Abstract
The present study sought to evaluate the central nervous system (CNS) depressant, antioxidant, and cytotoxicity activity of methanol and aqueous extract of Trametes versicolor (METV and AETV). The CNS activity was assessed by the open field, hole-cross, forced swimming, thiopental sodium-induced sleeping time, hole-board, and rotarod tests in Swiss albino mice. For both extracts, a substantial decrease in locomotion was observed in open field and hole-cross tests. In addition, the molecular docking study has been implemented through Maestro V11.1. The higher dose of METV (400 mg/kg) and the lower dose of AETV (200 mg/kg) exhibited a significant decrease in immobility time in forced swimming test and increased prolongation of sleep in thiopental sodium-induced sleeping time test, respectively. In contrast, a moderate finding was observed for the hole-board and rotarod tests. Additionally, a significant DPPH scavenging assay and a high toxicity effect in brine shrimp lethality assay were observed. Besides, five phenolic compounds, namely baicalin, quercetin, catechin, p-hydroxybenzoic acid, and quinic acid, were used for the molecular docking study, whereas catechin demonstrated the highest binding affinity towards the targets. The findings conclude that the T. versicolor could be an alternative source for CNS anti-depressant and antioxidant activity.
Keywords: Trametes versicolor, Central nervous system, Antioxidant, Cytotoxicity, Molecular docking
1. Introduction
Depression, which leading cause remains unresolved, is a common mental or psychiatric ailment. Studies have suggested that the imbalance of immunity and enhancements in proinflammatory cytokines might associate with depression (Rana, Behl et al. 2021). Another study based on the monoaminergic system does not proclaim a full perception of depression (Sultana, Mannan et al. 2018). Oxidative stress is related to the development of depression, which is the most accepted hypothesis for depression (Michel, Frangou et al., 2007). The reports of the World Health Organization (WHO), 121 million people were suffered from clinical depression, the second largest in the world after heart disease (Cryan and Lucki, 2000, Smith et al., 2008). Both secondary metabolites and the active drug compounds are prominently derived from medicinal plants. It features prominently in the discovery of new drug molecules, which possibly will be an alternative source of depression treatment (Zhang et al., 2015, Uddin Mazumdar et al., 2017). Antioxidants protect the physiological system of our body by protecting the cell against reactive oxygen species (ROS), whereas it also protects the cell from oxidative stress (OS) (Uddin Mazumdar, Islam et al. 2017). OS is caused due to the production of free radicals resulting in cell and DNA damage and various disorders (Festa et al., 2001, Uddin Mazumdar et al., 2017). OS causes changes in genes' expressions and induces abnormal proteins, which lead to several human disorders, including the development of atherosclerosis, heart disease, and central nervous system (CNS) diseases, cancer, and acquired immunodeficiency syndrome (AIDS) (Hela and Abdullah, 2010, Khatun et al., 2016). The human body's physiology can neutralize the OS by its antioxidant mechanism. But somehow it may fail because of the free radicals overproduction. Free radicals are neutralized by antioxidants, whereas plants and vegetables are a natural source of antioxidants. As a result, people are more efficient against OS-related health problems (Nahar et al., 2012, Rahman et al., 2015).
Nature is the best reliable source for potential drugs molecule. The wide range of structural molecules derived naturally leads to new medicines to attain better pharmacologic activity with the least side effects (Muhammad et al. 2015). In contrast, the mushroom has significant nutritional values (fibre, proteins, and vitamins) along with rich antioxidant-property phytochemicals (Puia et al., 2018). There is a substantial need for the development of potential drug molecules for new antioxidants, cytotoxic, and CNS depressants from natural resources. Trametes versicolor (L.) is one of the plants of the Polyporaceae family, which is also acknowledged as Turkey tail. Though it is not edible, it is useful in folk medicine for its various pharmacological effects. T. versicolor has been reported to have antioxidant (Kamiyama et al., 2013, Puia et al., 2018), anticancer (Cfr Ferreira et al., 2010, Puia et al., 2018), anti-microbial (Puia et al., 2018, ÖZgÖR.et.all., 2016), anti-inflammatory (Kamiyama et al. 2013), immune system boosters (Li et al. 2011) and antidiabetic effects (Puia et al., 2018, Shokrzadeh et al., 2017). In terms of bioactive compounds, it has been identified polysaccharides, 18-type of amino acids, and 28 phenolic compounds (Puia et al., 2018). However, there are no studies that scrutinized the CNS depressant activity of T. versicolor.
Thus, in the present study, methanol and aqueous extracts of T. versicolor have been used to assess the antioxidant, cytotoxic, and CNS activities. The study of CNS activity was performed on Swiss albino mice. Additionally, the molecular docking study was performed on phenolic compounds of T. versicolor against four different receptors.
2. Materials and methods
2.1. Chemicals and reagents
1, 1-diphenyl, 2-picryl hydrazyl (DPPH) and ascorbic acid was brought from Sigma Chemical Co., USA. And SD Fine Chem. Ltd., Biosar, India, respectively. Imipramine (Square Pharmaceuticals Limited, Dhaka) and diazepam (Square Pharmaceuticals Limited, Dhaka) and vincristine sulfate (Beacon Pharmaceuticals Limited, Dhaka) were procured from the Bangladeshi pharmaceutical company. The Department of Pharmacy, University of Chittagong, Chittagong, Bangladesh have supplied additional analytical-grade chemicals.
2.2. Mushroom collection and extraction
T. versicolor collected from the areas of the University of Chittagong campus. Mr. Sajib Rudra, Taxonomist, Department of Botany, University of Chittagong identified the mushrooms under the accession number of CTGUH SR 2798. The mushroom has been dried and ground in fine powder. The powder was mixed with methanol and distilled water for seven days with a ratio of 1:4 in a glass container. It was then filtered by filtering paper (Whatman size #1). A rotary evaporator has been used to evaporate the solvent to yield the methanol and aqueous extract. These methanol extract T. versicolor (METV) and aqueous extract T. versicolor (AETV) of were kept in closely sealed glass vials and stored (4 °C) until further use.
2.3. Animals
Either sex of Swiss albino mice at the age of 4–5 weeks old (20–25 g) were procured from BCSIR, Chattogram, Bangladesh. The mice were kept in room temperature conditions (relative humidity: 55–65% and 12 h light/dark cycle) for seven days to acclimatize with the laboratory conditions. The standard food pellet and water were supplied for the mice through the experiment. However, the study followed the animal guidelines declared by the Swiss Academy of Medical Sciences and the Swiss Academy of Sciences. Animals have euthanized according to the Guidelines for the Euthanasia of Animals: 2013 edition (Leary et al. 2013) and approved by the Institutional Ethics Committee of Department of Pharmacy, University of Chittagong approved this research study under the ethical number.
2.4. Experimental design
Either sex of Swiss albino mice was divided into twenty-six groups (control, standard, MEPTV, and AETV) (n = 5). The METV and AETV groups were administered with 200 and 400 mg/kg according to the body weight (BW), and 1% Tween-80 solution (10 mL/kg, BW) was treated for the control group. The diazepam (1 mg/kg/kg, BW, IP) were administrated as the standard drug for the open field test, hole cross test, forced swimming test, thiopental sodium induced sleeping test, hole board test and rotarod test. For the forced swim test, the imipramine (10 mg/kg, BW) was administrated orally.
