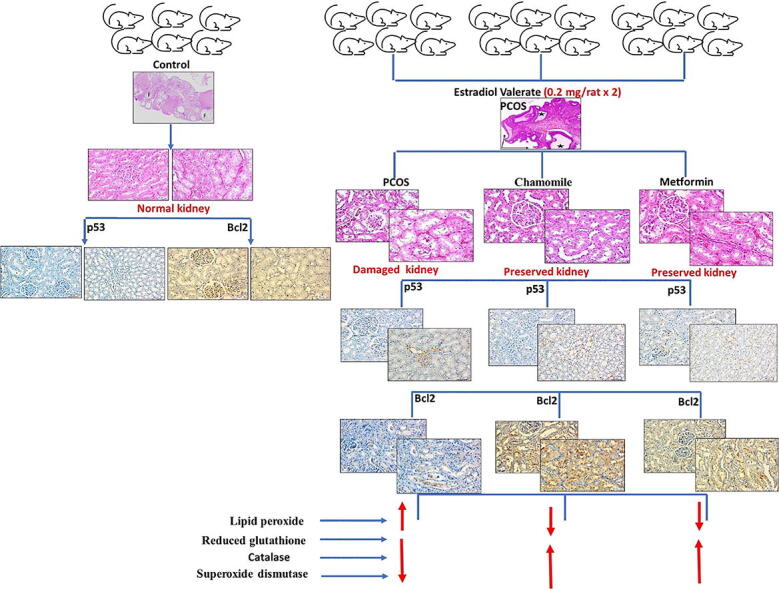
Graphical abstract
Keywords: PCOS, Kidney function, Apoptosis, Oxidative stress, Chamomile, Metformin
Abstract
Polycystic ovary syndrome (PCOS) is the most prevalent endocrine disorder in females of childbearing age and research findings have revealed a potential association between PCOS and renal dysfunction. This study aimed to investigate renal dysfunction that might be associated with PCOS in rats and to evaluate the potential protective effect of chamomile against PCOS complicated by kidney damage. A rat model of PCOS was induced by injecting estradiol valerate (0.2 mg/rat × 2) into adult virgin female rats. Rats were treated with either ethyl alcohol extract of chamomile flower (75 mg/kg/day) or metformin (Met) (500 mg/kg/day). Induction of PCOS was associated with increased relative right kidney weight percentage and increased serum levels of urea, lipid peroxide product, and testosterone. PCOS was also associated with increased p53 expression in kidney glomeruli and medullary tubules with decreased Bcl2 expression in kidney glomeruli. Administration of chamomile extract significantly decreased levels of serum urea, testosterone, and lipid peroxide product, and p53 expression in kidney glomeruli and tubules. The extract significantly increased levels of antioxidant markers levels (reduced glutathione, catalase, and superoxide dismutase) and the expression of the anti-apoptotic gene Bcl2. Conversely, administration of Met did not improve serum levels of urea. Met also exerted no pronounced effect on p53 gene expression. The results of this study highlight the importance of monitoring kidney function in patients with PCOS and investigating the associated underlying mechanism. Chamomile extract was found to ameliorate kidney damage associated with PCOS through antioxidant, testosterone-lowering, and anti-apoptotic mechanisms.
1. Introduction
Polycystic ovary syndrome (PCOS) is the most prevalent type of endocrine disorders in females of childbearing age. Globally, PCOS affects up to 20% of females of reproductive age (Azziz et al., 2016, Cooney and Dokras, 2018, Li et al., 2013, Lizneva et al., 2016, Teede et al., 2010). It has a drastic impact on reproductive capacity and sometimes results in female’s infertility. PCOS is characterized by oligomenorrhea and sometimes amenorrhea, oligoovulation, or anovulation as well as excess androgen production (dos Reis and Honorato-Sampaio, 2018, Showell et al., 2018, Wang et al., 2018). Although PCOS is distinguished by ovarian dysfunction and infertility, it is a metabolic disorder also accompanied by many systemic complications (Behboudi-Gandevani et al., 2018). PCOS has shown strong association with obesity, hyperlipidemia, fatty liver, type 2 diabetes, hypertension, anxiety, depression (Cooney et al., 2017), obstructive sleep apnea, endometrial cancer, and cardiovascular diseases (Barry et al., 2014, Behboudi-Gandevani et al., 2018, Cooney et al., 2017, Cooney and Dokras, 2018, Jakubowicz et al., 2013, Kahal et al., 2017, Teede et al., 2010, Yang et al., 2016).
Chronic kidney disease (CKD) is a progressive reduction of renal capacity over time. Without early prevention, management, and treatment, CKD may advance into uremia, the end-stage kidney failure with a dramatically increased death rate (Nigam and Bush, 2019). CKD mortality rates are strongly correlated with metabolic syndrome features, particularly obesity, diabetes, and cardiovascular disorders (Castro and Coresh, 2009, Chadban et al., 2010, Piccoli et al., 2018). Interestingly, most of these metabolic conditions seem to commonly occur alongside PCOS, suggesting an association between PCOS and renal damage. Research has shown a potential association between PCOS and renal dysfunction; in previous studies, more than 50% of patients with PCOS exhibited premicroalbuminuria linked to metabolic syndrome (Caglar et al., 2011, Patel et al., 2008, Ziaee et al., 2013). Additionally, there is a significant possibility of age-linked CKD in PCOS-induced experimental animals (Patil et al., 2017).
Hypothyroidism (HT) is a medical condition identified by thyroid hormone depletion that leads to impaired metabolism (Nagarajappa et al., 2014). Our research group recently reported an association of HT with PCOS in rats (Alahmadi et al., 2020, Alzahrani et al., 2019). HT involves multiple biochemical irregularities, including elevated serum creatinine and uric acid concentrations (Khan and Majumder, 2013, Mohamedali et al., 2014).
Matricaria chamomilla L. (family Asteraceae), commonly known as chamomile is among the most popular herbal medicines in Southern and Eastern Europe (Singh et al., 2011). The extract of chamomile flowers has been reported to ameliorate ovarian histological alteration in rats with PCOS and to improve thyroid function (Alahmadi et al., 2020, Farideh et al., 2010). This study aimed to investigate the renal dysfunction that might be associated with PCOS in rat and to examine the potential protective effect of chamomile against PCOS associated kidney damage in rats.
