Abstract
Chemotherapy-induced side effects affect the quality of life and efficacy of treatment of cancer patients. Current approaches for treating the side effects of chemotherapy are poorly effective and may cause numerous harmful side effects. Therefore, developing new and effective drugs derived from natural non-toxic compounds for the treatment of chemotherapy-induced side effects is necessary. Experiments in vivo and in vitro indicate that Panax ginseng (PG) and its ginsenosides are undoubtedly non-toxic and effective options for the treatment of chemotherapy-induced side effects, such as nephrotoxicity, hepatotoxicity, cardiotoxicity, immunotoxicity, and hematopoietic inhibition. The mechanism focus on anti-oxidation, anti-inflammation, and anti-apoptosis, as well as the modulation of signaling pathways, such as nuclear factor erythroid-2 related factor 2 (Nrf2)/heme oxygenase-1 (HO-1), P62/keap1/Nrf2, c-jun N-terminal kinase (JNK)/P53/caspase 3, mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinases (ERK), AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR), mitogen-activated protein kinase kinase 4 (MKK4)/JNK, and phosphatidylinositol 3-kinase (PI3K)/AKT. Since a systemic review of the effect and mechanism of PG and its ginsenosides on chemotherapy-induced side effects has not yet been published, we provide a comprehensive summarization with this aim and shed light on the future research of PG.
Keywords: Chemotherapy, Side effects, PG, Ginsenosides, Mechanism, Pharmacological effects
Abbreviations: 5-FU, 5-fluorouracil; AMPK, AMP-activated protein kinase; ADM, Adriamycin; AMO, Atractylodes macrocephala volatile oil; ARE, antioxidant response element; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMNC, bone marrow nucleated cells; CP, cisplatin; CY, cyclophosphamide; CK, compound K; CYP2E1, Cytochrome P450 E1; CIA, chemotherapy-induced hair loss; DAC, doses of docetaxel, doxorubicin as well as cyclophosphamide; ERK, extracellular signal-regulated kinases; ERG, enzyme-treated eRG; eRG, 50% ethanol-extracted RG; FBG, fermented black ginseng; FRGE, fermented red ginseng extract; FRG, probiotic-fermented eRG; GM-CSF, granulocyte macrophage colony-stimulating factor; HO-1, heme oxygenase-1; HSPCS, haematopoietic stem and progenitor cells; HEI-OC1, House Ear Institute-Organ of Corti 1; IL, interleukin; JNK, c-jun N-terminal kinase; KG-KH, the mixture of ginsenosides Rk3 and Rh4; LLC-PK1, porcine renal proximal epithelial tubular; LSK, Lin−Sca-1+c-kit+; MEK, mitogen activated protein kinase; MKK4, mitogen activated protein kinase kinase 4; mTOR, mammalian target of rapamycin; MDA, malonaldehyde; MAPK, mitogen-activated protein kinase; Nrf2, nuclear factor erythroid related factor 2; NF-κB, nuclear factor-kappa B p65; NQO, NAD (P) H quinone oxidoreductase; PG, Panax ginseng; PPD, protopanaxadiol; PPT, protopanaxatriol; PGS, PG total saponins; PGRT, PG root; PGLF, PG leaf; PGFR, PG flower; PGSM, PG stem; PGSD, PG seeds; PI3K, phosphatidylinositol 3-kinase; ROS, reactive oxygen species; RG, red ginseng; RGE, red ginseng extract; SREBP-1, sterol regulatory element binding protein 1; TNF-α, tumor necrosis factor-α; wRG, water-extracted RG
Graphical abstract
1. Introduction
As an effective strategy to treat cancer, chemotherapy is widely applied in the treatment of tumors that have a tendency for systemic spread and middle and advanced metastatic tumors. However, chemotherapeutic drugs are considered to be a high-risk treatment because of the narrow dose range of treatments that offer the maximum benefit with minimum severe side effects [1]. Among these chemotherapeutic drugs, cisplatin (CP), 5-fluorouracil (5-Fu), cyclophosphamide (CY), and Adriamycin (ADM) are commonly used but can cause several adverse reactions. According to the incidence of these adverse reactions, the decreasing order of incidence is nephrotoxicity, gastrointestinal mucosal injury, neurotoxicity, hematopoiesis inhibition, cardiotoxicity, immunosuppression, hepatotoxicity, and hair loss [[2], [3], [4], [5], [6], [7], [8]]. These side effects result from drug-derived damage to human tissues, cells, and the regulation of multiple targets and signaling pathways. The mechanisms behind these side effects focus on oxidative stress, inflammation, apoptosis, and autophagy.
Although some Western medicines can reduce the side effects of chemotherapy, their limitations are often ignored. For example, amifostine can ameliorate cumulative myelotoxicity, acute and chronic neutropenia, and/or thrombocytopenia induced by CY, but it can lead to adverse reactions such as dizziness and vomiting [9]. Additionally, mannitol and furosemide do not significantly relieve CP-induced nephrotoxicity, and patients will still be troubled by nephrotoxicity [10]. Hence, discovering non-toxic and effective drugs from ethnic or natural medicine is important.
In Eastern countries, side effects induced by chemotherapy are considered to be a deficiency syndrome of the human body. The treatment of deficiency disease often requires the use of tonic medicine. Among them, Panax ginseng (PG), usually refers to the dried root and rhizome of PG CA Meyer of the family Araliaceae, is regarded as a typical tonic medicine and has a long history of use in China, Korea, and Japan. Recently, clinical studies have shown that compounds or medicines containing different forms of PG have a promising effect on side effects caused by chemotherapy [11,12]. Moreover, ginsenosides, which are the main active ingredient of PG, have a pivotal role in the pharmacological actions of PG. Shenyi capsules, which is a Chinese patented drug that comprised ginsenoside Rg3, could effectively attenuate the side effects induced by chemotherapy in patients, such as nausea and vomiting [13]. Meta-analysis revealed that ginsenoside Rg3 alone reduced chemotherapy-induced myelosuppression in clinic [14]. Thus, scientists all over the world have given attention to the ameliorative effects of PG and its ginsenosides on the side effects caused by chemotherapy and have accumulated considerable discoveries. However, the pharmacological actions and potential mechanisms of PG and its ginsenosides on chemotherapy-induced side effects have not been systematically summarized.
This article reviews the medicinal potential and underlying mechanism of PG and its ginsenosides on the adverse reactions of chemotherapy and puts forward the view that PG and its ginsenosides can be used as adjuvants to reduce the side effects of chemotherapy. We hope this review will lay the foundation for an in-depth investigation of the biochemical mechanisms and pharmacological properties of PG and its ginsenosides to derive benefits from the further development and utilization of PG in treating the side effects of chemotherapy.
2. Different types of PG and its ginsenosides
According to different processing techniques, PG can be developed into several types, including white ginseng, red ginseng, and black ginseng (which is also called black-red ginseng). White ginseng is the product obtained from peeled and naturally dried PG. Red ginseng is obtained by steaming PG once. Black ginseng is the product generated by steaming PG nine times [15].
Ginsenosides are dammarane triterpenoid saponins and are composed of 17 carbons. Protopanaxadiol (PPD) and protopanaxatriol (PPT) are aglycones that have been isolated from PG. According to the number of hydroxyl groups in aglycone components, dammarane saponins are composed of two species, namely, the PPD type and the PPT type. Common PPD type ginsenosides are Rg3, Rb1, Rd, Rb3, Rh2, and compound K (CK), whereas PPT type ginsenosides are Re and Rg1.
3. Effects of PG and its ginsenosides on chemotherapy-induced side effects
3.1. Effects of PG on chemotherapy-induced nephrotoxicity
3.1.1. Chemotherapy-induced nephrotoxicity
Nephrotoxicity is a common side effect of chemotherapy and ultimately impacts the therapeutic efficacy of chemotherapy treatment of cancer patients. Studies have revealed that 90% of cancer patients receiving chemotherapy suffer from nephrotoxicity [2]. For example, CP, CY, 5-Fu, and ADM may lead to serious nephrotoxicity, including tubular damage and renal failure [16]. Among them, CP exhibits the strongest nephrotoxicity [17]. The pathogenic mechanism of chemotherapy-induced nephrotoxicity includes oxidative stress damage, cell apoptosis, and inflammation. Presently, PG and its ginsenosides can reduce levels of blood urea nitrogen and creatinine, thus promoting the recovery of the renal function and subsequently reducing kidney damage [18,19]. Therefore, PG and its ginsenosides are potential drugs for the treatment of CP-induced nephrotoxicity (Table 1).
Table 1.
Therapeutic effects of PG and its ginsenosides on chemotherapy-induced nephrotoxicity in vitro or in vivo.
| Treatment | Experimental model | Effects | Monitored indices | Mechanisms | Reference |
|---|---|---|---|---|---|
| Ginsenoside Re | CP-treated male ICR mice | Improvement of renal function and renal histology; antioxidant effect; anti-apoptotic effect; anti-inflammatory effect | (↓) BUN, CRE, kidney index and histopathological changes | (↓) MDA, 4-HNE, CYP2E1; Bax, COX-2, iNOS (↑) GSH, CAT, Bcl-2 |
[37] |
| KG-KH | CP-treated Male Sprague Dawley rats CP-treated LLC-PK1 cells |
Improvement of renal function and renal histology; antioxidant effect Antioxidant effect |
(↓) BUN, CRE, kidney index, the injury score of cortex and corticomedullary region (↑) Cell viability |
(↑) CAT, SOD (↓) ROS (↑) GR, SOD |
[39] |
| Ginsenoside Rg5 | CP-treated male ICR mice | Improvement of renal function and renal histology; antioxidant effect; anti-apoptotic effect; anti-inflammatory effect | (↓) BUN, CRE, kidney index and histopathological changes | (↓) MDA, 4-HNE; TNF-α, IL-1β, NF-κB p65, COX-2; Bax, CYP2E1 (↑) GSH, SOD, Bcl-2 |
[40] |
| Ginsenoside Rh2 | CP-treated male SPF grade ICR mice | Improvement of renal function and renal histology; antioxidant effect; anti-apoptotic effect; anti-inflammatory effect | (↓) BUN, CRE, kidney histopathological changes | (↓) HO-1, MDA; P53, Bax, cytochrome c, caspase-8, caspase-9, caspase-3, CYP2E1; TNF-α, NF-κB, iNOS, COX-2 (↑) GSH, CAT, SOD, Bcl-2 |
[41] |
| Ginsenoside Rg3 | CP-treated LLC-PK1 cells | Improvement of renal function and renal histology; antioxidant effect | (↓) BUN, CRE, kidney histopathological changes | (↓) ROS, NO, MDA, (↑) CAT, SOD, GSH-Px |
[42] |
| PG | CP-treated male Sprague Dawley rats |
Improvement of renal function and renal histology; antioxidant effect; anti-apoptotic effect; anti-inflammatory effect | (↓) BUN, CRE, kidney histopathological changes | (↓) P53, DNA fragmentation, TNF-α, IL-6, TBARS, XO, NO (↑) GST, GPX, CAT, SOD, ATPase, GSH |
[43] |
| Ginsenoside Rd | CP-treated male Wistar rats CP-treated LLC-PK1 cells |
Improvement of renal function and renal histology; antioxidant effect | (↓) BUN, CRE, kidney histopathological changes | (↑) SOD, CAT (↓) MDA, LDH |
[44] |
| Ginsenoside Rd | CP-treated LLC-PK1 cells | Improvement of renal function; anti-apoptotic effect | (↓) BUN, CRE (↑) Cell viability |
(↓) LDH, DNA fragmentation | [45] |
| Ginsenoside Rk1 | CP-treated HEK-293 cells | Antioxidant effect; anti-apoptotic effect | (↓) The percentage of apoptotic cells (↑) Cell viability |
(↓) MDA, ROS; Bax, caspase-3, caspase-9, (↑) GSH, Nrf2, OH-1; Bcl-2 | [46] |
| FBG and its ginsenoside 20(S)-Rg3 | CP-treated LLC-PK1 cells | Anti-apoptotic effect | (↓) The percentage of apoptotic cells (↑) Cell viability |
(↓) P53, JNK, caspase-3 | [47] |
| MG and its ginsenosides | CP-treated LLC-PK1 cells CP-treated male C57/BL6 mice |
Improvement of renal function; anti-apoptotic effect | (↑) Cell viability (↓) CRE |
(↓) P53, JNK, caspase-3 | [48] |
| Ginsenoside Rh3 | CP-treated LLC-PK1 cells | Anti-apoptotic effect | (↓) The percentage of apoptotic cells (↑) Cell viability |
(↓) JNK, ERK, p38, caspase-3 | [49] |
| Ginsenoside Rb3 | CP-treated male adult ICR mice CP-treated HEK293 cell |
Antioxidant effect; anti-apoptotic effect, regulation of autophagy Antioxidant effect; anti-apoptotic effect, regulation of autophagy |
(↓) BUN, CRE, kidney histopathological changes (↓) The percentage of apoptotic cells (↑) Cell viability |
(↓) MDA; Bax, Bad, caspase 3, caspase 9; ATG3, ATG5, ATG7; AMPK-/mTOR (↑) GSH, SOD; Bcl-2 (↓) ROS; Bax, Bad; ATG3, ATG5, mTOR, caspase 3 (↑) Bcl-2, Bcl-xl |
[51] |
4-HNE, 4-hydroxynonenal; ATG3, autophagy related 3; ATG5, autophagy related 5; ATG7, autophagy related 7; AMPK, AMP-activated protein kinase; BUN, blood urea nitrogen; CP, cisplatin; CRE, creatinine; CYP2E1, Cytochrome P450 E1; CAT, catalase; COX-2, cyclooxygenase 2; ERK, extracellular signal-regulated kinases; FBG, fermented black ginseng; GST, glutathione S-transferase; GPX, glutathione peroxidase; GSH, glutathione; GR, glutathione reductase; HO-1, heme oxygenase-1; iNOS, inducible nitric oxide synthase; IL-1β, interleukin-1β; IL-6, interleukin-6; JNK, c-jun N-terminal kinase; KG-KH, the mixture of ginsenosides Rk3 and Rh4; LDH, lactate dehydrogenase; LLC-PK1, porcine renal proximal epithelial tubular; mTOR, mammalian target of rapamycin; MG, microwave-processed ginseng; MDA, malonaldehyde; Nrf2, nuclear factor erythroid related factor 2; NF-κB, nuclear factor-kappa B p65; NO, nitric oxide; NAD (P) H quinone oxidoreductase-1; PG, Panax ginseng; ROS, reactive oxygen species; SOD, superoxide dismutase; TNF-α, tumor necrosis factor-α; TBARS, thiobarbituric acid reactive substances; XO, xanthine oxidase.
