Abstract
Aedes (Ae.) albopictus is an important vector for many pathogens. Previous studies have revealed a role for midgut bacteria during pathogen infection in mosquitoes; however, studies of Ae. albopictus midgut bacteria are limited. We examined the diversity of midgut bacteria in female laboratory-colonized and field-collected Ae. albopictus. A total of 31 bacterial genera were identified representing 10 and 28 genera of laboratory-colonized and field-collected Ae. albopictus, respectively. The predominant bacterial genera in the laboratory-colonized Ae. albopictus were Staphylococcus and Micrococcus, whereas the bacterial diversity in the field-collected Ae. albopictus exhibited a higher proportion of Rhizobium and Agrobacterium as the dominant genera. However, only Staphylococcus showed a significant difference between laboratory-colonized and field-collected Ae. albopictus. The midgut bacterial species were identified from 30 laboratory-colonized Ae. albopictus mosquitoes. A total of 16 bacterial species were identified and the predominant bacterial species was Micrococcus luteus, followed by Staphylococcus epidermidis and Agrobacterium tumefaciens. Field mosquitoes were collected from the Sing Buri, Chumphon, and Yala Provinces of Thailand. The midgut bacterial species identified from the 10 Ae. albopictus collected from the Sing Buri Province included Bacillus subtilis, Staphylococcus haemolyticus, Staphylococcus hominis, and Serratia marcescens. Serratia marcescens was the only bacteria identified from this area. Midgut bacterial species were identified from 40 filed-collected Ae. albopictus from Chumphon Province. A total of 25 bacterial species were identified and the predominant species were Enterobacter cloacae, Micrococcus luteus, and Providencia rettgeri. Only 15 bacterial species were identified from the mosquitoes collected from Chumphon Province. A total of 18 bacterial species were identified from 30 Ae. albopictus collected from Yala Province and the predominant species were Rhizobium pusense and Agrobacterium tumefaciens. Only 12 bacterial species were found in mosquitoes collected from Yala Province. These findings indicate changes in the midgut bacteria population in Ae. albopictus from various locales, which may result from variability in the blood-meal source, diet, or habitat. A comprehensive survey of the midgut bacteria community prevalence in wild populations is critical for not only gaining a better understanding of the role of this bacterium in shaping the microbial community in Ae. albopictus, but also for informing current and future mosquito and disease control programs.
Keywords: Asian tiger mosquito, Bacterial community, Culturable bacteria, Host-parasite interaction, Midgut epithelial surface, Pathogen infection
Asian tiger mosquito, bacterial community, culturable bacteria, host-parasite interaction, midgut epithelial surface, pathogen infection.
1. Introduction
Aedes (Ae.) albopictus (Skuse) or the Asian tiger mosquito is a mosquito that acts as a potential disease vector for the transmission of many filarial, protozoan, and viral pathogens including canine heartworm, avian malaria, chikungunya, dengue, West Nile, and yellow fever virus (Tiawsirisup et al., 2004, 2005; Tiawsirisup and Kaewthamasorn, 2007; Thavara et al., 2009; Chompoosri et al., 2016; Tuanudom et al., 2017; Yurayart et al., 2017). This mosquito is native to the tropical and subtropical areas of Southeast Asia and has spread to many countries by modern transportation (Craven et al., 1988). It does not exhibit any specific ecological specialization and has succeeded in colonizing temperate zones, such as in the United States and Europe (McHugh and Hanny, 1990; Dalla Pozza et al., 1994). Ae. albopictus exhibits both distinct cold tolerant and tropical strains, and it overwinters in the egg stage in temperate climates, but is active throughout the year in tropical and subtropical habitats (Hanson and Craig, 1995).
The digestive tract of the mosquito consists of three parts which include the foregut, midgut, and hindgut. The midgut serves as the first contact area between pathogens and the epithelial surface. Midgut microbiota are bacteria that are harbored or colonize the midgut of mosquitoes. Recently, studies have focused on the role of bacterial communities on the fitness and competence of various mosquito vectors and pathogen transmission (Pumpuni et al., 1996; Diallo et al., 1999; Dillon and Dillon, 2004; Azambuja et al., 2005; Gusmao et al., 2007). Some midgut bacteria play an important role in disease transmission, host-parasite interaction, and vector competence (Apte-Deshpande et al., 2014; Mohlmann et al., 2020). They may increase or decrease vector competence through various mechanisms including enhancement of the immune response or by obstructing the development of pathogens (Kalappa et al., 2018; Monteiro et al., 2019). Midgut bacteria may attenuate the expression of molecules directed against infecting pathogens, which could be used as a novel strategy for disease control (Shane et al., 2018). They are also known to increase the immune response of mosquitoes (Pumpuni et al., 1996; Meister et al., 2005; Dong et al., 2009), in which immunocompetent mosquitoes are less likely to transmit pathogens, which could also be useful for disease control (Abdul-Ghani et al., 2012).
The diversity of the midgut microbiota has been examined in different species of mosquitoes (Tiawsirisup et al., 2008, 2018; Guegan et al., 2020; Zoure et al., 2020). Previous studies have evaluated the influence of colonized microbiota on the susceptibility of mosquitoes to virus and parasitic infection (Pumpuni et al., 1996; Dillon and Dillon, 2004; Azambuja et al., 2005; Gusmao et al., 2007). However, studies on Ae. albopictus regarding the midgut microbiota identification and their interaction with pathogens are limited. In the present study, we examined the diversity of midgut bacteria in laboratory-colonized and field-collected Ae. albopictus from different locations in Thailand. The results provide important information for other basic and advanced studies on mosquito biology, midgut microbiota, and pathogen infection.
2. Materials and methods
2.1. Mosquitoes
Female laboratory-colonized and field-collected Ae. albopictus were examined. The laboratory-colonized mosquitoes were originally collected from Nonthaburi Province and colonized at the Department of Medical Sciences, Ministry of Public Health, Thailand. They were subsequently maintained in the Parasitology Unit, Department of Veterinary Pathology, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand for more than 10 generations. Field-collected mosquitoes were collected from the Sing Buri, Chumphon, and Yala Provinces of Thailand. This study was conducted in 2014, at which time mosquitoes were not included as experimental animals that required approval from the Chulalongkorn University Animal Care and Use Committee.
2.2. Mosquito dissection and bacterial isolation
Bacteria were isolated from the midguts of laboratory-colonized and field-collected Ae. albopictus. The mosquitoes were dissected and bacterial isolation was performed within 24 h. The mosquitoes were euthanized at −20 °C and dissected under sterile conditions. Each mosquito was washed with 70% ethanol for 5 min and then twice with phosphate-buffered saline before midgut dissection and bacterial cultivation. The midgut was homogenized in 300 μL of 60% glycerol and a 100 μL aliquot of the suspension was spread on tryptone soya agar supplemented with 5% sheep blood and incubated at 37 °C for 24 h. Pure bacterial isolates from the midgut of each mosquito were subcultured in 2 mL of tryptone soya broth and incubated at 37 °C for 24 h. The bacteria were collected by centrifugation at 20,000 rcf for 15 min and the bacterial pellet was washed with distilled water.
