Abstract
Microscopic examination of respiratory specimens for acid-fast bacilli (AFB) plays a key role in the initial diagnosis of tuberculosis, monitoring of treatment, and determination of eligibility for release from isolation. The objective of this study was to compare the sensitivity obtained with smears for detection of AFB (AFB smears) made directly from respiratory specimens (direct AFB smears) to that obtained with parallel smears made from concentrates of the specimens (concentrated AFB smears). A total of 2,693 specimens were evaluated; 1,806 were from the University of California Irvine Medical Center Medical Microbiology Laboratory (UCIMC), which serves a tertiary-care hospital with outpatient clinics, and 887 were from the Microbial Disease Laboratory at the California Department of Public Health (MDL), which receives specimens from outpatient facilities and clinics on Pacific islands. Of the 353 AFB culture-positive specimens at UCIMC, there was a statistically significant difference in the sensitivity of the direct AFB smear (34%) and that of the smear made from the concentrated specimen (58%) (P < 0.05). This was also true for the 208 specimens positive for Mycobacterium tuberculosis, for which the sensitivity of the direct smear was 42% (87 of 208) and that for the smear made from the concentrated specimen was 74% (154 of 208). At MDL, where all but 1 of the 45 culture-positive specimens grew M. tuberculosis, the sensitivity of the smear made from the concentrated specimen was 93% (42 of 45) and was not significantly higher than the sensitivity of the direct smear, which was 82% (37 of 45). By combining the results from both laboratories, 42 patients from whom at least three specimens were received were culture positive for M. tuberculosis. The cumulative results for the initial three specimens from these patients showed that the direct smear detected M. tuberculosis in 81% of these patients, whereas the smear made from the concentrate detected M. tuberculosis in 91% of these patients. In summary, when all culture-positive specimens are considered, the sensitivity of the direct smear compared to that of a smear made from the concentrated specimen was significantly different overall in the two different laboratory settings. However, this difference was reduced only if the cumulative results for the initial three specimens received from patients who were culture positive for M. tuberculosis were evaluated.
Microscopic examination of respiratory specimens for acid-fast bacilli (AFB) plays an important role in the initial diagnosis of tuberculosis. In addition, in some settings a smear must be negative for AFB before a patient can be released from isolation and, in some cases, the hospital (2). Therefore, from the standpoint of the public health concern about transmission as well as medical economics, smears for detection of AFB (AFB smears) play a central role.
It has been shown that concentration and liquefaction along with the use of fluorescent stains improve the sensitivities of AFB smears (5, 10, 13). In 1993, in an effort to combat the resurgence of Mycobacterium tuberculosis, the Centers for Disease Control and Prevention (CDC) issued recommendations for the rapid testing and the reporting of results for respiratory samples (11). Included in the recommendations was reporting of results for AFB smears within 24 h of specimen collection. However, surveys conducted by the College of American Pathologists in 1992, 1993, and 1995 indicated that only 26, 30, and 43% of diagnostic laboratories, respectively, concentrated specimens for mycobacteriology 7 days a week (12, 13). This would mean that in laboratories that do not concentrate specimens daily, in order to meet the 24-h smear reporting recommendation of CDC, a smear would need to be made directly with the original unprocessed specimen or with a specimen concentrated by some other rapid concentration method such as that proposed by Saceanu et al. (9).
Since the maintenance of the level of staffing needed to concentrate specimens on a daily basis remains a problem in many laboratories, the purpose of the evaluation described here was to determine the sensitivity of detection of AFB-positive patients when smears were made directly from the specimen (direct AFB smears) rather than from the concentrated material (concentrated AFB smears). We evaluated this in two laboratory settings, namely, a public health laboratory, the Microbial Diseases Laboratory (MDL; Berkeley, Calif.), and a university hospital laboratory, the University of California Irvine Medical Center Medical Microbiology Laboratory (UCIMC).
MATERIALS AND METHODS
Specimen processing.
Only respiratory specimens submitted to the mycobacteriology laboratories of MDL and UCIMC with a culture request were included in the evaluation. At MDL the specimens evaluated were mainly from an outpatient population from the Pacific Basin (including Palau, Saipan, Federated States of Micronesia, American Samoa, and the Republic of the Marshall Islands). Specimens entered into the evaluation at UCIMC were obtained from both outpatients and inpatients. At both UCIMC and MDL, direct smears were made prior to processing of the specimen. The specimens were then processed by a standard N-acetyl-l-cysteine NaOH digestion-decontamination method (7). Specimens were centrifuged at 3,200 × g (UCIMC) and 2,500 × g (MDL). The resulting sediments were then resuspended in 1.5 ml of phosphate-buffered saline (0.01 M; pH 7.2) containing penicillin (50 U/ml), and the suspensions were used to prepare the concentrated smear and to inoculate the culture media.
