Abstract
Background
MS is unpredictable regarding clinical symptoms; however, certain symptoms represent the preferred localization of white matter lesions such as brainstem, spinal cord; or optic nerve.
Objectives
To investigate the epidemiological, clinical, and imaging characteristics of MS patients in a national referral center in Peru, and to evaluate whether the type of symptom at onset relates with the time to making an MS diagnosis.
Methods
Retrospective study of MS patients at the Instituto Nacional de Ciencias Neurológicas between January 2010 and December 2018. Four different syndromes were selected for analysis as symptom onset (optic neuritis, brainstem syndrome, myelitis, and others).
Results
we identified 268 patients for whom a diagnosis of MS had been given; after excluding misdiagnosed patients (33 Neuromyelitis optica), lost or incomplete records, 121 patients were included. The majority of patients (46.6%) were born in Lima. Female to male ratio was 1.37 to 1, mean age at diagnosis was 31 years. At onset, myelitis was present in 35% of RRMS patients, followed by brainstem syndrome (25%) and optic neuritis (18%). Brainstem syndrome was statistically significant predictor for earlier diagnosis (adjusted HR: 2.09; p = 0.015).
Conclusion
Brainstem syndrome as an initial presentation of MS in Peru is related to an earlier diagnosis.
Keywords: Multiple sclerosis, Brainstem syndrome, Early diagnosis, Peru, Clinical characteristics
Highlights
-
∙
In Peru, the epidemiology of MS is still a major neglected topic.
-
∙
Brainstem syndrome as an initial presentation of MS in Peru is related to an early diagnosis.
-
∙
Our population represents different regions of our country.
-
∙
Similar clinical, epidemiological and imaging characteristic to other Latin American countries were found.
-
∙
We found a misdiagnosis rate of 36.2%, which remains a contemporary problem in MS diagnosis.
Multiple sclerosis, Brainstem syndrome, Early diagnosis, Peru, Clinical characteristics
1. Introduction
Multiple sclerosis (MS) is a chronic disease of the central nervous system (CNS) characterized by disseminated loss of motor and sensory function that results from immune-mediated inflammation, demyelination and subsequent axonal damage [1, 2, 3]. More than two million people worldwide suffer from MS [1]. In Peru, epidemiological data of the disease are scarce. The estimated prevalence of MS in Lima is 7.69 x 100000 inhabitants [4], corresponding to a medium to low frequency area [5].
MS may have different clinical patterns over time, with either subacute episodic periods of worsening, followed by variable improvement; gradual progressive decline of neurological function; or a combination of both [6]. The clinical spectrum of MS ranges from an asymptomatic course to rapid progression and permanent disability within a few years [7]. Furthermore, disease-modifying therapies (DMT) have convincingly altered the short- and intermediate-term natural history of the disease [8].
Regarding the clinical course of MS, according to the 2013 revisions by Lublin and colleagues, clinically isolated syndrome (CIS) was added to the phenotypes. Moreover, this new classification defines two phenotypes, relapsing remitting and progressive phenotype, and it further divides the progressive phenotype according to progression and activity [9, 10].
MS is unpredictable regarding clinical symptoms; however, certain signs and symptoms are frequently seen that represent the preferred localization of white matter lesions. The structures that typically are affected during clinical exacerbations of MS are the optic nerves, the spinal cord, and the brainstem. Symptoms due to lesions of cerebral hemispheres, cerebellum, or multifocal are less frequent [11, 12]. CIS can be attributed to a single inflammatory CNS lesion in approximately 85% [7, 13], including optic neuritis (38.4%), partial myelitis (27.8%) or a brainstem syndrome (24.4%) [7, 14].
This retrospective study was designed to investigate the epidemiological, clinical, and imaging characteristics of MS patients in Peru, and to evaluate if the location of symptom onset relates to the time of making an MS diagnosis.
2. Methods
2.1. Patients and methods
We conducted a retrospective study, enrolling patients with the diagnosis of MS who were treated at the Instituto Nacional de Ciencias Neurológicas (INCN) in Lima – Peru. We retrospectively reviewed all medical record databases of patients who were evaluated between 2010 and 2018. This institution is a government-run, tertiary level neurological center that treats persons with neurological disorders referred from a wide geographic and socio-demographic area.
Clinical and demographical data (at disease diagnosis) reviewed were age, sex, place of birth, current residence, disease phenotype, year and age at first symptom, year and age at diagnosis, recalled clinical symptoms (motor, sensitive, cerebellar, visual, oculomotor, cranial nerves, autonomic, gait, cognitive), number of relapses before diagnosis, EDSS score, first immunomodulatory treatment and follow up time until chart reviewed.
