Abstract
Objective
Taking leads from the available research, we aimed to develop a synergy-based herbal combination of Tinospora cordifolia (TC), Phyllanthus emblica (PE), and Piper nigrum (PN). Also, evaluating their synergistic effect on CP-induced immunosuppression in mice model and exploring the possible mechanisms involved in reversing the damage.
Methodology
The immunomodulatory activity of combination, of TC stem, PE fruits, and PN dried fruits, was determined by in vitro assays (splenocyte proliferation and pinocytic activity of peritoneal macrophages of mice) and in vivo study using CP-induced immunosuppression model in Swiss Albino mice. The ratio was optimized for combining three by in vitro MTT assay. The combination was further evaluated for anti-oxidant activity by DPPH scavenging method and quantified for its bioactive metabolites by HPTLC. Serum collected on day 0, 4, 7 and 14 was employed for estimation of haematogram (haematocrit, TLC, DLC, and haemoglobin, etc) and immune parameters (IL-10, IL-6 and TNF-α) by ELISA.
Results
The study demonstrated, that combination of herbal extracts at an intermediate dose could inhibit the proliferation of spleen cells and peritoneal macrophages (P ≤ 0.0001) and induce suppression of pro-inflammatory mediators, and also certified that combination exerts synergized effects. The results showed that the combination possess potential antioxidant activity by DPPH scavenging method (IC50-113.5 µg/ml). It was identified that combination significantly (P ≤ 0.0001) improved the immune markers, haematogram parameters, and histological parameters, with maximum protection offered by an intermediate dose.
Conclusion
The results suggested that present combination could be further explored clinically as potent synergy-based therapeutic approach for immune modulation.
Keywords: Immunomodulation, Herbal, In vivo, Immunity, Pinocytic assay, Synergy-based combination
1. Introduction
The human immune system is responsible for protection against pathogens, microbes and several diseases by producing immediate response through specific receptors. Infections, external harms, or pathogenic microbes lead to interaction of host with microorganism and disruption of homeostasis. Therefore, immunomodulation of immune response could provide as a substitute for a variety of disease conditions with immunodeficiency (Khodadadi, 2015).
Currently available immunomodulators such as levamisole, glucans, telerones, and L-fucose further causes immunosuppression, leading to adverse effects on human health, especially flu-like symptoms, fatigue, nausea, neutropenia, thrombocytopenia, depression, bone marrow damage and agranulocytosis that can also lead to other diseases. Due to the occurrence of chemical drugs-related adverse effects, natural immunomodulators are being looked upon as the potential agents to replace the chemical-based agents in therapeutic regimens (Jantan et al., 2019). The prevention and treatment of disease using plant-based medicines can be traced in human history, in all cultures and through all ages against all kinds of ailments. Several types of immunomodulators have been identified, including substances isolated and purified from plants. It is estimated by World Health Organization (WHO) that approximately 80% of the world’s population rely on natural remedies or herbal drugs, mainly plant origin drugs, for their primary health care (Makare et al., 2001). Immunomodulation by herbal drugs is known to stimulate both specific and non-specific immunity and thereby possess immunomodulatory property.
In this study, three plants have been using traditionally for therapeutic and neutraceutical purposes since decades namely, Phyllanthus emblica (PE), Tinospora cordifolia (TC) and Piper nigrum (PN) were chosen amongst eight plants screened for in vitro immunomodulatory potential.
Aamla, P. emblica (PE) (syn. Emblica officinalis), commonly known as Indian gooseberry, (family: Euphorbiaceae) is an important medicinal plant in the traditional system of Indian medicine. The fruit of Amla, rich in ascorbic acid possess immunomodulatory properties that enhances lymphocytes cells development (Sharma et al., 2017) due to presence of bioactive phytochemicals mainly polyphenols, tannins, flavonoids, glycosides and proanthocyanidins.
Giloy T. cordifolia (Willd.) (TC) commonly known as the Guduchi, (family: Menispermaceae) Various chemical constituents derived from T. cordifolia such as glycosides, phenolics, alkaloids, diterpenoid lactones, steroids, sesquiterpenoid, polysaccharides and aliphatic compounds (Bishayi et al., 2002) responsible for its pharmacological activities like anti-oxidant, anti-inflammatory, anti-periodic, anti-spasmodic, anti-arthritic, anti-allergic and anti-diabetic properties (Singh et al., 2003, Sharma et al., 2012).
Kali mirch, P. nigrum (PN) (family: Piperaceae) commonly known as Black pepper, has traditionally been used and its anti-hypertensive, anti-oxidant, anti-platelet, immunomodulatory, anti-tumor, anti-spasmodic, analgesic, anti-inflammatory, anti-depressants, anti-fungal, anti-bacterial, hepatoprotective activities are exhibited due to numerous bioactive compounds such as lignans derivatives, alkaloids, phenolics, flavonoids, terpenes, chalcones, steroids, piper-amine, dihydropipericide etc. It increases the bioavailability of many nutrients and drugs by inhibiting various metabolizing enzymes (Sabina et al. 2013).
Cyclophosphamide (CP), a widely used chemotherapeutic drug results in a lot of adverse effects including immunosuppression, bone marrow suppression, leucopenia and oxidative stress (Pass et al., 2005, Wang et al., 2018). It has been reported that CP generates oxidative stresses and lead to biochemical and physiological disturbances. Further, administration of CP can damage the Th1/Th2 balance and induce a reduction in the absolute counts of T cells and B cells (Qi et al., 2018).
The synergy-based herbal formulations composed of multiple herbal extracts have gathered attention of researchers in recent years. Synergistic effect of polyherbal extract-based formulation is associated with its improved efficacy. The approach for synergy-based formulations is on the fact that various diseases are caused by numerous factors and have complicated underlying pathophysiology. Therefore, such formulations are believed capable of enhancing the bioavailability of active constituents, amplifying therapeutic effects, and diminishing their toxic effects (Zhou et al., 2016). The adverse effects are neutralized by multiple components present in the extract formulation (Wagner and Marzenich, 2009, Yuan et al., 2017). Technologies and the excessive research on immunomodulatory natural products, plants, their extracts with immunomodulatory potential, may provide us with valuable entities to develop novel synergy-based immunomodulatory formulation to complement current chemotherapies.
Therefore, taking leads from the available research, we used a murine model of cyclophosphamide-induced immunosuppression in Swiss Albino mice to investigate the immunomodulatory effect of the synergy-based polyherbal combination.
2. Materials and methods
2.1. Chemicals and solvents
Cyclophosphamide (CP) injection (IP, 500 mg), Endoxan-N (Cadila Healthcare Limited AEU1025), was used as standard immunosuppressant. Levamisole tablet 50 mg, (Khandelwal Laboratories) and Septilin tablet (comprising of many ingredients including T. cordifolia, P. emblica, Licorice, Indian bdellium, P. nigrum etc), 324 mg (The Himalaya Drug Company), were used as allopathic and herbal standard drugs, respectively. All the other reagents and chemicals used in in vitro as well as in vivo studies were of analytical grade. The kits for IL-6 (Cat. no. E0049Mo), IL-10 (Cat no. E0022Mo) and TNF-α (Cat no. E0117Mo), were procured from Shanghai Korain Biotech Co., Ltd, China and the analysis were carried out in Molecular Biology Laboratory, Jamia Hamdard.
2.2. Plant material and preparation of extracts
The PE fruits, TC stem and PN dried fruits were obtained from Universal Trading Company, New Delhi, India and authenticated as per the standard protocol specified in Unani Pharmacopoeia of India. The authenticated plant materials have been deposited in the Bioactive Natural Product Laboratory for future reference with a voucher specimen number JH/SIST/BNPL/ABIDA/2017/PE, TC and PN, respectively. The plant sample was washed, shade dried, and coarsely powdered. The powdered drug material (100 g) of each plant was transferred into a flask containing water (1L) and was macerated for 24 h and extracted through reflux. Then, the suspension was filtered and the filtrate was subjected to dryness under reduced pressure. The extractive value and % yield of extracts were calculated and stored at 4 °C for bioactivity and quantitative analysis.
2.3. Determination of flavonoid and phenolic contents
Total phenols were determined based on a colorimetric assay (Parveen et al., 2019). A dilute extract of combination (0.5 ml of 10 mg/ml) and gallic acid (standard phenolic compound) was mixed with Folin Ciocalteu reagent (5 ml; Flavonoid 1:10 diluted with deionized water) and aqueous Na2CO3 (4 ml, 1 M). The mixture was allowed to stand for 15 min and the total phenols were determined by colorimetry at 765 nm. The standard curve was prepared using 25, 50, 100, 150, 200, 250 and 300 µg/ml solutions of gallic acid in methanol. Flavonoid content was determined using aluminum chloride colorimetric method. Combination of extracts (0.5 ml of 10 mg/ml) in methanol were separately mixed with 1.5 ml of methanol, 0.1 ml of 10% aluminum chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water incubated at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm. Rutin dissolved in methanol at concentrations of 10 to 100 µg/ ml was used as standard (Amir et al., 2011).
2.4. In vitro antioxidant (2, 2-diphenyl-1-picrylhydrazyl) DPPH free radical scavenging activity
The DPPH free radical scavenging activity of herbal combination was determined as per the previous protocol with slight modifications (Parveen et al., 2019). The prepared DPPH solution of 1 ml (0.3 mM in methanol) was mixed with 1 ml of obtained combination dose dissolved in water. The stock solution of different concentrations of obtained combination (1000, 500, 250, 125, 62.5, 31.25, 15.62 and 7.81 μg/ml) were prepared by serial dilution method. The reaction mixture was shaken intensely and incubated in dark for 30 min, and samples were analyzed in triplicate. Optical density was measured with a microplate reader at 517 nm using a spectrophotometer, and the percentage inhibition was calculated using the following formula:
where, Acontrol is the absorbance of the DPPH solution without extract; Asample is the absorbance of the sample with the DPPH solution.
