Abstract
Ovarian cancer is one of the deadliest gynecological malignancies and lacks treatments that do not significantly impact patient health-related quality of life. Exercise has been associated with reduced cancer risk and improved clinical outcomes; however the underlying molecular mechanisms are unknown. In this study, we utilized a treadmill-running exercise model to investigate the effects of exercise on high-grade serous ovarian carcinoma (HGSOC) progression and chemotherapy outcomes. We found that treadmill-running suppressed peritoneal colonization of tumors in a syngeneic mouse ovarian cancer model. Acute exercise stimulated the production of CCL2 and IL-15 in the peritoneal microenvironment while downregulating CCL22, VEGF, and CCL12. Using a co-culture model, we demonstrated the role of CCL2 in mediating the activity of peritoneal cells to inhibit cancer cell viability. We showed that the activation of M1 macrophages may contribute to the exercise-induced changes in the peritoneal microenvironment. We identified that chronic exercise modulates gene expression of intraperitoneal fat tissues related to lipid formation, thermogenesis, browning, and inflammation, which can contribute to inhibiting the colonization of metastatic ovarian cancer. Treadmill running also lowered blood urea nitrogen levels and reduced incidence of neutropenia and thrombocytopenia during chemotherapy in a mouse model, suggesting the potential beneficial effects of exercise in improving chemotherapy outcomes. Our data provided new insights into the acute and chronic effects of physical activity on ovarian cancer at the molecular and in vivo levels.
Keywords: Ovarian cancer, exercise, treadmill, peritoneal microenvironment, adipocytes, chemotherapy
Introduction
Ovarian cancer is the deadliest gynecological malignancy and is often diagnosed at advanced stages [1,2]. Current screening and early detection methods have little impact on the morbidity and mortality of ovarian cancer [3]. Debulking surgery followed by cytotoxic platinum-based chemotherapy is the standard treatment for ovarian cancer, which can lead to both short-term and long-term adverse effects therefore significantly impacting the health-related quality of life (HRQL) of ovarian cancer patients [4,5].
Physical activity is associated with a lower risk of at least 13 types of cancers and has also been associated with a lower risk of cancer mortality [6,7]. Low levels of physical inactivity are associated with all histotypes of epithelial ovarian cancer [8,9], and higher levels of physical activity reduce adverse effects of ovarian cancer treatments. We previously conducted a randomized controlled clinical trial titled Women’s Activity and Lifestyle Study in Connecticut (WALC), in which a six-month home-based exercise intervention improved patients’ HRQL based on the results from 113 ovarian cancer patients [10]. Other smaller exercise intervention trials with fewer ovarian cancer survivors also found improvements in reduced fatigue, cardiopulmonary function, muscle strength, and quality of life [11-13]. Despite these promising clinical findings, there is limited research on mechanisms mediating the effect of exercise on improving ovarian cancer outcomes. Due to the lack of mechanistic studies, it remains unclear whether physical activity directly or indirectly impacts ovarian cancer incidence and mortality.
In this study, we used treadmill-based mouse models to investigate the effects of exercise on ovarian cancer progression and chemotherapy outcomes. High-grade serous ovarian carcinoma (HGSOC) is the most common and lethal type of ovarian cancer. We focused on HGSOC colonization in the peritoneal microenvironment, which is a critical step of the tumorigenesis and progression. Now we know that the majority of “primary” ovarian tumors actually originated from outside the ovary. HGSOCs can originate from the fallopian tube epithelium--specifically, the serous tubal intraepithelial carcinomas (STICs) and the morphologically normal cells carrying TP53 mutations, also called “p53 signature” [14-21]. The fallopian tube tumor precursors disseminate to the ovary and peritoneal organs and transform into invasive HGSOCs. Our previous finding showed that the mutations in matched ovarian tumors and metastatic tumors were extremely similar [22], suggesting they develop from the same origin and the genetic components enabling metastasis had been hardwired into the ovarian tumors. This is also supported by evidence from transgenic mouse models of HGSOCs, which suggests that metastatic potential of ovarian cancer cells is determined at the stage of primary tumor development in the fallopian tube [23]. The early molecular and cellular events in the peritoneal microenvironment have profound impacts on the development and colonization of HGSOCs. Exercise that influences these early events may significantly impact the risk of developing ovarian cancer as well as disease progression.
Exercise can influence the peritoneal microenvironment through reducing visceral fat and modulating the inflammatory factors produced by adipocytes and immune cells [24-27]. The underlying molecular and cellular processes have been investigated in the context of metabolic syndrome [28-30]. Yet the mechanisms behind how these exercise-modulated processes affect ovarian cancer initiation and dissemination in the peritoneal microenvironment are still unclear. We hypothesized that peritoneal microenvironmental changes determine the impact of exercise on ovarian cancer development. The adipose-rich peritoneal microenvironment has been demonstrated to provide the “soil” for ovarian cancer cells to colonize. In particular, the adipocyte-abundant omentum tissues usually contain the largest tumors in the peritoneal cavity. The omental adipocytes secrete interleukin-8 to promote the homing of ovarian cancer cells and provide an energy source for ovarian cancer cells [31]. Adipocytes also secrete lipid factors that promote chemoresistance in ovarian cancer cells [32].
In this study, we used treadmill running in a syngeneic mouse model of ovarian cancer to identify exercise-induced microenvironmental changes in the peritoneal cavity and to understand the molecular mechanisms responsible for the impact of exercise on ovarian cancer development and progression.
Materials and methods
Cell lines
The triple mutant mouse ovarian cancer (OVC) cell line was derived from the high-grade serous carcinomas formed in the Dicer1flox/flox-Ptenflox/flox-Tpr53LSL-R172H/+ Amhr2cre/+ triple mutant Amhr2cre/+ mice [33,34]. The cell line was labeled with LV-CMV-red fluorescence protein (RFP) lentivirus or LV-CMV-firefly luciferase lentivirus (FCT068 and FCT005, Kerafast, Boston, MA). Cells were cultured in DMEM/F12 with 10% FBS, 1% non-essential amino acids, 1% Penicillin streptomycin, 1% sodium pyruvate, and 1% HEPES (Thermo Fisher Scientific, Waltham, MA) and stored in an incubator at 37°C with 5% CO2. In the co-culture models, OVC cells were labeled with luciferase lentiviral vector. They were co-cultured with peritoneal cells at 1:5 ratio or with adipocytes at 1:3 ratio. OVC cells were quantified by luciferase activity assay system (Promega, Madison, WI).
Mouse models
Animal experiments were approved by the Yale University Institutional Animal Care and Use Committee (Protocol #2017-20135). Female C57BL/6 aged 5-6 weeks were obtained from Envigo (Indianapolis, IN). Animals were housed in 12-hour light/dark cycle (7am to 7pm) at a temperature of 18-22°C. Mice had free access to food and water. An automated 5-lane mouse treadmill (Maze Engineers, Skokie, IL) was used in the forced running model. In the chronic exercise model, mice first received 1 week of treadmill training. The speed and duration gradually increased from 2 to 10 m/min and from 10 to 30 min. After the mice were all acclimated to treadmill running, they were randomized into the exercise and control groups. Mice in the control group were placed on a stopped treadmill for 45 min each day, 5 days per week. The mice in the exercise group ran on the treadmill at 10-12 m/min speed for the same duration. To avoid stress, we adjusted the treadmill speed when mice showed any signs of exhaustion (e.g. freezing) and cleaned the treadmill surface prior to running the next group. Four weeks later, mouse OVC labeled with RFP lentivirus (1×107 cells/mouse) were intraperitoneally (IP) injected to the mice of both groups. Mice were imaged weekly using an In Vivio Imagining System (IVIS, Perkins-Elmer, Waltham, MA). After the injection, mice were exposed to daily running or staying on the treadmill for 4 more weeks and euthanized. In the acute exercise model, after one week of training mice were randomized into exercise and control groups. All mice were injected with OVC (1×107 cells/mouse), after which they rested or ran on the treadmill at 10-12 m/min for 45 min. At 24 hours and 48 hours after the first session, two more sessions of treadmill activity were performed. At the end of the third session of treadmill activity, mice were euthanized to collect tissue and body fluid samples. In the chemo-treatment experiment, mouse OVC (1.5×107/mouse) were subcutaneously (SC) injected to the flank, which allowed the tumors to be measured with a vernier caliper every three days. When tumors reached 100 mm3, mice were treated with cisplatin (4 mg/kg, IP, once per week) for 4 weeks. Before the initiation of treatment, mice first received 1 week of treadmill training. After the mice were all acclimated to treadmill running, they were randomized into the exercise and control groups. Mice in the control group were placed on a stopped treadmill for 30 min each day, 5 days per week. The mice in the exercise group ran on the treadmill at 5-10 m/min speed for the same duration.
Peritoneal fluid collection, peritoneal cell (PC) isolation, and coculture with OVCs
Peritoneal lavage was collected as previously described [35] one hour after acute exercise. The peritoneal lavage was spun down at 500 rpm for 5 min at 4°C to collect the cells. When assessing the cytokines in the peritoneal fluid, 1 mL sterile PBS was IP injected into the mouse. The peritoneal lavage fluid was collected into a clean microcentrifuge tube to spin down the cells and collect the supernatant. The isolated cells were seeded as 1:5 (OVCs:PCs) ratio with OVCs for the coculture experiments.