Cultures.
At UCIMC, 0.5 ml of the concentrated specimen was used to inoculate a BACTEC 12B vial (Becton Dickinson, Sparks, Md.) supplemented with antimicrobial agents (polymyxin B, 50 U/ml; amphotericin B, 5 μg/ml; nalidixic acid, 20 μg/ml; trimethoprim, 5 μg/ml; azlocillin, 5 μg/ml), and 0.1 ml was placed on each side of a biplate of Middlebrook 7H11 agar consisting of media with and without carbenicillin (50 μg/ml) and trimethoprim lactate (20 μg/ml) (Remel, Lenexa, Kans.). The biplates were incubated at 37°C in a 5 to 10% CO2 atmosphere and were read weekly for 8 weeks. AFB were identified by DNA-RNA hybridization (AccuProbe; Genprobe, San Diego, Calif.) or, if necessary, by conventional methods (7). The BACTEC vials were read twice a week for the first 2 weeks after inoculation and weekly thereafter for 6 weeks. An aliquot of vials with a growth index of ≥100 was used to prepare an AFB smear, and if AFB were present, 1.8 ml of the culture was centrifuged and the pellet was resuspended and tested directly as described previously (3) by using nucleic acid probes specific for either M. tuberculosis or M. avium-M. intracellulare complex (Genprobe). If the direct hybridization of the BACTEC pellet was negative, the pellet was subcultured onto Middlebrook 7H11 agar and was identified as described above.
The same culture procedure described above was used at MDL, but in addition to the Middlebrook 7H11 agar biplate, Lowenstein-Jensen medium and a Middlebrook 7H10 plate were inoculated.
AFB smears.
Smears made from the original specimen or the concentrated specimen were heat fixed and were stained with auramine-rhodamine at MDL and with auramine O at UCIMC (7). At MDL and UCIMC smears were scanned at ×200 with an Olympus BH2 microscope (Japan). Before a smear was called negative, 30 to 50 fields were examined. To confirm the morphology of fluorescent material, smears were examined at ×400 (UCIMC) or ×600 (MDL). All smears positive by auramine staining were overstained with either Ziehl-Neelsen stain at MDL or Kinyoun's stain at UCIMC (7). These smears were then examined under oil immersion (×1,000), and the following quantitation was used at MDL: <3 AFB/smear were considered negative; 3 to 9 AFB/smear, rare (1+); >9 AFB/smear, few-moderate (2+); and >1 AFB/field, numerous (3+). At UCIMC smears stained with the Kinyoun method were reported as follows: <3 AFB/100 fields, negative; 3 to 9 AFB/100 fields, 1+; 1 to 9 AFB/10 fields, 2+; 1 to 9 AFB/field, 3+; ≥9 AFB/field, 4+.
Statistics.
Fischer's exact test was used to evaluate the differences between smears made directly from the specimen and those made from the concentrated material. Statistical significance was defined at a confidence level of >95% (P < 0.05).
RESULTS
A total of 2,693 respiratory specimens were included in the study (Table 1). Of these 67% were evaluated at the UCIMC laboratory, which serves inpatients in a tertiary-care hospital and several local outpatients clinics, and the remaining 33% were from MDL and came from clinics on Pacific islands. While 20% of all the respiratory specimens evaluated at UCIMC grew a Mycobacterium sp. only 5% of the specimens from MDL were culture positive. Of the specimens culture positive for Mycobacterium spp., 89% were from UCIMC and the remaining 11% were from specimens evaluated at MDL. Of the 353 culture-positive specimens at UCIMC, 59% were positive for M. tuberculosis. In contrast, all except 1 of the 45 culture-positive specimens at MDL grew M. tuberculosis. Therefore, the profile of the specimens from the two settings were clearly different in terms of the rate of positivity for a Mycobacterium sp.
TABLE 1.
