Abstract
The blood-brain barrier (BBB) is usually impermeable to several drugs, which hampers treatment of various brain-related diseases/disorders. There have been several approaches to open the BBB, including intracarotid infusion of hyperosmotic concentrations of arabinose, mannitol, oleic or linoleic acids, or alkylglycerols, intravenous infusion of bradykinin B2, administration of a fragment of the ZO toxin from vibrio cholera, targeting specific components of the tight junctions (e.g. claudin-5) with siRNA or novel peptidomimetic drugs, or the use of ultrasound with microbubbles. We propose the use of a low molecular weight (MW), nitrone-type compound, OKN-007, which can temporarily open up the BBB for 1-2 hours. Gadolinium (Gd)-based compounds assessed ranged in MW from 546 (Gd-DTPA) to 465 kDa (β-galactosidase-Gd-DOTA). We also included an albumin-based CA (albumin-Gd-DTPA-biotin) for assessment, as well as an antibody (Ab) against a neuron-specific biomarker conjugated to Gd-DOTA (anti-EphB2-Gd-DOTA). For the anti-EphB2 (goat Ab)-Gd-DOTA assessment, we utilized an anti-goat Ab conjugated with horse radish peroxidase (HRP) for confirmation of the presence of the anti-EphB2-Gd-DOTA probe. In addition, a Cy5 labeled anti-EphB2 Ab was co-administered with the anti-EphB2-Gd-DOTA probe, and assessed ex vivo. This study demonstrates that OKN-007 may be able to temporarily open up the BBB to augment the delivery of various compounds ranging in MW from as small as ~550 to as large as ~470 kDa. This compound is an investigational new drug for glioblastoma (GBM) therapy in clinical trials. The translational capability for human use to augment the delivery of non-BBB-permeable drugs is extremely high.
Keywords: OKN-007, BBB, in vivo, mice, contrast-enhanced MRI, molecular-targeted MRI, Eph-B2, β-galactosidase, Gd-DTPA, Gd-DTPA-albumin-biotin, Gd-DOTA
Introduction
The blood-brain barrier (BBB) is a boundary of the brain microvasculature, consisting predominantly of endothelial cells (ECs), astrocyte end-feet, and pericytes [1,2]. Tight junctions between the cerebral ECs form a diffusion barrier that prevents blood-borne components from entering the brain [1,2]. Astrocytic end-feet firmly encircle the vessel wall, and are thought to play a key role in maintaining the tight junction barrier [1,2]. Pericytes are cells of microvessels, which incorporate capillaries, venules, and arterioles, that surround the ECs [3]. These components divide the peripheral circulation from contact with the central nervous system (CNS). This boundary is made up of numerous parallel barriers that consist of the capillary bed of the CNS and choroid plexus [4]. The BBB also controls the crossing of some solutes into different compartments [5]. The choroid plexus structures the blood-cerebrospinal fluid barrier, and along with other barriers, including the blood-retinal barrier, these create both barrier and permeability elements that add to the total BBB [6]. Solute transfer from blood-to-brain or brain-to-blood arises via only a few processes, allowing small molecules, such as electrolytes, to large immune cells to cross [4,5].
Under physiological conditions, the BBB is somewhat impermeable [1,2]. However, in pathologic conditions, there are a number of chemical mediators that can be released to increase BBB permeability [7]. Examples of these mediators consist of glutamate, taurine, aspartate, NO (nitric oxide), ATP, tumor necrosis factor-α (TNF-α), endothelin-1, IL-β, and MIP-2, which are produced by astrocytes [7]. Other mediators that have been reported to increase BBB permeability include bradykinin, histamine, thrombin, 5HT, UTP, UMP, platelet-activating factor, substance P, quinolinic acid, and free radicals [7]. Furthermore, the expression of tight junction proteins and/or localization of individual principal proteins can be altered in response to pathophysiological stressors [8].
In this study, we report on the use of the nitrone compound, OKN-007 (OKlahoma Nitrone 007; also known as 2,4-disulfonyl-phenyl-N-tert-butyl nitrone), to temporarily open up the BBB in normal mouse brain. BBB permeability was measured using contrast-enhanced magnetic resonance imaging (CE-MRI) and molecular-targeted MRI (mtMRI). Compounds as small as Gd-DTPA (gadolinium diethylenetriaminepentacetate) and as large as Gd-DOTA (gadolinium 1,4,7,10-tetraazacyclododecane-N,N’,N’’,N’’’-tetraacetate)-labeled β-galactosidase were assessed in vivo. Other compounds assessed in vivo and ex vivo included an antibody against the neuron-specific marker, EphB2, attached to either Gd-DOTA or a fluorescent label, and Gd-DTPA-albumin-biotin. This approach could augment the delivery of drugs to the brain that usually are unable to cross an impermeable BBB or result in low concentrations in the brain due to hindrance by the BBB.
Methods
Ethics statement
Animal experiments were performed with the approval and strict adherence to the policies of the Oklahoma Medical Research Foundation Institutional Animal Care and Use Committee, which specifically approved this study, with adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize suffering.
Animals
Rats (Sprague-Dawley; 8-10 weeks old; male; n = 5) and mice (C57BL/6J; 8-10 weeks old; male; n = 47) were either treated with saline as controls or OKN-007 (i.v. via a tail-vein; 18 mg/kg body weight for rats, or 125 mg/kg body weight for mice).
