Abstract
Background
Irritable bowel syndrome (IBS), the most common functional gastrointestinal disorder, is characterized by chronic abdominal pain and bowel habit changes. Although diverse complicated etiologies are involved in its pathogenesis, a dysregulated gut–brain axis may be an important factor. Red ginseng (RG), a traditional herbal medicine, is proven to have anti-inflammatory effects and improve brain function; however, these effects have not been investigated in IBS.
Methods
Three-day intracolonic zymosan injections were used to induce post-infectious human IBS-like symptoms in mice. The animals were randomized to receive either phosphate-buffered saline (CG) or RG (30/100/300 mg/kg) for 10 days. Amitriptyline and sulfasalazine were used as positive controls. Macroscopic scoring was performed on day 4. Visceral pain and anxiety-like behaviors were assessed by colorectal distension and elevated plus maze and open field tests, respectively, on day 10. Next-generation sequencing of gut microbiota was performed, and biomarkers involved in gut–brain axis responses were analyzed.
Results
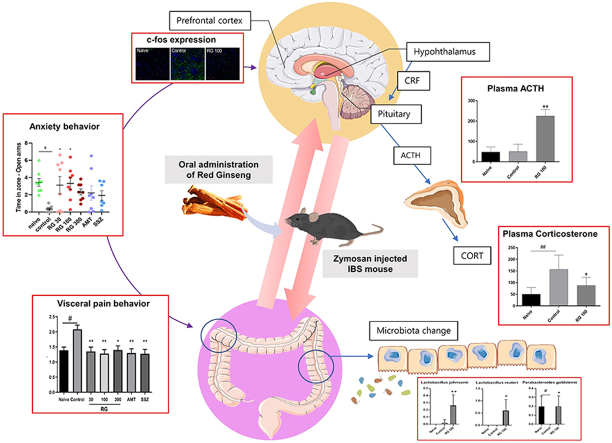
Compared to CG, RG significantly decreased the macroscopic score, frequency of visceral pain, and anxiety-like behavior in the IBS mice. These effects were comparable to those after sulfasalazine and amitriptyline treatments. Moreover, RG significantly increased the proliferation of beneficial microbes, including Lactobacillus johnsonii, Lactobacillus reuteri, and Parabacteroides goldsteinii. RG significantly suppressed expression of IL-1β and c-fos in the gut and prefrontal cortex, respectively. Further, it restored the plasma levels of corticosterone to within the normal range, accompanied by an increase in adrenocorticotropic hormone.
Conclusion
RG may be a potential therapeutic option for the management of human IBS.
Keywords: Irritable bowel syndrome, Gut–brain axis, Microbiota, Red ginseng
Graphical abstract
1. Introduction
Irritable bowel syndrome (IBS) is one of the most prevalent functional gastrointestinal disorders characterized by chronic or recurrent abdominal pain associated with changes in bowel habit. As yet, there are no IBS-specific diagnostic biomarkers or effective therapeutics. The current treatment comprises merely the simultaneous administration of multiple drugs, including anti-diarrheal, laxatives, analgesics, antidepressants, and anti-inflammatory agents, to relieve the symptoms.
Although the complex pathogenesis of IBS is not fully understood [1], an altered gut–brain axis homeostasis has been considered as an important etiology. The gut–brain axis is a bidirectional communication network between the gut and the brain that includes the enteric nervous system (ENS), central nervous system (CNS), brain, spinal cord, autonomic nervous system, and hypothalamic–pituitary–adrenal (HPA) axis [2,3]. Various factors, including intestinal infection, gut inflammation, and psychological stress, contribute to gut–brain axis dysfunction. It is known that post-infectious (PI)-IBS develops after severe enteritis caused by infection. This persistent low-grade inflammation sends detrimental signals to the brain and adversely affects intestinal physiology [4]. Traumatic stress, such as early maternal separation, is also associated with development of IBS [5]. Subjects with IBS exhibit higher depression and anxiety levels compared to those without; 30%–40% of patients with IBS have depression or anxiety disorder as comorbidities [6,7]. Furthermore, the HPA axis responds to stressors by releasing corticotropin-releasing hormone from the hypothalamus that stimulates the pituitary gland to secrete adrenocorticotropic hormone (ACTH), which in turn stimulates cortisol release [8]. Increased cortisol exacerbates the IBS-related abdominal symptoms via alterations in gut-associated immune tissue and the ENS [9]. Patients with IBS reportedly show excess levels of serum cortisol [8]. Another communication pathway between the gut and the brain is via the microbiota. Enteric microbiota interact with intestinal immune cells, ENS, and CNS through neuroendocrine pathways [2] and thereby play a pivotal role in the pathophysiology of IBS [1].
Ginseng, a perennial plant (genus Panax, family Araliaceae), is a source of popular herbal medicine that has been used for thousands of years [10]. It is reported to have ameliorative effects on inflammation, and is thought to help combat depression by effectively suppressing stress [11,12]. Therefore, it is a promising candidate to treat IBS.
Based on the understanding that IBS is a multifaceted disease affecting not just the gastrointestinal (GI) tract, but also the changes in mood and systemic endocrine responses [13], this study aimed to analyze the efficacy of red ginseng (RG) extract as a natural medicine for human PI-IBS using a zymosan-induced IBS mouse model presenting IBS-like symptoms [[14], [15], [16]].
2. Materials and methods
2.1. Preparation of RG
RG extract was manufactured and kindly provided by the Korea Ginseng Corporation (Daejeon, Korea). The procedure for RG extraction followed the international standard production process (ISO 19610), and the constituents of RG have been reported in previous studies [17,18].
2.2. Animal experiments
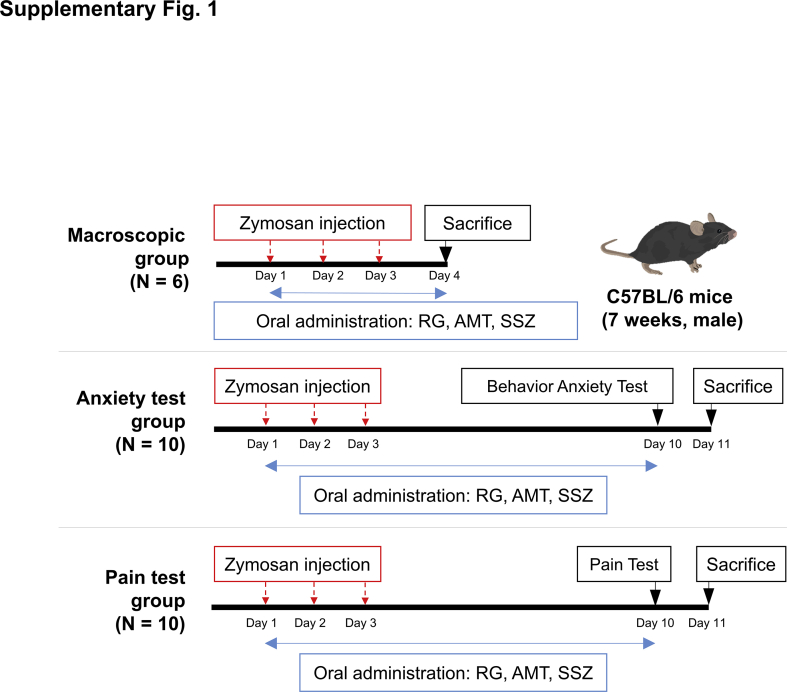
Seven-weeks-old C57BL/6 male mice were purchased from Daehan Biolink, Inc. (Chungbuk, Korea). All animal experiments in the present study were approved by the Gachon University Center of Animal Care and Use (LCDI-2019-0137). Mice were adapted to the controlled conditions of 12:12 h light/dark cycle, temperature of 22 ± 2°C, and humidity of 40–60%, with free access to standard food (LabDiet 5053, Orientbio) and sterilized water. After acclimating the mice for 1 week, they were randomly divided into 7 groups (n = 6/group, 3/cage for macroscopic, n = 10/group, 5/cage for behavior and pain tests) as follows: naïve, control (zymosan injection), RG (30, 100, or 300 mg/kg), amitriptyline (AMT; 10 mg/kg, Sigma-Aldrich, St. Louis, USA), and sulfasalazine (SSZ; 30 mg/kg, Sigma-Aldrich). Based on previous studies, the number of animals, dosage [[20], [21], [22]], and duration of RG treatment [13,19] were determined. IBS was induced in all mice except those in the naïve group by transcolonic injections of 0.1-mL suspension of zymosan [30 mg/mL in phosphate buffered saline (PBS), Sigma-Aldrich] for three consecutive days [23]; the naïve mice were injected with PBS. Three hours post zymosan injection, the mice were orally administered RG, AMT, or SSZ in 100 μL PBS. On days 4 and 10, the mice were anesthetized using isoflurane and sacrificed by cardiac puncture, and were subsequently biopsied for analysis. The animal experimental scheme is presented in supplementary Fig. 1.
