) and H. crispum ethanolic extract (eight compounds) as compared to the other extracts (Table 2). It can be concluded that increase in the number of compounds depends on polarity of extraction solvents. These phenolic compounds detected in selected extracts have various pharmacological uses. The medicinal value of selected plant species is further potentiated by the identification of all these polyphenols. Quercetin and coumaric acid were found to be dose-dependent inhibitors of multiple cancer cell lines (Zhang et al., 2008). In particular, Gallic acid has various therapeutic uses as an antiangiogenic, anticancer, anti-inflammatory and antimicrobial agent (Choubey et al., 2015). Antioxidant properties of caffeic acid are extensively tested, exceeding numerous other antioxidants in curbing the production of aflatoxin by more than 95 percent in one such research (Dai and Mumper, 2010). These polyphenols have ability to hinder the proliferation of cancer cells, activate pro-carcinogens and suppress growth signaling pathways (Hussain et al., 2016, Amin et al., 2015). Hence, this study confirmed the potential involvement of polyphenols in G. hispida and H. crispum for pharmacological operation.
Table 2.
Polyphenolic compounds detected at different retention time in the methanolic, ethanolic and ethyl acetate extracts of G. hispida and H. crispum by using HPLC technique.
| Polyphenols | Retention time | GHM | GHE | GHEA | HCM | HCE | HCEA |
|---|---|---|---|---|---|---|---|
| Gallic acid | 3.72 | + | + | + | – | + | – |
| Catechin | 7.33 | – | + | – | – | – | – |
| Gentisic acid | 8.08 | + | + | – | – | – | – |
| Vanillic acid | 9.54 | + | – | + | + | + | – |
| Caffeic acid | 9.59 | + | – | + | + | + | – |
| Syringic acid | 10.00 | + | – | – | – | + | + |
| Cinnamic acid | 13.08 | – | – | – | + | + | – |
| Coumaric acid | 14.76 | + | – | + | + | + | – |
| Myrcetin | 15.51 | + | + | – | – | – | – |
| Quercetin | 18.51 | + | – | – | + | – | – |
| Luteolin | 19.58 | + | – | + | – | – | – |
| Kaempferol | 21.20 | – | – | – | – | – | – |
| Apigenin | 22.00 | – | – | – | – | – | – |
| Plumbagin | 22.39 | + | + | – | + | + | + |
| Thymoquinone | 22.65 | + | – | + | + | + | + |
| Emodin | 29.05 | – | – | – | – | – | + |
*Data is representing compounds obtained from minimum to maximum retention time. ‘+’ and ‘-’ indicates the presence and absence of polyphenolic compounds in plant extracts (GHM: G. hispida methanolic extract; GHE: G. hispida ethanolic extract; GHEA: G. hispida ethyl acetate extract HCM: H. crispum methanolic extract; HCE: H. crispum ethanolic extract; HCEA: H. crispum ethyl acetate extract).
In proposed study, the plant extracts were passed through the FTIR and functional groups of the components get separated depending on peaks ratio. The obtained spectra confirmed the presence of alkanes, carboxylic acids, aldehydes, alcohols, ketones and amides in selected species. As both species belong to the same family, so they displayed the presence of almost similar kind of functional groups. Our study correlate with the earlier findings of Ahmad et al. (2016) who reported the presence of similar functional groups in the relative member of genus Heliotropium i.e. H. bacciferum. This is the first report on the FTIR analysis of selected species and is initial step towards biological research to explore H. crispum and G. hispida. Moreover, LIBS technique was used to determine the elements existing in any material including plants. Previously, many studies have been conducted on different medicinal plants reporting the presence of elements using LIBS (Zafar et al., 2019, Rai et al., 2013). Our studies confirmed the presence of eight elements (Si, Fe, Ba, Mg, Ti, Ca, Mg and Cr) indicating that the selected species can be effectively used in industries as a major source of elements.
