Abstract
Worldwide, tumors are one of the most common causes of death. Every year 3.7 million new cases occur in Europe and more than 1.9 million patients die (WHO data). Most of the fields of research are focused on developing new therapeutic strategies that will be effective in eliminating the tumor, preventing its remission, and avoiding or reducing the side effects of therapy. In the past, generally classical 2D cell cultures or immunodeficient animal models had been used to cultivate and test drugs on human cancer cell lines. Nowadays, there are increasing interests in three-dimensional (3D) cell cultures, a method with significant differences from flat cultured cells, both considering gene expressions and cell-cell interactions. Various evidence suggests that high tumorigenic properties might be dependent on the occurrence of a small cell population, pointed out to be responsible for metastasis and recurrence. This population is called cancer stem cells (CSCs), hinted to have a lot of similarities with normal stem cells. CSCs are the main reason for chemotherapy failure as well as multi-drug resistance (MDR). CSCs can also interact through the cytokine network, with other cells like the macrophages of the inflammatory system. The big advantage of a 3D culture is the possibility to isolate and investigate the CSCs population surrounded by its environment. This article aims to sum up known 3D cell cultures, especially in the field of CSCs research due to the importance of the tumor’s environment on stem cell’s markers expression and their development.
Keywords: Cancer stem cells (CSC), 3D cell cultures, anticancer drug discovery, cancer organoids, ultra-low attachment plates (ULA), hanging drop (HD), matrix, micro fluidics, lab-on-a-chip, solid-phase spheroids
Hypothesis and characteristics of CSCs
Despite many years of research, the answer to what leads to cancer recurrences and what is the cause of metastasis remains unsolved. Tumors can be described as inhomogeneous and molecularly complex structures. While some cancerous cells die after chemo- or radiotherapy, some of the tumor cells’ mass will survive and disseminate as metastases [1,2]. These facts are the basis of the assumption that some part of the tumor mass is constituted by cancer-initiating cells called cancer stem cells (CSCs). According to CSCs hypothesis, they are closely related to normal stem cells (SC) as they have similar features, such as the ability to self-renew and differentiate into other cell lines to regulate tissue functions [3]. Moreover, the CSCs constitute a specific population of cells that cumulate genetic mutations with divergent carcinogenic activity. That means that CSCs can generate new tumors after transplantation into an animal organism (this does not apply to regular stem cells) [1,4].
CSCs plasticity
Multilineage differentiation capacity of CSCs gives rise to the phenotypic and functional heterogeneity in cancer cells within the same tumor. However, the newer current dynamic CSC models assume a highly plastic behaviour of CSCs [5,6]. The plasticity is defined as a process by which cancer cells gain the dynamic ability to switch from non-cancer stem cells (non-CSCs) to CSCs and vice versa [5]. That means that any differentiated cancer cell can be reprogrammed to become CSCs through dedifferentiation and reacquiring of self-renewal capacity; on the other hand, each CSC has the ability to give rise to the differentiated tumor cells [6]. Thus, the plasticity of CSCs also contributes to the heterogeneity observed in cancer [5]. This conversion of CSCs into differentiated non-CSCs depends on several exogenous and endogenous factors such as cellular interactions, specific microenvironmental signals, exosomes, tumor-stroma interactions, the composition of the extracellular matrix, gene expression. These factors vary upon time and space in tumor progression which might explain the variation in the frequency of CSCs in different stages of diseases and various types of cancer. In addition, the rate of conversion of CSCs to non-CSCs influences tumor behavior, i.e. the lower the differentiation tendency, the greater the CSCs frequency within a tumor and the more aggressive cancer with higher capacity to metastasis and the resistance to chemotherapy [5,6].
CSCs in the same type of cancer are phenotypically and functionally heterogeneous [6-8]. CSCs heterogeneity was found in many neoplasms like breast, colon, brain, prostate, lung, and many other organs (liver, pancreas, kidney, bladder, ovary) tumors/cancers [6,7]. The most ‘primitive’ slow-cycling CSCs (quiescent CSCs) develop into more mature tumor progenitors (proliferative CSCs), which in turn develop into much less tumorigenic cells (differentiated CSCs) or undergo trans-differentiation into cells of different lineages (non-CSCs) [6,7,9].
CSCs metastatic ability
CSCs are able to detach from the primary tumor site and spread to distant tissues and organs where they can proliferate and give rise to secondary tumors. This happens when CSCs undergo epithelial to mesenchymal transition (EMT) that promotes the invasion of tumor cells [10]. CSCs that exhibit EMT characteristics remain after anticancer treatment, leading to cancer recurrence and drug resistance. The EMT displays a huge role in migration, alteration of the extracellular matrix, and apoptosis, and drives CSCs plasticity contributing to intra-tumor heterogeneity [8].
CSCs, cancer resistance, and recurrence
It is well documented that CSCs are highly resistant to chemotherapeutic agents, radiation, and cell death which is the main cause of the ineffectiveness of classical anticancer therapies [4]. The frequency of CSCs in tumors increases after anticancer treatment confirming their persistence during such therapy [10,11]. In addition, CSCs can be generated by epigenetic plasticity due to drug-induced dedifferentiation or conversion of non-CSCs to CSCs [4].
The resistance of CSCs is a very complex, multifactorial, inherent characteristic of these cells and is a result from radiation and chemotherapy. Various internal and external factors are involved in this phenomenon. For instance, CSCs represent slow-cycling cell population equipped with protective autophagy mechanisms that maintain their stemness, resistance, and low concentrations of reactive oxygen species ROS [12]. CSC resistance is also defined by high expression of multidrug resistance proteins (P-gp, BCRP, MRPs), efficient DNA repair systems, enhanced ROS scavenging capacity, and upregulation of anti-apoptotic proteins (Bcl-2, Bcl-X, c-FLIP). Elevated activity of detoxifying [13] signaling pathway components are implicated in the resistance of CSCs, including NOTCH, Wnt/β-catenin, Hedgehog, transforming growth factor-β (TGF-β), phosphoinositide 3-kinase PI3K/AKT, STAT, BMP, Bmi) and transcription nuclear factor-κB (NF-κB) [4,12,14].
Besides, in tumors CSCs reside in peculiar niches that provide specific microenvironments (CSCs-ME) protecting them against cell death and cancer therapy [4]. CSCs-ME is characterized by a complex network of autocrine and paracrine cross-communications involving activated fibroblasts, endothelial cells, macrophages, immune cells, and adipocytes. In addition, local and biochemical factors such as hypoxia, cytokines, exosomes, and extracellular matrix components play a role in a specific characteristic of the CSCs niche [15,16]. Mutual communication between CSC and surrounding niche involve adaptation of CSCs to the environmental changes. The CSCs themselves can also modulate their niche. In turn, the CSCs niche plays a huge role in CSCs behavior by enhancing stemness, self-renewal, invasion capacity, metastasis, and drug resistance [8,15,16]. Moreover, the CSCs-ME can revert non-CSCs into CSCs by among others EMT-associated processes, increasing tumor invasiveness and metastasis [8].
Conventional anticancer therapies target rapidly proliferating cancer cells, but cannot prevent resistance, metastasis, and tumor relapse due to the presence of CSCs. It has been postulated that complete eradication of malignancy can only be achieved by targeting CSCs [15]. However, a dynamic interchange between CSCs and non-CSCs populations as well as CSCs and CSCs-ME suggests that therapies that are only active against CSCs may result in cancer recurrence or resistance, in part because the residual differentiated cancer cells could repopulate the niche of CSCs [8].
Properties of CSCs-novel target therapies quest
The research on CSCs is not easy, mainly because of the difficulty of identification and determination of its origin. The first proof of CSCs presence was the discovery of CD34+/CD128- cells that initiate cancer in acute myeloid leukemia. Subsequent research showed the same trend for different solid tumors and other blood cancers. It was demonstrated that CSCs in hepatocellular carcinoma (HCC) originate from liver progenitor cells (LPC), and thus CSCs might be generated by differentiated mature liver cells under the influence of genetic and/or epigenetic alterations [17]. Those ambiguities indicate that research into the origin of CSCs needs to be pursued.