2.3. Changes in body weight and food intake
The body weights of mice in all groups were measured at the same time each day, prior to drug administration during the experiment. The individual food intake of mice was calculated as {(initial serving + additional serving)-remainder}/each cage mouse number [24].
2.4. Macroscopic scoring of zymosan-induced colon changes
The colon length was determined by measuring from the aboral end of the cecum to the anus, and colon weight was measured after removing the stools. Stool conditions were scored by five blinded researchers. The colon weight (increase vs. control), colon length (decrease vs. control) (score 0, < 5%; 1, 5%–14%; 2, 15%–24%; 3, 25%–35%; and 4, > 35%), and stool scores (0, normal; 1, loose/moist; 2, amorphous/sticky; and 3, diarrhea) were graded individually for all experimental animals. Total macroscopic score index for the severity of changes in the colon was defined as sum of the individual colon macroscopic score indices (0, normal; 1-11, increasing severity of affected) by Kimball et al. [24,25].
2.5. Histological examination
The colon tissues were fixed and embedded in paraffin, 4-μm thick sections were cut, and stained with hematoxylin and eosin to detect infiltrated inflammatory cells [25]. The cells were observed under a high resolution up-light fluorescence microscope (Zeiss, Oberkochen, Germany). The mucosa thickness (μm) was measured vertically from the abutting part of the submucosa and mucosa to the end of the mucosa. The proportion of infiltrated inflammatory cells was measured as the area (mm2) in the mucosa using Viewpoint software (Viewpoint, Inc., Chatswood, NSW, Australia).
2.6. Pain-related behavior test
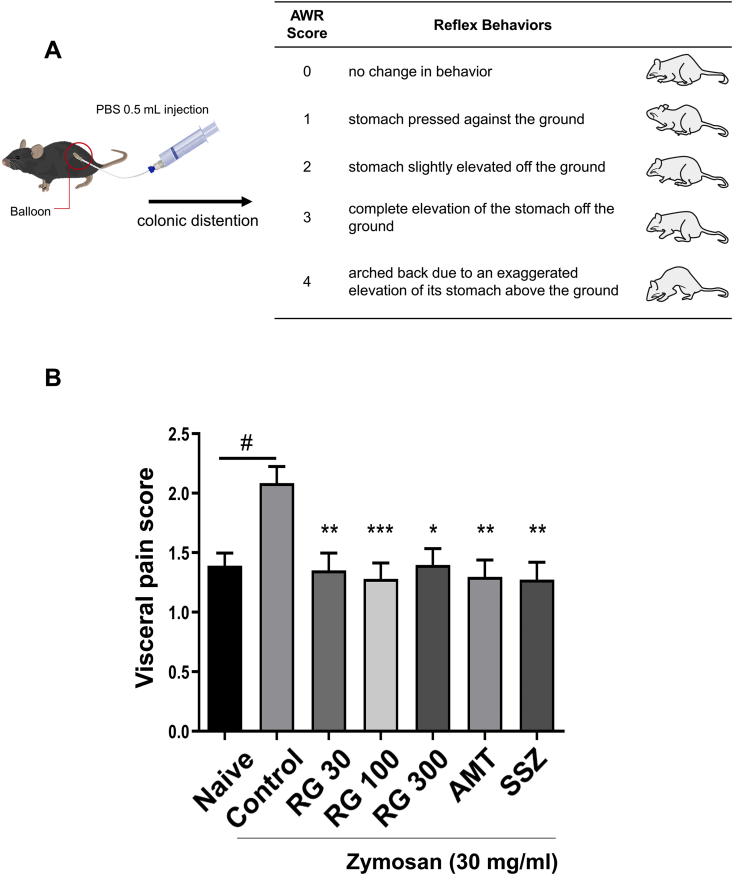
To investigate visceral pain in zymosan-induced PI-IBS, the following method and scoring were used to evaluate colorectal distension [26]: A thin tube (6-inch-long with 1.4 mm diameter) with a balloon at the end was inserted into a syringe filled with 0.5 mL PBS. The balloon was then inserted into the mice colon via the anus and as much pressure was applied as the PBS volume. Abdominal withdrawal reflex (AWR) scores (0–4) were used to assess the reaction of visceral stimuli [17] (Fig. 3A). The total visceral pain-related behaviors were counted and recorded over 10 min. The scores were scored by 5 blind testers [19,27].
Fig. 3.
Effects of RG on visceral pain-related behavior. (A) Visceral pain-related behavior test and abdominal withdrawal reflex (AWR) scoring method. (B) Pain-related behavior test results scored by visceral pain score via blind testing. Data are presented the means ± SD values. (One-way ANOVA; #p<0.05 vs. Naive; ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001 vs. Cotrol). AWR, abdominal withdrawal reflex; RG, red ginseng; AMT, amitriptyline; SSZ, sulfasalzine
2.7. Anxiety-related behavior test
In the open field test, mice were tested individually by transferring them to the test field and recording their behaviors for 10 min. For the elevated plus maze test, the structure consisted of an elevated plus-shaped acrylic maze (50 cm from the floor) with two open and two closed arms (50 cm × 5 cm × 40 cm walls). After placing each mouse in the maze center, its performance was recorded, including time spent in the open and closed arms throughout a 5-min session [28]. The recordings were analyzed using SMART 3.0 video tracking software (Panlab S.I., Barcelona, Spain).
2.8. Microbiota next-generation sequencing (NGS) analysis
Microbiota analysis was performed by Macrogen, Inc. (Seoul, South Korea) using the Illumina platform to sequence various species.
2.9. Real-time quantitative polymerase chain reaction (RT-qPCR)
Colon total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, USA), and cDNA synthesis using a PrimeScript RT reagent kit (TaKaRa, Shiga, Japan). mRNA expression of interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were quantified using a C1000 Thermal Cycler CFX384 Real-Time System (Bio-rad, Hercules, USA) with PowerSYBR® Green PCR Master Mix (Applied biosystems, Foster City, USA). The following primers were used: mouse IL-1β forward; 5′-AGTTGACGGACCCCAAAAGAT-3′, reverse; 5′-GTTGATGTGCTGCTGCGAGA-3′, mouse TNF-α forward; 5′-CTGTAGCCCACGTCGTAGC-3′, reverse; 5′-TTGAGATCCATGCCGTTG-3′, mouse GAPDH forward; 5′-CGACTTCAACAGCAACTCCCACTCTTCC-3′, reverse; and 5′-TGGGTGGTCCAGGGTTTCTTACTCCTT-3′. The reaction was performed at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 55 °C for 15 s, and 72 °C for 20 s.