Overall distribution of specimens at two sites
| Site | No. of specimens | No. of patients | Culture-positive specimens onlya
|
M. tuberculosis culture-positive specimens only
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of patients | No. of specimens | No. of specimens direct smear positive | No. of specimens concentrated smear positive | No. of patients | No. of specimens | No. of specimens direct smear positive | No. of specimens concentrated smear positive | |||
| MDL | 887 | 198 | 23 | 45 | 37 | 42 | 22 | 44 | 37 | 42 |
| UCIMC | 1,806 | 367 | 136 | 353 | 99 | 181b | 40 | 208c | 87 | 154b |
| Both | 2,693 | 565 | 159 | 398 | 136 | 223b | 62 | 252 | 124 | 196b |
Culture positive for any Mycobacterium sp.
P < 0.05 compared to direct smear results for the same specimen.
Includes 195 pure cultures of M. tuberculosis and 12 mixed cultures with M. tuberculosis.
Of the 398 culture-positive specimens, 65% grew M. tuberculosis, 20% grew a member of the M. avium-M. intracellulare complex, and 15% grew other Mycobacterium spp. (Table 2). Twenty cultures were mixed in that they grew more than one species of Mycobacterium. Eighteen specimens were positive for AFB with one of the smears but were culture negative. Although these specimens were from patients who had provided other specimens that were positive by culture during the evaluation period, data for these specimens were excluded from the data analysis for the smear comparison. Of the culture-positive specimens, AFB smears made from the concentrated specimen detected 56% (224 of 398) of the positive specimens. This was in contrast to the direct smear, which detected only 34% (136 of 398) of the positive specimens (P < 0.05). Of the 146 specimens culture positive for Mycobacterium spp. that were not M. tuberculosis, 19% (28 of 146) were positive when the concentrated smear was used and 8% (12 of 146) were positive when the direct smear was used. When only specimens that were culture positive for M. tuberculosis are considered, there was a significant difference in the abilities of the concentrated and direct smears to detect positive specimens (P < 0.05). Here, 78% (196 of 252) were positive when the concentrated smear was used, while 49% (124 of 252) were positive when the direct smear was used.
TABLE 2.
Overall results for direct and concentrated smears and culture
| Organism | No. of specimens with the following results (direct smear, concentrated smear, culture)c:
|
||||||
|---|---|---|---|---|---|---|---|
| +++ | −++ | +−+ | −−+ | ++− | −+− | Total | |
| M. tuberculosis | 123 | 73 | 1 | 55 | 8a | 10a | 270 |
| M. avium-M. intracellulare complex | 10 | 14 | 60 | 84 | |||
| M. gordonae | 1 | 28 | 29 | ||||
| Other | 2 | 23 | 25 | ||||
| Mixedb | 1 | 7 | 8 | ||||
| Total | 135 | 89 | 1 | 173 | 8 | 10 | 416 |
From patients with other specimens culture positive for M. tuberculosis within the same time period.
Except for mixed cultures that contained M. tuberculosis isolates that are counted as positive in the row for M. tuberculosis, cultures that grew more than one Mycobacterium spp. that was not M. tuberculosis are counted under mixed.
+, positive; −, negative
Because of differences in patient populations and positivity rates, the smear results from the two settings were analyzed separately. At UCIMC, in parallel with the overall results presented above, there was a significant difference (P < 0.05) in the abilities of the concentrated and direct smears to detect all culture-positive specimens as well as those positive for M. tuberculosis. In contrast, at MDL, while the concentrated smear was able to detect more culture-positive specimens than the direct smear, the difference was not significant. In comparing the quantities of AFB in the two smears, the majority of smears from both settings were scored as having more AFB on the smear from the concentrated specimen (Table 3).
TABLE 3.
