Abstract
Applications of various positron emission tomography (PET) tracers for assessing atherosclerosis have been evolving over the years. 18F-fluorodeoxyglucose (FDG)-PET was introduced in 2001 as a probe for this purpose. During the past decade, numerous papers have described a major role for sodium 18F-fluoride (NaF) as another tracer for assessing this vascular disease. We have reviewed the existing data about the merits of both techniques for assessing atherosclerosis. We have to emphasize that our team has been actively involved in conducting research with both tracers over many years. In this review, we have relied upon the data from the CAMONA study which has become a gold standard for defining the role of PET imaging in atherosclerosis. This study was one of the largest of any in recent years and has allowed comprehensive comparison between these two tracers in detecting and quantifying atherosclerosis. Based on what we have learned from this major undertaking, we believe the role of FDG-PET will be limited in assessing atherosclerosis in clinical work-up. This is relevant to both major and coronary arteries. In contrast to NaF-PET, the role of FDG-PET in assessing coronary artery atherosclerosis is almost non-existent. Based on the existing data in this domain, NaF-PET is an ideal imaging modality for both research and clinical assessment of atherosclerosis. The aim of this review is to describe the pros and cons of both approaches based on the existing data in the literature.
Keywords: Atherosclerosis, NaF, FDG, cardiovascular disease, PET quantification
Introduction
The underlying cause of coronary heart disease, peripheral artery disease and cerebrovascular accidents is atherosclerosis. These cardiovascular diseases count for the majority of mortality and morbidity in the Western countries [1,2]. Effective strategies to identify atherosclerotic disease early in the course of the disease, as well as to quantify the extent disease burden are therefore of great interest in this setting. The atherosclerotic process is complex and multi-factorial and begins in early decades of life and progresses in the ensuing years [3].
Endothelial cell dysfunction is believed to underlie the pathogenesis of atherosclerosis [4]. Briefly, both hyperlipidemia and hypertension promote an upregulation of endothelial cell adhesion molecules [5], which leads to the recruitment of inflammatory cells and the activation of inflammatory cascade, including platelet activation, deposition of lipid plaques, smooth muscle proliferation, vessel micro-calcification- and, ultimately, macro-calcifications (Figure 1) [2,6]. Oxidation of modified lipoproteins in the atheromatous plaque and secretion of pro-inflammatory cytokines by macrophages promote osteogenesis and formation of hydroxyapatite crystals lead to the vulnerability of the plaque to further damage and possible rupture [7,8]. The calcification of atheromatous plaques starts as areas of molecular micro-calcification that may further progress to structural macro-calcification. The underlying inflammatory mechanisms and the promoted activated molecular calcification of plaques are asymptomatic and progress gradually. Thus, diagnostic methods that can detect atherosclerosis early prior to the incidence of cardiovascular diseases and while the disease is still treatable are of great importance [9,10].
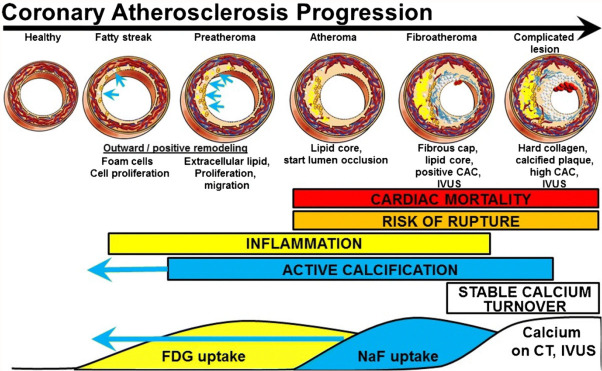
Figure 1.
Progression from healthy arteries to complicated lesions. FDG and NaF uptake have long been known to precede vascular calcification evident on CT and intravascular ultrasonography (IVUS). The paradigm shift is the stronger predictive power of NaF uptake and the occurrence of active calcification measured by NaF uptake in early coronary fatty streaks and preatheroma (CAC coronary artery calcium) (Reproduced with permission from McKenney-Drake ML et al.) [39].
Modern imaging modalities, such as ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI) angiography, are all widely used clinically to identify gross symptomatic plaques but have significant limitations in the detection of early stages of atherosclerosis when the plaques are biologically highly active [8,11]. Positron emission tomography (PET) allows examining the pathological and biologically active features of atherosclerotic disease at the molecular level [6]. 18F-fluorodeoxyglucose (FDG) and sodium 18F-fluoride (NaF) are the most commonly used PET tracers for detecting atherosclerosis. FDG is taken up by the activated macrophages in the plaques [12-14], while NaF is deposited at the sites of micro-calcification due to physicochemical exchange of the 18F- ion with the hydroxyl group in hydroxyapatite [15-17]. Thus, PET/CT imaging with FDG and NaF has the ability to assess atherosclerotic disease at the molecular phase of the disease when the process may still be reversible.
FDG-PET as a molecular probe in atherosclerosis
FDG-PET imaging for detecting atherosclerotic plaques was reported in 2001 by investigators at the University of Pennsylvania [1,2,18]. This observation was interpreted to reflect the presence of activated macrophages in the atherosclerotic plaques which are highly glycolytic. This observation eventually led to the adoption of FDG-PET/CT imaging by various groups to detect and characterize atherosclerotic plaques [19-29]. During the past 2 decades, numerous publications have reported some evidence for FDG-PET’s sensitivity in detecting plaques, particularly in major arteries such as the aorta [30-33]. However, FDG’s non-specificity and its uptake in other tissues in the arterial wall such as smooth muscles has raised some concerns about the role of this approach in assessing suspected atherosclerotic plaques [34,35]. Also, the fact that plaques are very small in size and are subject to constant motion due to cardiac cycle, have led to decreasing level of enthusiasm for adopting FDG-PET imaging as an optimal modality for assessing atherosclerotic plaques [36]. Furthermore, the significant uptake of FDG in the myocardium has prevented using this technology in detecting atherosclerosis in the coronary arteries, which is a main cause of morbidity and mortality in this population. Therefore, what is visualized by FDG in the arterial wall likely reflects uptake by a mixture of cells in addition to the macrophages in the plaques.
NaF-PET as a molecular probe in atherosclerosis
NaF imaging was introduced in the early 1960s as a radiotracer for examining osseous lesions in the skeleton but was abandoned soon after the introduction of technetium labeled phosphates in the early 1970s [37]. During the past decade, the interest in NaF has been revived due to its ability to detect molecular calcification in the plaques [17,38,39] and possibly in other organ structures [40-42]. This tracer is only taken up at the sites of active calcification/ossification and no other organs or disease processes are the targets for this tracer [43]. Also, NaF is rapidly cleared from the circulation and by 60-90 minutes, the content of this tracer in the circulation is very minimal and therefore a high contrast is reached between the sites of calcification and the background activity (Figure 2) [6].
Figure 2.
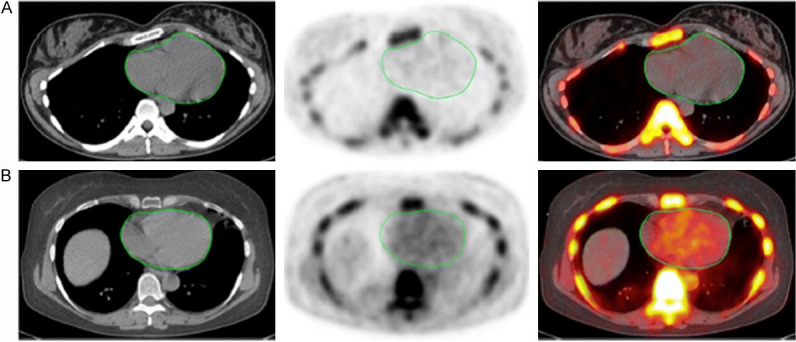
Transverse images (left CT, middle PET, right PET/CT) of the heart (green circles) in two clinically normal subjects (A, 25 years old, B, 61 years old). The global cardiac calcification scores were 12,492.44 in subject A and 18,424.70 in subject B. Normalizing the values to background NaF uptake increases the discrepancy between the subjects, resulting in 2.18 times the uptake in subject B than in subject A. Corresponding to the sites of NaF uptake in subject B, no structural calcification is seen on the corresponding CT scan and there is significant disparity between the PET and CT results. This is not an uncommon observation in this setting and clearly demonstrates the basis for assessment of cardiovascular calcification with these two different imaging modalities. While molecular imaging with NaF detects the earliest evidence for vascular calcification, evidence for calcification on CT largely reflects an end-stage disease process and therefore may be an irreversible pathologic state. Disparity between these two observations provides evidence for stage of calcification and has implications for the irreversibility of macrocalcification (Reproduced with permission from McKenney-Drake ML et al.) [39].
