Abstract
Solitary necrotic nodule of the liver (SNNL) is an uncommon disease in clinical practice, and its pathogenesis is still unclear. Here, we report the case of a 35-year-old woman. After physical examination, the patient was found to have a liver neoplasm, and there were no other physical complaints. Abdominal contrast-enhanced computed tomography (CT) showed the presence of a hypodense lesion. The patient opted for surgery to eliminate the lesion. Pathologic examination revealed an isolated necrotic nodular lesion with a size of 12 cm×10 cm×10 cm. The patient had a history of hepatitis B infection. To our knowledge, this is the largest SNNL ever reported and the first case with a history of hepatitis B infection.
Keywords: Liver tumor, solitary necrotic nodule, pathology
Introduction
Solitary necrotic nodule of the liver (SNNL) is a rare lesion in the clinic. A patient with SNNL usually has no symptoms or only mild discomfort in the liver. Most patients are found to have liver nodules on physical examination and seek further treatment. At present, the pathogenesis of SNNL remains unclear. An accurate diagnosis is difficult with current medical imaging. The vast majority of cases are supported by histopathology after surgical resection. Pathologic features of SNNL are a central necrotic core surrounded by a fibrotic capsule infiltrated by inflammatory cells [1,2].
Here, we report a case with a rare occurrence of a huge solitary necrotic nodule of the liver. The patient had a history of hepatitis B and a large SNNL lesion. Through the study of this case, we will have a more complete understanding of SNNL, and propose the hypothesis that HEPATITIS B virus may promote the enlargement of SNNL lesions, along with increasing the nodular volume.
Case report
A 39-year-old female patient without any symptoms came to the Department of Hepatobiliary Surgery with complains of a mass in the right lobe of the liver.
Ultrasound showed an oval mass with ill-defined margins and measuring 97 mm×74 mm. Abdominal contrast-enhanced computed tomography (CT) showed the presence of a hypodense lesion. In contrast-enhanced scan, uneven enhancement was observed in the arterial phase, and weakened in the portal and delayed phases. Laboratory tests verified that the patient had a history of hepatitis B, but all other tests were normal including serum carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), and alpha-fetoprotein.
The patient underwent surgery. The specimen was sent for histopathologic examination. There was a gray-yellow mass of 12 cm×10 cm×10 cm in the liver. Microscopically, the mass was a large sheet of coagulated, denatured and necrotic tissue surrounded by multinucleated giant cells (Figure 1). There were several small cavities in the necrotic foci and cystic mural structures around the cavities (Figure 2). Local areas of lymphocyte and eosinophil aggregation were also observed in the necrotic region. The special staining results showed: PAS (+), PASM (+), acid-fast (-), Congo red (-), reticular fibers (-), and elastic fibers (-). These microscopic manifestations confirmed the diagnosis of SNNL and we considered it as a lesion after parasitic infection.
Figure 1.
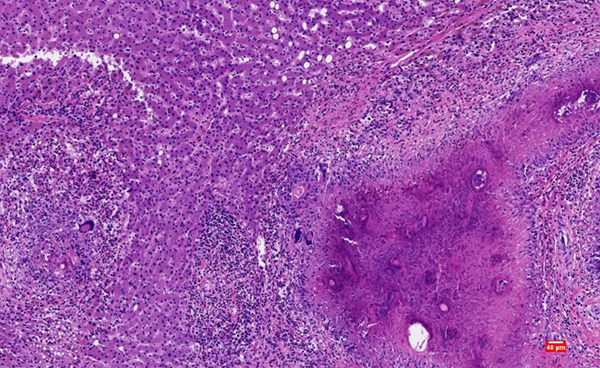
Multinucleated giant cells appeared at the edge of the necrotic area. (Hematoxylin and eosin staining, magnification ×100).
Figure 2.
