Abstract
The basement membrane (BM)-related factors, including collagen IV, are important for the maintenance and recovery of skeletal muscles. Aging impairs the expression of BM-related factors during recovery after disuse atrophy. Muscle activity facilitates collagen synthesis that constitutes the BM. However, the effect of endurance exercise on the BM of aged muscles is unclear. Thus, to understand the effect of endurance exercise on the BM of the skeletal muscle in aged rats, we prescribed treadmill running in aged rats and compared the differences in the expression of BM-related factors between the aged rats with and without exercise habits. Aged rats were subjected to endurance exercise via treadmill running. Exercise increased the mRNA expression levels of the BM-related factors, the area and intensity of collagen IV-immunoreactivity and the width of lamina densa in the soleus muscle of aged rats. These finding suggests that endurance exercise promotes BM construction in aged rats.
Keywords: aging, basement membrane, endurance exercise, soleus muscle
I. Introduction
Skeletal muscle fibers are coated by a layer of extracellular matrix (ECM) material called the basement membrane (BM). The BM is composed of a felt-like basal lamina directly linked to the plasma membrane and fibrillar reticular lamina and plays a role in protecting muscle fibers [3, 38, 46]. Collagen IV is one of the principal ingredients of BM and is encoded by Col4a1 and Col4a2. Collagen IV comprises 400 nm-long helical trimeric polypeptides regulated by HSP47, a collagen-specific molecular chaperone [4, 24, 36, 42, 43]. In contrast, MMP14 is a degradative factor of collagen IV [40]. The BM is maintained by a balance between the synthesis and degradation of collagen IV. During the recovery process following disuse atrophy or muscle injury, the gene expression of collagen IV and other BM-related factors promotes muscle recovery [17, 18]. Skeletal muscles in collagen IV knockout mice are fragile [13, 20, 25, 26]. Furthermore, in aged rats, collagen IV gene expression is reduced during delayed muscle recovery [17]. Thus, from the above studies, it is probable that the expression of BM-related factors is important for the maintenance and recovery of skeletal muscles.
Our previous studies suggested that the BM construction capacity declines with age. Aged rats exhibited a decrease in the levels of BM-related factors in the steady state [19]. In addition, the expression of BM-related factors in the process of muscle recovery after disuse atrophy was suppressed in aged rats in comparison with young rats [17]. In the previous study, we found that two weeks of hindlimb unloading resulted in disuse atrophy in the aged and young rats’ soleus muscle and reloading promoted the recovery of the atrophied muscle in the young rats [17]. On the other hand, during the recovery period, BM-related factors were poorly induced in the aged rats’ soleus muscle and the number of necrotic fibers increased. The BM structure of the necrotic fibers was disrupted at a high rate, resulting in delayed muscle recovery. Based on these findings, we hypothesized that interventions that elicit increased expression of BM-related factors may promote the recovery of disuse atrophy and damaged aged muscles.
Exercise may affect the structure of the BM. Eccentric contraction and treadmill running reportedly induce BM-related factors in young rats [22, 23]. On the contrary, the expression of BM-related factors is poor in aged muscle [19], suggesting that aged animals hardly show the induction of BM-related factors in response to exercise [17]. Nevertheless, the effects of exercise on the BM of aged animals remain to be elucidated. To clarify this, we performed endurance exercise experiments on a treadmill and examined the levels of BM-related factors and morphological alterations in BM in the muscles of aged rats.
II. Materials and Methods
Animals
Nine 18-month-old male Wistar rats were used in this study (Japan SLC, Shizuoka, Japan). All animals housed in standard clear plastic cages were always allowed free range intake of both food and water. The room temperature was kept at 22°C ± 2°C with a 12-hour:12-hour light: dark cycle. This research was conducted with the approval of the Animal Care and Use Committee of Osaka University of Human Sciences (#2), and all experimental procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Grouping and exercise protocol
The present study was designed to examine the effectiveness of endurance exercise on BM-related factors in aged rats, following a previous study [16]. Nine 18-month-old male Wistar rats were divided into sedentary aged rats (Aged Ex (−); n = 5) and those that underwent exercise program for 10 weeks (Aged Ex (+); n = 4). Treadmill running was used as an endurance exercise according to previous studies [16, 32]. Rats in the Aged Ex (+) were familiarized with walking on a motor-driven treadmill exercise three times a week at a slope of 0° at a speed of 10 m/min, and a running time of 10 min. In the first week of the exercise program, exercise with a treadmill grade of 8.5°, speed of 12 m/min, and running time of 15 min was performed three times a week. The treadmill grade and exercise frequency were not changed, and the speed and running time gradually increased weekly (Table 1). From the 7th to the 10th week of the exercise program, exercises with a treadmill grade of 8.5°, speed of 16 m/min, and running time of 45 min were performed three times a week.
Table 1. .
Protocol for exercise
| Exercise week | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Duration time [min/day] | 15 | 30 | 45 | 45 | 45 | 45 | 45 | 45 | 45 | 45 |
| Treadmill grade [ °] | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 |
| Treadmill speed [m/min] | 12 | 12 | 12 | 14 | 14 | 15 | 16 | 16 | 16 | 16 |
Rats in Aged Ex (+) were exercised 3 days per week.
