Abstract
Thymus vulgaris L. (thyme), Origanum majorana L. (marjoram), and Origanum vulgare L. (oregano) were used to determine whether light modification (plants grown under nets with 40% shaded index or in un-shaded open field) could improve the quantity and quality of essential oils (EOs) and antioxidant activity. The yield of EOs of thyme, marjoram, and oregano obtained after 120 min of hydrodistillation was 2.32, 1.51, and 0.27 mL/100 g of plant material, respectively. At the same time under shading conditions plants synthetized more EOs (2.57, 1.68, and 0.32 mL/100 g of plant material). GC/MS and GC/FID analyses were applied for essential oils determinations. The main components of the thyme essential oil are thymol (8.05–9.35%); γ-terpinene (3.49–4.04%); p-cymene (2.80–3.60%) and caryophyllene oxide (1.54–2.15%). Marjoram main components were terpinene 4-ol (7.44–7.63%), γ-terpinene (2.82–2.86%) and linalool (2.04–2.65%) while oregano essential oil consisted of the following components: caryophyllene oxide (3.1–1.93%); germacrene D (1.17–2.0%) and (E)-caryophyllene (1.48–1.1%). The essential oil from thyme grown under shading (EC50 value after 20 min of incubation) have shown the highest antioxidant activity – 0.85 mg mL−1 in comparison to marjoram and oregano (shaded plants EC50 19.97 mg mL−1 and 7.02 mg mL−1 and unshaded, control plants EC50 54.01 mg mL−1 and 7.45 mg mL−1, respectively). The medicinal plants are a good source of natural antioxidants with potential application in the food and pharmaceutical industries. For production practice, it can be recommended to grow medicinal plants in shading conditions to achieve optimal quality parameters.
Keywords: Thymus vulgaris L.; Origanum majorana L.; Origanum vulgare L.; Shading, Quality components
1. Introduction
Aromatic plants such as Thymus vulgaris L(common thyme), Origanum majorana L. (sweet marjoram), and Origanum vulgare L. (oregano) have a long tradition of use in both folk and conventional medicine (Mimica-Dukić et al., 2004). They are native plants from the Mediterranean region, widely distributed in Balkan countries, as well as in Serbia, with great health importance (Ilić et al., 2021). These plants can also be said to belong to culinary herbs due to their characteristic odor, and they are added as a spice in the preparation of stews, soups, meat, fish, and vegetable dishes. Thyme is considered one of the fine herbs of French cuisine, oregano in Italian and marjoram in all Mediterranean regions (Wiese et al., 2018).
The essential oils and their main components such as carvacrol, thymol, and linalool isolated from medicinal plants, contained phenols and other components, are being implemented in pharmaceutical and cosmetology industries as the carriers of antibacterial (De Falco et al., 2013), antiviral and antifungal activity (Liu et al., 2019). At the same time, they are distinguished as anti-inflammatory, antidiabetic and cancer suppressor agents (Leyva-López et al., 2017). Common thyme, sweet marjoram, and oregano essential oils - natural and liquid secondary plant metabolites-are gaining importance for their use in the protection of foods, since they are accepted as safe and healthy (Gavarić et al., 2015). The use of essential oils or plant extracts of thyme as a significant source of natural additives affects stability and reduces lipid oxidation during storage of foods such as meat, meat products, milk, fish and their products (Nieto, 2020).
The accumulation and the final composition of essential oils in the plants are highly influenced by the cultivation conditions, climate, and growth stage at the time of harvest (Murillo-Amador et al., 2013). Light quantity and quality play an important role in the plant production and synthesis of essential oils and have been shown to affect volatile compounds in herbs (Carvalho et al., 2016). Shading nets are characterized by different mechanical, physical, and optical properties, which allow for the modulation of light (quality and quantity of sunlight radiation), but at the same time they affect temperature regulation, humidity, and wind velocity levels around the crops, allowing the greater efficiency of herbs production and quality parameters of medicinal plants in screen-house cultivation (Milenković et al., 2019).
Studies about the cultivation of aromatic plants have found different responses concerning the content and composition of essential oil, according to the light spectrum control during cultivation (Martins et al., 2008, Costa et al., 2012, Oliveira et al., 2016, Milenković et al., 2019). The spectral changes provided by colored shade nets resulted in increments of 30% in the yield of essential oil in lemon balm plants. These plant's qualities make the use of blue net a cultivation practice suitable for commercial use (Oliveira et al., 2016). These results are in agreement with Martins et al. (2008), who showed that Origano gratissimum plants under the blue net were taller and had higher essential oil content. Buthelezi et al. (2016) present in their research that aromatic herbs grown under black nets achieve higher antioxidant content than herbs from open field and other shade nets.
The present paper aims to investigate the effect of light intensity modification on the yield, chemical composition of essential oils, and antioxidant activity of thyme, marjoram and oregano cultivated under different light conditions. It is important to determine what conditions the plants need for their optimum quality parameters.
2. Material and methods
2.1. Plant material and growing conditions
The experiment was conducted during 2019–2020 in an experimental garden at the village Moravac in South Serbia (21°42′E, 43°30′N, altitude 159 m). Thymus vulgaris L. (thyme), Origanum majorana L. (marjoram), and Origanum vulgare L. (oregano) were used to determine whether shading conditions (plants cover by color nets) could improve essential oils and antioxidant activity in plants.
The production and establishment of medicinal plants meant sowing seeds in the field at a distance between rows of 40 cm on a plot of only 3–6 mm deep on 25 May in raised beds (20 cm high), 1.2 m wide and 3 m long (3.6 m2 plot size). in 2019.After 6–8 days, the plants began to germinate, and then thinning was performed (at 5 cm distance of plants in the row) to achieve an optimal plant density (50 plants/m2). According to soil analyses, all quantities of phosphorus and potassium (50 kg ha−1) and 50% of total nitrogen (80 kg ha−1) is incorporated into the soil before sowing. The other half of nitrogen fertilizers were applied the first time 8 weeks (25 % N) after sowing and the second time after the first harvest (25 % N) using calcium ammonium nitrate (28 % N). Combinations of plant species treatments were replicated 3 times with shading (net house with shade nets from an Israel company Polysack Plastics Industries, with a shade index of 40%) and un-netted control treatment in a split-plot design. The shade nets were mounted on a structure placed about 2.0 m above the plants (net house) at middle of June until end of August.
Thyme, marjoram, and oregano plants in the second year (2020) after the establishment of the crop were harvested at the stage of commercial maturity (in full bloom stage). The plants were harvested in early August. Uniform shoots without disease with leaves without any injuries or defects were selected and dried without the presence of light and ventilation at room temperature (about 25–30 °C) as air-dry plants for analysis.
2.2. Light interception by nets
The Sun Scan probe SS1-UM-1.05 (Delta-T Devices Ltd., UK) was used for light intensity measurement (PAR-photosynthetically active radiation - μmol m-2 s-1) under the pearl nets and open field condition (control). Solar radiation was measured at different intervals during the days with Solarimeter-SL 100 (KIMO, France).
2.3. Clevenger-hydrodistillation
Preparation of plant material involves choping (milled air-dried aerial part of thyme, marjoram, and oregano plants: Thymi herba, Origani majoranae herba, and Origani herba was used for essential oil isolation by Clevenger-type hydrodistillation, with hydromodulus (ratio of plant material:water) 1:10 m/V during 120 min. The content of essential oil is displayed in % (v/m), which conforms to mL/100 g of air-dried plant material.
2.4. Gas chromatography/mass spectrometry (GC/MS) and gas chromatography/flame ionization detection (GC/FID) analysis
Injected in split/splitless injector set at 250 °C in 40:1 split mode. Oil constituents identification was based on the comparison of their retention indices (RIexp) with those available in the literature (Adams, 2007) (RIlit); their mass spectra with those of authentic standard as well as with those from Willey 6, NIST2011, and RTLPEST3 libraries and wherever possible, by co-injection with an authentic standard (Co-I). Quantification was done by external standard method using standards in the concentration ranges as follows: β-pinene (0.125–2 mg/mL), 1,8-cineole (0.25–3 mg/mL), citral (0.56–10 mg/mL), limonene (0.5–4 mg/mL), linalool (1.67–15 mg/mL), thymol (1.78–16 mg/mL) and γ-terpinene (0.75–5 mg/mL).
The response factor (RF) for each standard used was calculated as follows:
where Areastd is the peak area of the analyte standard and Cstd is the concentration of the standard used. Then the mean of the RFs was calculated (RFmean) and used to quantitate each of the analytes in the samples using the equation:
where Cx is the concentration of the analyte in the sample, Areax is the peak area of the analyte, and RFmean is the mean response factor (Sparkman et al., 2011).
2.5. DPPH assay
The ability of the essential oil to scavenge free DPPH radicals was determined using the DPPH assay. Absorption was measured at 517 nm immediately after adding the DPPH radical and after 20 min of incubation with the radical. Free radical scavenging activity was calculated according to the formula (Stanojević et al., 2015):
AS – Absorption of the “sample” at 517 nm. “Sample” – ethanolic solution of the essential oil treated with DPPH radical solution
AB – Absorption of the “blank” at 517 nm. “Blank” – ethanolic solution of the essential oil which is not treated with DPPH radical solution
AC – Absorption of the ”control” at 517 nm. ”Control” – ethanolic solution of the DPPH radical
All absorptions were measured on Perkin Elmer Lambda 25, Spectrophotometer.
The essential oil concentration needed for the neutralization of 50% of the initial DPPH radical concentration is called EC50 value. This value was determined by using linear regression analysis of the different concentrations range of essential oil added to the reaction mixture.
2.6. Statistical analysis
Statistical analysis of obtained data was performed using software Statistica (TIBCO Software Inc. 2018, version 13). ANOVA was used to analyse the significance of influence of shading conditions on medicinal plants with Duncan’s multiple range test used for analysis of significance of differences between means.
3. Results
3.1. Climatic conditions
The climatic conditions of southern Serbia are very favorable for the production of thyme, marjoram, and oregano throughout the growing season (Table 1a).
Table 1a.
Parameters of climatic conditions during the growing season in south Serbia (Aleksinac).
| Month | Temperature |
MSR | RH | PR | ||||
|---|---|---|---|---|---|---|---|---|
| TX | TM | TS | EMd | EMnd | MJ/m2 | % | mm | |
| May | 26.6 | 12.6 | 19.6 | 31.0 | 9.0 | 279.0 | 62 | 66.8 |
| June | 26.9 | 14.4 | 20.6 | 34.0 | 7.0 | 223.0 | 68 | 83.1 |
| July | 28.4 | 16.7 | 22.5 | 32.0 | 13.0 | 218.3 | 72 | 99.3 |
| August | 32.7 | 16.7 | 24.7 | 34.1 | 13.2 | 283.1 | 65 | 51.6 |
| September | 26.7 | 10.5 | 18.6 | 34.3 | −3.0 | 230.8 | 64 | 9.8 |
TX- temperature maximum; TM-temperature minimum; TS-mean monthly air temperature (oC); EMd-extreme maximum daily temperture; EMnd- extreme minimun daily temperyture; MSR, mean daily solar radiation (number of hours); MSR, mean daily solar radiation (MJ/m2); RH -Relative humidity (%); PR precipitation amount (mm).
