Abstract
Immune checkpoint inhibitors have a significant role in oncology. One of these immune checkpoints is cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). Inhibition of the CTLA-4 pathway has already led to the FDA approval of Ipilimumab (anti-CTLA-4), a targeted therapy for melanoma and other malignancies. CD137 is an inducible, costimulatory receptor of the tissue-necrosis-factor-receptor superfamily expressed on the activated immune cells. Clinical trials have also been set for anti-CD137 in several malignancies. We assessed CTLA-4 and CD137 expression on a tissue microarray (TMA) comprising of 99 core tissues which included normal, non-neoplastic, and neoplastic cervical lesions. When detected as strong granular cytoplasmic reaction in the epithelial cells, CTLA-4 expression was scored as positive. For CD137, the results were recorded based on the presence or absence of staining reaction on the cell membranes of the lymphoplasmacytic infiltrates. Overall, CTLA-4 was positive in 30% (30/100) of the cervical malignancies. Sub-categorically, 20% of invasive endocervical adenocarcinomas, 63% of adenosquamous carcinomas, and 31% of squamous cell carcinomas were positive for CTLA-4 with a tendency toward lower grade squamous cell carcinomas (SCCs). CD137 was positive in 100% lymphoplasmacytic infiltrates of endocervical adenocarcinomas, 90.5% of SCCs, and 87.5% of adenosquamous carcinomas. This study has found a significant expression of CTLA-4 in cervical cancer cells and CD137 positivity of lymphoplasmacytic infiltrates with potential for future targeted immunotherapy.
Keywords: Immune checkpoint, cytotoxic T-lymphocyte-associated protein 4, CD137, PD-L1, cervix, carcinoma, cervix
Introduction
Among gynecologic malignancies, cervical carcinoma is the fourth most common cancer cause of death worldwide with an estimated 570,000 cases diagnosed and 311,000 deaths in 2018 [1]. Human papillomavirus (HPV) is central to the development of the cervical neoplasia and can be detected in most cervical cancers. The most common histologic types of cervical cancer include squamous cell carcinoma which is the major type (70%), and endocervical adenocarcinoma which is less common (about 25%); the other types or variants constitute 5% [2].
Due to improved and increased availability of the cervical cancer screening tests, the precancerous lesions are detected and treated at earlier stages. Early-stage cervical cancer can be cured with surgery, while concurrent chemoradiation is the treatment for locally advanced disease. Patients with recurrent or metastatic cancer, however, have limited treatment options (NCCN). In recent years, there have been significant developments in anti-cancer immunotherapies targeting certain immune checkpoints which have shown to be a promising therapeutic strategy against advanced malignancies. Unlike radiation and standard chemotherapies, which aim to directly interfere with tumor cell growth and survival, immunotherapies target the tumors indirectly by enhancing the anti-tumor immune response that may spontaneously arise in the tumors [3]. The programmed death-1/programmed death-ligand-1 (PD-1/PD-L1) immune regulatory axis has emerged as a major target of immunotherapy in several malignancies. Our group and others have shown that PD-L1 is positive in a significant number of cervical cancers [4,5].
Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) represents a crucial immune checkpoint, the blockade of which can potentiate anti-tumor immunity. The anti-CTLA-4 antibody, ipilimumab, was the first immune checkpoint inhibitor to be tested and approved by the FDA for the treatment of cancer, including melanoma and prostate carcinoma. It has already resulted in significant favorable outcomes, representing a major milestone in cancer immunotherapy [6-11]. CTLA-4 acts as a negative regulator of the T-cell activation by indirectly diminishing signaling through the co-stimulatory receptor CD28 [12-14]. Studies have demonstrated that CTLA-4 expression, on certain tumor cell lines, causes apoptosis of CTLA-4-expressing tumor cells after interaction with soluble CD80 or CD86 recombinant ligands [15]. CTLA-4 expression has been shown in various neoplasms including nasopharyngeal carcinoma, esophageal carcinoma, non-small cell lung cancer, and breast cancer [16-21].
In addition to checkpoint blockade agents such as ipilimumab for CTLA-4 expressing tumors, agents targeting the tumor necrosis factor (TNF) superfamily of costimulatory receptors have gained attention in recent years [22]. CD137 is an inducible, costimulatory receptor of the TNF receptor superfamily expressed on activated immune cells, including effector and regulatory T cells, natural killer (NK) cells, and dendritic cells (DCs) [23,24]. Recent studies indicate that the addition of anti-CD137 antibodies can supplement the antitumor efficacy of immune checkpoint inhibitors by enhancing cytotoxic T-cell and NK-cell activities, with effective antitumor response [25-28].
The aims of this investigation were to observe and compare the expression of CTLA-4 and CD137 in the cervical tissues and evaluate the CTLA-4 immunohistochemistry (IHC) reactions as we had previously done in breast cancer [21].
Materials and methods
Ethics statement
This study was reviewed and approved by the Institutional Review Board at the David Geffen School of Medicine at UCLA (IRB#15-001035).