Sampling
The soleus muscle is a slow muscle with high aerobic metabolic activity and is advantageous for sustained muscle activity. The effect of endurance exercise tends to occur in slow muscles [12]. Therefore, in this study, we targeted this muscle. Six days after the last exercise, sodium pentobarbital was injected intraperitoneally into rats, and the soleus muscle was harvested as a sample. The central portion of the harvested soleus muscle was partially collected and stored in RNAlater (Thermo Fisher Scientific, Hanover Park, IL), and other portions of the soleus muscle were rapidly frozen with dry ice cooled isopentane to create frozen samples. Samples were stored in a freezer at −80°C until analysis.
Histochemical analysis
Transverse sections were prepared using a cryostat (CM1950; Leica, Wetzlar, Germany). The sections (10 μm thick) were made from the central portion of the soleus muscle at −25°C. Succinate dehydrogenase (SDH) staining and myofibrillar adenosine triphosphate (ATPase) staining were performed to confirm the effect of endurance exercise on aerobic metabolic activity in each muscle fiber type. SDH staining, an indicator of aerobic metabolic activity, was performed according to the procedure reported in previous studies [29, 31]. The reagent used for incubation was 0.2 M phosphate buffer (pH 7.6) containing 0.2 M sodium succinate and 0.05% nitroblue tetrazolium. Sections were incubated with the reagents for 60 min at 37°C. To distinguish between type I fibers and type II fibers, ATPase staining was performed using the calcium method [1]. First, serial sections of the sections used for SDH staining were subjected to ATPase staining following preincubation in alkaline (pH 10.7). Next, the sections were washed with 1% CaCl2, allowed to react with 2% CoCl2. Finally, the sections washed with 0.005 M sodium barbital and distilled water, and were subsequently stained by 2% ammonium sulfide. In addition, other sections were stained with hematoxylin and eosin (HE) to count the number of necrotic fibers, an indicator of muscle damage. After treating them with these histochemical stains, the sections were dehydrated and cleared by ethanol and xylene, and embedded in Permount (Thermo Fisher Scientific, Hanover Park, IL).
Immunohistochemical analysis
Transverse sections (10 μm thick) were fixed using 4% paraformaldehyde. After washing with phosphate-buffered saline (PBS) (pH 7.4), the sections treated with 3% hydrogen peroxide solution. Following washing with PBS, the samples were incubated with PBS containing 1% normal goat serum and 0.3% Triton X-100 at 4°C for 60 min. Samples were then incubated at 4°C for 24 hr in PBS containing 0.3% Triton X-100 supplemented with rabbit polyclonal anti-collagen IV antibody (ab6586; Abcam, Cambridge, MA) at a ratio of 1:500. The sections were incubated with PBS containing biotin-labeled anti-rabbit IgG (Vectastain ABC kit; Vector Laboratories, Burlingame, CA) at a ratio of 1:1000 for 60 min at 25°C. Then, the sections were incubated in the avidin-biotin complex (Vectastain ABC kit) for 60 min at 4°C. Sections were washed with PBS and then with Tris-HCl buffer (pH 7.4), then at 25°C with Tris-HCl buffer (pH 7.4) containing 0.035% DAB and 0.003% hydrogen peroxide solution. The cells were then incubated for 15 min. Finally, the sections were counterstained using hematoxylin, and dehydrated and cleared by ethanol and xylene, and embedded in Permount (Thermo Fisher Scientific, Hanover Park, IL). As a negative control for immunohistochemistry, tissue sections were also incubated with PBS instead of the primary antibody, and the results showed no staining. Furthermore, previous studies have reported that the primary antibody specifically localizes to the BM [17, 18].
Morphological analysis
SDH activity and fiber cross-sectional area (FCSA) of type I and type II fibers were measured using SDH staining images and ATPase staining images. First, based on the ATPase staining images, each myofiber was classified as type I or type II. Darkly stained myofibers were classified as type II myofibers, and lightly stained myofibers were classified as type I myofibers. The optical density of SDH staining and FCSA for each myofiber type were measured using Image J Fiji [39]. More than 200 myofibers were randomly selected from each section. The SDH activity of Aged Ex (+) was determined as changes relative to that of Aged Ex (−).
The number of necrotic fibers was counted as a marker of muscle damage. According to previous studies, necrotic myofibers is characteristic of the presence of pallor or hypercontraction, and changing the shape of the fibers, and phagocytosis in HE-stained tissues [10, 41]. Three images were randomly photographed, and more than 200 muscle fibers were analyzed per sample using the BZ-Analysis application (BZ-X700; Keyence, Osaka, Japan).
The area and intensity of collagen IV immunoreactivity (IR) was measured to evaluate the effect of endurance exercise on the BM. Three images were randomly photographed, and the area subjected to analysis was 1,181,640 μm2 per sample. More than 200 muscle fibers were analyzed per sample using the BZ-Analysis application and Image J Fiji [5, 39]. The area of collagen IV-IR was calculated as a percentage of the analysis area per sample, and the intensity of collagen IV-IR was determined as changes relative to the intensity of Aged Ex (−).
