Abstract
Aim
To determine the patterns of distribution of refractive errors in a clinical sample of patients examined in an optometry practice.
Method
In this retrospective study, the clinic records of 6687 patients aged 6 to 85 years comprising 2168 (32.2%) males and 4519 (67.5%) females were reviewed. Refractive error were analysed according to gender, age, as well as types and categories including axis of astigmatism using the vector power analysis method where the traditional sphero-cylinder was transformed into J0 (primary) and J45 (oblique) astigmatic components.
Results
Only the analysis for right eye was reported as right and left spherical equivalent were similar. The mean with standard deviations for refractive errors were: Myopia: −1.95 ± 2.6, hyperopia: 1.04 ± 0.9, astigmatism: −1.22 ± 0.71 and anisometropia: −0.01 ± 2.5 DS. The distributions with significant changes included males that were significantly more myopic and astigmatic, while females were more hyperopic across the age groups. Furthermore, myopia decreased, while hyperopia, astigmatism and anisometropia increased with increasing age. Unique findings from this study include: myopia peaked earlier, second hyperopic shift commenced after age 82 years and the distribution of severity of astigmatism contrasts with previous understanding.
Conclusion
Although the patterns of distribution of refractive errors in patients aged 6 to 85 years corroborates previous findings, myopia and hyperopia peak, as well as severity of astigmatism were unique to the present study. Results from non-clinic populations will be useful to confirm trends reported in this study.
Keywords: Patterns of distribution, Refractive errors, Optometry practice, Clinical population
Introduction
Uncorrected refractive error (URE) being one of the most prevalent visual anomalies across age groups and the second leading cause of treatable blindness worldwide,1, 2, 3 is a major public health concern.1, 2, 3 Uncorrected refractive error is associated with several symptoms such as impair distance and near vision, and can cause binocular vision anomalies with associated symptoms of asthenopia.4 Visual impairment from myopic pathologies such as glaucoma, retinal tears and detachments,4 as well as high economic and financial consequences of URE have been reported.3, 4 Reduced vision-related quality of life, decreased performance and productivity, as well as loss of independence,5 especially amongst the elderly population5 are also associated with URE.
Given the clinical and economic impact of URE, continued research from various practice settings will advance knowledge on URE and the associated concerns. Although studies on non-clinical settings give more precise prevalence estimates than clinic-based studies, large data from clinical settings enable extensive study of the characteristics of vision anomalies of interest.6 Studies on clinical settings include University optometry clinics, hospital eye clinics and independent optometric practices. For reviews and discussions in the present study, our focus is on clinic-based studies7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 which reported on age and gender patterns of distribution of refractive error. Available clinic-based studies include Queiros et al.8 who analyzed records of 4288 patients aged 4 to 89 years who were examined from five ophthalmologic and four optometric clinics in Portugal. With comparable age range to Queiros et al.,8 Gomez-Salazar et al.9 studied records of 6 to 90 years old patients collected from optometry clinics in 14 states of Mexico. In Canada, Hrynchak et al.10 analyzed 5885 records of patients aged between two months and 92 years who were examined at the University of Waterloo optometry clinic. Four available studies on African populations are reviewed.11, 12, 13, 14 In South Africa, Raliavhegwa and Oduntan analysed records of patients aged between four and 110 years who attended the University of Limpopo optometry clinic.11 With a similar study setting, Kragha13 analysed records of 822 patients aged between three and 62 year old patients who attended the University of Benin optometry clinic in Nigeria. Malu and Ojiabo12 analysed records of 601 patients aged between 5-85 years who attended the eye clinic of a tertiary hospital. Clinic-based studies from University clinics are prone to additional demerit as data were collected by various student clinicians and findings could differ markedly.
The consistent findings from studies reviewed7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 include that myopia was the most prevalent in school-aged children and its prevalence decreased with increasing age, hyperopia was most prevalent in adults aged older than 40 years, increased in prevalence with advancing age and similar trend reported for astigmatism.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 However, the specific age at which the URE peaked and shifted (changes in trend) occurred differed across studies.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 The unique aspects of the present study is that limitations of previous studies which included a lack of scientific rigour in analysis are addressed. For example, the pattern of distribution of types, severity and categories of myopia, hyperopia, anisometropia and astigmatism are presented extensively across a broad age range.
Also, several approaches were applied to establish the trend of distribution of refractive errors including the vector method was applied to analyse astigmatism. Furthermore, given that refractive error is influenced by factors such as race and ethnicity, data from this Black population collected from an independent optometry practice could give a new perspective to existing literature. Therefore, the aim of the present study was to determine the pattern of distribution of refractive errors in children and adults. Findings will be useful in epidemiology – teaching, clinical practice and in translational research.
Methods
Study design and setting
This was a cross sectional retrospective review of 6687 eligible case records of patients seen in the author’s optometry practice in Empangeni, South Africa from 2017 to 2019. Empangeni is a town in the uMhlathuze municipality which is an administrative area in the uThungulu district of KwaZulu-Natal, South Africa. The City of uMhlathuze is situated on the north-east coast of the province of KwaZulu-Natal, approximately 170 kilometers north-east of Durban and borders a coastline that spans approximately 45 kilometers long.4
Routinely, we request patients consent by requiring them to sign on the patient cards and it is explained to them that their names, telephone numbers and details of eye examination are not revealed to anybody for any reason and were assured that their data could be used only for research purposes. Parents or guardians consented for minors. The study protocol was approved by the Biomedical Research Ethics Committee ((BE096) of the University of KwaZulu-Natal, South Africa and the conduct of the study complied with guidelines in accordance with the Declaration of Helsinki.
Sample and participants
The participants comprise consecutive patients, mainly (approximately 99.5%) Black South Africans of the Zulu ethnic group who consulted at the first author’s (SOW) practice. The patients who attended the optometry practice for routine eye care are persons who resided around the town and surrounding villages. Analysis of the practice’s residential demography reveals that patients come from about 25 residential areas including “townships” and villages. The patients studied comprised 2152 males and 4527 females. Patients’ records were excluded from the study if they contained records of any eye disease or systemic conditions such as diabetes mellitus that could have influence on refractive findings. Blood pressure and blood sugar levels were routinely checked at the practice and patients whose range of blood glucose was abnormal were excluded and referred to physicians. Patients who had cataract surgeries were also excluded as it is intended to avoid the possible influence of cataract surgery-induced astigmatism.
Data collection procedure
In the first author (SOW) practice, visual acuity (VA) was routinely assessed using the projected Snellen chart ocular, and health status was evaluated using the direct ophthalmoscope (Welch Allyn) and the slit lamp biomicroscope (Zeiss SL120/130). Refractive errors were assessed objectively using an autorefractor (MRK-3100; Huvitz), the Welch Allyn streak retinoscope and refined subjectively to the best visual acuity with maximum convex (positive) and minimum concave (negative) lenses, monocularly and binocularly. Children younger than 13 years were routinely cyclopleged with a subsequent subjective refraction which was used for analysis. Astigmatic power and axis was refined using the Jackson cross cylinder. Accommodative and vergence measures were assessed using the routine optometric techniques and the reports on accommodative and vergence anomalies and associations with patient-reported symptoms are in preparation.
A trained employee of the practice extracted relevant cases based on outlined criteria and data were transferred into Microsoft excel. For patients who consulted more than once during the survey period, only the data from the most recent visit was recorded thus ensured that no patient’s data was duplicated. For all the cases extracted for analysis, patients’ demographic information – gender, age, occupation, residential areas, as well as the best corrected visual acuities and final subjective refraction were extracted. The refractive measures extracted were the sphere, cylinder power and axis and the subjective findings were analysed.
Data analysis
All data were reviewed by the authors and analyzed by a statistician using the Statistical Package for Social Sciences (SPSS) version 21 (SPSS for Windows Inc., Chicago, IL, USA). Descriptive statistics were presented with means and standard deviation. Distributions of variables were presented using tables, and histograms. Percentages and corresponding 95% confidence intervals were presented as an estimate of the prevalence. Pearson Chi-Squared test was used to analyze percentage differences in refractive error in relation to gender and age groups while the Analysis of Variance (ANOVA) and the t-test were used to analyze differences in means for two or more groups respectively. In all analyses, a p value of ≤ 0.05 was considered statistically significant.
The age groups were stratified into age intervals of 10, except for the oldest age group with fewer patients’ numbers.