Net houses have the potential to create an appropriate microclimate that positively affects plants productivity and quality. Photosynthetically active radiation (PAR) was significantly lower under pearl nets with 40% shading (1100 µmol s–1m−2) compared to the control - open field condition (2242 µmol s–1m−2). Shading with pearl nets affects the reduction of photosynthetically active radiation, slightly lower in the morning (31.2%), grows during the day, and provides the highest reduction (53.9%) in the afternoon. Results from Table 1b show that the maximum solar radiation in the open field, during a sunny day in July reached 874 Wm−2. Compared to control, the solar radiation at noon was significantly reduced under pearl nets (459 W m−2).
Table 1b.
Influence of shading on growing environment (average day in July).
| Time (h) | PAR* (μmol m−2 s−1) |
Solar radiation (W m−2) |
Temperature °C |
Relative Humidity % |
||||
|---|---|---|---|---|---|---|---|---|
| Non-shading | Shading Reduction % | Non-shading | Shading | Non-shading | Shading Reduction % | Non-shading | Shading Reduction % | |
| 6:00 | 182.5 | 31.2 | 162.5 | 40.5 | 16.7 | 0.0 | 74.7 | −4.1 |
| 9:00 | 1325.6 | 46.0 | 513.8 | 281.0 | 24.7 | −0.4 | 71.8 | 0.0 |
| 12:00 | 2242.2 | 49.1 | 874.5 | 459.5 | 31.4 | −2.2 | 47.3 | −2.1 |
| 15:00 | 1684.1 | 51.9 | 790.5 | 351.0 | 31.5 | −3.4 | 48.2 | −1.2 |
| 18:00 | 672.0 | 53.9 | 375.5 | 90.9 | 28.3 | −1.0 | 50.4 | −0.2 |
PAR.-Photosynthetically active radiation.
3.1.1. Thyme essential oil yield
Thyme has a characteristic odor of thymol and is used as a culinary herb with typical spicy aroma.
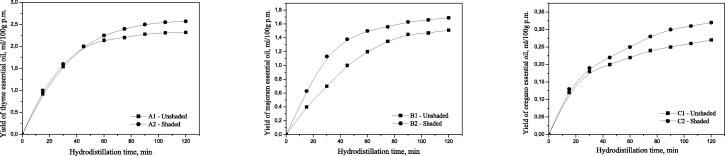
The yield of essential oils (EOs) from thyme was 2.32 mL/100 g of plant material from shaded condition and 2.57 mL/100 g of plant material from unshaded open field Table 2.
Table 2.
Yield of essential oil yield obtained after 120 min of hydrodistillation (hydromodule 1:10 m/v) and EC50 values of essential oil from the aerial part (herb) of thyme, marjoram and oregano.
| Plant species | Shading | Essential oil yield, mL/100 g p.m. | EC50, mg mL−1/20 min incubation |
|---|---|---|---|
| Thyme | Unshaded | 2.32b ± 0.03 | 0.944a ± 0.001 |
| Shaded | 2.57a ± 0,09 | 0.852a ± 0.005 | |
| Marjoram | Unshaded | 1.51d ± 0.03 | 54.012e ± 1.051 |
| Shaded | 1.68c ± 0.03 | 19.972d ± 0.199 | |
| Oregano | Unshaded | 0.27e ± 0.01 | 7.449c ± 0.018 |
| Shaded | 0.32e ± 0.01 | 7.023b ± 0.054 | |
| ANOVA (p values) | |||
| Plant species | 0.00000 | 0.00000 | |
| Shading | 0.00000 | 0.00000 | |
| Plan species · shading | 0.00518 | 0.00000 | |
Values followed by the same letter do not significantly differ between the treatments, at P = 0.05 according to Duncan’s multiple range test.
EC50, mg∙mL−1 = concentration of extract necessary to neutralize 50% of initial concentration of DPPH radicals.
3.1.2. Sweet marjoram essential oil
The yield of essential oil (EOs) from the aerial part (herb) of marjoram obtained after 120 min of hydrodistillation was 1.51 mL/100 g of plant material. Shaded plants showed higher EOs content (1.68 mL/100 g of plant material) than unshaded-control plants (Table 2).
The dependence of the yield of marjoram essential oil from the hydrodistillation time was presented in Fig. 1.
Fig. 1.
The dependence of the yield of thyme, marjoram and oregano essential oil from the hydrodistillation time.
3.1.3. Oregano essential oil yield
The yield of essential oil (EOs) from the aerial part (herb) of oregano obtained after 120 min of hydrodistillation was 0.27 mL/100 g of plant material. Shaded plants showed higher EOs content (0.32 mL/100 g of plant material) than un shaded-control plants. The yield of essential oils in oregano is much lower than that of thyme and marjoram (Table 2).
The dependence of the yield of oregano essential oil from the hydrodistillation time was presented in Fig. 1.
3.2. Essential oils composition
3.2.1. Thyme essential oils composition
The composition of thyme essential oils depends on the geographical origin, variety, growth phase, watering, harvest time and method of drying the plant mass. The chemical profile of thyme essential oils constituents determines their interactions and activity. Thyme EOs vary, which influences the physicochemical properties of the oil.
In our research the main components of the thyme essential oil are thymol (8.05–9.35 mg/mL); γ-terpinene (3.49–4.04%); p-cymene (2.80–3.60%) and caryophyllene oxide (1.54–2.15%) - Table 3 and Fig. 2.
Table 3.
Chemical composition of thyme essential oil.
| No. | tret, min | Compound | Molecular formula | RIexp | RIlit | Method of identification | Area% |
Content % |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Unshade | Shade | Unshade | Shade | |||||||
| 1 | 7.10 | α-Thujene | C10H16 | 927 | 924 | RI, MS | 0.8 | 1.1 | 0.18 | 0.28 |
| 2 | 7.32 | α-Pinene | C10H16 | 934 | 932 | RI, MS, Co-I | 0.5 | 0.7 | 0.12 | 0.17 |
| 3 | 7.78 | Camphene | C10H16 | 949 | 946 | RI, MS, Co-I | 0.3 | 0.3 | 0.07 | 0.07 |
| 4 | 8.52 | Sabinene | C10H16 | 974 | 969 | RI, MS | 0.1 | tr | 0.03 | 0.01 |
| 5 | 8.59 | 1-Octen-3-ol* | C8H16O | 979 | 974 | RI, MS | 1.2 | 1.7 | 0.27 | 0.44 |
| 6 | 8.64 | β-Pinene* | C10H16 | 978 | 974 | RI, MS, Co-I | ||||
| 7 | 8.85 | 3-Octanone | C8H16O | 985 | 979 | RI, MS | 0.1 | 0.1 | 0.01 | 0.02 |
| 8 | 9.01 | Myrcene | C10H16 | 991 | 988 | RI, MS | 1.5 | 1.7 | 0.34 | 0.44 |
| 9 | 9.12 | 3-Octanol | C8H18O | 994 | 988 | RI, MS | 0.1 | 0.1 | 0.02 | 0.03 |
| 10 | 9.53 | α-Phellandrene | C10H16 | 1006 | 1002 | RI, MS | 0.2 | 0.2 | 0.04 | 0.05 |
| 11 | 9.74 | δ-3-Carene | C10H16 | 1012 | 1008 | RI, MS | 0.1 | 0.1 | 0.02 | 0.02 |
| 12 | 9.96 | α-Terpinene | C10H16 | 1018 | 1014 | RI, MS | 2.0 | 2.2 | 0.45 | 0.55 |
| 13 | 10.28 | p-Cymene | C10H14 | 1026 | 1020 | RI, MS | 12.4 | 14.2 | 2.80 | 3.60 |
| 14 | 10.41 | Limonene* | C10H16 | 1029 | 1024 | RI, MS, Co-I | 0.6 | 0.6 | 0.05 | 0.06 |
| 15 | 10.43 | β-Phellandrene* | C10H16 | 1030 | 1025 | RI, MS | ||||
| 16 | 10.51 | 1,8-Cineole | C10H18O | 1032 | 1026 | RI, MS, Co-I | 0.5 | 0.4 | 0.05 | 0.03 |
| 17 | 11.07 | (E)-β-Ocimene | C10H16 | 1047 | 1044 | RI, MS | 0.1 | 0.1 | 0.02 | 0.02 |
| 18 | 11.58 | γ-Terpinene | C10H16 | 1060 | 1054 | RI, MS, Co-I | 14.3 | 14.8 | 3.49 | 4.04 |
| 19 | 11.85 | cis-Sabinene hydrate | C10H18O | 1068 | 1065 | RI, MS | 1.1 | 0.9 | 0.24 | 0.24 |
| 20 | 12.67 | Terpinolene | C10H16 | 1089 | 1086 | RI, MS | 0.3 | 0.2 | 0.07 | 0.04 |
| 21 | 13.09 | Linalool | C10H18O | 1100 | 1095 | RI, MS, Co-I | 3.1 | 2.3 | 0.32 | 0.21 |
| 22 | 14.01 | dehydro-Sabina ketone | C9H12O | 1122 | 1117 | RI, MS | 0.2 | 0.1 | 0.05 | 0.02 |
| 23 | 14.74 | trans-p-Menth-2-en-1-ol | C10H18O | 1140 | 1136 | RI, MS | 0.2 | tr | 0.04 | 0.01 |
| 24 | 15.00 | Camphor | C10H16O | 1146 | 1141 | RI, MS,Co-I | 0.1 | 0.1 | 0.01 | 0.02 |
| 25 | 15.86 | Borneol | C10H18O | 1167 | 1165 | RI, MS, Co-I | 1.0 | 0.8 | 0.23 | 0.19 |
| 26 | 16.38 | Terpinen-4-ol | C10H18O | 1179 | 1174 | RI, MS | 3.7 | 0.8 | 0.85 | 0.20 |
| 27 | 16.98 | α-Terpineol | C10H18O | 1194 | 1186 | RI, MS, Co-I | 0.3 | 0.1 | 0.06 | 0.03 |
| 28 | 17.72 | Linalool formate | C11H18O2 | 1211 | 1214 | RI, MS | 0.1 | – | 0.01 | – |
| 29 | 18.73 | Thymol, methyl ether | C11H16O | 1235 | 1232 | RI, MS | 0.3 | 0.4 | 0.07 | 0.10 |
| 30 | 19.13 | Carvacrol, methyl ether | C11H16O | 1244 | 1241 | RI, MS | 0.3 | 0.3 | 0.07 | 0.09 |
| 31 | 19.76 | Linalool acetate | C12H20O2 | 1259 | 1254 | RI, MS, Co-I | 0.2 | 0.1 | 0.04 | 0.03 |