3.1.2. Anti-oxidative effect of PG and ginsenosides on chemotherapy-induced nephrotoxicity
Several studies showed that anti-oxidative effects have partially contributed to the protection of PG and its ginsenosides against CP-induced nephrotoxicity. First, ginsenosides attenuate CP-induced nephrotoxicity by directly reducing the excessive production of reactive oxygen species (ROS) or indirectly alleviating the excessive production of ROS via inhibiting levels of Cytochrome P450 E1 (CYP2E1) and HO-1. Ginsenoside Rk3 and Rh4, which are different isomers at the double bond positions (D20e21 and D20e22), possessed similar renoprotective effects against the cytotoxicity of CP in cultured renal tubular cells, which was characterized by a reduction of lactate dehydrogenase and an increase in cell viability [18]. In CP-treated porcine renal proximal epithelial tubular (LLC-PK1) cells, the mixture of ginsenosides Rk3 and Rh4 (KG-KH) increased cell viability and decreased ROS production [20]. Interestingly, in CP-treated male ICR mice, ginsenosides Re and Rg5 played an anti-oxidative role by reducing CYP2E1 level and thus down-regulating the ROS level [19,21]. Additionally, the overexpression of HO-1 is one of the causes of excessive ROS production. Ginsenoside Rh2 could partly reduce CP-induced nephrotoxicity by down-regulating CYP2E1 and HO-1 levels [22]. Moreover, ginsenoside Rg3 could reduce the excessive production of ROS by decreasing the level of HO-1/NAD (P) H quinone oxidoreductase (NQO)-1 mediated by Nrf2, thus protecting CP-treated LLC-PK1 cells [23]. Second, the renoprotective effect of PG and its ginsenosides was achieved by increasing the activity of antioxidant enzymes and inhibiting lipid peroxidation. For example, Yousef et al. found that the activities of superoxide dismutase, catalase, glutathione S-transferase, glutathione peroxidase, ATPase, and renal non-enzymatic antioxidant glutathione were increased by PG in CP-treated rats. Furthermore, PG decreased the levels of renal thiobarbituric acid reactive substances, xanthine oxidase, and nitric oxide [24]. Moreover, ginsenosides can reduce the nephrotoxicity induced by chemotherapy by recovering the levels of antioxidant enzymes and suppressing lipid peroxidation. Ginsenosides such as ginsenoside Rg5, Rh2, Rg3, Rd, and KG-KH also increased antioxidant enzyme levels and consequently attenuated CP-induced renal damage [[21], [22], [23],25]. Additionally, ginsenosides Re, Rg5, Rh2, and Rd inhibited the excessive production of CP-induced lipid peroxidation products, such as 4-hydroxynonenal and malonaldehyde (MDA), in vivo.
3.1.3. Anti-apoptotic and anti-inflammatory effects of PG and ginsenosides on chemotherapy-induced nephrotoxicity
PG and its ginsenosides attenuate CP-induced nephrotoxicity by producing an anti-apoptotic effect. Initially, PG and its ginsenosides inhibit apoptosis mainly by regulating the related factors of the endogenous apoptosis pathway. PG improved nephrotoxicity by reducing P53 activity and DNA fragmentation while inducing DNA repair in CP-treated rats [25]. Next, ginsenoside Rd inhibited DNA fragmentation in CP-treated LLC-PK1 cells [26]. Moreover, Qi et al. studied the anti-apoptotic properties of ginsenoside Rh2 by systematically assessing its effects on the generation of a pro-apoptotic mediator induced by CP in ICR mice [22]. They found that ginsenoside Rh2 attenuated pro-apoptotic mediator production and enhanced the Bcl-2 level in ICR mice. Similarly, the anti-apoptotic effect of ginsenoside Rk1 included reducing the level of pro-apoptotic factors and increasing the Bcl-2 level in CP-treated HEK-293 cells [27]. Other ginsenosides, such as ginsenoside Re and Rg5, also exhibited an anti-apoptotic effect by adjusting the levels of Bcl-2 and Bax [19,21]. Second, the anti-apoptotic mechanisms of ginsenosides are related to the modulation of JNK-related pathways. Recently, studies have reported that fermented black ginseng and its active component ginsenoside 20 (S)-Rg3 can reduce CP-induced oxidative cytotoxicity by decreasing the protein levels of phosphorylated JNK, P53, and cleaved caspase-3, which might lead to a blockage of the JNK/P53/caspase-3 signaling cascade in LLC-PK1 cells [28]. Similarly, in CP-induced LLC-PK1 cells, microwave-processed ginseng, and its ginsenosides also alleviated cell damage by cisplatin by decreasing the expressions of P53, JNK, and caspase-3 [29]. Notably, Lee et al. [30] compared the action of ginsenoside Rh3 and its positional double bond isomer ginsenoside Rk2 in CP-induced apoptotic damage and found that ginsenoside Rh3 elicited a better protective effect than ginsenoside Rk2. Furthermore, ginsenoside Rh3 protected kidney cells from apoptotic damage by inhibiting the JNK and ERK signaling pathways, which was evidenced by decreases in the levels of JNK, ERK, p38, and cleaved caspase-3. Meanwhile, autophagy is the key pathway of CP-induced nephrotoxicity. A current study by Xing et al. claimed that suppression of autophagy led to apoptotic inactivation, which suggests that autophagy acted adversely in the kidney injury model for CP [31]. Further research reported that ginsenoside Rb3 inhibited autophagy through the AMPK/mTOR-dependent signaling pathway in CP-treated mice. Furthermore, ginsenoside Rb3 suppressed cell apoptosis and autophagy by inactivating the AMPK signaling pathway in HEK293 cells.
Moreover, the anti-inflammatory effects of ginsenosides alleviated CP-induced nephrotoxicity to some extent. In CP-treated rats, ginsenoside Rh2 elicited anti-inflammatory effects, which were manifested as decreased expressions of TNF-α, NF-κB, inducible nitric oxide synthase, and cyclooxygenase 2 [22]. What’s more, ginsenoside Rg5 [21] and Re [19] exerted the same anti-inflammatory effects by inhibiting the generation of pro-inflammatory cytokines and inflammation-related enzymes.
3.2. Effect of PG on chemotherapy-induced gastrointestinal mucosa injury
3.2.1. Chemotherapy-induced gastrointestinal mucosa injury
Gastrointestinal mucosal injury is a common side effect induced by chemotherapy. For example, vomiting after chemotherapy may be associated with gastric mucosal damage, which appears in 90% of patients receiving chemotherapy [3]. Diarrhea associated with intestinal mucosal damage also affects the progress of chemotherapy, with an incidence rate of 50% to 80% of patients receiving chemotherapy (e.g., 5-Fu) [4]. Moreover, oral mucositis is one of the common complications of chemotherapy, which often occurs in 18% to 40% of patients in the first stage of chemotherapy [5]. Generally, adverse reactions caused by gastrointestinal mucosal injury have a higher incidence rate. Overall, PG and its ginsenosides have exhibited promising therapeutic effects on the above-mentioned adverse reactions.
3.2.2. Therapeutic effect of PG and ginsenosides on chemotherapy-induced hamster oral mucositis and diarrhea
In one study, ginsenoside Rb1 was contained in the chitosan–sodium alginate membrane (G-Rb1film) and applied to 5-Fu-induced hamster oral mucositis. The results revealed that the G-Rb1film had a curative effect and anti-inflammatory properties in chemotherapy-induced severe oral mucositis [32]. In 5-Fu-induced oral mucositis hamsters, ginsenoside Rb1 reduced the mucositis area and alleviated inflammatory cell infiltration, myeloperoxidase activity, ulceration, and abscess formation.
Additionally, our study has revealed that Shenzhu Capsule, which contains PG total saponins (PGS), PG polysaccharides, Atractylodes macrocephala volatile oil (AMO), and Atractylodes macrocephala polysaccharides (AMP), alleviated diarrhea effectively in 5-Fu-treated mice [33]. It reversed colonic histopathological changes, restored the overall structure of the intestinal microflora, and inhibited potential pathogens that might be related to diarrhea. PGS combined with AMO, not AMO or PGS alone, could also improve diarrhea caused by 5-Fu. The mechanism was related to the inhibition of intestinal inflammatory cytokines caused by chemotherapy and a reduction of potential pathogens, indicating that together with AMO, ginsenosides separated from PG might have the potential to treat diarrhea caused by chemotherapy [34].
3.3. Effects of PG on chemotherapy-induced neurotoxicity
3.3.1. Chemotherapy-induced neurotoxicity
Chemotherapy is a systemic treatment strategy, and its side effects are distributed throughout the body. Among these side effects, the nervous system is the main target of chemotherapy-induced side effects, and 50% of cancer patients receiving chemotherapy suffer from neurological side effects, such as neuropathic pain and cognitive impairment [6]. Further, PG and its ginsenosides have significant potential therapeutic effects on chemotherapy-induced adverse neurological reactions.
3.3.2. Therapeutic effect of PG and ginsenosides on chemotherapy-induced neuropathic pain
Oxaliplatin is widely used due to its promising anticancer effects. However, it often causes peripheral neuropathy, including cold and mechanical hyperalgesia. Therefore, Suzuki et al. evaluated the in vivo effect of PG on neuropathic pain induced by oxaliplatin in rats and found that PG reduced the increase of cold allodynia and mechanical hyperalgesia [35]. They also found that the PG extract exhibited a preventive effect in L–OH–treated differentiated PC12 cells and claimed that the most active component of PG was ginsenoside F2 [36]. Similarly, in murine primary dorsal root ganglia cells, they found that PG exhibited a protective effect on neuronal injury, and its main active component was ginsenoside Rg3.
3.3.3. Therapeutic effect of PG and ginsenosides on chemotherapy-induced cognitive impairment
Ginsenosides significantly affected chemotherapy-induced cognitive impairment. In CP-treated rats, Chen et al. [37] found that ginsenoside Rb1 could significantly improve cognitive impairment and neuronal loss by suppressing oxidative stress and hippocampal neuronal inflammation and recovering the cholinergic neuron function. Furthermore, ginsenoside Rg1 could prevent cognitive impairment caused by chemotherapy, which might be correlated with the regulation of microglia-related cytokines and mediators, protection of neuronal activity, promotion of neural plasticity in specific brain regions related to cognitive processing, and inhibition of neuroinflammation [38]. Shi et al. developed a chemo brain mouse model with a combination of three doses of docetaxel, doxorubicin, and cyclophosphamide (DAC) at 2 day intervals and found that after the administration of ginsenoside Rg1, the decrease of prefrontal and hippocampal neuronal activity induced by DAC was reversed, and the elimination of dendritic spines of cortical neurons was improved in vivo. Meanwhile, ginsenoside Rg1 suppressed microglia polarization from the M2 to M1 phenotype as well as the increase of pro-inflammatory factors, including IL-6, but up-regulated IL-4 and IL-10 while modulating cytokine mediators. In in vitro experiments, ginsenoside Rg1 also inhibited the increased levels of pro-inflammatory cytokines in BV-2 microglial cells and increased cell viability in PC12 cells.
3.4. Effects of PG on chemotherapy-induced hematopoiesis inhibition
3.4.1. Chemotherapy-induced hematopoiesis inhibition
Of all the side effects caused by chemotherapy, hematopoiesis inhibition, is a formidable problem in clinic because of its toxicity in cells. A previous study indicated that 40%–47% of patients who received chemotherapy suffered from the side effects of decreased blood and bone marrow cells [7]. Myelosuppression often affects bone marrow cell lines, causing an initial decrease in white blood cells and subsequent hematopoietic stem cell/hematopoietic progenitor cell damage [39]. In clinic, serious hematopoiesis inhibition usually induces potentially life-threatening infections. Considering the importance of natural products as major contributors to the therapeutic properties of chemoprotective agents, the potential roles of PG and its ginsenosides against chemotherapy-induced hematopoiesis inhibition were studied (Table 2).
