Abstract
Transarterial chemoembolization (TACE) is the mainstay of treatment for patients with intermediate/advanced stage or unresectable hepatocellular carcinoma (HCC). Despite the palliative nature of TACE treatment, embolizing the tumor feeding vessels and leading to progressive tumor necrosis, complete response (CR) after TACE could still be observed in a certain population. Thus, this study aimed to investigate both the predictors for CR and the long-term prognosis of the patients with CR after TACE. The study recruited new diagnosed HCC patients initially treated with TACE from 2010 to 2013. Post TACE response was assessed by scheduled image studies according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST). Then, pre-TACE factors were compared between patients with and without CR. After the first session of TACE, 22.3% of the 669 TACE treated patients achieved CR. During a median of 26.6 months follow-up, patients with CR had better overall survival than those without (median: 35.8 vs. 24.0 months, P<0.001). By multivariate logistic regression analysis, Child-Turcotte-Pugh class B (OR: 0.419, P=0.005), tumor burden beyond up-to-7 criteria (OR: 0.118, P<0.001), bilobar tumor extent (OR: 0.236, P<0.001), higher alpha-fetoprotein (AFP) level (≥20 ng/ml, OR: 0.614, P=0.039) and higher platelet counts (>150 k/μl, OR: 0.482, P=0.002) were unfavorable predictors for CR after first TACE. In addition, macrovascular invasion (HR: 3.113, P=0.001) and higher AFP levels (≥15 ng/ml, HR: 2.601, P=0.007) were predictors for early HCC recurrence whereas diabetes mellitus (DM) (HR: 2.166, P=0.006) was the only significant predictor for late HCC recurrence in CR patients. In conclusion, more than one-fifth of HCC patients achieved CR after first TACE and these patients had favorable prognosis. Furthermore, tailored post-TACE follow-up strategies shall be considered in patients with different risk factors of early or late recurrence after CR.
Keywords: Complete response, TACE, predictors, prognosis
Introduction
Hepatocellular carcinoma (HCC), ranked the fourth leading cause of cancer-related death in the world, is an imperative global health issue [1]. In spite of the rapid development of target therapy and immunotherapy for patients with advanced tumors (Barcelona Clinic Liver Cancer (BCLC) stage C), transarterial chemoembolization (TACE) remains the first-line treatment for patients with intermediate tumor stages (BCLC stage B) [2,3].
Mounting evidence suggests that TACE improves the survival rates and the quality of life for patients with unresectable HCC in contrast to supportive treatment alone [4,5]. The reported median survival, however, ranges widely in the literatures, from 6 months to 5 years, owing to the heterogeneity of patients undergoing TACE [6]. The majority of the prediction models for TACE treatment efficacy are derived from Western cohorts, in which the HCC etiologies are different from those found by Asian studies [7-10]. In spite of the palliative nature of TACE treatment, a proportion of TACE treated HCC patients do achieve complete response (CR) from single or repeated TACE treatment sessions. Thus, this study aims to describe the characteristics of such CR populations, investigating the predictors for CR at first TACE as well as factors correlated with early and late recurrence after CR.
Materials and methods
Patient selection
The study enrolled 1073 fresh diagnosed HCC adult patients (>18 year-old) who treated by conventional TACE or drug eluting bead (DEB)-TACE in a single medical center between January 2010 and December 2013. All these patients’ HCC status were diagnosed by histology or by dynamic computer tomography (CT)/Magnetic resonance imaging (MRI) according to EASL diagnostic criteria [11]. Patients with prior treatments for HCC (N=71), down-staging before resection (N=15), combined treatment strategies (principally radiofrequency ablation or Sorafenib, N=107), tumor rupture (N=28), BCLC stage D (N=38), presence of an additional primary malignancy apart from HCC (N=9) and those lost to follow-up (N=136 patients) were excluded. Host factors including age, gender, cirrhotic status, liver function, tumor staging, pre-TACE biochemistry exams, along with radiological response after the first session of TACE, and subsequent follow-up, including adverse events after TACE were documented. Patients with segmental vein thrombosis were included in the analysis because this is not considered as an absolute contraindication for TACE in most hospital and this at-risk population has shown favorable survival rates with TACE comparing to conservative treatment [12].
Treatment procedure
In advance of the TACE, an angiography including SMA, celiac trunk, common hepatic artery, and tumor feeding vessel was employed to evaluate the patency of portal vein, vascular anatomy, and tumor vascularity. Throughout the TACE, an emulsion of contrast medium (1 cc), lipoidol (4 cc), and adriamycin (20 mg) was used, followed by gelatin sponge particle infusion till stasis. Radiological assessment including CT or MRI was arranged 4-12 weeks after TACE treatment, and every 3 months afterwards if no evidence of viable or recurrence HCC. Repeated TACE was performed on-demand when viable tumor was detected by scheduled image study follow-up [13], provided there was no evidence of extrahepatic metastases, major portal vein invasion, or deterioration in clinical conditions.