In case of brain tumors, CSCs exhibit the same markers as physiological stem cells (SCs). However, this is not a general rule and not all tumors are characterized by this dependency. In some cases, CSCs show different, genuine markers not found on the surface of the corresponding SCs. For example on colon cells, a marker like Dclk1, present on differentiated cells, will be lost within two weeks. On the other hand, the same marker Dclk1 appears on CSCs of adenomas and intestinal polyps signifying rapidly proliferating cells with tumor growth stimulation [18]. While surface markers analysis is not an ideal method for identifying and isolating CSCs, many studies of novel target therapies, capable of inhibiting the expansion of chemoresistant CSCs, use specific patterns of surface markers that identify these cells. Of equal importance is research focused on specific proteins and RNA, responsible for the development and functions of CSCs [17].
It is generally admitted that the reappearance of a tumor is the result of CSCs. One of the mechanisms responsible for the migration ability and increased drug resistance is the epithelial-mesenchymal transition (EMT). Some reports, focused on breast cancer and non-small cell lung cancer, showed that abnormal activation of the TGF-β pathway mediates tumor metastasis and recurrence, and is responsible for promoting EMT. Current studies on TGF-β pathway inhibitors are designed to reveal its role in the mediation of the EMT process [18].
Seeking CSCs and studying the derivation should not be the only goal. More and more reports indicate that it is necessary to study the microenvironment and thus the signaling pathways for elaborating new therapies. Both are responsible for regulating cells’ growth and proliferative potential. Several key pathways such as Wnt/β-catenin, Notch, and STAT3 affect CSCs characteristics [17,19]. Furthermore, the oxygen concentration within the tumors also influences the properties and growth of CSCs. It has been shown that the hypoxic environment stimulates CSCs and that hypoxia increases the cancerous potential of cells. However, both hypoxia and re-oxidation seem to be essential for CSCs growth and the ability to create new tumor outbreaks. Factors induced by hypoxia, like HIF 1a and HIF 2a expressed on glioblastoma CSCs, are correlated with poor prognosis in brain tumors; additionally, their decrease reduces cell self-renewal [1]. The use of an epigenetic modification of gene expression through histone modification and methylation seems to be promising. Moreover, in various cancers the CSCs express immune markers and display specific immune characteristics. These properties can be used in the development of immunotherapies to target CSC in the tumor microenvironment. Recent research has focused on developing a vaccine for immunotherapy and CSCs stimulation to enter the resting state of the cell cycle. Various reports have also shown that some hormones, e.g. thyroid hormones and dopamine, affect CSCs. In case of hepatocellular carcinoma, thyroid hormone increases the amount of CD90+ CSC, promoting CSCs auto-regeneration and carcinogenicity [17].
The importance of 3D culture to study CSCs properties
Today the superiority of 3D cell cultures over traditional 2D cell cultures is well established. First of all, 3D systems reflect the tumor microenvironment and cell-cell interactions much more accurately than cells growing in a single layer in plastic flasks. Moreover, there is a big difference between the two systems in response to an in vitro drug. Nowadays, searching for CSCs generally relies on surface markers monitored by flow cytometry. CSCs typically exhibit a high expression of CD44+, although each tumor has a slightly different composition of cell surface antigenic CSCs [20]. In colorectal cancer, various markers have been proposed for CSCs identification, including CD166, CD133, CD44, EpCAM, CD29, CD24, and the Lgr5 protein [21]. In ovarian cancer, CSCs shows high expression of markers such as CD133, CD44, ALDHhigh, Lgr5 and form side population cells (SP) in flow cytometric analysis [18]. Melissaridou et al. monitored the expression of some antigens (CD44, SOX2, and NANOG) and genes (CDH1, CDH2, VIM, FN1, TWIST, FOXC2) on head and neck cancer cells [20]. After 7 days of spheroid growth, the increased expression of CDH1 mRNA was observed when compared to 2D culture. The CDH1 gene codes for E-cadherin [22], the protein playing an important role in cell adhesion, and thus forming organized tissues, transmitting chemical signals within cells, controlling cell maturation and movement, and regulating the activity of certain genes. Increased expression of CSCs-related transcription factors such as NANOG and SOX2 was also demonstrated in 3D cultured cells compared to cells cultured by 2D methods. Moreover, cells cultured in spheroids showed greater viability and resistance to increased doses of cisplatin or cetuximab [20]. Thus, the doses of drugs used in 2D culture do not translate into a 3D culture. Therefore, the first stages of research on modern anticancer drugs should be carried out in three-dimensional, rather than on two-dimensional, cultures as they better reflect the tumor environment.
In addition to surface markers, it has been suggested to use intracellular markers involved in epithelial development, e.g. Wnt signaling cascades [23]. One of the most important markers is ß-catenin, which plays a crucial role in homeostatic intestinal renewal and carcinogenesis. It was noted that the analysis of CSCs proteins and metabolic pathways is important in the development of modern therapies. They are significant in acquiring resistance to treatment and stimulating tumor cell migration [21]. The study of the metastatic potential of the CSCs highlighted the importance of factors other than markers. Louie et al. evaluated the effects of hypoxia and re-oxygenation on breast cancer cell lines MDA-MB 231 and BCM2 [24]. The research consisted of subjecting the cells to hypoxia and re-oxygenation several times, and then selecting both adherent and floating surviving cell populations, which tended to form spheres. The latter cells were injected into immunosuppressed mice. The study showed that the non-adherent cells subjected to hypoxia and re-oxidation were highly carcinogenic compared to the adherent cells. Cells undergoing hypoxia cycles were also shown to have a slightly different phenotype. New cells showed EMT features, e.g. had an increased percentage of cells not expressing cadherin and the population of CD44+/CD24-. EMT is responsible for the increased migration potential of cancer cells. The important features of EMT are loss of E-cadherin and increased expression of fibronectin and vimentin [24]. Thus, it is extremely important to analyze the complex processes influencing the development of CSCs, including their environment. This is only possible in 3D cultures [25]. A nucleus composed of cells dying due to lack of oxygen and nutrients is a potential area for CSCs growth in developed spheroids as only CSCs can survive in such conditions. Subjecting cells to hypoxia and re-oxygenation cycles can also be used to select CSCs cells [24].
Methods used to engineer 3D cell cultures
Creating 3D cultures is becoming more common in cancer research. Different 3D culture systems are developed to achieve better and more accurate methods of recreating a microenvironment as similar as possible to the natural tumor surroundings. Reduced costs of cultivating cells, better production, growth, and later visualization are goals to be achieved. In 2D, the contact with the surface of a plastic bottle might change cellular morphology and have an impact on distribution of oxygen and nutrients. In contrast to 2D methods, where cells cover the flat bottom, in 3D methods cell cultures form complex structures such as organoids or spheroids. Generally, in vitro 3D culture techniques are categorized into: 1. anchorage-independent systems (non-scaffold based) ex. hanging drop, low attachment plate; 2. anchorage dependent (scaffold-based) ex. hydrogels; and 3. specialized 3D culture platforms ex. microfluidic devices, solid-phase spheroids [26,27]. In addition, in vivo or ex vivo models can be distinguished. An example of in vivo model is patient-derived xenografts (PDX). Breifly, patient tumor fragment is implemented into the immunodeficient mouse, which allows to preserve tumor morphology. Patient-derived explants (PDEs) is a known ex vivo study model, where the excised tumor is immediately used to test targeted therapy. Both methods are appreciated due to the possibility of preserving tumor architecture, chromosomal instability and resistance to treatment [28-30]. As in 2D cultures, it is possible to co-cultivate different cell phenotypes, like tumor cells and fibroblasts. The 3D co-cultures allows direct contact between cells (cell-cell intereaction), by secretion of signaling mediators, which at least partially reflect the multiple and simultaneous interactions that occur in the tumor microenvironment. Moreover, 3D cultures reflect properties of solid tumors with a close dependency of cancer cells to epithelial tissue, i.e. circulation of nutrients. This provides, as for in vivo tumors, a cell model with a necrotic core formed at the center of the tumor where the cells grow under unfavorable conditions, such as hypoxia and low concentration of nutrients. In such suboptimal environmental conditions reside CSCs [26,27]. Such a 3D microenvironment facilitates the study of the function, behavior, morphology or gene expression of CSCs, and cellular responses to the drug treatment in a manner more representative of in vivo conditions. It also allows the search for new drugs that could be effective in combating CSC and chemoresistant cells.