Brain and spinal MRI data was collected from the radiology report (closest to diagnosis). Radiology reports came from different MRI centers and we didn't have access to the images. Data collected was lesion localization, presence of black holes (hypointense lesions in T1 weighted imaging), and contrast enhancement.
Paraclinical data collected (at disease diagnosis) were cerebrospinal fluid (CSF) protein level, CSF cellularity, oligoclonal bands (determined by means of isoelectric focusing), and 25-hydroxyvitamin D. AQP4-IgG or MOG-IgG were not performed.
The onset syndrome (first symptom recalled by the patient at the time of diagnosis) was defined as follows: optic neuritis (ON), brainstem syndrome (BSS), myelitis and other (cerebellar, hemispheric or polyregional syndromes). Time to diagnosis was calculated from the recalled date of the first symptom and the date of diagnosis.
Included were medical records from adult MS patients diagnosed according to the 2010 or 2017 McDonald used at the time of diagnosis. Medical records with incomplete data were excluded. The Institutional Review Board (Comité Institucional de Ética en Investigación del INCN) approved the study.
2.2. Statistical analysis
The study included a descriptive analysis of data from all medical records (n: 121) and a multivariate analysis using Cox regression for the relapsing-remitting Multiple Sclerosis (RRMS) group (n: 71). The descriptive analysis of the data was presented as percentages, mean values, and standard deviation or medians. The distribution of data was assessed with the Shapiro-Wilk test. Comparisons of continuous data among the groups were analyzed using the Kruskal-Wallis test or the Mann-Whitney U test, and categorical data were analyzed using the Pearson chi-square test or Fisher's exact test.
The exploratory analysis of time to diagnosis in the RRMS group was performed using Kaplan-Meier curves and the differences in time to diagnosis were compared using the log-rank test. For the construction of the Cox Regression model, we considered all covariates that had a p value less than 0.2 in the bivariate analysis (Table 1) and the possible interactions between variables, evaluating the different factors that can modify the time until diagnosis. Risks were adjusted for age and sex, it was modeled using Cox proportional hazards regression analysis that shows results for hazard ratio (HR) with 95% confidence intervals (CI). The proportionality assumptions of the Cox hazard regression models were evaluated based on the Schoenfeld residuals.
Table 1.
Bivariate analysis of time to diagnosis for all study variables.
| Variables | p∗ | |
|---|---|---|
| Smoking (yes/no) | 0.044 | |
| Gender (M/F) | 0.820 | |
| Relapses (0–2/≥3) | 0.003 | |
| Symptoms | Motor | 0.117 |
| Sensitive | 0.533 | |
| Cerebellum | 0.758 | |
| Visual | 0.633 | |
| Oculomotor | 0.030 | |
| Cognitive/Psychiatric | 0.426 | |
| Autonomic | … | |
| Gait | 0.160 | |
| Fatigue | … | |
| Paroxysms | 0.755 | |
| Pain | …. | |
| Onset syndrome | Brainstem | 0.0179 |
| Optic neuritis | 0.265 | |
| Myelitis | 0.346 | |
| Other syndromes | 0.145 | |
| Brain hemispheres | Periventricular | 0.118 |
| Corpus callosum | 0.089 | |
| Juxtacortical | 0.602 | |
| Cortical | 0.616 | |
| Optic Nerve | 0.181 | |
| Brainstem | Midbrain | 0.090 |
| Protuberance | 0.612 | |
| Bulb | 0.324 | |
| Cerebellum | Cerebellar Hemisphere | 0.216 |
| Vermis | … | |
| Cerebellar peduncle | 0.050 | |
| Spinal cord | Cervical | 0.155 |
| Thoracic | 0.136 | |
| Lumbar | 0.857 | |
| Oligoclonal bands | 0.256 | |
| MRi | T2 lesions | … |
| Number of lesions | 0.642 | |
| Black holes | 0.102 | |
p values <0.05, statistically significant.
Log rank test.
All analyzes were performed using two-sided p values, values below 0.05 within the model were considered statistically significant. Statistical analyses were carried out with Stata/SE 14.2 for Windows.