Peritoneal adipocytes isolation and coculture
Peritoneal mesenteric and epididymal fat tissues were removed, minced and digested using collagenase I at 37°C for 2 hours. After filtering to remove the tissue debris, cells were washed extensively with Dulbecco’s modified Eagle’s medium containing 5% bovine serum albumin. The isolated cells were seeded as 1:3 (OVCs:adipocytes) ratio with OVCs for the coculture experiments. GapmeRs were used to knock down the expression of Cidea, Saa3, or Cxcl12 in the mesenteric adipocytes (Qiagen, Germantwon, MD). GapmeRs are locked nucleic acid-conjugated chimeric single-strand antisense oligonucleotides that are used for gene silencing similar to siRNA [36]. They have a phosphorothioate-modified backbone that makes them resistant to enzymatic degradation and more stable than siRNAs. GapmeRs are highly potent and stable both in vivo and in cell culture medium. They were taken up by cells through gymnosis, a natural unassisted delivery [37,38]. After freshly isolated adipocytes were incubated with GapmeRs for 48 hours, they were cocultured with OVCs.
Flow cytometry detection of intracellular TNFα in peritoneal macrophages
The intracellular staining of TNFα was combined with cell surface staining of Cd11b following the protocol provided by the manufacturer of the antibodies, including Alexa Fluor 488 anti-mouse TNFα antibody (MP6-XT22, BioLegend, San Diego, CA) and APC anti-mouse Cd11b antibody (M1/70, BioLegend).
Quantitative real-time PCR (QPCR)
Fat tissues were minced on ice and then homogenized in a Beadbug homogenizer (Benchmark Scientific, Sayreville, NJ) for 60 seconds. After removing large tissue debris, remaining homogenized tissues were used for RNA extraction with Monarch Total RNA Miniprep kit (New England Biolabs Ipswich, MA). Concentration of resulting RNA was determined using a Nanodrop (Thermo Fisher, Waltham, MA). cDNA was transcribed using qScript cDNA SuperMix (Quanta Bio, Beverly, MA) and a Multigene Optimax thermal cycler (Labnet International, Edison, NJ). QPCR was performed using SYBR Green Supermix and CFX Connect detection system (Bio-Rad, Hercules, CA). GAPDH was used as a reference gene in the ΔΔCT expression analysis. All reactions were performed with 3 technical replicates and 3-10 biological replicates. Gene list and primer sequences are included in supplemental information.
Multiplex-cytokine assay
Plasma samples were shipped to Eve Technologies on dry ice (Calgary, AB, Canada) to be analyzed using a Multiplexing LASER Bead Assay (Mouse Cytokine Array/Chemokine Array 31-Plex, Eve Technologies).
Blood urea nitrogen (BUN), glucose, corticosterone, white blood cell count and platelet count
Blood was collected through the retro-orbital sinus using capillary tubes once every two weeks, and then transferred into EDTA coated microcentrifuge tubes using a syringe. Tubes were centrifuged at 13,000 rpm for 15 min at 4°C to isolate plasma. Plasma was transferred into a new tube and stored at -80°C. Plasma samples were diluted to measure the concentration of urea nitrogen using BUN ELISA kit (MyBioSource, San Diego, CA) according to the manual provided by the manufacturer. Blood glucose levels of mouse tail-tip blood was determined using a OneTouch Ultra 2 blood glucose monitor (LifeScan, Milpitas, CA). The glucose level was measured once per week before mice were placed on the treadmill to evaluate the chronic effects of treadmill running. Measurements were taken twice and averaged. Blood corticosterone was determined using Corticosterone ELISA kit (ADI-900-097, Enzo Life Sciences, Farmingdale, NY). White blood cell count and platelet count were assessed as previously described [39,40].
Statistics
Numerical values are presented as mean ± SD. Prism 7 (GraphPad Software, La Jolla, CA, USA) was used to perform statistical analyses. For comparisons between two groups, P values were calculated using paired or unpaired two-tailed Student’s t-tests. One-way ANOVA was used to analyze more than two independent groups. Two-way ANOVA was used to compare the difference in experiments with two independent variables. P<0.05 was considered significantly different if not specified. The results of statistical analysis were indicated in the figures.
Results
Treadmill-running increases blood erythropoietin (EPO) and reduces glucose levels without changing body weight in mouse model
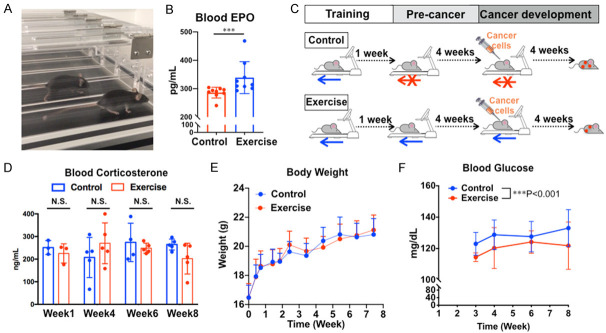
By comparing mice in the control and treadmill-running groups (Figure 1A), we identified that a single bout of treadmill running increased levels of EPO in the plasma (a marker for exercise response in humans) in the exercise group comparing to the control group (Figure 1B). During 8-week treadmill running and injection of cancer cells (Figure 1C), we examined blood corticosterone levels as a stress marker [41,42] and found no statistically significant difference between the control and exercise groups, nor was there a significant difference within groups at different time points (Figure 1D). We did not find statistical significance in body weight between two groups throughout the entire trial (Figure 1E), indicating that differences between groups was not caused by differences in body weight. By contrast, the random blood glucose levels were lower in the exercise group than the control group (Figure 1F), suggesting exercise improves glucose metabolism as expected.
Figure 1.
Mouse treadmill-running exercise model. A. C57BL/6 mice running on the treadmill. B. Blood erythropoietin (EPO) level was assessed by ELISA, n=10, ***P<0.005. C. Chronic exercise experimental design flowchart. D. Corticosterone level in mouse plasma was assessed by ELISA, n=5, N.S., the difference between groups was not statistically significant. E. Body weight of mice during the 8-week chronic exercise experiment, n=15. F. Blood glucose level measured using a glucose meter before the daily exercise. Two-way ANOVA test, n=10, ***P<0.001.
Exercise inhibits ovarian cancer colonization in mouse model
In the chronic exercise model, tumor colonization in the peritoneal cavity was reduced in the exercise group comparing to the control group (Figure 2A). We started to observe the difference between control and exercise groups at about 3 weeks after injecting the cancer cells (Figure 2B). At the time of necropsy, the numbers of peritoneal tumors were lower in the exercise group than the control group, with statistical significance (Figure 2C). The average tumor weight of the exercise group was lower than the control group, however, the difference was not statically significant (Figure 2D). We documented the location and size of the peritoneal tumors and found that the control group mice had more widespread tumors than exercise group mice (Figure 2E).
Figure 2.
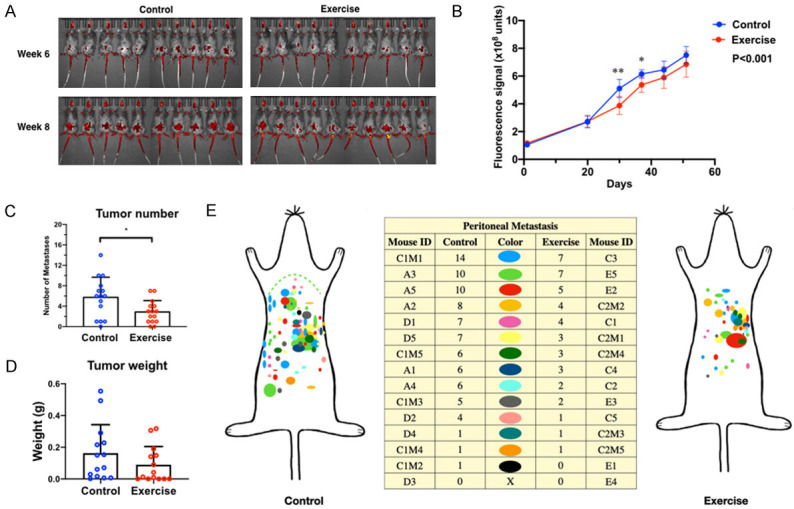
Exercise suppresses ovarian cancer colonization in mice. A. Representative images of tumors in C57BL/6 mice. B. Tumor growth curves assessed by live imaging of tumor fluorescence signals. Two-way ANOVA and Sidak’s test, n=15, P<0.001 between control and exercise groups. *P<0.05 and **P<0.005 for comparision between groups at the same time point. C. Tumor numbers were compared using student’s t-test, n=15, *P<0.05. D. Total tumor weight per animal was compared using student’s t-test, n=15. The difference between two groups was not statistically significant. E. Tumor distribution at necropsy. Each color represents the tumors from one mouse, n=15.
