Abstract
Gastric cancer (GC) patients with Epstein-Barr virus (EBV) positivity have demonstrated promising response with immunotherapy. We assessed the efficacy and safety of camrelizumab as salvage treatment in EBV-positive mGC. In this single-arm, phase 2 prospective clinical trial (NCT03755440), stage IV EBV-positive GC patients who failed/could not tolerate previous lines of chemotherapy were given intravenous camrelizumab 200 mg every 2 weeks until disease progression or unacceptable toxicity. The primary endpoint was objective response rate. Secondary endpoints were progression-free survival (PFS), overall survival (OS), disease control rate (DCR), duration of response, and toxicity. Exploratory analysis included the associations between treatment response and tumor mutation burden (TMB), programmed cell death ligand-1 (PD-L1) expression. Six eligible patients were enrolled in the first stage of the study. No patient achieved an objective response; thus, the study did not proceed to the second stage. The DCR was 67% (4/6). The median PFS rate was 2.2 months (95% CI: 1.5-not reached [NR]) and median OS was 6.8 months (95% CI: 1.7-NR). All treatment-related adverse events were grade 1-2, with reactive cutaneous capillary endothelial proliferation (n=4 [67%]) being the most commonly observed event. The only patient with PD-L1 combined positive score >1 had disease progression. Two stable disease and one disease progression were observed in three patients with TMB >10 Mut/Mb. EBV positivity may not be a good predictor for response to camrelizumab in mGC. Newer biomarkers are needed to identify EBV-positive mGC respondents who might benefit from immunotherapy.
Keywords: Epstein-Barr virus, gastric cancer, PD-1 inhibitor, camrelizumab
Introduction
Gastric cancer (GC) is one of the most common malignancies in the world, especially in China [1]. Treatment options for metastatic gastric cancer (mGC) are limited, especially for patients with resistance to chemotherapy beyond the first- or second-line settings. Several studies have reported the efficacy and safety of programmed cell death-1 (PD-1) inhibitors in mGC patients, with an objective response rate (ORR) ranging from 11% to 23.3% [2-6]. The phase 2 KEYNOTE-059 and phase 3 ATTRACTION-2 studies included mGC patients, regardless of the expression of programmed cell death ligand-1 (PD-L1), with ORR of 11.6% and 11.2% [2,3], respectively. Currently used biomarkers to predict response to anti-PD-1 therapy include microsatellite instability (MSI), PD-L1 expression, tumor mutation burden (TMB), and Epstein-Barr virus (EBV) positivity [7].
EBV positivity was identified as a distinct molecular subtype of gastric cancer by The Cancer Genome Atlas (TCGA) Research Network [8]. Clinically, in situ hybridization (ISH) targeting EBV-encoded RNA (EBER) is used to test the presence of EBV in GC cells, which generally defines EBV-associated GC (EBVaGC) [9], with an incidence rate of 9% according to the TCGA study and approximately 5% in China [10-12].
Previously, Panda et al. reported that an EBV-positive GC patient, with microsatellite stability (MSS) and low TMB, had achieved partial response after anti-PD-L1 therapy [13]. In another study, a 100% response rate was reported in EBV-positive mGC patients treated with PD-1 inhibitor, though there were only 6 cases included [14]. However, some recent studies showed that the response to immune checkpoint inhibitors (ICBs) in EBVaGC was not as promising as might had been hoped for, with an ORR of 16.7%-28.6% [4,7,15,16]. However, the aforementioned results were from observational studies or subgroup analyses, and the actual predictive role of EBV positivity for response to immunotherapy needs to be fully evaluated in a prospective clinical setting.
Camrelizumab (also known as SHR-1210) is a humanized, selective, high-affinity immunoglobulin G4 κ monoclonal PD-1 antibody. Camrelizumab was reported to have encouraging efficacy in mGC patients in China, with an ORR of 23.3% [6], similar to other PD-1 antibodies in mGC [2-4]. Here, we prospectively investigated the clinical safety and efficacy of camrelizumab in patients with EBV-positive advanced GC, and assessed the reliability of biomarkers for identifying potential respondents.
Methods
Study design
This was a single-center, single-arm, open-label, prospective phase 2 study (ClinicalTrials.gov, Identifier NCT03755440) conducted at the Sun Yat-sen University Cancer Center (Guangzhou, China).