2.3. Bacterial DNA extraction
A total of 40 μL of distilled water was added to the bacterial pellet, resuspended by vortexing, incubated at 100 °C for 10 min, cooled on ice, and centrifuged at 20,000 rcf for 10 min. The supernatant (extracted DNA) was stored at −80 °C until further use.
2.4. Midgut bacterial identification
The small ribosomal RNA (16S) gene was amplified by PCR from the extracted DNA of the isolates using two pairs of eubacteria-specific primers. The first primer pair was 16SF (5′–AGTTTGATCCTGGCTCAG–3′) and 16SR (5′–GCTACCTTGTTACGACTT C-3′) (Dinparast Djadid et al., 2011), whereas the second pair was 63F (5′–CAGGCCTAACACATGCAAGTC–3′) and 1387R (5′–GGGCGGWGTGTACAAGGC–3′) (Marchesi et al., 1998), which yielded amplicons of expected sizes of 1.5 and 1.3 kb, respectively. The amplified fragments were purified using the Gel/PCR DNA Fragments Extraction Kit (Geneaid, Taiwan) and submitted to First BASE Laboratories (Singapore) for sequencing.
All partial 16S rRNA gene sequences were assembled and analyzed using the Lasergene package version 5.03 (DNASTAR, Inc., Madison, Wisconsin, USA). The sequences obtained were compared against GenBank using the BLAST algorithm. Homologous sequences were retrieved from GenBank (BLASTn search) and aligned using the ClustalW program. Phylogenetic relationships were determined by tree reconstruction using the Neighbor-Joining (NJ) method with the Kimura-2 parameter for distance calculation, which is incorporated into the MEGA 7.0.26 package. The robustness of the phylogenetic tree was examined through 1,000 bootstrap replicates and the consensus tree was used for analysis. All sequences were submitted to the National Centre for Biotechnology and Information GenBank sequence database with accession numbers, SUB3724128: MG996794-MG996888, SUB3733025: MG997080-MG997092, SUB3782911: MH050409-MH050425, and SUB3782990: MH050699-MH050738.
2.5. Data analysis
Differences in the midgut bacterial genera infection rates between female laboratory-colonized and field-collected Ae. albopictus were determined by a Chi-square test. Differences in the proportion of the community of Actinobacteria, Firmicutes, and Proteobacteria phyla isolated from the midgut between female laboratory-colonized and field-collected Ae. albopictus were determined by a Chi-square test. Differences in the proportion of the community of Actinobacteria, Firmicutes, and Proteobacteria phyla isolated from the midgut among female laboratory-colonized and field-collected Ae. albopictus from Sing Buri, Chumphon, and Yala Provinces were determined by ANOVA. P values less than 0.05 were considered statistically significant.
3. Results
3.1. Diversity of cultured bacterial genera from laboratory-colonized and field-collected Ae. albopictus
The midgut bacterial genera identified from female laboratory-colonized and field-collected Ae. albopictus are summarized in Table 1. A total of 31 bacterial genera were identified which included 10 and 28 genera in laboratory-colonized and field-collected Ae. albopictus, respectively. The majority of the isolated bacteria were Gram-negative bacteria. The predominant bacterial genera in the laboratory-colonized Ae. albopictus were Staphylococcus (26.7%) and Micrococcus (16.7%), whereas the bacterial genera diversity in field-collected Ae. albopictus was much greater with Rhizobium (8.8%) and Agrobacterium (7.5%) representing the slightly dominant genera. Only Staphylococcus exhibited a statistically significant difference between laboratory-colonized and field-collected Ae. albopictus (P < 0.05) (Table 1). Besides, most of the identified bacterial genera belong to the Proteobacteria phylum, and there were only two bacterial genera, Bacillus and Staphylococcus, in the Firmicutes phylum.
Table 1.
Comparison of midgut bacterial infection rates between female laboratory-colonized and field-collected Aedes albopictus.
| Bacterial phylum | Bacterial genus (gram) | Infection rate (infected/tested mosquitoes) |
|
|---|---|---|---|
| Laboratory-colonized | Field-collected | ||
| Actinobacteria | Actinomyces (+) | 0 (0/30) | 1.3 (1/80) |
| Brachybacterium (+) | 0 (0/30) | 1.3 (1/80) | |
| Leucobacter (+) | 3.3 (1/30) | 0 (0/80) | |
| Microbacterium (+) | 13.3 (4/30) | 3.8 (3/80) | |
| Micrococcus (+) | 16.7 (5/30) | 6.3 (5/80) | |
| Nocardioides (+) | 0 (0/30) | 1.3 (1/80) | |
| Firmicutes | Bacillus (+) | 0 (0/30) | 6.3 (5/80) |
| Staphylococcus (+)∗ | 26.7 (8/30) | 3.8 (3/80) | |
| Proteobacteria | Acinetobacter (-) | 3.3 (1/30) | 5.0 (4/80) |
| Agrobacterium (-) | 13.3 (4/30) | 7.5 (6/80) | |
| Beijerinckia (-) | 0 (0/30) | 1.3 (1/80) | |
| Brevundimonas (-) | 0 (0/30) | 1.3 (1/80) | |
| Burkholderia (-) | 0 (0/30) | 2.5 (2/80) | |
| Candidatus Rhizobium (-) | 0 (0/30) | 2.5 (2/80) | |
| Chryseobacterium (-) | 0 (0/30) | 2.5 (2/80) | |
| Enhydrobacter (-) | 3.3 (1/30) | 0 (0/80) | |
| Enterobacter (-) | 0 (0/30) | 5.0 (4/80) | |
| Erwinia (-) | 0 (0/30) | 1.3 (1/80) | |
| Klebsiella (-) | 3.3 (1/30) | 3.8 (3/80) | |
| Massilia (-) | 0 (0/30) | 1.3 (1/80) | |
| Moraxella (-) | 0 (0/30) | 1.3 (1/80) | |
| Novosphingobium (-) | 0 (0/30) | 1.3 (1/80) | |
| Pandoraea (-) | 3.3 (1/30) | 0 (0/80) | |
| Pantoea (-) | 0 (0/30) | 2.5 (2/80) | |
| Pectobacterium (-) | 0 (0/30) | 1.3 (1/80) | |
| Providencia (-) | 0 (0/30) | 3.8 (3/80) | |
| Pseudomonas (-) | 6.7 (2/30) | 3.8 (3/80) | |
| Rahnella (-) | 0 (0/30) | 1.3 (1/80) | |
| Rhizobium (-) | 0 (0/30) | 8.8 (7/80) | |
| Serratia (-) | 0 (0/30) | 1.3 (1/80) | |
| Sphingomonas (-) | 0 (0/30) | 2.5 (2/80) | |
∗Significant difference in the midgut bacterial infection rates between female laboratory-colonized and field-collected Aedes albopictus was determined by a Chi-square test (P < 0.05).