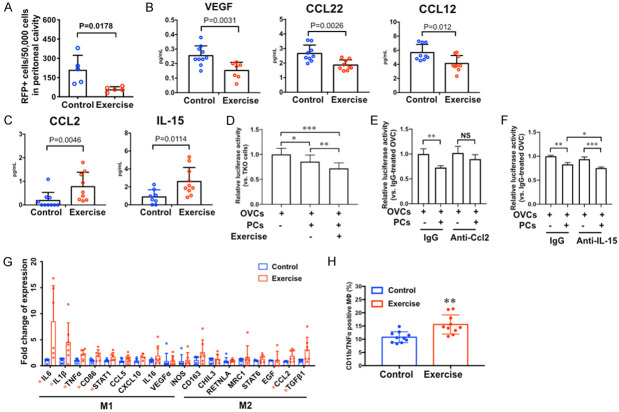
Acute exercise modulates peritoneal immune microenvironment
In the acute exercise model, the peritoneal lavage collected from mice in the exercise group contained fewer RFP+ cells than that of the control mice (Figure 3A). This suggests that the acute effects of exercise reduced surviving cancer cell numbers in the peritoneal cavity. We postulate that exercise induces peritoneal microenvironmental changes that impact ovarian cancer cell survival in the peritoneal cavity. In order to examine exercise-induced changes in the peritoneal microenvironment, we collected peritoneal fluid from the control mice and the mice that completed 3 sessions of 45-minute treadmill running exercise within 48 hours without injecting cancer cells. Using a 45-cytokine multiplex assay, we identified the down-regulation of VEGF (vascular endothelial growth factor), CCL12 (C-C motif chemokine ligand 12), and CCL22 (C-C motif chemokine ligand 22) in the peritoneal fluid of exercise group mice compared to the control group with statistical significance (Figure 3B). We also identified the statistically significant upregulation of CCL2 (C-C motif chemokine ligand 2) and IL-15 (interleukin-15) in the peritoneal fluid of mice in the exercise group comparing to that of the control group (Figure 3C).
Figure 3.
Effects of acute exercise on ovarian cancer cells and the peritoneal microenvironment. A. In acute exercise experiment, RFP positive OVCs in the peritoneal lavage were detected using flow cytometry, n=5, p value of student’s t-test is labeled in the graph. B and C. The levels of cytokines in peritoneal fluid collected 1 h after acute exercise, n=10. P values of student’s t-test are labeled in the graphs. D. Viability of OVCs determined by luciferase activity. Luciferase-labeled OVCs were co-cultured with freshly isolated peritoneal cells (PCs) from C57BL/6 mice that underwent 3 sessions of exercise in 48 hours, n=5, *P<0.05, **P<0.005, ***P<0.0005. E. Viability of OVCs co-cultured with PCs and neutralizing antibody against Ccl2 (anti-Ccl2) or the control antibody (IgG), n=5 **P<0.005. NS, not significant. F. Viability of OVCs co-cultured with PCs and neutralizing antibody against IL-15 (anti-IL-15) or the control antibody (IgG), n=5 *P<0.05, **P<0.005, ***P<0.0005. G. Gene expression of acute exercise-stimulated peritoneal cells analyzed by RT-QPCR, n=7, *P<0.05. H. Flow cytometry analysis of Cd11b/Tnfα positive peritoneal cells after acute exercise, n=10, **P<0.005.
Peritoneal cell-secreted CCL2 inhibits the viability of OVCs in coculture model
We established an in vitro coculture model to test the hypothesis that the acute effects of exercise can enhance the anti-cancer activity of peritoneal cells through CCL2 and IL-15. The cocultured OVCs and freshly isolated peritoneal cells (PCs) showed lower levels of luminescence comparing to the single culture of OVCs (Figure 3D), which suggests that PCs from mice in the control group inhibited the viability of OVCs. Moreover, the PCs isolated from mice in the exercise group further decreased the viability of OVCs than the control group (Figure 3D). When we added the neutralizing antibody against CCL2 to OVCs cocultured with PCs from the exercise group, the neutralizing antibody dampened the inhibitory effect of PCs on OVC cell viability but did not affect the viability in the single culture (Figure 3E). We conducted the same experiment with a neutralizing antibody against IL-15, but we did not observe similar inhibitory effect on the anti-cancer activity of exercise-stimulated PCs (Figure 3F).
Acute exercise activates M1 macrophages in the peritoneal microenvironment
Gene expression analysis of peritoneal cells showed that the level of CCL2 mRNA was higher in the exercise group with statistical significance (Figure 3G). This result is consistent with the exercise-stimulated upregulation of CCL2 at protein level that we identified using cytokine-multiplex assay (Figure 3C). Furthermore, we found that the M1 macrophage activation signature genes were upregulated in the exercise group relative to the control group, which include the statistically significant increase in the mRNA expression of Il6, Il1b, Tnf-α, and Cd86 (Figure 3G). Using flow cytometry, we determined that the CD11b (a macrophage marker) and TNF-α double-positive cell population was increased in the peritoneal cells of mice in the exercise group comparing to that of the control group (Figure 3H), which again indicates the activation of M1 macrophage.
Identification of differently expressed genes in the peritoneal adipose tissues of tumor-bearing mice subjected to chronic exercise
To determine the effect of chronic exercise on the relationship between cancer cells and peritoneal adipose tissues, we collected the peritoneal mesenteric white adipose tissues (mWAT) and the peritoneal epididymal white adipose tissue (eWAT) from the tumor-bearing mice after 8-weeks of exercise (Figure 1C). We examined the RNA expression of 24 genes that are involved in the adipocyte functions of lipid formation, thermogenesis, adipose tissue browning, and adipocyte inflammation via RT-qPCR. The data demonstrated that the tumor-bearing mice in the exercise group expressed statistically significant lower levels of Cidea (cell death-inducing DFFA-like effector A), Saa3 (serum amyloid A3), and Cxcl12 (C-X-C motif chemokine ligand 12) mRNAs in the mWAT comparing to that of the control group with (Figure 4A). In the eWAT of the mice in the exercise group, the relative mRNA levels of Cidea, Dio2, and Elovl3 were lower than that of the control group (Figure 4B).
Figure 4.
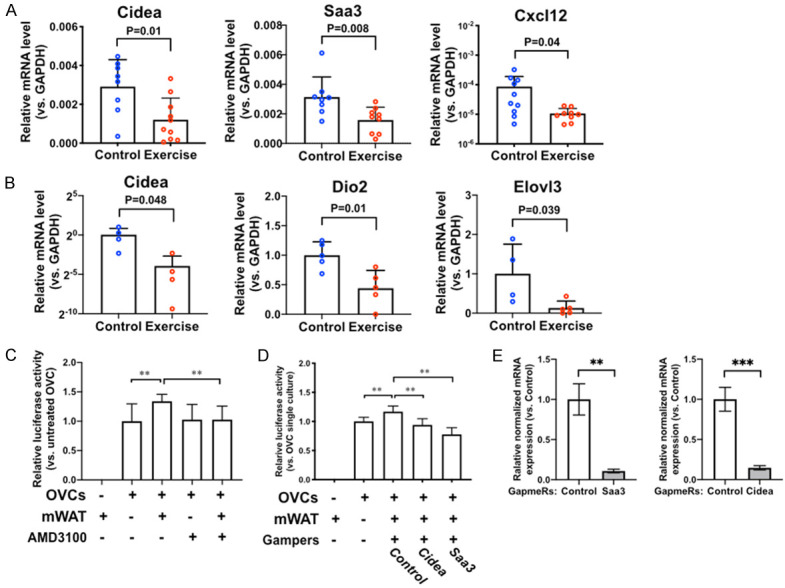
Chronic exercise affects peritoneal mesenteric and epididymal adipose tissue gene expression. A. Gene expression of mesenteric white adipose tissues (mWAT) analyzed by RT-QPCR, n=10. P values of student’s t-test are labeled in the graphs. B. Gene expression of epididymal fat pads analyzed by RT-QPCR, n=5. P values of student’s t-test are labeled in the graphs. C. Viability of OVCs co-cultured with freshly isolated mWAT cells assessed by luciferase activity. AMD3100 (a Cxcl12 antagonist) was added to the media. Two-way ANOVA test, **P<0.005. D. Viability of OVCs co-cultured with GapmeRs-pretreated mWAT cells assessed by luciferase activity. Control, mWAT pretreated with non-targeting negative control Gapmers. Cidea, mWAT pretreated with Gapmers targeting Cidea. Saa3, mWAT pretreated with Gapmers targeting Saa3. One-way ANOVA test, n=5, **P<0.005. E. Expression levels of Cidea and Saa3 in GapmeRs-pretreated mWAT cells assessed by RT-QPCR to confirm the gene knockdown. Student’s t-test, n=3, **P<0.005, ***P<0.0005.
Inhibition of Cxcl12, Cidea, and Saa3 in mWAT suppresses its ability to support OVCs
In a co-culture model of adipocytes and OVCs, cells isolated from mWAT increased the viability of OVCs in the co-culture, comparing to the single culture of OVCs (Figure 4C). When we added AMD3100, an antagonist of CXCR4 (the receptor of CXCL12), to the co-cultured OVCs and mWAT, AMD3100 inhibited the effect of mWAT in promoting the proliferation of OVCs (Figure 4C). The knockdown of CIDEA or SAA3 in the mWAT inhibited the ability of mWAT to promote the proliferation of cancer cells (Figure 4D and 4E). These results suggest that the exercise-induced decrease of Cidea, Saa3, or Cxcl12 expression can reduce the ability of peritoneal adipose tissues to support ovarian cancer cell survival and colonization in the peritoneal cavity.