Table 2.
Therapeutic Effects of PG and its ginsenosides on chemotherapy-induced hematopoiesis inhibition in vitro or in vivo
| Treatment | Experimental model | Effects | Monitored indices | Mechanisms | Reference |
|---|---|---|---|---|---|
| PG | CY-treated male BALB/c mice | Recovery of hematopoietic function, improvement of cell cycle, and hematopoiesis-related cytokines | (↑) Thymus and spleen indices, peripheral blood cells count, BMNCS | (↑) CFU-GM, CFU-E, BFU-E, CFU-Meg, TPO, EPO, GM-CSF, amount of G2/M and S phase cells | [70] |
| PG | CY-treated male BALB/c mice | Recovery of hematopoietic function | (↑) Thymus indices, peripheral blood cells count, BMNCS | [71] | |
| PG | 5-Fu-treated male C57BL/6 mice | Recovery of hematopoietic function, anti-myelotoxicity activity, promotion of myelopoiesis, improvement of hematopoietic progenitor cells and hematopoiesis-related cytokines | (↑) Thymus and spleen indices, peripheral blood cells count, BMNCS, body weight | (↑) CFU-GM, SCF, c-Kit, GM-CSF, IL-3, IL-1, IL-6, TNF-α | [72] |
| Ginsenoside Rg3 | CY-treated male ICR mice | Recovery of hematopoietic function, improvement of hematopoiesis-related cytokines | (↑) Bone marrow and spleen cells | (↑) SCF, GM-CSF, IL-3, IL-1, TPO | [73] |
| Ginsenoside Rh2 | CY-treated male SPF C57BL/6 mice | Anti-myelotoxicity activity | (↓) Micronucleus formation in polychromatic erythrocytes, DNA strand breaks in white blood cells | [74] | |
| TSPG | CY-treated male Swiss-Kunming mice | Antioxidant effect, anti-apoptotic effect; reduction of genotoxicity | (↓) DNA damage in peripheral lymphocytes (↑) T-SOD, GPx, CAT, GSH |
[75] | |
| Ginsenoside Rb1 | CY-treated male Swiss-Kunming mice | Antioxidant effect, anti-apoptotic effect; reduction of genotoxicity | (↓) MDA, DNA damage and apoptosis in peripheral lymphocytes and marrow cells (↑) T-SOD, GPx |
[76] | |
| 20(S)-ginsenoside Rg (3) | CY-treated male Swiss-Kunming mice | Antioxidant effect, anti-apoptotic effect | (↓) MDA, DNA damage and apoptosis in peripheral lymphocytes and marrow cells (↑) T-SOD, GPx |
[77] | |
| CK | CY-treated male BALB/c mice | Recovery of hematopoietic cells, improvement of cell cycle, and hematopoiesis-related cytokines, anti-apoptotic effect | (↑) Peripheral blood cells count, thymus indices | (↑) MEK/ERK, the radio of bcl-2/bax, TPO, EPO, GM-CSF | [78] |
| PDS-C | CY-treated male Kunming mice | Recovery of hematopoietic cells, improvement of hematopoiesis-related cytokines and hematopoietic progenitor cells | (↑) Peripheral blood cells count, overall bone marrow cellularity, hematopoietic cells count | (↑) MEK and ERK protein kinases, and C-kit and GATA-1 transcription factors | [79] |
| Ginsenoside Re and Rk3 | CY-treated male BALB/c mice | Recovery of hematopoietic cells, improvement of cell cycle, and hematopoiesis-related cytokines, anti-apoptotic effect | (↑) Peripheral blood cells count, thymus and spleen indices, BMNCS | (↓) Bax, caspase-3, the ratio of G0/G1phase cells (↑) CFU-GM, CFU-E, BFU-E, CFU-Meg, Bcl-2 |
[80] |
| Dammarane sapogenins | CY-treated male BALB/c mice | Recovery of hematopoietic function, improvement of hematopoiesis-related cytokines | (↑) Thymus and spleen indices, peripheral blood cells count | (↑) CFU-GM, CFU-E, BFU-E, CFU-Meg, CFU-GEMM | [81] |
| Ginsenoside Rg1 | CY-treated Kunming mice | Recovery of hematopoietic function | (↑) Bone marrow cell, femoral bone morphology | (↓) CaSR mRNA (↑) LSK positive cells, lymphoid CD3+ positive cells |
[82] |
| Ginsenoside Rg1 | CY-treated female C57BL/6J mice | Amelioration of bone marrow, alleviation of EMH | (↓) The cellularity and weight of the spleen | (↓) c-Kit + HSPCs in spleen (↑) The fraction of c-Kit+/CD45+HSPCs |
[83] |
5-FU, 5-fluorouracil; BMNC, bone marrow nucleated cells; BFU-E, burst-forming uniterythroid; BM, bone marrow; CY, cyclophosphamide; CK, compound K; CAT, catalase; CFU-E, colony-forming uniterythroid; CFUGM, colony-forming unitegranulocyte macrophage; CFU-Meg, colony-forming unitmegakaryocyte; CSF, colony stimulating factors; CFU-GEMM, colony forming unit-granulocyte, erythrocyte, monocyte and megakaryocyte; ERK, extracellular signal-regulated kinases; EPO, erythropoietin; EMH, extramedullary hematopoiesis; GATA-1, GATA binding protein 1; GPX, glutathione peroxidase; GSH, glutathione; GM-CSF, granulocyte macrophage colony-stimulating factor; HSPCS, haematopoietic stem and progenitor cells; IL-6, interleukin-6; IL-3, interleukin-3; IL-1, interleukin-1; LSK, Lin−Sca-1+c-kit+; MEK, mitogen activated protein kinase; MDA, malonaldehyde; SCF, stem cell factor; TNF-α, tumor necrosis factor-α; TPO, thrombopoietin.
3.4.2. Hematopoietic function regulation of PG and ginsenosides on chemotherapy-induced hematopoiesis inhibition
Accumulating evidence indicates that PG and its ginsenosides are effective in treating chemotherapy-induced myelosuppression. Attention has been given to the effect of PG on blood cells and hematopoietic function, especially hematopoietic stem cells, and hematopoietic progenitor cells. Han et al. [40] and Zhang et al. [41] reported that CY-induced myelosuppression could be greatly improved by PG. In CY-treated mice, PG could increase the amount of peripheral blood cells, bone marrow cells, bone marrow nucleated cells (BMNCs), and the spleen/thymus index. Hematopoiesis-related cytokines, such as thrombopoietin, erythropoietin, granulocyte macrophage colony-stimulating factor (GM-CSF), hematopoietic progenitors including burst-forming unit erythroid, colony-forming unit erythroid, colony-forming unit granulocyte macrophage, and colony-forming unit megakaryocyte, and the amount of G2/M and S phase cells were increased in the mice model after PG was administrated. Furthermore, when co-decocted with Ligustrum lucidum Ait or Ophiopogon japonicas, the protective action of PG against myelotoxicity was extremely enhanced. HPLC revealed that rare saponins might be effective chemical components for treating bone marrow suppression. Additionally, PG exhibited similar pharmacological activity in the 5-Fu-treated mice, which was reflected in an improvement of the hematopoietic cytokines, including colony-forming unit granulocyte macrophage and GM-CSF [42]. Furthermore, as a biological response modifier, the ginsenoside Rg3 treatment usually promoted proliferation of the total spleen and bone marrow cells and led to an increase in colony-stimulating factors, such as IL-3, GM-CSF, and thrombopoietin in CY-treated and normal mice [43]. A study performed by Wang et al. [44] illustrated that ginsenoside Rh2 resisted micronucleus formation in bone marrow polychromatic erythrocytes and reduced DNA strand breakage in white blood cells.
3.4.3. Anti-oxidative and anti-apoptotic effects of PG and ginsenosides on chemotherapy-induced hematopoiesis inhibition
The protective effects of PG against CY-induced genotoxicity contribute to its anti-oxidative and anti-apoptotic activity. Qiu and his coworkers showed that DNA damage and bone marrow cell apoptosis stimulated by CY were alleviated by the total saponins from the stem and leaf of PG. As an antioxidant agent, the total saponins from the stem and leaf of PG increased the activities of antioxidant enzyme contents in the CP-induced myelosuppressive model [45]. Similarly, the therapeutic effects of ginsenoside Rb1 and ginsenoside Rg3 on cyclophosphamide-induced myelosuppression are also related to the inhibition of DNA damage and apoptosis. Additionally, Rb1 and Rg3 each reversed the reduction of antioxidant enzyme activities and the increase of MDA content caused by CP [46,47]. Ginsenoside CK also promoted cell proliferation and differentiation and reduced the apoptosis of BMNCs through the Bcl-2/Bax and the MEK/ERK signaling pathways, which may result in reversing ginsenoside-CK-induced changes in the peripheral blood cells, BMNCs counts, hematopoiesis-related cytokines, and colony yield of hematopoietic progenitor cells in mice [48]. Meanwhile, a fraction separated from PG and was the termed panaxadiol saponins component, which contained five ginsenosides and enhanced proliferation and differentiation of hematopoietic progenitor cells in myelosuppressive mice, with an underlying mechanism mediated by the intracellular MAPK signaling pathway in bone marrow cells [49]. Han et al. compared the effects of ginsenoside Re and its secondary metabolite ginsenoside Rk3 on chemotherapy-induced myelosuppression [50]. The result indicated that, compared with ginsenoside Re, ginsenoside Rk3 more easily passed through biofilms, promoted a higher proliferation of bone marrow cells, and improved the hematopoietic function of bone marrow in CY-induced myelosuppressive mice. This was associated with the regulation of the cytokine level, normal cell cycle recovery, and prevention of BMNC apoptosis.
3.4.4. Immunogenic effect of PG and ginsenosides on chemotherapy-induced hematopoiesis inhibition
The immunogenic activity of PG is a necessary factor that is responsible for its role in myelopoiesis to some extent. Korean PG could improve the recovery of hematopoiesis by increasing cytokine release in 5-Fu-treated mice. The levels of IL-3 and GM-CSF in serum and bone marrow and the expressions of c-Kit, SCF, and IL-1 in the spleen were elevated after uptake of Korean PG. Ginsenoside Rg1 stimulated the expressions of cytokines in bone marrow cells and partially directed this effect of Korean PG. Dammarane sapogenins (DS), which was an active fraction mainly containing PPT and PPD, showed remarkable immunological action in CY-treated mice, as evidenced by stimulated ConA-induced splenocyte proliferation, lipopolysaccharide-induced proliferation, and the hematopoiesis recovery function. Notably, reduced red blood cells, which were hardly changed by PG and its active compounds in the myelosuppressive model, could be reversed by DS [51].
3.4.5. Extramedullary hematopoiesis regulation of ginsenoside Rg1 on chemotherapy-induced hematopoiesis inhibition
Additionally, studies have demonstrated that the protective action of ginsenoside Rg1 against CY-induced myelosuppression stems from the recovery of the bone marrow hematopoietic function and alleviation of extramedullary hematopoiesis in the spleen. Xu et al. reported that, apart from the elevation of bone marrow cells and alleviation of bone marrow damage, the up-regulation of the percentage of Lin−Sca-1+c-kit+ (LSK)-positive cells in bone marrow and peripheral blood cells, as well as the percentage of lymphoid CD3+-positive cells, were observed in the in vivo model of CY treatment after ginsenoside Rg1 treatment. Furthermore, this agent also inhibited the overexpression of the calcium-sensing receptor caused by CY, which indicated the beneficial effects of ginsenoside Rg1 on hematopoietic stem cell proliferation and mobilization into the peripheral blood. However, bone marrow hematogenesis in normal mice was not altered by ginsenoside Rg1 [52]. Another study [53] revealed that, without any effects on cell apoptosis, ginsenoside Rg1 elevated the proliferative activity of c-Kit + hematopoietic stem and progenitor cells (HSPCs) and decreased the absolute number of c-Kit + HSPCs, which indicated that a reduction of c-Kit + HSPCs stimulated by ginsenoside Rg1 resulted from the mobilization of HSPCs from the spleen to the bone marrow. Furthermore, ginsenoside Rg1 was proven to increase the frequency of HSPCs and LSK HSPCs, which demonstrated that the effect of ginsenoside Rg1 on CY-induced myelosuppression was reflected by the promoted proliferation of HSPCs in splenic extramedullary hematopoiesis and the migration HSPCs from the spleen to the bone marrow.
3.5. Effects of PG on chemotherapy-induced cardiotoxicity
3.5.1. Chemotherapy-induced cardiotoxicity
Cardiotoxicity is a common side effect induced by chemotherapy, which usually leads to severe heart failure and eventually cardiomyopathy. Presently, many chemotherapy drugs can cause cardiotoxicity, including ADM, paclitaxel, and CP. Among them, cardiotoxicity caused by ADM is the most prominent, and 8% to 26% of cancer patients treated with ADM suffer from cardiotoxicity, which is attributed to oxidative damage, apoptosis, autophagic death of cardiomyocytes, and, ultimately, abnormal heart function [8]. PG and its ginsenosides show obvious cardioprotective effects, such as improved electrocardiographic changes and reversing the decrease in the ejection fraction and fraction shortening induced by ADM [54]. These results indicate that PG and its ginsenosides may be candidates for the treatment of cardiotoxicity (Table 3).
