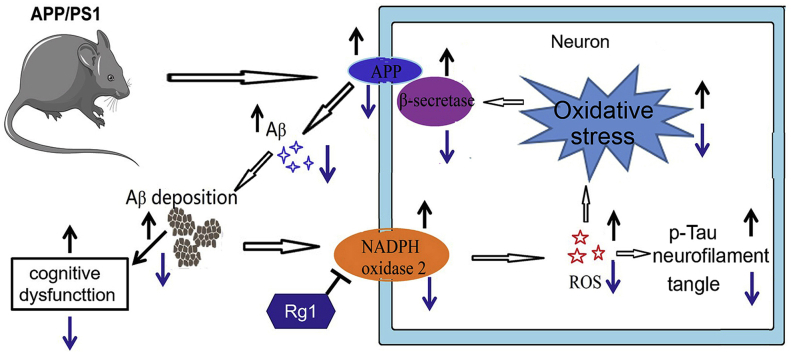
Abstract
Background
Ginsenoside Rg1 (Rg1), an active ingredient in ginseng, may be a potential agent for the treatment of Alzheimer’s disease (AD). However, the protective effect of Rg1 on neurodegeneration in AD and its mechanism of action are still incompletely understood.
Methods
Wild type (WT) and APP/PS1 AD mice, from 6 to 9 months old, were used in the experiment. The open field test (OFT) and Morris water maze (MWM) were used to detect behavioral changes. Neuronal damage was assessed by hematoxylin and eosin (H&E) and Nissl staining. Immunofluorescence, western blotting, and quantitative real-time polymerase chain reaction (q-PCR) were used to examine postsynaptic density 95 (PSD95) expression, amyloid beta (Aβ) deposition, Tau and phosphorylated Tau (p-Tau) expression, reactive oxygen species (ROS) production, and NAPDH oxidase 2 (NOX2) expression.
Results
Rg1 treatment for 12 weeks significantly ameliorated cognitive impairments and neuronal damage and decreased the p-Tau level, amyloid precursor protein (APP) expression, and Aβ generation in APP/PS1 mice. Meanwhile, Rg1 treatment significantly decreased the ROS level and NOX2 expression in the hippocampus and cortex of APP/PS1 mice.
Conclusions
Rg1 alleviates cognitive impairments, neuronal damage, and reduce Aβ deposition by inhibiting NOX2 activation in APP/PS1 mice.
Keywords: Ginsenoside Rg1, Aβ deposition, NADPH oxidase 2, APP/PS1 mice, Alzheimer’s disease
Graphical abstract
Highlights
-
•
Rg1 treatment significantly alleviated cognitive dysfunction and neuronal damage in APP/PS1 mice.
-
•
Rg1 treatment significantly reduced APP expression and Aβ deposition in APP/PS1 mice.
-
•
The expression of NOX2 and ROS production were significantly increased in APP/PS1 mice.
-
•
Rg1 treatment significantly decreased NOX2 expression and ROS accumulation in APP/PS1 mice.
1. Introduction
Alzheimer’s disease (AD) is a common neurodegenerative disease in the elderly population. Clinically, the main pathological features of AD are extracellular amyloid beta (Aβ) deposition, intracellular neurofibrillary tangles formed by highly phosphorylated Tau (p-Tau), and neurodegenerative changes accompanied by synaptic dysfunction and neuronal damage [1]. Among them, Aβ deposition has been considered to play crucial roles in the progression of AD. However, the pathogenesis of AD is still incompletely understood, and there are currently no effective agents to prevent the generation and progression of AD.
In recent years, natural medicines with fewer side effects and safer performance have been widely used to treat many diseases, including AD [2,3]. Ginseng has been used safely since ancient times to improve health and delay aging [4]. Ginsenoside Rg1 (Rg1), an active ingredient in ginseng, is a tetracyclic triterpenoid saponin [5]. Rg1 is thought to be a potential neuroprotective agent in several animal models of neurological diseases and cognitive impairments [6,7]. Our previous study demonstrated that Rg1 alleviated hydrogen peroxide (H2O2)-induced neuronal damage by inhibiting NADPH oxidase 2 (NOX2) activation and reactive oxygen species (ROS) production in hippocampal neurons in vitro [8]. Hence, Rg1 might delay the occurrence and development of AD. However, the precise effect and mechanism of Rg1 on neurodegeneration in AD are still unclear.
Growing evidence suggests that ROS play important roles in the pathogenesis of many neurodegenerative diseases. Excessive ROS generation can induce oxidative stress damage, which is considered to contribute to the progression of AD [9]. NOXs are the main enzymes that contribute to ROS elevation in many tissues and are also involved in the pathological process of AD [10]. NOX2 is constitutively expressed in the brain, particularly in neurons [11]. A recent study suggested that NOX2 contributes to aging-related neuronal oxidative stress damage and loss of brain function [12]. Our previous research indicated that NOX2 expression was significantly upregulated in prolonged culture of hippocampal neurons [13]. Moreover, treatment with Rg1 significantly reduced the NOX2 expression in H2O2-treated hippocampal neurons [8]. Therefore, Rg1 might alleviate cognitive impairment and neurodegeneration by inhibiting NOX2-mediated neuronal oxidative stress in AD.
The APP/PS1 mouse model of AD exhibits remarkable elevation of Aβ production associated with certain behavioral abnormalities. In this research, we hypothesized that Rg1 treatment alleviates Aβ generation and deposition by inhibiting NOX2 activation in APP/PS1 mice. Apocynin, a NOX inhibitor, has been reported to possess antioxidant effects that are beneficial as a treatment for neurological diseases [14,15]. In this study, we investigated the protective effects and mechanism of Rg1 and apocynin on locomotor activity, cognitive ability, neuronal damage, Aβ generation, and NOX2 expression in APP/PS1 mice. We found that Rg1 shows the potential to alleviate the cognitive impairments and Aβ generation through inhibiting NOX2 expression and ROS accumulation in APP/PS1 mice.
2. Materials and methods
2.1. Animals and treatment
Male APP/PS1 mice were used in the experiment, and age- and gender-matched wild type (WT) mice were used as controls. Mice were housed in standard laboratory conditions. Six-month-old (6M) WT and APP/PS1 mice were divided into 6 groups: WT-9M control, APP/PS1-6M control, APP/PS1-9M control, APP/PS1-9M Apocynin (50 mg/kg body weight), APP/PS1-9M Rg1 (5 mg/kg body weight), and APP/PS1-9M Rg1 (10 mg/kg body weight). Apocynin (Merck Millipore) and Rg1 (content > 98%; Chengdu Desite Biotechnology Co., China) were dissolved in water and were intragastrically administered (0.1 mL/10 g body weight) from 6 to 9 months. The WT-9M control and the APP/PS1-9M control received the same amount of water for 12 weeks. After behavioral testing, the brains were removed for other experiments.
2.2. Open field test (OFT)
The OFT equipment (Shanghai Biotechnology Co., Ltd.) includes a video-tracked cage (60 × 60 × 50 cm) and a behavior tracking system (ANY-maze, Stoelting Co., Wood Dale, IL, USA). The test was performed as described in a previous study [16]. The moving distance (m), the movement speed (m/s), and the number of lines crossed and stand up were calculated by the software to indicate the motor activity behavior.
2.3. Morris water maze (MWM) test
The test includes four training trials (one per day) and exploration trials on day 5 [17]. The mean escape latency (MEL, s) on days 1–4 was used to assess the learning ability. On day 5, each mouse performed a 60 s swimming probe trial without a platform. The swimming time in the quadrant of the platform, the latency of first entry to the platform, and the number of times crossing the platform were calculated to assess memory.