Tissue microarray
Tissue microarray (TMA) glass slides of formalin-fixed paraffin embedded human cervical tissues were obtained from Abcam Inc. (Cambridge, MA). The TMA included 99 cores of both normal and neoplastic cervical tissues. The average size of each core, after fixation and paraffin embedding, was about 1 mm. Each core had been derived from one patient with her respective age listed in the table (Supplementary Tables 1, 2, 3, 4, 5 and 6). Hematoxylin and eosin (H&E) stain was applied to one slide for histopathologic assessment. Two of the authors (AK and NAM) evaluated the cores for immunohistochemistry (IHC) grading and diagnostic accuracy. Histopathologic diagnoses were made per established criteria and nomenclature published by the World Health Organization [2]. Per Abcam’s specifications, all tissues had been fixed in 10% buffered formalin solution for 24 hours and had been further processed using identical standard procedures. Sections were freshly cut upon order and were placed on Superfrost-Plus or Starfrost adhesive glass slides. The sections were 4-6 μm in thickness.
CTLA-4 immunohistochemistry
Mouse anti-human CTLA-4 monoclonal antibody (clone F8) was obtained from Santa Cruz Biotechnology (Dallas, TX). IHC was carried out on one of the TMA slides employing the anti-CTLA-4 antibody at 1:100 dilution along tonsil tissue as control. The results were recorded based on the intensity of the staining reaction on the cytoplasm, as well as the estimated percentage of positive tumor cells as was previously described and photomicrographed in detail on female breast tissues [21].
Intensity 0: If there was no reaction in the cytoplasm.
Intensity 1+: If a low number of cytoplasmic granules had a reaction.
Intensity 2+: If a moderate number of cytoplasmic granules had a reaction.
Intensity 3+: If a high number of cytoplasmic granules had a reaction.
For staining evaluation, the same scoring system previously developed by our group, for PD-L1 in cervical [5] and CTLA-4 in breast [21] tissues, was used. Three categories of expression were designated for CTLA-4 staining, “Negative”, “Low-Positive (LoPos)”, and “Positive” as defined in the scoring system below.
Score “0”-100% of cells with Intensity of 0; Expression: Negative.
Score “1a”-<50% of cells with Intensity of 1+; Expression: Low-positive.
Score “1b”-<50% of cells with Intensity of 2+ and/or 3+; Expression: Low-Positive.
Score “2a”-≥50% of cells with Intensity of 1+; Expression: Positive.
Score “2b”-≥50% of cells with Intensity of 2+ and/or 3+; Expression: Positive.
Since 1+ intensity had been observed in normal epithelial tissue (squamous epithelial lining of tonsil), only “1b” and “2b” reactions were interpreted as “positive”.
CD137 immunohistochemistry
Rabbit anti-human CD137 polyclonal antibody (ab197942) was obtained from Abcam. It was applied to one of the TMA slides for IHC staining at 1:100 dilution using tonsil as control. The results were recorded based on presence or absence of the staining reactions on the cell membranes of the lymphoplasmacytic cells. Any expression intensity on ≥5% of cells within the lymphoplasmacytic infiltrate of the tumors was considered as “Positive”.
Statistical analysis
2×2 tables for nonparametric Fisher Exact testing were employed to compare the selected sets of data. Also, “Negative” and “Positive” interpreted results for CTLA-4 were converted to binary “zeros” and “ones” for Student’s t-test analysis. A result of less than 5% positivity for CD137 among the lymphoplasmacytic cells population was considered as negative and designated as “0” otherwise “1”. Microsoft (Redmond, WA) Office-365 Excel sheets and StatPlus, version7 (https://www.analystsoft.com) were used for tabulation of the data and the statistical analyses and the results are shown in Table 1.
Table 1.
Summary of cores with interpretive positivity of the immune checkpoints in the groups and subgroups of uterine cervical carcinoms and related statistical analysis results
| Group | Carcinoma | Median Age | Total (n) | CTLA-4 | CTLA-4 | CD137 | |||
|
| |||||||||
| Positive (n) | Positive (%) | Positive (%) | |||||||
|
| |||||||||
| Control | Benign | 57 | 4 | 0 | 0% | 25% | |||
| Group I | |||||||||
| Squamous cell carcinomas, all | 44 | 74 | 23 | 31% | 91% | ||||
| IA-SCC, grade I | 46 | 7 | 3 | 43% | 86% | ||||
| IB-SCC, grade II | 44 | 52 | 16 | 31% | 96% | ||||
| IC-SCC, grade Ill | 39 | 15 | 4 | 27% | 73% | ||||
| Group II | |||||||||
| Adenosquamous carcinoma | 40 | 8 | 5 | 63% | 88% | ||||
| Group III | |||||||||
| Endocervical adenocarcinoma | 40 | 13 | 2 | 15% | 100% | ||||
| Groups I, II, and III | 44 | 95 | 30 | 32% | 91% | ||||
|
| |||||||||
| Student’s t-test: “Benign” versus Group I, II, and III (two-tailed, unequal variance): CTLA-4: P-value <0.0001, CD137: P-value =0.075. | |||||||||
|
| |||||||||
| 2×2 table Fisher Exact statistical tests (CTLA-4) | Negative (n) | Positive (n) | 2-Tailed P-Value | ||||||
|
| |||||||||
| SCC, grade I | 4 | 3 | 0.67 | ||||||
| SCC, grade II | 36 | 16 | |||||||
| SCC, grade II | 36 | 16 | 1.0 | ||||||
| SCC, grade III | 11 | 4 | |||||||
| SCC, grade I | 4 | 3 | 0.63 | ||||||
| SCC, grade III | 11 | 4 | |||||||
| SCC, all grades | 51 | 23 | 0.12 | ||||||
| Adenosquamous carcinoma | 3 | 5 | |||||||
| SCC, all grades | 51 | 23 | 0.33 | ||||||
| Endocervical adenocarcinoma | 11 | 2 | |||||||
| Adenosquamous carcinoma | 3 | 5 | 0.056 | ||||||
| Endocervical adenocarcinoma | 11 | 2 | |||||||
SCC, squamous cell carcinoma; Positive includes “1b”, and “2b” scores.