2. Materials and methods
2.1. Animals and chemicals
Estradiol valerate (EV) (Abcam Inc, USA), metformin (Met) (Sigma-Aldrich Co, USA), and chamomile flowers (World of Herbs, Egypt) were used in this study. The crushed chamomile flowers were repeatedly extracted with ethyl alcohol (70%), and the extract was dried under a vacuum to produce chamomile extract powder. This study also utilized 24 mature, virgin, female Wistar rats purchased from King Fahad Research Centre, King Abdulaziz University, Jeddah, Saudi Arabia. The body weight of rats was 217.92 ± 12.57 g. After a one-week adaptation period, the experiment was performed under standard temperature and moisture conditions and a 12:12 h light/dark cycle. No limitations were applied to the rats with regard to water and food. The study’s protocol was accepted by the Biomedical Ethics Research Council, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia (168–19).
2.2. Study design
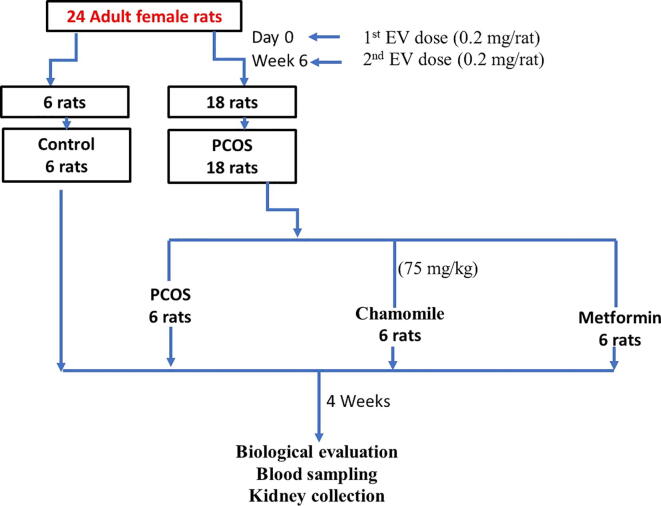
The rats were equally divided into 4 groups (n = 6 for each). Group 1 contained control rats; Group 2 contained PCOS-induced rats; Group 3 contained PCOS-induced rats treated with chamomile flower extract; and Group 4 contained PCOS-induced rats treated with metformin (Met). PCOS was generated by injecting 2 doses of EV (0.2 mg × 2) 6 weeks apart. This design was formally documented by Farideh et al. (2010) and clearly described in a previous study performed to produce PCOS associated with hypothyroidism (Alahmadi et al., 2020, Alzahrani et al., 2019). Rats treated with chamomile flower extract received 75 mg/kg/day, and rats treated with Met received 500 mg/kg/day. Treatments were given daily and continued for one month after induction of PCOS (Alahmadi et al., 2020) (Fig. 1).
Fig. 1.
Flowchart demonstrating the study methodology.
2.3. Calculation of % body weight change
The initial and final body weights were recorded for each rat. The percent change in body weight was calculated using the following equation:
2.4. Collection of serum and kidney samples
Blood samples were obtained by heart puncture. The serum was then isolated and held frozen at − 80 °C for assessment of kidney function, oxidative stress (OS), and antioxidant indicators. The ovaries and both right and left kidneys were then extracted and kept in 10% buffered formalin to confirm PCOS development and assess the histopathological alterations and immunohistochemical expressions of apoptotic/anti-apoptotic markers in the kidneys.
2.5. Calculation of relative kidney weight %
The final kidney weight (for both right and left kidneys) was recorded for each rat. The relative kidney weight % was calculated using the following equation:
2.6. Determination of kidney function markers
Serum creatinine, urea, and blood urea nitrogen (BUN) levels were determined using detection kits of Spectrum Diagnostics, Cairo, Egypt.
2.7. Histopathological investigation
Formalin-fixed ovary and kidney specimens were processed and embedded in paraffin, cut into 4 μm slices, hematoxylin and eosin (H&E)-stained, examined, and photographed under a light microscopy. Histological processing was done according to the method previously documented by Suvarna et al. (2019).
2.8. Determination of serum testosterone concentration
Serum testosterone concentration was measured using a testosterone ELISA kit (ab 108666), (Abcam, USA).
2.9. Determination of serum oxidative stress markers
Serum concentration of lipid peroxide product, reduced glutathione, catalase, and superoxide dismutase were measured using detection kits of Bioiagnostic, Giza, Egypt.
2.10. Immunohistochemistry investigation of apoptotic markers
An immunoperoxidase (peroxidase-anti-peroxidase) protocol was utilized to stain the kidney segments for p53 and Bcl2 genes. The antibodies (Lab Vision, Fremont, CA) were diluted 1:200. The segments were analyzed and photographed under light microscope.
2.11. Statistical analysis of data
Data were analyzed using analysis of variance (ANOVA) test followed by Tukey's multiple comparison test. Box plots were constructed using GraphPad Prism version 5. A p-value of ≤ 0.05 was considered to indicate statistical significance. A p-value of ≤ 0.05 was considered to indicate statistical significance.
3. Results
3.1. Histological evidence of PCOS induction
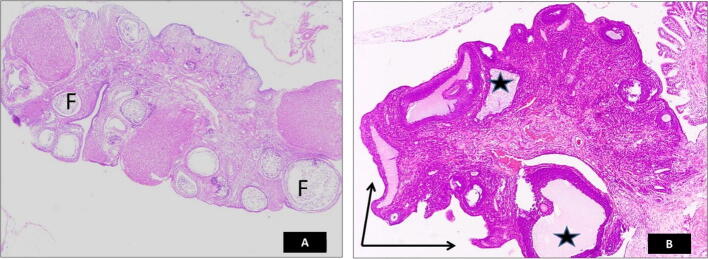
Upon examination, H&E-stained ovaries from rats with PCOS showed deformities and irregular surfaces. Compared to ovaries from the control group, the follicles were transformed into a cystic structure (Fig. 2).
Fig. 2.