| 32 | 21.38 | Thymol | C10H14O | 1298 | 1289 | RI, MS, Co-I | 47.4 | 49.2 | 8.05 | 9.35 |
| 33 | 21.64 | Carvacrol | C11H16O | 1304 | 1298 | RI, MS | 2.9 | 2.9 | 0.65 | 0.73 |
| 34 | 23.77 | Thymol acetate | C12H16O2 | 1355 | 1349 | RI, MS | 0.1 | 0.1 | 0.01 | 0.02 |
| 35 | 23.91 | Eugenol | C10H12O2 | 1358 | 1356 | RI, MS, Co-I | – | 0.1 | – | 0.01 |
| 36 | 26.54 | (E)-Caryophyllene | C15H24 | 1423 | 1417 | RI, MS | 1.2 | 1.3 | 0.27 | 0.33 |
| 37 | 27.90 | α-Humulene | C15H24 | 1457 | 1452 | RI, MS | 0.1 | 0.1 | 0.02 | 0.02 |
| 38 | 28.64 | Geranyl propanoate | C11H22O2 | 1475 | 1476 | RI, MS | 0.2 | 0.1 | 0.04 | 0.03 |
| 39 | 28.79 | γ-Muurolene | C15H24 | 1479 | 1478 | RI, MS | – | 0.1 | – | 0.02 |
| 40 | 29.60 | Bicyclogermacrene | C15H24 | 1499 | 1500 | RI, MS | 0.2 | 0.1 | 0.04 | 0.01 |
| 41 | 30.25 | γ-Cadinene | C15H24 | 1516 | 1513 | RI, MS | 0.1 | 0.1 | 0.02 | 0.03 |
| 42 | 30.62 | δ-Cadinene | C15H24 | 1526 | 1522 | RI, MS | 0.1 | 0.2 | 0.02 | 0.04 |
| 43 | 32.92 | Caryophyllene oxide | C15H24O | 1586 | 1582 | RI, MS | 0.3 | 0.2 | 0.06 | 0.06 |
| 44 | 35.02 | epi-α-Cadinol | C15H26O | 1644 | 1638 | RI, MS | 0.1 | 0.1 | 0.03 | 0.03 |
| Total identified (%) | 98.4 | 99.0 | ||||||||
| Grouped components (%) | ||||||||||
| Monoterpene hydrocarbons (1–4, 6, 8, 10–12, 14, 15, 17, 18, 20) | 20.8 | 22.0 | ||||||||
| Oxygen-containing monoterpenes (16, 19, 21, 23–28, 31, 38) | 10.5 | 5.6 | ||||||||
| Sesquiterpene hydrocarbons (36, 37, 39–42) | 1.7 | 1.9 | ||||||||
| Oxygen-containing sesquiterpenes (43, 44) | 0.4 | 0.3 | ||||||||
| Aromatic compounds (13, 29, 30, 32–35) | 63.4 | 67.2 | ||||||||
| Others (5, 7, 9, 22) | 1.6 | 2.0 | ||||||||
tret.: Retention time; RIlit-Retention indices from literature (Adams, 2007); RIexp: Experimentally determined retention indices using a homologous series of n-alkanes (C8-C20) on the HP-5MS column. MS: constituent identified by mass-spectra comparison; RI: constituent identified by retention index matching; Co-I: constituent identity confirmed by GC co-injection of an authentic sample; tr = trace amount (<0.05%); *co-eluting compounds. Compounds marked in italic are present only in sample from unshade plants Compounds marked in under line are present only in sample from shade plants
Fig. 2.
Structures of the most abundant components identified in thyme essential oil.
The majority of the compounds in the thyme essential oil in unshading and shading conditions were aromatic compounds (63.4–67.2%), followed by oxygen-containing monoterpenes (20.8–22.0%), oxygen-containing sesquiterpenes (10.5–5.6%), and sesquiterpene hydrocarbons (1.7–1.9%) respectively.
It has been noticed that only one component (linalool formate) has been registered in unshaded plants.
3.2.2. Marjoram essential oils composition
The majority of EO compounds in the marjoram cultivated in unshaded and shaded conditions were terpinene 4-ol (7.44–7.63%), γ-terpinene (2.82–2.86%) and linalool (2.04–2.65%), Table 4 and Fig. 3.
Table 4.
Chemical composition of marjoram essential oil.
| No. | tret, min | Compound | Molecular formula | RIexp | RIlit | Method of identification | Area% |
Content % |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Unshade | Shade | Unshade | Shade | |||||||
| 1 | 7.11 | α-Thujene | C10H16 | 927 | 924 | RI, MS | 0.4 | 0.3 | 0.07 | 0.08 |
| 2 | 7.33 | α-Pinene | C10H16 | 934 | 932 | RI, MS, Co-I | 0.3 | 0.4 | 0.06 | 0.08 |
| 3 | 8.53 | Sabinene | C10H16 | 974 | 969 | RI, MS | 4.4 | 4.8 | 0.89 | 1.06 |
| 4 | 8.66 | β-Pinene | C10H16 | 979 | 974 | RI, MS, Co-I | 0.3 | 0.3 | 0.18 | 0.21 |
| 5 | 9.02 | Myrcene | C10H16 | 991 | 988 | RI, MS | 1.4 | 1.5 | 0.28 | 0.33 |
| 6 | 9.54 | α-Phellandrene | C10H16 | 1006 | 1002 | RI, MS | 0.2 | 0.2 | 0.04 | 0.04 |
| 7 | 9.99 | α-Terpinene | C10H16 | 1018 | 1014 | RI, MS | 6.9 | 6.6 | 1.41 | 1.47 |
| 8 | 10.25 | p-Cymene | C10H14 | 1025 | 1020 | RI, MS | 0.6 | 0.6 | 0.12 | 0.12 |
| 9 | 10.43 | Limonene* | C10H16 | 1030 | 1024 | RI, MS, Co-I | 2.4 | 2.5 | 0.36 | 0.42 |
| 10 | 10.44 | β-Phellandrene* | C10H16 | 1030 | 1025 | RI, MS | ||||
| 11 | 10.51 | 1,8-Cineole | C10H18O | 1032 | 1026 | RI, MS, Co-I | 0.1 | 0.1 | 0.05 | 0.03 |
| 12 | 10.68 | (Z)-β-Ocimene | C10H16 | 1037 | 1032 | RI, MS | – | tr | – | 0.01 |
| 13 | 11.08 | (E)-β-Ocimene | C10H16 | 1047 | 1044 | RI, MS | 0.1 | 0.1 | 0.01 | 0.01 |
| 14 | 11.58 | γ-Terpinene | C10H16 | 1060 | 1054 | RI, MS, Co-I | 13.0 | 12.0 | 2.82 | 2.86 |
| 15 | 11.87 | cis-Sabinene hydrate | C10H18O | 1068 | 1065 | RI, MS | 3.0 | 3.3 | 0.60 | 0.74 |
| 16 | 12.70 | Terpinolene | C10H16 | 1090 | 1086 | RI, MS | 2.9 | 2.6 | 0.58 | 0.57 |
| 17 | 13.14 | Linalool | C10H18O | 1102 | 1095 | RI, MS, Co-I | 12.9 | 14.8 | 2.04 | 2.65 |
| 18 | 14.04 | cis-p-Menth-2-en-1-ol | C10H18O | 1123 | 1118 | RI, MS | 1.9 | 2.2 | 0.38 | 0.48 |
| 19 | 14.77 | trans-p-Menth-2-en-1-ol | C10H18O | 1141 | 1136 | RI, MS | 1.3 | 1.5 | 0.25 | 0.33 |
| 20 | 16.55 | Terpinen-4-ol | C10H18O | 1183 | 1174 | RI, MS | 36.8 | 34.4 | 7.44 | 7.63 |
| 21 | 16.98 | α-Terpineol | C10H18O | 1194 | 1186 | RI, MS, Co-I | 4.6 | 4.4 | 0.94 | 0.98 |
| 22 | 17.14 | γ-Terpineol | C10H18O | 1198 | 1199 | RI, MS | 0.4 | 0.5 | 0.08 | 0.11 |
| 23 | 17.21 | cis-Dihydro carvone | C10H16O | 1199 | 1191 | RI, MS | 0.3 | 0.2 | 0.06 | 0.04 |
| 24 | 17.51 | trans-Dihydro carvone | C10H16O | 1206 | 1200 | RI, MS | 0.2 | 0.2 | 0.03 | 0.03 |
| 25 | 17.63 | Linalool formate | C11H18O2 | 1209 | 1214 | RI, MS | 0.6 | 0.7 | 0.13 | 0.16 |
| 26 | 18.47 | Nerol | C10H18O | 1229 | 1227 | RI, MS | 0.1 | 0.1 | 0.02 | 0.02 |
| 27 | 19.64 | Linalool acetate | C12H20O2 | 1257 | 1254 | RI, MS, Co-I | 0.9 | 1.0 | 0.19 | 0.21 |
| 28 | 21.14 | Thymol | C10H14O | 1292 | 1289 | RI, MS, Co-I | – | 0.4 | – | 0.06 |
| 29 | 21.54 | Terpinen-4-ol acetate | C12H20O2 | 1301 | 1299 | RI, MS | 0.4 | 0.4 | 0.08 | 0.08 |
| 30 | 24.18 | Neryl acetate | C12H20O2 | 1365 | 1359 | RI, MS | 0.1 | 0.1 | 0.01 | 0.02 |
| 31 | 24.99 | Geranyl acetate | C12H20O2 | 1384 | 1379 | RI, MS | 0.1 | 0.2 | 0.02 | 0.04 |
| 32 | 26.54 | (E)-Caryophyllene | C15H24 | 1423 | 1417 | RI, MS | 1.3 | 1.5 | 0.25 | 0.34 |
| 33 | 27.89 | α-Humulene | C15H24 | 1457 | 1452 | RI, MS | 0.1 | 0.1 | 0.01 | 0.02 |
| 34 | 29.60 | Bicyclogermacrene | C15H24 | 1500 | 1500 | RI, MS | 0.8 | 0.9 | 0.16 | 0.20 |
| 35 | 32.69 | Spathulenol | C15H24O | 1581 | 1577 | RI, MS | 0.2 | 0.1 | 0.05 | 0.01 |
| 36 | 32.92 | Caryophyllene oxide | C15H24O | 1586 | 1582 | RI, MS | 0.3 | 0.1 | 0.07 | 0.02 |
| Total identified (%) | 99.3 | 99.1 | ||||||||
| Grouped components (%) | ||||||||||
| Monoterpene hydrocarbons (1–7, 9, 10, 12–14, 16) | 32.3 | 31.3 | ||||||||
| Oxygen-containing monoterpenes (11, 15, 17–27, 29–31) | 63.7 | 64.1 | ||||||||
| Sesquiterpene hydrocarbons (32–34) | 2.2 | 2.5 | ||||||||
| Oxygen-containing sesquiterpenes (35, 36) | 0.5 | 0.2 | ||||||||
| Aromatic compounds (8, 28) | 0.6 | 1.0 | ||||||||
tret.: Retention time; RIlit-Retention indices from literature (Adams, 2007); RIexp: Experimentally determined retention indices using a homologous series of n-alkanes (C8-C20) on the HP-5MS column. MS: constituent identified by mass-spectra comparison; RI: constituent identified by retention index matching; Co-I: constituent identity confirmed by GC co-injection of an authentic sample; tr = trace amount (<0.05%); *co-eluting compounds. Compounds marked in italic are present only in sample from unshade plants Compounds marked in under line are present only in sample from shade plants
Fig. 3.