Study design
Based on the histopathology diagnoses, the cores were categorized into three groups: Group I, squamous cell carcinoma; Group II, adenosquamous carcinoma; Group III, endocervical adenocarcinoma. Group I was further subcategorized as IA, carcinoma grade I; 1B, carcinoma grade II; and 1C, carcinoma grade III. Using the designed scoring method, CTLA-4 expression findings were recorded for each group and tabulated in their respective supplementary tables. The available clinical and demographic information was extracted from the Abcam product datasheet and added alongside the findings. Statistical tests were carried out to compare two sets of data at a time. To reject the null hypothesis, a two-tailed p-value ≤ 0.05 was considered asignificant difference between two groups.
Results
All CTLA-4 reactive cores showed cytoplasmic granules as had been observed previously [21], all with 1+ or 2+ intensities. We did not observe any reaction with 3+ intensity. All CD137 reactions were cytoplasmic (Figure 1) or on cell membranes of the lymphoplasmacytic cells which had been similarly observed by others [29]. No such reaction was observed on the tumor cells.
Figure 1.
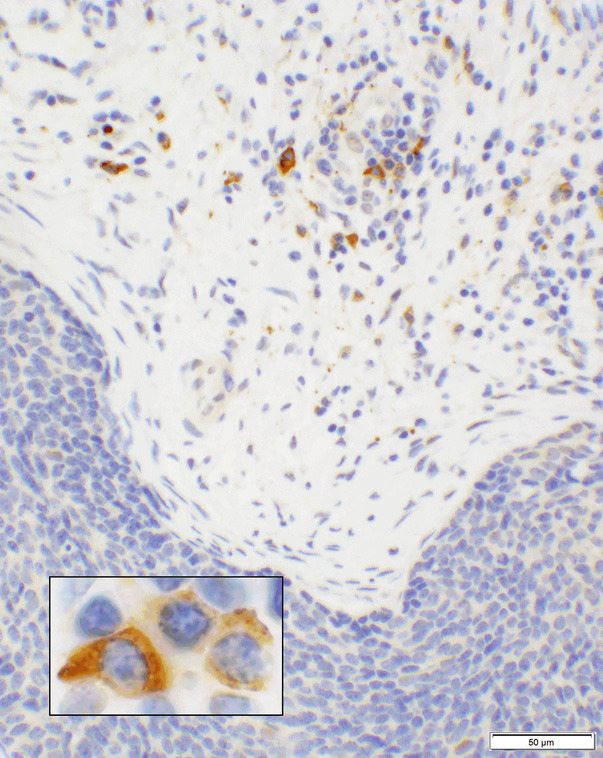
CD137 expression in lymphoplasmacytic cell infiltrates. A photomicrograph of a case showing squamous cell carcinoma (blue cells in lower segment of the picture) that are negative for CD137 while the infiltrating cells in the stroma show positive granular cytoplasmic staining of lymphoplasmacytic cells (20× objective). A high magnification of the lymphoplasmacytic cells is shown in the inset (100× objective).
Of the 99 TMA cores, four cases were benign cervical tissues (2 normal and 2 with cervicitis) listed in Supplementary Table 1. In one case (case # 2, Supplementary Table 1), 40% of the cells had a 1+ intensity reaction (Figure 2), however, all 4 cases were interpreted as “Negative” per established criteria. Except for the same case which had a few positive lymphoid cells, the CD137 reaction was negative in the other 3 cases (Supplementary Table 1). Remaining 95 cores were malignant which will be discussed below.
Figure 2.