Photomicrograph of hematoxylin and eosin (H&E)-stained ovarian section confirming the development of polycystic ovary syndrome (PCOS). (A) ovary from the control group, (B) ovary from the PCOS group. The PCOS ovary shows deformity and irregular surface (arrows) and the transformation of the follicles into the cystic structures (stars) compared to normal ovaries from the control group (F).
3.2. Hormonal evidence of PCOS induction and the effect of chamomile and Met treatment on serum testosterone concentration
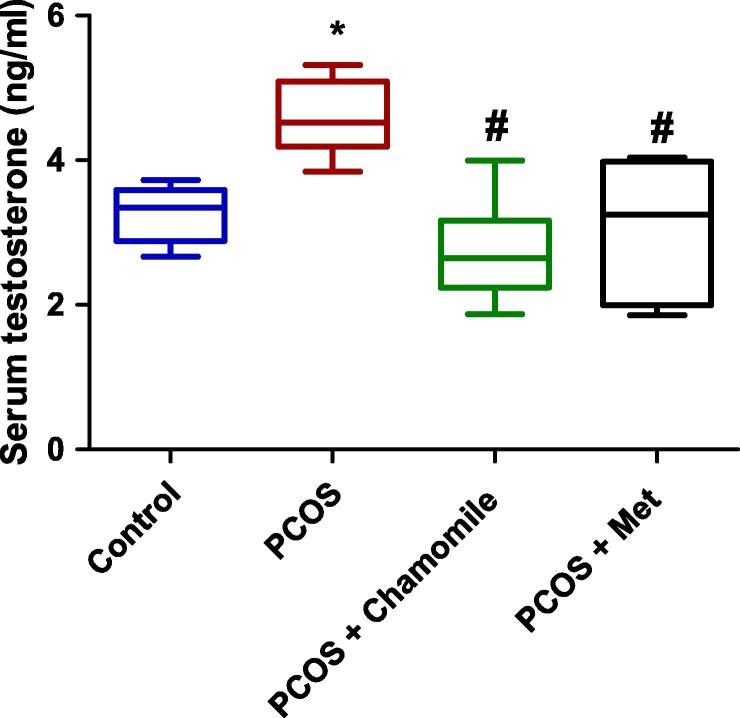
PCOS-induced rats exhibited a statistically significant association with increased serum testosterone concentration as compared to control rats (p ≤ 0.05). Treatment of PCOS-induced rats with either chamomile flower extract or Met significantly decreased serum testosterone (p ≤ 0.001 and p ≤ 0.01, respectively) compared to untreated rats with PCOS (Fig. 3).
Fig. 3.
Box plot showing the effect of polycystic ovary syndrome (PCOS), chamomile flower extract, and metformin (Met) treatments on serum testosterone concentration. The data represent the distribution of 6 rats in each group. Significant differences between groups were analyzed using analysis of variance (ANOVA), followed by Tukey’s multiple comparison test. *Significant difference compared to the control group (p ≤ 0.05). #Significant difference compared to the untreated PCOS group (p ≤ 0.05).
3.3. Effect of PCOS, chamomile, and Met on % body weight change and relative kidney weight %
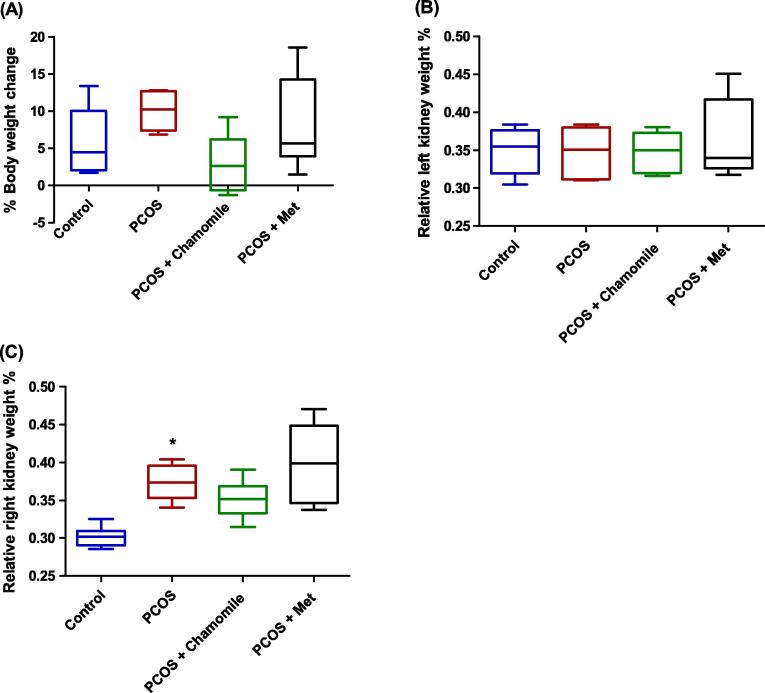
The results showed that neither induction of PCOS nor treatment with chamomile flower extract or Met affected the % body weight change calculated 12 weeks after PCOS induction (Fig. 4A).
Fig. 4.
Box plot showing the effect of polycystic ovary syndrome (PCOS), chamomile flower extract, and metformin (Met) treatments on (A) % body weight change, (B) relative left kidney weight %, and (C) relative right kidney weight %. The data represent the distribution of 6 rats in each group. Significant differences between groups were analyzed using analysis of variance (ANOVA), followed by Tukey’s multiple comparison test. *Significant difference compared to the control group (p ≤ 0.05).
Furthermore, neither PCOS induction nor treatment with chamomile flower extract or Met affected the relative left kidney weights % calculated 12 weeks after PCOS induction (Fig. 4B).
Conversely, in comparison with control rats, PCOS-induced rats exhibited significantly increased relative right kidney weights % as calculated 12 weeks after PCOS induction (p ≤ 0.01) (Fig. 4C). However, PCOS-induced rats treated with either chamomile flower extract or Met did not exhibit altered relative right kidney weights % compared to untreated PCOS-induced rats (Fig. 4C).