Structures of the most abundant components identified in marjoram essential oil.
Thymol (0.06 % mg/mL) and (Z)-β-Ocimene (0.01%) were detected only in shaded plants (Table 4).
3.2.3. Oregano essential oil composition
In our research, the presence of as many as 111 components of oregano essential oils was detected. Oregano EOs consist of the following components: caryophyllene oxide (3.1–1.93%); germacrene D (1.17–2.0%) and (E)-caryophyllene (1.48–1.1%) Table 5 and Fig. 4.
Table 5.
Chemical composition of oregano essential oil.
| No. | tret, min | Compound | Molecular formula | RIexp | RIlit | Method of identification | Area% |
Content % |
||
|---|---|---|---|---|---|---|---|---|---|---|
| Unshade | Shade | Unshade | Shade | |||||||
| 1 | 7.10 | α-Thujene | C10H16 | 927 | 924 | RI, MS | – | 0.1 | – | 0.02 |
| 2 | 8.10 | Benzaldehyde | C7H6O | 960 | 952 | RI, MS | 0.1 | – | 0.01 | – |
| 3 | 8.52 | Sabinene | C10H16 | 974 | 969 | RI, MS | 1.3 | 4.5 | 0.24 | 0.71 |
| 4 | 8.60 | 1-Octen-3-ol* | C8H16O | 977 | 974 | RI, MS | 2.2 | 1.8 | 0.41 | 0.29 |
| 5 | 8.65 | β-Pinene* | C10H16 | 979 | 974 | RI, MS, Co-I | – | 0.2 | – | 0.03 |
| 6 | 8.86 | 3-Octanone | C8H16O | 986 | 979 | RI, MS | 0.2 | 0.2 | 0.04 | 0.03 |
| 7 | 9.02 | Myrcene | C10H16 | 991 | 988 | RI, MS | 0.2 | 0.6 | 0.04 | 0.09 |
| 8 | 9.14 | 3-Octanol | C8H18O | 995 | 988 | RI, MS | 0.3 | 0.2 | 0.06 | 0.03 |
| 9 | 9.75 | δ-3-Carene | C10H16 | 1012 | 1008 | RI, MS | 0.1 | 0.1 | 0.02 | 0.02 |
| 10 | 9.96 | α-Terpinene | C10H16 | 1018 | 1014 | RI, MS | – | 0.2 | – | 0.04 |
| 11 | 10.25 | p-Cymene | C10H14 | 1025 | 1020 | RI, MS | 1.4 | 2.3 | 0.27 | 0.36 |
| 12 | 10.40 | Limonene | C10H16 | 1029 | 1024 | RI, MS, Co-I | 0.4 | 0.6 | 0.01 | 0.01 |
| 13 | 10.52 | 1,8-Cineole | C10H18O | 1032 | 1026 | RI, MS, Co-I | 0.5 | 0.7 | 0.04 | 0.05 |
| 14 | 10.68 | (Z)-β-Ocimene | C10H16 | 1037 | 1032 | RI, MS | 1.1 | 2.8 | 0.21 | 0.44 |
| 15 | 10.93 | Benzene acetaldehyde | C8H8O | 1043 | 1036 | RI, MS | 0.2 | 0.3 | 0.04 | 0.04 |
| 16 | 11.07 | (E)-β-Ocimene | C10H16 | 1047 | 1044 | RI, MS | 0.4 | 1.1 | 0.07 | 0.17 |
| 17 | 11.52 | γ-Terpinene | C10H16 | 1059 | 1054 | RI, MS, Co-I | 0.1 | 1.4 | 0.01 | 0.21 |
| 18 | 11.85 | cis-Sabinene hydrate | C10H18O | 1068 | 1065 | RI, MS | 0.4 | 0.4 | 0.08 | 0.07 |
| 19 | 12.06 | cis-Linalool oxide (furanoid) | C10H18O2 | 1073 | 1067 | RI, MS | 0.1 | 0.1 | 0.02 | 0.02 |
| 20 | 12.26 | 1-Nonen-3-ol | C9H18O | 1079 | 1083c | RI, MS | 0.2 | 0.1 | 0.03 | 0.02 |
| 21 | 12.68 | Terpinolene | C10H16 | 1090 | 1086 | RI, MS | 0.1 | 0.2 | 0.02 | 0.04 |
| 22 | 12.99 | Rose furan | C10H14O | 1098 | 1093 | RI, MS | 0.2 | – | 0.03 | – |
| 23 | 13.10 | Linalool | C10H18O | 1102 | 1095 | RI, MS, Co-I | 2.8 | 2.9 | 0.16 | 0.11 |
| 24 | 13.58 | 1-Octen-3-yl acetate | C10H18O2 | 1112 | 1110 | RI, MS | 0.1 | 0.1 | 0.02 | 0.01 |
| 25 | 14.02 | cis-p-Menth-2-en-1-ol | C10H18O | 1123 | 1118 | RI, MS | 0.2 | 0.2 | 0.04 | 0.04 |
| 26 | 14.27 | trans-p-Mentha-2,8-dien-1-ol | C10H16O | 1129 | 1119 | RI, MS | 0.1 | 0.1 | 0.03 | 0.01 |
| 27 | 14.75 | (Z)-Myroxide | C10H16O | 1132 | 1131 | RI, MS | 0.4 | 0.4 | 0.08 | 0.07 |
| 28 | 14.99 | trans-Verbenol | C10H16O | 1146 | 1137 | RI, MS | 0.1 | 0.1 | 0.02 | 0.01 |
| 29 | 15.52 | Sabina ketone | C9H14O | 1159 | 1154 | RI, MS | 0.4 | 0.4 | 0.07 | 0.07 |
| 30 | 15.87 | Borneol | C10H18O | 1167 | 1165 | RI, MS | 1.0 | 0.2 | 0.13 | 0.03 |
| 31 | 15.95 | Viridene | C11H16 | 1169 | 1163 | RI, MS | – | 0.2 | – | 0.03 |
| 32 | 16.23 | Rosefuran epoxide | C10H14O2 | 1176 | 1173 | RI, MS | 0.1 | – | 0.02 | – |
| 33 | 16.37 | Terpinen-4-ol | C10H18O | 1179 | 1174 | RI, MS | 2.2 | 2.9 | 0.42 | 0.45 |
| 34 | 16.63 | Thuj-3-en-10-al | C10H14O | 1185 | 1181 | RI, MS | 0.4 | 0.3 | 0.06 | 0.05 |
| 35 | 16.74 | Cryptone | C9H14O | 1188 | 1183 | RI, MS | – | 0.1 | – | 0.01 |
| 36 | 16.91 | α-Terpineol | C10H18O | 1192 | 1186 | RI, MS, Co-I | 1.0 | 1.2 | 0.19 | 0.18 |
| 37 | 17.02 | Myrtenol | C10H16O | 1195 | 1194 | RI, MS | 0.2 | 0.3 | 0.04 | 0.04 |
| 38 | 17.16 | Myrtenal | C10H14O | 1198 | 1195 | RI, MS | 0.2 | 0.2 | 0.03 | 0.03 |
| 39 | 17.61 | γ-Terpineol | C10H18O | 1209 | 1199 | RI, MS | 0.2 | 0.2 | 0.04 | 0.03 |
| 40 | 17.96 | Coahuilensol, methyl ether | C10H12O | 1217 | 1219 | RI, MS | 0.1 | – | 0.01 | – |
| 41 | 18.07 | trans-Carveol | C10H18O | 1220 | 1215 | RI, MS | 0.1 | 0.1 | 0.01 | 0.01 |
| 42 | 18.25 | Dihydro myrcenol acetate | C12H22O2 | 1224 | 1214 | RI, MS | 0.1 | – | 0.02 | – |
| 43 | 18.46 | cis-Sabinene hydrate acetate | C12H20O2 | 1229 | 1219 | RI, MS | 0.2 | – | 0.02 | – |
| 44 | 18.68 | Citronellol | C10H20O | 1234 | 1223 | RI, MS | 0.1 | – | 0.01 | – |
| 45 | 18.74 | Thymol, methyl ether | C11H16O | 1235 | 1232 | RI, MS | 0.1 | – | 0.01 | – |
| 46 | 18.99 | Cumin aldehyde* | C10H12O | 1241 | 1238 | RI, MS | – | 0.4 | – | 0.07 |
| 47 | 19.04 | Neral* | C10H16O | 1241 | 1235 | RI, MS | 3.4 | – | 0.53 | – |
| 48 | 19.17 | Carvone | C10H14O | 1245 | 1239 | RI, MS | 0.1 | 0.2 | 0.03 | 0.03 |
| 49 | 19.57 | Geraniol | C10H18O | 1255 | 1249 | RI, MS | 0.4 | 0.1 | 0.06 | 0.02 |
| 50 | 20.29 | Geranial | C10H16O | 1272 | 1264 | RI, MS | 4.2 | 0.3 | 0.80 | 0.05 |
| 51 | 20.48 | dihydro-Linalool acetate | C12H22O2 | 1276 | 1272 | RI, MS | 0.1 | 0.1 | 0.01 | 0.01 |
| 52 | 20.71 | cis-Verbenyl acetate | C12H18O2 | 1282 | 1280 | RI, MS | – | 0.2 | – | 0.03 |
| 53 | 20.87 | α-Terpinen-7-al | C10H14O | 1286 | 1283 | RI, MS | 0.1 | 0.2 | 0.01 | 0.02 |
| 54 | 20.96 | Bornyl acetate | C12H20O2 | 1288 | 1287 | RI, MS | 0.1 | – | 0.01 | – |
| 55 | 21.09 | Dihydroedulan II* | C13H22O | 1291 | 1288b | RI, MS | 1.3 | 1.7 | 0.25 | 0.27 |
| 56 | 21.13 | Thymol* | C10H14O | 1292 | 1289 | RI, MS, Co-I | – | – | – | – |
| 57 | 21.55 | Carvacrol | C10H14O | 1302 | 1298 | RI, MS | 0.2 | 1.0 | 0.03 | 0.16 |
| 58 | 22.09 | (3E)-Hexenyl tiglate | C11H18O2 | 1315 | 1315 | RI, MS | 0.1 | – | 0.02 | – |
| 59 | 22.72 | p-Mentha-1,4-dien-7-ol | C10H16O | 1330 | 1325 | RI, MS | 0.1 | 0.6 | 0.02 | 0.09 |
| 60 | 24.32 | Piperitenone oxide | C10H14O2 | 1368 | 1366 | RI, MS | 1.9 | 0.1 | 0.35 | 0.01 |
| 61 | 24.51 | cis-Carvyl acetate | C12H18O2 | 1373 | 1365 | RI, MS | 0.1 | – | 0.02 | – |
| 62 | 24.74 | α-Copaene | C15H24 | 1379 | 1374 | RI, MS | 0.4 | 0.3 | 0.07 | 0.04 |
| 63 | 24.98 | Geranyl acetate | C12H20O2 | 1384 | 1379 | RI, MS | 0.3 | 0.1 | 0.04 | 0.01 |
| 64 | 25.14 | β-Bourbonene | C15H24 | 1388 | 1387 | RI, MS | 2.2 | 0.7 | 0.42 | 0.11 |