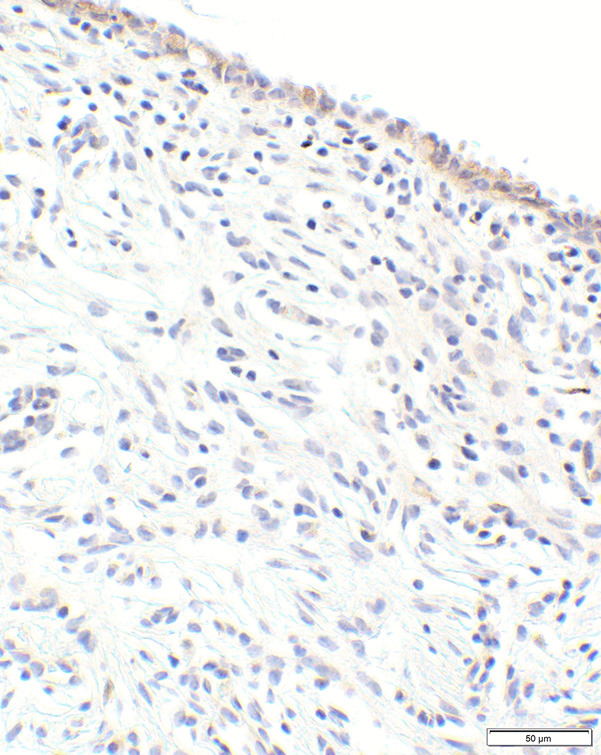
CTLA-4 expression in normal endocervical glands. Photomicrograph of benign endocervical tissue (case #2, Supplementary Table 1) with 1+ (light/weak) cytoplasmic reaction of the epithelial lining which was interpreted as “Negative” (20× objective).
Group I, Squamous cell carcinoma (SCC)
Of the 99 cores, 74 were SCC. Twenty-nine cores (39.2%) had no CTAL-4 expression (score “0”), 22 cores (29.7%) had 1+ intensity (scores: “1a” and “2a”), and 23 cores (31.1%) had 2+ intensity (scores: “1b” and “2b”) reactions. The cores with the scores of “1b” and “2b” were interpreted as “positive”. An example of the reaction in this group is shown in Figure 3. Sixty-seven (90.5%) cores showed positive expression of CD137 in the lymphoplasmacytic infiltrates in the tumor stroma (Figure 1).
Figure 3.
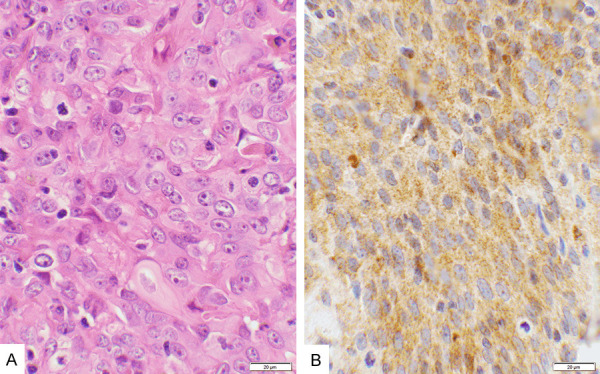
Score 2b in squamous cell carcinoma. A. An example of squamous cell carcinoma stained with hematoxylin & eosin, showing the malignant cell nuclei with prominent nucleoli. B. CTLA-4 stain of the same case showing a moderate cytoplasmic granular reaction (2+ intensity) involving more than 90% of the cells, scored as “2b”, and interpreted as “Positive” (40× objective).
Subgroup IA, SCC Grade I
Among the 74 SCC cores, 7 cores were grade I tumors, of which 2 (28.6%) had no CTLA-4 expression and scored as “0”. Two additional cores (28.6%) had expressions with 1+ intensity (score “1a” and “2a”). The remaining 3 cores (42.9%) had 2+ intensity reaction for CTLA-4 scored as “1b” and “2b” which were interpreted as “Positive”. All but one core had expression of CD137 (85.7%) within the lymphoplasmacytic infiltration (Supplementary Table 2).
Subgroup IB, SCC Grade II
Fifty-two cores had grade-II SCC lesions of which 21 (40.4%) had no reaction for CTLA-4, and thus scored as “0”. Fifteen cores had the reactions for CTLA-4 (28.9%) with 1+ intensity (score of “1a” and “2a”). The remaining 16 cores (30.8%) were positive for CTLA-4 with a score of either “1b” or “2b”, collectively interpreted as “Positive”. All 52 cores had lymphoplasmacytic infiltration which had expressed CD137 except for one (Supplementary Table 3).
Subgroup IC, SCC Grade III
This subgroup was comprised of 15 cores of which 6 (40%) had no reaction for CTLA-4 and thus scored as “0”. Five cores (33.3%) had 1+ intensity with score of “1a” and “2a”. Only four cores (26.7%) were interpreted as “Positive” for CTLA-4 with scores of “1b” and “2b”. Although all 15 cores had lymphoid infiltrates, only 11 (73.3%) had expressed CD137 (Supplementary Table 4).