Diagnostic criteria and classifications of outcome variables
The refractive status was assigned based on the eye with the greater ametropia and following the classification applied to define the types of refractive errors.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Absolute values (not spherical equivalent) of refraction were used for analysis. The criteria for the refractive errors are indicated as used in previous studies.7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 31 (Table 1).
Table 1.
Diagnostic criteria for myopia, hyperopia, anisometropia and astigmatism.
| Types of refractive error and criteria |
|---|
| Myopia (−0.50 DS) |
| ● Mild: −0.5 to −3.0 DS |
| ● Moderate: −3.25 to −6.0 DS |
| ● High: >−6. 25 DS |
| Hyperopia (≥0.50 DS) |
| ● Mild: 0.50 to 2.0 DS |
| ● Moderate: 2.25 to 4.00 DS |
| ● High: ≥4.25 DS |
| Anisometropia (− 0.75 or 0.75) |
| − 0.75 or 0.75 REM difference between both eyes23 |
| ● low: 0.75 to 2 |
| ● Moderate: 2.25 to3 |
| ● High: >3 |
| Astigmatism ≥/−0.75/DC |
| Astigmatism is presented in negative power notation and was further categorized as: |
| a) Magnitude-astigmatism |
| ● Low astigmatism (LA): 0.25 to 0.50 DC |
| ● Moderate astigmatism (MA): 0.75 to 2 DC |
| ● High astigmatism (HA): >2.00 DC |
| b) Axis astigmatism#### |
| Any amount of astigmatism- with With-the-rule (WTR), against-the-rule (ATR) and oblique (OA) |
| c) Sphero-astigmatism |
| a) Simple myopic astigmatism (SMA): when one ray comes into focus in front of the retina and one ray comes into focus on the retina. (Plano/≥− 0.50 D). |
| b) Compound myopic astigmatism (CMA): occurs when both point of light come into focus in front of the retina. (≥ −0.5 D/≥ − 0.5 D). |
| c) Hyperopic astigmatism (HPA): occurs when one ray comes into focus in front of the cornea and the other ray comes into focus behind the retina. (+0.5 D/≥ − 0.5 D). |
| Emmetropia: ≤ ± 0.50 spherical equivalent refraction (SER), defined as sphere + half cylinder power |
REM = right eye spherical equiuvalent.
Vector method analysis for astigmatism####
The traditional clinical representations of refraction using sphere, cylinder, and axis, are not adequate for quantitative analysis.10, 16, 24, 29,30 Unlike the traditional notation, a major advantage of the vector method is that it enables the means of cylinders with different axis to be summarised and added without losing values10, 16, 24, 29,30 In addition, the approach is useful in vision research and clinical practice.10, 16, 24, 29,30 Therefore, the transformations, J0 and J45 in the vector notation10, 16, 24, 29,30 was used to analyze axis-astigmatism. The conversion of the conventional sphere, cylinder and axis into vector transformation was achieved using the formula: 10, 16, 24, 29,30
| M = S + C/2J0 = −1/2 C cos (2 α) J 45 = − ½ C sin (2 α) where M = spherical equivalent, |
| S = sphere power, |
| C = negative cylinder power and |
| α = cylinder axis in degrees. |
The J0 component may be considered a Jackson cross cylinder (JCC) with axes at 180° and 90°. Positive values of J0 indicate With-The-Rule (WTR) astigmatism while negative values indicate Against-The-Rule astigmatism (ATR). The J45 describes a cross-cylinder set at 45° and 135°, representing oblique astigmatism. A positive value for J45 denotes that the negative cylinder axis of the JCC is at 45° and is termed levo-oblique astigmatism.10, 16, 24, 29,30 A negative value of J45 denotes that the negative cylinder axis of the JCC is at 135° and is referred to as dextro-oblique.10, 16, 24, 29,30 Furthermore, for axes of 90 and 180, J45 would be zero and for axes from 1 to 89, J45 would have a positive value. For axes from 91 to 179, J45 would be a negative number.10, 16, 24, 29,30 The means were obtained using the vector method and used for analysis.
Results
Patients’ demographics
During the study period, data for 6687 patients’ records which met the inclusion criteria were analysed. The descriptive data which included the means and standard deviation (SD) for age (years) for males were 38.7 ± 17.10 years (median: 39, frequency: 2168, 32.2%) and 37.7 ± 17.1 years (median: 39, frequency: 4519, 67.8%) To summarize findings, data for the sub-age groups 6–40 (hypothetically pre-presbyopic) and 40–85 (hypothetically, presbyopic) is presented. Additional demographic information is shown in Fig. 1.
Figure 1.
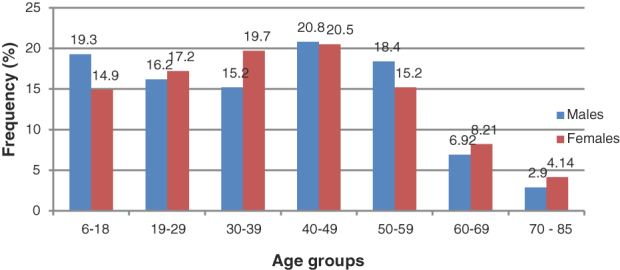
Frequency distribution of the patients’ age groups.
Descriptive statistics for outcome variables
Outcome variables were all refractive parameters. There was no significant difference between right (RE) and left eye (LE) (0.49, p = 0.62), therefore only the RE is presented. The descriptive statistics for right eye visual acuity, rsphere, spherical equivalent (REM), cylinder power and axis are indicated in Table 2.
Table 2.
Descriptive statistics for visual acuity and refractive variables
| Clinical measures | Mean | SD | Median | Min | Max | 1st quart | 3rd quart | Skew | Kurtosis |
|---|---|---|---|---|---|---|---|---|---|
| Right eye distance visual acuity | 0.72 | 0.32 | 0.80 | 0.10 | 1.00 | 0.51 | 1.00 | −0.89 | −0.65 |
| Right eye sphere | 0.17 | 1.44 | 0.25 | −25.00 | 16.02 | 0.02 | 0.50 | −4.17 | 55.26 |
| REM | −0.054 | 1.51 | 0.00 | −25.01 | 12.01 | −0.25 | 0.38 | −4.79 | 55.94 |
| Right eye cylinder | −0.52 | 0.52 | −0.50 | −6.00 | 1.30 | −0.50 | −0.25 | −3.33 | 18.86 |
| J45 | 0.02 | 0.25 | 0.05 | −2.49 | 15.39 | −0.02 | −0.03 | 34.25 | 2088.50 |
| J0 | 0.05 | 0.38 | 0.04 | −2.51 | 19.70 | −0.13 | 0.13 | 21.05 | 1085.18 |
REM = right eye spherical equivalent, J45=oblique. J0=primary axis, SD = standard deviation, min = minimum, max = maximum, quart = quartile. All values are expressed in diopters.
Overall descriptive statistics for spherical and cylinder errors
For all outcome variables, descriptions are presented for means and SD, as well as in percentage of prevalence. Regarding gender, the overall mean sphere was positive and significantly higher in females (0.22 ± 1.46 D) than in males (0.06 ± 1.39 D), (t = 46.55, p = 0.01). For spherical equivalent (REM), males were myopic (−0.18 ± 0.45 D) while females were hyperopic (0.02 ± 1.54 D) (t = 53.4, p = 0.001). Males had significantly higher mean cylinder power (−0.56 ± 0.45 D) than females (0.50 ± 0.49 D) (t = 15.1, p = 0.001), J45 axis orientation was also significantly higher in males (0.03 ± 0.20 D) than in females (0.01 ± 0.27 D) (t = 2.46 p = 0.01) ,whereas J0 axis did not differ between males (−0.05 ± 0.31 D) and females (0.03 ± 0.41 D) (t = 0.29, p = 0.58).