2.4. Histological examination
Paraffin-embedded brain tissues were sectioned at 5 μm using a microtome (Leica, Nussloch, Germany). Hematoxylin and eosin (H&E) and Nissl staining were performed to examine the pathological changes in cortex and hippocampus using a digital slice scanning and analysis system (Pannoramic MIDI, 3DHISTECH, Hungary). The density of the Nissl bodies was analyzed double-blindly with Image-Pro Plus 6.0 automatic analysis mode to assess the amount of Nissl bodies.
2.5. Determination of ROS production
Dihydropyridine (DHE) staining was used to examine the ROS production in the brain [4]. Briefly, DHE (Beyotime Biotechnology, Shanghai, China) was injected via the tail vein (100 μM, 0.1 mL/10 g body weight). Thirty minutes later, the brains were removed and then embedded in OCT (Sakura, Torrance, CA, USA), and were sectioned at 10 μm with a freezing microtome (Leica, Germany). The slices were washed and stained with DAPI for 5 min and were observed and photographed with a fluorescence microscope with an attached camera (Olympus IX71, Japan). The mean fluorescence density was analyzed double-blindly from the hippocampal CA1 and CA3 and cortex in each section to assess the ROS production.
2.6. Immunofluorescence
Tissue sections were treated with primary antibodies against postsynaptic density 95 (PSD95; 1:100, Servicebio Technology, China, GB11277) overnight at 4°C. The next day, the sections were treated with secondary antibody conjugated to rhodamine (1:200, ZSGB-BIO, China). The sections were then viewed and photographed with a fluorescence microscope with an attached camera (Olympus IX71). The fluorescence density was analyzed double-blindly from the hippocampal CA1 and CA3 and cortex to evaluate PSD95 expression.
2.7. Thioflavin-S staining
The sections were immersed in 1% thioflavin-S (HY-D0972, MedChemExpress USA) for 5 min, hydrated in 70% ethanol, and washed 2 × 5 min with phosphate-buffered saline (PBS). After staining with Hoechst 33258 for 10 min, the sections were sealed with a fluorescent mounting media. Pannoramic MIDI was used to scan the images and analyze the data. The percentage of thioflavin-S-positive areas was determined to evaluate amyloid deposition.
2.8. Immunoblot analysis
Total protein in the hippocampus and cortex was extracted using a cryogenic tissue grinder (Shanghai Jingxin Industrial Development Co., Ltd., Shanghai, China) at 4°C and 60 Hz for 50s. The protein concentration was determined using the BCA Protein Assay Kit (Beyotime Biotechnology). The samples (n = 4 per group) were separated and transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA). The membranes were incubated with 5% non-fat milk to block nonspecific protein binding and then incubated with primary antibodies (Table S1) overnight at 4°C. Subsequently, the membranes were incubated in horseradish-peroxidase-conjugated secondary antibody (ZSGB-BIO, ZF-2301, 1:10,000) for 1 h. The bands were visualized by using a mini imaging system (Chemi Q4800, Shanghai Bioshine Technology, Shanghai, China). The band densities were measured using Image J. The relative density divided by the WT control was calculated to show the changes of target proteins.
2.9. Quantitative real-time polymerase chain reaction (q-PCR) analysis
Total RNA was extracted from the cortex and hippocampus (n = 4 per group) as previously described [18]. Complementary DNA (cDNA) was synthesized using PrimeScript™ Reverse Transcriptase Kit (Takara Bio, RR037A, Japan). The SYBR®Premix Ex Taq™II RTPCR kit (Takara Bio, RR820A) was used for q-PCR of amyloid precursor protein (APP), beta-site APP-cleaving enzyme (BACE), nicastrin (NCSTN), NOX2, p22phox, p47phox, and β-actin. Primers are listed in Table S2. The transcript levels were assessed using the 2−ΔΔCT method.
2.10. Statistical analysis
All data are expressed as the mean ± standard deviation (SD). GraphPad Prism 6.0 was used to perform statistical analysis. Statistical significance was defined as P < 0.05. Repeated-measures analysis of variance (ANOVA) was used to analyze the results of OFT from 6 to 9 months and MWM training from days 1 to 4. Other data were analyzed by using one-way ANOVA, and Student’s t-test was performed to compare the difference between groups.
3. Results
3.1. Rg1 treatment ameliorated abnormal behaviors in APP/PS1 mice
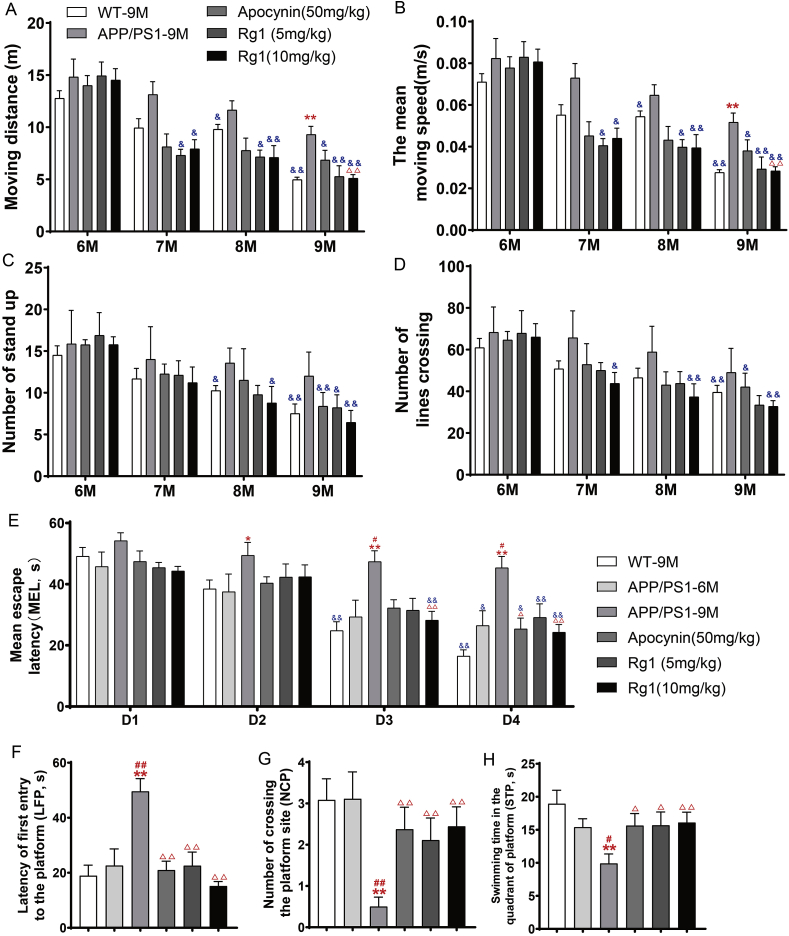
The OFT results showed that there were no obvious differences between the WT-9M and APP/PS1-6M groups before Rg1 and apocynin treatment. However, the moving distance and mean moving speed were significantly increased in the APP/PS1-9M group compared with the WT-9M group, and APP/PS1 mice treated with 10 mg/kg Rg1 showed a significantly decreased moving distance and mean moving speed compared with the APP/PS1-9M group (Fig. 1A and B). Hence, APP/PS1 mice showed significant motor hyperactivity as they aged, and Rg1 treatment reduced this motor hyperactivity.
Fig. 1.