2.5. CNS depressant activity
2.5.1. Open field test
The mushroom extract's CNS activity was assessed using the protocol of Saleem et al. with the minor modification (Saleem et al. 2011). The four-sided box was (highlighted in black and white) consisted of 60 × 60 × 60 cm3 with 25 equal squares (5 × 5 cm2). As per Section 2.4, each group was treated. After each group treatment, the mouse from each group was immediately employed on the board and observed for 3 min, where the number of squares movement was recorded. Similarly, the square movement was recorded at 30 and 60 min interval.
2.5.2. Hole-cross test
The hole-cross test for CNS activity was assessed by Takagi et al. with a small modification (Takagi et al., 1971). The hole-cross apparatus are made of wood which consists of 30 × 20 × 14 cm3 dimensions and 7.5 cm in height. A hole was located in the centre of the box (3 cm). As per Section 2.4, each group was treated. After each group treatment, the mice were immediately placed on the board (0, 30 and 60 min interval) and observed for 3 min, where the number of hole crossed was recorded.
2.5.3. Forced swimming test
The forced swimming test method was considered for the CNS depressant activity (Emon et al. 2021). As per Section 2.4, each group was treated. Sixty minutes after the treatment, the mice individually placed and positioned on the glass apparatus, which consist of 25 × 15 × 25 cm3 and filled with water (15 cm, 25 ± 2 °C). Each mouse was recorded for six minutes, where the first two minutes are initial adjustments and the last four minutes mentioned as immobile time.
2.5.4. Thiopental sodium-induced sleeping time test
The thiopental sodium-induced sleeping test was used for CNS activity of the METV and AETV (File and Pellow 1985). As per Section 2.4, each group was treated. After Twenty minutes, each mouse individually administered thiopental sodium (40 mg/kg, BW) intraperitoneally (i.p.), which induced sleep. The onset of sleep and prolongation of sleep was recorded for each mouse.
2.5.5. Hole-board test
The hole-board apparatus evaluated the anxiolytic activity of METV and AETV (Yao et al. 2010). The wood apparatus consists of 16 holes (Diameter: 3 cm) in a square chamber (40 × 40 × 25 cm3), and raised from the floor at 25 cm. According to Section 2.4., the dosing treatment of each mouse was followed. After 30 min of the administration, each mouse was individually placed and positioned in the centre of the apparatus and recorded the head dipping in the hole for 5 min.
2.5.6. Rotarod test
The rotarod apparatus evaluated the CNS activity by following the earlier described Dunham et al. (Dunham et al. 1957). This research was conducted with a rotarod device, which has a four-section design. According to Section 2.4., the dosing treatment of each mouse was followed. After treatment, each mouse was individually placed in the apparatus at 30, 60 and 90 min at a speed of 12 rpm.
2.6. Antioxidant activity by DPPH scavenging assay
DPPH scavenging assay was incorporated for the Antioxidant activity of METV and AETV (Alam et al. 2020). Two millilitres of serially diluted concentrations (12.5, 25, 50, 100, 200 and 400 μg/mL) of METV, AETV and ascorbic acid were mixed with 3 mL of a 0.004% DPPH solution (4 mg DPPH in 100 mL of 95% methanol) and kept them in the incubator for 30 min at 25 °C. The study was followed in a triplicate manner and the absorbance was taken in 517 nm.
2.7. Cytotoxic activity
The cytotoxicity activity of METV and AETV were assessed by brine shrimp lethality bioassay as described by Meyer et al. 1982 (Meyer et al. 1982). By mixing 38 g of sodium chloride (NaCl) in one litre of distilled water (DW), the artificial seawater was prepared and pH maintained at 8.0. A serially diluted concentration (50, 100, 250, 500 and 700 mg/mL) was prepared for METV and AETV, while vincristine sulfate used as a standard in different concentrations (0.25, 0.50, 1 and 5 mg/mL). Then, ten mature nauplii were taken in every test tube for each concentration. Every test tube was assessed after 24 h, whereas the number of alive nauplii was counted and recorded.
2.8. Molecular docking study
2.8.1. Protein preparation
The three dimensional (3D) structures of four different receptors are downloaded from the Protein Data Bank server in PDB format (Berman et al. 2002). They are potassium channel receptor (PDB ID: 4UUJ) (Lenaeus et al. 2014), human serotonin receptor (PDB ID: 5I6X) (Coleman et al., 2016), urate oxidase receptor (PDB ID: 1R4U) (Retailleau et al. 2004) and human estrogen receptor (PDB ID: 1ERR) (Brzozowski et al. 1997). Then, the four receptors were refined and optimized using the force field (OPLS3) of Maestro V11.1 (Roos et al. 2019, Kotha and Kulkarni 2020).
2.8.2. Ligand preparation
Baicalin, quercetin, catechin, p-hydroxybenzoic acid and quinic acid were used for the molecular docking study which was identified in earlier reports (Puia et al., 2018). The compound saved from the PubChem database and converted to three dimensional (3D) structures by a force field (OPLS3) of Maestro V11.1 (LigPrep) (Emon et al., 2020a, Kotha and Kulkarni, 2020).
2.8.3. Grid generation and molecular docking
The specific grids for four different receptors were generated using Schrödinger Glide the default parameters were followed (Kotha and Kulkarni 2020). After grid generation, the molecular docking was followed by the standard precession (SP) ligand docking, whereas the Van der Waals scaling factor was 0.80 along with the partial charge cutoff was 0.15 (Friesner et al., 2004, Friesner et al., 2006). The interacted score was recorded for each ligand against each receptor.
3. Statistical analysis
The results were indicated in mean ± standard error mean (SEM). Here, p < 0.05 was measured as statistically significant, where two-way analysis of variance (ANOVA, Dunnett’s test) was followed by GraphPad Prism (V.8.4. Besides, one-way ANOVA (Dunnett’s test) was used for the hole board and forced swimming test.