Table 3.
Therapeutic Effects of ginsenosides on chemotherapy-induced cardiotoxicity in vitro or in vivo
| Treatment | Experimental model | Effects | Monitored indices | Mechanism | Reference |
|---|---|---|---|---|---|
| Ginsenoside Rh2 | ADM-treated male swiss mice ADM-injured H9C2 cells ADM-injured male Sprague-Dawley rats |
Antioxidant effect Improvement of the electrocardiographic changes |
(↓) AST, creatine kinase, histopathological changes in left ventricles (↑) Cell viability (↓) Elevated heart rates and widened QRS complex |
(↑) CAT, GSH, SOD, (↓) MDA, LDH |
[88] |
| Ginsenoside Rg3 | ADM-treated cardiac microvascular endothelial cells ADM-treated male SD rats |
Antioxidant effect; anti-apoptotic effect; anti-inflammatory effect Improvement of cardiac function and endothelium function |
(↑) Cell viability (↑) EF, FS, left ventricular outflow; vasoconstriction of endothelial aorta (↓) Vasodilation of endothelial aorta |
(↑) Endothelial nitric oxide synthase, Nrf2-ARE, SOD, GPx; Bcl-2 (↓) ROS, LDH, endothelin-1, MDA, Akt; annexin V binding, Bax, Fas mRNA expression, calcium influx; ICAM-1, TGF-β, VEGF, TIMP-1 |
[90] |
| Ginsenoside Rg3 micelles | ADM-treated male SD rats | Improvement of solubility, oral bioavailability and cardiac function; antioxidant effect; anti-apoptotic effect | (↑) EF, FS (↓) Creatine kinase, creatine kinase-MB |
(↑) Mitochondrial function, metabolic function, calcium handling; Bcl-2 (↓) ROS, LDH; Bax, caspase3, caspase9 |
[91] |
| PG | ADM-treated male wistar rats | Improvement of heart failure; myocardial effects; antioxidant effect | (↑) Heart weight, body weight, mortality rate and ascites, arterial pressure | (↑) GSH-x, CAT, SOD, macromolecular biosynthesis (↓) MDA |
[93] |
| Ginsenoside Rg1 | ADM-treated male C57BL/6 mice | Improvement of cardiac function; anti-apoptotic effect; inhibition of cardiac inflammation and fibrosis | (↑) EF, FS | (↑) Phosphorylation of Akt and Erk, the ratio of Bcl-2 and Bax (↓) Cytochrome c, LDH, the infiltration of inflammatory cells |
[96] |
| Ginsenoside Rb1 | ADM-treated H9C2 cells | Anti-apoptotic effect | (↓) The percentage of apoptotic cells (↑) Cell viability |
(↓) Caspase-3, caspase-8, PARP, CYP1A1 and CYP1A2 | [99] |
| Ginsenoside Rb1 | ADM-treated H9C2 cells | Regulation of autophagy | (↑) Cell viability | (↑) P62 (↓) Autophagic structure and LC3-II/LC3-I ratio |
[102] |
| Ginsenoside Rg1 | ADM-treated in male C57BL/6J mice | Improvement of cardiac function; regulation of autophagy; inhibition of endoplasmic reticulum stress | (↑) EF, FS | (↑) GRP78, XBP1, GFAT1 (↓) LC3, ATG5, cleaved ATF6, IRE1, TIF1, p-P70S6K, JNK1 and Beclin1 |
[104] |
ADM, Adriamycin; AST, aspartate aminotransferase; ARE, antioxidant response element; ATG5, autophagy related 5; ATF6, activating transcription factor 6; CK, compound K; CRE, creatinine; CAT, catalase; EF, ejection fraction; FS, fraction shortening; GPX, glutathione peroxidase; GSH, glutathione; GRP78, glucose-regulated protein 78; GFAT1, glutamine: fructose-6-phosphate amidotransferase 1; ICAM-1, intercellular cell adhesion molecule-1; JNK1, c-jun N-terminal kinase1; LDH, lactate dehydrogenase; LC3-I, light chain 3-I; LC3-II, light chain 3-II; MDA, malonaldehyde; p-P70S6K, phosphorylated pibosomal protein S6 kinase beta-1; PARP, poly ADP-ribose polymerase; ROS, reactive oxygen species; SOD, superoxide dismutase; TIF1, transcriptional intermediary factor 1; TIMP-1, tissue inhibitor of metalloproteinase 1; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor; XBP1, X-box binding protein 1.
3.5.2. Anti-oxidative effect of PG and ginsenosides on chemotherapy-induced cardiotoxicity
The anti-oxidative effects of PG and its ginsenosides are responsible for their cardioprotective effect. First, the anti-oxidative effect of ginsenosides is manifested by reducing the overproduction of ROS. Wang et al. [55] indicated that ginsenoside Rg3 attenuated ADM-induced cardiotoxicity by improving oxidative stress-induced endothelial dysfunction. The mechanism involved reducing oxidative stress by activating AKT and up-regulating Nrf2/ARE and then reducing the ROS level. However, the use of ginsenoside Rg3 was limited because of its low aqueous solubility and oral bioavailability. To improve the medical use of ginsenoside Rg3, Li et al. encapsulated ginsenoside Rg3 through spontaneous self-assembly of Pluronic F127 and found that they could alleviate cardiotoxicity by improving the calcium treatment and mitochondrial and metabolic functions while reducing the ROS [56]. Moreover, PG and its ginsenosides demonstrated antioxidant effects by augmenting the levels of antioxidant biomolecules and reducing lipid peroxidation products. You et al. found that PG attenuated ADM-induced heart failure by increasing myocardial glutathione peroxidase, macromolecular biosynthesis, and superoxide dismutase activity as well as reducing lipid peroxidation [57]. Similarly, 20(S)-ginsenoside Rh2 increased antioxidant biomolecule levels and reduced the content of MDA in ADM-treated mouse heart tissue [54].
3.5.3. Anti-apoptotic and autophagy regulation of PG and ginsenosides on chemotherapy-induced cardiotoxicity
The cardioprotective mechanisms of PG and its ginsenosides are closely related to their anti-apoptotic and autophagy regulation properties. First, ginsenosides attenuate ADM-induced cardiotoxicity by inhibiting apoptosis. Reportedly, ADM treatment of cardiomyocytes can result in the activation of caspase3, PARP, and P53 and the release of cytochrome c from the mitochondria [58,59]. Ginsenoside Rg1 inhibited ADM-induced cardiomyocyte apoptosis by increasing the phosphorylation of Akt and Erk pathways and the ratio of Bcl-2 to Bax while reducing the release of mitochondrial cytochrome c [60]. Additionally, Zhang et al. reported that ginsenoside Rb1 also alleviated ADM-induced cardiomyocyte apoptosis by reducing the expressions of caspase-3, caspase-8, and PARP in ADM-treated H9C2 cells [61]. Further study found that ginsenoside Rb1 inhibited ADM-induced cell apoptosis, at least in part by competing with ADM for the aryl hydrocarbon receptor to reduce the induction of CYP1A, as evidenced by decreases in CYP1A1 and CYP1A2. Similarly, the cardioprotective effect of ginsenosides is achieved by regulating autophagy. Ginsenoside Rb1 could attenuate the ADM-induced decrease of cardiomyocyte viability and inhibit the increasement of the autophagy-related structure, the conversion of light chain 3-I to light chain 3-II, and the decrease of p62 protein expression [62]. Additionally, endoplasmic reticulum stress is another cause of ADM-induced cardiac dysfunction and is closely associated with autophagy activation [63]. Xu et al. reported that the inhibition of endoplasmic reticulum stress and autophagy might be the mechanism by which ginsenoside Rg1 improved ADM-induced cardiac dysfunction. The results illustrated that this agent decreased ADM-induced autophagy-related 5; cleaved activating transcription factor 6, inositol-requiring enzyme 1, transcriptional intermediary factor 1, phosphorylated ribosomal protein S6 kinase beta-1, JNK 1, and Beclin1; and increased glucose-regulated protein 78 expression [64].
3.6. Effects of PG on chemotherapy-induced immunotoxicity
3.6.1. Chemotherapy-induced immunotoxicity
Chemotherapy drugs usually cause serious immunotoxicity, which seriously weakens the effect of the treatment and reduces the patient’s quality of life. Of these chemotherapy drugs, CY, which is an inducer of immunosuppression, can cause the production of serum cytokines, such as interferon-γ, TNF-α, IL-2, IL -10, and IL-12 [65]. Moreover, CY-induced oxidative stress and free radical damage induced immunosuppression. Recently, PG and its ginsenosides were confirmed to be immunomodulators after chemotherapy in numerous studies (Table 4).
Table4.
Therapeutic Effects of PG and ginsenosides on chemotherapy-induced immunosuppression in vitro or in vivo
| Treatment | Experimental mode | Effects | Monitored indices | Mechanism | Reference |
|---|---|---|---|---|---|
| PGRT | CY-treated male BALB/c mice | Recovery of immunosuppression; anti-inflammatory effect; antioxidant effect | (↑) Viability of NK cells, immune organ index, CD4+ cells, ratio of CD4+/CD8+ cells, carbon clearance, phagocytic rate, phagocytic index | (↑) Nrf2, HO-1, NQO1, SOD1, SOD2, CAT, IL-1β, IL-4, IL-6, IFN-γ, TNF-α | [106] |
| PGLF | CY-treated male BALB/c mice | Recovery of immunosuppression; anti-inflammatory effect | (↑) Viability of NK cells, immune organ index, CD4+, ratio of CD4+/CD8+, carbon clearance, phagocytic rate, phagocytic index | (↑) IL-1β, IL-4, IL-6, IFN-γ, TNF-α | |
| PGFR | CY-treated male BALB/c mice | Recovery of immunosuppression; anti-inflammatory effect; antioxidant effect | (↑) Viability of NK cells, immune organ index, CD4+, ratio of CD4+/CD8+, carbon clearance, phagocytic rate, phagocytic index | (↑) Nrf2, HO-1, NQO1, SOD1, SOD2, CAT, IL-1β, IL-4, IL-6, IFN-γ, TNF-α | |
| PGSM | CY-treated male BALB/c mice | Recovery of immunosuppression; anti-inflammatory effect; antioxidant effect | (↑) Thymus indices, carbon clearance, splenocyte proliferation, NK cell activities | (↑) IL-1β, IL-4, IL-6, IFN-γ, TNF-α | |
| PGSD | CY-treated male BALB/c mice | Recovery of immunosuppression; anti-inflammatory effect | (↑) Thymus indices, carbon clearance, splenocyte proliferation, NK cell activities | (↑) IL-1β, IL-4, IL-6, IFN-γ, TNF-α | |
| Ginsenoside Rh2 and its metabolites | CY-treated male BALB/c mice | Immunomodulating effects; anti-inflammatory effect | (↑) Thymus and spleen indices, white blood cells, spleen lymphocyte proliferation, macrophage phagocytosis, carbon clearance, CD4+ cells, CD4+/CD8+ cells | (↑) IL-1β, IL-4, IL-6, IFN-γ, TNF-α | [107] |
| Red ginseng extract | CY-treated male BALB/c mice | Immunestimulating effects | (↑) White blood cells, T and B lymphocytes, antibody-forming cells, CD4+/CD8+ cells, CD4+CD25+ cells, neutrophils | [109] | |
| RG FRG |
CY-treated male BALB/c mice CY-treated male BALB/c mice |
Immunomodulating effects Immunomodulating effects |
(↑) body weight, NK cells (↑) body weight, NK cells |
(↑) IFN-γ, Treg cell differentiation (↑) IFN-γ, Th1 and Treg cell differentiation, macrophage activation |
[110] [110] |
| Ginsenoside Rg3 | CY-treated male BALB/c mice | Immunoenhancement effects | (↑) Thymus and spleen indices (↓) histopathological changes in thymus and spleen |
(↑) LDH, ACP, IgG, IL-2, G-CSF, IFN-γ (↓) GATA-3, IL-4 |
[111] |
| WRG | CY-treated male BALB/c mice | Immunomodulating effects; anti-inflammatory effect | (↑) T and B lymphocytes, NK cells | (↑) NO, iNOS, CXCL10, CXCL11, IL-1α, IL-23, IL-6, TNF-α | [112] |
| Ginsenoside Rh2 | CY-treated BALB/c T cells-deficient nude mice | Immunoenhancement effects; regulation of fatty acid metabolism | (↓) CORT, FASN, FA, SREBP-1, PI3K-AKT pathway (↑) Ketone |
[114] |
ACP, acid phosphatase; CY, cyclophosphamide; CAT, catalase; CXCL, chemokine (C-X-C motif) ligand; CORT, glucocorticoid; FRG, probiotic-fermented eRG; FASN, fatty acid synthase; FA, fatty acids; G-CSF, granu1ocyte colony-stimu1ating factor; GATA-3, GATA binding protein 3; HO-1, heme oxygenase-1; iNOS, inducible nitric oxide synthase; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-4, interleukin-4; IFN-γ, interferon-γ; IgG, immunoglobulin G; IL-2, interleukin-2; IL-1α, interleukin-1α; IL-23, interleukin-23; LDH, lactate dehydrogenase; Nrf2, nuclear factor erythroid related factor 2; NQO-1, NADPH quinineoxidoreductase-1; NO, nitric oxide; NADPH, nicotinamide adenine dinucleotide phosphate; NK, natural killer; PGRT, PG root; PGLF, PG leaf; PGFR, PG flower; PGSM, PG stem; PGSD, PG seeds; PI3K, phosphatidylinositol 3-kinase; RG, red ginseng; SOD, superoxide dismutase; SREBP-1, sterol regulatory element binding protein 1; TNF-α, tumor necrosis factor-α; wRG, water-extracted RG.