3.2. Diversity of cultured bacterial species from laboratory-colonized Ae. albopictus
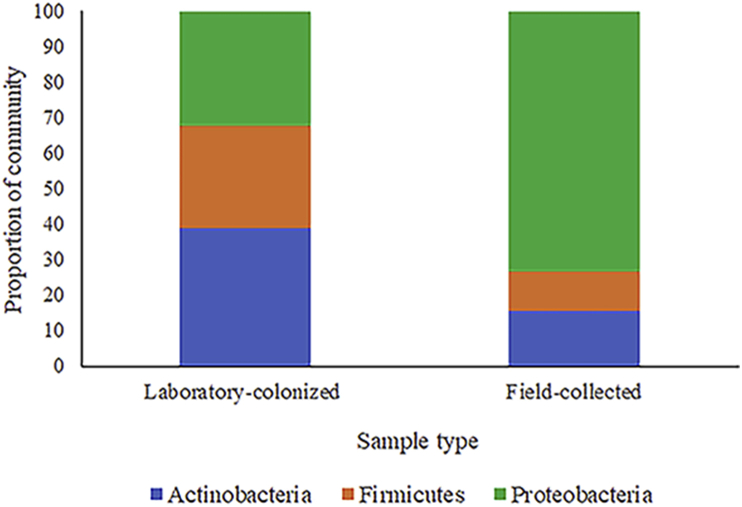
The midgut bacterial species identified from the 30 laboratory-colonized Ae. albopictus are summarized in Table 2. Most of the identified bacterial species from laboratory-colonized Ae. albopictus belong to the Actinobacteria phylum (Figure 1). A total of 16 bacterial species were identified and the dominant bacterial species were Micrococcus luteus (16.7%), followed by Staphylococcus epidermidis (13.3%) and Agrobacterium tumefaciens (13.3%). The highest average number of total bacterial colonies per mosquito was found in Microbacterium dextranolyticum at 212 (4–621).
Table 2.
The midgut bacterial infection rates in female laboratory-colonized Aedes albopictus.
| Bacterial phylum | Closest related bacterial species∗ | Infection rate (infected/tested mosquitoes) | Average number of total colonies per mosquito (range) |
|---|---|---|---|
| Actinobacteria | Leucobacter chironomi | 3.3 (1/30) | 12 |
| Microbacterium dextranolyticum | 10.0 (3/30) | 212 (4–621) | |
| Microbacterium laevaniformans | 3.0 (1/30) | 2 | |
| Micrococcus luteus | 16.7 (5/30) | 44 (16–86) | |
| Micrococcus yunnanensis | 6.7 (2/30) | 22 (12–32) | |
| Firmicutes | Staphylococcus arlettae | 6.7 (2/30) | 11 (3–18) |
| Staphylococcus epidermidis | 13.3 (4/30) | 45 (6–130) | |
| Staphylococcus pasteuri | 3.3 (1/30) | 12 | |
| Staphylococcus warneri | 6.7 (2/30) | 92 (3–180) | |
| Proteobacteria | Acinetobacter variabilis | 3.3 (1/30) | 14 |
| Agrobacterium tumefaciens | 13.3 (4/30) | 78 (6–258) | |
| Enhydrobacter aerosaccus | 3.0 (1/30) | 10 | |
| Klebsiella pneumoniae | 3.3 (1/30) | 14 | |
| Pandoraea sputorum | 3.3 (1/30) | 292 | |
| Pseudomonas aeruginosa | 3.3 (1/30) | 16 | |
| Pseudomonas luteola | 3.3 (1/30) | 92 |
All bacterial species were identified based on a 16S DNA sequence similarity of greater than 99%.
Figure 1.
Proportion of the community of identified bacterial species belonging to their respective phylum isolated from the midgut of female laboratory-colonized and field-collected Aedes albopictus from Thailand. There were differences in the proportion of the community of Actinobacteria, Firmicutes, and Proteobacteria phyla isolated from the midgut between laboratory-colonized and field-collected Ae. albopictus from Thailand, as determined by a Chi-square test (P < 0.05).
3.3. Diversity of cultured bacteria species from field-collected Ae. albopictus
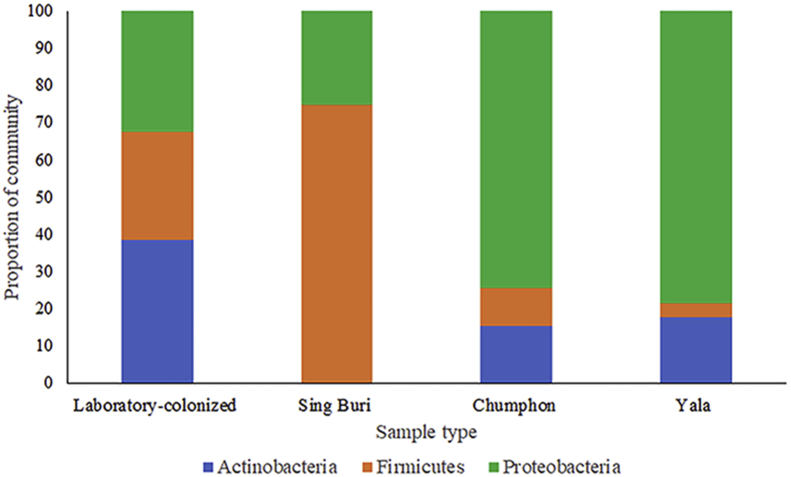
The field mosquitoes were collected from Sing Buri, Chumphon, and Yala Provinces, which are representative of the central, upper southern, and lower southern areas of Thailand, respectively. Midgut bacterial species were identified from 10 Ae. albopictus collected from the Sing Buri Province. The predominant bacterial species belonged to the Firmicutes phylum (Figure 2) and included Bacillus subtilis (10%), Staphylococcus haemolyticus (10%), Staphylococcus hominis (10%), and Serratia marcescens (10%). Serratia marcescens was the only species identified from this area. The highest average number of total bacterial colonies per mosquito found in Staphylococcus hominis was 66 (Table 3).
Figure 2.
