Abstract
IL-1β plays critical roles in the priming and effector phases of immune responses such as the differentiation, commitment, and memory formation of T cells. In this context, several reports have suggested that the IL-1β signal is crucial for CTL-mediated immune responses to viral infections and tumors. However, little is known regarding whether IL-1β acts directly on CD8+ T cells and what the molecular mechanisms underlying expression of IL-1 receptors (IL-1Rs) on CD8+ T cells and features of IL-1R+CD8+ T cells are. Here, we provide evidence that the expression of IL-1R type I (IL-1RI), the functional receptor of IL-1β, is preferentially induced by IL-21 on TCR-stimulated CD8+ T cells. Further, IL-1β enhances the effector function of CD8+ T cells expressing IL-21-induced IL-1RI by increasing cytokine production and release of cytotoxic granules containing granzyme B. The IL-21-IL-1RI-IL-1β axis is involved in an augmented effector function through regulation of transcription factors BATF, Blimp-1, and IRF4. Moreover, this axis confers a unique effector function to CD8+ T cells compared to conventional type 1 cytotoxic T cells differentiated with IL-12. Chemical inhibitor and immunoprecipitation assay demonstrated that IL-21 induces a unique pattern of STAT activation with the formation of both STAT1:STAT3 and STAT3:STAT5 heterodimers, which are critical for the induction of IL-1RI on TCR-stimulated CD8+ T cells. Taken together, we propose that induction of a novel subset of IL-1RI-expressing CD8+ T cells by IL-21 may be beneficial to the protective immune response against viral infections and is therefore important to consider for vaccine design.
Keywords: IL-1 Receptor, IL-1 Beta, IL-21, Cytotoxic T Lymphocyte, STAT transcription factors, Tc1 cells
INTRODUCTION
IL-1β is a pivotal proinflammatory cytokine that exerts a variety of effects on cells involved in the induction of local and systemic inflammation (1). In T-cell immunity, IL-1β acts as an important mediator to promote the development, commitment, and effector function of T cells (2,3). Considering its multipotent effects on immune responses, it makes sense that IL-1 signaling is tightly regulated through expression of its receptor (4). Two types of IL-1 receptors have been identified: IL-1 receptor type I (IL-1RI) is a functional receptor of IL-1 expressed at various levels on most cell types including immune cells, whereas IL-1 receptor type II (IL-1RII) serves as a decoy receptor that suppresses the signaling of IL-1 (5). Spatiotemporal regulation of IL-1RI and IL-1RII expression is important for an optimal immune response since the response to IL-1β is dependent on the expression of these 2 IL-1Rs. For example, it has been reported that the expression of IL-1RII, especially its soluble form, is negatively correlated with disease severity in rheumatoid arthritis (RA) (6). In human CD4+ T cells, the expression of IL-1RI and IL-1RII is tightly and dynamically regulated in response to environmental factors including TCR stimulation or cytokines such as IL-7, IL-15, and TGF-β, and this regulation of IL-1RI and IL-1RII affects Th17 cell responses (7). Prostaglandin E2, IL-21, and IL-6 induce IL-1RI expression during Th17 differentiation and favor the production of IL-17 (7,8,9), suggesting a critical role of cytokines and soluble factors for regulating the expression of IL-1Rs in immune cells.
Cytokines play a vital role in the differentiation of T cells. IL-2, IL-7, IL-12, and IL-21 have been known as important mediators for development of potent CD8+ T-cell-mediated immune responses for controlling acute and chronic viral infection (10,11,12,13). In particular, IL-2 and IL-21, primarily secreted by activated CD4+ T cells, have been shown to promote survival or memory formation of CD8+ T cells and also to enhance cytotoxic granule formation (14,15,16). Thus, this suggests that CD4+ T cells are requisite for the establishment of a protective CD8+ T-cell response (17). On the other hand, potent effects of proinflammatory cytokines such as IL-1β, which is released by Ag-presenting cells (APCs), on CD8+ T cell-mediated immune responses has also been recognized (18). The mechanisms underlying how IL-1β intensifies the effector functions of CD8+ T cells have been previously explored (19,20). IL-1β significantly enhances the expansion, effector function, and tissue localization of CD8+ T cells through increased formation of cytotoxic granules and cytokine production similar to IL-1β-mediated responses of CD4+ T cells (19). Murine virus infection models have shown that the IL-1R signal is associated with CTL-mediated virus clearance through an increase in granzyme and perforin (20). Furthermore, IL-1R−/− knockout mice have impaired CD8+ T cell-mediated immune responses against viruses such as lymphocytic choriomeningitis virus (LCMV), vaccinia, and influenza A (20,21,22). Thus, IL-1β has been suggested as a mucosal vaccine adjuvant to raise a protective immune response against the influenza A virus (23). Taken together, IL-1R-mediated signaling is critical for potent CD8+ T cell-mediated immunity. However, little is known about the molecular mechanisms underlying the expression of IL-1Rs on CD8+ T cells and the IL-1-mediated enhancement of their effector function.
In the present study, we provide novel insight into the critical role of IL-21 for induction of functional IL-1RI on human CD8+ T cells by exploring the molecular mechanisms of action of this cytokine and analyzing its effect on the effector function of CD8+ T cells. We found that IL-21 selectively upregulates the expression of IL-1RI on CD8+ T cells in response to TCR stimulation. Our biochemical analysis demonstrates that IL-21-mediated induction of IL-1RI is due to a unique STAT phosphorylation profile that results in both STAT1:STAT3 and STAT3:STAT5 heterodimer formation in CD8+ T cells. Enhanced IL-1 signaling potentiates robust production of the cytotoxic granules containing granzyme B (GzmB) and secretion of IFN-γ by CD8+ T cells, probably via regulation of Blimp-1, BATF, and IRF4 transcription factors (TFs). Of note, TCR-activated CD8+ T cells expressing IL-1RI preferentially express higher CXCR3 and CCR5 than do IL-1RI− CD8+ T cells, suggesting that IL-1RI+ CD8+ T cells have greater capability to migrate into infected tissue than do IL-1RI- CD8+ T cells. These findings underscore a novel role of the IL-21-IL-1RI-IL-1β axis on TCR-activated CD8+ T cells, contributing to their enhanced effector function and potentially important function in protective viral immunity.
MATERIALS AND METHODS
Cell preparation
The study protocols were approved by the Institutional Review Board of Seoul National University (No.1109-055-378, 1306-002-491, and 1403-049-564 for Seoul National University College of Medicine/Seoul National University Hospital). The methods were performed in accordance with the approved guidelines. PBMCs were isolated from blood healthy controls by density gradient centrifugation method (Bicoll separating solution; BIOCHROM Inc., Cambridge, UK). Naive and total CD8+ T cells were negatively purified from PBMC using Mojosort human naive CD8+ T cells and Mojosort human CD8+ T cells isolation kit (both from BioLegend, San Diego, CA, USA) followed by manufacture's instruction, respectively. Murine CD8+ T cells were isolated from spleen of c57BL/6 mice using Mojosort murine CD8+ T cells isolation kit (BioLegend).
Cell culture
Purified naive and total human CD8+ T cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 1% penicillin/streptomycin, and 1% L-glutamine, respectively. Cells were seeded at 5 to 10×104 cells into 96-well U bottom plate and stimulated with anti-CD3/CD28 coated microbeads (Dynabeads® T-Activator CD3/CD28; Thermo Fisher Scientific, Waltham, MA, USA) in the absence or presence of the indicated cytokines; recombinant human (rh) IL-1β (25 ng/ml), rhIL-6 (6.25 to 100 ng/ml), rhIL-21 (0.1 to 100 ng/ml), rhIL-7 (50 ng/ml), IFN-α (50 or 100 ng/ml), rhIL-10 (6.25 to 100 ng/ml; all from R&D systems, Minneapolis, MN, USA), rhIL-12 (50 ng/ml), rhIL-15 (50 or 100 ng/ml), rhIL-2 (100 IU/ml, all from PeproTech, Rocky Hill, NJ, USA). In some experiments, cells were stimulated with anti-CD3/CD28 coated microbeads with chemical inhibitors: STAT1 inhibitor Fludarabin, STAT3 inhibitor 5,15-DPP, or STAT5 inhibitor CAS 285986-31-4 (all from Sigma-aldrich, St. Louis, MO, USA).