Study population
Eligible patients were ≥18 years old, had histologically confirmed metastatic or recurrent unresectable GC; were EBER-positive according to ISH; and failed from first-line platinum- and fluorouracil-based chemotherapy, and second-line chemotherapy, or could not tolerate systemic chemotherapy. Patients should not receive immune checkpoint inhibitors before. The full eligibility criteria are detailed in the study protocol in the Supplementary Materials.
Ethics approval and consent to participate
This study was conducted at Sun Yat-sen University Cancer Center (Guangzhou, China). It was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Sun Yat-sen University Cancer Center (No. B2018-058-01). Written informed consent was obtained from each participant.
Treatments
Patients were treated with 200 mg camrelizumab intravenously over 30 min once every 2-week. The treatment was continued until disease progression, unacceptable toxicity or withdrawal of consent. Contrast computed tomography scans of the chest, abdomen, and pelvis were performed at baseline and repeated every 8 weeks. Tumor response was assessed by the investigator according to the Response Evaluation Criteria In Solid Tumors criteria version 1.1 (RECIST 1.1). Response was confirmed with a second scan at least 4 weeks after the criteria for objective response was met. Dose modifications of camrelizumab were not allowed, but dose delays for adverse events (AEs) up to 8 weeks were permitted. Patients who discontinued treatment because of AEs were followed at 8 weeks after the last dose of the study drug, until disease progression or initiation of subsequent therapy. Laboratory tests were performed during screening and on the first day of every 2 cycles of therapy. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Study endpoints
The primary endpoint of this study was ORR. The secondary endpoints were progression-free survival (PFS), overall survival (OS), disease control rate (DCR), duration of response (DoR), and safety.
ORR was defined as the proportion of patients who achieved complete response (CR) or partial response (PR). PFS was defined as the time from initiation of treatment to disease progression, or any-cause death, whichever came first. OS was defined as the time from treatment initiation to any-cause death. The DCR was defined as the proportion of patients who achieved CR, PR, and stable disease (SD). DoR was defined as the time from the first documented objective response to the first documented disease progression or any-cause death, whichever came first.
Biomarker analysis
Patients provided either newly biopsy-obtained or archival tumor samples for the assessment of PD-L1 expression, mismatch repair (MMR) status, and whole-exome sequencing.
PD-L1 expression was detected by immunohistochemistry staining with SP142, an antihuman PD-L1 monoclonal antibody [17]. The specimens were scored by two senior pathologists using a combined positive score (CPS), defined as the proportion of viable tumor cells and tumor-infiltrating immune cells with partial or complete membrane staining at any intensity. Positive PD-L1 status was defined as CPS ≥1.
Both whole-exome sequencing and ultra-deep target sequencing (HaploX HapOncoWESPlus panel, Roche NimbleGen) on tumor tissue and baseline cfDNA specimens were carried out. TMB was determined by analyzing somatic mutations per mega-base (Mb).
Bioinformatics analysis
Analysis ready reads were obtained from raw sequencing reads with a quality control process using the fastp software [18]. Alignment steps were then performed using the bwa software [19] followed by a series of procedures including samTobam, MarkDuplicates, BaseRecallberation, and ApplyBQSR recommended by GATK best practice (GATK v4.0) [20]. The genome build used in this study was hg38 from UCSC. Somatic mutations were identified by the Mutect2 algorithm and only those marked with high-confidential variants were retained for further analysis. We annotated the SNVs and InDels with VEP software [21]. In addition, CNVkit was applied for evaluating the somatic copy-number variations (SCNV) in each sample.
Statistical analysis
Sample size was determined using Simon’s optimal two-stage design, with a significance level of 5% and power of 80%. P0=15% (null hypothesis) was based on the data of previously published studies using ORR with anti-PD-1 antibody in mGC (11-23%) [2-4,6]. P1=45% or higher (alternative hypothesis) was considered as a success in EBV-positive mGC. In the 6 patients enrolled in the first stage, the presence of at least 1 response (CR or PR) allowed the study to proceed to the second stage, in which another 13 patients would be enrolled. Considering a drop-out rate of 5%, a total of at least 20 patients were needed for this two-stage trial.