2.5. Experimental animals, housing and husbandry
BALB/c mice of either sex (Grade II, 6 weeks old) weighing 18–22 g were procured for in vitro immunomodulatory study (Animal approval Number 1551) whereas Swiss albino mice of either sex weighing between 30 to 45 g were employed for in vivo study (Animal approval number-1575). Animals were procured from CAHF, Jamia Hamdard (173/GO/Re/S/2000/CPCSEA, 20th January 2000), New Delhi. Animals were allowed to acclimatize to the laboratory environment for 3 days and were bred and maintained under standard laboratory conditions (temperature 25 °C ± 2 °C and humidity of 50 ± 10% RH, and 12/12 h light–dark cycle). The experiments were performed in light cycle (09:00–16:00 h) in awaken conditions. A commercial pellet diet and tap water were given ad libitum.
2.6. Ethical statement
The protocol of study was approved by the Institutional Animal Ethics Committee (IAEC), Jamia Hamdard, New Delhi, India and the experimental procedures strictly followed the guidelines of CPCSEA and international standards on the Care and use of Experimental Animals (CCAC, 1993). Animal Research Reporting of in vivo Experiments (ARRIVE) guidelines were followed for reporting the data.
2.7. Proliferation assay of mouse spleen cells
Bagg Albino (BALB/c) mice were killed by cervical dislocation. The spleens of BALB/c mice were excised, cut into small pieces and homogenized with 5 ml phosphate buffer solution (PBS), passed through a mesh of 0.4 µm and centrifuged for 5 mins, to obtain a suspension of isolated spleen cells of mice. Red cells of spleen suspension were lysed by adding 4 ml solution of Tris hydrochloride ammonium chloride (tris- HCl –NH4Cl) (pH 7.2) and vortexed for 2 min. Roswell Park Memorial Institute (RPMI) medium (RPMI-1640, Millipore Sigma, India) was added and washed twice to remove any debris present. Further, cell pellets were re-suspended in 15 ml of RPMI-1640 medium with 15 ml 10% Foetal Bovine serum (FBS) centrifuged at 4000 rpm to get 3.0 × 106 cells/mL. Then, 100 µL of cell pellets were seeded in 96 well plates, 0.4 × 106 cells per well and 25 µL of mitogen Con A (2.0 μg/mL) were added to each well and 25 µL of drug extract was added with already having spleen cells in presence of Con A. Negative control (media treated with drug extract) and positive control (splenocytes treated with levamisole) were treated with RPMI-1640 medium and LNT, respectively and cultures were incubated at 37 °C in a CO2 incubator for 72 h. After 72 h incubation, 20 µL of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was added in each well plate and incubated further for 6 h (Shi and Fu, 2011). The optical density of splenocytes was measured at 490 nm using ELISA reader and the stimulation index (SI) was calculated by the following formula
2.8. Pinocytic assay of mice peritoneal macrophages
Peritoneal macrophages were isolated from two BALB/ c mice and 3 ml of thioglycolate, phosphate buffer saline (PBS) was injected after 3 days; and sterile peritoneal lavage was performed with 10 ml of PBS. Finally, 4 ml of peritoneal macrophages were collected. The isolated macrophages were washed twice with media and re-suspended at 1.0 x106 cells per mL in RPMI medium containing 10% FBS. RPMI medium served as negative control, collected cells were seeded and 200 µL were transferred in each well plate of 96 well plates and 25 µL of antibiotics (Penicillin + Streptomycin) were added and then the culture was incubated at 37 °C in a CO2 incubator for 3 h to allow macrophages to adhere to the plate. The non-adherent cells were gently washed with RPMI medium, and 100 μL of fresh RPMI media was added to each well. After which, 25 µL of each drug extract was added and incubated for 48 h in 5% CO2 at 37 °C. Further, 100 µL of neutral red solution (0.1% in 10 mM PBS) was added and kept for incubation for 2 h. The supernatant was discarded and the cells were washed with PBS twice to remove the neutral red that was not phagocytized by macrophage. Then, cell lysate (ethanol and 0.01% acetic acid at the ratio of 1:1, 100 μL/well) was added. The mixture was incubated at room temperature overnight, and the optical density was measured next day at 540 nm (Meng et al., 2018).
2.9. Combination index
The combined effects of TC, PE and PN were evaluated using combination index. The ratio was obtained for combining three drugs by in vitro pinocytic and spleen proliferation assay. The cell viability and inhibition percentage were determined by in vitro MTT assay. Combination index was calculated as:
where, PE- Phylanthus emblica, TC- Tinospora cordifolia, PN- Piper nigrum
The concentration of PE, TC and PN were used in combination to achieve 50% drug effect. Inhibitory concentration of each drug is the concentration for each single drug to achieve the same effect. CI value < 1, =1 and > 1 indicate synergistic, additive and antagonistic effect, respectively (Chou and Talalay, 1983, Chou and Talalay, 1984).
The concentration demonstrated best outcome was selected from in vitro immunomodulatory assays conducted on each drug and was combined with other five concentrations of each drug to study combined effects as given in Table 1.
Table 1.
The concentration demonstrating best outcome from in vitro immunomodulatory assays conducted on single drugs selected and combination of drugs.
| Drug | Selected Conc. (mg) | Varied concentrations | Best Outcome | Selected Conc. (mg) | Varied concentrations | Best Outcome |
|---|---|---|---|---|---|---|
| In vitro pinocytic assay | In vitro spleen proliferation assay | |||||
| PE | 0.02 | 0.01 0.015 0.02 0.05 0.1 |
PE + TC + PN 0.05 + 0.05 + 0.05 |
1 | 0.1 0.5 1 1.5 2 |
PE + TC + PN 1 + 1 + 1 |
| TC | 0.2 | 0.05 0.1 0.2 0.5 1 |
1 | 0.1 0.5 1 1.5 2 |
||
| PN | 0.02 | 0.01 0.015 0.02 0.05 0.1 |
1 | 0.1 0.5 1 1.5 2 |
Therefore, 25 combinations were prepared for each selected concentration and total 75 combinations were developed for five different concentrations of each drug. Also, percentage viability and % inhibition was calculated. Combination showing highest % inhibition was selected then IC50 was calculated for all three drugs. Hence, the optimized ratio obtained was 0.05: 0.05: 0.05 from pinocytic assay while 1:1:1 from spleen proliferation assay.
2.10. Dose calculation and conversion
The three escalating doses of all three drugs were calculated on the basis of their extractive values and Pharmacopeial dose mentioned in Unani Pharmacopeia of India.
Since, the highest extractive yield was found in aqueous extract, therefore aqueous extracts of all three drugs were chosen. The value for TC, PE and PN extracts were 12.6%, 51.4% and 9.6%, respectively. Although, the extractive yield was higher in hydro-alcoholic extract of PN among all three types of extract but aqueous extract is usually preferred to be used in the formulation of the traditional system and is considered much safer as compared to other extracts. The pharmacopeial doses for TC, PE and PN are 4, 7.5 and 1.5 g, respectively (Anonymous, 2007, Anonymous, 2009).
Therefore, the low, intermediate and high dose of PE and TC were calculated as 400, 600 and 800 mg, respectively. They were combined at a ratio of 1:1 while PN was fixed at 50 mg for all three varying doses as it comes under category 4 drugs or toxic drugs as per the Unani literature but still incorporated in the combination as a bioavailability enhancer. Finally, animal equivalent dose (AED) was calculated by allometric scaling (Nair and Jacob, 2016).
2.11. Quality control analysis by High-performance thin layer chromatography (HPTLC) finger printing
HPTLC was performed on silica gel 60F254, 20 × 10 cm HPTLC plates (Merck, Germany-#5642), with different suitable solvent as a mobile phase. Combination of extracts were applied to the plates as 10 mm bands, sample application with CAMAG-Linomat V (CAMAG, Switzerland) automated spray on band applicator equipped with a 100 µL syringe and operated with following settings. Band length 10 mm, application rate 10 sec/ µL, distance between 4 mm, distance from the plate side edge 1.5 cm and distance from the bottom of the plate 2 cm were optimized. Plates were transferred into a CAMAG twin glass tank with pre-saturated mobile phase for 40 min. After 40 min the plate was gently removed and air dried. Further, the dried plate was scanned at different wavelengths such as 254 and 366 nm. The developed solvent system was toluene: ethyl acetate: formic acid 3:6:1 (v/v/v) for PE and toluene: ethyl acetate: formic acid 6:3:1 (v/v/v) for PN while toluene: ethyl acetate: formic acid 6:10:4 (v/v/v) for TC. Whereas, the developed solvent system for synergy-based combination was toluene: ethyl acetate: formic acid 6:3:1 (v/v/v). The quality control evaluation of combination was performed as per the WHO guidelines (www.ich.org, Nair et al., 2017).
2.12. In vivo experimental study
2.12.1. Study design
The study was conducted in 42 Swiss albino mice. After three days of adaptation, the animals were randomly grouped into seven groups (each, n = 6), where group I and II served as the normal control and the cyclophosphamide-induced immunosuppressed (CPI) control, respectively, group III to V served as treatment groups and received herbal formula with different doses (low, intermediate and high doses), group VI and VII, served as the standard allopathic and herbal standard controls, respectively. The normal control received only phosphate buffered saline (PBS) for 14 days. Animals of group II to VII were induced immunosuppression by CP, intra-peritoneally for three consecutive days in a dose of 80 mg/kg B.W/day. Group III to V received combination of extracts at dose of 145.71 mg/kg B.W., 214.28 mg/kg B.W. and 282.85 mg/kg B.W., respectively for 14 days. Group VI received levamisole HCl at a dose of 10 mg/kg for 14 days started from day 3. Similarly, Group VII received herbal standard (Tab septilin as a suspension dissolved in distilled water) at 85.7 mg/kg for 14 days regularly.