3.4. Effect of PCOS, chamomile, and Met on kidney function markers
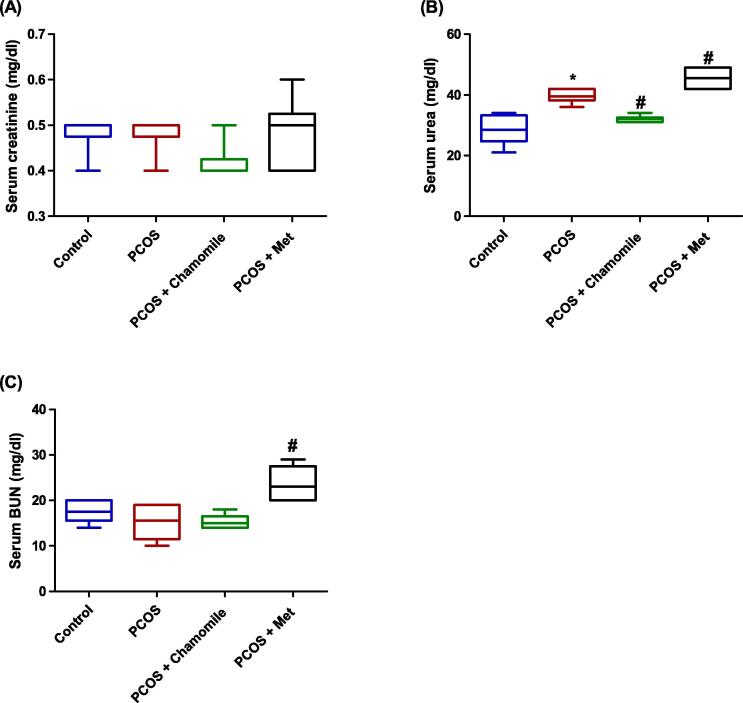
Our results revealed that neither PCOS induction nor treatment with chamomile flower extract or Met affected serum creatinine concentration measured 12 weeks after PCOS induction (Fig. 5A).
Fig. 5.
Box plot showing the effect of polycystic ovary syndrome (PCOS), chamomile flower extract, and metformin (Met) treatments on (A) serum creatinine concentration, (B) serum urea concentration, and (C) serum blood urea nitrogen (BUN) concentration. The data represent the distribution of 6 rats in each group. Significant differences between groups were analyzed using analysis of variance (ANOVA), followed by Tukey’s multiple comparison test. *Significant difference compared to the control group (p ≤ 0.05). #Significant difference compared to the untreated PCOS group (p ≤ 0.05).
However, compared to control rats, PCOS-induced rats demonstrated significantly increased serum urea concentration measured 12 weeks after PCOS induction (p ≤ 0.001) (Fig. 5B). Furthermore, compared to untreated PCOS-induced rats, treatment of PCOS-induced rats with chamomile flower extract yielded significantly decreased serum urea concentration (p ≤ 0.01) (Fig. 5B).
Additionally, neither PCOS induction nor treatment with chamomile flower extract affected serum BUN concentration measured 12 weeks after PCOS induction (Fig. 5C). However, PCOS-induced rats treated with Met exhibited significantly increased serum concentration of BUN as compared to untreated PCOS-induced rats (p ≤ 0.001) (Fig. 5C).
3.5. Effect of PCOS, chamomile, and Met on kidney histology
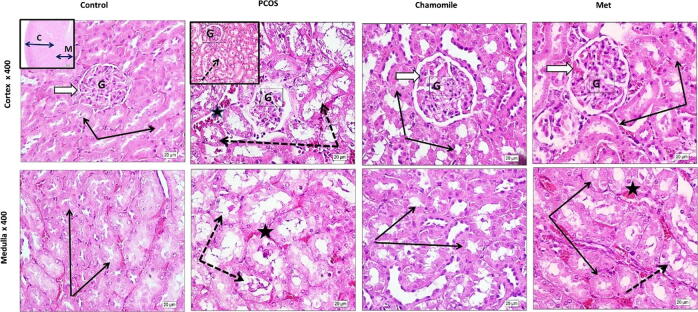
Examination of H&E-stained kidney sections from PCOS-induced rats revealed marked deformity of renal corpuscles, atrophy, and decreased glomerular cellular density. The kidney tubules in both the cortex and medulla showed dilated luminal cavities; degenerated lining cells with many tubules showed intra-luminal casts; and some peritubular capillaries were dilated and congested. Treatment of PCOS-induced rats with chamomile flower extract produced marked preservation of normal cortical and medullary histological architecture. Although treatment of PCOS-induced rats with Met showed restoration of normal structures of cortical renal corpuscles, glomeruli, and tubules, there were still some residual tubules that showed mild degenerative changes (Fig. 6).
Fig. 6.
Hematoxylin and eosin (H&E)-photos showing the effect of polycystic ovary syndrome (PCOS), chamomile flower extract, and metformin (Met) treatments on rat kidney histology. Control photos show the typical architecture of cortical renal corpuscles (white arrow) and glomeruli (G) with average cell density. Renal tubules in both cortex and medulla regions look normal with normal high cuboidal lining epithelium (black arrows). PCOS photos show deformity of renal corpuscles (white arrow) and marked atrophy and decreased glomerular cellular density (G). The kidney tubules (dotted arrows) in both cortex and medulla show dilated lumina; degenerated lining cells with many tubules showed intra-luminal casts (insert, dotted arrow); and some peritubular capillaries were dilated and congested (star). Chamomile photos show marked preservation of typical cortical and medullary histological architecture. Metformin (Met) photos show restoration of typical structures of cortical renal corpuscles, glomeruli (white arrow), and tubules (black arrows) with only a few residual tubules with mild degenerative changes (dotted arrows).