Flow cytometry
Human PBMC or cultured T cells were stained for 30 min at 4°C with following fluorochrome-conjugated Abs: APC-Cy7-anti-CD3, v500-anti-CD8, PE-Cy5-anti-CD25, APC-anti-CD27, PE-Cy7-anti-CD45RA, PE-Cy5-anti-CD45RA, Alexa Fluor 700-anti-CCR5, (7 from BD Bioscience, San Jose, CA, USA), PE-Cy7-anti-CXCR3 (BioLegend), PE-anti-IL-1RI (R&D systems). For intracellular cytokine staining, cultured T cells were re-stimulated for 6 h with PMA (50 ng/ml) and ionomycin (1 μg/ml) in the presence of BFA for last 4 h, followed by staining with PE-anti-IL-1RI and PE-Cy7-anti-CD25 Abs. The stained cells were fixed and permeabilized using Fix/Perm buffer (BioLegend), followed by staining with FITC-anti-IL-2 (BioLegend), PE-Cy7-anti-TNF (BD bioscience), V450-anti-IFN-γ (eBioscience, San Diego, CA, USA) Abs. For intracellular GzmB and Perforin staining, cultured cells were fixed and permeabilized using Fix/Perm buffer (BD bioscience), followed by staining with V450-anti-GzmB and FITC-anti-Perforin (both from BD bioscience). For phosphor flow, human PBMC were stimulated with indicated concentration of IL-2, IL-6, IL-10, IL-15, IFN-α, or IL-21 for 12, 60, or 120 min at 37°C. The cells were immediately fixed after stimulation, using pre-warmed BD Phosflow™ Lyse/Fix Buffer (BD bioscience) for 10 min at 37°C and then permeabilized using 87.68% methanol for 30 min at 4°C. After Fix/Perm, the cells were stained for 30 min at 4°C with following fluorochrome-conjugated Abs: PE-anti-STAT1 (pY701), PE-anti-STAT5 (pY694), APC-anti-STAT3 (pY705), APC-Cy7-anti-CD3, and v500-anti-CD8 (all from BD bioscience). The cells were acquired using a BD LSRFortessa (BD Bioscience) and analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
ELISA
The amounts of TNF, IFN-γ, and IL-10 in culture supernatant were quantified using commercially available human ELISA kits according to manufacturer's instructions (both TNF ELISA and IL-10 ELISA kits from eBioscience and Human IFN-γ ELISA MAX™ Deluxe from BioLegend). Measurement of OD was performed using Biorad microplate reader (Bio-Rad, Hercules, CA, USA).
Quantitative real-time PCR
Total RNA was extracted from cultured CD8+ T cells with TRIzol reagent (Invitrogen, Waltham, MA, USA) and cDNA was synthesized using GoScript™ Reverse Transcription System (Promega, Madison, WI, USA). Real-time quantitative RT-PCR was performed on CFX system (Bio-Rad) using SYBR green master mix (Bio-Rad). Primers were designed with Primer designing tool-NCBI (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) or adopted from previously published primer sequences; IFN-γ, 5′-TTTGGGTTCTCTTGGCTGTT-3, and 5′-TCTTTTGGATGCTCTGGTCA-3′, Blimp-1, 5′-TACATACCAAAGGGCA-3, and 5′-TGAAGCTCCCCTCTGGAA-3′, IRF4, 5′-ACAGCAGTTCTTGTCAGAG-3, and 5′-GAGGTTCTACGTGAGCTG-3′, TCF1 (also known as TCF7), 5′-TCCATGTATTCACACACCCCTC-3, and 5′-TTCTCAAGACTTCTTGCAGTGTTAC-3′, BATF, 5′-AGCGAAGACCTGGAGAAACA-3, and 5′-TTCAGCACCGACGTGAAGTA-3′, SOCS3, 5′-GGAGACTTCGATTCGGGACC-3, and 5′-GAAACTTGCTGTGGGTGACC-3′, TBX21, 5′-GATCATCACCAAGCAGGGACG-3, and 5′-TCCACACTGCACCCACTTGC-3′, eomesodermin (Eomes), 5′-AGCTCTCCAAGGAGAAAGTG-3, and 5′-GCCTTCGCTTACAAGCACTG-3′. The levels of gene expression were normalized to the expression of ACTINB. The comparative Ct method (ΔΔCt) was used for quantification of gene expression.
Immunoprecipitation (IP) and Immunoblotting
Human CD8+ T cells were treated indicated cytokines and anti-CD3/28 crosslinking for 30 min at 37°C and then lysed in Cell Lysis Buffer (Cell Signaling Technology, Danvers, MA, USA) and Protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific) for 10 min at 4°C. IP was performed using anti-STAT3 (#4904S, 1:50, Cell signaling Technology) Ab or nomal rabbit IgG (#2729, Cell Signaling Technology) on a rotor for 16 h at 4°C. Protein A/G PLUS-Agarose beads (Santa-Cruz Biotechnology, Heidelberg, Germany) were added into the samples and incubated on a rotor for 4 h at 4°C. After 5 times of washing with the lysis buffer, immunoprecipitates were eluted by boiling with the SDS-loading buffer. Precipitated proteins were analyzed by SDS-PAGE, followed by transferring to PVDF membranes, immunoblotted with the indicated Abs, and developed using a chemiluminescent detection method. The Abs with the indicated dilutions were as follows: anti-STAT1 (#9172, 1:1,000), anti-STAT3 (#4904S, 1:2,000), and anti-STAT5 (#94205, 1:1,000, all from Cell Signaling Technology).
Statistical analysis
Two-tailed paired or unpaired t-test was done to analyze data using Prism 8 software (Software, La Jolla, CA, USA) or and Microsoft Excel 2016 as indicated in the figure legends. The p-values of less than 0.05 were considered statistically significant.
RESULTS
IL-21 induces the expression of IL-1RI on TCR-stimulated CD8+ T cells
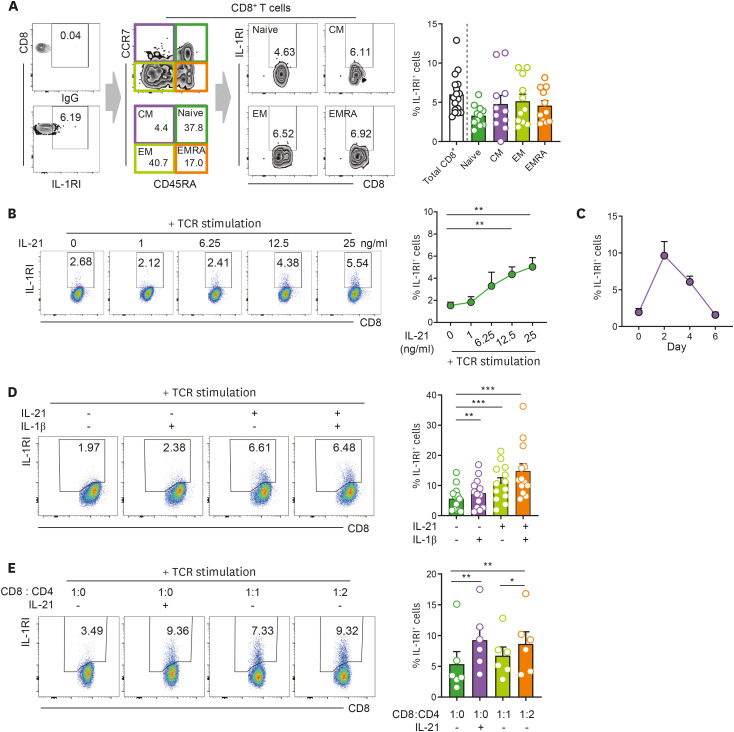
Recent studies have shown that IL-1β-mediated T-cell responses are coordinately regulated by the spatiotemporal expression of IL-1Rs (7,24,25). In this context, we first sought to analyze the expression of functional IL-1RI and decoy IL-1RII on CD8+ T cells. Approximately 5% of ex vivo CD8+ T cells express IL-1RI, whereas no obvious expression of IL-1RII was observed on the same cells, similar to what was previously described for CD4+ T cells (Fig. 1A and Supplementary Fig. 1) (7,24). In contrast to CD4+ T cells, IL-1RI expression was comparable among four different functional CD8+ T-cell subsets (Fig. 1A) (24). It has been demonstrated that cytokines play a pivotal role in the induction of IL-1Rs in various immune cells. Thus, a literature search was undertaken to get an idea of which cytokines are involved in the induction of IL-1Rs in CD8+ T cells. Re-analysis of previously published microarray data (GSE58262) revealed that the expression of IL-1RI of murine, naive OT-1 CD8+ T cells was obviously increased following OVA stimulation in the presence of exogenous IL-21 (Supplementary Fig. 2A) (26). This finding was verified at the protein level in murine CD8+ T cells purified from the spleens of C57BL/6 mice (Supplementary Fig. 2B). Furthermore, the expression of IL-1RI on human CD8+ T cells was significantly upregulated by exogenous IL-21 in a dose-dependent manner in response to TCR stimulation (Fig. 1B). IL-21-mediated induction of IL-1RI was similarly observed in four different functional T-cells subsets (Supplementary Fig. 2C). Kinetic analysis of IL-1RI expression on CD8+ T cells showed that the induction of IL-1RI peaked at 48 h post-stimulation and was then gradually downregulated (Fig. 1C). No apparent induction of IL-1RI expression was seen in CD8+ T cells following stimulation with IL-21 alone without TCR activation (Supplementary Fig. 3). Further, IL-21-mediated induction of IL-1RI was comparable on TCR-stimulated CD8+ T cells regardless of IL-1β treatment (Fig. 1D). IL-21 has been reported to be mainly produced in vivo by activated CD4+ T cells or NKT cells (27,28). Our co-culture experiments showed that upon TCR stimulation, CD8+ T cells cultured with autologous CD4+ T cells at a 1:2 ratio exhibited increased expression of IL-1RI to a level similar to that induced by exogenous IL-21 treatment (Fig. 1E). Collectively, our findings demonstrate that IL-21 induces the expression of IL-1RI on TCR-stimulated CD8+ T cells.