2.12.2. Experimental procedures
Dosing was continued for a period of 14 days, starting at 3rd day after CP-administration. The doses of allopathic and herbal standards were translated from human dose for each drug, using human equivalent dose conversion formula as also described previously during calculation of dose for herbal formula. Blood samples were collected from each animal on 1st day before induction of immunosuppression, on 3rd day i.e. after CP-induction, on 7th day and finally on 14th day i.e. post treatment period, just after 1 h of last treatment dose. Blood of 1 ml was collected through saphenous vein. The body weight was measured before and post treatment and organ indexes were also determined post experiment (Anjum et al., 2017). Finally, animals were sacrificed by CO2 inhalation.
2.12.3. Sample size
The sample size was calculated based on mean values of among the control and herbal treated group from a previous study (Devi et al., 2020). The power of the study was taken to be 95% at α = 0.05 and 95% confidence interval (C.I.), allocation ratio was assigned as 1 and the effect size was calculated to be 4.582. The sample size was calculated to be 3 per group using G power software version 3.2.90. However, we included six animals per group in the study.
2.12.4. Biochemical estimation of hematological parameters
Blood samples were collected in ethylenediamine tetraacetic acid (EDTA) vial from each animal. The complete blood count (CBC) including haemoglobin %, total leucocyte count (TLC), differentiating leucocyte count (DLC) haematocrit value were analysed by using a fully automated haematology analyzer (XP 100, Sysmex, Japan).
2.12.5. Determination of immunological parameters
The blood was collected in plain vial and allowed to stand for 1 h and centrifuged at 3000 rpm for 10 min. The serum was then isolated and stored at − 20 °C until later use. The levels of tumor necrosis factor-α (TNF-α), interleukins 6 and 10 (IL-6 & IL-10) in the supernatants were determined by commercial enzyme linked immunosorbent assay (ELISA), (PowerWave XS2, BioTek Instruments Inc., USA)
2.12.6. Measurement of organ indices and histopathological observation
After 1 h of the blood sampling, the mice were anesthetized with ether and sacrificed by CO2 inhalation. The liver and spleen of the mice were excised, and the excess tissues and fascia were stripped off and weighed. The liver and spleen indices were calculated according to the following equation:
Eventually, the liver and spleen were fixed with 10% formalin solution at room temperature for 24 h. The pathological specimens were routinely sliced (5 µm), embedded in paraffin and stained with haematoxylin and eosin for 10 min at room temperature. Pathological changes were observed under a light microscope (magnification, x100) and images were captured using Moticam 3.0 MP (Hongkong) (Yu et al., 2018).
2.13. Statistical analysis
All values were expressed as mean ± standard deviation (SD). Statistical analysis was performed using One Way Analysis of Variance (ANOVA), and two-way ANOVA (Graph Pad prism version 8.4.3, San Diego, CA) followed by post hoc multiple comparison Tukey’s test. Bonferroni multiple comparison test was used to evaluate the difference over the time. Values of P < 0.05 were considered statistically significant.
3. Results
The percentage yield was calculated for different extracts of the synergy-based combination, where the weight of crude TC, PE and PN was 50 g each. The obtained extractive percentage yield for aqueous extract of PE, TC and PN were 12.6, 51.4, and 9.6%, respectively.
3.1. Exploration of effective extracts combination by combination index
A synergistic immunomodulatory activity was observed when spleen cells as well as peritoneal macrophages were treated by TC, PE and PN. It was found that the immunomodulatory activity of three cell-bound extracts combination surpassed single extracts. Integration effects such as synergism, additive and antagonism may occur simultaneously when multiple bioactive compounds coincide.
In this study, the integration effects of herbal aqueous extracts of TC, PE and PN were evaluated firstly by using various concentrations of cell-bound extracts combinations. The proliferation enhanced significantly when treated with three-extract combinations in comparison with single-extract. Although, the combination of three extracts demonstrated the best inhibitory activity, alike the results of MTT assay above. However, the synergistic effect was observed for TC + PE + PN aqueous extracts combination at most of the concentrations with Confidence interval (CI) values <1.
3.2. In vitro antioxidant activity
3.2.1. Total flavonoid and phenolic contents
Flavonoids and phenolics were measured by aluminium chloride method and Folin-Ciocalteu method, respectively and were found to be as 130.13 ± 2.10 equivalent to gallic acid and 111.16 ± 3.90 µg/ml equivalent to rutin, respectively.
3.2.2. Scavenging effect on 2, 2-diphenyl-1-picryl hydrazyl radical
It is the most widely accepted method used for estimation of antioxidant activity. The present synergy-based combination demonstrated a concentration-dependent scavenging potential by inhibiting of DPPH radical with an IC50 value of 113.6 µg/ml, and 74.7 µg/ml for the standard (Ascorbic acid). The maximum inhibition was found at 300 µg/ml, and remains consistent at 350 µg/ml. The percentage inhibition of scavenging activity of the combination revealed 58.9% DPPH inhibition at 150 µg/mL. Antioxidant activity depends on the presence of amount of total polyphenolic compounds. The scavenging effect increased with rising concentration.
3.3. In vitro immunomodulatory activity
3.3.1. Effect of herbal synergy-based combination on splenocytes proliferation
The spleen contains a relatively homogenous fraction of B and T lymphocytes, consisting of 60% B cells and 40% T cells. The MTT-based splenocytes proliferation assay was performed on specific immune cells and the proliferation effects of various extract combinations of low, intermediate and high doses were analyzed using various stock concentrations, between 10 and 100 μg/ml. The T cell mitogen, concanavalin A (Con A) and levamisole were used as a positive control in the present study.
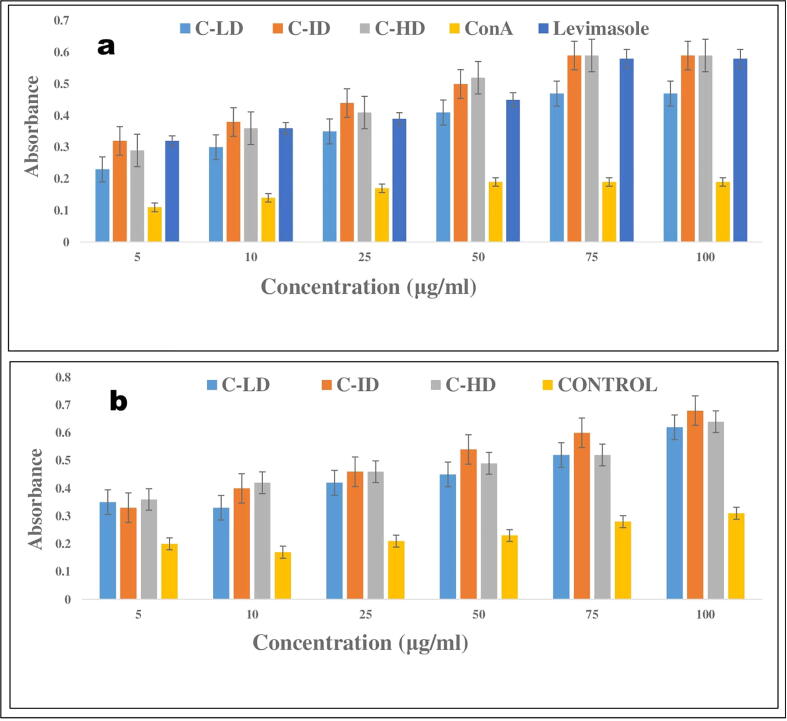
The different combination doses at various concentrations stimulated the spleen cells proliferation of the mice however; highest splenocytes proliferation was demonstrated by combination intermediate dose, which is equivalent to high dose group as well as positive control levamisole group. However, treatment groups and positive control indicated an improved stimulatory effect, exhibiting almost 40% more proliferation than the Con A groups, respectively. Furthermore, the predominant immunostimulatory effect of present herbal synergy-based combination on the mice splenocytes was observed at a concentration of 75 and 100 µg/ml as seen in Fig. 1a. There was no more difference in spleen proliferation found at 75 and 100 µg/ml concentrations.
Fig. 1.
a: The highest proliferation of splenocytes was observed in Combination intermediate dose (C-ID) than other comparative groups of doses combination low dose and high dose (C-LD and C-HD), negative control (Concalavine A) & positive control (Levamisole). The differences between the control and treatment group were measured by using one-way analysis of variance. * P ≤ 0.0001 indicated a statistically highly significant difference vs. treated cells. 1b: Values shown are mean ± SD (triplicate samples per dose). The highest neural red dye uptake was observed in combination of intermediate dose group (C-ID) which is almost equivalent to high dose group (C-HD). The peritoneal macrophages activity is extremely significant statistically in all treatment groups than Con. A group (P < 0.0009). The data was analyzed by one-way of ANOVA variance.
3.3.2. Extracts combination effects on pinocytic activity of mouse peritoneal macrophages
Peritoneal macrophageal pinocytic activity was assessed by neutral red dye uptake. As shown in Fig. 1b, the level of dye uptake by the cells accelerated with increasing concentrations of either combination extract. Pinocytic activity was expressed in terms of absolute optical density (OD) values (reflecting dye uptake) seemingly dose-related manner. The significant difference was observed between the treated and control specifically in intermediate dose. While control cells had average OD540 values of 0.31 ± 0.045 at 100 µg/ml concentration, cells treated with the maximal level at intermediate dose of the combination (C-ID) and combination of high dose (C-HD) had values of 0.68 ± 0.002 and 0.64 ± 0.02 respectively. As displayed in Fig. 1, while the treatment with intermediate dose of synergy-based combination significantly increased the phagocytosis activity of the macrophages (P ≤ 0.05).