Quantitative polymerase chain reaction (PCR)
Total soleus RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA, USA). After confirming the quality, the total RNA amount was unified to 1 μg between the samples and reverse transcribed using a random primer and ReverTra Ace (Toyobo, Osaka, Japan). TB Green Premix Ex Taq II (Takara Bio, Shiga, Japan) was used as the reagent, and Step One Plus Real-Time PCR System (Applied Biosystems, Carlsbad, CA) was used for quantitative PCR. A calibration curve was created using the template, and the amount of each target gene was measured. It was confirmed that the expression of Gapdh used as the housekeeping gene did not change significantly with exercise. The expression level of the target gene was normalized to the expression level of Gapdh. Upregulation or downregulation of the target gene was calculated as the rate of change compared to the values in the Aged Ex (−). The primer sets were as follows.
Col4a1, 5′-ATGCCAGGAAGAGCAGGAAC-3′ (Forward) and 5′-CGACTACCAGGAAAGCCAACTC-3′ (Reverse);
Hsp47, 5′-CGCAGCAGTAAGCAACACTACA-3′ (Forward) and 5′-TCCACATCCTTGGTGACCTCT-3′ (Reverse);
Mmp14, 5′-GGATACCCACTTTGATTCTGCTG-3′ (Forward) and 5′-GGAGGGGTCGTTGGAATGT-3′ (Reverse);
Col6a1, 5′-GACACTCAGCGGGACACTACAC-3′ (Forward) and 5′-GCGACAAAGCCAAACACATC-3′ (Reverse);
Gapdh, 5′-TGCACCACCAACTGCTTA-3′ (Forward) and 5′-GGATGCAGGGATGATGTTC-3′ (Reverse).
Electron microscopy analysis
Tissue sections (1 mm thick) were cut from the central portion of soleus muscle at −25°C. The sections were fixed in 4% paraformaldehyde/2% glutaraldehyde. Then, the sections were treated with osmium tetroxide and dehydrated with ethanol, and embedded in Epon. Longitudinal ultrathin sections (90 nm thick) were stained with 4% uranyl acetate and 1% lead citrate and were observed using transmission electron microscopy (TEM) (HT7700; Hitachi, Tokyo, Japan). We measured the width of lamina densa, myofiber and sarcomere using Image J Fiji [39]. Twenty images were randomly photographed per sample, and the area subjected to analysis was 84.5 μm2 for lamina densa, 211,832 μm2 for muscle fiber and 4,762 μm2 for sarcomere. The width of each measurement site was measured randomly at three points per image.
Statistical analysis
Data are presented as the mean ± standard error of the mean. A t-test was executed on the difference between the mean values for the Aged Ex (−) and Aged Ex (+). (KaleidaGraph statistical analysis software version 4.5.1; Synergy Software, Reading, PA). Significance level was set at 0.05.
III. Results
SDH activity and FCSA
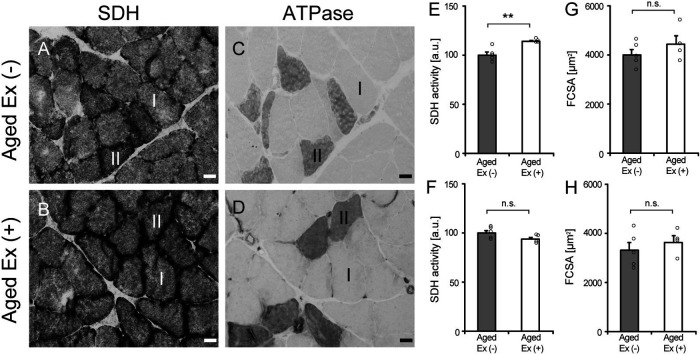
SDH activity and FCSA were measured to confirm whether the exercise prescribed in this study acted on the muscle fibers as an endurance exercise. Resistance exercise generally manifests muscle hypertrophy [7, 45], but endurance exercise does not result in muscle hypertrophy [16]. Moreover, endurance exercise improves aerobic metabolic activity [34]. SDH is one of enzymes in the tricarboxylic acid cycle which occurs in the mitochondrial matrix and contribute to synthesis of ATP [33, 37]. SDH activity was measured as an indicator of aerobic metabolism in myofibers. The activity of type I fibers of Aged Ex (+) was higher than that of Aged Ex (−) (Fig. 1). In addition, there were no significant differences in the FCSA of both type I and type II fibers between Aged Ex (−) and Aged Ex (+). These results confirmed that the exercise load used in this study had an endurance exercise effect on muscle fibers.
Fig. 1.
Succinate dehydrogenase (SDH) activity and fiber cross-sectional area (FCSA). Serial cross sections of the soleus muscles of Aged Ex (−) (A and C) and Aged Ex (+) (B and D) rats were stained with SDH (A and B) and myofibrillar adenosine triphosphate (ATPase) (C and D) stains (×20). Bar = 25 μm. SDH activity of type I fiber (E), type II fiber (F), and FCSA of type I (G) and type II fiber (H) were measured in Aged Ex (−) (n = 5) and Aged Ex (+) (n = 4) rats using the stained images. Upregulation or downregulation of the SDH activity of type I and type II fiber was calculated as the rate of change compared to the values in Aged Ex (−). **p < 0.01 vs. Aged Ex (−); n.s.: not significant. Data are shown as mean ± standard error of the mean (SEM).