4. Results
4.1. CNS depressant activity
4.1.1. Open field and hole-cross test
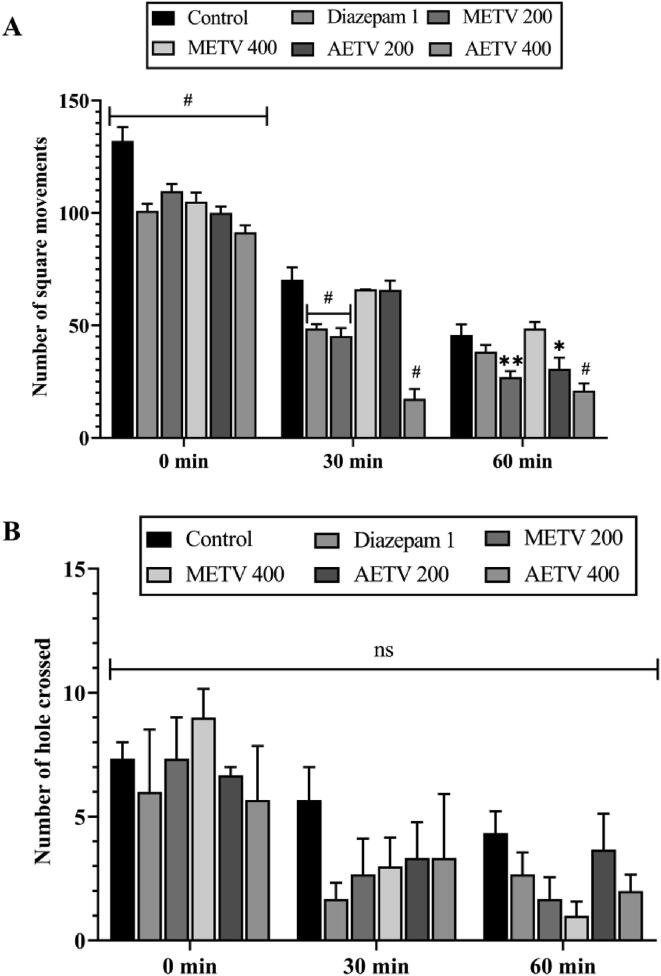
The METV and AETV dose (200 and 400 mg/kg) exposed significant (p < 0.05) decreased locomotion effect of mice in the open field, which was marked from the initial observation (0 min), and continuous to last observation (60 min), with the similar remark of standard Diazepam (Fig. 1A). While the hole-cross test unveiled a non-significant (p > 0.05) dose-dependent manner decreased hole crossing for both extracts (METV and AETV) and standard Diazepam (Fig. 1B).
Fig. 1.
Effect of methanol and aqueous extract of T. versicolor (METV & AETV) on Open field test (A) and Hole-cross test (B). Values are represented as Mean ± SEM (n = 5). * p < 0.01, ** p < 0.05, #p < 0.001 are statistically significant in comparison to Control (10 mL/kg) followed by ANOVA (Dunnett’s test).
4.1.2. Forced swimming test
The result of the forced swimming test was presented in table 1. The AETV at a lower dose (200 mg/kg) exposed significant (p < 0.05) decreases in the immobility times after oral administration when compared to a control group (Table 1). In contrast, 400 mg/kg dose of METV showed the maximal effect. Similarly, a significant decrease in immobility times (p < 0.05) was observed by standard diazepam (80.71%).
Table 1.
Effect of methanol and aqueous extract of T. versicolor (METV & AETV) on forced swimming test in mice.
| Groups (mg/kg) | Onset of sleep(min) | % Effect |
|---|---|---|
| Control | 70.0 ± 7.0 | – |
| Imipramine 10 | 13.5 ± 1.5# | 80.71 |
| METV 200 | 54.5 ± 12.5 | 22.14 |
| METV 400 | 48.0 ± 1.0 | 31.43 |
| AETV 200 | 40.5 ± 1.50** | 42.14 |
| AETV 400 | 52.5 ± 2.50 | 25.0 |
Values are represented as Mean ± SEM (n = 5). ** p < 0.01, #p < 0.001 are statistically significant in comparison to Control (10 mL/kg) followed by ANOVA.
4.1.3. 4.1.3.Thiopental sodium-induced sleeping time test
Like the forced swimming test, METV (400 mg/kg) and AETV (200 mg/kg) exhibited decreased behaviour for the onset of sleep. The findings were summarized in table 2. The AETV (200 mg/kg) and METV (400 mg/kg) showed 154.35% and 136.38% of prolongation of sleeping, while the diazepam was 325.09%.
Table 2.
Effect of methanol and aqueous extract of T. versicolor (METV & AETV) on thiopental sodium induced sleeping time in mice.
| Groups (mg/kg) | Onset of sleep(min) | Duration of sleep(min) | % Effect |
|---|---|---|---|
| Control | 30 ± 4.79 | 44.11 ± 2.13 | – |
| Diazepam 1 | 7 ± 2.48 # | 143.4 ± 5.69# | 325.09 |
| METV 200 | 16.31 ± 6.03 | 18.28 ± 4.58# | 12.75 |
| METV 400 | 10.71 ± 5.21** | 24.93 ± 3.24** | 136.38 |
| AETV 200 | 32.39 ± 1.92 | 38.48 ± 2.14 | 154.35 |
| AETV 400 | 9.68 ± 4.41** | 20.63 ± 2.73# | 53.61 |
Values are represented as Mean ± SEM (n = 5). ** p < 0.01, #p < 0.001 are statistically significant in comparison to Control (10 mL/kg) followed by ANOVA (Dunnett’s test).
4.1.4. Hole-board and rotarod test
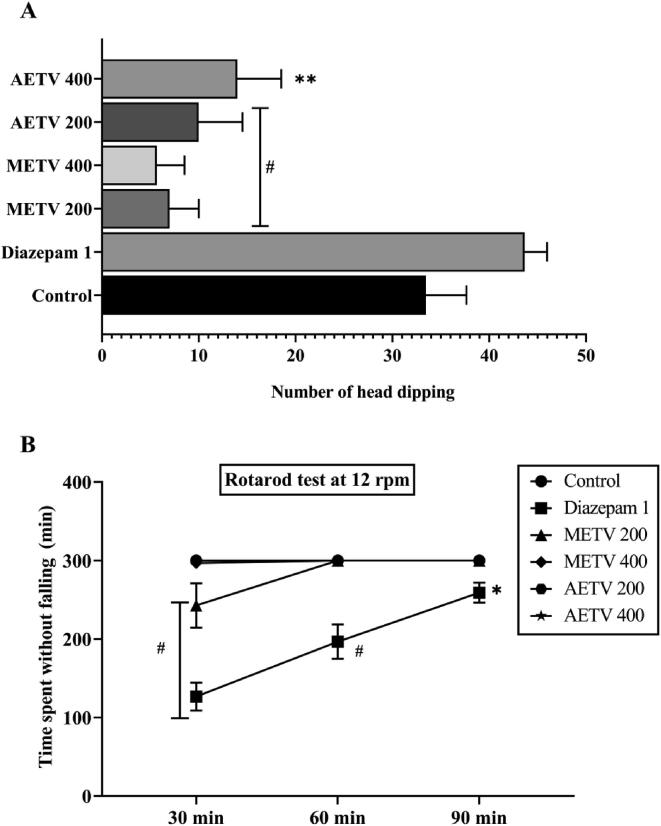
For METV and AETV, the hole-board test exhibited a significant decrease in head dipping compared to the control group, which is a depressive behaviour of mice (Fig. 2A). A similar observation was exposed by the rotarod test, where a non-significant reduction in time spent by the mice in the rotarod apparatus was observed (Fig. 2B). For both tests, the result of standard diazepam was statistically highly significant.
Fig. 2.
Effect of methanol and aqueous extract of T. versicolor (METV & AETV) on Hole-board test (A) and Rotarod test (B). Values are represented as Mean ± SEM (n = 5). * p < 0.01, ** p < 0.05, #p < 0.001 are statistically significant in comparison to Control (10 mL/kg) followed by ANOVA (Dunnett’s test).