3.3.3. Quantitative estimation of biomarkers in synergy-based herbal combination by HPTLC
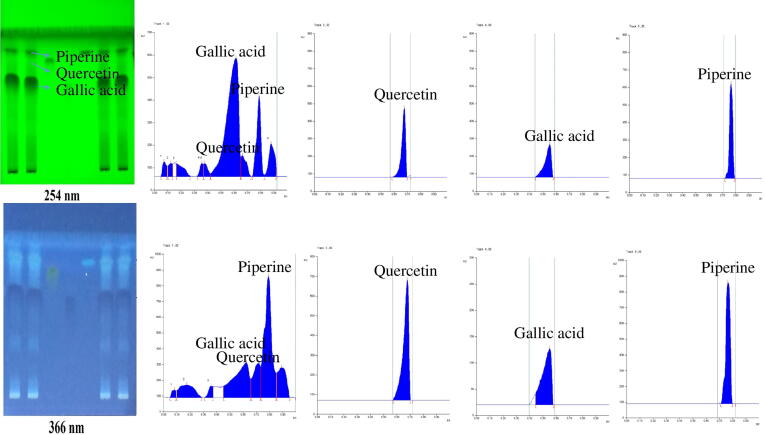
In the present study, quantitative estimation of specific bioactive metabolites, gallic acid, quercetin, and piperine components were conducted in the developed combination using HPTLC. As gallic acid is a common bioactive metabolite; so, it was perfectly represented in the developed chromatogram (Fig. 2) and relatively smaller peak was observed for piperine and quercetin. We used various compositions of mobile phase in order to get better separation and the method was optimized. Among the various solvent systems tried, the solvent system containing toluene: ethyl acetate: formic acid in the volume ratio of 6:3:1 (v/v/v) resulted in good separation of the gallic acid. Thin layer chromatography (TLC) plate was observed under Ultra violet (UV) light for the presence of gallic acid, quercetin and piperine, which was detected by prominent dark brown spots shown in Fig. 5B. The spots developed were dense, compact and typical peaks of gallic acid were obtained. The Rf value (0.85) for gallic acid in both sample and reference standard was found comparable under UV light at 254 and 366 nm. Peaks were symmetrical in nature and no tailing was observed when plates were scanned at 254 nm.
Fig. 2.
HPTLC fingerprinting demonstrated spots and chromatograms for developed combination of extracts, showing piperine, gallic acid and quercetin at 254 and 366 nm, respectively.
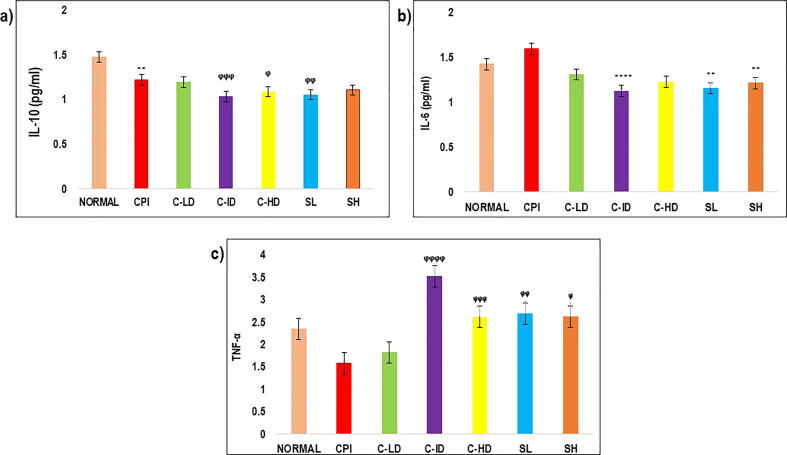
Fig. 5.
(a, b, c): Value are expressed as mean, n = 6, P < 0.01 when normal compared with CPI control (day 7); P < 0.001 when compared CPI with treatment groups (day 7 & 14).
3.3.4. In vivo study
The animal experiment was carried out for 14 days, mice were fed with pellets and water ad libitum. The quantity of pellets and water was measured at an alternate days, where the feed and water intake of the animals were affected immediately post-induction with CP and improved by the end of treatment. While, there was no significant difference recognized for food and water intake among the treated and control groups.
3.4. Body weight
The body weight was declined in all groups post induction by CP. Whereas, the body weight remarkably reversed in treatment and control groups but declined further in CPI group by the end of experiment. There was mild alteration in percentage body weight noticed in combination group of an intermediate dose as compared with other treatment doses group, which was equivalent to levamisole standard group, as shown in Table 2. Thus, it indicated that body weight at day 0 and day 14, didnot affect much in groups received combination doses of synergy-based combination or standard controls, whereas, marked change was recognized in CPI control from day 0 to day 14 due to CP-induction.
Table 2.
Effect of different drugs on organ indices in CP-induced immunosuppression in mice, expressed in mean ± SD (n = 6). Alteration in body weight percentage from day 0 to day 14 in different groups.
| Groups (n = 6) | Change in body weight (%) | Spleen Index (mg/g) | Liver Index (mg/g) |
|---|---|---|---|
| Normal Control | 0.9 | 0.498 ± 0.113 | 4.353 ± 0.253 |
| CPI Control | 16.26 | 0.126 ± 0.060 | 3.842 ± 0.458 |
| Combination low dose | 3.09 | 0.453 ± 0.048 | 4.433 ± 0.121 |
| Combination intermediate dose | 0.38 | 0.488 ± 0.051 | 4.5 ± 0.366 |
| Combination high dose | 2.63 | 0.465 ± 0.046 | 4.4 ± 0.374 |
| Standard Levimasole | 0.77 | 0.483 ± 0.033 | 4.333 ± 0.374 |
| Standard Septilin | 0.97 | 0.448 ± 0.075 | 4.4 ± 0.433 |
3.4.1. Organ indices
The organ weight such as liver and spleen was measured on 15th day of the experiment. Their relative index was calculated to estimate the immunomodulatory effect of synergy-based combination. The organ indices (spleen and liver) are demonstrated in Table 2, when compared with the normal group, the spleen and liver indices significantly decreased (p < 0.001) in CPI group. While, significant recovery of spleen and liver indices was observed on administration of herbal drug extract combinations in different doses as compared with CPI group (p < 0.001). The degree of recovery was more pronounced in mice of an intermediate group (p < 0.0037)
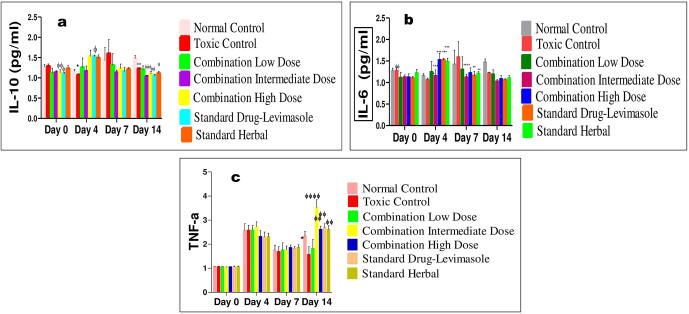
3.4.2. Effect of herbal synergy-based combination on cytokine levels in CP-treated mice serum
The effect of herbal combination was investigated on both splenic cytokines release, i.e. Th1 (TNF-α) and Th2 (IL-6 and IL-10) in mice. It was observed that CP caused a significant reduction in TNF-α (p < 0.01) in CPI group, while the expressions of IL-6 (p < 0.001) and IL-10 (p < 0.001) were higher in CPI group as compared with normal control group. As demonstrated in Fig. 3a, and b, developed combination remarkably reversed the CP-induced increment in IL-10 at day 14 and IL-6 at day 7. All treatment groups with present synergy-based combination (group III, IV and V) and with standard drugs (group VI and VII) induced significant increase in TNF-α when compared with the CPI group (p < 0.0001). Both C-ID (group IV) and SL (group VI)-treated groups specifically showed significant increase in TNF-α as compared with other treatment groups. As demonstrated in Fig. 3c, the combination significantly prevented the CP-induced reduction in the Th1 type cytokines (TNF-α) in serum at day 14. While the comparative effect of herbal combinations on the serum levels of cytokines at different days (0, 4, 7 and 14) is given in Fig. S1.
Fig. 3.
a: Interleukin-10 levels were elevated post-CP induction. The level was diminished by treatment with combination doses and standard controls. On day 14, significant marked reduction was identified with intermediate combination dose and levamisole group with P > 0.0006 and P > 0.002 respectively. 3b: The level of IL-6 was reduced on 7th day with most significant reduction seen in group IV and VI with P < 0.0001 3c: TNF-α level over a time period of 14 days, represented in various treatments, standard and control groups. On 14th day, the levels were significantly attenuated in group IV with P < 0.0001, also found to be higher in group V, VI and VII groups with P < 0.0023, P < 0.0012 & P < 0.0020, respectively. The results represented means ± SD (n = 6).