In order to overcome the shortcomings of FDG-PET imaging in the assessment of atherosclerotic plaques, efforts have been made to determine the role of NaF-PET imaging in this domain [44]. Extensive data generated by many investigators around the world have shown the superiority of NaF-PET as a molecular probe over FDG-PET with regards to its sensitivity and specificity in detecting and characterizing this serious arterial disease [39,45-48]. The fact that NaF can be used to detect plaques in the coronary arteries is a major advantage for this tracer [6,49]. Uptake of NaF can be quantified more accurately and precisely than that of FDG, and this is another significant advantage of this radiotracer for assessing atherosclerosis (Figure 1) [39]. Therefore, the future of PET based molecular assessment of atherosclerotic plaques will heavily rely upon NaF based imaging techniques [50].
Limitations and challenges of PET imaging in assessing atherosclerosis
The challenges that we face assessing atherosclerotic plaques are related to the limited spatial resolution of PET in detecting submillimeter lesions in various arterial structures in the body. While the spatial resolution of PET in phantom studies is in the range of 3-5 mm, in the human body it deteriorates substantially to 8-10 mm [51]. This poses a major challenge in detecting and characterizing a variety of diseases including atherosclerosis. In the early stages of the disease, these plaques are no more than a few hundred microns in size in most arteries and, therefore, PET imaging techniques will fail to detect such subtle abnormalities anywhere in the arterial system [36]. Furthermore, many tracers including FDG that have been proposed for detecting plaques remain in the circulation for an extended period of time [14,52]. The degree of uptake of tracers that have been proposed for assessing atherosclerosis is also of great importance in determining the role of PET as a molecular probe to detect and characterize the plaques. Therefore, efforts must be made to employ tracers that have high affinity for the ingredients of the plaques and minimal uptake in the adjacent structures. FDG scanning in particular is prone to poor contrast resolution due to nonspecific uptake in many tissues that are adjacent to arteries and this significantly affects the sensitivity and specificity of this tracer in this domain [6,53,54]. Accordingly, tracers with significant nonspecific uptake in the arterial wall structures and slow clearance from the circulation will be of limited value in detecting and characterizing atherosclerotic plaques (Figure 3) [35].
Figure 3.
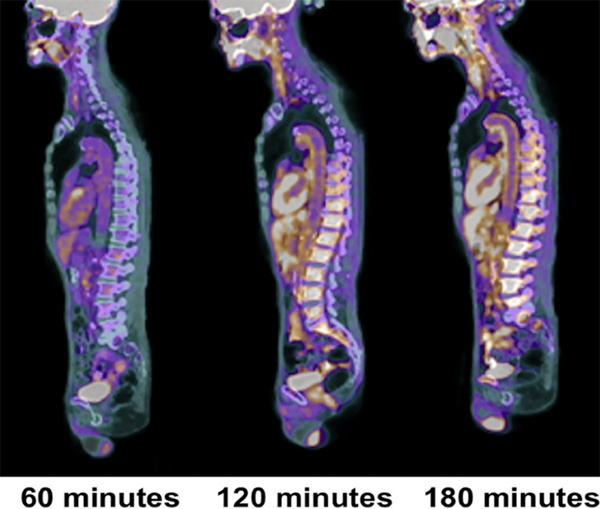
Changes in aortic wall and luminal blood FDG activity at different imaging time-points as seen on sagittal FDG PET images of the thoracic aorta. With time, luminal blood activity decreases while the aortic wall activity increases, which improves the arterial wall-to-blood contrast (superior target-to-background ratio) (Reproduced with permission from Moghbel M et al.) [35].
Molecular imaging of coronary artery atherosclerosis is particularly a challenging territory. The main challenge relates to the combined effects of cardiac and respiratory motions during data acquisition over an extended period of time. The constant and combined movements make it almost impossible to assess focal uptakes of the intended tracers (including FDG and NaF) at the targeted sites. While cardiac and respiratory gating has been proposed as a possible solution to correct for these undesirable movements, the success of such approaches is unproven. The questionable role of such efforts is particularly applicable to diaphragmatic movement, which is very irregular and cannot be corrected by adopting standard gating approaches [55,56]. Therefore, improving the spatial resolution of PET for imaging focal plaques in the coronary arteries is almost impossible and cannot be achieved with the current PET imaging modalities. Based on the limitations enumerated, the published reports in the literature about detecting focal atherosclerotic plaques in the coronaries with FDG, NaF and other tracers have to be viewed with great caution and skepticism.
Hybrid PET/MRI has added a new dimension to medical imaging with PET and has enhanced its role in domains where MRI has been successfully employed over the past 3 decades [57-59]. Unfortunately, there are still concerns about the accuracy of the quantitative data generated by this instrument due to the lack of optimal attenuation correction of the emitted gamma rays [60,61].
Questionable validity of Target-to-Background Ratio (TBR) correction
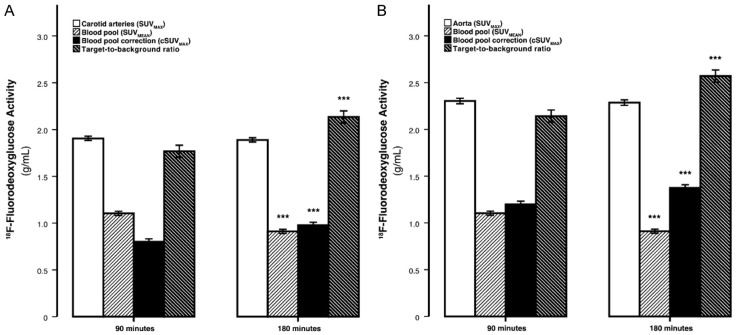
Attempts have been made to correct for blood pool activity by measuring target-to-background ratio (TBR) [62]. However, based on experience gained, this correction attempt appears to be unreliable and cannot be employed successfully for this purpose. Data from our own center reveals that when this correction is applied from images acquired over 1-3 hours, the numbers generated are totally different among different time points (Figure 4) [33]. Therefore, such “correction” schemes are suboptimal and of limited value for this purpose [63].
Figure 4.
The dependence of SUVMAX, blood-pool SUVMEAN, cSUVMAX, and the TBR on FDG circulating time-The average maximum carotid (A) and aortic (B). Arterial FDG activity was invariant to time, whereas blood-pool activity decreased and blood-pool corrected values and the target-to-background ratio significantly increased with time. Error bars represent the 95% confidence interval of the mean. ***P<.0001 decline or increase compared to previous time-point established by the paired Student’s t test (Reproduced with permission from Blomberg BA et al.) [33].
CAMONA study as a model for future research in atherosclerosis
In recent years, a major research study (CAMONA, which stands for Cardiovascular Molecular Calcification Assessed by NaF PET/CT) was conducted to compare the performance of FDG- and NaF-PET for assessing atherosclerosis [33,46,64]. This comprehensive study included a large number of normal controls as well as patients at risk for atherosclerosis. Normal subjects and angina pectoris patients underwent the same imaging protocol with these two tracers and the data generated from both groups were compared. In sub-studies, FDG-PET imaging was performed at 90 and 180 minutes, while NaF-PET was performed at 45, 90, and 180 minutes.
The data from this research study have been published extensively in the literature and clearly reflects the potential for future use of these tracers as molecular probes for detection and characterization of atherosclerosis [17,33,45,46,64-77]. Based on the results from the CAMONA project, it has become increasingly clear that the performance of FDG is substantially inferior to that of NaF (Figure 5) [67,78-80]. These data clearly demonstrate that NaF as a PET tracer will play a critical role in detecting atherosclerosis in both normal aging as well as in patients with low or high risk for this potentially fatal disease [67].