Necrotic fiber
Collagen production has been identified as a repair response after muscle injury and as a response after exercise without muscle damage [40]. The purpose of this study was to examine the effect of endurance exercise without muscle damage, on BM-related factors and collagen IV in aged rats. To exclude the possibility that the exercise we utilized produced damage, the number of necrotic fibers was counted as a marker of muscle damage. Necrotic fibers were rarely observed in Aged Ex (−) and Aged Ex (+) (Fig. 2). This result suggests that exercise did not cause muscle damage under the study conditions.
Fig. 2.
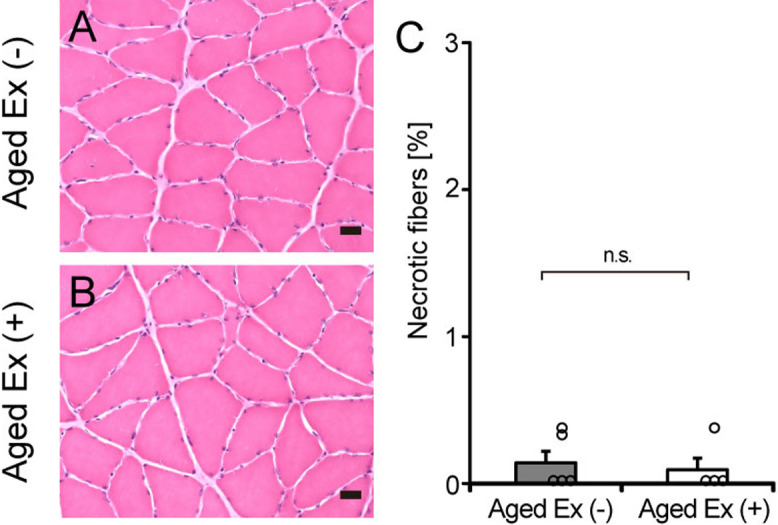
Necrotic fibers. Cross sections of the soleus muscles of the Aged Ex (−) (A) and Aged Ex (+) (B) rats were stained with HE (×20). Bar = 25 μm. The number of necrotic fibers (C) was analyzed in Aged Ex (−) (n = 5) and Aged Ex (+) (n = 4) rats using the stained images. n.s.: not significant. Data are shown as mean ± standard error of the mean (SEM).
BM related factors
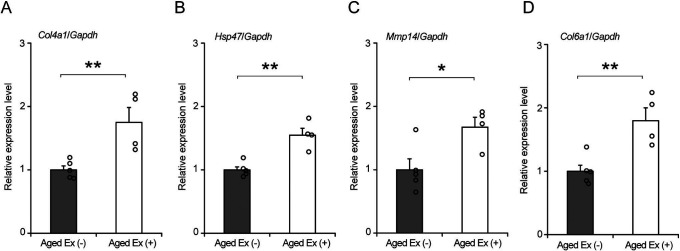
Collagen IV, the main component of the BM, is formed by the trimers of Col4a1 and Col4a2 and is regulated by HSP47, which is a folding factor [4, 24, 36, 42, 43]. In contrast, MMP14 is an enzyme that degrades collagen IV [40]. Furthermore, Col6a1 is a collagen VI gene that is localized in the BM, endomysium, perimysium, and epimysium. BM construction is regulated by collagen IV synthesis and degradation, and other constituents such as collagen VI [6]. To confirm the effect of exercise on the BM-related factors in aged rats, the expression levels of Col4a1, Hsp47, Mmp14, and Col6a1 were measured using quantitative PCR (Fig. 3). The expression levels of Col4a1, Hsp47, Mmp14, and Col6a1 in Aged Ex (+) were significantly higher than those in Aged Ex (−) (Fig. 3). This result suggests that the BM construction reaction occurred at the gene expression level.
Fig. 3.
Basement membrane (BM)-related factors. Comparison of the relative expression levels of Col4a1 (A), Hsp47 (B), Mmp14 (C), and Col6a1 (D) between the Aged Ex (−) (n = 5) and Aged Ex (+) (n = 4) using quantitative polymerase chain reaction (PCR). **p < 0.01 vs. Aged Ex (−); *p < 0.05 vs. Aged Ex (−). Data are shown as the mean ± standard error of the mean (SEM).
BM structure
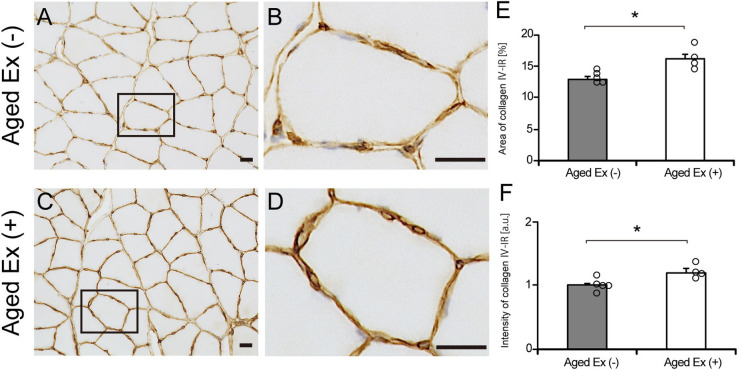
To investigate the effect of exercise on the BM structure in aged rats, we measured the area and intensity of collagen IV-IR. The area and intensity of the collagen IV-IR region of Aged Ex (+) was significantly increased compared with that of Aged Ex (−) (Fig. 4). These results suggest that exercise increased the area and intensity of the collagen IV-IR and change the structure of BM.