Rg1 treatment ameliorated abnormal behaviors in APP/PS1 mice. OFT: (A) The total moving distance (m); (B) The mean moving speed (m/s); (C-D) The number of stand up and lines crossing. MWM: (E) The mean escape latency (MEL, s); (F) The latency of first entry to the platform (LFP, s); (G) The number of crossing the platform (NCP); (H) The swimming time in the quadrant of platform (STP, s). Results are expressed as mean ± SD. APP/PS1-6M group, n = 9; orther groups, n = 12. ∗P < 0.05, ∗∗P < 0.01 vs WT-9M group; #P < 0.05, ##P < 0.01 vs APP/PS1-6M group; ΔP<0.05, ΔΔP<0.01 vs APP/PS1-9M group; &P < 0.05, &&P < 0.01 vs 6M group in OFT and D1 in MWM.
We also used the MWM to observe the effect of Rg1 on learning and memory impairments in APP/PS1 mice. Based on the training trial results, the escape latency was not significantly different on the first day (D1) among the experimental groups. However, compared with the escape latency on D1, the escape latency in the WT-9M group was significantly reduced on D3 and D4 (Fig. 1E); the APP/PS1-6M group also had a decreasing trend and a significantly reduced escape latency on D4 (Fig. 1E). However, the APP/PS1-9M group showed no difference in escape latency (Fig. 1E). Similarly to the WT-9M group, the escape latency in the APP/PS1 groups treated with apocynin or Rg1 showed a decreasing trend and was significantly reduced on D4 (Fig. 1E). However, the escape latency was significantly increased on D2, D3, and D4 in the APP/PS1-9M compared with the WT-9M group (Fig. 1E). Compared with the APP/PS1-9M group, the APP/PS1 groups treated with apocynin or 10 mg/kg Rg1 showed a significantly reduced escape latency on D4 (Fig. 1E). For the probe test (D5), the latency of the first entry to the platform (LFP, s) was significantly prolonged (Fig. 1F), and the number of crossing platform(NCP) (Fig. 1G) and swimming time in the quadrant of the platform (STP, s) (Fig. 1H) were significantly decreased in the APP/PS1-9M group compared with the WT-9M or APP/PS1-6M groups. Apocynin or Rg1 (5 and 10 mg/kg) treatment significantly reduces the LFP and increased the NCP and STP compared with the APP/PS1-9M group (Fig. 1F–H). Hence, Rg1 improved the learning and memory impairments in APP/PS1 mice.
3.2. Rg1 treatment alleviated neuronal damage in APP/PS1 mice
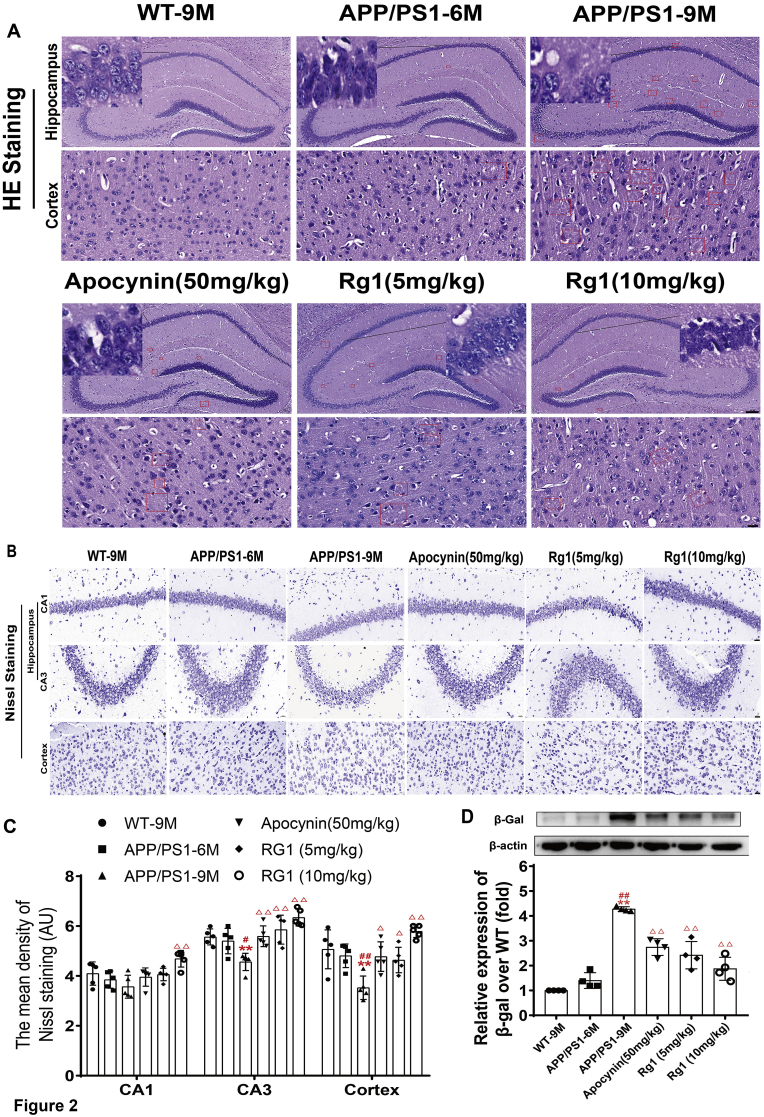
To determine the effect of Rg1 treatment on neuronal damage in APP/PS1 mice, we further observed the pathological changes using H&E and Nissl staining. The H&E results indicated no abnormal changes in the cortex and hippocampus in WT-9M mice. Compared with WT-9M mice, there were a few neuronal pathological changes in APP/PS1-6M mice, such as unclear nucleoli, karyopyknosis, and amyloid plaques (shown in the red square), suggesting that there were slight neuronal injuries in hippocampus and cortex. In APP/PS1-9M mice, there was obvious neuronal damage and amyloid plaques in the hippocampus and cortex. When APP/PS1 mice were treated with apocynin or Rg1, there was markedly less neuronal damage and amyloid plaque in the hippocampus and cortex (Fig. 2A). Thus, Rg1 alleviated the neuronal damage in APP/PPS1 mice.
Fig. 2.
Rg1 treatment alleviated neuronal damage in cortex and hippocamppus in APP/PS1 mice. (A) The results of H&E staining (Hippocampus 100 × , the bar = 100 μm; Cortex 400 × , the bar = 20 μm, n = 5). The red boxes indicate Aβ deposition areas. (B) The results of Nissl staining in the cortex, hippocampus CA1 and CA3 (400 × , the bar = 20 μm, n = 5); (C) The mean density of Nissl bodies in the cortex, hippocampus CA1 and CA3. (D) The bands and relative expression of β-Gal over WT-9M (Western blot, n = 4). Results are expressed as mean ± SD. ∗P < 0.05, ∗∗P < 0.01 vs WT-9M group; #P < 0.05, ##P < 0.01 vs APP/PS1-6M group; ΔP<0.05, ΔΔP<0.01 vs APP/PS1-9M group.
There were abundant Nissl-positive bodies in the hippocampus and cortex in WT-9M and APP/PS1-6M mice. Compared with WT-9M mice, the number of Nissl-positive bodies was significantly reduced in the hippocampal CA3 area and cortex in APP/PS1-9M mice (Fig. 2B and C). Compared with APP/PS1-9M mice, APP/PS1 mice treated with apocynin or Rg1 showed a significantly increased number of Nissl-positive bodies in the hippocampal CA3 area and cortex, particularly in the group treated with 10 mg/kg Rg1 (Fig. 2B and C). The results indicated that Rg1 treatment had a protective effect on neuronal damage in APP/PS1 mice.
β-gal activity is often measured to evaluate age-related neuronal damage [19]. The results showed that there was little β-gal expression in WT-9M and APP/PS1-6M mice, but β-gal expression was significantly increased in APP/PS1-9M mice (Fig. 2D). β-gal expression was significantly reduced in APP/PS1 mice treated with apocynin or Rg1 (Fig. 2D). Overall, Rg1 ameliorated aging-associated neuronal damage in APP/PS1 mice.