2.10. Immunofluorescence (IF) analysis
Animals were anesthetized and trans-cardially perfused with 4% paraformaldehyde (PFA) in PBS. Mice brains were pre-fixed with 4% PFA overnight and kept overnight in 30% sucrose in PBS. The prefrontal cortex (PFC) (1.78 mm prior to bregma) were frozen-sectioned into 20-μm thick serial coronal sections using an optimal cutting temperature compound. The sectionalized tissues on slide glass were permeabilized for 20 min at 23 °C with 0.1% Triton X-100 in PBS and blocked with 2% bovine serum albumin for 1 h at 23 °C. Then, they were incubated with c-fos antibody (1:50, Santa Cruz Biotechnology, Santa Cruz, USA) overnight at 4 °C, washed with PBS for 10 min, and incubated with fluorescein isothiocyanate-conjugated (FITC) AffiniPure goat anti-mouse IgG (H+L) (1:200, Jackson ImmunoResearch, Inc., West Grove, USA) for 1 h at 23 °C. C-fos expression was evaluated using confocal microscopy (LSM T-PMT; Zeiss, Oberkochen, Germany) and ZEN software (Zeiss). ImageJ software (National Institutes of Health) was used to quantify the fluorescence intensity of c-fos expression.
2.11. Western blotting
Brain samples were homogenized; equal amounts (40 μg) of proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF membranes (Merck, Darmstadt, Germany). The membranes were probed overnight with c-fos-specific antibodies (Santa Cruz Biotechnology, Inc.). The band density was compared using β-actin as control and measured using ImageJ software.
2.12. Enzyme-linked immunosorbent assay (ELISA)
ELISA kits were used to detect adrenocorticotropic hormone (Enzo life science, Farmingdale, USA), and corticosterone (Enzo life science) in mice plasma.
2.13. Statistical analyses
All data are represented as mean ± standard deviation. One-way analysis of variance (ANOVA) was performed using Prism GraphPad software (San Diego, CA, USA) to analyze differences between the groups. Multiple group comparisons were performed using one-way ANOVA, followed by post-hoc Tukey’s tests; p < 0.05, p < 0.01, and p < 0.001 were considered statistically significant.
3. Results
3.1. Effects of RG on body weight changes and food intake
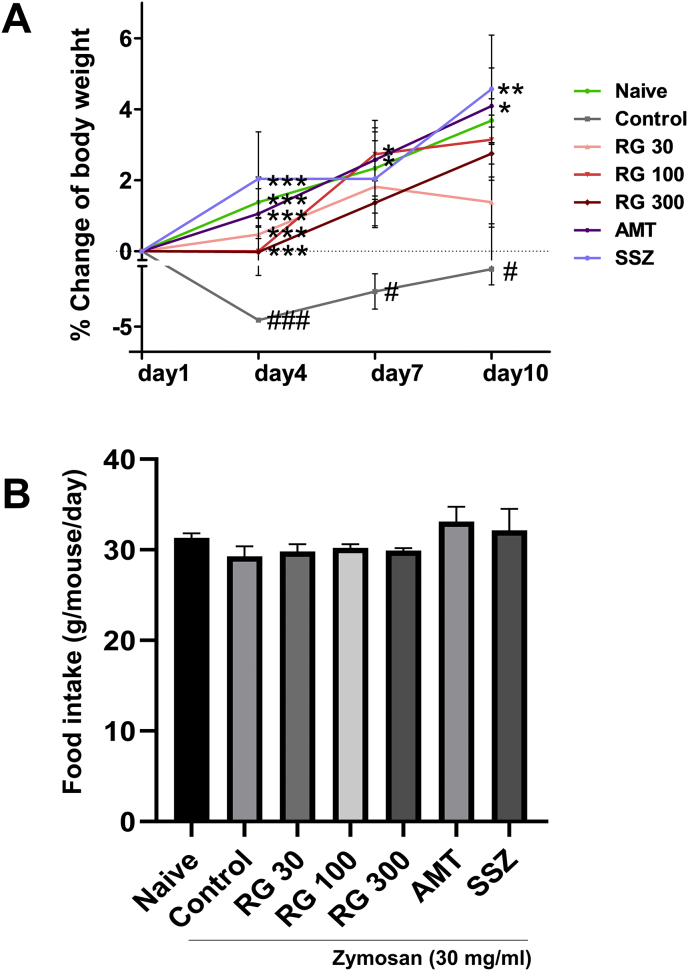
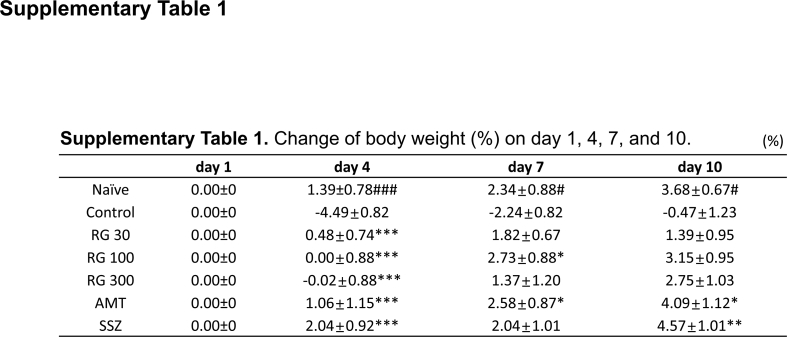
The body weight and food intake were compared across the different groups (Fig. 1A and B). Mean body weights were comparable across the 7 groups. Notably, zymosan injection was associated with significant decrease in body weight on days 4, 7, and 10 in the control mice (−4.49 ± 0.82%, −2.24 ± 0.82%, and −0.47 ± 1.23%) compared to that in the naïve mice. However, RG administration suppressed the loss of body weight on day 4 (RG 30: 0.4 8 ± 0.74%, RG-100: 0.00 ± 0.88%, and RG-300: −0.02 ± 0.88%) (Supplementary Table 1). Moreover, all groups were no difference observed in food intake (Fig. 1B). These results suggested that RG inhibited the decrease in body weight of mice with zymosan-induced IBS mice without affecting their food intake.
Fig. 1.