In vitro generated cancer organoids
Creating organoids is one of the established methods to explore cancer environment and help to generate a similar environment as close as possible to in vivo tumors. Organoids are 3D structures composed of cells from a given tumor or tissue. The sample is collected from a patient or animal and after proper dissolution can be seated on a Matrigel in presence of media that contain specific growth factors. As a result after few days, cells form organoids [31-33]. Several studies indicated that different organoids (like murine bladder organoids, lung cancer organoids) can be passaged weekly for a long period without any karyotypic and spherical morphology changes. Because organoids are made from different cell types and the presence of stem cells is a hallmark of these 3D structures, it might be one of the best tools to investigate CSCs forming tumor mass. Additionally, patient organoids are a good platform to investigate the properties of CSCs and cellular heterogeneity of tumors [34].
H. Zhao et al. created organoids from a patient with oral squamous cell carcinoma (OSCC) [35]. The HE staining showed that OSCC organoids reflect the structure of the primary cancer sample. Besides, the immunofluorescence staining of CD133 (a marker of OSCC CSCs) and C18 (a marker of differentiated cells) confirmed that in both cases expressions of these markers were similar. That might suggest, that a patient-derived organoid can capture the cellular heterogeneity. The same study pointed out the importance of the tumor environment. Lactate, which is aimed to be a waste product, may promote the CSCs. The administration of lactate was correlated with organoids formation, higher Wnt activity (pathway correlated with tumor-initiating capacity), and the CD133+ cells concentration. While Wnt pathway activity and CD133 protein expression were lower for the control group (with PBS) and group treated with lactate in addition to α-cyano-4-hydroxycinnamate (CHC-an inhibitor of lactate uptake), comparing to organoids treated with lactate [35]. Organoids of human urothelium carcinoma were successfully established and provided some interesting information. Hematoxylin-eosin staining indicated that some organoids grew as a solid tumor structure, while organoids from different patients contained lumen inside. There was a difference between the expression of CK20+ (luminal) cells as well. So in fact, the researchers created two subtypes-luminal and basal bladder cancer. Interestingly, two organoids lines from a tumor from one patient, expressed different markers (only one contained Ck20+ cells) [34].
The research on organoids from dog urine samples was carried out to study prostate cancer [36]. Organoids contained epithelial cells, leucocytes, cancer stem cells, myofibroblasts, and basal cells, all of which are also proposed to be responsible for organoids formation and tumor growth. The study showed that organoids formed from basal cells grew faster than the luminal cells organoids. This might be a sign that in case of prostate cancer basal cells might pose a reservoir of a cell origin and cancer progression. Previously proposed markers of stem cells (CD44, CD133, and active aldehyde dehydrogenase (ALDH)) expressions were investigated as an association with tumor formation and progression. Expression of the studied markers was higher in organoids than in urine cells. CD44 positive cells constituted the biggest group of stem cell markers groups. Also the expression of CD44 was higher in urine samples from PC dogs than in normal cells. This might suggested that CD44 positive cells may be responsible for regulation and initiation of a dog’s prostate cancer [36].
All reports indicate that organoids may be a good 3D culturing technique to obtain and study CSCs, tumor variability, and the influence of tumor environment. One of the advances is undoubtedly the possibility to create personalized therapy as organoids are derived from parental tissue. Besides, it is a good platform to investigate drug activity and toxicity. The limitation of organoids is lack of some tumor surrounding cells, like muscle cells, immune cells or nerve cells. Initially, it is necessary to optimize the ratio of existing cells to induce appropriate functions of the organoid. The contamination of normal epithelial cells may reflect the organoid growth rate. In addition, the presence of small inhibitors of some of the pathways found in the culture medium may affect the expression of certain genes and drug sensitivity [29,31].
Single cell-derived spheroids
Although the 3D cultures method is now overflowing with different techniques and it might be relatively cheap and efficient to study tumor properties, some limitations do not allow to study the CSCs characteristics accurately. Because of the tumor heterogeneity and very little percentage of tumor cells able to initiate tumor growth and self-renewal, the acquisition of CSCs from traditional spheroid forming methods and 2D cultures is difficult. Single cell-derived spheroids (SCDS) are a good platform to investigate CSCs, by observing the expression of important genes, the surface markers expression, and the multilineage potential to differentiate into a variety of cancer cells. Different subpopulations of CSCs might show various responses for some inducers and exhibit different proliferative potential [37].
A study on a lung cancer CSCs provided that there are at least two types of CSCs-symmetric division for self-renewal and asymmetric division for differentiation. Both were able to be stably passaged, demonstrated stem cell markers, and had the ability to initiate tumor growth and form spheroids. While culturing in traditional spheroids (aggregation spheroid cultures) it was not possible to obtain a pure CSCs population. However, single cell-derived spheroids allow to distinguish two different subpopulations of Lewis lung cancer CSCs and demonstrate variant characteristics [38]. Thanks to FACS or the limiting dilution protocol, it is possible to obtain one cell type of spheroid to investigate exact population behavior under different microenvironment conditions. The study on gastric cancer cells pointed out SCDS spheroids had round and clear boundaries morphology, in contrast to irregularly shaped aggregated spheroids. Besides, SCDS were highly adherent and compact (the distinction of an individual cell was difficult), while traditional spheroids made agglomerates and their self-renewal ability was decreased. For SCDS, the expressions of main transcription factors, like SOX2 and NANOG, and gastric CSCs markers CD44 and CD54 were up-regulated comparing to parental cells (2D cell line culture) and tumor spheroids. In conclusion, the SCDS better reflect CSCs characteristic. The same study indicated that SCDC were more resistant to apoptosis and necrosis, because only 25% of the cells in the spheroid were apoptotic/necrotic, while 40% of the cells from tumor spheroids expressed cell death markers [39]. That confirms the CSCs feature-resistance to apoptosis.
One of the important investigated aspects is the question of whether the CSCs can generate different cancer cell types under different conditions. The multilineage potential can be examined on SCDS in properly selected microenvironment conditions. Induction with various conditioned culture media allowed to transform specific markers negative cells into positive cells after differentiation. And this way, in the study of H. Liu et al., previously PAS (a marker of prostate cancer) and CD10 (a marker of lymphoma) negative T3A-A3 cells have changed and expressed a given marker, while treated with an appropriate tissue-derived conditioned medium. This finding was confirmed with other CSCs clone experiments [37]. For high-throughput experiments, the microfluid chips or special PDMS microplate provide significant efficiency, easy medium exchange, and similar size spheroids [39,40].
Hanging drop
Hanging drop (HD) is a scaffold-free technique. Creating spheroids inside hanging drop is known as one of the easiest methods to cultivate cells in 3D. A technology generated by droplets of cell suspension dispensed onto the underside of a petri dish lid from which they hang due to surface tension and gravitation. The cell suspension is contained in a liquid drop hanging onto/from a plate covered by amphiphilic surfactant coating [26,41]. For this method, special welled plates have to be used with a hole at the bottom of the upper well and the lower well filled up with water to avoid drying. The cells in suspension spontaneously aggregate into spheroids under gravity (Figure 1). For some of the brands of plates, a cell suspension on the cover lead should be applied, which, after inverting the plate, will create a hanging drop supported by adhesive forces. Due to gravitation, cells are dropping to the bottom of the drop and form spheroids at the water-air interface [41]. It is essential to protect the plate from drying out by ensuring adequate humidity. Usually, the droplets formed are in a volume of 20 µl (never exceeding 50 µl). It is possible to suspend a small number of cells, what can be useful for growing CSCs cells as they remain a small percentage of tumor cells [26,41,42].
Figure 1.