Comparison of quantitation of AFB from direct and concentrated smears from specimens culture positive for M. tuberculosis
| Site | Direct smear result | No. of specimens with the following concentrated smear result:
|
||||
|---|---|---|---|---|---|---|
| Negative | 1+ | 2+ | 3+ | 4+ | ||
| UCIMC | Negative | 52 | 40 | 21 | 8 | |
| 1+ | 1 | 4 | 16 | 23 | 6 | |
| 2+ | 2 | 8 | 14 | |||
| 3+ | 6 | |||||
| 4+ | 1 | 5 | ||||
| MDL | Negative | 3 | 3 | 1 | 1 | |
| 1+ | 5 | 14 | ||||
| 2+ | 5 | 7 | ||||
| 3+ | 5 | |||||
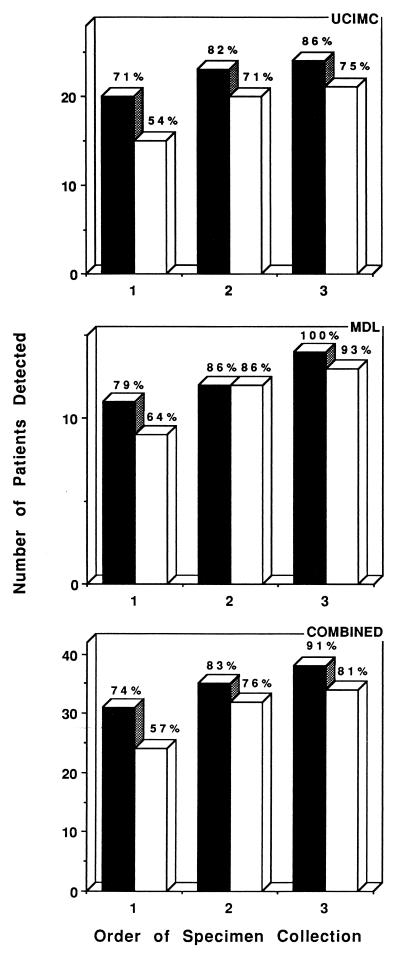
At UCIMC multiple specimens for culture were available from several of the patients infected with M. tuberculosis, with the average number of specimens that were either smear or culture positive being 5.2; the range was from 1 to 20 specimens. Therefore, the results were analyzed separately for the first three specimens received from a patient since for most patients these would be the specimens that would aid in the initial diagnosis of the infection and that would be obtained either before or shortly after the institution of antimicrobial therapy. There were 28 patients who were culture positive for M. tuberculosis and from whom three or more specimens were received. Among these patients, the concentrated smear of the first specimen identified 20 patients who were infected with M. tuberculosis with an additional 3 patients and 1 patient found to be positive by use of concentrated smears of the second and third samples, respectively (Fig. 1). The parallel direct smear for these patients was positive for 15 of the initial specimens, with the second direct smear identifying five more infected patients and the third one detecting one additional infected patient. No significant difference in the number of patients found to be positive with the first three samples was found between the concentrated and direct smears (P > 0.05).
FIG. 1.
AFB smear results for the initial three specimens received from patients culture positive for M. tuberculosis. The results are cumulative and are for the individual settings as well as the two laboratories combined. ■, concentrated smear; □, direct smear.
Three or more specimens were received from 15 patients at MDL. The smear of the concentrated specimen identified 11, 12, and 14 infected patients from the cumulative results for the first, second, and third specimens, respectively (Fig. 1). With the direct smear the cumulative results for the three specimens identified 9, 12, and 13 infected patients, respectively. As with the data from UCIMC, the difference between the initial direct and concentrated smears for the detection of patients culture positive for M. tuberculosis was not significant (P > 0.05).
By combining the results from the two settings for the initial three specimens, the concentrated smear identified more of the 42 infected patients for each of the three specimens submitted; however, the difference was not significant. The difference between the two smears was greatest with the first specimen; however, this difference was diminished with inclusion of a second and a third specimen. When comparing the abilities of the concentrated and direct smears to identify culture-positive patients, there was a significant difference in the ability of the initial three specimens to identify patients culture positive for M. tuberculosis in comparison to that of the first specimen alone (P < 0.05).
DISCUSSION
One purpose of the investigation described here was to determine the ability of a smear made directly from a respiratory sample and one made from a concentrated sample to identify which specimens would be culture positive for a Mycobacterium sp. If we considered all respiratory specimens evaluated in our study, it was clear that in a tertiary-care hospital, UCIMC, the direct smear was significantly less sensitive than the concentrated smear in detecting culture-positive specimens (28 and 51%, respectively) (P < 0.05). However, with the samples from outpatients in the Pacific islands used in this evaluation by MDL, while the direct smear was less sensitive than that made from the concentrated specimen (82 versus 93%, respectively), the difference was not significant. Two main factors most likely contribute to this difference in the overall abilities of the concentrated and direct smears to detect positive cultures in the two different laboratory settings. The average numbers of samples received from culture-positive patients were similar in the two settings (2.6 and 2.7 for UCIMC and MDL, respectively). However, at UCIMC, 59% of the positive cultures were for specimens from patients with M. tuberculosis, and from these patients the average number of specimens received was 5.2, whereas at MDL, with the exception of one patient, all culture-positive specimens were from patients infected with M. tuberculosis. It is logical to assume that at UCIMC more specimens than the initial three usually obtained for diagnostic purposes were obtained from patients and that some specimens were obtained while the patients were receiving therapy. Specimens from patients who are receiving therapy are likely to contain a smaller numbers of organisms. Corroborating this, as would be expected, the smaller the number of the organisms seen in the concentrated smears, the more likely it was for the direct smear to be negative. Also at UCIMC, 41% of the positive cultures grew a Mycobacterium sp. that was not M. tuberculosis. It has previously been reported by others that specimens infected with nontuberculous mycobacteria were usually smear negative due to the smaller number of organisms present (14). Of the specimens in this category, only 10% were positive by the direct smear method, whereas 23% were positive by use of the smear made from the concentrated specimen, thus widening the overall difference in the performance of the two smears. Another factor that may have contributed to the differences in the results obtained with the direct and concentrated smears between the two laboratories was the higher centrifugal force used at UCIMC (3,200 × g) in comparison to that used at MDL (2,500 × g). However, Ratman and March (8) compared centrifugation of specimens at 2,500 and 3,895 × g and did not find any difference in the smear result-culture result correlation.