Definitions and assessment of treatment responses
For evaluation of tumor response, we adopted modified Response Evaluation Criteria in Solid Tumors (mRECIST) guidelines, where tumor responses were classified into four categories: complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) [14]. A lesion, showing arterial enhancement and early wash-out in the dynamic CT or MRI, was regarded as a viable portion of tumor, while lesions with lipiodol or necrosis without arterial enhancement were considered to be a necrotic area. The tumor size was measured in the equilibrium (delayed) phase of post contrasted CT images. CR was defined as the complete disappearance of measurable lesions. PR was defined as at least a 30% decrease from the baseline sum of the longest diameter. PD was defined as a 20% increase compared with the baseline, and SD was defined as neither sufficient decrease as PR nor sufficient increase as PD. The images were reviewed by two independent radiologists to reduce the misclassifications. Overall survival (OS) was defined as the interval between the date of the first session of TACE and the date of last follow-up or death. Body weight loss was defined as reducing 5% of initial body weight in last 6 months [15]. To investigate the risk factors of recurrence at different time frame, early recurrence was defined as initial recurrence within 1 year following complete response while late recurrence was defined as recurrence occurred after complete remission for 1 year.
Statistical analysis
The continuous variables with normal distribution were reported as mean ± standard deviation (SD) and compared by Student’s t test. Those without normal distribution were reported as median [Interquartile Range (IQR)] and evaluated by Mann-Whitney U test. The category variables were presented as percentage and assessed by Chi-square test. Univariate and multivariate logistic regression analyses were applied for the predictors to CR after 1st TACE. Kaplan-Meier method and Log-Rank test were used for survival analysis. Cox regression model was adopted for investigating the correlation between baseline variables and time to recurrence after CR. For predictors of early recurrence, events occurred after one year were censored. For predictors of late recurrence, those followed up less than one year or those events occurred within one year from CR were excluded from the analysis. A P-value <0.05 was regarded as statistical significance. The statistical analyses were computed by SPSS version 20.0 (IBM, SPSS Inc. Chicago, IL).
Results
Patients’ characteristics
The baseline demography is shown in Table 1. Among 669 patients, 489 (73.1%) were male and the median age was 62.5 (IQR: 54.5-71.9) years. Most patients were diagnosed as BCLC stage B and C (n=498, 74.4%) initially. The most common etiology was chronic viral infection (including HBV or HCV infection, n=578, 86.4%), with 116 patients (17.3%) receiving antiviral therapy prior to the first TACE and 109 patients (16.3%) receiving adjuvant antiviral therapy. All patients had preserved liver function with Child-Turcotte-Pugh (CTP) class A or B and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. One hundred and seven patients (16.0%) had tumor invasion to peripheral branches of their portal veins. The median AFP level was 41 (IQR 10-546) ng/mL. The median diameter of the largest HCC was 3.5 (IQR 2.0-6.2) cm. 456 patients (68.2%) had 3 or fewer tumors.
Table 1.
Baseline clinical characteristics of patients with complete response at first TACE
| Variables | Overall (N=669) | Response (N=149) | No response (N=520) | P value |
|---|---|---|---|---|
| Age (years)* | 62.5 (54.5-71.9) | 62.5 (54.6-71.9) | 62.5 (54.2-71.8) | 0.811 |
| Gender (male, %) | 489 (73.1) | 105 (70.5) | 384 (73.8) | 0.413 |
| Body weight loss, n (%) | 87 (13.0) | 11 (6.8) | 76 (14.6) | 0.021 |
| Virus/Alcoholic/Cryptogenic, n (%) | 578/46/45 (86.4/6.9/6.7) | 131/12/6 (87.9/8.1/4.0) | 447/34/39 (86.0/6.5/7.5) | 0.284 |
| Anti-viral medication use, n (%) | 225 (38.9) | 55 (42.3) | 170 (37.9) | 0.369 |
| BMI (Kg/m2)* | 25.0 (22.6-27.8) | 25.2 (22.9-28.6) | 24.8 (22.6-27.7) | 0.121 |
| DM, n (%) | 197 (29.7) | 45 (30.2) | 152 (29.2) | 0.819 |
| CTP class A/B, n (%) | 543/126 (81.2/18.8) | 131/18 (87.9/12.1) | 412/108 (79.2/20.8) | 0.017 |
| BCLC stage 0/A/B/C, n (%) | 31/140/206/292 (4.6/20.9/30.8/43.6) | 22/62/18/47 (14.8/41.6/12.1/31.5) | 9/78/188/245 (1.7/15.0/36.2/47.1) | <0.001 |
| Ascites, n (%) | 127 (19.0) | 20 (13.4) | 107 (20.6) | 0.049 |
| NLR* | 1.96 (1.37-2.96) | 1.90 (1.33-2.86) | 1.95 (1.41-3.12) | 0.136 |
| PT (INR)* | 1.2 (1.1-1.3) | 1.2 (1.1-1.3) | 1.2 (1.1-1.3) | 0.061 |
| Total bilirubin (mg/dL)* | 0.8 (0.5-1.2) | 0.8 (0.5-1.1) | 0.8 (0.5-1.2) | 0.591 |
| AST (U/L)* | 52 (37-81) | 48 (33-69) | 55 (39-83) | 0.028 |
| ALT (U/L)* | 39 (26-64) | 37 (25-58) | 43 (26-66) | 0.290 |
| Albumin (g/dL)* | 3.59 (3.19-3.93) | 3.68 (3.27-4.00) | 3.56 (3.18-3.92) | 0.464 |
| AFP (ng/mL)* | 41 (10-546) | 18 (7-171) | 46 (10-682) | <0.001 |
| Platelet (1000/μL)* | 112 (77-180) | 94 (65-144) | 125 (81-192) | <0.001 |
| Creatinine (mg/dL)* | 0.79 (0.66-0.96) | 0.73 (0.59-0.92) | 0.75 (0.61-0.95) | 0.545 |
| Sodium (mEq/L)* | 140 (138-142) | 141 (138-142) | 140 (137-142) | 0.031 |
| Tumor numbers, n (%) 1/2&3/>3 | 228/228/213 (34.1/34.1/31.8) | 94/42/13 (63.1/28.2/8.7) | 134/186/200 (25.8/35.8/38.5) | <0.001 |
| Target lesion size (cm)* | 3.5 (2.0-6.2) | 2.4 (1.6-3.5) | 4.1 (2.4-7.4) | <0.001 |
| Within up-to-seven criteria, n (%) | 370 (55.3) | 136 (91.3) | 234 (45.0) | <0.001 |
| Tumor extent unilobar, n (%) | 388 (58.0) | 122 (81.9) | 266 (51.2) | <0.001 |
| Macrovascular invasion, n (%) | 107 (16.0) | 10 (6.7) | 97 (18.7) | <0.001 |
| Fever, n (%) | 205 (30.6) | 30 (20.1) | 175 (33.7) | 0.002 |
| Mortality, n (%) | 397 (59.3) | 48 (32.2) | 349 (67.1) | <0.001 |
| Follow-up time (months)* | 26.6 (14.8-38.9) | 35.8 (26.1-44.9) | 24.0 (11.7-38.1) | <0.001 |
Demonstrated as median (IQR).