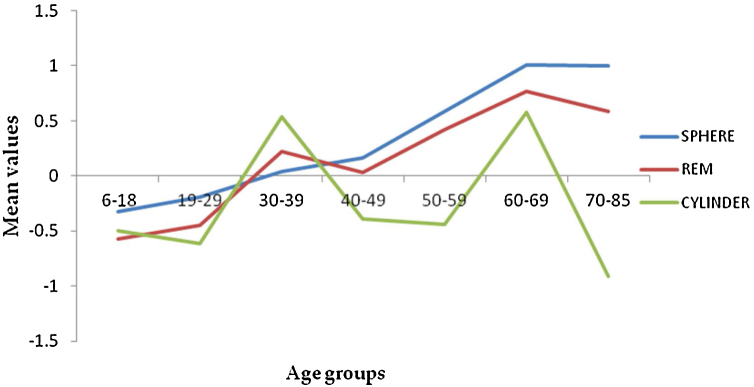
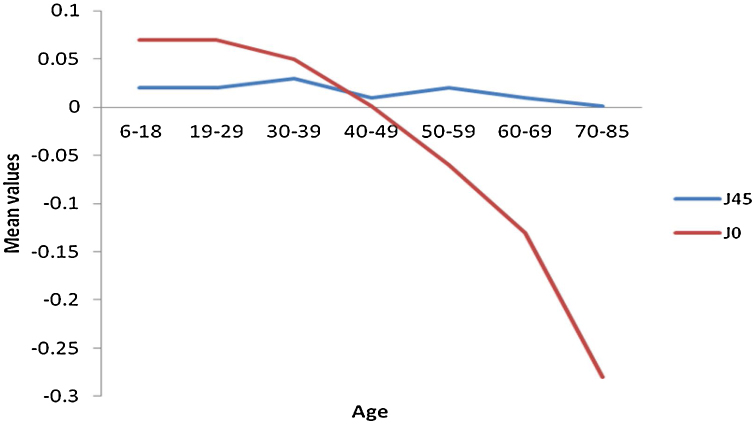
The frequency distribution for spheres and REM shows a myopic to hyperopic shift from age group 30-39 years with the second myopic shift at age group 70–85 years (Fig. 2) (specifically on ages 81 and 82) and a subsequent hyperopic trend from 83 years (Appendix A). There was a decrease in correcting cylinder power at age group 30–39 years followed by a consistent increased frequency from age group 40–49 to 70–85 years. (Fig. 2). Axis J45 was consistently at levo-oblique (positive) position, decreasing in values across all age groups whereas (J0) changed from WTR to ATR from age 50–59 till above 70–85 years (Fig. 3).
Figure 2.
Mean values for sphere, spherical equivalent (REM) and cylinder power.
Figure 3.
Mean values of vector refractive components in cylinder axis J45 and J0.
Distribution of refractive error
Spheres
Myopia was significantly more prevalent in males than in females (p = 0.01) and peaked (18.7%) at age group 6–18 years. (Table 3). The prevalence of myopia decreased significantly with increasing age with a subsequent increase to 15.8% (second myopic peak) at age 70–85 years. (Table 3). On severity (Table 4), low myopia was significantly most frequent (83.9%) and only the prevalence of high myopia was consistent and it decreased consistently with increasing age. (Table 4).
Table 3.
Gender and age distribution of refractive error: means with standard deviations and percentages.
| Myopia |
Hyperopia |
Emmetropia |
Astigmatism |
Anisometropia |
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | n | % | Mean | SD | n | % | Mean | SD | n | % | Mean | SD | n | % | Mean | SD | n | % | |||||||
| ALL | 6687 | −1.95 | 2.60 | 871 | 12.9 | 1.04 | 0.95 | 2373 | 35.2 | 0.11 | 0.17 | 3480 | 51.7 | −1.22 | 0.71 | 1202 | 21.0 | − 0.011 | 2.56 | 482 | 7.1 | ||||||
| 95% CI | 12.1–13.2 | 34.1–36.4 | 50.5–52.9 | 22.0–23.3 | 6.5–7.8 | ||||||||||||||||||||||
| Gender | |||||||||||||||||||||||||||
| Males | 2168 | −1.92 | 2.41 | 331 | 15.2 | 0.32 | 0.47 | 680 | 31.2 | 0.09 | 0.18 | 1139 | 52.9 | −1.22 | 0.75 | 461 | 21.4 | −0.12 | 2.35 | 200 | 9.2 | ||||||
| Females | 4519 | −1.99 | 2.73 | 532 | 11.0 | 0.37 | 0.48 | 1668 | 36.1 | 0.12 | 0.17 | 2320 | 51.2 | −1.22 | 0.69 | 729 | 16.1 | 0.05 | 2.70 | 311 | 6.3 | ||||||
| t- test/ χ2 | 0.38 (0.70) | 16.6 (0.01)a | 4.23 (0.01)a | 17.5 (0.01)a | 5.12 (0.01)a | 1.52 (0.21) | 0.01(0.99) | 28.7(0.01)a | 0.69 (0.48) | 11.7 (0.01)a | |||||||||||||||||
| Ages groups | |||||||||||||||||||||||||||
| 6-18 | 1088 | −2.53 | 3.65 | 204 | 18.7 | 0.80 | 1.32 | 156 | 14.3 | 0.07 | 0.19 | 720 | 66.1 | −1.40 | 0.86 | 164 | 15.0 | −0.57 | 5.44 | 68 | 6.2 | ||||||
| 19-29 | 1132 | −1.98 | 2.28 | 208 | 18.3 | 0.59 | 0.26 | 166 | 14.6 | 0.13 | 0.17 | 757 | 66.8 | −1.45 | 1.00 | 181 | 15.9 | −0.49 | 2.11 | 73 | 6.4 | ||||||
| 30-39 | 1221 | −1.68 | 1.88 | 159 | 13.0 | 0.60 | 0.38 | 342 | 28.3 | 0.16 | 0.16 | 719 | 58.8 | −1.22 | 0.78 | 163 | 13.3 | -0.15 | 1.60 | 55 | 4.5 | ||||||
| 40-49 | 1382 | −1.87 | 2.72 | 118 | 8.5 | 0.80 | 0.90 | 459 | 33.2 | 0.10 | 0.15 | 805 | 58.2 | −1.03 | 0.45 | 188 | 18.6 | -0.21 | 1.89 | 73 | 5.2 | ||||||
| 50-59 | 1091 | −1.59 | 2.33 | 90 | 8.2 | 1.14 | 0.69 | 658 | 60.3 | 0.11 | 0.16 | 343 | 31.4 | −1.04 | 0.41 | 203 | 18.6 | 0.02 | 1.72 | 96 | 8.7 | ||||||
| 60-69 | 521 | −1.51 | 1.21 | 43 | 8.2 | 1.14 | 0.69 | 393 | 75.4 | 0.10 | 0.17 | 84 | 16.1 | −1.12 | 0.50 | 154 | 29.5 | 0.19 | 2.32 | 81 | 15.5 | ||||||
| 70-85 | 252 | −1.76 | 1.02 | 40 | 15.8 | 1.78 | 1.54 | 179 | 71.2 | 0.05 | 0.16 | 33 | 13.0 | −1.31 | 0.67 | 138 | 54.7 | 0.02 | 2.57 | 62 | 17.0 | ||||||
| ANOVA/ χ2 | 2.50 (0.02)a | 1209.0 (0.01)a | 69.19 (0.01)a | 1256.6 (0.01)a | 20.44 (0.01)a | 844.1 (0.01)a | 10.75 (0.01)a | 355.5 (0.01)a | 1.17 (0.30) | 184 (0.01)a | |||||||||||||||||
| 6-40 | 3564 | −2.09 | 2.75 | 586 | 16.4 | 0.64 | 0.74 | 707 | 19.8 | 0. 12 | 0.18 | 2261 | 65.3 | −1.36 | 0.89 | 528 | 44.3 | -0.08 | 3.55 | 203 | 39.9 | ||||||
| 41-85 | 3123 | −1.71 | 2.27 | 276 | 8.8 | 1.21 | 0.99 | 1646 | 52.7 | 0.10 | 0.16 | 1200 | 34.6 | −1.11 | 0.51 | 663 | 55.6 | 0.02 | 1.65 | 305 | 60.4 | ||||||
| t-test/p- | 6687 | 1.96 (0.05) | 85.7 (0.01)a | 14.0(0.01) | 782.9(0.01) | 3.1 (0.02) | 421.1(0.01)a | 6.2 ( 0.01) | 96.4 (0.01) | −0.4 (0.67) | 38.5 (0.01)a | ||||||||||||||||
= significant association CI = confidence interval, SD = standard deviation, n = frequency in numbers, % = percent.
Table 4.