Exercise improves the outcomes of chemotherapy
We evaluated the effects of exercise on the outcomes of chemo-treatment using a syngeneic subcutaneous tumor model of C57BL/6 mice (Figure 5A). Considering that the mice might experience the side effects of chemotherapy, the running speed and duration were reduced from the standard conditions. Exercise improved the overall survival rate of the tumor-bearing mice (P=0.0069, Figure 5B). The cisplatin treatment caused significant weight loss in mice of both groups (Figure 5C). The average body weight of the exercise group was slightly lower than that of the control group, but not statistically significant. Mice in the exercise group showed increased white blood cell count (Figure 5D) and platelet count (Figure 5E) compared to the control mice with statistical significance, indicating the protection of bone marrow functions. (Figure 5F). The exercise group also showed lower levels of blood urea nitrogen (BUN, a marker of renal toxicity) comparing to the control group.
Figure 5.
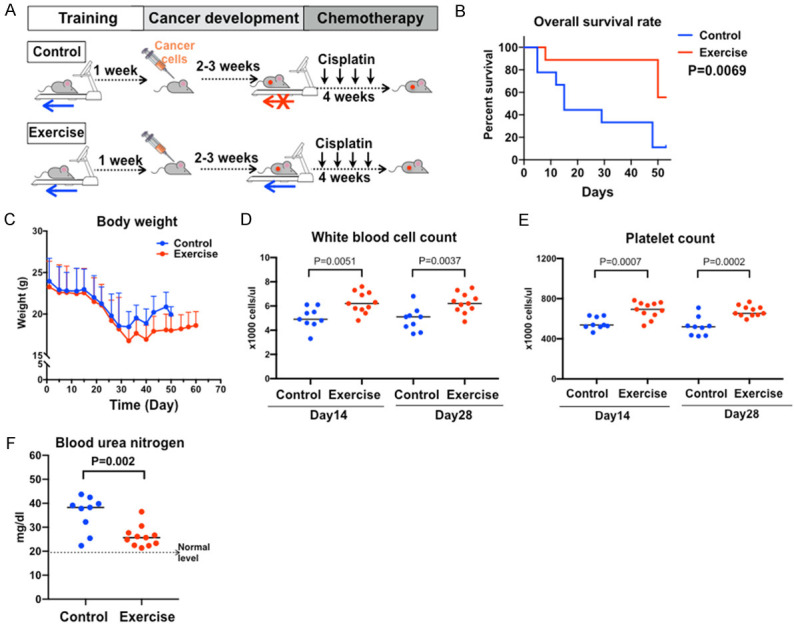
Exercise benefits cisplatin-treated mice by increasing over all survival and decreasing side effects. A. Experimental design flowchart. B. Kaplan-Meier survival curves of cisplatin-treated tumor bearing mice, n=10. Log rank test, P=0.0069. C. Bodyweight curves. No statistical difference, n=10. D. White blood cell count. E. Platelet count. F. Blood urea nitrogen. P values of student’s t-test are labeled in the graphs.
Discussion
In this study, we demonstrated that daily treadmill running limited the metastatic colonization of ovarian cancer cells in peritoneal cavity in a mouse model of ovarian cancer (Figure 6). We identified the exercise-induced changes in the peritoneal microenvironment that are involved in modulating the development and progression of ovarian cancer. We also subjected the tumor-bearing mice to cisplatin treatment and demonstrated that treadmill-running exercise reduced the common adverse side effects of chemotherapy, including neutropenia, thrombocytopenia, and kidney toxicity.
Figure 6.
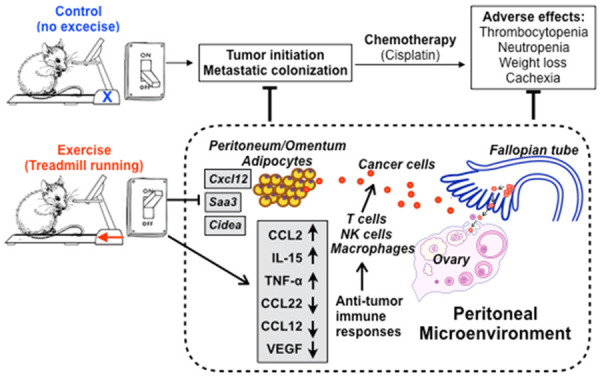
Study summary: Exercise decreases cancer progression, the expression of pro-cancer cytokines, and adverse effects of chemotherapy, while increasing anti-tumor immune responses.
Mouse ovarian cancer models for exercise study
We generated syngeneic mouse models of ovarian cancer using an ovarian cancer cell line that was derived from tumors of the Dicer1flox/flox-Ptenflox/flox-Tpr53LSL-R172H/+ Amhr2cre/+ triple mutant mice [33,34]. This transgenic mouse model resembles the initiation of HGSOCs from the fallopian tube and ovary. More importantly, these triple mutant mice form metastatic tumors throughout the peritoneal cavity accompanied by accumulation of hemorrhagic ascites with complete penetrance. Besides the phenotypic and histopathological similarities, these mouse HGSOCs also display marked chromosomal instability, impaired DNA repair, and reduced responses to chemotherapies [34]. These features recapitulate molecular and genomic features of human HGSOC. The cell line that we derived to form these tumors have the ability to form both intraperitoneal tumors and subcutaneous tumors in the wild-type C57BL/6 mice, which allowed us to study the effects of exercise on tumor development and the host with normal immune system.
Treadmill-running model for exercise study
Mouse models have been used to investigate the effects of exercise on lung cancer, liver cancer, melanoma, colorectal cancer, and breast cancer [43-45]. We utilized forced treadmill running in our model, as the treadmill device has speed control settings that ensures all mice received the same “dosage” of exercise between each other and between sessions. We developed our model to avoid stress by adjusting the treadmill speed when mice showed signs of exhaustion (e.g. freezing). Our stress-reducing strategies and the dosages of exercise are similar to a quantifiable running wheel model previously described by Melo and Hagar [45,46]. Additionally, we monitored the effectiveness and stress level by testing the blood levels of EPO, glucose, and corticosterone [41,42,47-49]. The increased levels of EPO [42,47] and reduced glucose levels [48,49] resembled the exercise response in humans and demonstrated the effectiveness of this exercise modality in mice. Our stress-preventing strategy was effective as evidenced by the similar blood corticosterone levels in the control and treadmill-running groups. No significant difference in body weight was observed between exercise and control groups. These results ensure that any differences between control and exercise mice were not caused by the stress of treadmill running or body weight changes. These features are critical for establishing reliable animal models of exercise, in particular for studying the effects of exercise on cancers.
Mechanisms underlying the anticancer effect of exercise
A number of biological processes have been linked to the beneficial effects of exercise on cancer prevention and patients’ survival, including lowering the levels of pro-cancer hormones and growth factors [50-54], reducing chronic inflammation [55], strengthening anticancer immune response [56-58], and preventing obesity, which is a risk factor for many types of cancer. These hypotheses are to be further tested to determine the underlying mechanisms. The focus of this study is to understand how exercise-related effects modulate the peritoneal microenvironment to influence the colonization of ovarian cancer. Our data supports previous hypotheses by demonstrating that treadmill running inhibited the peritoneal colonization of ovarian cancer cells and reduced the adverse effects of chemotherapy. Furthermore, we revealed the acute effects of exercise in stimulating the pro-inflammatory (M1) activation of peritoneal macrophages and their production of TNF-α. This result is consistent with the common view that acute exercise induces inflammatory responses and elevates pro-inflammatory cytokines [59]. This result also suggests that molecular mechanisms underlying the acute and chronic effects of exercise ovarian cancer development are different. Regular exercise can induce acute pro-inflammatory effects that stimulate anti-tumor immune responses. And repeated occurrence of this stimulation creates an anti-tumor peritoneal microenvironment, leading to a long-term inhibition of chronic inflammation, of which molecular mechanisms still need to be uncovered. The systematic and local tissue effects of exercise may also differ. Since tumor colonization in the peritoneal cavity is a critical step of the tumorigenesis and progression of HGSOCs, the knowledge of these exercise-modulated local biological processes will lead to the identification of promising molecular targets and biomarkers for developing exercise-based intervention.
Cells and factors in the peritoneal fluid
To understand how treadmill-running affected the peritoneal environment, we identified exercise-induced changes produced by the host in the absence of cancer cells. It is important to consider the host, cancer cells, and exercise in a whole system. However, cancer cells also secrete many cytokines and growth factors, and the reciprocal regulations between cancer cells and host cells actively mediate the process of tumor formation. In order to dissect the mechanisms of their complex signal exchanges, we first focused on identifying the effects of treadmill-running on the peritoneal environment, and then tested whether these exercise-induced changes can affect cancer cells. With this knowledge, we can further investigate how their reciprocal signal exchanges affect tumor development. Our findings suggest that acute exercise can stimulate the anti-cancer activity of peritoneal cells through CCL2. The limitation of this experiment is that we did not identify which specific cell populations were responsible for inhibiting cancer cell viability. Peritoneal fluid contains many immune cells, including macrophages, B cells, T cells, and natural killer cells. The changes in their cellular functions and interactions in response to exercise will need to be further investigated.