Abbreviations: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; BMI, body mass index; CTP, Child-Turcotte-Pugh; DM, Diabetes mellitus; INR, international normalized ratio; NLR, neutrophil-lymphocyte ratio; TACE, transarterial chemoembolization.
Initial and final response after first TACE session
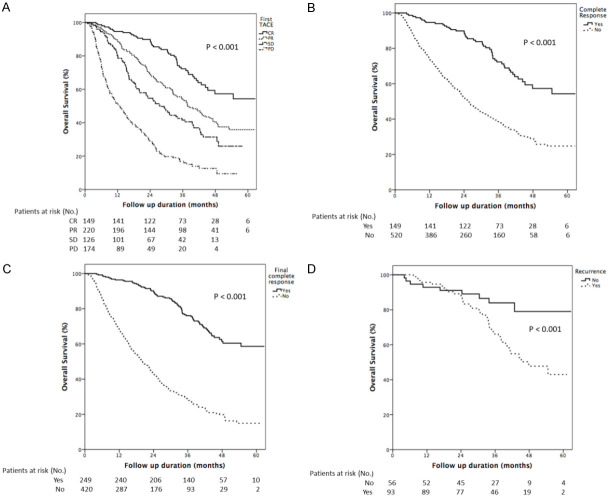
Figure 1 shows initial and final responses documented in patients from the first cycle of TACE until after multiple repeated procedures. After the first session of TACE, the number of patients with CR, PR, SD, and PD was 149 (22.3%), 220 (32.9%), 126 (18.8%), and 174 (26.0%) respectively. In 220 patients with PR at the first TACE, 72 (32.7%) patients eventually achieved CR. In patients with SD at the first TACE, 19 (15.1%) patients achieved CR later, while only 10 (5.7%) patients with initial PD achieved CR ultimately. Overall, the number of patients with CR rose from 149 (22.3%) to 249 (37.2%) after repeated TACE sessions. The median interval between the 1st cycle of TACE and first radiological evaluation among CR, PD, PR groups were 65 days, 64 days, 72 days, and 70 days, respectively (P=0.162). Figure 2 shows Kaplan-Meier curves of overall survival (OS) for all patient responses to the first TACE (A), for complete response at the first TACE (B), and for final complete response after repeated TACE sessions (C). The median OS was 35.8, 32.4, 26.0, and 12.6 months in patients with CR, PR, SD, and PD respectively (Figure 2A). Patients who achieved CR after the first TACE had much longer median OS than whose without (35.8 and 24.0 months, P<0.001, Figure 2B). Patients who had CR after repeated TACE sessions also had better prognoses and longer survival (median OS: 37.7 and 20.0 months, P<0.001) (Figure 2C).
Figure 1.
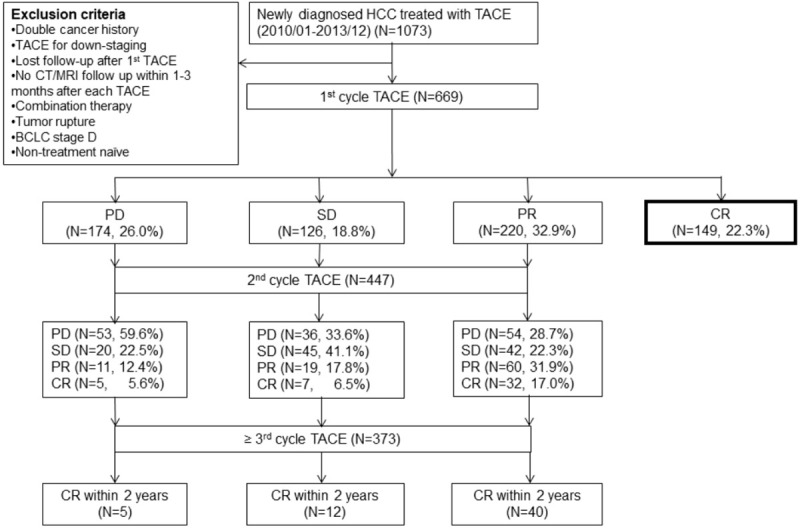
Flowchart of patients admitted to our hospital for hepatocellular carcinoma and selection of the study. TACE= transarterial chemoembolization, CT/MRI= computed tomography/magnetic resonance imaging, BCLC= Barcelona Clinic Liver Cancer, PD= progressive disease, SD= stable disease, PR= partial response, CR= complete response.