Gender and age distribution of severity of myopia, hyperopia and anisometropia
| Myopia |
Hyperopia |
Anisometropia |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mild |
Moderate |
High |
Mild |
Moderate |
Severe | Mild |
Mod |
Severe |
|||||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||||||
| Gender | |||||||||||||||||||||||
| Males | 278 | 12.9 | 16 | 0.7 | 500 | 23.2 | 635 | 29.5 | 40 | 1.8 | 5 | 0.2 | 76 | 3.5 | 8 | 0.3 | 10 | 0.4 | |||||
| Females | 445 | 9.8 | 18 | 0.3 | 32 | 0.7 | 1515 | 70.4 | 139 | 3.0 | 14 | 0.3 | 149 | 3.2 | 14 | 0.3 | 7 | 0.1 | |||||
| χ2 (p-value) | 25 (0.01)a | 0.05 (0.94) | 298 (0.01)a | 243.0 (0.01)a | 36.6 (0.01)a | 2.02(0.15) | 15.0(0.21) | 0.6 (0.43) | 0.09 (0.77) | ||||||||||||||
| Age groups | |||||||||||||||||||||||
| 6–18 | 158 | 14.5 | 8 | 0.7 | 25 | 2.3 | 150 | 3.7 | 3 | 0.2 | 3 | 0.2 | 28 | 2.5 | 4 | 0.3 | 1 | 0.0 | |||||
| 19–29 | 174 | 15.4 | 8 | 0.7 | 13 | 1.1 | 165 | 14.1 | 3 | 0.0 | 0 | 0.0 | 29 | 2.5 | 7 | 0.6 | 7 | 0.6 | |||||
| 30–39 | 135 | 11.0 | 8 | 0.6 | 6 | 0.4 | 337 | 27.6 | 4 | 0.3 | 1 | 0.0 | 20 | 1.6 | 1 | 0.0 | 2 | 0.1 | |||||
| 40–49 | 98 | 17.0 | 4 | 0.2 | 7 | 0.5 | 446 | 32.2 | 10 | 0.7 | 3 | 0.2 | 28 | 2.0 | 3 | 0.6 | 2 | 0.1 | |||||
| 50–59 | 82 | 7.5 | 3 | 0.2 | 2 | 0.1 | 608 | 55.7 | 48 | 4.3 | 2 | 0.1 | 44 | 4.0 | 4 | 0.3 | 2 | 0.1 | |||||
| 60–69 | 39 | 7.4 | 2 | 0.3 | 0 | 0.0 | 317 | 60.8 | 72 | 13.8 | 4 | 0.7 | 45 | 8.6 | 2 | 0.3 | 2 | 0.3 | |||||
| 70–85 | 36 | 14.2 | 1 | 0.4 | 0 | 0.0 | 134 | 53.1 | 38 | 15.0 | 7 | 2.7 | 29 | 11.5 | 2 | 0.7 | 1 | 0.4 | |||||
| χ2/p-value | 86.25 (0.01)a | 97.00 (0.01)a | 131.70 (0.01)a | 1126.1(0.01 | 1099 ( 0.01)a | 862.41 (0.01)a | 12.17(0.01)a | 0.07 (0.79) | 0.09 (0.76) | ||||||||||||||
| 6–40 | 479 | 13.4 | 25 | 0.7 | 44 | 1.2 | 694 | 19.4 | 9 | 5.11 | 4 | 0.11 | 2456 | 68.9 | 450 | 12.6 | 78 | 2.1 | |||||
| 41–85 | 243 | 7.7 | 9 | 0.2 | 9 | 0.2 | 1463 | 46.8 | 167 | 94.8 | 16 | 0.5 | 1079 | 34.5 | 635 | 20.3 | 28 | 0.8 | |||||
| χ2/p-value | 51.3 (0.01)a | 4.2 (0.04)a | 14.8 (0.01)a | 184.6(0.01)a | 101.9 (0.01)a | 3.8 (0.05)a | 362.9 (0.01)a | 20.7 (0.01)a | 15.1(0.01) | ||||||||||||||
= significant association. CI = confidence interval, SD = standard deviation, n = frequency in numbers, % = percent.
The prevalence of hyperopia and its categories were significantly higher in females (p = 0.001) and hyperopia was least prevalent (14.3%) at age group 6–18 years and most prevalent (75%) at age 60–69 years, existing then a decrease to 71.2% at age 70–82 years (Table 3 and Appendix A) and an increase in hyperopia again at 83 to 85 years. (Appendix A). Low hyperopia was the most prevalent category in terms of severity and remarkably all the severities of hyperopia increased significantly with advancing age (Table 4).
Anisometropia
Anisometropia was significantly more prevalent in males. (11.7, p = 0.01). The frequency of anisometropia increased consistently across the ages, with a shift in the mean values (Table 4) from myopia to hyperopia at age 50–59 years till 70–85 years. All categories of anisometropia increased with increased age. (Table 4).
Astigmatism
The frequency of right and left eye astigmatism were similar (p = 0.99) and therefore only the RE cylinder was reported to avoid duplication of findings. The overall prevalence of astigmatism was 21% (1202) while 79% of eyes (4521) did not have astigmatism (NA). The prevalence of astigmatism increased consistently with advancing age from 13.3% at age 30–39 years to the highest frequency of 54.7% at age 70–85 years (Table 3).
Types of astigmatism
Distribution of astigmatism data is presented according to axis (WTR, ATR and OA), spherical component associated (simple myopic, compound and hyperopic) and the amount/severity (low, moderate and high) inin Table 5. For axis astigmatism, gender was not associated with WTR astigmatism (p = 0.45) and it decreased significantly and consistently with advancing age (p = 0.01) (Table 5). With- the-rule astigmatism was the most frequent, with a prevalence of 33.9%, and the WTR to ATR shift occurred at age group 40–49 years, with no difference in gender (Table 5). Against-the rule astigmatism was significantly more frequent in males than in females (p = 0.001) and significantly with age. Likewise, its prevalence consistently increased from 40–49 years (17.4%) to more than 70 years (47.2%). Oblique astigmatism was significantly more frequent in females than in males. The frequency of oblique axis orientation decreased consistently with age in all age groups (p = 0.01) (Table 5).
Table 5.
Gender and age distribution of types of astigmatism.
| No astigmatism |
Axis - astigmatism |
Sphero-astigmatism |
Amount-astigmatism |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J45 (oblique) |
J0 positive (WTR) |
J0 Negative (ATR) |
SMA |
CMA |
HPA |
LA |
MA |
HA |
|||||||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||||||
| All | 4521 | 79.1 | 1532 | 32.9 | 1575 | 33.9 | 1537 | 33.1 | 213 | 11.2 | 529 | 27.9 | 1152 | 60.8 | 3548 | 74.6 | 1096 | 23.0 | 106 | 2.23 | |||||
| 95% CI | 77.9–80.4% | 31.8–34.6% | 32.5–35.2% | 31.5–34.2% | 9.8–12.7% | 25.9–30.0% | 58.8–63% | 73.4–75.9% | 21.8–24.3% | 1.8–2.7% | |||||||||||||||
| Gender | |||||||||||||||||||||||||
| Males | 1369 | 63.6 | 451 | 20.9 | 504 | 23.4 | 536 | 24.9 | 80 | 38.3 | 197 | 37.5 | 360 | 31.2 | 1057 | 30.0 | 421 | 38.8 | 40 | 38.1 | |||||
| Females | 3116 | 68.8 | 1081 | 23.0 | 1071 | 23.6 | 1001 | 22.1 | 126 | 61.1 | 328 | 62.4 | 780 | 68.4 | 2463 | 69.9 | 664 | 61.2 | 65 | 61.9 | |||||
| χ2/p-value | 175.1(0.01)a | 1.58 (0.45) | 94.1(0.01) | 155.7(0.01)a | 22.0(0.001)a | 103.9(0.01)a | 30.5(0.01)a | 30.0(0.01)a | 11.0(0.01) | ||||||||||||||||
| Age groups | |||||||||||||||||||||||||
| 6–18 | 911 | 20.2 | 319 | 29.3 | 314 | 28.0 | 230 | 21.0 | 66 | 31.1 | 102 | 19.4 | 78 | 6.8 | 150 | 6.9 | 135 | 12.4 | 29 | 27.3 | |||||
| 19–29 | 819 | 18.2 | 331 | 29.2 | 430 | 37.2 | 199 | 17.5 | 50 | 23.5 | 135 | 25.7 | 90 | 7.8 | 165 | 7.6 | 148 | 13.6 | 33 | 31.3 | |||||
| 30–39 | 897 | 19.9 | 373 | 30.4 | 375 | 30.4 | 279 | 22.2 | 33 | 15.5 | 96 | 19.3 | 189 | 16.5 | 337 | 15.6 | 149 | 13.7 | 14 | 13.2 | |||||
| 40–49 | 865 | 19.2 | 217 | 15.3 | 249 | 18.2 | 240 | 17.4 | 38 | 17.9 | 75 | 14.3 | 157 | 13.7 | 446 | 20.6 | 181 | 16.6 | 7 | 6.6 | |||||
| 50–59 | 637 | 14.1 | 160 | 15.0 | 135 | 12.3 | 282 | 25.3 | 10 | 4.7 | 58 | 11.0 | 278 | 24.3 | 608 | 28.1 | 196 | 33.4 | 7 | 6.6 | |||||
| 60–69 | 275 | 6.1 | 91 | 17.0 | 60 | 11.5 | 181 | 34.7 | 8 | 3.7 | 30 | 5.7 | 212 | 18.5 | 317 | 14.7 | 147 | 13.5 | 7 | 6.6 | |||||
| ≥70 | 89 | 1.9 | 57 | 22.1 | 16 | 6.3 | 120 | 47.2 | 7 | 3.2 | 28 | 5.3 | 137 | 12.0 | 134 | 6.21 | 129 | 11.8 | 9 | 8.4 | |||||
| Total | 4493 | 1548 | 1579 | 1531 | 212 | 212 | 524 | 1141 | 1085 | ||||||||||||||||
| χ2/p-value | 526.1 (0.01)a | 234.9 (0.01) | 341.7 (0.01 | 126.6 (0.01) | 126.6 (0.001)a | 377.1(0.01) | 1126 (0.01)a | 667 (0.01) | 324 (0.01) | ||||||||||||||||
| Sub- groups | |||||||||||||||||||||||||
| 6–40 | 2706 | 75.9 | 1053 | 29.5 | 1151 | 25.6 | 733 | 16.3 | 157 | 74.6 | 342 | 65.7 | 370 | 32.4 | 1079 | 30.5 | 450 | 41.4 | 78 | 73.5 | |||||
| 41–85 | 1787 | 57.2 | 495 | 15.2 | 428 | 9.5 | 798 | 17.7 | 55 | 25.9 | 182 | 34.7 | 771 | 67.5 | 2456 | 69.4 | 635 | 58.5 | 28 | 26.4 | |||||
| χ2/p-value | 135.3 (0.01)a | 225.03(0.01)a | 1.76(0.18) | 32.6 (0.01) | 362(0.01)a | 95.4 ( 0.01) | 362(0.01)a | 20.76(0.01)a | 15.17(0.01)a | ||||||||||||||||
significant association. CI = confidence interval, SD = standard deviation, n = frequency in numbers, %=percent.