3.6. Effect of PCOS, chamomile, and Met on serum oxidative stress markers
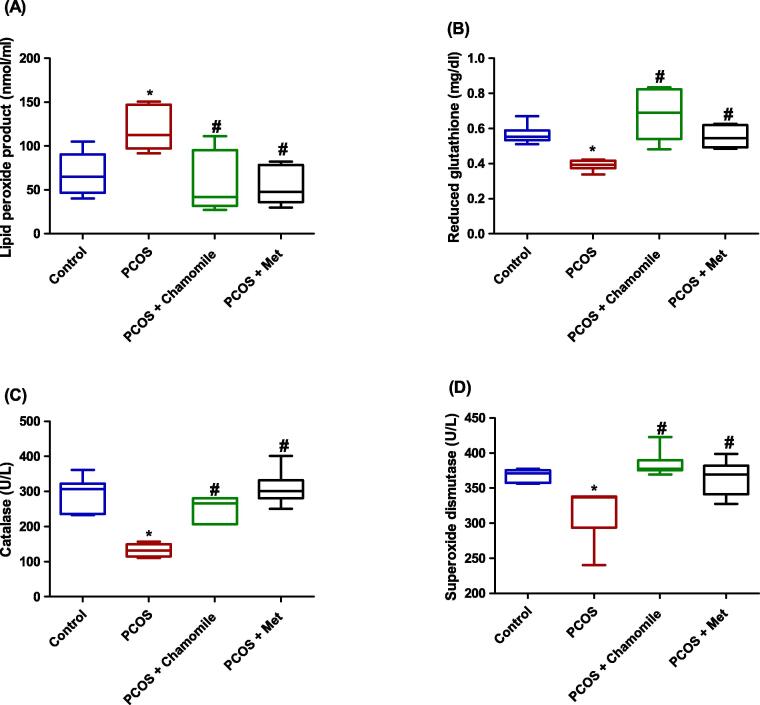
Rats with induced PCOS demonstrated significantly increased serum concentrations of lipid peroxide product as compared to the control rats (p ≤ 0.05) (Fig. 7A). In addition, rats with induced PCOS demonstrated significantly decreased serum reduced glutathione (p ≤ 0.05), catalase (p ≤ 0.001), and superoxide dismutase (p ≤ 0.05) as compared to untreated PCOS-induced rats (Fig. 7B–D).
Fig. 7.
Box plot showing the effect of polycystic ovary syndrome (PCOS), chamomile flower extract, and metformin (Met) treatments on (A) serum lipid peroxide product concentration, (B) serum reduced glutathione concentration, (C) serum catalase concentration, and (D) serum superoxide dismutase concentration. The data represent the distribution of 6 rats in each group. Significant differences between groups were analyzed using analysis of variance (ANOVA), followed by Tukey’s multiple comparison test. *Significant difference compared to the control group (p ≤ 0.05). #Significant difference compared to the untreated PCOS group (p ≤ 0.05).
PCOS-induced rats treated with either chamomile flower extract or Met showed significantly decreased serum lipid peroxide product as compared to untreated PCOS-induced rats (p ≤ 0.01) (Fig. 7A). Furthermore, PCOS-induced rats treated with either chamomile flower extract or Met exhibited significantly increased levels of serum reduced glutathione (p ≤ 0.001 and p ≤ 0.05, respectively), catalase (p ≤ 0.001), and superoxide dismutase (p ≤ 0.001 and p ≤ 0.05, respectively) as compared to untreated PCOS-induced rats (Fig. 7B–D).
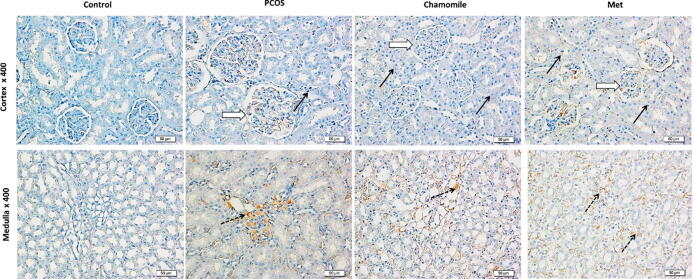
3.7. Effect of PCOS, chamomile, and Met on kidney p53 immunoexpression
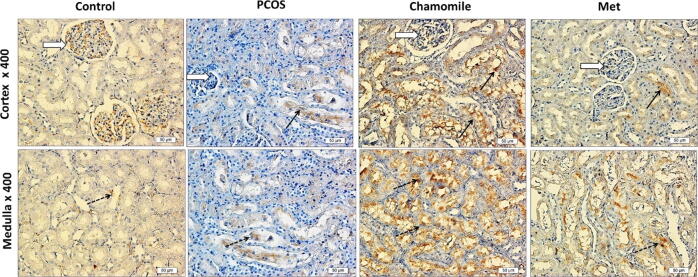
Kidneys from PCOS induced rats showed increased positive p53 staining in cortical glomerular capillaries and medulla loops of Henle compared to kidneys from control rats. PCOS-induced rats treated with chamomile demonstrated a marked decrease in p53 gene expression as compared to untreated PCOS-induced rats. On the other hand, treatment with Met caused only a moderate decrease in p53 gene expression as compared to untreated PCOS-induced rats (Fig. 8).
Fig. 8.
Immunohistochemical photos of rat kidney showing the effect of polycystic ovary syndrome (PCOS), chamomile flower extract, and metformin (Met) treatments on kidney apoptosis gene p53. Control photos show nil p53 gene expression in both the kidney cortex and medulla. PCOS photos show positive p53 staining in cortical glomerular capillaries (white arrow) and medulla loops of Henle (dotted black arrows) and no stain in other tubules (black arrows). Chamomile photos show a marked decrease in p53 gene expression. Metformin (Met) photos show a moderate decrease in p53 gene expression.
3.8. Effect of PCOS, chamomile, and Met on kidney Bcl2 immunoexpression
Kidneys from PCOS induced rats showed decreased Bcl2 staining in cortical glomerular capillaries, and medulla loops of Henle compared to kidneys from control rats. PCOS-induced rats treated with chamomile demonstrated a marked increase in Bcl2 immunoexpression in both cortex and medulla as compared to untreated PCOS-induced rats. On the other hand, treatment with Met caused weak Bcl2 expression in the cortex and moderate Bcl2 gene expression in the medulla as compared to untreated PCOS-induced rats (Fig. 9).
Fig. 9.