3.3. Rg1 treatment increased PSD95 expression in APP/PS1 mice
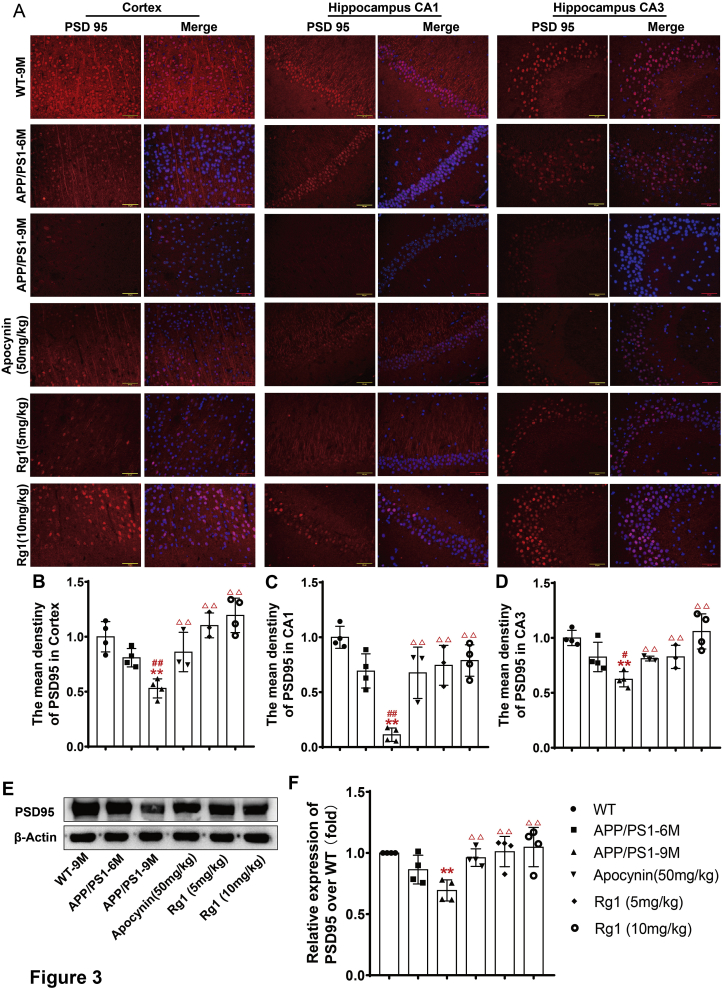
Behavioral dysfunction is usually accompanied by changes in synaptic structure and function [20]. The immunofluorescence results showed that there was abundant PSD95 expression in the WT-9M group. Compared with the WT-9M group, PSD95 expression was reduced, albeit not significantly, in the APP/PS1-6M group. In the APP/PS1-9M group, the PSD95 expression was significantly reduced compared with the WT-9M group (Fig. 3A–D). Compared with the APP/PS1-9M groups, the APP/PS1 groups treated with apocynin or Rg1 presented significantly increased PSD95 expression (Fig. 3A–D). The immunoblot results were similar to the immunofluorescence findings (Fig. 3E and F). The results indicated that Rg1 treatment might improve the synaptic dysfunction in APP/PS1 mice.
Fig. 3.
Rg1 treatment increased the expression of PSD95 in APP/PS1 mice. (A) The expression of PSD95 in the cortex, hippocampus CA1 and CA3 (immunofluorescence, 400 × , the bar = 20 μm. n = 4); (B-D) The mean density of PSD95 in cortex, hippocampus CA1 and CA3; (E) The bands of PSD95 and β-actin (Western blot, n = 4); (F) The relative expression of PSD95 over WT-9M. Results are expressed as mean ± SD. ∗P < 0.05, ∗∗P < 0.01 vs WT-9M group; #P < 0.05, ##P < 0.01 vs APP/PS1-6M group; ΔP<0.05, ΔΔP<0.01 vs APP/PS1-9M group.
3.4. Rg1 treatment reduced Aβ deposition and p-Tau in APP/PS1 mice
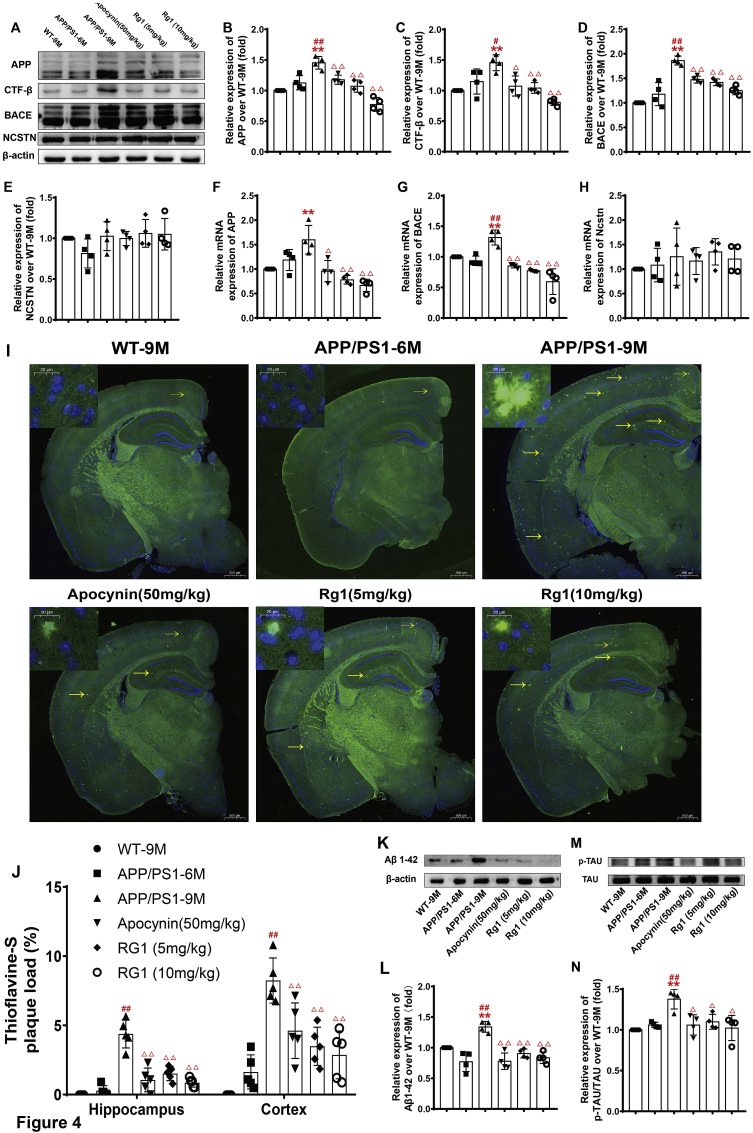
Aβ deposition is closely related to the generation and progression of pathological AD characteristics in APP/PS1 mice [21]. Using q-PCR, the expression levels of APP, CTF-β, and BACE in the APP/PS1-6M and WT-9M groups were similar, but the levels were significantly increased in the APP/PS1-9M group (Fig. 4A–D). Compared with the APP/PS1-9M group, the APP/PS1 groups treated with apocynin or Rg1 showed significantly decreased expression of APP, CTF-β, and BACE (Fig. 4A–D). NCSTN was not significantly different among the groups (Fig. 4A and E). The changes in APP and BACE mRNA expression were similar to the changes in protein expression (Fig. 4F–H). Treatment with Rg1 decreased the expression of APP and BACE but did not affect NCSTN expression.
Fig. 4.