Baseline characteristics, ORR, DCR, and AEs were analyzed using descriptive statistics. Survivals were estimated using the Kaplan-Meier method and the corresponding 95% confidence intervals (CIs) were calculated. Statistical analysis was performed using the Intercooled Stata 13.0 (Stata Corporation, College Station, TX). All the P-values were two-sided, and statistical significance was set at P<0.05.
Results
Basic features
From November 15, 2018, to September 18, 2020, a total of 6 patients with chemotherapy-refractory, metastatic EBVaGC consented to the treatment protocol and were enrolled in the first stage of the study. All analyses were performed after the last patient had disease progression from camrelizumab monotherapy. None of the 6 patients got CR or PR, therefore, the study did not proceed to the second stage.
The clinical and pathological characteristics of the enrolled patients are summarized in Table 1. The median age was 41.5 (range, 32-69) years and 5 (83.3%) of the 6 patients were male. One (16.7%) patient had lymphoepithelioma-like carcinoma and the rest (n=5, 83.3%) were adenocarcinoma. Four (66.7%) patients had stage IV disease and two (33.3%) had stage III at the time of diagnosis. In the patients diagnosed at stage III, one received radical resection but developed multiple bone and lymph node metastases 30 months after the surgery, and the other developed multiple lymph node metastases 5 months after the gastrectomy. The most common sites of metastases included distant lymph nodes, bone, and peritoneum. Four (66.7%) patients had Eastern Cooperative Oncology Group (ECOG) performance status of 1. All the 6 patients were human epidermal growth factor receptor 2 (HER2) negative and MMR proficient (pMMR). Three (50%) patients had an elevation of baseline EBV-DNA, defined as ≥100 copies/mL according to our previous report [10]. Two of these three patients had progressive disease (PD).
Table 1.
Demographic and clinicopathological characteristics of patients at baseline
| Features | Patients, N (%) |
|---|---|
| Age/median (range) | 41.5 (32-69) yrs |
| Gender | 5 (83.0) |
| Male | |
| Performance status | |
| 0 | 2 (33.3) |
| 1 | 4 (66.7) |
| Tumor location | |
| Body | 3 (50.0) |
| Antrum | 2 (33.3) |
| Fundus | 1 (16.7) |
| Pathological type | |
| Adenocarcinoma | 5 (83.3) |
| Lymphoepithelioma-like carcinoma | 1 (16.7) |
| Lauren classification | |
| Diffuse | 1 (16.7) |
| Intestine | 2 (33.3) |
| Mixed | 3 (50.0) |
| Prior surgery | |
| No | 2 (33.3) |
| Radical surgery | 2 (33.3) |
| Palliative surgery | 2 (33.3) |
| Metastasis | |
| Synchronous | 5 (83.3) |
| Metachronous | 1 (16.7) |
| No. of metastatic sites | |
| 0-2 | 5 (83.3) |
| >2 | 1 (16.7) |
| Site of metastatic disease | |
| Liver | 1 (16.7) |
| Bone | 2 (33.3) |
| Peritoneum | 3 (50.0) |
| Lymph node | 4 (66.7) |
| Ascites | |
| No | 4 (66.7%) |
| Yes | 2 (33.3) |
| HER2 status, negative no. (%) | 6 (100) |
| ERBR, Positive no. (%) | 6 (100) |
| PD-L1 CPS (%) | |
| <1 | 5 (83.3) |
| 1-5 | 1 (16.7) |
| TMB (Mut/Mb) | |
| <10 | 3 (50.0) |
| ≥10 | 3 (50.5) |
| Previous treatment | |
| Platinum and fluorouracil | 6 (100) |
| Taxales | 5 (83.3) |
| Apatinib | 2 (33.3) |
| Ramucirumab | 1 (16.7) |
Survival analysis and safety
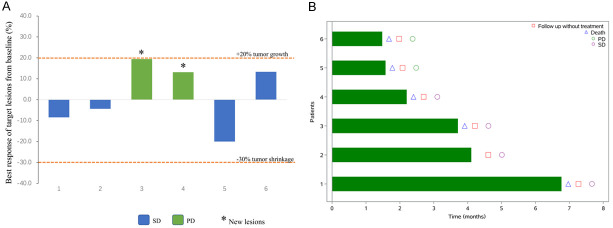
The median PFS was 2.2 months (95% CI: 1.4-not reached [NR]) (Figure 1A). By the analysis cut-off date, January 25, 2021, only one patient (17%) was alive but with disease, and the remaining 5 (83%) patients died due to disease progression. The median OS was 6.8 months (95% CI: 1.7-NR) (Figure 1B). Of all 6 patients, 4 achieved SD with a DCR of 67% (Figure 2). Since no objective response was observed, DoR could not be calculated.
