Abstract
Respiratory epithelial cells form a selective barrier between the outside environment and underlying tissues. Epithelial cells are polarized and form specialized cell-cell junctions, known as the apical junctional complex (AJC). Assembly and disassembly of the AJC regulates epithelial morphogenesis and remodeling processes. The AJC consists of tight and adherens junctions, functions as a barrier and boundary, and plays a role in signal transduction. Endothelial junction proteins play important roles in tissue integrity and vascular permeability, leukocyte extravasation, and angiogenesis. Air pollutants such as particulate matter, ozone, and biologic contaminants penetrate deep into the airways, reaching the bronchioles and alveoli before entering the bloodstream to trigger airway inflammation. Pollutants accumulating in the lungs exacerbate the symptoms of respiratory diseases, including asthma and chronic obstructive lung disease. Biological contaminants include bacteria, viruses, animal dander and cat saliva, house dust mites, cockroaches, and pollen. Allergic inflammation develops in tissues such as the lung and skin with large epithelial surface areas exposed to the environment. Barrier dysfunction in the lung allows allergens and environmental pollutants to activate the epithelium and produce cytokines that promote the induction and development of immune responses. In this article, we review the impact of environmental pollutants on the cell barrier in respiratory diseases.
Keywords: Air pollution, environmental pollutants, particulate matter, ozone, barrier, epithelium, asthma, chronic obstructive pulmonary disease, house dust mites
INTRODUCTION
Environmental pollution adversely affects human health and contributes to the disease burden. Air pollution is the fourth largest risk factor for disability-adjusted life years and was estimated to be responsible for approximately 5 million deaths worldwide in 2017, 70% of which were caused by outdoor environmental air pollution.1 The impact of air pollution on human health should not be underestimated. In-depth mechanistic studies, alongside clinical imaging studies, have shown that air pollution exposure is involved in the onset and progression of respiratory diseases.2,3,4
Air pollutants constitute a toxic mixture of particles and gases emitted in large quantities from many different combustion sources, including vehicles and factories. Indoor pollutants include smoke from tobacco, cooking, burning of wood and other materials in stoves and fireplaces, dust particles resuspended during cleaning, and outdoor particles that infiltrate the indoor environment.5 Major pollutants include particulate matter (PM), ozone, nitrogen dioxide, and sulfur dioxide. Biological contaminants include bacteria, viruses, animal dander and cat saliva, house dust mites (HDM), cockroaches, and pollen. Some biological contaminants trigger allergic reactions, including hypersensitivity pneumonitis, allergic rhinitis (AR), and certain types of asthma.6
PM is the main component of indoor and outdoor air pollution. PM comprises a complex mixture of materials, such as organic compounds, acids, and fine metal particles, along with a carbonaceous core.7 PM includes coarse, fine, and ultrafine particles. Particles larger than 10 μm are generally trapped in the nose and throat, and do not enter the lungs.8,9 Particles less than 10 μm but greater than 2 μm in size reach the tracheobronchial tree and are subject to mucociliary clearance. Smaller particles can pass through the airways, and are deposited in the alveolar and centriacinar regions of the lungs, where they induce respiratory diseases such as asthma and chronic obstructive pulmonary disease (COPD).8,9 In this region, phagocytic cells, including neutrophils and macrophages, are recruited by cytokines and chemokines to engulf the particles by phagocytosis.8,9 Oxidant-mediated cellular damage, including the production of reactive oxygen species (ROS) and oxidative stress, innate immunity and adaptive immunity can lead to PM-mediated adverse health effects.10
Ambient ozone is a common environmental air pollutant that has a considerable impact on public health. Ozone is highly reactive and oxidizes proteins and lipids in the fluid-lined compartments of the lungs. This oxidization initiates inflammation and increases lung permeability through cytotoxic mediators including pro-inflammatory cytokines and ROS as well as nitrogen intermediates such as peroxynitrite.11 Distal structures of the lungs, including the terminal bronchioles, bronchiole-alveolar duct junction, and proximal alveolar regions, are considered the primary targets of ozone.11 Epidemiological data indicate that individuals with chronic airway diseases are highly sensitive to ozone, exhibiting increased morbidity and mortality.12 Acute inhalation of ozone causes structural alterations in the lung, including disruption of the alveolar epithelial barrier; this leads to alveolar epithelial type II cell hypertrophy and hyperplasia.13,14 Recruitment of inflammatory cells into the lung following ozone exposure is another potential avenue through which tissue damage can occur, where toxic mediators are released from activated macrophages and neutrophils, including cytokines, ROS and nitrogen species, and proteolytic enzymes.13,14