3. Results
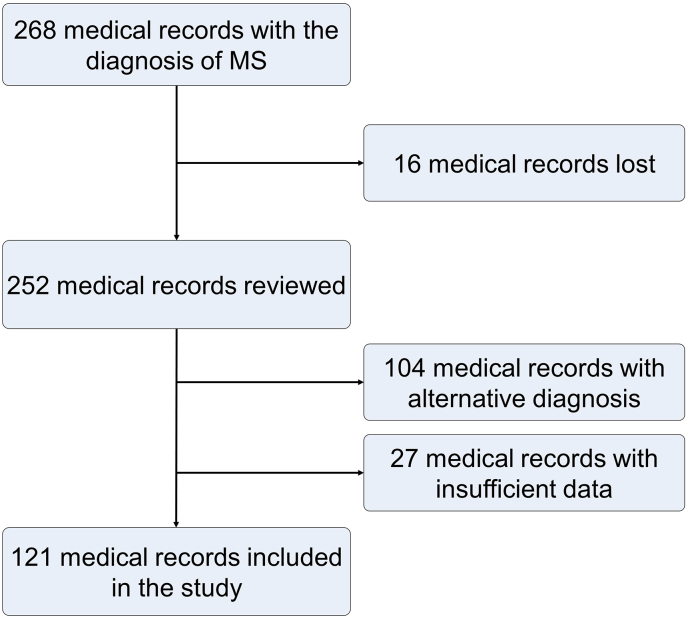
There were 268 medical records identified with the diagnosis of MS. Among these, 16 records were lost, 104 records corresponded to an alternative diagnosis (33 Neuromyelitis Optica, 23 to another CNS demyelinating disease), and 27 records had insufficient data. Among the other CNS demyelinating disease, 10 records corresponded to MS variants, 7 records to tumefactive demyelinating lesions, 4 records to isolated myelitis and 2 records to isolated optic neuritis. Finally, we included 121 records in this study (Figure 1).
Figure 1.
Flow chart of the study.
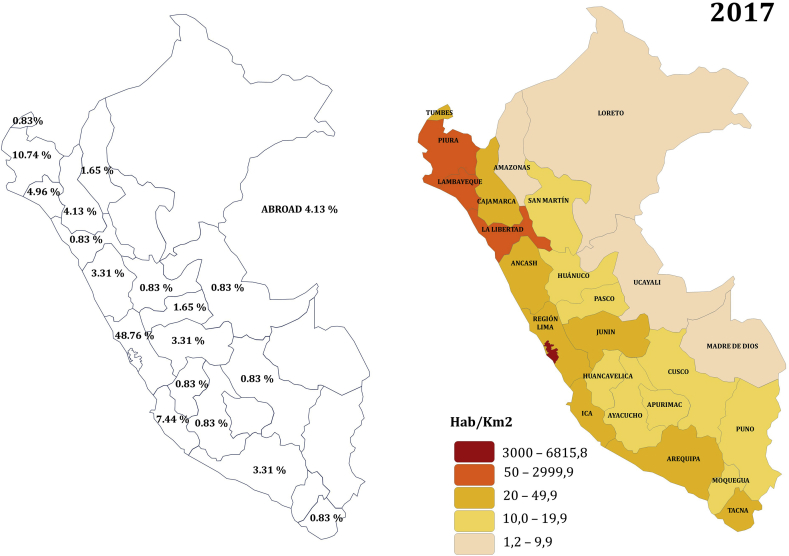
The largest percentages of patients (48.76%) were born in Lima (parallel 12°02′35″S); 27.31% of the patients were born in the north of the country (from parallel 0°01′48″S -9°31′40″S) and 13.24% in the south of the country (from parallel 14°4′4″S - 18°21′03″S). Figure 2 shows the distribution of patients in the country, and shows the population density of the country, data taken from the Instituto Nacional de Estadística e Informatica INEI (“National Institute of Statistics and Informatics”) [15].
Figure 2.
Distribution of patients (%) in the study. Population density of the country (2017). Source: Instituto Nacional de Estadística e Informática (INEI), reprinted with permission.
Women represent 57.85% of the cohort. The female/male ratio was 1.37/1, the mean age at diagnosis was 31.25 years (SD 11.2), and mean disease duration at the time of chart review was 30.32 months (SD 43.62). Disease phenotypes were not registered in 30.58% of the medical records, and misclassification of phenotypes were found in 36.36% of cases. The principal epidemiological and clinical characteristics are shown in Table 2. Almost half of the patients (46.28%) were receiving DMT by the time the chart was reviewed. Beta interferons were the most prescribed DMT (69.64%), followed by Glatiramer Acetate (5.36%), Teriflunomide (3.57%), Fingolimod (1.79%), Natalizumab (1.79%); and Cladribine (1.79%). Nearly a fifth of patients (17.86%) were receiving off-label medications (rituximab, cyclophosphamide, azathioprine, etc.).