Proportion of the community of identified bacterial species belonging to their respective phylum isolated from the midgut of female laboratory-colonized and field-collected Aedes albopictus from Sing Buri, Chumphon, and Yala Provinces, Thailand. There were differences in the proportion of the community of Firmicutes and Proteobacteria phyla isolated from the midgut among laboratory-colonized and field-collected Ae. albopictus from Sing Buri, Chumphon, and Yala Provinces, Thailand, as determined by ANOVA (P < 0.05).
Table 3.
The midgut bacterial infection rates in female field-collected Aedes albopictus from Sing Buri Province.
| Bacterial phylum | Closest related bacterial species∗ | Infection rate (infected/tested mosquitoes) | Average number of total colonies per mosquito (range) |
|---|---|---|---|
| Firmicutes | Bacillus subtilis | 10.0 (1/10) | 3 |
| Staphylococcus haemolyticus | 10.0 (1/10) | 3 | |
| Staphylococcus hominis | 10.0 (1/10) | 66 | |
| Proteobacteria | Serratia marcescens# | 10.0 (1/10) | 3 |
Represents the bacterial species that were only found in this group.
All bacterial species were identified based on a 16S DNA sequence similarity of greater than 99%.
The midgut bacterial species were identified from 40 filed-collected Ae. albopictus from Chumphon Province, and most identified bacterial species belonged to the Proteobacteria phylum (Figure 2). A total of 25 bacterial species was identified, with the dominant bacterial species being Enterobacter cloacae (7.5%), Micrococcus luteus (7.5%), and Providencia rettgeri (7.5%). Fifteen bacterial species were identified from mosquitoes collected from Chumphon Province. The highest average number of total bacterial colonies per mosquito found in Enterobacter cloaca was 98 (3–273) (Table 4).
Table 4.
The midgut bacterial infection rates in female field-collected Aedes albopictus from Chumphon Province.
| Bacterial phylum | Closest related bacterial species∗ | Infection rate (infected/tested mosquitoes) |
Average number of total colonies per mosquito (range) |
|---|---|---|---|
| Actinobacteria | Actinomyces oris# | 2.5 (1/40) | 24 |
| Microbacterium dextranolyticum | 2.5 (1/40) | 3 | |
| Microbacterium yannicii# | 2.5 (1/40) | 15 | |
| Micrococcus luteus | 7.5 (3/40) | 8 (3–15) | |
| Firmicutes | Bacillus kochii# | 5.0 (2/40) | (3–6) |
| Bacillus pocheonensis# | 2.5 (1/40) | 9 | |
| Staphylococcus epidermidis | 2.5 (1/40) | 3 | |
| Proteobacteria | Acinetobacter lwoffii# | 2.5 (1/40) | 3 |
| Acinetobacter variabilis | 5.0 (2/40) | 3 | |
| Agrobacterium tumefaciens | 5.0 (2/40) | 3 | |
| Chryseobacterium taklimakanense# | 5.0 (2/40) | 8 (3–12) | |
| Enterobacter cancerogenus# | 2.5 (1/40) | 279 | |
| Enterobacter cloacae# | 7.5 (3/40) | 98 (3–273) | |
| Enterobacter hormaechei# | 2.5 (1/40) | 3 | |
| Enterobacter mori# | 2.5 (1/40) | 3 | |
| Erwinia tasmaniensis# | 2.5 (1/40) | 3 | |
| Klebsiella pneumoniae | 5.0 (2/40) | 5 (3–6) | |
| Klebsiella quasipneumoniae# | 2.5 (1/40) | 30 | |
| Klebsiella variicola# | 2.5 (1/40) | 18 | |
| Moraxella osloensis | 2.5 (1/40) | 9 | |
| Novosphingobium panipatense | 2.5 (1/40) | 3 | |
| Pantoea dispersa# | 5.0 (2/40) | 8 (3–12) | |
| Providencia rettgeri | 7.5 (3/40) | 9 (3–15) | |
| Pseudomonas psychrotolerans# | 5.0 (2/40) | 8 (6–9) | |
| Rhizobium pusense | 5.0 (2/40) | 47 (12–81) |
Represents the bacterial species that were only found in this group.
All bacterial species were identified based on a 16S DNA sequence similarity of greater than 99%.
A total of 18 bacterial species were identified from 30 Ae. albopictus collected from the Yala Province and most of the identified bacterial species belonged to the Proteobacteria phylum (Figure 2). The predominant bacterial species were Rhizobium pusense (16.7%) and Agrobacterium tumefaciens (13.3%). A total of 12 bacterial species were found only in the mosquitoes collected from Yala Province. The highest average number of total bacterial colonies per mosquito found in Pectobacterium carotovorum was 546 (Table 5).
Table 5.
The midgut bacterial infection rates in female field-collected Aedes albopictus from Yala Province.
| Bacterial phylum | Closest related bacterial species∗ | Infection rate (infected/tested mosquitoes) | Average number of total colonies per mosquito (range) |
|---|---|---|---|
| Actinobactira | Brachybacterium nesterenkovii | 3.3 (1/30) | 9 |
| Microbacterium aoyamense# | 3.3 (1/30) | 9 | |
| Micrococcus luteus | 3.3 (1/30) | 3 | |
| Micrococcus yunnanensis | 3.3 (1/30) | 3 | |
| Nocardioides zeae# | 3.3 (1/30) | 9 | |
| Firmicutes | Bacillus altitudinis# | 3.3 (1/30) | 3 |
| Proteobacteria | Acinetobacter radioresistens | 3.3 (1/30) | 3 |
| Agrobacterium tumefaciens | 13.3 (4/30) | 4.5 (3–6) | |
| Beijerinckia fluminensis# | 3.3 (1/30) | 3 | |
| Brevundimonas aurantiaca# | 3.3 (1/30) | 3 | |
| Burkholderia seminalis# | 6.7 (2/30) | 3 | |
| Candidatus Rhizobium massiliae# | 6.7 (2/30) | 9 | |
| Massilia timonae# | 3.3 (1/30) | 18 | |
| Pectobacterium carotovorum# | 3.3 (1/30) | 546 | |
| Pseudomonas oleovorans# | 3.3 (1/30) | 6 | |
| Rahnella aquatilis# | 3.3 (1/30) | 15 | |
| Rhizobium pusense | 16.7 (5/30) | 3 | |
| Sphingomonas sanguinis# | 6.7 (2/30) | 3 |
Represents the bacterial species that were only found in this group.
All bacterial species were identified based on a 16S DNA sequence similarity of greater than 99%.