Gd-based contrast agents and molecular-targeted probes
The contrast agent, biotin-albumin-Gd-DTPA, was synthesized as previously described by our group [9,10], based on the modification of the method developed by Dafni et al. [11]. The approximate molecular weight for the biotin-albumin-Gd-DTPA moiety is ~80 kDa. It is approximated that there are 1.3 biotin and 23 Gd-DTPA groups bound to each albumin molecule [11]. Anti-EphB2 mAb (Cell Signaling Technology, Inc., MA, USA) was conjugated to Gd-DOTA based on a protocol outlined by Chen et al. [12]. Each mouse was injected with 20 μl anti-EphB2-Gd-DOTA (EphB2 probe) intravenously (i.v.) via the tail vein with an amount estimated to be 20 μg (1 mg/kg) anti-EphB2. The estimated molecular weight of the EphB2 probe is 150 kDa [10]. β-Galactosidase-Gd-DOTA was synthesized in a similar process to that of the EphB2 probe.
MRI experiments
MRI experimentations were done on a Bruker Biospec 7.0 Tesla/30 cm horizontal-bore imaging system. Multiple brain 1H-MR image slices were taken using a RARE multi-slice (repetition time (TR) 1.3 s, echo time (TE) 9 ms, 256×256 matrix, 4 steps per acquisition, 4×4 cm2 (rats) or 3×3 cm2 (mice) field-of-view (FOV), 1.0-mm slice thickness) imaging sequence.
For contrast-enhanced MRI (CE-MRI), multi-slice spin echo T1-weighted images (TR = 1000.0 ms, TE = 14 ms, FOV = 4×4 cm2 (rats) or 3×3 cm2 (mice), averages = 2, slices = 16, matrix size = 256×256) were also executed and acquired before and 10, 20, and 30 min following intravenous contrast agent injection (Gd-DTPA, Magnevist, Bayer Inc., Wayne, NY, USA; 0.4 mmol/kg) [13]. Whole brain evaluations in multiple regions-of-interest (ROIs) (10-15) were made regarding MRI signal intensity measurements. For larger Gd-based contrast agents and the EphB2 probe, a variable-TR RARE sequence (rapid acquisition with refocused echoes, with multiple TRs of 200, 400, 800, 1200 and 1600 ms, TE of 15 ms, FOV of 3×3 cm2, matrix size of 256×256 and a spatial resolution of 0.137 mm) was used to obtain T1-weighted images before and after administration of probe or contrast agents. The changes in signal intensities were calculated from regions of interest (ROIs) (n = 5) obtained from T1-weighted images. Pixel-by-pixel T1 relaxation maps were reconstructed from a series of T1-weighted images using a nonlinear two-parameter fitting procedure. Regional T1 values were obtained from ROIs (n = 5). ROI averages (SI or T1 values) were then obtained for each animal, and means ± standard deviations (SD) were obtained for each treatment group. Surrounding muscle SI or T1 values (n = 5) were used for normalization for each animal assessment. All ROIs were obtained using Paravision software (ver. 6.0.1).
Fluorescence staining
The mouse brains were extracted after the 90 min. MRI scan, and fixed in 4% paraformaldehyde over night at 4°C. The tissue was then washed with PBS and incubated with 15% sucrose before embedding in an Optimal Cutting Temperature (O.C.T.) compound and freezing in liquid nitrogen. For the EphB2 component, mice treated either with saline or OKN-007 were administered an anti-EphB2 antibody (0.5 mg/kg) labeled with Cy5. Tissues were embedded in cryo-gel, and 35 µm cryostat sections were collected unto glass slides and immuno-fluorescently stained. The nucleus was stained with DAPI (blue). Stained tissue slices were examined with a Nikon C1 confocal laser scanning microscope (Nikon Instruments, USA). Image collection parameters (neutral density filters, pinhole, and detector gains) were kept constant during image acquisition, to make reliable comparisons between specimens. For the albumin-Gd-DTPA-biotin component, the brains were divided into hemispheres and subsequently fixed in 4% paraformaldehyde, tissues were also embedded in cryo-gel, and 35 µm cryostat sections were collected unto glass slides for subsequent immuno-fluorescence staining. Primary staining was done with anti-Endomucin (rat monoclonal, Millipore; 1:75), visualized using secondary Alexa Fluor 568 (anti rat, Invitrogen, 1:500). The albumin-Gd-DTPA-biotin contrast agent was visualized with FITC conjugated Avidin (Biolegend, 1:100) added as a secondary antibody, where the Avidin targeted the biotin moeity. DAPI was added at a concentration of 0.5 µg/ml for 5 minutes. Sections were cover slipped with Prolong Gold Antifade (Invitrogen). Images were acquired with the SP8 confocal LEICA microscope and analyzed with IMAGEJ software (NIH).
Histology and immunohistochemistry (IHC)
Brain tissues were obtained and fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned in 5 μm sections for routine staining. Sections were deparaffinized, rehydrated and washed in Tris Buffered Saline (TBS). Antigen retrieval (pH 6 Citrate buffer, Vector Labs, Burlingame, CA) was achieved via twenty minutes in a steamer, followed by thirty minutes cooling at room temperature. Sections were treated with a peroxidase blocking reagent (Bloxall, Vector Labs, Burlingame, CA) to inhibit endogenous peroxidase activity, followed by 2.5% normal horse serum (Vector Labs, Burlingame, CA) to inhibit nonspecific binding. Suitable washes were in TBS. Tissue sections were incubated in humidified chambers with the Goat Probe or diluent for 3 hours at room temperature followed by TBS washes. The Goat-on-Rodent HRP polymer (Goat Polymer kit to detect the EphB2 goat antibody) (GHPS-H; Biocare Medical, Pacheco, CA) was applied to each section for 15 minutes followed by TBS washes. Slides were incubated with NovaRed®Red (Vector Labs, Burlingame, CA) chromogen for visualization. Counterstaining was carried out with Hematoxylin QS Nuclear Counterstain (Vector Labs, Burlingame, CA).
