Abstract
Affect-biased attention may play a fundamental role in early socioemotional development, but factors influencing its emergence and associations with typical versus pathological outcomes remain unclear. Here, we adopted a nonhuman primate model of early social adversity (ESA) to: (1) establish whether juvenile, pre-adolescent macaques demonstrate attention biases to both threatening and reward-related dynamic facial gestures; (2) examine the effects of early social experience on such biases; and (3) investigate how this relation may be linked to socioemotional behaviour. Two groups of juvenile macaques (ESA exposed and non-ESA exposed) were presented with pairs of dynamic facial gestures comprising two conditions: neutral-threat and neutral-lipsmacking. Attention biases to threat and lipsmacking were calculated as the proportion of gaze to the affective versus neutral gesture. Measures of anxiety and social engagement were also acquired from videos of the subjects in their everyday social environment. Results revealed that while both groups demonstrated an attention bias towards threatening facial gestures, a greater bias linked to anxiety was demonstrated by the ESA group only. Only the non-ESA group demonstrated a significant attention bias towards lipsmacking, and the degree of this positive bias was related to duration and frequency of social engagement in this group. These findings offer important insights into the effects of early social experience on affect-biased attention and related socioemotional behaviour in nonhuman primates, and demonstrate the utility of this model for future investigations into the neural and learning mechanisms underlying this relationship across development.
Subject terms: Psychology, Animal behaviour
Introduction
In the face of limited perceptual and cognitive resources, attention mechanisms enable the brain to manage competing demands in the everyday environment by prioritizing a subset of stimuli for dedicated processing. Such mechanisms guide behaviour from the earliest months postpartum, serving as a fundamental base for learning, self-regulation, and memory1,2. Affect-biased attention specifically is posited to play a broad and pervasive role in early socioemotional development3,4, with emerging affect biases shaping an infant’s experience of their environment via preferential processing of threat- and reward-related information. This, in turn, is thought to support the emergence of adaptive approach and avoidance behaviour5,6. However, specific affect-biases have also been linked to poor socioemotional functioning later on in development (e.g.7,8), and many questions remain concerning the mechanisms through which affect-biased attention arises and may relate to both typical and pathological outcomes.
Biased attention towards threat-relevant information serves an essential survival function9. It is unsurprising, therefore, that most affect-bias studies have focused on threatening stimuli such as angry or fearful versus neutral faces. An attention bias to threat (ABT) emerges during the first year postpartum5,10, and has been linked to positive socioemotional outcomes in the form of secure infant attachment6. Conversely, ABT has also been associated with emotion regulation difficulties, social withdrawal, and anxiety in both adults and younger populations11,12, with cognitive models of anxiety attributing a causal relation to ABT in the development and/or maintenance of anxiety13–15. This apparent contradiction suggests that any increased vulnerability conferred by threat-biased attention may arise from an early-emerging, normative threat bias16,17, with excessive ABT or a failure to inhibit ABT exacerbating the risk for psychopathology18. Inconsistent findings concerning a link between ABT and anxiety in childhood, with no relation often found despite ABT presence (e.g.17), is in keeping with this idea, but so far, very little is known about how ABT and socioemotional function interact across development.
An attention bias to positive stimuli (ABP) such as happy faces may also play an important role in early socioemotional functioning. Such positive biases are often conceptualized as a bias towards rewarding stimuli3. Relatively little is known about the emergence of ABP, but evidence suggests this also arises at an early stage in development10,19. ABP has been associated with several aspects of positive socioemotional functioning in children and adults, including social engagement and prosocial behaviour20, adaptive emotion regulation skills21, and positive affect22. Notably, ABP has also been linked to lower levels of anxiety (e.g.23), and may act as a protective factor in developmental populations at increased risk for poor socioemotional outcomes. This includes risk for anxiety and internalizing problems in behaviourally inhibited and previously institutionalized children20,24–26. Nevertheless, findings linking ABP to anxiety in younger populations are again mixed, with some studies failing to find any relation between ABP and anxiety in childhood8,27.
Nonhuman primate (NHP) models could add significantly to our understanding of affect-biased attention and its role in early socioemotional functioning. Adopting a comparative developmental approach can provide unique insights into the origins of human cognition, highlighting similarities and divergences in our evolutionary history28,29. However, it is currently unclear whether affect biases comparable to those in humans are present in early NHP development, and how these may relate to other specific developmental outcomes. Rhesus macaque (Macaca mulatta) monkeys are very similar to humans in terms of cognition, socio-affective characteristics, and brain organization, and are thus commonly utilized to investigate the aetiology of various psychiatric and neurodevelopmental disorders, including anxiety (see30,31). Macaques also live in large social groups, have an extended period of development comprising distinct infant, juvenile (pre-adolescent and adolescent), and adult periods, and the early macaque mother-infant relationship shares many commonalities with humans32. The macaque model is, therefore, especially well-suited to developmental studies, and could provide particularly valuable information concerning the mechanisms underlying ABT and ABP emergence. Accordingly, we adopted a macaque model in the current study with the goal of furthering our understanding of affect-biased attention in NHPs, and investigating the suitability of this as a translational model of its development and related socioemotional outcomes.
Early-emerging ABT also appears normative in macaques, assessed via presentation of threatening versus neutral faces29,33,34. To our knowledge, only one macaque study has investigated ABP specifically, with no bias towards a positive, affiliative facial gesture (‘lipsmacking’) versus a neutral face revealed at any stage in development29. Nevertheless, this lack of bias may have resulted from difficulty in discriminating between the two static images, as lipsmacking, a highly rhythmic and dynamic facial gesture, is very difficult to portray in a static stimulus. To date, the majority of both human and NHP studies that have utilized paired affective versus neutral facial stimuli to investigate affect-biased attention have used static images. The use of dynamic facial stimuli, however, improves various aspects of perception and enhances attention biases in human adults35–37, and recruits dissociable neural pathways from those involved in the perception of static faces38,39. This issue of static versus moving faces is especially pertinent for developmental research. Dynamic stimuli can enhance neural and behavioural discrimination of emotional versus neutral faces from early infancy40,41, and attention to emotional expressions is modulated by stimulus motion across childhood, adolescence, and into adulthood42. Altogether, this highlights the importance of adopting more ecologically valid, dynamic facial stimuli in studies of affect-biased attention, and indeed, there is a growing movement within the wider research community towards the use of more naturalistic stimuli in studies of social attention with humans and NHPs (e.g.43).