As a rich source of antioxidants, medicinal plants can reduce oxidative stress, thereby helping to cure various diseases (Ulewicz-Magulska and Wesolowski, 2019, Thatoi et al., 2014). The medicinal values of plants were assessed by its antioxidant potentials using different antioxidant assays as these are widely used assays owing to the benefits such as ease of use, inexpensive reagents used in these experiments, non-laboratory, and the ability to easily evaluate the antioxidant properties of large numbers of samples at a time (Gülçin et al., 2005). Our results revealed that the H. crispum and G. hispida extracts made in polar solvents showed more antioxidant potential than in non-polar extracts. Previously, Shahat et al. (2019) revealed that bioactive compounds (Rosmarinic acid and β-sitosterol) are present in G. hispida that are responsible for its antioxidant activity. However, antioxidant activity of H. crispum have been evaluated for the first time. It can be inferred that the phytochemicals such as phenolics, flavonoids, anthocyanin and coumarin present in these species are predominately responsible for radical scavenging potential (Aqil et al., 2006).
The continuous increase in resistance of microbial agents resulted in mortality worldwide. Bacteria have genetic capability to gain resistance against therapeutically active drugs and thus natural products with less toxicity and more antibacterial activity are usually explored (Maleki et al., 2008). Results revealed that selected species exhibit significant antibacterial potential, however, these extracts were found to be ineffective against the selected fungal strains. In case of antibacterial activity, K. pneumonia and B. subtilis were found to be highly resistant as most of the extracts formed no zones against them. Conversely, S. aureus, P. aeruginosa and E. coli were considered as the most sensitive bacterial strains. Present data also correlate with the earlier findings of Ihtesham et al. (2019) who reported that H. crispum showed highest activity against S. aureus (i.e. 30 ± 0.09 mm). Previously, Khan et al. (2016) also reported strong antibacterial activity of synthesized silver nanoparticles using H. crispum. Moreover, present study revealed that the extracts prepared in non-polar solvent (n-Hexane) exhibited lowest antibacterial potential. It can be suggested that the crude extracts of H. crispum and G. hispida prepared in polar solvents can be effectively utilized to treat different diseases caused by resistant microbes.
Selected plant extracts showed significant toxicity potential against shrimp’s. LC50 in H. crispum extracts ranged from 8.3640 ppm to 93.162 ppm while among G. hispida extracts ranged from 42.380 ppm to 178.16 ppm respectively (Table 5). Additionally, selected plant extracts were screened using the MTT reduction assay for anti-proliferative activity against HepG2 (liver cancer) and HT144 (skin cancer) cells lines. Our findings showed that all extracts suppressed HepG2 (liver cancer) and HT144 (skin cancer) cancer cell line proliferation, and the relative viability of HepG2 (liver cancer) and HT144 (skin cancer) cells in various solvents increased significantly. All-inclusive, G. hispida revealed lowest viability percentage in methanolic extract (8.08 % and 27.43 %) against HepG2 and HT144 cancer cell lines (Fig. 6). It is a known fact that plants contain a wide variety of bioactive compounds that are reported helpful in the treatment of cancer (Saddiqa et al., 2017). Hence, it can be suggested that the phenolic and flavonoid compounds present in these extracts might be responsible for the biological activities of selected plants.
Hydrogen peroxide and many other free radicals also damage the erythrocyte membrane causing hemolysis. Thus, it is important to eliminate H2O2 from our food systems. In plant extracts, organic compounds are present which scavenge H2O2 by neutralizing H2O2 to H2O (Escher et al., 2018, Ebrahimzadeh et al., 2010). Similarly, the cornerstone of diabetes regulation is blood glucose level management, which can be accomplished by the use of oral hypoglycaemic agents and inhibitors of carbohydrate hydrolysis enzymes (Patel et al., 2012). The tested extracts had lower mechanical hemolysis when compared with the control, indicating that these extracts exhibit minimum erythrocyte toxicity. The increase in antihemolytic activity was proportional to the extracts concentrations. Present results of alpha-amylase assay indicated highest activity in the H. crispum methanolic extracts (13.5 %). Present study is coherent with the earlier findings of Di Sotto et al. (2019) and Ahmed et al. (2017) who revealed alpha-amylase inhibitory potential in medicinal plants such as Punica granatum and Quercus dilatata. It can be suggested that the polyphenols present in plant extracts provide protection to the erythrocytes from hemolysis and can also be used as antidiabetic agent.