Immunohistochemical photos of rat kidney showing the effect of polycystic ovary syndrome (PCOS), chamomile flower extract, and metformin (Met) treatments on kidney apoptosis gene Bcl2. Control photos show marked Bcl2 gene expression in the kidney cortex and weak Bcl2 gene expression in the medulla. PCOS photos show weak Bcl2 staining in cortical glomerular capillaries (white arrow), medulla loops of Henle (dotted black arrows), and other tubules (black arrows). Chamomile photos show a marked increase in Bcl2 gene expression in both cortex and medulla. Metformin (Met) photos show weak Bcl2 gene expression in the cortex and moderate Bcl2 gene expression in the medulla.
4. Discussion
PCOS has shown strong correlation with the development of metabolic disorders, including obesity, diabetes mellitus, and increased blood pressure, which constitute risk factors for renal diseases (Castro and Coresh, 2009, Chadban et al., 2010, Cooney and Dokras, 2018). This study provided histological and biochemical evidences that PCOS is associated with decreased kidney function in the EV-PCOS rat model. In addition, the results of this study proved that chamomile flower extract improved kidney function in PCOS rats, both biochemically and histologically. Unfortunately, although Met ameliorated kidney histology alteration, it did not improve PCOS-induced biochemical functional deterioration; the aggravated kidney function’s biochemical markers (urea and BUN) even worse than PCOS rats themselves. The results from the present study are in agreement with those from a previous study that revealed an association between EV-induced PCOS in rats and increased serum urea levels, although serum creatinine was normal. That study also showed agreement with the present results in the histopathological finding in kidneys from rats-induced with PCOS (Sadrefozalayi and Farokhi, 2014). In another study, the ratio of microalbuminuria to urinary creatinine (UACR), a glomerular function predictor, was reported to be significantly increased in women with PCOS, indicating a strong connection between PCOS and renal dysfunction (Song et al., 2019). Furthermore, little available literature suggests linkages between PCOS and albuminuria (Patel et al., 2008, Ziaee et al., 2013). In another previous study, women with PCOS had increased glomerular filtration rate, serum uric acid, and urine albumin concentration compared to healthy women (Gozukara et al., 2015).
This study showed a significant increase in testosterone concentration in rats induced with PCOS compared to control rats. Testosterone plays a crucial function in the pathogenesis of PCOS-related renal dysfunction, particularly tubular damage. In women with PCOS, a strong positive association has been shown between blood testosterone level and UACR (a tubular damage marker) (Song et al., 2019). Researchers have shown that females with chronic hyperandrogenemia, like those with PCOS, are at higher risk of developing advanced-age kidney problems (Patil et al., 2017). Previous studies have also documented that testosterone can cause apoptosis of the kidney tubules (Verzola et al., 2004). The present study showed that PCOS was associated with increased expression of the p53 gene, a marker of apoptotic cell death in both kidney glomeruli and medullary tubules, and decreased expression of the Bcl2 gene, an anti-apoptotic marker in kidney glomeruli. These results indicate that apoptosis might contribute to the kidney dysfunction associated with PCOS due to testosterone elevation.
Another suggested mechanism that might link PCOS with renal dysfunction involves HT. Previous studies have suggested that HT could impact renal tissue, circulation, glomerular filtration rate, and tubular function in addition to water and salt reabsorption (Kimmel et al., 2012, Lippi et al., 2008). According to our recently published articles, the EV-PCOS model was associated with hypothyroid disease in rats (Alahmadi et al., 2020, Alzahrani et al., 2019). Therefore, HT may result in renal injury associated with PCOS. Several studies have linked the occurrence of OS and PCOS. This is due to the obesity, insulin resistance, and hyperlipidemia associated with this syndrome (Enechukwu et al., 2019). The present study showed increased OS markers and decreased antioxidants factors that might play an essential role in producing renal injury.
This study demonstrated the preventive action of chamomile flower extract against kidney damage associated with PCOS in rats. Chamomile improved testosterone levels, OS, and apoptotic cell death. Similarly, several prior studies have documented the protective role of chamomile against various kidney toxicity models via its antioxidant and anti-apoptotic actions (Kaseb et al., 2018, Mahdi, 2016, Salama, 2012). In contrast to treatment with chamomile, we observed marked increase in kidney function markers after treatment with Met. Met, to some extent, improved pathological changes in the kidney tissue, and it also reduced the signs of oxidative stress. However, it did not effectively reduce markers of apoptosis. In line with these results, a previous cohort study observed mild-to-moderate renal dysfunction among the initiators of Met (Christiansen et al., 2015). Health professionals reported that 1 out of 22 (4.5%) patients receiving Met showed increased serum creatinine concentrations that exceeded safe levels (Kennedy and Herman, 2005). This is in line with results from other publications (Calabrese et al., 2002, Horlen et al., 2002, Sulkin et al., 1997). In type 2 diabetics and moderate CKD patients, Met can be harmful to kidney function (Hsu et al., 2018). Lactic acidosis related to Met could induce metabolic acidosis in patients with moderate CKD. This has been shown to impair kidney function leading to reductions in glomerular filtration rate and CKD deterioration (Chen and Abramowitz, 2014).
5. Conclusion
This study demonstrated that PCOS might be accompanied by impaired kidney function, characterized by an increase in serum urea level. Chamomile extract improves kidney damage associated with PCOS through antioxidant, testosterone-lowering, and anti-apoptotic mechanisms. Serum concentrations of urea and BUN may increase with Met therapy.