Figure 1. IL-21 induces the expression of IL-1RI on TCR-stimulated CD8+ T cells. (A) Representative flow cytometric plot of IL-1RI expression on total CD8+ T cells, naive (CD45RA+CCR7+), CM (CD45RA−CCR7+), EM (CD45RA−CCR7−), and EMRA (CD45RA+CCR7−) cell subsets from healthy controls. Frequency (%) of IL-1RI+ cells in indicated T-cell populations. (B) Representative flow cytometric plot and the frequency (%) of IL-1RI expression on TCR-stimulated CD8+ T cells in the presence of the indicated concentration of rhIL-21. (C) Time kinetics of IL-21-induced IL-1RI expression on TCR-stimulated CD8+ T cells. (D) Representative flow cytometric plot and frequency (%) of IL-1RI on TCR-stimulated CD8+ T cells treated with rhIL-21 (25 ng/ml) and/or rhIL-1β (25 ng/ml) at day 2 post-stimulation. (E) Co-culture of purified human CD4+ and CD8+ T cells in the presence of TCR stimulation. Purified cells were seeded at the indicated ratio and cultured with anti-CD3/28 coated microbeads in the absence or presence of rhIL-21 (50 ng/ml) for 2 days. Bar graphs show the mean±SEM. The statistical significance was measured by 2-tailed paired t-test.
CM, central memory; EM, effector memory; EMRA, CD45RA+ effector memory.
*p<0.05, **p<0.01, ***p<0.005.
STAT3 activation is necessary, but not sufficient, for induction of IL-1RI on TCR-stimulated CD8+ T cells
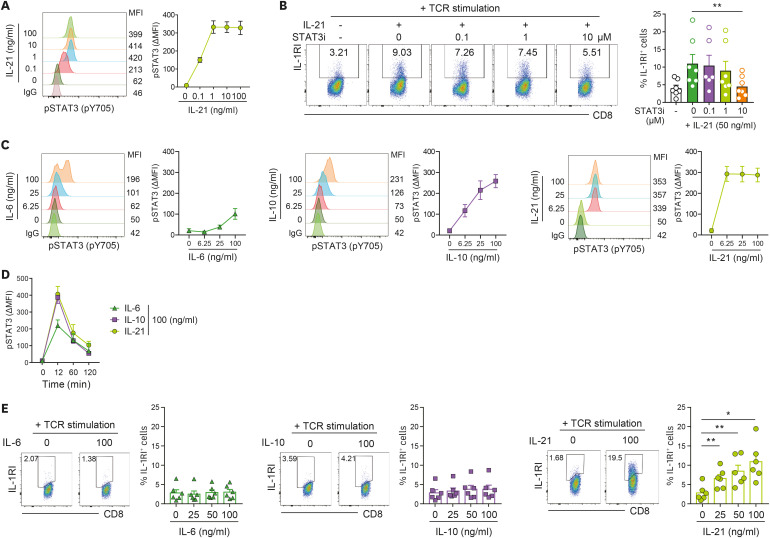
The IL-21 receptor consists of 2 different subunits, the IL-21 receptor (IL-21R) and the common cytokine receptor γ chain (γc). IL-21-mediated signaling transduction occurs via the JAK-STAT pathway and primarily activates STAT3 to form cell-type-specific complexes for signaling (27,29). In agreement with previous reports, we found that at a concentration as low as 0.1 ng/ml, IL-21 efficiently elicits phosphorylation of STAT3 (pY705) in TCR-stimulated human CD8+ T cells (Fig. 2A). To examine the direct role of IL-21-mediated activation of STAT3 on induction of IL-1RI, TCR-stimulated CD8+ T cells were treated with 5,15-DPP, a small molecule inhibitor of STAT3 (STAT3i). It was found that 10 μM of this STAT3i suppresses IL-21-mediated induction of IL-1RI on CD8+ T cells (Fig. 2B). STAT3 is activated in response to stimuli from a variety of cytokines. Thus, we next tested whether other STAT3-activating cytokines induce IL-1RI on TCR-stimulated CD8+ T cells. To this end, CD8+ T cells were stimulated with anti-CD3/CD28 coated beads in the presence of IL-6 or IL-10 and the level of phosphorylated STAT3 was compared with that of IL-21-treated CD8+ T cells (Fig. 2C). The kinetics of STAT3 phosphorylation were similar among these cytokine-treated cells, with marked phosphorylation of STAT3 (pY705) in CD8+ T cells at 12 min post-stimulation (Fig. 2D). However, STAT3 phosphorylation reached a plateau at the lowest concentration of IL-21 (Fig. 2C), while IL-10 was required to be at least 15 times higher in concentration to obtain the same degree of activation of STAT3. IL-6 induced phosphorylation of STAT3 in only a proportion of CD8+ T cells, likely the subset expressing IL-6 receptor α chain (30). Of note, IL-21, but not IL-6 or IL-10, provoked expression of IL-1RI on TCR-stimulated CD8+ T cells (Fig. 2E). These findings suggest that STAT3 activation is necessary, but not sufficient, for induction of IL-1RI on TCR-stimulated CD8+ T cells.
Figure 2. STAT3 activation is necessary, but not sufficient, for induction of IL-1RI on TCR-stimulated CD8+ T cells. (A) Phosphorylation of STAT3 (pY705) in IL-21-stimulated CD8+ T cells. The cells were stimulated with the indicated concentration of rhIL-21 for 12 min. A phospho-protein specific flow cytometric analysis (Phosflow assay) was used to detect pSTAT3. (B) Representative flow cytometric plot and the frequency (%) of IL-1RI expression on TCR-stimulated CD8+ T cells. Cells were treated with rhIL-21 (50 ng/ml) in the presence of the indicated concentrations of the 5,15-DPP STAT3i. (C) Flow cytometric analysis of pSTAT3 (pY705) in IL-6 (100 ng/ml), IL-10 (100 ng/ml), or IL-21 (100 ng/ml)-stimulated CD8+ T cells. Cells were stimulated with the indicated concentration of cytokines for 12 min (n=4). (D) Kinetics of STAT3 phosphorylation in CD8+ T cells treated with IL-6, IL-10, or IL-21 (n=4). (E) Representative flow cytometric plot and the frequency (%) of IL-1RI expression on TCR-stimulated CD8+ T cells treated with exogenous IL-6, IL-10, or IL-21 for 2 days. Bar graphs and line graphs show the mean±SEM. The statistical significance was measured by 2-tailed paired t-test.
MFI, mean fluorescence intensity.
*p<0.05, **p<0.01.
An IL-21-specific STAT signaling pathway is preferentially involved in the induction of IL-1RI on CD8+ T cells
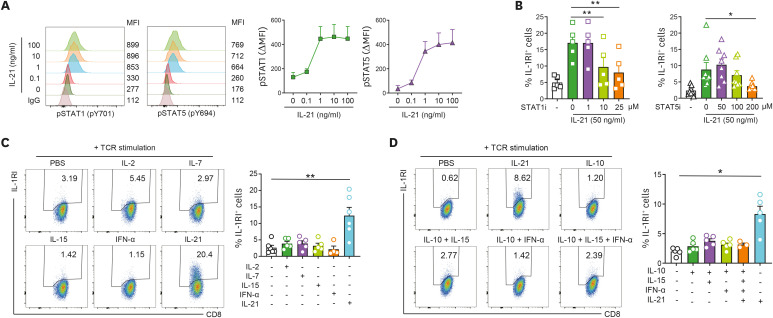
To explore the molecular mechanism underlying IL-21-mediated induction of IL-1RI on TCR-activated CD8+ T cells, the phosphorylation profile of signaling molecules in IL-21 treated CD8+ T cells was analyzed using Phosflow assay. Of note, we found that IL-21 efficiently elicits phosphorylation STAT1 (pY701) and STAT5 (pY694) as well as STAT3 (pY705), even at a concentration as low as 1 ng/ml (Figs. 2A and 3A). A direct role of IL-21-mediated activation of STAT1 and STAT5 for induction of IL-1RI was confirmed by treatment with Fludarabine or CAS 285986-31-4, inhibitors of STAT1 and STAT5, respectively. The results show almost complete suppression of IL-21-mediated induction of IL-1RI on CD8+ T cells (Fig. 3B) at 25 μM of Fludarabine and 200 μM of CAS 285986-31-4. It should be noted that these highest doses of inhibitors did not cause cell death in CD8+ T cells (data not shown).
Figure 3. An IL-21-specific STAT signaling pathway is preferentially involved in the induction of IL-1RI on CD8+ T cells. (A) Phosphorylation of STAT1 (pY701) and STAT5 (pY694) in IL-21-stimulated CD8+ T cells. (B) Frequency (%) of IL-1RI expression on TCR-stimulated CD8+ T cells treated with rhIL-21 (50 ng/ml) in the presence of the indicated concentrations of Fludarabine, STAT1i, or CAS 285986-31-4, a STAT5i, at day 2 post-stimulation. (C) Representative flow cytometric plot and the frequency (%) of IL-1RI expression on TCR-stimulated CD8+ T cells treated with various STAT1- or STAT5-activating cytokines (rhIL-2 [250 IU/ml], rhIL-7 [50 ng/ml], rhIL-15 [50 ng/ml], IFN-α [50 ng/ml], or rhIL-21 [50 ng/ml]). (D) Representative flow cytometric plot and the frequency (%) of IL-1RI expression on CD8+ T cells stimulated with anti-CD3/28 coated microbeads and various combinations of STAT1, STAT3, and STAT5-activating cytokines (rhIL-10 [50 ng/ml], rhIL-15 [50 ng/ml], IFN-α [50 ng/ml], or rhIL-21 [50 ng/ml]). Bar graphs and line graphs show the mean±SEM of independent experiments. The statistical significance was measured by 2-tailed paired t-test.