Table 2.
Epidemiological and clinical characteristics.
| CIS (n:12) | RRMS (n:71) | SPMS (n:15) | PPMS (n:23) | TOTAL | p | ||
|---|---|---|---|---|---|---|---|
| Age at diagnosis | Mean (SD) | 32 (14.1) | 28 (9.8) | 37 (12.9) | 40 (9.9) | 31.24 (11.19) | 0.0002∗ |
| Gender | Female (70) | 7 (58.33 %) | 38 (53.52 %) | 12 (80.00 %) | 13 (56.52 %) | 70 (57.85 %) | 0.324∗∗ |
| Male (51) | 5 (41.67 %) | 33 (46.48 %) | 3 (20.00 %) | 10 (43.48 %) | 51 (42.15 %) | Reference group | |
| Onset syndrome | Optic neuritis | 6 (50%) | 13 (18.31%) | 3 (20.00%) | --- | 22 (18.18) | 0.005∗∗ |
| Brainstem syndrome | 4 (33.33%) | 18 (25.35%) | 2 (13.33%) | 3 (13.04%) | 27 (22.31) | 0.059∗∗ | |
| Myelitis | --- | 25 (35.21%) | 4 (26.67%) | 8 (34.78%) | 37 (30.58) | 0.130∗∗ | |
| Other syndromes | 2 (16.67%) | 15 (21.13%) | 6 (40.00%) | 12 (52.17%) | 35 (28.93) | Reference group | |
| Relapses at diagnosis | Median (IR) | 1 (1–1) | 2 (1–3) | 2 (2–3) | 0 (0–0) | 2 (1–3) | 0.0001∗ |
| Time to Diagnosis (months) Median (IR) |
Optic neuritis | 2.5 (2–5) | 12 (1–24) | 73 (24–859) | --- | 7 (2–24) | 0.059∗ |
| Brainstem syndrome | 1.5 (1–37.5) | 7 (2–13) | 48 (24–73) | 31 (14–61) | 8 (2–24) | 0.068∗ | |
| Myelitis | --- | 23 (3–49) | 43 (25–85) | 41 (16–55) | 28 (4–49) | 0.297∗ | |
| Other syndromes | 67 (24–110) | 24 (1–52) | 79 (24–122) | 18 (10–40) | 24 (11–61) | 0.128∗ | |
(∗) Kruskal-Wallis test; (∗∗) Chi2 test. p-value represents the comparison between different phenotypes for baseline variables. p values <0.05, statistically significant.
Brain MRI reports showed a predominance of periventricular demyelinating lesions (95.73%) followed by brainstem lesions (54.7%) and cerebellar lesions (43.59%). Cortical lesions were seen in 8 cases (6.84%), but not all MRI scans included double inversion recovery (DIR) imaging. Spinal cord MRIs were performed in 69 patients (57.02%), of them 81.16% reported cervical lesions, 50.72% thoracic lesions, and only 7.25% lumbar lesions. Contrast enhancement was reported in 65.28% of RRMS and 68.18% of PPMS cases; and only 18.8% of brain MRIs reported black holes.
Oligoclonal bands (OCB) were tested in the CSF and serum of 59 patients (48.76%), and 74.58% of them showed isolated OCB in the CSF. From the BSS patients, only 9 (33.3%) have been tested for CSF-OCB, from them 5 reported isolated OCB in CSF. Mean CSF cellularity and protein levels were within normal ranges (mean cell number 4.97 leucocytes/μl, mean protein level 33.93 mg%). Vitamin D was tested in 36.36% of patients (44 cases), and we found decreased levels in 81.82% of them (mean vitamin D level 27.07 ng/mL, normal range >30 ng/mL).