The results of this study may contribute to the use of chamomile flower extract as an adjuvant therapy to treat kidney damage associated with PCOS. It is also recommended to monitor the serum concentrations of urea and BUN in women with PCOS during treatment with Met in a long-term study to determine its actual efficacy and avoid possible associated side effects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia, under Grant No. G-1356-247-1440. The authors, therefore, acknowledge with thanks the DSR for technical and financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alahmadi A.A., Alzahrani A.A., Ali S.S., Alahmadi B.A., Arab R.A., El-Shitany N.A.E.-A. Both matricaria chamomilla and metformin extract improved the function and histological structure of thyroid gland in polycystic ovary syndrome rats through antioxidant mechanism. Biomolecules. 2020;10 doi: 10.3390/biom10010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzahrani A.A., Alahmadi A.A., Ali S.S., Alahmadi B.A., Arab R.A., Wahman L.F., El-Shitany N.A. Biochemical and histological evidence of thyroid gland dysfunction in estradiol valerate model of the polycystic ovary in Wistar rats. Biochem. Biophys. Res. Commun. 2019;514:194–199. doi: 10.1016/j.bbrc.2019.04.126. [DOI] [PubMed] [Google Scholar]
- Azziz R., Carmina E., Chen Z., Dunaif A., Laven J., Legro R., Lizneva D., Natterson-Horowtiz B., Teede H. Polycystic ovary syndrome. Rev. Dis. Prim. Nat. 2016 doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- Barry J., Azizia M., Hardiman P. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Reprod. Update Hum. 2014 doi: 10.1093/humupd/dmu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behboudi-Gandevani S., Amiri M., Bidhendi Yarandi R., Noroozzadeh M., Farahmand M., Rostami Dovom M., Ramezani Tehrani F. The risk of metabolic syndrome in polycystic ovary syndrome: A systematic review and meta-analysis. Clin. Endocrinol. (Oxf). 2018 doi: 10.1111/cen.13477. [DOI] [PubMed] [Google Scholar]
- Caglar G., Oztas E., Karadag D., Pabuccu R., Eren A. The association of urinary albumin excretion and metabolic complications in polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;154:57–61. doi: 10.1016/j.ejogrb.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Calabrese A., Coley K., Dapos S., Swanson D., Rao R. Evaluation of prescribing practices: Risk of lactic acidosis with metformin therapy. Arch. Intern. Med. 2002;162:434–437. doi: 10.1001/archinte.162.4.434. [DOI] [PubMed] [Google Scholar]
- Castro A., Coresh J. CKD Surveillance Using Laboratory Data From the Population-Based National Health and Nutrition Examination Survey (NHANES) Am. J. Kidney Dis. 2009;53:S46–S55. doi: 10.1053/j.ajkd.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadban S., Howell M., Twigg S., Thomas M., Jerums G., Cass A., Campbell D., Nicholls K., Tong A., Mangos G., Stack A., MacIsaac R., Girgis S., Colagiuri R., Colagiuri S., Craig J. Assessment of kidney function in type 2 diabetes. Nephrology. 2010 doi: 10.1111/j.1440-1797.2010.01239.x. [DOI] [PubMed] [Google Scholar]
- Chen W., Abramowitz M.K. Metabolic acidosis and the progression of chronic kidney disease. BMC Nephrol. 2014 doi: 10.1186/1471-2369-15-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen C., Ehrenstein V., Heide-Jørgensen U., Skovbo S., Nørrelund H., Sørensen H., Li L., Jick S. Metformin initiation and renal impairment: A cohort study in Denmark and the UK. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2015-008531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney L., Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Steril. Fertil. 2018 doi: 10.1016/j.fertnstert.2018.08.021. [DOI] [PubMed] [Google Scholar]
- Cooney L., Lee I., Sammel M., Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. 2017;32:1075–1091. doi: 10.1093/humrep/dex044. [DOI] [PubMed] [Google Scholar]
- dos Reis A., Honorato-Sampaio K. C-type natriuretic peptide: A link between hyperandrogenism and anovulation in a mouse model of polycystic ovary syndrome. Sci. Clin. 2018 doi: 10.1042/CS20171491. [DOI] [PubMed] [Google Scholar]
- Enechukwu C., Onuegbu A., Olisekodiaka M., Eleje G., Ikechebelu J.I., Ugboaja J., Amah U., Okwara J.E., Igwegbe A. Oxidative stress markers and lipid profiles of patients with polycystic ovary syndrome in a Nigerian tertiary hospital. Obstet. Gynecol. Sci. 2019;62:335–343. doi: 10.5468/ogs.2019.62.5.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farideh Z., Bagher M., Ashraf A., Akram A., Kazem M. Effects of chamomile extract on biochemical and clinical parameters in a rat model of polycystic ovary syndrome. J. Reprod. Infertil. 2010;11:169–174. [PMC free article] [PubMed] [Google Scholar]
- Gozukara I., Gozukara K., Kucur S., Karakılıc E., Keskin H., Akdeniz D., Aksoy A., Carlıoglu A. Association of glomerular filtration rate with inflammation in polycystic ovary syndrome. Int. J. Fertil. Steril. 2015;9:176–182. doi: 10.22074/ijfs.2015.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horlen C., Malone R., Bryant B., Dennis B., Carey T., Pignone M., Rothman R. Frequency of inappropriate metformin prescriptions [3] J. Am. Med. Assoc. 2002 doi: 10.1001/jama.287.19.2504-a. [DOI] [PubMed] [Google Scholar]
- Hsu W., Hsiao P., Lin P., Chen S., Lee M., Shin S. Effect of metformin on kidney function in patients with type 2 diabetes mellitus and moderate chronic kidney disease. Oncotarget. 2018;9:5416–5423. doi: 10.18632/oncotarget.23387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowicz D., Barnea M., Wainstein J., Froy O. Effects of caloric intake timing on insulin resistance and hyperandrogenism in lean women with polycystic ovary syndrome. Clin. Sci. 2013;125:423–432. doi: 10.1042/CS20130071. [DOI] [PubMed] [Google Scholar]
- Kahal H., Kyrou I., Tahrani A., Randeva H. Obstructive sleep apnoea and polycystic ovary syndrome: A comprehensive review of clinical interactions and underlying pathophysiology. Clin. Endocrinol. (Oxf). 2017 doi: 10.1111/cen.13392. [DOI] [PubMed] [Google Scholar]