Effects of RG on body weight change and food intake. (A) Body weight changes compared across different treatment groups followed by (B) food intake assessment. Data are presented the means ± SD values. (One-way ANOVA; #p<0.05, ###p<0.001 vs. Naive; ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001 vs. Cotrol). RG, red ginseng; AMT, amitriptyline; SSZ, sulfasalzine
3.2. Effects of RG on macroscopic scoring
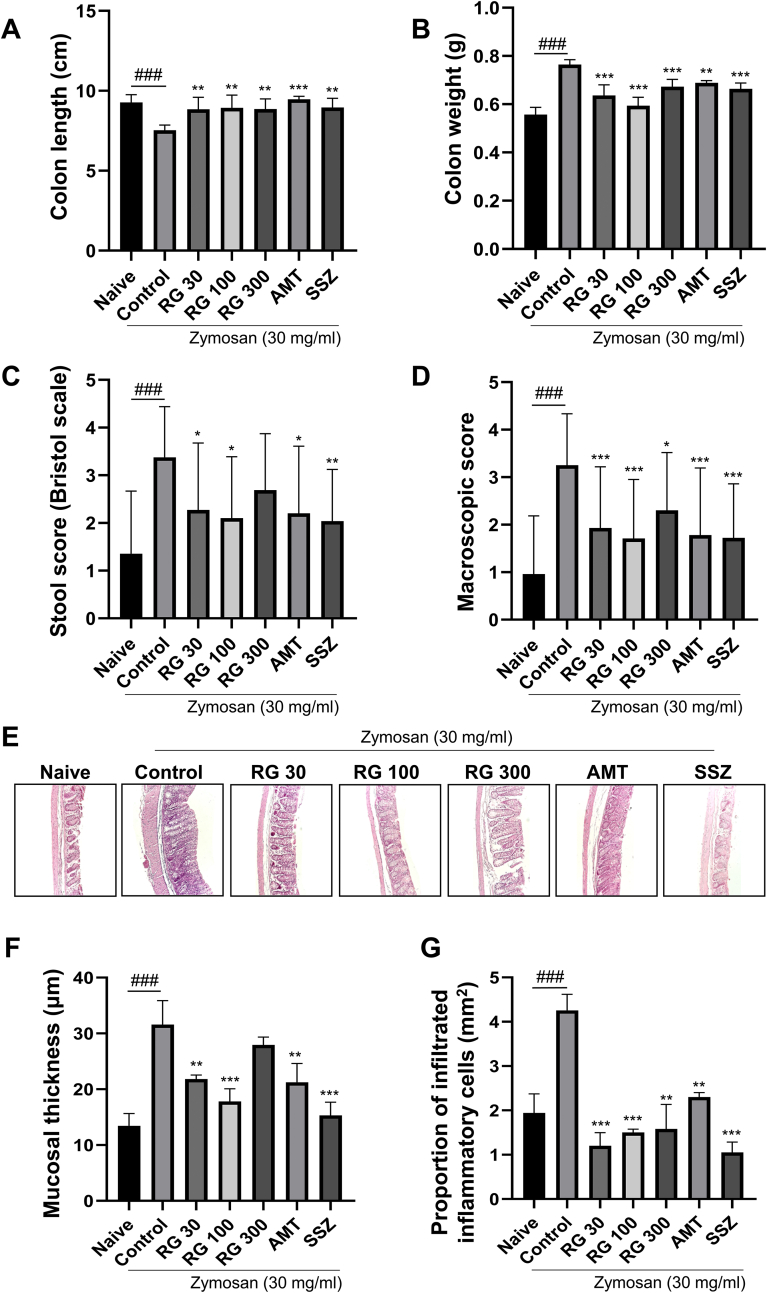
To determine the differences in gut physiology among the treatment groups, macroscopic scores using three parameters (colon length, colon weight, and stool score) were calculated and analyzed. Zymosan injections induced distal colon shortening in control mice (7.5 ± 0.33 cm) compared to the naïve mice (9.3 ± 0.49 cm), which indicated colitis. However, RG-administered groups showed increased colon length compared to that in the control group (RG-30: 8.9 ± 0.74 cm, RG-100: 8.9 ± 0.80 cm, and RG-300: 8.9 ± 0.80 cm; Fig. 2A). Furthermore, colon weights were significantly increased in the control (0.764 ± 0.021 g) relative to that in the naïve mice (0.557 ± 0.030 g). However, RG administration significantly decreased the colon weights (RG-30: 0.636 ± 0.044 g, RG-100: 0.594 ± 0.035 g, and RG-300: 0.673 ± 0.030 g), such that the weights approached those of the naïve group (Fig. 2B). The naïve mice defecated dark brown rigid feces, whereas the control mice had watery, sticky masses of bright brown or yellow-colored feces, indicating moderate diarrhea. The stool score increased in the control mice (3.38 ± 1.065) compared to that in the naïve mice (1.35 ± 1.314), but RG administration significantly reduced the stool score (RG-30: 2.27 ± 1.406, RG-100: 2.10 ± 1.285; Fig. 2C). The macroscopic score was significantly increased by zymosan injection and resulted in low-to-moderate colitis and diarrhea. Scores in the RG-administered groups were significantly reduced, counteracting the effects of zymosan injection (Fig. 2D).
Fig. 2.
Effects of RG on gut macroscopic changes. Macroscopic gut assessment of different treatment groups showing (A) colon length, (B) colon weight, (C) stool score, and (D) a comprehensive overall macroscopic score. (E) H&E staining showing histological changes across groups (X20) (F) quantitative results of colon mucosa thickness and (G) proportion of infiltrated inflammatory cells. Data are presented the means ± SD values. (One-way ANOVA; ###p<0.001 vs. Naive; ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001 vs. Cotrol). RG, red ginseng; AMT, amitriptyline; SSZ, sulfasalzine
3.3. Effects of RG on colon histological changes
To examine the RG effects on colonic inflammation, histological examinations of zymosan-injected colons by H&E staining were performed. The control group showed increased epithelial thickening and proportion of submucosal inflammatory cell infiltration, indicating low-to-moderate-grade inflammation (Fig. 2E) [24]. In contrast, mice in RG-30 and RG-100 groups showed decreased intestinal mucosal hyperplasia and inflammatory cell infiltration and decreased mucosal thickness to near normal levels (Fig. 2F and G). These results were comparable to that observed with administration of SSZ, an anti-inflammatory drug.
3.4. Effects of RG on visceral pain-related behavior
To investigate the effects of RG on visceral pain, mice were subject to colorectal distension to induce pain. On day 10, the control mice showed pain-related behaviors and the AWR was significantly higher in the control group (2.085 ± 0.940) than that in the naïve group (1.391 ± 0.659). In contrast, RG-30/100/300 groups showed significant decrease in AWR (1.351 ± 1.02, 1.280 ± 1.027, and 1.396 ± 1.000, respectively; Fig. 3B). These data suggested that RG administration improved visceral pain in mice with PI-IBS.
3.5. Effects of RG on anxiety-like behavior
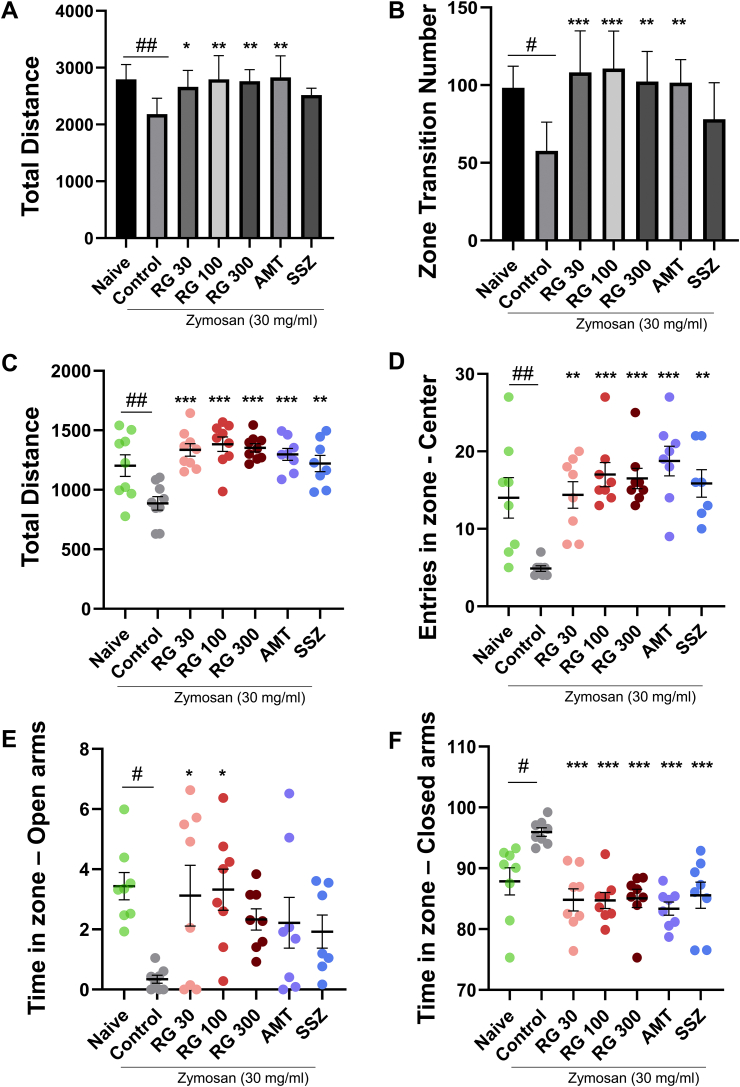
To quantify anxiety-like behaviors in mice, they were subject to both open field test (OFT) and elevated plus maze (EPM) test. The OFT results indicate the exploring ability, with decreased search activity indicating increased anxiety. In zymosan-induced IBS mice, total distance and the number of zone transitions (indicators of search activity) were significantly reduced in the control group (2180.63 ± 281.30 cm; Fig. 4A) compared to that in the naïve group. However, they were increased in the RG-30/100/300 groups (2660.76 ± 289.52, 2793.80 ± 418.90, and 2762.34 ± 202.22 cm, respectively) to levels similar to that in the naïve (2793.68 ± 261.50 cm) and AMT-treated groups (2826.88 ± 120.20 cm; Fig. 4A).