Respiratory epithelial cells function as a selective barrier between the outside environment and underlying tissues. Epithelial cells are polarized and consist of membrane-bound lipids and proteins.15 This polarity is partly based on the formation of specialized cell-cell junctions, which together comprise the apical junctional complex (AJC).15 Adherens junctions (AJs) and tight junctions (TJs) are dynamic structures that undergo constitutive turnover and recycling. The cytosolic face of the AJC is composed of a large array of distinct proteins that direct assembly of the AJC.16 Assembly and disassembly of the AJC are thought to regulate epithelial morphogenesis and remodeling processes.16,17 TJ form the apical-most constituents of the AJC in epithelial cell sheets, and are also present in vascular endothelial and mesothelial cells.18 TJ act as semipermeable barriers to the paracellular transport of ions, solutes, and water, and function as boundaries between apical and basolateral domains of plasma membranes.19,20 TJ coordinate a variety of signaling and trafficking molecules that regulate cell differentiation, proliferation, and polarity, thereby serving as multifunctional complexes.19,21 These TJ functions are critical for epithelial and endothelial cell sheets to establish distinct tissue compartments within the body and maintain homeostasis. Moreover, disturbance of the functions of TJ causes or contributes to a variety of pathological conditions, such as infections, cancers, and blood-borne metastasis.22 The barrier function of TJ also restricts drug delivery to underlying tissues. Overcoming this paracellular barrier is critical for the treatment of human diseases.23 Endothelial cells are the main cellular constituents of blood vessels; one of their critical functions is to separate blood from the underlying tissues. Endothelial junction proteins play important roles in tissue integrity and vascular permeability, as well as leukocyte extravasation and angiogenesis. Endothelial junctions contain AJ, TJ, and gap junctions that have important roles in tissue integrity, barrier functions, and cell-cell communication. Cell-cell junctions constitute attachment sites between endothelial cells, and function as signaling structures that communicate cell position, limit growth and apoptosis, and regulate vascular homeostasis.19
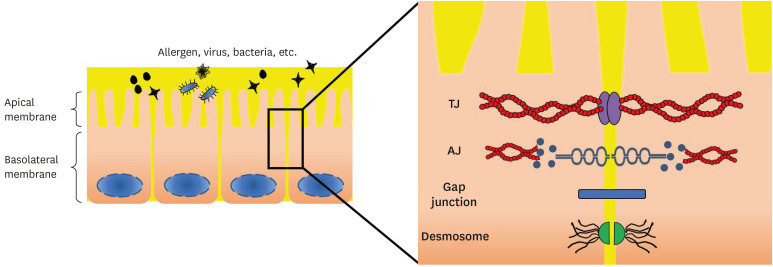
Junctional complexes trigger intracellular signaling directly (by engaging signaling proteins or growth-factor receptors) or indirectly (by tethering and retaining transcription factors at the cell membrane, thereby limiting their nuclear translocation).16 Junctional adhesion proteins bind to cytoskeletal and signaling proteins through their cytoplasmic tails, enabling anchoring of the AJ proteins to actin microfilaments and transferring intracellular signals inside the cell.24 An association with actin is required for junction stabilization, and for dynamic regulation of junction opening and closing. In addition, the interaction of AJ proteins with the actin cytoskeleton might be important in the maintenance of cell shape and polarity.24 Besides acting as adaptors that mediate the binding of adhesion proteins to actin, some intracellular junction proteins, when released from junctions, translocate to the nucleus and modulate transcription (Fig. 1, Table).19 Junction proteins might also function as scaffolds, binding several effector proteins and facilitating reciprocal interactions.25,26
Fig. 1. Structure of the AJC. The AJC consisting of the TJ and AJ resides at the interface between the apical and basolateral membrane.
AJC, apical junctional complex; TJ, tight junction; AJ, adherens junction.
Table. The apical junctional components.
| Junctional components | Binding moieties | Function19,24,25,26 | |
|---|---|---|---|
| Tight Junctions | |||
| Claudins | ZO-1, ZO-2, ZO-3, Afadin, Cingulin | Regulation of paracellular permeability | |
| JAMs | ZO-1, ZO-2, ZO-3, PAR3/PAR6/aPKC, MAGI-1, Afadin, Cingulin | Regulation of cell polarity, permeability, and transendothelial migration | |
| Homophilic and heterophilic trans-dimers | |||
| Occludin | ZO-1, ZO-2, ZO-3, JEAP | Sealing neighboring cells | |
| Adherens Junctions | |||
| Cadherins | α-Catenin, β-Catenin, γ-Catenin, P120 catenin | Ca2+-dependent cell adhesion | |
| Homophilic trans-dimers | |||
| Nectins | Afadin | Homophilic and heterophilic trans-dimers | |
| PAR3/PAR6/aPKC | Regulation of cell polarity | ||
| Formation of primordial junctions | |||
ZO, zonula occludens; JAMs, junctional adhesion molecules; PAR, partitioning-defective; aPKC, atypical protein kinase C; MAGI-1, membrane associated guanylate kinase, WW and PDZ domain containing 1; JEAP, junction-enriched and associated protein.