Statistical analysis
Statistical analyses were performed by using a Student’s two-tailed, unpaired t-test for comparison of MR signal intensity increase percentages or differences in T1 relaxation changes between saline- or OKN-007-treated mice administered Gd-based contrast agents or probes. For the Gd-DTPA time-course study, statistical analyses were done using both a two-way ANOVA and individual time-point Student’s t-test (unpaired) for comparison of % differences in MRI SI. Data were represented as mean ± S.D. and P-values <0.05 (*), <0.01 (**), <0.0001 (***) were considered statistically significant. All data were used with no experimental failures.
Results
Figure 1 illustrates the structure (Figure 1A) of OKN-007, and its’ ability to be quickly cleared from the blood over a period of less than an hour (Figure 1B), as well as its’ ability to be taken up into normal rodent brain (Figure 1C) through the BBB for a transitory period of 2-3 hours (Figure 1D). OKN-007 is a disulfonyl derivative of the parent nitrone, phenyl-N-tert-butyl nitrone or PBN (Figure 1A). Pharmacokinetics assessment of OKN-007, administered i.v., in normal rat blood has established that the elimination rate constant, Kel, is 0.00364 min-1 with a mean resident time (MRT) of 1,058 min (17.6 hours). However, as can be seen in Figure 1B, the majority of OKN-007 is cleared from the blood in less than 4 hours. We have detected OKN-007 in normal rat brain tissue lysates one hour following i.v. administration of OKN-007, as detected by HPLC (Figure 1C). With the use of the MRI contrast agent, Gd-DTPA, which does not cross the BBB, an increase in MRI signal intensity (SI) was observed over a period of 1-3 hours in mouse brains given OKN-007 via i.v. administration prior to Gd-DTPA, compared to saline controls where saline was administered 1 hour prior to the contrast agent (Figure 1D). There was a statistical increase in percent MRI signal intensity (SI) for the OKN-007-treated group compared to controls (P<0.001, two-way ANOVA; or P<0.05 for 1 and 3 hours or P<0.001 2 hours post OKN-007 administration, individual Student’s t-test).
Figure 1.
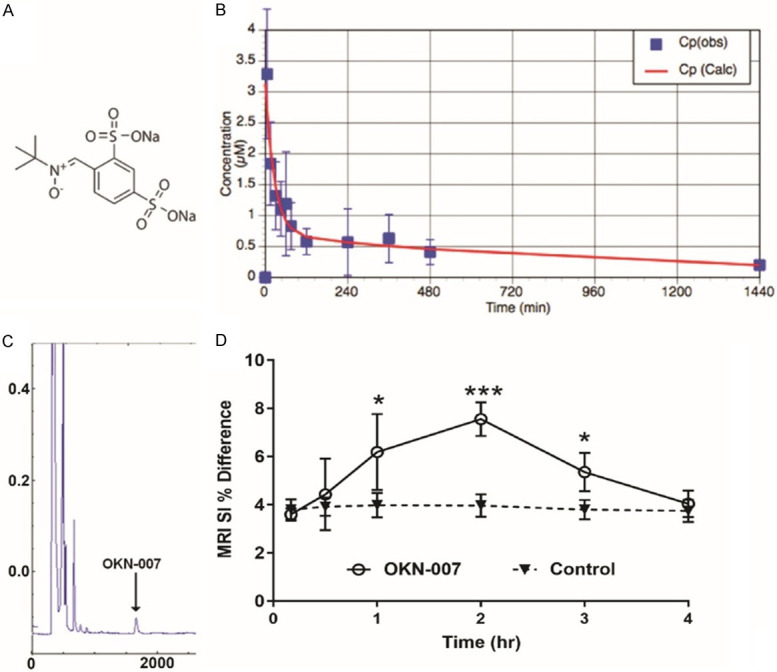
A. Chemical structure of OKN-007. B. Plasma concentration of OKN-007 from rat blood following administration of 18 mg OKN-007/kg body weight (i.v. via a tail-vein catheter), over a period of 1440 min (24 hours) (n = 5). C. HPLC chromatogram of OKN-007 from a rat brain lysate. D. Percent (%) difference in MRI signal intensities (SI) (post-Gd-DTPA contrast minus pre-contrast) in mouse brains (n = 5/group) administered OKN-007 (125 mg/kg) via i.v. administration (tail-vein catheter), compared to saline controls. A two-way ANOVA indicated a significant difference (***P<0.001) between the two treatment groups. Student t-tests at 1, 2 and 3 hours indicated that OKN-007-treated mouse brains were significantly higher (*P<0.05 for both 1 and 3 hours, and ***P<0.001 for 2 hours), compared to controls.