Fig. 4.
Effects of RG on anxiety-like behavior. Open field test results for different treatment groups showing the (A) total travel distance (cm) and (B) the number of transition zones crossed. Elevated plus maze test results show the (C) total travel distance (cm), (D) number of entries into the center zone, (E) total time spent in open arms (seconds), and (F) closed arms (seconds). Data are presented the means ± SD values. (One-way ANOVA; #p<0.05, ##p<0.01 vs. Naive; ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001 vs. Cotrol). RG, red ginseng; AMT, amitriptyline; SSZ, sulfasalzine
In the EPM test, anxiety was analyzed by measuring total distance, entries in zone-center, time spent in open/closed arms. The total distance in the control group (885.64 ± 171.87 cm) decreased whereas that in the RG-30/100/300 groups (1335.59 ± 157.63, 1383.32 ± 184.35, 1352.46 ± 102.76 cm, respectively) was similar to that in the naïve group (1202.98 ± 272.10 cm) (Fig. 4C). Control group showed fewer center zone entries compared to the naïve group (4.88 ± 0.99% vs. 14.00 ± 7.37%). RG treatment groups showed increased center zone entries (14.38 ± 4.87%, 17.00 ± 4.44%, 16.50 ± 3.74%, respectively; Fig. 4D). The duration of staying in the open arms was significantly reduced in the control mice compared to that in the naïve mice on day 10 (naïve: 3.44 ± 1.28% vs. control: 0.34 ± 0.37%). The frequency of staying in the open arms markedly increased in the RG group compared to that in the control group on day 10 (RG-30: 3.12 ± 2.86%, RG-100: 3.32 ± 1.94%, RG-300: 2.33 ± 1.00%; Fig. 4E). RG showed similar effects to AMT (2.22 ± 2.38%). Furthermore, the duration spent in the closed arm increased significantly in the control group compared to that in the naïve group (naïve: 87.86 ± 6.33% vs. control: 95.95 ± 1.98%), and decreased in the RG group (RG-30: 84.81 ± 5.14%, RG-100: 84.72 ± 3.72%, RG-300: 85.04 ± 4.23%, all p < 0.001), similar to that in the naïve group and the AMT group (83.36 ± 3.06%, vs. control, p < 0.001). (Fig. 4F). These data indicated that zymosan-induced PI-IBS mice showed decreased mobility, most likely due to anxiety-like behavior, and RG administration improved these symptoms in a dose-dependent manner.
3.6. Effects of RG on gut microbiome changes
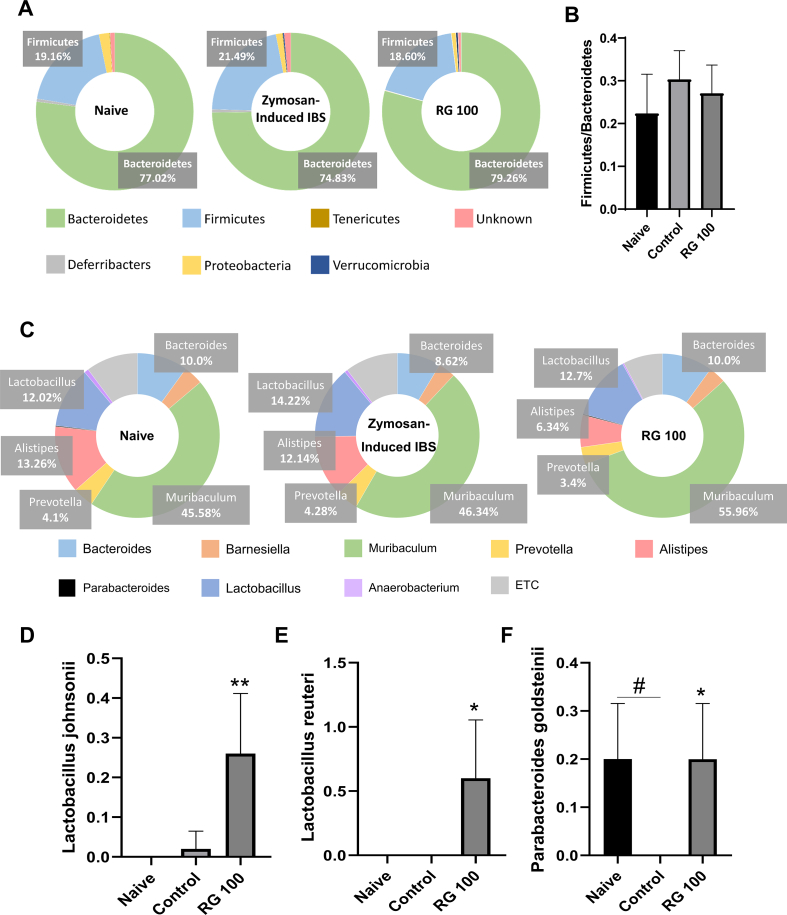
To determine whether zymosan-induced PI-IBS altered gut microbiota, a general microbiome assessment was done across all groups. At the phylum level of the gut microbiome, the ratio of the two most common bacteria, Firmicutes and Bacteroidetes changed [29]. The percentage ratio in the naïve group was 19.16/77.02, and that in the control group was 21.49/74.83. In the RG-100 group, this percentage ratio (18.60/79.26) was similar to that in the naïve group (Fig. 5A and B). Changes in the ratio at the genus level were also observed (Fig. 5C). At the species level, Lactobacillus johnsonii and Lactobacillus reuteri increased significantly in the RG-100 group. Parabacteroides goldsteinii was significantly decreased in the control group, but similar levels were found in both the RG-100 and naïve groups (Fig. 5D–F). These data indicated that zymosan-induced PI-IBS altered the gut microbiota at the phylum and species level, which may play a role in the overall pathophysiology of PI-IBS.
Fig. 5.