IMPACT OF ENVIRONMENTAL POLLUTANTS ON BARRIER DYSFUNCTION IN RESPIRATORY DISEASE
Allergic diseases
TJ are essential components of the barrier between the mucosa or skin and the environment, connecting adjacent epithelial cells.27 TJ prevent particles and pathogens from penetrating tissues. They control extracellular fluid and the paracellular flux of molecules, and are involved in the establishment of antigen-presenting cells (APC).28 Epithelial barrier defects due to disruptions in TJ have been reported in several allergic and inflammatory diseases, including atopic dermatitis (AD), asthma, and chronic rhinosinusitis.29 However, research is lacking on the interplay between air pollutants and the bronchial epithelial barrier, and on the impact of PM on epithelial barrier function. PM-induced disruption of barrier function in a bronchial epithelial cell line provided some insight into the role of epithelial barrier dysfunction in asthma.30 It remains unclear whether TJ are affected by PM in patients with asthma. Nanoparticles (NPs) such as titanium dioxide (TiO2) may cause cell and tissue damage, leading to local and systemic inflammatory responses and adverse effects on asthma due to the inhalation of PM.31
Protease-activated receptor-2 (PAR-2), a major receptor of activated factor X, is known to contribute to the pathogenesis of inflammatory diseases. PAR-2-modulated TJ were involved in the pathogenesis of chronic inflammatory diseases, and can downregulate the expression of zonula occludens-1 (ZO-1) and claudin (CLDN) 1, which are involved in epithelial barrier dysfunction in AR.26 Increased epithelial permeability has been reported in AR, with histamine and type-2 inflammation being responsible for TJ dysfunction. Epithelial barrier dysfunction facilitates transepithelial allergen passage, allergic sensitization, and allergen-induced mast cell degranulation even in the absence of an inflammatory environment. These results emphasize the crucial role of an intact epithelial barrier for the prevention of allergy.32 Mucin 1 (MUC1)-deficiency in the nasal epithelium of patients with AR exacerbates symptoms and affects nasal epithelial barrier functions in AR. Depletion of MUC1 suppresses the expression of epithelial cell junction proteins, which may be a promising therapeutic target in patients with AR.33 Changes in epithelial barrier functions are also documented in AR, including in the physical epithelial barrier, mucus production, antimicrobial defense, microbiome, and immune responses.34
Children with a disrupted skin barrier become allergic to peanuts through environmental exposure to peanut protein in household dust.35 Environmental and climatic factors can also adversely impact skin barrier integrity, increasing the risk and severity of AD. Mechanical damage caused by repetitive scratching, detergents, humidity, exogenous proteases, and air pollution negatively impacts filaggrin expression.36 Subjects from 5 to 15 years old whom were sensitized to environmental allergens demonstrate that the levels for occludin, CLDN, ZO-1, e-cadherin and β catenin of intra-epithelial junctional proteins in the lymphoepithelium of the adenoid tissue in the upper respiratory tract are lower in subjects with sensitization to inhalant antigens.37 Skin barrier dysfunctions are complex and driven by a combination of genetic, environmental, and immunologic factors that affect gene expression, structural proteins, and lipid profiles.38 Impaired skin barrier function is caused by changes in the expression of key structural cornified barrier proteins and skin barrier lipids.39 Barrier dysfunction is an important feature of AD that involves interleukin (IL)-4 and IL-13. Periostin, a matricellular protein induced by IL-4 or IL-13, plays a crucial role in the onset of allergic skin inflammation, including barrier dysfunction. The IL-13/periostin pathway induces IL-24 production in keratinocytes and plays an important role in barrier dysfunction in AD.40
Asthma
The bronchial epithelium is constantly exposed to a wide range of environmental materials present in inhaled air, including noxious gases and anthropogenic and natural particulates, such as gas and particles from car emissions, tobacco smoke, pollens, animal dander, and pathogens.41 The airway epithelium not only provides a physical barrier, but also modulates allergic responses and airway inflammation.42 Epithelial barrier dysfunction is involved in allergic inflammation and asthma due to increased exposure of sub-epithelial tissues to inhaled allergens and air pollutants. The TJ CLDN proteins are important regulators of paracellular permeability. PM contributes to airway epithelial barrier dysfunction, which results in airway inflammation and responsiveness.43
Impaired airway epithelium allows pathogens to remain in situ, which induces aberrant immunological reactions.44 Airway epithelium is the first line of defense between the external air and internal body. It acts as a physical barrier, and senses signals to coordinate the immunological responses associated with signal transduction, thus excluding pathogens such as bacteria, viruses, fungi, and allergens.45 Airway epithelial cells also play pivotal roles in the immunological coordination of defense mechanisms, by transmitting signals to immunologic cells to eliminate external pathogens from the airways.46