*p<0.05, **p<0.01.
It has been demonstrated that the IL-10/IL-10R interaction leads to Jak1 and Tyk2 phosphorylation and consequent STAT3 and STAT1 phosphorylation (31). We found that while STAT1 phosphorylation was dose-dependently induced by IL-10 (Supplementary Fig. 4A), IL-10 did not induce expression of IL-1RI on TCR-stimulated CD8+ T cells (Fig. 2E), indicating that STAT1 and STAT3 activation is not sufficient to induce IL-1RI expression. IL-21 is a member of the γc family of cytokines, which also includes IL-2, IL-7, and IL-15, that share signal transduction pathways (27). To investigate whether γc cytokine signaling is associated with the induction of IL-1RI expression on CD8+ T cells, freshly purified CD8+ T cells were treated with exogenous IL-2, IL-7, IL-15, or IL-21 in the presence of anti-CD3/28 coated microbeads for 48 h. As shown in Fig. 3C, the expression of IL-1RI was not induced by any γc cytokines except IL-21. As expected from the data in Supplementary Fig. 4B, IFN-α, a type I interferon that activates STAT1 and acts as an important cytokine for the proliferation of CTLs (32), also did not enhance IL-1RI expression on TCR-stimulated CD8+ T cells (Fig. 3C). Considering that IL-21 concurrently phosphorylates STAT1, STAT3, and STAT5 in CD8+ T cells (Figs. 2A and 3A) (29), IL-1RI expression on CD8+ T cells was analyzed upon treatment with cytokine combinations that potentiate phosphorylation of STAT1, STAT3, and STAT5; however, only IL-21 treatment resulted in significant upregulation (Fig. 3D). Our findings clearly illustrate that the IL-21-specific STAT signaling pathway is preferentially involved in the induction of IL-1RI on CD8+ T cells, implicating critical spatiotemporal interactions among IL-21-activated STAT molecules in CD8+ T cells.
The induction of IL-1RI expression is attributable to the IL-21-mediated formation of STAT1:STAT3 and STAT3:STAT5 heterodimers
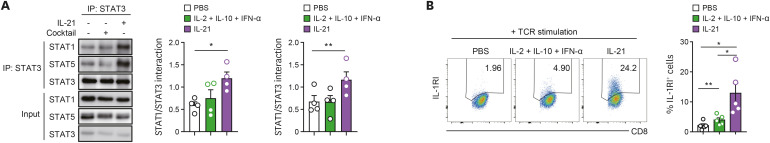
Activated STAT3 interacts with other STAT proteins and forms heterodimers with STAT1 or STAT5 (33). Since IL-21 signaling concurrently induces phosphorylation of STAT1, STAT3, and STAT5 in CD8+ T cells, we hypothesized that STAT3 forms a heterodimer complex with STAT1 and STAT5 in IL-21 treated CD8+ T cells and that these heterodimers play a critical role in expression of IL-1RI. To test this hypothesis, purified primary CD8+ T cells were stimulated with anti-CD3/28 Abs in the absence or presence of exogenous IL-21 (100 ng/ml) for 30 min, followed by IP using an anti-STAT3 Ab. Immunoblotting after the IP assay clearly showed that IL-21 allowed STAT3 to physically form heterodimers with STAT1 or STAT5 in TCR-stimulated CD8+ T cells (Supplementary Fig. 5). Although TCR-stimulated CD8+ T cells were treated with a cytokine cocktail composed of IL-2, IL-10, and IFN-α that is able to activate STAT5, STAT3, and STAT1 (Supplementary Fig. 4A and B, Fig. 2C), respectively, STAT3 heterodimers with STAT1 or STAT5 were not established (Fig. 4A) and IL-1RI expression was not induced (Fig. 4B). These findings demonstrate that IL-21 induces a unique STAT phosphorylation profile comprised of both STAT1:STAT3 and STAT3:STAT5 heterodimers in CD8+ T cells, which contribute to the expression of IL-1RI on TCR-stimulated CD8+ T cells.
Figure 4. The induction of IL-1RI expression is attributable to the IL-21 mediated formation of STAT1:STAT3 and STAT3:STAT5 heterodimers. (A) STAT3 interacts with both STAT1 and STAT5 in IL-21-stimulated CD8+ T cells. Purified human CD8+ T cells were stimulated for 30 min with anti-CD3/CD28 and rhIL-21 (100 ng/ml) or a combination of STAT1, STAT3, and STAT5-activating cytokines (IFN-α [100 ng/ml], IL-10 [100 ng/ml], and IL-2 [500 IU/ml]). Anti-STAT1, STAT3, or STAT5 Ab immunoblot of anti-STAT3 Ab immunoprecipitates. (B) Representative flow cytometric plot and the frequency (%) of IL-1RI expression on TCR-stimulated CD8+ T cells in the presence of the indicated cytokines at day 2 post-stimulation. Bar graphs show the mean±SEM of independent experiments. The statistical significance was measured by 2-tailed paired t-test.
*p<0.05, **p<0.01.
IL-1β signaling mediated by IL-21-induced IL-1RI enhances the effector function of human CD8+ T cells
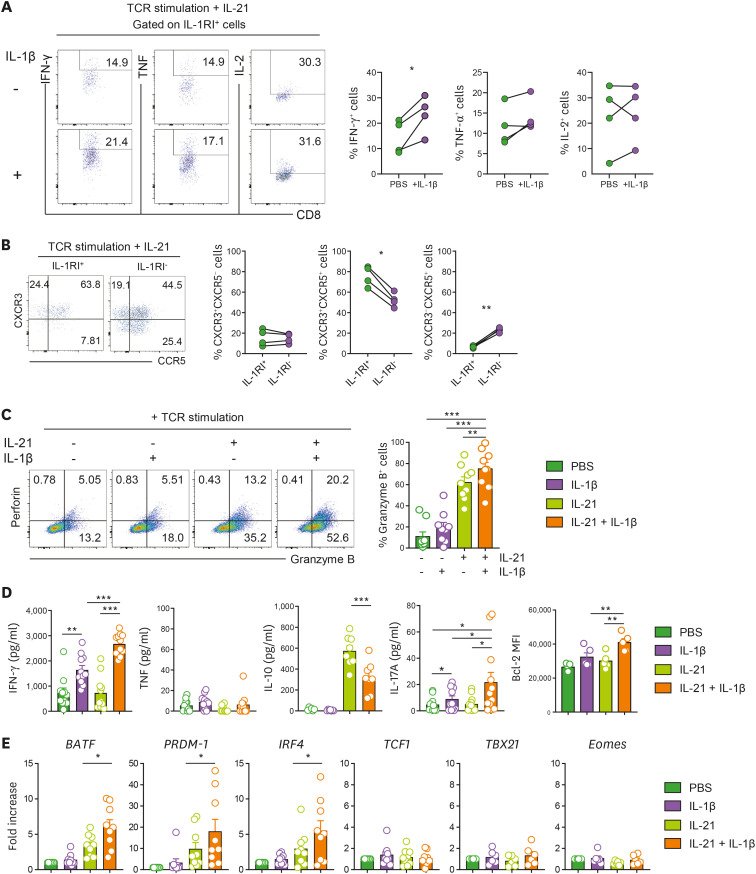
Several reports have demonstrated that the IL-1β signal plays a critical role in protection from viral infections by enhancing the effector function of CTLs (19,20,34). To investigate the effect of IL-1β on IL-1RI-expressing CD8+ T cells, the cytokine-production profile was analyzed in IL-1RI+CD8+ T cells stimulated with anti-CD3/CD28 and IL-21 for 3 days in the absence or presence of IL-1β (Fig. 5A). The frequency of IFN-γ-producing cells was significantly increased in IL-1RI+CD8+ T cells with IL-1β treatment. Moreover, approximately 75% of induced IL-1RI+CD8+ T cells expressed both CXCR3 and CCR5 chemokine receptors (Fig. 5B), suggesting that IL-1RI+CD8+ T cells have an augmented potential to migrate into tissues compared with IL-1RI-CD8+ T cells. We next sought to examine whether IL-1β causes increased effector function of CD8+ T cells through IL-21-induced IL-1RI. The frequency of GzmB+ CD8+ T cells was markedly increased in the cells treated with IL-21 and IL-1β compared with groups treated with IL-21 or IL-1β alone (Fig. 5C). In addition, the amount of IFN-γ and IL-17A in the culture supernatant was significantly upregulated in CD8+ T cells treated with IL-21 and IL-1β, whereas anti-inflammatory cytokine IL-10 was significantly reduced in the presence of IL-21 and IL-1β, suggesting an important role of IL-1β signaling in intensifying the effector function of CD8+ T cells (Fig. 5D). Our findings were corroborated by analyzing gene expression of TFs. TCR-stimulated CD8+ T cells treated with IL-21 and IL-1β had increased levels of several CTL-related TF mRNAs, such as PRDM1 (gene encoding the Blimp1 transcription factor), BATF, and IRF4 (Fig. 5E). However, expression of TCF1, a memory CD8+ T-cell-related TF, as well as TBX21 and Eomes, key TFs governing CD8+ T cell differentiation, were not changed in CD8+ T cells treated with IL-21 and IL-1β compared with the other groups (Fig. 5E).