| 65 | 25.33 | β-Cubebene | C15H24 | 1393 | 1387 | RI, MS | 0.1 | – | 0.02 | – |
| 66 | 25.40 | β-Elemene | C15H24 | 1394 | 1389 | RI, MS | 0.2 | 0.2 | 0.04 | 0.03 |
| 67 | 25.65 | (Z)-Jasmone | C11H16O | 1400 | 1392 | RI, MS | 0.1 | – | 0.02 | – |
| 68 | 25.88 | Italicene | C15H24 | 1406 | 1405 | RI, MS | 0.2 | 0.1 | 0.02 | 0.02 |
| 69 | 26.58 | (E)-Caryophyllene | C15H24 | 1424 | 1417 | RI, MS | 7.8 | 7.1 | 1.48 | 1.1 |
| 70 | 26.91 | β-Copaene | C15H24 | 1432 | 1430 | RI, MS | 0.4 | 0.2 | 0.08 | 0.03 |
| 71 | 27.15 | α-trans-Bergamotene | C15H24 | 1438 | 1432 | RI, MS | 0.1 | 0.1 | 0.02 | 0.01 |
| 72 | 27.52 | Aromadendrene | C15H24 | 1447 | 1439 | RI, MS | 0.2 | – | 0.03 | – |
| 73 | 27.90 | α-Humulene | C15H24 | 1457 | 1452 | RI, MS | 1.1 | 1.0 | 0.21 | 0.16 |
| 74 | 28.19 | allo-Aromadendrene | C15H24 | 1464 | 1458 | RI, MS | 1.0 | 0.7 | 0.18 | 0.11 |
| 75 | 28.27 | cis-Cadina-1(6),4-diene | C15H24 | 1466 | 1461 | RI, MS | – | 0.1 | – | 0.02 |
| 76 | 28.41 | cis-Muurola-4(14),5-diene | C15H24 | 1470 | 1465 | RI, MS | 0.1 | – | 0.02 | – |
| 77 | 28.82 | γ-Muurolene | C15H24 | 1480 | 1478 | RI, MS | 0.3 | 0.3 | 0.05 | 0.04 |
| 78 | 29.03 | Germacrene D | C15H24 | 1485 | 1487 | RI, MS | 6.3 | 12.6 | 1.17 | 2.0 |
| 79 | 29.13 | (E)-β-Ionone | C13H20O | 1488 | 1487 | RI, MS | 0.2 | – | 0.04 | – |
| 80 | 29.29 | β-Selinene | C15H24 | 1492 | 1489 | RI, MS | 0.1 | 0.1 | 0.02 | 0.02 |
| 81 | 29.60 | Bicyclogermacrene | C15H24 | 1499 | 1500 | RI, MS | 0.6 | 3.2 | 0.12 | 0.50 |
| 82 | 29.72 | α-Muurolene | C15H24 | 1501 | 1500 | RI, MS | 0.3 | 0.3 | 0.05 | 0.05 |
| 83 | 29.99 | (E,E)-α-Farnesene | C15H24 | 1510 | 1505 | RI, MS | 1.1 | 2.1 | 0.21 | 0.33 |
| 84 | 30.27 | γ-Cadinene | C15H24 | 1509 | 1513 | RI, MS | 0.4 | 0.5 | 0.07 | 0.08 |
| 85 | 30.39 | endo-1-Bourbonanol | C15H26O | 1510 | 1518 | RI, MS | 0.2 | – | 0.03 | – |
| 86 | 30.51 | Myristicin | C11H12O3 | 1512 | 1517 | RI, MS | 0.2 | 0.1 | 0.03 | 0.01 |
| 87 | 30.60 | trans-Calamenene* | C15H22 | 1526 | 1521 | RI, MS | – | – | – | – |
| 88 | 30.62 | δ-Cadinene* | C15H24 | 1513 | 1522 | RI, MS | 1.3 | 1.7 | 0.24 | 0.26 |
| 89 | 31.17 | α-Cadinene | C15H24 | 1513 | 1522 | RI, MS | 0.1 | 0.1 | 0.02 | 0.02 |
| 90 | 31.37 | α-Colacorene | C15H20 | 1545 | 1544 | RI, MS | 0.1 | 0.1 | 0.02 | 0.02 |
| 91 | 31.80 | Salviadienol | C15H24O | 1557 | 1560d | RI, MS | 1.6 | 1.2 | 0.27 | 0.17 |
| 92 | 32.16 | β-Colacorene | C15H20 | 1567 | 1564 | RI, MS | 0.1 | – | 0.02 | – |
| 93 | 32.29 | 1,5-Epoxysalvial-4(14)-ene | C15H24O | 1570 | 1562b | RI, MS | 0.3 | 0.2 | 0.04 | 0.02 |
| 94 | 32.82 | Spathulenol | C15H24O | 1584 | 1577 | RI, MS | 6.8 | 7.6 | 1.28 | 1.19 |
| 95 | 33.04 | Caryophyllene oxide | C15H24O | 1586 | 1582 | RI, MS | 16.8 | 12.3 | 3.1 | 1.93 |
| 96 | 33.24 | Guaiol | C15H26O | 1595 | 1600 | RI, MS | 0.8 | 0.4 | 0.02 | 0.07 |
| 97 | 33.34 | Salvial-4(14)-en-1-one | C15H24O | 1598 | 1594 | RI, MS | – | 0.2 | – | 0.03 |
| 98 | 33.67 | Ledol | C15H26O | 1607 | 1602 | RI, MS | 0.4 | 0.3 | 0.06 | 0.05 |
| 99 | 33.92 | Humulene epoxide II | C15H24O | 1613 | 1608 | RI, MS | 2.2 | 2.0 | 0.42 | 0.31 |
| 100 | 34.09 | 1,10-di-epi-Cubenol | C15H26O | 1618 | 1618 | RI, MS | 0.2 | 0.2 | 0.04 | 0.03 |
| 101 | 34.57 | 1-epi-Cubenol | C15H26O | 1631 | 1627 | RI, MS | 0.3 | 0.3 | 0.02 | 0.03 |
| 102 | 35.07 | epi-α-Cadinol | C15H26O | 1645 | 1638 | RI, MS | 1.7 | 1.4 | 0.25 | 0.21 |
| 103 | 35.22 | α-Muurolol | C15H26O | 1644 | 1644 | RI, MS | 0.5 | 0.4 | 0.08 | 0.07 |
| 104 | 35.54 | α-Cadinol | C15H26O | 1658 | 1652 | RI, MS | 1.7 | 2.2 | 0.31 | 0.34 |
| 105 | 35.70 | cis-Calamenen-10-ol | C15H22O | 1663 | 1660 | RI, MS | 0.3 | – | 0.05 | – |
| 106 | 35.99 | trans-Calamenen-10-ol | C15H22O | 1670 | 1668 | RI, MS | 1.2 | – | 0.21 | – |
| 107 | 36.13 | 14-hydroxy-9-epi-(E)-Caryophyllene | C15H24O | 1674 | 1668 | RI, MS | 1.1 | 1.1 | 0.21 | 0.17 |
| 108 | 36.66 | Eudesma-4(15),7-dien-1β-ol (impure) | C15H24O | 1694 | 1687 | RI, MS | 0.7 | 0.5 | 0.13 | 0.08 |
| 109 | 36.83 | 5-neo-Cedranol | C15H26O | 1694 | 1684 | RI, MS | 0.6 | 0.8 | 0.10 | 0.13 |
| 110 | 39.44 | Methyl ester of 2,2,5,6-tetramethylbenzotetrahydrofuran-3-carboxylic acid | C14H18O3 | 1770 | – | MS | 0.4 | 0.4 | 0.06 | 0.06 |
| 111 | 42.04 | Hexahydrofarnesyl acetone (phytone) | C18H36O | 1847 | 1846e | RI, MS | 0.4 | 0.3 | 0.07 | 0.05 |
| Total identified (%) | 95.2 | 96.2 | ||||||||
| Grouped components (%) | ||||||||||
| Monoterpene hydrocarbons (1, 3, 5, 7, 9, 10, 12, 14, 16, 17, 21) | 3.7 | 11.8 | ||||||||
| Oxygen-containing monoterpenes (13, 18, 19, 23, 25–28, 30, 33, 34, 36–39, 41–44, 47–54, 59–61, 63) | 21.1 | 12.2 | ||||||||
| Sesquiterpene hydrocarbons (62, 64–66, 68–78, 80–84, 88, 89) | 24.3 | 31.4 | ||||||||
| Oxygen-containing sesquiterpenes (85, 91, 93–104, 107–109) | 35.9 | 31.1 | ||||||||
| Aromatic compounds (2, 11, 15, 40, 45, 46, 56, 57, 86, 87, 90, 92, 105, 106) | 4.0 | 4.2 | ||||||||
| Others (4, 6, 8, 20, 22, 24, 29, 31, 32, 35, 55, 58, 67, 79, 110, 111) | 6.2 | 5.5 | ||||||||
tret.: Retention time; RIlit-Retention indices from literature (Adams, 2007; bMilosevic et al., 2010, dĐorđević et al., 2011; eBalogun et al., 2017); RIexp: Experimentally determined retention indices using a homologous series of n-alkanes (C8-C20) on the HP-5MS column. MS: constituent identified by mass-spectra comparison; RI: constituent identified by retention index matching; Co-I: constituent identity confirmed by GC co-injection of an authentic sample; tr = trace amount (<0.05%); *co-eluting compounds. Compounds marked in italic are present only in sample from unshade plants Compounds marked in under line are present only in sample from shade plants.
Fig. 4.
Structures of the most abundant components identified in oregano essential oil.
Among monoterpenes, hydrocarbons ranged between 3.7 and 11.8% whereas oxygenated monoterpenes (i.e., monoterpenoids) showed a range of 12.2–21.1%. Total sesquiterpenes were always represented in the highest level (24.3–31.4%). Aromatic compound are present in quate small levels (4.0–4.2%). The other compounds always reached amounts lower than 1%.
Even though no literature data could be found to compare with our EO profile, the characterizing and new compounds of the species were also detected in our study. It is very interesting to point out that some components of essential oils are present only in plants that are shaded, while some others are present only in un-shaded plants that grow in full light.