Dysregulated functions of asthmatic airway epithelium have been reported in the context of impaired wound repair, fragile TJ, and excessive proliferation, which lead to airway remodeling (i.e., aberrant airway responses caused by external pathogens).44 Increased sensitivity of asthmatic airway epithelia to environmental stressors and oxidative stress reduces the threshold for epithelial damage.47 Increased barrier permeability in asthma promotes allergic sensitization, reduces the threshold for epithelial damage, activates type 2 responses, and affects the microbial diversity of asthmatic airways.48 Impaired epithelial barrier repair in asthmatics leads to a failure to resolve inflammatory responses.46,49,50 Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections in children worldwide. While most develop a mild, self-limiting illness, some develop severe acute lower respiratory infection and persistent airway disease. Exposure to ambient PM has been linked to asthma, bronchitis, and viral infection in multiple epidemiological studies. TiO2-NP exposure exacerbates RSV-induced APC dysfunction and increases inflammation via mechanisms that involve the generation of ROS.51
Plasma CLDN7 levels are lower in patients with asthma compared to healthy individuals. CLDN7 levels are associated with the forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio; the proportion of blood eosinophils in patients with asthma and the expression of CLDN7 were elevated in the lungs of mice with asthma, and in normal human bronchial epithelial cells treated with HDM extracts. CLDN7 expression was suppressed by exposure to TiO2, suggesting that PM contributes to airway epithelial barrier dysfunction, and results in airway inflammation and responsiveness.43 CLDN4, 5, and 17 were affected in the nose and lung of mice exposed to diesel exhaust particles,43 suggesting that cell barrier integrity changed similarly between the upper and lower airways; this raises the possibility that modulation of cell barriers in the nose and lung could be a useful alternative treatment for airway disease.52
N-acetylcysteine (NAC) affects the signaling pathways involved in apoptosis, angiogenesis, cell growth and arrest, redox-regulated gene expression, and the inflammatory response. NAC diminished ovalbumin (OVA)-induced airway hyperresponsiveness and inflammation. Levels of CLDN18 protein were higher in lung tissue from OVA-induced asthma mice than control and were increased by treatment with NAC or dexamethasone. Soluble CLDN18 levels were lower in patients with asthma than controls, and were correlated with the percentage of neutrophils, the FEV1/FVC ratio, and FEV1% predicted. Those results suggest that CLDN18 is involved in the pathogenesis of asthma.53
Acrolein, an α/β-unsaturated aldehyde, is volatile at room temperature. It is a respiratory irritant found in tobacco smoke and can be generated during cooking or endogenously at injury sites. Acrolein induces reactive airway dysfunction syndrome, ROS, and angiogenesis, and TJ proteins such as CLDN4 and CLDN17 were involved in reactive airways dysfunction syndrome in a mouse model.54
CLDNs are major transmembrane protein components of TJs in endothelia and epithelia. CLDNs are essential in the role of TJs in cell permeability and cell signaling, through protein-protein interactions. Ozone induces oxidative stress and lung inflammation in humans and animal models, but the impact of ozone on CLDNs remains poorly understood. CLDNs such as CLDN3, CLDN4, and CLDN14 are involved in airway inflammation following ozone exposure, suggesting that ozone affects TJ proteins through oxidative mechanisms.55
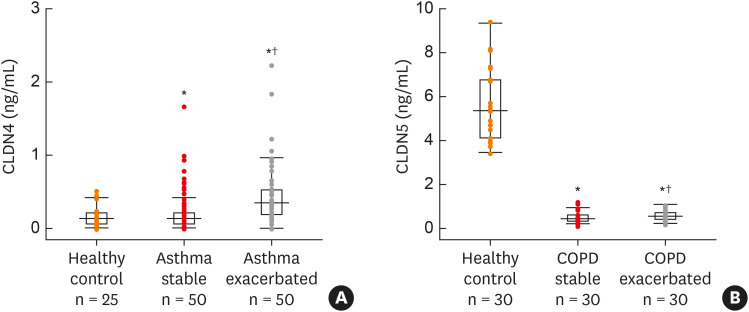
CLDN4 has been reported to function as a paracellular sodium barrier and is one of three major CLDNs expressed in lung alveolar epithelial cells. Blood from asthmatic patients showed increased CLDN4 levels compared to healthy control subjects (Fig. 2A). Plasma CLDN4 levels were significantly higher in patients with bronchial asthma than in control patients (Fig. 2A). Plasma CLDN4 levels correlated with eosinophils and total IgE level, and inversely correlated with FEV1% predicted and the FEV1/FVC ratio. Moreover, lung tissues from OVA-OVA mice showed significant increases in CLDN4 staining, transcripts, and proteins, as well as TJ breaks. Following CLDN4 knockdown by siRNA transfection, the expression of inflammatory cytokines induced by Der p1 treatment were significantly increased. These findings raise the possibility that regulation of lung epithelial barrier proteins may constitute a therapeutic approach for asthma.56
Fig. 2. (A) Plasma CLDN4 level between stable and exacerbated states of asthmatic subjects and control subjects. (B) Plasma CLDN5 levels in patients with COPD and control subjects, and in patients with stable or exacerbated COPD.
COPD, chronic obstructive pulmonary disease; CLDN, claudin.
*P < 0.05, compare to control; †P < 0.05, stable vs. exacerbated (modified from references56,63).
Inhaled corticosteroids are currently the most effective anti-inflammatory therapy for persistent asthma. Corticosteroid therapy might also suppress the increase in bronchial vascularity and edema observed in asthmatic patients. In our mouse model of airway inflammation, airway responsiveness and cytokine levels were reduced by corticosteroid treatment, and CLDN5 expression recovered (in terms of transcript and protein levels, and endothelial integration), supporting the idea that regulation of endothelial TJ could be a therapeutic target to decrease airway inflammation and responsiveness.57
Chronic obstructive pulmonary disease (COPD)
Airway epithelial barrier function is maintained by the formation of TJ and AJ. Inhalation of cigarette smoke (CS) causes airway epithelial barrier dysfunction and may contribute to the pathogenesis of chronic lung diseases, such as asthma and COPD.