Group II, adenosquamous carcinoma
Eight of the cores in the TMA fell into this group (Supplementary Table 5). One core had no reaction for CTLA-4, and thus scored as “0”. Two cores had 1+ intensity reaction (score of “1a” and “2a”) and were counted as “Negative” in the Interpretation column. Five cores were positive (62.5%) for CTLA-4 with an intensity of 2+ and were scored as “1b” and “2b” and interpreted as “Positive”. All but one core had expression of CD137 (87.5%) within the lymphoplasmacytic infiltrate (Supplementary Table 5).
Group III, endocervical adenocarcinoma
This group comprised of 13 cores. One core was scored as “0” since it had no reaction for CTLA-4. Additional 10 cores had 1+ intensity (score of “1a” and “2a”) and subsequently interpreted as “Negative”. Only 2 cores were positive (62.5%) with an intensity 2+, scored as “1b” and “2b”, and interpreted as “Positive”. An example of a positive case is shown in Figure 4. All cores had expression of CD137 (100%) within the lymphoplasmacytic infiltrate (Supplementary Table 6).
Figure 4.
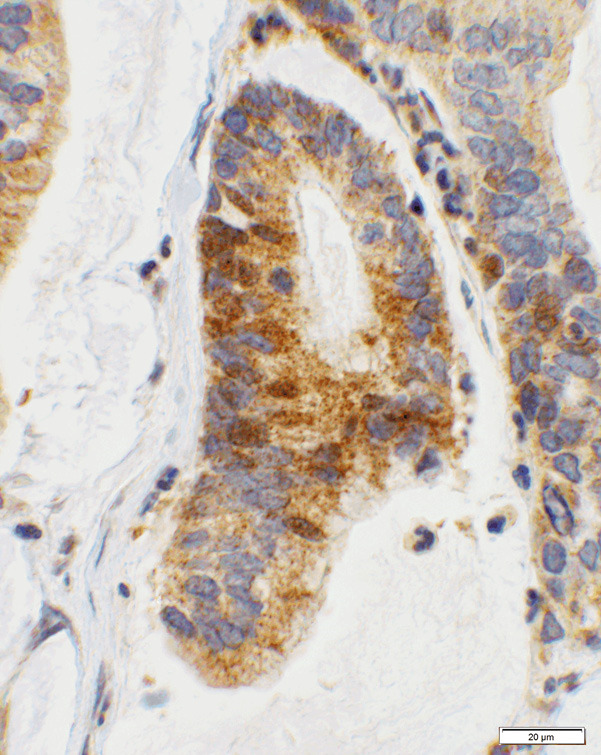
Score 2b in endocervical adenocarcinoma. An example of endocervical adenocarcinoma showing 2+ staining with CTLA-4 where there are a moderate number of granules in the cytoplasm (40× objective).
Statistical results
Student’s t-tests were performed on 4 benign versus 95 malignant cores using the “Negative” and “Positive” interpretive criteria as described for CTLA-4 and CD137. Since the compared core numbers were 4 versus 95, the analyses used unequal variance. The obtained two-tailed P-values were <0.0001 and 0.075 for CTLA-4 and CD137, respectively (Table 1). Fisher Exact (2×2 table) analysis was used to identify the differences between the groups and the subgroups. “Positive” CTLA-4 expression was present in 31% of SCCs, where the expressions were 43%, 31%, and 27% in grades I, II, and III, respectively (Table 1). Regardless of higher rates of expressions in the lower grades, the grade comparison in SCC resulted in insignificant P-values of 0.67, 1.0, and 0.63 (Table 1). Comparing SCC to adenosquamous-carcinoma and endocervical-adenocarcinoma yielded P-values of 0.12 and 0.33. Although a higher percentage of the adenosquamous carcinomas (63%) had expressed CTLA-4, when tested against endocervical adenocarcinoma, the resulting P-value was 0.056 (Table 1).
Discussion
At the outset, the results of this study indicate that CTLA-4 is expressed in 32% of cervical carcinomas which is significantly (P-value <0.0001) higher than occurring in normal or benign cervical tissues. Expression of CTLA-4 has a propensity for lower grade SCCs; however, the difference between grades did not reached statistical significance (Table 1). Endocervical adenocarcinomas exhibit the lowest (15%) and adenosquamous carcinoma the highest (63%) rates of “Positive” CTLA-4 expression with a Fisher Exact P-value of 0.056 which indicates a near-significant difference (Table 1). The rate of the reactivity of CTLA-4 immune checkpoint is similar, if not identical, to that of PD-L1 under similar experimental conditions [5]. On the other hand, CD137 is expressed in more than 90% of the lymphoplasmacytic infiltrates of the tumor microenvironment, although it is also expressed in benign cervical tissues (Supplementary Table 1), for which, the Student’s t-test returned a P-value of 0.075 when benign cores compared to the malignant ones. This statistically insignificant P-value may be due to the small size of the benign cores or may also be due to its common expression in both benign and malignant conditions.
Expression of PD-L1 and CTLA-4 in more than 30% of the cervical cancers helps in expanding the arsenal in the immunotherapy against this neoplasm. Also, with emergence of the monoclonal antibody, CD137 can serve as an adjuvant to the immune checkpoints in immunotherapy of the cancer since it is expressed in more than 90% of the cancer cases (Table 1).