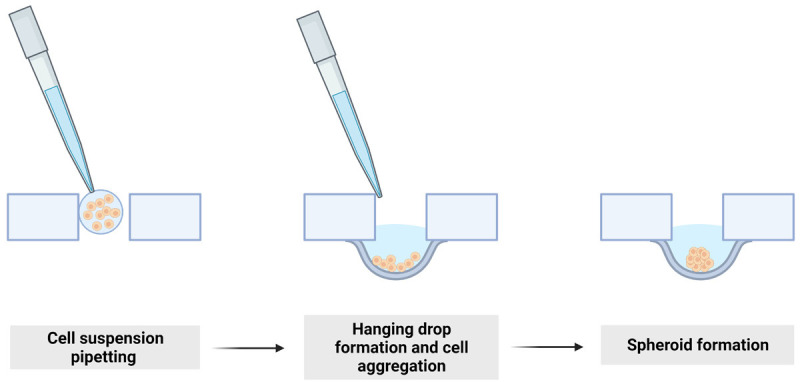
Hanging drop-spheroid formation. Small drops hang thanks to surface tension and gravity. On the bottom of the hanging drop single cells aggregate and create spheroid after few days of cultivating.
Through hanging drops, spheroids of set dimensions can be created in a series of experiments, thus obtaining reliable results. In addition, repeatability is achieved owing to other characteristics, the culture conditions for creating drops are a scaffold-free technique with no support system or matrix [42]. The hanging drop 3D system can be used for drug discovery, CSCs studies, and even to study angiogenesis of co-cultures of two phenotypes [26,43]. Some companies are fully specialized in such systems of spheroid production, e.g. 3D Biomatrix and InSphero [41]. Despite the apparent advantages of hanging drops, many limitations have prevented their widespread use. First of all, the small droplet volume is an obstacle for testing a large number of putative drugs as the technique is relatively laborious and time-consuming. This is due to manual pipetting, subsequent collection of spheroids, and/or media changes [41,42].
Aggregation in U or V bottomed ultra-low attachment plates
3D cell culture methods include scaffold-free techniques and scaffold-based techniques. Aggregation in U or V bottomed ultra-low attachment plates (ULA) is the forced-floating method, carried out using uncoated plates or plates coated with a hydrophilic polymer. Spheroids are self-assembled, created under appropriate conditions with the ability to be cultivated as a free-floating culture. This method is suitable for high-throughput screening because of automated research. The fact that no scaffold could interact with cells and change their characteristics and ability to grow is one of the main advantages [44,45].
Spheroids can be formed from as little as 500 to 20,000 cells. Their detection is possible within a few days, however, the formation of a spheroid is noticeable after 24 hours [44,46]. Any contact of the pipette with the bottom of the plate is not recommended as damage to the plate surface distorts the spheroids. The cultured spheroids are homogeneous and show a similar morphology. The size of the colonies is therefore a big advantage compared to other 3D methods. Cells cultured on ultra-low adhesion plates with a U-shaped bottom can be used for tumor cell migration and invasion assays, and immunohistochemical staining [47]. In the study of Vinci et al., spheroids obtained from ULA plates were transferred into a gelatin-coated flat-bottom 96-well plate to carry out the high-throughput migration assay. After a few hours diffusion of a single cells forming a spheroid structure was observed. The 72-hour record allowed for evaluation of tumor cells migration efficiency and the difference between migration trend (dependent on cell culture line)-more rapid and ameboid in contrast to slower and collective. To generate informative data, the inverted microscope and the proper software can be used to estimate cell migration. Likewise, the invasion assay can be accomplished. After four days of sphere cultivation in ULA, the Matrigel was added to the wells. It is a kind of matrix equivalent, so cell movement and local proteolysis may be observed. The invasion is accomplished after 72 hours and monitored every few hours. The images showing cell invasion were acquired thanks to a Colegio cytometer or inverted microscope [47]. In addition, this method enables to observe the development of spheroid formations using transmitted light imaging platforms. Many automated platforms and software are available to analyze results [46].
Comparative studies of tumor spheroid generation techniques revealed higher chemoresistance to cisplatin of cells in ULA and hanging drop (HD) plates compared to 2D methods. The spheroids generated using the ULA and HD plates presented a round-type morphology that is related to strong cell-cell adhesion. However, the size was different and spheres were bigger when grown on ULA, what might indicate that HD promotes higher cellular aggregation. The circularity of spheroids was similar in both methods. The differences in the colony sizes were most noticeable after 3rd day of cultivating. However, ULA was demonstrated to be more robust and reliable than the HD method, and therefore, can be considered as the most suitable and straightforward method to generate spheroids for cytotoxicity assays [48].
The CSCs analysis is also possible using the ULA plates. The tumor-initiating capacity was verified by in vivo serial dilution tumorigenesis assay, as a golden standard of evaluating CSCs properties. Only 500 sphere cells were able to induce the forming of a tumor after transferring to NOD/SCID mice, comparing to parenting cells where 105 cells were not able to initiate tumor in immunodeficient mice. Injected 1000 cells (from spheroid) were sufficient to develop tumors after less than two weeks (shorter time of tumor formation). These data confirm that hepatocellular carcinoma (HCC) sphere cells have an efficient tumor-initiating capacity. Besides, the colony-forming assay pointed out that sphere-forming cells proliferated significantly faster and formed bigger colonies than parental cells after 3 weeks of culture. Similar tests were performed with the patient-derived spheroids and similar results were obtained. It was found that tissues from patients with larger tumors, multiple lesions, satellite lesions, or advanced tumor stage had more efficient sphere-forming capacity under serum-free conditions [49].
Granger et al. investigated the property of proline rich polypeptide 1 (PRP-1) in the chondrosarcoma therapy. Previously, the immunomodulatory effect on the cancer cells have been confirmed in the monolayer. PRP-1 treatment of ULA optained chondrosarcoma spheroids confirmed its ability to target CSCs together with the capacity to penetrate the ouer proliferaive layer. Besides, the study confirmed that higher dose of PRP-1 was needed to reduce maximum spheroid growth, which might be because of the dens structure of spheroid [50].
Embedding in a matrix: hydrogels or polymers
For 3D cultures, polymers are another choice in the search for new, low-cost, and efficient solutions. Like other solutions, they provide a microenvironment to the cell in which cell-cell interactions are possible. Currently, various substances, both natural and synthetic, are used for matrix formulation. Attempts are being made to develop the best biopolymers and to miniaturize the system that would enable more efficient, stable, and reproducible conditions [51].
Biopolymers are known to be stable, but also biodegradable, of suitable pore size, and non-immunogenic. Modern materials and synthetic modifications of known biopolymers are being developed to obtain the best results. Every modification of the properties of the polymers affects the cultured cells and the expression of their genes. However, refinement is still sought to obtain an interior environment as close to in vivo as possible [52]. It has been noted that alginate hydrogels encapsulating the human cancer cells significantly reduce the time needed to create CSCs-containing spheroids. Thus spheroids cultured for only two days showed higher expression of stem cell surface markers and increased cell pluripotency than spheroids grown without those biomaterials [53]. Other studies confirmed an increase in the expression of markers and genes characteristic of the epithelial-mesenchymal transition (EMT). However, the presence of these markers and CSCs markers seem to be strongly dependent on the stiffness of the drop. Experiments conducted with alginate beads of varying gel stiffness show that the most efficient in spheroid formation are those with a moderate gel stiffness [54,55]. These reports are the kernel of developing the right material with specific stiffness and pore size ideal for test standardization. Unquestionably, hydrogels appear to be among the best solutions for the future of 3D culture with the widespread use of large-scale, thanks to miniaturization systems.
Microfluidics, lab-on-a-chip
The greatest benefit of developing microfluidic devices is the ability to create and control a specific micro-environment for cell growth, including its in vivo environments such as adhesion molecules and extracellular matrix (ECM). The creation of 3D culture using microfluids is possible through the use of devices named “lab-on-a-chip”: computer-controlled valves in an integrated circuit, pumps, and analytical systems, allowing programming of any automated media flow. In the center of the culture plate is the site of cell adhesion, growth, and proliferation (culture microcell). During culture formation cells are introduced through dedicated cavities, intended exclusively for the addition of cells. From both sides along the growth zone and through separate cavities flows a continuous sheath of medium (Figure 2). This method is based on a continuous flow of media thanks to a microchannel network, providing the cell with nutrients and oxygen, while efficiently draining unnecessary metabolites [56].
Figure 2.