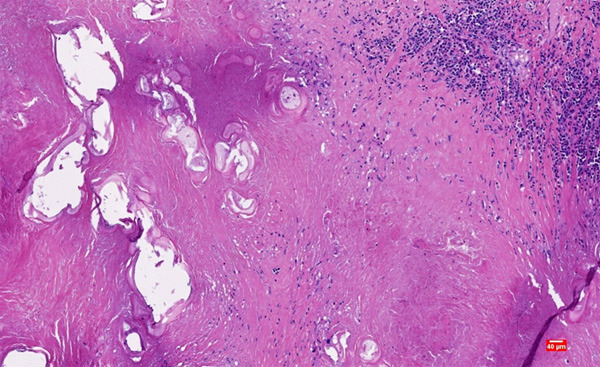
There were several cavities in the necrotic area, and the surrounding cavities showed cystic mural structures. (Hematoxylin and eosin staining, magnification ×100).
Discussion
Currently, only a few cases of SNNL have been reported. By conventional CT, solitary necrotic nodules usually appear as hypodense or iso-dense nodules. In most cases, the lesion does not show contrast enhancement, but may show enhancement in thin marginal tissue [3]. Calcification may be present in some lesions [4]. Such imaging findings make it difficult to distinguish SNNL from hepatic metastases [5]. Therefore, SNNL can be diagnosed by only pathology after surgery at present. SNN shows a significant necrotic core which may contain eosinophils, calcification, cholesterol, foam cells, and some inflammatory cells [2].
It was generally believed that SNNL was mostly a benign presence, and it was believed to be the final stage of a natural process caused by infection and degeneration [6]. However, according to recent reports, some cases of SNNL are associated with metastatic cancer, so we should consider the possibility of metastatic necrotic tumor lesions when evaluating SNNL [7]. It is important to bear in mind that “solitary” necrotic nodules may not occur in isolation [8]. In exploration of the formation mechanism of SNNLs, the sclerosis and evolution of small blood vessels [9], parasites [10], trauma, and other reasons are all hypothesized to cause its formation, and may even be caused by a combination of the above reasons [2,11]. In the hypothesis of small-vessel sclerosis, the pathologic findings are based on the phenomena that there are some hemangiomatous parts in the lesion, which are filled with blood vessels that supply nutrients. The lesion also contains more sclerotic tissue, some of which is calcifying. Sclerosis is also a major feature [9]. The presence of “feeding vessels” and the absence of cystic spaces or other signs of parasite erosion represent the hypothesized mechanism for the evolution of sclerosis in small hemangiomas [2]. However, granulomatous tissue is similar to parasitic granulomatous and eosinophilic nematode - like material in the mechanism of parasitic infection. This microscopic appearance suggests that parasitic infection may also be responsible for SNNL [8]. In the case we reported, the necrotic area had complete coagulative necrosis with only a few inflammatory cells such as eosinophils and lymphocytes. A large number of inflammatory cells such as eosinophils and multinucleated giant cells were seen at the junction with normal liver tissue. This lesion is therefore thought to be altered by parasitic infection.
Article reports of SNNL are relatively rare in China. A total of 25 cases have been reported in the English literature (Table 1). Of the 25 patients reported, 20 were female and 5 were male, of whom 11 patients had clinical symptoms of abdominal pain. One patient had a history of rectal cancer and two patients had a history of gastric cancer. In one case, the SNNL lesions increased in volume over a period of 7 months. In another of these cases, however, the lesion disappeared 7 months later without surgical treatment. As we learn about the causes of SNNL, we find that patients in SNNL cases could have no clinical symptoms. After all, some cases are even identified and defined at autopsy [9,10]. Most of the patients chose surgical treatment, which resulted in no recurrence of the lesion.
Table 1.