Figure 1.
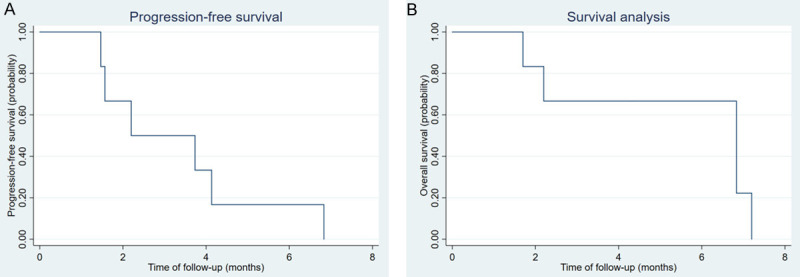
Kaplan-Meier plots of median (A) progression-free survival (PFS) and (B) overall survival (OS) of EBV positive gastric cancer patients treated with camrelizumab monotherapy.
Figure 2.
Tumor response in camrelizumab monotherapy assessed by investigator per the RECIST v1.1 criteria. A. Maximal change of tumor size from baseline in target the lesion(s). *The patient was characterized as progressive disease (PD) due to the development of new lesion(s) or progression of non-target lesion(s). B. Duration of treatment exposure.
By the cut-off date, none of the patients remained on treatment. All 6 patients received a total of 29 cycles of treatment with a median treatment duration of 3.0 months (interquartile range [IQR], 1.6-4.1) and a median treatment cycle of 4 (IQR, 3-8). All the treatment-related AEs (TRAEs) are presented in Table 2 (detailed descriptions in Supplementary Table 1). TRAEs included reactive cutaneous capillary endothelial proliferation (4 [66.7%]), fatigue (2 [33.3%]), hyperthyroidism (1 [16.7%]), hypothyroidism (1 [16.7%]) and rash (1 [16.7%]). Hyperthyroidism and hypothyroidism occurred in the same patient who had hyperthyroidism but 1 month later changed to hypothyroidism. No grade ≥3 TRAEs were found.
Table 2.
Treatment-related adverse events
| Events | Patients N (%) |
|---|---|
| Reactive cutaneous capillary endothelial proliferation (RCCEP) | 4 (66.7) |
| Grade 2 | 1 (16.7) |
| Grade 1 | 3 (50.0) |
| Fatigue | 2 (33.3) |
| Hypothyroidism | 1 (16.7) |
| Hyperthyroidism | 1 (16.7) |
| Rash | 1 (16.7) |
All the side effects were grade 1 except for one patient with grade 2 RCCEP.
The two patients who had PD were all intestinal sub-type (Lauren classification). Immunohistochemical evaluation of PD-L1 expression was available in pretreatment tumor samples from the 6 patients. Only one (16.7%) patient was PD-L1 positive (CPS=5) but developed disease progression after 3 cycles of camrelizumab monotherapy. Three (50%) patients had TMB >10 Mb but <12 Mb, and two of them achieved SD while the other one developed PD.