3.1. Time to diagnosis analysis in the RRMS group
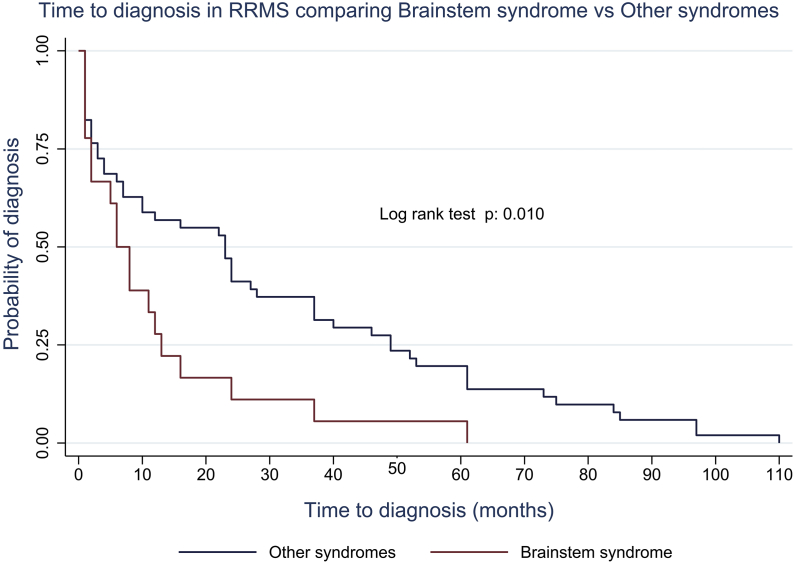
Variables included in the multivariable Cox regression model were all the variables described in the methods part. We presented only the variables that were significant when adjusting for age and sex. We analyzed the time to diagnosis for BSS versus other onset syndromes, we found a p value of 0.010 (Figure 3).
Figure 3.
Exploratory Kaplan-Meier analysis of time to diagnosis in RRMS comparing Brainstem syndrome vs other syndromes.
When observing the risk ratios within the Cox proportional hazards model, it's evidenced that those with BBS as an onset syndrome have much faster time to diagnosis compared to other syndromes (adjusted HR: 2.09; CI: 1.15–3.79). Additionally, the model was also significant regarding the presence of juxtacortical lesions, cerebellar peduncle lesions, and number of relapses at diagnosis (Table 3).
Table 3.
Cox proportional Hazard model for time to diagnosis in RRMS.
| Hazard ratio (crude) | Hazard ratio (adjusted) | p∗ | 95% CI | |
|---|---|---|---|---|
| BSS vs other syndromes | 1.93 | 2.36 | 0.029 | 1.15–3.79 |
| ON vs other syndromes | 0.96 | 1.27 | 0.549 | |
| Myelitis vs other syndromes | 1.0 | 1.26 | 0.526 | |
| Juxtacortical lesion | 1.10 | 2.32 | 0.021 | 1.15–6.13 |
| Cerebellar peduncle lesion | 0.60 | 0.55 | 0.033 | 0.29–0.94 |
| Number of relapses | 0.60 | 0.59 | 0.000 | 0.39–0.71 |
p values <0.05, statistically significant.
Adjusted HR for age and sex.
Although the Cox regression model showed that juxtacortical and cerebellar peduncle lesions were significant, the small number of patients with such lesions leads to a biased result. This is evident in the confidence interval, which is very close to no difference.
4. Discussion
The main finding from this study is that Peruvian patients presenting with a brainstem syndrome as the first manifestation were diagnosed as MS earlier than patients with presentations affecting other regions of the CNS. This suggest that it’s easier to fulfill dissemination in time and space when the first manifestation of the disease is a BSS, or the conversion rate after a CIS involving the brainstem is higher.
Tintoré and colleagues have investigated the conversion to MS on different topographies of CISs, the rate of conversion was 28% for ON, 36% for myelitis, 42% for BSS and 40% for other topographies [14, 16]. In a combined clinical and MRI follow-up study, Millner showed that after an interval of 16 months, conversion to MS was seen in 57% of patients with a brainstem syndrome and 42% of patients with a myelitis onset [17]. These previous studies show that the conversion rate for brainstem syndrome is higher than other topographies, which could be related to our findings and could explain why in our patients a brainstem syndrome is related to an early diagnosis.
Another explanation is that patients are more prone to seek neurologic evaluation when brainstem symptoms occur. Lesions appearing in infratentorial and spinal cord regions are usually more prone to affect clinically eloquent areas and are frequently associated with relapses in the same topography [18]. This could have led our patients with BSS to seek neurological attention faster than patients with other presentations, thereby reducing time to diagnosis.
It is also possible that Peruvian neurologist are more reassured of MS being the correct diagnosis when the onset is in the brainstem versus other locations, particularly since NMO, which classically affects the spinal cord and/or optic nerves, is one of the principal differential diagnoses when MS is suspected. Nevertheless, demyelinating lesions in the brainstem can be frequently found in other diseases such as NMOS spectrum disorders and Myelin Oligodendrocyte Glicoprotein antibody associated encephalomyelitis (MOGEM) [19, 20, 21, 22]. Making antibody testing very important in the differential diagnosis of such lesions. Finally, it is possible that this predisposition to diagnosis MS relates to unmeasured confounding.