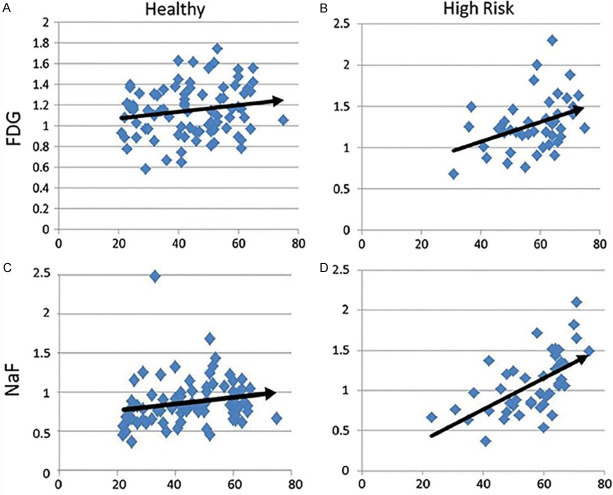
Figure 5.
The graphic data shown above reveal the correlation between age and evidence for inflammation (FDG) and calcification (NaF) in healthy subjects (A, C) and subjects with high risk for atherosclerosis (B, D). Based on these molecular data, NaF-PET imaging appears to be more sensitive in detecting evidence for atherosclerosis in the arch of the aorta than FDG-PET scanning (Reproduced with permission from Alavi A et al.) [78].
PET tracers beyond FDG and NaF to detect atherosclerosis
The role of other tracers that have been proposed as potential probes for imaging atherosclerosis is unknown but does not appear promising based on what has been published in the literature [35]. For example, similar to FDG, tracers that have been used for this purpose are nonspecific in nature and therefore lead to generating images with low contrast between the plaques and the surrounding structures [81,82].
Based on what has been described above, it is increasingly clear that the role of FDG-PET imaging to detect atherosclerosis is very limited. Therefore, the future of this approach is uncertain at this time. While inflammation is considered as the beginning of the disease, its assessment by PET or other imaging modalities will encounter substantial challenges that will be difficult to overcome. Attempts are being made to use radiolabeled nanoparticles, particularly with positron-emitting radionuclides, which could play a major role in detecting only the inflammatory process in the plaques [83]. This type of research is in its early stages and is conducted only in animals but could eventually lead to applications in human studies in the near future (Figure 6) [84]. With the nanoparticle methodology and global disease assessment, it is conceivable that we will be able to detect and quantify inflammatory components of the plaques more successfully than what is achievable by FDG-PET. This approach may prove to be of some value in detecting coronary artery disease as a diffuse process.
Figure 6.
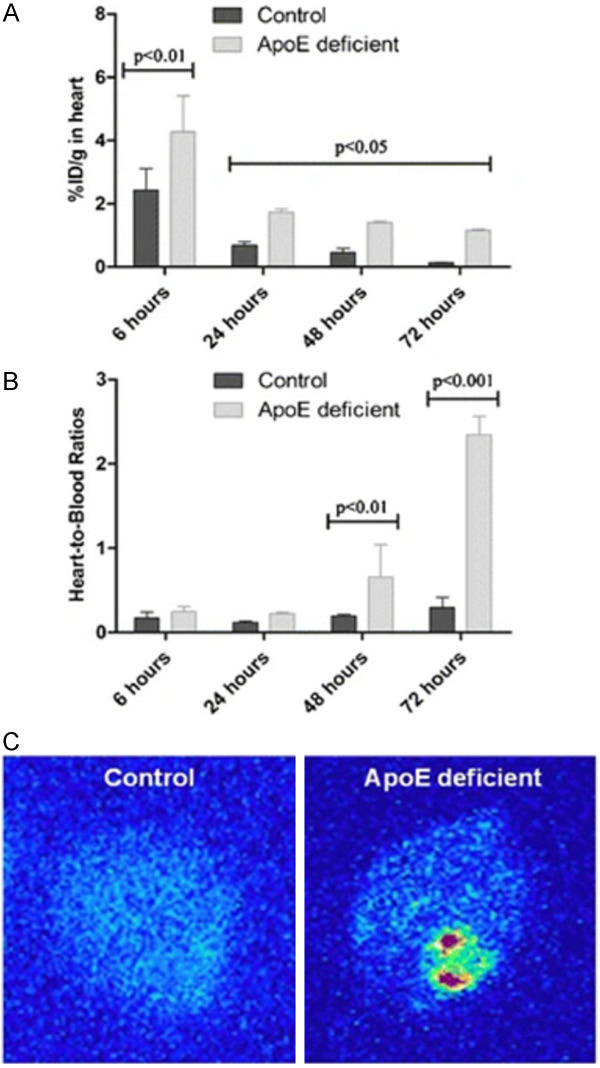
Heart uptake and digital autoradiography (DAR) from control and apolipoprotein-E-deficient (apoE-/-) mice. Mean heart uptake obtained after intravenous administration of [125I]-labeled iron oxide nanoparticles (IONPs) into healthy and atherosclerotic mice (n=4) (A). Mean heart/blood ratios obtained after intravenous administration of [125I]IONPs into healthy and atherosclerotic mice (n=4) (B). DAR obtained from heart of healthy and atherosclerotic mice, respectively, at 72 h post-injection of [125I]IONPs (20 μCi, 0.8 mg Fe/kg) (C). Increased uptake in apoE-/- mouse likely represent atherosclerotic plaques in coronary arteries (Reproduced with permission from de Barros AL et al.) [84].
Global disease assessment (Alavi-Carlsen Score) and total body PET
Atherosclerotic plaques are diffuse in nature and involve most of the arteries in their entirety with different degrees [85]. As such, the disease does not present itself as a focal process and this should be taken into consideration in quantitative assessment of its extent. Based on the data that we and others have published, it is increasingly clear that global disease measurement is substantially superior to approaches that focus on detecting the disease process as focal lesions [86-90]. Since atherosclerosis is treated as a systemic disease, providing a global value will be the optimal means for guiding the management of these patients. Also, global assessment allows clinicians to take advantage of the diffuse nature of atherosclerotic processes throughout the body and therefore treat the disease as a systemic process (Figures 7 and 8) [39]. Performing CT coronary angiogram along with gated PET imaging is also of limited value in assessing global disease activity [91].
Figure 7.
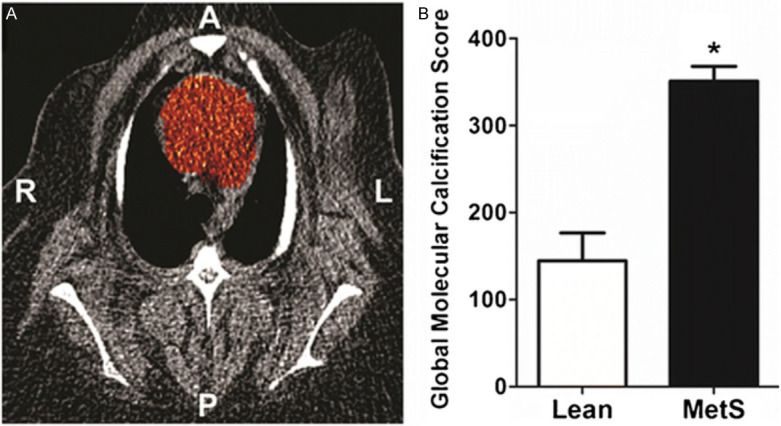
Coronary artery global molecular calcification score (GMCS) and percent injected dose per gram body weight NaF uptake. A region of interest was drawn around the heart on each cardiac CT slice from which GMCS was calculated (A). Pigs with metabolic syndrome (MetS; n=11) had a GMCS almost 2.5-fold higher than lean pigs (n=2; *P<0.05) (B) (Reproduced with permission from McKenney-Drake ML et al.) [39].
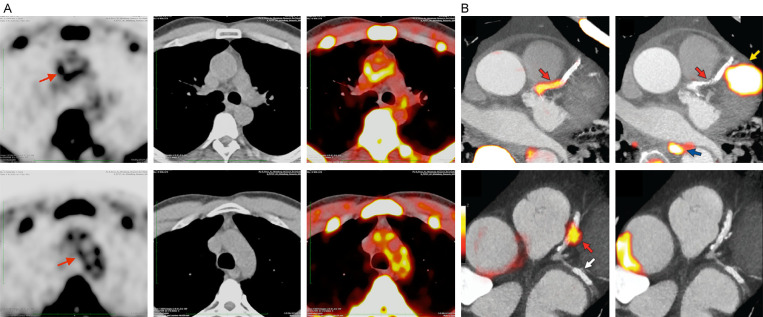
Figure 8.