Figure 5. IL-1β signaling mediated by IL-21-induced IL-1RI enhances the effector function of CD8+ T cells. (A) Representative flow cytometric plot and the frequency of IFN-γ, TNF, or IL-2-producing IL-1RI+CD8+ T cells. Cells were stimulated for 3 days with anti-CD3/28 coated microbeads and rhIL-21 (50 ng/ml) in the absence or presence of IL-1β (50 ng/ml). (B) Representative flow cytometric plot and the frequency (%) of CXCR3 and CCR5-expressing IL-1RI+CD8+ and IL-1RI-CD8+ T cells at day 2 post-stimulation. (C-E) Enhanced effector function of CD8+ T cells by IL-21 and IL-1β. Purified naive CD8+ T cells were stimulated for 5 days with anti-CD3/28 coated microbeads and rhIL-21 (50 ng/ml) in the absence or presence of rhIL-1β (50 ng/ml). Representative flow cytometric plot and the frequency (%) of GzmB+ cells (C). The amount of IFN-γ, TNF, IL-10, and IL-17A in the culture supernatant (ELISA) (D). Relative gene expression of transcription factors BATF, PRDM-1 (Blimp-1), IRF4, TCF1, TBX21, and Eomes. (E). Quantitative PCR analysis of target gene expression by CD8 T cells under the indicated conditions. Expression was normalized to β-actin, and the comparative Ct method was used for the quantification of gene expression. Bar graphs show the mean±SEM of independent experiments. The statistical significance was measured by 2-tailed paired t-test.
PRDM1, encoding the Blimp1 transcription factor.
*p<0.05, **p<0.01, ***p<0.005.
Lastly, we compared the features of CD8+ T cells cultured with IL-21 and IL-1β with classical type 1 cytotoxic T cells (Tc1) cultured with IL-12 (Supplementary Fig. 6). Treatment with IL-21 and IL-1β significantly increased the frequency of GzmB+CD8+ T cells and their expression of Blimp-1, BATF, and IRF4 upon TCR stimulation compared to IL-12-treated CD8+ T cells. However, the production of IFN-γ and the expression of TBX21 and Eomes were significantly higher in Tc1 cells differentiated with IL-12 than in those treated with IL-21 and IL-1β (Supplementary Fig. 6). These findings suggest that induction of the IL-1β signal via IL-21-induced IL-RI leads to enhanced effector function of CD8+ T cells with different features from classic Tc1 differentiated with IL-12.
DISCUSSION
IL-21 is a cytokine with multifaceted actions that influence the differentiation and functions of adaptive and innate immune cells and is mainly produced by activated CD4+ T cells, especially T follicular helper cells, as well as Th17 cells and NKT cells (28,35). The binding of IL-21 to the IL-21 receptor (IL-21R) stabilizes its heterodimeric complex with the common cytokine receptor γc and initiates signaling transduction via the JAK/STAT, MAPK, and PI3K-AKT signaling pathways. Since functional IL-21R is broadly expressed on a variety of cells and its signal transduction involves major signaling pathways, IL-21 exerts a broad range of immunoregulatory effects (27,28,35) through direct involvement of recently identified IL-21 target genes.
In the present study, we found that IL-21 leads to an increase of functional IL-1RI expression on activated CD8+ T cells and indirectly regulates the effector function of these cells via boosting responsiveness to IL-1β (Figs. 1 and 5). Complicated cytokine cues dictate the differentiation of naive T cells into lineage-committed effector T cells (36). In this process, the cytokine binds to its receptor, which is constitutively expressed on target cells, whereas certain cytokines selectively induce the expression of receptors for other cytokines. IL-6, and less potently IL-21, are critical cytokines for the induction of IL-1RI expression on naive CD4+ T cells in response to TCR stimulation (37), whereas a combination of IL-7, IL-15, and TGF-β upregulate IL-1RI expression on naive CD4+ T cells even without TCR stimulation (7). Given that IL-1β is an important cytokine for pathogenic Th17 generation, cytokine-induced IL-1RI expression has been found to contribute to the pathogenesis of autoimmune diseases such as RA (24,38,39). However, most studies have focused on the functional IL-1RI and decoy, IL-1RII, expressed by CD4+ T cells and little is known about features of IL-1RI expressing CD8+ T cells and the molecular mechanisms underlying its expression.
The re-analysis of previously published microarray data suggested that of common γc family cytokines, only IL-21 induces expression of IL-1RI on activated CD8+ T cells in mice (Supplementary Fig. 2A) (26). Similar to other common γc family cytokines, IL-21 activates tyrosine kinases JAK1 and JAK3, which recruit and phosphorylate STAT1, STAT 3, and STAT 5 (40,41). It is established that the IL-21 signal is mediated by more potent and sustained activation of STAT3 compared to other common γc family cytokines, such as IL-2, IL-7, and IL-15, which predominantly activate STAT5A and STAT5B. Thus, this suggests the existence of a distinct IL-21-mediated signaling pathway (29,41). A comprehensive Phosflow assay illustrated that IL-21 synchronously phosphorylates STAT1, STAT3, and STAT5. Moreover, even at IL-21 concentrations as low as 1 ng/ml, phosphorylation of these STATs reached almost the maximal level (Figs. 2A and 3A). Consistent with previous findings (29,41), IL-2 and IL-15 treatment potentiate STAT5 phosphorylation in human CD8+ T cells compared to treatment with IL-21, but no activation of STAT3 was observed in IL-2 or IL-15 treated CD8+ T cells (Supplementary Fig. 4B). We found that IL-21-induced expression of IL-1RI on CD8+ T cells requires phosphorylation of STAT1, STAT3, and STAT5 as illustrated through use of individual STAT inhibitors Fludarabine, 5,15-DPP, and CAS 285986-31-4 (Figs. 2B and 3B). However, a cytokine cocktail that induces phosphorylation of STAT1, STAT3, and STAT5 in CD8+ T cells was incapable of inducing IL-1RI expression (Figs. 2C and 4B, Supplementary Fig. 4). Thus, this strongly suggests a critical role of IL-21-mediated spatiotemporal activation of these 3 STAT molecules.
The JAK-STAT pathway is the principal signaling mechanism for a broad range of cytokines and growth factors (42). Binding of receptors by cytokines activates JAKs associated with their cytoplasmic regions. As a result of JAK-STAT signal transduction, phosphorylated STAT proteins form either homodimers or heterodimers which in turn, translocate into the nucleus to bind to target sequences and function as TFs (33,40). Our result indicated that efficient and concurrent activation of 3 STAT molecules, STAT1, STAT3 and STAT5, is necessary for induction IL-1RI on CD8+ T cells (Figs. 2A 3A, and 4D). Therefore, we hypothesized that the formation of STAT heterodimers is critical for IL-RI expression. Several studies have demonstrated that STAT heterodimers play an important role in certain cytokine signals (33). M-CSF signal leads to formation of STAT5 homodimers and STAT3:STAT5 heterodimers, the latter of which is able to bind particular consensus sequences (43). IL-6 also utilizes STAT1:STAT3 heterodimers, especially in its late signaling (44). One of the important findings in the present study is that the IL-21 signal leads to the formation of distinctive STAT heterodimers including STAT1:STAT3 and STAT3:STAT5 complexes (Fig. 4A). An IP assay using anti-STAT3 Ab clearly illustrates that while an IFN-α, IL-10, and IL-2 cytokine cocktail robustly activates STAT1, STAT 3, and STAT5, it is unable to induce the formation of the STAT3:STAT5 heterodimer and to a lesser degree, the STAT1:STAT3 heterodimer and to cause the expression of IL-1RI on activated CD8+ T cells (Fig. 4B). This implies that IL-21-mediated spatiotemporal activation of STAT1, STAT3, and STAT5 is critical for the formation of unique STAT3 heterodimers necessary for the transcription of IL-1RI. On the contrary, no obvious phosphorylation of Erk1/2 or p38, IL-21R signaling components, was observed in IL-21 stimulated CD8+ T cells (data not shown).
IL-1β plays critical and pleiotropic roles in orchestrating innate and adaptive immune responses. In T-cell immunity, it has been known that IL-1β greatly enhances Ag-driven responses of CD4+ and CD8+ T cells by directly increasing cell expansion and survival (45). Especially, Ag-specific Th17 differentiation of naive CD4+ T cells is markedly promoted by IL-1β (46). Lee and colleagues have recently reported that IL-1β improves the antitumor function of adoptively transferred CD8+ T cells by enhancing their tissue homing and survival (47). Administration of IL-1β controls the cytotoxicity of CD8+ T cells indirectly via its actions on radio-resistant host cells in an IL-2 and IL-15 dependent manner (47). In the present study, we provide evidence that IL-1β potentiates the effector function of human CD8+ T cells via the induction of IL-1RI by IL-21. Further, TCR-activated IL-1RI+CD8+ T cells exhibit a higher frequency of IFN-γ producing cells under exogenous IL-1β stimulation (Fig. 5A), and IL-21 alone was not sufficient for CD8+ T cells to produce IFN-γ. TCR stimulation of cells treated with IL-1β and IL-21 resulted in robust secretion of IFN-γ and production of cytotoxic granules containing GzmB (Fig. 5C). Thus, this suggests a critical role of IL-1β signaling for the induction of IL-1R1 mediated cytotoxic functions by IL-21. A recent study showed that in the acute LCMV infection mouse model, IL-1 signaling directly promotes the expansion and polyfunctionality of CD8+ T cells via the MyD88-IRAK1/4 axis (48). In agreement with previous studies using this mouse model (20,49), we also found that the IL-1R signal robustly increases the expression of GzmB and Bcl-2 (Fig. 5C and D). Of note, the combination of IL-21 and IL-1β was a more potent trigger of cytotoxic granule production by CD8+ T cells than was stimulation with the classical Tc1 promoting cytokine, IL-12 (Supplementary Fig. 6). Based on our findings, increased IL-1β responsiveness through induction of IL-IRI by CD4-derived IL-21 may contribute to protective immune responses against pathogens by vigorously enhancing the effector function of CD8+ T cells.