Effects of RG on gut microbiota changes. (A) Phylum level assessment of gut microbiota across different treatment groups showing overall distribution, (B) Firmicutes/Bacteroidetes ratio. (C) Genus level distribution and species-specific levels for (D) L. johnsonii, (E) L. reuteri, and (F) P. goldsteinii. Data are presented the means ± SD values. (One-way ANOVA; #p<0.05 vs. Naive; ∗p<0.05, ∗∗p<0.01 vs. Cotrol). IBS, irritable bowel syndrome; RG, red ginseng
3.7. Effects of RG on gut pro-inflammatory cytokine expression
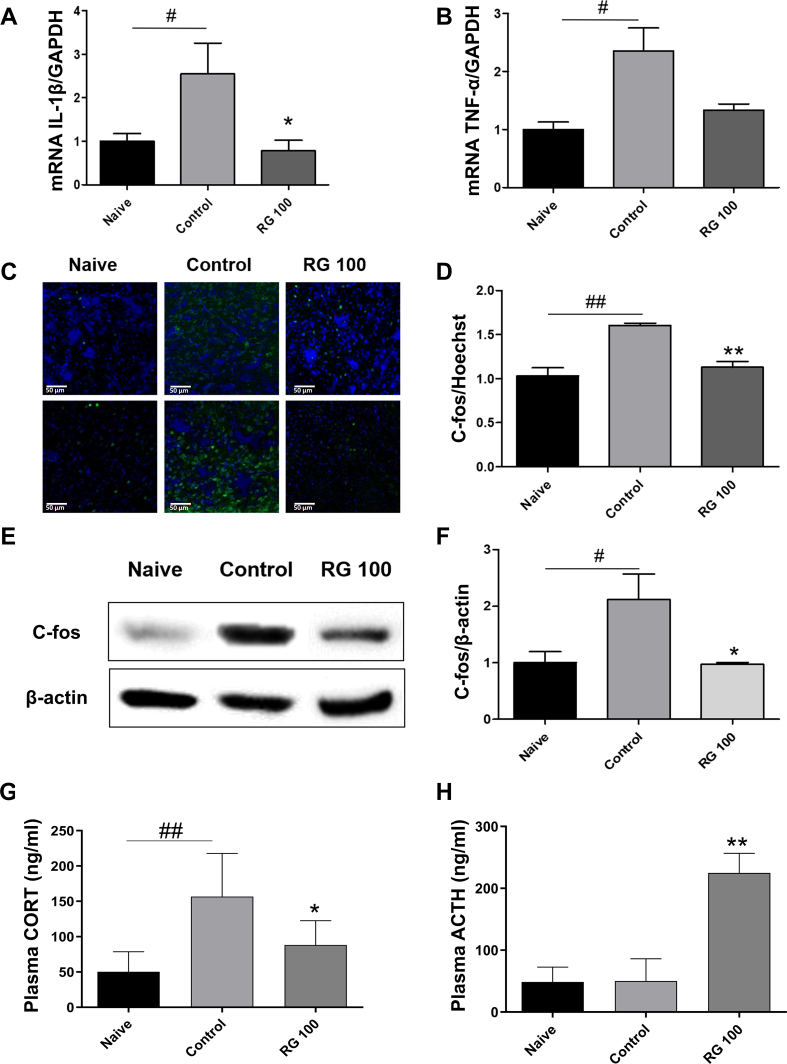
To evaluate the changes in inflammatory cytokine levels of the colon, mRNA levels of IL-1β and TNF-α were measured. The control group showed increased levels of both IL-1β and TNF-α compared to the naïve group. Contrastingly, the IL-1β levels were significantly reduced and TNF-α levels tended to decrease in the RG-100 group (Fig. 6A and B).
Fig. 6.
Effects of RG on various biomarkers involved in gut and brain responses. The mRNA levels of (A) IL-1β and (B) TNF-α in the colon were measured using RT-qPCR. (C-D) PFC sections were stained with c-fos antibodies (green) and Hoechst (blue) and observed by confocal microscopy (scale bar: 50 μm). The c-fos/Hoechst level was quantified using ImageJ software. (E-F) Isolated PFC in the brain lysates was analyzed by western blotting. Band densities were analyzed with Image J software. Beta-actin was used as the loading control. (G) Plasma corticosterone, and (H) ACTH levels measured by ELISA kit. Data are presented the means ± SD values. (One-way ANOVA; #p<0.05, ##p<0.01 vs. Naive; ∗p<0.05, ∗∗p<0.01 vs. Cotrol). RG, red ginseng; CORT, corticosterone; ACTH, adrenocorticotropic hormone.
3.8. Effects of RG on prefrontal cortex c-fos expression
The elevated c-fos level in the prefrontal cortex indicates neural hyperactivation in IBS [30,31]. To determine changes in c-fos levels across the groups, extracted prefrontal cortices were analyzed. IF showed an increase in c-fos expression in the control group and a decrease in the RG-100 group. Quantitative analysis of c-fos protein showed an increase in the control group and a significant decrease in the RG-100 group (Fig. 6C and D). Furthermore, western blot confirmed that the c-fos levels in the RG-100 group were significantly reduced compared to those in the control group (Fig. 6E and F).
3.9. Effects of RG on plasma corticosterone and ACTH levels
To investigate whether zymosan-induced PI-IBS altered the HPA axis, the concentration of corticosterone and ACTH in mouse plasma were measured by ELISA. Plasma corticosterone levels were significantly increased in the control group [vs. naive group] and significantly decreased in the RG-100 group [vs. control group]. The plasma ACTH levels in the control group were similar to that in the naïve group, while levels in the RG-100 group were significantly increased (Fig. 6G and H). These data suggested that RG treatment can decrease the induced corticosterone levels and normalize the HPA axis dysfunction in mice with PI-IBS induced by zymosan treatment.
4. Discussion
In this study, we assessed the potential of RG as a new treatment for IBS. The repeated colonic zymosan injections successfully induced PI-IBS-like symptoms and pathophysiology in mice, evident from the low-grade inflammation in the colon, diarrhea, abdominal pain, and anxiety-like behaviors [13] as well as altered gut microbiota [32]. Additionally, there were significant abnormalities in the levels of various biomarkers including ACTH and corticosterone in the plasma, inflammatory cytokines such as TNF-α and IL-1β in the colon, and c-fos expression in the brain. However, oral RG administration in zymosan-treated mice mostly prevented the development of symptoms and restored pathophysiological alterations to normal levels.
First, the body weight transiently decreased until peak inflammation at day 4 post zymosan injection. However, the body weight in RG-treated mice did not decrease, despite there being no differences in the food intake. The colon length was significantly decreased while the colon weight increased in IBS mice, along with intestinal mucosal thickening and inflammatory cell infiltration. RG treatment showed a marked improvement in the macroscopic score. Thus, RG seems to decrease the moisture content within the stool, thereby decreasing the likelihood of diarrhea in the affected mice.
Second, visceral pain and hypersensitivity are the main symptoms of IBS, whose pathophysiological mechanism is not well understood. RG treatment attenuated abdominal pain-related behaviors, which could be a result of decreased visceral hypersensitivity. TNF-α and IL-1β activation in the gut wall is associated with visceral pain in IBS [31,[33], [34], [35]]. Consistent with the previous report that RG suppresses IL-1β and NF-κB activation [36], RG treatment in our study significantly inhibited IL-1β expression in the colon. It also suppressed TNF-α expression, similar to that observed in the group treated with the anti-inflammatory SSZ compound.
An increase in the level of c-fos, a well-known biomarker for neural activation, in CNS, is linked to visceral hypersensitivity [31], especially with the paraventricular nucleus projecting to the prefrontal cortex (PFC) in rodents [37]. We observed increased c-fos expression in the PFC of PI-IBS mice, while the RG groups showed reduced expression. Taken together, we surmised that RG attenuated visceral sensitivity by decreasing IL-1β in the colon and c-fos levels within the brain.
Third, RG significantly controlled anxiety-like behaviors. Total distance traveled in the OFT is a benchmark for normal behavior. While IBS mice showed less travel distance, the RG-treated mice displayed similar results as that displayed by the naïve mice, indicating that anxiety-induced behaviors were alleviated by RG administration. The EPM test works under the principle that healthier, normal mouse will remain in the center of the maze for as long as possible and visit the center as often as it can. The more anxious the mouse, the longer it will remain in the closed arms section [38,39]. All RG treatment groups showed progressively significant difference over the control group in terms of total distance, number of times the mice entered the maze center, as well as duration of time spent in the closed arms section, similar or superior to the mice treated with AMT, an anxiolytic medicine.
