Abstract
The emergence of multidrug resistance in pathogenic bacteria limits the utilization of available antibiotics. The development of alternate options to treat infectious diseases is the need of the day.The present study was aimed to synthesize, characterize and evaluate the bioactive properties of silver nanoparticles. Endophytic bacterium Bacillus cereus (MT193718) isolated from Berberis lycium was used to synthesize biocompatible silver nanoparticles. Antibacterial properties of AgNPs were evaluated against clinically isolated multidrug-resistant strains of Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae. AgNPs indicated significant antibacterial activity against S. aureus and K. pneumoniae fwith a zone of inhibition of 17 and 18 mm at a concentration of 1000 µg/ mL with minimum inhibitory concentration of 15.6 and 62.5 µg/mL respectively. Significant antioxidant activity with an IC50 value of 9.5 µg/mL was recorded. Biosynthesized AgNPs were found compatible with red blood cells at a concentration of 31.5 µg/ml with no clumping of erythrocytes. The study suggested that AgNPs synthesized by the endophytic bacterium Bacillus cereus are biologically active and can be used as antioxidant and antibacterial agents against drug-resistant bacteria.
Keywords: Biosynthesis, MDRs, Antioxidant activity, XRD, FTIR, Hemolysis
1. Introduction
Multidrug resistance (MDRs) is a phenomenon, where a single bacterium develops resistance to more than one antibiotic (Orsi et al., 2011). Infections caused by MDRs are hard to treat due to limited choice of available antibiotics and prolonged stay at hospitals leading to economical burden. Antibiotic resistance is a significant threat to human health and is responsible for approximately 0.7 million annual deaths worldwide (Clifford et al., 2018, Alexander and MacLean, 2020). Antibiotic resistant bacteria harbor plasmids coding for antibiotic resistant genes that can be transferred to other bacteria and hence can also make them antibiotic resistant. In recent years, an alarming rise in bacterial infections has been observed owing to MDRs (Rosenberger et al., 2011, Lin et al., 2015). Antibiotic-resistant mechanisms include increased expression of genes coding for multidrug efflux, changed cell permeability, enzymes inactivation, protection of target sites, biofilm formation, and quorum sensing (Munita and Arias, 2016). Numerous bacterial strains have been isolated that have adopted resistance to several antibiotics. Among them, Methicillin-Resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter baumannii and Escherichia coli are of major concern (Basak et al., 2016). According to an estimate, these drug-resistant infections would cause a loss of up to 100 trillion US dollars per year (Aidara-Kane et al., 2018). To prevent the spread of antibiotic-resistant strains, efforts should be diverted for the development and discovery of new antibacterial agents (Qiao et al., 2018). Many investigations have suggested silver nanoparticles (AgNPs) as an alternative to cope with the problem of antibiotic resistance as their efficiency has been reported against various Gram-positive and Gram-negative bacteria (Liu et al., 2015).
AgNPs have various applications in nanotechnology, nanomedicine, biosensing, nano-medical imaging, skin burns, and cancer treatment (Zhang et al., 2016). Physicochemical binding of silver nanoparticles on the cell surface leads to membrane damage resulting inmembrane destabilization and leakage of cytoplasm. The release of free radicals and the generation of reactive oxygen species (ROS) cause subcellular structure damage and inactivation of microbialenzymes, proteins, and nucleotides (Akter et al., 2018, Zheng et al., 2018).
AgNPs also plays important role in minimizing oxidative stress in the human body (Nagaich et al., 2016). Free radicals are also responsible for various kinds of degenerative ailments in body like cardiovascular diseases, mutations, aging, and carcinogenesis. The utilization of AgNPs as an alternative to antibiotics can be beneficial as they also scavenge free radicals (Moteriya et al., 2017, Moteriya and Chanda, 2014). Different chemical, physical and biological methods have been used for the synthesis of AgNPs. These methods have various limitations such as consumption of high energy, toxicity, high cost, heat generation, and use of expensive chemicals (Iravani et al., 2014). Therefore biological/green methods for nanoparticle synthesis are more preferable as they result in the synthesis of less toxic, stable, and soluble nanoparticles (Gurunathan et al., 2015). Green synthesis involves the use of various biological entities ranging from prokaryotes to eukaryotic fungi and plants (Mohanpuria et al., 2008). Problems associated with plant utilization are harvesting the endangered species that pose a risk to plant diversity (Baker and Satish, 2012). Bacteria can be alternate for the most stable synthesis of silver nanoparticles that lead to the reduction of silver ions to NPs in an aqueous solution (Singh et al., 2020). The use of stabilizing and reducing agents can be avoided while working with bacteria. Biomolecules and pathways intermediates can act as capping and reducing agents (Liu et al., 2015).
Many studies have been conducted for the synthesis of AgNPs using bacterial culture but few studies are available for the synthesis of silver nanoparticles, using endophytic bacteria (Syed et al., 2016, Singh et al., 2017, Silambarasan and Abraham, 2012, Sunkar and Nachiyar, 2012). Endophytic bacteria are microorganisms that live inside the living tissues of plants without any obvious negative effect (Afzal et al., 2019). Endophytic bacteria can produce a variety of antibacterial, antifungal, anticancer, and antioxidant compounds like alkaloids, flavonoids, isoprenoids and indoles (Barman and Bhattacharjee, 2020, Monnanda et al., 2020).
Based on the importance of green synthesized silver nanoparticles present study was aimed to develop AgNPs as an alternative to conventional antibiotics, using endophytic bacterium Bacillus cereus isolated from a medicinally important plant Berberis lyceum. B. cereus was selected on the base of production of bioactive secondary metabolites and pharmaceutical potential (Unpublished data). The antibacterial activity of AgNPs was evaluated against multidrug-resistant bacterial strains. Moreover antioxidant properties of AgNPs were also investigated. Despite of pharmaceutical properties of AgNPs there is a need to analyze their biocompatibility through in vitro experiments to ensure biosafety thus toxicity of biosynthesized AgNPs was investigated against human red blood cells.