The results of coculture experiments suggest that exercise-stimulated peritoneal cells can inhibit the viability of the OVCs through CCL2 signaling, but IL-15 may not be responsible for the direct interaction between the peritoneal cells and OVCs. It is also possible that the functions of IL-15 are not fully recapitulated in this coculture model and other cellular components may interact with cancer cells through IL-15 signaling. CCL2 is a potent monocyte-attracting chemokine [60]. It is essential for the recruitment of monocytes to the peritoneal cavity [61]. CCL2 also can prime macrophages to increase their cytotoxicity [62]. IL-15 can enhance the reactivity of peritoneal natural killer (NK) cells against cancer cells in ovarian cancer patients [63]. It also enhances the cytolytic activity of CD8+ T cells [64] and sustains CD8+CD44 high memory T cells [65]. Thus, exercise may increase the levels of CCL2 and IL-15 in the peritoneal environment to help recruit monocytes and enhance the activity of macrophages, NK cells and T cells to eliminate the disseminating cancer cells. Besides increasing the levels of CCL2 and IL-15, acute exercise downregulates the levels of peritoneal CCL22, VEGF, and CCL12. The roles of these cytokines in the exercise-induced anticancer effects have yet to be determined. CCL22 has been shown to recruit regulatory T cells to promote an immunosuppressive microenvironment in ovarian cancer [66]. VEGF promotes angiogenesis and the development of peritoneal carcinomatosis associated with malignant ascites in ovarian cancer [67]. A mouse model of lung cancer has shown that the downregulation of CCL12 can inhibit the recruitment of myeloid-derived suppressor cells to premetastatic lungs and attenuate tumor metastasis [68]. By decreasing the levels of these cytokines in the peritoneal microenvironment, exercise may inhibit their pro-cancer functions and contribute to ovarian cancer prevention.
Peritoneal fat and ovarian cancer
Human intra-abdominal fat tissues are the visceral adipose tissue surrounding the inner organs [69]. They include the omental, mesenteric, retroperitoneal, gonadal, and pericardial fat tissues. HGSOCs often metastasize to the omental and mesenteric fat. The mouse mesenteric fat pad (i.e. mWAT) alongside the intestinal tract is the most analogous to human visceral adipose tissue with respect to its location and biology. However, the mouse mesenteric fat pad lacks detailed studies due to technical challenges in its surgical manipulation and isolation. The largest and most accessible peritoneal fat pads in mice, the epididymal (or periovarian) fat pads (i.e. eWAT) are the most frequently studied in the literature [70-72]. However, they are considered as peri-visceral, not true visceral fat pads. Humans do not harbor fat tissues that are analogous to these fat pads. The omental depot in mice is present in very small quantities and not as clearly defined as in humans. The anatomical differences between humans and mice were considered during our study. In this study we examined the gene expression in both eWAT and mWAT, the most frequently studied and the most analogous to human visceral fat, and used the mWAT for functional studies in the co-culture experiments.
We identified that exercise lowered the levels of Cidea, Saa3, Cxcl12 mRNA in mWAT, and the levels of Cidea, Dio2, and Elovl3 mRNA in eWAT. CIDEA is predominantly expressed by the adipocytes and regulates lipid droplet formation [73]. It is implicated in cancer cachexia due to its overexpression in the white fat tissues of cachectic cancer patients and mice [74,75]. The role of CIDEA in ovarian cancer is unclear. Our data suggests that exercise may inhibit CIDEA-regulated lipid formation to modulate adipocytes-cancer cells interaction and adjust metabolic balance in cancer patients. CXCL12 and SAA3 both have been demonstrated to promote metastasis [76,77]. CXCL12 is an independent predictor of poor survival in ovarian cancer patients [78] and a promising therapeutic target for ovarian cancer [79-81]. SAA3 overexpression in tumor cells potently promotes widespread metastasis [82]. High circulating serum amyloid A level is a prognostic marker for poor clinical outcomes in cancers and has been associated with the increased risks of cancer [83,84]. Very little is known about the roles of DIO2 and ELOVL3 in cancers. DIO2 plays a role in adipose tissue lipid accumulation, mitochondrial function, inflammation, and metabolism in humans [85], and it may promote tumor growth through thyroid hormone signaling [86]. ELOVL3 may play a role in metastasis of prostate cancer [87]. The mechanisms of how these genes in peritoneal fat tissues mediate the development and progression of ovarian cancer will be pursued in our future study.
Exercise and chemotherapy
We previously conducted the WALC clinical trial [10], in which a six-month home-based exercise intervention improved ovarian cancer patients’ HRQL. This study showed the beneficial effects of exercise in ovarian cancer patients who underwent chemotreatments. The results from our animal model resembled the beneficial effects of exercise in ovarian cancer patients. These results not only demonstrated the effectiveness of our exercise model but also allow us to further investigate how exercise can improve patient clinical outcome by reducing the adverse effects of chemotherapies. Chemotherapeutic agents are known for their toxicity of reducing blood cell production and damaging kidney functions. We used our mouse model to further evaluate the signs of side effects caused by chemotherapy and identified that mice in exercise group had improved blood cell production. The lower levels of BUN in the exercise group than the control group also indicate that exercise can help alleviate renal toxicity associated with chemotherapy. Our result suggests that exercise can reduce common side effects of chemotherapy, such as neutropenia, thrombocytopenia, and nephrotoxicity, which also mirrors the beneficial effects of exercise in ovarian cancer patients as demonstrated in our previous exercise intervention trial [10]. The molecular mechanisms of these effects are complex and need to be further investigated. One important aspect is that exercise can improve mitochondrial function [88]. Many chemotherapy-induced side effects are mediated by mitochondrial damage [89]. Exercise improves bone marrow functions [90,91] and alleviates renal injury through restoring mitochondrial function [92,93] in several chronic disease models. Therefore, exercise is a promising non-pharmacological approach for improving mitochondrial function and reducing chemotherapy-induced side effects.
Translation to humans
A major challenge in studying exercise with mouse models is to accurately translate the results to human settings. The low translational success rates from animal studies to clinical testing are common issues in cancer therapeutic development. In terms of exercise research, unique challenges involve the significant physiological differences between mice and humans. For example, mice are natural runners and can perform high intensity running all night long at all ages [94]. However, humans can usually only endure high intensity running for up to 30 min with a significant decrease associated with aging [95]. We should not simply translate the 8-week duration of daily treadmill exercise in mice to 5-7 years in humans by comparing their life span and growth rate [96]. The development of reliable methods for translating the doses and duration of exercise in mice to human equivalents is needed for the clinical translation of exercise research. Before these methods are established, rodent exercise models remain a valuable tool for mechanistic studies and dose-response studies as they can be utilized in a more controlled fashion than human studies.
Conclusion
Our study provided evidence that exercise can positively change the peritoneal microenvironment to prevent or suppress the dissemination of ovarian cancer cells (Figure 6). Understanding how physical activity can modulate the peritoneal microenvironment will contribute to designing the most optimal exercise-based interventions for preventing and inhibiting ovarian cancer.
Acknowledgements
The research is supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Ovarian Cancer Research Program under Award No. W81XWH-15-1-0221, NIH/NCI R03 CA216127, Discovery To Cure Ovarian Cancer Research Grant, and Colleen’s Dream Foundation Research Grant.