4.2. Antioxidant activity
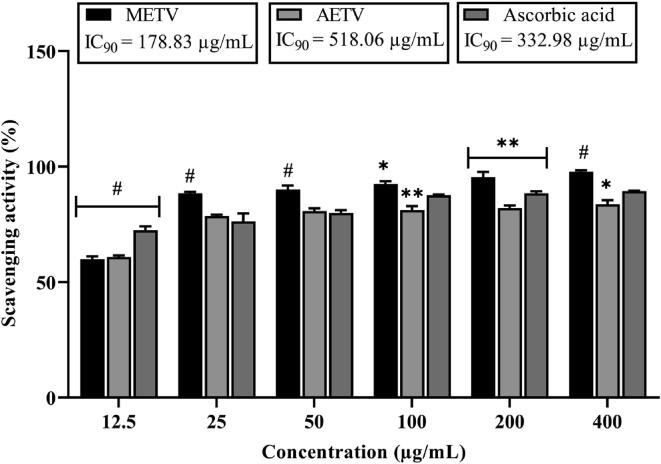
Both METV and AETV extract showed a dose-dependent response in the DPPH scavenging assay (Fig. 3). The IC90 value was 178.83 μg/mL, 518.06 μg/mL and 332.98 μg/mL, for METV, AETV and ascorbic acid respectively.
Fig. 3.
DPPH scavenging assay of ascorbic acid, methanol and aqueous extract of T. versicolor (METV & AETV) on different concentrations. Values are represented as Mean ± SEM (n = 3). * p < 0.01, ** p < 0.05, #p < 0.001 are statistically significant in comparison to Control (10 mL/kg) followed by ANOVA (Dunnett’s test).
4.3. Cytotoxic activity
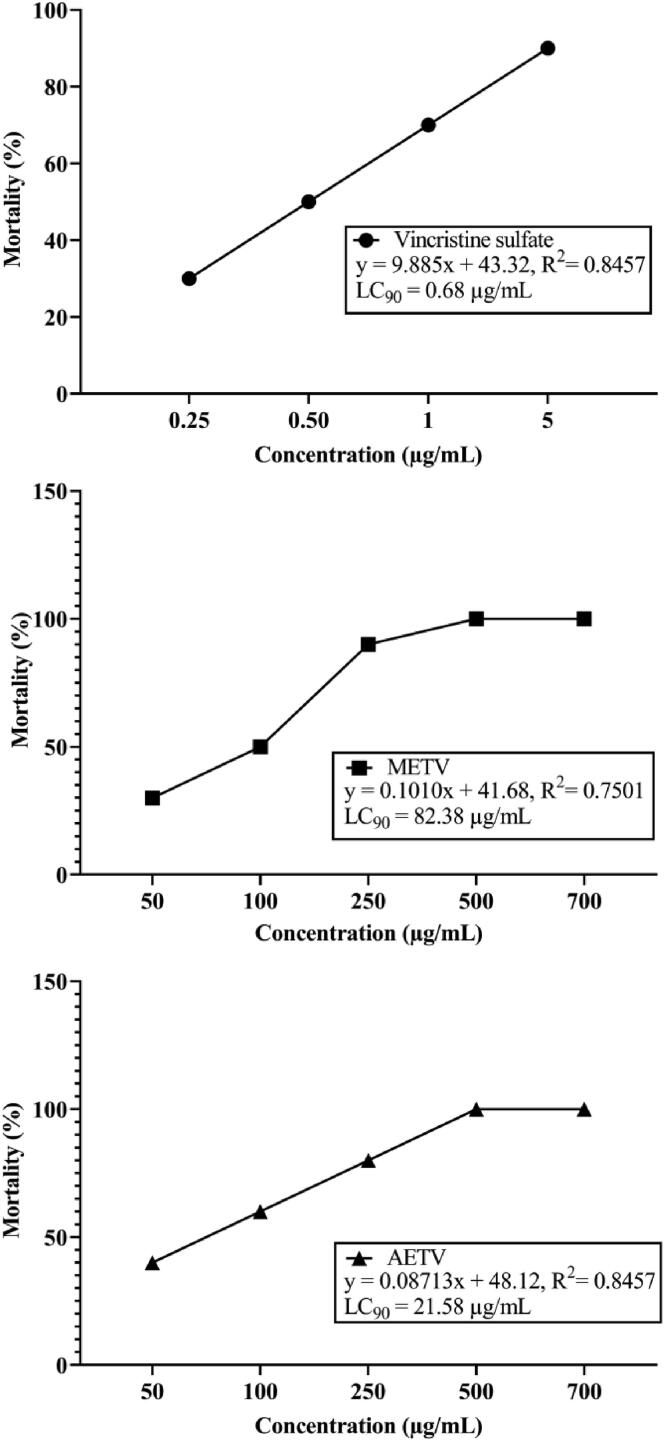
METV and AETV extract presented a dose-dependent percentage mortality rate in brine shrimp lethality assay (Fig. 4). The extracts LC50 value was METV = 82.38 μg/mL and AETV = 21.58 μg/mL, while the vincristine sulfate was 0.68 μg/mL.
Fig. 4.
Brine shrimp lethality bioassay of Vincristine sulfate, methanol and aqueous extract of T. versicolor (METV & AETV) on different concentrations.
4.4. Molecular docking study
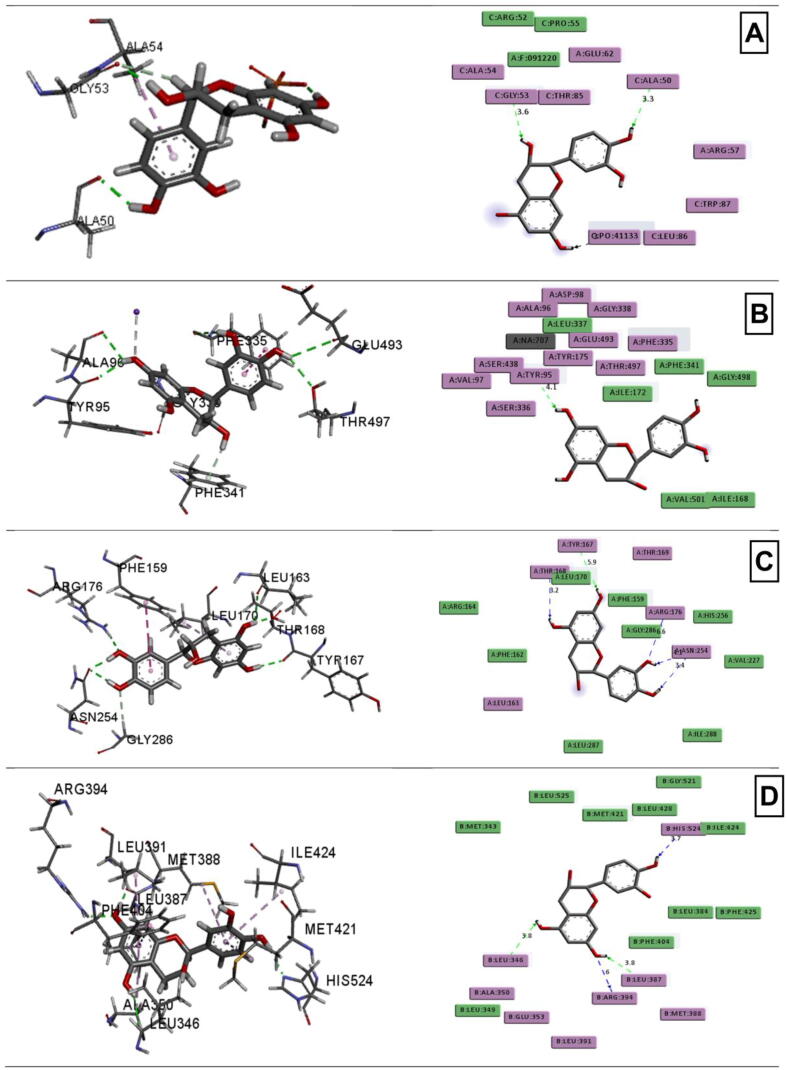
Catechin demonstrated the highest docking score towards the potassium channel receptor (−5.607 kcal/mol), human serotonin transporter receptor (−8.26 kcal/mol), urate oxidase receptor (−5.847 kcal/mol) and human estrogen receptor (−9.921 kcal/mol). The docking score of Catechin was higher than the standard drugs (Table 3 and Fig. 5).