Our results highlight the relevance of BSS as the initial manifestation and allowed us to have an earlier diagnosis of MS in such patients. Earlier diagnosis is important for the optimal timing for initiating DMT, thus identification of patients with a higher probability of conversion to MS is a key step in the decision of initiating treatment. Our findings emphasize the preferred localization of demyelinating lesions in MS. Infratentorial lesions have been considered specific for MS, and the presence of such lesions ranged from 6% in patients with optic neuritis to 40% in patients with CIS [23, 24, 25]. Tintoré et al. have showed that the presence of infratentorial lesions in CIS patients increased the risk of conversion to MS [18]. Therefore, MRI is the most informative predictive marker for conversion to MS [7, 16, 18].
Another result that caught our attention was that the majority of primary progressive multiple sclerosis (PPMS) patients showed gadolinium-enhancing lesions in T1-weighted imaging. This result differs with results from clinical trials, in the ORATORIO study [26] the percentage of patients with gadolinium-enhancing lesions was 27.5% for the treatment group and 24.7% for the placebo group. In the OLYMPUS study [27], 24.5% of patients showed gadolinium-enhancing lesions. Ingle et al. reported 42% gadolinium-enhancing brain lesions in PPMS patients whose duration of disease was less than 5 years [28]. One explanation for our results could be the younger age at diagnosis of PPMS patients (40 years) showing that younger patients present more inflammatory lesions; also a misclassification of patients as PPMS may play a confounding factor.
We found a higher proportion of patients in the North of the country compared with the proportion in the South. We expected to find more patients in the South of the county due to the latitudinal prevalence gradient. Risco et al. have shown a latitudinal prevalence gradient of MS in Latin American countries between Panama and Argentina, and determined an increase in the prevalence of 0.33 per 100000 per degree latitude [29]. This result caught our attention due to a possible inversion in the latitudinal gradient of MS, but when we analyzed this proportions with the population density of the country (Figure 2), we found that the proportion of cases was directly related to population density. The majority of patients belonged to Lima because the population density is the highest in this metropolitan city area, which represents 1/3 of Peruvian population. Furthermore, access to medical care and diagnostic methods is easier in the capital city. Our result is similar to a descriptive study that found that 65% of MS patients were born in Lima, and 11% in the North of Peru (Piura), where they postulated an explanation due to exposure to chemical substances in that region due to petrochemical industry and organic solvents [30]. Another explanation could be that there is a great deal of genetic difference between Lima and the other cities of the country, probably finding more Spanish ancestry in Lima.
Of the 268 patients initially identified as MS, 104 patients had an incorrect assignment of diagnosis. We found a misdiagnosis rate of 36.2%. Of these patients, 31.7% had neuromyelitis optica (NMO), 22.1% had another CNS demyelinating disease, and 46.15% corresponded to non-demyelinating disease (stroke in 7.69%, neoplasm in 4.81%, migraine in 2.88%, tropical spastic paraparesis in 1.92%, among the more representative). This rate can be compared with multiple studies that have indicated that 30–67% of referrals to MS subspecialty centers ultimately do not have MS [31, 32, 33]. Solomon et al. found 110 misdiagnosed patients from 4 academic MS centers, of them 6% were NMO spectrum disorders [32]. Kaisey et al. retrospectively reviewed new MS patients in two centers in USA and identified 36% of misdiagnosed patients [33]. Our results are consistent with the literature; interestingly we found a significant proportion of NMO among misdiagnosed patients, which could be related to ancestry. Misdiagnosis of MS remains a contemporary problem and is associated with long-term risk and morbidity for patients and considerable cost for health care systems [34].
The main limitation of our work is the retrospective recollection of data. The MS unit in our institution started in 2017, since then cases are systematically diagnosed and followed. Data before 2017 could be inexact due to the lack of standardized evaluation of demyelinating diseases. Recall bias for symptoms may also be one big limitation of our study, since the recalled first symptom, and date of presentation may be inaccurate. MRI data is also a limitation, since our results are based on radiology reports and we didn't have access to images. Moreover our cohort of patients is a small group from which to make big conclusion.
5. Conclusions
The present study findings show that brainstem syndrome as an initial presentation of MS in Peru is related to an earlier diagnosis. The population of this study has a wide representation of patients from different regions of our country. We found similar clinical, epidemiological, and imaging characteristics to other countries in Latin America.
Declarations
Author contribution statement
César Caparó-Zamalloa, Sheila Castro-Suarez, Jaqueline Cortez-Escalante, Wilfor Aguirre-Quispe, Erik Guevara-Silva, Victor Osorio-Marcatinco, María Meza-Vega: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to express their gratitude to Prof. Ellen Mowry (John's Hopkings University) and Prof. Orhan Aktas (Heinrich Heine Universität Düsseldorf) for giving intellectual advice to the present study.