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A, Siegel RL. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Park EY, Kim O, Schilder JM, Coffey DM, Cho CH, Bast RC Jr. Cell origins of high-grade serous ovarian cancer. Cancers (Basel) 2018;10:433. doi: 10.3390/cancers10110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK, Amso NN, Apostolidou S, Benjamin E, Cruickshank D, Crump DN, Davies SK, Dawnay A, Dobbs S, Fletcher G, Ford J, Godfrey K, Gunu R, Habib M, Hallett R, Herod J, Jenkins H, Karpinskyj C, Leeson S, Lewis SJ, Liston WR, Lopes A, Mould T, Murdoch J, Oram D, Rabideau DJ, Reynolds K, Scott I, Seif MW, Sharma A, Singh N, Taylor J, Warburton F, Widschwendter M, Williamson K, Woolas R, Fallowfield L, McGuire AJ, Campbell S, Parmar M, Skates SJ. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet. 2016;387:945–956. doi: 10.1016/S0140-6736(15)01224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews S, von Gruenigen VE. Management of the late effects of treatments for gynecological cancer. Curr Opin Oncol. 2013;25:566–570. doi: 10.1097/CCO.0b013e328363e11a. [DOI] [PubMed] [Google Scholar]
- 5.Ezendam NP, Pijlman B, Bhugwandass C, Pruijt JF, Mols F, Vos MC, Pijnenborg JM, van de Poll-Franse LV. Chemotherapy-induced peripheral neuropathy and its impact on health-related quality of life among ovarian cancer survivors: results from the population-based PROFILES registry. Gynecol Oncol. 2014;135:510–517. doi: 10.1016/j.ygyno.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 7.Moore SC, Lee IM, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami HO, Blair CK, Borch KB, Boyd E, Check DP, Fournier A, Freedman ND, Gunter M, Johannson M, Khaw KT, Linet MS, Orsini N, Park Y, Riboli E, Robien K, Schairer C, Sesso H, Spriggs M, Van Dusen R, Wolk A, Matthews CE, Patel AV. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannioto R, LaMonte MJ, Risch HA, Hong CC, Sucheston-Campbell LE, Eng KH, Brian Szender J, Chang-Claude J, Schmalfeldt B, Klapdor R, Gower E, Minlikeeva AN, Zirpoli GR, Bandera EV, Berchuck A, Cramer D, Doherty JA, Edwards RP, Fridley BL, Goode EL, Goodman MT, Hogdall E, Hosono S, Jensen A, Jordan S Australian Ovarian Cancer Study Group. Kjaer SK, Matsuo K, Ness RB, Olsen CM, Olson SH, Leigh Pearce C, Pike MC, Anne Rossing M, Szamreta EA, Thompson PJ, Tseng CC, Vierkant RA, Webb PM, Wentzensen N, Wicklund KG, Winham SJ, Wu AH, Modugno F, Schildkraut JM, Terry KL, Kelemen LE, Moysich KB. Chronic recreational physical inactivity and epithelial ovarian cancer risk: evidence from the ovarian cancer association consortium. Cancer Epidemiol Biomarkers Prev. 2016;25:1114–1124. doi: 10.1158/1055-9965.EPI-15-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cannioto RA, LaMonte MJ, Kelemen LE, Risch HA, Eng KH, Minlikeeva AN, Hong CC, Szender JB, Sucheston-Campbell L, Joseph JM, Berchuck A, Chang-Claude J, Cramer DW, DeFazio A, Diergaarde B, Dork T, Doherty JA, Edwards RP, Fridley BL, Friel G, Goode EL, Goodman MT, Hillemanns P, Hogdall E, Hosono S, Kelley JL, Kjaer SK, Klapdor R, Matsuo K, Odunsi K, Nagle CM, Olsen CM, Paddock LE, Pearce CL, Pike MC, Rossing MA, Schmalfeldt B, Segal BH, Szamreta EA, Thompson PJ, Tseng CC, Vierkant R, Schildkraut JM, Wentzensen N, Wicklund KG, Winham SJ, Wu AH, Modugno F, Ness RB, Jensen A, Webb PM, Terry K, Bandera EV, Moysich KB. Recreational physical inactivity and mortality in women with invasive epithelial ovarian cancer: evidence from the Ovarian Cancer Association Consortium. Br J Cancer. 2016;115:95–101. doi: 10.1038/bjc.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Y, Cartmel B, Gottlieb L, Ercolano EA, Li F, Harrigan M, McCorkle R, Ligibel JA, von Gruenigen VE, Gogoi R, Schwartz PE, Risch HA, Irwin ML. Randomized trial of exercise on quality of life in women with ovarian cancer: women’s activity and lifestyle study in connecticut (WALC) J Natl Cancer Inst. 2017;109:djx072. doi: 10.1093/jnci/djx072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnelly CM, Blaney JM, Lowe-Strong A, Rankin JP, Campbell A, McCrum-Gardner E, Gracey JH. A randomised controlled trial testing the feasibility and efficacy of a physical activity behavioural change intervention in managing fatigue with gynaecological cancer survivors. Gynecol Oncol. 2011;122:618–624. doi: 10.1016/j.ygyno.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 12.Hwang KH, Cho OH, Yoo YS. The effect of comprehensive care program for ovarian cancer survivors. Clin Nurs Res. 2016;25:192–208. doi: 10.1177/1054773814559046. [DOI] [PubMed] [Google Scholar]
- 13.Moonsammy SH, Guglietti CL, Santa Mina D, Ferguson S, Kuk JL, Urowitz S, Wiljer D, Ritvo P. A pilot study of an exercise & cognitive behavioral therapy intervention for epithelial ovarian cancer patients. J Ovarian Res. 2013;6:21. doi: 10.1186/1757-2215-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J. Clin. Oncol. 2007;25:3985–3990. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 15.Carcangiu ML, Radice P, Manoukian S, Spatti G, Gobbo M, Pensotti V, Crucianelli R, Pasini B. Atypical epithelial proliferation in fallopian tubes in prophylactic salpingo-oophorectomy specimens from BRCA1 and BRCA2 germline mutation carriers. Int J Gynecol Pathol. 2004;23:35–40. doi: 10.1097/01.pgp.0000101082.35393.84. [DOI] [PubMed] [Google Scholar]
- 16.Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross AL, Kurman RJ, Vang R, Shih IeM, Visvanathan K. Precursor lesions of high-grade serous ovarian carcinoma: morphological and molecular characteristics. J Oncol. 2010;2010:126295. doi: 10.1155/2010/126295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, Garber JE, Cramer DW, Crum CP. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 19.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P, Verheijen RH. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 20.Saleemuddin A, Folkins AK, Garrett L, Garber J, Muto MG, Crum CP, Tworoger S. Risk factors for a serous cancer precursor (“p53 signature”) in women with inherited BRCA mutations. Gynecol Oncol. 2008;111:226–232. doi: 10.1016/j.ygyno.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Folkins AK, Jarboe EA, Saleemuddin A, Lee Y, Callahan MJ, Drapkin R, Garber JE, Muto MG, Tworoger S, Crum CP. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol Oncol. 2008;109:168–173. doi: 10.1016/j.ygyno.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C, Bonazzoli E, Bellone S, Choi J, Dong W, Menderes G, Altwerger G, Han C, Manzano A, Bianchi A, Pettinella F, Manara P, Lopez S, Yadav G, Riccio F, Zammataro L, Zeybek B, Yang-Hartwich Y, Buza N, Hui P, Wong S, Ravaggi A, Bignotti E, Romani C, Todeschini P, Zanotti L, Zizioli V, Odicino F, Pecorelli S, Ardighieri L, Silasi DA, Litkouhi B, Ratner E, Azodi M, Huang GS, Schwartz PE, Lifton RP, Schlessinger J, Santin AD. Mutational landscape of primary, metastatic, and recurrent ovarian cancer reveals c-MYC gains as potential target for BET inhibitors. Proc Natl Acad Sci U S A. 2019;116:619–624. doi: 10.1073/pnas.1814027116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim O, Park EY, Kwon SY, Shin S, Emerson RE, Shin YH, DeMayo FJ, Lydon JP, Coffey DM, Hawkins SM, Quilliam LA, Cheon DJ, Fernandez FM, Nephew KP, Karpf AR, Widschwendter M, Sood AK, Bast RC Jr, Godwin AK, Miller KD, Cho CH, Kim J. Targeting progesterone signaling prevents metastatic ovarian cancer. Proc Natl Acad Sci U S A. 2020;117:31993–32004. doi: 10.1073/pnas.2013595117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, Barrett EJ, Gaesser GA, Weltman A. Effect of exercise training intensity on abdominal visceral fat and body composition. Med Sci Sports Exerc. 2008;40:1863–1872. doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You T, Berman DM, Ryan AS, Nicklas BJ. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J Clin Endocrinol Metab. 2004;89:1739–1746. doi: 10.1210/jc.2003-031310. [DOI] [PubMed] [Google Scholar]