Image set (A) is reproduced exactly from the original data (without processing) generated in a patient with rheumatoid arthritis with evidence for significant molecular calcification in the aortic arch (Reproduced with permission from Moghbel M et al.) [35]. The left column shows significant NaF uptake in the aortic wall (arrow) on PET images alone, the middle column shows CT images, and the right column shows fused NaF-PET/CT images. The latter clearly shows the sites of molecular calcification on PET correspond to aortic wall which reveals no evidence for structural calcification. These images were generated without modifying original data provided by the PET/CT instrument. Image set (B) shows selected sites of NaF (left column) and FDG (right column) uptake superimposed on coronary angiogram from the contrast enhanced CT scan (Reproduced with permission from Joshi NV et al.) [109]. These images were generated based on selected NaF uptake sites at the corresponding segments of coronary artery. As such, the scans do not correspond to the original images reconstructed by the conventional software provided by the PET/CT instruments. Therefore, the reproducibility and reliability of such results are of some concern and may not be as accurate and realistic as conventional approaches in optimal applications of this modality. As such, detection and quantification of coronary artery calcification should be based upon reliable and reproducible approaches for convincing applications of NaF-PET imaging in this setting.
Conventional PET studies with instruments with a limited field of view (FOV) of approximately 20-30 cm in the axial direction requires imaging the body in segments over a period of 20-30 minutes with acquisitions time of 2-3 minutes for each bed position. Because of this limitation, most 18F-based imaging studies are performed at 60-90 minutes following the administration of the related compounds, which is suboptimal for the detection of atherosclerosis [92,93]. This major shortcoming has been overcome by the introduction of total body PET instruments over the past 2 years which allow imaging the entire body with a single image acquisition over a few minutes [94,95]. The sensitivity of this technique is substantially higher (theoretically about 40 times) than that of conventional instruments with limited FOV, allowing for delayed imaging and ensuring clearance of the tracer from circulation. We believe one of the major applications of total body imaging is going to involve detection of atherosclerotic plaques throughout the body [96-98]. With this approach, it is likely that sensitivity of PET imaging with either FDG or NaF will substantially improve and this will further enhance the role of these tracers in assessing atherosclerosis. Significant clearance of background activity in the blood and other tissues will result in enhanced contrast between the plaques and surrounding structures [96].
Questionable validity of organ interplay in genesis and course of atherosclerosis
In recent years, a very complicated and convoluted process has been proposed that claims to play a major role among atherogenic plaques, brain function and hematopoietic cells in the bone marrow and spleen [99]. A large body of animal and human data has been introduced in an effort to convince the community about a strong relation among these organs as the underlying factor for genesis and progression of atherosclerotic plaques in brain disorders due to depression and inflammatory disorders [100-103]. Although there is relatively clear-cut evidence for high incidence of atherosclerosis in patients with psoriasis, rheumatoid arthritis, HIV-AIDS and other diseases such as cancer, the claim that neuropsychiatric disorders have a role in causing atherosclerosis via direct and/or indirect interactions with the hemopoietic system is theoretical and unclear at this time [103]. These investigators do not consider the non-specific nature of FDG accumulation in the bone marrow in such self-claimed hypotheses. FDG uptake in bone marrow is due to metabolically active cells that eventually lead to producing blood cells [104]. Furthermore, bone marrow activity as visualized by FDG is extremely variable among subjects as noted on standard FDG-PET scans and is significantly different between younger and older populations [105,106]. Therefore, using FDG for assessing bone marrow as the source of inflammatory cells that eventually migrate to atherosclerotic plaques is very speculative. Similarly, uptake of FDG in the spleen is extremely variable since this organ, like the bone marrow, is subject to many ongoing activities in the rest of the body [107]. Therefore, hypothesizing the spleen as the source of inflammatory cells for atherosclerosis is also questionable and unjustified.
Future prospects for PET imaging in atherosclerosis
Finally, the future of molecular imaging for detection of atherosclerosis appears very promising and it is likely that this approach will replace structural imaging techniques for medical management of this very common and potentially fatal disease. We believe changes that are detected by CT, MRI or ultrasound are of limited value since they represent late or end stages of the disease, whereas PET depicts primarily its early, molecular and active phase [39]. Detection of plaques as focal abnormalities such as structural calcification is of limited value in the management of patients with atherosclerosis [80]. The introduction of PET/CT and PET/MRI has demonstrated the critical the role of combined molecular and structural techniques in medicine and theses innovative advances will substantially enhance the overall performance of medical imaging in treating patients with atherosclerosis [11,108]. However, among the various molecular imaging probes, NaF-PET may become the technique of choice for the early detection of atherosclerosis [6]. Although the data are limited at this time, it is conceivable that the natural course of the disease and the efficacy of systemic medical treatment and other interventions will be best served by this approach in the future [44,50].
Disclosure of conflict of interest
None.
References
- 1.Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. 2016;118:535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, Tokgözoğlu L, Lewis EF. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 3.Shah PK. Inflammation and plaque vulnerability. Cardiovasc Drugs Ther. 2009;23:31–40. doi: 10.1007/s10557-008-6147-2. [DOI] [PubMed] [Google Scholar]
- 4.Crowther MA. Pathogenesis of atherosclerosis. Hematology Am Soc Hematol Educ Program. 2005:436–441. doi: 10.1182/asheducation-2005.1.436. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Cybulsky MI, Gimbrone MA Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993;13:197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- 6.Raynor WY, Borja AJ, Rojulpote C, Hoilund-Carlsen PF, Alavi A. 18F-sodium fluoride: an emerging tracer to assess active vascular microcalcification. J Nucl Cardiol. 2020 doi: 10.1007/s12350-020-02138-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Hutcheson JD, Goettsch C, Bertazzo S, Maldonado N, Ruiz JL, Goh W, Yabusaki K, Faits T, Bouten C, Franck G, Quillard T, Libby P, Aikawa M, Weinbaum S, Aikawa E. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15:335–343. doi: 10.1038/nmat4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syed MB, Fletcher AJ, Forsythe RO, Kaczynski J, Newby DE, Dweck MR, van Beek EJ. Emerging techniques in atherosclerosis imaging. Br J Radiol. 2019;92:20180309. doi: 10.1259/bjr.20180309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grammaticos PC. Diagnosing atherosclerosis makes nuclear medicine a tissue imaging modality. Hell J Nucl Med. 2014;17:12. [PubMed] [Google Scholar]