3.6.2. Immunomodulation of PG and ginsenosides on chemotherapy-induced immunotoxicity
The treatments of PG and its ginsenosides can improve chemotherapy-induced immunotoxicity by regulating the function of immune-related organs and cells and the levels or balance of immune-related cells and cytokines. Chen et al. reported that different parts of PG usually had a different immunomodulatory effect [66]. PG root (PGRT), PG leaf (PGLF), and PG flower (PGFR) could improve the spleen and thymus indices, increase natural killer cell activities, recover macrophage function, and enhance the cell-mediated immune response in CY-injected mice. Conversely, PG stem (PGSM) and PG seeds (PGSD) showed similar immunomodulatory effects, but the effect was not as strong as that of the other groups. In CY-injected mice, PGRT, PGLF, and PGFR increased the CD4+ cell count, CD4+/CD8+ ratio, and serum cytokines levels. Generally, the immune effect of PGRT, PGLF, and PGFR was better than that of PGSM and PGSD, which may be because the contents of ginsenoside Rc, Rb1, Rg1, Rb2, and Re in PGRT, PGFR, and PGSD were significantly higher than those in PGSM and PGSD. Similarly, the therapeutic mechanism of (20S)-ginsenoside Rh2 and its main metabolites (24R)-pseudo-ginsenoside HQ (R-PHQ) and (24S)-pseudo-ginsenoside HQ (S-PHQ) on CY-treated immunosuppressive mice is highly consistent with that of PG. This had been demonstrated in the white blood cell count, cellular immunity, and macrophage phagocytosis [67]. Moreover, Lin et al. found that 20(S)-PPD reduced CY-induced toxicity by increasing the white blood cell count, cellular immunity, and immune-related cytokines [68]. Additionally, red ginseng extract (RGE) could increase antibody cell numbers and activate neutrophil migration in CY-induced immunosuppressive mice [69]. Furthermore, black-red ginseng extract and fermented RGE showed similar immunostimulatory activity to that of RGE, but the effect was not as strong as that of RGE. This action may be related to the processing time of RGE. Apart from processing time, different treatment methods will also affect the immunomodulatory effect of RG. Kim et al. [70] observed the immunomodulatory effects of probiotic-fermented eRG (FRG), water-extracted RG (WRG), enzyme-treated eRG (ERG), and 50% ethanol-extracted RG (eRG) on CY-induced immunosuppressive mice. WRG, eRG, ERG, and FRG increased the levels of blood IFN-γ and white blood cells in CY-induced immunosuppressive mice and promoted the differentiation of Treg cells (except for WRG) and Th1 cells (especially FRG and WRG). Among them, FRG showed the strongest activity, which may have been caused by RG fermentation by probiotics, which promoted a better absorption of ginsenoside Rd and CK in the blood than that of unfermented RG. Ginsenoside Rg3, which is the most active natural biological component extracted from red ginseng, may be responsible for the immunostimulatory effect of red ginseng. The mechanism of the reversal effect of ginsenoside Rg3 on CY-induced immunosuppressive mice is related to the enhancement of immune organs indexes, phagocytes, and T lymphocytes [71]. Notably, the chemotherapy-induced imbalance of Th1/Th2 cells, which caused an imbalance of immune-related cytokines and eventually led to an abnormal immune system, was reversed by ginsenoside Rg3. This effect was caused by increased expression of T-bet and interferon-γ and decreased expression of the GATA-binding protein 3 and IL-4 in the thymus and the spleen. Similarly, (20S)-ginsenoside Rh2 and its main metabolites including (24R)-pseudo-ginsenoside HQ and (24S)-pseudo-ginsenoside HQ balanced spleen T lymphocyte subsets and serum cytokines levels in CY-treated immunosuppressive mice [67].
3.6.3. Immunomodulation mechanisms of PG and ginsenosides on chemotherapy-induced immunotoxicity
The mechanisms of PG and its ginsenosides in alleviating immunotoxicity are related to anti-oxidative, anti-inflammatory, and fatty acids-regulatory activities. Chen et al. reported that the protein expressions of Nrf2, HO-1, NQO1, and other antioxidant enzymes were increased by PGRT and PGFR treatments in the spleen, which indicated that Nrf2/HO-1 and Nrf2/NQO1 signaling played an essential role in alleviating immunosuppression in CY-treated mice [66]. Meanwhile, the anti-inflammatory effect is an important way for white ginseng extract to restore CY-induced immunosuppression, and the underlying mechanism is related to the activation of the mitogen-activated protein kinase kinase 4(MKK4)/JNK pathway [72]. The experimental results revealed that WRG not only increased the NO level by up-regulating inducible nitric oxide synthase expression and the expression of cytokines in INF-γ-induced macrophages but also enhanced natural killer cell activity and the proliferation of B- and T lymphocytes in the CY-induced immunosuppressive mouse model. Furthermore, the phosphorylation of MKK4 and JNK in the spleen was observed in INF-γ-induced macrophages. Additionally, because studies have found that metabolic dysregulation is one of the triggers leading to immune disorders [73], Qian et al. investigated the relationship between the immunoregulative effect and the metabolism-boosting benefits of ginsenoside Rh2 [74] and found that ginsenoside Rh2 modulated fatty acid metabolism by targeting the PI3K/AKT pathway to improve immunodeficiency, thus blocking the transcriptional regulation of fatty acid synthase by the sterol regulatory element binding protein 1 (SREBP-1). Moreover, the results revealed that ginsenoside Rh2 triggered the oxidative decomposition of fatty acids, inhibited fatty acids synthesis, increased ketone levels, and reduced corticosteroid secretion.
3.7. Effects of PG on chemotherapy-induced hepatotoxicity
The central role of the liver in drug metabolism makes it vulnerable to toxic injury. Chemotherapeutic drugs, such as CP and CY, can cause hepatotoxicity, and the mechanisms involved include oxidative stress, apoptosis, and inflammation. Notably, PG and its ginsenosides with enhanced antioxidant, anti-apoptosis, and anti-inflammatory activities were proven to attenuate chemotherapeutic hepatotoxicity (Table 5).
Table 5.
Therapeutic Effects of PG and its ginsenosides on chemotherapy-induced hepatotoxicity in vivo
| Treatment | Experimental mode | Effects | Monitored indices | Mechanism | Reference |
|---|---|---|---|---|---|
| PG | CP-treated male albino rats | Improvement of hepatic dysfunctions and damage | (↓) ALT, AST; histological changes of liver | [119] | |
| PG | CY-treated male albino rats | Improvement of hepatic dysfunctions and damage; antioxidant effect; anti-apoptotic effect; anti-inflammatory effect | (↓) ALT, AST, ALP; histological changes of liver | (↓) MDA, TNF-α, IL-1β, caspase3 (↑) CAT, SOD, GPX, Bcl-2 |
[120] |
| PG | CY-treated male Sprague-Dawley rats | Improvement of hepatic dysfunctions and damage; antioxidant effect; anti-inflammatory effect; regulation of the disordered homeostasis of GSH and bile acid | (↓) ALT, AST, ALP; histological changes of liver | (↓) MDA; IL-6, IL-10 (↑) GSH, CAT, SOD, GPO, Nrf2, bile acids homeostasis, GCLC, GCLM, GS, GST, FXR, CYP7A1, NTCP, MRP 3 |
[121] |
| Ginsenoside Rg1 | CP-treated C57BL/6 male mice | Improvement of hepatic dysfunctions and damage; antioxidant effect | (↓) ALT, AST, ALP; histological changes of liver | (↓) LDH, ROS; Keap1 activity (↑) GSH, Nrf2, p62, JNK |
[122] |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; CP, cisplatin; CY, cyclophosphamide; CAT, catalase; CYP7A1, cholesterol 7-alpha-hydroxylase; FXR, farnesoid X receptor; GPX, glutathione peroxidase; GSH, glutathione; GPO, glutathione peroxidase; GCLC, glutamate cysteine ligase catalytic subunit; GCLM, glutamate cysteine ligase modifier subunit; GS, GSH synthase; GST, GSH transfer enzyme; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-10, interleukin-10; JNK, c-jun N-terminal kinase; LDH, lactate dehydrogenase; MRP 3, multi-drug resistance-associated protein 3; NTCP, Na + -taurocholate-cotransporting protein; Nrf2, nuclear factor erythroid related factor 2; PG, Panax ginseng; ROS, reactive oxygen species; SOD, superoxide dismutase; TNF-α, tumor necrosis factor-α
Based on experimental evidence, PG and its ginsenosides exhibit a promising hepatoprotective feature. Alrashed et al. discovered that CP-induced hepatotoxicity was alleviated by PG. In CP-induced liver-damaged rats, PG decreased the levels of serum alanine aminotransferase and aspartate aminotransferase while alleviating hepatic damage [75]. Another study discovered that the hepatoprotective potential of PG was correlated with its antioxidant, anti-apoptotic, and anti-inflammatory effects in CY-treated rats [76]. PG decreased pro-inflammatory genes, caspase-3, and MDA levels while enhancing the expressions of Bcl-2 and antioxidant enzymes. Notably, other researchers have conducted studies on the antioxidant mechanism of PG and its ginsenosides and found that the Nrf2 pathway provided a vital role in their anti-oxidative effect. Zhu et al. [77] revealed that PG protected against CY-induced hepatotoxicity by increasing Nrf-2 expression and regulating the dynamic balance of glutathione and bile acid. In addition, Gao and his coworkers [78] reported that ginsenoside Rg1 effectively protected against CP-induced hepatotoxicity mainly by inhibiting the binding of Keap1 and Nrf2, partly through p62 accumulation. More importantly, ginsenoside Rg1 could prevent CP-induced hepatotoxicity by increasing the production of Nrf2-related antioxidant proteins in mice.
3.8. Effects of PG and ginsenosides on other chemotherapy-induced side effects
3.8.1. Effects of PG and ginsenosides on ototoxicity caused by chemotherapy
Chemotherapy-induced ototoxicity is characterized by irreversible sensorineural hearing loss. Korean PG showed protective effects against CP-induced ototoxicity by exhibiting anti-apoptotic, antioxidant, and anti-inflammatory effects. In the ototoxicity model of CP-induced auditory cell line House Ear Institute-Organ of Corti 1 (HEI-OC1), Korean PG reduced the CP-induced increase of ROS and suppressed the CP-stimulated expressions of caspase-3 and poly-ADP-ribose polymerase, which reflected the antioxidant and anti-apoptotic effects of Korean PG [79]. Kim et al. further studied the mechanism of Korean PG on the toxicity of CP to HEI-OC1 auditory cells in vitro and found that Korean PG did not have antioxidant, anti-apoptotic effects, and anti-inflammatory effects [80]. In an in vivo study, CP-induced hearing threshold changes in mice and damage to the hair cell array in primary Corti explants in rats could be prevented by Korean PG. In an in vitro study, Korean PG suppressed ROS production, the activation of the pro-apoptotic factor, and pro-inflammatory factors in HEI-OC1 auditory cell lines. Interestingly, one study reported that Korean PG at a low dose has the most obvious antagonistic effect on CP-induced ototoxicity in rats, especially in spiral ganglion neurons and brainstem neurons [81]. In an in vitro study on the CP-treated HEI-OC1 cell line, Korean PG alleviated CP-induced ototoxicity by decreasing apoptotic gene expression while increasing anti-apoptotic gene expression. In the rat model of CP-induced ototoxicity, low dose Korean PG could significantly antagonize CP-induced ototoxicity in rats, especially in spiral ganglion neurons and brainstem neurons, which reveals its potential protection activity against ototoxicity after chemotherapy treatment.
3.8.2. Effects of PG on adverse reactions in testicular toxicity caused by chemotherapy
Testicular toxicity is one of the reasons that clinical chemotherapy is restricted and may lead to azoospermia and oligospermia, thus affecting fertility. ADM is a common chemotherapeutic drug with various side effects, including testicular toxicity. Kang et al. reported that ginseng intestinal metabolite-I had a protective effect against ADM-induced testicular toxicity induced by [82]. Doxorubicin-induced reduction in body weight, spermatogenic activity (Sertoli cells, re-proliferation, and the epididymal index), serum lactate dehydrogenase, and creatine phosphokinase levels can be restored by GIM. Additionally, GIM attenuated germ cell injury and decreased the mRNA of phospholipid hydroperoxide glutathione peroxidase.