The PD-1 pathway involves PD-L1 and PD-L2 by binding to the PD-1 receptors of the T-cells which shield the cells from the attack by the inactivating killer T-cells. Pembrolizumab, a humanized monoclonal antibody, binds to the PD-1 receptors, shielding it from the ligands. When unable to bind to the receptor, PD-L1 and/or PD-L2 expressing cells will be exposed to the attack by the immune cells. In this process, not only the tumor cells will be the subject of the strikes, but also normal cells may get damaged during the process, leading to the autoimmune side effects [30].
The cytotoxic T-lymphocyte-associated antigen 4 or CTLA-4 molecules negatively regulate T-cell activation by triggering inhibitory pathways which dampen T-cell activity when bound to their ligands (CD80/CD86) [31]. CTLA-4 is an immune checkpoint inhibitor, best known for its activity and survival effects on metastatic melanoma, as well as other solid tumors [32,33]. Blocking CTLA-4 results in promotion of T-cell activation and proliferation [10,19]. Ipilimumab, a recombinant human monoclonal antibody, acts as such blocking agent. Therefore, by blocking, the overall immune responses are enhanced leading to subsequent tumor killing by the T-cells [34]. The side effects of this treatment include fatigue, diarrhea, pruritis, rash, and colitis [35].
CD137 (4-1BB, TNFRSF9) molecule which is a member of tumor necrosis factor family is expressed on a variety of inflammatory cells including lymphocytes of B & T lineage, NK cells, dendritic cells, and neutrophils [24]. Its expression is triggered by activated CD4+ and CD8+ T-cells [36]. CD137 signals are mediated by activation of NF-κB (nuclear factor-kappa B) and by TRAF2 (TNF receptor-associated factor 2) [37]. By binding, urelumab, a humanized IgG4 monoclonal agonist antibody, crosslinks CD137 molecules which results in further activation and proliferation of the T-cells leading to inteleukin-2 secretion, apoptosis, and cytolytic activities [38-41]. These processes will enhance immune activity to eliminate tumor cells [42]. A significant side-effect of the treatment is liver toxicity of the antibody which caused a clinical trial to be stopped [43].
Thus, combination therapies of low dose anti-CD137 with other FDA-approved immunomodulators and antibody therapeutics including anti-PD-1 and anti-CTLA-4 can have great anti-tumor activity and minimize the possibility of systemic toxicity. It has already been shown that agonistic CD137 antibody with combination of anti-CTLA-4 therapy increases the survival of mice in the setting of glioblastoma, colonic, and prostate cancer [42,44-47]. Immune checkpoint and costimulatory pathways have different mechanisms with potential for therapeutic synergy approach [48]. This study highlights the importance of understanding the expression levels of these immune checkpoint regulators in cervical cancers for effective translational and clinical research.
Acknowledgements
CTLA-4 findings of this work were presented at United States and Canadian Academy of Pathologists (USCAP) annual meeting, National Harbor, Maryland, USA in March of 2019.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Longacre TA, Bell DA, Malpica A, Prat J, Ronnett BM, Seidman JD, Vang R. Tumours of the ovary: mucinous tumours. In: Kurman RJ, Carcangiu ML, Herrington CS, Young RH, editors. WHO Classification of tumours of female reproductive organs. Lyon: IARC; 2014. pp. 25–38. [Google Scholar]
- 3.Dougan M, Dranoff G, Dougan SK. Cancer immunotherapy: beyond checkpoint blockade. Annu Rev Cancer Biol. 2019;3:55–75. doi: 10.1146/annurev-cancerbio-030518-055552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol. 2015;28:1594–1602. doi: 10.1038/modpathol.2015.108. [DOI] [PubMed] [Google Scholar]
- 5.Reddy OL, Shintaku PI, Moatamed NA. Programmed death-ligand 1 (PD-L1) is expressed in a significant number of the uterine cervical carcinomas. Diagn Pathol. 2017;12:45. doi: 10.1186/s13000-017-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, Davis T, Henry-Spires R, MacRae S, Willman A, Padera R, Jaklitsch MT, Shankar S, Chen TC, Korman A, Allison JP, Dranoff G. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 9.Fong L, Kwek SS, O’Brien S, Kavanagh B, McNeel DG, Weinberg V, Lin AM, Rosenberg J, Ryan CJ, Rini BI, Small EJ. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PubMed] [Google Scholar]
- 10.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 13.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 14.Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009;229:12–26. doi: 10.1111/j.1600-065X.2009.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Contardi E, Palmisano GL, Tazzari PL, Martelli AM, Fala F, Fabbi M, Kato T, Lucarelli E, Donati D, Polito L, Bolognesi A, Ricci F, Salvi S, Gargaglione V, Mantero S, Alberghini M, Ferrara GB, Pistillo MP. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int J Cancer. 2005;117:538–550. doi: 10.1002/ijc.21155. [DOI] [PubMed] [Google Scholar]