References
- 1.Karussis D. The diagnosis of multiple sclerosis and the various related demyelinating syndromes: a critical review. J. Autoimmun. 2014 Mar;48–49:134–142. doi: 10.1016/j.jaut.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Frohman E.M., Racke M.K., Raine C.S. Multiple sclerosis--the plaque and its pathogenesis. N. Engl. J. Med. 2006 Mar 2;354(9):942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 3.Steinman L. Multiple sclerosis: a two-stage disease. Nat. Immunol. 2001 Sep;2(9):762–764. doi: 10.1038/ni0901-762. [DOI] [PubMed] [Google Scholar]
- 4.Vizcarra Escobar D., Kawano Castillo J., Castañeda Barba C., Chereque Gutierrez A., Tipismana Barbarán M., Bernabé Ortiz A. Prevalencia de Esclerosis Múltiple en Lima - perú. Rev. Méd. Hered. 2009 Jul;20(3):146–150. [Google Scholar]
- 5.Browne P., Chandraratna D., Angood C., Tremlett H., Baker C., Taylor B.V. Atlas of Multiple Sclerosis 2013: a growing global problem with widespread inequity. Neurology. 2014 Sep 9;83(11):1022–1024. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lublin F.D., Reingold S.C. Defining the clinical course of multiple sclerosis: results of an international survey. National multiple sclerosis society (USA) advisory committee on clinical trials of new agents in multiple sclerosis. Neurology. 1996 Apr;46(4):907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 7.Sellner J., Lüthi N., Bühler R., Gebhardt A., Findling O., Greeve I. Acute partial transverse myelitis: risk factors for conversion to multiple sclerosis. Eur. J. Neurol. 2008 Apr;15(4):398–405. doi: 10.1111/j.1468-1331.2008.02088.x. [DOI] [PubMed] [Google Scholar]
- 8.Wingerchuk D.M., Carter J.L. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin. Proc. 2014 Feb;89(2):225–240. doi: 10.1016/j.mayocp.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Lublin F.D., Reingold S.C., Cohen J.A., Cutter G.R., Sørensen P.S., Thompson A.J. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014 Jul 15;83(3):278–286. doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lublin F.D. New multiple sclerosis phenotypic classification. Eur. Neurol. 2014;72(Suppl 1):1–5. doi: 10.1159/000367614. [DOI] [PubMed] [Google Scholar]
- 11.Tintoré M., Rovira A., Auger C., Vidal A. Master in Neuroimmunology. eleventh ed. Viguera Editores SLU; Barcelona: 2018. Esclerosis Múltiple: clínica y diagnóstico; pp. 49–173. [Google Scholar]
- 12.Efendi H. Clinically isolated syndromes: clinical characteristics, differential diagnosis, and management. Noro Psikiyatr. Ars. 2015 Dec;52(Suppl 1):S1–11. doi: 10.5152/npa.2015.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller D.H., Chard D.T., Ciccarelli O. Clinically isolated syndromes. Lancet Neurol. 2012 Feb;11(2):157–169. doi: 10.1016/S1474-4422(11)70274-5. [DOI] [PubMed] [Google Scholar]
- 14.Montalban X. The importance of long-term data in multiple sclerosis. J. Neurol. 2006 Nov 1;253(6):vi9–15. [Google Scholar]
- 15.INEI Instituto Nacional de Estadística e Informática. Perú: Crecimiento y distribución de la población . 2017. Primeros Resultados [Internet]. Censos Nacionales 2017: XII de Población y VII de Vivienda. Lima - Perú; 2018; p. 48.https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1530/libro.pdf Available from: [Google Scholar]
- 16.Tintoré M., Rovira A., Rio J., Nos C., Grivé E., Téllez N. Is optic neuritis more benign than other first attacks in multiple sclerosis? Ann. Neurol. 2005 Feb;57(2):210–215. doi: 10.1002/ana.20363. [DOI] [PubMed] [Google Scholar]
- 17.Miller D.H., Ormerod I.E., Rudge P., Kendall B.E., Moseley I.F., McDonald W.I. The early risk of multiple sclerosis following isolated acute syndromes of the brainstem and spinal cord. Ann. Neurol. 1989 Nov;26(5):635–639. doi: 10.1002/ana.410260508. [DOI] [PubMed] [Google Scholar]
- 18.Tintore M., Rovira A., Arrambide G., Mitjana R., Río J., Auger C. Brainstem lesions in clinically isolated syndromes. Neurology. 2010 Nov 23;75(21):1933–1938. doi: 10.1212/WNL.0b013e3181feb26f. [DOI] [PubMed] [Google Scholar]