Table 3.
Docking score of phenolic compounds from T. versicolor.
| Compounds | Docking score (kcal/mol) |
||||||
|---|---|---|---|---|---|---|---|
| 4UUJ | 5I6X | 1R4U | 1ERR | ||||
| Baicalin | −4.694 | −7.858 | −4.939 | −5.159 | |||
| Quercetin | −5.36 | −7.131 | −5.214 | −7.991 | |||
| Catechin | −5.607 | −8.26 | −5.847 | −9.921 | |||
| p-Hydroxybenzoic acid | −3.904 | −4.642 | −4.712 | −5.89 | |||
| Quinic acid | – | −6.595 | −5.195 | −5.728 | |||
| Standard drug | |||||||
| Diazepam | Imipramine | AA | VS | −3.035 | −7.358 | −4.512 | – |
Fig. 5.
Molecular docking interaction of phenolic compound catechin towards different receptors. (A) Potassium channel receptor interaction with catechin, (B) human serotonin transporter receptor with catechin, (C) urate oxidase receptor with catechin, and (D) human estrogen receptor with catechin.
5. Discussion
The current study was carried out to evaluate the CNS depressant activities of methanol and aqueous extract of T. versicolor in mice using six neuropharmacological methods, which include open field, hole-cross, forced swimming, thiopental sodium-induced sleeping time, hole-board and rotarod tests. These methodologies are commonly used for neuropharmacological screening models.
The methanol and aqueous extract of T. versicolor demonstrated a noticeable decreased in the square movements and crossing of the hole in the open field, and hole-cross tests in mice. The findings indicated that the extract reduced locomotives' activity, which confirms their CNS depressing effects, whereas the open field, and hole-cross test used to assess the locomotor activity. Locomotive behaviour is considered a guide of alertness, and any reduction in locomotion indicates the effect of CNS-depressant (Sousa et al. 2004; Gahlot et al. 2013). This measured the excitability level of the CNS and can be attributable to the CNS depressing effect of the plant extract by reducing motor activity (Mansur et al. 1980; Rakotonirina et al. 2001). Both extracts on the open field, and hole-cross tests reduced the locomotion in the mice substantially. The METV and AETV-treated mice demonstrated a significant decrease in the head‐dipping from the hole-board test, suggesting an increased fearfulness. Here, the hole board apparatus assesses the exploratory behaviour of mice individually from the locomotor assay (Takeda et al., 1998, Brown and Nemes, 2008). Earlier, decreased head-dipping behaviour has been described as anxiety behaviour in mice (Saitoh et al., 2006). Several neuro-system (gamma‐aminobutyric acid, serotonin, and dopamine) dysregulation might be responsible for anxiety disorders (Murrough et al., 2015). Motor coordination effect of the extract was observed by rotarod test, whereas the extracts showed a non-significant motor coordination effect which suggesting the antagonistic effect on GABA receptor (Vikas and Payal, 2010, Tirumalasetti et al., 2015), indicating CNS-depressant effect.
The forced swimming test is typically used in rodent models to evaluate antidepressant activity. The decreased percentage in immobility time denotes antidepressant activity, while extended immobility reflects a CNS-depression response (Subarnas et al., 1993). The lowered concentration of neurochemicals such as dopamine, norepinephrine and dopamine are responsible for depression. Concurrently, any of the antidepressant drugs act on at least one of these chemical transmitters and increase their activity (Southwick et al., 2005, Berton and Nestler, 2006, Osanloo et al., 2016). The forced swimming test study, in which METV and AETV extract after administration decreased the immobility time in mice, the label that extracts from this plant might increase at least one of the neurotransmitters intricate in depression (Beck and Alford, 2009, Osanloo et al., 2016), while standard diazepam also showed CNS depressant effects on the forced swimming test on mice model. The test for the sleeping time was used to observe sedative-hypnotic drugs in the Swiss albino mouse, while thiopental sodium refers to the barbiturate groups which induce sleep in human and rodent groups (Huang et al. 2007). As such, thiopental induce hypnosis through postsynaptic GABA mediated inhibition initiated by the allosteric modification of the GABAA receptors. CNS depressant components reduce the onset or duration of sleeping or both (Nyeem et al., 2006, Hasan et al., 2009). Our results unveiled that the higher dose of METV and lower dose of AETV has maximal CNS depressant effect.
Many studies have shown that phenols, flavonoids, saponins, and tannins containing plants help many CNS disorders (Bhattacharya and Satyan 1997), whereas the T. versicolor reported 28 phenolic compounds (Puia et al., 2018). This phenolic compound may therefore be mainly accountable for its antioxidant activity. Oxidative imbalance by overproduction of ROS leads to impair the protein and nucleic acids (Zhao et al. 2008). As a result, it causes neuronal dysfunction associated with depressive disorder development (Atmaca et al., 2004, Ng et al., 2008). Although the oxidative condition of antidepressants is improved, their mechanisms of action remain unclear. According to the hypothesis, antidepressants act as a primary or direct effect to restore noradrenergic and serotoninergic neurotransmitter systems to normal levels, while antioxidant efforts are a secondary effect (Maes et al., 2000, Tsuboi et al., 2006, Sarandol et al., 2007). Antioxidants are an amazing resource for neutralizing free radicals in lipid chains, which directly convert phenolic groups into stable free radicals that do not trigger further lipid oxidation (Uttara et al., 2009). The METV and AETV have a substantial IC90 value, whereas the METV was higher than the standard ascorbic acid, which could be an alternative approach to treat depression-related disorders.
The polysaccharides from the T. versicolor were reported to have a significant toxicity level in the cancer cell. At the same time, they exposed a cell growth inhibitory and apoptosis effect on the cancer cell. According to the recent review by Habtemariam, 2020, the T. versicolor has a significant impact on a different cancer cell line, which was studied in several in vitro cancer/tumour cell models and also in tumour-bearing mice models (Habtemariam 2020). Generally, the LC50, which is lower than 100 µg/mL, is signified as highly toxic (Nguta et al., 2011), while in our present study, the METV and AETV reported high cytotoxicity in brine shrimp lethality bioassay.