- 26.van Gemert WA, Peeters PH, May AM, Doornbos AJH, Elias SG, van der Palen J, Veldhuis W, Stapper M, Schuit JA, Monninkhof EM. Effect of diet with or without exercise on abdominal fat in postmenopausal women - a randomised trial. BMC Public Health. 2019;19:174. doi: 10.1186/s12889-019-6510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vissers D, Hens W, Taeymans J, Baeyens JP, Poortmans J, Van Gaal L. The effect of exercise on visceral adipose tissue in overweight adults: a systematic review and meta-analysis. PLoS One. 2013;8:e56415. doi: 10.1371/journal.pone.0056415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziegler AK, Damgaard A, Mackey AL, Schjerling P, Magnusson P, Olesen AT, Kjaer M, Scheele C. An anti-inflammatory phenotype in visceral adipose tissue of old lean mice, augmented by exercise. Sci Rep. 2019;9:12069. doi: 10.1038/s41598-019-48587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wedell-Neergaard AS, Lang Lehrskov L, Christensen RH, Legaard GE, Dorph E, Larsen MK, Launbo N, Fagerlind SR, Seide SK, Nymand S, Ball M, Vinum N, Dahl CN, Henneberg M, Ried-Larsen M, Nybing JD, Christensen R, Rosenmeier JB, Karstoft K, Pedersen BK, Ellingsgaard H, Krogh-Madsen R. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: a randomized controlled trial. Cell Metab. 2019;29:844–855.e3. doi: 10.1016/j.cmet.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Paley CA, Johnson MI. Abdominal obesity and metabolic syndrome: exercise as medicine? BMC Sports Sci Med Rehabil. 2018;10:7. doi: 10.1186/s13102-018-0097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt MR, Romero IL, Carey MS, Mills GB, Hotamisligil GS, Yamada SD, Peter ME, Gwin K, Lengyel E. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J, Zaman MM, Vlasakov I, Roy R, Huang L, Martin CR, Freedman SD, Serhan CN, Moses MA. Adipocytes promote ovarian cancer chemoresistance. Sci Rep. 2019;9:13316. doi: 10.1038/s41598-019-49649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J, Coffey DM, Ma L, Matzuk MM. The ovary is an alternative site of origin for high-grade serous ovarian cancer in mice. Endocrinology. 2015;156:1975–1981. doi: 10.1210/en.2014-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim O, Park EY, Klinkebiel DL, Pack SD, Shin YH, Abdullaev Z, Emerson RE, Coffey DM, Kwon SY, Creighton CJ, Kwon S, Chang EC, Chiang T, Yatsenko AN, Chien J, Cheon DJ, Yang-Hartwich Y, Nakshatri H, Nephew KP, Behringer RR, Fernandez FM, Cho CH, Vanderhyden B, Drapkin R, Bast RC Jr, Miller KD, Karpf AR, Kim J. In vivo modeling of metastatic human high-grade serous ovarian cancer in mice. PLoS Genet. 2020;16:e1008808. doi: 10.1371/journal.pgen.1008808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. J Vis Exp. 2010:1488. doi: 10.3791/1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fazil MH, Ong ST, Chalasani ML, Low JH, Kizhakeyil A, Mamidi A, Lim CF, Wright GD, Lakshminarayanan R, Kelleher D, Verma NK. GapmeR cellular internalization by macropinocytosis induces sequence-specific gene silencing in human primary T-cells. Sci Rep. 2016;6:37721. doi: 10.1038/srep37721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soifer HS, Koch T, Lai J, Hansen B, Hoeg A, Oerum H, Stein CA. Silencing of gene expression by gymnotic delivery of antisense oligonucleotides. Methods Mol Biol. 2012;815:333–346. doi: 10.1007/978-1-61779-424-7_25. [DOI] [PubMed] [Google Scholar]
- 38.Stein CA, Hansen JB, Lai J, Wu S, Voskresenskiy A, Hog A, Worm J, Hedtjarn M, Souleimanian N, Miller P, Soifer HS, Castanotto D, Benimetskaya L, Orum H, Koch T. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 2010;38:e3. doi: 10.1093/nar/gkp841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aurbach K, Spindler M, Haining EJ, Bender M, Pleines I. Blood collection, platelet isolation and measurement of platelet count and size in mice-a practical guide. Platelets. 2019;30:698–707. doi: 10.1080/09537104.2018.1528345. [DOI] [PubMed] [Google Scholar]
- 40.O’Connell KE, Mikkola AM, Stepanek AM, Vernet A, Hall CD, Sun CC, Yildirim E, Staropoli JF, Lee JT, Brown DE. Practical murine hematopathology: a comparative review and implications for research. Comp Med. 2015;65:96–113. [PMC free article] [PubMed] [Google Scholar]
- 41.Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, Luo MJ, Tan JH. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One. 2015;10:e0117503. doi: 10.1371/journal.pone.0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts D, Smith DJ. Erythropoietin concentration and arterial haemoglobin saturation with supramaximal exercise. J Sports Sci. 1999;17:485–493. doi: 10.1080/026404199365795. [DOI] [PubMed] [Google Scholar]
- 43.Kelly SA, Zhao L, Jung KC, Hua K, Threadgill DW, Kim Y, de Villena FP, Pomp D. Prevention of tumorigenesis in mice by exercise is dependent on strain background and timing relative to carcinogen exposure. Sci Rep. 2017;7:43086. doi: 10.1038/srep43086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, Nielsen J, Gehl J, Pedersen BK, Thor Straten P, Hojman P. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23:554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Hagar A, Wang Z, Koyama S, Serrano JA, Melo L, Vargas S, Carpenter R, Foley J. Endurance training slows breast tumor growth in mice by suppressing Treg cells recruitment to tumors. BMC Cancer. 2019;19:536. doi: 10.1186/s12885-019-5745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Melo L, Hagar A. How to train a mouse-methodological issues in pre-clinical exercise oncology. Am J Cancer Res. 2019;9:1246–1253. [PMC free article] [PubMed] [Google Scholar]
- 47.Schwandt HJ, Heyduck B, Gunga HC, Rocker L. Influence of prolonged physical exercise on the erythropoietin concentration in blood. Eur J Appl Physiol Occup Physiol. 1991;63:463–466. doi: 10.1007/BF00868079. [DOI] [PubMed] [Google Scholar]
- 48.Adams OP. The impact of brief high-intensity exercise on blood glucose levels. Diabetes Metab Syndr Obes. 2013;6:113–122. doi: 10.2147/DMSO.S29222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colberg SR, Hernandez MJ, Shahzad F. Blood glucose responses to type, intensity, duration, and timing of exercise. Diabetes Care. 2013;36:e177. doi: 10.2337/dc13-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ennour-Idrissi K, Maunsell E, Diorio C. Effect of physical activity on sex hormones in women: a systematic review and meta-analysis of randomized controlled trials. Breast Cancer Res. 2015;17:139. doi: 10.1186/s13058-015-0647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kossman DA, Williams NI, Domchek SM, Kurzer MS, Stopfer JE, Schmitz KH. Exercise lowers estrogen and progesterone levels in premenopausal women at high risk of breast cancer. J Appl Physiol (1985) 2011;111:1687–1693. doi: 10.1152/japplphysiol.00319.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meneses-Echavez JF, Jimenez EG, Rio-Valle JS, Correa-Bautista JE, Izquierdo M, Ramirez-Velez R. The insulin-like growth factor system is modulated by exercise in breast cancer survivors: a systematic review and meta-analysis. BMC Cancer. 2016;16:682. doi: 10.1186/s12885-016-2733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie L, Wang W. Weight control and cancer preventive mechanisms: role of insulin growth factor-1-mediated signaling pathways. Exp Biol Med (Maywood) 2013;238:127–132. doi: 10.1177/1535370213477602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu M, King B, Ewert E, Su X, Mardiyati N, Zhao Z, Wang W. Exercise activates p53 and negatively regulates IGF-1 pathway in epidermis within a skin cancer model. PLoS One. 2016;11:e0160939. doi: 10.1371/journal.pone.0160939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, Schauer DB, Dedon PC, Fox JG, Samson LD. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meneses-Echavez JF, Correa-Bautista JE, Gonzalez-Jimenez E, Schmidt Rio-Valle J, Elkins MR, Lobelo F, Ramirez-Velez R. The effect of exercise training on mediators of inflammation in breast cancer survivors: a systematic review with meta-analysis. Cancer Epidemiol Biomarkers Prev. 2016;25:1009–1017. doi: 10.1158/1055-9965.EPI-15-1061. [DOI] [PubMed] [Google Scholar]
- 57.Nilsson MI, Bourgeois JM, Nederveen JP, Leite MR, Hettinga BP, Bujak AL, May L, Lin E, Crozier M, Rusiecki DR, Moffatt C, Azzopardi P, Young J, Yang Y, Nguyen J, Adler E, Lan L, Tarnopolsky MA. Lifelong aerobic exercise protects against inflammaging and cancer. PLoS One. 2019;14:e0210863. doi: 10.1371/journal.pone.0210863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang HL, Hsieh PL, Hung CH, Cheng HC, Chou WC, Chu PM, Chang YC, Tsai KL. Early moderate intensity aerobic exercise intervention prevents doxorubicin-caused cardiac dysfunction through inhibition of cardiac fibrosis and inflammation. Cancers (Basel) 2020;12:1102. doi: 10.3390/cancers12051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hennigar SR, McClung JP, Pasiakos SM. Nutritional interventions and the IL-6 response to exercise. FASEB J. 2017;31:3719–3728. doi: 10.1096/fj.201700080R. [DOI] [PubMed] [Google Scholar]
- 60.Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169:1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu B, Rutledge BJ, Gu L, Fiorillo J, Lukacs NW, Kunkel SL, North R, Gerard C, Rollins BJ. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Q, Ren J, Morgan S, Liu Z, Dou C, Liu B. Monocyte chemoattractant protein-1 (MCP-1) regulates macrophage cytotoxicity in abdominal aortic aneurysm. PLoS One. 2014;9:e92053. doi: 10.1371/journal.pone.0092053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoogstad-van Evert JS, Maas RJ, van der Meer J, Cany J, van der Steen S, Jansen JH, Miller JS, Bekkers R, Hobo W, Massuger L, Dolstra H. Peritoneal NK cells are responsive to IL-15 and percentages are correlated with outcome in advanced ovarian cancer patients. Oncotarget. 2018;9:34810–34820. doi: 10.18632/oncotarget.26199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu J, Giuntoli RL 2nd, Omiya R, Kobayashi H, Kennedy R, Celis E. Interleukin 15 promotes antigen-independent in vitro expansion and long-term survival of antitumor cytotoxic T lymphocytes. Clin Cancer Res. 2002;8:3877–3884. [PubMed] [Google Scholar]