It has been noticed that a number of components have only been registered in unshaded plants. Among these arebenzaldehyde (0.01%); rose furan (0.03%); rosefuran epoxide (0.02%); coahuilensol, methyl ether (0.01%); cis-sabinene hydrate acetate (0.02%); citronellol (0.01%); β-cubebene (0.02%); (Z)-jasmone (0.02%); aromadendrene (0.03%); cis-muurola-4(14),5-diene (0.02%); endo-1-bourbonanol (0.03%) and β-colacorene (0.02%).
Conversely, α-thujene (0.02%); α-terpinene (0.04%); viridene (0.03%); cryptone (0.01%); cis-verbenyl acetate (0.03%) and cis-cadina-1(6),4-diene (0.02%) were present only in shaded plants.
3.3. Antioxidant activity of thyme, marjoram, and oregano EOs
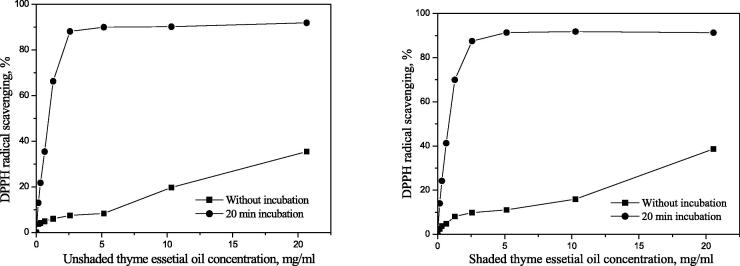
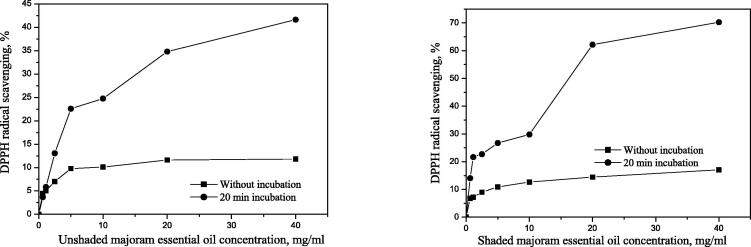
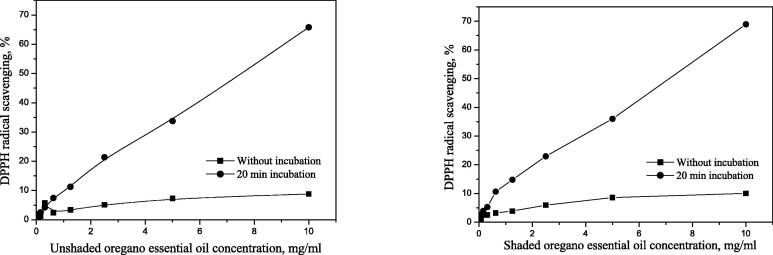
Efficient concentration – EC50 values of essential oil during 20 min incubation from plants covered by shade nets were: 0.852 mg mL−1, 19.97 mg mL−1, and 7.023 mg mL−1 of thyme, marjoram, and oregano essential oil, respectively. The efficient concentration (EC50) values from the unshaded plants were: 0.944, 54.01, and 7.450 mg mL−1, respectively (Table 2).
Medicinal plant (thyme, marjoram and oregano) essential oils samples from shaded plants showed higher antioxidant activity compared to the unshaded control plants. The results in Table 2 revealed that the highest antioxidant activity was seen in thyme EOs from shaded plants.
Comparing it with the activity after 20 min incubation with a radical (when compared all the samples), the activity decreases in the following order (the smaller the EC50 value, the better the antioxidant): shaded thyme (0.852) > unshaded thyme (0.944) > shaded oregano 7.023) > unshaded oregano (7.450) > shaded marjoram (19.97) > unshaded marjoram (54.01). Based on the results given in Table 6 the highest antioxidant activity was observed in a thyme EOs from plants covered by nets (Fig. 5). Marjoram and oregano essential oils samples from shaded plants showed higher antioxidant activity than the unshaded control plants (Fig. 6, Fig. 7).
Fig. 5.
Antioxidant activity of unshaded (A1) and shaded (Sample A2) thyme essential oil.
Fig. 6.
Antioxidant activity of unshaded (B1) and shaded (B2) marjoram essential oil.
Fig. 7.
Antioxidant activity of unshaded (C1) and shaded (C2) oregano essential oil.
4. Discussion
Thyme, marjoram, and oregano were cultivated by direct sowing to the open field, but different biotic and abiotic challenges during the summer (high temperatures, hail, wind, pathogens, pests, birds) forced producers to protect plants by covering and shading with nets. Unless additional protective measures are taken, high temperatures and solar radiation may affect crop growth and performance including the appearance of abiotic disorders, limited metabolism and productivity (Ilić and Fallik, 2017).
Net houses have the potential to create a appropriate microclimate that positively affects plants productivity and quality. Growth, development, and accumulation of the secondary metabolites of medicinal plants may be significantly influenced by genetic (internal) and environmental (external) factors. Light intensity play an important role in oil biosynthesis and accumulation. The effect of the light spectrum transmitted by shade nets confirms the effect of the accumulation of secondary metabolites of medicinal plants (Stagnari et al., 2018). Therefore, it is possible to alter the composition of the bioactive compound in basil and other plants, through the exposure of plants to a specific light spectrum (Hosseini et al., 2018, Milenković et al., 2019).
Light intensity can affect the EOs content, the EOs compounds, and the antioxidant activity of medicinal plants. The growing condition and cultivation methods during production of many herbs can improve the yield and quality of EOs more than gathering them from wild nature.
According to some literature data, the content of essential oil in dry herb of thyme ranges from 0.3% (Ozguven and Tansi, 1998) to 4.0% (Carlen et al., 2010). Results from our study are in agreement with Gavarić et al. (2015) who showed that the presence of essential oil in thyme leaves from Serbia also noticeably with a relatively low amount (0.8–2.6%).
Similarly to these results, also in our previous studies, we have observed significantly different yields of the EOs between shaded and unshaded plants. Thus, the lowest accumulation of essential oils in sweet basil was observed in the unshaded, control plants (1.02 mL/100 g of plant material) while the highest oil accumulation was achieved in plants from red shade nets (3.23 mL/100 g) (Milenković et al., 2019).
Method of production (open field or protected area) and environmental conditions greatly affects the quality and composition of the medicinal plants. Similarly to our research and in the results of other colleagues we can recognize the positive impact of shading on the content of EOs.
The fresh or dried highly aromatic leaves and flowering tops of marjoram are widely used to flavor many foods. Usually, marjoram contains 0.5–3.5% of essential oil in the dry herb (Tabanca et al., 2014). EO content in oregano of approximately 3.7% on dry weight obtained by Azizi et al. (2009), corresponding to approximately 1.52% on a fresh weight basis obtained by Tibaldi et al. (2011). The much higher EO content than our result (0.27–0.32%) could partially be due to the different cultural practices and environmental conditions, and also to the different distillation method and material used by Azizi et al. (2009).
At the same time, we can find in the literature a lower yield of oregano EOs than in our research. Thus, EO content (0.16%) of oregano plants grown in the pot exposed to full light was around 50% higher than the EO from the plants grown in the soil exposed with full light or the pot plants cover with 50% shade nets (Tibaldi et al., 2011).
Light conditions and solar radiation can affect the differently to the accumulation of essential oils EO and EO profile in plants. Thus, Li et al. (1996) found that Salvia officinalis L., grown in shading condition (shade index-55%), reached the highest total EO content (0.38%) compared to the plants grown in the open field with full light (0.34%) conditions. EO content decreased with shading intensity by 55% to 85% shade. Opposite, some herbs like Thymus vulgaris L. the maximum total EO content obtained at full light (0.49%).
Thyme EO contains at least 60 bioactive compounds with powerful antioxidant properties. The antioxidant properties of thyme extract from Spain has been analyzed by Rota et al. (2008), and they concluded that main phenolic compounds are thymol (68.1%), p-cymene (11.2%), γ-terpinene (4.8%), and carvacrol (3.5%). In Hungary, the dominant compounds also was thymol (32.2%). In the agro-climacteric condition of Serbia the main compound of essential oil in thyme were thymol (25–50%) and carvacrol (3–10%) (Gavarić et al., 2015).
Marjoram essential oil (MEO) is obtained by steam distillation of dry marjoram leaves which contain 0.7–3.5% essential oil (Kumar et al., 2011). Considerable variations in the content and compositional pattern of MEO are observed depending on the species, growth stages, the origin of herb, climatic and drying conditions (Baatour et al., 2012).
The characteristic compounds of the commercially exploited marjoram essential oils are terpinen-4-ol, α-terpinene, γ-terpinene, α-terpineol, and cis-sabinene hydrate, occurring in variable quantities. Marjoram also produces essential oils with different compositions, which are either rich in linalool or p-cymene and its biosynthetically related compounds thymol and carvacrol (Kokini et al., 2003).
It was postulated that the marjoram essential oils exist in two forms. In the first chemotype, terpinene-4-ol either alone or together with sabinene hydrate, α- terpineol, α- and γ-terpinene were found to be main constituents of the essential oils (Vera and Chane-Ming, 1999, Banchio et al., 2008), and the other chemotype with thymol and/or carvacrol as predominant compounds (Daferera et al., 2003). Marjoram volatile oil is rich in terpinen-4-ol, sabinene hydrate, γ-terpinene, p-cymene, α-terpinene, and α-terpineol (Lis et al., 2007). However, terpinene-4-ol alone or along with sabinene hydrate is responsible for the characteristic flavor and fragrance of marjoram oil (Vági et al., 2005). Spicy “marjoramy” aroma derived from compound like cis-sabinene hydrate (Raina and Negi, 2012).
The essential oil composition of marjoram showed terpinen-4-ol (31.15%), cis-sabinene hydrate (15.76%), p-cymene (6.83%), sabinene (6.91%), trans-sabinene hydrate (3.86%), and α-terpineol (3.71%) as the main constituents. Precursors of phenolic components like p-cymene and γ-terpinene were much higher in O. vulgare compared to O. majorana, whereas sabinene, cis- and trans-sabinene hydrate, and α-terpineole were much higher in O. majorana. The amounts of oxygenated monoterpenes were higher in O. majorana (Raina and Negi, 2012).
Egyptian marjoram oil belonged to terpinen-4-ol /sabinene-hydrate chemotype. Studies also indicated that the oil was dominated by monoterpene-hydrocarbons (75.79%), followed by oxygenated-monoterpenes (21.50%) and sesquiterpene-hydrocarbons (2.34%), (Badee et al., 2013). Terpinen-4-ol (29.13–32.57%), cis-sabinene hydrate (19.9–29.27%) and trans-sabinene hydrate (3.5–11.61%) were the main components of this fraction in O. majorana essential oils harvested in Tunisia, (Sellami et al., 2009) whereas 1,8-cineole (58.59 ± 0.85%), linalool (13.05 ± 0.04%) and α-terpineol (3.33 ± 0.10%) were the main compounds in commercial natural marjoram analyzed in Spain (Ibáñez and Blázquez, 2017). The main components of the essential oil from marjoram collected from Greece were terpinen-4-ol (37.1%), p-cymene (12.05%), α-terpineol (7.15%), carvacrol (3.60%), trans-sabinene hydrate (2.41%), cis-sabinene hydrate (1.43%) and thymol (0.7%), (Komaitis, 1992).