Computer-Aided Drug Design (CADD) is now an essential tool in the complex process of new drug discovery and development. Simultaneously, molecular docking is a widely known CADD approach that predicts the interaction between the ligands and receptors (Emon et al., 2020b, Rudra et al., 2020). In our present molecular docking study, potassium channel receptor, human serotonin transporter receptor, urate oxidase receptor and human estrogen receptor are used for the CNS, antioxidant and cytotoxicity activities against five phenolic compounds of T. versicolor, whereas the catechin showed better and highest docking interaction than other four phenolics and standard drugs. Catechin is a phenolic compound that has neuroprotective effects (Wang et al. 2016). Molecular docking of phenolic compounds of T. versicolor towards the different receptors was executed to correlate the observed pharmacological effects with the computational study.
6. Conclusion
The present study displays the analysis of different solvents of T. versicolor on CNS depressant activity in mouse models and a significant antioxidant and cytotoxic activity. Five phenolic compounds have interacted with the various receptors, and catechin has shown a strong binding affinity. These effects might be responsible for the phenolic compounds, which require further research.
CRediT authorship contribution statement
S. M. Moazzem Hossen: Conceptualization, Research design, Acquisition, Supervision, Writing - review & editing. Mohammad Akramul Hoque Tanim: Research design, Investigation, Formal analysis, Data curation, Software, Writing - review & editing. Mohammad Shahadat Hossain: Formal analysis, Data curation, Writing - review & editing. Saad Ahmed Sami: Formal analysis, Data curation, Writing - review & editing. Nazim Uddin Emon: Research design, Methodology, Validation, Software, Writing - original draft, Writing - review & editing. Finally, all the authors reviewed and agreed to publish this research.
Acknowledgments
Acknowledgements
We are thankful to the Department of Pharmacy, University of Chittagong for technical and laboratory support.
Funding
Not applicable.
Competing interests
The authors have no known competing interests.
Ethical Consideration
In keeping with the ethical principles outlined in the Helsinki 2013 Declaration (Emanuel, 2013, Ndebele, 2013) all biological activity evaluation was carried out. The animals were treated in compliance with the values of the Swiss Academy of Sciences and Swiss Academy of Medical Sciences and have been euthanized according to the recommendations for animal euthanasia: 2013 edition.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alam S., Emon N.U., Shahriar S., Richi F.T., Haque M.R., Islam M.N., Sakib S.A., Ganguly A. Pharmacological and computer-aided studies provide new insights into Millettia peguensis Ali (Fabaceae) Saudi Pharm. J. 2020 doi: 10.1016/j.jsps.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmaca M., Tezcan E., Kuloglu M., Ustundag B., Tunckol H. Antioxidant enzyme and malondialdehyde values in social phobia before and after citalopram treatment. Eur. Arch. Psychiatry Clin. Neurosci. 2004;254(4):231–235. doi: 10.1007/s00406-004-0484-3. [DOI] [PubMed] [Google Scholar]
- Beck, A.T., Alford, B.A., 2009. Depression: Causes and treatment, University of Pennsylvania Press.
- Berman H.M., Battistuz T., Bhat T., Bluhm W.F., Bourne P.E., Burkhardt K., Feng Z., Gilliland G.L., Iype L., Jain S. The protein data bank. Acta Crystall. Section D: Biol. Crystall. 2002;58(6):899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- Berton O., Nestler E.J. New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 2006;7(2):137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S.K., Satyan K.S. Experimental methods for evaluation of psychotropic agents in rodents: I-Anti-anxiety agents. Indian J. Exp. Biol. 1997;35(6):565–575. [PubMed] [Google Scholar]
- Brown G.R., Nemes C. The exploratory behaviour of rats in the hole-board apparatus: is head-dipping a valid measure of neophilia? Behav. Process. 2008;78(3):442–448. doi: 10.1016/j.beproc.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzozowski A.M., Pike A.C.W., Dauter Z., Hubbard R.E., Bonn T., Engström O., Öhman L., Greene G.L., Gustafsson J.-Å., Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389(6652):753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- Cfr Ferreira I., Vaz J.A., Vasconcelos M.H., Martins A. Compounds from wild mushrooms with antitumor potential. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 2010;10(5):424–436. doi: 10.2174/1871520611009050424. [DOI] [PubMed] [Google Scholar]
- Coleman J.A., Green E.M., Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature. 2016;532(7599):334–339. doi: 10.1038/nature17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Lucki I. Antidepressant-like behavioral effects mediated by 5-hydroxytryptamine2C receptors. J. Pharmacol. Exp. Ther. 2000;295(3):1120–1126. [PubMed] [Google Scholar]
- Dunham N.W., Miya T.S., Edwards L.D. The pharmacological activity of a series of basic esters of mono-and dialkylmalonic acids. J. Am. Pharm. Assoc. 1957;46(1):64–66. doi: 10.1002/jps.3030460119. [DOI] [PubMed] [Google Scholar]
- Emanuel E.J. Reconsidering the declaration of Helsinki. The Lancet. 2013;381(9877):1532–1533. doi: 10.1016/s0140-6736(13)60970-8. [DOI] [PubMed] [Google Scholar]
- Emon N.U., Alam S., Rudra S., Chowdhury S., Rajbangshi J.C., Ganguly A. Evaluation of pharmacological potentials of the aerial part of Achyranthes aspera L.: in vivo, in vitro and in silico approaches. Adv. Traditional Med. 2020:1–14. [Google Scholar]
- Emon N.U., Alam S., Rudra S., Riya S.R., Paul A., Hossen S.M., Kulsum U., Ganguly A. Antidepressant, anxiolytic, antipyretic, and thrombolytic profiling of methanol extract of the aerial part of Piper nigrum: In vivo, in vitro, and in silico approaches. Food Sci. Nutrit. 2021;9(2):833–846. doi: 10.1002/fsn3.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emon N.U., Jahan I., Sayeed M.A. Investigation of antinociceptive, anti-inflammatory and thrombolytic activity of Caesalpinia digyna (Rottl.) leaves by experimental and computational approaches. Adv. Tradit. Med. 2020:1–9. [Google Scholar]
- Festa F., Aglitti T., Duranti G., Ricordy R., Perticone P., Cozzi R. Strong antioxidant activity of ellagic acid in mammalian cells in vitro revealed by the comet assay. Anticancer Res. 2001;21(6A):3903–3908. [PubMed] [Google Scholar]