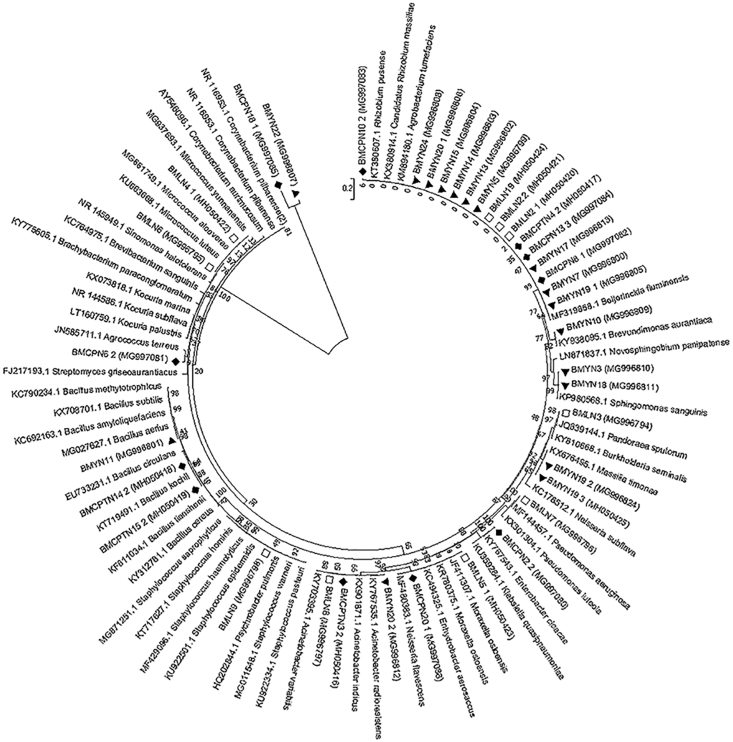
There were differences in the proportion of the community of Actinobacteria, Firmicutes, and Proteobacteria phyla isolated from the midgut between laboratory-colonized and field-collected Ae. albopictus from Thailand, as determined by a Chi-square test (P < 0.05) (Figure 1). In addition, there were differences in the proportion of the community of Firmicutes and Proteobacteria phyla isolated from the midgut among laboratory-colonized and field-collected Ae. albopictus from Sing Buri, Chumphon, and Yala Provinces of Thailand, as determined by ANOVA (P < 0.05) (Figure 2). A total of 53 phylotypes were observed in the NJ phylogenetic tree using a 99% DNA sequence similarity as the cut-off, and the 16S rRNA gene sequences from a variety of phylogenetic groups are shown in Figure 3.
Figure 3.
Phylogenetic tree (NJ) constructed from the partial 16S rRNA gene fragment sequences (1,500 bp) of isolates cultured from laboratory-colonized and field-collected Ae. albopictus with BS values provided at the nodes. Entries with a black square represent reference names and accession numbers (in parentheses). Entries from this study are represented as strain number and accession number (in parentheses) (■ reference names, □ laboratory-colonized Ae. albopictus, △ field-collected from Chumphon, ▲ field-collected from Yala).
4. Discussion
The diversity of midgut microbiota has been studied and identified from various species of mosquitoes, primarily Anopheles mosquitoes (Tainchum et al., 2020; Zoure et al., 2020). In contrast, studies of Aedes and Culex mosquitoes are limited (Tiawsirisup et al., 2018; Muturi et al., 2020; Seabourn et al., 2020). In the present study, we examined the diversity of midgut bacteria in female laboratory-colonized and field-collected Ae. albopictus, the latter of which was sampled from three different locations in Thailand. There were concerns that the field-collected mosquitoes were only tested for CHIKV, but none of them were infected with CHIKV. However, they were not tested for dengue virus, Zika virus, and other pathogens, which may affect the microbiota of field-collected mosquitoes. Because of the limitation of the bacterial culture method, this study focused only on the characterization of culture-dependent aerobic bacteria from the mosquito midguts. Unfortunately, the effect of bacteria on inducing or inhibiting pathogen infection was not determined. Wolbachia is one of the most important symbiont bacteria found in different species of mosquitoes [e.g., Ae. albopictus and Culex (Cx.) gelidus] (Kitrayapong et al., 2002; Tiawsirisup et al., 2008); however, it was not examined in this study because of the limitation of the culture method and these bacteria are primarily found in the reproductive system of mosquitoes.
Proteobacteria was the predominant phylum found in field-collected Ae. albopictus, whereas Actinobacteria was the predominant phylum found in the laboratory-colonized Ae. albopictus. The predominant bacterial genera in the laboratory-colonized Ae. albopictus were Staphylococcus and Micrococcus, whereas the bacterial genera diversity in field-collected Ae. albopictus was much greater and included Rhizobium and Agrobacterium. However, only Staphylococcus showed a statistically significant difference between laboratory-colonized and field-collected Ae. albopictus.
A high diversity in the microbiota of the midgut was observed in the field-collected Ae. albopictus compared with those colonized in the laboratory. These discrepancies may result from differences in the source of the blood meal, diet, or the environment in which they live (Chen et al., 2020; Lee et al., 2020; Scolari et al., 2021). Acinetobacter, Bacillus, Enterobacter, Pseudomonas, and Raoultella bacterial isolates were found in larval feeding and sugar solution and Enterobacter was found to successfully immigrate to both larval and adult stages of Ae. albopictus (Chen et al., 2020). Bacillus is one genus of the midgut bacteria that was isolated from mosquitoes collected from all three locations, but none from the laboratory-colonized mosquitoes. Bacillus also dominated the isolated bacterial taxa from wild Ae. albopictus and Ae. aegypti collected in Madagascar, with Acinetobacter, Agrobacterium, and Enterobacter following closely behind (Zouache et al., 2011).
The midgut microbiota community may experience interference and undergo change because of the blood meal and food received at each stage of the mosquitoes, which subsequently affects pathogen infection, dissemination, and transmission in the mosquitoes (Muturi et al., 2019). The microbiota composition is affected by the developmental stage in mosquitoes (Scolari et al., 2021). However, trans-stadial passage of some bacteria from the larva to the adult stage has been shown in Ae. albopictus and Anopheles (An.) albimanus (Yadav et al., 2016; Chen et al., 2020; Galeano-Castaneda et al., 2020).
Previous research using bacterial culture and denaturing gel electrophoresis revealed that Proteobacteria and Firmicutes were the dominant bacterial communities associated with Ae. albopictus in the Indian Ocean, and that bacterial diversity and composition were influenced by the mosquitoes' habitat (Zouache et al., 2009, 2011). A taxonomic microarray targeting a broader range of bacterial taxa revealed that the bacterial community in Ae. albopictus, which originated on La Réunion, was more diverse than previously described, and that the various endosymbionts could interact with one another and with the chikungunya virus within the mosquitoes (Zouache et al., 2009).