To address outstanding questions concerning the mechanisms through which affect-biased attention emerges and relates to both typical and atypical functioning, it is critical to consider which factors may contribute to individual differences in ABT and ABP, and how such differences may confer vulnerability or resilience. It is well established that early social adversity can increase risk for a number of adverse socioemotional outcomes, including anxiety and reduced social engagement in humans and macaques (e.g.44–47). A small number of studies have linked elevated ABT specifically to early social deprivation20,25 and maternal anxiety10 in children and human infants, with maternal abuse and over-protectiveness associated with ABT magnitude in infant and adolescent macaques33,34. Early social deprivation may also impact ABP, with ‘care-as-usual’ versus foster home placement related to a reduced or absent bias towards happy faces, and greater ABP to more social engagement and fewer internalizing problems20,25 in the context of early institutionalization. It remains unknown whether early social adversity has similar effects on the relation between affect-biased attention and comparable socioemotional outcomes in NHPs.
The current study was designed to investigate both ABT and ABP in pre-adolescent macaques using dynamic facial stimuli, and to examine whether such biases are linked to socioemotional functioning; specifically, anxiety-like behaviour and social engagement. To consider the effects of early social adversity, we assessed two groups of juvenile macaques (aged 2.5 years), one mother-reared and one peer-reared. Peer-rearing is often adopted in macaque models of early social adversity, and has been associated with both increased anxiety and decreased social behaviour45,47. Our hypotheses were: (1) although all animals will demonstrate attention biases, peer-reared animals will demonstrate greater ABT, and mother-reared animals will demonstrate greater ABP; (2) greater ABT will be related to more anxiety-like behaviour, but greater ABP will be related to less; and (3) greater ABP will be related to more social engagement.
Methods
Subjects and housing conditions
The sample consisted of 21 juvenile rhesus macaques (Macaca mulatta), 11 mother-reared (six female) and 10 peer-reared (5 female). Subjects were aged around 2.5 years at the time of this study (mother-reared; M = 943.18 days, SD = 17.98: peer-reared; M = 956.2 days, SD = 20.24). Subjects were housed at the Institut des Sciences Cognitives Marc Jeannerod, CNRS, in mixed mother- and peer-reared social groups of 5–6 animals. All housing and procedures conformed to current guidelines concerning the care and use of laboratory animals (European Community Council Directive No. 86-609), and were approved by our local ethics board, ‘Comité d’Ethique Lyonnais pour les Neurosciences Expérimentales’ (CELYNE) C2EA #42 (03.10.18), and the French Ministry of Research (10.10.18); project reference APAFIS#15091_2018071014483295_v2. All reporting here conforms to the recommendations in the ARRIVE Guidelines for Reporting Animal Research.
All subjects were born and raised at the Laboratory of Comparative Ethology at the National Institutes of Health, US. Peer-reared animals were raised in a nursery with access to same-aged peers; see48 for more rearing protocol details. Rearing procedures were approved by the NICHD and the University of Maryland Animal Care and Use Committee, and adhered to the NIH Guide for the Care and Use of Laboratory Animals. Animals were relocated to the Rousset Primatological Station, CNRS, France at two years of age. More information about the rearing protocol and housing can be found in the SI.
Facial gesture stimuli
Stimuli for the attention bias task consisted of short, dynamic video clips (5 s) of an unfamiliar adult female macaque performing three types of facial movements: (1) neutral facial movements (i.e. no gesture but with small movements of, for example, the nose and mouth); (2) lipsmacking (LPS), comprised of the rapid, rhythmic opening and closing of the mouth and pursing of the lips; and (3) open-mouth threat, comprised of the wide opening of the mouth and lowering of the jaw, with lips held in a tense position covering the teeth. The onset and duration of movement, size, brightness, contrast, spatial frequency, and overall motion levels were controlled for (see SI) to ensure that neutral, lipsmacking, and threat video stimuli did not differ in terms of low-level visual features. All videos started with a 500 ms static period showing the first frame of the video (with a neutral facial expression), followed by 4500 ms of movement (i.e. two consecutive instances of each gesture or neutral movement sequence).
Attention bias task
Each subject was temporarily separated from their social group and placed into the testing area in another section of the room; 87 × 100 × 120 cm enclosure with a clear panelled front. Before commencing the task, a widescreen computer monitor (35 × 61 cm; 2560 × 1440 resolution) was placed 60 cm from the front of the enclosure, and animals were given five minutes to habituate to the enclosure once separated. Note, all animals had already been well familiarized with this process of separation into the testing enclosure and presentation of non-social video stimuli before the day of assessment. Animals were recorded during the task using a webcam (30 fps) placed on the top-centre of the monitor.
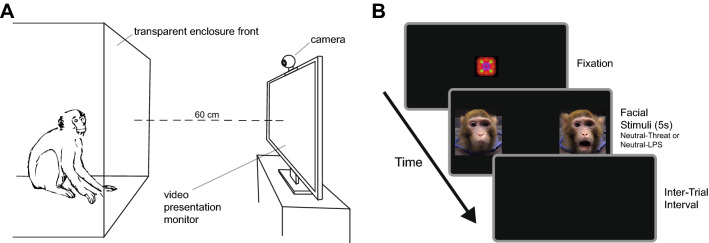
Animals were presented with pairs of neutral-affective gesture stimuli comprising two conditions: (1) Neutral-Threat (five trials per subject); and (2) Neutral-LPS (five trials per subject), i.e. the positive or reward condition. Video pairs were presented for 5 s per trial, with condition order and position (left or right) of neutral-affective gesture videos counterbalanced across subjects. Before the stimuli appeared, a moving geometric pattern accompanied by a non-social sound was presented in the centre of the screen to attract the subject’s attention, with stimuli presentation then triggered by an experimenter watching the animal live on a separate monitor (not in view of the subject). Additionally, a calibration procedure was conducted before presentation of experimental stimuli, whereby images of objects (e.g. ball, toy car) were presented on the right, centre, and left of the screen. Each image was jittered up and down slightly to attract attention and was accompanied by a non-social sound. Psychopy v1.90.249 was used for stimulus (calibration and experimental) presentation, with video recording onset and offset automatically triggered at the start and end of each presentation. This sequence and the experimental set-up is illustrated in Fig. 1.
Figure 1.