- 10.Buscombe JR. Exploring the nature of atheroma and cardiovascular inflammation in vivo using positron emission tomography (PET) Br J Radiol. 2015;88:20140648. doi: 10.1259/bjr.20140648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takx RA, Partovi S, Ghoshhajra BB. Imaging of atherosclerosis. Int J Cardiovasc Imaging. 2016;32:5–12. doi: 10.1007/s10554-015-0730-y. [DOI] [PubMed] [Google Scholar]
- 12.Skagen K, Johnsrud K, Evensen K, Scott H, Krohg-Sørensen K, Reier-Nilsen F, Revheim ME, Fjeld JG, Skjelland M, Russell D. Carotid plaque inflammation assessed with (18)F-FDG PET/CT is higher in symptomatic compared with asymptomatic patients. Int J Stroke. 2015;10:730–736. doi: 10.1111/ijs.12430. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury MM, Tarkin JM, Evans NR, Le E, Warburton EA, Hayes PD, Rudd JHF, Coughlin PA. 18F-FDG uptake on PET/CT in symptomatic versus asymptomatic carotid disease: a meta-analysis. Eur J Vasc Endovasc Surg. 2018;56:172–179. doi: 10.1016/j.ejvs.2018.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer M, Borja AJ, Hancin EC, Auslander T, Revheim ME, Moghbel MC, Werner TJ, Alavi A, Rajapakse CS. Imaging atherosclerosis by PET, with emphasis on the role of FDG and NaF as potential biomarkers for this disorder. Front Physiol. 2020;11:511391. doi: 10.3389/fphys.2020.511391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKenney-Drake ML, Territo PR, Salavati A, Houshmand S, Persohn S, Liang Y, Alloosh M, Moe SM, Weaver CM, Alavi A, Sturek M. (18)F-NaF PET imaging of early coronary artery calcification. JACC Cardiovasc Imaging. 2016;9:627–628. doi: 10.1016/j.jcmg.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Nakahara T, Narula J, Strauss HW. NaF uptake in unstable plaque: what does fluoride uptake mean? Eur J Nucl Med Mol Imaging. 2018;45:2250–2252. doi: 10.1007/s00259-018-4177-y. [DOI] [PubMed] [Google Scholar]
- 17.Paydary K, Revheim ME, Emamzadehfard S, Gholami S, Pourhassan S, Werner TJ, Hoilund-Carlsen PF, Alavi A. Quantitative thoracic aorta calcification assessment by (18)F-NaF PET/CT and its correlation with atherosclerotic cardiovascular disorders and increasing age. Eur Radiol. 2020;31:785–794. doi: 10.1007/s00330-020-07133-9. [DOI] [PubMed] [Google Scholar]
- 18.Yun M, Yeh D, Araujo LI, Jang S, Newberg A, Alavi A. F-18 FDG uptake in the large arteries: a new observation. Clin Nucl Med. 2001;26:314–319. doi: 10.1097/00003072-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Haim S, Kupzov E, Tamir A, Israel O. Evaluation of 18F-FDG uptake and arterial wall calcifications using 18F-FDG PET/CT. J Nucl Med. 2004;45:1816–1821. [PubMed] [Google Scholar]
- 20.Dunphy MP, Freiman A, Larson SM, Strauss HW. Association of vascular 18F-FDG uptake with vascular calcification. J Nucl Med. 2005;46:1278–1284. [PubMed] [Google Scholar]
- 21.Bural GG, Torigian DA, Chamroonrat W, Houseni M, Chen W, Basu S, Kumar R, Alavi A. FDG-PET is an effective imaging modality to detect and quantify age-related atherosclerosis in large arteries. Eur J Nucl Med Mol Imaging. 2008;35:562–569. doi: 10.1007/s00259-007-0528-9. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa AL, Subramanian SS, Cury RC, Truong QA, Gardecki JA, Tearney GJ, Hoffmann U, Brady TJ, Tawakol A. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5:69–77. doi: 10.1161/CIRCIMAGING.110.959478. [DOI] [PubMed] [Google Scholar]
- 23.Abdelbaky A, Corsini E, Figueroa AL, Fontanez S, Subramanian S, Ferencik M, Brady TJ, Hoffmann U, Tawakol A. Focal arterial inflammation precedes subsequent calcification in the same location: a longitudinal FDG-PET/CT study. Circ Cardiovasc Imaging. 2013;6:747–754. doi: 10.1161/CIRCIMAGING.113.000382. [DOI] [PubMed] [Google Scholar]
- 24.Pasha AK, Moghbel M, Saboury B, Gharavi MH, Blomberg BA, Torigian DA, Kwee TC, Basu S, Mohler Iii ER, Alavi A. Effects of age and cardiovascular risk factors on (18)F-FDG PET/CT quantification of atherosclerosis in the aorta and peripheral arteries. Hell J Nucl Med. 2015;18:5–10. doi: 10.1967/s002449910161. [DOI] [PubMed] [Google Scholar]
- 25.Hetterich H, Rominger A, Walter L, Habs M, Volpers S, Hacker M, Reiser MF, Bartenstein P, Saam T. Natural history of atherosclerotic disease progression as assessed by (18)F-FDG PET/CT. Int J Cardiovasc Imaging. 2016;32:49–59. doi: 10.1007/s10554-015-0660-8. [DOI] [PubMed] [Google Scholar]
- 26.Cho SG, Park KS, Kim J, Kang SR, Kwon SY, Seon HJ, Jabin Z, Kim YJ, Jeong GC, Song M, Song HC, Min JJ, Bom HS. Prediction of coronary artery calcium progression by FDG uptake of large arteries in asymptomatic individuals. Eur J Nucl Med Mol Imaging. 2017;44:129–140. doi: 10.1007/s00259-016-3523-1. [DOI] [PubMed] [Google Scholar]
- 27.Kim JM, Lee ES, Park KY, Seok JW, Kwon OS. Analysis of (18)F-fluorodeoxyglucose and (18)F-fluoride positron emission tomography in korean stroke patients with carotid atherosclerosis. J Lipid Atheroscler. 2019;8:232–241. doi: 10.12997/jla.2019.8.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Omarjee L, Mention PJ, Janin A, Kauffenstein G, Pabic EL, Meilhac O, Blanchard S, Navasiolava N, Leftheriotis G, Couturier O, Jeannin P, Lacoeuille F, Martin L. Assessment of inflammation and calcification in pseudoxanthoma elasticum arteries and skin with 18F-flurodeoxyglucose and 18F-sodium fluoride positron emission tomography/computed tomography imaging: the GOCAPXE trial. J Clin Med. 2020;9:3448. doi: 10.3390/jcm9113448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Zaghal A, Aras M, Borja AJ, Moghbel M, Demir Y, Houshmand S, Ciftci E, Werner TJ, Hoilund-Carlsen PF, Torigian DA, Han Y, Alavi A. Detection of pulmonary artery atherosclerosis by FDG-PET/CT: a new observation. Am J Nucl Med Mol Imaging. 2020;10:127–134. [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa M, Ishino S, Mukai T, Asano D, Teramoto N, Watabe H, Kudomi N, Shiomi M, Magata Y, Iida H, Saji H. (18)F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med. 2004;45:1245–1250. [PubMed] [Google Scholar]
- 31.Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V, Fayad ZA. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892–896. doi: 10.1016/j.jacc.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Bucci M, Aparici CM, Hawkins R, Bacharach S, Schrek C, Cheng S, Tong E, Arora S, Parati E, Wintermark M. Validation of FDG uptake in the arterial wall as an imaging biomarker of atherosclerotic plaques with 18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET/CT) J Neuroimaging. 2014;24:117–123. doi: 10.1111/j.1552-6569.2012.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blomberg BA, Thomassen A, Takx RA, Hildebrandt MG, Simonsen JA, Buch-Olsen KM, Diederichsen AC, Mickley H, Alavi A, Hoilund-Carlsen PF. Delayed (1)(8)F-fluorodeoxyglucose PET/CT imaging improves quantitation of atherosclerotic plaque inflammation: results from the CAMONA study. J Nucl Cardiol. 2014;21:588–597. doi: 10.1007/s12350-014-9884-6. [DOI] [PubMed] [Google Scholar]
- 34.Brammen L, Palumbo B, Lupattelli G, Sinzinger H. Is (18)F-FDG PET really a promising marker for clinically relevant atherosclerosis? Hell J Nucl Med. 2014;17:62–63. [PubMed] [Google Scholar]