2. Materials and methods
2.1. Propagation of endophytic bacteria
Bacillus cereus (MT193718) identified earlier was collected from Plant Microbiology Lab, The University of Haripur. The strain was inoculated on nutrient agar (Merck) and incubated at 37 °C for colony formation. After 24 hr a single colony was sub-cultured in nutrient broth (Merck) and incubated in a rotating shaker at 37 °C for 24 hr. Bacterial culture was then centrifuged at 12000 rpm (Remi RM12C) for 10 min to separate the biomass from the supernatant.
2.2. Synthesis of silver nanoparticles
Bacterial biomass was used for the intracellular synthesis of silver nanoparticles as initial experiments conducted on intercellular and intracellular biosynthesis indicated better peak intensity for intracellular synthesis. A standard method of silver nanoparticle synthesis was adopted as reported earlier with little modification (Silambarasan and Abraham, 2012). Bacterial biomass was washed twice with autoclaved distilled water to remove impurities and media adhered to bacterial biomass. Biomass (2 g) was added to 20 mL of silver nitrate (2.5 mM) solution and incubated for 72 h at 37 °C in a rotating shaker at 200 rpm. The synthesis of silver nanoparticles was monitored by a change in the color of the solution. Nanoparticles were separated by centrifugation at 10000 rpm for 15 min followed by washing with sterile deionized water. Silver nanoparticles were dried under vacuum and stored till further use.
2.3. Characterization of green synthesized silver nanoparticles
Characterization of nanoparticles was carried out using different analytical techniques such as UV–Vis spectrophotometer, Fourier transform infrared (FTIR) (Schimadzu Fourier transform infrared spectrophotometer, model 270), X-Ray Diffraction (X'pert PRO of PANalytical company), and Scanning Electron Microscopy (JOEL-JSM-6490LA™ SEM). Nanoparticles were suspended in 1 mL of distilled water and absorbance was recorded from 200 to 800 nm.
For Fourier transform, infrared NPs were mixed with potassium bromide (KBr) at the ratio of 1:100. Absorbance was recorded from 400 to 4000 cm−1. Different modes of vibration predict different functional groups. The size of nanoparticles was analyzed using an X-Ray diffractometer at 40 KV and 30 mA at 37 °C. Silver nanoparticles were coated on an X-Ray grid and analyzed by an X-Ray diffractometer. Peaks' width and length were used to calculate the diameter and size of silver nanoparticles. X-Ray analysis was done in a different range of Bragg angles Φ at a rate of 2Φ angles/min. The morphology of the synthesized AgNPs was performed using scanning electron microscopy operating at 20 kV with a counting rate of 2838 cps.
2.4. Antibacterial activity
The AgNPs were tested against clinically isolated human pathogenic MDR strains of Klebsiella pneumoniae, Acinetobacter baumannii, Methicillin-resistant Staphylococcus aureus, and Pseudomonas aeruginosa by agar well diffusion method (Sikder et al., 2018). In short MDR strains were inoculated in nutrient broth for 24 hr at 37 °C. Each of the MDR strains was maintained at OD of 600 nm and then swabbed on an agar plate. Wells (5 mm) were made in each plate with the aid of a sterilized cork borer. Two concentrations of nanoparticles 500 and 1000 µg/mL were loaded in wells and then incubated at 37 °C for 24 hr. Bactericidal activity was determined by measuring the zone of inhibition against tested bacterial strains.
2.5. Minimum inhibitory concentration
MIC of AgNPs was evaluated by performing a 96-well microtiter plate assay. Different concentrations (250, 125, 62.5, 31.2, 7.8, and 3.9 μg/ml) of nanoparticles were made in DMSO and further two-fold serial dilution was made and tested against different MDR strains (Yu et al., 2019). Briefly, 5 μL of bacterial suspension was inoculated in each well of the microplate. This plate was incubated at 37 °C for 24 hr. The optical density of each plate was measured at 600 nm by a multi-detection microplate reader.
2.6. Antioxidant activity by DPPH free radical scavenging assay
The antioxidant activity of biosynthesized nanoparticles was evaluated using DPPH (2,2-diphenyl-1-picryl-hydrazyl) free radical scavenging assay as it is easy, fast, reliable and does not require special device (Aksoy et al., 2013, Qasim et al., 2018). Different dilutions of silver nanoparticles (10–100 µg/mL) were prepared in DMSO. Silver nanoparticles solution (1 mL) from each tube was mixed with 1 mL of DPPH and vortexed. The solution was then incubated in darkness at 37 °C for 30 min. The absorbance of the reaction mixture was recorded at 517 nm using a UV–Vis spectrophotometer. DPPH was used as negative control while different concentrations of ascorbic acid were used as a positive control. The free radical scavenging activity was calculated as follow
Free radical scavenging activity (%) = absorbance of control-absorbance of sample/absorbance of control × 100
2.7. Hemolytic activity
The biocompatibility of silver nanoparticles was evaluated by performing a hemolytic assay (Loo et al., 2018). Fresh human blood was mixed with phosphate buffer solution (PBS) and centrifuged at 9000 rpm for 10 min. Red blood cells were washed three times with PBS at 9000 rpm. Washed red blood cells were diluted with PBS at the ratio of 1:10.
Different serial dilutions of silver nanoparticles (7–250 µg/mL) were prepared in DMSO and gently mixed with red blood cells (1 mL). The solution was then incubated at 37 °C for 1 hr and centrifuged at 1000 rpm for 5 min. The supernatant was separated and absorbance was checked at 540 nm. Triton x100 and PBS were used as positive and negative control respectively.