(A) Schematic illustration of the attention bias task set-up. (B) Illustration of a trial in the attention bias task. An experimenter triggered the appearance of fixation and facial stimuli screens when the subject looked towards the monitor.
Subjects’ gaze (left, right, other, offscreen) was manually coded offline, frame-by-frame, by a researcher blind to the condition being presented and the position of the neutral-gesture stimuli. A random 15% of videos were coded by a second researcher to establish reliability, with very good reliability scores obtained (ĸ = 0.84).
Behavioural observation
Video recordings of the social group (50 fps) were made two times per week, once in the morning and once in the afternoon, for three weeks. Two cameras were used to capture the whole home enclosure and were synchronized offline for the manual coding of behaviour. Each animal was coded second-by-second for five minutes per recording session, totalling 30 min per animal. One experimenter coded all of the videos, with a second experimenter coding a random 15% to establish reliability (ĸ = 0.93).The following behaviours were coded using the focal sampling method: (a) self-scratching, self-grooming, yawns, and body shakes (i.e. behaviours reflecting anxiety in macaques; see30); and (b) social grooming (both give and receive), which is a primary means by which macaques maintain and strengthen social relationships50.
Data analysis
To calculate attention bias to threat (ABT), the proportion of time looking at the neutral and threat stimuli (out of total time looking onscreen) was calculated separately for each Neutral-Threat trial, with the neutral proportion then subtracted from the threat proportion. The equivalent approach was used to calculate attention bias to LPS stimuli, i.e. positive stimuli (ABP). A linear mixed model was then utilized to investigate potential differences between rearing groups and attention bias type at the trial level, with group (mother-reared or peer-reared), condition (threat or LPS), and their interaction included as fixed effects, and subject-specific intercepts as a random effect. Social rank (randomized Elo-ratings) was also included as a covariate, and z-scored for analysis. Model residuals were checked for normality and homogeneity. Before analysis, trials where animals failed to look at the screen during facial gesture presentation or were 2.5 SDs above or below the mean were excluded. More details about rank calculation and trial exclusion can be found in the SI.
The following behavioural indices were computed based on the behavioural observation coding: (1) anxiety frequency, obtained by summing occurrences of self-scratching, self-grooming, yawns, and body shakes; and (2) both duration and frequency of social engagement, obtained by calculating total time spent in social grooming interactions and the frequency of social grooming interactions, respectively. To explore the relation between attention biases and anxiety or social engagement at the observation session level, generalized linear mixed models were run separately for average ABT and ABP per subject, with negative binomial error distribution and a log link function. This was done with either frequency or duration of behaviour as the outcome variable. Group (mother-reared or peer-reared), ABT or ABP, and their interaction were included as fixed effects, rank (randomized Elo-ratings) as a covariate, and subject-specific intercepts as a random effect. Elo-ratings and attention biases were z-scored for analysis.
R v3.6.351 was utilized to conduct these analyses (see SI for package information). P-values for fixed effects and interactions were obtained using Type III F tests for linear models, and Type III Wald χ2 tests for generalized linear models. Significant interactions between factors were followed up by planned pairwise comparisons of estimated marginal means which were Tukey-corrected for multiple comparisons. Significant interactions between factors and continuous variables (i.e. ABT or ABP) were followed up by planned comparison of the estimated marginal means of the linear trends of the continuous variable to 0 at each level of the factor. Effect sizes are reported as unstandardized model parameter estimates (in the scale of the model response variable). All animals were included in these analyses (n = 21; 11 mother-reared, 10 peer-reared). Descriptive statistics can be found in Table 1.
Table 1.
Gaze measures and socioemotional behaviours.
| Group | Mother-reared | Peer-reared |
|---|---|---|
| Gaze measures | ||
| Proportion onscreen | 0.60 (0.15) | 0.44 (0.19) |
| Proportion threat (Neutral-Threat trials) | 0.455 (0.1) | 0.536 (0.17) |
| Proportion neutral (Neutral-Threat trials) | 0.264 (0.103) | 0.145 (0.065) |
| Proportion LPS (Neutral-LPS trials) | 0.456 (0.094) | 0.324 (0.1) |
| Proportion neutral (Neutral-LPS trials) | 0.225 (0.069) | 0.292 (0.133) |
| Attention bias to threat (ABT) | 0.19 (0.164) | 0.386 (0.151) |
| Attention bias to LPS (ABP) | 0.231 (0.086) | 0.032 (0.14) |
| Socioemotional behaviour | ||
| Anxiety frequency | 1.561 (0.743) | 2.383 (1.114) |
| Social groom frequency | 1.106 (0.987) | 0.983 (0.713) |
| Social groom duration (s) | 50.606 (48.466) | 37.117 (31.096) |
Proportion onscreen is the proportion (M and SD) of trial time spent attending to the screen. Proportion threat, neutral, and LPS are the proportions (M and SD) of time attending to the screen spent attending to the threat, neutral, and LPS stimuli, respectively. ABT and ABP (i.e. affective versus neutral stimuli) is the difference between proportions to threat (or LPS) and neutral in the neutral-threat, and neutral-LPS trials, respectively. Anxiety and social groom frequency are the frequencies (M and SD) of anxiety-like behaviour and social grooming during the behavioural observation period. Social groom duration (M and SD) is the total amount of time (seconds) spent in grooming interactions during the behavioural observation period.