Severity (amount) of astigmatism
All severity of astigmatism was significantly associated with age and low astigmatism (LA) was the most frequent (74.6%), while high astigmatism (HA) was the least frequent (2.2%) (Table 4). The frequency of LA increased with increasing age, the 50–59 years age group had the highest frequency (28%) while 6–18 years age group had the lowest (6.9%). In contrast, the frequency of HA decreased with increasing age, most frequent (27%) at the younger age groups (6–18 &19–29 years) while the older age groups had the least frequency (8.4%) of HA (Table 4).
Sphero-astigmatism
All types of sphero-astigmatism were more frequent in females and decreased with age significantly. (Table 4). Hyperopic astigmatism was the most frequent, with prevalence of 60.8% and it decreased while hyperopic astigmatism increased with advancing age (Table 4).
Discussion
We studied the gender and age-related pattern of distribution of refractive errors across a broad age range of patients examined in an optometry practice. Our findings show that the low categories of refractive errors were the most prevalent and males were significantly more myopic, astigmatic and anisometropicwhile females were more hyperopic across the age groups. Other significant findings included: myopia decreased, while hyperopia, astigmatism and anisometropia increased with increasing age. Unique findings from this study which has not been reported in a South African population or elsewhere in Africa included that myopia peaked earlier, and second hyperopic shift commenced after the age of 82 years (Appendix A). In addition, the frequency of low astigmatism was low and increased with age (instead of high and decreased with age) while the frequency of high astigmatism was relatively high and decreased with age instead of the opposite. Overall, findings from our study suggest that aspects of the pattern of distribution of refractive errors may be different from those from other populations. This study findings are discussed with focus on the peak prevalence, changes in trends, as well as contextualisation of findings.
Refractive errors, gender and age groups
Males were significantly more myopic and this finding agrees with several studies.9, 11 The decreasing prevalence of myopia with advancing age is similar to findings from other studies.7, 8, 9, 10, 11, 12, 13, 14, 15 Myopia prevalence peaked at various age ranges across studies with the present study having the lowest age range for myopia peak (Table 3). Differences in study design especially criteria and how age groups were stratified could influence the prevalence and distribution of refractive errors across studies. However, we may speculate that the relatively lower peak age range from the present study may be the increased awareness for vision care among school-aged children as most students in the province undergo vision screening at first grade. Beside the different prevalence peaks, the myopia-to-hyperopia shift as well as the second myopia shift commenced at various age ranges.
Such shift occurred at age group 30–39 years in the present study, except for anisometropia, for which the shift occurred at age group 50–59 years which may be due to a sharp increase in the mean and frequency of hyperopia at that age range. In general, variations in the age ranges where refractive shifts occurred may be related to cohort effects which could vary across studies. The myopia to hyperopia shift has been linked to changes to the cornea curvature, refractive indices, and the distances between the optical components of the human eye throughout life.10
In general, our findings on myopia (Table 3) relates to earlier concepts which suggest that the myopia which is present during school years continue throughout school years till around late teens (approximately 20 years old) and early adulthood.16, 17 This description corresponds with the period in the mid to late teenage years when eye growth ceases and corresponds roughly with the time of the cessation of general body growth.16, 17 Consequently, myopia stabilises and decreases from around late 30 s towards emmetropia or becoming hyperopic.17 In adult onset myopia, the myopia continues to decline (in the present study from 13.0% at age 30–39 years to 8.2% at age 60–69), with a sharp increase to 15. 8% at age group 70 to 85 years (second myopic shift). Table 6 this second myopic peak has been reported in several studies9, 10, 11, 15 (Table 3) and it is attributed to the incidence of nuclear sclerosis cataract in older adults.9, 10, 11, 15 In the present study, the relatively high frequency of overall myopia and mild myopia at the second myopic shift (Table 4) may be related to the fact that older patients hesitate or sometimes fear to have cataract surgeries. In South Africa, cataract surgeries are performed free of charge to elderly patients in government hospitals and there are often numerous patients on the waiting list for operation. Due to fear of surgery and in some cases several months of waiting periods, elderly patients often consult optometrists requesting for spectacle lenses that can give any appreciable vision improvement. These factors may increase the frequency of patients presenting with late onset and second myopic shift.
Table 6.
Myopia peak and second shift here.
| Authors | Country of study | Sample size | Patients age range (years) | Peak (age/years) | myopia to hyperopia shift (years) | Second myopia shift (years) |
|---|---|---|---|---|---|---|
| Present study | South Africa | 6687 | 6–85 | 6–18 | 30–39 | 70–85 |
| Raliavhegwa and Oduntan11 | South Africa | 4193 | 4–110 | 21–30 | 31–40 | 81–110 |
| Kragha13 | Nigeria | 822 | 3–62 | 20–25 | 40–44 | 50 |
| Maju and Ojiabo 12 | Nigeria | 601 | 5-85 | 41–50 | 51–60 | 71-80 |
| Koomson et al.14 | Ghana | 6027 | 0–70 | 20–29 | 30–39 | ≥70 |
| Hrynchak et al.10 | Canada | 5885 | 5–90 | 20–25 | 25–30 | N/A |
| Natung et al.15 | India | 2546 | 5–88 | 20–39 | 40–59 | N/A |
| Gomez-Salazar et al.9 | Mexico | 676,856 | 6–90 | 20–29 | 30–39 | 80–90 |
| Leung et al.24 | Hong Kong | 2759 | 3–84 | 21–30 | 31–40 | N/A |
For hyperopia, the findings of females being more hyperopic agrees with other studies11, 13 and the findings on hyperopic shift and the increase of the prevalence of hyperopia with advancing age is consistent with findings from several studies.8, 9, 10, 11 These trends may be due to actual changes in the refraction of the ageing eye, associated with a reduction of lens and corneal curvature or decrease in the axial length of the eye.20 Age-related increases in hyperopia may also be due to loss of accommodative tone associated with latent hypermetropia and presbyopia.20 Noteworthy is the fact that most studies on clinical populations including the present study applied low criteria to define hyperopia. This is appropriate given that even corrections for low hyperopia are useful to alleviate symptoms of asthenopia which may be due to refractive error, as well as binocular anomalies such as accommodate insufficiency (Table 6).