Figure 2 depicts the increase in MRI signal intensity in a representative mouse brain administered OKN-007 (Figure 2A), compared to a control mouse brain administered saline (Figure 2B). The percent (%) difference in MRI SI between post-contrast and pre-contrast, Gd-DTPA contrast-enhanced MRI (CE-MRI) in OKN-007 and saline-treated (i.v. administration) mouse brains, one hour post-contrast, is shown in Figure 2C. There was a significant increase in the % difference in MRI SI for OKN-007-treated mouse brains, compared to saline controls (P<0.001). Due to the small MW and rapid clearance rate of Gd-DTPA, only MRI SI values were obtained. For larger MW GD-based contrast agents with longer clearance rates, both MRI SI and T1 values were obtained.
Figure 2.
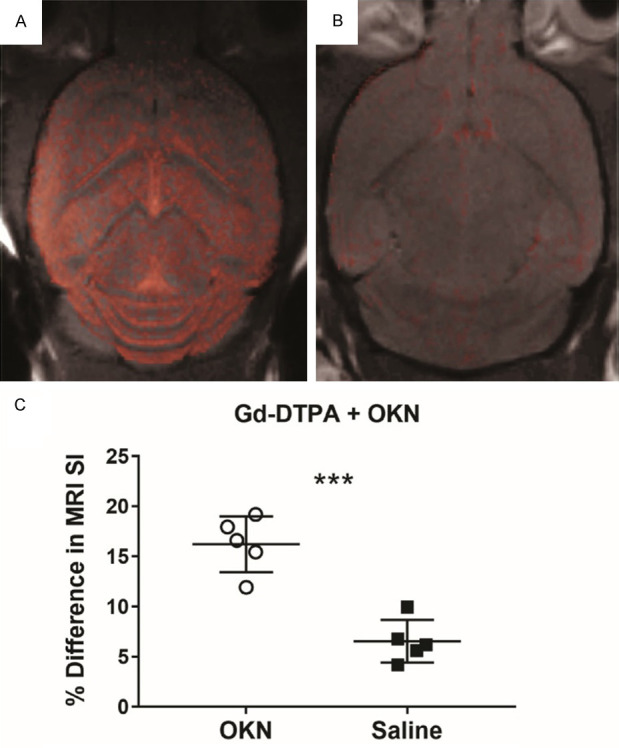
Representative mouse brain difference images (post-contrast minus pre-contrast), overlaid on top of morphological images, indicating an increase in MRI signal intensities in a mouse brain administered OKN-007 (A), compared to a control mouse brain administered saline (B), both 1 hour prior to Gd-DTPA. (C) Percent (%) differences in MRI SI between post-contrast and pre-contrast, Gd-DTPA contrast-enhanced MRI (CE-MRI) in OKN-007 (n = 5) and saline-treated (i.v. administration) (n = 5) mouse brains, one hour post-contrast. There was a significant increase in the % difference in MRI SI for OKN-007-treated mouse brains, compared to saline controls (P<0.001).
Figure 3 demonstrates that a large molecule, such as Gd-DOTA-labelled β-galactosidase (MW 465 kDa), is able to cross the BBB when administered one hour post-OKN-007 treatment, as detected by an increase in MRI signal intensity from the Gd-based contrast agent after OKN-007 (Figure 3A), compared to a saline control also administered the Gd-DOTA-labelled β-galactosidase (Figure 3B). MRI SI, which is increased in the presence of Gd-based contrast agents, was found to be significantly increased in % difference in MRI SI for OKN-007-treated mouse brains administered the Gd-DOTA-β-galactosidase (Gd-DTPA-B-Gal), compared to saline controls (P<0.0001) (Figure 3C). T1 relaxation, which decreases in the presence of Gd-based contrast agents, was found to have a significant increase in % decrease in T1 in OKN-007-treated mouse brains administered the Gd-DOTA-β-galactosidase (Gd-DTPA-B-Gal), compared to saline controls (P<0.001) (Figure 3D).
Figure 3.
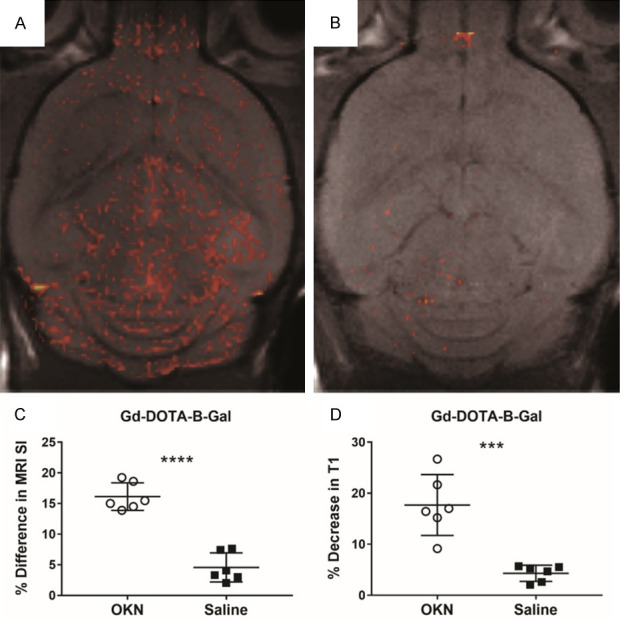
Gd-DOTA-labelled β-galactosidase (MW 465 kDa), is able to cross the BBB. Gd-DOTA-β-galactosidase was administered one hour post-OKN-007 treatment, and an increase in MRI signal intensity from the Gd-based contrast agent after administration of OKN-007 (1 hour prior) (A) was detected, compared to a saline control (1 hour prior) also administered the Gd-DOTA-labelled β-galactosidase (B). (C) Percent difference in MRI SI was found to be significantly increased in OKN-007-treated mouse brains administered the Gd-DOTA-β-galactosidase (Gd-DTPA-B-Gal) (n = 6), compared to saline controls (n = 6) (P<0.0001). (D) The percent (%) decrease in T1 relaxation was found to be significantly increased in OKN-007-treated mouse brains administered the Gd-DOTA-β-galactosidase (Gd-DTPA-B-Gal) (n = 6), compared to saline controls (n = 6) (P<0.001).