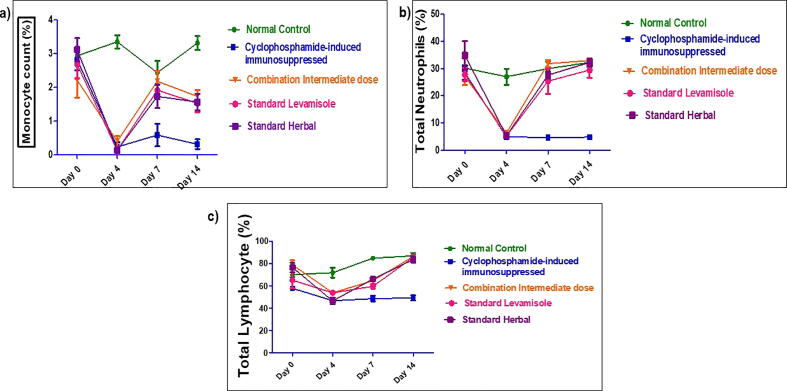
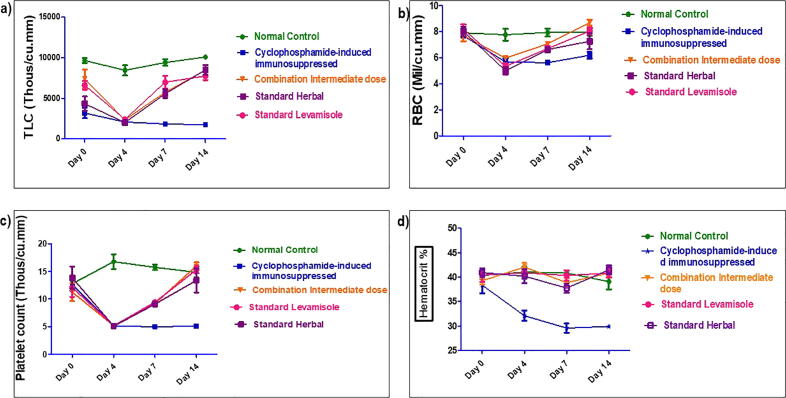
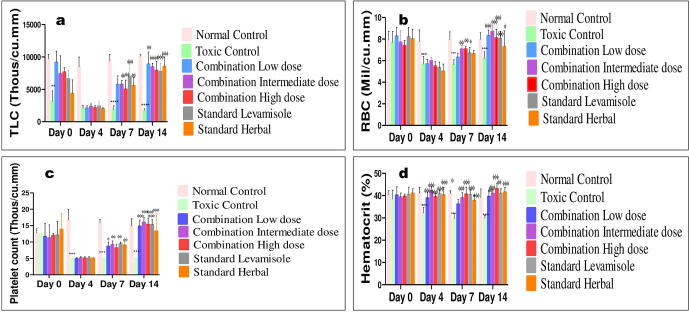
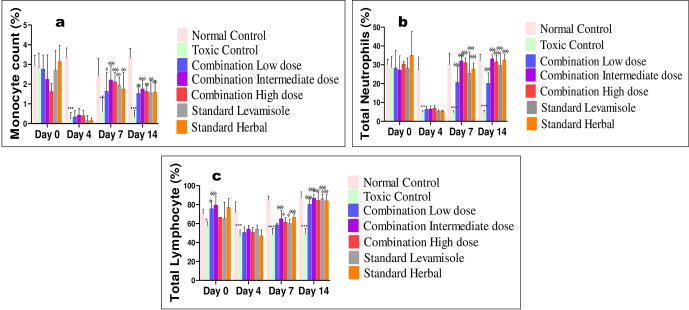
3.4.3. Hematological parameters
The effect of combination dose showing significant and most promising effects i.e. group IV (C-ID) among all tested dose combinations was determined on hematological parameters including hemoglobin percentage, TLC, DLC, RBC, hematocrit and platelet counts on day 0, 4, 7 and 14, as shown in Fig. 4, Fig. 5. There was significant reduction in hemoglobin percentage, total RBC, TLC, neutrophils, lymphocytes and platelet counts post-CP treatment in all groups but reversed in combination and standard groups whereas, remain low in CPI group till day 14. The standard reference drugs, levamisole and septilin, significantly increased most of the parameters respectively when compared with negative control groups. Similarly, combination of an intermediate dose group significantly raised TLC counts, neutrophils and lymphocytes compared to control group. However, there was no much variation in the RBC, hematocrit, platelet counts and hemoglobin concentration.
Fig. 4.
(a, b, c, d): Value are expressed as mean n = 6 , P < 0.001 when normal compared with CP control. p < 0.01 when RBC and Pt count of treatment and standard groups on day 4 when compared with CPI. ***p < 0.001, **p < 0.01, *P < 0.05 vs Normal Control; ффф p < 0.001, фф p < 0.01, ф p < 0.05 vs CPI control as analyzed by Two way ANOVA followed by Multiple comparison Bonferroni test.
Furthermore, all three doses of combination tested, remarkably improved the blood parameters (Fig. S2). Among them, group IV intermediate dose (214 mg/kg) was found to be more effective in ameliorating the haemato-suppressive effect induced by CP (Fig. 4, Fig. 5). Hence, the effect of intermediate combination at a dose of 214 mg/kg was almost similar to standard drugs levamisole and septilin.
Severe destruction in DLC was apparent specifically on day 4, post-CP treatment and treatment doses reversed the CP toxicity. On day 7 and 14, significant increase in differentiating count was observed in group IV, VI and VII. The effect of different tested doses of herbal combination on hematological parameters is represented in Fig. S2
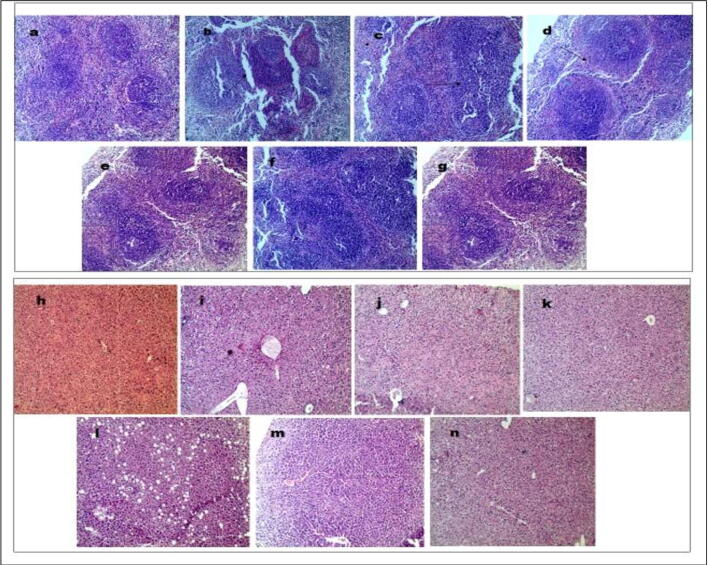
3.4.4. Histological observations in spleen and liver
In the present study, the histopathology of the spleen and liver were examined with an optical microscope. The spleens (Fig. 6a-g) and livers (Fig. 6h-n) of normal control (I) mice displayed massive closely arranged and deeply stained splenocytes with an obvious nucleus. While in the CPI (II) group, there was decrease in the number of spleen cells i.e. due to depletion of white pulp. Necrotic areas without any cell structure were also seen in the haematoxylin and eosin (HE) stained histopathological images. In the treatment groups, low dose (group III) nuclei was clearly seen, in intermediate dose (IV) and high dose (V) groups, there was well arranged of spleen cells compactly in an accurate sequence with clear nuclei and less intercellular space, which was similar to the normal group. Also, splenomegaly was marked due to extra medullary hematopoiesis in red pulp. Similarly, in standard group’s viz., standard levamisole (VI) and standard herbal (VII), proper order of splenocytes was observed with marked prominent nuclei. The results indicated that our synergy-based combination significantly prevent the damage to the spleen cells in CP-induced Swiss albino mice.
Fig. 6.
(a-g): Haematoxylin and eosin stained spleen tissues with 10x magnification (I) Normal control spleen section showing normal echo texture with an obvious nucleus, (II) showing necrotic changes with depletion of white pulp, (III) apparently visible nuclei, (IV) marked prominent nuclei with minimum intercellular space and healthy lymphoid tissue as normal, (V) well defined spleen section with compacted spleen cells, (VI) proper order of splenocytes were observed, (VII) well defined spleen with clear nuclei. 6 (h-n): Haematoxylin- and eosin-stained liver tissues with 10x magnification (I) Normal mice liver section showing normal architecture, (II) prominent pathological changes characterized by focal necrosis and edema, (III) kupffer cells are reactivated, (IV) looks apparently healthy alike normal, (V) apparently healthy with bi-nucleated cells, (VI and VII) necrotic changes were reversed with clearly visible of nuclei.
The histopathology of liver tissue in normal control group (I) was showing normal architecture with distinct hepatic lobules, cords of kupffer cells were separated with sinusoids. While in CPI group (II) treated with CP, engorgement of the central and portal veins of liver was seen. Hepatic structure was altered as well as infiltration of acute inflammatory cells along with focal necrosis and hepatocytes edema was seen and vacuolar degeneration in the perivascular tissue was also noticed. In addition, the albino mice treated by present combination in different doses have been recovered to larger extent especially at intermediate doses (IV). Microscopically, the necrotic changes were diminished, with substantial reduction in neutrophils infiltration in the vicinity of hepatic portal and cellular edema was seen low. Similarly in low dose group (III) bi-nucleated cells are seen demonstrating regeneration of kupffer cells. While in high dose combination group (V) apparently healthy with presence of bi-nucleated cells and activated kupffer cells Also, in standard groups (SL & SH), necrotic changes were reversed and hepatic cells were clearly visible and apparent.
4. Discussion
In this study, herbal immunomodulatory combination was prepared by synergized combination of herbal extracts, scientifically quantified for their bioactive metabolites, tested in vitro and evaluated in CP-induced immunosuppression model in Swiss Albino mice.