English literature reports of cases of Solitary necrotic nodule of the liver
| Case | Age/gender | Location (lobe) | Clinical symptoms | Medical History | laboratory examination | Size (mm) | Therapies | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 22/F | Right lobe (S6) | Fever | Epilepsy | hepatocyte dysfunction | 10 | Surgery | N0 recurrence |
| 2 | 76/F | Right lobe (S6) | Normal | NA | Normal | 12 | Surgery | No recurrence |
| 3 | 74/F | Right lobe (S8) | Normal | Rectal cancer | Normal | 16 | Surgery | No recurrence |
| 4 | 55/M | NA | Normal | NA | NA | 20 | NA | NA |
| 5 | 55/F | Right lobe (S8) | Abdominal pain | NA | NA | 80 | NA | NA |
| 6 | 41/F | Right lobe (S8) | Normal | NA | NA | 21 | NA | NA |
| 7 | 62/F | Left lobe (S3) | epigastric pain | Chronic renal failure | The count of CEA and CA199 increased slightly | 80 | Surgery | No recurrence |
| 8 | 28/F | NA | Right abdomen pain | NA | NA | 10 | Surgery | NA |
| 9 | 32/F | NA | Right abdomen pain | NA | NA | 12 | Surgery | NA |
| 10 | 48/F | Right lobe (S8) | Normal | NA | The count of CEA and CA199 increased | 20 | Surgery | No recurrence |
| 11 | 30/M | Right lobe (S5) | Right abdomen pain | NA | Leukocytosis and dysfunction of liver | 70 | Surgery | No recurrence |
| 12 | 48/F | Right lobe (S6) | Abdominal pain | NA | Normal | 15 | Surgery | No recurrence |
| 13 | 35/F | NA | Normal | NA | Normal | 40 | Surgery | No recurrence |
| 14 | 46/M | NA | Normal | Type 2 diabetes | Hyperlipidemia | 18 | Surgery | No recurrence |
| 15 | 40/F | Right lobe (S5) | Normal | NA | Normal | 30 | Surgery | No recurrence |
| 16 | 59/F | Left lobe (S4) | Right abdomen pain | Hypertension, osteoarticular pathology and penicillin allergy | Normal | 42 | Surgery | No recurrence |
| 17 | 52/M | Right lobe (S7) | Normal | Gastric cancer | NA | 8 | Surgery | No recurrence |
| 18 | 30/F | Right lobe (S6) | dyspeptic symptoms | Gastric cancer | NA | 15 | No surgery | unchanged |
| 19 | 58/F | Right lobe (S5) | Right abdomen pain | NA | NA | 20 | Surgery | No recurrence |
| 20 | 84/F | Right lobe (S7) | Anemia | carcinoma of cecum | NA | NA | Surgery | No recurrence |
| 21 | 64/F | NA | Right abdomen pain | NA | NA | NA | Surgery | NA |
| 22 | 35/M | Right lobe (S6) | Normal | Fatty liver | The serum level of alanine aminotransferase rose | 20 | No surgery | The mass disappeared |
| 23 | 33/F | Right lobe (S6) | Right abdomen pain | Normal | Normal | NA | Surgery | No recurrence |
| 24 | 51/F | Right lobe (S6) | Right abdomen pain | Cervical cancer | mild transaminitis and an eosinophilia | 8 | Surgery | No recurrence |
| 25 | 64/F | Left lobe (S3) | Normal | Diarrhea | A slightly raised white cell count | 12 | Surgery | No recurrence |
M: Male; F: Female; NA: not available.
We reported SNNL in a patient with a history of hepatitis B and it has the largest lesion volume among the reported cases. The patient is also the only one with a history of hepatitis B reported so far. In most cases, SNNL nodules were found to be less than 20 mm in size [6]. A few lesions may be larger than 50 mm in size, but all are smaller than 100 mm. Patients with SNNL previously reported also had no history of hepatitis B. Does hepatitis B virus infection affect the size of SNNL lesions? Hepatitis B virus-encoded X protein (HBx) up-regulates special AT-rich binding protein 1 (SATB1) and promotes hepatic fibrosis through paracrine activation of stellate cells [12]. HBx also induces the production of interleukin 8 (IL-8), tumor necrosis factor α (TNF-α), and chemokine ligand 2 (CXCL-2) [13]. These three cytokines can promote inflammation. In addition to inducing the production of inflammatory factors, chronic HBV infection also promotes hepatocyte cloning. During chronic HBV infection, the number of hepatocyte clones detected using integrated HBV DNA as a specific marker was greater than the number of clones where random hepatocyte death and proliferation occurred [14].
We still need to further explore the occurrence and growth mechanism of SNNL. We followed up on the patient in the report. Three months after the operation, the patient was healthy and had no recurrence. Certainly, longer follow-up time is required.