While one objective of the present study was to establish the overall sensitivity of a direct smear, more specifically, we wanted to determine the ability of a direct smear of the initial three specimens obtained from a patient to identify those individuals culture positive for M. tuberculosis. For the most part, it is this group of patients who need to be promptly identified for treatment and isolation (1). These initial specimens are the main focus of the CDC recommendation that a smear report be available within 24 h of specimen collection (11). Staffing of a laboratory 7 days a week for concentration of these specimens to achieve this objective of smear reporting has been problematic for some laboratories. Therefore, we examined the sensitivity of a direct smear because in some laboratory settings, on certain days, e.g., weekends and holidays, the only smear report generated may be one for a direct smear. If we combine the data from both sites, there was a 17% difference between direct and concentrated smears in identifying positive patients with the initial specimen; this was reduced to 7 and 10% with the second and third specimens, respectively. Therefore, 81 and 91% of patients for whom at least three specimens were submitted were found to be positive with the direct and concentrated smears, respectively. In our study this meant that among the 42 patients examined, if only a direct smear was used for the initial three samples, 8 patients would have been missed, whereas 4 would have missed if the smear made from the concentrate was used. While this number did not reach significance in this study, this may be due merely to a limitation of the number of samples included in this evaluation.
The practice of obtaining three specimens to aid in the initial diagnosis of tuberculosis has been challenged by some (6). In our study the third specimen was the first to be positive with the smear made from the concentrated specimen for 7.0% (3 of 43) of the patients; this rate was 4.7% (2 of 43) for the direct smear. These data were similar to those of Nelson et al. (6), who found that the third concentrated specimen submitted from a subsequently culture positive patient was the first smear-positive specimen for 13% (7 of 56) of the patients examined. These data led the authors to question whether the third specimen was of diagnostic value. However, in our study, while the difference in the abilities of the concentrated smear and the direct smear to identify positive patients was not significant when the cumulative results for the first and second specimens were analyzed, the difference was significant when the initial result was compared to the cumulative results for three specimens.
Another goal of the study was to determine whether a direct smear could be substituted for one made from a concentrated specimen at times when a patient was being considered to be released from isolation after antimycobacterial therapy had been instituted. From our results from consideration of all specimens culture positive for M. tuberculosis at UCIMC, where serial samples were obtained from patients who were receiving therapy, this would not be achieved by using the direct smear results since there was, overall, a significant difference in the results for the direct and concentrated smears, especially for samples with smaller numbers of AFB, as would be the case for treated patients. Prior to the issuance of the CDC guidelines for removal of patient from isolation, which call for three consecutive negative smears of sputum, the generally accepted rule was to wait for 2 weeks after the initiation of chemotherapy before releasing the patient from isolation (1, 4). This was based on a series of studies that have provided evidence that once a patient is treated for this time period, unless the isolate is resistant to treatment, there is a minimal chance of transmission. Therefore, even though the direct smear appeared to be inadequate for the identification of specimens that may eventually grow M. tuberculosis, some may argue that patients who have been receiving therapy for at least 2 weeks are not infectious, even though small numbers of M. tuberculosis remain in their respiratory samples (4).