Figure 4 shows that an antibody (150 kDa) against the neuron-specific biomarker, Eph-B2, which is labelled with Gd-DOTA, has an increased MRI SI in the OKN-007-treated mouse brain (Figure 4A), compared to a saline control (Figure 4B). The Gd-DOTA-labelled antibody against Eph-B2, significantly increased the % difference in MRI SI (Figure 4C; P<0.001) and % decrease in T1 relaxation (Figure 4D; P<0.01) for OKN-007-treated mouse brains, compared to saline controls. A fluorescent-labelled antibody against Eph-B2 was found to be elevated in an OKN-007-treated mouse brain (Figure 4E), compared to a saline control (Figure 4F). An anti-goat antibody labelled with horse radish peroxidase (HRP) was found to be increased in the OKN-007-treated mouse brain administered the Gd-DOTA-labeled goat antibody against Eph-B2 (Figure 4Gi and 4Gii, compared to a saline control (Figure 4H).
Figure 4.
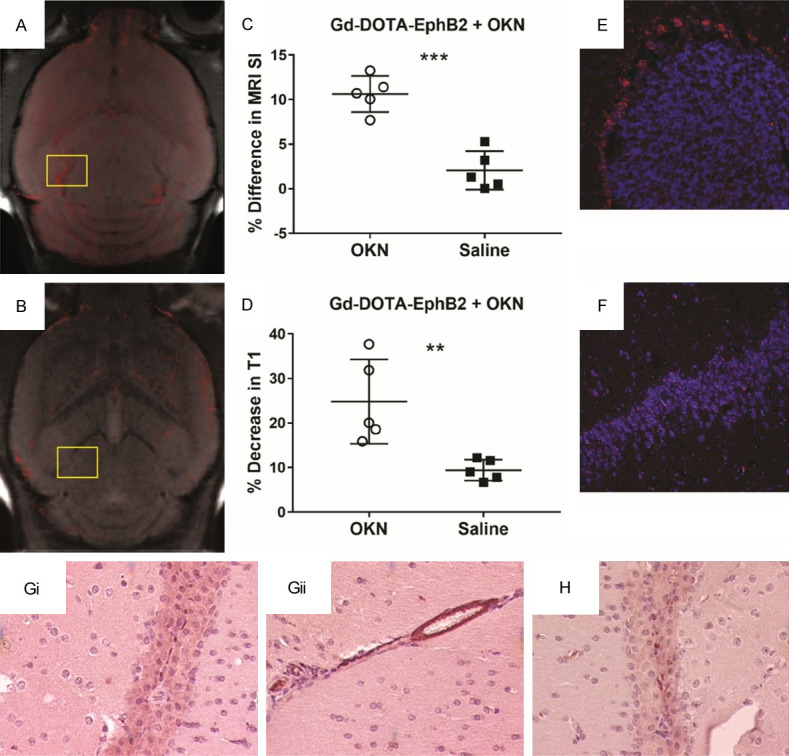
An antibody (150 kDa) against the neuron-specific biomarker, EphB2, which is labelled with Gd-DOTA, has an increased MRI SI in an OKN-007-treated mouse brain (A), compared to a saline control (B). (C) The percent (%) difference in MRI SI was found to be significantly increased for the Gd-DOTA-labelled antibody against EphB2, in OKN-007-treated mouse brains (P<0.001), compared to saline controls. (D) The percent (%) decrease in T1 relaxation was found to be significantly increased for OKN-007-treated mouse brains (P<0.01) (n = 5), compared to saline controls (n = 5). A fluorescent-labelled (Cy5) antibody against Eph-B2, co-administered in vivo with the anti-EphB2-Gd-DOTA, was found to be elevated in an OKN-007-treated mouse brain, particularly the corpus callusum region (highlighted in panel A) (E), compared to a saline control (highlighted in panel B) (F). An anti-goat antibody labelled with horse radish peroxidase (HRP) was found to be increased in the OKN-007-treated mouse brain administered the Gd-DOTA-labeled goat antibody against Eph-B2 (Gi and Gii), compared to a saline control (H).
Figure 5 demonstrates that the MRI signal intensity (SI) in a mouse brain administered Gd-DTPA-albumin-biotin, one hour following OKN-007-treatment is increased (Figure 5A), compared to a saline control (Figure 5B). There was a significant increase in the % difference in MRI SI for the OKN-007-treated mouse brains, compared to saline controls (P<0.001), both administered the Gd-DTPA-albumin-biotin contrast agent (Figure 5C). Confirmation of the presence of the Gd-DTPA-albumin-biotin contrast agent was determined by tagging the biotin in ex vivo mouse brain tissues with FITC-labelled avidin (Figure 5E). Endomucin was also used to delineate cerebral vasculature, and a merged image shows the presence of the avidin-FITC with cell nuclei (DAPI) and vasculature in the cerebral cortex. Quantitative assessment of the number of avidin specks detected are shown in Figure 5D, where there was a significant increase (P<0.05) in the OKN-007-treated mouse brains, compared to saline controls.