2.8. Statistical analysis
All the experiments were performed in triplicate and acquired data were expressed as mean SD. The data were analyzed by one-way analysis of variance using IBM SPSS for Windows, Version 20. Results were considered statistically significant at p ≤ 0.05.
3. Results
3.1. UV–Vis spectrophotometer analysis
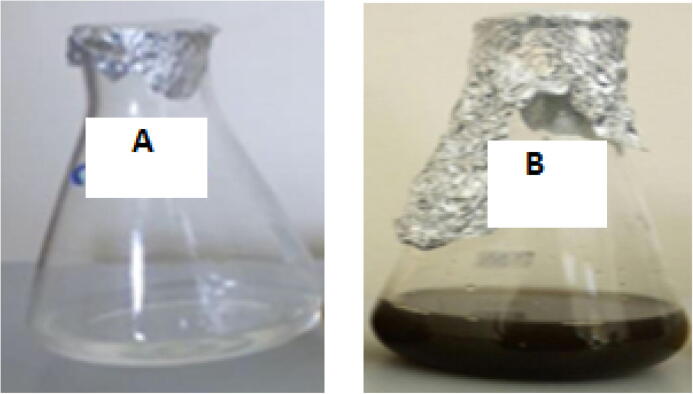
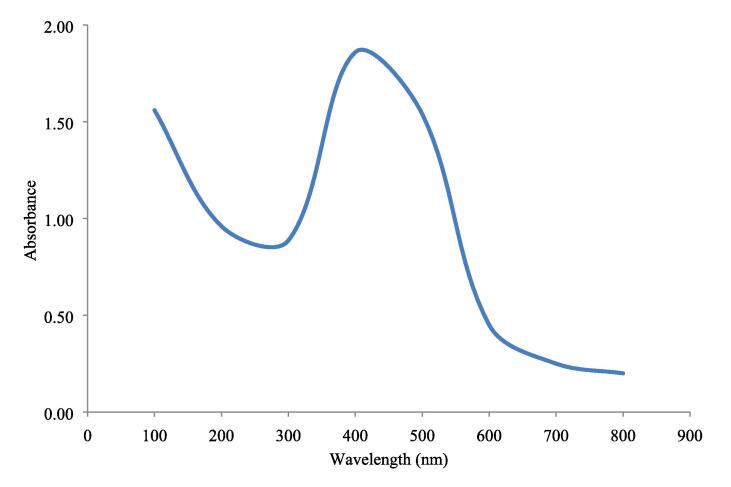
Silver nanoparticle synthesis was preliminarily confirmed by a color change from yellow to deep brown. A UV–Visible spectrum of the aqueous medium containing silver nanoparticles indicated a peak at 425 nm corresponding to the plasmon absorbance of silver nanoparticles (Fig. 1, Fig. 2).
Fig. 1.
Synthesis of silver nanoparticles by endophytic bacteria i.e. Control (A); Nanoparticles indicated by color change (B).
Fig. 2.
UV–Vis spectrophotometric analysis of silver nanoparticles.
3.2. XRD analysis
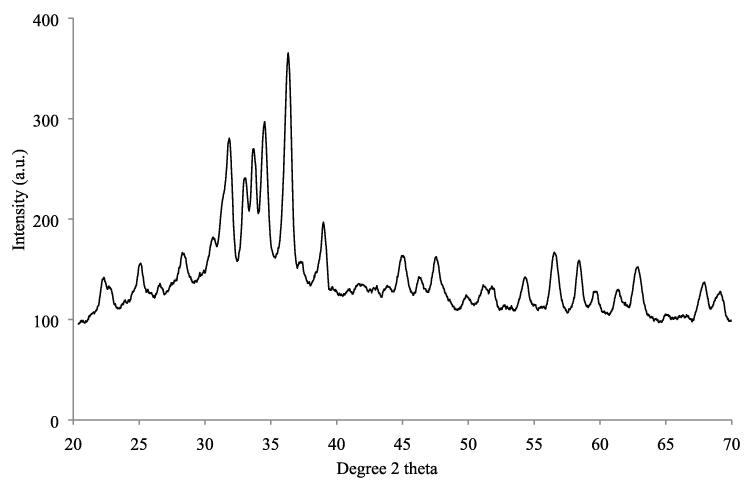
XRD diffraction analysis indicated major peaks at 31.7, 32.8, 33.6, 34.3, 36.1, 38.1°, 45.71° and 64.64° corresponding to different planes as reported in the literature. The number of peaks indicates that NPs synthesized were diverse in size but crystalline in nature. The average size calculated by the Scherrer equation was 21.5 nm (Fig. 3).
Fig. 3.
XRD spectrum of biosynthesized nanoparticles.
3.3. FTIR analysis
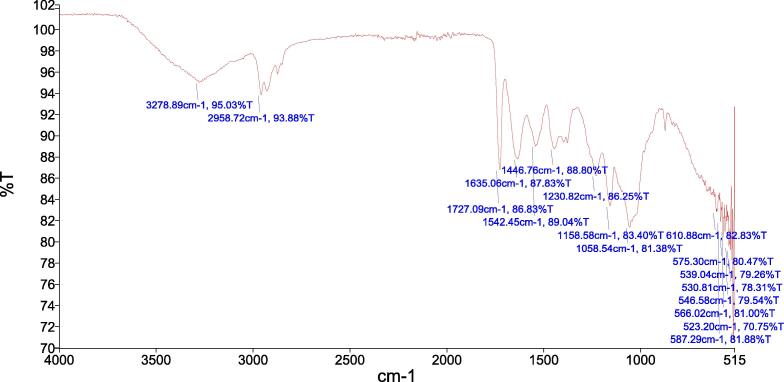
FTIR analysis showed a broad characteristic peak at 3278 cm−1 which is assigned to O—H and N—H vibrations. The peak observed at 2958 cm−1 indicated CH2 symmetric and asymmetric stretching. FTIR spectra of AgNPs showed sharp peaks at 1635 cm−1 which were assigned to C O stretching and indicator of amide. A stretch at 1727 cm−1 indicated presence of C O and band at 1542 cm−1 corresponded to the presence of carboxylic acid chain (Fig. 4).