In conclusion, in the two different laboratory settings used in the present study, there were different findings with respect to the relative sensitivity of an AFB smear made directly from a specimen compared with the sensitivity of an AFB smear made from a concentrated specimen in their ability to detect specimens which were culture positive for M. tuberculosis. In the tertiary-care setting, where there were large numbers of nontuberculous mycobacteria and where specimens from treated patients were received for follow-up, the concentrated smear was significantly more sensitive than the direct smear. For the specimens received from outpatient clinics on Pacific islands, there were few nontuberculous mycobacteria, and very few specimens were received after receipt of the initial set of specimens submitted for establishment of the diagnosis. For these Pacific island specimens, the sensitivity of detection with the concentrated smears was not significantly greater than that of detection with the direct smears. However, the direct smear was always less sensitive than the smear made from the concentrated specimens overall, and achievement of statistical significance is most likely a function of the numbers of specimens entered into the study. Therefore, caution must be exerted in laboratory settings when a direct smear is substituted for a smear made from a concentrated specimen. During those times when a laboratory is unable to adhere to the requirement to submit within 24 h a smear report obtained by using a concentration method, it should explore alternate methods such as that described by Saceanu et al. (9) or at least consider the direct AFB smear report to be preliminary and to confirm the result once the specimen is concentrated.
ACKNOWLEDGMENTS
We thank the technical staff at both UCIMC and MDL that contributed to this work.
REFERENCES
- 1.American Thoracic Society. Control of tuberculosis in the United States. Am Rev Respir Dis. 1992;146:1623–1633. doi: 10.1164/ajrccm/146.6.1623. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities. Morbid Mortal Weekly Rep. 1994;43(Suppl. RR-13):1–132. [PubMed] [Google Scholar]
- 3.Evans K D, Nakasone A S, Sutherland P A, de la Maza L M, Peterson E M. Identification of Mycobacterium tuberculosis and Mycobacterium avium-M. intracellulare directly from primary BACTEC cultures by using acridinium-ester-labeled DNA probes. J Clin Microbiol. 1992;30:2427–2431. doi: 10.1128/jcm.30.9.2427-2431.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iseman M. An unholy trinity-three negative sputum smears and release from tuberculosis isolation. Clin Infect Dis. 1997;25:671–672. doi: 10.1086/513774. . (Editorial response.) [DOI] [PubMed] [Google Scholar]
- 5.Miorner H, Ganlov G, Yohannes Z, Adane Y. Improved sensitivity of direct microscopy for acid-fast bacilli: sedimentation as an alternate to centrifugation for concentration of tubercle bacilli. J Clin Microbiol. 1996;34:3206–3207. doi: 10.1128/jcm.34.12.3206-3207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson S N, Deike M A, Cartwright C P. Value of examining multiple specimens in the diagnosis of pulmonary tuberculosis. J Clin Microbiol. 1998;36:467–469. doi: 10.1128/jcm.36.2.467-469.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolte F S, Metchock B. Mycobacterium. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 400–437. [Google Scholar]
- 8.Ratman S, March S B. Effect of centrifugal force and centrifugation time on sedimentation of mycobacteria in clinical specimens. J Clin Microbiol. 1986;23:582–585. doi: 10.1128/jcm.23.3.582-585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saceanu C A, Pfeiffer N C, McLean T. Evaluation of sputum smears concentrated by cytocentrifugation for detection of acid-fast bacilli. J Clin Microbiol. 1993;31:2371–2374. doi: 10.1128/jcm.31.9.2371-2374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinnick T M, Good R C. Diagnostic mycobacteriology laboratory practices. Clin Infect Dis. 1995;21:291–299. doi: 10.1093/clinids/21.2.291. [DOI] [PubMed] [Google Scholar]
- 11.Tenover F C, Crawford J T, Huebner R E, Geiter L J, Horsburgh C R, Jr, Good R C. The resurgence of tuberculosis: is your laboratory ready? J Clin Microbiol. 1993;31:767–770. doi: 10.1128/jcm.31.4.767-770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woods G L, Long T A, Witebsky F G. Mycobacterial testing in clinical laboratories that participate in the College of American Pathologists mycobacteriology surveys: changes in practices based on responses to 1992, 1993 and 1995 questionnaires. Arch Pathol Lab Med. 1996;120:429–435. [PubMed] [Google Scholar]
- 13.Woods G L, Witebsky F G. Mycobacterial testing in clinical laboratories that participate in the College of American Pathologists' mycobacteriology E survey: results of a 1993 questionnaire. J Clin Microbiol. 1995;33:407–412. doi: 10.1128/jcm.33.2.407-412.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yajko D M, Nassos P S, Sanders C A, Jadej J J, Hadlem W J. High predictive value of the acid-fast smear for Mycobacterium tuberculosis despite the high prevalence of Mycobacterium avium complex in respiratory specimens. Clin Infect Dis. 1994;19:334–336. doi: 10.1093/clinids/19.2.334. [DOI] [PubMed] [Google Scholar]