Our marjoram essential oil composition was found to be close to that reported by Badee et al. (2013) except for some minor variations. The oil quality was close to the one produced in Europe and southern India.
Diverse concentrations of the main constituents are reported in oregano essential oils (OEOs) from different Origanum variety, geographic region, and origin, environmental conditions, harvest time, etc. The carvacrol content of different chemotypes of O. vulgare is variable and it can be up to 95% (Gounaris et al., 2002). Thymol (36.91–60.14%), γ-terpinene (11.59–24.14%), and p-cymene (2.56–9.38%) were the major components in all OEOs (Napoli et al., 2020).
The effect of light intensity through alteration in photosynthesis, physiological, and morphological processes of plants and methods of production in oregano plants affect essential oil constituents. The soil full-light treatment plant gave the essential oil mainly composed of 4-terpineol, γ-terpinene, carvacrol, and p-cymene. The pot 50%-shade treatment of the plant gave the essential oil mainly composed of γ-terpinene, 4-terpineol, carvacrol and p-cymene (Tibaldi et al., 2011).
Origanum species shared a similar aroma profile, described as spicy, phenolic and minty, as many of them contain thymol and carvacrol in varying amounts (Meyers, 2005).
Phenolic compounds (thymol and carvacrol) and their biogenetic precursors γ-terpinene and p-cymene are the main compounds in oregano essential oils, but with great variability in the percentage depending on the geographical origin. The Origanum species from Saudi and Jordanian indicate that the cymyl chemotype should predominate from these regions. Saudi Origanum contain carvacrol as the major component (79.5%-71.9%) while Jordanian Origanum contain thymol (68.7%) as the main constituents (Khan et al., 2018). High content of carvacrol and thymol has been determined also for oregano EO from Italy (De Martino et al., 2009) while, Armenian oregano consisted mainly of sesqui- and monoterpenes (β-caryophyllene epoxide − 13.3%; β-caryophyllene − 8.2%; ο-cymene − 5.2%), (Moghrovyanet al., 2019).
In certain regions of India, O. vulgare produces an essential oil rich in p-cymene (6.7–9.8%), γ-terpinene (12.4–14.0%), thymol (29.7–35.1%), and carvacrol (12.4–20.9%) (Pande et al., 2012), whereas Turkish oregano essential oil together with the phenolic compounds thymol (15.66%) and carvacrol (24.52%) contain high amounts of linalool (50.53%) (Ozkan & Erdoğan, 2011). Carvacrol (72.06%) and thymol (4.98%) were the major oil constituents in the EO of O. vulgare from Serbia too, followed by trans‐caryophyllene (3.61%) and p‐cymene (2.08%) (Karaman et al., 2017).
Many herbs like thyme, sweet marjoram, oregano, and their extracts have been added to a variety of foods to improve their sensory characteristics and extend shelf-life (Burt, 2004). In recent years, the use of natural plant preservatives to increase the shelf-life of food products is promising technology since they derived substances having antioxidant and antimicrobial properties.
In our research thyme EO was reported to be the best antioxidant in a comparison of the antioxidant activity with other plants from Fam. Lamiaceae in the following order: thyme > oregano > marjoram. Our results are consistent with research of Roby et al. (2013).
Thyme, compared to other medicinal plant species, has better antioxidant properties of volatile oils with an inhibitory effect similar to the effect of α-tocopherol or BHT (butylhydroxytoluene), (Lee and Shibamoto, 2002).
Thymol and carvacrol, are main constituents of the thyme and oregano essential oil with antioxidant activity (Rodriguez-Garcia et al., 2016). Several Thymus species were found rich in both carvacrol and thymol and observed to have a high antioxidant activity including Thymus vulgaris L. (Alsaraf et al., 2020). The different position of the phenolic group in thymol relative to that in carvacrol makes it most likely that thymol is a better antioxidant and more efficient in oxidizing lipids at room temperature (Yanishlieva et al.,1999).
Shade nets provide greater presence and biosynthesis of polyphenolic compounds known to exhibit antioxidant properties (Milenković et al., 2019).
Thyme essential oil, thymol and carvacrol, are generally recognized as safe (GRAS status) and have been registered by the European Commission for use as flavoring agents in foods (FAD, 2010). These compounds are also potent antioxidants, EOs could be directly used in food products with some novel applications such as encapsulation, edible films, and edible coatings (Mutlu-Ingok et al., 2020).
Essential oils, especially from thyme and oregano are a potential source of natural antioxidants, as a possible alternative to synthetic antioxidants in food products and can prevent their oxidative deterioration. Most of these phytochemicals from the mentioned medicinal plants should soon be included in the regular dietary procedure and side effects on human health that can be caused by classic drugs and antibiotics should be avoided (Roychoudhury and Bhowmik, 2020). More abundant use of natural antiviral bioactive supplements in the form of hot drinks like tea, can help strengthen the immune system in protection against the current COVID-19 pandemic, as well as against possible newer viruses (Roychoudhury and Bhowmik, 2020).
5. Conclusion
Our data show that shade nets can be incorporated into the protected cultivation practices currently used for producing of medicinal plants. All three plant species under shade nets increase the yield of essential oils, but thyme and marjoram resulting in statisticaly higher levels of essential oil yield. In the light of this investigation, it is evident that the modification of ligh intensity can act as a physiological tool via the shade nets to improve the phytochemical quality and antioxidant activity of these plants. Our observation confirms that the antioxidant scavenging activity of different herbs in this study depends on the light modification to a greater and lesser level depending on the plant species. Among the three plant species investigated in this work, thyme plants are characterized by the highest level of antioxidant activity. Marjoram and oregano tolerate shading well, so it is recommended to grow them under shading nets.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
The authors extend their appreciation through the project number: TR-31027, TR-34012 (Program for financing scientific research work, number 451-03-9/2021-14/200133) was financially supported by the Ministry of Education Science and Technological Development of the Republic of Serbia.
Author contributions
Z.S.I., and Lj. S.Head of the research group planned the research, analyzed, and wrote the manuscript; L.M. N.T. and L.S conducted the experiment in the field; and J.C. and D.C. performed analyses of physical properties and chemical composition in the laboratory. All authors have read and agree to the published version of the manuscript.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adams R.P. fourth ed. Allured Publishing Corporation; Illinois: 2007. Identifcation of essential oil components by gass chromatography/ mass spectrometry. [Google Scholar]
- Alsaraf S., Hadi Z., Al-Lawati W.M., Al Lawati A.A., Khan S.A. Chemical composition, in vitro antibacterial and antioxidant potential of Omani Thyme essential oil along with in silico studies of its major constituent. J. King Saud Univ. Sci. 2020;32(1):1021–1028. [Google Scholar]
- Azizi, A., Yan, F., Honermeier, B., 2009. Herbage yield, essential oil content and composition of three oregano (Origanum vulgare L.) populations as affected by soil moisture regimes and nitrogen supply. Ind. Crops Prod. 29, 554-561.
- Badee A.Z.M., Moawad R.K., El Noketi M.M., Gouda M.M. Antioxidant and antimicrobial activities of marjoram (Origanum majorana L.) essential oil. J. App Sci. Res. 2013;9(2):1193–1201. [Google Scholar]
- Baatour O., Tarchoune I., Mahmoud H., Nassr N.W., Kaddour R., Hamdaou G., Ayachi M.B.N., Ben Nasri M., Lachaal M., Marzouk B. Culture conditions and salt effects on essential oil composition of sweet marjoram (Origanum majorana) from Tunisia. Acta Pharm. 2012;62:251–261. doi: 10.2478/v10007-012-0019-9. [DOI] [PubMed] [Google Scholar]
- Banchio E., Bogino P.C., Zygadlo J., Giordano W. Plant growth promoting Rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem. Syst. Ecol. 2008;36(10):766–771. [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in food-A review. Inter. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Buthelezi M.N.D., Soundy P., Jifon J., Sivakumar D. Spectral quality of photoselective nets improves phytochemicals and aroma volatiles in coriander leaves (Coriandrum sativum L.) after postharvest storage. J. Photoch. Photob. B: Biol. 2016;161:328–334. doi: 10.1016/j.jphotobiol.2016.05.032. [DOI] [PubMed] [Google Scholar]
- Carlen C., Schaller M., Carron C.A., Vouillamoz J.F., Baroffio C.A. The new Thymus vulgaris L. hybrid cultivar (Varico 3) compared to five established cultivars from Germany, France and Switzerland. Acta Hort. 2010;(860):161–166. doi: 10.17660/ActaHortic.2010.860.23. [DOI] [Google Scholar]
- Carvalho S.D., Schwieterman M.L., Abrahan C.E., Colquhoun T.A., Folta K.M. Light quality dependent changes in morphology, antioxidant capacity, and volatile production in sweet basil (Ocimum basilicum) Front. Plant Sci. 2016;7:1328. doi: 10.3389/fpls.2016.01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A.G., Chagas J.H., Pinto J.E.B.P., Bertolucci S.K.V. Crescimento vegetativo e produção de óleo essencial de hortelãpimenta cultivada sob malhas. Pesq Agrop. Brasil. 2012;47(4):534–540. [Google Scholar]
- Daferera D.J., Ziogas B.N., Polissiou M.G. The effectiveness of plant essential oils on the growth of Botrytis cinerea, Fusarium sp. and Clavibacter michiganensis subsp. michiganensis. Crop Prot. 2003;22(1):39–44. [Google Scholar]
- De Falco E., Mancini E., Roscigno G., Mignola E., Taglialatela-Scafati O., Senatore F. Chemical composition and biological activity of essential oils of Origanum vulgare subsp. vulgare L under different growth conditions. Molecules. 2013;18(12):14948–14960. doi: 10.3390/molecules181214948. PMID: 24304588; PMCID: PMC6270476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino L., De Feo V., Formisano C., Mignola E., Senatore F. Chemical composition and antimicrobial activity of the essential oils from three chemotypes of Origanum vulgare L. ssp. hirtum (Link) letswaart growing wild in Campania (Southern Italy) Molecules. 2009;14(8):2735–2746. doi: 10.3390/molecules14082735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAD - Food additives European Commission for use as flavouring agents in foods, 2010. Europa Food Safety.
- Gavarić, N., Kovač, J., Kretschmer, N., Kladar, N., Smole Možina, S., Bucar, F., Bauer, R., Božin, B., 2015. Natural products as antibacterial agents - antibacterial potential and safety of post-distillation and waste material from Thymus vulgaris L., Lamiaceae concepts, compounds and the alternatives of antibacterials. Varaprasad Bobbarala, Intech Open, pp. 123–151. doi: 10.5772/60869.