CS extract (CSE) causes airway epithelial barrier dysfunction and simultaneously downregulates multiple TJ and AJ proteins.58 Combining corticosteroids and long-acting bronchodilators had no additive effect on reducing CSE-induced transepithelial electrical resistance (TEER).59 LL-37 counteracted CSE-induced TEER reduction and prevented the disruption of occludin and ZO-1. The use of LL-37 to counteract airway epithelial barrier dysfunction may have significant benefits for respiratory diseases, such as asthma and COPD.59
Wildfire smoke induces acute pulmonary distress and is of particular concern to at-risk groups, such as the sick and elderly. Woodsmoke (WS) contains many of the same toxic compounds as CS, including polycyclic aromatic hydrocarbons, carbon monoxide, and free radicals. CS is a well-established risk factor for respiratory diseases such as asthma and COPD. WS may contribute to the breakdown of alveolar structure and function through a p44/42 MAPK-dependent pathway, with chronic exposure leading to the development and/or exacerbation of respiratory pathologies.60 Wildfire smoke extract inhibits autophagic flux and induces barrier dysfunction in the airway epithelium. As autophagy is a central regulator of cellular repair, viability, and inflammation, targeting autophagic flux blockade may ameliorate the consequences of wildfire smoke exposure for individuals with pre-existing respiratory conditions.61
The barrier function of airway epithelium in COPD can be potentiated by dysregulating TJ protein complexes.62 Zinc (Zn) is a vital cytoprotective factor for the airway epithelium, and its depletion by CS produces disease-related modifications consistent with the inflammatory changes seen in COPD. Zn deficiency, alongside CSE, potentiates the leaky barrier phenotype and increased airway epithelial permeability exhibited in COPD. Zn dyshomeostasis has been reported in airway epithelium exposed to chronic CS and inflammation. Therefore, targeting these mechanisms has promise for ameliorating the leaky barrier phenotype synonymous with COPD.62
The epithelial lining of the airway is the first barrier against environmental attack, such as inhaled CS, which is the primary risk factor for COPD development. Destruction of the epithelial barrier exposes the subepithelial layers to hazardous agents in the inspired air, and alters the normal function of epithelial cells, which increases the likelihood of COPD. Disruption of the epithelial junctions may modulate signaling pathways involved in differentiation, repair, and pro-inflammatory responses.58
CLDN5 is a critical TJ in the control of endothelial cell polarity and pericellular permeability. Mean plasma CLDN5 levels of patients with COPD are often lower than in healthy controls and are increased in exacerbated versus stable COPD patients (Fig. 2B). Plasma CLDN5 levels among COPD subjects were correlated with the amount of smoking. Plasma CLDN5 levels in stable COPD patients were correlated with the FEV1% predicted. These results suggest that CLDN5 may be involved in the pathogenesis of COPD.63
CLDN5 is an integral membrane protein that constitutes a critical component of endothelial TJ, controlling pericellular permeability. Acrolein is a major irritant in smoke that can induce acute lung injury (ALI), possibly by altering CLDN5 expression. Sensitive and resistant mouse strains showed different levels of CLDN5 expression following acrolein exposure, suggesting that preservation of endothelial CLDN5 may be a novel clinical approach for treating ALI.64
THERAPEUTIC TARGETS FOR CELL BARRIERS
Strategies to repair epithelial barrier function include the use of prescription emollients that mimic skin barrier lipid composition, biologics such as anti-IL-4R, anti-IgE, anti-IL 13, and environmental interventions such as dietary changes, avoidance of allergens and detergents, the use of humidifiers, protective measures against pollution, and various microbiomes.34 HDM disrupts the airway barrier function through the PAR2/TLR4/PGC-1α-dependent pathway. Thus, modulation of this pathway could represent a new approach to treat asthma. To date, no medication has shown efficacy for restoring airway barrier function.65
The airway epithelial layer is disrupted in asthma, as indicated by the detachment of ciliated cells, presence of epithelial cell aggregates in the sputum, increased permeability to allergens, and reduced expression of the cell-cell adhesion molecule E-cadherin.66,67 The inability to repair epithelial barrier function has significant pathophysiological consequences, and results in increased permeability to allergens and the propagation of pro-inflammatory and abnormal repair responses in the airways, in turn leading to airway hyperresponsiveness and remodeling.68 Therapies targeting epithelial barrier defects are attractive. Current approved therapies are aimed at restoring or improving epithelial permeability in chronic airway diseases.69,70 However, novel experimental in vitro and in vivo mouse models have reported reconstitution of the epithelial barrier.72 These approaches focus on TJ regulation and have the potential to prevent the onset of upper and lower airway pathologies, such as asthma and respiratory disease.71