- 65.Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170:5018–5026. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 66.Wertel I, Surowka J, Polak G, Barczynski B, Bednarek W, Jakubowicz-Gil J, Bojarska-Junak A, Kotarski J. Macrophage-derived chemokine CCL22 and regulatory T cells in ovarian cancer patients. Tumour Biol. 2015;36:4811–4817. doi: 10.1007/s13277-015-3133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Masoumi Moghaddam S, Amini A, Morris DL, Pourgholami MH. Significance of vascular endothelial growth factor in growth and peritoneal dissemination of ovarian cancer. Cancer Metastasis Rev. 2012;31:143–162. doi: 10.1007/s10555-011-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shi H, Zhang J, Han X, Li H, Xie M, Sun Y, Liu W, Ba X, Zeng X. Recruited monocytic myeloid-derived suppressor cells promote the arrest of tumor cells in the premetastatic niche through an IL-1beta-mediated increase in E-selectin expression. Int J Cancer. 2017;140:1370–1383. doi: 10.1002/ijc.30538. [DOI] [PubMed] [Google Scholar]
- 69.Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes. 2011;2011:490650. doi: 10.1155/2011/490650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abreu-Vieira G, Fischer AW, Mattsson C, de Jong JM, Shabalina IG, Ryden M, Laurencikiene J, Arner P, Cannon B, Nedergaard J, Petrovic N. Cidea improves the metabolic profile through expansion of adipose tissue. Nat Commun. 2015;6:7433. doi: 10.1038/ncomms8433. [DOI] [PubMed] [Google Scholar]
- 71.Buzelle SL, Przygodda F, Rossi-Valentim R, Ferreira GN, Garofalo MAR, Alves VM, Chaves VE, Navegantes LCC, Kettelhut IDC. Activation of adipose tissue glycerokinase contributes to increased white adipose tissue mass in mice fed a high-fat diet. Endocrine. 2020;69:79–91. doi: 10.1007/s12020-020-02288-3. [DOI] [PubMed] [Google Scholar]
- 72.van der Stelt I, Hoevenaars F, Siroka J, de Ronde L, Friedecky D, Keijer J, van Schothorst E. Metabolic response of visceral white adipose tissue of obese mice exposed for 5 days to human room temperature compared to mouse thermoneutrality. Front Physiol. 2017;8:179. doi: 10.3389/fphys.2017.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gummesson A, Jernas M, Svensson PA, Larsson I, Glad CA, Schele E, Gripeteg L, Sjoholm K, Lystig TC, Sjostrom L, Carlsson B, Fagerberg B, Carlsson LM. Relations of adipose tissue CIDEA gene expression to basal metabolic rate, energy restriction, and obesity: population-based and dietary intervention studies. J Clin Endocrinol Metab. 2007;92:4759–4765. doi: 10.1210/jc.2007-1136. [DOI] [PubMed] [Google Scholar]
- 74.Laurencikiene J, Stenson BM, Arvidsson Nordstrom E, Agustsson T, Langin D, Isaksson B, Permert J, Ryden M, Arner P. Evidence for an important role of CIDEA in human cancer cachexia. Cancer Res. 2008;68:9247–9254. doi: 10.1158/0008-5472.CAN-08-1343. [DOI] [PubMed] [Google Scholar]
- 75.Rohm M, Schafer M, Laurent V, Ustunel BE, Niopek K, Algire C, Hautzinger O, Sijmonsma TP, Zota A, Medrikova D, Pellegata NS, Ryden M, Kulyte A, Dahlman I, Arner P, Petrovic N, Cannon B, Amri EZ, Kemp BE, Steinberg GR, Janovska P, Kopecky J, Wolfrum C, Bluher M, Berriel Diaz M, Herzig S. An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nat Med. 2016;22:1120–1130. doi: 10.1038/nm.4171. [DOI] [PubMed] [Google Scholar]
- 76.Ahirwar DK, Nasser MW, Ouseph MM, Elbaz M, Cuitino MC, Kladney RD, Varikuti S, Kaul K, Satoskar AR, Ramaswamy B, Zhang X, Ostrowski MC, Leone G, Ganju RK. Fibroblast-derived CXCL12 promotes breast cancer metastasis by facilitating tumor cell intravasation. Oncogene. 2018;37:4428–4442. doi: 10.1038/s41388-018-0263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Djurec M, Grana O, Lee A, Troule K, Espinet E, Cabras L, Navas C, Blasco MT, Martin-Diaz L, Burdiel M, Li J, Liu Z, Vallespinos M, Sanchez-Bueno F, Sprick MR, Trumpp A, Sainz B Jr, Al-Shahrour F, Rabadan R, Guerra C, Barbacid M. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc Natl Acad Sci U S A. 2018;115:E1147–E1156. doi: 10.1073/pnas.1717802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Popple A, Durrant LG, Spendlove I, Rolland P, Scott IV, Deen S, Ramage JM. The chemokine, CXCL12, is an independent predictor of poor survival in ovarian cancer. Br J Cancer. 2012;106:1306–1313. doi: 10.1038/bjc.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhong C, Wang J, Li B, Xiang H, Ultsch M, Coons M, Wong T, Chiang NY, Clark S, Clark R, Quintana L, Gribling P, Suto E, Barck K, Corpuz R, Yao J, Takkar R, Lee WP, Damico-Beyer LA, Carano RD, Adams C, Kelley RF, Wang W, Ferrara N. Development and preclinical characterization of a humanized antibody targeting CXCL12. Clin Cancer Res. 2013;19:4433–4445. doi: 10.1158/1078-0432.CCR-13-0943. [DOI] [PubMed] [Google Scholar]
- 80.Mao TL, Fan KF, Liu CL. Targeting the CXCR4/CXCL12 axis in treating epithelial ovarian cancer. Gene Ther. 2017;24:621–629. doi: 10.1038/gt.2017.69. [DOI] [PubMed] [Google Scholar]
- 81.Gil M, Komorowski MP, Seshadri M, Rokita H, McGray AJ, Opyrchal M, Odunsi KO, Kozbor D. CXCL12/CXCR4 blockade by oncolytic virotherapy inhibits ovarian cancer growth by decreasing immunosuppression and targeting cancer-initiating cells. J Immunol. 2014;193:5327–5337. doi: 10.4049/jimmunol.1400201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hansen MT, Forst B, Cremers N, Quagliata L, Ambartsumian N, Grum-Schwensen B, Klingelhofer J, Abdul-Al A, Herrmann P, Osterland M, Stein U, Nielsen GH, Scherer PE, Lukanidin E, Sleeman JP, Grigorian M. A link between inflammation and metastasis: serum amyloid A1 and A3 induce metastasis, and are targets of metastasis-inducing S100A4. Oncogene. 2015;34:424–435. doi: 10.1038/onc.2013.568. [DOI] [PubMed] [Google Scholar]
- 83.Zhou J, Sheng J, Fan Y, Zhu X, Tao Q, He Y, Wang S. Association between serum amyloid A levels and cancers: a systematic review and meta-analysis. Postgrad Med J. 2018;94:499–507. doi: 10.1136/postgradmedj-2018-136004. [DOI] [PubMed] [Google Scholar]
- 84.Lin HY, Tan GQ, Liu Y, Lin SQ. The prognostic value of serum amyloid A in solid tumors: a meta-analysis. Cancer Cell Int. 2019;19:62. doi: 10.1186/s12935-019-0783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bradley D, Liu J, Blaszczak A, Wright V, Jalilvand A, Needleman B, Noria S, Renton D, Hsueh W. Adipocyte DIO2 expression increases in human obesity but is not related to systemic insulin sensitivity. J Diabetes Res. 2018;2018:2464652. doi: 10.1155/2018/2464652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kojima Y, Kondo Y, Fujishita T, Mishiro-Sato E, Kajino-Sakamoto R, Taketo MM, Aoki M. Stromal iodothyronine deiodinase 2 (DIO2) promotes the growth of intestinal tumors in Apc(Delta716) mutant mice. Cancer Sci. 2019;110:2520–2528. doi: 10.1111/cas.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang Y, Liu L, Li M, Cheng X, Fang M, Zeng Q, Xu Y. The chromatin remodeling protein BRG1 links ELOVL3 trans-activation to prostate cancer metastasis. Biochim Biophys Acta Gene Regul Mech. 2019;1862:834–845. doi: 10.1016/j.bbagrm.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Menshikova EV, Ritov VB, Fairfull L, Ferrell RE, Kelley DE, Goodpaster BH. Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J Gerontol A Biol Sci Med Sci. 2006;61:534–540. doi: 10.1093/gerona/61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorini S, De Angelis A, Berrino L, Malara N, Rosano G, Ferraro E. Chemotherapeutic drugs and mitochondrial dysfunction: focus on doxorubicin, trastuzumab, and sunitinib. Oxid Med Cell Longev. 2018;2018:7582730. doi: 10.1155/2018/7582730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Baker JM, De Lisio M, Parise G. Endurance exercise training promotes medullary hematopoiesis. FASEB J. 2011;25:4348–4357. doi: 10.1096/fj.11-189043. [DOI] [PubMed] [Google Scholar]
- 91.Maredziak M, Smieszek A, Chrzastek K, Basinska K, Marycz K. Physical activity increases the total number of bone-marrow-derived mesenchymal stem cells, enhances their osteogenic potential, and inhibits their adipogenic properties. Stem Cells Int. 2015;2015:379093. doi: 10.1155/2015/379093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang LX, Wang B, Wu ZK. Aerobic exercise training alleviates renal injury by interfering with mitochondrial function in type-1 diabetic mice. Med Sci Monit. 2018;24:9081–9089. doi: 10.12659/MSM.912877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y, Liu Y, Bi X, Hu C, Ding F, Ding W. Therapeutic approaches in mitochondrial dysfunction, inflammation, and autophagy in uremic cachexia: role of aerobic exercise. Mediators Inflamm. 2019;2019:2789014. doi: 10.1155/2019/2789014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goh J, Ladiges W. Voluntary wheel running in mice. Curr Protoc Mouse Biol. 2015;5:283–290. doi: 10.1002/9780470942390.mo140295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, Carter RE, Lanza IR, Nair KS. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017;25:581–592. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flurkey KCJ, Harrison DE. The mouse in aging research. ELSEVIER. 2007 [Google Scholar]