- File S.E., Pellow S. The effects of PK 11195, a ligand for benzodiazepine binding sites, in animal tests of anxiety and stress. Pharmacol. Biochem. Behav. 1985;23(5):737–741. doi: 10.1016/0091-3057(85)90064-4. [DOI] [PubMed] [Google Scholar]
- Friesner R.A., Banks J.L., Murphy R.B., Halgren T.A., Klicic J.J., Mainz D.T., Repasky M.P., Knoll E.H., Shelley M., Perry J.K. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004;47(7):1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- Friesner R.A., Murphy R.B., Repasky M.P., Frye L.L., Greenwood J.R., Halgren T.A., Sanschagrin P.C., Mainz D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein− ligand complexes. J. Med. Chem. 2006;49(21):6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- Gahlot K., Lal V.K., Jha S. Anticonvulsant potential of ethanol extracts and their solvent partitioned fractions from Flemingia strobilifera root. Pharmacognosy Res. 2013;5(4):265. doi: 10.4103/0974-8490.118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habtemariam S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: targets and efficacy. Biomedicines. 2020;8(5) doi: 10.3390/biomedicines8050135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.M., Hossain M., Akter R., Jamila M., Mazumder M., Hoque E., Rahman S. Sedative and anxiolytic effects of different fractions of the Commelina benghalensis Linn. Drug Discoveries & Therapeutics. 2009;3(5) [PubMed] [Google Scholar]
- Hela A.E., Abdullah A. Antioxidant and antimicrobial activities of methanol extracts of some Verbena species: In vitro evaluation of antioxidant and antimicrobial activity in relation to polyphenolic content. J. Appl. Sci. Res. 2010;6:683–689. [Google Scholar]
- Huang F., Xiong Y., Xu L., Ma S., Dou C. Sedative and hypnotic activities of the ethanol fraction from Fructus Schisandrae in mice and rats. J. Ethnopharmacol. 2007;110(3):471–475. doi: 10.1016/j.jep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Kamiyama M., Horiuchi M., Umano K., Kondo K., Otsuka Y., Shibamoto T. Antioxidant/anti-inflammatory activities and chemical composition of extracts from the mushroom Trametes versicolor. Int. J. Nutr. Food Sci. 2013;2(2):85–91. [Google Scholar]
- Khatun A., Rahman M., Rahman M.M., Hossain H., Jahan I.A., Nesa M.L. Antioxidant, antinociceptive and CNS activities of Viscum orientale and high sensitive quantification of bioactive polyphenols by UPLC. Front. Pharmacol. 2016;7:176. doi: 10.3389/fphar.2016.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotha S., Kulkarni V.M. An in-silico approach: identification of PPAR-γ agonists from seaweeds for the management of alzheimer’s disease. J. Biomol. Struct. Dyn. 2020:1–20. doi: 10.1080/07391102.2020.1747543. [DOI] [PubMed] [Google Scholar]
- Leary S.L., Underwood W., Anthony R., Cartner S., Corey D., Grandin T., Greenacre C., Gwaltney-Brant S., McCrackin M., Meyer R. 2013 edition. American Veterinary Medical Association Schaumburg; IL: 2013. AVMA guidelines for the euthanasia of animals. [Google Scholar]
- Lenaeus M.J., Burdette D., Wagner T., Focia P.J., Gross A. Structures of KcsA in complex with symmetrical quaternary ammonium compounds reveal a hydrophobic binding site. Biochemistry. 2014;53(32):5365–5373. doi: 10.1021/bi500525s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Wen H., Zhang Y., Aa M., Liu X. Purification and characterization of a novel immunomodulatory protein from the medicinal mushroom Trametes versicolor. Sci. China Life Sci. 2011;54(4):379–385. doi: 10.1007/s11427-011-4153-2. [DOI] [PubMed] [Google Scholar]
- Maes M., De Vos N., Pioli R., Demedts P., Wauters A., Neels H., Christophe A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000;58(3):241–246. doi: 10.1016/s0165-0327(99)00121-4. [DOI] [PubMed] [Google Scholar]
- Mansur R.M., Martz W., Carlini E.A. Effects of acute and chronic administration of Cannabis satis and (-) 9-trans tetrahydro cannabinaol on the behaviour of rats in open field arena. Psychopharmacol. 1980;2:5–7. doi: 10.1007/BF00404383. [DOI] [PubMed] [Google Scholar]
- Meyer, B.N., Ferrigni, N.R., Putnam, J.E., Jacobsen, L.B., Nichols, D.E.j., McLaughlin, J.L., 1982. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Medica 45(05), 31–34. [PubMed]
- Michel T.M., Frangou S., Thiemeyer D., Camara S., Jecel J., Nara K., Brunklaus A., Zoechling R., Riederer P. Evidence for oxidative stress in the frontal cortex in patients with recurrent depressive disorder—a postmortem study. Psychiatry Res. 2007;151(1–2):145–150. doi: 10.1016/j.psychres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Muhammad G., Hussain M.A., Anwar F., Ashraf M., Gilani A.H. Alhagi: a plant genus rich in bioactives for pharmaceuticals. Phytotherapy Res. 2015;29(1):1–13. doi: 10.1002/ptr.5222. [DOI] [PubMed] [Google Scholar]
- Murrough J.W., Yaqubi S., Sayed S., Charney D.S. Emerging drugs for the treatment of anxiety. Expert Opin. Emerging Drugs. 2015;20(3):393–406. doi: 10.1517/14728214.2015.1049996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar L., Zahan R., Morshed M.T.I., Haque A., Alam Z., Mosaddik A. Antioxidant, analgesic and CNS depressant effects of Synedrella nodiflora. Pharmacognosy Journal. 2012;4(31):29–36. [Google Scholar]
- Ndebele P. The Declaration of Helsinki, 50 years later. JAMA. 2013;310(20):2145–2146. doi: 10.1001/jama.2013.281316. [DOI] [PubMed] [Google Scholar]
- Ng F., Berk M., Dean O., Bush A.I. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 2008;11(6):851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Nguta J.M., Mbaria J.M., Gathumbi P.K., Kabasa J.D., Kiama S.G. Biological screening of Kenya medicinal plants using Artemia salina (Artemiidae) Pharmacologyonline. 2011;2:458–478. [Google Scholar]
- Nyeem, A.B., Alam, M.A., Awal, M.A., Mostafa, M., Uddin, S.J., Islam, N. Rouf, R., 2006. CNS depressant effect of the crude ethanolic extract of the flowering tops of Rosa damascena.
- Osanloo N., Najafi-Abedi A., Jafari F., Javid F., Pirpiran M., Memar Jafari M.-R., Mousavi Khosravi S.A., Rahimzadeh Behzadi M., Ranjbaran M., Sahraei H. Papaver Rhoeas L. hydroalcoholic extract exacerbates forced swimming test-induced depression in mice. Basic Clin. Neurosci. 2016;7(3):195–202. doi: 10.15412/J.BCN.03070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ÖZgÖR, E., M. Ulusoy, İ. ÇElebİEr, S. Yildiz and N. KeskİN (2016). “Investigation of antimicrobial activity of different Trametes versicolor extracts on some clinical isolates.” Biological Chemistry 43: 267-272.