Figure 5.
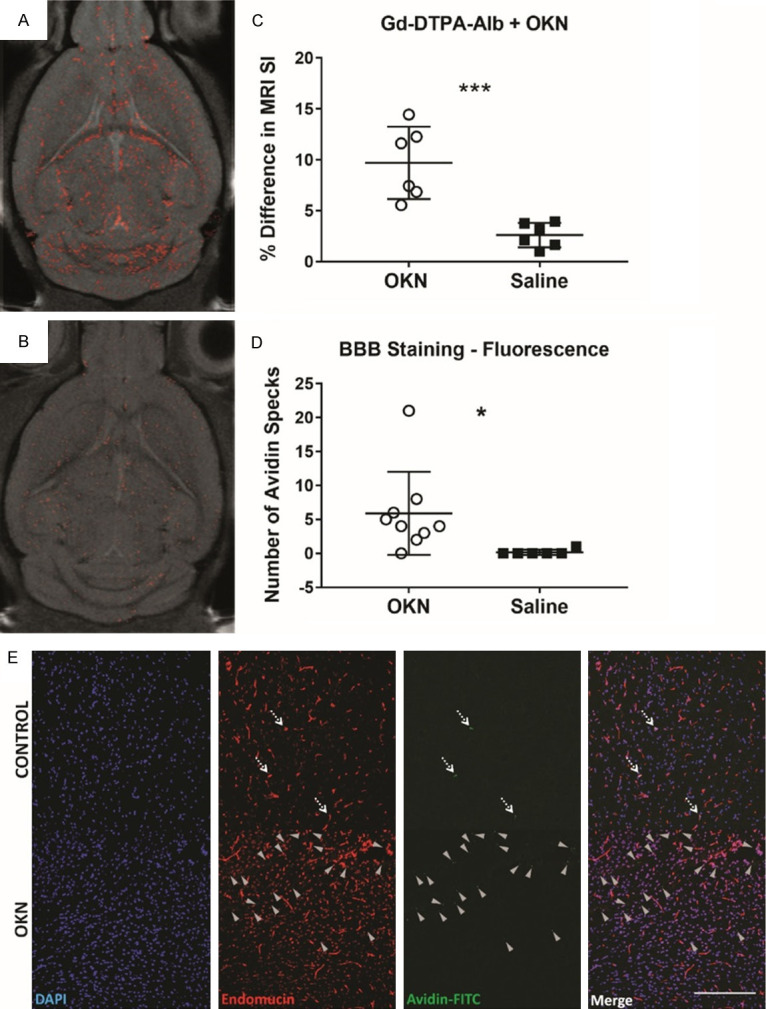
Albumin-Gd-DTPA-biotin (MW 80 kDa), is able to cross the BBB. Albumin-Gd-DTPA-biotin was administered one hour post-OKN-007 treatment, and an increase in MRI signal intensity from the Gd-based contrast agent after administration of OKN-007 (A) was detected, compared to a saline control also administered albumin-Gd-DTPA-biotin (B). (C) The percent (%) difference in MRI signal intensity (SI) was found to be significantly increased in OKN-007-treated mouse brains administered the albumin-Gd-DTPA-biotin contrast agent (n = 6), compared to saline controls (n = 6) (***P<0.001). Confirmation of the presence of the albumin-Gd-DTPA-biotin contrast agent was obtained ex vivo by tagging the biotin moiety with avidin-FITC. (D) Number of avidin specks counted in the OKN-007-treated mouse brains (n = 5) in various regions-of-interest (ROIs) throughout the brain were found to be significantly increased (*P<0.05), compared to saline control brains (n = 4). (E) Representative fluorescence images from the cerebral cortex regions of an OKN-007-treated mouse brain (bottom panel), compared to a saline control (top panel) are also shown. DAPI (blue) stains the nuclei. Endomucin (red) delineates the cerebral vasculature. Avidin-FITC (green) depicts the presence of the albumin-Gd-DTPA-biotin contrast agent in the brain parenchyma. A merged image is also presented. Dashed arrows point out the albumin-Gd-DTPA-biotin in the vascular lumen, and grey arrowheads point out the extravasated albumin-Gd-DTPA-biotin. Scale: 200 µm.
Discussion
What is currently known about the BBB is that it consists of: (1) tight junctions, which consist of membrane proteins, occludin, claudins, junction adhesion molecules, and cytoplasmic accessory proteins (e.g. such as zonula occludens (ZO) 1-3, and cingulin); and (2) adherens junctions, made up of the membrane protein cadherin, which connects the actin cytoskeleton via intermediary proteins such as catenins, that forms adhesive contacts between cells [7]. Claudins attach homotypically to claudins on neighboring ECs that effect the formation of a primary seal of the tight junction, and the carboxy terminal of claudins attaches to cytoplasmic proteins that consist of ZO 1-3 [14]. Occludin is additionally a regulatory protein that can modify paracellular permeability [15]. Junctional adhesion molecules are associated with the immunoglobulin superfamily, and are implicated in cell-to-cell adhesion and monocyte transmigration through the BBB [16]. The cytoplasmic accessory proteins, which includes ZO-1 and ZO-2 attach to actin cross-linking transmembrane elements and provide structural support for the ECs [17].