Results
Attention bias to threat and LPS
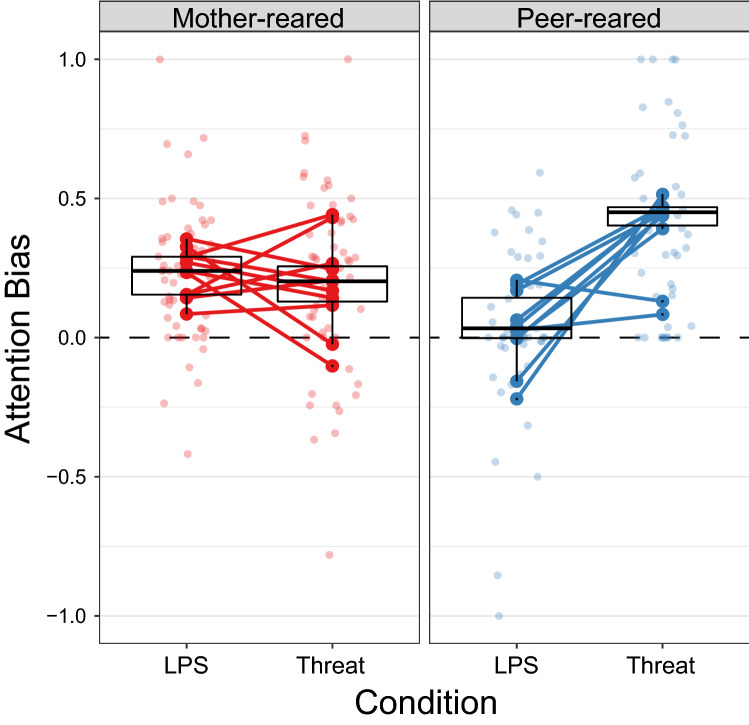
First we compared the attention biases to affective stimuli in the Neutral-LPS and Neutral-Threat conditions between the mother- and peer-reared groups. This revealed a significant main effect of group [F(1) = 8.288, p = 0.007, effect size (mother-peer) = 0.036], as well as a significant group × condition interaction [F(1) = 22.101, p < 0.0001, mother-reared effect size (LPS-threat) = 0.036, peer-reared effect size (LPS-threat) = − 0.364] (Fig. 2). Attention bias to threat (ABT) was greater in the peer-reared compared to mother-reared group [t(33.1) = − 2.079, p = 0.045], and attention bias to LPS (i.e. positive bias; ABP) was greater in the mother-reared compared peer-reared group [t(34.6) = 2.879, p = 0.007]. In the mother-reared group, ABT and ABP were not significantly different from each other [t(176) = 0.628, p = 0.531] but in the peer-reared group, ABT was significantly greater that ABP [t(178) = − 5.827, p < 0.0001].
Figure 2.
Attention biases in the Neutral-LPS and Neutral-threat conditions, for the mother-reared group (red; left) and peer-reared group (blue; right). Zero indicates no bias, positive values a bias towards LPS or threat versus neutral, and negative values a bias towards neutral versus LPS or threat. Light-coloured dots represent the bias in each trial, large dark-coloured dots indicate the average bias for each subject, and lines connect the average bias in the two conditions per subject. The median bias per group, first and third quartiles, and + or − 1.5 times the inter-quartile range from the first and third quartiles are also shown in the box plots.
One sample t-tests also confirmed that in the mother-reared group, ABT [t(10) = 3.841, p = 0.003] and ABP [t(10) = 8.905, p < 0.0001] were significantly different from zero, whereas in the peer-reared group, only ABT [t(9) = 8.093, p < 0.0001] was significantly different from zero.
Relation between attention biases and socioemotional behaviour
Having established a difference in attention biases between groups and conditions, we then sought to determine if attention biases were related to the frequency of anxious behaviour in the two rearing groups. We found a group × ABT interaction [χ2(1) = 6.256, p = 0.012, mother-reared effect size = − 0.193, peer-reared effect sized = 0.506] (Fig. 3), with greater ABT related to more frequent anxiety-like behavior in the peer-reared group [z = 2.261, p = 0.024]. There was no significant main effect of ABP or the relation between ABP and frequency of anxious behaviour in either rearing group (both p > 0.458).
Figure 3.
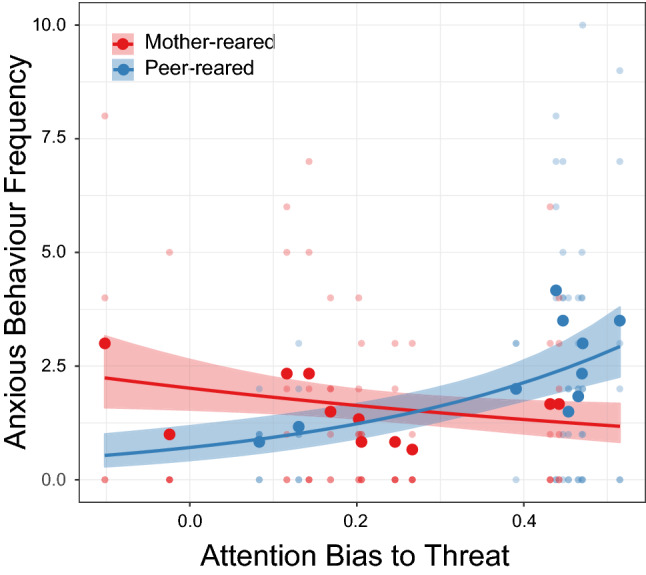
The relation between attention bias to threat (ABT) and frequency of anxiety-like behaviour in the mother-reared (red) and peer-reared (blue) group. Each light-coloured dot represents an individual behavioural observation session, large dark-coloured dots represent the subject mean, dark lines indicate the model fit, and shaded regions around the lines denote + or − SE.
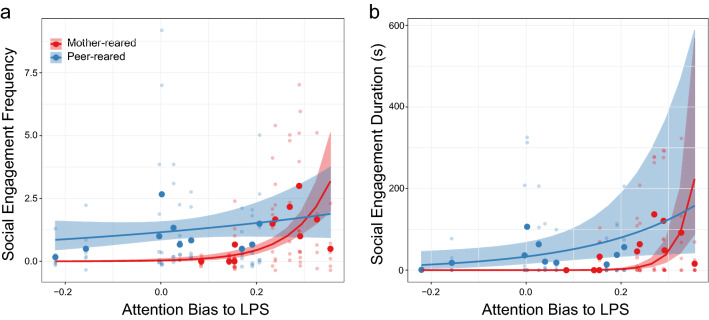
We then went on to investigate relations between attention biases and the frequency and duration of social engagement. There were significant main effects of ABP [χ2(1) = 10.064, p = 0.002, effect size = 1.070], group [χ2(1) = 7.445, p = 0.006, effect size (mother-peer) = − 1.199], and an ABP × group interaction [χ2(1) = 6.194, p = 0.013, mother-reared effect size = 1.931, peer-reared effect size = 0.211] for social engagement frequency (Fig. 4a), with greater ABP related to more frequent social engagement in the mother-reared group [z = 3.172, p = 0.002]. For social engagement duration, we found significant main effects of both ABP [χ2(1) = 18.397, p ≤ 0.0001, effect size = 2.56] and group [χ2(1) = 12.746, p ≤ 0.001, effect size (mother-peer) = − 60.590], and an ABP × group interaction [χ2(1) = 9.793, p = 0.002, mother-reared effect size = 4.456, peer-reared effect size = 0.665]; greater ABP was related to a longer duration of social engagement [z = 4.289, p < 0.0001 in the mother-reared group (Fig. 4b). There were no significant main effects of ABT or relations between ABT and frequency or duration of social engagement (all p > 0.236).