Fig. 4.
Fourier Transform Infrared (FTIR) spectra of green synthesized AgNPs.
3.4. SEM analysis
Results of SEM analysis are indicated in Fig. 5. The SEM analysis of biosynthesized AgNPs indicated the spherical shape and small size of the nanoparticles corresponding to the results of XRD analysis.
Fig. 5.
Scanning Electron Micrograph (SEM) of biosynthesized silver nanoparticles.
3.5. Antibacterial activity
Antibacterial sensitivity test of bacterial strains was performed to confirm their resistance profile. The results of antibiotic sensitivity are presented in Table 1. Results confirmed the multidrug resistance status as they were found resistant to most of the antibiotics tested.
Table 1.
Antibiotic sensitivity pattern of clinical isolates of bacteria.
| S. No. | Name of organism | Antibiotic sensitivity pattern |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | Gentamicin | Ciprofloxacin | Cotrimoxazole | Imipenem | Minocycline | Aztreonam | Augmentin | Chloramphenicol | ||
| 1 | Methicillin Resistant Staphylococcus aureus |
R | R | R | S | R | – | R | R | S |
| 2 | Klebsiella pneumoniae | R | R | R | R | R | – | R | R | – |
| 3 | Pseudomonas aeruginosa | – | R | R | – | R | R | R | – | – |
| 4 | Acinetobacter baumannii | R | R | R | R | R | – | R | R | – |
| 5 | Escherichia coli | – | R | R | – | R | R | R | – | – |
The Abbreviations: R = Resistant, S = Sensitive.
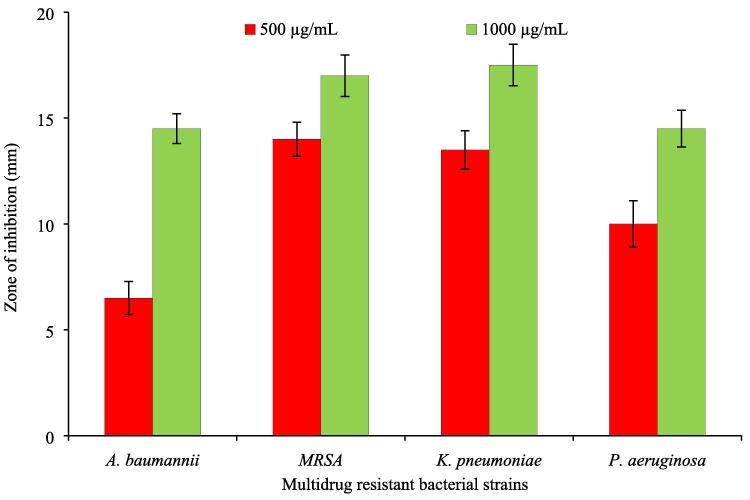
Nanoparticles exhibited a different range of activities against tested MDR strains with varying zones of inhibition (mm). Dose-dependent response of bacterial strains was observed on exposure to different concentrations of nanoparticles. Among these MDR strains, Klebsiella pneumoniae and MRSA were found most sensitive by exhibiting zones of inhibition of 18 mm. Moderate antibacterial effects were recorded against Pseudomonas aeruginosa with a zone of inhibition of 14 mm (Fig. 6).
Fig. 6.
Antibacterial activity of green synthesized silver nanoparticles.
3.6. Minimum inhibitory concentration
The minimum inhibitory concentration of biosynthesized silver nanoparticles was determined and marked as the least diluted silver nanoparticles at which no visible microbial growth was detected (Guntur et al., 2018, Laloy et al., 2014). Silver nanoparticles exhibited antibacterial effects against Acinetobacter baumannii with MIC of 15.6 μg/mL. While against Klebsiella pneumoniae MIC was recorded at a concentration of 62.5 μg/mL. MIC values of 62.5 and125 μg/mL were recorded against Pseudomonas aeruginosa and MRSA, respectively.
3.7. Antioxidant activity
Results of antioxidant activity indicated that bacterial-based AgNPs had remarkable free radical scavenging activity. Maximum scavenging activity (65.5 %) was observed at a concentration of 100 μg/mL followed by 64, 62.2, 60.3 and 57.6 % at a concentration of 75, 50, 40 and 30 μg/mL concentrations respectively. The IC 50 value of AgNPS was 9.5 μg/mL in comparison to ascorbic acid that showed an IC 50 value of 11.2 μg/mL (Table 2).
Table 2.
DPPH free radical scavenging activity of silver nanoparticles.