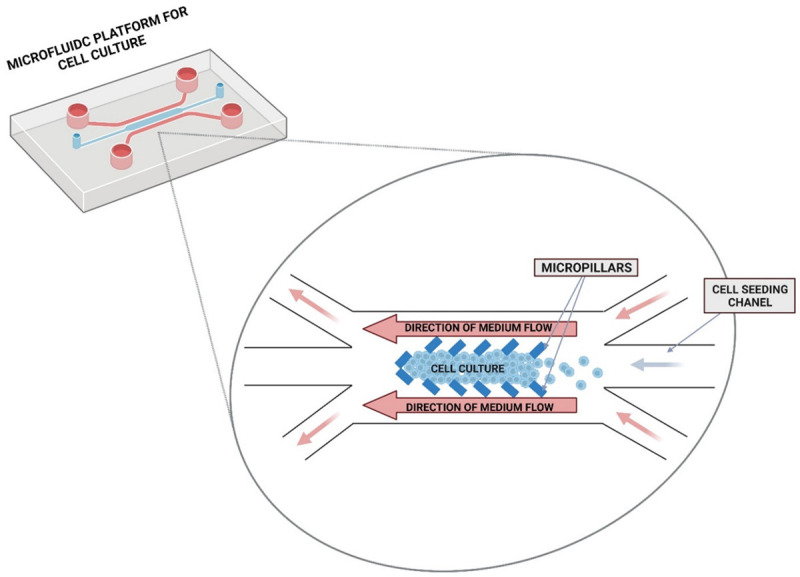
Scheme of microfluidics cell culture. Culture cells are kept inside the microchip cell chamber by micropillars. Computer controlled medium flow allows to provide nutrients and waste products removal.
Transparent microfluidic platforms are compatible with in situ optical monitoring by fluorescent reporters followed by high-resolution imaging, such as confocal microscopy, to study events resulting from cell responses [57]. Such a system can also be used for 2D cell cultures, however the biological relevance of microfluidic 3D cell culture systems offers more applications in cell-based research. There are many modifications to this method available, for example, microflows providing constant diffusion to spheroids.
New micro scaffolds consisting of nanoparticles and biopolymers are created to provide the cell with the best diffusion and adhesion [58,59]. Polydimethylsiloxane (PDMS) deserves a special interest among polymeric materials as it has many remarkable properties such as biocompatibility, gas permeability, flexibility, and water impermeability. PDMS is also stable over a wide temperature range and is characterized by extremely high hydrophobicity, which excludes its use as a surface intended for the growth of adherent cells [60]. However, it is successfully used for spheroid culture. The most common designs of this type of microchips are based on the use of so-called micropillars. These are three-dimensional microstructures based on an ellipse, rhombus, square, or triangle, arranged and oriented in a microchannel to allow diffusion while retaining cells in the culture microcell. Their diameter is several dozen micrometers, they are arranged so that the cells create 3D aggregates while taking into account their proliferative potential [61]. Micropillars are used in microchips mainly made of PDMS. The main flow microsystem has a microchannel with perfect dimension: length =1 cm, width =600 µm, height =100 µm [59]. Setting the microchips at an angle to the longitudinal axis of the microchannel additionally increases the efficiency of cell immobilization in its central part. The use of cell suspension with a density of fewer than 1.5×106 cells/ml allows cells to be placed side by side to form a monolayer.
In addition to microchips allowing three-dimensional cell cultures, the surface of the microchannel can be modified by microgrooves, thus providing appropriate cell-cell organization. This is very important for the cultivation of muscle (myocardial, skeletal, or smooth muscle) and nerve cells, as they grow parallelly to each other in the human body to form fibers. The presence of microgrooves affects the orientation of cells, which in turn allows to reflect the in vivo conditions of such cell phenotypes more accurately [62].
Another way to obtain 3D cell cultures in microsystems, without requiring the use of complex microchannel geometries, is to use hydrogels. The liquid form of hydrogel turns into a gel by the influence of various external factors. The gel forms a three-dimensional network of fiber with a specific diameter and pore size, structurally similar to the extracellular matrix. Before the gelation, the cell suspension is mixed with liquid hydrogel. Then, the prepared mixture is subjected to a gelling agent [63]. As the result, the liquid hydrogel solidifies with cells inside [64]. In the flow microsystems, hydrogels are often used to obtain 3D cell cultures.
This method is the closest to the physiology of the human body and uses human fibroblasts in co-culture with tested cells [57,65]. Even in 2D cultures, the introduction of fibroblasts causes a phenotypic change of tumor cells. Thus, the creation of a scaffold with surrounding fibroblasts should be studied further [66,67].
These methods require the use of specialized equipment which makes growing cell culture using Microfluidics and Lab-on-chip techniques very costly. The culture provides a relatively small number of cells which is cumbersome if further analysis of protein expression is required, e. g. ELISA or Western-Blot. Additionally, the adhesion of cells to the columns makes it difficult to collect cells for experiments. However, these techniques provide control over the size and parameters of the cell culture, and by establishing the flow rate of the medium, we can accelerate the digestion time of the cell culture [68,69].
Solid-phase spheroids
An example of solid-phase spheroids is liquid marbles, i.e. liquid drops enclosed in a hydrophobic powder. When grown with a medium inside liquid marbles, cells spontaneously form spherical agglomerates (spheroids) with no further need for a supporting scaffold [70,71]. Liquid marbles have been found in nature for centuries, e.g. the first drop of rain after a long drought spontaneously covered with solid soil particles [72]. Another example of natural liquid marbles are aphids and their honeydew drops covered with a wax layer, giving them hydrophobic properties [73].
Preparation of liquid marbles is not very complicated-a small amount of liquid (20 to 300 µl) is pipetted onto a layer of hydrophobic powder that propagates spontaneously at the liquid-air interface (Figure 3). Liquid marbles have the same properties as a liquid drop and also behave as a soft solid [74]. When rolling on a hydrophobic surface, a liquid marble does not lose any of its liquid content. It is possible to flatten a liquid marble on solid surfaces; it can even float on water or other liquid surfaces. Liquid marbles behave like deformable balls in movement.
Figure 3.
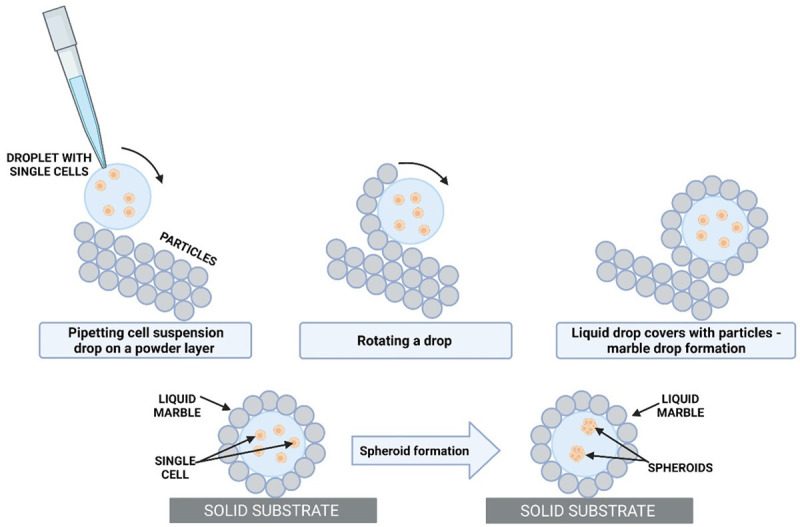
Formation of a spheroid by coating cells suspension in culture medium with a hydrophobic powder. Spheroids form from the cells enclosed in the liquid marble after few days (3-4 days), depends on cell line.
In vivo studies of graphene and its derivatives are very promising in the biological assessment as they show long-term stability in aqueous solution and controlled systemic toxicity. New bifunctional graphene nanoparticles were successfully synthesized in the presence of Herceptin, a natural antibody, using an ultrasound-assisted method. Graphite layers were efficiently created, obtaining excellent stability of the separated layers in the Herceptin solution. The concentration of graphene in the aqueous solution was controlled by changing the content of Herceptin and the time of sonication. The graphene toxicity in 3D spheroid cultures is significantly reduced compared to 2D cultures. In addition, graphene toxicity is dependent on the graphene concentration and significantly decreases when the graphene concentration is below 1 µg/ml. Thanks to this quality, it is used in drug therapy, regenerative medicine, and tissue engineering, including in cell cultures in spheroids [75,76].