5. Conclusion and future prospective
The goal of the present study was to investigate the phytochemicals, elements and the biological profiling of G. hispida and H. crispum extracts. For this purpose, the use of a wide-ranging polarity of the solvent system is proved as an effective and reliable source. Identifying essential polyphenols and evaluating the antimicrobial activity of selected plants in different crude extracts helps to clarify some of their common uses. The plant extracts also displayed strong anticancer activity against HepG2 and HT144 cancer cell line. Biochemical profiling and elemental analysis revealed that H. cispum and G. hispida might be a potential source of bioactive compounds and essential elements which needs to be isolated and characterized so that they could be further exploited in drug development. From the present study, it can be recommended to conduct a systematic in vivo study targeting the anti-cancer and anti-diabetic aspects of the selected extracts. The existence of significant secondary metabolites including phenolics and flavonoids, which causes antioxidant and antimicrobial properties in plants, further encourages to undertake research in detail.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/350), King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Javed Iqbal, Email: javed89qau@gmail.com.
Tariq Mahmood, Email: tmahmood.qau@gmail.com.
References
- Ahmad S., AbdEl-Salam N.M., Ullah R. In vitro antimicrobial bioassays, DPPH radical scavenging activity and FTIR spectroscopy analysis of Heliotropium bacciferum. Biomed Res. Int. 2016;2016:1–12. doi: 10.1155/2016/3818945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbasi B.A., Iqbal J., Ahmad R., Bibi S., Mahmood T., Kanwal S., Bashir S., Gul F., Hameed S. Potential phytochemicals in the prevention and treatment of esophagus cancer: A green therapeutic approach. Pharmacol. Rep. 2019;71(4):644–652. doi: 10.1016/j.pharep.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Abbasi B.A., Iqbal J., Khan Z., Ahmad R., Uddin S., Shahbaz A., Mahmood T. Phytofabrication of cobalt oxide nanoparticles from Rhamnus virgata leaves extract and investigation of different bioactivities. Microsc. Res. Tech. 2021;84(2):192–201. doi: 10.1002/jemt.23577. [DOI] [PubMed] [Google Scholar]
- Abbasi B.A., Iqbal J., Kiran F., Ahmad R., Kanwal S., Munir A., Uddin S., Nasir J.A., Chalgham W., Mahmood T. Green formulation and chemical characterizations of Rhamnella gilgitica aqueous leaves extract conjugated NiONPs and their multiple therapeutic properties. J. Mol. Struct. 2020;1218:128490. doi: 10.1016/j.molstruc.2020.128490. [DOI] [Google Scholar]
- Ahmed M., Fatima H., Qasim M., Gul B. Polarity directed optimization of phytochemical and in vitro biological potential of an indigenous folklore: Quercus dilatata Lindl. ex Royle. BMC Complement. Altern. Med. 2017;17(1):1–16. doi: 10.1186/s12906-017-1894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Khan M.R., Iqbal J., Batool R., Naz I., Yaseen T., El-Serehy H.A. Chemical composition and pharmacological bio-efficacy of Parrotiopsis jacquemontiana (Decne) Rehder for anticancer activity. Saudi J Biol Sci. 2021;28(9):4969. doi: 10.1016/j.sjbs.2021.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S., Khan M.R., Batool R., Shah S.A., Iqbal J., Abbasi B.A., Yaseen T., Zahra N., Aldhahrani A., Althobaiti F. Characterization and phytochemical constituents of Periploca hydaspidis Falc crude extract and its anticancer activities. Saudi Journal of Biological Sciences. 2021 doi: 10.1016/j.sjbs.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aïssaoui H., Mencherini T., Esposito T., De Tommasi N., Gazzerro P., Benayache S., Benayache F., Mekkiou R. Heliotropium bacciferum Forssk. (Boraginaceae) extracts: chemical constituents, antioxidant activity and cytotoxic effect in human cancer cell lines. Nat. Prod. Res. 2019;33(12):1813–1818. doi: 10.1080/14786419.2018.1437433. [DOI] [PubMed] [Google Scholar]