CONCLUSION
In this review, we discussed the impact of environmental pollutants on cell barriers in respiratory diseases. Airway epithelial cells, which form the initial airway barrier, play pivotal roles in asthma and COPD pathogenesis, and are potential therapeutic targets to maintain airway integrity. Their disruption enables environmental pollutants to remain in contact with the airway epithelium, leading to airway inflammation, bronchoconstriction, and airway remodeling. Airway epithelial cells also contribute to the coordination of immune responses, to eliminate pathogens. Further basic and clinical research is warranted to gather data on environmental pollutants and airway cell barriers, which would aid the identification of novel therapeutic targets for respiratory diseases.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2020R1A2C1006506) and Soonchunhyang University.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
References
- 1.GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sly PD, Cormier SA, Lomnicki S, Harding JN, Grimwood K. Environmentally persistent free radicals linking air pollution and poor respiratory health? Am J Respir Crit Care Med. 2019;200:1062–1063. doi: 10.1164/rccm.201903-0675LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thurston GD, Kipen H, Annesi-Maesano I, Balmes J, Brook RD, Cromar K, et al. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J. 2017;49:1600419. doi: 10.1183/13993003.00419-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack MC, Breysse PN, Hansel NN, Matsui EC, Tonorezos ES, Curtin-Brosnan J, et al. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environ Res. 2008;106:148–155. doi: 10.1016/j.envres.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Indoor air quality: biological contaminants. Report on a WHO meeting. WHO Reg Publ Eur Ser. 1990;31:1–67. [PubMed] [Google Scholar]
- 7.Schäfer T, Ring J. Epidemiology of allergic diseases. Allergy. 1997;52(Suppl):14–22. doi: 10.1111/j.1398-9995.1997.tb04864.x. [DOI] [PubMed] [Google Scholar]
- 8.Jang AS. In: Air pollution: a comprehensive perspective. Haryanto B, editor. London: IntechOpen Limited; 2012. Particulate air pollutants and respiratory diseases; pp. 153–174. [Google Scholar]
- 9.Leikauf GD, Kim SH, Jang AS. Mechanisms of ultrafine particle-induced respiratory health effects. Exp Mol Med. 2020;52:329–337. doi: 10.1038/s12276-020-0394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 11.Al-Hegelan M, Tighe RM, Castillo C, Hollingsworth JW. Ambient ozone and pulmonary innate immunity. Immunol Res. 2011;49:173–191. doi: 10.1007/s12026-010-8180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaudel C, Mackowiak C, Maillet I, Fauconnier L, Akdis CA, Sokolowska M, et al. Ozone exposure induces respiratory barrier biphasic injury and inflammation controlled by IL-33. J Allergy Clin Immunol. 2018;142:942–958. doi: 10.1016/j.jaci.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Jang AS, Choi IS, Koh YI, Park CS, Lee JS. The relationship between alveolar epithelial proliferation and airway obstruction after ozone exposure. Allergy. 2002;57:737–740. doi: 10.1034/j.1398-9995.2002.23569.x. [DOI] [PubMed] [Google Scholar]
- 14.Jang AS, Choi IS, Yang SY, Kim YG, Lee JH, Park SW, et al. Antioxidant responsiveness in BALB/c mice exposed to ozone. Respiration. 2005;72:79–84. doi: 10.1159/000083405. [DOI] [PubMed] [Google Scholar]
- 15.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 16.Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- 17.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. Bioessays. 2005;27:356–365. doi: 10.1002/bies.20203. [DOI] [PubMed] [Google Scholar]
- 18.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JM, Cereijido M. In: Tight junctions. Cereijido M, Anderson JM, editors. Boca Raton (FL): CRC Press; 2001. Introduction: evolution of ideas on the tight junction; pp. 1–18. [Google Scholar]
- 21.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 22.Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, et al. Tight junctions and human diseases. Med Electron Microsc. 2003;36:147–156. doi: 10.1007/s00795-003-0219-y. [DOI] [PubMed] [Google Scholar]
- 23.Mullin JM, Agostino N, Rendon-Huerta E, Thornton JJ. Keynote review: epithelial and endothelial barriers in human disease. Drug Discov Today. 2005;10:395–408. doi: 10.1016/S1359-6446(05)03379-9. [DOI] [PubMed] [Google Scholar]
- 24.Stevens T, Garcia JG, Shasby DM, Bhattacharya J, Malik AB. Mechanisms regulating endothelial cell barrier function. Am J Physiol Lung Cell Mol Physiol. 2000;279:L419–L422. doi: 10.1152/ajplung.2000.279.3.L419. [DOI] [PubMed] [Google Scholar]
- 25.Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev. 2005;57:815–855. doi: 10.1016/j.addr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Wang Ms J, Kang Ms X, Huang Ms ZQ, Shen Ms L, Luo Md Q, Li Ms MY, et al. Protease-activated receptor-2 decreased zonula occlidens-1 and claudin-1 expression and induced epithelial barrier dysfunction in allergic rhinitis. Am J Rhinol Allergy. 2021;35:26–35. doi: 10.1177/1945892420932486. [DOI] [PubMed] [Google Scholar]