Spontaneous spheroid formation techniques are easy to use and relatively inexpensive, allowing high throughput if simple 96-well plates are used. Special adhesive plates are commercially available to help form and maintain the spherical structure. Spheroids can be cultured for long periods and can be recovered after culture. The difficulty of the method lies in the lack of precise control over the size and composition of the resulting spheroids and the exchange of culture medium so as not to damage their structure. Due to the small size of the culture environment, the number of cells inside is greatly reduced. It is also a challenge to determine the correct ratio of two different cell types in spheroids when performing co-cultures [68,69].
Conclusion-challenges for the future of 3D cultures
Cell cultures remain an important element of any research of new drug discovery as well as cell signaling pathways studies. The 2D culture methods used so far have many limitations. As such, the various 3D culture techniques have a good chance of supplanting single-layer cultures. Three-dimensional cultures have many advantages, however still require some improvements (Table 1). Firstly, the cost of such cultures exceeds the cost of traditional ones. Specific scaffolds, hydrogels, plates, or even the appropriate medium used for organoid cultivation significantly increase the final expense. Another problem seems to be the amount of work needed to create such a system. Some methods require manual pipetting of small volumes of fluids. However, improvement of this methods are observed. Nowadays, more and more companies provide modifications, that allow investigators to cultivate spheroids on the high-throughput systems. Supplying growing spheroids with nutrients is an additional challenge, which has been partly solved in the case of microflows cultivation. The later stage of 3D culture research, i.e. their evaluation and imaging, is also becoming important. Flow cytometry, used especially in CSC studies, is associated with the termination of the culture, as it requires enzymatic destruction of the 3D structure. Thus image cytometry is a solution, which now brings the question of accessibility of the markers inside the spheroid structure [77].
Table 1.
Comparison of 3D CSCs culture methods (Advantages and disadvantages)
| Method | Advantages | Disadvantages |
|---|---|---|
| Organoids | • The possibility of creating tumor-specific therapy | • The need to collect material from the patient |
| • They reflect tumor morphology and heterogeneity | • Establishment of specific cell ratio at the beginning | |
| • Relatively simple to maintain | • Selection of appropriate medium with cell growth factors or inhibitors | |
| • Ready-made protocols for various cancer types | • Lack of immune, muscle, nerve, and vascular cells to completely recreate tumor environment | |
| • The ability to derive high-throughput drug testing | ||
| • A good tool to investigate CSCs | ||
| • Organoids bio-bank-currently in development | ||
| Single cell derived spheroids | • Possible to detect CSC and distinguish subpopulations | • The necessity to obtain a single cell type by using FACS or limiting dilution protocol |
| • Possible to study only one type of cell reaction for a specific drug | • The optimal effect, while using advanced technology-microflow technique and special plates | |
| • Thanks to a microchip, the medium flow can be preserved and high-throughput drug screening is possible | ||
| Hanging drop | • One of the easiest method to obtain spheroids | • Might dry if the humidity is not well controlled |
| • Relatively cheap technique | • Time-consuming pipetting and medium change | |
| • The scaffold-free technique allows to avoid some cell-material interactions | • A small size of droplets and a small number of cells can be used | |
| ULA plate | • Ready for automated method and high-throughput research | • Lack of microenvironment reflection |
| • Wide range of cell quantity used for spheroid formation | • Might be difficult in drug distribution and imaging because of high compact spheroid | |
| • Homogenous spheroids with similar morphology | • Possible damage of formed spheroid during medium changing | |
| Hydrogels and polymers | • Opportunity to adjust pore size and stiffness of a drop | • Different results for different hydrogels/polymers |
| • A low-cost and efficient platform for spheroid forming | • Might interact with cells | |
| • Stable and biodegradable form | • CSCs markers expression depends on drop stiffness | |
| • Modification of a hydrogel or polymer composition might change gene expression | ||
| Solid-phase spheroids | • Easy to maintain 3D culture | • Some hydrophobic powder might be cytotoxic |
| • No need for supporting scaffold (only liquid drop is covered by hydrophobic powder) | • Difficulties in real-time imaging | |
| • Low-cost technique | • Might be difficult to provide medium exchange without structural damage |
Unquestionably 3D cultures in CSCs research provide much greater opportunities and better reflect their phenotype or mechanisms for stimulating tumor formation. It remains necessary to further develop and improve the current culture techniques so that 3D cultures can be an easy model for preclinical research on precursor cells in targeted cancer therapy. With further development of 3D cultures, it will be possible to eliminate certain drugs and/or modify them already at the initial screenings what can significantly reduce the time and cost of anticancer research.
Acknowledgements
This review was supported by the Wroclaw Medical University Grant no. SUB.D.130.19.040.
Disclosure of conflict of interest
None.
Abbreviations
- CSC
cancer stem cells
- SC
stem cells
- 3D
three dimensional
- 2D
two dimensional
- Dclk1
Doublecortin-like kinase 1
- HCC
hepatocellular cancer
- LPC
liver progenitor cell
- EMT
epithelial mesenchymal transition
- CDH1
the gene encodes cadherin-1
- CDH2
the gene encodes cadherin-2
- VIM
the gene encodes vimentin
- FN1
the gene encodes fibronectin-1
- TWIST
the gene encodes Twist Family BHLH Transcription Factor 1
- FOXC2
the gene encodes Forkhead Box C2
- HNSCC
head and neck squamous cell carcinoma
- CHC
α-cyano-4-hydroxycinnamate
References
- 1.Dawood S, Austin L, Cristofanilli M. Cancer stem cells: implications for cancer therapy. Oncology (Williston Park) 2014;28:110–1107. 1110. [PubMed] [Google Scholar]
- 2.El-Ashmawy NE, Salem ML, Khedr EG, El-Zamarany EA, Ibrahim AO. Dual-targeted therapeutic strategy combining CSC-DC-based vaccine and cisplatin overcomes chemo-resistance in experimental mice model. Clin Transl Oncol. 2020;22:1155–1165. doi: 10.1007/s12094-019-02242-4. [DOI] [PubMed] [Google Scholar]
- 3.Scott JG, Dhawan A, Hjelmeland A, Lathia J, Chumakova A, Hitomi M, Fletcher AG, Maini PK, AAnderson ARA. Recasting the cancer stem cell hypothesis: unification using a continuum model of microenvironmental forces. Curr Stem Cell Rep. 2019;5:22–30. [Google Scholar]
- 4.Safa AR. Resistance to drugs and cell death in cancer stem cells (CSCs) J Transl Sci. 2019;5:10. doi: 10.15761/jts.1000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Das PK, Pillai S, Rakib MA, Khanam JA, Gopalan V, Lam AKY, Islam F. Plasticity of cancer stem cell: origin and role in disease progression and therapy resistance. Stem Cell Rev Rep. 2020;16:397–412. doi: 10.1007/s12015-019-09942-y. [DOI] [PubMed] [Google Scholar]
- 6.Moreira H, Barg E. Cancer stem cells-current knowledge and targeting with natural compounds. Postepy Biologii Komorki. 2019;46:43–62. [Google Scholar]