Staphylococcus (i.e., S. arlettae, S. epidermidis, S. pasteuri, and S. warneri) was the predominant genus found in the laboratory-colonized Ae. albopictus, which was significantly different from the field-collected Ae. albopictus. Mixed infection of S. arlettae and S. epidermidis was also found in one laboratory-colonized mosquito. S. haemolyticus and S. hominis were found in mosquitoes collected from Sing Buri Province and S. epidermidis was found in mosquitoes collected from Chumphon Province. In contrast, there was no Staphylococcus isolated from mosquitoes collected from Yala Province. Staphylococcus has also been identified from other species of mosquitoes (e.g., Ae. aegypti, An. albimanus, and Cx. quinquefasciatus) (Tiawsirisup et al., 2018; Galeano-Castaneda et al., 2020).
Our study found Enterobacter at 5% of the midguts of field-collected Ae. Albopictus, whereas E. cloacae was found in the Ae. albopictus collected from Chumphon Province. The previously reported data indicate the involvement of E. cloacae in the inhibition of Plasmodium berghei development in Anopheles stephensi (Eappen et al., 2013). Both Micrococcus luteus and M. yunnanensis were identified from laboratory-colonized and field-collected Ae. albopictus. Previous studies showed that Micrococcus produces proteins for antibiotic tolerance, re-emergence from latent infections and even quorum sensing and biofilm formation (Mali et al., 2017). Another important bacterial genus identified from Ae. albopictus was Acinetobacter, which is known to take part in blood digestion by mosquitoes (Gaio Ade et al., 2011). These bacteria have been isolated from various environments, including soil samples, potato plants, and dried seaweed, as well as from the air (Groth et al., 1996; Zhang et al., 2010). The mosquitoes may receive these bacteria with food that the mosquito larvae feed upon or in the sheep blood provided to the adult mosquitoes.
The mosquito midgut microbiota may decrease or facilitate the development of a pathogen in mosquitoes (Mohlmann et al., 2020). In the present study, Serratia marcescens were only isolated from Ae. albopictus collected from Sing Buri Province; however, our previous study indicated there was no S. marcescens isolated from laboratory-reared and field-collected Ae. aegypti and Cx. quinquefasciatus from Bangkok, Thailand (Tiawsirisup et al., 2018). These bacteria have also been isolated from lab-reared and field-caught adult females and larvae of Anopheles stephensi (Rani et al., 2009). Serratia marcescens are important because they facilitate arboviral infection and enhance viral dissemination through a secreted protein that digests membrane-bound mucins on the mosquito gut epithelium (Wu et al., 2019). The susceptibility of Ae. aegypti to CHIKV and dengue viruses increases in the presence of Serratia odorifera because of the suppression of the immune response of Ae. aegypti (Apte-Deshpande et al., 2012, 2014). Ae. aegypti are more susceptible to DENV-2 when fed with Aeromonas and Escherichia coli (Apte-Deshpande et al., 2014). However, these bacteria reduce the Plasmodium parasite load in Anopheles mosquitoes (Bando et al., 2013).
A thorough study of the role of midgut bacteria may result in a better understanding of the microbiota's direct or indirect involvement in mosquito immune response and reproduction. This may contribute to the enhancement of current vector and disease control efforts. Certain bacteria that live in the midgut are critical for disease transmission, host-parasite interactions, and vector competence. They can reduce or improve vector competence in a variety of ways, including by enhancing the immune response or by inhibiting parasite development. The midgut is the first point of contact between parasites and the epithelial surface, where parasite populations are significantly reduced (Azambuja et al., 2005). The midgut microbiota may be able to genetically alter the expression of anti-parasite compounds, which might be employed as a novel vector control method. They are also known to enhance the immune response of mosquitoes (Pumpuni et al., 1996; Meister et al., 2005; Dong et al., 2009), in which the immunocompetent mosquitoes are less likely to transmit parasites and could be useful for disease control (Abdul-Ghani et al., 2012). Given the well-established link between specific bacterial taxa and vector susceptibility to a variety of mosquito-borne pathogens, changes in gut microbial communities in response to host blood meal source may have a profound effect on pathogen transmission and may be a key determinant of variation in vector competence (Muturi et al., 2019). Further studies are needed to assess the role of each midgut bacteria and specific pathogen infection, dissemination, and transmission in Ae. albopictus mosquitoes, which may improve vector and disease control strategies.
5. Conclusions
This study examined the diversity of midgut bacteria in female laboratory-colonized and field-collected Ae. albopictus. A total of 31 bacterial genera were identified which included 10 and 28 genera in laboratory-colonized and field-collected mosquitoes, respectively. A total of 16 bacterial species were identified from the laboratory-colonized mosquitoes and a total of 4, 25, and 18 midgut bacterial species were identified from the mosquitoes collected from Sing Buri, Chumphon, and Yala Province, respectively. These discrepancies may result from differences in the source of the blood meal, diet, or environment, all of which may affect pathogen infection, dissemination, and transmission in mosquitoes. An extensive survey of the midgut bacteria community prevalence in wild populations is necessary to not only improve our understanding of the role of bacteria in shaping the microbial community in mosquitoes, but also for providing essential information for current and future mosquito and disease control programs.
Declarations
Author contribution statement
Ranida Tuanudom: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Nichapat Yurayart: Performed the experiments.
Channarong Rodkhum: Analyzed and interpreted the data.
Sonthaya Tiawsirisup: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the 90th Anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot Endowment Fund), the Thailand Science Research and Innovation and Chulalongkorn University (RSA 5680030), the Thailand Science Research and Innovation for its financial support to the TRF Senior Scholar (RTA6080012), and the Chulalongkorn University Research Unit (GRU 6203331007-1).
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors thank Dr. Usavadee Thavara for mosquito collection from Sing Buri Province.