A growing body of evidence has documented the presence of IL-17-producing CD8+ T cells (Tc17) in various conditions (50,51). Their ability to secrete proinflammatory cytokine IL-17 and plasticity to convert CTL or Tc2 phenotype allow Tc17 cells for contributing to protective as well as pathologic immune responses (50,51). IL-17-producing CD8+ T cells protect against lethal influenza virus challenge via promotion of inflammation, bacterial skin infections, and lethal fungal pneumonia (52,53,54). On the other hand, IL-17-producing CD8+ T cells are involved in pathogenic roles in autoimmune inflammation such as multiple sclerosis and psoriasis (55,56). The role of IL-17-producing CD8+ T cells in tumors remains controversial (57). A tumor-promoting function of IL-17-producing CD8+ T cells associates with poor patient survival in gastrointestinal cancers including gastric cancer and hepatocellular carcinoma (58), whereas the anti-tumor effect of IL-17-producing CD8+ T cells has been reported in a mouse B16 melanoma model (59). More recently, it has been demonstrated that Tc17 cells serve as one of the suppressive immune cells via promoting the exhaustion of CD8+ T cells in tumors under CD4-depleted conditions (60). The differentiation of Tc17 cells, analogous to IL-17-producing CD4+ (Th17) cells, is regulated by multiple cytokines including TGF-β, IL-6, IL-21, IL-23, and IL-1β and transcriptional factor RORγt. IRF4 and STAT3 positively regulate RORγt, whereas Blimp1, T-bet, Eomes, FOXP3, and IRF3 repress RORγt and thereby Tc17 differentiation (50,51).
One of the interesting findings in the present study is that stimulation with IL-1β and IL-21 in concert promotes Tc17 responses. TCR-activated CD8+ T cells treated with IL-1β and IL-21 secret a higher amount of IL-17 than do cells treated with IL-21 or IL-1β alone (Fig. 5D). Although IL-21 has been well known as an important cytokine for IL-17+CD8+ cell differentiation (51,61), our data underscore the importance of IL-1β for the production of IL-17 in CD8+ T cells via IL-21-mediated IL-1RI expression. Considering that IL-21 is expressed by CD4+ T cells early during influenza infection (62), our finding may give another explanation on Tc17-mediated protection of influenza or other viral infections by enhanced CTL function via IL-21-IL-1RI-IL-1β axis. Xin et al. have reported that STAT3-induced BATF is crucial for acquiring optimal CD8+ T-cell effector function (63) through cooperation with TCR-induced IRF4, which leads to the expression of Blimp-1 and sustains CD8+ T-cell effector function. Further, it appears that IL-1RI signaling reinforces the IL-21-mediated transcription of BATF, IRF4, and Blimp-1 compared to treatment with IL-21 alone (Fig. 5E). Interestingly, IL-21 and IL-1β do not affect the expression of TCF1, a marker of self-renewing memory CD8+ T cells (64), but enhance survival of CD8+ T cells by increasing expression of anti-apoptotic Bcl-2 protein (Fig. 5D). These data indicate that the IL-1R signal enhances the effector phenotype of CD8+ T cells. Moreover, this may provide another explanation of how CD4+ T cells provide help to CD8+ T cells to augment effector function via the IL-21/IL-1RI/IL-1β axis.
Only a limited number of studies have described the role of IL-1β in CD8+ T-cell responses to viral infection and tumors compared to the role of this cytokine in the response of innate and CD4 T cells. However, several recent studies have shown that IL-1β directly enhances Ag-driven CD8+ T cell responses and boosts the protectivity of weak or ineffective vaccines, implying the expression of IL-1RI on CD8+ T cells (18,19). CD4+ T cells secret IL-21 during the early phase of infection in secondary lymphoid organs (65), and IL-21 signaling plays a critical role in CD8+ T cell responses to acute viral infections (16,66). Thus, our findings suggest a model in which CD4+ T cells provide help through the IL-21-IL-1RI-IL-1β axis to robustly enhance effector CD8+ T cell responses.
In summary, the current study provides new insight into the regulatory role of the IL-21/IL-1RI/IL-1β axis resulting in robust enhancement of the effector function of CD8+ T cells. IL-21 is a unique cytokine that has the potential to induce expression of functional IL-1RI on human CD8+ T cells upon TCR stimulation. This induction of IL-1RI expression is closely dependent on the formation of STAT3:STAT1 and STAT3:STAT5 heterodimers as a consequence of IL-21 signaling. IL-1β signal leads to an increase in expression of the effector function-related genes BATF, IRF4 and Blimp-1, and an increase in cytotoxic granules and IFN-γ in CD8+ T cells. Taken together, these findings increase understanding of the modulatory mechanisms regulating CTLs and the contribution of the IL-21/IL-1RI/IL-1β axis to protective immunity against viruses and tumors.
ACKNOWLEDGEMENTS
The authors thank Jiyeon Jang (Seoul National University College of Medicine) for assisting in the recruitment of human subjects and thank Core Lab, Clinical Trials Center, Seoul National University Hospital for drawing blood. This work was supported by a grant (NRF-2018R1A2B2006310 to W.-W. Lee) from the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (MSIT), Republic of Korea.
Abbreviations
- APC
Ag-presenting cell
- Eomes
eomesodermin
- γc
γ chain
- GzmB
granzyme B
- IL-1R
IL-1 receptor
- IL-1RI
IL-1 receptor type I
- IL-1RII
IL-1 receptor type II
- IP
immunoprecipitation
- LCMV
lymphocytic choriomeningitis virus
- RA
rheumatoid arthritis
- rh
recombinant human
- STAT3i
STAT3 inhibitor
- TBX21
T-box transcription factor 21
- Tc1
type 1 cytotoxic T cell
- TCF1
T cell factor 1
- TF
transcription factor
- CM
central memory
- EM
effector memory
- EMRA
CD45RA+ effector memory
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Lee WW.
- Data curation: Kim DH, Lee WW.
- Funding acquisition: Lee WW.
- Investigation: Kim DH, Kim HY.
- Methodology: Kim DH, Kim HY.
- Project administration: Lee WW.
- Resources: Kim DH.
- Supervision: Lee WW.
- Writing - original draft: Kim DH, Lee WW.
SUPPLEMENTARY MATERIALS
The expression of IL-1RI and IL-RII on ex vivo CD8+ T cells in humans. Representative flow cytometric plot and the frequency (%) of IL-1RI and IL-RII expression on CD8+ T cells of freshly isolated PBMC. Bar graphs show the mean±SEM of independent experiments.
IL-21 induces IL-1RI expression on murine and human CD8+ T cells. (A) Relative gene expression of various cytokine receptors (heat map) and IL-1RI (bar graph) in IL-2, IL-4, IL-7, IL-15, or IL-21-stimulated CD8+ T cells based on re-analysis of previously published microarray data (GSE58262). (B) Representative flow cytometric plot and the frequency (%) of IL-1RI expression on murine CD8+ T cells, which were stimulated for 3 days with plate-bound anti-CD3 and soluble anti-CD28 in the absence or presence of rmIL-21 (50 ng/ml). (C) Representative flow cytometric plot and the frequency (%) of IL-1RI expression on IL-21-stimulated naive (CD45RA+CD27+), memory (CD45RA−CD27+), effector (CD45RA−CD27−), CD45RA+ effector (CD45RA+CD27−) CD8+ T-cell subsets after 2 days of TCR-stimulation. Bar graphs show the mean±SEM of independent experiments.
TCR stimulation is a prerequisite for IL-21-mediated IL-1R induction. Representative flow cytometric plot and the frequency (%) of IL-1RI on CD8+ T cells that were stimulated for 48 h with or without anti-CD3/CD28 Abs in the presence or absence of exogenous IL-21. Bar graphs show the mean±SEM. The statistical significance was measured by a 2-tailed paired t-test.
Cytokine-specific STAT activation profile. IL-6, IL-10, IL-2, IL-15, or IFN-α stimulation does result in concurrent phosphorylation of STAT1, STAT3, and STAT5. Human PBMCs were stimulated with the indicated concentration of cytokines for 12 min. Delta MFI (mean fluorescence intensity) of phosphorylated STAT proteins in CD8+ T cells was measured by phospho-protein specific flow cytometric analysis (Phosflow assay). (A) Phosphorylation of STAT1 (pY701) of IL-6 or IL-10-stimulated CD8+ T cells (n=3). (B) Phosphorylation of STAT1 (pY701), STAT3 (pY705), and STAT5 (pY694) of CD8+ T cells stimulated with the indicated cytokines (n=3).
IL-21 induces the formation of STAT3:STAT1 and STAT3:STAT5 heterodimers. Purified human CD8+ T cells were stimulated for 30 min with anti-CD3/CD28 and rhIL-21 (100 ng/ml), followed by immunoprecipitation with anti-STAT3 Ab (1:50) or normal rabbit IgG and immunoblot performed using anti-STAT1, STAT3, or STAT5 Abs.