The foremost categories of mechanisms that allow transfer between the blood and CNS have also been recognized, which consist of: (1) transmembrane diffusion, which depends on the physiochemical properties of a compound in its capacity to cross cell membranes (e.g. lipid solubility, molecular weight, molecular charge); (2) saturable transport, which comprises facilitated diffusion, receptor-mediated transcytosis, and energy-requiring pore-dependent transport; (3) facilitated diffusion, which is a non-energy supported mechanism transferring a ligand from a high concentration side to a lower concentration compartment; (4) receptor-mediated transport, which is typically energy-dependent, but not vesicular-dependent; (5) receptor-mediated transcytosis, which is a saturable, energy-dependent and vesicular-dependent mechanism, transferring ligands either bidirectionally or unidirectionally; (6) adsorptive transcytosis, a vesicular-dependent transport depending on the binding between glycoprotein receptors and ligands; (7) fluid phase pinocytosis, a kind of vesicular transfer that is not necessarily ligand-specific, and is similarly synonymous with transcellular diffusion; (8) paracellular diffusion, which involves diffusion between cells that form the BBB, frequently caused by tight junction dysregulation; (9) diapedesis, which involves the process how immune cells cross the BBB; and (10) extracellular pathways, which account for residual or functional permeability of the BBB resulting from low concentrations of albumin in the cerebrospinal fluid (CSF) [4].
Our approach was to use a small, nitrone-based molecule, OKN-007, which we found to be effective in momentarily opening up the BBB, and permitting compounds ranging in molecular weight from ~550 (Gd-DTPA, a commonly used MRI contrast agent to assess in vivo BBB permeability in various neurological diseases) to ~470 kDa (the relatively large enzyme, β-galactosidase) to cross the BBB into mouse brain tissues. The temporary opening is more likely associated with the fairly rapid clearance profile for OKN-007, when administered i.v. (Figure 1). Gd-DTPA, which normally is unable to cross the BBB, was found to be increased in OKN-007 treated mouse brains, measured as an increase in MRI signal intensity (SI), over a period of 1-3 hours, reaching an optimum SI maximum at 2 hours. We also attempted to enhance the delivery of a large enzyme, β-galactosidase (MW 465 kDa), which we conjugated to Gd-DOTA. The OKN-007-treated mouse brains were found to significantly decrease T1 relaxation, measured as a percent decrease in T1 relaxation, indicating the presence of the Gd-labeled contrast agent, compared to saline controls. Next, we used an antibody against a neuronal-specific marker, Eph-B2, to confirm that an antibody-sized molecule can also cross the BBB when administered 1 hour following OKN-007 treatment. There was both a significant increase in MRI SI and a significant decrease in % change in T1 relaxation in the OKN-007-treated mouse brains, compared to saline controls, both administered the anti-EphB2-Gd-DOTA molecular-targeted MRI probe. Verification of the presence of the anti-EphB2 (goat-derived antibody) mtMRI probe was obtained ex vivo in mouse brains treated with OKN-007 using an anti-goat antibody against EphB2. We also attached a fluorescence label to the anti-EphB2 antibody, which was also co-administered with the mtMRI anti-EphB2-Gd-DOTA probe (used to obtain the in vivo data) (Figure 4). Lastly, we also used an albumin-Gd-DTPA-biotin contrast agent, and found that the OKN-007-treated mouse brains had a significant increase in MRI SI, compared to saline controls. Confirmation of the albumin-Gd-DTPA-biotin was also obtained by fluorescence staining of the biotin moiety with avidin (Figure 5), which demonstrated a significant increase in avidin specks for the OKN-007-treated mouse brains, compared to saline controls. Our rationale for choosing the 1 hour time-point to administer compounds following OKN-007 was based on selecting a time-point where there was an increasing signal intensity and/or change in T1 relaxation. This would provide time for a larger compound to reach the brain via i.v. administration, or provide time for a drug/compound to reach the brain via an oral administration route. One and a half to 2 hours may also be just as effective, but would need to be further investigated. The focus of the study was a proof-of-concept that pre-administration of OKN-007 could temporarily open the BBB to allow a range of molecules/compounds to cross the BBB into brain tissue. Although we generated some novel Gd-based compounds (e.g. anti-EphB2-Gd-DOTA, and Gd-DOTA-labelled β-galactosidase), the focus was not to characterize their pharmacokinetics/pharmacodynamics and mean residence time (MRT) further, which could be done in future studies.
Other common approaches used to moderate BBB permeability in order to enhance drug delivery include: (1) intracarotid infusion of hyperosmotic concentrations of arabinose, oleic or linoleic acids, alkylglycerols, or mannitol; (2) intravenous infusion of bradykinin B2; (3) administration of the 12 kDa fragment obtained from ZO toxin (ΔG) of vibrio cholera; (4) targeting explicit components of the tight junctions (such as claudin-5) with siRNA or novel peptidomimetic treatments; and (5) ultrasound with microbubbles using focused ultrasound (FUS) [8].