Fig. 4.
Basement membrane (BM) structure. The BM structures of the soleus muscle were observed in the Aged Ex (−) (A and B) and the Aged Ex (+) (C and D) using immunohistochemistry with collagen IV (×20). Bar = 25 μm. B and D are extended images of black rectangles in A and C. Enlargement of the area of collagen IV-IR is observed in Aged Ex (+) rats (D) compared to Aged Ex (−) (B). The area (E) and intensity (F) of collagen IV-IR was measured in the Aged Ex (−) (n = 5) and the Aged Ex (+) (n = 4) using the stained images. *p < 0.05 vs. Aged Ex (−). Data are shown as the mean ± standard error of the mean (SEM).
Electron microscopic analysis
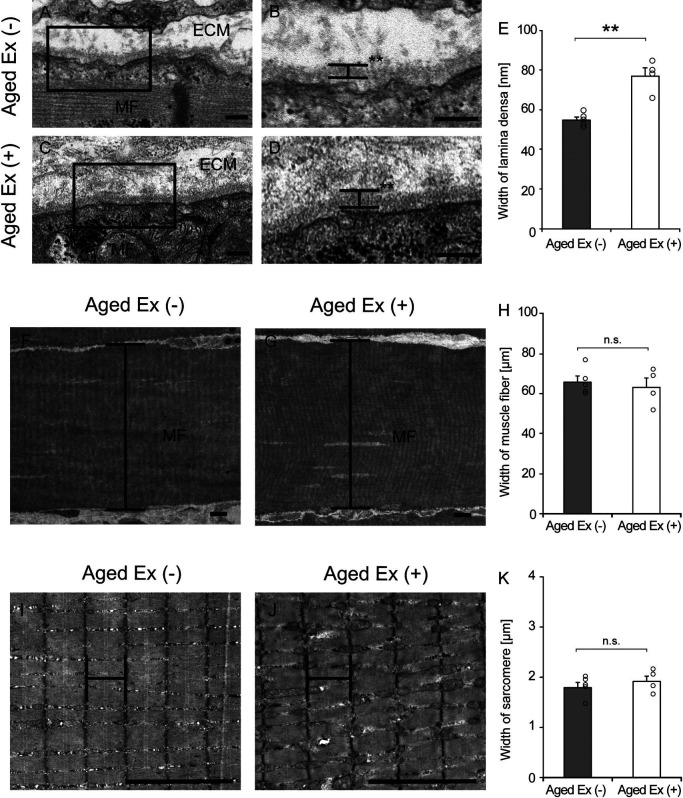
To further observe the BM structures in detail, electron microscopy was performed. The BM consists of lamina fibroreticularis, lamina densa, and lamina lucida as seen by electron microscopy [28], and we measured the width of lamina densa, which is always stably observed and has high electron density. The lamina densa region of the BM was thickened in response to exercise (Fig. 5). The width of the lamina densa in Aged Ex (+) was significantly increased in comparison to that in Aged Ex (−) (Fig. 5). On the other hand, the width of muscle fibers and sarcomeres was not significantly changed by exercise. These results suggest that the BM-specific structural changes were caused by exercise.
Fig. 5.
Electron microscopy analysis. Longitudinal ultrathin sections of the soleus muscles were examined using transmission electron microscopy (TEM) in Aged Ex (−) (n = 5) and Aged Ex (+) (n = 4) rats. Muscle fiber (MS) and extracellular matrix (ECM) images of Aged Ex (−) (A and B) and Aged Ex (+) (C and D) rats are presented. B and D are extended images of the black rectangles in A and C (×7000). Bar = 200 nm (A–D). An increase in the width of the lamina densa was observed in Aged Ex (+) rats (D) compared to that in Aged Ex (−) (B). The width of the lamina densa (E) was measured in the Aged Ex (−) (n = 5) and the Aged Ex (+) (n = 4) rats. MS images of Aged Ex (−) (F) and Aged Ex (+) (G) rats are presented (×300). The width of the MS (H) was measured in the Aged Ex (−) (n = 5) and the Aged Ex (+) (n = 4) rats. Sarcomere images of Aged Ex (−) (I) and Aged Ex (+) (J) rats are presented (×2000). The width of the sarcomere (K) was measured in the Aged Ex (−) (n = 5) and the Aged Ex (+) (n = 4) rats. Bar = 5 μm (F, G, I and J). **p < 0.01 vs. Aged Ex (−). Data are shown as mean ± standard error of the mean (SEM).
IV. Discussion
Our previous study revealed that BM-related factors are not induced in aged muscles and recovery is delayed even during the muscle recovery period when BM damage occurs and repair is required [17]. This finding motivated us to search for interventions that induce BM-related factors in aged muscles. Therefore, in this study, we examined the effect of endurance exercise on BM in the skeletal muscles of aged rats. The results showed that exercise increased the expression levels of Col4a1, Hsp47, Mmp14, and Col6a1, the area and intensity of collagen IV-IR, and the width of the lamina densa in the soleus muscles of aged rats. These findings suggest that endurance exercise induces a BM-construction response in aged muscles. Furthermore, there were no noticeable changes in the number of necrotic muscle fibers after endurance exercise, suggesting that an appropriate exercise load can lead to the induction of BM-related factors in aged muscles without causing muscle damage. These results prove that the proposed intervention induces BM-related factors in aged muscles, which is the major goal of this study.