| Sample | Concentration in μg/mL | DPPH free radial scavenging activity % | IC 50 value in μg/mL |
|---|---|---|---|
| Biosynthesized AgNPS | 100 | 65.50 | 9.5 |
| 75 | 64 | ||
| 50 | 62.20 | ||
| 40 | 60.30 | ||
| 30 | 57.60 | ||
| 20 | 56 | ||
| 10 | 53 | ||
| Ascorbic acid | 100 | 76.60 | 11.2 |
| 75 | 74.60 | ||
| 50 | 73.60 | ||
| 40 | 71 | ||
| 30 | 68.60 | ||
| 20 | 63 | ||
| 10 | 50 |
3.8. Hemolytic activity of silver nanoparticles
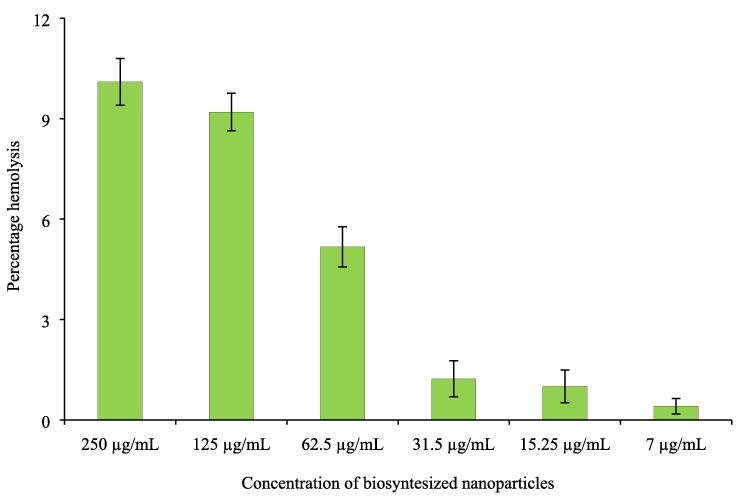
Different concentrations of AgNPs were used to determine hemolysis of red blood cells. Results indicated dose-dependent hemolytic activity with a maximum of 10.1 % hemolysis of red blood cells at a concentration of 250 μg/mL followed by 9.2% at a concentration of 125 μg/mL. At a concentration of 31.5 μg/mL and below least hemolysis was observed as 1.23, 1, and 0.4 % respectively (Fig. 7). The result of hemolysis indicates the biocompatibility of green synthesized silver nanoparticles at lower concentrations.
Fig. 7.
Hemolytic activity of silver nanoparticles.
4. Discussion
In the present era, great interest has been developed in the synthesis and applications of nanoparticles. Biosynthesis of nanoparticles using natural products is an environment-friendly technique. Bacteria can synthesize metallic nanoparticles due to the presence of biomolecules that reduce ions into particles. The use of bacterial strains for nanoparticle synthesis is convenient as they are easy to culture and undergo genetic modifications. The current study was designed to use endophytic Bacillus cereus for the synthesis of silver nanoparticles and to check their effectiveness against multidrug-resistant bacteria. The synthesis of silver nanoparticles was confirmed by a color change from yellow to deep brown when exposed to silver nitrate. The color change is the visual confirmation of the synthesis of nanoparticles (Chen and Schluesener, 2008). Change in the color intensity can be linked with excitation of surface plasmon resonance of synthesized nanoparticles (Kim et al., 2007). The synthesis of silver nanoparticles was further confirmed by a characteristic peak at 430 nm, as indicated by the results of UV visible spectroscopy (Saravanan et al., 2017, Gopinath et al., 2012). Different studies report the utilization of bacteria for the synthesis of biocompatible silver nanoparticles. The endophytic Bacillus cereus isolated from Garcinia xanthochymus has also been reported for the synthesis of nanoparticles (Silambarasan and Abraham, 2012).
XRD analysis of silver nanoparticles indicated three diffraction peaks corresponding to 111, 200, and 202 planes. The presence of peaks at these positions was consistent with earlier reports where nanoparticles synthesized by endophytic bacteria exhibited the same pattern. Data confirmed the crystalline nature of nanoparticles with an average size of 21.5 nm (Elamawi et al., 2018).
FTIR analysis was carried out to determine the capping agent, functional groups, and possible mechanism behind AgNPs formation (Rahman et al., 2019, Netala et al., 2016). It showed broad characteristic peaks at 3278 cm−1, 2958 cm−1 and 1635 cm−1 corresponding to O-H and N-H vibrations predicting the involvement of protein molecules in the stabilization of nanoparticles. It has been reported that biological molecules can perform dual functions (formation and stabilization of silver nanoparticles) in an aqueous medium (Adur et al., 2018). Results of the FTIR spectrogram are in agreement with previous findings that bacterial proteins act as a capping agent for nanoparticle synthesis (Siddiqi et al., 2018). The shape and morphological characteristics of nanoparticles were determined by SEM analysis. Results indicated the spherical shape of nanoparticles with an average size of 20–40 nm. Results were found to be consistent with the previous reports (Dong et al., 2017, Spence et al., 2014).
The antibacterial activity of nanoparticles was determined against drug-resistant clinical bacteria isolates. Results indicated a dose-dependent response of bacteria towards nanoparticles. Various levels of antibacterial effects were observed with the highest activity (18 mm) against K. pneumoniae and MRSA. These results are following the previous finding where the broad-spectrum antibacterial activity of biosynthesized silver nanoparticles from endophyticBacillus cereus was observed against human pathogens (Dong et al., 2017, Banu and Balasubramanian, 2015). Similarly, silver nanoparticles synthesized from Aloe vera exhibited antibacterial activity against Gram-positive and Gram-negative bacterial strains (Sulaiman et al., 2015). However, the mechanism of antibacterial activity of nanoparticles is not well documented.Studies propose possible mechanisms of antibacterial effects including damage to the cell wall, destabilization and rupture of the plasma membrane, blockage of respiratory enzymes, and DNA damage (Abalkhil et al., 2017, Manikprabhu and Lingappa, 2013). MIC of silver nanoparticles was recorded against multidrug-resistant bacteria as 15.6 μg/mL against Acinetobacter baumanni, 62.5 μg/mL against Pseudomonas aeruginosa and MRSA while 125 μg/mL against Klebsiella pneumoniae. Results of the study are in agreement with the previous finding where silver nanoparticles exhibited MIC of 125 μg/mL is against MRSA (Kumar et al., 2016).
Antioxidant activity of silver nanoparticles was conducted by using DPPH free radical scavenging assay. Significant free radical scavenging activity was observed ranging from 53 to 65 %. Strong antioxidant activity of silver nanoparticles synthesized by Cestrum nocturnum has been reported (Keshari et al., 2020). Antioxidant activity is a phenomenon helping to reduce oxidative stress and hence involved in the prevention of various degenerative diseases. To neutralize the free radicals, antioxidants themselves donate electrons and undergo oxidation. Silver nanoparticles are active and stable enough to undergo the oxidation process hence can be used for the treatment of many diseases caused by oxidative stress (Mahmoud et al., 2017).