- 16.Bouwhuis MG, Gast A, Figl A, Eggermont AM, Hemminki K, Schadendorf D, Kumar R. Polymorphisms in the CD28/CTLA4/ICOS genes: role in malignant melanoma susceptibility and prognosis? Cancer Immunol Immunother. 2010;59:303–312. doi: 10.1007/s00262-009-0751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvi S, Fontana V, Boccardo S, Merlo DF, Margallo E, Laurent S, Morabito A, Rijavec E, Dal Bello MG, Mora M, Ratto GB, Grossi F, Truini M, Pistillo MP. Evaluation of CTLA-4 expression and relevance as a novel prognostic factor in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2012;61:1463–1472. doi: 10.1007/s00262-012-1211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H, Yang J, Jiao S, Li Y, Zhang W, Wang J. Cytotoxic T lymphocyte antigen 4 expression in human breast cancer: implications for prognosis. Cancer Immunol Immunother. 2015;64:853–860. doi: 10.1007/s00262-015-1696-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang PY, Guo SS, Zhang Y, Lu JB, Chen QY, Tang LQ, Zhang L, Liu LT, Zhang L, Mai HQ. Tumor CTLA-4 overexpression predicts poor survival in patients with nasopharyngeal carcinoma. Oncotarget. 2016;7:13060–13068. doi: 10.18632/oncotarget.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang XF, Pan K, Weng DS, Chen CL, Wang QJ, Zhao JJ, Pan QZ, Liu Q, Jiang SS, Li YQ, Zhang HX, Xia JC. Cytotoxic T lymphocyte antigen-4 expression in esophageal carcinoma: implications for prognosis. Oncotarget. 2016;7:26670–26679. doi: 10.18632/oncotarget.8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassardjian A, Shintaku PI, Moatamed NA. Expression of immune checkpoint regulators, cytotoxic T lymphocyte antigen 4 (CTLA-4) and programmed death-ligand 1 (PD-L1), in female breast carcinomas. PLoS One. 2018;13:e0195958. doi: 10.1371/journal.pone.0195958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao S, Zhu Y, Chen L. Advances in targeting cell surface signalling molecules for immune modulation. Nat Rev Drug Discov. 2013;12:130–146. doi: 10.1038/nrd3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melero I, Murillo O, Dubrot J, Hervas-Stubbs S, Perez-Gracia JL. Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol Sci. 2008;29:383–390. doi: 10.1016/j.tips.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Vinay DS, Kwon BS. Immunotherapy of cancer with 4-1BB. Mol Cancer Ther. 2012;11:1062–1070. doi: 10.1158/1535-7163.MCT-11-0677. [DOI] [PubMed] [Google Scholar]
- 25.Li SY, Liu Y. Immunotherapy of melanoma with the immune costimulatory monoclonal antibodies targeting CD137. Clin Pharmacol. 2013;5(Suppl 1):47–53. doi: 10.2147/CPAA.S46199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yonezawa A, Dutt S, Chester C, Kim J, Kohrt HE. Boosting cancer immunotherapy with anti-CD137 antibody therapy. Clin Cancer Res. 2015;21:3113–3120. doi: 10.1158/1078-0432.CCR-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S, Lee LF, Fisher TS, Jessen B, Elliott M, Evering W, Logronio K, Tu GH, Tsaparikos K, Li X, Wang H, Ying C, Xiong M, VanArsdale T, Lin JC. Combination of 4-1BB agonist and PD-1 antagonist promotes antitumor effector/memory CD8 T cells in a poorly immunogenic tumor model. Cancer Immunol Res. 2015;3:149–160. doi: 10.1158/2326-6066.CIR-14-0118. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava RM, Trivedi S, Concha-Benavente F, Gibson SP, Reeder C, Ferrone S, Ferris RL. CD137 stimulation enhances cetuximab-induced natural killer: dendritic cell priming of antitumor t-cell immunity in patients with head and neck cancer. Clin Cancer Res. 2017;23:707–716. doi: 10.1158/1078-0432.CCR-16-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson MW, Zhao S, Freud AG, Czerwinski DK, Kohrt H, Alizadeh AA, Houot R, Azambuja D, Biasoli I, Morais JC, Spector N, Molina-Kirsch HF, Warnke RA, Levy R, Natkunam Y. CD137 is expressed in follicular dendritic cell tumors and in classical Hodgkin and T-cell lymphomas: diagnostic and therapeutic implications. Am J Pathol. 2012;181:795–803. doi: 10.1016/j.ajpath.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wendel Naumann R, Leath CA 3rd. Advances in immunotherapy for cervical cancer. Curr Opin Oncol. 2020;32:481–487. doi: 10.1097/CCO.0000000000000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolchok JD, Chan TA. Cancer: antitumour immunity gets a boost. Nature. 2014;515:496–498. doi: 10.1038/515496a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mansh M. Ipilimumab and cancer immunotherapy: a new hope for advanced stage melanoma. Yale J Biol Med. 2011;84:381–389. [PMC free article] [PubMed] [Google Scholar]