- 35.Moghbel M, Al-Zaghal A, Werner TJ, Constantinescu CM, Hoilund-Carlsen PF, Alavi A. The role of PET in evaluating atherosclerosis: a critical review. Semin Nucl Med. 2018;48:488–497. doi: 10.1053/j.semnuclmed.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Alavi A, Werner TJ, Hoilund-Carlsen PF. What can be and what cannot be accomplished with PET to detect and characterize atherosclerotic plaques. J Nucl Cardiol. 2018;25:2012–2015. doi: 10.1007/s12350-017-0977-x. [DOI] [PubMed] [Google Scholar]
- 37.Blake GM, Fogelman I. Bone radionuclide imaging, quantitation and bone densitometry. In: McCready R, Gnanasegaran G, Bomanji JB, editors. a history of radionuclide studies in the UK: 50th Anniversary of the British Nuclear Medicine Society. Cham (CH): 2016. pp. 111–120. [Google Scholar]
- 38.Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, Joshi FR, Gallagher FA, Warburton EA, Bennett MR, Brindle KM, Newby DE, Rudd JH, Davenport AP. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun. 2015;6:7495. doi: 10.1038/ncomms8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenney-Drake ML, Moghbel MC, Paydary K, Alloosh M, Houshmand S, Moe S, Salavati A, Sturek JM, Territo PR, Weaver C, Werner TJ, Hoilund-Carlsen PF, Sturek M, Alavi A. (18)F-NaF and (18)F-FDG as molecular probes in the evaluation of atherosclerosis. Eur J Nucl Med Mol Imaging. 2018;45:2190–2200. doi: 10.1007/s00259-018-4078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Zaghal A, Raynor W, Khosravi M, Guermazi A, Werner TJ, Alavi A. Applications of PET imaging in the evaluation of musculoskeletal diseases among the geriatric population. Semin Nucl Med. 2018;48:525–534. doi: 10.1053/j.semnuclmed.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Suh MS, Park SH, Kim YK, Yun PY, Lee WW. (18)F-NaF PET/CT for the evaluation of temporomandibular joint disorder. Clin Radiol. 2018;73:414.e7–414.e13. doi: 10.1016/j.crad.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Son SM, Kim K, Pak K, Kim SJ, Goh TS, Lee JS. Evaluation of the diagnostic performance of (18)F-NaF positron emission tomography/computed tomography in patients with suspected ankylosing spondylitis according to the Assessment of SpondyloArthritis International Society criteria. Spine J. 2020;20:1471–1479. doi: 10.1016/j.spinee.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 43.Raynor W, Houshmand S, Gholami S, Emamzadehfard S, Rajapakse CS, Blomberg BA, Werner TJ, Hoilund-Carlsen PF, Baker JF, Alavi A. Evolving role of molecular imaging with (18)F-sodium fluoride PET as a biomarker for calcium metabolism. Curr Osteoporos Rep. 2016;14:115–125. doi: 10.1007/s11914-016-0312-5. [DOI] [PubMed] [Google Scholar]
- 44.Hoilund-Carlsen PF, Sturek M, Alavi A, Gerke O. Atherosclerosis imaging with (18)F-sodium fluoride PET: state-of-the-art review. Eur J Nucl Med Mol Imaging. 2020;47:1538–1551. doi: 10.1007/s00259-019-04603-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blomberg BA, Thomassen A, de Jong PA, Lam M, Hess S, Olsen MH, Mali W, Alavi A, Hoilund-Carlsen PF. Reference values for fluorine-18-fluorodeoxyglucose and fluorine-18-sodium fluoride uptake in human arteries: a prospective evaluation of 89 healthy adults. Nucl Med Commun. 2017;38:998–1006. doi: 10.1097/MNM.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 46.Blomberg BA, de Jong PA, Thomassen A, Lam MGE, Vach W, Olsen MH, Mali W, Narula J, Alavi A, Hoilund-Carlsen PF. Thoracic aorta calcification but not inflammation is associated with increased cardiovascular disease risk: results of the CAMONA study. Eur J Nucl Med Mol Imaging. 2017;44:249–258. doi: 10.1007/s00259-016-3552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujimoto K, Norikane T, Yamamoto Y, Takami Y, Mitamura K, Okada M, Hatakeyama T, Kawanishi M, Nishiyama Y. Association between carotid (18)F-NaF and (18)F-FDG uptake on PET/CT with ischemic vascular brain disease on MRI in patients with carotid artery disease. Ann Nucl Med. 2019;33:907–915. doi: 10.1007/s12149-019-01403-3. [DOI] [PubMed] [Google Scholar]
- 48.Guaraldi G, Milic J, Prandini N, Ligabue G, Esposito F, Ciusa G, Malagoli A, Scaglioni R, Besutti G, Beghetto B, Nardini G, Roncaglia E, Mussini C, Raggi P. (18)Fluoride-based molecular imaging of coronary atherosclerosis in HIV infected patients. Atherosclerosis. 2020;297:127–135. doi: 10.1016/j.atherosclerosis.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 49.Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW. Coronary artery calcification: from mechanism to molecular imaging. JACC Cardiovasc Imaging. 2017;10:582–593. doi: 10.1016/j.jcmg.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Hoilund-Carlsen PF, Piri R, Constantinescu C, Iversen KK, Werner TJ, Sturek M, Alavi A, Gerke O. atherosclerosis Imaging with (18)F-sodium fluoride PET. Diagnostics (Basel) 2020;10:852. doi: 10.3390/diagnostics10100852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaidi H, Alavi A. Current trends in PET and combined (PET/CT and PET/MR) systems design. PET Clin. 2007;2:109–123. doi: 10.1016/j.cpet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Cheng G, Alavi A, Lim E, Werner TJ, Del Bello CV, Akers SR. Dynamic changes of FDG uptake and clearance in normal tissues. Mol Imaging Biol. 2013;15:345–352. doi: 10.1007/s11307-012-0600-0. [DOI] [PubMed] [Google Scholar]
- 53.Wootton R, Doré C. The single-passage extraction of 18F in rabbit bone. Clin Phys Physiol Meas. 1986;7:333–343. doi: 10.1088/0143-0815/7/4/003. [DOI] [PubMed] [Google Scholar]
- 54.Hawkins RA, Choi Y, Huang SC, Hoh CK, Dahlbom M, Schiepers C, Satyamurthy N, Barrio JR, Phelps ME. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med. 1992;33:633–642. [PubMed] [Google Scholar]
- 55.Teo BK, Saboury B, Munbodh R, Scheuermann J, Torigian DA, Zaidi H, Alavi A. The effect of breathing irregularities on quantitative accuracy of respiratory gated PET/CT. Med Phys. 2012;39:7390–7397. doi: 10.1118/1.4766876. [DOI] [PubMed] [Google Scholar]
- 56.Salavati A, Borofsky S, Boon-Keng TK, Houshmand S, Khiewvan B, Saboury B, Codreanu I, Torigian DA, Zaidi H, Alavi A. Application of partial volume effect correction and 4D PET in the quantification of FDG avid lung lesions. Mol Imaging Biol. 2015;17:140–148. doi: 10.1007/s11307-014-0776-6. [DOI] [PubMed] [Google Scholar]
- 57.Kothekar E, Raynor WY, Al-Zaghal A, Jonnakuti VS, Werner TJ, Alavi A. Evolving role of PET/CT-MRI in assessing muscle disorders. PET Clin. 2019;14:71–79. doi: 10.1016/j.cpet.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Borja AJ, Hancin EC, Khosravi M, Ghorpade R, Koa B, Miao X, Werner TJ, Newberg AB, Alavi A. Applications of hybrid PET/magnetic resonance imaging in central nervous system disorders. PET Clin. 2020;15:497–508. doi: 10.1016/j.cpet.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Hancin EC, Borja AJ, Nikpanah M, Raynor WY, Haldar D, Werner TJ, Morris MA, Saboury B, Alavi A, Gholamrezanezhad A. PET/MR imaging in musculoskeletal precision imaging-third wave after X-Ray and MR. PET Clin. 2020;15:521–534. doi: 10.1016/j.cpet.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Lillington J, Brusaferri L, Klaser K, Shmueli K, Neji R, Hutton BF, Fraioli F, Arridge S, Cardoso MJ, Ourselin S, Thielemans K, Atkinson D. PET/MRI attenuation estimation in the lung: a review of past, present, and potential techniques. Med Phys. 2020;47:790–811. doi: 10.1002/mp.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Musafargani S, Ghosh KK, Mishra S, Mahalakshmi P, Padmanabhan P, Gulyas B. PET/MRI: a frontier in era of complementary hybrid imaging. Eur J Hybrid Imaging. 2018;2:12. doi: 10.1186/s41824-018-0030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osborn EA, Jaffer FA. The advancing clinical impact of molecular imaging in CVD. JACC Cardiovasc Imaging. 2013;6:1327–1341. doi: 10.1016/j.jcmg.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blomberg BA, Akers SR, Saboury B, Mehta NN, Cheng G, Torigian DA, Lim E, Del Bello C, Werner TJ, Alavi A. Delayed time-point 18F-FDG PET CT imaging enhances assessment of atherosclerotic plaque inflammation. Nucl Med Commun. 2013;34:860–867. doi: 10.1097/MNM.0b013e3283637512. [DOI] [PubMed] [Google Scholar]