Figure 2.
Shows Kaplan-Meier curves of overall survival (OS) according to the response at first TACE in overall patients (A), complete response at 1st TACE (B), and final complete response after repeated TACE sessions (C) and tumor recurrence after complete response at 1st TACE (D) (both P<0.001).
Predictive factors to achieve complete response at the first TACE session
Since the patients achieving CR at the first TACE tended to have the best survival, baseline variables were evaluated to explore possible factors correlated with achieving CR. By univariate analysis, absence of bodyweight loss, CTP class A, unilobar tumor extent, within up-to-7 criteria (combines the number of tumors and the size of the largest tumor, with the sum being no more than 7), lack of macrovascular invasion, lower aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (AAR), lower AFP level, lower platelet counts, and absence of fever after first TACE session were correlated with the CR after the first TACE. In the multivariate analysis, CTP class B [Odds ratio (OR): 0.419, P=0.005], beyond up-to-7 criteria (OR: 0.118, P<0.001), bilobar tumors extent (OR: 0.236, P<0.001), macrovascular invasion (OR: 0.440, P<0.001), higher AFP level (≥20 ng/ml, OR: 0.614, P=0.039) and higher platelet counts (≥150 k/μl, OR: 0.482, P=0.002) were unfavorable predictors for CR at the first TACE session (Table 2).
Table 2.
Logistic regression of risk factors associated with complete response at first TACE
| Predictor Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age (per year increase) | 1.004 | 0.989-1.019 | 0.615 | |||
| Male (vs. female) | 0.845 | 0.565-1.264 | 0.413 | |||
| Body weight loss (vs. no) | 0.466 | 0.241-0.901 | 0.023 | 0.797 | 0.390-1.627 | 0.533 |
| Viral hepatitis infection (vs. non-viral hepatitis) | 1.189 | 0.685-2.063 | 0.539 | |||
| DM (vs. no) | 1.048 | 0.704-1.559 | 0.819 | |||
| CTP class B (vs. A) | 0.524 | 0.307-0.896 | 0.018 | 0.419 | 0.229-0.767 | 0.005 |
| Beyond up-to-7 criteria (vs. within) | 0.078 | 0.043-0.142 | <0.001 | 0.118 | 0.060-0.230 | <0.001 |
| Bilobar tumor extent (vs. unilobar) | 0.232 | 0.148-0.364 | <0.001 | 0.236 | 0.147-0.379 | <0.001 |
| Macrovascular invasion (vs. no) | 0.314 | 0.159-0.618 | 0.001 | 0.440 | 0.213-0.909 | 0.027 |
| AAR≥1.7 (vs. <1.7) | 0.531 | 0.324-0.870 | 0.012 | 0.728 | 0.424-1.251 | 0.250 |
| AFP≥20 ng/ml (vs. <20 ng/ml) | 0.525 | 0.342-0.805 | 0.003 | 0.614 | 0.386-0.975 | 0.039 |
| Platelet≥150 K/μL (vs. <150 K/μL) | 0.423 | 0.278-0.644 | <0.001 | 0.482 | 0.301-0.770 | 0.002 |
| Fever after 1st TACE (vs. no) | 0.497 | 0.320-0.772 | 0.002 | 0.650 | 0.404-1.045 | 0.075 |
Abbreviations: AAR, aspartate aminotransferase to alanine aminotransferase ratio; AFP, alpha-fetoprotein; CTP, Child-Turcotte-Pugh; DM, diabetes mellitus; TACE, transarterial chemoembolization.
Analysis for tumor recurrence after complete response at first TACE
Among 149 CR patients after first session of TACE, 93 (62.4%) patients had tumor recurrence during follow-up (median time to recurrence: 13.1 (IQR 8.3-19.6) months). Most patients (n=57, 61.3%) had local tumor recurrence, followed by intra-hepatic distant metastasis (n=44, 47.3%) and distant metastasis (n=21, 22.6%), principally to the lung (n=14, 66.7%). Patients with tumor recurrence had an overall worse prognosis, with median OS 45.2 vs. 54.6 months (log-rank test, P<0.001) (Figure 2D). The baseline demography of patients with tumor recurrence are shown in Table 3. By multivariate Cox regression analysis, only higher AFP level [≥15 ng/ml, adjusted Hazard ratio (aHR): 1.628, P=0.023] and macrovascular invasion [aHR: 2.084, P=0.05] were the two independent predictors for tumor recurrence in 149 patients achieving complete response after the first TACE session (Table 4). Considering the factors contributing to early (<1 year) and late (>1 year) recurrence may be different, cox regression analysis showed macrovascular invasion (HR: 3.113, P=0.001) and higher AFP level (≥15 ng/ml, HR: 2.601, P=0.007) were the independent risk factors of early recurrence (Supplementary Table 1), whereas the comorbidity of diabetes mellitus (DM) (HR: 2.166, P=0.006) was the only risk factor associated with late recurrence (Supplementary Table 2).