- Amin, A.R., Karpowicz, P.A., Carey, T.E., Arbiser, J., Nahta, R., Chen, Z.G., Dong, J.T., Kucuk, O., Khan, G.N., Huang, G.S., 2015. Evasion of anti-growth signaling: a key step in tumorigenesis and potential target for treatment and prophylaxis by natural compounds. In: Semin. Cancer Biol. S55-S77. [DOI] [PMC free article] [PubMed]
- Apak R., Guclu K., Ozyurek M., Karademir S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agr. Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Aqil F., Ahmad I., Mehmood Z. Antioxidant, and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turk. J. Biol. 2006;30(3):177–183. [Google Scholar]
- Aslam H., Khan A.-U., Rehman N.-u., Ali F., Nadeem H., Shah S.M. Anti-hyperglycemic activity of Heliotropium strigosum (Boraginaecae) whole plant extract in alloxan-induced diabetic mice. Trop. J. Pharm. Res. 2017;16(10):2425–2430. [Google Scholar]
- Báidez A.G., Gómez P., Del Río J.A., Ortuño A. Dysfunctionality of the xylem in Olea europaea L. plants associated with the infection process by Verticillium dahliae Kleb. Role of phenolic compounds in plant defense mechanism. J. Agr. Food Chem. 2007;55(9):3373–3377. doi: 10.1021/jf063166d. [DOI] [PubMed] [Google Scholar]
- Bazzaz B.S., Haririzadeh G. Screening of Iranian plants for antimicrobial activity. Pharm. Biol. 2003;41(8):573–583. [Google Scholar]
- Batool R., Aziz E., Salahuddin H., Iqbal J., Tabassum S., Mahmood T. Rumex dentatus could be a potent alternative to treatment of micro-bial infections and of breast cancer. J. Tradit. Chin. Med. 2019;39:772–779. [PubMed] [Google Scholar]
- Batool R., Aziz E., Iqbal J., Salahuddin H., Tan B.-H., Tabassum S., Mahmood T. In vitro antioxidant and anti-cancer activities and phytochemical analysis of Commelina benghalensis L. root extracts. Asian Pac. J Trop. Biomed. 2020;10(9):417. doi: 10.4103/2221-1691.290133. [DOI] [Google Scholar]
- Casciaro B., Calcaterra A., Cappiello F., Mori M., Loffredo M., Ghirga F., Mangoni M., Botta B., Quaglio D. Nigritanine as a new potential antimicrobial alkaloid for the treatment of Staphylococcus aureus-induced infections. Toxins. 2019;11(9):511. doi: 10.3390/toxins11090511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.C., Yang M.H., Wen H.M., Chern J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Chlopicka J., Pasko P., Gorinstein S., Jedryas A., Zagrodzki P. Total phenolic and total flavonoid content, antioxidant activity and sensory evaluation of pseudocereal breads. LWT-Food Sci. Technol. 2012;46(2):548–555. [Google Scholar]
- Choubey S., Varughese L.R., Kumar V., Beniwal V. Medicinal importance of gallic acid and its ester derivatives: a patent review. Pharm. Pat. Anal. 2015;4(4):305–315. doi: 10.4155/ppa.15.14. [DOI] [PubMed] [Google Scholar]
- Dai J., Mumper R.J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler S., Szymczak G., Wójcik M. Comparison of some secondary metabolite content in the seventeen species of the Boraginaceae family. Pharm. Biol. 2017;55(1):691–695. doi: 10.1080/13880209.2016.1265986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimzadeh M.A., Nabavi S.F., Nabavi S.M., Eslami B. Anti-hemolytic and antioxidant activities of Allium paradoxum. Cent. Eur. J. Biol. 2010;5:338–345. [Google Scholar]
- Escher G.B., Santos J.S., Rosso N.D., Marques M.B., Azevedo L., do Carmo M.A.V., Daguer H., Molognoni L., Prado-Silva L.d., Sant'Ana A.S., da Silva M.C., Granato D. Chemical study, antioxidant, anti-hypertensive, and cytotoxic/cytoprotective activities of Centaurea cyanus L. petals aqueous extract. Food Chem. Toxicol. 2018;118:439–453. doi: 10.1016/j.fct.2018.05.046. [DOI] [PubMed] [Google Scholar]