The immunomodulatory activity of plant extracts that stimulate or suppress the immune system, may be of paramount importance in regulating diseases that originate from immune cell disturbances. Approximately, >40 single herbal drugs in AYUSH system have been reported to present immunomodulatory activity (Basu et al., 1947, Nagarathna et al., 2013). For this study, eight drugs (PE, TC, WS, OS, PN, AM, AI and CL) showing potent immunomodulatory activity were selected based on previously reported literature and were further screened by performing anti-oxidant (DPPH scavenging) and in vitro immunomodulatory assays (pinocytic activity of peritoneal macrophages of mice and proliferation of spleen cells) to get three potent drugs possessing immunomodulatory property. Hence, the three herbal aqueous extracts namely, TC, PE and PN, that demonstrated best in vitro immunomodulatory activities with IC50 values of 0.438, 0.190 and 0.869 respectively, were employed further in the study.
The purpose of combining PN with other two drugs in the present combination was due to its bioavailability enhancer activity. A study by Jawalkar et al. (2014) revealed that aqueous extract of P. nigrum significantly enhances splenocyte proliferation in a synergistic dose-dependent manner and significantly modulates T helper (Th)1 cytokine release by spleen cells. Piperine and P. nigrum also reported to maintain superoxide dismutase, catalase, glutathione peroxidase, glutathione-s-transferase, glutathione levels and reduction of oxidative stress (Joshi et al., 2018).
Drug combinations have been used in AYUSH herbal formulations since centuries, as they have potential to enhance efficacy (Berenbaum, 1977). Sometimes, the active metabolites present in various herbs responsible for producing pharmacological actions become significant when potentiated by that of other plants and could not produce desirable therapeutic effects when used alone (Gayathri et al., 2005). But, a lack of proper understanding and scientific rationale for combining them in an appropriate ratio and method for dose calculations, hinders their acceptability.
In the present study, the combination of these herbal extracts was exerting synergistic and complementary effects to modulate immune system. Furthermore, the integration effects of PE, TC and PN were evaluated firstly by using the different concentrations of the cell-bound compounds combinations. In addition, ratio for combining three drugs was obtained by in vitro MTT assay as 1:1:1. The synergistic effects from the herbal combination was obtained by combination index demonstrated through the pinocytic and spleen proliferation activities, where CI was found to be<1, indicative of synergism among the drugs (Chou, 2010). Moreover, dose for each drug was calculated by extractive yield obtained and pharmacopoeial dose given in UPI. The highest extractive yield was found in aqueous extract for most of the selected eight drugs.
The phenolics and flavonoids are well known phytochemical molecules from plants, having antioxidant properties. The herbal extracts present in the synergy-based combination are PE, TC and PN, which are abundant source of phenolics and flavonoid metabolites and are responsible for their bioactivity. These content help in regulating the immune system by identifying the foreign invaders and further stimulating the immune cells such as monocytes, natural killer cells and macrophages, through chelating and scavenging property which is attributed to their hydroxyl groups and scavenges mostly oxidizing molecules and various free radicals (Tungmunnithum et al., 2018). In this study, the flavonoid and phenolic content in the combination of TC, PE and PN was estimated by aluminum chloride colorimetric method and Folin-Ciocalteu reagent where gallic acid and rutin were used as standrads to express the phenolic and flavonoid content, respectively. Amir et al. (2011) also reported the flavonoid and phenolic content for polyherbal formulation containing amla as chief ingredient as 135.83 and 103.33, respectively.
The results obtained from antioxidant activity by DPPH scavenging indicate that our combination possess potent antioxidant potential even better than their individual drug extracts. It is already evinced that plants rich in polyphenolic compounds have marvelous antioxidant potential. The inhibitory concentration of well-known polyherbal immunomodulatory formulation, Rumalaya Forte (Subhash et al., 2012) is reported to be 315.92 µg/ml, which is greater than our herbal combination having IC50 value of 113.6 µg/ml. A lower IC50 value is usually indicative of greater antioxidant activity. The metabolites present in the combination are responsible for the antioxidant activities as reported in literature (Sharma et al., 2009) by creating conjugation free radicals and limiting the amount of free radicals present, so prevents the cellular damage. Similarly, TC exhibited the flavonoid compounds such as quercetin and rutin responsible for antioxidant activities by terminating action of free radicals (Begum and Ramamurthy, 2019). While, piperine and P. nigrum have also been reported to maintain superoxide dismutase, catalase, glutathione peroxidase, glutathione-s-transferase, glutathione levels and reduction of oxidative stress (Joshi et al., 2018).
The combination was evaluated for phagocytic activity of macrophages by pinocytic assay as macrophages phagocytosis is the first line of defence against microbial attack by immune system (Ang et al, 2014). Our results demonstrated a potent increment in phagocytic activity of macrophages especially by intermediate dose combination. Hence, it indicated that our combination plays an important role in modulating the nonspecific immune responses regulated by cytokines and other mediators released by macrophages, which is in accordant with a study reported by Meng et al. (2018) which also showed significant increment in the macrophage phagocytosis and lymphocyte proliferation activity by L. plantarum. Further, our herbal combination was evaluated for activity of spleen cells (lymphocytes, T, B, dendritic cells), which are vital for adaptive immune responses. The ConA mitogen was used to induce the proliferation of T and B lymphocytes, and evaluated the effects of combination on the proliferation of spleen cells in immunosuppressed mice with varying doses and compared with levimasole. Hence, C-ID markedly enhanced the splenocytes proliferation of immunosuppressive mice induced by CP. The results are in accordance with previous study reported by Ang et al. (2014), which demonstrated the augmentation in thymocytes and splenocytes proliferation by P. indica and D. pentandra as compared with untreated cells.
The quantitative estimation of all biologically active metabolites (gallic acid, piperine, quercetin) present in synergy-based herbal combination was carried out through HPTLC. The chromatograms of gallic acid, piperine and quercetin were well represented in their quantitative estimation. The Rf values of all three components, was found comparable in both sample and reference standard under UV light at 254 and 366 nm. It is reported in many studies that PE contains phytochemicals like gallic acid, ellagic acid, responsible for antioxidant, anti-inflammatory and immunomodulatory activities (Sheikh et al., 2015). Similarly, quercetin is a flavonoid compound that possess anti-oxidant property (Polu et al., 2017). Whereas, piperine is the alkaloid obtained from PN and known for its bioenhancer activity as it increases the bioavailability and permeability of epithelial membranes as reported by many studies (Gorgani et al., 2017).
The pre-clinical study was carried out in CP-induced Swiss albino mice to evaluate the synergistic activity of the present combination in varied doses and to explore the underlying mechanism of modulating innate and adaptive immune responses (Nfambi et al., 2015, Sakthivel and Guruvayoorappan, 2015, Ahirwal et al., 2015, Sharififar et al., 2009).
Since, organ indices are an important indicator of immune response, we evaluated the spleen and liver index to have a proper reflection on immune function (Zhang et al., 2017). An increment in indices observed with the treatment groups especially in intermediate dose group suggests modulation of immunity. Also, changes in body weight percentage was calculated to obtain the difference of pre and post treatment, where the combination treatment groups showed significant improvement demonstrating the maximum change suggesting the improvement of overall well-being and immune functions.
The hematological parameters were assessed for any variation indicating the modulation in the immune responses and to determine the detrimental and beneficial effects of foreign materials on immune cells (Ajibade and Soetan, 2012). In this study, we observed that, most of the haematological parameters (Hb level, DLC, TLC, RBC, platelet count) were significantly decreased in all groups after CP-administration to mice. This is due to the fact that most chemotherapeutic agents have immunosuppressive effects which certainly affect the dividing hematopoietic cells resulting in leucopenia, neutropenia and lymphopenia and ultimately weakens the immunity (Salem et al., 2012). In the present study, we noticed that TLC, neutrophils, monocytes and lymphocytes count were augmented with all the doses of the test combination especially on 7th and 14th day, when compared with standard groups. The augmentation of TLC and DLC counts represent the enhanced immune responses of the host while raised TLC counts indicate immunostimulant activity. Leukocytes are an important part of immune system (Lammermann and Germain, 2014) and a reduction in the number of leukocytes leads to immune disorders. The results of the present study indicated that intermediate combination dose significantly inhibits the attenuation of leukocytes in CP-induced immunocompromised mice. Further, macrophages are major immune cell and plays a key role in the cellular nonspecific defence mechanism by phagocytosis. Metabolites present in TC, PE and PN have shown their immunostimulatory function by enhancing the major innate immune cells like lymphocytes and monocytes (Shruthi and Vijayalaxmi, 2016). Monocytes count were significantly higher on 7th day specifically in intermediate dose treated group than other dose groups. Also, it was markedly higher in all doses when compared with CPI control, as monocytes are the first immune cell released against any foreign invader and initiates the phagocytosis as well as secretion of pro-inflammatory markers. Devi et al. (2020) also studied the same effect, which revealed significant increment in lymphocyte count in K. parviflora treated immunosuppressed mice. While, our data showed that level of Hb percentage was not significantly different in any group including CPI group even on 4th day i.e. immediately after CP injection, which was similar to results obtained by El-Naggar et al. (2015).
Cytokines are generally classified as interleukins, TNF, IFN, chemokines, colony-stimulating factors, growth factors and many more. The production of IL-2, IL-12, IFN-γ and TNF-α leads to T helper1 type immune response, while the release of IL-4, IL-5, IL-6 and IL-10 leads to T helper2 type immune response (Turner et al., 2014, Dirchwolf et al., 2016). Cytokines play an important role in regulating adaptive and innate immune responses through signal transducing between cells, mediating inflammatory cascades, contributing in the development of immune cell differentiation, and stimulating hematopoiesis (Cuneo and Autieri, 2009). They are produced especially by TH cells and lead to various functions of the two types of T cell. Generally, TH2 cells assist in B-cell antibody secretion while TH1 cells induce delayed-type hypersensitivity reactions (Zhou et al., 2018). In this study, IL-10, IL-6 and TNF-α were determined to ascertain the regulation of immune responses by treatment doses. The level of anti-inflammatory cytokines (IL-6 and IL-10) were raised till 7th day in all the groups, while in CPI group, they were elevated till 14th day after CP injection, illustrating their immunosuppressive effect. This effect was reversed after giving the combination doses reflecting that the test combination used could ameliorate the CP-induced immunosuppression, which is in accordance with other reports (Zhang et al., 2017).