It is important to determine the biosafety and biocompatibility of silver nanoparticles before their clinical applications. Various features like the size and surface area of nanoparticles affect their hemolytic ability. Hemolysis is caused by leakage of hemoglobin by rupturing of erythrocyte membranes on exposure to nanoparticles (Hamouda et al., 2019). Results indicate dose-dependent hemolysis, with no or negligible hemolytic activity at low concentration. Results of the present investigation are following previous reports where green synthesized silver nanoparticles were found compatible with red blood cells at lower concentrations (Shah et al., 2018, Liu et al., 2013).
5. Conclusion
An environment-friendly, intracellular synthesis of silver nanoparticles using endophytic bacterium Bacillus cereus was reported. UV–visible spectrophotometer confirmed the synthesis of silver nanoparticles. XRD analysis further confirmed the crystalline nature of nanoparticles. FTIR spectrum indicated the involvement of biomolecules present in the extract of bacterial endophyte as capping and reducing agents. Under experimental conditions biosynthesized nanoparticles exhibited significant antibacterial activity against multidrug-resistant pathogenic bacteria. The nanoparticles exhibited free radical scavenging activity, comparable with ascorbic acid. Initial hemolytic analysis indicated biosafety of nanoparticles at low concentrations. It is concluded that bacterial endophyte Bacillus cereus, based nanoparticles have the potential to be developed as promising antibacterial and antioxidant agents. However further in vivo studies are required to confirm their applications as a safe therapeutic agent in the current era of developing multidrug resistance.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors would like to acknowledge the support of Prince Sultan University for paying the Article Processing Charges of this publication.
Funding
Higher Education Commission, Islamabad, Pakistan for financial support (HEC-NRPU#4695).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Yamin Bibi, Email: dryaminbibi@uaar.edu.pk.
Abdul Qayyum, Email: aqayyum@uoh.edu.pk.
References
- Abalkhil T.A., Alharbi S.A., Salmen S.H., Wainwright M. Bactericidal activity of biosynthesized silver nanoparticles against human pathogenic bacteria. Biotechnol. Biotechnol. Equip. 2017;31:411–417. doi: 10.1080/13102818.2016.1267594. [DOI] [Google Scholar]
- Adur A.J., Nandini N., Mayachar K.S., Ramya R., Srinatha N. Bio-synthesis and antimicrobial activity of silver nanoparticles using anaerobically digested parthenium slurry. J. Photochem. Photobiol. B: Biol. 2018;183:30–34. doi: 10.1016/j.jphotobiol.2018.04.020. [DOI] [PubMed] [Google Scholar]
- Afzal I., Shinwari Z.K., Sikandar S., Shahzad S. Plant beneficial endophytic bacteria: Mechanisms, diversity, host range and genetic determinants. Microbiol. Res. 2019;221:36–49. doi: 10.1016/j.micres.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Aidara-Kane A., Angulo F.J., Conly J.M., Minato Y., Silbergeld E.K., McEwen S.A., Collignon P.J. World health organization (WHO) guidelines on use of medically important antimicrobials in food-producing animals. Antimicrob. Resist. Infect. Control. 2018;7:1–8. doi: 10.1186/s13756-017-0294-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy, L., Kolay, E., Ağılönü, Y., Aslan, Z. and Kargıoğlu, M., 2013. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica. Saudi J. Biol. Sci. 20(3), 235-239. https://dx.doi.org/10.1016%2Fj.sjbs.2013.02.003 [DOI] [PMC free article] [PubMed]
- Akter M., Sikder M.T., Rahman M.M., Ullah A.A., Hossain K.F.B., Banik S., Hosokawa T., Saito T., Kurasaki M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018;9:1–16. doi: 10.1016/j.jare.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander H.K., MacLean R.C. Stochastic bacterial population dynamics restrict the establishment of antibiotic resistance from single cells. Proc. Natl. Acad. Sci. 2020;117(32):19455–19464. doi: 10.1073/pnas.1919672117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S., Satish S. Endophytes: Toward a vision in synthesis of nanoparticle for future therapeutic agents. Int. J. Bio-Inorg. Hybd. Nanomat. 2012;1(2):67–77. [Google Scholar]
- Banu A.N., Balasubramanian C. Extracellular synthesis of silver nanoparticles using bacillus megaterium against malarial and dengue vector (diptera: Culicidae) Parasitol. Res. 2015;114:4069–4079. doi: 10.1007/s00436-015-4635-4. [DOI] [PubMed] [Google Scholar]
- Barman, D., and Bhattacharjee, K., 2020. Endophytic bacteria associated with medicinal plants: the treasure trove of antimicrobial compounds, in: Egamberdieva, D., Tiezzi, A.(Eds), Medically Important Plant Biomes. Springer, Singapore, pp. 153–187.