Rg1 treatment reduced Aβ deposition in APP/PS1 mice. (A) The bands of APP, CTF-β, BACE, NCSTN and β-actin (Western blot); (B-E) The relative expression of APP, CTF-β, BACE and NCSTN over WT-9M; (F–H) The relative mRNA expressions of APP, BACE and NCSTN (q-PCR); (I) The Aβ deposition in cortex and hippocamus (Thioflavin-S staining, 20 × , the bar = 500 μm, yellow arrows indicate Aβ deposition); (J) The ratio of Aβ plaque load (%) in cortex and hippocampus; (K) The bands of Aβ1-42 and β-actin (Western blot); (L) The relative expression of Aβ1-42 over WT-9M. (M) The bands of TAU and p-TAU (Western blot); (N) The relative expression of p-TAU/TAU over WT-9M. Results are expressed as mean ± SD, Thioflavin-S staining, n = 5; other experiments, n = 4. ∗P < 0.05, ∗∗P < 0.01 vs WT-9M group; #P < 0.05, ##P < 0.01 vs APP/PS1-6M group; ΔP<0.05, ΔΔP<0.01 vs APP/PS1-9M group.
We further examined the Aβ deposition by thioflavin-S staining and Aβ1–42 expression by immunoblot. Compared with the WT-9M group, Aβ deposition was slightly increased in the APP/PS1-6M group, while in the APP/PS1-9M group, the Aβ deposition was markedly increased in the hippocampus and cortex (Fig. 4I and J). Compared with the APP/PS1-9M group, the APP/PS1 groups treated with apocynin or Rg1 had significantly decreased Aβ deposition both in the hippocampus and cortex (Fig. 4I and J). The expression of Aβ1–42 was similar to the thioflavin-S staining (Fig. 4K and L). Thus, Rg1 inhibited Aβ generation and deposition and reduced the formation of amyloid plaque.
We also observed the effect of Rg1 treatment on the levels of Tau and p-Tau. Tau expression was not different among the groups. Compared with the WT-9M group, the level of p-Tau was obviously increased in the APP/PS1-9M group (Fig. 4M and N). Compared with the APP/PS1-9M group, the APP/PS1 groups treated with apocynin or Rg1 presented a significantly reduced level of p-Tau (Fig. 4M and N). The results suggest that Rg1 treatment might alleviate the neurofilament tangle induced by hyperphosphorylation of Tau.
3.5. Rg1 treatment reduced ROS generation and NOX2 expression in APP/PS1 mice
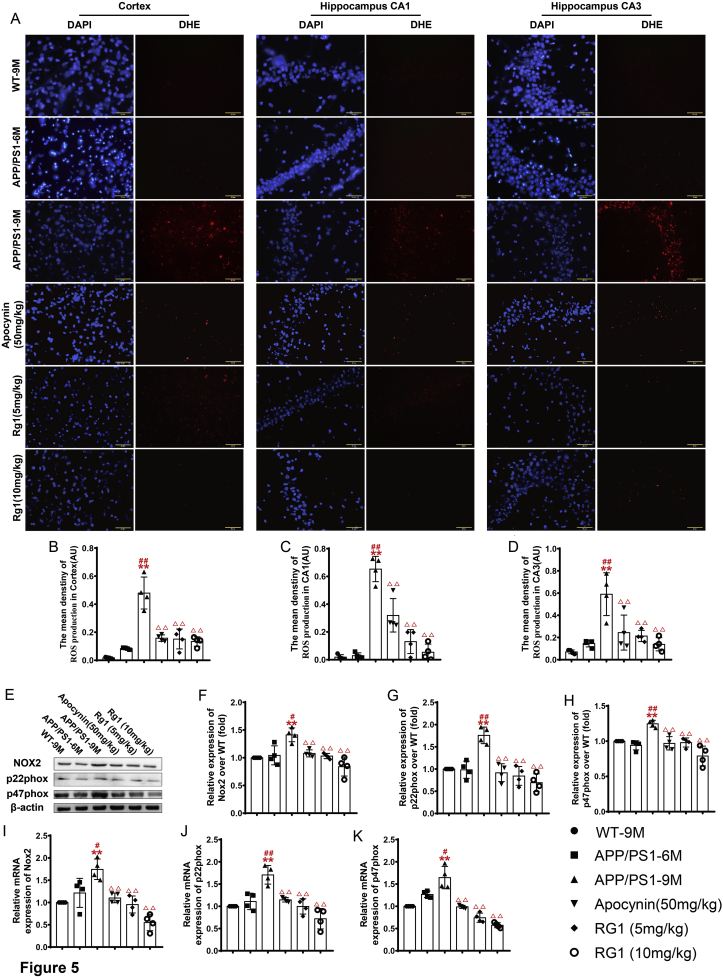
ROS accumulation plays vital roles in promoting the pathogenesis of AD [22,23]. Our results showed that there was slight ROS accumulation in the cortex and hippocampus in the WT-9M and APP/PS1-6M groups. There was marked ROS production in the cortex and hippocampus in the APP/PS1-9M compared with the WT-9M group (Fig. 5A–D). Compared with the APP/PS1-9M group, the APP/PS1 groups treated with apocynin or Rg1 showed significantly reduced ROS production in the cortex and hippocampal CA1 and CA3 areas (Fig. 5A–D). The results suggest that ROS accumulation might exacerbate the pathogenesis of AD and Rg1 reduced ROS production in the APP/PS1 mice.
Fig. 5.
Rg1 treatment decreased the level of ROS and the expressions of NADPH oxidase 2 in APP/PS1 mice. (A) The ROS production in the cortex, hippocampus CA1 and CA3 (DHE fluorescence staining, 400 × , the bar = 50 μm); (B-D) The mean density of ROS production in the cortex and hippocampus CA1, CA3. (E) The bands of NOX2, p22phox, p47phox and β-actin (Western blot); (F–H) The relative expression of NOX2, p22phox and p47phox over WT-9M; (I–K) The relative mRNA expression of NOX2, p22phox and p47phox (q-PCR). Results are expressed as mean ± SD, n = 4. ∗P < 0.05, ∗∗P < 0.01 vs WT-9M group; #P < 0.05, ##P < 0.01 vs APP/PS1-6M group; ΔP<0.05, ΔΔP<0.01 vs APP/PS1-9M group.
We further detected the expression of NOX2-related proteins and mRNAs. Regarding protein expression, compared with the WT-9M group, NOX2, p47phox, and p22phox were similar to the levels in the APP/PS1-6M group, while they were significantly upregulated in the APP/PS1-9M group (Fig. 5E–H). Compared with the APP/PS1-9M group, the APP/PS1 groups treated with apocynin or Rg1 had significantly decreased levels of NOX2, p22phox, and p47phox (Fig. 5E–H). Similarly to their proteins, the expression of NOX2, p22phox, and p47phox mRNA was also significantly increased in the APP/PS1-9M compared with the WT-9M group (Fig. 5I–K), and apocynin or Rg1 treatment significantly reduced their levels (Fig. 5I–K). The results suggest that Rg1 treatment might reduce ROS generation by inhibiting the expression of NOX2 in APP/PS1 AD mice.
4. Discussion
AD is characterized by progressive cognitive dysfunction, Aβ deposition, Tau hyperphosphorylation, and neurodegeneration in the hippocampus and cortex. The pathogenesis of AD is still not completely understood, but growing evidence has focused on ROS-mediated oxidative stress. There are currently no completely effective medicines for the treatment of AD. Since ancient times, ginsenoside Rg1 (Rg1), a bioactive component in ginseng, has been used to delay senescence. Our previous studies demonstrated that Rg1 ameliorates H2O2-induced hippocampal neuron damage in vitro [8]. In this study, we hypothesized that Rg1 treatment improves learning and memory and alleviates Aβ deposition and neuronal damage by inhibiting NOX2 activation and reducing ROS-mediated oxidative stress in APP/PS1 mice. We found that Rg1 treatment alleviated the learning and memory impairments and neuronal damage; reduced the expression of APP, BACE, and Aβ1–42; decreased Aβ deposition and p-Tau; and reduced ROS accumulation and NOX2 expression in APP/PS1 mice. Hence, Rg1 treatment might be a potential therapeutic agent for preventing the progression of AD.