Genomic landscape of the EBVaGC patients
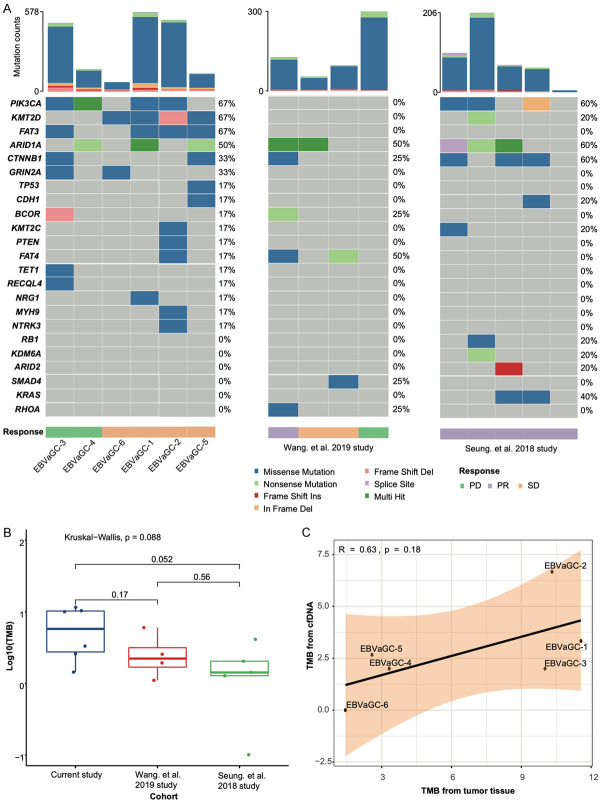
For the genomic landscape from tumor tissue, we identified 1,962 somatic variations including 1,634 missense, 235 truncating mutations, and 93 other types (Supplementary Table 2). PIK3CA, KMT2D, FAT1, and ARID1A were the most common mutated genes in our cohort (Figure 3A). According to the TCGA GC research network, both PIK3CA and ARID1A were highly enriched in EBV positive GC subgroup [8]. Therefore, our cohort may present the most comparable mutational profile to the TCGA EBV-positive GC cohort.
Figure 3.
A. Oncoplot showing the genomic landscape of patients with EBV-positive gastric cancer in both our dataset and two public datasets. The top bar summarizes the total mutation count in each sample. Annotation bar at the bottom presents the clinical features. Colors from the heatmap indicate mutational classification identified in each gene. B. Comparison of TMB among the three cohorts. P values were calculated with the Kruskal-Wallis test. C. Correlation analysis between tumor-derived TMB (tTMB) and cfDNA derived TMB (bTMB).
To further compare the genomic alterations between our cohort and others, known ICB-related predictors were evaluated in our cohort and in two additional EBV-positive gastric cohorts who received ICBs treatment [4,14]. However, we did not find any significant difference of the genomic features among these three cohorts. For TMB evaluation, all the six patients in our cohort were TMB-L with a median TMB 6.67 Mut/Mb (1.44-11.56 Mut/Mb), comparable to those from the other 2 cohorts (Figure 3B). For cfDNA based mutations, we totally identified 50 somatic variants including 24 missense, 24 truncating, and 2 other type mutations, respectively. Correlation analysis showed that the bTMB was positively correlated with the tTMB (R=0.63, Spearman correlation coefficient, Figure 3C), indicating a potential application of bTMB on treatment monitoring of mGC patients.
Discussion
To our knowledge, this is the first prospective study evaluating the safety and efficacy of anti-PD-1 antibody in patients with EBV-positive advanced GC. Though there was no responder in the first six patients, the DCR was as high as 66.7%. Moreover this group of patients had a meaningful PFS and OS after the treatment of PD-1 inhibitor.
Patient enrollment into the first stage of this study was relatively slow, ~2 years, possibly due to, first, the low incidence of EBVaGC. According to our previous report, the incidence rate of EBVaGC was 5.1% in the all stage GC population and 1.4% in the stage IV GC patients [10]. Second, majority of EBVaGC patients (>80%) are usually diagnosed in stage I-III and are associated with relatively high 3- and 5-year DFS rates, at 83.7% and 73.8%, respectively [15]; thus, contributing to the long enrollment process.
This current prospective phase 2 clinical trial was conducted according to Simon’s optimal two-stage design. The study did not proceed to the second stage because no responder was identified in the first stage of the study. EBV positivity only may thus not be a good predictor for response for the use of PD-1 inhibitors in mGC patients.