Astigmatism was significantly more prevalent in males and the increased prevalence of astigmatism with increasing age agrees with some studies10, 20, 24 and contrasts with others.9, 10, 11, 12, 13 Astigmatism is a dynamic phenomenon and in general, its magnitude and axis are influenced by several factors which include ethnicity and race, genetic factors, eyeball-extraocular structures interaction, visual tasks, age, as well as the changes that the cornea undergoes with time.26, 28 Astigmatism has a unique functional significance as its impact may be greater than for other forms of refractive error for which altering the accommodative state or viewing distance may alleviate the optical defect.22, 24 Although the types of astigmatism are not mutually exclusive, its classifications are relevant in understanding the relation of astigmatism to unaided visual acuity, visual perception and symptoms.4, 26 Low astigmatism is the most prevalent (74.6%) amount of astigmatism which corroborates findings from other authors11, 13 Of the three severities of amount-astigmatism, LA is reported to be the most common cause of ocular headaches.19, 31, 33
Regarding our unique findings on LA and HA (Table 4), Gwiazda et al.32 reported that the high magnitude of astigmatism seen in infancy is eliminated or greatly reduced by school age and Shih et al.21 reported that school-aged children have 0.5 D of astigmatism consistently. Our findings differ from Gwiazda et al.32 and others which suggest that LA is the most prevalent while the prevalence of HA is low in school school-aged and early adult populations.21, 25, 28, 32,33 Regardless of these reports21, 25, 28, 32,33 a possible explanation for the low frequency of LA in younger age groups may be related to the idea that LA is more symptomatic than the other amount of astigmatism.31, 33 Ideally, the more symptoms should warrant more visits to the optometrist with a possible higher prevalence of LA. However, that is not the case in the present study and may be due to the hypothesis that younger children may be unable to quantify or define symptoms appropriately. In some cases, they may ignore symptoms, and not report to their parents and not consult the optometrist.
Alternatively, it may be that the early adults in the present study did not engage in extensive near tasks that may cause symptoms of asthenopia which could warrant consulting the optometrist. Some populations including East Asians and Native American have uniquely high prevalence and magnitude of astigmatism.26, 28 These have been attributed to thicker or tighter eyelids among these populations. The higher frequency of HA in our study agrees with those by Marasisni18 in Maldives and Pensyl on the (Sioux Indian populations)34 and contrasts previous reports.21, 25, 28, 32,33 However, we may speculate that young children who manifest high severity of astigmatism in our study are more likely to consult the optometrist due to impaired distance and near vision than those with LA which may not impair vision markedly. Poor nutrition has been hypothesized to cause reduced pressure of the eyelid on the cornea which may increase the magnitude of astigmatism.26, 28 The patients from our study were mainly from rural settlements who may be prone to nutritional deficiencies and we are unable to make interferences with our data. Furthermore, we may cautiously speculate that the findings on LA and HA could be unique patterns of distribution of astigmatism among the Zulu tribe of South Africa.
Axis astigmatism is the most reported and most frequent type of astigmatism10, 11, 13, 22,24 and the WTR-ATR shift increased with age.10, 11, 13, 22,24 The most common hypothesis proposed for changes in axis orientation with age relates to the effect of eyelid pressure on the cornea. Teenage and early adulthood are characterised by steep vertical cornea meridian (the axis of the cylinder is horizontal, thus the curvature is vertical) and firm eyelids which exerts much pressure on the cornea. With advancing age, eyelid tension decreases, cornea curvature flattens and may thus create the possibility of the shift from WTR to ATR astigmatism.26, 27, 28 Another hypothesis relates to the action of medial rectus in convergence which flattens the horizontal meridian of the cornea, causes the vertical meridian to be more curved, with a subsequent increase in WTR astigmatism –and these decrease with aging.27, 28, 32
Study strengths, implications, applications and limitations
Findings from this study will be relevant in epidemiology-in teaching, translational research and policy decisions. Specifically, findings will guide the clinical optometrists on the types of refractive errors, expected frequencies and prescription ranges to expect for different age groups and gender. In addition, especially for a country such as South Africa where eye surgeries and spectacles are provided free to persons who receive social grants, findings will guide the authorities to know at what age ranges that some types of lenses prescriptions are common thereby will guide the supply chains units on negotiating pricing for contracts awarded to suppliers.
The strengths of this study include low cost study design, large sample size, as well as the extensive analysis of various categories of the refraction error which were not adequately addressed in previous studies. Furthermore, for the period studied, patients were examined by only one optometrist. Although the study provides useful findings, the retrospective study design, as well as the non-probability sampling approach may limit extrapolation of the findings.
Conclusion
The prevalence of myopia decreased whereas hyperopia, astigmatism and anisometropia increased with increasing age. The unique findings are:
-
•
Most changes in the trend of distribution in the present study such as myopia peak commenced earlier than in previous studies.
-
•
Frequency of low astigmatism was low and high astigmatism was uniquely high in younger patients.
-
•
Second hyperopia shift occurs after the age of 82 years and this has not been reported previously.
The findings from this study add to existing knowledge on refractive error in this population. Further studies to reproduce this study will be useful.
Conflict of interest
The authors have no conflicts of interest to declare.
Contributor Information
Samuel Otabor Wajuihian, Email: swajuihian@mweb.co.za.
Khathutshelo Percy Mashige, Email: mashigek@ukzn.ac.za.
Appendix A.
| Age | Freq (n = 6684) | (%) | Sphere |
Cylinder |
||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | |||
| 6 | 22 | 0.33 | −1.1477 | 3.2675 | 0.0000 | −0.7614 | 1.2063 | −0.5000 |