References
- Abdul-Ghani R., Al-Mekhlafi A., Alabsi M. Microbial control of malaria: biological warfare against the parasite and its vector. Acta Trop. 2012;121:71–84. doi: 10.1016/j.actatropica.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Apte-Deshpande A., Paingankar M., Gokhale M., Deobagkar D. Serratia odorifera a midgut inhabitant of Aedes aegypti mosquito enhances its susceptibility to dengue-2 virus. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte-Deshpande A., Paingankar M., Gokhale M., Deobagkar D. Serratia odorifera mediated enhancement in susceptibility of Aedes aegypti for chikungunya virus. Indian J. Med. Res. 2014;139:762–768. [PMC free article] [PubMed] [Google Scholar]
- Azambuja P., Garcia E., Ratcliffe N. Gut microbiota and parasite transmission by insect vectors. Trends Parasitol. 2005;21:568–572. doi: 10.1016/j.pt.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Bando H., Okado K., Guelbeogo W.M., Badolo A., Aonuma H., Nelson B. Intra-specific diversity of Serratia marcescens in Anopheles mosquito midgut defines Plasmodium transmission capacity. Sci. Rep. 2013;3:1641. doi: 10.1038/srep01641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhang D., Augustinos A., Doudoumis V., Bel Mokhtar N., Maiga H. Multiple factors determine the structure of bacterial communities associated with Aedes albopictus under artificial rearing conditions. Front. Microbiol. 2020;11:605. doi: 10.3389/fmicb.2020.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chompoosri J., Thavara U., Tawatsin A., Boonserm R., Phumee A., Sangkitporn S. Vertical transmission of Indian Ocean Lineage of chikungunya virus in Aedes aegypti and Aedes albopictus mosquitoes. Parasites Vectors. 2016;9:227. doi: 10.1186/s13071-016-1505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven R.B., Eliason D.A., Francy D.B., Reiter P., Campos E.G., Jakob W.L. Importation of Aedes albopictus and other exotic mosquito species into the United States in used tires from Asia. J. Am. Mosq. Control Assoc. 1988;4:138–142. [PubMed] [Google Scholar]
- Dalla Pozza G.L., Romi R., Severini C. Source and spread of Aedes albopictus in the Veneto region of Italy. J. Am. Mosq. Control Assoc. 1994;10:589–592. [PubMed] [Google Scholar]
- Diallo M., Thonnon J., Traore-Lamizana M., Fontenille D. Vectors of Chikungunya virus in Senegal: current data and transmission cycles. Am. J. Trop. Med. Hyg. 1999;60:281–286. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- Dillon R., Dillon V. The gut bacteria of insects: non-pathogenic interactions. Annu. Rev. Entomol. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- Dinparast Djadid N., Jazayeri H., Raz A., Favia G., Ricci I., Zakeri S. Identification of the midgut microbiota of An. stephensi and An. maculipennis for their application as a paratransgenic tool against malaria. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Manfredini F., Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009;5:1–10. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eappen A.G., Smith R.C., Jacobs-Lorena M. Enterobacter-activated mosquito immune responses to Plasmodium involve activation of SRPN6 in Anopheles stephensi. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaio Ade O., Gusmao D.S., Santos A.V., Berbert-Molina M.A., Pimenta P.F., Lemos F.J. Contribution of midgut bacteria to blood digestion and egg production in Aedes aegypti (Diptera: culicidae) (L.) Parasites Vectors. 2011;4:105. doi: 10.1186/1756-3305-4-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeano-Castaneda Y., Bascunan P., Serre D., Correa M.M. Trans-stadial fate of the gut bacterial microbiota in Anopheles albimanus. Acta Trop. 2020;201:105204. doi: 10.1016/j.actatropica.2019.105204. [DOI] [PubMed] [Google Scholar]
- Groth I., Schumann P., Weiss N., Martin K., Rainey F. Agrococcus jenensis gen. nov., sp. nov., a new genus of Actinomycetes with diaminobutyric acid in the cell wall. Int. J. Syst. Bacteriol. 1996;46:234–239. doi: 10.1099/00207713-46-1-234. [DOI] [PubMed] [Google Scholar]
- Guegan M., Martin E., Valiente Moro C. Comparative analysis of the bacterial and fungal communities in the gut and the crop of Aedes albopictus mosquitoes: a preliminary study. Pathogens. 2020;9 doi: 10.3390/pathogens9080628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusmao D., Santos A., Marini D., de Souza Russo E., Dias Peixoto A., Bacci Júnior M. First isolation of microorganisms from the gut diverticulum of Aedes aegypti (Diptera: Culicidae): new perspectives for an insect-bacteria association. Mem. Inst. Oswaldo Cruz. 2007;102:919–924. doi: 10.1590/s0074-02762007000800005. [DOI] [PubMed] [Google Scholar]
- Hanson S.M., Craig G.B., Jr. Aedes albopictus (Diptera: Culicidae) eggs: field survivorship during northern Indiana winters. J. Med. Entomol. 1995;32:599–604. doi: 10.1093/jmedent/32.5.599. [DOI] [PubMed] [Google Scholar]
- Kalappa D.M., Subramani P.A., Basavanna S.K., Ghosh S.K., Sundaramurthy V., Uragayala S. Influence of midgut microbiota in Anopheles stephensi on Plasmodium berghei infections. Malar. J. 2018;17:385. doi: 10.1186/s12936-018-2535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitrayapong P., Baimai V., O'Neill S.L. Field prevalence of Wolbachia in the mosquito vector Aedes albopictus. Am. J. Trop. Med. Hyg. 2002;66:108–111. doi: 10.4269/ajtmh.2002.66.108. [DOI] [PubMed] [Google Scholar]
- Lee J.M., Yek S.H., Wilson R.F., Rahman S. Characterization of the Aedes albopictus (Diptera: Culicidae) holobiome: bacterial composition across land use type and mosquito sex in Malaysia. Acta Trop. 2020;212:105683. doi: 10.1016/j.actatropica.2020.105683. [DOI] [PubMed] [Google Scholar]
- Mali S., Mitchell M., Havis S., Bodunrin A., Rangel J., Olson G. A proteomic signature of dormancy in the Actinobacterium micrococcus luteus. J. Bacteriol. 2017;199 doi: 10.1128/JB.00206-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi J., Sato T., Weightman A., Martin T., Fry J., Hiom S. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 1998;64:795–799. doi: 10.1128/aem.64.2.795-799.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh C.P., Hanny P.A. Records of Aedes albopictus, Ae. aegypti and Ae. triseriatus from the U.S. Air Force ovitrapping program--1989. J. Am. Mosq. Control Assoc. 1990;6:549–551. [PubMed] [Google Scholar]