The advantage of using OKN-007 to temporarily open the BBB is that this compound has been shown in two indications (stroke >3,000 human patients (previous phase I-III stroke clinical trials [18,19]) and GBM (current phase II clinical trials)) to have no adverse effects, and has a beneficial human safety profile. The timing for pre-administration of OKN-007 is crucial as the window is quite narrow (due to a fairly rapid clearance), and subsequent drug delivery needs to be ideally coordinated. The short time-frame is an advantage, as leaving the BBB open for too long could result in unwanted complications. A disadvantage, at this point, is that the mechanism-of-action (MOA) of how OKN-007 is temporarily opening up the BBB is currently unknown, and will need to be studied in future experiments. Upon establishing the MOA, the range of compounds that could be delivered post-OKN-007 administration may be further expanded.
OKN-007 is currently in combination therapy with temozolomide (TMZ) [20,21], a chemotherapeutic agent for GBM, in multi-institutional phase 2 clinical trials, due to its effect on TMZ-resistant cancer cells, but also in part to the augmented delivery of more TMZ to diffuse GBM tissues that have an intact BBB.
Overall, for this study, the data provided shows that prior administration of OKN-007 (one hour beforehand) can temporarily open up the BBB and allow the delivery of various compounds with a large range of MW into normal mouse brain in different regions.
Conclusions
OKN-007 can readily be translated for human use as an agent that can momentarily open up the BBB and enhance the delivery of a wide-range of compounds from as small as ~550 to as large as ~470 KDa in MW. As this compound is currently in clinical trials and has an advantageous safety profile, the use of OKN-007 to augment the delivery of drugs through the BBB should be considered.
Acknowledgements
Funding was obtained by the Oklahoma Medical Research Foundation and the Presbyterian Health Foundation (both to RAT).
Disclosure of conflict of interest
RAT holds a patent regarding the use of OKN-007 to temporarily open up the BBB. All other co-authors have no conflicts of interest.
References
- 1.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schroeter ML, Mertsch K, Giese H, Muller S, Sporbert A, Hickel B, Blasig IE. Astrocytes enhance radical defense in capillary endothelial cells constituting the blood-brain barrier. FEBS Lett. 1999;449:241–244. doi: 10.1016/s0014-5793(99)00451-2. [DOI] [PubMed] [Google Scholar]
- 3.Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 4.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, Pardgridge W, Rosenberg GA, Smith Q, Drewes LR. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84–96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- 6.Johanson CE, Duncan III JA, Kling PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview; structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Lochhead JJ, Yang J, Ronaldson PT, Davis TP. Structure, function, and regulation of the blood-brain barrier tight junction in central nervous disorders. Front Physiol. 2020;11:914. doi: 10.3389/fphys.2020.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Towner RA, Smith N, Doblas S, Garteiser P, Watanabe Y, He T, Saunders D, Herlea O, Silasi-Mansat R, Lupu F. In vivo detection of inducible nitric oxide synthase in rodent gliomas. Free Radic Biol Med. 2010;48:691–703. doi: 10.1016/j.freeradbiomed.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 10.He T, Smith N, Saunders D, Pittman BP, Lerner M, Lightfoot S, Silasi-Mansat R, Lupu F, Towner RA. Molecular MRI differentiation of VEGF receptor-2 levels in C6 and RG2 glioma models. Am J Nucl Med Mol Imaging. 2013;3:300–11. [PMC free article] [PubMed] [Google Scholar]
- 11.Dafni H, Landsman L, Schechter B, Kohen F, Neeman M. MRI and fluorescence microscopy of the acute vascular response to VEGF165: vasodilation, hyper-permeability and lymphatic uptake, followed by rapid inactivation of the growth factor. NMR Biomed. 2002;15:120–131. doi: 10.1002/nbm.724. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. MicroPET and autoradiographic imaging of breast cancer alpha v-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug Chem. 2004;15:41–9. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- 13.Towner RA, Saunders D, Smith N, Towler W, Cruz M, Do S, Maher JE, Whitaker K, Lerner M, Morton KA. Assessing long-term neuroinflammatory responses to encephalopathy using MRI approaches in a rat endotoxemia model. Geroscience. 2018;40:49–60. doi: 10.1007/s11357-018-0009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junctions. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110:1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 16.Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98:3699–3707. doi: 10.1182/blood.v98.13.3699. [DOI] [PubMed] [Google Scholar]
- 17.Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occluding. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyden PD, Shuaib A, Lees KR, Davalos A, Davis SM, Diener HC, Grotta JC, Ashwood TJ, Hardemark HG, Svensson HH, Rodichok L, Wasiewski WW, Ahlberg G CHANT Trial Investigators. Safety and tolerability of NXY-059 for acute intracerebral hemorrhage: the CHANT trial. Stroke. 2007;38:2262–9. doi: 10.1161/STROKEAHA.106.472746. [DOI] [PubMed] [Google Scholar]
- 19.Diener HC, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Shuaib A, Ashwood T, Wasiewski W, Alderfer V, Hårdemark HG, Rodichok L SAINT I and II Investigators. NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II trials. Stroke. 2008;39:1751–8. doi: 10.1161/STROKEAHA.107.503334. [DOI] [PubMed] [Google Scholar]
- 20. NCT04388475. ClinicalTrials.gov. NIH U.S. National Library of Medicine. open-label study investigating of OKN-007 combined with temozolomide in patients with recurrent glioblastoma. https://clinicaltrials.gov/ct2/show/ NCT04388475.
- 21.NIH National Cancer Institute. OKN-007 and temozolomide in treating patients with grade III-IV glioblastoma multiforme undergoing adjuvant concomitant radiotherapy. https://www.cancer.gov/about-cancer/treatment/clinical-trials/search/v?id=NCI-2018-03791.