Figure 4.
The relations between attention bias to LPS (i.e. to positive stimuli; ABP) and social engagement in the mother–reared (red) and peer-reared (blue) group. Each light-coloured dot represents an individual behavioural observation session, large dark-coloured dots represent the subject mean, dark lines indicate the model fit, and shaded regions around the lines denote + or − SE.
Discussion
In this study, juvenile macaques demonstrated biased attention to both threatening and affiliative dynamic facial gestures. This is in keeping with a previously demonstrated bias towards static images of open-mouth threat29,33,34, and provides evidence for a bias towards positive or reward-related facial gestures also. Early social deprivation was associated with the degree of bias towards both types of affective stimuli, with a greater attention bias to threat (ABT) found in peer-reared animals, and a greater attention bias to positive stimuli (ABP) found in animals reared by their mothers. Notably, ABT was also linked to anxiety-like behaviour in peer-reared animals, and ABP to levels of social engagement in mother-reared animals. These findings offer novel insights into the effects of early social adversity on affect-biased attention, and suggest that such biases could play an important role in early macaque socioemotional functioning.
The presence of a bias towards dynamic open-mouth threat in both our rearing groups provides some support for ABT emergence being part of typical macaque development. This bias was, however, greater in the peer-reared compared to mother-reared group. Therefore, although ABT emergence is likely normative in macaques29,33, it is possible that very early social deprivation can exacerbate this bias, even into the preadolescent juvenile period. Interestingly, such effects of early social experience on ABT are similar to those seen in human children in the context of early institutionalization20,25. Mother-reared animals also showed a bias towards dynamic lip-smacking (LPS) stimuli. No previous macaque study has investigated potential effects of early adversity on an attention-bias to dynamic LPS specifically, but in line with our results, there is some evidence that early rearing status can affect infants’ social responses to LPS performed by a human experimenter52. As a whole, the peer-reared group did not show significant ABP, again paralleling results from the available human literature concerning the effects of early social deprivation on ABP in pre-adolescence20,25.
Here, a greater magnitude of ABT was related to more frequent anxiety-like behaviour in the peer-reared group, suggesting that early social adversity can confer greater risk for anxiety via exaggerated or uninhibited ABT. This finding aligns with evidence suggesting that in humans, the ABT-anxiety link is not found consistently during childhood, and may be found more reliably in the context of early adverse experience. A relation between ABP and social engagement (i.e. social grooming) was also revealed in the mother-reared group, with more frequent and a longer time spent in grooming interactions associated with greater ABP. Therefore, it is possible that a bias towards reward-related stimuli also serves a positive function in macaque development. In contrast to some research with human children, we did not find a relation between greater ABP and reduced anxiety. There are a number of possible explanations for this result. For instance, some human research suggests that ABP is present in institutionalized children and linked to fewer internalizing problems only after stable fostering placement25. Additionally, in community samples, it may be that ABP only serves as a protective factor against anxiety in behaviourally inhibited children53,54.
In the case of atypical early parenting input, it is possible that uninhibited, exacerbated, or diminished affective attention biases are adaptive in the short-term, increasing offspring survival rates. Longer term, however, this may tie individuals to maladaptive trajectories of development3,55, increasing the risk for psychopathology and social difficulties. Reduced attention to threat has already been associated with insecure and disorganized attachment in infants6,56. Parental sensitivity impacts the formation of infant-caregiver attachment (e.g.57), with insecure and disorganized attachment implicated in the development of numerous adverse outcomes (see58). In the case of early social deprivation, an attachment figure is completely absent. Therefore, while it may be adaptive to avoid threat in the presence of, for example, an abusive caregiver, it may be adaptive for an infant with no social buffering to be hypervigilant towards threat. This highlights an important outstanding issue, with general versus specific mechanisms linking early adversity to negative socioemotional functioning being poorly understood59. Clarifying these mechanisms requires consideration of how specific types of adversity may increase risk for specific adverse outcomes. In terms of a positive bias, it may be that a lack of learning opportunities to associate positive facial gestures with reward in the absence of an attachment figure influences the emergence of a positive affect bias and related social outcomes. This idea needs to be explored more explicitly in subsequent studies, but it is in keeping with evidence for atypical reward processing in the context of early social deprivation (e.g.60).
Although we found a link between ABT and anxious behaviour in juvenile macaques, it remains unclear whether ABT actually played a causal or maintaining role, or simply reflected current levels of anxiety. This remains a key unanswered question in the literature. Very little research thus far has investigated how ABT relates to or predicts anxiety longitudinally, or even the developmental trajectory of ABT itself in typical or at-risk populations. There is some evidence that ABT is unstable across childhood25,53, with ABT links to anxiety variable across this period53,54,61. ABP may also vary across childhood25,53. Research focused on how early ABP predicts subsequent anxiety and social behaviour17,53 suggests that greater ABP is linked to more positive outcomes and may serve as a protective factor against anxiety from very early childhood, but again, a lack of studies measuring both ABP and socioemotional functioning at more than one time-point limits understanding of the exact role ABP serves.
Clearly, longitudinal studies assessing both affect-biased attention and socioemotional functioning at multiple time points are now needed to better understand the role of attention biases in healthy and pathological development. Such studies will be vital to determine how increased vulnerability associated with atypical ABT might arise from a normative threat bias, and how the presence or stability of affect-biased attention across development relates to positive and negative outcomes in different populations. As childhood and adolescence represent the core period of developmental risk for anxiety disorders62, longitudinal studies across this period are of particular importance. Examining the neural learning mechanisms through which attention biases and related outcomes arise will also be critical to address these outstanding questions, and to clarify the processes underlying these relations across different points in development. Our results suggest that the macaque model is ideal for such longitudinal research, and could add considerably to our understanding on whether universal mechanisms of development underlie affect-biased attention, and whether these evolved in primates to help offspring adapt to differences in their early social environment.