TNF–α plays important part in immune cells differentiation and regulation, so reduction in its level causes disruption of immune factors. It has also been revealed that CP significantly attenuates TNF–α levels in mice (Yu et al., 2018). The present study demonstrated that intermediate combination doses inhibited the CP–induced reduction in TNF–α levels, thus improved the immune functions in mice. Therefore, our combination especially intermediate dose manifested satisfactory effect in CP-induced immunosuppression in mice and improved the symptoms of low immunity by modifying the immune imbalance.
It has been reported that CP can cause reduction in weight and atrophy of the immune organs (Chen et al., 2006). Spleen is an important immune organ and liver is a major metabolic organ, both participate in maintaining immune homeostasis (Qi et al., 2018). Tissue inflammation is usually observed by histological observation of spleen and liver. So, any pathology in these tissues, reflected by lesions of immune organs may cause weakening of immune function (Yu et al., 2018, Bhat et al., 2018). In the present study, the histology revealed that CP caused destruction in spleen and liver tissues, which was significantly inhibited by combination doses. The results of HE staining in the spleen and liver demonstrated that combination treatment could prevent the damage to spleen and liver induced by CP, indicating that present combination at intermediate dose specifically is able to counteract the effect of immunosuppression on immune organs (Kraus, 2003).
The herbal standard used in our study, Himalaya-Septilin, a well-known clinically used polyherbal formulation having amla and giloy as main ingredient was evaluated on albino rats immune system and showed that it reduces phagocytic activity of the Polymorphonuclear leucocytes (PMN) cells/reticuloendothelial system and increases the percentage and absolute number of circulating neutrophils (Daswani and Yegnanarayan, 2002). However, to be noted, that the percentage of neutrophils increased was significantly higher by our combination, as demonstrated in our study. Also, Dabur-Chayawanprash, a polyherbal preparation used for immunostimulation since decades, reported to be immunoprotective role by enhancing the TNF-α and IL-1 levels (Madaan et al., 2015).
5. Conclusion
Our study demonstrated normalization of both TH (Th1 and Th2) immune responses by increasing TNF-α and decreasing IL-10 and IL-6 levels in CP-induced immunosuppressed mice. The present study indicated that the developed synergy-based herbal combination is enriched with biologically active phenolics and flavonoids which are responsible for their antioxidant and immunomodulatory activity as demonstrated through in vitro assays and in vivo models. The immunomodulatory effect is mediated by activation of both innate and acquired arms of the immune system and exhibited dual effects on the immune system, the humoral immunity and the cellular immunity both were enhanced. Hence, the present synergy-based herbal combination possess promising immunomodulatory activity by enhancing non-specific immunity, while repressing pro-inflammatory cytokines. The results suggested that combination of TC, PE and PN in an intermediate dose could be further explored clinically as potential synergy-based therapeutic approach for immune modulation. Further studies are needed to isolate and identify pure active compounds from this to fully understand their immunomodulatory effects, so that the combination can be translated from bedside to bench.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The present study was partially supported by CSIR (Council of Scientific and Industrial Research (09/591(0165)/2019 EMR-I) fellowship grant for financial support. The authors also acknowledge BNPL (Bioactive Natural Product Laboratory) for HPTLC, extraction, animal work and other laboratory related activities.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2021.06.076.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
Supplementary figure 3.
References
- Ahirwal L., Singh S., Dubey M.K., Bharti V., Mehta A., Shukla S. In vivo immunomodulatory effects of the methanolic leaf extract of Gymnema sylvestre in swiss albino mice. Arch. Biol. Sci. Belgrade. 2015;67(2):561–570. [Google Scholar]
- Ajibade T.O., Soetan K.O. Evaluation of hematological and plasma biochemical effects of aqueous extracts of Parkia biglobosa seeds in rats. Afr J. Biotechnol. 2012;11:15446–15450. [Google Scholar]
- Amir M., Khan A., Mujeeb M., Ahmad M.A., Siddiqui N.A. Phytochemical screening and in vitro antioxidant activity of Jawarishe Amla- A polyherbal formulation. PHCOG J. 2011;3(26):54–60. [Google Scholar]
- Ang H.Y., Subramani T.S., Yeap S.K., Omar A.R., Ho W.Y., Abdullah M.P., Alitheen N.B. Immunomodulatory effects of Potentilla indica and Dendrophthoe pentandra on mice splenocytes and thymocytes. Experim. Therapeut. Med. 2014;7:1733–1737. doi: 10.3892/etm.2014.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum V., Arora P., Ansari S.H., Najmi A.K., Ahmad S. Antithrombocytopenic and immunomodulatory potential of metabolically characterized aqueous extract of Carica papaya leaves. Pharm. Biol. 2017;55(1):2043–2056. doi: 10.1080/13880209.2017.1346690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. The Unani Pharmacopoeia of India, Part I, Vols I–IV, Department of Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homeopathy (AYUSH), Ministry of Health and Family Welfare, New Delhi, India. 2007.
- Anonymous. The Unani Pharmacopoeia of India, Part I, Vols I–IV, Department of Ayurveda, Yoga and Naturopathy, Unani, Siddha and Homeopathy (AYUSH), Ministry of Health and Family Welfare, New Delhi, India. 2009.
- Basu N.K., Lal S.B., Sharma S.N. Investigations on Indian medicinal plants. Quart J. Pharm. Pharmacol. 1947;20:38–42. [PubMed] [Google Scholar]
- Begum H.J., Ramamurthy V. Evaluation of secondary metabolites and antioxidant activity of ethanolic leaves extract of Tinospora cordifolia. Int. J. Basic Appl. Res. 2019;9(2):256–263. [Google Scholar]
- Berenbaum M. Synergy, additivism and antagonism in immunosuppression - critical review. Clin. Exp. Immunol. 1977;28:1–18. [PMC free article] [PubMed] [Google Scholar]
- Bhat N., Kalthur S.G., Padmashali S., Monappa V. Toxic Effects of Different Doses of Cyclophosphamide on Liver and Kidney Tissue in Swiss Albino Mice: A Histopathological Study. Ethiop J. Sci. 2018;28(6):711. doi: 10.4314/ejhs.v28i6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayi B., Roychowdhury S., Ghosh S., Sengupta M. Hepatoprotective and immunomodulatory properties of Tinospora cordifolia in CCl 4 intoxicated mature albino rats. J Toxicol Sci. 2002;27:139–146. doi: 10.2131/jts.27.139. [DOI] [PubMed] [Google Scholar]
- CCAC, 1993. https://www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf.