- 19.Wingerchuk D.M., Banwell B., Bennett J.L., Cabre P., Carroll W., Chitnis T. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015 Jul 14;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borisow N., Mori M., Kuwabara S., Scheel M., Paul F. Diagnosis and treatment of NMO spectrum disorder and MOG-encephalomyelitis. Front. Neurol. 2018;9:888. doi: 10.3389/fneur.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fan S., Xu Y., Ren H., Guan H., Feng F., Gao X. Comparison of myelin oligodendrocyte glycoprotein (MOG)-antibody disease and AQP4-IgG-positive neuromyelitis optica spectrum disorder (NMOSD) when they co-exist with anti-NMDA (N-methyl-D-aspartate) receptor encephalitis. Mult. Scler. Relat. Disord. 2018 Feb;20:144–152. doi: 10.1016/j.msard.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Jarius S., Paul F., Aktas O., Asgari N., Dale R.C., de Seze J. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J. Neuroinflammation. 2018 May 3;15(1):134. doi: 10.1186/s12974-018-1144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs L., Munschauer F.E., Kaba S.E. Clinical and magnetic resonance imaging in optic neuritis. Neurology. 1991 Jan;41(1):15–19. doi: 10.1212/wnl.41.1.15. [DOI] [PubMed] [Google Scholar]
- 24.Sailer M., O’Riordan J.I., Thompson A.J., Kingsley D.P., MacManus D.G., McDonald W.I. Quantitative MRI in patients with clinically isolated syndromes suggestive of demyelination. Neurology. 1999 Feb;52(3):599–606. doi: 10.1212/wnl.52.3.599. [DOI] [PubMed] [Google Scholar]
- 25.Brex P.A., O’Riordan J.I., Miszkiel K.A., Moseley I.F., Thompson A.J., Plant G.T. Multisequence MRI in clinically isolated syndromes and the early development of MS. Neurology. 1999 Oct 12;53(6):1184–1190. doi: 10.1212/wnl.53.6.1184. [DOI] [PubMed] [Google Scholar]
- 26.Montalban X., Hauser S.L., Kappos L., Arnold D.L., Bar-Or A., Comi G. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 2017 19;376(3):209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 27.Hawker K., O’Connor P., Freedman M.S., Calabresi P.A., Antel J., Simon J. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann. Neurol. 2009 Oct;66(4):460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 28.Ingle G., Sastre-Garriga J., Miller D., Thompson A. Is inflammation important in early PPMS? a longitudinal MRI study. J. Neurol. Neurosurg. Psychiatry. 2005 Sep;76(9):1255–1258. doi: 10.1136/jnnp.2004.036590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Risco J., Maldonado H., Luna L., Osada J., Ruiz P., Juarez A. Latitudinal prevalence gradient of multiple sclerosis in Latin America. Mult. Scler. 2011 Sep;17(9):1055–1059. doi: 10.1177/1352458511405562. [DOI] [PubMed] [Google Scholar]
- 30.Vizcarra-Escobar D., Cava-Prado L., Tipismana-Barbarán M. Multiple sclerosis in Peru. A clinical-epidemiological description of a series of patients. Rev. Neurol. 2005 Nov 16;41(10):591–595. [PubMed] [Google Scholar]
- 31.Solomon A.J., Naismith R.T., Cross A.H. Misdiagnosis of multiple sclerosis: impact of the 2017 McDonald criteria on clinical practice. Neurology. 2019 01;92(1):26–33. doi: 10.1212/WNL.0000000000006583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solomon A.J., Bourdette D.N., Cross A.H., Applebee A., Skidd P.M., Howard D.B. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology. 2016 Sep 27;87(13):1393–1399. doi: 10.1212/WNL.0000000000003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaisey M., Solomon A.J., Luu M., Giesser B.S., Sicotte N.L. Incidence of multiple sclerosis misdiagnosis in referrals to two academic centers. Mult. Scler. Relat. Disord. 2019 May;30:51–56. doi: 10.1016/j.msard.2019.01.048. [DOI] [PubMed] [Google Scholar]
- 34.Solomon A.J. Diagnosis, differential diagnosis, and misdiagnosis of multiple sclerosis. Continuum (Minneap Minn) 2019 Jun;25(3):611–635. doi: 10.1212/CON.0000000000000728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.