In terms of neural bases, the emergence of affect-biased attention is thought to involve attentional networks supporting alerting, orienting, and executive functions, mediated by emotion processing circuitry. This includes brain structures such as the amygdala, orbital frontal cortex (OFC), regions of prefrontal cortex, and anterior cingulate cortex3,4, but evidence from developmental populations is severely lacking. The amygdala and related circuitry may play a particularly crucial role in the processes underlying a relation between early adversity and affect-biases, with a wealth of evidence from the animal and human literature linking adversity to atypical amygdala function and development63. Although the amygdala is classically described as a central node of the fear network, this region probably supports both ABT and ABP3, with more recent studies demonstrating a large overlap of fear and reward networks (e.g.64,65). Investigating links between development in these networks and a relation between affect-biased attention and socioemotional functioning over time will be key in future studies.
Strengths of the current study include the use of ecological dynamic stimuli and investigation of both ABT and ABP links to socioemotional behaviour. In addition, the use of a macaque model allowed for very well-controlled consideration of early environmental effects. In human research, it often difficult to distinguish effects of the early social environment from other factors such as physical neglect, but results here provide support for the effects of very early social deprivation specifically on affect-biased attention in the pre-adolescent period. However, there are some limitations to this study that should be noted. First, the modest sample size could represent a limitation, and it is important that the results are confirmed in larger samples Second, these results do not speak to the issue of trait versus state anxiety, both of which have been linked to affect-biased attention (see11), which will require the measurement of anxiety-like behaviour in different contexts across an extended time scale. Third, atypical ABP may also play a role in adverse behavioural outcomes in human children (e.g.66), therefore prospective macaque research should consider this also.
There are three additional points concerning the group differences revealed here that need to be considered. Firstly, although animals in the mother-reared group remained in their natal group for the first eight months of life, they were also removed from their natal group post-weaning. Therefore, it is possible that our results were affected by ‘earlier vs later’ maternal separation. Future studies would ideally include another group of animals that were not removed from their natal social group at any point. However, our results do demonstrate clear differences between the two rearing groups, and are in keeping with previous findings concerning development of affect biased attention in individuals who have been exposed to early psychosocial adversity versus not (e.g.20,25). Behaviour in our peer-reared group (e.g. increased anxiety-like behaviour) does suggest that their risk for such long-term poor outcomes is greater than in the mother-reared group, perhaps highlighting the first months of life as a sensitive period for the impact of social adversity on certain aspects of socioemotional development. Secondly, peer-reared animals often obtain a lower social rank than mother-reared animals (e.g.67), and hypothetically, exposure to more threat in the environment as a result of low rank may have a direct effect on anxiety, or indirectly via effects on attention biases. In our sample, peer-reared animals did tend to be lower-ranking (see SI), though the inclusion of rank as a covariate in our models controlled for this factor. This suggests that early social adversity affects rank and gaze bias/behaviour independently, but is still possible that rank moderates the mediating effects of attention biases. Indeed, some previous work with adult macaques has found that social dominance can influence attention towards social stimuli (e.g.68). This possibility is an interesting avenue for future longitudinal work with larger samples, especially in the case of natural variation in early social experience. And thirdly, while social grooming is often used as a measure of prosocial engagement in macaques, grooming is a complex behaviour with context-dependent effects of hormonal and neural responses in NHPs69,70. Elucidating these context-dependent effects such as the relative rank of the actor and receiver, and their relationship to affect-biased attention will be important in future research.
To conclude, this study demonstrates that juvenile, pre-adolescent macaques are biased towards looking at both threatening and affiliative dynamic facial gestures, but that the degree of attention bias is influenced by early social adversity. Furthermore, links between ABT and anxiety in the context of early adversity, and an absence of ABP links with social engagement, suggests that affect-biased attention could play an important role in rhesus macaque development. Longitudinal research concerning the mechanisms underlying these relations is now required to determine the factors conferring greatest risk for anxiety, as well as those implicated in positive social outcomes and resilience.
Supplementary Information
Acknowledgements
We would like to thank Annika Paukner and Ruth Woodward for all their involvement in the early care of the animals included in this project and their transport from the US. We would also like to thank Romain Lacoste for his involvement in the care of the animals once relocated to France, and the CNRS for supporting the costs of this relocation.
Author contributions
H.R. and P.F.F. designed the study; H.R., A.M., and M.B. collected the data; H.R. analysed the data; H.R. and P.F.F. wrote the manuscript; H.R., A.M., M.B., S.B.H., and P.F.F. all reviewed the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 841210 (AnxNPS); the Fondation de France (No. 00079331); the National Institute of Child Health and Human Development (P01HD064653); and the Agence Nationale de la Recherche (ANR-19-CE37-0023).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-00620-z.