- 27.Kast JI, Wanke K, Soyka MB, Wawrzyniak P, Akdis D, Kingo K, et al. The broad spectrum of interepithelial junctions in skin and lung. J Allergy Clin Immunol. 2012;130:544–547.e4. doi: 10.1016/j.jaci.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 28.Schulzke JD, Günzel D, John LJ, Fromm M. Perspectives on tight junction research. Ann N Y Acad Sci. 2012;1257:1–19. doi: 10.1111/j.1749-6632.2012.06485.x. [DOI] [PubMed] [Google Scholar]
- 29.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol. 2007;120:1233–1244. doi: 10.1016/j.jaci.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Sweerus K, Lachowicz-Scroggins M, Gordon E, LaFemina M, Huang X, Parikh M, et al. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017;139:72–81.e1. doi: 10.1016/j.jaci.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim BG, Lee PH, Lee SH, Park MK, Jang AS. Effect of TiO2 nanoparticles on inflammasome-mediated airway inflammation and responsiveness. Allergy Asthma Immunol Res. 2017;9:257–264. doi: 10.4168/aair.2017.9.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kortekaas Krohn I, Seys SF, Lund G, Jonckheere AC, Dierckx de Casterlé I, Ceuppens JL, et al. Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy. 2020;75:1155–1164. doi: 10.1111/all.14132. [DOI] [PubMed] [Google Scholar]
- 33.Zhou LB, Zheng YM, Liao WJ, Song LJ, Meng X, Gong X, et al. MUC1 deficiency promotes nasal epithelial barrier dysfunction in subjects with allergic rhinitis. J Allergy Clin Immunol. 2019;144:1716–1719.e5. doi: 10.1016/j.jaci.2019.07.042. [DOI] [PubMed] [Google Scholar]
- 34.Zhang N, Van Crombruggen K, Gevaert E, Bachert C. Barrier function of the nasal mucosa in health and type-2 biased airway diseases. Allergy. 2016;71:295–307. doi: 10.1111/all.12809. [DOI] [PubMed] [Google Scholar]
- 35.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J Allergy Clin Immunol. 2015;135:164–170. doi: 10.1016/j.jaci.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol. 2014;134:792–799. doi: 10.1016/j.jaci.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Yılmaz Ö, Şimşek Y, İnan S, Buga Ö, Eskiizmir G, Pınar E, et al. Significant changes in trans-epithelial barrier proteins of adenoid tissue with atopic status in children. Turk Thorac J. 2020;21:242–247. doi: 10.5152/TurkThoracJ.2019.18207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung DY, Berdyshev E, Goleva E. Cutaneous barrier dysfunction in allergic diseases. J Allergy Clin Immunol. 2020;145:1485–1497. doi: 10.1016/j.jaci.2020.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goleva E, Berdyshev E, Leung DY. Epithelial barrier repair and prevention of allergy. J Clin Invest. 2019;129:1463–1474. doi: 10.1172/JCI124608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitamura Y, Nunomura S, Nanri Y, Ogawa M, Yoshihara T, Masuoka M, et al. The IL-13/periostin/IL-24 pathway causes epidermal barrier dysfunction in allergic skin inflammation. Allergy. 2018;73:1881–1891. doi: 10.1111/all.13437. [DOI] [PubMed] [Google Scholar]
- 41.Global Initiative for Asthma. Global strategy for asthma management and prevention [Internet] Fontana (WI): GINA; 2020. [cited 2021 Feb 23]. Available from: http://ginasthma.org/2020-gina-report-global-strategy-for-asthma-management-and prevention/ [Google Scholar]
- 42.Davies DE. Epithelial barrier function and immunity in asthma. Ann Am Thorac Soc. 2014;11(Suppl 5):S244–S251. doi: 10.1513/AnnalsATS.201407-304AW. [DOI] [PubMed] [Google Scholar]
- 43.Lee YG, Lee SH, Hong J, Lee PH, Jang AS. Titanium dioxide particles modulate epithelial barrier protein, claudin 7 in asthma. Mol Immunol. 2021;132:209–216. doi: 10.1016/j.molimm.2021.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Inoue H, Akimoto K, Homma T, Tanaka A, Sagara H. Airway epithelial dysfunction in asthma: relevant to epidermal growth factor receptors and airway epithelial cells. J Clin Med. 2020;9:3698. doi: 10.3390/jcm9113698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 46.Grainge CL, Davies DE. Epithelial injury and repair in airways diseases. Chest. 2013;144:1906–1912. doi: 10.1378/chest.12-1944. [DOI] [PubMed] [Google Scholar]
- 47.Bucchieri F, Puddicombe SM, Lordan JL, Richter A, Buchanan D, Wilson SJ, et al. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol. 2002;27:179–185. doi: 10.1165/ajrcmb.27.2.4699. [DOI] [PubMed] [Google Scholar]
- 48.Durack J, Lynch SV, Nariya S, Bhakta NR, Beigelman A, Castro M, et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol. 2017;140:63–75. doi: 10.1016/j.jaci.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loxham M, Davies DE. Phenotypic and genetic aspects of epithelial barrier function in asthmatic patients. J Allergy Clin Immunol. 2017;139:1736–1751. doi: 10.1016/j.jaci.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011;242:205–219. doi: 10.1111/j.1600-065X.2011.01030.x. [DOI] [PubMed] [Google Scholar]