3.8.3. Effects of PG on fatigue, hair loss, and cachexia caused by chemotherapy
A variety of “disease behaviors” of CP in rats, including weight loss, hypothermia, fatigue, and hyperalgesia, mimic several aspects of cachexia as experienced by patients receiving chemotherapy. The PG extract had a protective effect on cachexia model rats, showing that it prevented CP-induced weight loss, hypothermia, hyperalgesia, and reduced running time [83]. Additionally, a study on cancer-related fatigue was conducted, and it was found that BST204, a PG-purified dry extract, might improve cancer-related fatigue by regulating inflammation and hematopoiesis [84]. In the behavioral data, running wheel activity, and forced swimming time increased because of the BST204 treatment. Additionally, BST204 not only increased the level of muscle glycogen activity and blood routine index but also decreased the serum levels of pro-inflammatory cytokines.
Chemotherapy-induced hair loss (CIA) is one of the most disturbing adverse reactions because it causes negative psychological perceptions in patients. The characteristic signs of CIA include decreased proliferation and increased apoptosis of hair matrix keratinocytes, suppression of hair growth, and premature catagen development. Korean PG suppressed these characteristic signs of CIA in the 4-hydroperoxycyclophosphamide-induced alopecia model. Additionally, Korean PG restored apoptosis-related protein expression induced by 4-hydroperoxycyclophosphamide in hair follicle cells, which means that the inhibitory effect of Korean PG on CIA might be related to the anti-apoptotic property [85].
4. Discussion
As a traditional Chinese medicine, PG has been used for thousands of years. Ginsenosides may be the efficacy basis of PG. In this paper, the therapeutic effects of PG and ginsenosides on adverse reactions of chemotherapy are reviewed (Fig. 1). The underlying mechanism may be related to an increase of antioxidant enzymes and anti-apoptotic, anti-inflammatory signals and immunostimulatory factors and a decrease in the pro-apoptotic, pro-inflammatory, immunosuppressive, and pro-oxidative indexes. Further study found that the mechanism involved multiple signal pathways, such as Nrf2/HO-1, P62/keap1/Nrf2, JNK/P53/caspase3, MEK/ERK, AMPK/mTOR, MKK4/JNK, and PI3K/Akt (Fig. 2). Notably, among the ginsenosides reviewed in this paper, ginsenoside Rg3, as a rare product produced during processing, exhibits promising therapeutic efficiency against various adverse chemotherapy reactions, including nephrotoxicity, cardiotoxicity, immunotoxicity, and hematopoietic inhibition, which is in line with the clinical results [14,15]. Ginsenoside Rg3, as an approved drug, is applicable in the treatment of chemotherapy-induced side effects.
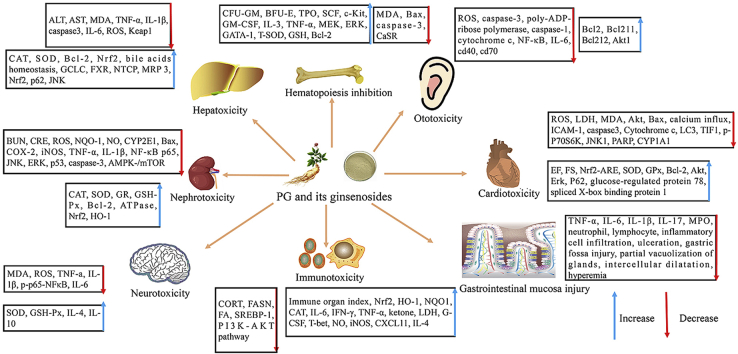
Fig. 1.
Effects of PG and its ginsenosides on the side effects caused by chemotherapy. PG and its ginsenosides were used to treat chemotherapy-induced side effects, including nephrotoxicity, gastrointestinal mucosa injury, cognitive impairment, myelosuppression, cardiotoxicity, immunotoxicity, hepatoxicity, and ototoxicity, mainly through the regulation of factors and signaling pathways related to oxidation, apoptosis, inflammation, autophagy, and immunity.
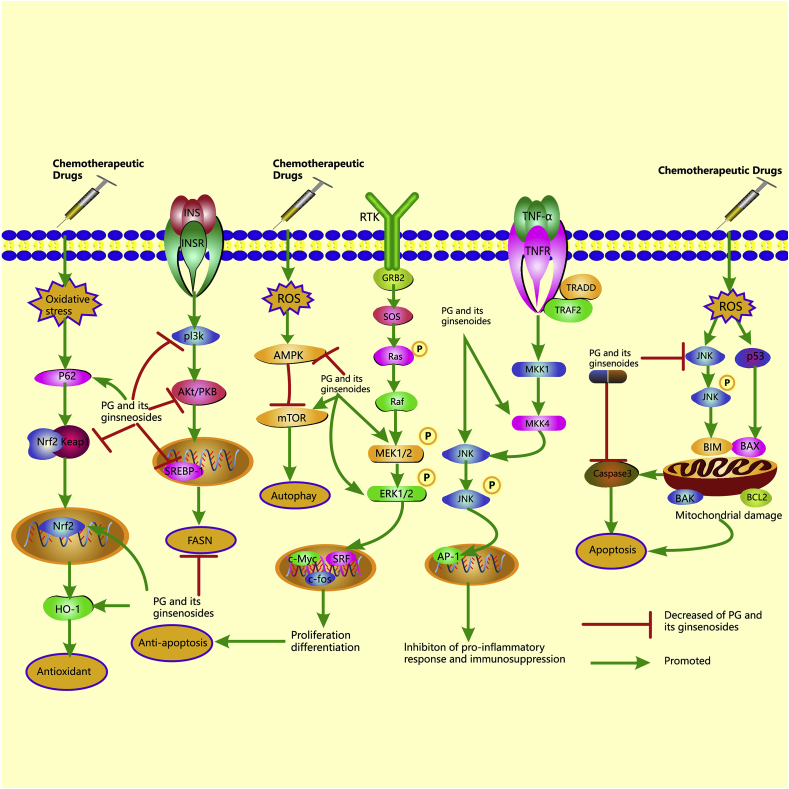
Fig. 2.
The molecular protective mechanisms of PG and its ginsenosides against chemotherapy-induced side effects. PG or its ginsenosides can regulate Nrf2/HO-1, P62/keap1/Nrf2, JNK/P53/caspase3, MEK/ERK, AMPK/mTOR, MKK4/JNK, and PI3K/Akt signaling pathways. Nrf2/HO-1 and P62/keap1/Nrf2 signaling pathways are classic antioxidant pathways. PG or its ginsenosides exert antioxidant effects by up-regulating P62, Nrf2, and HO-1 expressions and down-regulating keap1 expression. JNK/P53/caspase3 and MEK/ERK signaling pathways are related to cell apoptosis. PG or its ginsenosides show anti-apoptosis effects by inhibiting the activation of JNK, P53, and caspase3 and activating the expressions of MEK and EKR. AMPK/mTOR signaling pathway plays a crucial role in the genesis and progression of autophagy. PG or its ginsenosides regulate AMPK/mTOR pathways by increasing mTOR expressions and suppressing AMPK expressions. MKK4/JNK is an essential pathway by which PG and its ginsenosides alleviate chemotherapy-induced immunosuppression. PG and its ginsenosides regulate MKK4/JNK pathways by promoting MKK4 and JNK expressions. The regulation of fatty acid synthase (FASN) is associated with immunomodulatory effects. PG or its ginsenosides regulate FASN by inhibiting the activation of PI3K, AKT, and SREBP-1.
Additionally, although PG and ginsenosides can alleviate the side effects caused by chemotherapy, some shortcomings still cannot be ignored. The exact mechanism of PG and its ginsenosides is unclear, which may be attributed to the underlying mechanism involving multiple pathways and targets. Therefore, additional research methods, such as omics technologies (e.g., genomics, metabolomics, proteomics, transcriptomics), should be applied. By using these methods, researchers can study tissue and cellular structures, genes, proteins, and their interactions with molecules and then elucidate the exact mechanism of PG and its ginsenosides. In addition, attention should be paid to molecular docking technology. A recent study [86] simulated the docking of ginsenosides and anti-apoptotic proteins and found that ginsenosides, especially Rg1, Rg3, Rh2, and Rf, could be effective compounds for reducing the overexpression of anti-apoptotic proteins (such as BCL-2, BCL-XL, and MCL-1). However, this novel method has not been applied to help us understand effect of PG and its ginsenosides on chemotherapy-caused side effects. Moreover, the intestinal flora has become a hotspot in research on drug mechanisms. Several studies have shown that natural products can treat diseases by affecting the intestinal flora [87,88]. However, so far, whether PG and its ginsenosides regulate the side effects of chemotherapy through intestinal flora is unclear. Notably, the oral bioavailability of ginsenosides is low because of their high molecular weight, low water solubility, and poor gastrointestinal stability. Thus, the bioavailability of ginsenosides should be improved by establishing effective drug delivery systems and various drug delivery routes, including micelles and emulsion drug delivery systems. Moreover, studies have reported that the inhibitory actions of PG and its ginsenosides on the side effects of chemotherapy were mostly limited to animals and cells. To elucidate the role and mechanism of PG and its ginsenosides in inhibiting the side effects induced by chemotherapy in humans, longitudinal human studies are warranted to evaluate their effectiveness, safety, and tolerance.
5. Conclusion
This review briefly summarizes the therapeutic potential of PG and its ginsenosides in reducing the side effects of chemotherapy in vivo and in vitro and explains the mechanisms by which PG and its ginsenosides alleviate chemotherapy-induced side effects. The underlying molecular mechanism can be partially elucidated by the roles of the Nrf2/HO-1, P62/keap1/Nrf2, JNK/P53/caspase3, MEK/ERK, AMPK/mTOR, MKK4/JNK, and PI3K/Akt signaling pathways. In conclusion, because of their less desirable side effects and anti-oxidative, anti-inflammatory, and anti-apoptotic actions, PG and its ginsenosides are potential agents for the prevention and treatment of chemotherapy-induced side effects.
Funding
This work was supported by Program of National Natural Science Foundation of China (81503272,81630101), Application Foundation Research Project of Sichuan Provincial Department of Science and Technology (2017JY0187), and Xinglin Scholar Research Premotion Project of Chengdu University of TCM (2018016).
Author contributions
Yan Wan contributed to the drafting of the manuscript. Hui Ao and Cheng Peng obtained funding, designed, conceived and supervised process, and revised the manuscript. Others were involved in searching, screening the search results, translation, and data collection. All the authors have read and approved the final manuscript.
Declaration of competing interest
All authors declare no conflict of interest regarding the present work and they have no involvements that might raise the question of bias in the work reported or in the conclusions, implications or opinions stated.
Contributor Information
Yan Wan, Email: 15592038985@163.com.
Jing Wang, Email: wangjing2018@foxmail.com.
Jin-feng Xu, Email: 15008423324@163.com.
Fei Tang, Email: tangfei0227@163.com.
Lu Chen, Email: chenlu@cdutcm.edu.cn.
Yu-zhu Tan, Email: tanyuzhu@cdutcm.edu.cn.
Chao-long Rao, Email: 184950883@qq.com.
Hui Ao, Email: aohui2005@126.com.
Cheng Peng, Email: pengchengcxy@126.com.