References
- 1.Buss AT, Ross-Sheehy S, Reynolds GD. Visual working memory in early development: A developmental cognitive neuroscience perspective. J. Neurophysiol. 2018;120:1472–1483. doi: 10.1152/jn.00087.2018. [DOI] [PubMed] [Google Scholar]
- 2.Rueda MR, Posner MI, Rothbart MK. The development of executive attention: Contributions to the emergence of self-regulation. Dev. Neuropsychol. 2005;28:573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- 3.Morales S, Fu X, Pérez-Edgar KE. A developmental neuroscience perspective on affect-biased attention. Dev. Cogn. Neurosci. 2016;21:26–41. doi: 10.1016/j.dcn.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todd RM, Cunningham WA, Anderson AK, Thompson E. Affect-biased attention as emotion regulation. Trends Cogn. Sci. 2012;16:365–372. doi: 10.1016/j.tics.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Peltola MJ, Hietanen JK, Forssman L, Leppänen JM. The emergence and stability of the attentional bias to fearful faces in infancy. Infancy. 2013;18:905–926. doi: 10.1111/infa.12013. [DOI] [Google Scholar]
- 6.Peltola MJ, Forssman L, Puura K, van IJzendoorn MH, Leppänen JM. Attention to faces expressing negative emotion at 7 months predicts attachment security at 14 months. Child Dev. 2015;86:1321–1332. doi: 10.1111/cdev.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-Edgar K, et al. Patterns of sustained attention in infancy shape the developmental trajectory of social behavior from toddlerhood through adolescence. Dev. Psychol. 2010;46:1723–1730. doi: 10.1037/a0021064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy AK, et al. Attention bias toward threat in pediatric anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:1189–1196. doi: 10.1097/CHI.0b013e3181825ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pourtois G, Schettino A, Vuilleumier P. Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biol. Psychol. 2013;92:492–512. doi: 10.1016/j.biopsycho.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Morales S, et al. Maternal anxiety predicts attentional bias towards threat in infancy. Emotion. 2017;17:874–883. doi: 10.1037/emo0000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol. Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Dudeney J, Sharpe L, Hunt C. Attentional bias towards threatening stimuli in children with anxiety: A meta-analysis. Clin. Psychol. Rev. 2015 doi: 10.1016/j.cpr.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Clark DA. An information processing model of anxiety: Automatic and strategic processes. Behav. Res. Ther. 1997;35:49–58. doi: 10.1016/S0005-7967(96)00069-1. [DOI] [PubMed] [Google Scholar]
- 14.MacLeod C, Mathews A. Cognitive bias modification approaches to anxiety. Annu. Rev. Clin. Psychol. 2012;8:189–217. doi: 10.1146/annurev-clinpsy-032511-143052. [DOI] [PubMed] [Google Scholar]
- 15.Mathews A, MacLeod C. Induced processing biases have causal effects on anxiety. Cogn. Emot. 2002;16:331–354. doi: 10.1080/02699930143000518. [DOI] [Google Scholar]
- 16.Field AP, Lester KJ. Is there room for ‘development’ in developmental models of information processing biases to threat in children and adolescents? Clin. Child Fam. Psychol. Rev. 2010;13:315–332. doi: 10.1007/s10567-010-0078-8. [DOI] [PubMed] [Google Scholar]
- 17.Dodd H, et al. Anxiety and attentional bias in preschoolaged children: An eyetracking study. J. Abnorm. Child Psychol. 2015 doi: 10.1007/s10802­014­9962­x. [DOI] [PubMed] [Google Scholar]
- 18.Kindt M, Van Den Hout M. Selective attention and anxiety: A perspective on developmental issues and the causal status. J. Psychopathol. Behav. Assess. 2001;23:193–202. doi: 10.1023/A:1010921405496. [DOI] [Google Scholar]
- 19.Burris JL, Barry-Anwar RA, Rivera SM. An eye tracking investigation of attentional biases towards affect in young children. Dev. Psychol. 2017;53:1418–1427. doi: 10.1037/dev0000345. [DOI] [PubMed] [Google Scholar]
- 20.Troller-Renfree S, McDermott JM, Nelson CA, Zeanah CH, Fox NA. The effects of early foster care intervention on attention biases in previously institutionalized children in Romania. Dev. Sci. 2015;18:713–722. doi: 10.1111/desc.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J. Abnorm. Psychol. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- 22.Grafton B, Ang C, MacLeod C. Always look on the bright side of life: The attentional basis of positive affectivity. Eur. J. Pers. 2012;26:133–144. doi: 10.1002/per.1842. [DOI] [Google Scholar]
- 23.Taylor CT, Bomyea J, Amir N. Malleability of attentional bias for positive emotional information and anxiety vulnerability. Emotion. 2011;11:127–138. doi: 10.1037/a0021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dodd, H. F. et al. Trajectories of anxiety when children start school: The role of Behavioural Inhibition and attention bias to angry and happy faces. J. Abnorm. Psychol.129, 701. [DOI] [PubMed]
- 25.Troller-Renfree S, et al. The beneficial effects of a positive attention bias amongst children with a history of psychosocial deprivation. Biol. Psychol. 2017;122:110–120. doi: 10.1016/j.biopsycho.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanTieghem MR, et al. Positive valence bias and parent-child relationship security moderate the association between early institutional caregiving and internalizing symptoms. Dev. Psychopathol. 2017;29:519. doi: 10.1017/S0954579417000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waters AM, Henry J, Mogg K, Bradley BP, Pine DS. Attentional bias towards angry faces in childhood anxiety disorders. J. Behav. Ther. Exp. Psychiatry. 2010;41:158–164. doi: 10.1016/j.jbtep.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Rosati AG, Wobber V, Hughes K, Santos LR. Comparative developmental psychology: How is human cognitive development unique? Evol. Psychol. 2014;12:147470491401200. doi: 10.1177/147470491401200211. [DOI] [PubMed] [Google Scholar]
- 29.Rosati AG, Arre AM, Platt ML, Santos LR. Developmental shifts in social cognition: Socio-emotional biases across the lifespan in rhesus monkeys. Behav. Ecol. Sociobiol. 2018;72:1–20. doi: 10.1007/s00265-018-2573-8. [DOI] [Google Scholar]
- 30.Coleman K, Pierre PJ. Assessing anxiety in nonhuman primates. ILAR J. 2014;55:333–346. doi: 10.1093/ilar/ilu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalin, N. H. & Shelton, S. E. Nonhuman Primate Models to Study Anxiety, Emotion Regulation, and Psychopathology. in Annals of the New York Academy of Sciences, 1008, 189–200 (New York Academy of Sciences, 2003). [DOI] [PubMed]
- 32.Ferrari P, Paukner A, Ionica C, Suomi SJ. Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Curr. Biol. 2009;19:1768–1772. doi: 10.1016/j.cub.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandalaywala TM, Parker KJ, Maestripieri D. Early experience affects the strength of vigilance for threat in rhesus monkey infants. Psychol. Sci. 2014;25:1893–1902. doi: 10.1177/0956797614544175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morin EL, Howell BR, Meyer JS, Sanchez MM. Effects of early maternal care on adolescent attention bias to threat in nonhuman primates. Dev. Cogn. Neurosci. 2019;38:100643. doi: 10.1016/j.dcn.2019.100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ambadar Z, Schooler JW, Conn JF. Deciphering the enigmatic face the importance of facial dynamics in interpreting subtle facial expressions. Psychol. Sci. 2005;16:403–410. doi: 10.1111/j.0956-7976.2005.01548.x. [DOI] [PubMed] [Google Scholar]