Our results suggest that muscular activity through endurance exercise may promote the production of collagen IV in aged muscles. In contrast, in our previous study, collagen IV was not induced during the recovery period after disuse muscle atrophy in aged rats, even though myofibers and BM structures were disrupted and collagen IV was required for their repair [17]. This difference suggests that an endurance exercise regimen, not muscle damage, is suitable for the induction of collagen IV in aged muscles. In previous studies, it has been reported that collagen IV production is stimulated during the recovery period of disuse muscle atrophy and muscle injury in young rats [17]. Other previous studies using young rats have reported that high-intensity exercise and eccentric contractions induce collagen IV [22, 23]. Therefore, it is likely that collagen IV is induced either by muscle damage or exercise load in young rats, but is induced in aged rats only by an appropriate exercise load. This hypothesis should be investigated in future studies.
In this study, collagen IV-IR area and intensity in the soleus muscle of aged rats was increased with exercise compared with those without exercise. As collagen IV is mainly expressed in the BM, this finding suggests BM construction in the soleus muscle of aged rats by exercise. A previous study supports the hypothesis that certain exercises increase the collagen IV-IR [30]. Collagen IV-IR was increased in the vastus lateralis muscles of aged patients with knee osteoarthritis and an exercise regime for 12 weeks [30]. Exercise promotes BM remodeling to strengthen the ECM surrounding individual muscle fibers [30]. As the BM protects muscle fibers from mechanical stress, such as contraction and elongation [3, 38, 46], a positive collagen IV turnover in this study may have occurred to adapt to the mechanical stress by treadmill running in aged rats.
In this study, we found that exercise affected the BM microstructure in aged rats. Using TEM, we observed that the lamina densa region of Aged Ex (+) was significantly increased compared to that of Aged Ex (−). The BM is a multifunctional membrane that regulates epithelial growth and differentiation during embryogenesis and organogenesis [11, 27] and as well as cell migration during tissue regeneration [38, 44], and essentially protects cells by providing mechanical support [38]. Collagen IV is one of the primary elements of the lamina densa; it is a non-fibrillar collagen that can envelop muscle fibers in a sheet-like structure and has a protective effect on muscle fibers [8, 9, 28, 38]. In the present study, the soleus muscles of aged rats may have adapted to the continuous mechanical stresses of muscle contraction and elongation during endurance exercise. This indicates that an increase in the width of the lamina densa in muscle fibers might strengthen the protective function of the BM in aged rats.
The present study has some limitations. First, the effect of exercise on muscle function, such as muscle tension, in aged rats has not been confirmed. Second, the effects of exercise on young and adult rats have not been compared. Further verification is needed to determine whether the findings of our study are age-specific. Third, treadmill running is known to cause various stresses such as heat stress [32] and release of hormones, cytokines, and myokines [2, 35] as well as mechanical stress [14, 15, 21]. Therefore, stresses other than mechanical stress may trigger the expression of BM-related factors associated with treadmill running. In this study, the details of the triggers that induce the expression of BM-related factors have not been clarified.
In summary, treadmill running activates the expression of BM-related factors and changes BM structure in the soleus muscle of aged rats. Our findings suggest that this exercise may result in BM construction within the soleus muscles of aged rats.
V. Funding
This work was supported by JSPS KAKENHI under Grant [18K17776].
VI. Conflicts of Interest
No potential conflict of interest was reported by the authors.
VII. Acknowledgments
We would like to thank the Life Science Research Institute of Kindai University for their technical support.