Comparison of features of CD8+ T cells cultured with IL-21/IL-1β and classical Tc1 cultured with IL-12. Purified human naive CD8+ T cells were stimulated for 5 days with anti-CD3/28 coated microbeads and rhIL-1β/IL-21 (50 ng/ml of each) or rhIL-12 (50 ng/ml). (A) Representative flow cytometric plot and the frequency (%) of GzmB+CD8+ T cells. (B) The amount of IFN-γ and TNF-α in the culture supernatant (ELISA). (C) Relative gene expression of TFs BATF, PRDM1, IRF4, TBX21, and Eomes. Quantitative PCR analysis of target gene expression by CD8 T cells under the indicated conditions. Expression was normalized to β-actin, and the comparative Ct method was used for the quantification of gene expression. Bar graphs show the mean±SEM of independent experiments.
References
- 1.Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lasigliè D, Traggiai E, Federici S, Alessio M, Buoncompagni A, Accogli A, Chiesa S, Penco F, Martini A, Gattorno M. Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS One. 2011;6:e20014. doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 4.Sha Y, Markovic-Plese S. A role of IL-1R1 signaling in the differentiation of Th17 cells and the development of autoimmune diseases. Self Nonself. 2011;2:35–42. doi: 10.4161/self.2.1.15639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garlanda C, Riva F, Bonavita E, Gentile S, Mantovani A. Decoys and regulatory “receptors” of the il-1/toll-like receptor superfamily. Front Immunol. 2013;4:180. doi: 10.3389/fimmu.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee WW, Kang SW, Choi J, Lee SH, Shah K, Eynon EE, Flavell RA, Kang I. Regulating human Th17 cells via differential expression of IL-1 receptor. Blood. 2010;115:530–540. doi: 10.1182/blood-2009-08-236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boniface K, Bak-Jensen KS, Li Y, Blumenschein WM, McGeachy MJ, McClanahan TK, McKenzie BS, Kastelein RA, Cua DJ, de Waal Malefyt R. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J Exp Med. 2009;206:535–548. doi: 10.1084/jem.20082293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeste A, Mascanfroni ID, Nadeau M, Burns EJ, Tukpah AM, Santiago A, Wu C, Patel B, Kumar D, Quintana FJ. IL-21 induces IL-22 production in CD4+ T cells. Nat Commun. 2014;5:3753. doi: 10.1038/ncomms4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011;144:601–613. doi: 10.1016/j.cell.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Schurich A, Pallett LJ, Lubowiecki M, Singh HD, Gill US, Kennedy PT, Nastouli E, Tanwar S, Rosenberg W, Maini MK. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 2013;9:e1003208. doi: 10.1371/journal.ppat.1003208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janas ML, Groves P, Kienzle N, Kelso A. IL-2 regulates perforin and granzyme gene expression in CD8+ T cells independently of its effects on survival and proliferation. J Immunol. 2005;175:8003–8010. doi: 10.4049/jimmunol.175.12.8003. [DOI] [PubMed] [Google Scholar]
- 15.Duan MC, Huang Y, Zhong XN, Tang HJ. Th17 cell enhances CD8 T-cell cytotoxicity via IL-21 production in emphysema mice. Mediators Inflamm. 2012;2012:898053. doi: 10.1155/2012/898053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J Immunol. 2011;186:2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laidlaw BJ, Craft JE, Kaech SM. The multifaceted role of CD4(+) T cells in CD8(+) T cell memory. Nat Rev Immunol. 2016;16:102–111. doi: 10.1038/nri.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Sasson SZ, Wang K, Cohen J, Paul WE. IL-1β strikingly enhances antigen-driven CD4 and CD8 T-cell responses. Cold Spring Harb Symp Quant Biol. 2013;78:117–124. doi: 10.1101/sqb.2013.78.021246. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, Caucheteux S, Ratner-Hurevich M, Berzofsky JA, Nir-Paz R, et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med. 2013;210:491–502. doi: 10.1084/jem.20122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joeckel LT, Wallich R, Metkar SS, Froelich CJ, Simon MM, Borner C. Interleukin-1R signaling is essential for induction of proapoptotic CD8 T cells, viral clearance, and pathology during lymphocytic choriomeningitis virus infection in mice. J Virol. 2012;86:8713–8719. doi: 10.1128/JVI.00682-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian T, Jin MQ, Dubin K, King SL, Hoetzenecker W, Murphy GF, Chen CA, Kupper TS, Fuhlbrigge RC. IL-1R type 1-deficient mice demonstrate an impaired host immune response against cutaneous vaccinia virus infection. J Immunol. 2017;198:4341–4351. doi: 10.4049/jimmunol.1500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lapuente D, Storcksdieck Genannt Bonsmann M, Maaske A, Stab V, Heinecke V, Watzstedt K, Heß R, Westendorf AM, Bayer W, Ehrhardt C, et al. IL-1β as mucosal vaccine adjuvant: the specific induction of tissue-resident memory T cells improves the heterosubtypic immunity against influenza A viruses. Mucosal Immunol. 2018;11:1265–1278. doi: 10.1038/s41385-018-0017-4. [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Kim HY, Cho S, Yoo SJ, Kim WJ, Yeon HR, Choi K, Choi JM, Kang SW, Lee WW. Induction of the IL-1RII decoy receptor by NFAT/FOXP3 blocks IL-1β-dependent response of Th17 cells. Elife. 2021;10:e61841. doi: 10.7554/eLife.61841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlüter T, Schelmbauer C, Karram K, Mufazalov IA. Regulation of IL-1 signaling by the decoy receptor IL-1R2. J Mol Med (Berl) 2018;96:983–992. doi: 10.1007/s00109-018-1684-z. [DOI] [PubMed] [Google Scholar]
- 26.McNamara MJ, Kasiewicz MJ, Linch SN, Dubay C, Redmond WL. Common gamma chain (γc) cytokines differentially potentiate TNFR family signaling in antigen-activated CD8(+) T cells. J Immunother Cancer. 2014;2:28. doi: 10.1186/s40425-014-0028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard WJ, Wan CK. Il-21 signaling in immunity. F1000Res. 2016;5:F1000. doi: 10.12688/f1000research.7634.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov. 2014;13:379–395. doi: 10.1038/nrd4296. [DOI] [PubMed] [Google Scholar]
- 29.Ives ML, Ma CS, Palendira U, Chan A, Bustamante J, Boisson-Dupuis S, Arkwright PD, Engelhard D, Averbuch D, Magdorf K, et al. Signal transducer and activator of transcription 3 (stat3) mutations underlying autosomal dominant hyper-IgE syndrome impair human CD8(+) T-cell memory formation and function. J Allergy Clin Immunol. 2013;132:400–411.e9. doi: 10.1016/j.jaci.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang Y, Yu HT, Kim DH, Jang J, Kim HY, Kang I, Kim HC, Park S, Lee WW. Expansion of CD8(+) T cells lacking the IL-6 receptor α chain in patients with coronary artery diseases (CAD) Atherosclerosis. 2016;249:44–51. doi: 10.1016/j.atherosclerosis.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 31.Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155:1079–1090. [PubMed] [Google Scholar]
- 32.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo . Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 33.Delgoffe GM, Vignali DA. STAT heterodimers in immunity: a mixed message or a unique signal? JAKSTAT. 2013;2:e23060. doi: 10.4161/jkst.23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Z, Liu J, Wu W, Zhang E, Zhang X, Li Q, Zelinskyy G, Buer J, Dittmer U, Kirschning CJ, et al. The IL-1R/TLR signaling pathway is essential for efficient CD8+ T-cell responses against hepatitis B virus in the hydrodynamic injection mouse model. Cell Mol Immunol. 2017;14:997–1008. doi: 10.1038/cmi.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spolski R, Leonard WJ. Interleukin-21: basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 36.Mandraju R, Jain A, Gao Y, Ouyang Z, Norgard MV, Pasare C. Myd88 signaling in T cells is critical for effector CD4 T cell differentiation following a transitional T follicular helper cell stage. Infect Immun. 2018;86:e00791-17. doi: 10.1128/IAI.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ikeda S, Saijo S, Murayama MA, Shimizu K, Akitsu A, Iwakura Y. Excess IL-1 signaling enhances the development of Th17 cells by downregulating TGF-β-induced Foxp3 expression. J Immunol. 2014;192:1449–1458. doi: 10.4049/jimmunol.1300387. [DOI] [PubMed] [Google Scholar]
- 38.Sha Y, Markovic-Plese S. Activated IL-1RI signaling pathway induces Th17 cell differentiation via interferon regulatory factor 4 signaling in patients with relapsing-remitting multiple sclerosis. Front Immunol. 2016;7:543. doi: 10.3389/fimmu.2016.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koenders MI, Devesa I, Marijnissen RJ, Abdollahi-Roodsaz S, Boots AM, Walgreen B, di Padova FE, Nicklin MJ, Joosten LA, van den Berg WB. Interleukin-1 drives pathogenic Th17 cells during spontaneous arthritis in interleukin-1 receptor antagonist-deficient mice. Arthritis Rheum. 2008;58:3461–3470. doi: 10.1002/art.23957. [DOI] [PubMed] [Google Scholar]
- 40.Leonard WJ, Spolski R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nat Rev Immunol. 2005;5:688–698. doi: 10.1038/nri1688. [DOI] [PubMed] [Google Scholar]