References
- 1.Gaguski M.E., Karcheski T. Dosing done right: a review of common chemotherapy calculations. Clin J Oncol Nurs. 2011;15:471–473. doi: 10.1188/11.CJON.471-473. [DOI] [PubMed] [Google Scholar]
- 2.Joy J., Nair C.K. Amelioration of cisplatin induced nephrotoxicity in Swiss albino mice by Rubia cordifolia extract. J Cancer Res Ther. 2008;4:111–115. doi: 10.4103/0973-1482.43139. [DOI] [PubMed] [Google Scholar]
- 3.Navari R.M. Management of chemotherapy-induced nausea and vomiting: focus on newer agents and new uses for older agents. Drugs. 2013;73:249–262. doi: 10.1007/s40265-013-0019-1. [DOI] [PubMed] [Google Scholar]
- 4.Guo S., Guo Y., Ergun A., Lu L., Walker W.A., Ganguli K. Secreted Metabolites of Bifidobacterium infantis and Lactobacillus acidophilus protect immature human enterocytes from IL-1β-induced inflammation: a transcription profiling analysis. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan C.W., Chang A.M., Molassiotis A., Lee I.Y., Lee G.C. Oral complications in Chinese cancer patients undergoing chemotherapy. Support Care Cancer. 2003;11:48–55. doi: 10.1007/s00520-002-0413-9. [DOI] [PubMed] [Google Scholar]
- 6.Souglakos J., Mavroudis D., Kakolyris S., Kourousis Ch, Vardakis N., Androulakis N., Agelaki S., Kalbakis K., Tsetis D., Athanasiadis N. Triplet combination with irinotecan plus oxaliplatin plus continuous-infusion fluorouracil and leucovorin as first-line treatment in metastatic colorectal cancer: a multicenter phase II trial. J Clin Oncol. 2002;20:2651–2657. doi: 10.1200/JCO.2002.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Jodrell D.I., Stewart M., Aird R., Knowles G., Bowman A., Wall L., McLean C. 5-fluorouracil steady state pharmacokinetics and outcome in patients receiving protracted venous infusion for advanced colorectal cancer. Br J Cancer. 2001;84:600–603. doi: 10.1054/bjoc.2000.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magdy T., Burmeister B.T., Burridge P.W. Validating the pharmacogenomics of chemotherapy-induced cardiotoxicity: what is missing? Pharmacol Ther. 2016;168:113–125. doi: 10.1016/j.pharmthera.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alberts D.S. Protection by amifostine of cyclophosphamide-induced myelosuppression. Semin Oncol. 1999;26:37–40. [PubMed] [Google Scholar]
- 10.Launay-Vacher V., Rey J.B., Isnard-Bagnis C., Deray G., Daouphars M. European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European society of clinical pharmacy special interest group on cancer care. Cancer Chemother Pharmacol. 2008;61:903–909. doi: 10.1007/s00280-008-0711-0. [DOI] [PubMed] [Google Scholar]
- 11.Kim H.S., Kim M.K., Lee M., Kwon B.S., Suh D.H., Song Y.S. Effect of red ginseng on genotoxicity and health-related quality of life after adjuvant chemotherapy in patients with epithelial ovarian cancer: a randomized, double blind, placebo-controlled trial. Nutrients. 2017;9(7):772. doi: 10.3390/nu9070772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao Z., Wang C., Zhou M., Hu S., Jiang Y., Huang X., Li N., Feng J., Tang F., Chen X. Clinical efficacy and safety of Aidi injection plus paclitaxel-based chemotherapy for advanced non-small cell lung cancer: a meta-analysis of 31 randomized controlled trials following the PRISMA guidelines. J Ethnopharmacol. 2019;228:110–122. doi: 10.1016/j.jep.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Huang J.Y., Sun Y., Fan Q.X., Zhang Y.Q. Efficacy of Shenyi Capsule combined with gemcitabine plus cisplatin in treatment of advanced esophageal cancer: a randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2009;7:1047–1051. doi: 10.3736/jcim20091105. [DOI] [PubMed] [Google Scholar]
- 14.Pan L., Zhang T., Cao H., Sun H., Liu G. Ginsenoside Rg3 for chemotherapy-induced Myelosuppression: a meta-analysis and systematic review. Front Pharmacol. 2020;11:649. doi: 10.3389/fphar.2020.00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jo E.J., Kang S.J., Kim A.J. Effects of steam-and dry-processing temperatures on the benzo (a) pyrene content of black and red ginseng. The Korean Journal of Food And Nutrition. 2009;22:199–204. [Google Scholar]
- 16.Manohar S., Leung N. Cisplatin nephrotoxicity: a review of the literature. J Nephrol. 2018;31:15–25. doi: 10.1007/s40620-017-0392-z. [DOI] [PubMed] [Google Scholar]
- 17.Miller R.P., Tadagavadi R.K., Ramesh G., Reeves W.B. Mechanisms of cisplatin nephrotoxicity. Toxins. 2010;2:2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baek S.H., Piao X.L., Lee U.J., Kim H.Y., Park J.H. Reduction of cisplatin-induced nephrotoxicity by ginsenosides isolated from processed ginseng in cultured renal tubular cells. Biol Pharm Bull. 2006;29:2051–2055. doi: 10.1248/bpb.29.2051. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z., Li Y.F., Han X.Y., Sun Y.S., Zhang L.X., Liu W., Liu X.X., Li W., Liu Y.Y. Kidney protection effect of ginsenoside Re and its underlying mechanisms on cisplatin-induced kidney injury. Cell Physiol Biochem. 2018;48:2219–2229. doi: 10.1159/000492562. [DOI] [PubMed] [Google Scholar]
- 20.Baek S.H., Shin B.K., Kim N.J., Chang S.Y., Park J.H. Protective effect of ginsenosides Rk3 and Rh4 on cisplatin-induced acute kidney injury in vitro and in vivo. J Ginseng Res. 2017;41:233–239. doi: 10.1016/j.jgr.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W., Yan M.H., Liu Y., Liu Z., Wang Z., Chen C., Zhang J., Sun Y.S. Ginsenoside Rg5 ameliorates cisplatin-Induced nephrotoxicity in mice through inhibition of inflammation, oxidative Stress, and apoptosis. Nutrients. 2016;8:566. doi: 10.3390/nu8090566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi Z., Li W., Tan J., Wang C., Lin H., Zhou B., Liu J., Li P. Effect of ginsenoside Rh2 on renal apoptosis in cisplatin-induced nephrotoxicity in vivo. Phytomedicine. 2019;61:152862. doi: 10.1016/j.phymed.2019.152862. [DOI] [PubMed] [Google Scholar]
- 23.Lee C.K., Park K.K., Chung A.S., Chung W.Y. Ginsenoside Rg3 enhances the chemosensitivity of tumors to cisplatin by reducing the basal level of nuclear factor erythroid 2-related factor 2-mediated heme oxygenase-1/NAD(P)H quinone oxidoreductase-1 and prevents normal tissue damage by scavenging cisplatin-induced intracellular reactive oxygen species. Food Chem Toxicol. 2012;50:2565–2574. doi: 10.1016/j.fct.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Yousef M.I., Hussien H.M. Cisplatin-induced renal toxicity via tumor necrosis factor-α, interleukin 6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: protective effect of ginseng. Food Chem Toxicol. 2015;78:17–25. doi: 10.1016/j.fct.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Yokozawa T., Liu Z.W. The role of ginsenoside-Rd in cisplatin-induced acute renal failure. Ren Fai. 2000;22:115–127. doi: 10.1081/jdi-100100858. [DOI] [PubMed] [Google Scholar]
- 26.Yokozawa T., Dong E. Role of ginsenoside-Rd in cisplatin-induced renal injury: special reference to DNA fragmentation. Nephron. 2001;89:433–438. doi: 10.1159/000046116. [DOI] [PubMed] [Google Scholar]
- 27.Hu J.N., Xu X.Y., Jiang S. Protective effect of ginsenoside Rk1, a major rare saponin from black ginseng, on cisplatin-induced nephrotoxicity in HEK-293 cells. Kaohsiung J Med Sci. 2020;36:732–740. doi: 10.1002/kjm2.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han M.S., Han I.H., Lee D., An J.M., Kim S.N., Shin M.S., Yamabe N., Hwang G.S., Yoo H.H., Choi S.J. Beneficial effects of fermented black ginseng and its ginsenoside 20(S)-Rg3 against cisplatin-induced nephrotoxicity in LLC-PK1 cells. J Ginseng Res. 2016;40:135–140. doi: 10.1016/j.jgr.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J.Y., Choi P., Kim T., Ko H., Kim H.K., Kang K.S., Ham J. Protective effects of processed ginseng and its active ginsenosides on cisplatin-induced nephrotoxicity: in vitro and in vivo studies. J Agric Food Chem. 2015;63:5964–5969. doi: 10.1021/acs.jafc.5b00782. [DOI] [PubMed] [Google Scholar]
- 30.Lee H.L., Kang K.S. Protective effect of ginsenoside Rh3 against anticancer drug-induced apoptosis in LLC-PK1 kidney cells. J Ginseng Res. 2017;41:227–231. doi: 10.1016/j.jgr.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xing J.J., Hou J.G., Ma Z.N., Wang Z., Ren S., Wang Y.P., Liu W.C., Chen C., Li W. Ginsenoside Rb3 provides protective effects against cisplatin-induced nephrotoxicity via regulation of AMPK-/mTOR-mediated autophagy and inhibition of apoptosis in vitro and in vivo. Cell Prolif. 2019;52 doi: 10.1111/cpr.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe S., Suemaru K., Yamaguchi T., Hidaka N., Sakanaka M., Araki H. Effect of oral mucosal adhesive films containing ginsenoside Rb1 on 5-fluorouracil-induced oral mucositis in hamsters. Eur J Pharmacol. 2009;616:281–286. doi: 10.1016/j.ejphar.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Wang J., Feng W., Zhang S., Chen L., Tang F., Sheng Y., Ao H., Peng C. Gut microbial modulation in the treatment of chemotherapy-induced diarrhea with Shenzhu Capsule. BMC Complement Altern Med. 2019;19:126. doi: 10.1186/s12906-019-2548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J., Feng W., Zhang S., Chen L., Sheng Y., Tang F., He J., Xu X., Ao H., Peng C. Ameliorative effect of Atractylodes macrocephala essential oil combined with Panax ginseng total saponins on 5-fluorouracil induced diarrhea is associated with gut microbial modulation. J Ethnopharmacol. 2019;238:111887. doi: 10.1016/j.jep.2019.111887. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki T., Yamamoto A., Ohsawa M., Motoo Y., Mizukami H., Makino T. Effect of ninjin’yoeito and ginseng extracts on oxaliplatin-induced neuropathies in mice. J Nat Med. 2017;71:757–764. doi: 10.1007/s11418-017-1113-6. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T., Yamamoto A., Ohsawa M., Motoo Y., Mizukami H., Makino T. Ninjin’yoeito and ginseng extract prevent oxaliplatin-induced neurodegeneration in PC12 cells. J Nat Med. 2015;69:531–537. doi: 10.1007/s11418-015-0921-9. [DOI] [PubMed] [Google Scholar]
- 37.Chen C., Zhang H., Xu H., Zheng Y., Wu T., Lian Y. Ginsenoside Rb1 ameliorates cisplatin-induced learning and memory impairments. J Ginseng Res. 2019;43:499–507. doi: 10.1016/j.jgr.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi D.D., Huang Y.H., Lai C.S.W., Dong C.M., Ho L.C., Li X.Y., Wu E.X., Li Q., Wang X.M., Chen Y.J. Ginsenoside Rg1 prevents Chemotherapy-induced cognitive impairment: associations with microglia-mediated cytokines, neuroinflammation, and neuroplasticity. Mol Neurobiol. 2019;56:5626–5642. doi: 10.1007/s12035-019-1474-9. [DOI] [PubMed] [Google Scholar]
- 39.Molyneux G., Andrews M., Sones W., York M., Barnett A., Quirk E., Yeung W., Turton J. Haemotoxicity of busulphan, doxorubicin, cisplatin and cyclophosphamide in the female BALB/c mouse using a brief regimen of drug administration. Cell Biol Toxicol. 2011;27:13–40. doi: 10.1007/s10565-010-9167-1. [DOI] [PubMed] [Google Scholar]
- 40.Han J., Dai M., Zhao Y., Cai E., Zhang L., Jia X., Sun N., Fei X., Shu H. Compatibility effects of ginseng and Ligustrum lucidum Ait herb pair on hematopoietic recovery in mice with cyclophosphamide-induced myelosuppression and its material basis. J Ginseng Res. 2020;44:291–299. doi: 10.1016/j.jgr.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang S., Sun H., Wang C., Zheng X., Jia X., Cai E., Zhao Y. Comparative analysis of active ingredients and effects of the combination of Panax ginseng and Ophiopogon japonicus at different proportions on chemotherapy-induced myelosuppression mouse. Food Funct. 2019;10:1563–1570. doi: 10.1039/c8fo02354a. [DOI] [PubMed] [Google Scholar]
- 42.Raghavendran H.R., Sathyanath R., Shin J., Kim H.K., Han J.M., Cho J., Son C.G. Panax ginseng modulates cytokines in bone marrow toxicity and myelopoiesis: ginsenoside Rg1 partially supports myelopoiesis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joo S.S., Won T.J., Kim M.S., Lee D.I. Hematopoietic effect of ginsenoside Rg3 in ICR mouse primary cultures and its application to a biological response modifier. Fitoterapia. 2004;75:337–341. doi: 10.1016/j.fitote.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z., Zheng Q., Liu K., Li G., Zheng R. Ginsenoside Rh2 enhances antitumour activity and decreases genotoxic effect of cyclophosphamide. Basic Clin Pharmacol Toxicol. 2006;98:411–415. doi: 10.1111/j.1742-7843.2006.pto_348.x. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q.H., Wu C.F., Duan L., Yang J.Y. Protective effects of total saponins from stem and leaf of Panax ginseng against cyclophosphamide-induced genotoxicity and apoptosis in mouse bone marrow cells and peripheral lymphocyte cells. Food Chem Toxicol. 2008;46:293–302. doi: 10.1016/j.fct.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q.H., Wu C.F., Yang J.Y., Mu Y.H., Chen X.X., Zhao Y.Q. Reduction of cyclophosphamide-induced DNA damage and apoptosis effects of ginsenoside Rb (1) on mouse bone marrow cells and peripheral blood leukocytes. Environ Toxicol Pharmacol. 2009;27:384–389. doi: 10.1016/j.etap.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Q.H., Wu C.F., Duan L., Yang J.Y. Protective effects of ginsenoside Rg (3) against cyclophosphamide-induced DNA damage and cell apoptosis in mice. Arch Toxicol. 2008;82:117–123. doi: 10.1007/s00204-007-0224-3. [DOI] [PubMed] [Google Scholar]