EBV positivity could be confounded by other factors, and we, therefore, explored the relationship between EBV positivity and other known response predictors, namely, MSI-high (MSI-H), TMB, and PD-L1 expression. Though there are reports about the co-existence of EBV positivity and MSI-H in GC patients [10,22], EBV positivity and MSI-H are basically considered as mutually exclusive [8]. Both in the present study and Kim et al. study [14], all the EBV-positive patients were pMMR. As for TMB, we found no statistical difference between high TMB and EBV-positive or -negative GC in our previous study [23]. Similarly, no high TMB was found in the EBV-positive patients from Kim’s cohort [14]. Therefore, EBV-positive GC patients were basically pMMR with general TMB level. Though the cut-off value for PD-L1 positivity was controversial, results of several studies have suggested that patients with PD-L1-positive tumors had higher response rates than those with PD-L1-negative tumors [24,25]. A series of trials only focused on patients with PD-L1-positive GC [26-28]. In cohort 1 of the KEYNOTE-059 trial, the ORR of patients with PD-L1-negative tumors was 6.4% [2]. A general positive correlation was found between EBV-positive GC and PD-L1 expression [8,29]. We found that the 6 EBV-positive patients with PR from Kim et al. cohort [14] and the only one EBV-positive patient with PR from Wang et al. cohort [4] were all PD-L1 positive (CPS cut-off value of 1), while in the present study, 5 out of 6 patients were PD-L1 negative. This may partially explain the low response rate in the present study. It indicated that instead of EBV positivity, PD-L1 expression might be the underlying factor to predict response to PD-1 inhibitors.
Except for the low PD-L1 positivity rate, there were other factors that may lead to the low response in our cohort. First, 4 (67%) of the patients had ECOG performance status of 1. Second, 3 (50%) of the patients had peritoneum metastasis and 2 of them suffered from ascites. Previous study had found that poor performance status and peritoneum metastasis were negative predictors to PD-1 inhibitors [30].
Can the different response to ICB between our cohort and Seung’s cohort be caused by the different genomic background? However, we found that the mutational features and TMB levels were comparable among these three cohorts (current cohort, Kim et al. cohort and Wang et al. cohort) [4,14], signifying that the different response to ICB between our cohort and Seung’s cohort may not be explained by genomic background.
Another difference between our current study and Kim et al. study is the generic name of PD-1 inhibitors, camrelizumab in our study and pembrolizumab in Kim et al. study [14]. In the KEYNOTE-059 trial, the ORR of pembrolizumab for mGC patients in third-line setting was 11% [2]. Camrelizumab was reported to have encouraging efficacy in mGC patients, with an ORR of 23% [6]. Therefore, the different response cannot be explained by the kinds of PD-1 inhibitors.
Though the response rate to anti-PD-1 antibody was low, the DCR in our study was higher than that with pembrolizumab in the KEYNOTE-059 study (67% vs. 27.0%, respectively) [2] and the nivolumab arm in the ATTRACTION-02 study (67% vs. 40.3%, respectively) [3,30]. Furthermore, the DCR in the present study was similar to previous reports in EBVaGC patients (75.0% in Wang et al. study) [4]. Moreover, the median PFS (2.2 months) and OS (6.8 months) in our cohort was better, or at least comparable to the results in the KEYNOTE-059 (PFS: 2.0 months; OS: 5.6 months) and ATTRACTION-02 studies (PFS: 1.6 months; OS: 5.3 months) [2,3,30]. There could be two attributable reasons for the superior survival observed in our cohort. First, EBV-positive patients could be associated with better prognosis than EBV-negative patients [31], and second, high DCR in the current study could have contributed to PFS and OS. The present finding led us to consider the optimal endpoint for third-line PD-1 inhibitors in mGC patients. Compared with ORR, DCR or OS may be better primary endpoint for PD-1 inhibitors.
In our study, most toxicity of camrelizumab observed were similar to those previously reported [6]. Other immune-related AEs, such as skin disorder and hypothyroidism, were all grade 1 or 2 and successfully controlled with observation or complementary therapy.
The limitation of our present study is the small sample size. However, the sample size was calculated by the Simon’s optimal two-stage design. We did not move to the second stage study because no efficacy was found in the first 6 patients. This is the first prospective study to evaluate the efficacy and side effect of PD-1 antibody in metastatic EBV positive gastric cancer.
In conclusion, this single-arm, open-label phase 2 trial revealed that EBV positivity may not be an ideal biomarker to predict response to anti-PD-1 antibody in mGC. PD-L1 expression may be the confounding factor.
Acknowledgements
We gratefully thank the patients and their families for participating in this study. This work was supported by the National Key R&D Program of China (grant number: 2018YFC1313300), National Natural Science Foundation of China (grant number: 82073377 and 81772587), Science and Technology Program of Guangdong (grant number: 2021A1515012439), Guangdong Esophageal Cancer Institute Science and Technology Program (grant number: M201809), Chinese Society of Clinical Oncology-Hengrui Oncology Research Fund (grant number: Y-HR2018-184), Jiangsu Hengrui Pharmaceuticals Co., Ltd., the Third Outstanding Young Talents Training Plan and Medical Scientist Program of Sun Yat-sen University Cancer Center.