- 41.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135–4142. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 43.Novak U, Mui A, Miyajima A, Paradiso L. Formation of STAT5-containing DNA binding complexes in response to colony-stimulating factor-1 and platelet-derived growth factor. J Biol Chem. 1996;271:18350–18354. doi: 10.1074/jbc.271.31.18350. [DOI] [PubMed] [Google Scholar]
- 44.Haan S, Keller JF, Behrmann I, Heinrich PC, Haan C. Multiple reasons for an inefficient STAT1 response upon IL-6-type cytokine stimulation. Cell Signal. 2005;17:1542–1550. doi: 10.1016/j.cellsig.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Ben-Sasson SZ, Caucheteux S, Crank M, Hu-Li J, Paul WE. IL-1 acts on T cells to enhance the magnitude of in vivo immune responses. Cytokine. 2011;56:122–125. doi: 10.1016/j.cyto.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santarlasci V, Cosmi L, Maggi L, Liotta F, Annunziato F. IL-1 and T helper immune responses. Front Immunol. 2013;4:182. doi: 10.3389/fimmu.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee PH, Yamamoto TN, Gurusamy D, Sukumar M, Yu Z, Hu-Li J, Kawabe T, Gangaplara A, Kishton RJ, Henning AN, et al. Host conditioning with IL-1β improves the antitumor function of adoptively transferred T cells. J Exp Med. 2019;216:2619–2634. doi: 10.1084/jem.20181218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarkar S, Yuzefpolskiy Y, Xiao H, Baumann FM, Yim S, Lee DJ, Schenten D, Kalia V. Programming of CD8 T cell quantity and polyfunctionality by direct IL-1 signals. J Immunol. 2018;201:3641–3650. doi: 10.4049/jimmunol.1800906. [DOI] [PubMed] [Google Scholar]
- 49.Bartholdy C, Christensen JE, Grujic M, Christensen JP, Thomsen AR. T-cell intrinsic expression of MyD88 is required for sustained expansion of the virus-specific CD8+ T-cell population in LCMV-infected mice. J Gen Virol. 2009;90:423–431. doi: 10.1099/vir.0.004960-0. [DOI] [PubMed] [Google Scholar]
- 50.Lückel C, Picard FS, Huber M. Tc17 biology and function: novel concepts. Eur J Immunol. 2020;50:1257–1267. doi: 10.1002/eji.202048627. [DOI] [PubMed] [Google Scholar]
- 51.Srenathan U, Steel K, Taams LS. IL-17+ CD8+ T cells: differentiation, phenotype and role in inflammatory disease. Immunol Lett. 2016;178:20–26. doi: 10.1016/j.imlet.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hamada H, Garcia-Hernandez ML, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, Conlan S, Himmelfarb S, Byrd AL, Deming C, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520:104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nanjappa SG, McDermott AJ, Fites JS, Galles K, Wüthrich M, Deepe GS, Jr, Klein BS. Antifungal Tc17 cells are durable and stable, persisting as long-lasting vaccine memory without plasticity towards IFNγ cells. PLoS Pathog. 2017;13:e1006356. doi: 10.1371/journal.ppat.1006356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huber M, Heink S, Pagenstecher A, Reinhard K, Ritter J, Visekruna A, Guralnik A, Bollig N, Jeltsch K, Heinemann C, et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest. 2013;123:247–260. doi: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheuk S, Schlums H, Gallais Sérézal I, Martini E, Chiang SC, Marquardt N, Gibbs A, Detlofsson E, Introini A, Forkel M, et al. CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity. 2017;46:287–300. doi: 10.1016/j.immuni.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuen DS, Kim BS, Chung Y. IL-17-producing cells in tumor immunity: Friends or foes? Immune Netw. 2020;20:e6. doi: 10.4110/in.2020.20.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhuang Y, Peng LS, Zhao YL, Shi Y, Mao XH, Chen W, Pang KC, Liu XF, Liu T, Zhang JY, et al. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology. 2012;143:951–962.e8. doi: 10.1053/j.gastro.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski P, Restifo NP. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim BS, Kuen DS, Koh CH, Kim HD, Chang SH, Kim S, Jeon YK, Park YJ, Choi G, Kim J, et al. Type 17 immunity promotes the exhaustion of CD8+ T cells in cancer. J Immunother Cancer. 2021;9:e002603. doi: 10.1136/jitc-2021-002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen HW, Tsai JP, Yao TY, Hsieh CL, Chen IH, Liu SJ. TGF-β and IL-21 cooperatively stimulate activated CD8(+) T cells to differentiate into Tc17 cells. Immunol Lett. 2016;174:23–27. doi: 10.1016/j.imlet.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Karnowski A, Chevrier S, Belz GT, Mount A, Emslie D, D'Costa K, Tarlinton DM, Kallies A, Corcoran LM. B and T cells collaborate in antiviral responses via IL-6, IL-21, and transcriptional activator and coactivator, Oct2 and OBF-1. J Exp Med. 2012;209:2049–2064. doi: 10.1084/jem.20111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xin G, Schauder DM, Lainez B, Weinstein JS, Dai Z, Chen Y, Esplugues E, Wen R, Wang D, Parish IA, et al. A critical role of IL-21-induced BATF in sustaining CD8-T-cell-mediated chronic viral control. Cell Reports. 2015;13:1118–1124. doi: 10.1016/j.celrep.2015.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kratchmarov R, Magun AM, Reiner SL. TCF1 expression marks self-renewing human CD8+ T cells. Blood Adv. 2018;2:1685–1690. doi: 10.1182/bloodadvances.2018016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian Y, Zajac AJ. Il-21 and T cell differentiation: consider the context. Trends Immunol. 2016;37:557–568. doi: 10.1016/j.it.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yi JS, Ingram JT, Zajac AJ. IL-21 deficiency influences CD8 T cell quality and recall responses following an acute viral infection. J Immunol. 2010;185:4835–4845. doi: 10.4049/jimmunol.1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The expression of IL-1RI and IL-RII on ex vivo CD8+ T cells in humans. Representative flow cytometric plot and the frequency (%) of IL-1RI and IL-RII expression on CD8+ T cells of freshly isolated PBMC. Bar graphs show the mean±SEM of independent experiments.
IL-21 induces IL-1RI expression on murine and human CD8+ T cells. (A) Relative gene expression of various cytokine receptors (heat map) and IL-1RI (bar graph) in IL-2, IL-4, IL-7, IL-15, or IL-21-stimulated CD8+ T cells based on re-analysis of previously published microarray data (GSE58262). (B) Representative flow cytometric plot and the frequency (%) of IL-1RI expression on murine CD8+ T cells, which were stimulated for 3 days with plate-bound anti-CD3 and soluble anti-CD28 in the absence or presence of rmIL-21 (50 ng/ml). (C) Representative flow cytometric plot and the frequency (%) of IL-1RI expression on IL-21-stimulated naive (CD45RA+CD27+), memory (CD45RA−CD27+), effector (CD45RA−CD27−), CD45RA+ effector (CD45RA+CD27−) CD8+ T-cell subsets after 2 days of TCR-stimulation. Bar graphs show the mean±SEM of independent experiments.
TCR stimulation is a prerequisite for IL-21-mediated IL-1R induction. Representative flow cytometric plot and the frequency (%) of IL-1RI on CD8+ T cells that were stimulated for 48 h with or without anti-CD3/CD28 Abs in the presence or absence of exogenous IL-21. Bar graphs show the mean±SEM. The statistical significance was measured by a 2-tailed paired t-test.
Cytokine-specific STAT activation profile. IL-6, IL-10, IL-2, IL-15, or IFN-α stimulation does result in concurrent phosphorylation of STAT1, STAT3, and STAT5. Human PBMCs were stimulated with the indicated concentration of cytokines for 12 min. Delta MFI (mean fluorescence intensity) of phosphorylated STAT proteins in CD8+ T cells was measured by phospho-protein specific flow cytometric analysis (Phosflow assay). (A) Phosphorylation of STAT1 (pY701) of IL-6 or IL-10-stimulated CD8+ T cells (n=3). (B) Phosphorylation of STAT1 (pY701), STAT3 (pY705), and STAT5 (pY694) of CD8+ T cells stimulated with the indicated cytokines (n=3).
IL-21 induces the formation of STAT3:STAT1 and STAT3:STAT5 heterodimers. Purified human CD8+ T cells were stimulated for 30 min with anti-CD3/CD28 and rhIL-21 (100 ng/ml), followed by immunoprecipitation with anti-STAT3 Ab (1:50) or normal rabbit IgG and immunoblot performed using anti-STAT1, STAT3, or STAT5 Abs.
Comparison of features of CD8+ T cells cultured with IL-21/IL-1β and classical Tc1 cultured with IL-12. Purified human naive CD8+ T cells were stimulated for 5 days with anti-CD3/28 coated microbeads and rhIL-1β/IL-21 (50 ng/ml of each) or rhIL-12 (50 ng/ml). (A) Representative flow cytometric plot and the frequency (%) of GzmB+CD8+ T cells. (B) The amount of IFN-γ and TNF-α in the culture supernatant (ELISA). (C) Relative gene expression of TFs BATF, PRDM1, IRF4, TBX21, and Eomes. Quantitative PCR analysis of target gene expression by CD8 T cells under the indicated conditions. Expression was normalized to β-actin, and the comparative Ct method was used for the quantification of gene expression. Bar graphs show the mean±SEM of independent experiments.