- 36.Caudek C, Ceccarini F, Sica C. Facial expression movement enhances the measurement of temporal dynamics of attentional bias in the dot-probe task. Behav. Res. Ther. 2017;95:58–70. doi: 10.1016/j.brat.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 37.Kamachi M, et al. Dynamic properties influence the perception of facial expressions. Perception. 2013;42:1266–1278. doi: 10.1068/p3131n. [DOI] [PubMed] [Google Scholar]
- 38.Kilts CD, Egan G, Gideon DA, Ely TD, Hoffman JM. Dissociable neural pathways are involved in the recognition of emotion in static and dynamic facial expressions. Neuroimage. 2003;18:156–168. doi: 10.1006/nimg.2002.1323. [DOI] [PubMed] [Google Scholar]
- 39.Pitcher D, Duchaine B, Walsh V. Combined TMS and fMRI reveal dissociable cortical pathways for dynamic and static face perception. Curr. Biol. 2014;24:2066–2070. doi: 10.1016/j.cub.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 40.Ichikawa H, Kanazawa S, Yamaguchi MK. Infants recognize the subtle happiness expression. Perception. 2014;43:235–248. doi: 10.1068/p7595. [DOI] [PubMed] [Google Scholar]
- 41.Quadrelli E, Conte S, Macchi Cassia V, Turati C. Emotion in motion: Facial dynamics affect infants’ neural processing of emotions. Dev. Psychobiol. 2019;61:843–858. doi: 10.1002/dev.21860. [DOI] [PubMed] [Google Scholar]
- 42.Nelson NL, Mondloch CJ. Children’s visual attention to emotional expressions varies with stimulus movement. J. Exp. Child Psychol. 2018;172:13–24. doi: 10.1016/j.jecp.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Fan S, Dal Monte O, Chang SWC. Levels of naturalism in social neuroscience research. iScience. 2021;24:102702. doi: 10.1016/j.isci.2021.102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almas AN, et al. The effects of early institutionalization and foster care intervention on children’s social behaviors at the age of eight. Soc. Dev. 2015;24:225–239. doi: 10.1111/sode.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dettmer AM, Novak MA, Suomi SJ, Meyer JS. Physiological and behavioral adaptation to relocation stress in differentially reared rhesus monkeys: hair cortisol as a biomarker for anxiety-related responses. Psychoneuroendocrinology. 2012;37:191–199. doi: 10.1016/j.psyneuen.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGoron L, et al. Recovering from early deprivation: Attachment mediates effects of caregiving on psychopathology. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:683–693. doi: 10.1016/j.jaac.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 48.Shannon C, Champoux M, Suomi SJ. Rearing condition and plasma cortisol in rhesus monkey infants. Am. J. Primatol. 1998;46:311–321. doi: 10.1002/(SICI)1098-2345(1998)46:4<311::AID-AJP3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 49.Peirce J, et al. PsychoPy2: Experiments in behavior made easy. Behav. Res. Methods. 2019;51:195–203. doi: 10.3758/s13428-018-01193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dunbar. Grooming, Gossip, and the Evolution of Language—Robin Dunbar, Robin Ian MacDonald Dunbar—Google Books. https://books.google.fr/books?hl=en&lr=&id=nN5DFNT-6ToC&oi=fnd&pg=PA1&dq=Grooming,+gossip+and+the+evolution+of+language&ots=70M30YcLR8&sig=CEkFumlb_j5VgPE5yXI0xpr5mSc#v=onepage&q=Grooming%2Cgossipandtheevolutionoflanguage&f=false (1998).
- 51.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.Rproject.org/. (2020).
- 52.Vanderwert RE, et al. Early social experience affects neural activity to affiliative facial gestures in newborn nonhuman primates. Dev. Neurosci. 2015 doi: 10.1159/000381538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White LK, et al. Developmental relations among behavioral inhibition, anxiety, and attention biases to threat and positive information. Child Dev. 2017;88:141–155. doi: 10.1111/cdev.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dodd HF, et al. Trajectories of anxiety when children start school: The role of behavioral inhibition and attention bias to angry and happy faces. J. Abnorm. Psychol. 2020;129:701. doi: 10.1037/abn0000623. [DOI] [PubMed] [Google Scholar]
- 55.Pérez-Edgar K, Taber-Thomas B, Auday E, Morales S. Temperament and attention as core mechanisms in the early emergence of anxiety. Contrib. Hum. Dev. 2014;26:42–56. doi: 10.1159/000354350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peltola MJ, van IJzendoorn MH, Yrttiaho S. Attachment security and cortical responses to fearful faces in infants. Attach. Hum. Dev. 2020;22:174–188. doi: 10.1080/14616734.2018.1530684. [DOI] [PubMed] [Google Scholar]
- 57.McElwain NL, Booth-Laforce C. Maternal sensitivity to infant distress and nondistress as predictors of infant-mother attachment security. J. Fam. Psychol. 2006;20:247–255. doi: 10.1037/0893-3200.20.2.247. [DOI] [PubMed] [Google Scholar]
- 58.Ranson KE, Urichuk LJ. The effect of parent-child attachment relationships on child biopsychosocial outcomes: A review. Early Child Dev. Care. 2008;178:129–152. doi: 10.1080/03004430600685282. [DOI] [Google Scholar]
- 59.McLaughlin KA. Future directions in childhood adversity and youth psychopathology. J. Clin. Child Adolesc. Psychol. 2016;45:361–382. doi: 10.1080/15374416.2015.1110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goff B, et al. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nozadi SS, et al. The moderating role of attention biases in understanding the link between Behavioral Inhibition and anxiety. J. Exp. Psychopathol. 2016;7:451–465. doi: 10.5127/jep.052515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. Psychiatr. Clin. N. Am. 2009;32:483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Callaghan BL, Tottenham N. The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernando ABP, Murray JE, Milton AL. The amygdala: Securing pleasure and avoiding pain. Front. Behav. Neurosci. 2013;7:190. doi: 10.3389/fnbeh.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrison SE, Salzman CD. Re-valuing the amygdala. Curr. Opin. Neurobiol. 2010;20:221–230. doi: 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morales S, et al. Attention bias to reward predicts behavioral problems and moderates early risk to externalizing and attention problems. Dev. Psychopathol. 2020;32:397–409. doi: 10.1017/S0954579419000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dettmer AM, et al. Associations between early life experience, chronic HPA axis activity, and adult social rank in rhesus monkeys. Soc. Neurosci. 2017;12:92–101. doi: 10.1080/17470919.2016.1176952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schülke O, Dumdey N, Ostner J. Selective attention for affiliative and agonistic interactions of dominants and close affiliates in macaques. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-62772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shutt K, MacLarnon A, Heistermann M, Semple S. Grooming in Barbary macaques: Better to give than to receive? Biol. Lett. 2007;3:231. doi: 10.1098/rsbl.2007.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crockford C, et al. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. Biol. Sci. 2013;280:20122765–20122765. doi: 10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.