Table 3.
Baseline clinical characteristics of patients with and without recurrence after first TACE
| Variables | Overall (N=149) | Recurrence (N=93) | No recurrence (N=56) | P value |
|---|---|---|---|---|
| Age (years)* | 62.5 (54.6-71.9) | 62.5 (54.9-70.3) | 62.5 (54.5-72.8) | 0.697 |
| Gender (male, %) | 105 (70.5) | 63 (67.7) | 42 (75.0) | 0.347 |
| Body weight loss, n (%) | 11 (6.8) | 5 (5.4) | 6 (10.7) | 0.227 |
| Virus/Alcoholic/Cryptogenic, n (%) | 131/12/6 (87.9/8.1/4.0) | 84/5/4 (90.3/5.4/4.3) | 47/7/2 (83.9/12.5/3.6) | 0.300 |
| Anti-viral medication use, n (%) | 55 (42.3) | 37 (44.6) | 18 (38.3) | 0.486 |
| BMI (Kg/m2)* | 25.2 (22.9-28.6) | 25.2 (23.8-28.6) | 25.2 (22.6-28.9) | 0.256 |
| DM, n (%) | 45 (30.2) | 34 (36.6) | 11 (19.6) | 0.029 |
| CTP class A/B, n (%) | 131/18 (87.9/12.1) | 85/8 (91.4/8.6) | 46/10 (82.1/17.9) | 0.093 |
| BCLC stage 0/A/B/C, n (%) | 22/62/18/47 (14.8/41.6/12.1/31.5) | 12/38/12/31 (12.9/40.9/12.9/33.3) | 10/24/6/16 (17.9/42.9/10.7/28.6) | 0.800 |
| AST (U/L)* | 48 (33-69) | 49 (37-70) | 41 (29-69) | 0.034 |
| ALT (U/L)* | 37 (25-58) | 37 (26-71) | 30 (23-44) | 0.006 |
| AFP (ng/mL)* | 18 (7-171) | 40 (11-267) | 16 (6-502) | 0.037 |
| Platelet (1000/μL)* | 94 (65-144) | 100 (65-138) | 89 (64-134) | 0.046 |
| Creatinine (mg/dL)* | 0.73 (0.59-0.92) | 0.75 (0.58-0.92) | 0.72 (0.59-0.87) | 0.952 |
| Sodium (mEq/L)* | 141 (138-142) | 141 (139-143) | 141 (138-142) | 0.581 |
| Tumor numbers, n (%) 1/2&3/>3 | 94/42/13 (63.1/28.2/8.7) | 56/29/8 (60.2/31.2/8.6) | 38/13/5 (67.9/23.2/8.9) | 0.573 |
| Target lesion size (cm)* | 2.4 (1.6-3.5) | 2.6 (1.7-3.6) | 2.0 (1.5-3.5) | 0.044 |
| Within up-to-seven criteria, n (%) | 136 (91.3) | 84 (90.3) | 52 (92.9) | 0.595 |
| Tumor extent unilobar, n (%) | 122 (81.9) | 74 (79.6) | 48 (85.7) | 0.346 |
| Macrovascular invasion, n (%) | 10 (6.7) | 8 (8.6) | 2 (3.6) | 0.235 |
| Fever, n (%) | 30 (20.1) | 17 (18.3) | 13 (23.2) | 0.467 |
| Mortality, n (%) | 48 (32.2) | 39 (41.9) | 9 (16.1) | 0.001 |
| Disease free interval (months)* | 35.8 (26.1-44.9) | 13.5 (8.3-19.7) | 35.0 (24.1-43.7) | <0.001 |
Demonstrated as median (IQR).
Abbreviations: AFP, alpha-fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BCLC, Barcelona Clinic Liver Cancer; BMI, body mass index; CTP, Child-Turcotte-Pugh; DM, Diabetes mellitus; INR, international normalized ratio; NLR, neutrophil-lymphocyte ratio; TACE, transarterial chemoembolization.
Table 4.
Cox regression of risk factors associated with recurrence in CR patients after first TACE
| Predictor Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (per year increase) | 0.997 | 0.979-1.015 | 0.721 | |||
| Male (vs. female) | 1.053 | 0.681-1.628 | 0.818 | |||
| Body weight loss (vs. no) | 0.744 | 0.302-1.833 | 0.521 | |||
| Viral infection (vs. non-viral) | 1.556 | 0.782-3.098 | 0.208 | |||
| DM (vs. no) | 1.425 | 0.933-2.178 | 0.101 | |||
| CTP class B (vs. A) | 0.741 | 0.384-1.430 | 0.372 | |||
| Beyond up-to-7 criteria (vs. within) | 1.455 | 0.735-2.923 | 0.277 | |||
| Bilobar tumor extent (vs. unilobar) | 1.415 | 0.853-2.345 | 0.179 | |||
| Macrovascular invasion (vs. no) | 2.264 | 1.091-4.700 | 0.028 | 2.084 | 1.000-4.345 | 0.050 |
| AAR≥1.7 (vs. <1.7) | 0.703 | 0.374-1.319 | 0.272 | |||
| AFP≥15 ng/ml (vs. <15 ng/ml) | 1.675 | 1.104-2.541 | 0.015 | 1.628 | 1.071-2.474 | 0.023 |
| Platelet≥150 K/μL (vs. <150 K/μL) | 0.752 | 0.453-1.247 | 0.269 | |||
| Fever after 1st TACE (vs. no) | 0.622 | 0.364-1.062 | 0.081 | |||
Abbreviations: AAR, aspartate aminotransferase to alanine aminotransferase ratio; AFP, alpha-fetoprotein; CTP, Child-Turcotte-Pugh; DM, diabetes mellitus; TACE, transarterial chemoembolization.