- 64.Blomberg BA, Thomassen A, Takx RA, Vilstrup MH, Hess S, Nielsen AL, Diederichsen AC, Mickley H, Alavi A, Hoilund-Carlsen PF. Delayed sodium 18F-fluoride PET/CT imaging does not improve quantification of vascular calcification metabolism: results from the CAMONA study. J Nucl Cardiol. 2014;21:293–304. doi: 10.1007/s12350-013-9829-5. [DOI] [PubMed] [Google Scholar]
- 65.Blomberg BA, Thomassen A, de Jong PA, Simonsen JA, Lam MG, Nielsen AL, Mickley H, Mali WP, Alavi A, Hoilund-Carlsen PF. Impact of personal characteristics and technical factors on quantification of sodium 18F-fluoride uptake in human arteries: prospective evaluation of healthy subjects. J Nucl Med. 2015;56:1534–1540. doi: 10.2967/jnumed.115.159798. [DOI] [PubMed] [Google Scholar]
- 66.Blomberg BA, Thomassen A, de Jong PA, Lam MGE, Diederichsen ACP, Olsen MH, Mickley H, Mali W, Alavi A, Hoilund-Carlsen PF. Coronary fluorine-18-sodium fluoride uptake is increased in healthy adults with an unfavorable cardiovascular risk profile: results from the CAMONA study. Nucl Med Commun. 2017;38:1007–1014. doi: 10.1097/MNM.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 67.Arani LS, Gharavi MH, Zadeh MZ, Raynor WY, Seraj SM, Constantinescu CM, Gerke O, Werner TJ, Hoilund-Carlsen PF, Alavi A. Association between age, uptake of (18)F-fluorodeoxyglucose and of (18)F-sodium fluoride, as cardiovascular risk factors in the abdominal aorta. Hell J Nucl Med. 2019;22:14–19. doi: 10.1967/s002449910954. [DOI] [PubMed] [Google Scholar]
- 68.Asadollahi S, Rojulpote C, Bhattaru A, Patil S, Gonuguntla K, Karambelkar P, Borja AJ, Vuthaluru K, Seraj SM, Zhang V, Werner TJ, Gerke O, Hoilund-Carlsen PF, Alavi A. Comparison of atherosclerotic burden in non-lower extremity arteries in patients with and without peripheral artery disease using (18)F-NaF-PET/CT imaging. Am J Nucl Med Mol Imaging. 2020;10:272–278. [PMC free article] [PubMed] [Google Scholar]
- 69.Gonuguntla K, Rojulpote C, Patil S, Bhattaru A, Karambelkar P, Vuthaluru K, Raynor WY, Borja AJ, Zhang V, Werner TJ, Gerke O, Hoilund-Carlsen PF, Alavi A. Utilization of NaF-PET/CT in assessing global cardiovascular calcification using CHADS2 and CHADS2-VASc scoring systems in high risk individuals for cardiovascular disease. Am J Nucl Med Mol Imaging. 2020;10:293–300. [PMC free article] [PubMed] [Google Scholar]
- 70.Patil S, Rojulpote C, Gonuguntla K, Karambelkar P, Bhattaru A, Raynor WY, Borja AJ, Vuthaluru K, Zhang V, Werner TJ, Gerke O, Hoilund-Carlsen PF, Alavi A. Association of triglyceride to high density lipoprotein ratio with global cardiac microcalcification to evaluate subclinical coronary atherosclerosis in non-diabetic individuals. Am J Cardiovasc Dis. 2020;10:241–246. [PMC free article] [PubMed] [Google Scholar]
- 71.Rojulpote C, Patil S, Gonuguntla K, Karambelkar P, Bravo PE, Seraj SM, Asadollahi S, Raynor WY, Bhattaru A, Borja AJ, Zhang V, Werner TJ, Gerke O, Hoilund-Carlsen PF, Alavi A. NaF-PET/CT global assessment in detecting and quantifying subclinical cardiac atherosclerosis and its association with blood pressure in non-dyslipidemic individuals. Am J Cardiovasc Dis. 2020;10:101–107. [PMC free article] [PubMed] [Google Scholar]
- 72.Seraj SM, Raynor WY, Revheim ME, Al-Zaghal A, Zadeh MZ, Arani LS, Rojulpote C, Werner TJ, Gerke O, Hoilund-Carlsen PF, Baker JF, Alavi A, Hunt SJ. Assessing the feasibility of NaF-PET/CT versus FDG-PET/CT to detect abdominal aortic calcification or inflammation in rheumatoid arthritis patients. Ann Nucl Med. 2020;34:424–431. doi: 10.1007/s12149-020-01463-w. [DOI] [PubMed] [Google Scholar]
- 73.Zhang V, Borja AJ, Rojulpote C, Padmanabhan S, Patil S, Gonuguntla K, Revheim ME, Werner TJ, Hoilund-Carlsen PF, Alavi A. Global quantification of pulmonary artery atherosclerosis using (18)F-sodium fluoride PET/CT in at-risk subjects. Am J Nucl Med Mol Imaging. 2020;10:119–126. [PMC free article] [PubMed] [Google Scholar]
- 74.Borja AJ, Bhattaru A, Rojulpote C, Hancin EC, Detchou DK, Patil S, Gonuguntla K, Karambelkar P, Chinta S, Vuthaluru K, Werner TJ, Gerke O, Hoilund-Carlsen PF, Alavi A. Association between atherosclerotic cardiovascular disease risk score estimated by pooled cohort equation and coronary plaque burden as assessed by NaF-PET/CT. Am J Nucl Med Mol Imaging. 2020;10:312–318. [PMC free article] [PubMed] [Google Scholar]
- 75.Rojulpote C, Mehdizadeh Seraj S, Zirakchian Zadeh M, Yadav D, Raynor WY, Kothekar E, Al-Zaghal A, Werner TJ, Gerke O, Hoilund-Carlsen PF, Alavi A. Role of FDG-PET/CT in assessing the correlation between blood pressure and myocardial metabolic uptake. Asia Ocean J Nucl Med Biol. 2020;8:36–45. doi: 10.22038/aojnmb.2019.41530.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rojulpote C, Borja AJ, Zhang V, Aly M, Koa B, Seraj SM, Raynor WY, Kothekar E, Kaghazchi F, Werner TJ, Gerke O, Hoilund-Carlsen PF, Alavi A. Role of (18)F-NaF-PET in assessing aortic valve calcification with age. Am J Nucl Med Mol Imaging. 2020;10:47–56. [PMC free article] [PubMed] [Google Scholar]
- 77.Sorci O, Batzdorf AS, Mayer M, Rhodes S, Peng M, Jankelovits AR, Hornyak JN, Gerke O, Hoilund-Carlsen PF, Alavi A, Rajapakse CS. (18)F-sodium fluoride PET/CT provides prognostic clarity compared to calcium and Framingham risk scoring when addressing whole-heart arterial calcification. Eur J Nucl Med Mol Imaging. 2020;47:1678–1687. doi: 10.1007/s00259-019-04590-3. [DOI] [PubMed] [Google Scholar]
- 78.Alavi A, Werner TJ, Høilund-Carlsen PF. PET-based imaging to detect and characterize cardiovascular disorders: unavoidable path for the foreseeable future. J Nucl Cardiol. 2018;25:203–207. doi: 10.1007/s12350-017-1062-1. [DOI] [PubMed] [Google Scholar]
- 79.Alavi A, Werner TJ, Høilund-Carlsen PF. PET-based imaging to detect and characterize cardiovascular disorders: unavoidable path for the foreseeable future. J Nucl Cardiol. 2018;25:203–207. doi: 10.1007/s12350-017-1062-1. [DOI] [PubMed] [Google Scholar]
- 80.Hoilund-Carlsen PF, Moghbel MC, Gerke O, Alavi A. Evolving role of PET in detecting and characterizing atherosclerosis. PET Clin. 2019;14:197–209. doi: 10.1016/j.cpet.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 81.Hellberg S, Silvola JMU, Kiugel M, Liljenback H, Savisto N, Li XG, Thiele A, Lehmann L, Heinrich T, Vollmer S, Hakovirta H, Laine VJO, Yla-Herttuala S, Knuuti J, Roivainen A, Saraste A. 18-kDa translocator protein ligand 18F-FEMPA: biodistribution and uptake into atherosclerotic plaques in mice. J Nucl Cardiol. 2017;24:862–871. doi: 10.1007/s12350-016-0527-y. [DOI] [PubMed] [Google Scholar]
- 82.Hellberg S, Liljenback H, Eskola O, Morisson-Iveson V, Morrison M, Trigg W, Saukko P, Yla-Herttuala S, Knuuti J, Saraste A, Roivainen A. Positron emission tomography imaging of macrophages in atherosclerosis with (18)F-GE-180, a radiotracer for translocator protein (TSPO) Contrast Media Mol Imaging. 2018;2018:9186902. doi: 10.1155/2018/9186902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shimizu Y, Kuge Y. Recent advances in the development of PET/SPECT probes for atherosclerosis imaging. Nucl Med Mol Imaging. 2016;50:284–291. doi: 10.1007/s13139-016-0418-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Barros AL, Chacko AM, Mikitsh JL, Al Zaki A, Salavati A, Saboury B, Tsourkas A, Alavi A. Assessment of global cardiac uptake of radiolabeled iron oxide nanoparticles in apolipoprotein-E-Deficient mice: implications for imaging cardiovascular inflammation. Mol Imaging Biol. 2014;16:330–339. doi: 10.1007/s11307-013-0709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pahwa R, Jialal I. Atherosclerosis. Treasure Island (FL): StatPearls; 2020. [Google Scholar]