The APP/PS1 transgenic mouse model is the most commonly used AD model. These mice develop spatial memory dysfunction and show Aβ deposition, synapse loss, and neuronal death similar to the pathological features in AD [24,25]. It has been reported that amyloid plaque can occur in the neocortex at 6–8 weeks and significantly increases at 8 months of age [26], the synaptic dysfunction appears at about 3 months of age [27], and the cognitive disability starts to show at about 6 months of age [28]. Therefore, we observed the effect of Rg1 treatment on APP/PS1 mice from 6 to 9 months in this study. The behavioral results indicated that the APP/PS1-6M mice showed an increase of hyperactivity in the OFT, which is consistent with other studies [29], and slight learning and memory impairments in the MWM test. Of note, APP/PS1-9M mice showed significant hyperactivity and learning and memory impairments compared with the WT-9M mice. A recent study suggests that hyperactivity is an anxiety symptom, resulting in a more rapid decline in several cognitive functions [30]. We speculate that excessive Aβ generation in the APP/PS1 mice might overexcite the brain and thus induce hyperactivity and neuronal damage. In addition, Rg1 treatment significantly decreased the hyperactivity and alleviated learning and memory impairments, suggesting a protective effect of Rg1 on abnormal behavior in APP/PS1-9M mice.
AD-induced behavioral dysfunction is thought to be related to the structural and functional impairments of specific brain regions [31]. The hippocampus and cortex are especially vulnerable regions that are affected by Aβ [32]. In this study, APP/PS1-6M mice showed slight Aβ deposition and neuronal damage in the hippocampus and cortex, while APP/PS1-9M mice presented neuronal damage and Aβ deposition, and there were fewer Nissl bodies in the hippocampus and cortex. However, Rg1 treatment alleviated neuronal damage and Aβ deposition. To confirm the protective effect of Rg1 treatment on APP/PS1 mice, we detected the expression of senescence-associated β-gal, which is a critical biomarker of cell aging. The results showed that Rg1 treatment also reduced β-gal expression, which was also significantly increased in the APP/PS1-9M mice. PSD95 is crucial for synaptic plasticity and is widely accepted as a protein that can regulate synapse function [33]. It has been reported that PSD95 is significantly decreased in the hippocampus and cortex of AD patients and negatively correlated with the severity of cognitive deficits [34]. The present study showed that PSD95 expression was significantly decreased in the hippocampus and cortex of APP/PS1-9M mice, but these changes were reversed after Rg1 treatment for 12 weeks, suggesting that Rg1 treatment might reverse the synaptic dysfunction of AD mice. These data indicate that the changes in neuronal damage and Aβ deposition are associated with behavioral disorders in APP/PS1 mice, and Rg1 treatment could alleviate the Aβ deposition and neuronal damage in APP/PS1 mice.
Amyloid plaques are important pathological characteristics of AD. Aβ plays a crucial role in the progression of AD pathologies [35]. A recent study reported that excessive Aβ generation and deposition could change cell metabolism and trigger Tau hyperphosphorylation, synaptic dysfunction, oxidative stress, and neuroinflammation, further leading to progressive recognition dysfunction [1]. APP plays an important role during the occurrence of AD [35]. APP can be cleaved by β/γ-secretase into SAPPβ fragments and pernicious Aβ peptides [36]. APP can also be cleaved to produce a nonamyloidogenic peptide and a neuroprotective fragment of SAPPα by α/γ-secretase [37]. BACE is reported to possess β-secretase activity. BACE overexpression accelerates APP cleavage into CTF-β. NCSTN, one protein component of γ-secretase, reportedly possesses γ-secretase activity, which further cleaves CTF-β into Aβ. In the present study, Rg1 treatment decreased the expression of APP, BACE, and CTF-β, which were significantly increased in APP/PS1-9M mice, but had no effect on NCSTN expression. Our results also indicated that Rg1 treatment significantly reduced the expression of Aβ1–42 and the formation of amyloid plaque in APP/PS1 mice. The q-PCR results demonstrated that Rg1 treatment decreased the levels of APP and BACE mRNA in APP/PS1 mice. Rg1 treatment also reduced the levels of p-Tau and senescence-associated β-gal, which were increased in the APP/PS1-9M mice. These data suggest that Rg1 may not only decrease APP expression but also inhibit the β-secretase pathway to hinder pernicious Aβ formation and deposition. Moreover, Aβ degradation also plays an important role in ameliorating pathological characteristics of AD. A recent study indicated that treatment with transcrocetin, an active constituent of Crocus sativus L., significantly increased Aβ1-42 degradation in CD14+ monocytes of AD patients through the upregulation of the lysosomal protease cathepsin B [38]. From the current results, it is unclear whether Rg1 can promote the degradation of Aβ. This topic requires further study.
While the Aβ cascade has been considered the most important factor for AD pathogenesis, there are still no clinically effective drugs acting on Aβ that prevent AD. Hence, other mechanisms are likely involved in AD. Growing evidence suggests that ROS accumulation plays an important role in the progression of AD [39,40] and may even be the initial pathogenic event of AD [41]. NOX is the only enzyme family known to produce ROS as its sole function [42]. The NOX complex is composed of several isoforms such as NOX1–5. NOX2 is composed of the gp91phox (NOX2) and p22phox (membrane heterodimers) and p47phox, p40phox, p67phox, and RAC (cytosolic subunits) [43]. In the brain, NOX2 is mainly expressed in neurons and is closely involved in the progression of AD [44]. However, it is still unknown whether NOX2 activation is involved in regulation of Aβ generation and deposition in the progression of AD. Growing evidence suggests that Rg1 exerts significant antioxidant and protective effects in in vivo and in vitro neurological disease models [45,46]. Our previous study indicated that Rg1 treatment could reduce oxidative stress and neuroinflammation and delay neuronal senescence in primary cultured hippocampal neurons in vitro [13]. The present results demonstrated that Rg1 treatment decreased the levels of ROS in cortex and hippocampus. In addition, Rg1 treatment significantly reduced the protein and mRNA expression of NOX2-related factors, namely NOX2, p22phox, and p47phox. These data suggest that the protective effects of Rg1 on APP/PS1 mice might be closely related to NOX2 inhibition.
Rg1 is not the only drug that can effectively inhibit oxidative stress. The latest research suggests that green tea catechins can also inhibit the generation of ROS, improve spatial memory, and accelerate Aβ degradation [47,48]. However, the expected therapeutic effect has not been achieved in clinical trials. In fact, there is a big difference between murine and human microglial cells. Murine microglial cells promote inflammation in AD mice, which is closely related to ROS. However, human microglial cells do not express inducible nitric oxide synthase (iNOS), an enzyme that produces nitric oxide (NO), but do express neuroprotective and neuroregenerative growth/neurotrophic factors (GF/NF) [49]. In the present study, we found that Rg1 treatment significantly alleviated the neurodegenerotion in APP/PS1 mice. While there are few clinical studies of Rg1 on AD patients, and it is still unknown whether Rg1 can improve the occurrence and development of AD in humans.