- 33.Yun S, Vincelette ND, Green MR, Wahner Hendrickson AE, Abraham I. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: a systematic review and meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Med. 2016;5:1481–1491. doi: 10.1002/cam4.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simeone E, Ascierto PA. Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti-CTLA-4, anti-CD137, and anti-PD1. J Immunotoxicol. 2012;9:241–247. doi: 10.3109/1547691X.2012.678021. [DOI] [PubMed] [Google Scholar]
- 35.O’Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L, Verschraegen C, Humphrey R, Ibrahim R, de Pril V, Hoos A, Wolchok JD. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 36.Wehler TC, Karg M, Distler E, Konur A, Nonn M, Meyer RG, Huber C, Hartwig UF, Herr W. Rapid identification and sorting of viable virus-reactive CD4(+) and CD8(+) T cells based on antigen-triggered CD137 expression. J Immunol Methods. 2008;339:23–37. doi: 10.1016/j.jim.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Jang IK, Lee ZH, Kim YJ, Kim SH, Kwon BS. Human 4-1BB (CD137) signals are mediated by TRAF2 and activate nuclear factor-kappa B. Biochem Biophys Res Commun. 1998;242:613–620. doi: 10.1006/bbrc.1997.8016. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto K. CD137 as an attractive t cell co-stimulatory target in the TNFRSF for immuno-oncology drug development. Cancers (Basel) 2021;13:2288. doi: 10.3390/cancers13102288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho SK, Xu Z, Thakur A, Fox M, Tan SS, DiGiammarino E, Zhou L, Sho M, Cairns B, Zhao V, Xiong M, Samayoa J, Forsyth CM, Powers DB, Chao DT, Hollenbaugh D, Alvarez HM, Akamatsu Y. Epitope and Fc-mediated cross-linking, but not high affinity, are critical for antitumor activity of CD137 agonist antibody with reduced liver toxicity. Mol Cancer Ther. 2020;19:1040–1051. doi: 10.1158/1535-7163.MCT-19-0608. [DOI] [PubMed] [Google Scholar]
- 40.Geng T, Yan Y, Zhang Y, Xu L, Zang G, Yan JC. CD137 signaling promotes endothelial apoptosis by inhibiting Nrf2 pathway, and upregulating NF-kappaB pathway. Mediators Inflamm. 2020;2020:4321912. doi: 10.1155/2020/4321912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etxeberria I, Glez-Vaz J, Teijeira A, Melero I. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open. 2020;4(Suppl 3):e000733. doi: 10.1136/esmoopen-2020-000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannons JL, Choi Y, Watts TH. Role of TNF receptor-associated factor 2 and p38 mitogen-activated protein kinase activation during 4-1BB-dependent immune response. J Immunol. 2000;165:6193–6204. doi: 10.4049/jimmunol.165.11.6193. [DOI] [PubMed] [Google Scholar]
- 43.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37:508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 44.Youlin K, Li Z, Xiaodong W, Xiuheng L, Hengchen Z. Combination immunotherapy with 4-1BBL and CTLA-4 blockade for the treatment of prostate cancer. Clin Dev Immunol. 2012;2012:439235. doi: 10.1155/2012/439235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belcaid Z, Phallen JA, Zeng J, See AP, Mathios D, Gottschalk C, Nicholas S, Kellett M, Ruzevick J, Jackson C, Albesiano E, Durham NM, Ye X, Tran PT, Tyler B, Wong JW, Brem H, Pardoll DM, Drake CG, Lim M. Focal radiation therapy combined with 4-1BB activation and CTLA-4 blockade yields long-term survival and a protective antigen-specific memory response in a murine glioma model. PLoS One. 2014;9:e101764. doi: 10.1371/journal.pone.0101764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hebb JPO, Mosley AR, Vences-Catalan F, Rajasekaran N, Rosen A, Ellmark P, Felsher DW. Administration of low-dose combination anti-CTLA4, anti-CD137, and anti-OX40 into murine tumor or proximal to the tumor draining lymph node induces systemic tumor regression. Cancer Immunol Immunother. 2018;67:47–60. doi: 10.1007/s00262-017-2059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kroon P, Gadiot J, Peeters M, Gasparini A, Deken MA, Yagita H, Verheij M, Borst J, Blank CU, Verbrugge I. Concomitant targeting of programmed death-1 (PD-1) and CD137 improves the efficacy of radiotherapy in a mouse model of human BRAFV600-mutant melanoma. Cancer Immunol Immunother. 2016;65:753–763. doi: 10.1007/s00262-016-1843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tirapu I, Mazzolini G, Rodriguez-Calvillo M, Arina A, Palencia B, Gabari I, Melero I. Effective tumor immunotherapy: start the engine, release the brakes, step on the gas pedal,. ..and get ready to face autoimmunity. Arch Immunol Ther Exp (Warsz) 2002;50:13–18. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