| 7 | 21 | 0.31 | −0.2738 | 3.1364 | 0.0000 | −0.5238 | 0.5472 | −0.5000 |
| 8 | 36 | 0.54 | −0.3958 | 3.2018 | 0.0000 | −0.6111 | 0.7617 | −0.5000 |
| 9 | 42 | 0.63 | −0.3631 | 0.9803 | −0.2500 | −0.5595 | 0.6914 | −0.5000 |
| 10 | 72 | 1.08 | −0.7676 | 3.3094 | 0.0000 | −0.3486 | 0.3548 | −0.5000 |
| 11 | 83 | 1.24 | −0.5253 | 2.4271 | 0.0000 | −0.5772 | 0.7220 | −0.5000 |
| 12 | 83 | 1.24 | −0.3201 | 2.0925 | 0.0000 | −0.4695 | 0.4588 | −0.5000 |
| 13 | 80 | 1.20 | −0.7156 | 3.0621 | 0.0000 | −0.5096 | 0.5733 | −0.5000 |
| 14 | 110 | 1.65 | −0.3205 | 1.5329 | 0.0000 | −0.5432 | 0.6557 | −0.5000 |
| 15 | 125 | 1.87 | −0.2581 | 1.4535 | 0.0000 | −0.5102 | 0.4474 | −0.5000 |
| 16 | 134 | 2.00 | −0.0985 | 1.0042 | 0.2500 | −0.4773 | 0.4566 | −0.5000 |
| 17 | 144 | 2.15 | −0.1944 | 1.2224 | 0.2500 | −0.4773 | 0.4653 | −0.5000 |
| 18 | 136 | 2.03 | 0.0386 | 1.2442 | 0.2500 | −0.4519 | 0.3152 | −0.5000 |
| 19 | 128 | 1.92 | −0.4374 | 1.4792 | 0.0000 | −0.6319 | 0.7662 | −0.5000 |
| 20 | 130 | 1.94 | −0.1538 | 1.3888 | 0.2500 | −0.4979 | 0.3105 | −0.5000 |
| 21 | 110 | 1.65 | −0.0864 | 0.8496 | 0.2500 | −0.6531 | 0.5938 | −0.5000 |
| 22 | 96 | 1.44 | −0.1536 | 0.7917 | 0.0000 | −0.6603 | 0.7750 | −0.5000 |
| 23 | 74 | 1.11 | −0.2939 | 1.2629 | 0.2500 | −0.7097 | 0.7618 | −0.5000 |
| 24 | 83 | 1.24 | −0.3476 | 1.7672 | 0.2500 | −0.6014 | 0.5595 | −0.5000 |
| 25 | 101 | 1.51 | −0.0842 | 0.6994 | 0.0000 | −0.5989 | 0.5724 | −0.5000 |
| 26 | 85 | 1.27 | 0.0647 | 0.8175 | 0.2500 | −0.5034 | 0.2871 | −0.5000 |
| 27 | 102 | 1.53 | −0.1814 | 1.4051 | 0.2500 | −0.6563 | 0.7074 | −0.5000 |
| 28 | 113 | 1.69 | −0.1482 | 1.7971 | 0.2500 | −0.5469 | 0.4432 | −0.5000 |
| 29 | 110 | 1.65 | −0.2477 | 1.4528 | 0.2500 | −0.6485 | 0.5468 | −0.5000 |
| 30 | 123 | 1.84 | −0.0020 | 0.9748 | 0.2500 | −0.5621 | 0.5545 | −0.5000 |
| 31 | 128 | 1.92 | −0.0547 | 0.9510 | 0.2500 | −0.4792 | 0.2737 | −0.5000 |
| 32 | 119 | 1.78 | −0.0042 | 1.1099 | 0.2500 | −0.5291 | 0.4804 | −0.5000 |
| 33 | 110 | 1.65 | 0.0596 | 0.6355 | 0.2500 | −0.5025 | 0.2449 | −0.5000 |
| 34 | 112 | 1.68 | −0.1138 | 1.3452 | 0.2500 | −0.5313 | 0.3427 | −0.5000 |
| 35 | 125 | 1.87 | 0.0880 | 0.9637 | 0.2500 | −0.6045 | 0.6464 | −0.5000 |
| 36 | 130 | 1.94 | 0.0462 | 1.1605 | 0.2500 | −0.5565 | 0.3933 | −0.5000 |
| 37 | 117 | 1.75 | 0.0684 | 0.8633 | 0.2500 | −0.4926 | 0.2331 | −0.5000 |
| 38 | 125 | 1.87 | 0.1420 | 1.0002 | 0.2500 | −0.5991 | 0.5470 | −0.5000 |
| 39 | 131 | 1.96 | 0.1546 | 0.8142 | 0.2500 | −0.5405 | 0.4219 | −0.5000 |
| 40 | 122 | 1.83 | 0.0451 | 0.9415 | 0.2500 | −0.5530 | 0.5117 | −0.5000 |
| 41 | 113 | 1.69 | −0.2500 | 2.1454 | 0.2500 | −0.5054 | 0.4186 | −0.5000 |
| 42 | 117 | 1.75 | 0.1368 | 0.6627 | 0.2500 | −0.3579 | 0.3234 | −0.2500 |
| 43 | 124 | 1.86 | 0.1089 | 1.3295 | 0.2500 | −0.4703 | 0.4188 | −0.5000 |
| 44 | 152 | 2.27 | 0.1086 | 0.5357 | 0.1250 | −0.3242 | 0.3967 | −0.2500 |
| 45 | 121 | 1.81 | 0.1839 | 0.7208 | 0.2500 | −0.3075 | 0.3382 | −0.2500 |
| 46 | 148 | 2.21 | 0.4037 | 1.3651 | 0.2500 | −0.2894 | 0.3334 | −0.2500 |
| 47 | 163 | 2.44 | 0.1887 | 1.5434 | 0.2500 | −0.3571 | 0.3563 | −0.2500 |
| 48 | 156 | 2.33 | 0.2372 | 0.8195 | 0.2500 | −0.3794 | 0.3925 | −0.2500 |
| 49 | 165 | 2.47 | 0.3152 | 0.9407 | 0.2500 | −0.3774 | 0.4663 | −0.2500 |
| 50 | 139 | 2.08 | 0.4748 | 0.7879 | 0.5000 | −0.3536 | 0.3543 | −0.2500 |
| 51 | 154 | 2.30 | 0.4656 | 0.9425 | 0.5000 | −0.4212 | 0.4510 | −0.5000 |
| 52 | 126 | 1.89 | 0.3790 | 0.9310 | 0.5000 | −0.3892 | 0.4066 | −0.2500 |
| 53 | 123 | 1.84 | 0.5508 | 1.1028 | 0.5000 | −0.4438 | 0.4440 | −0.5000 |
| 54 | 116 | 1.74 | 0.6789 | 0.8715 | 0.5000 | −0.4601 | 0.4054 | −0.5000 |
| 55 | 92 | 1.38 | 0.7310 | 0.9516 | 0.7500 | −0.3801 | 0.3776 | −0.5000 |
| 56 | 95 | 1.42 | 0.6526 | 0.8326 | 0.7500 | −0.4857 | 0.5348 | −0.5000 |
| 57 | 82 | 1.23 | 0.6890 | 2.4822 | 0.7500 | −0.4924 | 0.4783 | −0.5000 |
| 58 | 80 | 1.20 | 0.8656 | 1.0606 | 0.7500 | −0.5212 | 0.4853 | −0.5000 |
| 59 | 84 | 1.26 | 0.6875 | 1.4777 | 0.7500 | −0.5571 | 0.4800 | −0.5000 |
| 60 | 83 | 1.24 | 0.8434 | 1.2345 | 1.0000 | −0.5156 | 0.3880 | −0.5000 |
| 61 | 62 | 0.93 | 0.8548 | 1.2816 | 1.0000 | −0.4104 | 0.4134 | −0.5000 |
| 62 | 70 | 1.05 | 0.9058 | 1.0450 | 0.5000 | −0.5637 | 0.4920 | −0.5000 |
| 63 | 52 | 0.78 | 0.8462 | 1.4143 | 1.0000 | −0.6033 | 0.5016 | −0.5000 |
| 64 | 51 | 0.76 | 0.9657 | 1.1769 | 0.7500 | −0.5417 | 0.5789 | −0.5000 |
| 65 | 52 | 0.78 | 1.1106 | 0.8141 | 1.1250 | −0.4611 | 0.4361 | −0.5000 |
| 66 | 34 | 0.51 | 0.9853 | 1.1009 | 1.2500 | −0.5431 | 0.4486 | −0.5000 |
| 67 | 43 | 0.64 | 1.3953 | 1.2645 | 1.5000 | −0.6944 | 0.6271 | −0.6250 |
| 68 | 47 | 0.70 | 1.5585 | 1.9892 | 1.5000 | −0.7829 | 0.6211 | −0.5000 |
| 69 | 26 | 0.39 | 0.8173 | 1.1652 | 1.1250 | −0.9688 | 0.9038 | −0.7500 |
| 70 | 34 | 0.51 | 1.1515 | 1.6452 | 1.2500 | −0.7250 | 0.5737 | −0.7500 |
| 71 | 27 | 0.40 | 1.0000 | 1.3781 | 1.0000 | −0.7065 | 0.5572 | −0.7500 |
| 72 | 18 | 0.27 | 0.8750 | 1.2784 | 1.0000 | −0.8833 | 0.6187 | −0.7500 |
| 73 | 20 | 0.30 | 2.2750 | 3.6273 | 1.2500 | −0.7647 | 0.3587 | −0.7500 |
| 74 | 27 | 0.40 | 0.6852 | 1.4653 | 1.0000 | −1.0100 | 0.7198 | −0.7500 |
| 75 | 19 | 0.28 | 1.0526 | 1.0979 | 1.2500 | −0.8382 | 0.5073 | −1.0000 |
| 76 | 14 | 0.21 | 0.6250 | 1.5620 | 0.3750 | −1.0893 | 1.1334 | −0.7500 |
| 77 | 13 | 0.19 | 0.0400 | 1.4176 | 0.2500 | −1.4000 | 1.3187 | −1.1250 |
| 78 | 15 | 0.22 | 0.4633 | 1.5540 | 0.5000 | −1.0536 | 0.6591 | −1.0000 |
| 79 | 16 | 0.24 | 1.4219 | 1.1609 | 1.3750 | −0.7000 | 0.4247 | −0.5000 |
| 80 | 9 | 0.13 | 1.2500 | 0.9270 | 1.0000 | −0.8125 | 0.4581 | −1.0000 |
| 81 | 12 | 0.18 | −0.1458 | 2.0183 | 0.5000 | −0.9375 | 0.5126 | −1.0000 |
| 82 | 7 | 0.10 | −0.3214 | 2.3572 | 0.0000 | −1.0357 | 0.6524 | −1.0000 |
| 83 | 6 | 0.09 | 1.5833 | 1.6176 | 1.8750 | −0.7917 | 0.6785 | −0.8750 |
| 84 | 5 | 0.07 | 0.4500 | 1.4076 | 0.7500 | −0.8500 | 0.9287 | −1.0000 |
| 85 | 2 | 0.03 | 1.5000 | 0.0000 | 1.5000 | −1.3750 | 0.8839 | −1.3750 |
| 86 | 2 | 0.03 | 5.8750 | 5.8336 | 5.8750 | −2.0000 | 0.0 | −2.0000 |
| 87 | 4 | 0.06 | 2.0000 | 0.9354 | 2.1250 | −1.6875 | 0.8004 | −2.0000 |
| 88 | 1 | 0.01 | 5.0000 | 0.0 | 5.0000 | −1.2500 | 0.0 | −1.2500 |
| 92 | 1 | 0.01 | 1.7500 | 0.0 | 1.7500 | −4.5000 | 0.0 | −4.5000 |
References
- 1.Resnikoff S., Pascolini D., Mariotti SP, Pokharel G.P. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull WHO. 2008;86:63–70. doi: 10.2471/BLT.07.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dandona R., Dandona L. Refractive error blindness. Bull WHO. 2001;79:237–243. [PMC free article] [PubMed] [Google Scholar]