- Chen X.T., Li J., Wang H.L., Cheng W.M., Zhang L., Ge J.F. Immunomodulating effects of fractioned polysaccharides isolated from Yu-Ping-Feng-Powder in cyclophosphamide-treated mice. Am. J. Chin. Med. 2006;34:631–641. doi: 10.1142/S0192415X06004168. [DOI] [PubMed] [Google Scholar]
- Chou T.C., Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci. 1983;4:450–454. [Google Scholar]
- Chou T.C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Chou T.C. Drug combination studies and their synergy quantification using the Chou-Talaly method. Can. Res. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- Cuneo A.A., Autieri M.V. Expression and function of anti-inflammatory interleukins: the other side of vascular response to injury. Current Vascular Pharmacol. 2009;7(3):267–276. doi: 10.2174/157016109788340721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daswani B.R., Yegnanarayan R. Immunomodulatory activity of Septilin, a polyherbal preparation. Phytotherapy Res. 2002;16(2):162–165. doi: 10.1002/ptr.996. [DOI] [PubMed] [Google Scholar]
- Devi A.R., Kariyil B.J., Raj N.M., Akhil G.H., Balakrishnan-Nair D.K. Immunomodulatory effect of Kaempferia parviflora against cyclophosphamideinduced immunosuppression in swiss albino mice. Phcog. Mag. 2020;16:S13–S21. [Google Scholar]
- Dirchwolf M., Podhorzer A., Marino M., Shulman C., Cartier M., Zunino M., Paz S., Munoz A., Bocassi A., Gimenez J., Di Pietro L., Romer G., Fainboim H., Fainboim L. Immune dysfunction in cirrhosis: Distinct cytokines phenotypes according to cirrhosis severity. Cytokine. 2016;77:14–25. doi: 10.1016/j.cyto.2015.10.006. [DOI] [PubMed] [Google Scholar]
- El-Naggar S.A., Alm-Eldeen A.A., Germoush M.O., El-Boray K.F., Elgebaly H.A. Ameliorative effect of propolis against cyclophosphamide-induced toxicity in mice. Pharm. Biol. 2015;53(2):235–241. doi: 10.3109/13880209.2014.914230. [DOI] [PubMed] [Google Scholar]
- Gayathri V., Asha V.V., Subramoniam A. Preliminary studies on the immuno-modulatory and antioxidant properties of Sellaginella species. Indian J. Pharmacol. 2005;37:381–385. [Google Scholar]
- Gorgani L., Mohammadi M., Najafpour G.D., Nikzad M. Piperine-the bioactive compound of black pepper: from isolation to medicinal formulations. Comprehensive Rev. Food Sci. Food Safety. 2017;16:124–140. doi: 10.1111/1541-4337.12246. [DOI] [PubMed] [Google Scholar]
- Jantan I., Haque M.A., Ilangkovan M., Arshad L. An Insight Into the Modulatory Effects and Mechanisms of Action of Phyllanthus Species and Their Bioactive Metabolites on the Immune System. Front. Pharmacol. 2019;10(878):1–19. doi: 10.3389/fphar.2019.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawalkar P.D., Bhujbal S.S., Bafna A.R. Immunomodulatory activity of herbal formulation (CE1 and CE2) containing Sphaeranthus indicus, Curculigo orchioides and Piper nigrum. Int J Pharm Chem Sci. 2014;3(1):175–180. [Google Scholar]
- Joshi D.R., Shrestha A.C., Adhikari N. A review on diversified use of the king of spices: Piper nigrum (black pepper) Int. J. Pharm. Sci. Res. 2018;5(9):4089–4101. [Google Scholar]
- Khodadadi S. Role of herbal medicine in boosting immune system. Immunopathologia Persa. Immunopathol Persa. 2015;1(1) [Google Scholar]
- Kraus M.D. Splenic histology and histopathology: An update. Semin. Diagn. Pathol. 2003;20:84–93. doi: 10.1016/s0740-2570(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Lammermann T., Germain R.N. The multiple faces of leukocyte interstitial migration. Semin. Immunopathol. 2014;36:227–251. doi: 10.1007/s00281-014-0418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaan A., Kanjilala S., Gupta A., Sastry J.L.N., Verma R., Singh A.T., Jaggi M. Evaluation of immunostimulatory activity of Chayawanprash using in vitro assays. Indian J. Exp. Biol. 2015;53:158–163. [PubMed] [Google Scholar]
- Makare N., Bodhankar S., Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J. Ethnopharmacol. 2001;78 doi: 10.1016/s0378-8741(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Meng, Y., Li, B., Jin, D., Zhan, M., Ji Lu, J., Huo, G., 2018. Immunomodulatory activity of Lactobacillus plantarum KLDS1.0318 in cyclophosphamide-treated mice. Food & Nutrition Research. 62, 1296. [DOI] [PMC free article] [PubMed]
- Nagarathna P.K.M., Reena K., Reddy S., Wesley J. Review on Immunomodulation and Immunomodulatory Activity of Some Herbal Plants. Int. J. Pharm. Sci. Rev. Res. 2013;22(1):76–89. [Google Scholar]
- Nair A.B., Jacob S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharma. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair C.V.J., Ahamad S., Khan W., Anjum V., Mathur R. Development and validation of high performance thin layer chromatography method for simultaneous determination of polyphenolics compounds in medicinal plants. Pharmacognosy. Res. 2017;9(1):S67–S73. doi: 10.4103/pr.pr_122_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nfambi J., Bbosa G.S., Sembajwe L.F., Gangunga J., Kasolo J.N. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in Wistar albino rats. J. Basic Clin. Physiol. Pharmacol. 2015;26(6):603–611. doi: 10.1515/jbcpp-2014-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen S., Ansari M.H.R., Parveen R., Khan W., Ahmad S., Husain S.A. Chromatography Based Metabolomics and In Silico Screening of Gymnema sylvestre Leaf Extract for Its Antidiabetic Potential. Evidence-Based Complementary Alternative Med. 2019;2019:1–14. doi: 10.1155/2019/7523159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass G.J., Carrie D., Boylan N. Role of hepatic cytochrome P450s in the pharmacokinetics and toxicity of cyclophosphamide: studies with the hepatic cytochrome P450 reductase null mouse. Cancer Res. 2005;65(10):4211–4217. doi: 10.1158/0008-5472.CAN-04-4103. [DOI] [PubMed] [Google Scholar]
- Polu P.R., Nayanbhirama U., Khan S., Maheswari R. Assessment of free radical scavenging and antiproliferative activities of Tinospora cordifolia Miers (Wild) BMC Complementary Med. Therapies. 2017;17(457):1–12. doi: 10.1186/s12906-017-1953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q., Dong Z., Sun Y., Li S., Zhao Z. Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice. Molecules. 2018;23:2–14. doi: 10.3390/molecules23102668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabina E.P., Nasreen A., Vedi M., Rasool M. Analgesic, antipyretic and ulcerogenic effects of piperine: An active ingredient of pepper. J. Pharm. Sci. and Res. 2013;5(10):203–206. [Google Scholar]
- Sakthivel K.M., Guruvayoorappan C. Acacia ferruginea inhibits cyclophosphamide-induced immunosuppression and urotoxicity by modulating cytokines in mice. J. Immunotoxicol. 2015;12:154–163. doi: 10.3109/1547691X.2014.914988. [DOI] [PubMed] [Google Scholar]
- Salem M.L., Al-Khami A.A., El-Nagaar S.A. Kinetics of rebounding of lymphoid and myeloid cells in mouse peripheral blood, spleen and bone marrow after treatment with cyclophosphamide. Cell Immunol. 2012;276:67–74. doi: 10.1016/j.cellimm.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharififar F., Pournourmohammadi S., Arabnejad M. Immunomodulatory activity of aqueous extract of Achillea wilhemsii C. Koch in mice. Indian J. Experim. Biol. 2009;47:668–671. [PubMed] [Google Scholar]
- Sharma U., Bala M., Kumar N., Singh B., Munshi R.K., Bhalerao S. Immunomodulatory active compounds from Tinospora cordifolia. J. Ethnopharmacol. 2012;141:918–926. doi: 10.1016/j.jep.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Sharma A., Sharma M.K., Kumar M. Modulatory role of Emblica officinalis fruit extract against arsenic induced oxidative stress in swiss albino mice. Chemico-Biol Interaction. 2009;180:20–30. doi: 10.1016/j.cbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Sharma P., Kumar P., Sharma R., Gupta G., Chaudhary A. Immunomodulators: Role of medicinal plants in immune system. Natl. J. Physiol. Pharm. Pharmacol. 2017;7(6):552–556. [Google Scholar]
- Sheikh Z.A., Shakeel S., Gul S., Zahoor A., Khan S.S., Zaidi F.H., Usmanghani K. A novel HPTLC method for quantitative estimation of biomarkers in polyherbal formulation. Asian Pacific J. Biomed. 2015;5(11):955–959. [Google Scholar]
- Shi L., Fu Y. Isolation, purification, and immunomodulatory activity in vitro of three polysaccharides from roots of Cudrania tricuspidata. Acta Biochim. Biophys. Sin. 2011;43:418–424. doi: 10.1093/abbs/gmr024. [DOI] [PubMed] [Google Scholar]
- Shruthi S., Vijayalaxmi K.K. Antigenotoxic effects of a polyherbal drug septilin against the genotoxicity of cyclophosphamide in mice. Toxicol. Rep. 2016;3:563–571. doi: 10.1016/j.toxrep.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.S., Pandey S.C., Srivastava S., Gupta V.S., Patro B., Ghosh A.C. Chemistry and medicinal properties of Tinospora cordifolia (guduchi) Indian J. Pharmacol. 2003;35:83–91. [Google Scholar]
- Subhash K.R., Cheriyan B.V., Parvathavarthini S., Bharaati G.M., Venogopal V. Effect of polyherbal formulation Rumalaya forte on adjuvant induced arthritis in rats. Ind. Drugs. 2012;49(10):1–24. [Google Scholar]
- Tungmunnithum D., Areeya Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines. 2018;5(93):1–16. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M.D., Nedjai B., Hurst T., Pennington D.J. Cytokines and chemokines: At the crossroads of cell signaling and inflammatory disease. Biochem et Biophy Acta. 2014;1843:2563–2582. doi: 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Wagner H., Marzenich G.U. Synergy research: approaching a new generation of phytopharmaceuticals. J. Natural Remedies. 2009;2(9):21–141. 1. [Google Scholar]
- Wang S., Huang S., Ye Q., Zeng X., Yu H., Qi D., Qiao S. Prevention of Cyclophosphamide-Induced Immunosuppression in Mice with the Antimicrobial Peptide. Jr. Immunol. res. 2018:1–11. doi: 10.1155/2018/4353580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- www.ich.org/quality/guidelines
- Yu J., Cong L., Wang C., Li H., Zhang C., Guan X., Liu P., Xie Y., Sun J. Immunomodulatory effect of Schisandra polysaccharides in cyclophosphamide–induced immunocompromised mice. Experim. Therapeutic Med. 2018;15:4755–4762. doi: 10.3892/etm.2018.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Ma Q., Cui H., Liu G., Zhao X., Li W., Pioa G. How can synergism of herbal medicines benefit from network pharmacology? Molecules. 2017;22(1135):1–19. doi: 10.3390/molecules22071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Cong R., Hu M., Zhu Y., Yang X. Immunoenhancement of Edible Fungal Polysaccharides (Lentinan, Tremellan, and Pachymaran) on Cyclophosphamide-Induced Immunosuppression in Mouse Model. Evidence-Based Complementary Alternative Med. 2017:1–7. doi: 10.1155/2017/9459156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Dong Q., Peng L., Xu X., Fang Y., Yang J. Immunomodulatory activity of a novel polysaccharide from Lonicera japonica in immunosuppressed mice induced by cyclophosphamide. PLoS ONE. 2018;13(10):1–15. doi: 10.1371/journal.pone.0204152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Seto S.W., Chang D., Kiat H., Razmovski-Naumovski V., Chan K., Bensoussan A. Synergistic Effects of Chinese Herbal Medicine: A Comprehensive Review of Methodology and Current Research. Front. Pharmacol. 2016;7(201):1–16. doi: 10.3389/fphar.2016.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]