In summary, we have demonstrated that Rg1 treatment alleviated learning and memory impairments, synaptic dysfunction, and neuronal damage, and reduced Aβ generation and deposition in APP/PS1 mice. Rg1 significantly decreased ROS production and inhibited NOX2 expression in the hippocampus and cortex of APP/PS1 mice. These data suggest that Aβ deposition may increase NOX2 expression and ROS generation, which further upregulate the expression of APP and BACE, resulting in a vicious circle in APP/PS1 mice. Rg1 treatment significantly decreases NOX2 expression and ROS production and thus breaks the vicious circle in APP/PS1 mice. However, we only evaluated the basic effects of Rg1 on preventing the progression of AD in vivo, and the precise mechanisms of Rg1 on the regulation of NOX2 and Aβ generation warrant further investigation. Furthermore, a therapeutic experiment may be needed to examine the effect of Rg1 treatment on Aβ degradation in APP/PS1 mice from 9 to 12 months of age. In conclusion, despite its limitations, the study provides valuable preclinical data for the use of Rg1 in AD.
Declaration of competing interest
The authors declared that they had no conflicts of interests.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81671384, 81970630) and the Major projects of Anhui Provincial Department of Education (KJ2020ZD14). The authors thank Bao Li and Dake Huang in the Synthetic Laboratory of Basic Medicine College for their technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2021.03.003.
Contributor Information
Weiping Li, Email: lwp19@126.com.
Weizu Li, Email: liweizu@126.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Zhang Z., Shen Q., Wu X., Zhang D., Xing D. Activation of PKA/SIRT1 signaling pathway by photobiomodulation therapy reduces Abeta levels in Alzheimer’s disease models. Aging Cell. 2020;19(1) doi: 10.1111/acel.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar H., More S.V., Han S.D., Choi J.Y., Choi D.K. Promising therapeutics with natural bioactive compounds for improving learning and memory--a review of randomized trials. Molecules. 2012;17(9):10503–10539. doi: 10.3390/molecules170910503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y., Kan H., Yin Y., Wu W., Hu W., Wang M. Protective effects of ginsenoside Rg1 on chronic restraint stress induced learning and memory impairments in male mice. Pharmacol Biochem Behav. 2014;120:73–81. doi: 10.1016/j.pbb.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Fan C., Song Q., Wang P., Li Y., Yang M., Yu S.Y. Neuroprotective effects of ginsenoside-rg1 against depression-like behaviors via suppressing glial activation, synaptic deficits, and neuronal apoptosis in rats. Front Immunol. 2018;9:2889. doi: 10.3389/fimmu.2018.02889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Shim J.S., Song M.Y., Yim S.V., Lee S.E., Park K.S. Global analysis of ginsenoside Rg1 protective effects in beta-amyloid-treated neuronal cells. J Ginseng Res. 2017;41(4):566–571. doi: 10.1016/j.jgr.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song X.Y., Hu J.F., Chu S.F., Zhang Z., Xu S., Yuan Y.H. Ginsenoside Rg1 attenuates okadaic acid induced spatial memory impairment by the GSK3beta/tau signaling pathway and the Abeta formation prevention in rats. Eur J Pharmacol. 2013;710(1–3):29–38. doi: 10.1016/j.ejphar.2013.03.051. [DOI] [PubMed] [Google Scholar]
- 8.Xu T.Z., Shen X.Y., Sun L.L., Chen Y.L., Zhang B.Q., Huang D.K. Ginsenoside Rg1 protects against H2O2induced neuronal damage due to inhibition of the NLRP1 inflammasome signalling pathway in hippocampal neurons in vitro. Int J Mol Med. 2019;43(2):717–726. doi: 10.3892/ijmm.2018.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butterfield D.A., Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20(3):148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma M.W., Wang J., Zhang Q., Wang R., Dhandapani K.M., Vadlamudi R.K. NADPH oxidase in brain injury and neurodegenerative disorders. Mol Neurodegener. 2017;12(1):7. doi: 10.1186/s13024-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park K.W., Baik H.H., Jin B.K. IL-13-induced oxidative stress via microglial NADPH oxidase contributes to death of hippocampal neurons in vivo. J Immunol. 2009;183(7):4666–4674. doi: 10.4049/jimmunol.0803392. [DOI] [PubMed] [Google Scholar]
- 12.Fan L.M., Geng L., Cahill-Smith S., Liu F., Douglas G., McKenzie C.A. Nox2 contributes to age-related oxidative damage to neurons and the cerebral vasculature. J Clin Invest. 2019;129(8):3374–3386. doi: 10.1172/JCI125173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu T., Sun L., Shen X., Chen Y., Yin Y., Zhang J. NADPH oxidase 2-mediated NLRP1 inflammasome activation involves in neuronal senescence in hippocampal neurons in vitro. Int Immunopharmacol. 2019;69:60–70. doi: 10.1016/j.intimp.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Li J., Yang J.Y., Yao X.C., Xue X., Zhang Q.C., Wang X.X. Oligomeric Abeta-induced microglial activation is possibly mediated by NADPH oxidase. Neurochem Res. 2013;38(2):443–452. doi: 10.1007/s11064-012-0939-2. [DOI] [PubMed] [Google Scholar]
- 15.Qiu L.L., Luo D., Zhang H., Shi Y.S., Li Y.J., Wu D. Nox-2-Mediated phenotype loss of hippocampal parvalbumin interneurons might contribute to postoperative cognitive decline in aging mice. Front Aging Neurosci. 2016;8:234. doi: 10.3389/fnagi.2016.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu W., Zhang Y., Wu W., Yin Y., Huang D., Wang Y. Chronic glucocorticoids exposure enhances neurodegeneration in the frontal cortex and hippocampus via NLRP-1 inflammasome activation in male mice. Brain Behav Immun. 2016;52:58–70. doi: 10.1016/j.bbi.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y.D., Pang W., He C.C., Lu H., Liu W., Wang Z.Y. The cognitive impairment induced by zinc deficiency in rats aged 0 approximately 2 months related to BDNF DNA methylation changes in the hippocampus. Nutr Neurosci. 2017;20(9):519–525. doi: 10.1080/1028415X.2016.1194554. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B., Zhang Y., Wu W., Xu T., Yin Y., Zhang J. Chronic glucocorticoid exposure activates BK-NLRP1 signal involving in hippocampal neuron damage. J Neuroinflammation. 2017;14(1):139. doi: 10.1186/s12974-017-0911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong W., Cheng S., Huang F., Fan W., Chen Y., Shi H. Mitochondrial dysfunction in long-term neuronal cultures mimics changes with aging. Med Sci Monit. 2011;17(4) doi: 10.12659/MSM.881706. BR91–B96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steeland S., Gorle N., Vandendriessche C., Balusu S., Brkic M., Van Cauwenberghe C. Counteracting the effects of TNF receptor-1 has therapeutic potential in Alzheimer’s disease. EMBO Mol Med. 2018;10(4) doi: 10.15252/emmm.201708300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao J., Li K., Wan K., Sun T., Zheng N., Zhu F. Organoplatinum-substituted polyoxometalate inhibits beta-amyloid aggregation for Alzheimer’s therapy. Angew Chem Int Ed Engl. 2019;58(50):18032–18039. doi: 10.1002/anie.201910521. [DOI] [PubMed] [Google Scholar]
- 22.Baldeiras I., Santana I., Proenca M.T., Garrucho M.H., Pascoal R., Rodrigues A. Oxidative damage and progression to Alzheimer’s disease in patients with mild cognitive impairment. J Alzheimers Dis. 2010;21(4):1165–1177. doi: 10.3233/jad-2010-091723. [DOI] [PubMed] [Google Scholar]