- 7.Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2020;22:457–472. doi: 10.1038/cr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanabe S, Quader S, Cabral H, Ono R. Interplay of EMT and CSC in cancer and the potential therapeutic strategies. Front Pharmacol. 2020;11:904. doi: 10.3389/fphar.2020.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee BWL, Ghode P, Ong DST. Redox regulation of cell state and fate. Redox Biol. 2019;25:101056. doi: 10.1016/j.redox.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dzobo K, Senthebane DA, Ganz C, Thomford NE, Wonkam A, Dandara C. Advances in therapeutic targeting of cancer stem cells within the tumor microenvironment: an updated review. Cells. 2020;9:1896. doi: 10.3390/cells9081896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreira H, Szyjka A, Gąsiorowski K. Chemopreventive activity of celastrol in drug-resistant human colon carcinoma cell cultures. Oncotarget. 2018;9:21211–21223. doi: 10.18632/oncotarget.25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781. doi: 10.1016/j.lfs.2019.116781. [DOI] [PubMed] [Google Scholar]
- 13.Sun HR, Wang S, Yan SC, Zhang Y, Nelson PJ, Jia HL, Qin LX, Dong QZ. Therapeutic strategies targeting cancer stem cells and their microenvironment. Front Oncol. 2019;9:1104. doi: 10.3389/fonc.2019.01104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao L, Niu HJ, Qu TL, Zhang XF, Du FY. Targeting cancer stem cells in drug discovery: current state and future perspectives. World J Stem Cells. 2019;11:398–420. doi: 10.4252/wjsc.v11.i7.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Francesco EM, Sotgia F, Lisanti MP. Cancer stem cells (CSCs): metabolic strategies for their identification and eradication. Biochem. 2018;475:1611–1634. doi: 10.1042/BCJ20170164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Y, Zhang J, Zhang X, Zhou H, Liu G, Li Q. Cancer stem cells: a potential breakthrough in HCC-targeted therapy. Front Pharmacol. 2020;11:198. doi: 10.3389/fphar.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen H, Qian M, He J, Li M, Yu Q, Leng Z. Inhibiting of self-renewal, migration and invasion of ovarian cancer stem cells by blocking TGF-β pathway. PLoS One. 2020;15:e0230230. doi: 10.1371/journal.pone.0230230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermawan A, Putri H. Bioinformatics studies provide insight into possible target and mechanisms of action of nobiletin against cancer stem cells. Asian Pac J Cancer Prev. 2020;21:611–620. doi: 10.31557/APJCP.2020.21.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melissaridou S, Wiechec E, Magan M, Jain MV, Chung MK, Farnebo L, Roberg K. The effect of 2D and 3D cell cultures on treatment response, EMT profile and stem cell features in head and neck cancer. Cancer Cell Int. 2019;19:16. doi: 10.1186/s12935-019-0733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajcevic U, Knol JC, Piersma S, Bougnaud S, Fack F, Sundlisaeter E, Søndenaa K, Myklebust R, Pham TV, Niclou SP, Jiménez CR. Colorectal cancer derived organotypic spheroids maintain essential tissue characteristics but adapt their metabolism in culture. Proteome Sci. 2014;12:39. doi: 10.1186/1477-5956-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDH1 Gene - GeneCards | CADH1 Protein | CADH1 Antibody. https://www.genecards.org/cgi-bin/carddisp.pl?gene=CDH1 (accessed Jun. 11, 2021)
- 23.Raghavan S, Mehta P, Xie Y, Lei YL, Mehta G. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J Immunother Cancer. 2019;7:190. doi: 10.1186/s40425-019-0666-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louie E, Nik S, Chen JS, Schmidt M, Song B, Pacson C, Chen XF, Park S, Ju J, Chen EI. Identification of a stem-like cell population by exposing metastatic breast cancer cell lines to repetitive cycles of hypoxia and reoxygenation. Breast Cancer Res. 2010;12:R94. doi: 10.1186/bcr2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betriu N, Semino CE. Development of a 3D co-culture system as a cancer model using a self-assembling peptide scaffold. Gels. 2018;4:65. doi: 10.3390/gels4030065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta P, Novak C, Raghavan S, Ward M, Mehta G. Self-renewal and CSCs in vitro enrichment: growth as floating spheres. Methods Mol Biol. 2018;169:61–75. doi: 10.1007/978-1-4939-7401-6_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishiguro T, Ohata H, Sato A, Yamawaki K, Enomoto T, Okamoto K. Tumor-derived spheroids: relevance to cancer stem cells and clinical applications. Cancer Sci. 2017;108:283–289. doi: 10.1111/cas.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AlMusawi S, Ahmed M, Nateri AS. Understanding cell-cell communication and signaling in the colorectal cancer microenvironment. Clin Transl Med. 2021;11:e308. doi: 10.1002/ctm2.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagle PW, Plukker JTM, Muijs CT, van Luijk P, Coppes RP. Patient-derived tumor organoids for prediction of cancer treatment response. Semin Cancer Biol. 2018;53:258–264. doi: 10.1016/j.semcancer.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Powley IR, Patel M, Miles G, Pringle H, Howells L, Thomas A, Kettleborough C, Bryans J, Hammonds T, MacFarlane M, Pritchard C. Patient-derived explants (PDEs) as a powerful preclinical platform for anti-cancer drug and biomarker discovery. Br J Cancer. 2020;122:735–744. doi: 10.1038/s41416-019-0672-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, Lyu X, Yi M, Zhao W, Song Y, Wu K. Organoid technology and applications in cancer research. J Hematol Oncol. 2018;11:116. doi: 10.1186/s13045-018-0662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devarasetty M, Mazzocchi AR, Skardal A. Applications of bioengineered 3D tissue and tumor organoids in drug development and precision medicine: current and future. BioDrugs. 2018;32:53–68. doi: 10.1007/s40259-017-0258-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oost KC, van Voorthuijsen L, Fumagalli A, Lindeboom RGH, Sprangers J, Omerzu M, Rodriguez-Colman MJ, Heinz MC, Verlaan-Klink I, Maurice MM, Burgering BMT, van Rheenen J, Vermeulen M, Snippert HJG. Specific labeling of stem cell activity in human colorectal organoids using an ASCL2-responsive minigene. Cell Rep. 2018;22:1600–1614. doi: 10.1016/j.celrep.2018.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullenders J, de Jongh E, Brousali A, Roosen M, Blom JPA, Begthel H, Korving J, Jonges T, Kranenburg O, Meijer R, Clevers HC. Mouse and human urothelial cancer organoids: a tool for bladder cancer research. Med Sci. 2019;116:4567–4574. doi: 10.1073/pnas.1803595116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao H, Hu CY, Chen WM, Huang P. Lactate promotes cancer stem-like property of oral sequamous cell carcinoma. Curr Med Sci. 2019;39:403–409. doi: 10.1007/s11596-019-2050-2. [DOI] [PubMed] [Google Scholar]
- 36.Usui T, Sakurai M, Nishikawa S, Umata K, Nemoto Y, Haraguchi T, Itamoto K, Mizuno T, Noguchi S, Mori T, Iwai S, Nakagawa T, Yamawaki H, Ohama T, Sato K. Establishment of a dog primary prostate cancer organoid using the urine cancer stem cells. Cancer Sci. 2017;108:2383–2392. doi: 10.1111/cas.13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Zhang W, Jia Y, Yu Q, Grau GE, Peng L, Ran Y, Yang Z, Deng H, Lou J. Single-cell clones of liver cancer stem cells have the potential of differentiating into different types of tumor cells. Cell Death Dis. 2013;4:e857. doi: 10.1038/cddis.2013.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Sun Z, Liu Y, Kong L, Zhou S, Tang J, Xing HR. Comparison of tumor biology of two distinct cell sub-populations in lung cancer stem cells. Oncotarget. 2017;8:96852–96864. doi: 10.18632/oncotarget.18451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JW, Sung JS, Park YS, Chung S, Kim YH. Isolation of spheroid-forming single cells from gastric cancer cell lines: enrichment of cancer stem-like cells. BioTechniques. 2018;65:197–203. doi: 10.2144/btn-2018-0046. [DOI] [PubMed] [Google Scholar]
- 40.Lin D, Li P, Feng J, Lin Z, Chen X, Yang N, Wang L, Liu D. Screening therapeutic agents specific to breast cancer stem cells using a microfluidic single-cell clone-forming inhibition assay. Small. 2020;16:1901001. doi: 10.1002/smll.201901001. [DOI] [PubMed] [Google Scholar]
- 41.Arya A, Forget A. Biomaterials based strategies for engineering tumor microenvironment. Advances in Biomaterials for Biomedical Applications. 2017;66:301–361. [Google Scholar]