- Puia I.C., Aida P., Chedea V.S., Leopold N., Bocsan I.C., Buzoianu A.D. Characterization of Trametes versicolor: Medicinal mushroom with important health benefits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2018;46(2):343–349. doi: 10.15835/nbha46211132. [DOI] [Google Scholar]
- Rahman M., Khatun A., Islam S., Akter A., Kawser U. Phytopharmacological evaluation of leaves of olive-Olea europaea. Pharmacologyonline. 2015;1:45–49. [Google Scholar]
- Rakotonirina V.S., Bum E.N., Rakotonirina A., Bopelet M. Sedative properties of the decoction of the rhizome of Cyperus articulatus. Fitoterapia. 2001;72(1):22–29. doi: 10.1016/s0367-326x(00)00243-4. [DOI] [PubMed] [Google Scholar]
- Rana T., Behl T., Mehta V., Uddin M.S., Bungau S. Molecular insights into the therapeutic promise of targeting HMGB1 in depression. Pharmacol Rep. 2021;73(1):31–42. doi: 10.1007/s43440-020-00163-6. [DOI] [PubMed] [Google Scholar]
- Retailleau P., Colloc'h N., Vivares D., Bonnete F., Castro B., El-Hajji M., Mornon J.P., Monard G., Prange T. Complexed and ligand-free high-resolution structures of urate oxidase (Uox) from Aspergillus flavus: a reassignment of the active-site binding mode. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 3):453–462. doi: 10.1107/S0907444903029718. [DOI] [PubMed] [Google Scholar]
- Roos K., Wu C., Damm W., Reboul M., Stevenson J.M., Lu C., Dahlgren M.K., Mondal S., Chen W., Wang L. OPLS3e: Extending force field coverage for drug-like small molecules. J. Chem. Theory Comput. 2019;15(3):1863–1874. doi: 10.1021/acs.jctc.8b01026. [DOI] [PubMed] [Google Scholar]
- Rudra S., Tahamina A., Emon N.U., Adnan M., Shakil M., Chowdhury M., Uddin H., Barlow J.W., Alwahibi M.S., Soliman Elshikh M. Evaluation of various solvent extracts of Tetrastigma leucostaphylum (Dennst.) Alston leaves, a Bangladeshi traditional medicine used for the treatment of Diarrhea. Molecules. 2020;25(21):4994. doi: 10.3390/molecules25214994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh A., Hirose N., Yamada M., Yamada M., Nozaki C., Oka T., Kamei J. Changes in emotional behavior of mice in the hole-board test after olfactory bulbectomy. J. Pharmacol. Sci. 2006;102(4):377–386. doi: 10.1254/jphs.fp0060837. [DOI] [PubMed] [Google Scholar]
- Saleem A.M., Hidayat M.T., Jais A.M.M., Fakurazi S., Moklas M., Sulaiman M.R., Amom Z. Antidepressant-like effect of aqueous extract of Channa striatus fillet in mice models of depression. Eur. Rev. Med. Pharmacol. Sci. 2011;15(7):795–802. [PubMed] [Google Scholar]
- Sarandol A., Kirli S., Akkaya C., Ocak N., Eroz E., Sarandol E. Coronary artery disease risk factors in patients with schizophrenia: effects of short term antipsychotic treatment. J Psychopharmacol. 2007;21(8):857–863. doi: 10.1177/0269881107077609. [DOI] [PubMed] [Google Scholar]
- Shokrzadeh M., Azdo S., Habibi E. Anti-diabetic effect of methanol extract of Trametes versicolor on male mice. J. Mazandaran Univ. Med. Sci. 2017;26(145):165–175. [Google Scholar]
- Smith A.J., Sketris I., Cooke C., Gardner D., Kisely S., Tett S.E. A comparison of antidepressant use in Nova Scotia, Canada and Australia. Pharmacoepidemiol. Drug Saf. 2008;17(7):697–706. doi: 10.1002/pds.1541. [DOI] [PubMed] [Google Scholar]
- Sousa F.C.F., Melo C.T.V., Monteiro A.P., Lima V.T.M., Gutierrez S.J.C., Pereira B.A., Barbosa-Filho J.M., Vasconcelos S.M.M., Fonteles M.F., Viana G.S.B. Antianxiety and antidepressant effects of riparin III from Aniba riparia (Nees) Mez (Lauraceae) in mice. Pharmacol. Biochem. Behav. 2004;78(1):27–33. doi: 10.1016/j.pbb.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Southwick S.M., Vythilingam M., Charney D.S. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu. Rev. Clin. Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- Subarnas A., Tadano T., Nakahata N., Arai Y., Kinemuchi H., Oshima Y., Kisara K., Ohizumi Y. A possible mechanism of antidepresant activity of beta-amyrin palmitate isolated from Lobelia inflata leaves in the forced swimming test. Life Sci. 1993;52(3):289–296. doi: 10.1016/0024-3205(93)90220-w. [DOI] [PubMed] [Google Scholar]
- Sultana T., Mannan M.A., Ahmed T. Evaluation of central nervous system (CNS) depressant activity of methanolic extract of Commelina diffusa Burm. in mice. Clin. Phytosci. 2018;4(1):5. [Google Scholar]
- Takagi K., Watanabe M., Saito H. Studies of the spontaneous movement of animals by the hole cross test; effect of 2-dimethyl-aminoethanol and its acyl esters on the central nervous system. Japanese J. Pharmacol. 1971;21(6):797–810. doi: 10.1254/jjp.21.797. [DOI] [PubMed] [Google Scholar]
- Takeda H., Tsuji M., Matsumiya T. Changes in head-dipping behavior in the hole-board test reflect the anxiogenic and/or anxiolytic state in mice. Eur. J. Pharmacol. 1998;350(1):21–29. doi: 10.1016/s0014-2999(98)00223-4. [DOI] [PubMed] [Google Scholar]
- Tirumalasetti J., Patel M., Shaikh U., Harini K., Shankar J. Evaluation of skeletal muscle relaxant activity of aqueous extract of Nerium oleander flowers in Albino rats. Indian J. Pharmacol. 2015;47(4):409–413. doi: 10.4103/0253-7613.161265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi H., Tatsumi A., Yamamoto K., Kobayashi F., Shimoi K., Kinae N. Possible connections among job stress, depressive symptoms, lipid modulation and antioxidants. J. Affect. Disord. 2006;91(1):63–70. doi: 10.1016/j.jad.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Uddin Mazumdar M.M., Islam M.A., Hosen M.T., Alam M.S., Alam M.N., Faruk M., Rahman M.M., Sayeed M.A., Rahman M.M., Uddin S.B. Estimation of in vivo neuropharmacological and in vitro antioxidant effects of Tetracera sarmentosa. Cogent Biology. 2017;3(1):1300990. [Google Scholar]
- Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikas G., Payal M. Phytochemical and pharmacological potential of Nerium oleander: a review. Int. J. Pharm. Sci. Res. (IJPSR) 2010;1(3):21–27. [Google Scholar]
- Wang Y.X., Engelmann T., Xu Y.F., Schwarz W. Catechins from green tea modulate neurotransmitter transporter activity in Xenopus oocytes. Cogent Biol. 2016;2(1):1261577. [Google Scholar]
- Yao Y., Jia M., Wu J.-G., Zhang H., Sun L.-N., Chen W.-S., Rahman K. Anxiolytic and sedative-hypnotic activities of polygalasaponins from Polygala tenuifolia in mice. Pharm. Biol. 2010;48(7):801–807. doi: 10.3109/13880200903280042. [DOI] [PubMed] [Google Scholar]
- Zhang J.-C., Wu J., Fujita Y., Yao W., Ren Q., Yang C., Li S.-X., Shirayama Y., Hashimoto K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 2015;18(4) doi: 10.1093/ijnp/pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]