- Kaseb F., Yazdanpanah Z., Biregani A., Yazdi N., Yazdanpanah Z. The effect of chamomile (Matricaria recutita L.) infusion on blood glucose, lipid profile and kidney function in Type 2 diabetic patients: A randomized clinical trial. Prog. Nutr. 2018;20:110–118. doi: 10.23751/pn.v20i1-S.5884. [DOI] [Google Scholar]
- Kennedy L., Herman W.H. Renal status among patients using metformin in a primary care setting. Diabetes Care. 2005;28:922–924. doi: 10.2337/diacare.28.4.922. [DOI] [PubMed] [Google Scholar]
- Khan A., Majumder I. Serum Creatinine and Uric Acid Levels of Hypothyroid Patients. Bangladesh J. Med. Biochem. 2013;3:61–63. doi: 10.3329/bjmb.v3i2.13814. [DOI] [Google Scholar]
- Kimmel M., Braun N., Alscher M. Influence of thyroid function on different kidney function tests. Kidney Blood Press. Res. 2012;35:9–17. doi: 10.1159/000329354. [DOI] [PubMed] [Google Scholar]
- Li R., Zhang Q., Yang D., Li S., Lu S., Wu X., Wei Z., Song X., Wang X., Fu S., Lin J., Zhu Y., Jiang Y., Feng H., Qiao J. Prevalence of polycystic ovary syndrome in women in China: A large community-based study. Hum. Reprod. 2013;28:2562–2569. doi: 10.1093/humrep/det262. [DOI] [PubMed] [Google Scholar]
- Lippi G., Montagnana M., Targher G., Salvagno G., Guidi G. Relationship between thyroid status and renal function in a general population of unselected outpatients. Clin. Biochem. 2008;41:625–627. doi: 10.1016/j.clinbiochem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Lizneva D., Suturina L., Walker W., Brakta S., Gavrilova-Jordan L., Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Steril. Fertil. 2016 doi: 10.1016/j.fertnstert.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Mahdi J. Histological study of the hot aqueous extracts of chamomile on renal toxicity Induced by- Methomyl 90%In male albino mice. Int. J. Sci. Eng. Res. 2016;7:519–526. [Google Scholar]
- Mohamedali M., Reddy Maddika S., Vyas A., Iyer V., Cheriyath P. Thyroid disorders and chronic kidney disease. J. Nephrol. Int. 2014 doi: 10.1155/2014/520281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajappa K., Sushma B., Hebbar S. Study of thyroid stimulating hormone, serum creatinine and uric acid levels in patients with hypothyroidism. Int. J. Pure App. Biosci. 2014;2(2):187–190. http://www.ijpab.com [Google Scholar]
- Nigam S., Bush K. Uraemic syndrome of chronic kidney disease: altered remote sensing and signalling. Rev. Nephrol. 2019 doi: 10.1038/s41581-019-0111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Bloomgarden Z., Futterweit W. Premicroalbuminuria in women with polycystic ovary syndrome: A metabolic risk marker. Endocr. Pract. 2008;14:193–200. doi: 10.4158/EP.14.2.193. [DOI] [PubMed] [Google Scholar]
- Patil C., Racusen L., Reckelhoff J. Consequences of advanced aging on renal function in chronic hyperandrogenemic female rat model: implications for aging women with polycystic ovary syndrome. Physiol. Rep. 2017;5 doi: 10.14814/phy2.13461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli G., Alrukhaimi M., Liu Z., Zakharova E., Levin A., Tao Li P., Garcia-Garcia G., Benghanem-Gharbi M., Kalantar-Zadeh K., Kernahan C., Kumaraswami L., Piccoli G., Saadi G., Fox L., Andreoli S. Women and kidney disease: Reflections on World Kidney Day 2018. Dial. Transplant Nephrol. 2018 doi: 10.1093/ndt/gfx358. [DOI] [Google Scholar]
- Sadrefozalayi S., Farokhi F. Effect of the aqueous extract of Foeniculum vulgare (fennel) on the kidney in experimental PCOS female rats. Avicenna J. phytomedicine. 2014;4:110–117. doi: 10.22038/ajp.2014.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama R. Matricaria chamomilla attenuates cisplatin nephrotoxicity. Saudi J. Kidney Dis. Transpl. 2012;23:765–772. doi: 10.4103/1319-2442.98158. [DOI] [PubMed] [Google Scholar]
- Showell M., Mackenzie-Proctor R., Jordan V., Hodgson R., Farquhar C. Inositol for subfertile women with polycystic ovary syndrome. Rev. Cochrane Database Syst. 2018 doi: 10.1002/14651858.CD012378.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh O., Khanam Z., Misra N., Srivastava M. Chamomile (Matricaria chamomilla L.): An overview. Pharmacogn. Rev. 2011 doi: 10.4103/0973-7847.79103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Ye W., Ye H., Xie T., Shen W., Zhou L. Serum testosterone acts as a prognostic indicator in polycystic ovary syndrome-associated kidney injury. Physiol. Rep. 2019;7 doi: 10.14814/phy2.14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkin T., Bosman D., Krentz A. Contraindications to metformin therapy in patients with NIDDM. Diabetes Care. 1997;20:925–928. doi: 10.2337/diacare.20.6.925. [DOI] [PubMed] [Google Scholar]
- Teede H., Deeks A., Moran L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010 doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzola D., Gandolfo M., Salvatore F., Villaggio B., Gianiorio F., Traverso P., Deferrari G., Garibotto G. Testosterone promotes apoptotic damage in human renal tubular cells. Kidney Int. 2004;65:1252–1261. doi: 10.1111/j.1523-1755.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang H., Liu W., Zhang Z., Zhang Y., Zhang W., Chen Z., Xia G., Wang C. High level of C-type natriuretic peptide induced by hyperandrogen-mediated anovulation in polycystic ovary syndrome mice. Clin. Sci. 2018;132:759–776. doi: 10.1042/CS20171394. [DOI] [PubMed] [Google Scholar]
- Suvarna, S.K., Layton, C., Bancroft, J.D., 2019. The hematoxylin and eosin. In: Bancroft’s Theory and Practice of Histological Techniques, Eighteen ed., 2019. 126-138. DOI: 10.1016/C2015-0-00143-5
- Yang R., Yang S., Li R., Liu P., Qiao J., Zhang Y. Effects of hyperandrogenism on metabolic abnormalities in patients with polycystic ovary syndrome: A meta-analysis. Biol. Endocrinol. Reprod. 2016 doi: 10.1186/s12958-016-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaee A., Oveisi S., Ghorbani A., Hashemipour S., Mirenayat M. Association between metabolic syndrome and premicroalbuminuria among Iranian women with Polycystic Ovary Syndrome: a case control study. Glob. J. Health Sci. 2013;5:187–192. doi: 10.5539/gjhs.v5n1p187. [DOI] [PMC free article] [PubMed] [Google Scholar]