- Basak S., Singh P., Rajurkar M. Multidrug resistant and extensively drug resistant bacteria: A study. J. Pathog. 2016 doi: 10.1155/2016/4065603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Schluesener H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008;176:1–12. doi: 10.1016/j.toxlet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Clifford K., Desai D., da Costa C.P., Meyer H., Klohe K., Winkler A.S., Rahman T., Islam T., Zaman M.H. Antimicrobial resistance in livestock and poor quality veterinary medicines. Bull. World Health Organ. 2018;96(9):662. doi: 10.2471/blt.18.209585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z.Y., Narsing Rao M.P., Xiao M., Wang H.-F., Hozzein W.N., Chen W., Li W.J. Antibacterial activity of silver nanoparticles against Staphylococcus warneri synthesized using endophytic bacteria by photo-irradiation. Front. Microbiol. 2017;8:1090. doi: 10.3389/fmicb.2017.01090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elamawi R.M., Al-Harbi R.E., Hendi A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest. Co. 2018;28(1):1–11. doi: 10.1186/s41938-018-0028-1. [DOI] [Google Scholar]
- Gopinath V., MubarakAli D., Priyadarshini S., Priyadharsshini N.M., Thajuddin N., Velusamy P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloids Surf. B. Biointerf. 2012;96:69–74. doi: 10.1016/j.colsurfb.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Guntur, S.R., Kumar, N.S., Hegde, M.M., Dirisala, V.R., 2018. In vitro studies of the antimicrobial and free-radical scavenging potentials of silver nanoparticles biosynthesized from the extract of Desmostachya bipinnata. Anal. Chem. Insights 13, 1-9 https://doi.org/10.1177%2F1177390118782877 [DOI] [PMC free article] [PubMed]
- Gurunathan S., Park J.H., Han J.W., Kim J.H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J. Nanomedicine. 2015;10:4203–4222. doi: 10.2147/ijn.s83953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda R.A., Hussein M.H., Abo-Elmagd R.A., Bawazir S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019;9:1–17. doi: 10.1038/s41598-019-49444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iravani S., Korbekandi H., Mirmohammadi S.V., Zolfaghari B. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res. Pharm. Sci. 2014;9(6):385–406. [PMC free article] [PubMed] [Google Scholar]
- Keshari A.K., Srivastava R., Singh P., Yadav V.B., Nath G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020;11:37–44. doi: 10.1016/j.jaim.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.S., Kuk E., Yu K.N., Kim J.H., Park S.J., Lee H.J., Kim S.H., Park Y.K., Park Y.H., Hwang C.Y., Kim Y.K., Lee Y.S., Jeong D.H., Cho M.H. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Kumar, T.V.R., Murthy, J., Rao, M.N., Bhargava, Y., 2016. Evaluation of silver nanoparticles synthetic potential of Couroupita guianensis Aubl., flower buds extract and their synergistic antibacterial activity. 3 Biotech. 6(1), 92. https://dx.doi.org/10.1007%2Fs13205-016-0407-9 [DOI] [PMC free article] [PubMed]
- Laloy, J., Minet, V., Alpan, L., Mullier, F., Beken, S., Toussaint, O., Lucas, S., Dogne, J.-M., 2014. Impact of silver nanoparticles on haemolysis, platelet function and coagulation. Nanobiomedicine. 1,4 https://doi.org/10.5772%2F59346 [DOI] [PMC free article] [PubMed]
- Lin, Y.T., Wang, Y.P., Wang, F.D., Fung, C.P., 2015. Community-onset Klebsiella pneumoniae pneumonia in Taiwan: Clinical features of the disease and associated microbiological characteristics of isolates from pneumonia and nasopharynx. Front. Microbiol. 6, 122. https://dx.doi.org/10.3389%2Ffmicb.2015.00122 [DOI] [PMC free article] [PubMed]
- Liu L., Yang J., Xie J., Luo Z., Jiang J., Yang Y.Y., Liu S. The potent antimicrobial properties of cell penetrating peptide-conjugated silver nanoparticles with excellent selectivity for gram-positive bacteria over erythrocytes. Nanoscale. 2013;5:3834–3840. doi: 10.1039/c3nr34254a. [DOI] [PubMed] [Google Scholar]
- Liu Z., Yan J., Miao Y.E., Huang Y., Liu T. Catalytic and antibacterial activities of green-synthesized silver nanoparticles on electrospun polystyrene nanofiber membranes using tea polyphenols. Compos. B. Eng. 2015;79:217–223. doi: 10.1016/j.compositesb.2015.04.037. [DOI] [Google Scholar]
- Loo Y.Y., Rukayadi Y., Nor-Khaizura M.A.R., Kuan C.H., Chieng B.W., Nishibuchi M., Radu S. In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens. Front. Microbiol. 2018;9:1555. doi: 10.3389/fmicb.2018.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud W., Elazzazy A.M., Danial E.N. In vitro evaluation of antioxidant, biochemical and antimicrobial properties of biosynthesized silver nanoparticles against multidrug-resistant bacterial pathogens. Biotechnol. Biotechnol. Equip. 2017;31:373–379. [Google Scholar]
- Manikprabhu D., Lingappa K. γ Actinorhodin a natural and attorney source for synthetic dye to detect acid production of fungi. Saudi. J. Biol. Sci. 2013;20(2):163–168. doi: 10.1016/j.sjbs.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanpuria P., Rana N.K., Yadav S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008;10:507–517. [Google Scholar]
- Monnanda, S. N., Harischandra, S. P., and Mysore, V. T., 2020. Bioactive potentials of novel molecules from the endophytes of medicinal plants, In: Egamberdieva, D., Tiezzi, A. (Eds), Medically Important Plant Biomes. Springer, Singapore, pp. 293–355. doi:10.1007/978-981-13-9566-6
- Moteriya P., Chanda S. Biosynthesis of silver nanoparticles using flower extract of Cassia roxburghii DC and its synergistic antibacterial efficacy. Sci. Iran. Trans. F Nanotechnol. 2014;21(6):2499–2507. [Google Scholar]