Discussion
This study highlights the importance of CR after the first TACE and identifies the independent predictors for achieving CR as well as risk factors for HCC recurrence among CR patients based on a large sample of TACE treated HCC patients. TACE embolizes the tumor feeding vessels, which majorly composed of hepatic artery, and leads to tumor necrosis. Since HCC may have several feeding vessels which can hardly be all embolized by TACE, TACE has been regarded as a palliative rather than curative anti-cancer modality. However, earlier studies have reported nearly 50% of the TACE patients could achieve CR after their first session of TACE [16,17], which the CR rates are much higher than that of 22.3% in the current study. The possible reasons are our current study includes patients with segmental vein thrombosis and 43.6% BCLC stage C patients. The greater tumor burden and deteriorated liver reserve both known unfavorable factors for TACE response.
In current study, patients who achieved CR after the first TACE have approximate median OS to those sustained CR after repeated TACE (35.8% vs. 37.7%). This phenomenon supports the index predictive value of first TACE response on overall survival. Patients who showed a CR at first TACE session and no detected viable tumor over at least two subsequent follow-ups with 3 month intervals had a better prognosis than those failed to achieve sustained CR (median OS: 35.3 months vs. 23.7 months, P<0.001). In general, patients who achieved CR immediately tended to have better preserved liver function, tumor loads within the up to-seven criteria, unilobar tumor extent, lower baseline AFP level and platelet counts. Therefore, in these patients, TACE is strongly indicated as first line therapy.
Different from previous existing prediction models which focus on predicting poor survival after repeated sessions of TACE (e.g. HAP (Hepatoma Arterial-embolization Prognostic) score and STATE (Selection for transarterial chemoembolization treatment) score [8,9] or on evaluating the risk of repeated TACE (e.g. the ART (Assessment for Retreatment with TACE) score and ABCR (AFP, BCLC, CTP, and response) score [7,10], the current study strengthens the evidence that a considerable subset of patients could benefit from CR after first and repeated TACE. Patients with unfavorable pre-TACE factors and fail to achieve CR after the first TACE shall be considered to combine other anti-cancer regimen such as target therapy or immunotherapy to increase their response rate or survival [18].
Interestingly, we found that patients with lower pre-TACE platelet count having higher probability of post-TACE complete response. Lee et al. reported that, in patients with early-stage HCC, lower pretreatment platelet count is significantly correlated with the lower incidence of extrahepatic metastasis [19]. This phenomenon is supported by in vitro experiment that platelets promote metastasis [20,21]. It has been reported that the inflammatory mediators released by activated platelets promote leukocyte migration into tissues [22]. We therefore suggest that a higher platelet count is a negative predictor in determining response after TACE.
Unlike surgical resection of tumor ablation result in removal or coagulative necrosis of 0.5-1.0 cm tumor free margin, TACE only affects tumor itself which explain the high recurrence rate of 62.4% among patients achieving CR in our study during a median 13-month follow-up. However, the recurrence rate in current study was higher than those of 21%~43.3% in previous studies [23-26], which may be explained by the different tumor etiology, tumor burden heterogeneity across different studies and relative low antiviral therapy rate in our current TACE cohort.
In the current study, the risk factors of early HCC recurrence differed from that of late recurrence. Macrovascular invasion and pre-therapeutic AFP level were predictors of early HCC recurrence after TACE, whereas DM was the only risk factor of late HCC recurrence. As in earlier studies [24-27], higher tumor burden is associated with tumor recurrence. Macrovascular invasion and pre-therapeutic AFP are correlated with the tumor burden along with its invasiveness, which resulting in the early recurrence after complete response by TACE. However, comorbidity with DM, which was not well addressed or reported in earlier research, also correlated with a higher late recurrence rate after achievement of CR at first TACE (36.6% vs. 19.6%, P=0.029) in our patients. In spite of the mounting epidemiology evidence that diabetes mellitus is an independent risk factor for HCC [28-30] and also a risk factor of poor prognosis [31-33], the exact mechanism between diabetes and its highly association with hepatocarcinogenesis is not well understood. Major dysregulations of insulin dependent pathways [34], and hyperglycemia may have direct carcinogenic effect via Wnt/beta-catenin pathway [35] while adipokines including tumor necrosis factor-alfa and interleukin-6 produced by adipose tissue contribute to microenvironment facilitate the ongoing carcinogenesis [36,37]. These findings suggest that more frequent surveillance for early detection of recurrence is required in CR patients with high pre-TACE AFP level and macrovascular invasion and also the stringent health education and glycemic control may be helpful to alleviate the probability of late recurrence.
There are some limitations in our study. First, we did not further evaluate the comparative effectiveness of conventional TACE and DEB-TACE. As unproven survival benefit DEB-TACE has, compared with conventional TACE, such treatment might have better response rate and less systemic toxicities [38]. Second, the study was conducted in a retrospective cohort and another study group (internal and external) might be necessary to validate our findings.