Disclosure of conflict of interest
None.
Supplementary Materials
Supplementary Table 1
Supplementary Table 2
References
- 1.Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, Yuan XL, Liu TS, Li GX, Wu Q, Xu HM, Ji JF, Li YF, Wang X, Yu S, Liu H, Guan WL, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond) 2019;39:10. doi: 10.1186/s40880-019-0349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, Yoon HH. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, Yuan XL, Chen Y, Yang SJ, Shi JH, Hu XC, Lin XY, Zhang QY, Feng JF, Ba Y, Liu YP, Li W, Shu YQ, Jiang Y, Li Q, Wang JW, Wu H, Feng H, Yao S, Xu RH. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol. 2019;30:1479–1486. doi: 10.1093/annonc/mdz197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu H. Safety and efficacy of toripalimab in advanced gastric cancer: a new clinical trial bringing hope for immunotherapy in gastric cancer. Cancer Commun (Lond) 2020;40:194–196. doi: 10.1002/cac2.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Mo H, Zhang W, Chen X, Qu D, Wang X, Wu D, Wang X, Lan B, Yang B, Wang P, Zhang B, Yang Q, Jiao Y, Xu B. Promising efficacy of SHR-1210, a novel anti-programmed cell death 1 antibody, in patients with advanced gastric and gastroesophageal junction cancer in China. Cancer. 2019;125:742–749. doi: 10.1002/cncr.31855. [DOI] [PubMed] [Google Scholar]
- 7.Mishima S, Kawazoe A, Nakamura Y, Sasaki A, Kotani D, Kuboki Y, Bando H, Kojima T, Doi T, Ohtsu A, Yoshino T, Kuwata T, Tsuji A, Shitara K. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer. 2019;7:24. doi: 10.1186/s40425-019-0514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer (review) Int J Oncol. 2015;46:1421–1434. doi: 10.3892/ijo.2015.2856. [DOI] [PubMed] [Google Scholar]
- 10.Qiu MZ, He CY, Lu SX, Guan WL, Wang F, Wang XJ, Jin Y, Wang FH, Li YH. Prospective observation: clinical utility of plasma Epstein-Barr virus DNA load in EBV-associated gastric carcinoma patients. Int J Cancer. 2020;146:272–280. doi: 10.1002/ijc.32490. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Liu YQ, Wang XH, Ji K, Li ZW, Bai J, Yang AR, Hu Y, Han HB, Li ZY, Bu ZD, Wu XJ, Zhang LH, Ji JF. Clinicopathological and molecular characteristics of Epstein-Barr virus associated gastric cancer: a single center large sample case investigation. Beijing Da Xue Xue Bao Yi Xue Ban. 2019;51:451–458. doi: 10.19723/j.issn.1671-167X.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang SC, Ng KF, Yeh TS, Cheng CT, Lin JS, Liu YJ, Chuang HC, Chen TC. Subtraction of Epstein-Barr virus and microsatellite instability genotypes from the Lauren histotypes: combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1,248 cases. Int J Cancer. 2019;145:3218–3230. doi: 10.1002/ijc.32215. [DOI] [PubMed] [Google Scholar]
- 13.Panda A, Mehnert JM, Hirshfield KM, Riedlinger G, Damare S, Saunders T, Kane M, Sokol L, Stein MN, Poplin E, Rodriguez-Rodriguez L, Silk AW, Aisner J, Chan N, Malhotra J, Frankel M, Kaufman HL, Ali S, Ross JS, White EP, Bhanot G, Ganesan S. Immune activation and benefit from avelumab in EBV-positive gastric cancer. J Natl Cancer Inst. 2018;110:316–320. doi: 10.1093/jnci/djx213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, Lee S, Park SH, Park JO, Park YS, Lim HY, Lee H, Choi M, Talasaz A, Kang PS, Cheng J, Loboda A, Lee J, Kang WK. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449–1458. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 15.Qiu MZ, He CY, Yang DJ, Zhou DL, Zhao BW, Wang XJ, Yang LQ, Lu SX, Wang FH, Xu RH. Observational cohort study of clinical outcome in Epstein-Barr virus associated gastric cancer patients. Ther Adv Med Oncol. 