- Meister S., Kanzok S., Zheng X., Luna C., Li T., Hoa N. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. PNAS. 2005;102:11420–11425. doi: 10.1073/pnas.0504950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohlmann T.W.R., Vogels C.B.F., Goertz G.P., Pijlman G.P., Ter Braak C.J.F., Te Beest D.E. Impact of gut bacteria on the infection and transmission of pathogenic arboviruses by biting midges and mosquitoes. Microb. Ecol. 2020;80:703–717. doi: 10.1007/s00248-020-01517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro V.V.S., Navegantes-Lima K.C., de Lemos A.B., da Silva G.L., de Souza Gomes R., Reis J.F. Aedes-chikungunya virus interaction: key role of vector midguts microbiota and its saliva in the host infection. Front. Microbiol. 2019;10:492. doi: 10.3389/fmicb.2019.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muturi E.J., Dunlap C., Caceres C.E. Microbial communities of container aquatic habitats shift in response to Culex restuans larvae. FEMS Microbiol. Ecol. 2020;96 doi: 10.1093/femsec/fiaa112. [DOI] [PubMed] [Google Scholar]
- Muturi E.J., Dunlap C., Ramirez J.L., Rooney A.P., Kim C.H. Host blood-meal source has a strong impact on gut microbiota of Aedes aegypti. FEMS Microbiol. Ecol. 2019;95 doi: 10.1093/femsec/fiy213. [DOI] [PubMed] [Google Scholar]
- Pumpuni C., Demaio J., Kent M., Davis J., Beier J. Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am. J. Trop. Med. Hyg. 1996;54:214–218. doi: 10.4269/ajtmh.1996.54.214. [DOI] [PubMed] [Google Scholar]
- Rani A., Sharma A., Rajagopal R., Adak T., Bhatnagar R.K. Bacterial diversity analysis of larvae and adult midgut microflora using culture-dependent and culture-independent methods in lab-reared and field-collected Anopheles stephensi-an Asian malarial vector. BMC Microbiol. 2009;9:96. doi: 10.1186/1471-2180-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolari F., Sandionigi A., Carlassara M., Bruno A., Casiraghi M., Bonizzoni M. Exploring changes in the microbiota of Aedes albopictus: comparison among breeding site water, larvae, and adults. Front. Microbiol. 2021;12:624170. doi: 10.3389/fmicb.2021.624170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabourn P., Spafford H., Yoneishi N., Medeiros M. The Aedes albopictus (Diptera: Culicidae) microbiome varies spatially and with Ascogregarine infection. PLoS Neglected Trop. Dis. 2020;14 doi: 10.1371/journal.pntd.0008615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane J.L., Grogan C.L., Cwalina C., Lampe D.J. Blood meal-induced inhibition of vector-borne disease by transgenic microbiota. Nat. Commun. 2018;9:4127. doi: 10.1038/s41467-018-06580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainchum K., Dupont C., Chareonviriyaphap T., Jumas-Bilak E., Bangs M.J., Manguin S. Bacterial microbiome in wild-caught Anopheles mosquitoes in Western Thailand. Front. Microbiol. 2020;11:965. doi: 10.3389/fmicb.2020.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thavara U., Tawatsin A., Pengsakul T., Bhakdeenuan P., Chanama S., Anantapreecha S. Outbreak of chikungunya fever in Thailand and virus detection in field population of vector mosquitoes, Aedes aegypti (L.) and Aedes albopictus Skuse (Diptera: Culicidae) Southeast Asian J. Trop. Med. Publ. Health. 2009;40:951–962. [PubMed] [Google Scholar]
- Tiawsirisup S., Kaewthamasorn M. The potential for Aedes albopictus (Skuse) (Diptera: Culicidae) to be a competent vector for canine heartworm, Dirofilaria immitis (Leidy) Southeast Asian J. Trop. Med. Publ. Health. 2007;38:208–214. [Google Scholar]
- Tiawsirisup S., Platt K.B., Evans R.B., Rowley W.A. Susceptibility of Ochlerotatus trivittatus (Coq.), Aedes albopictus (Skuse), and Culex pipiens (L.) to West Nile virus infection. Vector Borne Zoonotic Dis. 2004;4:190–197. doi: 10.1089/vbz.2004.4.190. [DOI] [PubMed] [Google Scholar]
- Tiawsirisup S., Platt K.B., Evans R.B., Rowley W.A. A comparision of West Nile virus transmission by Ochlerotatus trivittatus (COQ.), Culex pipiens (L.), and Aedes albopictus (Skuse) Vector Borne Zoonotic Dis. 2005;5:40–47. doi: 10.1089/vbz.2005.5.40. [DOI] [PubMed] [Google Scholar]
- Tiawsirisup S., Sirijutalak J., Sondang M., Supol M., Ansusinha N., Tuanudom R. A preliminary study on diversity of midgut microbiota in Aedes aegypti (Linnaeus) and Culex quinquefasciatus (Say) collected from Bangkok, Thailand. Thai. J. Vet. Med. 2018;48:613–622. [Google Scholar]
- Tiawsirisup S., Sripatranusorn S., Oraveerakul K., Nuchprayoon S. Distribution of mosquito (Diptera: Culicidae) species and Wolbachia (Rickettsiales: Rickettsiaceae) infections during the bird immigration season in Pathumthani province, central Thailand. Parasitol. Res. 2008;102:731–735. doi: 10.1007/s00436-007-0825-z. [DOI] [PubMed] [Google Scholar]
- Tuanudom R., Yurayart N., Tiawsirisup S. Effects of Chikungunya virus titers in blood meals on virus infection, dissemination, and transmission in Asian tiger mosquito: Aedes albopictus (Diptera: Culicidae) Thai. J. Vet. Med. 2017;47:233–240. [Google Scholar]
- Wu P., Sun P., Nie K., Zhu Y., Shi M., Xiao C. A gut commensal bacterium promotes mosquito permissiveness to arboviruses. Cell Host Microbe. 2019;25:101–112. doi: 10.1016/j.chom.2018.11.004. e105. [DOI] [PubMed] [Google Scholar]
- Yadav K.K., Datta S., Naglot A., Bora A., Hmuaka V., Bhagyawant S. Diversity of cultivable midgut microbiota at different stages of the Asian tiger mosquito, Aedes albopictus from Tezpur, India. PLoS One. 2016;11 doi: 10.1371/journal.pone.0167409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurayart N., Kaewthamasorn M., Tiawsirisup S. Vector competence of Aedes albopictus (Skuse) and Aedes aegypti (Linnaeus) for Plasmodium gallinaceum infection and transmission. Vet. Parasitol. 2017;241:20–25. doi: 10.1016/j.vetpar.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu X., Liu S. Agrococcus terreus sp. nov. and Micrococcus terreus sp. nov., isolated from forest soil. Int. J. Syst. Evol. Microbiol. 2010;60:1897–1903. doi: 10.1099/ijs.0.013235-0. [DOI] [PubMed] [Google Scholar]
- Zouache K., Raharimalala F., Raquin V., Tran-Van V., Raveloson L., Ravelonandro P. Bacterial diversity of field-caught mosquitoes, Aedes albopictus and Aedes aegypti, from different geographic regions of Madagascar. FEMS Microbiol. Ecol. 2011;75:377–389. doi: 10.1111/j.1574-6941.2010.01012.x. [DOI] [PubMed] [Google Scholar]
- Zouache K., Voronin D., Tran-Van V., Mavingui P. Composition of bacterial communities associated with natural and laboratory populations of Asobara tabida infected with Wolbachia. Appl. Environ. Microbiol. 2009;75:3755–3764. doi: 10.1128/AEM.02964-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoure A.A., Sare A.R., Yameogo F., Somda Z., Massart S., Badolo A. Bacterial communities associated with the midgut microbiota of wild Anopheles gambiae complex in Burkina Faso. Mol. Biol. Rep. 2020;47:211–224. doi: 10.1007/s11033-019-05121-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.