In conclusion, although TACE has been suggested as a palliative therapy for patients with unresectable HCC, more than one-fifth of the TACE HCC patients achieve complete response after first TACE. Patients with preserved CTP score, lower pre-TACE AFP level, lower platelet count, tumor burden within the up-to-seven criteria, and unilobar tumor are more inclined to achieve CR. However, half of these CR patients encounter HCC recurrence, especially those with DM and/or higher pre-TACE AFP level. It is therefore possible that combination with another anticancer modality might reduce HCC recurrence rate in patients with otherwise favorable predictors who have DM or higher pre-TACE AFP level. Suitable treatment combinations may also improve TACE efficacy in patients with less favorable indicators, allowing them to achieve CR after TACE.
Acknowledgements
The authors thank all the members of the Cancer Center, Chang Gung Memorial Hospital, for their invaluable help, including Ching-Ting Wang and Hsiu-Ying Chai. The authors also thank Mon-Tzy Lu, Pin-Hsiu Lin and Mei-Hsia Ku for their assistance in data acquisition and cleaning. This study was supported by grants from Chang Gung Medical Research Fund (CMRPG3J1341, CORPG3G0871, CORPG3H0641, CORPG3H0651, CORPG3H0661, CORPG3H0671), National Science Council, Taiwan (NMRPG3H0471).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, Al-Raddadi R, Alvis-Guzman N, Amoako Y, Artaman A. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hucke F, Sieghart W, Schoniger-Hekele M, Peck-Radosavljevic M, Muller C. Clinical characteristics of patients with hepatocellular carcinoma in Austria-is there a need for a structured screening program? Wien Klin Wochenschr. 2011;123:542–551. doi: 10.1007/s00508-011-0033-9. [DOI] [PubMed] [Google Scholar]
- 3.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380:1450–1462. doi: 10.1056/NEJMra1713263. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Sola R, Rodes J, Bruix J Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 5.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 6.Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64:106–116. doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 7.Sieghart W, Hucke F, Pinter M, Graziadei I, Vogel W, Müller C, Heinzl H, Trauner M, Peck-Radosavljevic M. The ART of decision making: retreatment with transarterial chemoembolization in patients with hepatocellular carcinoma. Hepatology. 2013;57:2261–2273. doi: 10.1002/hep.26256. [DOI] [PubMed] [Google Scholar]
- 8.Kadalayil L, Benini R, Pallan L, O’Beirne J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK, Palmer DH, Meyer T. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24:2565–2570. doi: 10.1093/annonc/mdt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hucke F, Pinter M, Graziadei I, Bota S, Vogel W, Muller C, Heinzl H, Waneck F, Trauner M, Peck-Radosavljevic M, Sieghart W. How to STATE suitability and START transarterial chemoembolization in patients with intermediate stage hepatocellular carcinoma. J Hepatol. 2014;61:1287–1296. doi: 10.1016/j.jhep.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Adhoute X, Penaranda G, Naude S, Raoul JL, Perrier H, Bayle O, Monnet O, Beaurain P, Bazin C, Pol B, Folgoc GL, Castellani P, Bronowicki JP, Bourliere M. Retreatment with TACE: the ABCR SCORE, an aid to the decision-making process. J Hepatol. 2015;62:855–862. doi: 10.1016/j.jhep.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 13.Cheng AL, Amarapurkar D, Chao Y, Chen PJ, Geschwind JF, Goh KL, Han KH, Kudo M, Lee HC, Lee RC, Lesmana LA, Lim HY, Paik SW, Poon RT, Tan CK, Tanwandee T, Teng G, Park JW. Re-evaluating transarterial chemoembolization for the treatment of hepatocellular carcinoma: consensus recommendations and review by an international expert panel. Liver Int. 2014;34:174–183. doi: 10.1111/liv.12314. [DOI] [PubMed] [Google Scholar]
- 14.Shim JH, Lee HC, Kim SO, Shin YM, Kim KM, Lim YS, Suh DJ. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708–718. doi: 10.1148/radiol.11110282. [DOI] [PubMed] [Google Scholar]
- 15.Bruggeman AR, Kamal AH, LeBlanc TW, Ma JD, Baracos VE, Roeland EJ. Cancer cachexia: beyond weight loss. J Oncol Pract. 2016;12:1163–1171. doi: 10.1200/JOP.2016.016832. [DOI] [PubMed] [Google Scholar]
- 16.Terzi E, Golfieri R, Piscaglia F, Galassi M, Dazzi A, Leoni S, Giampalma E, Renzulli M, Bolondi L. Response rate and clinical outcome of HCC after first and repeated cTACE performed “on demand”. J Hepatol. 2012;57:1258–1267. doi: 10.1016/j.jhep.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Kim BK, Kim SU, Kim KA, Chung YE, Kim MJ, Park MS, Park JY, Kim DY, Ahn SH, Kim MD, Park SI, Won JY, Lee DY, Han KH. Complete response at first chemoembolization is still the most robust predictor for favorable outcome in hepatocellular carcinoma. J Hepatol. 2015;62:1304–1310. doi: 10.1016/j.jhep.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Raoul JL, Forner A, Bolondi L, Cheung TT, Kloeckner R, de Baere T. Updated use of TACE for hepatocellular carcinoma treatment: how and when to use it based on clinical evidence. Cancer Treat Rev. 2019;72:28–36. doi: 10.1016/j.ctrv.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Lee CH, Lin YJ, Lin CC, Yen CL, Shen CH, Chang CJ, Hsieh SY. Pretreatment platelet count early predicts extrahepatic metastasis of human hepatoma. Liver Int. 2015;35:2327–2336. doi: 10.1111/liv.12817. [DOI] [PubMed] [Google Scholar]