It is evident that plasma cortisol levels are elevated in not only IBS but also in patients suffering from depression and anxiety. In a healthy HPA axis, elevated levels of cortisol would indicate elevated ACTH levels as well. However, prolonged stimulation of the HPA axis could disable the feedback mechanism of ACTH and cortisol, resulting in an extended period of downregulated ACTH levels [8]. This phenomenon not only provides proof that our PI-IBS model shows comparable physiology to that of real IBS [8], but also suggests that RG offers a possible restorative model for attenuating HPA axis dysregulation. Plasma ACTH levels remained comparable in both naïve and control groups, but showed a marked increase in the RG group. Finally, plasma corticosterone levels increased in the control group, but decreased in the RG group.
Fourth, it is common knowledge that GI disorders alter gut microbiota [38]. Zymosan-induced PI-IBS mice showed altered microbiota at both the phylum and species levels. There was an increase in the Firmicutes/Bacteroidetes ratio in the control mice compared to that in the naïve mice, indicating that the number of Bacteroidetes decreased, which is in line with the intestinal barrier break down and diarrhea in IBS patients [39,40]. At the genus level, the control mice showed a decrease in levels of Bacteroides, and Parabacteroides and an increase in Prevotella species. Overall, the control group showed similar enteric microbiotic structure as that observed in IBS physiology [41,42]. Bacteroides and Prevotella are known to have an antagonistic relationship, so having one decrease while the other increases further confirms the success of this IBS model [43]. Despite not being significant, RG administration seemed to show a consistent tendency to normalize enteric microbiota. At the species level, three species significantly stood out: L. johnsonii, L. reuteri, and P. goldsteinii. L. reuteri is probably one of the most famous probiotic species, with known association to alleviating pediatric diarrhea and abdominal pain [[44], [45], [46]]. L. reuteri was reported to reduce inflammatory cytokines and strengthen the intestinal barrier [47]. In addition, oral administration of L. reuteri alleviated ampicillin-induced anxiety and colitis [48]. L. johnsonii works similarly to L. reuteri as it has anti-inflammatory effects and alleviates pediatric diarrhea and abdominal pain by preventing pathogen proliferation [49,50]. It has been reported that the amelioration of gastrointestinal inflammation by treatment with L. johnsonii, can alleviate anxiety [51]. Consumption of L. johnsonii also triggers mucin secretion that strengthens the intestinal barrier [52]. Both L. johnsonii and L. reuteri were not present in the naïve group and increased only in the RG-100 treatment group. This suggests that RG treatment induces the proliferation of L. reuteri and L. johnsonii, which may be a mechanism through which RG promotes anti-inflammatory action. The absence of P. goldsteinii is commonly observed in patients with inflammatory bowel disease, while it is normally abundant in healthy enteric microbiota [53]. In addition, P. goldsteinii treatment increases the IL-10 expression level, and is thought to play a role in intestinal barrier formation via the anti-inflammatory pathway [54]. In contrast to L. johnsonii and L. reuteri, P. goldsteinii levels were restored to that in the naïve group by RG-100 treatment, indicating that restoring normal, healthy enteric function is associated with the presence of P. goldsteinii [55]. Thus, RG not only promotes the proliferation of non-abundant species (such as L. johnsonii & L. reuteri), but also restores the normally existing species (such as P. goldsteinii) to control gut-brain responses in IBS.
In summary, our study provides the possibility that RG may be a promising treatment for human IBS through its systematic action on the gut, enteric microbiota, and brain.
Declaration of competing interest
The authors declare that they have no conflict of interests regarding the contents of this article.
Acknowledgment
This work was supported by the (2019) grant from the Korean Society of Ginseng, Bio & Medical Technology Development Program of the National Research Foundation (NRF), funded by the Korean government (MSIT) (grant number: NRF-2019M3E5D5064771 and NRF-2018M3A9C4076473), and Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI20C0015).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgr.2021.03.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Halkjaer S.I., Boolsen A.W., Gunther S., Christensen A.H., Petersen A.M. Can fecal microbiota transplantation cure irritable bowel syndrome? World J Gastroenterol. 2017;23:4112–4120. doi: 10.3748/wjg.v23.i22.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carabotti M., Scirocco A., Maselli M.A., Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 3.Moloney R.D., Johnson A.C., O’Mahony S.N. Stress and the microbiota-gut-brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci Ther. 2016;22:102–117. doi: 10.1111/cns.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enck P., Aziz Q., Barbara G. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee A., Sarkhel S., Sarkar R., Dhali G.K. Anxiety and depression in irritable bowel syndrome. Indian J Psychol Med. 2017;39:741–745. doi: 10.4103/IJPSYM.IJPSYM_46_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee C., Doo E., Choi J.M. The increased level of depression and anxiety in irritable bowel syndrome patients compared with healthy controls: systematic review and meta-analysis. J Neurogastroenterol Motil. 2017;23:349–362. doi: 10.5056/jnm16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabra N., Nadkarni A. Prevalence of depression and anxiety in irritable bowel syndrome: a clinic based study from India. Indian J Psychiatry. 2013;55:77–80. doi: 10.4103/0019-5545.105520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang L., Sundaresh S., Elliott J. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugaya N., Izawa S., Saito K. Effect of prolonged stress on the adrenal hormones of individuals with irritable bowel syndrome. Biopsychosoc Med. 2015;9:4. doi: 10.1186/s13030-015-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J., Zhong W., Wang W. Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-kappaB activation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S., Rhee D.K. Effects of ginseng on stress-related depression, anxiety, and the hypothalamic-pituitary-adrenal axis. J Ginseng Res. 2017;41:589–594. doi: 10.1016/j.jgr.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H.J., Kim P., Shin C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J Ginseng Res. 2013;37:8–29. doi: 10.5142/jgr.2013.37.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun E., Yoon S., Parveen A., Jin M. Alleviation of irritable bowel syndrome-like symptoms and control of gut and brain responses with oral administration of Dolichos lablab L. In a mouse model. Nutrients. 2018;10 doi: 10.3390/nu10101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vannucchi M.G., Evangelista S. Experimental models of irritable bowel syndrome and the role of the enteric neurotransmission. J Clin Med. 2018;7 doi: 10.3390/jcm7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Bi Z., Wang E., Sun B., Zheng Y., Zhong L.L. Rodent model of irritable bowel syndrome. Int J Gastroenterol Disord Ther. 2017 [Google Scholar]