VIII. References
- 1.Brooke, M. H. and Kaiser, K. K. (1970) Muscle fiber types: how many and what kind? Arch. Neurol. 23; 369–379. [DOI] [PubMed] [Google Scholar]
- 2.Brown, D. A., Johnson, M. S., Armstrong, C. J., Lynch, J. M., Caruso, N. M., Ehlers, L. B., et al. (2007) Short-term treadmill running in the rat: what kind of stressor is it? J. Appl. Physiol. 103; 1979–1985. [DOI] [PubMed] [Google Scholar]
- 3.Campbell, K. P. and Stull, J. T. (2003) Skeletal muscle basement membrane-sarcolemma-cytoskeleton interaction minireview series. J. Biol. Chem. 278; 12599–12600. [DOI] [PubMed] [Google Scholar]
- 4.Chioran, A., Duncan, S., Catalano, A., Brown, T. J. and Ringuette, M. J. (2017) Collagen IV trafficking: the inside-out and beyond story. Dev. Biol. 431; 124–133. [DOI] [PubMed] [Google Scholar]
- 5.Crowe, A. R. and Wei, Y. (2019) Semi-quantitative Determination of Protein Expression Using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio Protoc. 3; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csapo, R., Gumpenberger, M. and Wessner, B. (2020) Skeletal muscle extracellular matrix—What do we know about its composition, regulation, and physiological roles? A narrative review. Front. Physiol. 11; 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dankel, S. J., Mattocks, K. T., Jessee, M. B., Buckner, S. L., Mouser, J. G., Counts, B. R., et al. (2017) Frequency: The overlooked resistance training variable for inducing muscle hypertrophy? Sports Med. 47; 799–805. [DOI] [PubMed] [Google Scholar]
- 8.Davis, W., Mahale, S., Carranza, A., Cox, B., Hayes, K., Jimenez, D. and Ding, Y. (2007) Exercise pre-conditioning ameliorates blood–brain barrier dysfunction in stroke by enhancing basal lamina. Neurol. Res. 29; 382–387. [DOI] [PubMed] [Google Scholar]
- 9.Ding, Y. H., Ding, Y., Li, J., Bessert, D. A. and Rafols, J. A. (2006) Exercise pre-conditioning strengthens brainmicrovascular integrity in a rat stroke model. Neurol. Res. 28; 184–189. [DOI] [PubMed] [Google Scholar]
- 10.Dobek, G. L., Fulkerson, N. D., Nicholas, J. and Schneider, B. S. P. (2013) Mouse model of muscle crush injury of the legs. Comp. Med. 63; 227–232. [PMC free article] [PubMed] [Google Scholar]
- 11.Ekblom, P. (1981) Formation of basement membranes in embryonic kidney: an immunohistological study. J. Cell Biol. 91; 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitts, R. H. and Widrick, J. J. (1996) Muscle mechanics: adaptations with exercise-training. Exerc. Sport Sci. Rev. 24; 427–473. [PubMed] [Google Scholar]
- 13.Hayashi, G., Labelle-Dumais, C. and Gould, D. B. (2018) Use of sodium 4-phenylbutyrate to define therapeutic parameters for reducing intracerebral hemorrhage and myopathy in Col4a1 mutant mice. Dis. Model. Mech. 11; 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphrey, J. D., Dufresne, E. R. and Schwartz, M. A. (2014) Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 15; 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Husse, B., Briest, W., Homagk, L., Isenberg, G. and Gekle, M. (2007) Cyclical mechanical stretch modulates expression of collagen I and collagen III by PKC and tyrosine kinase in cardiac fibroblasts. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293; 1898–1907. [DOI] [PubMed] [Google Scholar]
- 16.Joanisse, S., Nederveen, J. P., Baker, J. M., Snijders, T., Iacono, C. and Parise, G. (2016) Exercise conditioning in old mice improves skeletal muscle regeneration. FASEB J. 30; 3256–3268. [DOI] [PubMed] [Google Scholar]
- 17.Kanazawa, Y., Ikegami, K., Sujino, M., Koinuma, S., Nagano, M., Oi, Y., et al. (2017) Effects of aging on basement membrane of the soleus muscle during recovery following disuse atrophy in rats. Exp. Gerontol. 98; 153–161. [DOI] [PubMed] [Google Scholar]
- 18.Kanazawa, Y., Nagano, M., Koinuma, S., Sujino, M., Minami, Y., Sugiyo, S., et al. (2020) Basement membrane recovery process in rat soleus muscle after exercise-induced muscle injury. Connect. Tissue Res. 22; 1–12. [DOI] [PubMed] [Google Scholar]
- 19.Kanazawa, Y., Nagano, M., Koinuma, S., Sugiyo, S. and Shigeyoshi, Y. (2021) Effects of aging on basement membrane-related gene expression of the skeletal muscle in rats. Biomed. Res. 42; 115–119. [DOI] [PubMed] [Google Scholar]
- 20.Kelemen-Valkony, I., Kiss, M., Csiha, J., Kiss, A., Bircher, U., Szidonya, J., et al. (2012) Drosophila basement membrane collagen col4a1 mutations cause severe myopathy. Matrix Biol. 31; 29–37. [DOI] [PubMed] [Google Scholar]
- 21.Kjaer, M. (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 84; 649–698. [DOI] [PubMed] [Google Scholar]
- 22.Koskinen, S. O. A., Ahtikoski, A. M., Komulainen, J., Hesselink, M. K., Drost, M. R. and Takala, T. E. S. (2002) Short-term effects of forced eccentric contractions on collagen synthesis and degradation in rat skeletal muscle. Pflugers Arch. 444; 59–72. [DOI] [PubMed] [Google Scholar]
- 23.Koskinen, S. O. A., Wang, W., Ahtikoski, A. M., Kjær, M., Han, X. Y., Kovanen, V., et al. (2001) Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am. J. Physiol. 280; 1292–1300. [DOI] [PubMed] [Google Scholar]