- 20.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riedl J, Pabinger I, Ay C. Platelets in cancer and thrombosis. Hamostaseologie. 2014;34:54–62. doi: 10.5482/HAMO-13-10-0054. [DOI] [PubMed] [Google Scholar]
- 22.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 23.Park W, Chung YH, Kim JA, Jin YJ, Lee D, Shim JH, Lee D, Kim KM, Lim YS, Lee HC, Lee YS, Kim PN, Sung KB. Recurrences of hepatocellular carcinoma following complete remission by transarterial chemoembolization or radiofrequency therapy: focused on the recurrence patterns. Hepatol Res. 2013;43:1304–1312. doi: 10.1111/hepr.12083. [DOI] [PubMed] [Google Scholar]
- 24.Jin YJ, Chung YH, Kim JA, Park W, Lee D, Shim JH, Lee D, Kim KM, Lim YS, Lee HC. Predisposing factors of hepatocellular carcinoma recurrence following complete remission in response to transarterial chemoembolization. Dig Dis Sci. 2013;58:1758–1765. doi: 10.1007/s10620-013-2562-8. [DOI] [PubMed] [Google Scholar]
- 25.Rou WS, Lee BS, Moon HS, Lee ES, Kim SH, Lee HY. Risk factors and therapeutic results of early local recurrence after transcatheter arterial chemoembolization. World J Gastroenterol. 2014;20:6995–7004. doi: 10.3748/wjg.v20.i22.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Douhara A, Namisaki T, Moriya K, Kitade M, Kaji K, Kawaratani H, Takeda K, Okura Y, Takaya H, Noguchi R. Predisposing factors for hepatocellular carcinoma recurrence following initial remission after transcatheter arterial chemoembolization. Oncol Lett. 2017;14:3028–3034. doi: 10.3892/ol.2017.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng CW, Teng W, Hsieh YC, Jeng WJ, Huang CH, Lui KW, Hung CF, Chen YC, Lin CC, Lin CY, Lin SM, Sheen IS. Postembolization fever after transarterial chemoembolization is a sign of unfavorable therapeutic response in hepatocellular carcinoma patients. Advances in Digestive Medicine. 2020;7:83–92. [Google Scholar]
- 28.El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369–380. doi: 10.1016/j.cgh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–221. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 30.Koh WP, Wang R, Jin A, Yu MC, Yuan JM. Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer. 2013;108:1182–1188. doi: 10.1038/bjc.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huo TI, Wu JC, Lui WY, Huang YH, Lee PC, Chiang JH, Chang FY, Lee SD. Differential mechanism and prognostic impact of diabetes mellitus on patients with hepatocellular carcinoma undergoing surgical and nonsurgical treatment. Am J Gastroenterol. 2004;99:1479–1487. doi: 10.1111/j.1572-0241.2004.30024.x. [DOI] [PubMed] [Google Scholar]
- 32.Huo TI, Hsu CY, Huang YH, Hsia CY, Lin HC, Lee PC, Loong CC, Chiang JH, Chiou YY, Lee SD. Diabetes mellitus as an independent prognostic predictor and its association with renal dysfunction in patients with hepatocellular carcinoma. Liver Int. 2010;30:198–207. doi: 10.1111/j.1478-3231.2009.02143.x. [DOI] [PubMed] [Google Scholar]
- 33.Shau WY, Shao YY, Yeh YC, Lin ZZ, Kuo R, Hsu CH, Hsu C, Cheng AL, Lai MS. Diabetes mellitus is associated with increased mortality in patients receiving curative therapy for hepatocellular carcinoma. Oncologist. 2012;17:856–862. doi: 10.1634/theoncologist.2012-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chettouh H, Lequoy M, Fartoux L, Vigouroux C, Desbois-Mouthon C. Hyperinsulinaemia and insulin signalling in the pathogenesis and the clinical course of hepatocellular carcinoma. Liver International. 2015;35:2203–2217. doi: 10.1111/liv.12903. [DOI] [PubMed] [Google Scholar]
- 35.Chocarro-Calvo A, García-Martínez JM, Ardila-González S, De la Vieja A, García-Jiménez C. Glucose-induced β-catenin acetylation enhances Wnt signaling in cancer. Mol Cell. 2013;49:474–486. doi: 10.1016/j.molcel.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Garg SK, Maurer H, Reed K, Selagamsetty R. Diabetes and cancer: two diseases with obesity as a common risk factor. Diabetes Obes Metab. 2014;16:97–110. doi: 10.1111/dom.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujita K, Iwama H, Miyoshi H, Tani J, Oura K, Tadokoro T, Sakamoto T, Nomura T, Morishita A, Yoneyama H. Diabetes mellitus and metformin in hepatocellular carcinoma. World J Gastroenterol. 2016;22:6100. doi: 10.3748/wjg.v22.i27.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62:1187–1195. doi: 10.1016/j.jhep.2015.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.