- Espinosa-Leal C.A., Puente-Garza C.A., García-Lara S. In vitro plant tissue culture means for production of biological active compounds. Planta. 2018;248(1):1–18. doi: 10.1007/s00425-018-2910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney D.J. Probit analysis. J. Inst. Actuaries. 1952;78(3):388–390. [Google Scholar]
- Goli A.H., Barzegar M., Sahari M.A. Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chem. 2005;92(3):521–525. [Google Scholar]
- Gülçin İ., Berashvili D., Gepdiremen A. Antiradical, and antioxidant activity of total anthocyanins from Perilla pankinensis decne. J. Ethnopharmacol. 2005;101(1-3):287–293. doi: 10.1016/j.jep.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Harborne J.B. Chapman and Hall Ltd.; London, New York: 1973. Phytochemical methods. A guide to modern techniques of plant analysis; pp. 49–188. [Google Scholar]
- Hussain S.A., Sulaiman A.A., Balch C., Chauhan H., Alhadidi Q.M., Tiwari A.K. Natural polyphenols in cancer chemoresistance. Nutr. Cancer. 2016;68(6):879–891. doi: 10.1080/01635581.2016.1192201. [DOI] [PubMed] [Google Scholar]
- Ihtesham Y., Khan U., Ullah I., Khan W., Qureshi R.A. In vitro antibacterial and antifungal potential of methanolic crude extracts of some Heliotropium spp. Advanc. Med. Plant Res. 2019;7(3):79–84. [Google Scholar]
- Imran M., Bano S., Dawood M., Tarar M.A., Ali A. Climate change, poverty and agricultural resource degradation: a case study of district DG Khan. Pak. J. Agri. Sci. 2013;50(1):163–167. [Google Scholar]
- Iqbal K., Nawaz S.A., Malik A., Riaz N., Mukhtar N., Mohammad P., Choudhary M.I. Isolation and lipoxygenase-inhibition studies of phenolic constituents from Ehretia obtusifolia. Chem. Biodivers. 2005;2(1):104–111. doi: 10.1002/cbdv.200490161. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Abbasi B.A., Ahmad R., Batool R., Mahmood T., Ali B., Khalil A.T., Kanwal S., Afzal Shah S., Alam M.M., Bashir S., Badshah H., Munir A. Potential phytochemicals in the fight against skin cancer: Current landscape and future perspectives. Biomed. Pharmacother. 2019;109:1381–1393. doi: 10.1016/j.biopha.2018.10.107. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Abbasi B.A., Ahmad R., Mahmoodi M., Munir A., Zahra S.A., Shahbaz A., Shaukat M., Kanwal S., Uddin S., Mahmood T., Capasso R. Phytogenic synthesis of nickel oxide nanoparticles (NiO) using fresh leaves extract of Rhamnus triquetra (wall.) and investigation of its multiple in vitro biological potentials. Biomedicines. 2020;8(5):117. doi: 10.3390/biomedicines8050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J., Abbasi B.A., Ahmad R., Mahmood T., Kanwal S., Ali B., Khalil A.T., Shah S.A., Alam M.M., Badshah H. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomed. Pharmacother. 2018;108:752–756. doi: 10.1016/j.biopha.2018.09.096. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Abbasi B.A., Batool R., Mahmood T., Ali B., Khalil A.T., Kanwal S., Shah S.A., Ahmad R. Potential phytocompounds for developing breast cancer therapeutics: nature’s healing touch. Eur. J. Pharmacol. 2018;827:125–148. doi: 10.1016/j.ejphar.2018.03.007. [DOI] [PubMed] [Google Scholar]
- Iqbal J., Abbasi BanzeerAhsan, Khalil AliTalha, Ali B., Mahmood T., Kanwal S., Shah SayedAfzal, Ali W. Dietary isoflavones, the modulator of breast carcinogenesis: Current landscape and future perspectives. Asian Pac. J. Trop. Med. 2018;11(3):186. doi: 10.4103/1995-7645.228432. [DOI] [Google Scholar]
- Iqbal J., Abbasi B.A., Mahmood T., Kanwal S., Ali B., Shah S.A., Khalil A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017;7(12):1129–1150. [Google Scholar]