2020;12:1758835920937434. doi: 10.1177/1758835920937434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie T, Liu Y, Zhang Z, Zhang X, Gong J, Qi C, Li J, Shen L, Peng Z. Positive status of Epstein-Barr virus as a biomarker for gastric cancer immunotherapy: a prospective observational study. J Immunother. 2020;43:139–144. doi: 10.1097/CJI.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Büttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, Dietel M, Longshore JW, López-Ríos F, Penault-Llorca F, Viale G, Wotherspoon AC, Kerr KM, Tsao MS. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J. Clin. Oncol. 2017;35:3867–3876. doi: 10.1200/JCO.2017.74.7642. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Zhou Y, Chen Y, Gu J. Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. doi: 10.1038/ng.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, Cunningham F. The ensembl variant effect predictor. Genome Biol. 2016;17:122. doi: 10.1186/s13059-016-0974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewitt LC, Inam IZ, Saito Y, Yoshikawa T, Quaas A, Hoelscher A, Bollschweiler E, Fazzi GE, Melotte V, Langley RE, Nankivell M, Cunningham D, Allum W, Hutchins GG, Grabsch HI. Epstein-Barr virus and mismatch repair deficiency status differ between oesophageal and gastric cancer: a large multi-centre study. Eur J Cancer. 2018;94:104–114. doi: 10.1016/j.ejca.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He CY, Qiu MZ, Yang XH, Zhou DL, Ma JJ, Long YK, Ye ZL, Xu BH, Zhao Q, Jin Y, Lu SX, Wang ZQ, Guan WL, Zhao BW, Zhou ZW, Shao JY, Xu RH. Classification of gastric cancer by EBV status combined with molecular profiling predicts patient prognosis. Clin Transl Med. 2020;10:353–362. doi: 10.1002/ctm2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717–726. doi: 10.1016/S1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 27.Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, Fornaro L, Olesinski T, Caglevic C, Chung HC, Muro K, Goekkurt E, Mansoor W, McDermott RS, Shacham-Shmueli E, Chen X, Mayo C, Kang SP, Ohtsu A, Fuchs CS. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 28.Tabernero J, Van Cutsem E, Bang Y, Fuchs C, Wyrwicz L, Lee K, Kudaba I, Garrido M, Chung H, Castro Salguero H, Mansoor W, Braghiroli M, Goekkurt E, Chao J, Wainberg Z, Kher U, Shah S, Kang S, Shitara K. Pembrolizumab with or without chemotherapy versus chemotherapy for first-line treatment of advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: the phase 3 KEYNOTE-062 study. Ann Oncol. 2019;30(Suppl 4):iv152–iv153. [Google Scholar]
- 29.Nakano H, Saito M, Nakajima S, Saito K, Nakayama Y, Kase K, Yamada L, Kanke Y, Hanayama H, Onozawa H, Okayama H, Fujita S, Sakamoto W, Saze Z, Momma T, Mimura K, Ohki S, Goto A, Kono K. PD-L1 overexpression in EBV-positive gastric cancer is caused by unique genomic or epigenomic mechanisms. Sci Rep. 2021;11:1982. doi: 10.1038/s41598-021-81667-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satoh T, Kang YK, Chao Y, Ryu MH, Kato K, Cheol Chung H, Chen JS, Muro K, Ki Kang W, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Tanimoto M, Chen LT, Boku N. Exploratory subgroup analysis of patients with prior trastuzumab use in the ATTRACTION-2 trial: a randomized phase III clinical trial investigating the efficacy and safety of nivolumab in patients with advanced gastric/gastroesophageal junction cancer. Gastric Cancer. 2020;23:143–153. doi: 10.1007/s10120-019-00970-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K, Yu J, Sung JJ, Herrera-Goepfert R, Meneses-Gonzalez F, Kijima Y, Natsugoe S, Liao LM, Lissowska J, Kim S, Hu N, Gonzalez CA, Yatabe Y, Koriyama C, Hewitt SM, Akiba S, Gulley ML, Taylor PR, Rabkin CS. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014;63:236–243. doi: 10.1136/gutjnl-2013-304531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.