- 16.Fuentes I.M., Christianson J.A. Ion channels, ion channel receptors, and visceral hypersensitivity in irritable bowel syndrome. Neurogastroenterol Motil. 2016;28:1613–1618. doi: 10.1111/nmo.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.In G., Ahn N.G., Bae B.S. In situ analysis of chemical components induced by steaming between fresh ginseng, steamed ginseng, and red ginseng. J Ginseng Res. 2017;41:361–369. doi: 10.1016/j.jgr.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J., Park J., Lee Y.Y., Lee Y. Comparative transcriptome analysis of the protective effects of Korean Red Ginseng against the influence of bisphenol a in the liver and uterus of ovariectomized mice. J Ginseng Res. 2020;44:519–526. doi: 10.1016/j.jgr.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park B.K., Chun E., Choi J.J. Administration of wasabia koreana ameliorates irritable bowel syndrome-like symptoms in a zymosan-induced mouse model. J Med Food. 2017;20:474–484. doi: 10.1089/jmf.2016.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Z., Kim Y.W., Zhang J., Lee J.H. Korean Red Ginseng attenuates anxiety-like behavior during ethanol withdrawal in rats. J Ginseng Res. 2014;38:256–263. doi: 10.1016/j.jgr.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H.J., Won H., Im J. Effects of Panax ginseng CA Meyer extract on the offspring of adult mice with maternal immune activation. Mol Med Rep. 2018;18:3834–3842. doi: 10.3892/mmr.2018.9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang S.H., Park J., Kim S.H. Oral administration of red ginseng powder fermented with probiotic alleviates the severity of dextran-sulfate sodium-induced colitis in a mouse model. Chin J Nat Med. 2017;15:192–201. doi: 10.1016/S1875-5364(17)30035-3. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M.M., Liu S.B., Chen T. Effects of NB001 and gabapentin on irritable bowel syndrome-induced behavioral anxiety and spontaneous pain. Mol Brain. 2014;7:47. doi: 10.1186/1756-6606-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimball E.S., Palmer J.M., D’Andrea M.R., Hornby P.J., Wade P.R. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1266–G1273. doi: 10.1152/ajpgi.00444.2004. [DOI] [PubMed] [Google Scholar]
- 25.Kim Y.R., Volpert G., Shin K.O. Ablation of ceramide synthase 2 exacerbates dextran sodium sulphate-induced colitis in mice due to increased intestinal permeability. J Cell Mol Med. 2017;21:3565–3578. doi: 10.1111/jcmm.13267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi D.B., Li W.M. Effects of electroacupuncture on expression of c-fos protein in the spinal dorsal horn of rats with chronic visceral hyperalgesia. Zhong Xi Yi Jie He Xue Bao. 2012;10:1490–1496. doi: 10.3736/jcim20121224. [DOI] [PubMed] [Google Scholar]
- 27.Laird J.M., Martinez-Caro L., Garcia-Nicas E., Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 28.Jung J.H., Kim S.J. Anxiolytic action of taurine via intranasal administration in mice. Biomol Ther (Seoul) 2019;27:450–456. doi: 10.4062/biomolther.2018.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang T., Santisteban M.M. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/Hypertensionaha.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macsharry J., O’Mahony L., Fanning A. Mucosal cytokine imbalance in irritable bowel syndrome. Scand J Gastroenterol. 2008;43:1467–1476. doi: 10.1080/00365520802276127. [DOI] [PubMed] [Google Scholar]
- 31.Zhang R., Zou N., Li J. Elevated expression of c-fos in central nervous system correlates with visceral hypersensitivity in irritable bowel syndrome (IBS): a new target for IBS treatment. Int J Colorectal Dis. 2011;26:1035–1044. doi: 10.1007/s00384-011-1153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song L. Seoul National University; 2019. Effects of Lactobacillus plantarum and Lactobacillus paracasei for the prevention and alleviation of zymosan-induced irritable bowel syndrome in mice. [Google Scholar]
- 33.Shouval D.S., Biswas A., Kang Y.H. Interleukin 1beta mediates intestinal inflammation in mice and patients with interleukin 10 receptor deficiency. Gastroenterology. 2016;151:1100–1104. doi: 10.1053/j.gastro.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coccia M., Harrison O.J., Schiering C. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209:1595–1609. doi: 10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren K., Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang S.W., Park J.H., Seok H., Park H.J. The effects of Korea red ginseng on inflammatory cytokines and apoptosis in rat model with chronic nonbacterial prostatitis. Biomed Res Int. 2019;2019:2462561. doi: 10.1155/2019/2462561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pirnik Z., Mravec B., Kiss A. Fos protein expression in mouse hypothalamic paraventricular (PVN) and supraoptic (SON) nuclei upon osmotic stimulus: colocalization with vasopressin, oxytocin, and tyrosine hydroxylase. Neurochem Int. 2004;45:597–607. doi: 10.1016/j.neuint.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Raskov H., Burcharth J., Pommergaard H.C., Rosenberg J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. 2016;7:365–383. doi: 10.1080/19490976.2016.1218585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu H., Zeng D., Wang Q. Diarrhea-associated intestinal microbiota in captive sichuan golden snub-nosed monkeys (Rhinopithecus roxellana) Microbes Environ. 2018;33:249–256. doi: 10.1264/jsme2.ME17163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Braun T., Sengni A.D., BenShoshan M. Fecal microbial characterization of hospitalized patients with suspected infectious diarrhea shows significant dysbiosis. Sci Rep. 2017;7:1088. doi: 10.1038/s41598-017-01217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wick E.C., Sears C.L. Bacteroides spp. and diarrhea. Curr Opin Infect Dis. 2010;23:470–474. doi: 10.1097/QCO.0b013e32833da1eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forbes J.D., Van Domselaar G., Bernstein C.N. The gut microbiota in immune-mediated inflammatory diseases. Front Microbiol. 2016;7:1081. doi: 10.3389/fmicb.2016.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ley R.E. Gut microbiota in 2015: Prevotella in the gut: choose carefully. Nat Rev Gastroenterol Hepatol. 2016;13:69–70. doi: 10.1038/nrgastro.2016.4. [DOI] [PubMed] [Google Scholar]
- 44.Urbanska M., Gieruszczak-Bialek D., Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025–1034. doi: 10.1111/apt.13590. [DOI] [PubMed] [Google Scholar]
- 45.Jadresin O., Hojsak I. Lactobacillus reuteri DSM 17938 in the treatment of functional abdominal pain in children: RCT study. J Pediatr Gastroenterol Nutr. 2017;64:925–929. doi: 10.1097/MPG.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 46.Kamiya T., Wang L., Forsythe P. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in sprague-dawley rats. Gut. 2006;55:191–196. doi: 10.1136/gut.2005.070987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mu Q., Tavella V.J., Luo X.M. Role of Lactobacillus reuteri in human health and diseases. Front Microbiol. 2018;9:757. doi: 10.3389/fmicb.2018.00757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang H.M., Lee H.J., Jang S.E., Han M.J., Kim D.H. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 2018;11:1386–1397. doi: 10.1038/s41385-018-0042-3. [DOI] [PubMed] [Google Scholar]
- 49.Davoren M.J., Liu J., Castellanos J., Rodriguez-Malave N.I., Schiestl R.H. A novel probiotic, Lactobacillus johnsonii 456, resists acid and can persist in the human gut beyond the initial ingestion period. Gut Microbes. 2019;10:458–480. doi: 10.1080/19490976.2018.1547612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eftekhari K., Vahedi Z., Kamali Aghdam M., Noemi Diaz D. A randomized double-blind placebo-controlled trial of Lactobacillus reuteri for chronic functional abdominal pain in children. Iran J Pediatr. 2015;25 doi: 10.5812/ijp.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jang H.M., Lee K.E., Lee H.J., Kim D.H. Immobilization stress- induced Escherichia coli causes anxiety by inducing NF-kappa B activation through gut microbiota disturbance. Sci Rep-Uk. 2018;8 doi: 10.1038/s41598-018-31764-0. ARTN 13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujimura K.E., Demmor T., Rauch M. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A. 2014;111:805–810. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miranda P.M., Bertolini Francesca, Kadarmideen Haja N. 2019. Investigation of gut microbiome association with inflammatory bowel disease and depression: a machine learning approach. F1000Research. [Google Scholar]
- 54.Wu T.R., Lin C.S., Chang C.J. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut. 2019;68:248–262. doi: 10.1136/gutjnl-2017-315458. [DOI] [PubMed] [Google Scholar]
- 55.Chassaing B., Gewirtz A.T. Mice harboring pathobiont-free microbiota do not develop intestinal inflammation that normally results from an innate immune deficiency. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.