- 24.Kuo, D. S., Labelle-Dumais, C. and Gould, D. B. (2012) COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum. Mol. Genet. 21; 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo, D. S., Labelle-Dumais, C., Mao, M., Jeanne, M., Kauffman, W. B., Allen, J., et al. (2014) Allelic heterogeneity contributes to variability in ocular dysgenesis, myopathy and brain malformations caused by Col4a1 and Col4a2 mutations. Hum. Mol. Genet. 23; 1709–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labelle-Dumais, C., Dilworth, D. J., Harrington, E. P., deLeau, M., Lyons, D., Kabaeva, Z., et al. (2011) COL4A1 mutations cause ocular dysgenesis, neuronal localization defects, and myopathy in mice and Walker-Warburg syndrome in humans. PLoS Genet. 7; 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leivo, I. and Wartiovarra, J. (1989) Basement membrane matrices in mouse embryogenesis, teratocarcinoma differentiation and neuromuscular maturation. Int. J. Dev. Biol. 33; 81–89. [PubMed] [Google Scholar]
- 28.Mak, K. M. and Mei, R. (2017) Basement Membrane Type IV Collagen and Laminin: An Overview of Their Biology and Value as Fibrosis Biomarkers of Liver Disease. Anat. Rec. (Hoboken) 300; 1371–1390. [DOI] [PubMed] [Google Scholar]
- 29.Martin, T. P., Vailas, A. C., Durivage, J. B., Edgerton, V. R. and Castleman, K. R. (1985) Quantitative histochemical determination of muscle enzymes: biochemical verification. J. Histochem. Cytochem. 33; 1053–1059. [DOI] [PubMed] [Google Scholar]
- 30.Mattiello-Sverzut, A. C., Petersen, S. G., Kjaer, M. and Mackey, A. L. (2013) Morphological adaptation of muscle collagen and receptor of advanced glycation end product (RAGE) in osteoarthritis patients with 12 weeks of resistance training: influence of anti-inflammatory or glucosamine treatment. Rheumatol. Int. 33; 2215–2224. [DOI] [PubMed] [Google Scholar]
- 31.Nachlas, M. M., Tsou, K. C., De Souza, E., Cheng, C. S. and Seligman, A. M. (1957) Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J. Histochem. Cytochem. 5; 420–436. [DOI] [PubMed] [Google Scholar]
- 32.Naito, H., Powers, S. K., Demirel, H. A. and Aoki, J. (2001) Exercise training increases heat shock protein in skeletal muscles of old rats. Med. Sci. Sports Exerc. 33; 729–734. [DOI] [PubMed] [Google Scholar]
- 33.Nassan, S. A., Fujita, N., Kondo, H., Murakami, S. and Fujino, H. (2012) Chronic Exercise Training Down-Regulates TNF-α and Atrogin-1/MAFbx in Mouse Gastrocnemius Muscle Atrophy Induced by Hindlimb Unloading. Acta Histochem. Cytochem. 45; 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pattamaprapanont, P., Muanprasat, C., Soodvilai, S., Srimaroeng, C. and Chatsudthipong, V. (2016) Effect of Exercise Training on Signaling of Interleukin-6 in Skeletal Muscles of Type 2 Diabetic Rats. Rev. Diabet. Stud. 13; 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen, B. K., Åkerström, T. C. A., Nielsen, A. R. and Fischer, C. P. (2007) Role of myokines in exercise and metabolism. J. Appl. Physiol. 103; 1093–1098. [DOI] [PubMed] [Google Scholar]
- 36.Pöschl, E., Schlötzer-Schrehardt, U., Brachvogel, B., Saito, K., Ninomiya, Y. and Mayer, U. (2004) Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131; 1619–1628. [DOI] [PubMed] [Google Scholar]
- 37.Rutter, J., Winge, D. R. and Schiffman, J. D. (2010) Succinate dehydrogenase-assembly, regulation and role in human disease. Mitochondrion 10; 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanes, J. R. (2003) The basement membrane/basal lamina of skeletal muscle. J. Biol. Chem. 278; 12601–12604. [DOI] [PubMed] [Google Scholar]
- 39.Schindelin, J., Carreras, I. A., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., et al. (2012) Fiji: an open-source platform for biological-image analysis. Nat. Methods 9; 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snyman, C. and Niesler, C. U. (2015) MMP-14 in skeletal muscle repair. J. Muscle Res. Cell Motil. 36; 215–225. [DOI] [PubMed] [Google Scholar]
- 41.Stauber, W. T. (2004) Factors involved in strain-induced injury in skeletal muscles and outcomes of prolonged exposure. J. Electromyogr. Kinesiol. 14; 61–70. [DOI] [PubMed] [Google Scholar]
- 42.Sundaramoorthy, M., Meiyappan, M., Todd, P. and Hudson, B. G. (2002) Crystal structure of NC1 domains. Structural basis for type IV collagen assembly in basement membranes. J. Biol. Chem. 277; 31142–31153. [DOI] [PubMed] [Google Scholar]
- 43.Timpl, R., Wiedemann, H., van Delden, V., Furthmayr, H. and Kühn, K. (1981) A network model for the organization of type IV collagen molecules in basement membranes. Eur. J. Biochem. 120; 203–211. [DOI] [PubMed] [Google Scholar]
- 44.Vracko, R. (1974) Basal lamina scaffold: anatomy and significance for maintenance of orderly tissue structure. Am. J. Pathol. 77; 313–346. [PMC free article] [PubMed] [Google Scholar]
- 45.Wackerhage, H., Schoenfeld, B. J., Hamilton, D. L., Lehti, M. and Hulmi, J. J. (2019) Stimuli and sensors that initiate skeletal muscle hypertrophy following resistance exercise. J. Appl. Physiol. 126; 30–43. [DOI] [PubMed] [Google Scholar]
- 46.Yurchenco, P. D. (2011) Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 3; 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]