Acknowledgements
The study was supported by the grants from the National Natural Science Foundation of China (Grant number: 81960521).
Informed consent was obtained from the patient described in this study.
Disclosure of conflict of interest
None.
References
- 1.Colagrande S, Politi LS, Messerini L, Mascalchi M, Villari N. Solitary necrotic nodule of the liver: imaging and correlation with pathologic features. Abdom Imaging. 2003;28:41–44. doi: 10.1007/s00261-001-0181-x. [DOI] [PubMed] [Google Scholar]
- 2.Patti R, Cabibi D, Sparacello M, Di Vita G, Montalto G. Solitary necrotic nodule of the liver: different pathological findings express a different histogenesis. Case Rep Gastroenterol. 2008;2:149–154. doi: 10.1159/000128168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang LX, Liu K, Lin GW, Zhai RY. Solitary necrotic nodules of the liver: histology and diagnosis with CT and MRI. Hepat Mon. 2012;12:e6212. doi: 10.5812/hepatmon.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colagrande S, Paolucci ML, Messerini L, Schima W, Stadler A, Bartolotta TV, Vanzulli A, Brancatelli G. Solitary necrotic nodules of the liver: cross-sectional imaging findings and follow-up in nine patients. AJR Am J Roentgenol. 2008;191:1122–1128. doi: 10.2214/AJR.07.3488. [DOI] [PubMed] [Google Scholar]
- 5.Hwang JY, Lee JE, Jung MJ. A challenging case of solitary necrotic nodules of the liver mimicking hepatic metastases: CT, MRI, and PET-CT findings. J Belg Soc Radiol. 2020;104:16. doi: 10.5334/jbsr.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou Y, Li B, Xu F, Wang B, Li D, Liu P, Yang J. Clinical features of solitary necrotic nodule of the liver. Hepatobiliary Pancreat Dis Int. 2008;7:485–489. [PubMed] [Google Scholar]
- 7.Deniz K, Coban G. Solitary necrotic nodule of the liver: always benign? J Gastrointest Surg. 2010;14:536–540. doi: 10.1007/s11605-009-1120-3. [DOI] [PubMed] [Google Scholar]
- 8.Kondi-Pafiti AI, Grapsa DS, Kairi-Vasilatou ED, Voros DK, Smyrniotis VE. “Solitary” necrotic nodule of the liver: an enigmatic entity mimicking malignancy. Int J Gastrointest Cancer. 2006;37:74–78. doi: 10.1007/s12029-007-0002-8. [DOI] [PubMed] [Google Scholar]
- 9.Berry CL. Solitary “necrotic nodule” of the liver: a probable pathogenesis. J Clin Pathol. 1985;38:1278–1280. doi: 10.1136/jcp.38.11.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsui W, Yuen R, Chow L, Tse C. Solitary necrotic nodule of the liver: parasitic origin? J Clin Pathol. 1992;45:975–978. doi: 10.1136/jcp.45.11.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang HQ, Wu ZS, Li DW. Solitary necrotic nodule of the liver: a report of two cases and review of the literature. Case Reports Hepatol. 2011;2011:845406. doi: 10.1155/2011/845406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong J, Tu W, Han J, He J, Liu J, Han P, Wang Y, Li M, Liu M, Liao J, Tian D. Hepatic SATB1 induces paracrine activation of hepatic stellate cells and is upregulated by HBx. Sci Rep. 2016;6:37717. doi: 10.1038/srep37717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, He T, Wan B, Wang X, Zhang L. Orexin A ameliorates HBV X protein-induced cytotoxicity and inflammatory response in human hepatocytes. Artif Cells Nanomed Biotechnol. 2019;47:2003–2009. doi: 10.1080/21691401.2019.1614014. [DOI] [PubMed] [Google Scholar]
- 14.Mason WS, Jilbert AR, Litwin S. Hepatitis B virus DNA integration and clonal expansion of hepatocytes in the chronically infected Liver. Viruses. 2021;13:210. doi: 10.3390/v13020210. [DOI] [PMC free article] [PubMed] [Google Scholar]