- Jo C., Park B.J., Chung S.H., Kim C.B., Cha B.S., Byun M.W. Antibacterial and antifungal activity of citrus (Citrus unshiu) essential oil extracted from peel by-products. Food Sci. Biotechnol. 2004;13:384–386. [Google Scholar]
- Kallassy H. Phytochemistry and biological activities of selected lebanese plant species (Crataegus Azarolus L. and Ephedra Campylopoda) Med. Sci. Monit. Basic Res. 2019;25:88–99. doi: 10.12659/MSMBR.914741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmakar B., Halder G. Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies. Energy Convers Manag. 2019;182:307–339. [Google Scholar]
- Khalil M., Teunissen C.E., Otto M., Piehl F., Sormani M.P., Gattringer T., Barro C., Kappos L., Comabella M., Fazekas F., Petzold A., Blennow K., Zetterberg H., Kuhle J. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018;14(10):577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- Khan F., Hashmi M.U., Khalid N., Hayat M.Q., Ikram A., Janjua H.A. Controlled assembly of silver nano-fluid in Heliotropium crispum extract: a potent anti-biofilm and bactericidal formulation. Appl. Surf. Sci. 2016;387:317–331. [Google Scholar]
- Ložienė K., Venskutonis P.R., Šipailienė A., Labokas J. Radical scavenging and antibacterial properties of the extracts from different Thymus pulegioides L. chemotypes. Food Chem. 2007;103(2):546–559. [Google Scholar]
- Maleki S., Seyyednejad S.M., Damabi N.M., Motamedi H. Antibacterial activity of the fruits of Iranian Torilis leptophylla against some clinical pathogens. Pak. J. Biol. Sci. 2008;11(9):1286–1289. doi: 10.3923/pjbs.2008.1286.1289. [DOI] [PubMed] [Google Scholar]
- Meenambal M., Pughalendy K., Vasantharaja C., Prapakaran S., Vijayan P. Phytochemical information from FTIR and GC-MS studies of methanol extract of Delonix elat leaves. Int. J. Chem. Anal. Sci. 2012;3(6):1446–1448. [Google Scholar]
- Mehwish S., Islam A., Ullah I., Wakeel A., Qasim M., Khan M.A., Ahmad A., Ullah N. In vitro antileishmanial and antioxidant potential, cytotoxicity evaluation and phytochemical analysis of extracts from selected medicinally important plants. Biocatal. Agric. Biotechnol. 2019;19:101117. doi: 10.1016/j.bcab.2019.101117. [DOI] [Google Scholar]
- Mickymaray S. Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics. 2019;8(4):257. doi: 10.3390/antibiotics8040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munir H., Sarfraz R.A. Medicinal attributes of Aerva javanica native to Pothohar Plateau. Pak. J. Life Soc. Sci. 2014;12(2):80–86. [Google Scholar]
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. J. Nutr. 1986;44:307–315. [Google Scholar]
- Panthi M., Subba R.K., Raut B., Khanal D.P., Koirala N. Bioactivity evaluations of leaf extract fractions from young barley grass and correlation with their phytochemical profiles. BMC Complement. Med. Ther. 2020;20:64. doi: 10.1186/s12906-020-2862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D.K., Kumar R., Laloo D., Hemalatha S. Diabetes mellitus: An overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac. J. Trop. Biomed. 2012;2(5):411–420. doi: 10.1016/S2221-1691(12)60067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A.D.T., Netzel G., Chhim P., Netzel M.E., Sultanbawa Y. Phytochemical characteristics and antimicrobial activity of Australian grown Garlic (Allium sativum L.) cultivars. Foods. 2019;8:358. doi: 10.3390/foods8090358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto P., Pineda M., Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269(2):337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Qasim M., Abideen Z., Adnan M.Y., Gulzar S., Gul B., Rasheed M., Khan M.A. Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. S. Afr. J. Bot. 2017;110:240–250. [Google Scholar]