- 3.Smith T.S.T., Frick K.D., Holden B.A., Fricke T.R., Naidoo K.S. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull WHO. 2009;87:431–437. doi: 10.2471/BLT.08.055673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wajuihian S.O., Hansraj R. Refractive error in a sample of Black high school children in South Africa. Optom Vis Sci. 2017;94:1145–1152. doi: 10.1097/OPX.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 5.Nowak M.S., Jurowski P., Grzybowski A., Smigielski J. Characteristics of refractive errors in a population of adults in the central region of Poland. Int J Environ Res Public Health. 2018;5:4–10. doi: 10.3390/ijerph15010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO; Geneva: 2000. Vision 2020. Global Initiative for the elimination of avoidable blindness. In Fact Sheet No. 213. [Google Scholar]
- 7.Allison CL. Proportion of refractive errors in a Polish immigrant population in Chicago. Optom Vis Sci. 2010;87:588–592. doi: 10.1097/OPX.0b013e3181e61beb. [DOI] [PubMed] [Google Scholar]
- 8.Queirós A., Ferrer-Blasco T., Jorge J. Prevalence of refractive conditions in the general population attending eye care clinics in the north of Portugal. Atti della Fondazione Giorgio Ronchi. 2009;64:101–111. [Google Scholar]
- 9.Gomez-Salazar F., Campos-Romero A., Gomez-Campaña H. Refractive errors among children, adolescents and adults attending eye clinics in Mexico. Int J Ophthalmol. 2017;10:796–802. doi: 10.18240/ijo.2017.05.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hrynchak P.K., Mittelstaedt A., Machan C.M., Bun C., Irving E.L. Increase in myopia prevalence in clinic-based populations across a century. Optom Vis Sci. 2013;90:1331–1341. doi: 10.1097/OPX.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 11.Raliavhegwa M, Oduntan AO. A retrospective study of the refractive status of a South African black population. S Afr Optom. 2000;59:72–77. [Google Scholar]
- 12.Malu K, Ojabo C. Refractive errors in patients attending a private hospital in Jos, Nigeria. Niger J Clin Pract. 2014;17:106–111. doi: 10.4103/1119-3077.122863. [DOI] [PubMed] [Google Scholar]
- 13.Kragha IKO. The distribution of refractive errors in Nigeria. Ophthal Physiol Opt. 1987;7:241–244. [PubMed] [Google Scholar]
- 14.Koomson Y., Lartey Y., Adjah K. Prevalence of myopia amongst patients with refractive error in the Kumasi metropolis of Ghana. J Sc Techn. 2013;3:73–80. [Google Scholar]
- 15.Natung T., Taye T., Lyngdoh L.A., Dkhar B., Hajong R. Refractive errors among patients attending the ophthalmology department of a medical college in North-East India. J Fam Med Prim Care. 2017;6:543–548. doi: 10.4103/2249-4863.222023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goss D, Winkler RL. Progression of myopia in youth: age of cessation. Am J Optom Physiol Optics. 1983;60:651–658. doi: 10.1097/00006324-198308000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Grosvenor T. A review and suggested classification of myopia on the basis of age-related prevalence and age of onset. Am J Optom Physiol Opt. 1987;64:545–554. doi: 10.1097/00006324-198707000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Marasini S, Khadka J, Sthapit PR, Sharma R, Prasad BJ. Ocular morbidity on headache ruled out of systemic causes: A prevalence study carried out at a community based hospital in Nepal. J Optom. 2012;5:68–74. [Google Scholar]
- 19.Hirsch MJ. Changes in astigmatism after the age of forty. Am J Optom Arch Am Acad Optom. 1959;36:395–405. doi: 10.1097/00006324-195908000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Gudmundsdottir E., Jonasson F., Jonsson V., Stefánsson Sasaki H, Sasaki K., Iceland-Japan Co-Working Study Groups “With the rule” astigmatism is not the rule in the elderly. Reykjavik Eye Study: a population based study of refraction and visual acuity in citizens of Reykjavik 50 years and older. Acta Ophthalmol Scand. 2000;78:642–646. doi: 10.1034/j.1600-0420.2000.078006642.x. [DOI] [PubMed] [Google Scholar]
- 21.Shih Y.F., Hsiao C.K., Tung Y.L., Lin L.L., Chen C.J., Hung P.T. The prevalence of astigmatism in Taiwan schoolchildren. Optom Vis Sci. 2004;81:94–98. doi: 10.1097/00006324-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Sanfilippo P.G., Yazar S., Kearns L., Sherwin J.C., Hewitt A.W., Mackey DA. Distribution of astigmatism as a function of age in an Australian population. Acta Ophthalmol. 2015;93:377–385. doi: 10.1111/aos.12644. [DOI] [PubMed] [Google Scholar]
- 23.Linke S.J., Richard G., Katz T. Prevalence and associations of anisometropia with spherical ametropia, cylindrical power, age, and sex in refractive surgery candidates. Invest Ophthalmol Vis Sci. 2011;52:7538–7547. doi: 10.1167/iovs.11-7620. [DOI] [PubMed] [Google Scholar]
- 24.Leung T.W., Lam A.K., Deng L., Kee C.S. Characteristics of astigmatism as a function of age in a Hong Kong clinical population. Optom Vis Sci. 2012;89:984Y92. doi: 10.1097/OPX.0b013e31825da156. [DOI] [PubMed] [Google Scholar]
- 25.O’Donoghue L., Rudnicka A.R., McClelland JF, Logna N.S., Saunders KJ. Refractive and corneal astigmatism in White school children in Northern Ireland. Invest Ophthalmol Vis Sci. 2011;52:4048–4053. doi: 10.1167/iovs.10-6100. [DOI] [PubMed] [Google Scholar]
- 26.Read S.A., Collins M.J., Carney L.G. A review of astigmatism and its possible genesis. Clin Exp Optom. 2007;90:5–19. doi: 10.1111/j.1444-0938.2007.00112.x. [DOI] [PubMed] [Google Scholar]
- 27.Asano K., Nomura H., Iwano M. Relationship between astigmatism and aging in middle-aged and elderly Japanese. Jpn J Ophthalmol. 2005;49:127–133. doi: 10.1007/s10384-004-0152-1. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi Seyed-Farzad, Tahvildari M., Hadi Z-Mehrjardi H. 2012. Physiology of astigmatism, Astigmatism - Optics, Physiology and Management, intechopen.com. Last Accessed 5 May 2020. [Google Scholar]
- 29.Salmon T. Lecture 5. Power vector and Zernike Conversion. Available at: http://arapaho.nsuok.edu/~salmonto/VSII/Zernike2Rx.xls. Accessed November 2017.
- 30.Thibos L.N., Wheeler W., Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74:367–375. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Wajuihian SO. Frequency of asthenopia and its association with refractive errors. Afr Vision Eye Health. 2015;74:1–7. doi: 10.4102/aveh.v74i1.293. [DOI] [Google Scholar]
- 32.Gwiazda J., Scheiman M., Mohindra I., Held R. Astigmatism in children: changes in axis and amount from birth to six years. Invest Ophthalmol Vis Sci. 1984;25:88–92. [PubMed] [Google Scholar]
- 33.Grosvenor T. 5th ed. Butterworth Heinemann Elsevier; Philadelphia: 2007. Primary care optometry. [Google Scholar]
- 34.Pensyl C.D., Harrison R.A., Simpson P., Waterbor J.W. Distribution of astigmatism among Sioux Indians in South Dakota. J Am Optom Assoc. 1997;68:425–431. [PubMed] [Google Scholar]