- 48.Han J., Wang Y., Cai E., Zhang L., Zhao Y., Sun N., Zheng X., Wang S. Study of the effects and mechanisms of ginsenoside Compound K on myelosuppression. J Agric Food Chem. 2019;67:1402–1408. doi: 10.1021/acs.jafc.8b06073. [DOI] [PubMed] [Google Scholar]
- 49.Sun X., Zhao Y.N., Qian S., Gao R.L., Yin L.M., Wang L.P., Chong B.H., Zhang S.Z. Ginseng-derived panaxadiol saponins promote hematopoiesis recovery in cyclophosphamide-induced myelosuppressive mice: potential novel treatment of chemotherapy-induced cytopenias. Chin J Integr Med. 2018;24:200–206. doi: 10.1007/s11655-017-2754-8. [DOI] [PubMed] [Google Scholar]
- 50.Han J., Xia J., Zhang L., Cai E., Zhao Y., Fei X., Jia X., Yang H., Liu S. Studies of the effects and mechanisms of ginsenoside Re and Rk3 on myelosuppression induced by cyclophosphamide. J Ginseng Res. 2019;43:618–624. doi: 10.1016/j.jgr.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang Y., Xu S., Xu Q., Liu X., Gao Y., Steinmetz A., Wang N., Wang T., Qiu G. Protective effect of dammarane sapogenins against chemotherapy-induced myelosuppression in mice. Exp Biol Med. 2011;236:729–735. doi: 10.1258/ebm.2011.010369. [DOI] [PubMed] [Google Scholar]
- 52.Xu S.F., Yu L.M., Fan Z.H., Wu Q., Yuan Y., Wei Y., Fang N. Improvement of ginsenoside Rg1 on hematopoietic function in cyclophosphamide-induced myelosuppression mice. Eur J Pharmacol. 2012;695:7–12. doi: 10.1016/j.ejphar.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 53.Liu H.H., Chen F.P., Liu R.K., Lin C.L., Chang K.T. Ginsenoside Rg1 improves bone marrow haematopoietic activity via extramedullary haematopoiesis of the spleen. J Cell Mol Med. 2015;19:2575–2586. doi: 10.1111/jcmm.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang H., Yu P., Gou H., Zhang J., Zhu M., Wang Z.H., Tian J.W., Jiang Y.T., Fu F.H. Cardioprotective effects of 20(S)-ginsenoside Rh2 against doxorubicin-induced cardiotoxicity in vitro and in vivo. Evid Based Complement Alternat Med. 2012;2012:506214. doi: 10.1155/2012/506214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X., Chen L., Wang T., Jiang X., Zhang H., Li P., Lv B., Gao X. Ginsenoside Rg3 antagonizes ADM-induced cardiotoxicity by improving endothelial dysfunction from oxidative stress via upregulating the Nrf2-ARE pathway through the activation of akt. Phytomedicine. 2015;22:875–884. doi: 10.1016/j.phymed.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 56.Li L., Ni J., Li M., Chen J., Han L., Zhu Y., Kong D., Mao J., Wang Y., Zhang B. Ginsenoside Rg3 micelles mitigate doxorubicin-induced cardiotoxicity and enhance its anticancer efficacy. Drug Deliv. 2017;24:1617–1630. doi: 10.1080/10717544.2017.1391893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.You J.S., Huang H.F., Chang Y.L. Panax ginseng reduces adriamycin-induced heart failure in rats. Phytother Res. 2005;19:1018–1022. doi: 10.1002/ptr.1778. [DOI] [PubMed] [Google Scholar]
- 58.Volkova M., Palmeri M., Russell K.S., Russell R.R. Activation of the aryl hydrocarbon receptor by doxorubicin mediates cytoprotective effects in the heart. Cardiovasc Res. 2011;90:305–314. doi: 10.1093/cvr/cvr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu X., Chen K., Kobayashi S., Timm D., Liang Q. Resveratrol attenuates doxorubicin-induced cardiomyocyte death via inhibition of p70 S6 kinase 1-mediated autophagy. J Pharmacol Exp Ther. 2012;341:183–195. doi: 10.1124/jpet.111.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu C., Wang Y., Liu H., Mu H., Lu Y., Zhang J., Huang J. Oral administration of Ginsenoside Rg1 prevents cardiac toxicity induced by doxorubicin in mice through anti-apoptosis. Oncotarget. 2017;8:83792–83801. doi: 10.18632/oncotarget.19698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y., Wang Y., Ma Z., Liang Q., Tang X., Tan H., Xiao C., Gao Y. Ginsenoside Rb1 inhibits doxorubicin-triggered H9C2 cell apoptosis via aryl hydrocarbon receptor. Biomol Ther. 2017;25:202–212. doi: 10.4062/biomolther.2016.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li L.F., Ma Z.C., Wang Y.G., Tang X.L., Tan H.L., Xiao C.R., Gao Y. Protective effect of ginsenoside Rb1 on adriamycin-induced cardiomyocyte autophagy. China J Chin Mater Med. 2017;42:1365–1369. doi: 10.19540/j.cnki.cjcmm.20170222.009. [DOI] [PubMed] [Google Scholar]
- 63.Rashid H.O., Yadav R.K., Kim H.R., Chae H.J. ER stress: autophagy induction, inhibition and selection. Autophagy. 2015;11:1956–1977. doi: 10.1080/15548627.2015.1091141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu Z.M., Li C.B., Liu Q.L., Li P., Yang H. Ginsenoside Rg1 prevents doxorubicin-induced cardiotoxicity through the inhibition of autophagy and endoplasmic reticulum stress in mice. Int J Mol Sci. 2018;19:3658. doi: 10.3390/ijms19113658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Turk J.L., Parker D. Effect of cyclophosphamide on immunological control mechanisms. Immunol Rev. 1982;65:99–113. doi: 10.1111/j.1600-065x.1982.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 66.Chen L.X., Qi Y.L., Qi Z., Gao K., Gong R.Z., Shao Z.J., Liu S.X., Li S.S., Sun Y.S. A comparative study on the effects of different parts of Panax ginseng on the immune activity of cyclophosphamide-induced immunosuppressed mice. Molecules. 2019;24:1096. doi: 10.3390/molecules24061096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qi Z., Chen L., Li Z., Shao Z., Qi Y., Gao K., Liu S., Sun Y., Li P., Liu J. Immunomodulatory effects of (24R)-pseudo-ginsenoside HQ and (24S)-pseudo-ginsenoside HQ on cyclophosphamide-induced immunosuppression and their anti-tumor effects study. Int J Mol Sci. 2019;20:836. doi: 10.3390/ijms20040836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin G., Yu X., Wang J., Qu S., Sui D. Beneficial effects of 20(S)-protopanaxadiol on antitumor activity and toxicity of cyclophosphamide in tumor-bearing mice. Exp Ther Med. 2013;5:443–447. doi: 10.3892/etm.2012.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saba E., Lee Y.Y., Kim M., Kim S.H., Hong S.B., Rhee M.H. A comparative study on immune-stimulatory and antioxidant activities of various types of ginseng extracts in murine and rodent models. J Ginseng Res. 2018;42:577–584. doi: 10.1016/j.jgr.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim J.K., Kim J.Y., Jang S.E., Choi M.S., Jang H.M., Yoo H.H., Kim D.H. Fermented red ginseng alleviates cyclophosphamide-induced immunosuppression and 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice by regulating macrophage activation and T cell differentiation. Am J Chin Med. 2018;46:1879–1897. doi: 10.1142/S0192415X18500945. [DOI] [PubMed] [Google Scholar]
- 71.Liu X., Zhang Z., Liu J., Wang Y., Zhou Q., Wang S., Wang X. Ginsenoside Rg3 improves cyclophosphamide-induced immunocompetence in Balb/c mice. Int Immunopharmacol. 2019;72:98–111. doi: 10.1016/j.intimp.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 72.Lim T.G., Jang M., Cho C.W., Hong H.D., Kim K.T., Lee S.Y., Jung S.K., Rhee Y.K. White ginseng extract induces immunomodulatory effects via the MKK4-JNK pathway. Food Sci Biotechnol. 2016;25:1737–1744. doi: 10.1007/s10068-016-0265-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wellenstein M.D., de Visser K.E. Cancer-cell-intrinsic mechanisms shaping the tumor immune landscape. Immunity. 2018;48:399–416. doi: 10.1016/j.immuni.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 74.Qian Y., Huang R., Li S., Xie R., Qian B., Zhang Z., Li L., Wang B., Tian C., Yang J. Ginsenoside Rh2 reverses cyclophosphamide-induced immune deficiency by regulating fatty acid metabolism. J Leukoc Biol. 2019;106:1089–1100. doi: 10.1002/JLB.2A0419-117R. [DOI] [PubMed] [Google Scholar]
- 75.Alrashed A.A., El-Kordy E.A. Possible protective role of panax ginseng on cisplatin-induced hepatotoxicity in adult male albino rats (Biochemical and Histological Study) J Microsc Ultrastruct. 2019;7:84–90. doi: 10.4103/JMAU.JMAU_4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdelfattah-Hassan A., Shalaby S.I., Khater S.I., El-Shetry E.S., Abd El Fadil H., Elsayed S.A. Panax ginseng is superior to vitamin E as a hepatoprotector against cyclophosphamide-induced liver damage. Complementary Therapies in Medicine. 2019;46:95–102. doi: 10.1016/j.ctim.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 77.Zhu H., Long M.H., Wu J., Wang M.M., Li X.Y., Shen H., Xu J.D., Zhou L., Fang Z.J., Luo Y. Ginseng alleviates cyclophosphamide-induced hepatotoxicity via reversing disordered homeostasis of glutathione and bile acid. Sci Rep. 2015;5:17536. doi: 10.1038/srep17536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao Y., Chu S., Shao Q., Zhang M., Xia C., Wang Y., Li Y., Lou Y., Huang H., Chen N. Antioxidant activities of ginsenoside Rg1 against cisplatin-induced hepatic injury through Nrf2 signaling pathway in mice. Free Radical Research. 2017;51:1–13. doi: 10.1080/10715762.2016.1234710. [DOI] [PubMed] [Google Scholar]
- 79.Im G.J., Chang J.W., Choi J., Chae S.W., Ko E.J., Jung H.H. Protective effect of Korean red ginseng extract on cisplatin ototoxicity in HEI-OC1 auditory cells. Phytother Res. 2010;24:614–621. doi: 10.1002/ptr.3082. [DOI] [PubMed] [Google Scholar]
- 80.Kim S.J., Kwak H.J., Kim D.S., Choi H.M., Sim J.E., Kim S.H., Um J.Y., Hong S.H. Protective mechanism of Korean red ginseng in cisplatin-induced ototoxicity through attenuation of nuclear factor-κB and caspase-1 activation. Mol Med Rep. 2015;12:315–322. doi: 10.3892/mmr.2015.3396. [DOI] [PubMed] [Google Scholar]
- 81.Olgun Y., Kırkım G., Altun Z., Aktaş S., Kolatan E., Kiray M., Bağrıyanık A., Olgun A., Çakır Kızmazoğlu D., Özoğul C. Protective Effect of Korean red ginseng on cisplatin ototoxicity: is it effective enough? J Int Adv Otol. 2016;12:177–183. doi: 10.5152/iao.2016.1989. [DOI] [PubMed] [Google Scholar]
- 82.Kang J., Lee Y., No K., Jung E., Sung J., Kim Y., Nam S. Ginseng intestinal metabolite-I (GIM-I) reduces doxorubicin toxicity in the mouse testis. Reprod Toxicol. 2002;16:291–298. doi: 10.1016/s0890-6238(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 83.Lobina C., Carai M.A., Loi B., Gessa G.L., Riva A., Cabri W., Petrangolini G., Morazzoni P., Colombo G. Protective effect of Panax ginseng in cisplatin-induced cachexia in rats. Future Oncol. 2014;10:1203–1214. doi: 10.2217/fon.13.276. [DOI] [PubMed] [Google Scholar]
- 84.Park H.J., Shim H.S., Kim J.Y., Kim J.Y., Park S.K., Shim I. Ginseng purified dry extract, BST204, improved cancer chemotherapy-related fatigue and toxicity in mice. Evid Based Complement Alternat Med. 2015;2015:197459. doi: 10.1155/2015/197459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Keum D.I., Pi L.Q., Hwang S.T., Lee W.S. Protective effect of Korean Red Ginseng against chemotherapeutic drug-induced premature catagen development assessed with human hair follicle organ culture model. J Ginseng Res. 2016;40:169–175. doi: 10.1016/j.jgr.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sathishkumar N., Sathiyamoorthy S., Ramya M., Yang D.U., Lee H.N., Yang D.C. Molecular docking studies of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides from Panax ginseng. J Enzyme Inhib Med Chem. 2012;27:685–692. doi: 10.3109/14756366.2011.608663. [DOI] [PubMed] [Google Scholar]
- 87.Feng W., Ao H., Peng C., Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. 2019;142:176–191. doi: 10.1016/j.phrs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 88.Feng W., Liu J., Ao H., Yue S.J., Peng C. Targeting gut microbiota for precision medicine: focusing on the efficacy and toxicity of drugs. Theranostics. 2020 doi: 10.7150/thno.47289. [DOI] [PMC free article] [PubMed] [Google Scholar]