- Rai P.K., Srivastava A.K., Sharma B., Dhar P., Mishra A.K., Watal G. Use of laser-induced breakdown spectroscopy for the detection of glycemic elements in Indian medicinal plants. Evid-Based Complement. Altern. Med. 2013;2013:1–9. doi: 10.1155/2013/406365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman N. New Indian Publishing Agencies; New Delhi: 2006. Phytochemical technique; p. 19. [Google Scholar]
- Rodrigues T., Reker D., Schneider P., Schneider G. Counting on natural products for drug design. Nature Chem. 2016;8(6):531–541. doi: 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- Sadiqa A., Cakmak O., Usman M. Isocoumarins and 3,4-dihydroisocoumarins, amazing natural products: a review. Turk. J. Chem. 2017;41(2):153–178. [Google Scholar]
- Shahat A.A., Hidayathulla S., Khan A.A., Alanazi A.M., Al Meanazel O.T., Alqahtani A.S., Alsaid M.S., Hussein A.A. Phytochemical profiling, antioxidant and anticancer activities of Gastrocotyle hispida growing in Saudi Arabia. Acta Trop. 2019;191:243–247. doi: 10.1016/j.actatropica.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Shinwari Z.K. Medicinal plants research in Pakistan. J. Med. Plant Res. 2010;4(3):161–176. [Google Scholar]
- Steel R.G.D., Torrie J.H., Dickey D.A. 3rd ed. McGraw Hill Co.; New York: 1996. Principles and procedures of statistics: a biometrical approach. [Google Scholar]
- Tepe B., Daferera D., Sokmen A., Sokmen M., Polissiou M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae) Food Chem. 2005;90:333–340. [Google Scholar]
- Thatoi H.N., Patra J.K., Das S.K. Free radical scavenging and antioxidant potential of mangrove plants: a review. Acta Physiol. Plant. 2014;36(3):561–579. [Google Scholar]
- Toiu A., Vlase L., Vodnar D.C., Gheldiu A.M., Oniga I. Solidago graminifolia L. Salisb. (Asteraceae) as a valuable source of bioactive polyphenols: HPLC profile, in vitro antioxidant and antimicrobial potential. Molecules. 2019;24:2666. doi: 10.3390/molecules24142666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulewicz-Magulska B., Wesolowski M. Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Foods Hum. Nutr. 2019;74(1):61–67. doi: 10.1007/s11130-018-0699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.G., Sun H.X., Fang W.H. Haemolytic activities and adjuvant effect of Astragalus membranaceus saponins (Ams) on the immune responses to ovalb Sermakkani umin in mice. Vaccine. 2005;23:5196–5203. doi: 10.1016/j.vaccine.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Zafar A., Ahmed N., Umar Z.A., Baig M.A. Spectrochemical analysis of dates available in Pakistan using laser induced breakdown spectroscopy (LIBS) and laser ablation time-of-flight mass spectrometer (LA-TOF-MS) Laser Phys. 2019;29(8):1–7. [Google Scholar]
- Zahra S.A., Iqbal J., Abbasi B.A., Shahbaz A., Kanwal S., Shah S.L., Ahmad P., Mahmood T. Antimicrobial, cytotoxic, antioxidants, enzyme inhibition activities, and scanning electron microscopy of Lactuca orientalis (Boiss.) Boiss. Seeds. Microsc Res Tech. 2021;84(6):1284–1295. doi: 10.1002/jemt.23687. [DOI] [PubMed] [Google Scholar]
- Zahra S.A., Iqbal J., Abbasi B.A., Yaseen T., Hameed A., Shahbaz A., Kanwal S., Mahmood T., Ahmad P. Scanning electron microscopy of Sophora alopecuroides L. seeds and their cytotoxic, antimicrobial, antioxidant, and enzyme inhibition potentials. Microsc. Res. Tech. 2021;84(8):1809–1820. doi: 10.1002/jemt.23740. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Seeram N.P., Lee R., Feng L., Heber D. Isolation, and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 2008;56(3):670–675. doi: 10.1021/jf071989c. [DOI] [PubMed] [Google Scholar]