- 86.Rose S, Sheth NH, Baker JF, Ogdie A, Raper A, Saboury B, Werner TJ, Thomas P, Vanvoorhees A, Alavi A, Torigian DA, Gelfand JM, Mehta NN. A comparison of vascular inflammation in psoriasis, rheumatoid arthritis, and healthy subjects by FDG-PET/CT: a pilot study. Am J Cardiovasc Dis. 2013;3:273–278. [PMC free article] [PubMed] [Google Scholar]
- 87.Gholami S, Salavati A, Houshmand S, Werner TJ, Alavi A. Assessment of atherosclerosis in large vessel walls: a comprehensive review of FDG-PET/CT image acquisition protocols and methods for uptake quantification. J Nucl Cardiol. 2015;22:468–479. doi: 10.1007/s12350-015-0069-8. [DOI] [PubMed] [Google Scholar]
- 88.Hoilund-Carlsen PF, Edenbrandt L, Alavi A. Global disease score (GDS) is the name of the game! Eur J Nucl Med Mol Imaging. 2019;46:1768–1772. doi: 10.1007/s00259-019-04383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takx RAP, van Asperen R, Bartstra JW, Zwakenberg SR, Wolterink JM, Celeng C, de Jong PA, Beulens JW. Determinants of 18F-NaF uptake in femoral arteries in patients with type 2 diabetes mellitus. J Nucl Cardiol. 2020 doi: 10.1007/s12350-020-02099-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arani LS, Zirakchian Zadeh M, Saboury B, Revheim ME, Oestergaard B, Borja AJ, Samadi Samarin D, Mehdizadeh Seraj S, Kalbush E, Ayubcha C, Morris MA, Werner TJ, Abildgaard N, Hoilund-Carlsen PF, Alavi A. Assessment of atherosclerosis in multiple myeloma and smoldering myeloma patients using 18F- sodium fluoride PET/CT. J Nucl Cardiol. 2021 doi: 10.1007/s12350-020-02446-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 91.Kwiecinski J, Cadet S, Daghem M, Lassen ML, Dey D, Dweck MR, Berman DS, Newby DE, Slomka PJ. Whole-vessel coronary 18F-sodium fluoride PET for assessment of the global coronary microcalcification burden. Eur J Nucl Med Mol Imaging. 2020;47:1736–1745. doi: 10.1007/s00259-019-04667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cheng G, Torigian DA, Zhuang H, Alavi A. When should we recommend use of dual time-point and delayed time-point imaging techniques in FDG PET? Eur J Nucl Med Mol Imaging. 2013;40:779–787. doi: 10.1007/s00259-013-2343-9. [DOI] [PubMed] [Google Scholar]
- 93.Houshmand S, Salavati A, Segtnan EA, Grupe P, Hoilund-Carlsen PF, Alavi A. Dual-time-point imaging and delayed-time-point fluorodeoxyglucose-PET/computed tomography imaging in various clinical settings. PET Clin. 2016;11:65–84. doi: 10.1016/j.cpet.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 94.Saboury B, Morris MA, Farhadi F, Nikpanah M, Werner TJ, Jones EC, Alavi A. Reinventing molecular imaging with total-body PET, part I: technical revolution in evolution. PET Clin. 2020;15:427–438. doi: 10.1016/j.cpet.2020.06.012. [DOI] [PubMed] [Google Scholar]
- 95.Saboury B, Morris MA, Nikpanah M, Werner TJ, Jones EC, Alavi A. Reinventing molecular imaging with total-body PET, part II: clinical applications. PET Clin. 2020;15:463–475. doi: 10.1016/j.cpet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schmall JP, Karp JS, Alavi A. The potential role of total body PET imaging in assessment of atherosclerosis. PET Clin. 2019;14:245–250. doi: 10.1016/j.cpet.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 97.Borja AJ, Rojulpote C, Hancin EC, Hoilund-Carlsen PF, Alavi A. An update on the role of total-body PET imaging in the evaluation of atherosclerosis. PET Clin. 2020;15:477–485. doi: 10.1016/j.cpet.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 98.Hoilund-Carlsen PF, Piri R, Gerke O, Edenbrandt L, Alavi A. Assessment of total-body atherosclerosis by PET/computed tomography. PET Clin. 2021;16:119–128. doi: 10.1016/j.cpet.2020.09.013. [DOI] [PubMed] [Google Scholar]
- 99.Stiekema LCA, Schnitzler JG, Nahrendorf M, Stroes ESG. The maturation of a ‘neural-hematopoietic’ inflammatory axis in cardiovascular disease. Curr Opin Lipidol. 2017;28:507–512. doi: 10.1097/MOL.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 100.Fukuda D, Sata M, Ishizaka N, Nagai R. Critical role of bone marrow angiotensin II type 1 receptor in the pathogenesis of atherosclerosis in apolipoprotein E deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:90–96. doi: 10.1161/ATVBAHA.107.152363. [DOI] [PubMed] [Google Scholar]
- 101.Devaki M, Nirupama R, Yajurvedi HN. Chronic stress-induced oxidative damage and hyperlipidemia are accompanied by atherosclerotic development in rats. Stress. 2013;16:233–243. doi: 10.3109/10253890.2012.719052. [DOI] [PubMed] [Google Scholar]
- 102.Golbidi S, Frisbee JC, Laher I. Chronic stress impacts the cardiovascular system: animal models and clinical outcomes. Am J Physiol Heart Circ Physiol. 2015;308:H1476–1498. doi: 10.1152/ajpheart.00859.2014. [DOI] [PubMed] [Google Scholar]
- 103.Tawakol A, Ishai A, Takx RA, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJ, Calcagno C, Mani V, Tang CY, Mulder WJ, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. Lancet. 2017;389:834–845. doi: 10.1016/S0140-6736(16)31714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Blebea JS, Houseni M, Torigian DA, Fan C, Mavi A, Zhuge Y, Iwanaga T, Mishra S, Udupa J, Zhuang J, Gopal R, Alavi A. Structural and functional imaging of normal bone marrow and evaluation of its age-related changes. Semin Nucl Med. 2007;37:185–194. doi: 10.1053/j.semnuclmed.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 105.Basu S, Houseni M, Bural G, Chamroonat W, Udupa J, Mishra S, Alavi A. Magnetic resonance imaging based bone marrow segmentation for quantitative calculation of pure red marrow metabolism using 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography: a novel application with significant implications for combined structure-function approach. Mol Imaging Biol. 2007;9:361–365. doi: 10.1007/s11307-007-0112-5. [DOI] [PubMed] [Google Scholar]
- 106.Fan C, Hernandez-Pampaloni M, Houseni M, Chamroonrat W, Basu S, Kumar R, Dadparvar S, Torigian DA, Alavi A. Age-related changes in the metabolic activity and distribution of the red marrow as demonstrated by 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography. Mol Imaging Biol. 2007;9:300–307. doi: 10.1007/s11307-007-0100-9. [DOI] [PubMed] [Google Scholar]
- 107.Bural GG, Torigian DA, Chen W, Houseni M, Basu S, Alavi A. Increased 18F-FDG uptake within the reticuloendothelial system in patients with active lung cancer on PET imaging may indicate activation of the systemic immune response. Hell J Nucl Med. 2010;13:23–25. [PubMed] [Google Scholar]
- 108.Nikpanah M, Katal S, Christensen TQ, Werner TJ, Hess S, Malayeri AA, Gholamrezanezhad A, Alavi A, Saboury B. Potential applications of PET Scans, CT Scans, and MR imaging in inflammatory diseases: part II: cardiopulmonary and vascular inflammation. PET Clin. 2020;15:559–576. doi: 10.1016/j.cpet.2020.06.010. [DOI] [PubMed] [Google Scholar]
- 109.Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, Yeoh SE, Wallace W, Salter D, Fletcher AM, van Beek EJ, Flapan AD, Uren NG, Behan MW, Cruden NL, Mills NL, Fox KA, Rudd JH, Dweck MR, Newby DE. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–713. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]