- 42.Silvestri A, Schumacher D, Silvestrov M, Schäfer R, Reinhard C, Hoffmann C, Boehnke K, Regenbrecht CRA, Haybaeck J. In vitro three-dimensional cell cultures as tool for precision medicine. Mechanisms of Molecular Carcinogenesis. 2017;2:281–313. [Google Scholar]
- 43.Timmins NE, Dietmair S, Nielsen LK. Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis. 2004;7:97–103. doi: 10.1007/s10456-004-8911-7. [DOI] [PubMed] [Google Scholar]
- 44.Nath S, Devi GR. Three-dimensional culture systems in cancer research: focus on tumor spheroid model. Pharmacol Ther. 2016;163:94–108. doi: 10.1016/j.pharmthera.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amaral RLF, Miranda M, Marcato PD, Swiech K. Comparative analysis of 3D bladder tumor spheroids obtained by forced floating and hanging drop methods for drug screening. Front Physiol. 2017;8:605. doi: 10.3389/fphys.2017.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Close DA, Camarco DP, Shan F, Kochanek SJ, Johnston PA. The generation of three-dimensional head and neck cancer models for drug discovery in 384-well ultra-low attachment microplates. Methods Mol Biol. 2018;1683:355–369. doi: 10.1007/978-1-4939-7357-6_20. [DOI] [PubMed] [Google Scholar]
- 47.Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, Court W, Lomas C, Mendiola M, Hardisson D, Eccles SA. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biology. 2012;10:29. doi: 10.1186/1741-7007-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raghavan S, Mehta P, Horst EN, Ward MR, Rowley KR, Mehta G. Comparative analysis of tumor spheroid generation techniques for differential in vitro drug toxicity. Oncotarget. 2016;7:16948–16961. doi: 10.18632/oncotarget.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma XL, Sun YF, Wang BL, Shen MN, Zhou Y, Chen JW, Hu B, Gong ZJ, Zhang X, Cao Y, Pan BS, Zhou J, Fan J, Guo W, Yang XR. Sphere-forming culture enriches liver cancer stem cells and reveals Stearoyl-CoA desaturase 1 as a potential therapeutic target. BMC Cancer. 2019;19:760. doi: 10.1186/s12885-019-5963-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Granger CJ, Hoyt AK, Moran A, Becker B, Sedani A, Saigh S, Conway SA, Brown J, Galoian K. Cancer stem cells as a therapeutic target in 3D tumor models of human chondrosarcoma: an encouraging future for proline rich polypeptide-1. Mol Med Rep. 2020;22:3747–3758. doi: 10.3892/mmr.2020.11480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liebmann T, Rydholm S, Akpe V, Brismar H. Self-assembling Fmoc dipeptide hydrogel for in situ 3D cell culturing. BMC Biotechnology. 2007;7:88. doi: 10.1186/1472-6750-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Luca A, Raimondi L, Salamanna F, Carina V, Costa V, Bellavia D, Alessandro R, Fini M, Giavaresi G. Relevance of 3d culture systems to study osteosarcoma environment. J Exp Clin Cancer Res. 2018;37:2. doi: 10.1186/s13046-017-0663-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ordikhani F, Kim Y, Zustiak SP. The role of biomaterials on cancer stem cell enrichment and behavior. JOM. 2015;67:2543–2549. [Google Scholar]
- 54.Qiao SP, Zhao YF, Li CF, Yin YB, Meng QY, Lin FH, Liu Y, Hou XL, Guo K, Chen XB, Tian WM. An alginate-based platform for cancer stem cell research. Acta Biomaterialia. 2016;37:83–92. doi: 10.1016/j.actbio.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 55.Barralet JE, Wang L, Lawson M, Triffitt JT, Cooper PR, Shelton RM. Comparison of bone marrow cell growth on 2D and 3D alginate hydrogels. J Mater Sci Mater Med. 2005;16:515–519. doi: 10.1007/s10856-005-0526-z. [DOI] [PubMed] [Google Scholar]
- 56.Ong SM, Zhang C, Toh YC, Kim SH, Foo HL, Tan CH, van Noort D, Park S, Yu H. A gel-free 3D microfluidic cell culture system. Biomaterials. 2008;29:3237–3244. doi: 10.1016/j.biomaterials.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 57.Taylor DL, Woo ES, Giuliano KA. Real-time molecular and cellular analysis: the new frontier of drug discovery. Curr Opin Biotechnol. 2001;12:75–81. doi: 10.1016/s0958-1669(00)00180-4. [DOI] [PubMed] [Google Scholar]
- 58.Fang Y, Eglen RM. Three-dimensional cell cultures in drug discovery and development. SLAS Discov. 2017;22:456–472. doi: 10.1177/1087057117696795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Toh YC, Zhang C, Zhang J, Khong YM, Chang S, Samper VD, van Noort D, Hutmacher DW, Yu H. A novel 3D mammalian cell perfusion-culture system in microfluidic channels. Lab Chip. 2007;7:302–309. doi: 10.1039/b614872g. [DOI] [PubMed] [Google Scholar]
- 60.Mojsiewicz-Pieńkowska K, Łukasiak J. Polidimetylosiloksany w środowisku człowieka. Polimery. 2003;48:403–409. [Google Scholar]
- 61.Kim MS, Hwang H, Choi YS, Park JK. Microfluidic micropillar arrays for 3D cell culture. Open Biotechnol J. 2008;2:224–228. [Google Scholar]
- 62.Anene-Nzelu CG, Peh KY, Fraiszudeen A, Kuan YH, Ng SH, Toh YC, Leo HL, Yu H. Yu Scalable alignment of three-dimensional cellular constructs in a microfluidic chip. Lab Chip. 2013;13:4124–4133. doi: 10.1039/c3lc50730k. [DOI] [PubMed] [Google Scholar]
- 63.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 64.Chan BP, Leong KW. Scaffolding in tissue engineering: general approaches and tissue-specific considerations. Eur Spine J. 2008;17(Suppl 4):467–479. doi: 10.1007/s00586-008-0745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dolznig H, Rupp C, Puri C, Haslinger C, Schweifer N, Wieser E, Kerjaschki D, Garin-Chesa P. Modeling colon adenocarcinomas in vitro a 3D co-culture system induces cancer-relevant pathways upon tumor cell and stromal fibroblast interaction. Am J Pathol. 2011;179:487–501. doi: 10.1016/j.ajpath.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu T, Lin B, Qin J. Carcinoma-associated fibroblasts promoted tumor spheroid invasion on a microfluidic 3D co-culture device. Lab Chip. 2010;10:1671–1677. doi: 10.1039/c000022a. [DOI] [PubMed] [Google Scholar]
- 67.Shafira M, Pramono A, Nugrohowati N, Sahlan M. High tetragonula sp honey addition reduce cell proliferation on fibroblast preputium culture. IOP Conf Ser Mater Sci Eng. 2019;508:012146. [Google Scholar]
- 68.Lv D, Hu Z, Lu L, Lu H, Xu X. Three-dimensional cell culture: a powerful tool in tumor research and drug discovery. Oncol Lett. 2017;14:6999–7010. doi: 10.3892/ol.2017.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoarau-Véchot J, Rafii A, Touboul C, Pasquier J. Halfway between 2D and animal models: are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int J Mol Sci. 2018;19:181. doi: 10.3390/ijms19010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eshtiaghi N, Hapgood KP. A quantitative framework for the formation of liquid marbles and hollow granules from hydrophobic powders. Powder Technol. 2012;223:65–76. [Google Scholar]
- 71.Rychecký O, Majerská M, Král V, Štěpánek F, Čejková J. Spheroid cultivation of HT-29 carcinoma cell line in liquid marbles. Chem Pap. 2017;71:1055–1063. [Google Scholar]
- 72.Aussillous P, Quéré D. Liquid marbles. Nature. 2001;411:924–927. doi: 10.1038/35082026. [DOI] [PubMed] [Google Scholar]
- 73.Pike N, Richard D, Foster W, Mahadevan L. How aphids lose their marbles. Proc Biol Sci. 2002;269:1211–1215. doi: 10.1098/rspb.2002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aussillous P, Quéré D. Properties of liquid marbles. Proc Math Phys Eng Sci P ROY SOC A-MATH PHY. 2006;462:973–999. [Google Scholar]
- 75.Askari E, Naghib SM, Seyfoori A, Maleki A, Rahmanian M. Ultrasonic-assisted synthesis and in vitro biological assessments of a novel herceptin-stabilized graphene using three dimensional cell spheroid. Ultrason Sonochemistry. 2019;58:104615. doi: 10.1016/j.ultsonch.2019.104615. [DOI] [PubMed] [Google Scholar]
- 76.Konieczny K, Bodzek M. Nanoporowaty grafen-nowy materiał do wytwarzania półprzepuszczalnych membran. Instal. 2019;9:40–44. [Google Scholar]
- 77.Jensen C, Teng Y. Is it time to start transitioning from 2D to 3D cell culture? Front Mol Biosci. 2020;7:33. doi: 10.3389/fmolb.2020.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]