- Moteriya P., Padalia H., Chanda S. Characterization, synergistic antibacterial and free radical scavenging efficacy of silver nanoparticles synthesized using Cassia roxburghii leaf extract. J. Genet. Eng. Biotechnol. 2017;15(2):505–513. doi: 10.1016/j.jgeb.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munita J.M., Arias C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016;4(2) doi: 10.1128/microbiolspec.vmbf-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaich U., Gulati N., Chauhan S. Antioxidant and antibacterial potential of silver nanoparticles: Biogenic synthesis utilizing apple extract. J. Pharm. 2016 doi: 10.1155/2016/7141523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netala, V.R., Bethu, M.S., Pushpalatha, B., Baki, V.B., Aishwarya, S., Rao, J.V., Tartte, V., 2016. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int. J. Nanomedicine. 11: 5683–5696. https://dx.doi.org/10.2147%2FIJN.S112857 [DOI] [PMC free article] [PubMed]
- Orsi G.B., Falcone M., Venditti M. Surveillance and management of multidrug-resistant microorganisms. Expert. Rev. Anti. Infect. Ther. 2011;9(8):653–679. doi: 10.1586/eri.11.77. [DOI] [PubMed] [Google Scholar]
- Qasim M., Udomluck N., Chang J., Park H., Kim K. Antimicrobial activity of silver nanoparticles encapsulated in poly-n-isopropylacrylamide-based polymeric nanoparticles. Int. J. Nanomedicine. 2018;13:235–249. doi: 10.2147/ijn.s153485. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Qiao M., Ying G.G., Singer A.C., Zhu Y.G. Review of antibiotic resistance in china and its environment. Environ. Int. 2018;110:160–172. doi: 10.1016/j.envint.2017.10.016. [DOI] [PubMed] [Google Scholar]
- Rahman S., Rahman L., Khalil A.T., Ali N., Zia D., Ali M., Shinwari Z.K. Endophyte-mediated synthesis of silver nanoparticles and their biological applications. Appl. Microbiol. Biotechnol. 2019;103(6):2551–2569. doi: 10.1007/s00253-019-09661-x. [DOI] [PubMed] [Google Scholar]
- Rosenberger L.H., Hranjec T., Politano A.D., Swenson B.R., Metzger R., Bonatti H., Sawyer R.G. Effective cohorting and “superisolation” in a single intensive care unit in response to an outbreak of diverse multi-drug-resistant organisms. Surg. Infect. (Larchmt) 2011;12(5):345–350. doi: 10.1089/sur.2010.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanan C., Rajesh R., Kaviarasan T., Muthukumar K., Kavitake D., Shetty P.H. Synthesis of silver nanoparticles using bacterial exopolysaccharide and its application for degradation of azo-dyes. Biotechnol. Rep. 2017;15:33–40. doi: 10.1016/j.btre.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A., Lutfullah G., Ahmad K., Khalil A.T., Maaza M. Daphne mucronata-mediated phytosynthesis of silver nanoparticles and their novel biological applications, compatibility and toxicity studies. Green Chem. Lett. Rev. 2018;11(3):318–333. doi: 10.1080/17518253.2018.1502365. [DOI] [Google Scholar]
- Siddiqi K.S., Husen A., Rao R.A.K. A review on biosynthesis of silver nanoparticles and their biocidal properties. J Nanobiotechnol. 2018;16:14. doi: 10.1186/s12951-018-0334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikder M., Lead J.R., Chandler G.T., Baalousha M. A rapid approach for measuring silver nanoparticle concentration and dissolution in seawater by uv–vis. Sci. Total. Environ. 2018;618:597–607. doi: 10.1016/j.scitotenv.2017.04.055. [DOI] [PubMed] [Google Scholar]
- Silambarasan S., Abraham J. Biosynthesis of silver nanoparticles using the bacteria bacillus cereus and their antimicrobial property. Int. J. Pharm. Pharm. Sci. 2012;4:536–540. [Google Scholar]
- Singh A., Gautam P.K., Verma A., Singh V., Shivapriya P.M., Shivalkar S., Sahoo A.K., Samanta S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Rep. 2020;25 doi: 10.1016/j.btre.2020.e00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T., Jyoti K., Patnaik A., Singh A., Chauhan R., Chandel S. Biosynthesis, characterization and antibacterial activity of silver nanoparticles using an endophytic fungal supernatant of Raphanus sativus. J. Genet. Eng. Biotechnol. 2017;15:31–39. doi: 10.1016/j.jgeb.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C., Alff E., Johnson C., Ramos C., Donofrio N., Sundaresan V., Bais H. Natural rice rhizospheric microbes suppress rice blast infections. BMC Plant Biol. 2014;14:130. doi: 10.1186/1471-2229-14-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman G.M., Hussien H.T., Saleem M.M. Biosynthesis of silver nanoparticles synthesized by aspergillus flavus and their antioxidant, antimicrobial and cytotoxicity properties. Bull. Mater. Sci. 2015;38:639–644. [Google Scholar]
- Sunkar, S., Nachiyar, C.V., 2012. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium bacillus cereus isolated from Garcinia xanthochymus. Asian Pac. J. Trop. Biomed. 2, 953-959. https://dx.doi.org/10.1016%2FS2221-1691(13)60006-4 [DOI] [PMC free article] [PubMed]
- Syed B., Rao H.C.Y., Nagendra-Prasad M.N., Prasad A., Harini B.P., Azmath P., Rakshith D., Satish S. Biomimetic synthesis of silver nanoparticles using endosymbiotic bacterium inhabiting Euphorbia hirta L. and their bactericidal potential. Scientifica. 2016;9020239 doi: 10.1155/2016/9020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C., Tang, J., Liu, X., Ren, X., Zhen, M., Wang, L., 2019. Green biosynthesis of silver nanoparticles using. Eriobotrya japonica (Thunb.) leaf extract for reductive catalysis. Materials. 12(1), 189. https://dx.doi.org/10.3390%2Fma12010189 [DOI] [PMC free article] [PubMed]
- Zhang X.F., Liu Z.G., Shen W., Gurunathan S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016;17(9):1534. doi: 10.3390/ijms17091534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K., Setyawati M.I., Leong D.T., Xie J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018;357:1–17. doi: 10.1016/j.ccr.2017.11.019. [DOI] [Google Scholar]