- Gounaris Y., Skoula M., Fournaraki C., Drakakaki G., Makris A. Comparison of essential oils and genetic relationship of Origanum × intercedens to its parental taxa in the island of Crete. Bioch. System Ecol. 2002;30(3):249–258. [Google Scholar]
- Hosseini A., Mehrjerdi M.Z., Aliniaeifard S. Alteration of bioactive compounds in two varieties 368 of basil (Ocimum basilicum L.) grown under different light spectra. J Essen Oil Bear Plants. 2018;21:913–923. [Google Scholar]
- Ilić S.Z., Fallik E. Light quality manipulation improve vegetables quality at harvest and postharvest: a review. Environ Exper Bot. 2017;139:79–90. [Google Scholar]
- Ilić S.Z., Milenković L., Stanojević L., Šunić L., Lalević D., Savić N., Cvetković D., Kevrešan Ž., Mastilović J. Total phenolic, flavonoid contents and antioxidant activity in herb liquers from medical plants. J. Anim. Plant Sci. 2021 in press. [Google Scholar]
- Ibáñez M.D., Blázquez M.A. Herbicidal value of essential oils from oregano-like flavour species. Food Agric. Immun. 2017;28(6):1168–1180. [Google Scholar]
- Karaman M., Bogavac M., Radovanović B., Sudji J., Tešanović K., Janjušević L. Origanum vulgare essential oil affects pathogens causing vaginal infections. J. App. Microbiol. 2017;122(5):1177–1185. doi: 10.1111/jam.13413. [DOI] [PubMed] [Google Scholar]
- Khan M., Khan S.T., Khan N.A., Mahmood A., Al-Kedhairy A.A., Alkhathlan H.Z. The composition of the essential oil and aqueous distillate of Origanum vulgare L. growing in Saudi Arabia and evaluation of their antibacterial activity. Arab. J. Chem. 2018;11(8):1189–1200. [Google Scholar]
- Komaitis, M.E., Ifanti-Papatragianni N., Melissari-Panagiotou, E., 1992. Composition of the essential oil of marjoram (Origanum majorana L.). Food Chem. 45,(2),117-118.
- Kumar B.V.N., Rupesh K.M., Tamizhmani T., Fasalu R.O.M., Mohamed N.K. Marjoram hortensis (M.): a review. Pharma Sci. Mon. Inter J Pharmac Sci. 2011;2(4):59–74. [Google Scholar]
- Kokkini, R., Karousou, E., Hanlidou, 2003. Herbs. Herbs of the Labiatae. In: Benjamin Caballero (Ed.), Encyclopedia of Food Sciences and Nutrition, second ed., Academic Press, 2003, pp. 3082–3090.
- Liu Q.C., Qiao K., Zhang S.A. Potential of a small molecule carvacrol in management of vegetable diseases. Molecules. 2019;24(10):1932. doi: 10.3390/molecules24101932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Craker L.E., Potter T. Effect of light level on the essential oil production of sage (Salvia officinalis) and thyme (Thymus vulgaris) Acta Hortic. 1996;426:419–426. [Google Scholar]
- Lis A., Piter S., Gora J. A comparative study on the content and chemical composition of essential oils in commercial aromatic seasonings. Herba Polon. 2007;53(1):21–26. [Google Scholar]
- Leyva-López N., Gutiérrez-Grijalva E.P., Vazquez-Olivo G., Heredia J.B. Essential oils of oregano: biological activity beyond their antimicrobial properties. Molecules. 2017;22(6):989. doi: 10.3390/molecules22060989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.-G., Shibamoto T. Determination of antioxidant potential of volatile extracts isolated from various herbs and spices. J. Agric. Food Chem. 2002;50(17):4947–4952. doi: 10.1021/jf0255681. [DOI] [PubMed] [Google Scholar]
- Martins J.R., Alvarenga A.A., Castro E.M., Pinto J.E.B.P., Silva A.P.O. Avaliação do crescimento e do teor de óleo essencial em plantas de Ocimum gratissimum L. cultivadas sob malhas coloridas. Rev. Brasil Plant Medic. 2008;10:102–107. [Google Scholar]
- Mimica-Dukic N., Bozin B., Sokovic M., Simin N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 2004;52(9):2485–2489. doi: 10.1021/jf030698a. [DOI] [PubMed] [Google Scholar]
- Milenković L., Stanojević J., Cvetković D., Stanojević L., Lalević D., Šunić L., Fallik E., Ilić Z.S. New technology in basil production with high essential oil yield and quality. Ind. Crops Prod. 2019;140:111718. doi: 10.1016/j.indcrop.2019.111718. [DOI] [Google Scholar]
- Meyers, M. 2005. Oregano and Marjoram: An Herb Society of America Guide to the Genus Origanum- The Herb Society of America: Kirtland, OH, USA.
- Moghrovyan A., Sahakyan N., Babayan A., Chichoyan N., Petrosyan M., Trchounian A. Essential oil and ethanol extract of oregano (Origanum vulgare L.) from Armenian flora as a natural source of terpenes, flavonoids and other phytochemicals with antiradical, antioxidant, metal chelating, tyrosinase inhibitory and antibacterial activity. Curr. Pharm. Design. 2019;25(16):1809–1816. doi: 10.2174/1381612825666190702095612. [DOI] [PubMed] [Google Scholar]
- Murillo-Amador B., Nieto-Garibay A., López-Aguilar R., Troyo-Diéguez E., Rueda-Puente E.O., Flores-Hernández A., Ruiz-Espinoza F.H. Physiological, morphometric characteristics and yield of Origanum vulgare L. and Thymus vulgaris L. exposed to open-field and shade-enclosure. Ind. Crop. Prod. 2013;49:659–667. [Google Scholar]
- Mutlu-Ingok A., Devecioglu D., Dikmetas D.N., Karbancioglu-Guler F., Capanoglu E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: an updated review. Molecules. 2020;25(20):4711. doi: 10.3390/molecules25204711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli E., Giovino A., Carrubba A., How Yuen Siong V., Rinoldo C., Nina O., Ruberto G. Variations of essential oil constituents in oregano (Origanum vulgare subsp. viridulum (O. heracleoticum) over cultivation cycles. Plants. 2020;9(9):1174. doi: 10.3390/plants9091174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto G.A. Review on applications and uses of Thymus in the food industry. Plants. 2020;9(8):961. doi: 10.3390/plants9080961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira G.C., Vieira W.L., Bertolli S.C., Pacheco A.C. Photosynthetic behavior, growth and essential oil production of Melissa officinalis L. cultivated under colored shade nets. Chilean J. Agric Res. 2016;76(1):123–128. [Google Scholar]
- Ozguven M., Tansi S. Drug yield and essential oil of Thymus vulgaris L. as in influenced by ecological and ontogenetical variation. Tur. J. Agric. Forest. 1998;22:537–542. [Google Scholar]
- Ozkan A., Erdoğan A. A comparative evaluation of antioxidant and anticancer activity of essential oil from Origanum onites (Lamiaceae) and its two major phenolic components. Turk. J Biol. 2011;35:735–742. [Google Scholar]
- Pande C., Tewari G., Singh S., Singh C. Chemical markers in Origanum vulgare L. from Kumaon Himalayas: a chemosystematic study. Nat. Prod Res. 2012;26(2):140–145. doi: 10.1080/14786419.2010.535150. [DOI] [PubMed] [Google Scholar]
- Raina A.P., Negi K.S. Essential oil composition of Origanum majorana and Origanum vulgare ssp. hirtum growing in India. Xимиp пpиpниx coeдин. 2012;47(6):1015–1017. [Google Scholar]
- Roby M.H.H., Sarhan M.A., Selim K.A.H., Khalel K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013;43:827–831. [Google Scholar]
- Rodriguez-Garcia, I., Silva-Espinoza, B.A., Ortega-Ramirez, L.A., Leyva, J.M., Siddiqui, M.W., Cruz-Valenzuela, M.R., Gonzalez-Aguilar, G.A., Ayala-Zavala, J.F., 2016. Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit. Rev. Food Sci. Nutr. 56, 1717–1727. [DOI] [PubMed]
- Rota M.C., Herrera A., Martínez R.M., Sotomayor J.A., Jordán M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control. 2008;19(7):681–687. [Google Scholar]
- Roychoudhury A., Bhowmik R. Health benefits of plant derived bioactive secondary metabolites as dietary constituents. SF J. Clin. Pharm. Res. 2020;2(1):1002. [Google Scholar]
- Sellami I.H., Maamouri E., Chahed T., Wannes W.A., Kchouk M.E., Marzouk B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.) Indus. Crops Prod. 2009;30(3):395–402. [Google Scholar]
- Sparkman, D.O., 2011. In: Penton, Z.E., Fulton K.G., (Eds.), Gas chromatography and mass spectrometry: a practical guide, second ed. Elsevier Inc., Oxford, USA.
- Stanojevic J., Stanojevic L., Cvetkovic D., Danilovic B. Chemical composition, antioxidant and antimicrobial activity of the turmeric essential oil (Curcuma longa L.) Adv. Technol. 2015;4(2):19–25. [Google Scholar]
- Stagnari F., Di Mattia C., Galieni A., Santarelli V., D'Egidio S., Pagnani G., Pisante M. Light quantity and quality supplies sharply affect growth, morphological, physiological and quality traits of basil. Ind. Crops Prod. 2018;122:277–289. [Google Scholar]
- Tabanca N., Ozek T., Husnu Can Baser K., Tümen G. Comparison of the essential oils of Origanum majorana L. and Origanum x majoricum Cambess. J. Essen Oil Res. 2014;16(3):248–252. [Google Scholar]
- Tibaldi Giorgio, Fontana Emanuela, Nicola Silvana. Growing conditions and postharvest management can affect the essential oil of Origanum vulgare L. ssp. hirtum (Link) Ietswaart. Ind. Crops Prod. 2011;34(3):1516–1522. [Google Scholar]
- Vera R.R., Chane-Ming J. Chemical composition of the essential oil of marjoram (Origanum majorana L.) from Reunion Island. Food Chem. 1999;66(2):143–145. [Google Scholar]
- Vági E., Simándi B., Suhajda Á., Héthelyi É. Essential oil composition and antimicrobial activity of Origanum majorana L. extracts obtained with ethyl alcohol and supercritical carbon dioxide. Food Res. Int. 2005;38(1):51–57. [Google Scholar]
- Wiese N., Fischer J., Heidler J., Lewkowski O., Degenhardt J., Erler S. The terpenes of leaves, pollen, and nectar of thyme (Thymus vulgaris) inhibit growth of bee disease-associated microbes. Sci. Rep. 2018;8:14634. doi: 10.1038/s41598-018-32849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanishlieva N.V., Marinova E.M., Gordon M.H., Raneva V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999;64:59–66. [Google Scholar]