- 51.Smallcombe CC, Harford TJ, Linfield DT, Lechuga S, Bokun V, Piedimonte G, et al. Titanium dioxide nanoparticles exaggerate respiratory syncytial virus-induced airway epithelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2020;319:L481–L496. doi: 10.1152/ajplung.00104.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee PH, Kim BG, Park MK, Hong J, Lee YG, Jang AS. The impact of diesel exhaust particles on tight junctional proteins on nose and lung in a mouse model. Allergy Asthma Immunol Res. 2021;13:350–352. doi: 10.4168/aair.2021.13.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee PH, Hong J, Jang AS. N-acetylcysteine decreases airway inflammation and responsiveness in asthma by modulating claudin 18 expression. Korean J Intern Med. 2020;35:1229–1237. doi: 10.3904/kjim.2019.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim BG, Lee PH, Lee SH, Hong J, Jang AS. Claudins, VEGF, Nrf2, Keap1, and nonspecific airway hyper-reactivity are increased in mice co-exposed to allergen and acrolein. Chem Res Toxicol. 2019;32:139–145. doi: 10.1021/acs.chemrestox.8b00239. [DOI] [PubMed] [Google Scholar]
- 55.Kim BG, Lee PH, Lee SH, Park CS, Jang AS. Impact of ozone on claudins and tight junctions in the lungs. Environ Toxicol. 2018;33:798–806. doi: 10.1002/tox.22566. [DOI] [PubMed] [Google Scholar]
- 56.Lee PH, Kim BG, Lee SH, Lee JH, Park SW, Kim DJ, et al. Alteration in claudin-4 contributes to airway inflammation and responsiveness in asthma. Allergy Asthma Immunol Res. 2018;10:25–33. doi: 10.4168/aair.2018.10.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moon KY, Lee PH, Kim BG, Park CS, Leikauf GD, Jang AS. Claudin 5 in a murine model of allergic asthma: Its implication and response to steroid treatment. J Allergy Clin Immunol. 2015;136:1694–1696.e5. doi: 10.1016/j.jaci.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 58.Aghapour M, Raee P, Moghaddam SJ, Hiemstra PS, Heijink IH. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am J Respir Cell Mol Biol. 2018;58:157–169. doi: 10.1165/rcmb.2017-0200TR. [DOI] [PubMed] [Google Scholar]
- 59.Tatsuta M, Kan-O K, Ishii Y, Yamamoto N, Ogawa T, Fukuyama S, et al. Effects of cigarette smoke on barrier function and tight junction proteins in the bronchial epithelium: protective role of cathelicidin LL-37. Respir Res. 2019;20:251. doi: 10.1186/s12931-019-1226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zeglinski MR, Turner CT, Zeng R, Schwartz C, Santacruz S, Pawluk MA, et al. Soluble wood smoke extract promotes barrier dysfunction in alveolar epithelial cells through a MAPK signaling pathway. Sci Rep. 2019;9:10027. doi: 10.1038/s41598-019-46400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roscioli E, Hamon R, Lester SE, Jersmann HP, Reynolds PN, Hodge S. Airway epithelial cells exposed to wildfire smoke extract exhibit dysregulated autophagy and barrier dysfunction consistent with COPD. Respir Res. 2018;19:234. doi: 10.1186/s12931-018-0945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roscioli E, Jersmann HP, Lester S, Badiei A, Fon A, Zalewski P, et al. Zinc deficiency as a codeterminant for airway epithelial barrier dysfunction in an ex vivo model of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:3503–3510. doi: 10.2147/COPD.S149589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim BG, Lee PH, Lee SH, Baek AR, Park JS, Lee J, et al. Impact of the endothelial tight junction protein claudin-5 on clinical profiles of patients with COPD. Allergy Asthma Immunol Res. 2018;10:533–542. doi: 10.4168/aair.2018.10.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jang AS, Concel VJ, Bein K, Brant KA, Liu S, Pope-Varsalona H, et al. Endothelial dysfunction and claudin 5 regulation during acrolein-induced lung injury. Am J Respir Cell Mol Biol. 2011;44:483–490. doi: 10.1165/rcmb.2009-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saito T, Ichikawa T, Numakura T, Yamada M, Koarai A, Fujino N, et al. PGC-1α regulates airway epithelial barrier dysfunction induced by house dust mite. Respir Res. 2021;22:63. doi: 10.1186/s12931-021-01663-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hackett TL, Singhera GK, Shaheen F, Hayden P, Jackson GR, Hegele RG, et al. Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to respiratory syncytial virus and air pollution. Am J Respir Cell Mol Biol. 2011;45:1090–1100. doi: 10.1165/rcmb.2011-0031OC. [DOI] [PubMed] [Google Scholar]
- 67.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128:549–556.e1. doi: 10.1016/j.jaci.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 68.Boxall C, Holgate ST, Davies DE. The contribution of transforming growth factor-beta and epidermal growth factor signalling to airway remodelling in chronic asthma. Eur Respir J. 2006;27:208–229. doi: 10.1183/09031936.06.00130004. [DOI] [PubMed] [Google Scholar]
- 69.Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol. 2020;145:1499–1509. doi: 10.1016/j.jaci.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 71.Steelant B, Seys SF, Boeckxstaens G, Akdis CA, Ceuppens JL, Hellings PW. Restoring airway epithelial barrier dysfunction: a new therapeutic challenge in allergic airway disease. Rhinology. 2016;54:195–205. doi: 10.4193/Rhino15.376. [DOI] [PubMed] [Google Scholar]