- 23.Calsolaro V., Edison P. Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement. 2016;12(6):719–732. doi: 10.1016/j.jalz.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Li X.Y., Men W.W., Zhu H., Lei J.F., Zuo F.X., Wang Z.J. Age- and brain region-specific changes of glucose metabolic disorder, learning, and memory dysfunction in early Alzheimer’s disease assessed in APP/PS1 transgenic mice using (18)F-FDG-PET. Int J Mol Sci. 2016;17(10) doi: 10.3390/ijms17101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izco M., Martinez P., Corrales A., Fandos N., Garcia S., Insua D. Changes in the brain and plasma Abeta peptide levels with age and its relationship with cognitive impairment in the APPswe/PS1dE9 mouse model of Alzheimer’s disease. Neuroscience. 2014;263:269–279. doi: 10.1016/j.neuroscience.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Radde R., Bolmont T., Kaeser S.A., Coomaraswamy J., Lindau D., Stoltze L. Abeta42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006;7(9):940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volianskis A., Kostner R., Molgaard M., Hass S., Jensen M.S. Episodic memory deficits are not related to altered glutamatergic synaptic transmission and plasticity in the CA1 hippocampus of the APPswe/PS1deltaE9-deleted transgenic mice model of ss-amyloidosis. Neurobiol Aging. 2010;31(7):1173–1187. doi: 10.1016/j.neurobiolaging.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Blanch M., Mosquera J.L., Ansoleaga B., Ferrer I., Barrachina M. Altered mitochondrial DNA methylation pattern in alzheimer disease-related pathology and in Parkinson disease. Am J Pathol. 2016;186(2):385–397. doi: 10.1016/j.ajpath.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Pan Y., Short J.L., Newman S.A., Choy K.H.C., Tiwari D., Yap C. Cognitive benefits of lithium chloride in APP/PS1 mice are associated with enhanced brain clearance of beta-amyloid. Brain Behav Immun. 2018;70:36–47. doi: 10.1016/j.bbi.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 30.Pietrzak R.H., Lim Y.Y., Neumeister A., Ames D., Ellis K.A., Harrington K. Amyloid-beta, anxiety, and cognitive decline in preclinical Alzheimer disease: a multicenter, prospective cohort study. JAMA Psychiatry. 2015;72(3):284–291. doi: 10.1001/jamapsychiatry.2014.2476. [DOI] [PubMed] [Google Scholar]
- 31.Pereda D., Al-Osta I., Okorocha A.E., Easton A., Hartell N.A. Changes in presynaptic calcium signalling accompany age-related deficits in hippocampal LTP and cognitive impairment. Aging Cell. 2019;18(5) doi: 10.1111/acel.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris J.A., Devidze N., Verret L., Ho K., Halabisky B., Thwin M.T. Transsynaptic progression of amyloid-beta-induced neuronal dysfunction within the entorhinal-hippocampal network. Neuron. 2010;68(3):428–441. doi: 10.1016/j.neuron.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer D., Bonhoeffer T., Scheuss V. Balance and stability of synaptic structures during synaptic plasticity. Neuron. 2014;82(2):430–443. doi: 10.1016/j.neuron.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 34.Cui Y.Q., Wang Q., Zhang D.M., Wang J.Y., Xiao B., Zheng Y. Triptolide rescues spatial memory deficits and amyloid-beta aggregation accompanied by inhibition of inflammatory responses and MAPKs activity in APP/PS1 transgenic mice. Curr Alzheimer Res. 2016;13(3):288–296. doi: 10.2174/156720501303160217122803. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y.J., Mei Y., Qu Z.L., Zhang S.J., Zhao W., Fang J.S. Ligustilide ameliorates memory deficiency in APP/PS1 transgenic mice via restoring mitochondrial dysfunction. Biomed Res Int. 2018;2018:4606752. doi: 10.1155/2018/4606752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seubert P., Oltersdorf T., Lee M.G., Barbour R., Blomquist C., Davis D.L. Secretion of beta-amyloid precursor protein cleaved at the amino terminus of the beta-amyloid peptide. Nature. 1993;361(6409):260–263. doi: 10.1038/361260a0. [DOI] [PubMed] [Google Scholar]
- 37.Lammich S., Kojro E., Postina R., Gilbert S., Pfeiffer R., Jasionowski M. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci U S A. 1999;96(7):3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiribuzi R., Crispoltoni L., Chiurchiu V., Casella A., Montecchiani C., Del Pino A.M. Trans-crocetin improves amyloid-beta degradation in monocytes from Alzheimer’s Disease patients. J Neurol Sci. 2017;372:408–412. doi: 10.1016/j.jns.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Butterfield D.A., Swomley A.M., Sultana R. Amyloid beta-peptide (1-42)-induced oxidative stress in Alzheimer disease: importance in disease pathogenesis and progression. Antioxid Redox Signal. 2013;19(8):823–835. doi: 10.1089/ars.2012.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zotova E., Bharambe V., Cheaveau M., Morgan W., Holmes C., Harris S. Inflammatory components in human Alzheimer’s disease and after active amyloid-beta42 immunization. Brain. 2013;136(Pt 9):2677–2696. doi: 10.1093/brain/awt210. [DOI] [PubMed] [Google Scholar]
- 41.Tayler H., Fraser T., Miners J.S., Kehoe P.G., Love S. Oxidative balance in Alzheimer’s disease: relationship to APOE, Braak tangle stage, and the concentrations of soluble and insoluble amyloid-beta. J Alzheimers Dis. 2010;22(4):1363–1373. doi: 10.3233/JAD-2010-101368. [DOI] [PubMed] [Google Scholar]
- 42.Qin Y.Y., Li M., Feng X., Wang J., Cao L., Shen X.K. Combined NADPH and the NOX inhibitor apocynin provides greater anti-inflammatory and neuroprotective effects in a mouse model of stroke. Free Radic Biol Med. 2017;104:333–345. doi: 10.1016/j.freeradbiomed.2017.01.034. [DOI] [PubMed] [Google Scholar]
- 43.Masamune A., Watanabe T., Kikuta K., Satoh K., Shimosegawa T. NADPH oxidase plays a crucial role in the activation of pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G99–G108. doi: 10.1152/ajpgi.00272.2007. [DOI] [PubMed] [Google Scholar]
- 44.Ansari M.A., Scheff S.W. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med. 2011;51(1):171–178. doi: 10.1016/j.freeradbiomed.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu H., Fan C., Yang L., Yu S., Song Q., Wang P. Ginsenoside Rg1 prevents chronic stress-induced depression-like behaviors and neuronal structural plasticity in rats. Cell Physiol Biochem. 2018;48(6):2470–2482. doi: 10.1159/000492684. [DOI] [PubMed] [Google Scholar]
- 46.Zheng T., Jiang H., Jin R., Zhao Y., Bai Y., Xu H. Ginsenoside Rg1 attenuates protein aggregation and inflammatory response following cerebral ischemia and reperfusion injury. Eur J Pharmacol. 2019;853:65–73. doi: 10.1016/j.ejphar.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Bae H.J., Kim J., Jeon S.J., Kim J., Goo N., Jeong Y. Green tea extract containing enhanced levels of epimerized catechins attenuates scopolamine-induced memory impairment in mice. J Ethnopharmacol. 2020;258:112923. doi: 10.1016/j.jep.2020.112923. [DOI] [PubMed] [Google Scholar]
- 48.Chen T., Yang Y., Zhu S., Lu Y., Zhu L., Wang Y. Inhibition of Abeta aggregates in Alzheimer’s disease by epigallocatechin and epicatechin-3-gallate from green tea. Bioorg Chem. 2020;105:104382. doi: 10.1016/j.bioorg.2020.104382. [DOI] [PubMed] [Google Scholar]
- 49.Kim S.U., de Vellis J. Microglia in health and disease. J Neurosci Res. 2005;81(3):302–313. doi: 10.1002/jnr.20562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.